Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P. L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory
________________________
1This document was prepared by the AEGL Development Team composed of Sylvia Talmage (Summitec Corporation) and Jim Holler and William Bress (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold levels for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Methyl ethyl ketone (MEK) is a volatile solvent with a sweet and sharp acetone-like odor. MEK is widely used as a solvent in common household products, such as inks, paints, cleaning fluids, varnishes, and glues. In most industrial applications, it is used as a component of a mixture of organic solvents. It has also been detected in a wide variety of natural products and may be a minor product of normal mammalian metabolism. In 1999, U.S. production capacity was 675 million pounds.
The inhalation toxicity of MEK is low. In clinical studies, a constant concentration of 200 ppm and short exposures at 380 ppm were judged nonirritating. At high concentrations of several thousand parts per million, MEK causes reversible central nervous system (CNS) depression as evidenced by neurobehavioral effects in animals. Data on human exposures were available from clinical studies and workplace monitoring. Animal studies with a variety of species (baboon, rat, mouse, and guinea pig) addressed irritation, neurotoxicity, developmental toxicity, and lethality. Exposure durations ranged from acute to chronic. MEK is not teratogenic, but at high concentrations, it is mildly fetotoxic to rats and mice. Genotoxicity was also addressed. No information on a concentration-exposure duration relationship for a defined end point was found. In clinical studies of 4-h duration, uptake was rapid during the first hour of expo-
sure at 200 ppm, approaching steady state in the blood by the end of the exposure (Liira et al. 1988a,b).
Four well-conducted clinical studies indicated that MEK is not a sensory irritant, nor does it induce neurobehavioral changes at concentrations up to 200 ppm for 2 or 4 h (Dick et al 1992; Muttray et al 2002; Shibata et al. 2002) or at variable concentrations ranging from 10 to 380 ppm over 4 h (five 8-min peaks to 380 ppm) (Seeber et al. 2002). Seeber et al. (2002) tested healthy subjects as well as subjects with self-reported multiple chemical sensitivity (sMCS). Subjects with sMCS reported no adverse symptoms during the 8-min exposures to 380 ppm. Additional metabolism studies were conducted at concentrations of 25 to 400 ppm for 4 h, but these studies did not address sensory irritation or neurotoxic effects. In a clinical study with 24 male and female subjects, a concentration of 200 ppm was judged unobjectionable for an 8-h exposure (Dick et al. 1992). Therefore, 200 ppm was selected as the threshold for sensory irritation and was used to derive the AEGL-1. The selection of this value is supported by numerous clinical studies in which volunteers were routinely exposed to MEK at 200-400 ppm for up to 4 h and by the exposure of sMCS subjects to it at 380 ppm for short periods of time. Because effects were not different in sensitive subjects at the higher concentration of 380 ppm, an intraspecies uncertainty factor of 1 was applied. Because steady-state would be approached within 4 h at the 200-ppm concentration (Liira et al. 1988a,b) and because MEK is rapidly metabolized, the 200-ppm concentration was used across all AEGL-1 exposure durations.
The AEGL-2 was based on an exposure concentration that did not result in neurobehavioral effects on the first day of the subchronic study by Cavendar et al. (1983). Rats were exposed to MEK at 5,000 ppm for 6 h/day, 5 days/week, for 90 days. No lesions were reported in this study (specific neuropathologic studies were conducted on the medulla and peripheral nerves), and there were no neurofunctional deficits. Narcosis was not observed on the first day of exposure or on subsequent days. The concentration may be close to the threshold for narcosis, as evidenced by mild somnolence in a repeated exposure study in which rats were exposed at 6,000 ppm for several weeks (Altenkirch et al. 1978). Because uptake is dependent on the ventilatory rate and cardiac output, which are higher in rodents than in humans, an interspecies uncertainty factor of 1 was applied (at similar exposure concentrations, blood levels of MEK are higher in rats than in humans [Liira et al. 1990a]). Because the threshold for narcosis differs by no more than 2- to 3-fold among the general population (see Section 4.4.2), an intraspecies uncertainty factor of 3 was applied to protect sensitive individuals. At the 5,000-ppm concentration, steady-state in the blood is predicted to occur sometime after 4 h. Therefore, the 4- and 8-h AEGL-2 values were set equal to 1,700 ppm. The data show that for a common end point, higher concentrations can be tolerated at the shorter exposure durations. Therefore, the values for the shorter exposure durations were time-scaled from the 4-h time using the default n value of 3.
The AEGL-3 values were derived using different studies. The 10- and 30-min time periods were derived using the studies by Klimisch (1988) and Zakhari et al. (1977) with support from Hansen et al. (1992). The 1-, 4-, and 8-h values were derived from the study by Fowles et al. (1999) using data from La Belle and Brieger (1955). No deaths occurred in rats after a 30-min exposure to MEK at 92,239 ppm (Klimisch 1988), and no deaths occurred in mice after a 45-min exposure at 50,000 ppm (Zakhari et al. 1977). A projected value of 32 or 145 ppm for 30 min would decrease the respiratory rate of mice by 50% (Hansen et al. 1992). The highest tested concentration in the Hansen et al. (1992) study was 26,000 ppm. On the basis of these data it is thought that nearly all individuals could be exposed at 10,000 ppm for up to 30 min without developing life-threatening effects. Inter- and intraspecies uncertainty factors of 1 and 3, respectively, were applied for the AEGL-2. Additional studies support the 10,000-ppm value as being nonlethal: 10,000 ppm for 10 or 30 min was narcotic to mice in one study (Glowa and Dews 1987) but not in another (Hansen et al. 1992), 10,000 ppm was tolerated by rats for 8 h/day for several days (Altenkirch et al. 1978), and no deaths occurred in guinea pigs inhaling 10,000 ppm for 13.5 h (Patty et al. 1935).
The longer-term AEGL-3 values were based on a maximum likelihood estimate, with a 1% response (MLE01), of 7,500 ppm calculated by Fowles et al. (1999) from a 4-h study with rats exposed at several concentrations for 4 h (La Belle and Brieger 1955). In this study, the 4-h LC50 (concentration lethal to 50% of the exposed population) was 11,700 ppm, and the highest concentration resulting in no deaths was 7,850 ppm for 4 h. The 7,500-ppm MLE01 concentration was divided by an interspecies uncertainty factor of 1 and an intraspecies uncertainty factor of 3, using the same rationale as that for AEGL-1. The resulting value of 2,500 ppm was used for both the 4-h and 8-h AEGL-3 values. MEK may approach steady state in the blood by the end of 8 h. The 4-h 2,500 ppm value was time-scaled to the 1-h time using the default n value of 3 for scaling to shorter time intervals. The 8-h AEGL-3 of 2,500 ppm is low compared with 8-h nonlethal concentrations in animal studies cited above.
The calculated values are listed in Table 3-1 below.
