___________
The Secretary is charged to periodically update the essential health benefits (EHB) and to report to Congress and the public whether enrollees have had difficulty accessing needed services, whether advances in medicine and scientific evidence need to be accounted for, and whether updates will increase costs relative to actuarial limitations. With these considerations in mind, the committee sets a goal for the EHB package to become more fully evidence-based, specific, and value-promoting over time. To ensure that updates to the EHB do not accelerate health-spending growth beyond medical inflation, the committee recommends that any changes to the EHB be no more expensive than the actuarially equivalent future year cost of the base-year package. Further, to preserve the intent of Congress in extending coverage for a basic set of benefits to most Americans, the committee recommends that the Secretary, in collaboration with others, develop a strategy to restrain health care spending. A standing multidisciplinary National Benefits Advisory Council (NBAC) is also proposed to be established to advise the Secretary on updating the EHB.
The coming decades will bring advances in medical science, the emergence of new health problems, changes in the way health care services are delivered, changes in the way existing technologies are used, and new insights into how to help patients manage their health problems more effectively. As the environment in which health care services are delivered changes, the EHB will also have to change in order to continue to facilitate access to quality care for a broad population. Responsible stewardship of public funds must be a key consideration during updates to the EHB. Difficult choices will have to be made about the categories and specific services that are eligible for coverage under the EHB. In developing its recommended approach to updating, the committee was guided by the requirements in the Patient Protection and Affordable Care Act’s (ACA’s) charge for the Secretary to report to Congress and the public.
ACA DIRECTION TO THE SECRETARY ON UPDATING THE EHB
The Secretary is charged to periodically update the EHB to address any gaps in access to coverage or changes in the evidence base that the Secretary identifies during the review of the EHB for the mandated Department of
Health and Human Services (HHS) report to Congress and the public.1 Section 1302 of the ACA requires that the report contain
• An assessment of whether enrollees are facing any difficulty accessing needed services for reasons of coverage or cost;
• An assessment of whether the EHB need to be modified or updated to account for changes in medical evidence or scientific advancement;
• Information on how the EHB will be modified to address any such gaps in access or changes in the evidence base; and
• An assessment of the potential of additional or expanded benefits to increase costs and the interactions between the addition or expansion of benefits and reductions in existing benefits to meet actuarial limitations.2
In Chapter 7, the committee recommends developing a framework to guide the data collection and research infrastructure necessary to identify problems with access and advances in science (the first two required elements of the report to Congress). The information developed through this recommendation provides the starting point for the process of updating the EHB. In this chapter, the committee recommends an approach to updating the EHB, using criteria discussed earlier. The committee also recommends an approach to incorporating costs into the update process (responding to the fourth bullet in the report to Congress).
In its deliberations, the committee recognized that Congress intended the EHB to be similar in structure to existing employer benefit packages. However, the committee believes that over time, the Secretary will have an opportunity to provide leadership through the EHB updating process to improve the content and structure of the EHB to better reflect the scientific evidence base, to reflect societal priorities in providing a basic set of benefits, to ensure greater clarity about what services are and are not eligible for coverage in those policies covered by the EHB definition, and to promote high-value utilization.
Evidence-Based Science Should Be the Guiding Force
The committee concluded that the scope of benefits eligible for coverage should be guided by scientific evidence about which screening, diagnosis, treatment, management, and monitoring interventions are effective in improving or maintaining people’s health and functioning. For example, physician specialty societies, independent research organizations, health plans, and other organizations that focus on particular health problems increasingly incorporate the results of scientific studies into their treatment and payment guidelines. The Institute of Medicine (IOM) has recently released reports on strengthening standards for developing trustworthy clinical guidelines and systematic reviews, and the committee believes that these standards should inform the way medical evidence is used to shape future iterations of the EHB (IOM, 2011a,b). This means that the EHB would make eligible for coverage those interventions that are effective and would not make eligible for coverage those aspects of care that have not been shown to be effective. In Chapters 2 and 3, the committee discusses application of hierarchies of evidence in defining the scope of benefit inclusions and the need to strengthen medical practice to be more evidence-based.
Greater Specificity Required in Defining the EHB
If the EHB are to be guided by scientific evidence in the future, the definitions and descriptions of what is included and excluded must become more specific, consistent with the way scientific evidence is structured. There
1 Patient Protection and Affordable Care Act of 2010 as amended. § 1302(b)(4)(H), 111th Cong., 2d sess.
2 § 1302(b)(4)(G)(i)-(iv).
are few methods of diagnosis or treatment that are either always or never effective. More commonly, interventions such as medications, surgeries, or screening tests are investigated for their effectiveness with specific groups of patients such as those with a particular diagnosis (e.g., hypertension), a certain level of disease severity (e.g., Class IV heart failure), gender or age (e.g., women over 50), and so on. Thus, the committee concluded that as the EHB evolve, greater specificity than is typical of evidence of coverage documents today will be required along with more transparency in clinical policies.
Most health insurance plan documents today provide general statements about what is covered (e.g., ambulatory services) and what is not covered (e.g., experimental treatments). Increased specificity in defining the EHB (inclusions and exclusions) should be designed to reduce uncertainty for patients and doctors about the likelihood that insurance coverage will be available for a course of diagnosis or treatment (vs. the patient being responsible for paying for a selected therapeutic option).
The committee considered several options for improving the degree of specificity to guide the contents of the EHB package and data collection over time: having the Secretary make an increasingly more specific list of included services, making a more specific list of exclusions and nonessential services, and leaving inclusions more general and dependent on insurers’ publication of clinical policies and the application of medical necessity. These options are not mutually exclusive. With regard to specificity on inclusions, the Oregon prioritized list, organized by condition-treatment pairs (e.g., medical therapy for hypertensive disease), is the only one the committee encountered with a high degree of specificity of services that matches the way scientific evidence is structured (Oregon Health Services Commission, 2011). In general, the committee believes that the EHB should evolve toward the level of specificity characterized by the Oregon approach. Additionally, as discussed in Chapter 7, health plans should provide greater specificity and transparency around clinical policies and the operation of medical necessity determination processes.
Structure of Benefits Should Promote High-Value Utilization
Finally, the committee concluded that the financial structure of benefit packages should be consistent with and reinforce the use of high-value, necessary care. Today, it is common for policies to treat all interventions (e.g., medication) within the same cost structure (e.g., co-payments relate to whether the drug is generic or name brand but may not make distinctions between medications for different conditions). The use of value-based insurance design has been growing, and the committee believes that this trend should be incorporated into future EHB packages. The principle of value-based insurance design is to provide financial incentives to encourage people to use what is effective and to discourage people from using services when they are not effective. For example, research has demonstrated that cost sharing is associated with lower utilization of services than no cost sharing (Newhouse and the Insurance Experiment Group, 1993). This is suitable if one is seeking to control utilization, but these incentives affect both appropriate and inappropriate uses of services similarly. Thus, if a service is critical to maintain health (e.g., medications for the treatment of high blood pressure), a policy that creates a disincentive for appropriate use may result in poor adherence to a high-value intervention. Value-based insurance design would reduce or eliminate cost sharing in these instances in order to promote high levels of adherence to the use of necessary medications; in many instances, improved adherence to routinely required medications and other services may contribute to avoiding high cost services such as hospitalization. In implementing value-based insurance design packages, insurers will have to comply with the actuarial value limits specified in the ACA. In this regard, it is important that computation of actuarial value not impede inclusions of some services with high cost sharing (as opposed to excluding them).
