Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory
________________________
1This document was prepared by the AEGL Development Team composed of Claudia Troxel (Oak Ridge National Laboratory) and Loren Koller and George Woodall (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee concludes that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold levels for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
N,N-Dimethylformamide (DMF) is a clear-to-slightly yellow liquid with a faint amine (fishy) odor. Odor thresholds have been reported to range from 0.47 to 100 ppm. DMF is a polar compound used as a solvent in the manufacturing of many products. American manufacturers consumed 32 million pounds of DMF in 1993 (TURI 2001). The primary end-users of DMF are manufacturers of pharmaceuticals (12 million pounds), electronic components (10 million pounds), butadiene (3 million pounds), and urethanes (3 million pounds). It is also used as a resin cleanup solvent, reaction solvent, and processing solvent in the manufacture of polyimides, optical brightners, semipermeable membranes, and pesticides.
Human data were limited to controlled inhalation exposures or accidental workplace exposures. Although no adverse effects were reported in the controlled studies, these studies were designed to assess DMF metabolism, and follow-up physical evaluations of the volunteers were not carried out. Reports of both accidental and chronic daily workplace inhalation exposures to DMF describe signs and symptoms, including abdominal pain, nausea, and vomiting, and liver toxicity as indicated by elevated serum enzymes and histologic evaluation. Epidemiologic studies suggest a causal association between DMF exposure and testicular germ cell tumors.
Single inhalation exposures of mice and rats to high concentrations of DMF (approaching or at saturation of the chemical in air) resulted in mortality (Stasenkova 1961; Shell Oil Company 1982), and inhalation exposure of rats to low and intermediate concentrations resulted only in alterations of liver enzymes (Brondeau et al. 1983; Lundberg et al. 1986; Roure et al. 1996). The cause of death following acute inhalation exposure was not identified. Repeated inhalation exposure of rats, mice, and cats to DMF generally resulted in reduced body weight, and hepatotoxicity indicated by increased liver enzymes and histopathologic changes including degeneration and necrosis. However, repeated inhalation exposure of monkeys to DMF at 500 ppm for 6 h/day, 5 days per week, for up to 13 weeks failed to result in any measurable adverse effects (Hurtt et al. 1991, 1992). Inhalation developmental toxicity studies reported reduced maternal body weight. Developmental effects included reduced fetal weight; increases in the litter incidence of total external, skeletal, and visceral malformations and skeletal variations; and increased number and percentage of dead implants (BASF 1974a,b,c; Kimmerle and Machemer 1975; BASF 1989; Hellwig et al. 1991; Lewis et al. 1992). Genotoxicity testing of DMF has generally been negative (Antoine et al. 1983; NTP 1992). One study found no evidence of carcinogenicity when mice and rats inhaled DMF up to 400 ppm for 2 years (E.I. Dupont de Nemours & Co. 1992); a more recent study found that chronically inhaled DMF produced hepatocellular adenomas and carcinomas in rats at 400 ppm or 800 ppm, respectively, and hepatoblastomas and hepatocellular adenomas and carcinomas in mice at 200 ppm and above (Senoh et al. 2004).
An AEGL-1 value was not recommended because data pertaining to end points relevant to the AEGL-1 definition were not available.
The AEGL-2 derivation was based on the study in which groups of 15 pregnant Himalayan rabbits were exposed to DMF at 0, 50, 150, or 450 ppm for 6 h/day on gestation days (GD) 7-19 (Hellwig et al. 1991). Over GD 7-19, mean maternal body-weight gain was reduced in dams exposed to DMF at 150 ppm compared with controls, while dams in the 450-ppm group lost weight; mean maternal body-weight gain over the entire study period of GD 0-29 was also decreased in dams from the 150- and 450-ppm DMF groups compared with controls. Developmental toxicity was evident at 450 ppm as increases in external malformations and total malformations (external, soft tissue, and skeletal combined). Other effects included a reduction in fetal weight (86% of controls) and statistically significant increases in the litter incidence of skeletal variations, including splitting of skull bones, fused sternebrae, irregular shaped sternebrae, and bipartite sternebrae. An increase in fetal deaths did not occur. No developmental effects were observed at 150 ppm. To protect against irreversible developmental effects (malformations), the rabbit no-observed-adverse-effect level (NOAEL) of 150 ppm for 6 h was used as the point of departure for derivation of AEGL-2 values (Hellwig et al. 1991).
A total uncertainty factor of 3 was applied to the point of departure of 150 ppm for 6 h: 1 for interspecies variability and 3 for intraspecies variability. An interspecies uncertainty factor of 1 was applied because it appears that primates
are not as sensitive as rodents. Monkeys inhaled DMF at 500 ppm for 6 h/day, 5 days/week, for up to 13 weeks with no measurable adverse effects (parameters examined included clinical signs, body weight, hematology and serum chemistry analyses, urinalysis, semen analysis, and gross necropsy findings). In contrast, subchronic DMF inhalation exposure produced significant hepatic effects in rats at concentrations of 200 ppm (Senoh et al. 2003), 300 ppm (Craig et al. 1984), and 400 ppm (NTP 1992) and in mice at 100 ppm (Senoh et al. 2003), 150 ppm (Craig et al. 1984), and 200 ppm (NTP 1992). Indexes of toxicity after repeated DMF exposure ranged from elevated serum enzymes indicative of liver injury to hepatic degeneration and necrosis. From these exposure data, humans would be expected to be less sensitive than laboratory animals (rodents). Because the mechanism of hepatotoxicity is believed to be related to the metabolism of DMF to a reactive intermediate, fetal toxicity is expected to result from exposure to the parent DMF or metabolites. An oral study assessing the tissue and metabolite distribution of DMF in pregnant rats indicated that DMF and its metabolites were transferred across the placenta by passive diffusion and that maternal plasma, embryo or fetus, placenta, and amniotic fluid belonged to the same compartment (Saillenfait et al. 1997). Therefore, the fetus and placenta will not provide any additional protection or enhancement of DMF toxicity because exposure to DMF and its metabolites will depend on the metabolism by the mother.
An intraspecies uncertainty factor of 10 would normally be applied because a host of interindividual differences could affect the manifestation of DMF toxicity: (1) activity of CYP2E1, an enzyme that plays a pivotal role in the metabolism of DMF to reactive intermediates, can be induced by ethanol consumption, obesity, diabetes, and other lifestyle and genetic factors (Gonzalez 1990; Song et al. 1990; Lucas et al.1998; McCarver et al. 1998), and increased CYP2E1 levels increase the toxic metabolites of DMF; (2) prior consumption of ethanol can exacerbate DMF toxicity in individuals; (3) on the basis of the proposed mechanism of action, detoxification of the reactive intermediate is partly dependent on conjugation with glutathione; therefore, if glutathione levels are depleted for other reasons, the potential exists for greater exposure to the reactive intermediate; and (4) because DMF exposure can result in hepatotoxicity, individuals with chronic liver disease may be at increased risk. However, application of a total uncertainty factor of 10 produces AEGL-2 values that are inconsistent with the available human data. (Values for the 10-min, 30-min and 1-, 4-, and 8-h AEGL-2 using default time-scaling would be 49, 34, 27, 17, and 11 ppm, respectively.) Humans were exposed by inhalation to DMF at 87 ppm for 4 h or at 81 ppm for 2 h to assess the metabolism of DMF (Kimmerle and Eben 1975b; Eben and Kimmerle 1976). These single-exposure studies were conducted to assess DMF metabolism, and no adverse effects were reported; thus, the concentration can be considered an acute exposure concentration unlikely to result in adverse effects in healthy adults. Therefore, the intraspecies uncertainty factor is reduced to 3, resulting in a total uncertainty factor of 3.
The experimentally derived exposure value is scaled to AEGL time frames using the concentration-time relationship given by the equation Cn × t = k, where C = concentration, t = time, k is a constant, and n generally ranges from 1 to 3.5 (ten Berge et al. 1986). The value of n was not empirically derived because of inadequate data; therefore, the default value of n = 1 was used for extrapolating from shorter to longer exposure periods, and a value of n = 3 was used to extrapolate from longer to shorter exposure periods. The 30-min AEGL-2 value was set equal to the 10-min value because of the uncertainty in extrapolating from a 6-h exposure duration to a 10-min duration.
The AEGL-3 derivation was based on the study in which groups of three male and three female rats were exposed to DMF at 3,700 ppm for 1 or 3 h with no mortality, while exposure for 7 h resulted in 83% mortality (Shell Oil Company 1982). Clinical signs were limited to excess grooming in all exposure groups, with lethargy also noted in rats exposed for 7 h. The end point of no mortality in rats exposed at 3,700 ppm for 3 h was chosen for the derivation.
A total uncertainty factor of 10 was applied to the point of departure for the AEGL-3: 1 for interspecies variability and 10 for intraspecies variability. The total uncertainty factor of 10 should protect against all but hypersensitive human hepatotoxic effects. An interspecies uncertainty factor of 1 was applied because it appears that primates are not as sensitive as rodents. Monkeys inhaled DMF at 500 ppm for 6 h/day, 5 days/week, for up to 13 weeks with no measurable adverse effects (parameters examined included clinical signs, body weight, hematology and serum chemistry analyses, urinalysis, semen analysis, and gross necropsy findings). In contrast, subchronic DMF inhalation exposure produced significant hepatic effects in rats at concentrations of 200 ppm (Senoh et al. 2003), 300 ppm (Craig et al. 1984), and 400 ppm (NTP 1992) and in mice at 100 ppm (Senoh et al. 2003), 150 ppm (Craig et al. 1984), and 200 ppm (NTP 1992). Indexes of toxicity after repeated DMF exposure ranged from elevated serum enzymes indicative of liver injury to hepatic degeneration and necrosis. From these exposure data, humans would be expected to be less sensitive than laboratory animals (rodents). An intraspecies uncertainty factor of 10 is applied because a host of interindividual differences could affect the manifestation of DMF toxicity: (1) activity of CYP2E1, an enzyme that plays a pivotal role in the metabolism of DMF, to reactive intermediates, can be induced by ethanol consumption, obesity, diabetes, and other lifestyle and genetic factors (Gonzalez 1990; Song et al. 1990; Lucas et al. 1998; McCarver et al. 1998), and increased CYP2E1 levels increase the toxic metabolites of DMF; (2) prior consumption of ethanol can exacerbate DMF toxicity in individuals; (3) on the basis of the proposed mechanism of action, detoxification of the reactive intermediate is partly dependent on conjugation with glutathione; therefore, if glutathione levels are depleted for other reasons, the potential exists for greater exposure to the reactive intermediate; (4) because DMF exposure can result in hepatotoxicity, individuals with chronic liver disease may be at increased risk. Therefore, a total uncertainty factor of 10 is applied.
The experimentally derived exposure value is scaled to AEGL time frames using the concentration-time relationship given by the equation Cn × t = k, where C = concentration, t = time, k is a constant, and n generally ranges from 1 to 3.5 (ten Berge et al. 1986). The value of n was not empirically derived because of inadequate data; therefore, the default value of n = 1 was used for extrapolating from shorter to longer exposure periods, and a value of n = 3 was used to extrapolate from longer to shorter exposure periods.
There is a high potential for DMF to be absorbed dermally, so this route of exposure should be considered along with inhalation.
The calculated values are listed in Table 1-1 below.
1. INTRODUCTION
DMF is a clear-to-slightly yellow liquid with a faint amine (fishy) odor. It can be synthesized in a one-stage process by reacting dimethylamine in methanol with carbon monoxide in the presence of sodium methylate or with metal carbonyls; it also can be synthesized in a two-stage process from reacting methanol with carbon monoxide in the presence of sodium methylate, followed by reaction with dimethylamine (IARC 1989). DMF is a polar compound used as a solvent in manufacturing acrylic fibers, films, surface coatings, synthetic leather, polyurethane, and wire enamels based on polyimides or polyurethanes (Trochimowicz et al. 1994). It is also used as a solvent for certain epoxy resin curing agents. DMF has applications in hydrocarbon separations (such as recovery or removal of acetylene and extraction of butadiene from hydrocarbon streams) and in selective solvent extractions (such as separating nonparaffinic from paraffinic hydrocarbons in petroleum processing and in the separation of polycarboxylic acids) (IARC 1989; Trochimowicz et al. 1994).
TABLE 1-1 Summary of AEGL Values for DMFa
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| AEGL-1 (nondisabling) | NRb | NR | NR | NR | NR | |
| AEGL-2 (disabling) | 110 ppm (330 mg/m3) | 110 ppm (330 mg/m3) | 91 ppm (270 mg/m3) | 57 ppm (170 mg/m3) | 38 ppm (110 mg/m3) | 150 ppm for 6 h in rabbits to protect against irreversible effects (malformations) (Hellwig et al. 1991) |
| AEGL-3 (lethal) | 970 ppm (2,900 mg/m3) | 670 ppm (2,000 mg/m3) | 530 ppm (1,600 mg/m3) | 280 ppm (840 mg/m3) | 140 ppm (420 mg/m3) | No mortality in 6 rats exposed to 3,700 ppm for 3 h (Shell Oil Company 1982) |
aThere is a high potential for DMF to be absorbed dermally, so this route of exposure should be considered along with inhalation.
bNR, not recommended. Absence of an AEGL-1 does not imply that exposure below the AEGL-2 is without adverse effects.
American manufacturers used 32 million pounds of DMF in 1993 (TURI 2001). The primary end users of DMF are manufacturers of pharmaceuticals (12 million pounds), electronic components (10 million pounds), butadiene (3 million pounds), and urethanes (3 million pounds). DMF is also used as a resin cleanup solvent, reaction solvent, and processing solvent in the manufacture of polyimides, optical brightners, semipermeable membranes, and pesticides.
Human data are available from reports of accidental and controlled inhalation exposures and from epidemiologic studies investigating consequences of chronic exposure. Animal data consisted of acute inhalation studies with mice and rats and studies designed to examine the mode of action responsible for induction of hepatotoxicity. Repeat-exposure studies were available for monkeys, rats, mice, and cats.
The chemical and physical data on DMF are presented in Table 1-2.
TABLE 1-2 Chemical and Physical Data
| Parameter | Data | Reference |
| Synonyms | N,N-dimethylformamide, DMF | |
| CAS registry no. | 68-12-2 | |
| Chemical formula | C3H7NO | |
| Molecular weight | 73.09 | Budavari et al. 1996 |
| Physical state | Liquid | Budavari et al. 1996 |
| Color | Colorless to slightly yellow | Budavari et al. 1996 |
| Melting point | −61°C | Budavari et al. 1996 |
| Boiling point760 | 153°C | Budavari et al. 1996 |
| Solubility in water | Miscible with water and most common organic solvents | Budavari et al. 1996 |
| Vapor pressure | 2.6 mmHg (20°C) 3.7 mmHg (25°C) |
Trochimowicz et al. 1994 IARC 1989 |
| Saturated vapor pressure | 3,755 ppm at 20°C 5,000 ppm at at 25°C |
Shell Oil Company 1982 Lundberg et al. 1986 |
| Liquid density (water =1) | 0.9445 | Budavari et al. 1996 |
| Conversion factors | 1 ppm = 2.99 mg/m3 1 mg/m3 = 0.33 ppm |
NIOSH 2005 |
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
No acute lethality data in humans were found in the searched literature.
2.2. Nonlethal Toxicity
2.2.1. Controlled Exposures
DMF has a faint amine odor (Budavari et al. 1996). Odor thresholds range from 0.47 to 100 ppm (EPA 1992). The 0.47-ppm concentration was the threshold for recognition; no data were provided for the 100-ppm concentration. Trochimowicz et al. (1994) reported an odor threshold of 21.4 ppm, and Amoore and Hautala (1983) reported a threshold of 2.2 ppm; they stated that less than 50% of distracted individuals could perceive odor at the Threshold Limit Value (TLV) of 10 ppm.
A number of controlled human inhalation exposures to DMF are available, and these metabolism studies are discussed in Section 4.2. The studies were conducted to assess metabolism, and no adverse effects of inhaled DMF exposure were reported at the concentrations and durations of exposure examined. A summary of the following data is found in Table 1-3: 10 healthy volunteers (five males and five females, ages 25-56 years) were exposed to DMF at 3, 10, or 20 ppm for 8 h (Mraz and Nohova 1992); 10 healthy human volunteers (five males and five females, ages 26-56) were exposed at 20 ppm for 8 h (Mraz et al. 1989); four volunteers (three males and one female, ages 20-50) were exposed to DMF at 53 ± 32 ppm for 2 h (Eben and Kimmerle 1976); and four volunteers were exposed at 26 ± 8 ppm (four males, ages 25-50) or 87 ± 25 ppm for 4 h (three males and one female, ages 20-50) or 21 ± 4 ppm (four males, ages 25-50) for 4 h/day for 5 consecutive days (Kimmerle and Eben 1975b). Alcohol intolerance was not observed when four volunteers (three males and one females; ages 20-50) drank 19 g of ethanol (50 mL of a 38% schnaps or gin) followed by a 2-h exposure to DMF at 82 ± 20 ppm (Eben and Kimmerle 1976). This observation is significant in light of evidence that sufficiently high concomitant DMF and ethanol exposures can result in disulfiram-like symptoms (see Section 4.3).
2.2.2. Case Reports
Potter (1973) described an accidental DMF exposure in a 52-year-old man where DMF splashed on approximately 20% of the victim’s body, after which he washed the affected skin, put his clothes back on, and drove home (45 min). The intense odor of DMF was noted in the factory following the accident and in his car. Immediate symptoms were limited to dermal irritation and hyperemia, with anorexia developing 1-2 days later. Sixty-two hours after the accident, he
developed epigastric pain that spread throughout his abdomen, chest, and thighs, and episodes of vomiting followed. On admission to the hospital, he presented with hypertension, and he complained of weakness and incoordination of his legs, but no objective neurologic changes were apparent. Minimal abdominal tenderness was noted. Increased white blood cells and serum conjugated and total bilirubin, glutamic oxaloacetic transaminase, and glutamic pyruvic transaminase were observed. Urine tested positive for porphobilinogen for the 3 days the patient experienced abdominal pain. Minimal S-T segment and T-wave depressions were noted during electrocardiograms, but the abnormalities returned to normal before discharge. An aspiration biopsy of the liver 11 days after the exposure revealed minimal septal fibrosis and an accumulation of mononuclear cells. Upon discharge from the hospital 15 days postexposure, the patient was free of any symptoms.
A 21-year-old man was hospitalized following accidental exposure to DMF at work (exposure quantity and route not characterized) (Chary 1974). On hospital admission, he experienced upper abdominal pain radiating in his back. Nausea and vomiting, epigastric tenderness, and an erythematous rash on his hands and forearms (possibly suggesting direct skin contact with DMF) developed. Serum amylase levels were increased to 2,400 I.U./liter (L), but a cholecystogram and intravenous cholangiogram were normal. Following the accident, a search of factory records found that a 28-year-old male coworker had previously been admitted to the hospital following accidental exposure to DMF. Again, the exposure route was not characterized, but this patient too had an erythematous rash on his hands and forearms, and suffered from upper abdominal pain, nausea and vomiting, and epigastric tenderness. Serum amylase levels were not measured, but a cholecystogram was normal. Follow-up of the patient revealed continuing complaints of epigastric pain. The three remaining workers in the factory were then questioned about symptoms. All admitted intermittent gastrointestinal symptoms, erythema of exposed parts, and pruritus, particularly after consuming ethanol.