1. INTRODUCTION
MEK is a volatile solvent with a sweet and sharp acetone-like odor. It is commercially manufactured from n-butenes in a metal-catalyzed hydrogenation reaction that proceeds through the intermediate formation of 2-butanol. MEK is widely used as a solvent in industrial settings and common household products, such as protective coatings, adhesives, inks, paints, cleaning fluids, and dewaxing agents. It is a common ingredient in consumer products, such as varnishes and glues. In most applications, it is used as a component of a mixture of organic solvents. It has also been detected in a wide variety of natural
products and may be a minor product of normal mammalian metabolism (WHO 1993; Morgott et al. 2001). In 1999, U.S. production capacity was 675 million pounds (ChemExpo 2001). Global capacity in 2002 was about 1.3 million metric tons (Greiner and Funada 2009). Chemical and physical properties are listed in Table 3-2.
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
The relative toxicity of ketones is low (Morgott et al. 2001), and no studies were located regarding deaths of humans following inhalation, oral, or dermal exposure to MEK (ATSDR 1992; WHO 1993).
TABLE 3-1 Summary of AEGL Values for Methyl Ethyl Ketone
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| AEGL-1 (nondisabling) | 200 ppm (586 mg/m3) | 200 ppm (586 mg/m3) | 200 ppm (586 mg/m3) | 200 ppm (586 mg/m3) | 200 ppm (586 mg/m3) | NOAEL for subjective symptoms in humans (Dick et al. 1992; Muttray et al. 2002; Seeber et al. 2002 Shibata et al. 2002) |
| AEGL-2 (disabling) | 4,900 ppma (14,357 mg/m3) | 3,400 ppma (9,962 mg/m3) | 2,700 ppma (7,911 mg/m3) | 1,700 ppm (4,980 mg/m3) | 1,700 ppm (4,980 mg/m3) | Threshold for narcosis in rats (Cavender et al. 1983) |
| AEGL-3 (lethal) | b | b | 4,000 ppma (11,720 mg/m3) | 2,500 ppma (7,325 mg/m3) | 2,500 ppma (7,325 mg/m3) | Threshold for lethality, mouse, rat (La Belle and Brieger 1955; Zakhari et al. 1977; Klimisch 1988; Hansen et al. 1992) |
aThe 10- and 30-min and the 1-h AEGL-2 values and the 1-, 4-, and 8-h AEGL-3 values are higher than one-tenth of the lower explosive limit (LEL) of methyl ethyl ketone in air (LEL = 18,000 ppm). Therefore, safety considerations against the hazard of explosion must be taken into account.
bThe 10- and 30-min AEGL-3 value of 10,000 ppm (29,300 mg/m3) is higher than 50% of the LEL of methyl ethyl ketone in air (LEL = 18,000 ppm). Therefore, extreme safety considerations against the hazard of explosion must be taken into account. Abbreviation: NOAEL, no-observed-adverse-effect level.
TABLE 3-2 Chemical and Physical Data for Methyl Ethyl Ketone
| Parameter | Data | Reference |
| Synonyms | MEK, 2-butanone, ethyl methyl ketone, methyl acetone, 2-oxobutane | ATSDR 1992; O’Neil et al. 2001 |
| CAS registry no. | 78-93-3 | ATSDR 1992 |
| Chemical formula | CH3COCH2CH3 | O’Neil et al. 2001 |
| Molecular weight | 72.10 | O’Neil et al. 2001 |
| Physical state | Liquid | O’Neil et al. 2001 |
| Boiling point | 79.6°C | O’Neil et al. 2001 |
| Melting point | −86°C | O’Neil et al. 2001 |
| Solubility in water | 275,000 mg/L 353,000 mg/L | O’Neil et al. 2001 HSDB 2008 |
| Vapor pressure | 90.6 mmHg at 25°C | ATSDR 1992 |
| Vapor density (air =1) | 1.3814 2.41 | O’Neil et al. 2001 HSDB 2008 |
| Liquid density (water =1) | 0.805 | O’Neil et al. 2001 |
| Flash point | 6°C (closed cup) | O’Neil et al. 2001 |
| Explosive limits | ACGIH 2006 | |
| Upper | 12% by volume | |
| Lower | 1.8% by volume | |
| Conversion factors | 1 ppm = 2.93 mg/m3 | ATSDR 1992 |
| 1 mg/m = 0.341 ppm | ||
2.2. Nonlethal Toxicity
2.2.1. Odor Threshold
The odor of MEK has been described as sweet and sharp with the hedonic tone described as neutral to unpleasant (Leonardos et al. 1969; Hellman and Small 1974). The odor threshold has variously been reported as 0.25 to 147 ppm (Billings and Jonas 1981; Amoore and Hautala 1983; Ruth 1986); following standardization of results from different threshold studies, an odor detection threshold of 7.8 ppm was reported (Devos et al. 1990). In the Devos et al. (1990) study, odor thresholds were similar for male and female control subjects, 8.2 and 8.1 ppm, and for male and female subjects with multiple chemical sensitivities, 5.7 and 7.6 ppm. The odor recognition thresholds for trained panels of experts were similar, 6 ppm (Hellman and Small 1974) and 10 ppm (Leonardos et al. 1969). The threshold for irritation as reported by Ruth (1986) was 200 ppm. No data were provided for this value.
2.2.2. Clinical Studies
Five clinical studies addressed subjective symptoms during MEK exposure. These studies are summarized in Table 3-3 and discussed in the text below. Clinical studies that addressed neurotoxic end points are discussed in Table 3-3. During metabolism studies, groups of healthy subjects were exposed to MEK at 200 ppm (Liira et al. 1988a,b; 1990a,b; Shibata et al. 2002), 300 ppm (Tada et al. 1972; van Engelen et al. 1997), or 400 ppm (Liira et al. 1990a) for 2-4 h. A series of studies by Dick et al. (1984; 1988; 1989; 1992) and a study by Shibata et al. (2002) addressed sensory irritation and neurotoxicity as well as metabolism. Two studies involved coexposures to MEK and n-hexane (van Engelen et al. 1997; Shibata et al. 2002). No adverse symptoms were reported in these studies. In some cases exercise was incorporated into the study protocol.
Nelson et al. (1943) exposed 10 male and female volunteers to several concentrations of MEK for 3 to 5 min to determine a concentration that would be satisfactory for industrial exposures and a concentration that would be “unpleasant” or objectionable. Atmospheres were generated by adding a known quantity of vapor saturated air to the measured flow of air being forced into the chamber; there were no analytic measurements. The volunteers found that nose and throat irritation were slight at 100 ppm. Mild eye irritation was reported by some subjects at 200 ppm, and 350 ppm was considered objectionable for an 8-h exposure. The majority of subjects considered 200 ppm satisfactory for an 8-h exposure.