Table 9-1 illustrates how these three principles for updating could be incorporated into future versions of the EHB as implemented by health plans in response to guidance from the Secretary. The goals are that over time, the package becomes more evidence-based, defined with greater specificity, and constructed to encourage the use of high-value care. Plans could further these goals by including incentives for consumers to engage in other healthy behaviors.
Recommendation 4a: Beginning in 2015, for implementation in 2016 and annually thereafter, the Secretary should update the EHB package, with the goals that it becomes more fully evidence-based, specific, and value-promoting.
|
|
|
| Scope of Benefits Eligible for Coverage in Typical Policies Today | Illustration of How Scope of Benefits Might Be Described in Future Policies |
|
|
|
| Ambulatory care | Annual visit to a primary care or specialty physician for monitoring of hypertension |
| Prescription drugs | Antihypertensive medications for persons with an established diagnosis of hypertension |
| Cost sharing: $10 for generic, $25 for name brand medications, and $20 for an office visit | No cost sharing for annual visit |
| No co-payment for generic medications for treatment of hypertension for patients with JNC stage 1 hypertension or higher | |
|
|
|
NOTE: JNC = Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.
CONSIDERING TYPICAL EMPLOYER IN THE FUTURE
Section 1302 of the ACA also requires that the Secretary of HHS’ report to Congress on revising benefits contain a certification from the Chief Actuary of the Centers for Medicare & Medicaid Services (CMS) indicating the essential benefits are equal to the scope of benefits provided under a typical employer plan.3 The committee’s first recommendation includes guidance that the Secretary interpret “typical employer” as a small employer. Because the EHB will be incorporated into individual and small group policies both in and out of the exchanges, the typical small employer plan will be defined by the EHB, making an independent reference to typical employer less meaningful over time. As referenced in Chapter 1, how employers respond to the new options available through the ACA could change the way insurance is offered. In addition to employers, insurers may change their behavior and practices in response to experience with the exchanges and the EHB. It will be important to monitor the changes in insurer behavior and plan offerings as part of the monitoring recommended in Chapter 7. Further, states in the future will have the option to open exchanges to businesses of all sizes, and firms that choose to offer insurance through an exchange will have those policies defined by the EHB. Because the method for updating recommended by the committee is designed to keep the costs of the EHB in line with the expected growth in premiums for small employers, the committee believes that the Chief Actuary will have to rely primarily on the cost of the package to certify that the updated EHB is consistent with the intent of the ACA. The committee believes that in going forward, the cost of the EHB should continue to be built on the scope of benefits and premiums of small employers.
METHODS FOR INCORPORATING COSTS INTO UPDATES TO THE EHB
The committee concluded that the updating process must explicitly consider the cost of the EHB and its potential to escalate over time, whether due to technological advances or other medical price increases. The alternative would have been for the committee to recommend that the Secretary not address cost issues in making changes to the EHB over time. The committee believes that it is unacceptable for the Secretary to ignore the costs associated with proposed updates to the EHB. This section explores various options considered and selected for updating to maintain an affordable and sustainable premium level.
In considering how the costs associated with the EHB could be incorporated into updates, the committee discussed a number of design choices: the level at which costs would be considered, the unit by which costs are characterized, the way in which cost information would be used, the approach to enforcement, and the mechanism for achieving any cost target. The pros and cons of each of these are discussed in turn along with the committee’s understanding of the current authority available to the Secretary.
3 § 1302(b)(2)(A)-(B).
Level of Consideration of Costs
The committee considered three potential levels at which costs could be incorporated into decisions in subsequent years: (1) federal, (2) state, or (3) health plan.
At the federal level, costs would be considered at an aggregate level—without explicit efforts to address regional variation in wages, prices, population characteristics; variations in the intensity and use of technology; and other potential cost drivers. For example, the Secretary could obtain an actuarial estimate from the CMS Chief Actuary of the expected cost to purchase the EHB package in the coming year based on a national standard population,4 with explicit assumptions about the degree of medical management, network configuration, and other factors typically included in pricing plans for employers. The committee believes that taking cost into account at a national average level is analogous to the strategy used for setting the initial EHB and is within the purview of the Secretary.
If cost was considered at the state level, differences in the cost structure of health care delivery in states would have to be explicitly addressed (Branscome, 2011). For example, an actuarial estimate could be obtained for the cost of offering the EHB in each state based on a standard population that reflects the characteristics of state residents that are eligible to purchase policies defined by the EHB. The committee believes that an assessment of costs at the state level is consistent with the required guidance from the Secretary about rates of premium growth considered to be excessive (HHS, 2010). The committee also concluded that few states today have undertaken such assessments and that it might be difficult for most states to conduct this work.
If costs were considered at the health plan level, estimates would have to be derived for plans within states or for types of plans within states. These cost estimates would be designed to make explicit how tradeoffs are made among the prevailing costs of care, comprehensiveness of benefits, medical management, network design, and other factors. Plans might be asked to produce packages to offer on the exchanges that fall within the cost guidance and the required EHB design. Alternatively, the Secretary might consider a competitive bidding process similar to that conducted under Medicare Part D. The committee noted that the Secretary does not currently have clear authority to direct or influence pricing of plans, with the possible exception of plans that might be offered in a national or federally directed exchange.
The committee concluded that costs should be incorporated into updates at the federal level consistent with the level at which the EHB are defined in the committee’s first recommendation in this report.
Unit at Which Costs Are Characterized
The committee considered three possible ways in which costs might be characterized: (1) estimated premiums, (2) total federal spending (by category), or (3) rates of change.
The use of premiums as a budget mechanism to incorporate costs into updates is consistent with the committee’s recommended method for establishing the initial health benefits package. Estimated premiums are also one factor incorporated into the cost estimates produced by the Congressional Budget Office (CBO) during debate over the ACA.5 Premiums are the unit by which costs are characterized for most purchasers, whether employers or individuals. Premiums are a familiar way to express the average expected cost of a package of benefits being offered and are the “price” at which comparative shopping for plans takes place. There are, of course, many factors (e.g., benefit design, population) besides the EHB that go into actuarial calculations of premiums; the committee determined that all of these inputs should be accounted for to inform policy decisions around incorporating costs into the updates.