TABLE 1-3 Summary of Controlled Human Exposures to DMFa
| Number of Subjects | Duration | Concentration (ppm) | Reference |
| 10 (5 males, 5 females) | 8 h | 3 | Mraz and Nahova 1992 |
| 10 | |||
| 20 | |||
| 10 (5 males, 5 females) | 8 h | 20 | Mraz et al. 1989 |
| 4 (4 males) | 4 h | 26 | Kimmerle and Eben 1975b |
| 4 (3 males, 1 female) | 87 | ||
| 4 (4 males) | 4 h/d for 5 d | 21 | |
| 4 (3 males, 1 female) | 2 h | 53 | Eben and Kimmerle 1976 |
| 82b | |||
aBecause these studies were designed only to assess metabolism, clinical signs and symptoms were not evaluated by the study authors.
bExposure occurred following consumption of ethanol.
2.2.3. Epidemiologic Studies
Fiorito et al. (1997) conducted a cross-sectional study investigating the prevalence of liver function abnormalities in workers exposed to DMF in a synthetic leather factory. The study consisted of 75 exposed workers (average employment 3.8 years) and 75 unexposed individuals matched for age, sex, social status, and place of residence. Although these workers were generally exposed to less than 10 ppm DMF, biologic monitoring revealed that occasional overexposure was possible. Fifty percent of the DMF-exposed workers complained of gastrointestinal symptoms, and 40% of exposed workers also complained of disulfiram-like symptoms (facial flushing [38%], palpitation [30%], headache [22%], dizziness [22%], body flushing [15%], and tremors [14%]) after ethanol consumption. Covariance analysis of clinical chemistry parameters revealed increased alanine aminotransaminase (ALT), aspartate aminotransferase (AST), gamma glutamyl transpeptidase (GGT), and alkaline phosphatase (AP) in DMF-exposed workers compared with the reference group. Twenty-three percent of DMF-exposed workers had abnormal transaminase values, compared with 4% of controls. The study authors concluded that repeated occupational exposure to DMF at levels less than 10 ppm for 8-h TWAs can impair liver function.
In response to a case of suspected toxic hepatitis in a worker from a fabric coating factory, a clinical-epidemiologic investigation and environmental assessment of the patient’s workplace was conducted (Redlich et al. 1988). A total of 58 workers participated in the study: All had at least one liver function test; 46 completed a questionnaire addressing demographic background, job history, and symptoms; and 27 underwent an extensive clinical evaluations to assess liver function. Workers were exposed to DMF in the process of coating fabric in poorly ventilated areas, and little effort was made to control direct skin contact with the solvent. Results from the questionnaire and clinic interviews revealed complaints of gastrointestinal problems (31 of 46), headache and dizziness (18 of 46), and alcohol intolerance characterized by facial flushing and palpitations after drinking ethanol (11 of 46; total number consuming ethanol not provided). Clinical chemistry analyses revealed that 36 of 58 workers had increased AST or ALT levels, 19 having elevations greater than twice normal, and 9 of the 19 having increases greater than five times normal. All but one of these employees were production-line workers (35 of 46, vs. 1 of 12 nonproduction-line workers). Histologic examination of liver biopsies from four workers confirmed toxic liver injury. Serologic testing and a ratio of AST to ALT of less than one ruled out infectious hepatitis in all but two workers and alcoholic liver disease in all but one worker, respectively.
The cohort described by Redlich et al. (1988) was re-evaluated by Fleming et al. (1990). In the re-evaluation, the defined exposure population consisted of subjects who were male, Hispanic, and who worked in jobs with DMF exposure. An unexposed population of 111 individuals was chosen from a preemployment population for comparison. A complete liver enzyme profile was determined for each individual. Analysis of the data revealed a statistically sig-
nificant (p <0.0001) increase in ALT and a decrease in the AST:ALT ratio (ratio of <1.0) in the DMF-exposed group compared with the referent group, but there was no difference in AST levels. Continued surveillance of the workplace over the next 14 months failed to identify any additional cases of liver dysfunction; this observation was coincident with changes in several engineering and industrial hygiene changes and a reduction in the quantity of DMF used in the process. The study authors therefore concluded that the outbreak of liver damage was “almost certainly” causally related to workplace exposure to DMF.
Wrbitzky (1999) measured liver function in workers exposed to DMF alone or after ethanol consumption. The study involved 126 male workers exposed to DMF in their job and 54 comparable unexposed male employees. DMF concentrations measured in workplace air ranged from <0.1 to 37.9 ppm, and the concentrations of the DMF metabolite N-methylformamide (NMF)measured in the urine of exposed workers ranged from 0.05 to 22.0 mg/L preshift and 0.9 to 100.0 mg/L post shift. Facial flushing following ethanol consumption was noted by 70% of the DMF-exposed workers compared with 4% of unexposed controls. Exposed workers had significant increases in GGT and ALT activities. Exposed workers were further categorized as having high (0.1-100 ppm) or low exposures (0.1-13.7 ppm) to DMF, and alcohol consumption was assigned using the criteria of consuming no alcohol, consuming <50 g/day, or consuming >50 g/day. A ranking sum value based on GGT, AST, and ALT levels was determined for all groups. The results demonstrated that chronic occupational DMF exposure can impair liver function, and drinking alcohol was synergistic with the hepatotoxicity of DMF.
Catenacci et al. (1984) found no alterations in hepatic function in 54 workers employed for at least 5 years in an acrylic fiber plant and exposed to DMF at <10 ppm for 8-h TWAs. Hepatic parameters included assessment of serum ALT, AST, GGT, and AP.
A cohort study by E.I. Dupont de Nemours & Co. (1973) investigated the association between DMF exposure and adverse health effects. Workers at two DuPont plants (Waynesboro and Camden) were categorized into three groups based on work history: currently exposed to DMF, previously exposed to DMF, or never exposed to DMF. The DMF-exposed workers were compared with the referent group for history of chronic disease, findings at periodic health examinations, and sickness absenteeism over a 5-year period. Although all illnesses were investigated, the liver, gastrointestinal system, and cardiovascular system were of particular focus. Because differences were observed in the distribution of age and race among the DMF-exposed and the referent groups, comparisons were made by age categories and by computing age-adjusted rates. The study authors concluded that there was no significant excess in any of the parameters examined. However, a significant reduction in the prevalence of hypertension was found in workers currently exposed to DMF at the Waynesboro plant, but this finding was not observed in workers previously exposed to DMF. Although it appeared that a similar reduction in the prevalence of hypertension may have
occurred in employees at the Camden plant, this reduction was not confirmed by the data.
Evaluation of blood pressure in 12 workers exposed to DMF over a 3-month period in 1943 revealed that four individuals had abnormal readings (E.I. Dupont de Nemours & Co. 1944). Three of the four individuals had normal blood pressure readings in the morning but low blood pressure in the afternoon. The fourth individual had a high diastolic reading in the morning and normal blood pressure in the afternoon.
2.3. Developmental and Reproductive Toxicity
Three cases of third-trimester intrauterine death were reported over a period of 3 years (1979-1982) in women (ages 22, 26, and 28) working as quality control analysts in the same pharmaceutical laboratory (Farquharson et al. 1983). DMF was one of a number of unspecified chemicals to which the women were potentially exposed. No workplace air or biologic monitoring data were reported that could be used to document the extent of DMF exposure; therefore, the late deaths could not be attributed to DMF.
2.4. Genotoxicity
DMF failed to induce chromosomal aberrations or sister chromatid exchanges in vitro in lymphocytes from a healthy male donor (Antoine et al. 1983).
2.5. Carcinogenicity
Three cases of testicular germ cell tumors were reported among 153 white male workers employed in a Navy F4 jet airframes repair facility (Ducatman et al. 1986). These three cases prompted investigation of two other aircraft repair facilities: one facility where identical work was being performed and the other where major airframe structural repair of F4 aircraft was never done. A case was defined as “any employee working at an airframe repair facility at least 3 years before the onset of signs or symptoms leading to a documented histopathologic diagnosis of testicular germ-cell cancer.” No cases of testicular cancer were identified among 446 workers from the facility that had never performed structural F4 repair work. However, four cases were identified among 680 white male workers at the other facility (p <0.01, Poisson, compared with the expected number of cases based on national incidence rates). One additional worker with testicular cancer was identified at the original facility, but he developed symptoms within 1 year of his employment at the shop and, therefore, his case could not be included. However, he had been employed in another F4 airframe repair shop for over 20 years. An investigation of the work processes occurring in the
three facilities revealed that all three had similar exposures to various dusts and solvents with one exception: In the repair of F4 airframes, depotting (removal of embedded electrical components in elastomeric materials) was performed on the floor of the airframe repair area using a solvent containing 80% DMF, and this work was performed without the use of ventilators. Although a causal association between chronic occupational DMF exposure and the development of testicular cancer was not established, the authors considered the cluster “highly suspicious.”
Levin et al. (1987) reported three cases of testicular cancer in leather tannery workers with DMF inhalation and dermal exposure. The cases included a 32-year-old male exposed for 13 years, a 36-year-old male exposed for 14 years, and a 25-year-old male exposed for 8 years. Histologic analyses of the tumors revealed a metastatic embryonal cell carcinoma, a combined embryonal cell carcinoma and seminoma, and a metastatic embryonal cell carcinoma with foci of choriocarcinoma, respectively.
A case-control study of workers from four DuPont plants investigated whether a significant association existed between DMF exposure and development of cancer of the buccal cavity and pharynx (39 cases), liver (6 cases), prostate (43 cases), testis (11 cases), or malignant melanoma of the skin (39 cases) (Walrath et al. 1989). Cases were identified using the company Cancer Registry, in which cancer cases were reported by male employees active during 1956-1985. Each case was matched to two controls based on sex, salary, birth year, and plant location. Each job with possible DMF exposure was identified, and exposure rankings were assigned based on industrial hygiene monitoring of DMF, monitoring of urinary DMF metabolites, and knowledge of work practices and plant operations. Worker exposure patterns were then classified as ever-vs.never exposed to DMF or as highest DMF exposure experienced. No significant associations were observed between the identified cancers and having ever been exposed to DMF when considering the summary data of all four plants combined. When considering individual plants, three of four cases of prostate cancer from one plant were associated with DMF exposure. The authors discounted this association as being related to DMF exposure because this association was not observed in any of the other three plants where workers were also exposed to DMF, the DMF exposures were low, and the latency period was short (12-16 years). When evaluating the combination of the highest DMF exposure rank, duration of exposure, and latency, no causal association was observed between DMF exposure and cancer of the buccal cavity and pharynx, liver, malignant melanoma, prostate, or testis. The authors cautioned that this study was limited by the relatively small numbers of cases and the lack of data on workers who were no longer employed by Dupont.
IARC (1989) concluded that there was limited evidence for the carcinogenicity of DMF in humans and inadequate evidence in experimental animals. Therefore, the overall IARC evaluation was that DMF is possibly carcinogenic to humans (Group 2B).
2.6. Summary
Reports of accidental exposure described symptoms of DMF exposure, including abdominal pain, nausea, vomiting, and liver toxicity as indicated by increased serum enzymes and histologically confirmed hepatic damage. A local erythematous rash was also described, but it was most likely the result of direct dermal exposure to DMF. Daily exposure to lower concentrations of DMF resulted in gastrointestinal distress, disulfiram-like symptoms following ethanol ingestion, headache, dizziness, changes in blood pressure, and liver injury as indicated by increased liver enzymes and histologic evaluation. One case report suggested a link between DMF exposure and human developmental toxicity, but no data to confirm this association or quantitative measurements of DMF concentrations or descriptions of other chemical exposure in those instances were available. Two reports suggested an association between repeated occupational DMF exposure and testicular germ-cell tumors, but other larger studies in which industrial hygiene and biologic exposure data were collected found no such association. Human data with measured exposure concentrations were generally limited to acute controlled DMF exposures for the purpose of characterizing DMF metabolism. In these metabolism studies, no adverse effects were reported.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
3.1.1. Rats
Groups of three male or three female Wistar rats were exposed to DMF at approximately 3,700 ppm (3,755 ppm is the saturation concentration at 20°C) for 1, 3, or 7 h followed by an observation period of 14 days (Shell Oil Company 1982). DMF vapors were generated by passing compressed air through a flask by means of a glass frit, on top of which DMF was situated. The portion of the flask containing DMF was immersed in a water bath maintained at 20°C, and the resulting vapor was brought into the exposure chamber. The nominal concentration was estimated by considering the total weight loss of chemical from the flask, the airflow rate, and duration of exposure. In the animals that inhaled DMF for 7 h, all the females died 2 days postexposure, and two of the three males died 3 days postexposure. The remaining male survived to study termination. All rats survived exposure at 3,700 ppm for 1 or 3 h. All exposure groups responded with excessive grooming, and those that inhaled DMF for 7 h exhibited lethargy. The authors stated that “all animals appeared normal shortly after exposure and remained so even if subsequently dying.” Body weight at 0, 7, or 14 days postexposure was comparable in the male and female rats exposed for 1 or 3 h. Necropsy was not performed.
Ten Crl:CD male rats inhaled DMF at 2,523 ppm for 6 h/day for 5 days (information about exposure chamber not provided) and were observed for 10 days following cessation of exposure (Kennedy and Sherman 1986). Animals were observed and weighed daily. Histopathologic examination was conducted on all rats either upon death or at the termination of the 10-day period. Clinical signs among exposed rats consisted of progressive weakness, discomfort, and body-weight loss. Seven rats died 1 to 3 days after the last exposure with evidence of dehydration and acute liver necrosis. One rat died after the second exposure from acute pulmonary edema and congestion. The two surviving rats improved during the 10-day recovery period, and histopathologic examination of the liver revealed resolution of acute hepatic injury in one of the rats. No adverse effects were noted in the concurrent control group.
3.1.2. Mice
Groups of 20 mice were exposed for 2 h to air containing DMF vapor at concentrations of 0, 670, 1,300, 2,000, 3,100, 4,000, 4,700, 5,700, or 7,700 ppm (reported as 0, 2.0, 4.0, 6.0, 9.4, 12.0, 14.0, 17.0, or 23.0 mg/L) and were then observed for at least 15 days (Stasenkova 1961). Mortality data are summarized in Table 1-4. The LC50 (concentration of a substance that is lethal to 50% of the exposed population) as determined by probit analysis was 3,981 ± 187 ppm (calculated by reviewer). Details of experimental protocol, including the method of chamber concentration analysis, were not provided. It appears unlikely that concentrations of ≥ 5,700 ppm could have been attained without generation of DMF aerosols because atmospheric saturation occurs at 3,755 ppm at 20°C (Shell Oil Company 1982) and 5,000 ppm at 25°C (Lundberg et al. 1986).
TABLE 1-4 Mortality of White Mice Exposed for 2 h to Vapor from DMF at Various Concentrations
| Concentration (ppm) | Total Death (% mortality) |
| 0 | 0 (0) |
| 670 | 0 (0) |
| 1,300 | 1 (10) |
| 2,000 | 1 (10) |
| 3,100 | 10 (50) |
| 4,000 | 12 (60) |
| 4,700 | 12 (60) |
| 5,700 | 16 (80) |
| 7,700 | 20 (100) |
Source: Adapted from Stasenkova 1961.
3.2. Nonlethal Toxicity
3.2.1. Rats
Groups of 10 female, Sprague-Dawley rats (~200 g) were exposed to DMF by inhalation in a 60-L dynamic exposure chamber for 4 or 8 h (Lundberg et al. 1986). All of the actual DMF concentrations were not provided, but the authors stated that the experimental design involved a geometric concentration series. Solvent concentrations in the chamber were monitored by analysis of a stream of chamber air continuously drawn through an infrared analyzer, and exposure concentrations were adjusted accordingly. Animals were observed for mortality for 24 h after initiation of exposure. All animals survived a 4-h or an 8-h exposure at 5,000 ppm (reported as 13,440 mg/m3). Because this concentration represented the saturated air concentration at 25°C, an LC50 value for the vapor could not be determined.
In an additional study by Lundberg et al. (1986), groups of six rats inhaled air only or DMF for 4 h at approximate concentrations of 140, 280, 560, 1,120, or 2,250 ppm (420, 840, 1,680, 3,360, and 6,720 mg/m3, respectively; reported as 1/32, 1/16, 1/8, 1/4, or 1/2 of the saturation concentration). Hepatic damage was assessed 20 h later by measuring serum sorbitol dehydrogenase (SDH) in all rats and by histologic analysis of liver from rats exposed at 2,250 ppm (6,720 mg/m3). SDH concentrations were raised (p <0.05) in rats exposed at 280 or 560 ppm (1/16 or 1/8 of the saturation concentration; exact SDH values not provided) compared with controls, the greatest increase occurring in rats exposed at 560 ppm. SDH concentrations were comparable to control levels in all other exposure groups, including those that inhaled 1,120 or 2,250 ppm. No abnormalities were observed during histologic examination of livers from rats exposed at 2,250 ppm DMF for 4 h.
Groups of 10 male Sprague-Dawley rats inhaled DMF at measured concentrations of 0, 81, 153, 313, 441, or 991 ppm for 4 h in a 200-L dynamic inhalation chamber (with adjustable laminar airflow ranging from 10 to 20 m3/h) at 23°C (Roure et al. 1996). The concentration of DMF in the chamber was monitored continuously by gas-liquid chromatography, and periodic air samples were also collected with charcoal-packed glass tubes, desorbed with disulfide, and analyzed by gas-liquid chromatography. Serum SDH and glutamate dehydrogenase (GDH) were measured 24, 48, and 72 h postexposure (Table 1-5). Significant concentration-related increases in SDH and GDH were observed in rats exposed at 153-991 ppm, but there were marked variations in the results even among the concurrent controls. SDH and GDH levels were maximally increased by 24 h in rats exposed at 153, 313, or 441 ppm, with SDH levels increased approximately 2-fold in the 153-ppm group, and SDH and GDH levels increased approximately 6-fold and 10.5-fold in the 313- and 441-ppm group, respectively. In contrast, SDH and GDH levels in rats exposed at 991 ppm peaked at 48 h, having increases of 140-fold and 130-fold, respectively and no significant increases at 24 h. At 72-h postexposure, statistically significant increases in en-
zyme levels were seen only in GDH levels of rats exposed at 441 and 991 ppm (increased 1.5-fold and 20-fold, respectively). Assaying for SDH is generally difficult, and the results often vary (Tietz 1995), a fact reflected in the relatively large standard deviations among the controls (Table 1-5).
Brondeau et al. (1983) exposed groups of eight male Sprague-Dawley rats for 4 h to measured concentrations of DMF at 0, 66, 126, 281, or 314 ppm in a 200-L dynamic inhalation chamber (airflow rate of 10-12 m3/h). Chamber concentrations were measured at least three times by pumping 5-10 L of chamber air through a glass tube packed with activated charcoal to collect the vapors, desorbing with an appropriate solvent, and analyzing with gas liquid chromatography using internal standards (more specific details not provided because atmosphere sampling and analysis data were not presented specifically for DMF but rather for several test chemicals). Twenty-four hours following termination of DMF exposure, rats were killed and blood was collected to measure serum GDH, AST, and SDH. The minimally active DMF 4-h exposure for hepatotoxicity was 126 ppm based on significant differences (p <0.02) in at least two clinical chemistry parameters. When compared with the concurrent controls, rats that inhaled DMF at 126, 281, or 314 ppm had increased (p <0.05; 0.02) GDH (+38%, +516%, and +260%, respectively); ALT (+37%, +54%, and +50%, respectively); and SDH (+130%, +325%, and +379%, respectively). Rats exposed to DMF at 314 ppm developed increased AST (+38%) compared with controls.
Sherman rats exposed to a saturated vapor concentration of DMF for 4 h survived (no further experimental details provided (Smyth and Carpenter 1948). The concentration used was interpreted as 3,500 ppm (at 20°C) by the National Institute for Occupational Safety and Health (NIOSH 1996) in the original immediately dangerous to life or health (IDLH) derivation as described by Clayton et al. (1963).