In a combined metabolism and sensory irritation study, four healthy male subjects with no prior exposure to organic solvents inhaled MEK at 100 or 200 ppm for 2 h, both in combination with hexane at 50 ppm (Shibata et al. 2002). The subjects exercised on a ergometer bicycle at a constant workload of 50 watts. The subjects rated the severity of the following symptoms: discomfort in eye, running nose, discomfort in throat or airways, headache, fatigue, nausea, dizziness, feeling of intoxication, difficulty in breathing, and odor of solvents. The rating system ranged from “no effect at all” to “almost unbearable.” Except for odor, all symptoms were rated between “not at all” and “hardly at all” by the subjects. Solvent odor ratings increased with increasing exposure to MEK (rating not stated). Combined exposure to MEK and n-hexane depressed the metabolism of n-hexane. There were no differences in heart rate or performed workload among the different exposure conditions. Metabolism results are summarized in Section 4.1.2.
In a double-blind study, Dick et al. (1992) exposed 13 male and 11 female subjects, ages 18-32, to 200 ppm for 4 h in a test of neurobehavioral performance (summarized in Section 2.3) and sensory and irritant effects. The 4-h exposure session was composed of two 2-h periods. Additional subjects were exposed to methyl isobutyl ketone, a combination of MEK and methyl isobutyl
TABLE 3-3 Summary of Human Studies for Methyl Ethyl Ketone
| Concentration (ppm) | Exposure Duration | Effect and Type of Study | Reference |
| 90-270 (average 150) | 4 h | Concentrations not held constant; underestimation of times of 5 to 30 s by men and expansion of variation of time estimation in women; questionable results | Nakaaki 1974 |
| 100 | 3-5 min | Slight nose and throat irritation | Nelson et al. 1943 |
| 200 | 3-5 min | Mild eye irritation in some subjects; judged satisfactory for 8-h exposure | |
| 350 | 3-5 min | Judged objectionable for 8-h exposure | |
| 100, 200 | 2 h | Metabolism study; exposures in combination with n-hexane; constant workload of 50 watts; odor noticeable; no irritation and no subjective symptoms | Shibata et al. 2002 |
| 200 | 4 h | No significant difference in choice reaction time, visual vigilance, or pattern recognition tests | Dick et al. 1984 |
| 200 | 4 h | No significant difference in psychomotor tests of choice reaction time, visual vigilance, dual task of auditory tone discrimination and tracking, memory scanning, postural sway, profile of moods states | Dick et al. 1988; 1989 |
| 200 | 4 h | Noticeable strong, unobjectionable odor; subjective symptoms similar to control responses | Dick et al. 1992 |
| 200 | 4 h | No irritation; no subjective symptoms; strong odor; increase in mucociliary transport time; nonsignificant changes in proinflammatory cytokines | Muttray et al. 2002 |
| 10 | 4 h | No effect | Seeber et al. 2002; van Thriel et al. 2003a |
| 10-380 (five 8-min peaks to 380 ppm; time-weighted average ≈188) | 4 h | Intense odor; irritation rated “hardly at all”; subjects with self-reported multiple chemical sensitivity included in the study | |
| 25, 200, 400 | 4 h | Metabolism studies; exercise incorporated into some protocols | Liira et al. 1988a,b; 1990a,b |
| 300 | 2-4 h | Metabolism study; sensory and neurobehavioral effects not addressed | Tada et al. 1972 |
| 300-600 | Occupational | Central-nervous-system effects, possibly attributable to concurrent dermal exposure | Smith and Mayers 1944 |
| 33,000, 100,00 | Few breaths | Intolerable, irritation to eyes and nose | Patty et al. 1935 |
| 10,000 | Few breaths | Almost intolerable, irritation to eyes and nose | |
| 3,300 | Not given | Strong odor, moderately irritating to eyes and nose | |
ketone, or an alcoholic drink, which served as a positive control for the neurobehavioral tests. Two control groups were also used: a chemical-control group and an alcohol-control group. The chemical control group was exposed to a combination of MEK and methyl isobutyl ketone at 25 ppm for 5 min at the beginning of the control session. For the subjective part of the study, two questionnaires were used. The “Subjective I” questionnaire consisted of a yes/no format in response to the following items: (1) presence of odor, (2) strong odor, (3) objectionable odor, (4) headache, (5) nausea, (6) throat dryness or coughing, (7) tearing, and (8) unpleasant exposure. The “Subjective II” questionnaire also required yes/no responses to indicate whether the subjects had been exposed to a chemical or to the control atmosphere. The percentages of exposed subjects reporting yes to the eight items above involving odor and irritation were 96%, 48%, 48%, 7%, 19%, 50%, 17%, and 44%, respectively. Except for strong odor, similar numbers of positive responses were recorded for the chemical-control group: 94%, 22%, 40%, 12%, 6%, 34%, 24%, and 34%. As noted, 48% of subjects exposed to MEK reported a strong odor and 22% of the subjects in the chemical-control group reported a strong odor (p < 0.05). The authors, in comparing the headache response between the chemical-control and chemically exposed groups, suggested that test-taking for 4 h accounted for the headache effect. In response to the Subjective II questionnaire, 96% of the subjects exposed to MEK correctly reported that they had been exposed to a chemical.
Muttray et al. (2002) exposed 19 healthy nonsmoking males, ages 22-41, to MEK at 0 or 200 ppm for 4 h. The study was not blind in that subjects were aware of a chemical odor during the exposure to MEK. A questionnaire of 17 items relating to irritation of the mucous membranes, difficulties in breathing, and prenarcotic symptoms was administered before, after 2 h, and after 4 h of exposure. The nasal mucosa was examined. There was no subjective irritation of nasal mucosa. On a scale of 0 to 5, all median scores were 0 (no symptoms). Mucociliary transport time was statistically significantly higher, 660 vs. 600 s. Some cytokines were slightly, nonsignificantly increased, whereas others were unaffected. The authors considered any changes subclinical.