Another option the committee considered was to use the federal budget for subsidies and for Medicaid spending as the unit of cost focus. Similar to premiums, the total cost to the federal government was a significant element of the debate over the law, and design choices were made in order to achieve an overall spending target set by the
4 The national standard population in this case would be those eligible to purchase policies defined by the EHB, that is, the individual and small group market. This is the group that the RAND microsimulation used for its analyses (Eibner et al., 2010).
5 The others being the expected number of newly insured and their distribution by type of insurance, including the number eligible for federal subsidies (CBO, 2009).
President and Congress. This federal budget amount accounts both for the unit costs (e.g., premiums) of health insurance and for the number of people opting for Medicaid or public subsidies for private insurance. The committee concluded, however, that because the EHB definition is just one component of determining the total federal budget amount for subsidies and Medicaid, this is a less useful way of characterizing costs in updating the EHB.
A third option is to use the rates of increase in health care spending or in premiums in incorporating costs into updates to the EHB package. Because the growth in premium prices has exceeded growth in both the purchasing power of the individual (wages) and the economy (gross domestic product, GDP), health care spending is consuming an increasing share of economic output. To control total costs to firms, employers have introduced new products such as high-deductible health plans and other methods for increasing the share of costs paid by individual employees, have changed provider network design, and have reduced the scope of benefits (Claxton et al., 2010).
Within this option, the committee noted that the Secretary could either use information about trends to establish the new cost target within which the scope of benefits would be defined or establish targets around the rate of growth in premiums that are designed to help slow or reverse the growth in health spending (e.g., limiting the allowable growth of premiums to the growth in GDP plus 1 percentage point). In other parts of the ACA, Congress called for ways to address health spending growth. For example, the ACA requires that between 2014 and 2018 adjustments be made to the calculation of premium subsidies that reflect the relative change in premiums compared to changes in household income (known as the regular indexing approach).6 After 2018, an additional adjustment factor is included that accounts for the excess in premium growth compared to the growth in the Consumer Price Index in urban areas.7,8 These indexing methods are designed to ensure that the intent of the original subsidy structure is maintained. The report that the Secretary must deliver to Congress annually includes the impact of changes in the EHB on costs as well as the interaction between additions or expansions to the EHB and concomitant reductions in existing benefits in order to meet actuarial limits required by the law. The ACA also established an Independent Payment Advisory Board (IPAB) to identify mechanisms for keeping the growth in Medicare spending linked to the growth of the economy (GDP plus 1 percentage point).8
The committee noted that the Secretary could consider trends on an annual basis or within a longer timeframe but ultimately recommends an annual update. The committee concludes that premiums should be the way in which costs are characterized and that the Secretary should use the estimated premium required to buy the current year package in the next year as the budget constraint within which updates to the EHB are evaluated. In Recommendation 1, the committee recommends that the contents of the initial benefit package be constructed within the national average premium for a silver-level plan. Updates to premiums should account for trends in medical prices, utilization, new technologies, and population characteristics.
Use of Cost Information
The committee considered three ways in which information about cost could be used to inform updates to the EHB package: (1) for guidance, (2) to establish voluntary goals, or (3) as a binding constraint.
If cost information were used for guidance, the Secretary might issue an annual report noting the increased cost to purchase the EHB package and the cost implications of proposed changes to the package of benefits. There would be no explicit incorporation of costs into updates; changes would be based solely on evidence about the effectiveness of new categories of service. The Secretary could provide some guidance about how changes in the EHB package (e.g., the addition of a new category of coverage or a new set of services within an existing category) would affect changes in the premiums, total costs, or rates of change in health care spending.
Using cost information to establish voluntary goals would encourage states and health plans to take action to achieve the goal. For example, the Secretary could justify changes to the EHB in terms of a desire to voluntarily keep premium prices within some limit, to ensure federal spending is capped at a particular level, or to maintain a predetermined rate of growth in health care spending. Voluntary goals could be set for states or for health plans.
6 § 1401 adding § 36B(b)(3)(A)(ii)(I) to the Internal Revenue Code.
7 § 1401 adding § 36B(b)(3)(A)(ii)(II) to the Internal Revenue Code.
8 § 3403(c)(6)(C)(ii), amending § 1899 of the Social Security Act.
Voluntary goals have been used many times in the past in an effort to avoid more directed federal action in the area of cost containment. These efforts have largely been unsuccessful in achieving goals in the long run, although they have slowed growth of spending in the short run (Block et al., 1987; Raphaelson and Hall, 1978).
Using cost information as a binding constraint would be done in the context of authority to enforce a consideration of cost in future updates to the EHB package. In this case, the Secretary would impose a cost target as part of the update, and changes to the benefit package would have to fall within the established target. The target could range from one that reflects the current trend in medical spending to one that seeks to decelerate the rate of growth. The impact on the scope of the EHB depends on the extent to which the selected cost target is lower than general medical cost trends. For example, if medical cost trends are increasing at 8 percent annually and the Secretary were to set a goal of a 6 percent increase, then the EHB would have to be scaled back to achieve the target. In this case, a binding constraint should not be interpreted to mean that the Secretary is setting premium prices in local markets but rather that the actuarial price of updates to the EHB cannot exceed a pre-established target.
The committee has concluded that costs should be a binding constraint on updates to the EHB but that the cost target should be linked to the rate of premium increases so as not to create a relative disadvantage for the EHB. The committee recognizes that using cost as a binding constraint is challenging and later suggests some mechanisms to assist the Secretary in implementing this approach.
Approaches to Enforcement
The discussion around the use of cost information then led the committee to consider approaches to enforcement, including none, incentives (rewards), penalties, and a binding constraint.
Guidance and voluntary goals do not require enforcement. These approaches rely on the shared willingness of other actors in the health care system to pursue a joint set of goals; all actors means health insurers, hospitals, physicians, other care providers, manufacturers of drugs and devices, regulators, purchasers, employer sponsors, and consumers. The committee agreed that without enforcement, health care spending would continue to increase at a rate that exceeds growth in the economy.
If costs were incorporated at a level other than the federal level, using incentives or rewards would provide some financial benefit to complying with the policy goals. For example, cost-of-living increases could be tied to achieving some target cost growth; this is analogous to the approach taken to public reporting for hospitals under the Medicare “pay-for-reporting” initiative, which was successful in encouraging high rates of participation for a relatively small investment of money. Many pay-for-performance initiatives link annual cost increases to achieving specific performance or activity goals.
A variety of penalties from financial (fines) to restrictions (e.g., no new subsidized enrollees in a plan) could be considered to enforce consideration of cost targets at levels other than the federal level. Two key challenges were identified in this area: the statutory authority currently held by the Secretary and balancing an effective enforcement approach against ensuring an adequate number of plans offered in the market. If too few plans are available as a result of strict enforcement practices or if too few providers are willing to contract with these plans over time, people might encounter difficulties in complying with the individual mandate or in accessing care.