TABLE 1-5 Serum SDH and GDH Activities in Rats Following a 4-h Exposure to DMF
| Concentration (ppm) | SDH (U/l) | GDH (U/l) | ||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| 0 | 4.5 ± 1.4 | 3.8 ± 1.5 | 4.9 ± 2.6 | 4.3 ± 0.3 | 4.6 ± 0.6 | 4.4 ± 0.6 |
| 81 | 6.4 ± 2.3* a | 3.9 ± 1.4 | 4.2 ± 1.5 | 5.7 ± 1.6** | 4.7 ± 0.5 | 4.6 ± 0.5 |
| 0 | 7.1 ± 1.6 | 8.4 ± 3.0 | 11.5 ± 8.6 | 9.1 ± 14.9 | 4.9 ± 1.2 | 5.6 ± 2.2 |
| 153 | 16.1 ± 5.2** a | 9.2 ± 2.9 | 13.4 ± 7.4 | 11.4 ± 5.9 | 6.0 ± 2.7 | 8.7 ± 10.8 |
| 0 | 20.7 ± 29.3 | 7.9 ± 3.8 | 6.1 ± 1.6 | 5.8 ± 2.7 | 5.1 ± 3.3 | 4.2 ± 1.2 |
| 313 | 120.1 ± 89.7** | 12.8 ± 5.5* | 8.2 ± 3.6 | 35.7 ± 12.6** | 9.9 ± 3.0** | 4.6 ± 1.0 |
| 0 | 8.9 ± 2.7 | 10.5 ± 2.7 | 11.6 ± 7.1 | 3.9 ± 0.6 | 4.4 ± 0.7 | 4.6 ± 0.6 |
| 444 | 94.1 ± 57.6** | 28.9 ± 31.5 | 17.2 ± 7.1 | 41.7 ± 27.9** | 16.5 ± 7.3** | 7.3 ± 1.8** |
| 0 | 3.4 ± 0.8 | 3.0 ± 0.5 | 3.5 ± 0.9 | 11.2 ± 20.7 | 5.1 ± 0.7 | 4.4 ± 0.6 |
| 991 | 4.5 ± 1.8 | 422.7 ± 559.6* | 30.0 ± 41.3 | 4.8 ± 0.7 | 657.3 ± 744.1** | 87.5 ± 79.9** |
aStatistically significant compared with controls: *p <0.05; **p <0.01. Abbreviations: SDH, serum sorbitol dehydrogenase; GDH, glutamate dehydrogenase.
Source: Roure et al. 1996. Reprinted with permission; copyright 1996, Journal of Applied Toxicology.
3.2.2. Mice
To assess DMF-induced sensory irritation, respiration rates in groups of four male CD-1 mice were measured using whole-body plethysmographs during 10-min head-only inhalation exposures to DMF at 0, 55, 154, 550, 1,658, or 2,110 ppm (Kennedy and Sherman 1986). Sensory irritation as evidenced by reduced respiratory rate was observed at 1,658 and 2,110 ppm (decreases of 12.8% and 28.3% of controls, respectively). An RD50 (concentration required to produce a 50% decrease in respiration rate) could not be calculated because the maximum respiratory decrease was only 28%, and the test system could not generate vapor concentrations greater than 2,110 ppm.
3.3. Developmental and Reproductive Toxicity
Groups of 15 artificially inseminated female Himalayan rabbits inhaled air containing DMF at 0, 50, 150, or 450 ppm for 6 h/day on gestation days (GD) 7-19 (BASF 1989; Hellwig et al. 1991). Animals were sham-exposed for 4 days prior to actual exposure to DMF. Chamber concentrations were monitored by gas chromatographic analysis of hourly samples taken from the breathing zone. Animals were killed on GD 29 and laparatomy was performed. Gross necropsy was performed on each dam, and each fetus was examined for external, soft tissue, and skeletal malformations and variations. Selected maternal and fetal effects are summarized in Table 1-6. Over GD 7-19, mean maternal body-weight gain was reduced in dams exposed to DMF at 150 ppm compared with controls, and dams in the 450-ppm group lost weight. Mean maternal body-weight gain over the entire study period of GD 0-29 was also decreased in dams from the 150- and 450-ppm groups compared with controls. Developmental toxicity was evident in the 450-ppm group because of significant reductions in mean fetal body weight compared with weight in the concurrent controls and significant increases in the litter incidence of total external malformations, total skeletal variations, and total malformations (external, soft tissue, and skeletal combined). Significant increases in the litter incidence of skeletal variations included splitting of skull bones, fused sternebrae, irregularly shaped sternebrae, and bipartite sternebrae.
Groups of 17 or 18 impregnated Sprague-Dawley rats were exposed to DMF at 0, 221, or 522 ppm for 6 h/day on GD 4-8 (BASF 1974c). Dams were killed on GD 20 and caesarean section was performed. Dams underwent gross necropsy, and all fetuses were examined externally with two-thirds of the fetuses examined for skeletal abnormalities, and the remaining one-third examined for soft tissue abnormalities. Selected maternal and fetal observations are summarized in Table 1-7. The only treatment-related effect observed in DMF-exposed dams was reduced body-weight gain in those that inhaled 221 ppm over GD 4-11 (−26% compared with controls, respectively; statistical analysis not conducted) and in dams that inhaled 522 ppm over GD 4-11 (−55%), GD 15-20
(−19%) and GD 4-20 (−23%). Other observations noted among rats exposed to DMF at 522 ppm included an increased absolute number of dead implants and increased percentage of dead implants. The majority of loss occurred early in gestation (prior to GD 13) as evidenced by increases in early resorptions. Mean fetal body weight was reduced (p <0.05) in the 221- and 522-ppm groups. Mean fetal body length and placental weight were reduced (p <0.05) in 221-ppm fetuses, but no such changes were observed in the 522-ppm fetuses. No treatment-related external soft-tissue abnormalities or skeletal malformations and variations were observed.
TABLE 1-6 Selected Results of Pregnant Rabbits Exposed to DMF 6h/day on GD 7-19
| Parameter | Exposure concentration (ppm) | |||
| 0 | 50 | 150 | 450 | |
| Number of animals | 15 | 15 | 15 | 15 |
| Mean maternal body-weight change (g): | ||||
|
GD 7-19 |
31.0 | 42.4 | 3.1 | −34.3 |
|
GD 0-29 |
248.1 | 202.1 | 146.4 | 183.0 |
| Number of litters | 12 | 14 | 14 | 15 |
| Number of live fetuses | 67 | 71 | 72 | 86 |
| Mean corpora lutea | 8.3 | 8.2 | 8.2 | 8.6 |
| Mean implantation sites | 6.3 | 5.9 | 6.7 | 6.4 |
| Preimplantation loss (%) | 22.8 | 29.3 | 16.9 | 24.3 |
| Postimplantation loss (%) | 9.5 | 11.3 | 22.6 | 14.5 |
| Total resorptions | 8 | 12 | 19 | 10 |
| Mean fetal weight (g) | 43.7 | 42.1 | 41.7 | 37.7** a |
| Number of fetuses (litters) examined | 67 (12) | 71 (14) | 72 (14) | 86 (15) |
| External malformations: total | 0 (0) | 1 (1) | 1 (1) | 8*a (5)* |
|
Umbilical hernia: |
0 (0) | 0 (0) | 1 (1) | 7* (4) |
| External variation | 0 (0) | 0 (0) | 3 (2) | 6* (2) |
|
Pseudoanklosis of for limb |
0 (0) | 0 (0) | 3 (2) | 6* (2) |
| Soft-tissue malformations | 2 (2) | 2 (2) | 3 (3) | 7 (5) |
| Soft-tissue variations | 21 (11) | 17 (10) | 21 (10) | 30 (14) |
| Total skeletal malformations | 1 (1) | 1 (1) | 0 (0) | 4 (4) |
| Total skeletal retardations | 33 (11) | 30 (10) | 29 (14) | 23** (10) |
| Total skeletal variations | 10 (6) | 8 (7) | 16 (10) | 73** (15)** |
|
Splitting of skull bones |
2 (1) | 2 (2) | 2 (2) | 11* (7)* |
|
Fused sternebrae |
5 (4) | 2 (2) | 13 (8) | 51** (13)** |
|
Irregularily shaped sternebrae |
2 (2) | 3 (3) | 1 (1) | 34** (13)** |
|
Bipartite sternebrae |
0 (0) | 0 (0) | 0 (0) | 12** (8)** |
|
Accessory 13th rib |
1 (1) | 2 (2) | 2 (1) | 7 (6) |
| Total malformations | 3 (2) | 2 (2) | 4 (4) | 15* (9)* |
| Total variations | 29 (11) | 23 (12) | 32 (12) | 77** (15) |
aStatistically significant compared with controls: *p <0.05; **p <0.01. Abbreviation: GD, gestation day.
Sources: BASF 1989; Hellwig et al. 1991.
TABLE 1-7 Selected Results of Pregnant Rats Exposed to DMF for 6 h/day on GD 4-8
| Parameter | Exposure concentration (ppm) | ||
| 0 | 221 | 522 | |
| Number of animals | 18 | 18 | 19 |
| Number of pregnant animals | 18 | 17 | 18 |
| Mean maternal body-weight change (g):a | |||
|
GD 4-11b |
22.6 | 16.6 (−26)c | 10.2 (−55) |
|
GD 11-15 |
19.6 | 22.1 (+13) | 18.9 (−3) |
|
GD 15-20 |
66.1 | 67.4 (+2) | 53.7 (−19) |
|
GD 4-20 |
108.2 | 106.1 (−2) | 82.8 (−23) |
| No. litters | 18 | 17 | 16 |
| No. live fetuses | 201 | 215 | 168 |
| Mean number of live fetuses/dam | 11.17 | 12.65 | 9.33 |
| Total implantation sites | 208 | 224 | 212 |
| Mean implantation sites/dam | 11.56 | 13.18 | 11.83 |
| Percent live fetuses related to implantations | 96.6 | 96.0 | 78.9 |
| Dead implants as percent of total implantations | 7 | 9 | 45**e |
| 3.37 | 4.02 | 21.13 | |
| Total Resorptions: | |||
|
Early |
7 | 9 | 45 |
|
(number of resorptions/dam)d |
7 (0.4) | 8 (0.5) | 44 (2.75) |
|
Middle |
0 | 1 | 1 |
|
Late |
0 | 0 | 0 |
| Dams with all resorptions | 0 | 0 | 2 |
| Mean fetal weight, g | 3.77 | 3.55* e | 3.62* e |
aCalculated by reviewer using mean body-weight values.
bBody weights recorded on GD 0, 4, 11, 15, and 20; therefore, body-weight gain over the exposure interval of GD 4-8 could not be calculated.
cPercent decrease or increase compared with controls; calculated by reviewer.
dCalculated by reviewer: Number of resorptions and number of pregnant dams.
eStatistically significant compared with controls: *p <0.05; **p <0.01.
Abbreviation: GD, gestation day.
Source: BASF 1974c.
Groups of 30 impregnated female Sprague-Dawley rats were exposed to air or 287 ppm DMF for 6 h/day (BASF 1974a,b; Hellwig et al. 1991). Two discontinuous exposure regimes were tested: Rats were exposed on GD 0-1, 4-8, 11-15, and 18-19 (Group I) or on GD 0-3, 6-10, and 11-18 (Group II). The DMF concentration was analyzed 12 times during the exposure period (287 ± 50.2
ppm), and the constancy of the concentration in the chambers was monitored daily using an infrared photometer. Twenty rats/group were killed on GD 20. Gross necropsy and caesarean sections were performed; all fetuses were examined externally, and one-third were fixed and examined for soft tissue abnormalities and two-thirds were fixed and examined for skeletal abnormalities. The remaining 10 dams/group were allowed to deliver, and all dams and pups were killed and examined on day 20 after birth. Effects of exposure in animals from Groups I and II (killed on GD 20) included reduced mean maternal body-weight gain (rats in Groups I and II gained 56% and 39% less than controls, respectively), decreased (p <0.05) mean fetal weight (Group I: 3.34 vs. 3.77 g for controls; Group II: 3.35 vs. 3.70 g for controls), mean fetal length (Group I: 3.55 vs. 3.66 cm for controls; Group II: 3.49 vs. 3.62 cm for controls), and mean placental weight (Group I: 0.49 vs. 0.56 g for controls; Group II: 0.51 vs. 0.59 g for controls). The number of dead implants was statistically increased in dams from Group I (21 vs. 9 for controls; p <0.05). The percentage of total dead implants in Group I was increased after DMF exposure (10.8% vs. 4.43% for controls), but the increase did not attain statistical significance. An increased litter incidence of aplasia of some sternebrae was observed in exposed fetuses from both Groups I and II (Group 1: 12/18 vs. 3/19 for controls; Group II: 11/17 vs. 5/20 for controls). No treatment-related adverse effects were observed in dams or pups that were allowed to deliver their offspring.
Groups of 22 or 23 impregnated Long Evans rats inhaled DMF at measured concentrations of DMF at 0, 18, or 172 ppm dissolved in polyethylene glycol 400 (20 mm3/L air) for 6 h/day during GD 6-15 (Kimmerle and Machemer 1975). Dams were killed on GD 20, and caesarean sections were performed. All fetuses were examined for external malformations, one-third of the fetuses were examined for visceral malformations, and the remaining two-thirds of the fetuses were examined for skeletal malformations. No clinical signs or body-weight changes were observed in dams. The only effect observed in fetuses was reduced mean fetal body weight in the 172-ppm group (3.78 g vs. 4.07 g for controls; p <0.01), but the reduction in fetal weight was not accompanied by any other signs of growth retardation, such as delays in skeletal ossification.
Groups of 21 impregnated, Crl:CD rats inhaled DMF at 0, 30, or 300 ppm for 6 h/day during GD 6-15 (Lewis et al. 1992). Chamber concentrations were measured by infrared analysis at four intervals during each exposure day (measured concentrations were 0, 31.2 ± 4.6, and 297 ± 22 ppm, respectively). Dams were killed on GD 21 and subjected to gross necropsy. All fetuses were examined externally, two-thirds of the fetuses were examined for skeletal abnormalities, and the remaining one-third of the fetuses were examined for visceral abnormalities. The only effect observed in dams was reduced weight gain over GD 6-15 in those that inhaled 300 ppm (78% of controls; p <0.05). Decreased mean fetal body weight was observed in the 300-ppm group (96% of controls; p <0.05), but the reduced fetal weight was not accompanied by changes in skeletal ossification. No treatment-related malformations were noted.
3.4. Repeated Exposures
Repeated exposure data are discussed to place the acute toxicity data into perspective.
3.4.1. Nonhuman Primates
Adult male cynomolgus monkeys were exposed to DMF at a nominal concentration of 500 ppm for 6 h/day, 5 days/week, for 2 weeks (Hurtt et al. 1991). One monkey was exposed by whole-body inhalation (4.1 m3 chamber with airflow of 500 L/min), and a second monkey was exposed head-only (acrylic helmet with airflow rate of 10 L/min). Mean analytic concentrations for the 10-day period were 509 and 385 ppm, respectively. Clinical signs were recorded daily during exposure, and body weight was recorded 1 day before exposure, on study day 8, and after the last exposure. Blood was collected for hematology and clinical chemistry analysis 1 day before exposure at the end of the first exposure day and following the last exposure day. Neither monkey showed clinical signs of toxicity or abnormalities in standard hematology or serum chemistry parameters (included counts of red blood cells, platelets, white blood cells, and white-blood-cell differential; hematocrit; hemoglobin; mean corpuscular volume and hemoglobin concentration; total reticulocytes; SDH; ALT; AP; total bile acids; total bilirubin; urea; nitrogen; creatinine; creatine kinase; total protein; fasting glucose; albumin; cholesterol; isocitrate dehydrogenase; sodium; postassium; chloride; phosphorous; magnesium; and calcium).
In a subsequent study, Hurtt et al. (1992) exposed groups of five male and three female adult cynomolgus monkeys to measured concentrations of DMF at 0, 30, 100, or 500 ppm for 6 h/day, 5 days/week, for 13 weeks using a flow-through exposure chamber (15 air changes/h or 1,025 L/min). No exposure-related changes in clinical signs (assessed once/week; twice daily for morbidity or mortality); body weight (measured prior to first exposure, weekly during the study, and at study termination); hematology or serum chemistry analysis or urinalysis (measured prior to study initiation after first exposure; end of exposure weeks 2, 4, 8, and 12; and at study termination), semen analysis (volume, motility, count, or morphology; measured three times prior to study initiation and once/week during study), or gross necropsy findings were observed.
3.4.2. Rats
Groups of 10 Fischer 344 (F344)/DuCrj male and female rats were exposed to DMF for 6 h/day, 5 days/week at target concentrations of 0, 100, 200, 400, 800, or 1,600 ppm for 2 weeks or at 0, 50, 100, 200, 400, or 800 ppm for 13 weeks (Senoh et al. 2003). In the 2-week exposure group, exposure at 1,600 ppm resulted in the death of three males and seven females, death occurring after the third, fourth, fifth, and tenth exposure. Death was attributed to marked
centrilobular necrosis of the liver. No clinical signs were noted in any of the other groups. Reduced body-weight gain was observed in male rats exposed to DMF at ≥800 ppm and in female rats exposed at ≥ 400 ppm. Histopathologic examination of rats in the 1,600-ppm exposure group revealed massive hepatic necrosis extending over more than two lobules and centrilobular fibrosis in a more limited area. Centrilobular single-cell necrosis associated with fragmented nucleoli was also seen in male and female rats exposed at 800 ppm. In the 13-week exposure study, body weight was reduced in male and female rats exposed to DMF at 400 and 800 ppm, and feed consumption was reduced in rats exposed at 800 ppm. Relative liver weight was increased in male rats exposed at ≥100 ppm and in female rats exposed at ≥200 ppm. Histopathologic examination revealed increased hepatic single-cell necrosis in male and female rats exposed at ≥200 ppm and in centrilobular hypertrophy in male and female rats exposed at ≥400 ppm. Clinical chemistry analyses revealed increased ALT, AST, lactate dehydrogenase (LDH), GGT, total cholesterol, and phospholipid in male and female rats (generally occurring in males exposed at ≥50 ppm and females exposed at ≥200 ppm). Benchmark dose analysis found a repeat exposure BMDL10 (lower confidence limit on the benchmark dose corresponding to a 10% response) of 1.1 and 13.1 ppm for the increased relative liver weight in males and females, respectively, and of 68.5 and 191 ppm for cellular hypertrophy in males and females, respectively.
Groups of 30 male or 30 female F344/N rats were exposed to air containing measured concentrations of DMF at 0, 50, 100, 200, 400, or 800 ppm for 6 h/day, 5 days/week, for 13 weeks (NTP 1992; Lynch et al. 2003). Rats were subdivided into three groups: 10 rats/sex in a base study group, 10 rats/sex in a cardiovascular group, and 10 rats/sex in a renal function group. In the base group, rats were observed twice/day for mortality or moribundity, and body weight and clinical observations were recorded weekly. Blood was drawn for hematologic and clinical chemistry analysis at days 4, 24, and 91 of the study. Necropsy was conducted on all base group rats, and most tissues from the control and high-concentration groups and the livers from rats from all base groups were examined histologically. In the cardiovascular group, blood pressure and electrocardiograms were taken within 24 h of the last DMF exposure, and the hearts of these animals were examined microscopically. In the renal function group, animals were placed in metabolism cages and urine collected for 16 h at the end of the study, and kidneys were collected at necropsy and evaluated histologically. Sperm morphology was evaluated in males at necropsy, and vaginal cytology was investigated in females 2 weeks prior to necropsy by evaluating vaginal lavage fluid. All animals survived to study termination. It was noted that DMF was mildly irritating to rats exposed at 400 or 800 ppm, as indicated by occasional nasal and ocular discharges. Absolute body weight and body-weight gain were decreased in the 400- and 800-ppm males and females. Alterations in liver enzymes (ALT, SDH, and isocitrate dehydrogenase) and bile salts were present at the first analysis on day 4 in the 400- and 800-ppm males and females. Serum cholesterol was increased in all exposed rats; marginal increases in abso-
lute and relative liver weights were observed. Histologic evaluation revealed minimal-to-moderate necrosis of individual hepatocytes around the central veins and the presence of macrophages containing a golden-brown pigment in the 400- or 800-ppm male and female rats. High-dose female rats had an increased length of estrous cycle compared with controls. No definitive exposure-related effects were observed in renal function, blood pressure, or electrocardiogram readings or in male reproductive parameters.