Seeber et al. (2002, see also van Thriel et al. 2002, 2003b) evaluated psychologicl reactions related to chemosensory irritation. Specifically, the authors focused on relationships between irritation, odor, and annoyance in response to acute solvent exposure. They conducted 14 inhalation studies with 4-h exposures to each of eight chemicals. The subjects rated odor (scale of 0 [“not at all”] to 5 [“very strong”]), annoyance or well-being (scale of 1 [“not annoying”] to 7 [“very annoying”]), and eye and nose irritation (same scale as for odor) every half hour. For MEK, 24 paid naive subjects were exposed at a constant concentration of 10 ppm (near the odor threshold) or at five peaks of 380 ppm (initial exposure) with decreases to 10 ppm. The low and high concentrations were held for 8 min; they were linked by periods of increasing or decreasing concentrations for 22 min. The time-weighted average (TWA) in a similar study reported by van Thriel et al. (2003a) was 188 ppm. Rating surveys were taken during the maximum and minimum exposures and during the control exposure, and muco-
sal swelling as measured by anterior active rhinomenometry was measured. The study was single blind because the subjects were unaware of the exposures, but the staff had little interaction with the subjects. The exposure chamber was 28 m3, and concentrations were measured. Irritation, odor, and annoyance scores during exposure to clean air were 0.1, 0.1, and 1.3, respectively. The eye irritation score was 0.4 for the constant 10-ppm MEK concentration. For the changing conditions, odor ratings followed the peaks and valleys of the exposure concentrations, ratings of ≥ 3 ranging from 0-9% of respondents at 10 ppm to 55-91% of respondents at 380 ppm. The averaged ratings for eye and nose irritation were similar and were verbally scored “hardly at all.” Statistically, odor had the strongest effect, followed by annoyance and irritation. The authors (Seeber et al. 2002) concluded that there was no evidence of sensory irritation on a subjective level.
When subjects in the Seeber et al. (2002) study were divided into those with “self-reported multiple chemical sensitivity” (sMCS), measured by response to items on a questionnaire, and subjects who were not sensitive to chemicals (controls), the scores for the sMCS increased with time, whereas those for the controls did not. Each of the nine ratings for the sMCS subjects, taken during the 4-h exposure, was ≤ 1 (“hardly at all”) for nose and eye irritation, and the scores for the controls were all ≤ 0.25 (close to “not at all”). The 95% confidence interval for nose and eye irritation never rose above a score of 1.5. Inflammatory biomarkers—eosinophil cationic protein, myeloperoxidase, interleukin 1β, substance P, and neurokinin—were not affected by either exposure in either the control or the sMCS groups (van Thriel et al. 2003a). A weak dose-response increase in nasal symptoms was reported by the sMCS group; however, mean scores for nasal and eye irritation were never greater than 1 on a scale of 0-5; controls scored 0.2. There was no effect on nasal flow. (Compared with the controls, sMCS subjects had a significant decrease in the flow value in anterior rhinomanometry independent of dose [Wiesmuller et al. 2002]). Breathing rate and heart rate of the two groups of subjects reported in another paper (Haumann et al. 2003) were not changed appreciably by the exposures.
Patty et al. (1935) stated that 33,000 and 100,000 ppm were intolerable to humans because of irritation of the eyes and nasal passages. A concentration of 10,000 ppm was intolerable after a few inhalations because of irritation to the eyes and nose, and 3300 ppm had a moderate-to-strong odor and was moderately irritating to the eyes and nose (no exposure durations given). The raw data or the source of the data were not provided, but the exposures presumably took place during the authors’ exposure of guinea pigs to the same concentrations.
2.2.3. Monitoring Studies
Monitoring studies indicated that workers were routinely exposed to MEK at ≤ 100 ppm, as taken by instantaneous and 4-h passive samplers (Miyasaka et al. 1982; Brugnone et al. 1983; Perbellini et al. 1984), and to TWA exposures up
to 224 ppm (Yoshikawa et al. 1995) and 270 ppm (median value, 26 ppm) (Imbriani et al. 1989); in one case, 4-h TWA exposures ranged up to 950 ppm (Ghittori et al. 1987). Samples were taken by several methods, including instantaneous samples via glass tubes and 2- and 4-h passive samplers. In some cases, workers were exposed to a mixture of solvents. Health effects were not addressed in these studies.
2.2.4. Case Reports
In occupational settings, the primary routes of exposure are inhalation and skin contact. Symptoms incurred by workers during occupational exposures have been described. MEK is a strong degreasing agent, and contact with the skin might result in dermatitis. Workers handling MEK while manufacturing raincoat water-proofing material developed severe dermatosis with a complete lack of sensation in the digits and limbs (Smith and Mayers 1944). Workroom concentrations ranged from 300 to 600 ppm. Dermal contact with liquid MEK during the processes was highly likely because it was reported that workers tended to wash their hands in the solvent. Two workers in a similar plant where exposures were at 1,000 ppm measured as ketone vapors (acetone at 330-495 ppm plus MEK at 398-561 ppm) suffered episodes of CNS depression, and loss of consciousness (Smith and Mayers 1944).
2.2.5. Epidemiologic Studies
Available epidemiology studies involved a mixture of solvents and generally addressed neurotoxicity (Arlien-Soberg 1991). Adverse effects could not clearly be related to exposure to MEK alone and therefore are not discussed in this report. Epidemiology studies that addressed the potential carcinogenicity of MEK are discussed in Section 2.6 (Carcinogenicity).
2.3. Neurotoxicity
During 4-h exposures of male and female human subjects to MEK at 90 to 270 ppm, the subjects participated in time-estimation tests (Nakaaki 1974). The concentration increased over the 4-h periods; the average concentration for each exposure was 150 ppm. There were nine morning and nine afternoon sessions, and two males and two females participated in each session. Males tended to underestimate times of 5 to 30 s, and females showed more variable results compared with control estimates of time. The time-estimation values from this study were highly variable, and no statistical differences were presented between or among the exposure groups. The subjects reported a strong odor at 90 ppm. This study differs from recent well-conducted clinical studies in that symptoms of tears and sneezes were reported (see Table 3-3 for results of recent stud-
ies). Furthermore, when testing solvents, such as acetone, with similar mechanisms of action, time-estimation changes in males and females differed from those of MEK.
Dick et al. (1984) exposed groups of paid volunteers to MEK at 0 or 200 ppm for 4 h in a study of psychomotor performance. A group of 20 male and female volunteers were exposed at 200 ppm for 4 h. The control group consisted of 20 volunteers. The MEK concentration was continuously monitored with infrared analyzers and confirmed by gas chromatography. The average concentration over the 4-h period was 190 ppm. Two performance tests, reaction time and visual vigilance, were administered throughout the pre-exposure, exposure, and postexposure periods; a third test, pattern discrimination, was run only during the pre-exposure and exposure period. There were no statistically significant differences between the treated and the control groups on any test.
In a double-blind study, groups of 9-12 male and 10-13 female volunteers ranging in age from 18 to 32 years were exposed to MEK at 0 or 200 ppm or to 95% ethanol (0.84 mL/kg as a positive control) for 4 h (Dick et al. 1988, 1989). The computerized testing regimen consisted of 2-h sessions on each of 3 days: a practice session on day 1; tests prior to exposure, during exposure (two testing sessions) and postexposure on day 2; and tests postexposure on day 3. During each 2-h test session, four psychomotor tests (choice reaction time, visual vigilance, dual task, and short-term memory scanning); a neurophysiologic test (eye blink reflex); and one sensorimotor test (postural sway) were administered to the test subjects. A profile-of-mood states psychologic test was administered following exposure and on the following day. Exposure to MEK produced no statistically significant interpretable results. Exposure to MEK at 100 ppm and to either acetone at 125 ppm or toluene at 50 ppm (Dick et al. 1984) had no significant effect on behavioral tests. Ethanol, at a measured blood alcohol content of 0.07-0.08%, produced pronounced performance decrements in several tests.