Finally, if the Secretary required that a cost target be a binding constraint on updates to the EHB at the federal level, this would constitute an effective enforcement mechanism. The committee concluded that this approach is the preferred strategy given its focus on incorporating costs at the federal level although the committee recognizes the political and technical challenges in enforcing this constraint. Further, the committee notes that under this approach the constraint is applied only to the contents of the EHB package.
Mechanism for Achieving the Cost Target
The committee considered three mechanisms by which cost targets could be achieved: (1) no mechanism; (2) change only what is included in the essential health benefits; or (3) allow factors other than the scope of EHB to be adjusted.
The first approach acknowledges that for certain options, no mechanism to achieve the target is required.
The second approach is to focus on making adjustments to the EHB that are “cost neutral.” As updates are considered, in order for a new benefit area or new services to be added within an existing area, some other benefit or service would have to be reduced or eliminated to produce a package that falls within the cost target. In the future, the Secretary may be asked to consider categories other than those initially covered. Such inclusions would have to be considered within the context of the budget constraint. This approach is intended to be consistent with ensuring that the EHB package does not become an ever-expanding entitlement over time, resulting in a price that makes the package increasingly unaffordable. Because a major driver of cost increases is medical technology (Smith et al., 2009), whether new technologies are treated as part of the underlying trend or treated as a new benefit area will significantly affect how much this approach contributes to restraining cost growth. The committee notes that in the next few years, because the EHB is likely to lack specificity, there will be limited opportunities for the Secretary to explicitly consider whether certain new technologies should be included or not. They will likely be included in the general categories that define the EHB at the outset. Going forward, however, the committee intends that the specificity of the EHB will increase such that the Secretary can address the role of new technology more explicitly and require that inclusion of such changes meet the process below. The committee believes that this option is within the authority currently held by the Secretary.
The third approach is to allow all factors that can influence health care costs to be available for achieving the cost target. This would allow levers such as network design, value-based purchasing, medical management, and similar tools to be used to achieve a cost target. While benefit design would be one of those tools (although limited by the actuarial value rules), it would not be the only available option. The committee believes that this approach is most effectively executed at the state and health plan levels and would anticipate that the implementation of the EHB by health plans offering products would incorporate these strategies. Nonetheless, in estimating a national average premium, explicit assumptions will have to be made about benefit design and medical management.
The committee concluded that adjustments to the EHB should be cost neutral, that is, any changes could not result in a package that is more expensive than the estimated cost of the existing package in the next year. Putting the design choices together, the committee recommends that costs be incorporated into updates to the EHB at the federal level, using premiums as the unit at which the costs of the EHB are characterized, using cost information to create a binding constraint on the content of future year packages, enforcing a cost-neutral approach to updates, and achieving the premium target only through changes in the EHB. The committee believes that the public deliberative process described previously in this report should be used to inform priorities for making tradeoffs within the cost constraints.
Recommendation 4b: The Secretary should explicitly incorporate costs into updates to the EHB package.
• The Secretary should obtain an actuarial estimate of the national average premium for a silver-level plan with the existing EHB package in the next year; the estimate will account for trends in medical prices, utilization, new technologies, and population characteristics.
• Any changes to the EHB package should not result in a package that exceeds the actuarially estimated cost of the current package in the next year. A public deliberative process should be used to inform choices about inclusions to or exclusions from the updated package, with specific attention to how inclusion of new benefits could affect the availability of existing covered benefits.
CONSEQUENCES FOR THE EHB AND ACA OF FAILING TO ADDRESS RISING HEALTH CARE COSTS
Congress clearly signaled a concern about the impact of changes to the EHB over time on the costs of the package and on existing coverage by calling these items out as specific required aspects in the annual report to Congress and the public. From the beginning of its deliberations, the committee unanimously agreed that if the country does not address the problem of health care costs growing faster than the GDP, the effectiveness of the ACA in achieving its goal of substantially reducing the number of people without a basic level of health insurance
will be undermined. This is necessary to ensure the integrity of the EHB in the future. The committee offers two concrete illustrations of the problem.
As Figure 9-1 shows, U.S. health care spending is increasing in all sectors at an exponential rate. The Chief Actuary for CMS estimates that the ACA will not result in a deceleration in the spending growth rate, but likely will contribute to a small increase (0.9 percent between 2010 and 2019) (CMS, 2010a).
Rapid growth in health spending by itself would not be a problem if the U.S. economy were growing at the same rate, but this is not the case, as shown in Figure 9-2. Since 1990, the growth in national health care spending has generally exceeded the growth in GDP by 2 to 3 percentage points. The effect of this pattern is that an increasing share of national spending goes to health care, which crowds out spending on other goods and services by individuals, businesses, and governments, such as support for public education, investment in infrastructure (e.g., transportation and utilities), and provision of social services for vulnerable populations.
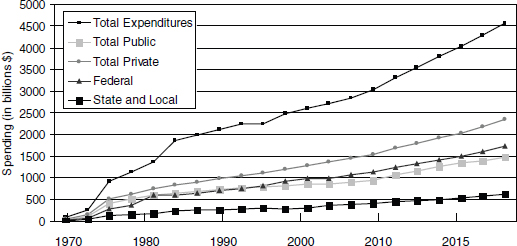
FIGURE 9-1 U.S. health care expenditure trends.
SOURCE: CMS, 2010b.
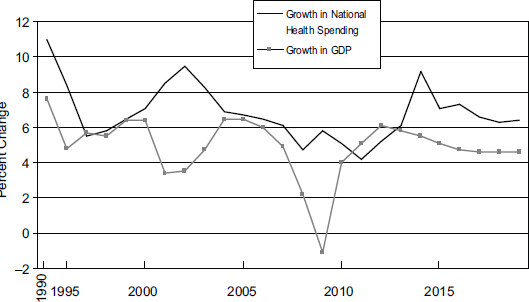
FIGURE 9-2 U.S. national health care spending relative to growth in gross domestic product (GDP).
NOTE: Actual expenditure and GDP figures used 1990-2008; projected figures used 2009-2019.
SOURCE: Adapted from Catlin et al., 2007; CMS, 2010b, 2011.
How does this affect the definition and updating of the EHB? Among other things, it means that each year it will cost relatively more of everyone’s income to purchase the same package of benefits, reducing both individual and social capacity to spend and invest in other areas. The committee’s Recommendation 4b is designed to ensure that the EHB not accelerate the increase in health spending for the 68 million Americans whose terms of purchase are governed by the EHB definition. However, the committee also recognized that the EHB should not be held to a different standard than the rest of the public and private health care sectors—for example, by limiting the growth in spending on the EHB alone. If the committee had recommended that the growth in the estimated average premium to purchase the EHB be held to a lower rate of growth than increases in premiums in all other sectors, the result would have been to make the EHB cover less and less over time—clearly at odds with the intent of Congress.