Groups of F344 rats (number and sex not specified) inhaled DMF at 0, 150, 300, 600, or 1,200 ppm (average measured concentrations of 149, 302, 587, and 1,184 ppm) in a 1.5-m3 dynamic chamber (airflow approximately 283 L/min) for 6 h/day, 5 days/week, for 12 weeks (Craig et al. 1984). Death occurred before study termination in one male at 300 ppm and in one male and one female at 1,200 ppm; the day of death was not provided. Nasal discharge described as “crusty nose” was observed most frequently at week 2 in all groups exposed to DMF, but this observation was not concentration-related. Body weight was reduced in males at 1,200 ppm beginning at week 4 and in the females at 1,200 ppm beginning at week 2. A concentration-related increase in serum cholesterol was observed in both males and females, the increases being statistically significant at 600 and 1,200 ppm. Females exposed at 1,200 ppm developed hepatocellular collapse near the central veins occasionally extending to the portal zones; fibrosis; accumulation of yellow-brown pigment in Kupffer cells, macrophages, and hepatocytes; variations in nuclear and cell size with the presence of large hepatocytes; and dark staining of the cytoplasm of hepatocytes. Examination of the livers from females exposed at 600 ppm revealed the presence of only a small amount of hepatic pigment and no collapse or fibrosis, but variations in nuclear size and cytoplasmic variations were present to a lesser degree than those at 1,200 ppm. The only hepatic lesions noted in females exposed at 300 ppm were barely discernible variations in nuclear size and cytoplasmic variations. The authors stated that similar hepatic changes were observed in exposed males except for there being little or no collapse and no fibrosis. Histopathologic evaluation of the liver from the one male and one female rat that died early in the 1,200-ppm group revealed widespread collapse, necrosis, and pigment accumulation. One liver also exhibited innumerable mitotic figures (animal affected not identified).
To determine whether age influences the toxicity of inhaled DMF, groups of 10 3-, 4-, 5-, 8-, or 12-week-old female Sprague-Dawley rats were subdivided into two groups of five each: One group at each age was exposed to DMF at 200 ppm in a 1.5 m3-chamber (ventilation rate of 200 L/min) for 8 h/day, 7 days/week, for 4 weeks, and the other rats served as control groups (Tanaka 1971). At study termination, biochemical tests (protein, AST, ALT, AP, and LDH) and histopathologic evaluations were performed. Significantly increased levels of ALT and AST were observed only in 3-week-old rats. No other biochemical changes were observed. All exposed rats exhibited histopathologic changes in the liver, generally in the central zone of the lobules. The primary alteration was degeneration, as indicated by cloudy swelling of liver cells. In
some cases, mild fatty change was also noted. It appeared that the younger the rat, the more pronounced the hepatic damage. A correlation was also found between the increase of transaminases and the extent of pathologic change in the liver.
In a second experiment by Tanaka (1971), two groups of 15 3-week-old female Sprague-Dawley rats inhaled DMF at 200 ppm in a 0.6 m3-gas tight chamber (ventilation rate of 180-200 L/h) for 1 h/day or 8 h/day for 4 weeks. A third group was not exposed and served as the concurrent control. Within each group, five animals were killed at the end of 1, 2, or 4 weeks of exposure, and biochemical and histopathologic evaluations were conducted as in the previous experiment (see previous paragraph). Increased AST and ALT were present in both exposure groups after 1 week of exposure. Histopathologic changes in the liver were the same as those noted in the previous experiment, the changes being more extensive in rats exposed for 8 h daily compared with rats exposed for only 1 h daily. Degeneration was most extensive in rats exposed 8 h daily for 1 week. After 2 and 4 weeks of exposure, hepatic degeneration and marked regeneration were present in both groups of exposed rats.
Groups of 16 rats were exposed to DMF at 100, 230, or 450 ppm in a 0.6-m3 gas tight chamber (ventilation rate of 180-200 L/h) for 8 h/day, 6 days/week, for up to 120 days (Massmann 1956). During the study, rats were said to have been unaffected by exposure to DMF, and there was no change in body weight. Necropsy revealed liver necrosis. Bronchopneumonic changes were noted in some animals, and hyperemia of the brain, cloudy swelling of renal tubules, and iron deposits in the spleen were observed. However, the report combined experiments on rats and cats, and it was not stated which species (cats or rats) and at what concentration these effects were noted. No other experimental details were provided.
3.4.3. Mice
Groups of 10 Crj:BDF1 male and female mice inhaled DMF for 6 h/day, 5 days/week, at target concentrations of 0, 100, 200, 400, 800, or 1,600 ppm for 2 weeks or concentrations of 0, 50, 100, 200, 400, or 800 ppm for 13 weeks (Senoh et al. 2003). All mice survived to study termination; no adverse clinical signs were reported. In the 2-week exposure group, reduced body-weight gain was observed in males and females exposed at 1,600 ppm (exact values not provided). Relative liver weights were increased in male mice exposed at ≥400 ppm and in female mice exposed at ≥200 ppm (values not reported). Histopathologic examination of mice exposed at 1,600 ppm revealed focal necrosis structured with small clusters of necrotic hepatocytes and inflammatory cell infiltrate with single-cell necrosis associated with fragmented nucleoli. Centrilobular degeneration was present in male mice exposed at ≥200 ppm and in female mice exposed at ≥800 ppm. In the 13-week study, males exhibited reduced body weight and increased relative liver weight at all DMF concentrations and a reduction in feed
consumption at 800 ppm, but no significant differences in these parameters were observed in any of the females. Histopathologic examination revealed increased centrilobular hypertrophy in males exposed at ≥50 ppm and in females exposed at 800 ppm. Single-cell necrosis was noted in the males and females exposed at 800 ppm. Focal necrosis was increased in male and female mice exposed at ≥100 ppm, but the increase was not related to concentration. Clinical chemistry analysis revealed increased ALT, LDH, and total cholesterol in male and female mice (at ≥100 ppm).
Groups of 10 male and 10 female B6C3F1 mice were exposed to air containing measured concentrations of DMF at 0, 50, 100, 200, 400, or 800 ppm for 6 h/day, 5 days/week, for 13 weeks (NTP 1992; Lynch et al. 2003). Mice were checked twice per day and body weight, and clinical observations were recorded weekly. Blood was drawn at days 4, 24, and 91 of the study. Sperm morphology was evaluated at necropsy, and vaginal cytology was investigated 2 weeks prior to necropsy by evaluating vaginal lavage fluid. Necropsy was conducted on all mice, and most tissues from the control and high-concentration groups and the livers from mice at all exposure concentrations were examined histologically. No treatment-related deaths were observed (five deaths in exposed male mice were considered incidental). Body-weight gain was decreased in male and female rats exposed at 800 ppm. A concentration-related increase in liver weight was evident in all exposure groups, although the increases were biologically significant only at 200 ppm and higher. Microscopic examination of the liver revealed minimal-to-mild centrilobular hepatocellular hypertrophy in all male exposure groups and in females exposed at 100 ppm or higher. No changes in reproductive tissue parameters were noted in exposed male mice, and female mice showed a significant trend toward an increase in the estrous cycle length.
Groups of 10 male and 10 female B6C3F1 mice were exposed to DMF at 0, 150, 300, 600, or 1,200 ppm (average measured concentrations of 149, 302, 587, or 1,184 ppm) in a 1.5-m3 dynamic inhalation chamber (airflow approximately 283 L/min) for 6 h/day, 5 days/week, for 12 weeks (Craig et al. 1984). One male at 150 ppm, two males at 600 ppm, and five males at 1,200 ppm were found dead or were killed in moribund condition, and three females at 1,200 ppm were killed in moribund condition (day of death not reported). Other animals that were accidentally killed or withdrawn for other reasons (further details not provided) were evenly distributed among all the groups. Body weight was not affected by exposure, and no clinical toxicity signs or abnormalities in hematology or clinical chemistry parameters were observed. Necropsy of the animals that died or were killed early revealed one male and one female mouse from the 1,200-ppm group with livers exhibiting single-cell necrosis. It is unclear from the study description if the other deaths or early kills were related to exposure. Necropsy of animals surviving to study termination revealed focal areas of discoloration and alterations in the consistency of livers from one male and one female at 600 ppm and from two females at 1,200 ppm. Histologic evaluation was conducted on the livers of nine, nine, nine, six, and five male mice in the 0-, 150-, 300-, 600-, or 1,200-ppm groups, respectively, and on the
livers of eight, eight, eight, 10, and five female mice in the 0-, 150-, 300-, 600-, or 1,200 ppm groups, respectively. Hepatic changes in males included areas of collapse with the presence of phagocytes containing yellow-brown pigment in three males at 600 ppm and two males at 1,200 ppm. One male at 300 ppm had a large area of coagulative necrosis. In females, liver necrosis was observed in one, one, and two mice from the 150-, 600-, and 1,200-ppm groups, respectively. Kupffer’s cells and phagocytes containing pigment were present in only three mice from the 600- and 1,200-ppm groups. Concentration-related hepatic cytomegaly around central veins was found in all groups exposed to DMF.
3.4.4. Cats
Groups of two cats inhaled air containing DMF at 100, 230, or 450 ppm in a 0.6-m3 gas tight chamber (ventilation rate of 180-200 L/h) for 8-h/day, 6 days/week, for up to 120 days (Massmann 1956). Cats had a poor appetite and lost weight during the course of the study. No other toxicity signs were observed. No changes were observed in hematology, urinalysis, or ECG recordings. Necropsy revealed fatty hepatic degeneration but no necrosis. Bronchopneumonic changes were noted in some animals, and hyperemia of the brain, cloudy swelling of renal tubules, and iron deposits in the spleen were observed. It was not clear in which animals (cats or rats) and at what concentrations and exposure durations these effects were noted. No other details were provided.
3.5. Genotoxicity
In vitro, DMF was not mutagenic in Salmonella typhimurium strains TA1535, TA100, TA1537, TA1538, and TA98 with or without metabolic activation (Antoine et al. 1983; NTP 1992) and did not induce sister chromatid exchanges or chromosomal aberrations in Chinese hamster ovary cells with or without metabolic activation (NTP 1992). In vivo, DMF was negative in the micronucleus test on mouse bone-marrow cells and did not cause mouse sperm abnormalities (Antoine et al. 1983). One laboratory found a marginal response in mouse lymphoma L5178Y/Tk+/− cells following exposure of DMF, but this response was not seen in two other laboratories (NTP 1992).
3.6. Carcinogenicity
Groups of 87 male and 87 female Crl:CD BR rats and 78 male and 78 female Crl:CD-1 (ICR) BR mice were exposed to DMF at 0, 25, 100, or 400 ppm for 6 h/day, 5 days/week, for up to 2 years (rats) or 18 months (mice) (E.I. Dupont de Nemours & Co. 1992; Malley et al. 1994). Female rats exposed at 400 ppm and male rats exposed at 100 or 400 ppm had decreased absolute body weight, and body-weight gain was reduced in males and females at 100 or 400
ppm. Males and females exposed at 100 or 400 ppm had exposure-related increases in SDH activity, relative liver weight, centrilobular hepatocellular hypertrophy, and centrilobular lipofuscin and hemosiderin accumulation. Males and females exposed at 400 ppm had an increased incidence of centrilobular single-cell necrosis of hepatocytes, males at 100 or 400 ppm had an increased incidence of hepatic focal cystic degeneration, males at 100 ppm and males and females at 400 ppm had an increased incidence of hepatic clear-cell foci, and females at 400 ppm had an increased incidence of eosinophilic foci. Under the conditions of this study, DMF was not oncogenic in rats, and exposure of up to 400 ppm failed to result in any exposure-related effects on the estrous cycle.
In mice, body weight and body-weight gain were not affected by DMF exposure at up to 400 ppm; males and females at 100 or 400 ppm had increased body weight and body-weight gain compared with controls (E.I. Dupont de Nemours & Co. 1992; Malley et al. 1994). Absolute and relative liver weights were increased in males at 100 ppm and 400 ppm and in females at 400 ppm. Male and female mice from all test groups also exhibited centrilobular hepatocellular hypertrophy, increased incidence of individual hepatocellular necrosis and Kupffer cell hyperplasia with accumulation of lipofuscin and hemosiderin, and a concentration-related increase in eosinophilic and mixed foci of cellular alteration. Under the conditions of this study, DMF was not oncogenic in mice, and exposure of up to 400 ppm failed to result in any exposure-related effects on the estrous cycle.
Groups of 50 male and 50 female F344/DuCrj (SPF) rats and 50 male and 50 female Crj:BDF1 (SPF) mice were exposed to DMF at 0, 200, 400, or 800 ppm for 6 h/day, 5 days/week, for 104 weeks (Senoh et al. 2004). Three male rats and 13 female rats from the 800-ppm group died by 13 and 21 weeks, respectively. A concentration-related reduction in absolute body weight was observed in DMF-exposed male and female rats, and females exposed at 800 ppm reduced their food consumption. Clinical chemistry revealed a concentration-related increase in AST, ALT, GGT, and AP activity in male and female rats, and males at 800 ppm had increased LDH. Relative liver weight was increased in all DMF-exposed groups. Gross necropsy of the liver of rats that died within the first 21 weeks revealed red zones or accentuated lobular structure, and histopathology revealed centrilobular necrosis in three of the males and all 13 of the females that died. Terminal necropsy of surviving rats revealed white or brown hepatic nodules in males and females exposed at 800 ppm. Histopathology of the males exposed to DMF found increased spongiosis hepatitis at 200 ppm and above, clear-cell foci and eosinophilic-cell foci at 400 ppm and above, and basophilic-cell, mixed-cell, and vacuolated-cell at 800 ppm. Histopathologic examination of the female rats revealed an increase in clear-cell foci at 200 ppm and above, and eosinophilic-cell foci at 400 ppm and above. Neoplastic lesions included an increase in hepatocellular adenomas at 400 ppm and above and hepatocellular carcinomas at 800 ppm. Data are summarized in Table 1-8.
TABLE 1-8 Incidences of Neoplastic and Non-neoplastic Liver Lesions in Rats and Mice Exposed to DMF for 2 Years
| Concentration, ppm | Males | Females | ||||||
| 0 | 200 | 400 | 800 | 0 | 200 | 400 | 800 | |
| Number of Rats | 50 | 50 | 50 | 50 | 49 | 50 | 50 | 50 |
| Neoplastic lesions | ||||||||
|
Hepatocellular adenoma |
1 | 3 | 13** a | 20** | 1 | 1 | 6 | 16** |
|
Hepatocellular carcinoma |
0 | 1 | 0 | 24** | 0 | 0 | 0 | 5*a |
|
Total hepatocellular tumors. |
1 | 4 | 13** | 33** | 1 | 1 | 6 | 19** |
| Pre-neoplastic lesions | ||||||||
|
Altered-cell foci |
23** | |||||||
|
Clear-cell foci |
11 | 21 | 35** | 40** | 3 | 4 | 33** | 33** |
|
Eosinophilic-cell foci |
13 | 14 | 34** | 40** | 0 | 27 | 10** | 22** |
|
Basophilic-cell foci |
24 | 26 | 29 | 42** | 23 | 0 | 15 | 29 |
|
Mixed-cell foci |
0 | 0 | 1 | 6* | 0 | 0 | 0 | 1 |
|
Vacuolated-cell foci |
6 | 0* | 7 | 16* | 0 | 0 | 1 | 3 |
|
Spongiosis hepatitis |
4 | 21** | 26** | 24** | 0 | 0 | 2 | |
| Non-neoplastic lesions | ||||||||
|
Necrosis: centrilobular |
1 | 5 | 0 | 5 | 0 | 3 | 2 | 13** |
|
Number of Mice |
50 | 50 | 49 | 50 | 49 | 50 | 50 | 49 |
| Neoplastic lesions | ||||||||
|
Hepatocellular adenoma |
6 | 36** | 41** | 41** | 1 | 42** | 47** | 48** |
|
Hepatocellular carcinoma |
2 | 12** | 16** | 16** | 3 | 25** | 32** | 35** |
|
Hepatoblastoma |
0 | 13** | 7** | 4 | 0 | 0 | 4 | 0 |
|
Total hepatocellular tumors |
8 | 42** | 46** | 44** | 3 | 45** | 49** | 49** |
| Pre-neoplastic lesions | ||||||||
|
Altered-cell foci |
||||||||
|
Clear-cell foci |
4 | 21** | 13** | 17** | 3 | 7 | 4 | 2 |
|
Eosinophilic-cell foci |
1 | 38** | 41** | 42** | 1 | 43** | 43** | 48** |
| Non-neoplastic lesions | ||||||||
|
Centrilobular hypertrophy |
0 | 39** | 41** | 48** | 2 | 11* | 5 | 16** |
|
Nuclear atypia: centrilobular |
0 | 33** | 42** | 45** | 2 | 7 | 3 | 16** |
|
Necrosis: single cell |
12 | 38** | 43** | 48** | 22 | 13 | 6** | 19 |
|
Inflammatory cell nest |
15 | 37** | 42** | 48** | 24 | 13* | 4** | 19 |
aStatistically significant compared with controls: *p <0.05; **p <0.01.
Source: Adapted from Senoh et al. 2004.
Overall, survival was unaffected in mice that inhaled DMF at up to 800 ppm for 2 years (Senoh et al. 2004). However, two males and 14 females in the 800-ppm group, six males and seven females in the 400-ppm group, and four males and four females from the 200-ppm group died of hepatocellular tumors. Body-weight gain of DMF-exposed mice was suppressed in a concentration-related manner, but food consumption was not affected. Clinical chemistry analyses revealed a concentration-related increase in AST, ALT, LDH, AP, and total cholesterol in male and female mice. Relative and absolute liver weights were increased in all DMF-exposed groups. Gross necropsy revealed multiple occurrences of white, brown, or red nodules in the livers of almost all of the DMF-exposed groups. Histopathology of the livers from male mice revealed an increase in clear-cell and eosinophilic-cell foci, centrilobular hypertrophy, centrilobular nuclear atypia, single-cell necrosis, and inflammatory cell nests at 200 ppm and above. Histopathologic examination of the livers from female mice revealed an increase in eosinophilic-cell foci at 200 ppm and above, and centrilobular hypertrophy at 800 ppm. Neoplastic lesions included an increase in hepatocellular adenomas and hepatocellular carcinomas at 200 ppm and above in both male and female mice. Hepatoblastomas were elevated above historical controls in males at 200 ppm and above and in females at 400 ppm. Data are summarized in Table 1-8.
IARC (1989) concluded that there was inadequate evidence for the carcinogenicity of DMF in experimental animals, but that there was limited evidence in humans. The overall evaluation was that DMF is possibly carcinogenic to humans (Group 2B). A carcinogenicity assessment was not conducted for DMF in the IRIS database (EPA 1990).