In a third double-blind study, Dick et al. (1992) exposed 13 male and 11 female volunteers, ages 18-32, to 200 ppm for 4 h while they performed five psychomotor tests—choice reaction time, simple reaction time, visual vigilance, dual task, and memory scanning—and one sensorimotor test—postural sway. The 4-h exposure session was composed of two 2-h periods. Sensory effects for this study are summarized in Section 2.2. As in the earlier study (Dick et al. 1988), ethanol (0.84 mL/kg) was used as a positive control substance. According to the authors, the exposure to MEK did not produce any conclusive, consistently interpretable effect of chemical treatment. In contrast, ethanol ingestion produced significant decrements on every performance test except memory scanning.
In contrast to acute exposures, chronic exposures may be neurotoxic. Workers in a cable factory exposed at 50-120 ppm over an 8-h work shift for an average of 14 years had increased mood disorders and reported more headaches, memory difficulties, and sensory irritation than controls. Although tested, decrements in neurobehavioral tests were not reported for MEK (Mitran et al. 1997).
2.4. Developmental and Reproductive Toxicity
No information was found specific to the developmental and reproductive toxicity of MEK in humans.
2.5. Genotoxicity
No studies regarding the genotoxicity of MEK in humans via the inhalation route were located in the available literature. In an in vitro study, MEK at concentrations of 10−2, 10−3, or 10−4 M was neither cytotoxic nor increased tritiated thymidine uptake in human lymphocytes (Perocco et al. 1983).
2.6. Carcinogenicity
In an early unpublished study of 306 male employees who had been employed in the lubricants dewaxing unit (a petroleum refining process) of a refinery, the overall mortality was less than expected, and there was no evidence of an excess of deaths from cancer (Enterline 1978). Five deaths from cancer occurred, and six were expected. Two of the deaths were from prostate cancer.
A retrospective epidemiology study was undertaken of 446 men who had worked at two MEK dewaxing plants for a period of at least 1 continuous year (Alderson and Rattan 1980). The solvent exposure consisted of MEK and benzene prior to 1971, after which benzene was replaced with toluene. There was a slight reduction in overall mortality: observed deaths, 46; expected deaths, 55.5; and a slight deficiency of deaths from neoplasms: observed, 13; expected, 14.4. There were more deaths than expected from buccal cavity and pharyngeal cancers: observed, 2; expected, 0.13; and fewer deaths than expected from lung cancer: observed, 1; expected, 6.0. From the results, the authors concluded that there was no clear evidence of a cancer hazard.
Wen et al. (1985) conducted a retrospective cohort mortality study of 1,008 men employed in solvent dewaxing units of a refinery between 1935 and 1978. Exposure was to benzene and MEK prior to 1945 and to MEK and toluene thereafter. Personal samples indicated that the 8-h TWA for MEK was approximately 1 ppm. Less than 5% of the samples were more than 5 ppm. The TWA toluene concentration was also approximately 1 ppm. Measurements of other solvents, including benzene, hexane, xylene, and methyl isobutyl ketone, were < 0.1 ppm. The standardized mortality ratio (SMR) (compared with the U.S. population) for all causes was 0.70, and the SMR for cancer was 0.86. Prostate cancers were nonsignificantly increased among maintenance workers but not among workers specifically assigned to the MEK units.
2.7. Summary
The relative toxicity of most ketones is low, and no information on deaths
attributable to exposure to MEK was located. The odor recognition threshold for MEK ranges from 6 to 10 ppm (Leonardos et al. 1969; Hellman and Small 1974). Volunteers exposed to MEK for 3-5 min judged 200 ppm as acceptable for an 8-h exposure and 350 ppm as objectionable for an 8-h exposure (Nelson et al. 1943). In a more recent 4-h study, 200 ppm was also judged as unobjectionable by healthy volunteers (Dick et al. 1992). Additional behavioral and metabolism studies with human volunteers conducted at 200 and 400 ppm for 4 h did not reveal either irritant or neurotoxic effects. The Dick et al. studies (1984, 1988, 1989, 1992) with exposures at 200 ppm for 4 h found no exposure-related changes in performance on psychomotor and mood tests or incidences of irritation. A 4-h exposure at 90-270 ppm caused minor disturbances in the conception of time (Nakaaki 1974). Several recent clinical studies (Dick et al 1992; Muttray et al. 2002; Seeber et al. 2002; Shibata et al. 2002) reported that MEK was associated with strong odor rather than irritation. This finding shows that odor rather that irritation was probably responsible for symptom complaints in earlier studies, such as Nelson et al. (1943). Subjects with sMCS found repeated 8-min exposures at a concentration of 380 ppm practically nonirritating (Seeber et al. 2002). Some workers exposed at higher concentrations, up to 1,000 ppm total ketones for unknown exposure durations, suffered CNS depression (Smith and Mayers 1944), but dermal exposure to the liquid in addition to inhalation exposure most likely contributed to the effects.
No studies regarding genotoxicity in humans were located. No information was found specific to the developmental and reproductive toxicity of MEK in humans. Two retrospective epidemiology studies of workers chronically exposed to MEK at petroleum refining plants reported that deaths due to cancers were fewer than expected (Alderson and Rattan 1980; Wen et al. 1985).
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
Data on acute lethality were available for the rat, mouse, and guinea pig. Data are summarized in Table 3-4.
3.1.1. Rats
Carpenter et al. (1949) reported that a 4-h exposure of Sherman rats to MEK at 2,000 ppm killed two to four of six rats (exact number not stated). However, in a later study, this same group (Pozzani et al. 1959; Smyth et al. 1962) reported that rats exposed at 2,000 ppm for 2 h showed no toxicity, an 8-h exposure at 8,000 ppm killed half of the rats, and an exposure at 16,000 ppm caused the deaths of all of the rats in the exposure group. All concentrations were nominal.