Because the committee’s Recommendation 4b allows the cost of the EHB to increase with the general increase in health care premiums, this enhances the likelihood of maintaining the initial level of comprehensiveness established by the Secretary. Unfortunately, this also means that the cost of the EHB will likely continue to increase faster than wages and faster than the growth in the economy. In turn, the number of people who will be able to afford to purchase the EHB-defined insurance will decline. As the premiums increase, subsidies will take a larger share of the federal budget. As premiums rise, many more people will choose to enroll in Medicaid, rather than a private plan on the exchange, increasing the strain on state budgets. All of these consequences violate one of the criteria established by the committee: the EHB should be affordable for consumers, employers, and taxpayers. The committee further envisioned that the pressure on federal and state budgets might lead to repeal of the EHB requirement. This threat to the long-term integrity of the EHB caused the committee to consider what could be done in order to mitigate these adverse consequences.
Serious efforts to change the rate of increase in health spending will have to go beyond the definition of essential health benefits—the cost trend cannot be moderated through this mechanism alone. Mindful of its focus on EHB integrity, the committee considered a variety of strategies for addressing this problem and came to some conclusions about desirable attributes of an approach the Secretary might adopt. First, the committee considered whether a Medicare-only strategy for reducing health spending growth might be enough to change the country’s health care spending trajectory. Although Medicare represents a significant portion of the U.S. health market (20 percent) (CMS, 2010b; Martin et al., 2011), Medicare is the only sector that is not directly subject to the EHB, and it is not clear that changes in this segment of the market alone would be enough to affect overall spending. The committee was also concerned that Medicare-only strategies might result in responses in other segments that would include higher rates of increase in prices in the non-Medicare market and changes in the quantity and quality of services offered to all patients, which might undermine the effectiveness of this particular cost control strategy. In fact, in a hearing on the 2011 Medicare Trustees Report, representatives from the American Academy of Actuaries indicated that “when evaluating proposals to improve Medicare’s financial condition, it is important to recognize that improving the sustainability of the health system as a whole requires slowing the growth in overall health spending rather than shifting costs from one payer to another” (American Academy of Actuaries, 2011, p. 2).
The committee then considered whether a federal-only or government-only strategy would be adequate to control health spending and preserve the intended scope of health benefits. Federal sources of spending (Medicare, Medicaid, Department of Defense, Department of Veterans Affairs, Federal Employees Health Benefits Program [FEHBP], Indian Health Service, community health centers, Title X clinics) combined represent a substantial portion of the health care market (27 percent according to the CMS Actuary). State spending, dominated by Medicaid, adds another 16 percent to the overall share accounted for by the public sector (CMS, 2010b; Martin et al., 2011). Although this approach would contribute to changes in a larger portion of the market, the committee expects that adverse effects on prices in the private sector are possible and that the failure to coordinate strategies with the private sector, which is also affected by the EHB, would limit the effectiveness of this approach.
There are a variety of payers and different payment mechanisms in the market today; changes in one payer can affect the others in many different ways including choices made about which plan to enroll in (Medicaid versus exchange), access to providers willing to take patients with different types of insurance, changes in the site of care (hospital vs. office or stand-alone center), changes in the quantity of services ordered, and changes in the quality of care provided. A private-public strategy can ensure that the approaches in each sector are not in conflict with one another.
Having concluded that a strategy that involves all payers is essential to ensure serious attention to the problem of rising health care costs and a set of strategies that would not result in market distortions, the committee considered whether any existing body could take on this charge. None of the examined entities explicitly engages the private sector although IPAB will ultimately make recommendations that relate to the private sector.
Much of the impetus for establishing IPAB was to provide an alternative to congressional deliberation on technical proposals for improving the sustainability of Medicare and this influenced the structure and function of IPAB. IPAB has not yet been constituted and has a full agenda. Without engagement of the private sector in a meaningful way, the committee believes that it is less likely that actions recommended by IPAB for the private sector will be accepted and acted upon. The committee believes that IPAB’s structure (i.e., full-time, federal employees) is not optimal for purposes of engaging the private sector in implementation. Furthermore, IPAB is not required to start making advisory recommendations about spending outside of Medicare until 2015. The committee believes that the legislative language for composition, function, and timeline of activities for IPAB would have to be changed at least in the following ways: (1) have private sector representation, (2) be able to address provider payments before 2020, and (3) have a unified approach addressing both Medicare and the private sector simultaneously (see Ebeler et al., 2011). Because IPAB is seen by some as taking away congressional authority, the committee was concerned that its future might be compromised by ongoing political debate over whether it should exist at all, and the prospect for legislative change is unpredictable at best.
Unless a strategy for containing costs throughout the health care system is adopted, the definition of an essential health benefits package will ultimately fail to achieve congressional intent to establish an appropriate basic package that is affordable and extends coverage to millions.
Recommendation 4c: To ensure over time that EHB-defined packages are affordable and offer reasonable coverage, the Secretary of HHS, working in collaboration with others, should develop a strategy for controlling rates of growth in health care spending across all sectors in line with the rate of growth in the economy.
The committee concluded that any meaningful approach to developing a strategy for controlling cost should include the following attributes: the approach should (1) be nonpartisan, (2) include public and private sector collaboration, (3) integrate activities across all sectors, and (4) be able to ensure action on the recommendations. For example, the Secretary could co-convene a commission with a representative of the private sector experienced in purchasing health services9 to develop and implement meaningful actions to control costs. Because coordinated federal action would increase the likelihood of success in the public sector, the Secretary of HHS could coordinate federal participation in a commission and oversee federal implementation of such a commission’s recommendations.
In summary, while it may appear that addressing the rate of growth in health care spending is beyond the scope of the EHB provisions, the committee views its Recommendations 4b and 4c as necessary complements. The committee’s Recommendation 4b is designed to preserve the scope of benefits over time and to ensure that the EHB package itself will not accelerate the increase in spending by keeping the package equivalent in content. But without making concerted progress in stemming rising health care costs (Recommendation 4c), it will cost more to purchase the same package of benefits each year, eroding the purchasing power of the estimated 68 million people who will depend on EHB coverage. Eventually, the EHB package will become a hollow promise of coverage. The committee’s charge was to develop a viable approach to defining the EHB that would work now and into the future, and this requires a two-pronged approach.