3.7. Summary
A summary of lethal and nonlethal effects of acute DMF exposure can be found in Table 1-9, and a summary of subchronic inhalation exposure is presented in Table 1-10. Acute exposures of mice and rats to high concentrations of DMF resulted in mortality, while low and intermediate concentrations resulted only in alterations of liver enzymes. Repeated exposure of rats, mice, and cats to DMF generally resulted in decreased body weight and hepatotoxicity, as indicated by increases in liver enzymes and histopathologic changes in the liver, including degeneration and necrosis. However, 6-h exposures of monkeys to DMF at 500 ppm did not result in any measurable effects. Developmental toxicity studies reported maternal effects of reduced maternal body weight, and developmental effects included reduced fetal weight; increases in the litter incidence of total external, skeletal, and visceral malformations and skeletal variations; and an increased number and percentage of dead implants. Genotoxicity testing of DMF has generally been negative. One study found no evidence of carcinogenicity when mice and rats inhaled DMF for 2 years; another study
found that chronically inhaled DMF was associated with hepatocellular adenomas and carcinomas in rats and hepatoblastomas and hepatocellular adenomas and carcinomas in mice.
TABLE 1-9 Summary of Acute Inhalation Data in Laboratory Animals
| Concentration (ppm) | Duration | Effect | Reference |
| Rat | |||
| 3,700 | 1, 3 h | 3/3 males and 3/3 females survived; excessive grooming | Shell Oil Company 1982 |
| 7 h | 2/3 males and 3/3 females died; excessive grooming, lethargy | ||
| 5,000 | 4 or 8 h | No mortality (within 24 h) | Lundberg et al. 1986 |
| 280, 560 | 4 h | SDH increased 20 h postexposure (no concentration response) | Lundberg et al. 1986 |
| 2,250 | SDH not affected; no histologic hepatic changes | ||
| 153 | 4 h | SDH increased (2-fold) 24 h postexposure | Roure et al. 1996 |
| 313 | SDH and GDH increased (6-fold) 24 h postexposure | ||
| 441 | SDH and GDH increased (10.5-fold) 24 h postexposure; at 72 h postexposure, only GDH increased 1.5 fold | ||
| 991 | SDH (140-fold) and GDH increased (130-fold) 48 h postexposure; at 72 h postexposure, only GDH increased 20-fold | ||
| 126, 281, 314 | 4 h | GDH increase: 38%, 516%, and 260%, respectively ALT increase: 37%, 54%, 50%, respectively SDH increase: 130%, 325%, 379%, respectively | Brondeau et al. 1983 |
| Mouse | |||
| 1,628, 2,110 | 10 min | Respiratory rate decrease of 12.8% and 28.3%, respectively; RD50 could not be determined | Kennedy and Sherman 1986 |
| 3,981 | 2 h | LC50 | Stasenkova 1961 |
| 670 | Highest no effect for mortality | ||
Abbreviations: SDH, sorbitol dehydrogenase; GDH, glutamate dehydrogenase; ALT, alanine aminotransferase; RD50, concentration of a substance that reduces the respiratory rate by 50%; LC50, concentration of a substance that is lethal to 50% of the exposed population.
TABLE 1-10 Summary of Repeated Exposure Inhalation Data in Laboratory Animals
| Concentration (ppm) | Duration | Effect | Reference |
| Monkey | |||
| 500 | 6 h/d, 5 d/wk, 2 wk | No adverse effects after first exposure | Hurtt et al. 1991 |
| 500 | 6 h/d, 5 d/wk, 13 wk | No adverse effects | Hurtt et al. 1992 |
| Rat | |||
| 2,523 | 6 h/d, 5 d | 8/10 died (7 of acute liver necrosis; 1 of acute pulmonary edema and congestion); progressive weakness, discomfort, weight loss | Kennedy and Sherman 1986 |
| 50, 100, 200 400, 800 | 6 h/d, 5 d/wk, 13 wk | Increased cholesterol in all exposed groups; mildly irritating (occasional nasal and ocular discharge); increased ALT, SDH, isocitrate dehydrogenase, bile salts on exposure day 4; decreased body weight; hepatocellular necrosis | NTP 1992 |
| 150, 300, 600 1,200 | 6 h/d, 5d/wk, 12 wk | No adverse effect Variations in hepatic nuclear size and cytoplasmic variations at 300 ppm and above; increased cholesterol at 600 ppm and above; 1 male and 1 female died, liver necrosis; surviving rats, decreased body weight, liver necrosis | Craig et al. 1984 |
| 100, 200, 400 800, 1,600 | 6 h/d, 5 d/wk, 2 wk | No clinical signs at any concentration Decreased growth rate at 400 ppm and above Centrilobular single-cell necrosis 3 males and 7 females died within 10 exposures; massive hepatic necrosis | Senoh et al. 2003 |
| 50, 100 200, 400, 800 | 6 h/d, 5 d/wk, 13 wk | Increased liver enzymes at 50 ppm and above Increased relative liver weight at 100 ppm and above | Senoh et al. 2003 |
| Increased hepatic single-cell necrosis at 200 ppm and above Centrilobular hypertrophy at 400 ppm and above, decreased body weight; decreased body weight and food consumption | |||
| 100, 230, 450 | 8 h/d, 6 d/wk, 120 d | Appeared unaffected by exposure | Massmann 1956 |
| Concentration (ppm) | Duration | Effect | Reference |
| Mouse | |||
| 100, 200, 400, 800, 1,600 | 6 h/d, 5 d/wk, 2 wk | No clinical signs at any concentration Increased relative liver weight at 200 ppm and above Decreased growth rate, focal hepatic necrosis at 1,600 ppm | Senoh et al. 2003 |
| 50, 100, 200, 400, 800 | 6 h/d, 5 d/wk, 13 wk | Decreased growth rate and increased liver weight in all exposed groups; centrilobular hypertrophy in males at 50 ppm and above Increased liver enzymes at 100 ppm and above Decreased food consumption, single-cell necrosis in liver, centrilobular hypertrophy in females at 800 ppm | Senoh et al. 2003 |
| 50, 100, 200, 400, 800 | 6 h/d, 5 d/wk, 13 wk | Mild centrilobular hepatocellular hypertophy at ≥50 ppm Significant increase in liver weight at ≥200 ppm Decreased growth rate at 800 ppm | NTP 1992 |
| 150, 300, 600, 1,200 | 6 h/d, 5d/wk, 12 wk | 1 died; hepatic cytomegaly around central vein at ≥150 ppm Histopathologic hepatic changes at ≥300 ppm 2 died at 600 pm 8 died or killed moribund; single-cell necrosis in 2 mice at 1,200 ppm | Craig et al. 1984 |
| Cat | |||
| 100, 230, 450 | 8 h/d, 6 d/wk, 120 d | Poor appetite, weight loss; no effects on clinical signs, blood analysis, urinalysis, ECG recordings; necropsy, fatty degeneration and no necrosis | Massmann 1956 |
4. SPECIAL CONSIDERATIONS
4.1. Absorption and Disposition
Mraz and Nohova (1992) determined that pulmonary retention of DMF was approximately 90% following exposure of groups of male or female volunteers to DMF at 3, 10, or 20 ppm (reported as 10, 30, or 60 mg/m3) for 8 h. Approximately 20% of excreted metabolites recovered in the urine were from absorption of DMF vapor through the skin.
Hurtt et al. (1991) also observed that dermal absorption accounts for a measurable amount of total DMF absorbed during an inhalation exposure in which one cynomolgus monkey was exposed by whole-body inhalation and another was exposed head only to DMF at 500 ppm for 6 h. Plasma taken 0.5 to
18 h postexposure revealed that the DMF area under the curve (AUC) value was three times greater in the monkey exposed by whole-body exposure compared with the monkey exposed by head only, indicating that dermal exposure to DMF vapor contributed significantly to the total DMF absorbed dose. The authors also investigated the amount of DMF absorbed by the respiratory tract by measuring the tidal volume and the DMF concentration going into and out of the head-only exposure unit and found that pulmonary absorption was approximately 100%. Lundberg et al. (1983) commented that only negligible amounts of DMF should be found in expired air based on the fact that DMF dissolves in water by hydrogen binding, resulting in a loss of vapor pressure in the respiratory system.
Mraz et al. (1989) reported that the absorbed dose by humans following inhalation exposure to DMF at 20 ppm for 8 h was one-half of that absorbed by male mice, rats, and hamsters after a single intraperitoneal injection of DMF at 0.1, 0.7, or 7 mmol/kg of body weight. However, it must be emphasized here that the rodents were exposed via a parenteral injection route, and the rodents received a much higher bolus dose than humans.
Distribution of DMF and its metabolites was fairly uniform among blood, liver, kidney, brain, and adrenals following a 4-h inhalation exposure of female rats to DMF at 565 or 2,250 ppm, the blood containing slightly higher concentrations (Lundberg et al. 1983). DMF and its metabolites were no longer detected in these tissues by 20 h postexposure to DMF at 565 ppm, or by 48 h postexposure at 2,250 ppm.
Quantitative data on the placental transfer of inhaled DMF were not available. Oral data assessing metabolic tissue profile, time-course disposition, and transfer into milk following a single dose of 14C-DMF at100 mg/kg by gavage were discussed by Saillenfait et al. (1997). Exposure to a single oral 100-mg/kg bolus produced maternal toxicity as evidenced by reductions in maternal body-weight gain, body-weight gain corrected for uterine weight, and feed consumption. Developmental toxicity was indicated by reduced weight. In the time-course disposition portion of the study, pregnant rats were dosed with 14C-DMF on GD 12 or GD 18 and examined over a 48-h period. Similar results were found at both GD 12 and GD 18; the results from GD 12 are described here. The radiolabel reached peak concentration in all tissues within 1 h after dosing, indicating rapid distribution, and remained elevated until 4 h, when the concentration declined. Total radioactivity in the placenta and embryo as a percentage of maternal plasma concentration was 64-70% and 79-93%, respectively, at 0.5-8 h after dosing. After 48 h, the ratio of placental and embryonic tissue radiolabel reached approximately three and four times the concentration in maternal plasma, respectively, suggesting a biphasic rate of distribution and elimination. Excluding the gastrointestinal tract, the highest percentage of the dose was found in the fetus, followed by amniotic fluid, maternal liver, and placenta, the percentages ranging from 6.52% to 2.41% of the administered dose. The metabolic profile of radioactivity and levels of parent DMF, N-(hydroxymethyl)-Nmethylformamide (HMMF) and N-methylformamide (NMF) were generally the same in maternal plasma, placenta, amniotic fluid, and fetuses from dams dosed
on GD 12 or GD 18. DMF levels were highest at 1 h postexposure, and HMMF and NMF were highest at 16 h postexposure. Negligible amounts of N-acetylS(N-methylcarbamoyl)cysteine (AMCC) and formamide were recovered. The authors concluded that DMF and its metabolites were transferred across the placenta by passive diffusion and that maternal plasma, embryo or fetus, placenta, and amniotic fluid belonged to the same compartment. Therefore, there is probably only minimal metabolic contribution, if any, of the placenta and fetus to metabolize DMF to HMMF, NMF, formamide, and AMCC. To assess the transfer of DMF into milk, lactating rats were dosed with 14C-DMF on postpartum day 14, and milk was collected up to 24 h after dosing. The concentrations of DMF, HMMF, and NMF in the milk were similar to those measured in maternal plasma.
4.2. Metabolism
DMF is metabolized primarily by hydroxylation of its methyl groups, and hepatic cytochrome P450 2E1 (CYP2E1) is important in the metabolism of DMF in both rats and humans (Mraz et al. 1993; Amato et. al. 2001). The primary urinary metabolite of humans, monkeys, dogs, rats, and mice is HMMF, which can decompose to NMF and formamide (reviewed in Gescher 1993). Many of the early studies report that NMF, a potent rodent hepatotoxicant, was the major DMF metabolite recovered in urine. However, it has since been determined that the conditions used in those early gas-chromatographic analyses resulted in thermolytic degradation of the HMMF metabolite to NMF. Studies measuring plasma concentrations of DMF and its metabolites in monkeys, rats, and mice following acute inhalation exposure reported that both HMMF and NMF are recovered when appropriate methods are used, and the concentrations of each in relation to the other vary; however, HMMF was the primary urinary metabolite recovered (Hundley et al. 1993a,b).
HMFF and NMF can be further metabolized to formamide. Another pathway for metabolism is the oxidation of the formyl group to unidentified reactive intermediates, which appear responsible for hepatotoxicity. The reactive intermediates can be conjugated with glutathione to S-(N-methylcarbamoyl) glutathione (SMG), ultimately forming the urinary metabolite AMCC (Mraz and Turecek 1987; Mraz et al. 1989).
Metabolism of DMF is a saturable process. The plasma AUC values for DMF and the metabolites NMF and HMMF (combined) were calculated using biologic material taken from groups of two male and two female cynomolgus monkeys inhaling DMF at 30, 100, or 500 ppm for 6 h in the Hurtt et al. (1992) study (Hundley et al. 1993b) or from groups of four male Crl:CD7BR rats and four male mice inhaling DMF at 10, 250, or 500 ppm for 1, 3, or 6 h (Hundley et al. 1993a). The DMF AUC in monkeys, rats, and mice increased disproportionately with exposure concentration; the DMF AUC increased 19- to 37-fold in male and 35- to 54-fold in female monkeys as the inhaled concentration in-
creased 5-fold (from 100 to 500 ppm), and the plasma AUCs increased 8- and 29-fold in rats and mice, respectively, as exposure concentration doubled (from 250 ppm to 500 ppm). Correspondingly, there was no increase in the NMF and HMMF AUC at the same exposure concentrations in rats or mice, supporting the hypothesis that metabolic saturation occurred. Through in vitro work in rat liver microsomes, Mraz et al. (1993) found that DMF actually inhibits the oxidation of NMF to SMG; the inhibition appears to be primarily competitive.
Mraz et al. (1989) exposed 10 healthy volunteers (five males and five females, age 26-56 years) to air containing a measured concentration of DMF at 20 ppm (reported as 60 mg/m3) for 8 h, and these investigators also gave male mice, rats, and hamsters DMF at 0.1, 0.7, or 7 mmol/kg of body weight via intraperitoneal injection. The absorbed dose by humans was one-half the lowest dose administered to the animals by injection. The major urinary metabolites recovered in humans over 72 h as a percentage of the administered dose were the following: 16-49% as HMMF, 8-24% as formamide (the precursor of which may be N-(hydroxymethyl)formamide (HMF) and 10-23% as AMCC. In rodents, the metabolites recovered in the urine expressed as a percentage of the administered dose were the following: 8-47% as HMMF, 8-38% as formamide, and 1-5% as AMCC. The authors concluded that “this is the first time that a quantitative difference has been observed in the metabolism of DMF between humans and rodents.” However, it must be emphasized here that the rodents were exposed via parenteral injection route, and the rodents received a much higher bolus dose than humans.
Excretion of DMF and its metabolites was almost exclusively via the urine. Following a single 4-h exposure of volunteers to DMF at 26 ppm (four men, age 25-50 years) or 87 ppm (three men and one woman, age 20-50 years), Kimmerle and Eben (1975b) found that DMF metabolites (reported as “NMF”) were detected in the urine following cessation of exposure, with approximately 50-70% of the metabolites recovered within 4 h postexposure. Formamide elimination was slightly delayed: Elimination occurred primarily 4-20 h postexposure, with significant amounts still detected 20-68 h postexposure. Low concentrations of unchanged DMF were found only in the urine of those exposed at 87 ppm. When volunteers (four men, age 25-50 years) were repeatedly exposed to DMF at 21 ppm for 4 h/day for 5 consecutive days, no accumulation of urinary metabolites was observed. Following each daily exposure, the DMF blood concentration decreased rapidly and was generally no longer detectable 4 h postexposure. Blood and urine analyses demonstrated that repeated exposure did not result in accumulation of the metabolite “NMF.”
In a follow-up study, Mraz and Nohova (1992) exposed volunteers (five males and five females, age 25-56) to DMF at 3.3, 10, or 20 ppm for 8 h and measured urinary metabolites over 120 h postexposure. Maximal excretion occurred between 6 and 8 h for DMF and HMMF, between 8 and 14 h for HMF and between 24-34 h for AMCC. The corresponding elimination half-lives were 2, 4, 7, and 23 h, respectively. Urinary metabolites were still present 120 h after exposure at 20 ppm. Mraz et al. (1993) demonstrated that DMF inhibits
CYP2E1 activity, thereby inhibiting its own metabolism. The authors propose that this inhibition could be the cause of the delayed urinary excretion of AMCC.
In comparing the metabolism of inhaled DMF following acute exposure in dogs and rats, Kimmerle and Eben (1975a) found species-specific differences in the time course of elimination. Groups of six male rats were exposed to DMF at 21, 146, or 2,005 ppm for 3 h or to 29 or 170 ppm for 6 h; two male dogs were exposed at 20 or 170 ppm for 6 h, and two female dogs were exposed at 31 or 134 ppm for 6 h. Although the chemical identity of the metabolites was no different between species, DMF metabolites were present longer in the blood and urine of dogs compared with rats. For example, following exposure at 170 ppm, metabolites were present in dog urine after 6 days, and DMF metabolites were found in rats only up to 24 h postexposure. A similar pattern was observed following exposure to DMF at 20 ppm. These differences in excretion might be related to body mass and metabolism rate: The smaller animals metabolize DMF at a higher rate and eliminated the chemical more quickly than larger animals.
4.2.1. Effect of CYP2E1 Polymorphisms on Metabolism
Nomiyama et al. (2001b) investigated human CYP2E1 PstI/RsaI polymorphism (CYP2E1*5B) in relation to urinary excretion of DMF and its metabolites. A group of 123 male Japanese workers were genotyped for CYP2E1. Of the 123 individuals, 77 were c1 homozygotes; 45 were c2 heterozygotes, and 1 was a c2 homozygote. From these individuals, 7, 5, and 1 of the c1 homozygotes, c2 heterozygotes, and c2 homozygote, respectively, were chosen for the exposure study. Volunteers were asked to refrain from drinking ethanol 24 h before or 72 h after exposure. Subjects were exposed once dermally to a vapor concentration of 6.2 ± 1.0 ppm and once via inhalation to 7.1 ± 1 ppm for a total of 8 h with at least 96 h separating the exposures. For the dermal exposure, 90% of the skin of the volunteers was exposed to vapors of DMF while the subjects sat in the exposure chamber and breathed fresh air through a respirator. During the inhalation exposure, the volunteers sat outside the exposure chamber and inhaled air containing DMF from the exposure chamber. Chamber DMF concentrations were monitored every 10 min using a gas chromatograph. Urine was collected up to 72 h postexposure. The half-lives of urinary NMF were assessed for the c1 homozygotes, c2 heterozygotes, and the c2 homozygote. Following dermal exposure, the urinary half-lives were 3.86 ± 1.90 h, 4.38 ± 1.53 h, and 4.20 h, respectively, and following respiratory exposure were 1.58 ± 0.42 h, 1.84 ± 0.61 h, and 3.20 h, respectively. No significant differences were noted. The authors noted that the urinary NMF half-life for the c2 homozygote following respiratory exposure was greater than the other two genotypes, but no rigorous conclusions could be drawn on the basis of only one data point.