TABLE 3-4 Summary of Acute Lethal Inhalation Data in Laboratory Animals
| Species | Concentration (ppm) | Exposure Duration | Effect | Reference |
| Rat | 92,239 | 3 h | LT50 | Klimisch 1988 |
| 92,239 | 30 min | No deaths | ||
| Rat | 8,000 | 8 h | LC50 | Pozzani et al. 1959; |
| 2,000 | 2 h | No signs of toxicity | Smyth et al. 1962 | |
| Rat | 20,200 | 4 h | 100% mortality | La Belle and Brieger 1955 |
| 18,100 | 4 h | 100% mortality | ||
| 13,750 | 4 h | 87.5% mortality | ||
| 12,200 | 4 h | 50% mortality | ||
| 9,260 | 4 h | 12.5% mortality | ||
| 9,090 | 4 h | 12.5% mortality | ||
| 7,850 | 4 h | No deaths | ||
| 11,700 | 4 h | LC50 | ||
| Mouse | 103,000 | 43 min | Mean survival time | La Belle and Brieger 1955 |
| Mouse | 100,000 | 45 min | 100% mortality | Zakhari et al. 1977 |
| 90,000 | 45 min | 80% mortality | ||
| 80,000 | 45 min | 50% mortality | ||
| 70 000 | 45 min | 20% mortality | ||
| 60,000 | 45 min | No deaths | ||
| 50,000 | 45 min | No deaths | ||
| 69,500 | 45 min | LC50 | ||
| Guinea | 100,000 | 45 min | Lethal | Patty et al. 1935 |
| pig | 33,000 | 200 min | Lethal | |
| 10,000 | 2-4 min | Irritation of eyes and | ||
| 40 min | nose | |||
| 90 min | Lacrimation | |||
| 4-4 7 h | Incoordination | |||
| 13.5 h | Narcosis | |||
| 3,300 | 13.5 h | No deaths | ||
| No overt signs of toxicity | ||||
Groups of eight adult male albino rats inhaled concentrations of 7,850, 9,090, 9,260, 12,200, 13,750, 18,100, or 20,200 ppm for 4 h (La Belle and Brieger 1955). Atmospheres were measured spectrophotometrically. Respective mortalities were 0%, 12.5%, 12.5%, 50%, 87.5%, 100%, and 100%. The calculated LC50 was 11,700 ± 2,400 ppm. Most deaths were “immediate”, narcosis having preceded death.
Klimisch (1988) exposed three male and three female rats (strain unspecified) to the saturated vapor of MEK at 20°C to determine the time to 50% mortality (LT50). The postexposure observation period was 14 days. The concentration that corresponded to a nominal LT50 of 3 h was 272 mg/L (approximately 92,239 ppm). No deaths occurred after a 30-min exposure at 92,239 ppm.
3.1.2. Mice
Adult white mice (strain not identified) exposed to a saturated vapor concentration of MEK, estimated at 103,000 ppm, had a mean survival time of 43 min (La Belle and Brieger 1955). Six groups of 10 male CF-1 mice were exposed at concentrations of 50,000, 60,000, 70,000, 80,000, 90,000, or 100,000 ppm for 45 min (Zakhari et al. 1977). Survivals, observed immediately after exposure, were 100%, 80%, 50%, 20%, 0%, and 0% at the respective exposure concentrations (see Table 3-4). The 45-min LC50 was 69,500 ppm. According to Zakhari et al. (1977), the progression of toxic signs was incoordination, narcosis, and respiratory depression followed by death.
3.1.3. Guinea Pigs
Patty et al. (1935) exposed groups of six guinea pigs to MEK at 3,300, 10,000, 33,000, or 100,000 ppm (the latter concentration was the air saturation concentration) for periods up to 13.5 h. The acute effects advanced through several distinct stages as the exposure proceeded: nose irritation (manifested by rubbing of the nose with the forepaws), eye irritation (squinting), lacrimation, incoordination, narcosis, labored breathing, and death. Vapor concentrations of 33,000 and 100,000 ppm produced death by 200 and 45 min, respectively. Gross pathology revealed slight congestion in the brain and marked congestion of the lung, liver, and kidneys of those animals that succumbed during the exposure or were killed immediately after exposure. These signs were absent in most animals that were killed 4 to 8 days later. Animals that survived exposure at 100,000 ppm for 30 min showed severe corneal opacity that regressed by 8 days postexposure. Although the authors could not clearly attribute the cause of death to either irritation of the lungs or a state of narcosis that terminated in death, the complete reversal of effects in all animals that survived the exposures indicated that the primary mode of action was narcosis. No abnormal signs were observed during a 13.5-h exposure at 3,300 ppm. At 10,000 ppm, progressive signs included irritation of the nose and eyes in 2 and 4 min, respectively, lacrimation in 40 min, incoordination in 90 min, and unconsciousness in 4-4.7 h. No gasping respiration or deaths occurred during a 13.5-h exposure at 10,000 ppm.
3.2. Nonlethal Toxicity
Studies with acute exposure durations are summarized in Table 3-5 and discussed below. Studies with neurotoxic end points are listed in Table 3-5 and discussed in Section 3.3. Studies with intermediate exposure durations are also discussed in the following sections.
TABLE 3-5 Summary of Nonlethal Inhalation Data in Laboratory Animals
| Species | Concentration (ppm) | Exposure Duration | Effect | Reference |
| Baboon | 100 | 24 h, 7 days | No effect on match-to-sample task during first day | Geller et al. 1979 |
| Rat | 10,000 | 8 h/day several days | Severe irritation, respiratory tract | Altenkirch et al. 1978 |
| 6,000 | 8 h/day for weeks | Mildly somnolent, but arousable | ||
| Mouse | 31,426 26,000 10,000 | 30 min 30 min 30 min | Calculated 50% decreased respiration Decrease in body movements Not anesthetic | Hansen et al. 1992 |
| Mouse | 10,745 | 5 min | RD50 | De Ceaurriz et al. 1981 |
| Mouse | 5,000 9,000 | 10 min 10 min | 15% decrease in respiratory rate RD50 | Stone et al. 1981 |
| Mouse | 2,891 | 9.5 min | EC50, schedule-controlled behavior | Glowa and Dews 1987 |
| 10,000 | 9.5 min | Mice unresponsive | ||
| 5,600 | 9.5 min | No response in most mice | ||
| 3,000 | 9.5 min | Response decreased by 75% | ||
| 1,000 | 9.5 min | Response slightly decreased | ||
| 300 | 9.5 min | No effect on response | ||
| Mouse | 2,065 | 4 h | 50% decrease in immobility in behavioral-despair swimming test | De Ceaurriz et al. 1983 |
3.2.1. Rats
Altenkirch et al. (1978) exposed a group of five male Wistar rats to MEK for 8 h/day, 7 days/week, for 7 weeks (a control group was not reported). Other groups were exposed to MEK and n-hexane or n-hexane alone. The initial exposure to MEK alone was at 10,000 ppm but had to be lowered to 6,000 ppm within a few days because of severe irritation of the respiratory tract. The rats exposed to MEK at 1,000 ppm and to n-hexane at 9,000 ppm became “somnolent within 5 to 10 min after a short excitation stage but remained arousable during the whole period of exposure.” This effect was “less marked” in rats exposed to MEK alone. The authors did not state the day on which this effect was first noted. Transient signs of ataxia and gait disturbances, primarily during the last weeks of exposure, were noted for 10-20 min after exposure. None of the MEK-exposed rats developed motor impairment, and neurohistologic examinations showed no treatment-related lesions. The exposure duration was planned for 15 weeks, but all rats died of bronchopneumonia during the seventh week of exposure.