NATIONAL BENEFITS ADVISORY COUNCIL
Having identified a set of recommendations for updating the EHB and addressing the sector-wide challenges with rising health care costs, the committee next considered whether the Secretary would benefit from forming a new advisory group focused on updates to the EHB. The committee refers to this advisory group as the National Benefits Advisory Council (NBAC). Public respondents to questions posed about updates to the EHB often noted
9 This should not be someone who is a delegate of a lobbying organization.
that this activity should not be solely a staff function within HHS because it requires making tradeoffs based on science, values, and diverse viewpoints and doing so in a transparent and fair manner. This led the committee to agree with numerous commenters that prefer a process to provide the Secretary with ongoing, structured external advice, such as through an advisory body. As envisioned by the committee, the advisory group, would be
• Comprised of a diverse group of stakeholders and
• Free from political influences (e.g., by ensuring that benefit decisions are evidence-based).
Public comments suggested two main advisory body approaches: an HHS panel or “an independent, nonpartisan” advisory body. An HHS panel was variously referred to as an oversight body or standing committee (Borelli, 2010; Keller, 2010; Metz, 2010). Before considering whether any existing entities could serve this function, the committee identified the functions of an external advisory committee.
Functions of the NBAC
The NBAC should advise the Secretary on (1) the research framework and scope of the data collection discussed earlier (Chapter 7), (2) updates to the overall benefit package and related benefit design issues, including assessments for the Report to Congress and whether adjustments need to be made for the entry of large employer groups in the exchanges, (3) changes to the premium target, and (4) appropriate mechanisms for evaluating new interventions. The IOM committee considered whether the NBAC should have a role in defining the initial EHB package; although the committee was not opposed to the NBAC having a role, the members thought that it would not be practical to get the NBAC appointed and operational in a timely enough fashion to be useful in the initial definition process. The committee views its own activities as contributing to this initial process and believes the initial EHB package will have to rely more on the scope of the typical employer plan than it will in the future. Additionally, the recommendation for structured public deliberation and making available the actuarial value of possible tradeoffs in rulemaking notices will contribute to a more transparent process during the initial definition of the EHB.
The NBAC approach to updating should conform to the criteria that the committee offers to guide the methods for defining and updating the EHB (Figure S-2 in the Summary). The policy foundations that the committee used to frame its approach reflect the need to find a balance among competing societal goals. The EHB package should ensure that the most vulnerable members of society are protected, the structure of the EHB encourages appropriate use of services, decisions are made on the best available science, available resources are used in the most cost-effective manner, and the process to define and apply decisions about the EHB is fair and transparent. The committee has defined an explicit approach to developing the updated national average premium by incorporating cost into the updating process. This approach is intended to ensure that changes to the EHB are cost-neutral. The NBAC can provide the Secretary guidance on the considerations in updating that premium. Over time, a variety of factors in the private and public health sectors and general economy may require re-examination of the IOM committee’s recommended approach. In addition, the NBAC should ensure that a national public deliberative process is conducted periodically to inform debates on tradeoffs. If state-based public deliberations are widespread, sufficient information may be gathered and there may be no need to duplicate the process.
The NBAC is to focus on what is appropriate for subsidized coverage in insurance programs that incorporate essential health benefits, not what should be covered in every public or private insurance program. Indeed, Section 1302 points out that the ACA does not prohibit a health plan from providing benefits in excess of the EHB if individuals and/or employers choose to pay the additional costs.
Consideration of Existing Entities
Having identified the functions of the external advisory committee, the IOM committee considered a variety of existing entities to see if they might serve this advisory function as summarized in Table 9-2.
The committee concluded that the U.S. Preventive Services Task Force (USPSTF),
Medicare Evidence Development and Coverage Advisory Committee (MEDCAC), and Patient-Centered Outcomes Research Institute (PCORI) would all likely produce scientific information that would be useful in considering updates but that none is constituted to provide the Secretary with the full range of advice required for updating. Thus, the committee recommends that a new body, a National Benefits Advisory Council, be established. The NBAC would be staffed by HHS.
|
|
||
| Existing Entity | Current Function | Ability to Advise Secretary on Updates to the EHB |
|
|
||
| U.S. Preventive Services Task Force (USPSTF) (Healthcare Research and Quality Act of 1999 § 915) | The USPSTF identifies preventive services for which there is adequate scientific evidence to recommend routine inclusion in primary care visits. | The USPSTF undertakes scientific evaluations that are consistent with part of the committee’s criteria for updating. |
| Focus is solely on preventive services; scope would have to be expanded to take on full range of benefits. | ||
| Function is not related to benefit coverage. Meetings are not open, and there is no mechanism for considering tradeoffs (services are evaluated independent of one another). | ||
| Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) (Public Health Service Act § 222) | The MEDCAC reviews and evaluates medical literature, technology assessments, and examines data and information on the effectiveness and appropriateness of medical items and services that are covered under Medicare, or that may be eligible for coverage under Medicare. The MEDCAC judges the strength of the available evidence and makes recommendations to CMS based on that evidence. | The MEDCAC undertakes evaluations of the science base for making coverage decisions for Medicare. The function is consistent with part of what the committee believes should be considered in updates but does not include a mechanism for making tradeoffs across the entire benefit package and would have to be expanded to consider coverage outside of Medicare. |
| Patient-Centered Outcomes Research Institute (PCORI) (Patient Protection and Affordable Care Act [PPACA] § 6301) | PCORI is a public-private body that is tasked with developing research on the comparative effectiveness of alternative approaches to screening, diagnosis, treatment, and monitoring, taking account of patients’ preferences, values, and experiences in order to inform patient decision making. | PCORI is constituted to develop evidence that might inform updates to the EHB but not to consider coverage issues per se. Focus is more on research and patient (or shared) decision making than on the structure of benefit packages. |
| Consumer Operated and Oriented Plan (CO-OP) Program Advisory Board (PPACA § 1322) | CO-OP’s purpose is to foster the creation of qualified nonprofit health insurance issuers to offer qualified health plans (QHPs) in the individual and small group markets in the states in which issuers are licensed to offer such plans. | CO-OP’s focus is on encouraging the development of qualified health plans and not on the benefit packages offered by those plans. Primary advice is on strategies for increasing the number of CO-OP offerings. |
|
|
||
Appointment, Conflict of Interest, and Composition
The IOM committee recommends that the appointment process for membership to this proposed NBAC be coordinated through a nonpartisan entity, such as the Comptroller General of the U.S. Government Accountability Office (GAO). As part of any selection process, consultation could take place with a range of stakeholders on possible nominees, including Congress and the White House, and should include a process for disclosure of any real or perceived conflict of interest related to the EHB, particularly any financial interests and professional or
intellectual biases (Guyatt et al., 2010; IOM, 2011a,b).10 The GAO should take potential conflicts into account in the selection process and attempt to select a council as free of conflicts as possible. The council should be balanced with respect to intellectual and professional biases.
The conflict of interest disclosure process should be repeated at the initial meeting of the NBAC and repeated periodically. Members should not participate as representatives of stakeholder organizations, but rather as independent individuals. In general, members should divest financial investments that could be affected by recommendations, but given the breadth of possible EHB recommendations this may not be possible in all circumstances. Allowances can be made for exceptions, such as when the group is not able to perform its work without members who have a conflict of interest. In all cases, members should recuse themselves from voting on selected topics with specific conflicts.