Nomiyama et al. (2001a) investigated the effect of the insertion polymorphism of CYP2E1 (CYP2E1*1C does not have the insertion; CYP2E1*1D has
the insertion) on the biotransformation of DMF and measured urinary NMF in Japanese workers following DMF exposure. It had been suggested that CYP2E1 polymorphism might be associated with up to a 3-fold increase in activity in obese people or after ethanol consumption (McCarver et al. 1998). The frequency of the insertion polymorphism ranges from 0.011 for the Swedish up to 0.389 in Pigmy populations, with Caucasians having a frequency of 0.02 to 0.034 (summarized by Nomiyama et al. 2001a). In the Nomiyama et al. (2001a) study, 22 subjects had the CYP2E1*1C/*1C genotype, 21 had the *1C/*1D genotype, and 1 had the *1D*/1D genotype, with an overall frequency of the insertion polymorphism of 0.261. The participating subjects wore diffusive passive samplers attached to their collars during the last 8-h work shift to assess breathing zone DMF concentrations, and urine was collected “just after” the work shift (no further details provided). Mean DMF exposure was 4.3, 1.8, and 21.9 ppm for the respective genotypes. The slope of the line correlating DMF exposure levels with NMF levels measured in the urine was comparable across all groups. A multivariate analysis investigating the interaction between the *D1 allele and obesity or ethanol intake failed to reveal any significant contributions to the variability of NMF values in urine. The authors concluded that the *D1 allele had no appreciable influence on the metabolism of inhaled DMF as assessed by measurement of urinary NFM concentrations.
4.3. Mechanism of Toxicity
The exact mechanism of DMF-induced hepatotoxicity is unknown, but the hepatotoxicity observed after repeated DMF exposure is related to its metabolites, as only those formamides and acetamides that undergo oxidation at their formyl moiety are hepatotoxic (Kestell et al. 1987). The identification of AMCC as a urinary metabolite following DMF exposure (Mraz et al. 1989) prompted the hypothesis that DMF is metabolized to a reactive intermediate responsible for the liver damage unless it is conjugated with thiol-containing molecules to form N-methylcarbamic acid thioesters (Gescher 1993; Mraz et al. 1993). The unidentified reactive intermediate(s) is (are) postulated to be formed during metabolism of NMF or HMMF to SMG. Because AMCC has also been identified as a metabolite of methyl isocyanate (MIC), it has been speculated that MIC might be a reactive intermediate in the disposition of DMF. Support that MIC might be a metabolite of DMF is found in the studies reporting identical hemoglobin (Hb) adducts (N-methylcarbamoylated valine-globin) in workers exposed to DMF and individuals exposed to MIC, as well as those derived from the in situ reaction between Hb and MIC (Angerer et al. 1998; Kafferlein and Angerer 2001). The identical Hb adduct has also been identified at much lower concentrations in blood from the general population, indicating the adduct is not unique to DMF or MIC exposure.
The precise mechanism of the disulfiram-like symptoms that occur with combined ethanol consumption and DMF exposure is not known. Disulfiram
(Antabuse) has been used in treatment of alcoholism, where it inhibits aldehyde dehydrogenase, resulting in increasing circulating acetaldehyde (Hardman and Limbird 2001). Individuals treated with disulfiram who consume sufficient quantities of ethanol develop facial flushing, a pulsing headache, respiratory difficulties, nausea, vomiting, chest pain, hypotension, weakness, vertigo, and confusion. The facial flushing is then replaced by pallor, and blood pressure may plummet. Subjects exposed to DMF following ethanol consumption report face flushing, palpitation, headache, dizziness, body flushing, and tremors (Redlich et al. 1988; Fiorito et al. 1997; Wrbitzky 1999). Because DMF does not inhibit alcohol or aldehyde dehydrogenase in vitro, a metabolite of DMF might be responsible for the enzyme inactivation (Mraz et al. 1993).
4.4. Other Relevant Information
4.4.1. Species Variability
The mode of DMF-induced hepatotoxicity is thought to be related to the metabolism of DMF to reactive intermediates, and CYP2E1 plays a pivotal role in the metabolism of DMF (Mraz et al. 1993; Amato et al. 2001). Mraz et al. (1993) reported that the in vitro Michaelis constant (Km) and the maximum rate of metabolism (Vmax) values for CYP2E1 are comparable between human and rat liver (Table 1-11).
Despite the similar properties of hepatic CYP2E1 in rats and humans, it appears that there are species differences regarding the response to DMF exposure, rodents being more sensitive than primates. Monkeys inhaled DMF at 500 ppm for 6 h/day, 5 days/week, for up to 13 weeks with no measurable adverse effects (parameters examined include clinical signs, body weight, hematology and serum chemistry analyses, urinalysis, semen analysis, and gross necropsy findings) (Hurtt et al. 1991, 1992). In contrast, subchronic DMF inhalation exposure produced significant hepatic effects in rats at concentrations of 200 ppm (Senoh et al. 2003), 300 ppm (Craig et al. 1984), and 400 ppm (NTP 1992) and in mice at 100 ppm (Senoh et al. 2003), 150 ppm (Craig et al. 1984), and 200 ppm (NTP 1992). Indexes of toxicity after repeated DMF exposure ranged from elevated serum enzymes indicative of liver injury to hepatic degeneration and necrosis. Because humans are more similar to primates than to rodents, humans are expected to be less sensitive than laboratory animals (rodents).
4.4.2. Susceptible Subpopulations
Interindividual differences could affect the manifestation of DMF toxicity. First, CYP2E1 activity can be induced by moderate ethanol consumption, obesity, and diabetes (Gonzalez 1990; Song et al. 1990; Lucas et al. 1998; McCarver et al. 1998). Increased CYP2E1 levels could increase generation of
TABLE 1-11 Rat and Human Liver Microsome Kinetic Parameters for Metabolic Oxidation of Formamides
| Apparent Km (mM) | Apparent Vmax, Nmol/(mg of microsomal protein)/min | ||||
| Substrate | Product | Rat | Human | Rat | Human |
| DMFa | HMMF | 0.20 ± 0.06 | 0.12 ± 0.06 | 0.54 ± 0.20 | 0.57 ± 0.49 |
| NMFb | SMG | 4.28 ± 1.35 | 3.92 ± 2.11 | 0.34 ± 0.08 | 0.24 ± 0.17 |
| HMMFb | SMG | 2.52 ± 0.34 | 1.25 | 0.016 ± 0.005 | 0.033 |
aSubstrate concentration range: 0.02-5 mM.
bSubstrate concentration range: 0.4-10 mM.
Abbreviations: DMF, dimethylformamide; HMMF, N-(hydroxymethyl)-N-methylformamide; NMF, N-methylformamide; SMG, S-(N-methylcarbamoyl)glutathione.
Source: Mraz et al. 1993. Reprinted with permission; copyright 1993, Chemical Research in Toxicology.
the metabolites of DMF. Second, ethanol consumption prior to DMF exposure results in disulfiram-type reactions. Third, detoxification of the reactive intermediate is partly dependent on conjugation with glutathione. If glutathione levels are depleted, the potential exists for greater exposure to the reactive intermediates. Last, because repeated DMF exposure has produced hepatotoxicity in exposed workers (Kennedy 1986; Scailteur and Lauwreys 1987), individuals with compromised liver function or prior DMF contact may be at an increased risk.
4.4.3. Concentration–Exposure-Duration Relationship
Experimentally derived exposure values are scaled to AEGL time frames using the concentration-time relationship given by the equation Cn × t = k, where C = concentration, t = time, k is a constant, and n generally ranges from 1 to 3.5 (ten Berge et al. 1986). The value of n could not be empirically derived due to inadequate data; therefore, the default value of n = 1 was used for extrapolating from shorter to longer exposure periods, and a value of n = 3 was used to extrapolate from longer to shorter exposure periods.
5. DATA ANALYSIS FOR AEGL-1
5.1. Summary of Human Data Relevant to AEGL-1
The controlled human exposures in which the metabolism of DMF was investigated were not designed to assess the toxicity of DMF exposure (Kimmerle and Eben 1975b; Mraz et al. 1989; Mraz and Nohova 1992). It is not clear whether any symptoms, including irritation, were present.
5.2. Summary of Animal Data Relevant to AEGL-1
No animal data were found that were relevant to derivation of an AEGL-1.
5.3. Derivation of AEGL-1
An AEGL-1 is not recommended because an appropriate AEGL-1 end point was not noted in any of the available studies (Table 1-12).
6. DATA ANALYSIS FOR AEGL-2
6.1. Summary of Human Data Relevant to AEGL-2
No human data relevant to derivation of an AEGL-2 were available.
6.2. Summary of Animal Data Relevant to AEGL-2
In a developmental toxicity study, groups of 15 pregnant Himalayan rabbits were exposed to DMF at 0, 50, 150, or 450 ppm for 6 h/day on GD 7-19 (Hellwig et al. 1991). Over GD 7-19, mean maternal body-weight gain was reduced in dams exposed at 150 ppm compared with controls, while dams in the 450-ppm group lost weight. Mean maternal body-weight gain over the entire study period of GD 0-29 was also decreased in dams from the 150- and 450-ppm DMF groups compared with controls. Developmental toxicity was evident at 450 ppm as increases in external malformations and total malformations (external, soft tissue, and skeletal combined). Other effects included a reduction in fetal weight (86% of controls), and statistically significant increases in the litter incidence of skeletal variations, including splitting of skull bones, fused sternebrae, irregular shaped sternebrae, and bipartite sternebrae. An increase in fetal deaths did not occur. No developmental effects were observed at 150 ppm.
TABLE 1-12 AEGL-1 Values for DMFa
| 10 min | 30 min | 1 h | 4 h | 8 h |
| NRb | NR | NR | NR | NR |
aThere is a high potential for DMF to be absorbed dermally, so this route of exposure should be considered along with inhalation.
bAbsence of an AEGL-1 does not imply that exposure below the AEGL-2 is without adverse effects.
Abbreviation: NR, not recommended.
6.3. Derivation of AEGL-2
The AEGL-2 derivation was based on the study in which groups of 15 pregnant Himalayan rabbits were exposed to DMF at 0, 50, 150, or 450 ppm for 6 h/day on GD 7-19 (Hellwig et al. 1991). Over GD 7-19, mean maternal body-weight gain was reduced in dams exposed at 150 ppm compared with controls, while dams in the 450-ppm group lost weight; mean maternal body-weight gain over the entire study period of GD 0-29 was also decreased in dams from the 150- and 450-ppm DMF groups compared with controls. Developmental toxicity was evident at 450 ppm as increases in external malformations and total malformations (external, soft tissue, and skeletal combined). Other effects included a reduction in fetal weight (86% of controls), and statistically significant increases in the litter incidence of skeletal variations, including splitting of skull bones, fused sternebrae, irregular shaped sternebrae, and bipartite sternebrae. An increase in fetal deaths did not occur. No developmental effects were observed at 150 ppm. To protect against irreversible developmental effects (malformations), the rabbit NOAEL of 150 ppm for 6 h was used as the point of departure for derivation of AEGL-2 values (Hellwig et al. 1991).
A total uncertainty factor of 3 was applied to the point of departure of 150 ppm for 6 h: 1 for interspecies variability and 3 for intraspecies variability. An interspecies uncertainty factor of 1 was applied because it appears that primates are not as sensitive as rodents. Monkeys inhaled DMF at 500 ppm for 6 h/day, 5 days/week, for up to 13 weeks with no measurable adverse effects (parameters examined included clinical signs, body weight, hematology and serum chemistry analyses, urinalysis, semen analysis, and gross necropsy findings). In contrast, subchronic DMF inhalation exposure produced significant hepatic effects in rats at concentrations of 200 ppm (Senoh et al. 2003), 300 ppm (Craig et al. 1984), and 400 ppm (NTP 1992) and in mice at 100 ppm (Senoh et al. 2003), 150 ppm (Craig et al. 1984), and 200 ppm (NTP 1992). Indexes of toxicity after repeated DMF exposure ranged from elevated serum enzymes indicative of liver injury to hepatic degeneration and necrosis. From these exposure data, humans are expected to be less sensitive than laboratory animals (rodents). Because the mechanism of hepatotoxicity is thought to be related to the metabolism of DMF to a reactive intermediate, fetal toxicity is expected to result from exposure to the parent DMF or metabolites. An oral study assessing the tissue and metabolite distribution of DMF in pregnant rats indicated that DMF and its metabolites were transferred across the placenta by passive diffusion and that maternal plasma, embryo or fetus, placenta, and amniotic fluid belonged to the same compartment (Saillenfait et al. 1997). Therefore, the fetus and placenta will not provide any additional protection or enhancement of DMF toxicity because exposure to DMF and its metabolites will depend on the metabolism by the mother. An intraspecies uncertainty factor of 10 would normally be applied because a host of interindividual differences could affect the manifestation of DMF toxicity: (1) activity of CYP2E1, an enzyme that plays a pivotal role in the metabolism of DMF to reactive intermediates, can be induced by ethanol con-
sumption, obesity, diabetes, and other lifestyle and genetic factors (Gonzalez 1990; Song et al. 1990; Lucas et al. 1998; McCarver et al. 1998), and increased CYP2E1 levels increase the toxic metabolites of DMF; (2) prior consumption of ethanol can exacerbate DMF toxicity in individuals; (3) on the basis of the proposed mechanism of action, detoxification of the reactive intermediate is partly dependent on conjugation with glutathione; therefore, if glutathione levels are depleted for other reasons, the potential exists for greater exposure to the reactive intermediate; and (4) because DMF exposure can result in hepatotoxicity, those individuals with chronic liver disease may be at increased risk.
Application of a total uncertainty factor of 10 produces AEGL-2 values that are inconsistent with the available human data (values for the 10-min, 30-min, 1-h, 4-h, and 8-h AEGL-2 using default time-scaling would be 49, 34, 27, 17, and 11 ppm, respectively). Humans were exposed by inhalation to DMF at 87 ppm for 4 h or at 81 ppm for 2 h to assess the metabolism of DMF (Kimmerle and Eben 1975b; Eben and Kimmerle 1976). Although these single exposure studies were conducted to assess DMF metabolism, no adverse effects were reported, and the concentration can be considered an acute exposure concentration unlikely to result in adverse effects in healthy adults. Therefore, the intraspecies uncertainty factor is reduced to 3, resulting in a total uncertainty factor of 3.
The experimentally derived exposure value is scaled to AEGL time frames using the concentration-time relationship given by the equation Cn × t = k, where C = concentration, t = time, k is a constant, and n generally ranges from 1 to 3.5 (ten Berge et al. 1986). The value of n was not empirically derived because of inadequate data; therefore, the default value of n = 1 was used for extrapolating from shorter to longer exposure periods and a value of n = 3 was used to extrapolate from longer to shorter exposure periods. The 30-min AEGL-2 value was set equal to the 10-min value because of the uncertainty in extrapolating from a 6-h exposure duration to a 10-min duration.
The AEGL-2 values are presented in Table 1-13.
7. DATA ANALYSIS FOR AEGL-3
7.1. Summary of Human Data Relevant to AEGL-3
No human data relevant to derivation of an AEGL-3 were available.
7.2. Summary of Animal Data Relevant to AEGL-3
Groups of six rats (three male and three female) survived a 1- or 3-h exposure to 3,700 ppm, while exposure for 7 h resulted in mortality in two of three males and three of three females (Shell Oil Company 1982). Mortality occurred 2 or 3 days postexposure. Although no mortality was observed in groups of 10
TABLE 1-13 AEGL-2 Values for DMFa
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 110 ppm (330mg/m3) | 110 ppm (330 mg/m3) | 91 ppm (270 mg/m3) | 57 ppm (170 mg/m3) | 38 ppm (110 mg/m3) |
aThere is a high potential for DMF to be absorbed dermally, so this route of exposure should be considered along with inhalation.
female rats exposed at 5,000 ppm for 4 or 8 h, animals were observed for only 24 h for mortality (Lundberg et al. 1986). Stasenkova (1961) reported a NOEL for mortality of 670 ppm and a LOEL of 1,300 ppm in mice exposed to DMF for 2 h. A corresponding LC50 of 3,981 ppm was calculated by the reviewer. The reported exposure concentrations in the Stasenkova study are not deemed reliable because the highest reported exposure concentration was 7,700 ppm, well above the saturation point for this chemical.
7.3. Derivation of AEGL-3
The AEGL-3 derivation was based on the study in which groups of three male and three female rats were exposed to DMF at 3,700 ppm for 1 or 3 h; no mortality occurred, but exposure for 7 h resulted in 83% mortality (Shell Oil Company 1982). Clinical signs were limited to excess grooming in all exposure groups; lethargy was noted in rats exposed for 7 h. The end point of no mortality in rats exposed at 3,700 ppm for 3 h was chosen for the derivation.
A total uncertainty factor of 10 was applied to the point of departure for the AEGL-3: 1 for interspecies variability and 10 for intraspecies variability. The total uncertainty factor of 10 should protect against all but hypersensitive human hepatotoxic effects. An interspecies uncertainty factor of 1 was applied because it appears that primates are not as sensitive as rodents. Monkeys inhaled DMF at 500 ppm for 6 h/day, 5 days/week, for up to 13 weeks with no measurable adverse effects (parameters examined included clinical signs, body weight, hematology and serum chemistry analyses, urinalysis, semen analysis, and gross necropsy findings). In contrast, subchronic DMF inhalation exposure produced significant hepatic effects in rats at concentrations of 200 ppm (Senoh et al. 2003), 300 ppm (Craig et al. 1984), and 400 ppm (NTP 1992) and in mice at 100 ppm (Senoh et al. 2003), 150 ppm (Craig et al. 1984), and 200 ppm (NTP 1992). Indexes of toxicity after repeated DMF exposure ranged from elevated serum enzymes indicative of liver injury to hepatic degeneration and necrosis. From these exposure data, humans are expected to be less sensitive than laboratory animals (rodents). An intraspecies uncertainty factor of 10 is applied because a host of interindividual differences could affect the manifestation of DMF toxicity: (1) activity of CYP2E1, an enzyme that plays a pivotal role in the metabolism of DMF to reactive intermediates, can be induced by ethanol consumption,
obesity, diabetes, and other lifestyle and genetic factors (Gonzalez 1990; Song et al. 1990; Lucas et al. 1998; McCarver et al. 1998), and increased CYP2E1 levels increase the toxic metabolites of DMF; (2) prior consumption of ethanol can exacerbate DMF toxicity in individuals; (3) on the basis of the proposed mechanism of action, detoxification of the reactive intermediate is partly dependent upon conjugation with glutathione; therefore, if glutathione levels are depleted for other reasons, the potential exists for greater exposure to the reactive intermediate; and (4) because DMF exposure can result in hepatotoxicity, those individuals with chronic liver disease may be at increased risk. Therefore, a total uncertainty factor of 10 is applied.
The experimentally derived exposure value is scaled to AEGL time frames using the concentration-time relationship given by the equation Cn × t = k, where C = concentration, t = time, k is a constant, and n generally ranges from 1 to 3.5 (ten Berge et al. 1986). The value of n was not empirically derived because of inadequate data; therefore, the default value of n = 1 was used for extrapolating from shorter to longer exposure periods, and a value of n = 3 was used to extrapolate from longer to shorter exposure periods.
AEGL-3 values are presented in Table 1-14.
8. SUMMARY OF AEGLS
8.1. AEGL Values and Toxicity End Points
The AEGL values for DMF are summarized in Table 1-15. An AEGL-1 is not recommended on the basis of inadequate data. The AEGL-2 was based on a NOAEL for irreversible developmental effects (malformations) in rabbits. The AEGL-3 was based on the highest concentration and longest exposure duration causing no mortality in rats.
A useful way to evaluate the AEGL values in context of existing empirical data is presented in Table 1-1. For this plot, the toxic response was placed into severity categories. The severity categories fit into definitions of the AEGL health effects: no effects, discomfort, disabling, lethal, and partially lethal (an experimental concentration at which some of the animals died and some did not). The effects that place an experimental result into a particular category vary according to the spectrum of data available on a specific chemical and the effects from exposure to that chemical. The doses often span a number of orders of magnitude, especially when human data exist. Therefore, the concentration is placed on a log scale. The graph in Figure 1-1 plots the DMF AEGL values along with the existing acute human and animal toxicity data for DMF in terms of the categories assigned to them. From this plot, it is apparent that the AEGL values are below any exposure concentration in animals resulting in any effects and should therefore be protective of human health.