In a 90-day study, groups of 15 male and 15 female Fischer 344 rats were exposed to MEK at 0, 1,250, 2,500, or 5,000 ppm for 6 h/day, 5 days/week, for 90 days (ToxiGenics 1981; Cavender et al. 1983). Analytically determined TWA concentrations were 0, 1,254, 2,518, or 5,041 ppm. No animals died during the study, and there were no adverse effects on the clinical health or growth of the rats, although body weights were transiently depressed early in the study for both sexes in the 5,000-ppm group. Daily observations and weekly clinical examinations revealed no nasal or eye irritation. Absolute and relative liver weights were significantly increased in both sexes in the 5,000-ppm group, and some serum hematology and clinical chemistry parameters were increased in females in the 5,000-ppm group. Evaluations of neurologic function (posture, gait, facial muscular tone, and neuromuscular reflexes) revealed no abnormalities. Special neuropathy studies of the medulla and peripheral nerves revealed no lesions attributable to MEK exposure. The U.S. Environmental Protection Agency (EPA 2003) noted the presence of chronic respiratory disease in both control and MEK-exposed rats.
In additional studies of intermediate duration, no clinical signs or deaths were reported during or following exposures of rats at 200 ppm, 12 h/day, for 24 weeks (Takeuchi et al. 1983) or at 1,125 ppm, 24 h/day, for 5 months (Saida et al. 1976).
3.2.2. Mice
Several studies addressed the sensory irritation of MEK. The concentration that depressed the respiratory rate by 50% (RD50) after 5 min for six male OF1 mice was 10,745 ppm (De Ceaurriz et al. 1981). Atmospheres were measured with gas chromatography. Stone et al. (1981) reported a similar value, 9,000 ppm, using male Swiss-Webster mice with a 10-min exposure period (exposure system not described), whereas Hansen et al. (1992) reported a much higher value, 31,426 ppm during 30-min exposures of male CF1 mice. The concentration of 31,426 ppm was projected from the data because test concentrations ranged only up to 26,416 ppm (range, 3,809-26,416 ppm). The differences among the studies may be due to the fact that for nonprimary irritants, the concentrations causing decreased respiration plateau at high concentrations. In this study (Hansen et al. 1992), atmospheres were monitored continuously by infrared spectroscopy. The threshold for respiratory rate depression was 3,589 ppm at the beginning of an exposure. Tidal volume was also decreased in this study; this response is not mediated by the trigeminal nerve (which mediates the irritant response). Body movements were unaffected at 10,000 ppm, slightly decreased at 15,000-20,000 ppm, and strongly depressed at 26,416 ppm. Tracheally cannulated mice were also tested at these concentrations and none died. No histopathologic examinations were conducted.
3.3. Neurotoxicity
Geller et al. (1979) exposed four baboons to MEK at 100 ppm continuously for 7 days. Operant conditioning behavior, a match-to-sample discrimination task, conducted during exposure was compared with pre-exposure test scores for each baboon. There was no significant effect on accuracy, but there was a decrease in mean response time and response during delay beginning on the second day. There was no effect during the first day of exposure. The same animals were used for several chemical tests, and MEK was tested second. At least 1 month elapsed between tests.
During exposure of five adult male Wistar rats to MEK at 10,000 or 6,000 ppm for 8 h/day, the animals were excitable for 5-10 min at the beginning of each exposure and then became mildly somnolent but arousable (Altenkirch et al. 1978). Exposure at 6,000 ppm continued for several weeks. Exposure of guinea pigs at 10,000 ppm caused incoordination in 90 min and narcosis in 4-4.7 h (Patty et al. 1935). Continuous exposure of young male Wistar rats at 750 ppm for 7 days reduced subsequent hexabarbital sleep times (16 min vs. 26 min for controls), (Couri et al. 1977). This effect is most likely due to induction of cytochrome P-450-2B and -2E, which would enhance metabolic clearance and reduce the hypnotic action.
In a schedule-controlled-response experiment that used milk as an incentive, a group of 12 mice was exposed at increasing concentrations of MEK (300, 1,000, 3,000, 5,600, or 10,000 ppm) for a series of eight food presentations or 9.5 min, whichever occurred first (Glowa and Dews 1987). There was a 30-min break between each exposure. There was no effect on behavior at 300 ppm. At 1,000 and 3,000 ppm, responses were decreased by approximately 25% and 75%, respectively. At 5,600 ppm, response in most mice ceased, and at 10,000 ppm, all response ceased. The EC50 (concentration that causes an effect in 50% of the exposed population) was 2,891 ppm. Responding completely recovered 30 min after exposure.
Exposure of male Swiss OF1 mice to 1,602, 1,848, 2,050, or 2,438 ppm for 4 h caused a dose-related reduction of immobility in a behavioral-despair swimming test (De Ceaurriz et al. 1983). The concentration associated with a 50% decrease in immobility during the 3-min test was 2,065 ppm. The authors did not interpret the meaning of the increase in swimming time (antidepressive effect of solvents).
During sensory irritation tests with mice that lasted 30 min, Hansen et al. (1992) observed body movements at 26,416 ppm and noted that this concentration did not cause “serious” depression of the CNS, that is, anesthesia or asphyxia.
Exposure of six male Sprague-Dawley rats to MEK at 500 ppm for 22 h/day, 7 days/week, for 6 months did not result in any significant clinical or histopathologic evidence of neurologic dysfunction (Egan et al. 1980). Exposure
of Fischer 344 rats at up to 5,000 ppm for 90 days did not result in any signs of neurotoxicity or lesions of the nervous system (ToxiGenics 1981; Cavender et al.1983). In the latter study, neurohistopathologic examination of the medulla and the sciatic and tibial nerves revealed no lesions that could be attributed to MEK exposure.
3.4. Developmental and Reproductive Toxicity
Groups of 21-23 pregnant Sprague-Dawley rats were exposed to MEK at nominal concentrations of 1,000 or 30,00 ppm for 7 h/day during gestation days 6 through 15 (Schwetz et al. 1974). Experimental exposures at 1,000 or 3,000 ppm were conducted separately, each with a control group. Analytically determined concentrations (infrared analysis) were 1,126 and 2,618 ppm. These concentrations were stated as being subanesthetic by the study authors. Neither concentration had an effect on the incidence of fetal resorptions. Fetal body measurements were reduced in the group exposed at 1,000 ppm but not in fetuses of rats exposed at 3,000 ppm. For the group exposed at 3,000 ppm, compared with the control group, there was an increase in the total number of litters containing fetuses with delayed ossification of the sternebrae and in the total number of litters containing fetuses with soft tissue anomalies (both, p < 0.05), although no soft-tissue anomaly occurred at a significantly increased incidence compared with the control group. (There were more litters with soft-tissue anomalies than were in the control group, but there were no more anomalies per litter.) There was no maternal toxicity as observed by clinical signs, food consumption, weight gain, conception rate, number of implantations or litter size, serum glutamic-pyruvic transaminase activity, or absolute or relative liver weights.