Various health care advisory bodies are appointed by the GAO because of its own nonpartisan status. It provides Congress with reports, testimonies, correspondence, and legal decisions and opinions (GAO, 2011b). At its head, the Comptroller General of the United States has also been mandated to appoint certain health care—related commissions, advisory groups, and governing boards (GAO, 2011a), and such responsibilities have increased substantially with the passage of the ACA. The GAO is responsible for the appointments of members of six different bodies, including the Medicare Payment Advisory Commission (MedPAC), the Medicaid and CHIP (Children’s Health Insurance Program) Payment and Access Commission (MACPAC), the PCORI Governing Board, the PCORI Methodology Committee, the National Health Care Workforce Commission, and the Consumer Operated and Oriented Plan (CO-OP) Program Advisory Board. The committee intends the NBAC to be advisory to the Secretary, not an operational body.11
The NBAC should be multidisciplinary and balanced, comprising a variety of methodological experts and clinicians, as well as populations expected to be affected. This body will need sufficient expertise to fulfill its functions, including, for example, persons knowledgeable about the concerns of small employers, consumers, health professionals, and the insurance industry and persons with sufficient understanding of technical aspects of the tasks (e.g., economics, health services research, actuarial science). Members’ terms of appointment should be staggered to avoid turnover of the entire council at one time.
Timeline
Annual updates of the scope of benefits in health insurance contracts are the norm. Such updates reflect continuous review processes conducted throughout the year by insurers to consider evidence in the peer-reviewed literature, guidelines, and consensus statements; changes in regulatory agency approvals or in medical protocols that may necessitate a modification in benefits; or other information that is material to the status of a medical technology such as the quantity of use and importance of questions that have arisen regarding specific technologies (McDonough, 2011).
Periodicity
The IOM considered whether an annual update or some other interval was preferable. Respondents to the committee’s online questionnaire offered a variety of suggestions—that HHS establish a “6-month monitoring process” (Wojcik, 2010), or that the review occur annually (Edgington, 2010; Monahan, 2010), every 2 to 3 years (Bocchino, 2010; Keller, 2010; Mahoney, 2010), or as infrequently as every 5 years (Hafner, 2010). On the one hand, the process needs to be responsive to changes in public priorities and advances in clinical medicine—for example, some noted the importance of any time line for review having “sufficient flexibility to incorporate new
10 Recent IOM standards for conflict of interest (COI) regarding clinical practice guideline development include the following elements: disclosure of potential conflicts prior to appointment, disclosure within the convened group, and divesture of financial investments whose interest could be affected by recommendations, but allowances made for exceptions, such as, when the group is not able to perform its work without members who have a COI; chairs or co-chairs should not have a COI (IOM, 2009, 2011a).
11 To be in compliance with the Appointments Clause of the U.S. Constitution. See Buckley v. Valeo, 424 U.S. 1 (1976).
information/breakthroughs” (Bocchino, 2010; Sacco, 2010). On the other hand, insurers, in particular, raised concerns that the frequency of reviews “should be balanced with administrative burdens related to the incorporation of new services in a benefit package” (Bocchino, 2010). “It will take time for insurers to adopt any new changes made by HHS, and consumers have an interest in stable, predictable benefits and costs. Unnecessarily adjusting benefit packages will only serve to increase costs for insurers and consumers” (Kelmar, 2010).
Other respondents suggested that updates correspond to updates in the FEHBP, which uses an annual contracting process. The committee concluded that an annual update was best to be consistent with standard contracting practices. It is not expected that the entire EHB must be updated annually; however, the Secretary would have the flexibility to update whatever should be updated within a reasonable time after finding credible evidence of the need for change.
The FEHBP cycle illustrates the amount of time required between identifying the need for change and getting that change implemented. In the context of updating, it suggests that 2016 may be the earliest year that a revised benefit package would be offered in exchanges, and annually thereafter. Reporting from states on their operations during 2014 will likely not occur until at least 2 months into 2015, leaving the balance of that calendar year for consideration of revisions. Thus, the first year in which the Secretary would have to identify a package revision within an updated premium target is 2016.
The NBAC provides an important forum for a transparent, public dialogue about how best to update the EHB in light of advances in science, information about the trends in health care spending, current societal assessments about the relative importance of newly available interventions compared to the existing set, and results from research about the effects of the current EHB on access to appropriate care.
Recommendation 5: As soon as is feasible, the Secretary should establish a National Benefits Advisory Council (NBAC), staffed by HHS but appointed through a nonpartisan process, such as the Office of the Comptroller General of the United States. The NBAC should
• By January 1, 2013, advise the Secretary on a research plan and data requirements for updating the EHB package;
• Starting in 2015 for implementation in 2016, make recommendations annually to the Secretary regarding (1) any changes to the EHB package by applying the committee’s recommended criteria (see Figure S-2 in the Summary), (2) any changes to the premium target, and (3) any mechanisms that would enhance the evidence base of the EHB package and its potential for promoting value; and
• Advise the Secretary on conducting and using the results of a periodic national public deliberative process to inform its recommendations around updates to the EHB.
The ACA provides for a comprehensive set of categories, within which there are many potential services and items eligible to be deemed essential and thereby qualify for public subsidy of coverage using pooled public resources. A tradeoff exists between the inclusiveness of benefits, and the cost of the insurance product for the consumer and the sustainability of subsidies for the taxpayer. If the appropriate balance between comprehensiveness and affordability is not attained, there are tangible repercussions:
• If the benefits are not affordable, fewer people will get adequate coverage.
• If the benefit design puts excessive impediments to access, people will not get the care they need.
• If health care spending continues to rise disproportionately to GDP, the EHB could end up being substantially cut.
The committee concluded that any determination of scope of the benefit package should be thought about within the context of national, state, and consumer budget constraints and public examination of priorities. The
committee’s aims for the EHB mirror the criteria for assessing the package’s content. The EHB must be affordable, maximize the number of people with insurance coverage, protect the most vulnerable individuals, protect against the greatest financial risks, promote better care, ensure stewardship of limited financial resources by focusing on high value services of proven effectiveness, promote shared responsibility for improving our health, and address the medical concerns of greatest importance to the nation.
American Academy of Actuaries. 2011. Statement of Thomas F. Wildsmith, Vice President, Health Practice Council and Cori E. Uccello, Senior Health Fellow, American Academy of Actuaries at the Committee on Ways and Means, Subcommittee on Health, U.S. House of Representatives Hearing on 2011 Medicare Trustees Report, June 22, 2011. http://waysandmeans.house.gov/UploadedFiles/American_Academy_of_Actuaries622.pdf (accessed September 14, 2011).