TABLE 1-14 AEGL-3 Values for DMFa
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 970 ppm (2,900 mg/m3) | 670 ppm (2,000 mg/m3) | 530 ppm (1,600 mg/m3) | 280 ppm (840 mg/m3) | 140 ppm (420mg/m3) |
aThere is a high potential for DMF to be absorbed dermally, so this route of exposure should be considered along with inhalation.
TABLE 1-15 Summary of AEGL Values for DMFa
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h |
| AEGL-1 (nondisabling) | NRa | NR | NR | NR | NR |
| AEGL-2 (disabling) | 110 ppm (330 m3) | 110 ppm (330 m3) | 91 ppm (270m3) | 57 ppm (170 m3) | 38 ppm (110 m3) |
| AEGL-3 (lethal) | 970 ppm (2,900 m3) | 670 ppm (2,000 m3) | 530 ppm (1,600 m3) | 280 ppm (840 m3) | 140 ppm (420 m3) |
aThere is a high potential for DMF to be absorbed dermally, so this route of exposure should be considered along with inhalation.
bNR, not recommended. Absence of an AEGL-1 does not imply that exposure below the AEGL-2 is without adverse effects.
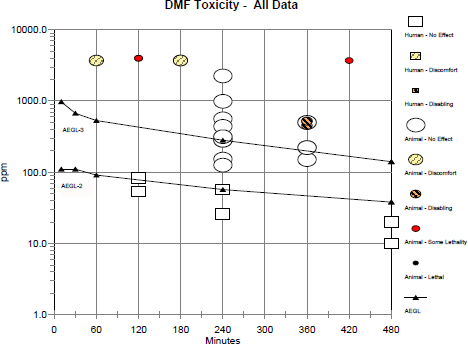
FIGURE 1-1 Category plot of animal toxicity data compared with AEGL values.
8.2. Comparison with Other Standards and Guidelines
Standards and guidelines for short-term exposures are listed in Table 1-16. The 1-h AEGL-2 values are comparable to the ERPG-2, while the AEGL-3 values are above the ERPG-3. The 30-min AEGL-3 is above the IDLH. Occupational workplace standards lie below the 8-h AEGL-2 levels.
TABLE 1-16 Extant Standards and Guidelines for DMF
| Guideline | Exposure Duration | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h | |
| AEGL-1 | NR | NR | NR | NR | NR |
| AEGL-2 | 110 ppm | 110 ppm | 91 ppm | 57 ppm | 38 ppm |
| AEGL-3 | 970 ppm | 670 ppm | 530 ppm | 280 ppm | 140 ppm |
| ERPG-1 (AIHA)a | 2 ppm | ||||
| ERPG-2 (AIHA) | 100 ppm | ||||
| ERPG-3 (AIHA) | 200 ppm | ||||
| IDLH (NIOSH)b | 500 ppm | ||||
| TLV-TWA (ACGIH)c | 10 ppm | ||||
| PEL-TWA (OSHA)d | 10 ppm (30 mg/m3) | ||||
| REL-TWA (NIOSH)e | 10 ppm | ||||
| MAK (Germany)f | 10 ppm (30 mg/m3) | ||||
| MAC (The Netherlands)g | 5 ppm (15 mg/m3) | ||||
aERPG (emergency response planning guidelines, American Industrial Hygiene Association, AIHA 2002).
The ERPG-1 is the maximum airborne concentration below which nearly all individuals could be exposed for up to 1 h without experiencing effects other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. An ERPG-1 for DMF was based on the approximate geometric mean of the odor threshold data. The ERPG-2 is the maximum airborne concentration below which nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protective action. The ERPG-2 for DMF is based primarily on human experience in which volunteers were exposed to concentrations up to 87 ppm for 4 h with no reported adverse effects (Kimmerle and Eben 1975b). The ERPG-3 is the maximum airborne concentration below which nearly all individuals could be exposed for up to 1 h without experiencing or developing life-threatening health effects. The ERPG-3 for DMF is based on animal data (2-h mouse LC50 calculated to be 3,140 ppm) in addition to reports of irritation in workers and of high concentrations and the fact that some members or the community might be more susceptible to DMF.
bIDLH (immediately dangerous to life or health, National Institute for Occupational Safety and Health, NIOSH 1996) represents the maximum concentration from which one could escape within 30 min without any escape-impairing symptoms or any irreversible health effects. The IDLH for DMF is based on acute inhalation toxicity data in animals (Stasenkova 1961).
cTLV-TWA (Threshold Limit Value-time-weighted average, American Conference of Governmental Industrial Hygienists, ACGIH 2009) is the TWA concentration for a normal 8-h work day and a 40-h work week to which nearly all workers may be repeatedly exposed, day after day, without adverse effect. DMF has an adopted urinary Biological Exposure Index (N-methylformamide at 15 mg/L at end of shift and N-acetyl-S-(N-methylcarbamoyl cysteine) at 40 mg/L prior to last shift of the last work week). DMF is among the chemical substances currently under study for possible revision.
dPEL-TWA (permissible exposure limits - time-weighted average, Occupational Health and Safety Administration, OSHA 29 CFR 1910.1000 [1999]) is analogous to the ACGIH TLV-TWA but is for exposures of no more than 10 h/day, 40 h/week.
eREL-TWA (recommended exposure limit-time-weighted average, National Institute for Occupational Safety and Health, NIOSH 2005) is analogous to the ACGIH TLV-TWA.
fMAK (maximale Arbeitsplatzkonzentration [maximum workplace concentration], Deutsche Forschungsgemeinschaft [German Research Association], DFG 2002) is analogous to the ACGIH TLV-TWA.
gMAC (maximaal aanvaarde concentratie [maximum accepted concentration], Dutch Expert Committee for Occupational Standards, The Netherlands, (MSZW 2004) is analogous to the ACGIH TLV-TWA.
8.3. Data Quality and Research Needs
Quality data for derivation of the AEGL values were very limited, and data meeting the definition of AEGL-1 end points were not available. Nonlethal acute inhalation effects in animals were limited to measurements of alterations in liver enzymes; livers from animals subjected to a single exposure were not examined histologically. Histologic analyses of tissues from animals that died following acute DMF exposure were generally not available. Studies addressing the acute lethal and nonlethal toxicity of inhaled DMF over exposure durations of 10 min up to 8 h would be useful to further elucidate the health effects.
9. REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 2009. TLVs and BEIs: Based on Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices: N,N-dimethylformamide. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
AIHA (American Industrial Hygiene Association). 2002. Pp. 1-7 in Emergency Response Planning Guidelines. Fairfax, VA: AIHA Press.
Amato, G., E. Grasso, V. Longo, and P.G. Gervasi. 2001. Oxidation of N,N-dimethylformamide and N,N-diethylformamide by human liver microsomes and human recombinant P450s. Toxicol. Lett. 124(1-3):11-19.
Amoore, J.E., and E. Hautala. 1983. Odor as an aid to chemical safety: Odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 3(6):272-290.
Angerer, J., T. Goen, A. Kramer, and H.U. Kafferlein. 1998. N-methylcarbamoyl adducts at the N-terminal valine of globin in workers exposed to N,N-dimethylformamide. Arch. Toxicol. 72(5):309-313.
Antoine, J.L., J. Arany, A. Leonard, J. Henrotte, G. Jenar-Dubuisson, and G. Decat. 1983. Lack of mutagenic activity of dimethylformamide. Toxicology 26(3-4):207-212.
BASF. 1974a. Report on the Study of the Prenatal, Perinatal, and Postnatal Toxicity of Dimethylformamide in Rats on Repeated Inhalation (1st Communication) with Attachments and Sheet Dated 06/12/89. EPA Document No. 86-890000648. Microfiche No. OTS 0521154. U.S. Environmental Protection Agency, Washington, DC.
BASF. 1974b. Report on the Study of the Prenatal, Perinatal, and Postnatal Toxicity of Dimethylformamide in Rats on Repeated Inhalation (2nd Communication) with Attachments and Sheet Dated 06/12/89. EPA Document No. 86-890000649. Microfiche No. OTS 0521155. U.S. Environmental Protection Agency, Washington, DC.
BASF. 1974c. Report on the Study of Dimethylformamide for a Teratogenic Effect on Rats after Repeated Inhalation with Attachments and Cover Sheet Dated 06/12/89. EPA Document No. 86-890000650. Microfiche No. OTS 0521156. U.S. Environmental Protection Agency, Washington, DC.
BASF. 1989. Prenatal Toxicity of Dimethylformamide in Rabbits after Inhalation, Volume I-II (Draft Report) with Attached Supplement to the Report and Cover Sheet Dated 06/12/89. EPA Document No.86-890000632. Microfiche No. OTS0521138. U.S. Environmental Protection Agency, Washington, DC.
Brondeau, M.T., P. Bonnet, J.P. Guenier, and J. de Ceaurriz. 1983. Short-term inhalation test for evaluating industrial hepatotoxicants in rats. Toxicol. Lett. 19(1-2):139-146.
Budavari, S., M.J. O'Neil, A. Smith, P.E. Heckelman, and J.F. Kinneary, eds. 1996. P. 549 in The Merck Index: An Encyclopedia of Chemicals, Drug, and Biologicals, 12th Ed. Whitehouse Station, NJ: Merck.
Catenacci, G., D. Grampella, R. Terzi, A. Sala, and G. Pollini. 1984. Hepatic function in subjects exposed to environmental concentrations of DMF lower than the actually proposed TLV. G. Ital. Med. Lav. 6(3-4):157-158.
Chary, S. 1974. Dimethylformamide: A cause of acute pancreatitis? Lancet 2(7876):356.
Clayton, J.W., Jr., J.R. Barnes, D.B. Hood, and G.W. Schepers. 1963. The inhalation toxicity of dimethylformamide (DMF). Am. Ind. Hyg. Assoc. J. 24:144-154.
Craig, D.K., R.J. Weir, W. Wagner, and D. Groth. 1984. Subchronic inhalation toxicity of dimethylformamide in rats and mice. Drug Chem. Toxicol. 7(6):551-571.
DFG (Deutsche Forschungsgemeinschaft). 2002. List of MAK and BAT Values 2002. Maximum Concentrations and Biological Tolerance Values at the Workplace Report No. 38. Weinheim, Federal Republic of Germany: Wiley VCH.
Ducatman, A.M., D.E. Conwill, and J. Crawl. 1986. Germ cell tumors of the testicle among aircraft repairmen. J. Urol. 136(4):834-836.
Eben, A., and G. Kimmerle. 1976. Metabolism studies of N,N-dimethylformamide. III. Studies about influence of ethanol in persons and laboratory animals. Int. Arch. Occup. Environ. Health 36(4):243-265.
E.I. Dupont de Nemours & Co. 1944. The Toxicity of Dimethylformamide with Cover Sheets and Dated 09/24/84 (sanitized). EPA Document 86-890000768S. Microfiche No. OTS 0520887. U.S. Environmental Protection Agency, Washington, DC.
E.I. Dupont de Nemours & Co. 1973. An Epidemiology Study of Workers Exposed to Dimethylformamide with Attachments and Cover Sheets Dated 09/24/84. EPA Document No. 86-890000788. Microfiche No. OTS 0521260. U.S. Environmental Protection Agency, Washington, DC.
E.I. Dupont de Nemours & Co. 1992. Long-Term Inhalation Oncogenicity Study with Dimethylformamide in Rats and Mice (11 Volumes) with Cover Letter Dated 01/04/93. EPA Document No. 86-930000085. Microfiche Number OTS 0544841. U.S. Environmental Protection Agency, Washington, DC.
EPA (U.S. Environmental Protection Agency). 1990. N,N-Dimethylformamide. Integrated Risk Information System, U.S. Environmental Protection Agency [online]. Available: http://www.epa.gov/iris/subst/0511.htm [accessed Oct. 22, 2010].
EPA (U.S. Environmental Protection Agency). 1992. Reference Guide to Odor Thresholds for Hazardous Air Pollutants Listed in the Clean Air Act Amendments of 1990. EPA/600/R-92/047. Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC. March 1992 [online]. Available: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=40610 [accessed Oct. 22, 2010].
Farquharson, R.G., M.H. Hall, and W.T. Fullerton. 1983. Poor obstetric outcome in three quality control laboratory workers. Lancet 1(8331):983-984.
Fiorito, A., F. Larese, S. Molinari, and T. Zanin. 1997. Liver function alterations in synthetic leather workers exposed to dimethylformamide. Am. J. Ind. Med. 32(3):255-260.
Fleming, L.E., S.L. Shalat, and C.A. Redlich. 1990. Liver injury in workers exposed to dimethylformamide. Scand. J. Work Environ. Health 16(4):289-292.
Gescher, A. 1993. Metabolism of N,N-dimethylformamide: Key to the understanding of its toxicity. Chem. Res. Toxicol. 6(3):246-251.
Gonzalez, F.J. 1990. Molecular genetics of the P-450 superfamily. Pharmacol. Ther. 45(1):1-38.
Hardman, J.G., and L.E. Limbird, eds. 2001. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 10th Ed. New York: McGraw-Hill Professional.
Hellwig, J., J. Merkle, H.J. Klimisch, and R. Jackh. 1991. Studies on the prenatal toxicity of N,N-dimethylformamide in mice, rats and rabbits. Food Chem.Toxicol. 29(3):193-201.
Hundley, S.G., P.H. Lieder, R. Valentine, L.A. Malley, and G.L. Kennedy, Jr. 1993a. Dimethylformamide pharmacokinetics following inhalation exposure to rats and monkeys. Drug Chem. Toxicol. 16(1):21-52.
Hundley, S.G., K.T. McCooey, P.H. Lieder, M.E. Hurtt, and G.L. Kennedy, Jr. 1993b. Dimethylformamide pharmacokinetics following inhalation exposure in monkeys. Drug Chem. Toxicol. 16(1):53-79.
Hurtt, M.E., K.T. McCooey, M.E. Placke, and G.L. Kennedy. 1991. Ten-day repeated-exposure inhalation study of dimethylformamide (DMF) in cynomolgus monkeys. Toxicol. Lett. 59(1-3):229-237.
Hurtt, M.E., M.E. Placke, J.M. Killinger, A.W. Singer, and G.L. Kennedy, Jr. 1992. Thirteen-week inhalation toxicity study of dimethylformamide (DMF) in cynomolgus monkeys. Fundam. Appl. Toxicol. 18(4):596-601.
IARC (International Agency for Research on Cancer). 1989. Dimethylformamide. Pp. 171-197 in Some Organic Solvents, Resin Monomers and Related Compounds, Pigments and Occupational Exposures in Paint Manufacture and Painting. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol. 47. Lyon, France: IARC.
Kafferlein, H.U., and J. Angerer. 2001. N-methylcarbamoylated valine of hemoglobin in humans after exposure to N,N-dimethylformamide: Evidence for the formation of methyl isocyanate? Chem. Res. Toxicol. 14(7):833-840.
Kennedy, G.L. 1986. Biological effects of acetamide, formamide, and their monomethyl and dimethyl derivatives. Crit. Rev. Toxicol. 17(2):129-182.
Kennedy, G.L., and H. Sherman. 1986. Acute and subchronic toxicity of dimethylformamide and dimethylacetamide following various routes of administration. Drug Chem. Toxicol. 9(2): 147-170.
Kestell, P., M.D. Threadgill, A. Gescher, A.P. Gledhill, A.J. Shaw, and P.B. Farmer. 1987. An investigation of the relationship between hepatotoxity and the metabolism of N-alkylformamides. J. Pharmacol. Exp. Ther. 240(1):265-270.
Kimmerle, G., and A. Eben. 1975a. Metabolism studies of N,N-dimethylformamide. I. Studies in rats and dogs. Int. Arch. Arbeitsmed. 34(2):109-126.
Kimmerle, G., and A. Eben. 1975b. Metabolism studies of N,N-dimethylformamide. II. Studies in persons. Int. Arch. Arbeitsmed. 34(2):127-136.
Kimmerle, G., and L. Machemer. 1975. Studies with N,N-dimethylformamide for embryotoxic and teratogenic effects on rats after dynamic inhalation. Int. Arch. Arbeitsmed. 34(3):167-175.
Levin, S.M, D.B. Baker, P.J. Landrigan, S.V. Monaghan, E. Frumin, M. Braithwaite, and W. Towne. 1987. Testicular cancer in leather tanners exposed to dimethylformamide. Lancet 2(8568):1153.
Lewis, S.C., R.E. Schroeder, and G.L. Kennedy, Jr. 1992. Developmental toxicity of dimethylformamide in the rat following inhalation exposure. Drug Chem. Toxicol. 15(1): 1-14.
Lucas, D., C. Farez, L.G. Bardou, J. Vaisse, J.R. Attali, and P. Valensi. 1998. Cytochrome P450 2E1 activity in diabetic and obese patients as assessed by chlorzoxazone hydroxylation. Fundam. Clin. Pharmacol. 12(5):553-558.
Lundberg, I., A. Pehrsson, S. Lundberg, T. Kronevi, and V. Lidums. 1983. Delayed dimethylformamide biotransformation after high exposures in rats. Toxicol. Lett. 17(1-2): 29-34.
Lundberg, I., M. Ekdahl, T. Kronevi, V. Lidums, and S. Lundberg. 1986. Relative hepatotoxicity of some industrial solvents after intraperitoneal injection or inhalation exposure in rats. Environ. Res. 40(2):411-420.
Lynch, D.W., M.E. Placke, R.L. Persing, and M.J. Ryan. 2003. Thirteen-week inhalation toxicity study of , N-dimethylformamide in F344/N rats and B6C3F1 mice. Toxicol. Sci. 72(2): 347-358.
Malley, L.A., T.W. Slone, C. Van Pelt, G.S. Elliott, P.E. Ross, J.C. Stadler, and G.L. Kennedy. 1994. Chronic toxicity/oncogenicity of dimethylformamide in rats and mice following inhalation exposure. Fundam. Appl. Toxicol. 23(2):268-279.
Massmann, W. 1956. Toxicological investigations on dimethylformamide. Br. J. Ind. Med. 13(1): 51-54.
McCarver, D.G., R. Byun, R.N. Hines, M. Hichme, and W. Wegenek. 1998. A genetic polymorphism in the regulatory sequences of human CYP2E1: Association with increased chlorzoxazone hydroxylation in the presence of obesity and ethanol intake. Toxicol Appl. Pharmacol. 152(1):276-281.
Mraz, J., and H. Nohova. 1992. Absorption, metabolism and elimination of N,N-dimethylformamide in humans. Int. Arch. Occup. Environ. Health 64(2):85-92.
Mraz, J., and F. Turecek. 1987. Identification of N-acetyl-S-(N-methylcarbamoyl) cysteine, a human metabolite of N,N-dimethylformamide and N-methylformamide. J. Chromatogr. 414(2):399-404.
Mraz, J., H. Cross, A. Gescher, M.D. Threadgill, and J. Flek. 1989. Differences between rodents and humans in the metabolic toxication of N,N-dimethylformamide. Toxicol. Appl. Pharmacol. 98(3):507-516.
Mraz, J., P. Jheeta, A. Gescher, R. Hyland, K. Thummel, and M.D. Threadgill. 1993. Investigation of the mechanistic basis of N,N-dimethylformamide toxicity. Metabolism of N,N-dimethylformamide and its deuterated isotopomers by cytochrome P450 2E1. Chem. Res. Toxicol. 6(2):197-207.
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: Dimethylformamide. Den Haag: SDU Uitgevers [online]. Available: http://www.lasrook.net/lasrookNL/maclijst2004.htm [accessed Oct. 21, 2010].
NIOSH (National Institute for Occupational Safety and Health). 1996. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLH): NIOSH Chemical Listing and Documentation of Revised IDLH Values (as of 3/1/95)-Dimethylformamide. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. August 1996 [online]. Available: http://www.cdc.gov/niosh/idlh/68122.html [accessed Oct. 21, 2010].
NIOSH (National Institute for Occupational Safety and Health). 2005. NIOSH Pocket Guide to Chemical Hazards: Dimethylformamide. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH. September 2005 [online]. Available: http://www.cdc.gov/niosh/npg/npgd0226.html [accessed Oct. 21, 2010].
Nomiyama, T., V. Haufroid, J.P. Buchet, H. Miyauchi, S. Tanaka, T. Yamauchi, S. Imamiya, Y. Seki, K. Omae, and D. Lison. 2001a. Insertion polymorphism of CYP2E1 and urinary N-methylformamide after N,N-dimethylformamide exposure in Japanese workers. Int. Arch. Occup. Environ. Health 74(7):519-522.
Nomiyama, T., H. Nakashima, Y. Sano, L.L. Chen, S. Tanaka, H. Miyauchi, T. Yamauchi, H. Sakurai, and K. Omae. 2001b. Does the polymorphism of cytochrome P-450 2E1 affect the metabolism of N,N-dimethylformamide? Comparison of the half-lives of urinary N-methylformamide. Arch. Toxicol. 74(12):755-759.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NTP (National Toxicology Program). 1992. Toxicology Studies of N,N-Dimethylformamide (CAS No. 68-12-2) Administered by Inhalation to F344/N Rats and B6C3F1 Mice. NTP TR 22. NIH 93-3345. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Toxicology Program, Research Triangle Park, NC [online]. Available: http://ntp.niehs.nih.gov/ntp/htdocs/ST_rpts/tox022.pdf [accessed Oct. 22, 2010].
Potter, H.P. 1973. Dimethylformamide-induced abdominal pain and liver injury. Arch. Environ. Health 27(5):340-341.
Redlich, C.A., W.S. Beckett, J. Sparer, K.W. Barwick, C.A. Riely, H. Miller, S.L. Sigal, S.L. Shalat, and M.R. Cullen. 1988. Liver disease associated with occupational exposure to the solvent dimethylformamide. Ann. Intern. Med. 108(5):680-686.
Roure, M.B., A.M. Lambert, C. Cour, P. Bonnet, and A.M. Saillenfait. 1996. Hepatotoxicity of N,N-dimethylformamide in rats following intraperitoneal or inhalation routes of administration. J. Appl. Toxicol. 16(3):265-267.
Saillenfait, A.M., J.P. Payan, D. Beydon, J.P. Fabry, I. Langonne, J.P. Sabate, and F. Gallissot. 1997. Assessment of the developmental toxicity, metabolism, and placental transfer of N,N-dimethylformamide administered to pregnant rats. Fundam. Appl. Toxicol. 39(1):33-43.
Scailteur, V., and R.R. Lauwreys. 1987. Dimethylformamide (DMF) hepatotoxicity. Toxicology 43(3):231-238.
Senoh, H., T. Katagiri, H. Arito, T. Nishizawa, K. Nagano, S. Yamamoto, and T. Matsushima. 2003. Toxicity due to 2- and 13-wk inhalation exposures of rats and mice to N,N-dimethylformamide. J. Occup. Health 45(6):365-375.
Senoh, H., S. Aiso, H. Arito, T. Nishizawa, K. Nagano, S. Yamamoto, and T. Matsushima. 2004. Carcinogenicity and chronic toxicity after inhalation exposure of rats and mice to N,N-dimethylformamide. J. Occup. Health 46(6):429-439.
Shell Oil Company. 1982. Test Standardization: Inhalation Toxicity Testing of 8 Chemicals According to the OECD Inhalation Hazard Test. EPA Document No. 878212113. Microfiche No. OTS0205969. U.S. Environmental Protection Agency, Washington, DC.
Smyth, H.F., and C.P. Carpenter. 1948. Further experience with the range finding test in the industrial toxicology laboratory. J. Ind. Hyg. Toxicol. 30(1):63-68.
Song, B.J., R.L. Veech, and P. Saenger. 1990. Cytochrome P450IIE1 is elevated in lymphocytes from poorly controlled insulin-dependent diabetics. J. Clin. Endocrinol. Metab. 71(4): 1036-1040.
Stasenkova, K.P. 1961. Toxicity of dimethylformamide [in Russian]. Toksikol. Nov. Prom. Khim. Vesh. 1:54-69.
Tanaka, K.I. 1971. Toxicity of dimethylformamide (DMF) to the young female rat. Int. Arch. Occup. Health 28(2):95-105.
ten Berge, W.F., A. Zwart, and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapors and gases. J. Hazard. Mater. 13(3): 301-309.
Tietz, N., ed. 1995. Clinical Guide to Laboratory Tests, 3rd Ed. New York: W.B. Saunders.
Trochimowicz, H.J., G.L. Kennedy, Jr., and N.D. Krivanek. 1994. Heterocyclic and miscellaneous nitrogen compounds: Aromatic Compounds- dimethylformamide. Pp. 3464-3521 in Patty’s Industrial Hygiene and Toxicology, Vol. II E, Toxicology, 4th Ed., G.D. Clayton, and F.E. Clayton, eds. New York: John Wiley & Sons.
TURI (Massachusetts Toxics Use Reduction Institute). 2001. Dimethylformamide. Massachusetts Chemical Fact Sheet. Toxics Use Reduction Institute, University of Massachusetts, Lowell [online]. Available: http://www.turi.org/library/turi_publications/massachusetts_chemical_fact_sheets/dimethylformamide [accessed Oct. 21, 2010].
Walrath, J., W.E. Fayerweather, P.G. Gilby, and S. Pell. 1989. A case-control study of cancer among DuPont employees with potential for exposure to dimethylformamide. J. Occup. Med. 31(5):432-438.
Wrbitzky, R. 1999. Liver function in workers exposed to N,N-dimethylformamide during the production of synthetic textiles. Int. Arch. Occup. Environ. Health 72(1):19-25.
APPENDIX A
DERIVATION OF AEGL VALUES FOR N,N-DIMETHYLFORMAMIDE
Derivation of AEGL-1 Values
An AEGL-1 value was not derived because it was not appropriate. No data pertaining to end points relevant to the AEGL-1 definition were available. 10- and 30-min and 1-, 4-, and 8-h AEGL-1: not recommended.
Derivation of AEGL-2 Values
| Key studies: | Hellwig et al. 1991; BASF 1989 |
| Toxicity end points: | No developmental effects seen in rabbits exposed to 150 ppm for 6 h; exposure at 450 ppm for 6 h resulted in irreversible developmental effects (malformations) |
| Time-scaling: | Cn × t = k (default of n = 3 for longer to shorter exposure periods; n = 1 for shorter to longer exposure periods) |
| [(150 ppm)/3]1 × 6 h = 300 ppm-h | |
| [(150 ppm)/3]3 × 6 h = 750,000 ppm-h | |
| Uncertainty factors: | 1 for interspecies variability |
| 3 for intraspecies variability Combined uncertainty factor of 3 | |
| Modifying factor: | Not applicable |
| Calculations: | |
| 10-min AEGL-2: | Set equal to 30-min value due to uncertainty in extrapolating from 6 h exposure duration to 10 min |
| 30-min AEGL-2: | C3 × 0.5 h = 750,000 ppm-h |
| C3 = 1,500,000 ppm | |
| C = 114 ppm = 110 ppm | |
| 1-h AEGL-2: | C3 × 1 h = 750,000 ppm-h |
| C3 = 91 ppm | |
| 4-h AEGL-2: | C3 × 4 h = 750,000 ppm-h |
| C2 = 187,500 ppm | |
| C = 57 ppm | |
| 8-h AEGL-2: | C1 × 8 h = 300 ppm-h |
| C1 = 37.5 ppm | |
| C = 38 ppm | |
| Derivation of AEGL-3 Values | |
| Key studies: | Shell Oil Company 1982 |
| Toxicity end points: | Goup of three male and three female rats survived a 3-h exposure to DMF at 3,700 ppm |
| Time-scaling: | Cn × t = k (default of n = 3 for longer to shorter exposure periods; n = 1 for shorter to longer exposure periods) |
| [(3,700 ppm)/10]1 × 3 h = 1,110 ppm-h | |
| [(3,700 ppm)/10]3 × 3 h = 151,959,000 ppm-h | |
| Uncertainty factors: | 1 for interspecies variability |
| 10 for intraspecies variability | |
| Combined uncertainty factor of 10 | |
| Modifying factor: | Not applicable |
| Calculations: | |
| 10-min AEGL-3: | C3 × 0.167 = 151 959 000 ppm-h |
| C3 = 909,934,132 ppm | |
| C = 969 ppm = 970 ppm | |
| 30-min AEGL-3: | C3 × 0.5 = 151,959,000 ppm-h |
| C3 = 303,918,000 ppm | |
| C = 672 ppm = 670 ppm | |
| 1-h AEGL-3: | C3 × 1 h = 151,959,000 ppm-h |
| C3 = 151,959,000 ppm | |
| C = 534 ppm = 530 ppm | |
| 4-h AEGL-3: | C1× 4 h = 1,110 ppm-h |
| C1 = 277.5 ppm | |
| C = 280 ppm | |
| 8-h AEGL-3: | C1× 8 h = 1,110 ppm-h |
| C1 = 138.8 ppm | |
| C = 140 ppm | |
APPENDIX B
ACUTE EXPOSURE GUIDELINE LEVELS FOR N,N-DIMETHYLFORMAMIDE
Derivation Summary N,N-Dimethylformamide
AEGL-1 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| Not | Not | Not | Not | Not |
| recommended | recommended | recommended | recommended | recommended |
| Reference: Not applicable | ||||
| Test species/Strain/Number: Not applicable | ||||
| Exposure route/Concentrations/Durations: Not applicable | ||||
| Effects: Not applicable | ||||
| End point/Concentration/Rationale: Not applicable | ||||
| Uncertainty factors/Rationale: Not applicable | ||||
| Modifying factor: Not applicable | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time-scaling: Not applicable | ||||
| Data adequacy: No human or animal data pertaining to end points relevant to the AEGL-1 definition were available. Absence of an AEGL-1 does not imply that exposures below the AEGL-2 values are without adverse effects. | ||||
AEGL-2 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 110 ppm | 110 ppm | 91 ppm | 57 ppm | 38 ppm |
| Key references: | ||||
| Hellwig, J., J. Merkle, H.J. Klimisch, and R. Jackh. 1991. Studies on the prenatal toxicity of N,N-dimethylformamide in mice, rats and rabbits. Food Chem. Toxicol. 29(3):193-201. | ||||
| BASF. 1989. Prenatal Toxicity of Dimethylformamide in Rabbits after Inhalation, Volume I-II (Draft Report) with Attached Supplement to the Report and Cover Sheet Dated 06/12/89. EPA Document No.86-890000632. Microfiche No. OTS0521138. | ||||
| U.S. Environmental Protection Agency, Washington, DC. | ||||
| Test species/Strain/Number: 15 Himalayan rabbits per group | ||||
| Exposure route/Concentrations/Durations: Inhaled DMF at 0, 50, 150, or 450 ppm for 6 h/d over GD 7-19 | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 110 ppm | 110 ppm | 91 ppm | 57 ppm | 38 ppm |
| Effects: (1) Maternal toxicity evident at 150 and 450 ppm as decreased body-weight gain or weight loss over GD 7-19 and GD 0-29. (2) Developmental toxicity evident at 450 ppm as increase in external malformations and total malformations (external, soft tissue, and skeletal combined), as decrease in fetal weight (86% of controls), and as increase in litter incidence of skeletal variations (splitting of skull bones; fused, irregular shaped, and bipartite sternebrae). No developmental effects were observed at 150 ppm. | ||||
| End point/Concentration/Rationale: 150 ppm for 6 h to protect against irreversible developmental effects (malformations) | ||||
| Uncertainty factors/Rationale: | ||||
| Total uncertainty factor: 3 | ||||
| Interspecies: 1was applied because it appears that primates are not as sensitive as rodents. Monkeys inhaled DMF at 500 ppm for 6 h/d, 5 d/wk, for up to 13 weeks with no measurable adverse effects. In contrast, subchronic DMF inhalation exposure produced significant hepatic effects in rats at concentrations of 200 ppm (Senoh et al. 2003), 300 ppm (Craig et al. 1984) and 400 ppm (NTP 1992) and in mice at 100 ppm (Senoh et al. 2003), 150 ppm (Craig et al. 1984), and 200 ppm (NTP 1992). Indexes of toxicity after repeated DMF exposure ranged from elevated serum enzymes indicative of liver injury to hepatic degeneration and necrosis. From these exposure data, humans are expected to be less sensitive than laboratory animals (rodents). Because the mechanism of hepatotoxicity is thought to be related to the metabolism of DMF to a reactive intermediate, fetal toxicity is expected to result from exposure to the parent DMF or metabolites. An oral study assessing the tissue and metabolite distribution of DMF in pregnant rats indicated that DMF and its metabolites were transferred across the placenta by passive diffusion and that maternal plasma, embryo or fetus, placenta, and amniotic fluid belonged to the same compartment (Saillenfait et al. 1997). Therefore, the fetus and placenta will not provide any additional protection or enhancement of DMF toxicity because exposure to DMF and its metabolites will depend on the metabolism by the mother. | ||||
| Intraspecies: 3, an intraspecies uncertainty factor of 10 would normally be applied because a host of interindividual differences could affect the manifestation of DMF toxicity: (1) activity of CYP2E1, an enzyme that plays a pivotal role in the metabolism of DMF to reactive intermediates, can be induced by ethanol consumption, obesity, diabetes, and other lifestyle and genetic factors (Gonzalez 1990; Song et al. 1990; Lucas et al. 1998; McCarver et al. 1998), and increased CYP2E1 levels increase the toxic metabolites of DMF;(2) prior consumption of ethanol can exacerbate DMF toxicity in individuals; (3) on the basis of the proposed mechanism of action, detoxification of the reactive intermediate is partly dependent on conjugation with glutathione; therefore, if glutathione levels are depleted for other reasons, the potential exists for greater exposure to the reactive | ||||
| Data adequacy: No human or animal data pertaining to end points relevant to the AEGL-1 definition were available. Absence of an AEGL-1 does not imply that exposures below the AEGL-2 values are without adverse effects. | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 110 ppm | 110 ppm | 91 ppm | 57 ppm | 38 ppm |
| intermediate; (4) because DMF exposure can result in hepatotoxicity, individuals with chronic liver disease may be at increased risk. However, application of a total uncertainty factor of 10 produces AEGL-2 values that are inconsistent with the available human data (values for the 10- and 30-min and1-, 4-, and 8-h AEGL-2 using default time-scaling would be 49, 34, 27, 17, and 11 ppm, respectively). Humans were exposed by inhalation of DMF at 87 ppm for 4 h or at 81 ppm for 2 h to assess the metabolism of DMF (Kimmerle and Eben 1975b; Eben and Kimmerle 1976). These single-exposure studies were conducted to assess DMF metabolism, and no adverse effects were reported; the concentration can be considered an acute exposure concentration unlikely to result in adverse effects in healthy adults. Therefore, the intraspecies uncertainty factor is reduced to 3. | ||||
| Modifying factor: Not applicable | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time-scaling: Default time-scaling using n = 3, 1. The 30-min AEGL-2 value was set equal to the 10-min value because of the uncertainty in extrapolating from a 6-h exposure duration to a 10-min duration. | ||||
| Data quality and support for the AEGL values: Data meeting the definition of an AEGL-2 end point were limited to developmental toxicity studies. Other nonlethal acute health effects in animals were limited to alterations in liver enzymes because livers from animals following a single exposure were not examined histologically. Histologic analysis of tissues from animals that died following acute exposure was not available to determine the cause of death. | ||||
AEGL-3 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 970 ppm | 670 ppm | 530 ppm | 280 ppm | 140 ppm |
| Key reference: Shell Oil Company. 1982. Test Standardization: Inhalation Toxicity Testing of 8 Chemicals According to the OECD Inhalation Hazard Test. EPA Document No. 878212113. Microfiche No. OTS0205969. U.S. Environmental Protection Agency, Washington, DC | ||||
| Test species/Strain/Number: groups of three male and three female Wistar rats | ||||
| Exposure route/Concentrations/Durations: exposed to 3,700 ppm DMF for 1, 3, or 7 h and observed for mortality for 14 days postexposure | ||||
| Effects: 1- or 3-h exposure at 3,700 ppm, no mortality; 7-h exposure at 3,700 ppm, killed 2/3 males and 3/3 females | ||||
| End point/Concentration/Rationale: exposure for 3 h to 3,700 ppm did not result in mortality | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 970 ppm | 670 ppm | 530 ppm | 280 ppm | 140 ppm |
| Uncertainty factors/Rationale: | ||||
| Total uncertainty factor: 10 | ||||
| Interspecies: 1 was applied because it appears that primates are not as sensitive as rodents. Monkeys inhaled DMF at 500 ppm for 6 h/d, 5 d/wk, for up to 13 weeks with no measurable adverse effects. In contrast, subchronic DMF inhalation exposure produced significant hepatic effects in rats at concentrations of 200 ppm (Senoh et al. 2003), 300 ppm (Craig et al. 1984), and 400 ppm (NTP 1992) and in mice at 100 ppm (Senoh et al. 2003), 150 ppm (Craig et al. 1984), and 200 ppm (NTP 1992). Indexes of toxicity after repeated DMF exposure ranged from elevated serum enzymes indicative of liver injury to hepatic degeneration and necrosis. From these exposure data, humans are expected to be less sensitive than laboratory animals (rodents). Because the mechanism of hepatotoxicity is thought to be related to the metabolism of DMF to a reactive intermediate, fetal toxicity is expected to result from exposure to the parent DMF or metabolites. An oral study assessing the tissue and metabolite distribution of DMF in pregnant rats indicated that DMF and its metabolites were transferred across the placenta by passive diffusion and that maternal plasma, embryo or fetus, placenta, and amniotic fluid belonged to the same compartment (Saillenfait et al. 1997). Therefore, the fetus and placenta will not provide any additional protection or enhancement of DMF toxicity because exposure to DMF and its metabolites will depend on the metabolism by the mother. | ||||
| Intraspecies: 10 was applied because a host of interindividual differences could affect the manifestation of DMF toxicity: (1) activity of CYP2E1, an enzyme that plays a pivotal role in the metabolism of DMF to reactive intermediates, can be induced by ethanol consumption, obesity, diabetes, and other lifestyle and genetic factors (Gonzalez 1990; Song et al. 1990; Lucas et al. 1998; McCarver et al. 1998), and increased CYP2E1 levels increase the toxic metabolites of DMF; (2) prior consumption of ethanol can exacerbate DMF toxicity in individuals; (3) on the basis of the proposed mechanism of action, detoxification of the reactive intermediate is partly dependent on conjugation with glutathione; therefore, if glutathione levels are depleted for other reasons, the potential exists for greater exposure to the reactive intermediate; and (4) because DMF exposure can result in hepatotoxicity, individuals with chronic liver disease may be at increased risk. | ||||
| Modifying factor: Not applicable | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time-scaling: Default time-scaling using n = 3, 1 | ||||
| Data quality and support for the AEGL values: Quality data for derivation of the AEGL-3 value were sparse. The AEGL-3 level is based on a study in which groups of only 3 rats of each sex were used, as opposed to 10 animals per group. The other studies investigating lethality following acute exposure to DMF did not observe animals for 14 days postexposure and did not report reliable exposure concentrations. However, the lethality data provided in the key study is consistent with the weight of evidence. | ||||



























