Block, J. A., D. I. Regenstreif, and P. F. Griner. 1987. A community hospital payment experiment outperforms national experience. Journal of the American Medical Association 257(2):193-197.
Bocchino, C. 2010. Online questionnaire responses submitted by Carmella Bocchino, Executive Vice President, America’s Health Insurance Plans to the IOM Committee on the Determination of Essential Health Benefits, December 6.
Borelli, C. 2010. Online questionnaire responses submitted by Claire Borelli, Manager, Health Policy, American Diabetes Association to the IOM Committee on the Determination of Essential Health Benefits, December 6.
Branscome, J. M. 2011. Statistical brief #329: State differences in the cost of job-related health insurance, 2010. http://www.meps.ahrq.gov/mepsweb/data_files/publications/st329/stat329.shtml (accessed July 22, 2011).
Catlin, A., C. Cowan, S. Heffler, B. Washington, and the National Health Expenditure Accounts Team. 2007. National health spending in 2005: The slowdown continues. Health Affairs 26(1):142-153.
CBO (Congressional Budget Office). 2009. Letter to the Honorable Evan Bayh, U.S. Senate from Douglas W. Elmendorf, Director, Congressional Budget Office, November 30.
Claxton, G., B. DiJulio, H. Whitmore, J. D. Pickreign, M. McHugh, A. Osei-Anto, and B. Finder. 2010. Health benefits in 2010: Premiums rise modestly, workers pay more toward coverage. Health Affairs 29(10):1942-1950.
CMS (Centers for Medicare & Medicaid Services). 2010a. Memorandum by Richard S. Foster, Chief Actuary, CMS on the estimated financial effects of the “Patient Protection and Affordable Care Act,” as amended. https://www.cms.gov/ActuarialStudies/Downloads/PPACA_2010-04-22.pdf (accessed July 11, 2011).
______. 2010b. National health expenditure projections 2009-2019. http://www.cms.gov/NationalHealthExpendData/downloads/NHEProjections2009to2019.pdf (accessed March 23, 2011).
______. 2011. Table 1: National health expenditures aggregate, per capita amounts, percent distribution, and average annual percent growth: Selected calendar years 1960-2009. https://www.cms.gov/NationalHealthExpendData/downloads/tables.pdf (accessed November 2, 2011).
Ebeler, J., T. Neuman, and J. Cubanski. 2011. The Independent Payment Advisory Board: A new approach to controlling Medicare spending. Menlo Park, CA: Kaiser Family Foundation.
Edgington, S. 2010. Online questionnaire responses submitted by Sabrina Edgington, Program and Policy Specialist, National Health Care for the Homeless to the IOM Committee on the Determination of Essential Health Benefits, December 6, 2010.
Eibner, C., F. Girosi, C. C. Price, A. Cordova, P. S. Hussey, A. Beckman, and E. A. McGlynn. 2010. Establishing state health insurance exchanges: Implications for health insurance enrollment, spending, and small businesses. Santa Monica, CA: RAND Corporation.
GAO (Government Accountability Office). 2011a. Health care advisory committees. http://www.gao.gov/about/hcac/ (accessed July 15, 2011).
______. 2011b. Our products. http://www.gao.gov/about/products/ (accessed July 15, 2011).
Guyatt, G., E. A. Akl, J. Hirsh, C. Kearon, M. Crowther, D. Gutterman, S. Z. Lewis, I. Nathanson, R. Jaeschke, and H. Schnemann. 2010. The vexing problem of guidelines and conflict of interest: A potential solution. Annals of Internal Medicine 152(11):738-741.
Hafner, J. 2010. Online questionnaire responses submitted by Jay Hafner, Clinic Director, Hafner Chiropractic to the IOM Committee on the Determination of Essential Health Benefits, December 2.
HHS (Department of Health and Human Services). 2010. Rate increase disclosure and review. Federal Register 75(246):81004-81029.
IOM (Institute of Medicine). 2009. Conflict of interest in medical research, education, and practice. Washington, DC: The National Academies Press.
______. 2011a. Clinical practice guidelines we can trust. Washington DC: The National Academies Press.
______. 2011b. Finding what works in health care: Standards for systematic reviews. Washington, DC: The National Academies Press.
Keller, K. 2010. Online questionnaire responses submitted by Kate Keller, Senior Program Officer, Health Foundation of Greater Cincinnati to the IOM Committee on the Determination of Essential Health Benefits, December 6.
Kelmar, S. 2010. Online questionnaire responses submitted by Steven Kelmar, Senior Vice President, Government Affairs & Public Policy, Aetna to the IOM Committee on the Determination of Essential Health Benefits, December 6.
Mahoney, K. 2010. Online questionnaire responses submitted by Katie Mahoney, Director, Health Care Regulations, U.S. Chamber of Commerce to the IOM Committee on the Determination of Essential Health Benefits, December 6.
Martin, A., D. Lassman, L. Whittle, A. Catlin, and the National Health Expenditure Accounts Team. 2011. Recession contributes to slowest annual rate of increase in health spending in five decades. Health Affairs 30(1):11-22.
McDonough, R. 2011. Determination of essential health benefits. PowerPoint Presentation to the IOM Committee on the Determination of Essential Health Benefits by Robert McDonough, Director, Clinical Policy Research and Development, Aetna, Washington, DC, January 13.
Metz, R. D. 2010. Online questionnaire responses submitted by R. Douglas Metz, EVP & Chief Health Services Officer, American Specialty Health to the IOM Committee on the Determination of Essential Health Benefits, December 20.
Monahan, C. 2010. Online questionnaire responses submitted by Christine Monahan, Health Policy Advisor, National Partnership for Women & Families to the IOM Committee on the Determination of Essential Health Benefits, December 6.
Newhouse, J. P., and the Insurance Experiment Group. 1993. Free for all? Lessons from the RAND health insurance experiment. Cambridge, MA: Harvard University Press.
Oregon Health Services Commission. 2011. Prioritized list of health services: April 1, 2011. http://www.oregon.gov/OHA/OHPR/HSC/docs/L/Apr11List.pdf (accessed May 10, 2011).
Raphaelson, A. H., and C. P. Hall, Jr. 1978. Politics and economics of hospital cost containment. Journal of Health Politics Policy and Law 3(1):87-111.
Sacco, R. 2010. Online questionnaire responses submitted by Ralph Sacco, President, American Heart Association to the IOM Committee on the Determination of Essential Health Benefits, December 21.
Smith, S., J. P. Newhouse, and M. S. Freeland. 2009. Income, insurance, and technology: Why does health spending outpace economic growth? Health Affairs 28(5):1276-1284.
Wojcik, S. 2010. Online questionnaire responses submitted by Steve Wojcik, Vice President, Public Policy, National Business Group on Health to the IOM Committee on the Determination of Essential Health Benefits, December 6.


