To confirm the fetotoxic effects observed in the Schwetz et al. (1974) study, Deacon et al. (1981) repeated the study by exposing groups of 25 pregnant Sprague-Dawley rats to MEK at 1,000 or 3,000 ppm for 7 h/day on gestation days 6 through 15. In addition to 35 controls, a group of 25 rats was also exposed at 400 ppm. Analytically determined concentrations were 412, 1,002, and 3,005 ppm, respectively. Slight maternal toxicity was observed in dams exposed at 3,000 ppm as evidenced by decreased weight gain and increased water consumption. In this study, there was no adverse effect on fetal body weight or crown-rump length among litters. No external or soft-tissue alterations were observed among fetuses at any exposure concentration. Slight fetotoxicity was observed among litters of rats exposed at 3,000 ppm as evidenced by an increased incidence of minor skeletal variants—delayed ossification of the skull, extra ribs, and delayed ossification of the cervical centra (all p < 0.05). According to the authors, there was no evidence of an embryotoxic or teratogenic response in any exposure group.
Groups of 10 virgin and 33 pregnant Swiss mice (Crl:CD-1) were exposed at 0 (filtered air), 400, 1,000, or 3,000 ppm for 7 h/day on gestation days 6-15
(Mast et al. 1989; Schwetz et al. 1991). Virgin females were included to assess the state of pregnancy on maternal toxicity. Analytically determined concentrations were 398, 1,010, and 3,020 ppm, respectively. Chamber atmospheres were monitored with a gas chromatograph. Body weights were obtained throughout the study, and uterine and fetal body weights were obtained at death on day 18. Uterine implants were enumerated, and live fetuses were sexed and examined for gross defects. There were no deaths or overt signs of toxicity in virgin or pregnant females during the exposures. Body weights and uterine weights were not affected by exposure, but the liver-to-body-weight ratio was significantly increased in pregnant females in the 3,000-ppm group. Compared with the controls, there was no effect of exposure on number and percentage of pregnant females, implantations/dam, live fetuses/litter, resorptions/litter, dead fetuses/litter, or litters with resorptions. Fetal weights of both males and females of dams exposed at 3,000 ppm were lower than control weights, the difference attaining significance in males (5%) and males and females combined (4%) (both, p < 0.05). The number of fetuses with malformations was slightly increased in the 3,000-ppm group, 323 vs. 310 in the control group, but the difference was not statistically significant on the basis of number of fetuses, litters affected, or number of fetuses per litter. However, several types of malformations observed in the exposed group were not present in the control group: cleft palate, fused ribs, missing vertebrae, and syndactyly. There was a significant trend for misaligned sternebrae in fetuses (p < 0.05) but not on the basis of litters. No significant signs of maternal or developmental toxicity were observed at 1,000 ppm. The authors considered 1,000 ppm to be a no-effect concentration for maternal and developmental toxicity and 3,000 ppm to be a concentration that caused mild developmental toxicity.
Exposure of pregnant female Wistar rats for 23 h/day, 7 days/week, at 800 or 1,000-1,500 ppm prenatally (21 days) or pre- and postnatally (52 days) resulted in concentration-related decreases in pregnancy and resorption rates (Stoltenburg-Didinger et al. 1990).
Decreased fetal body weight was also reported in pregnant rats administered 2-butanol (a metabolite of MEK) by the oral route in a multigeneration and developmental study (Cox et al. 1975). The no-observed-adverse-effect level (NOAEL) and the lowest-observed-adverse-effect level (LOAEL) in this study were 1,771 and 3,122 mg/kg/day.
No studies were located that specifically addressed reproductive toxicity. However, histologic examination of the reproductive organs of male and female rats exposed at 5,000 ppm for 90 days revealed no exposure-related lesions (Cavender et al. 1983).
3.5. Genotoxicity
Genotoxicity studies were reviewed in ATSDR (1992). In in vivo studies, no induction of micronuclei was found in erythrocytes of mice or hamsters in-
jected intraperitoneally with MEK. MEK was not mutagenic in several strains of Salmonella typhimurium or in Escherichia coli and Saccharomyces cerevisiae, but it induced aneuploidy and chromosome loss in S. cerevisiae. In mammalian cells, test results were negative for chromosomal aberrations and unscheduled DNA synthesis in rat liver cells, morphologic transformation in BALB/3T3 cells, and gene mutation in mouse lymphoma cells.
3.6. Chronic Toxicity and Carcinogenicity
No studies that addressed the carcinogenicity of MEK via the inhalation route were located in the available literature. No tumors were observed on the skin of mice exposed with dermal applications of MEK of 50 mg/application, 2 days/week for 1 year (Horton et al. 1965).
3.7. Summary
Data on lethality were available for the rat, mouse, and guinea pig. Lethal values ranged from a 45-min LC50 of 69,500 ppm for the mouse (Zakhari et al. 1977) to an 8-h LC50 of 8,000 ppm for the rat (Pozzani et al. 1959). However, data were conflicting because Altenkirch et al. (1978) reported no deaths (but severe irritation of the respiratory tract) in rats following exposure at 10,000 for 8 h/day, for several days. Slight narcosis was reported at 6,000 (rat) to 10,000 ppm (mouse) in some studies (Altenkirch et al. 1978; Glowa and Dews 1987) but not at 10,000 ppm in another study with the mouse (Hansen et al. 1992) or at 3,300 ppm for 13.5 h in the guinea pig (Patty et al. 1935). Subtle neurobehavioral changes were observed in the mouse at 2,438 and 2,891 ppm (De Ceaurriz et al. 1983; Glowa and Dews 1987). In a 90-day study, 5,000 ppm for 6 h/day was a no-effect level for neurobehavioral deficits and neurologic lesions in rats (Cavender et al. 1983). EPA (2003) stated that “animal studies provide no convincing evidence that exposure to MEK alone causes persistent neurotoxic effects.” Severe respiratory irritation as measured by the RD50 in the mouse ranged from 9,000 ppm (Stone et al. 1981) to 31,426 ppm (Hansen et al. 1992).
Results of a series of developmental studies with the mouse and rat determined that 3,000 ppm was toxic to the fetus as indicated by reduced fetal body weight and bone abnormalities; 1,000 ppm was a NOAEL (Deacon et al. 1981; Mast et al. 1989; Schwetz et al. 1991). In the Deacon et al. (1981) study, the developmental toxicity may be related to the concomitant overt maternal toxicity as evidenced by reduced body weights of the dams.
MEK was not genotoxic in a series of tests with S. typhimurium and mammalian cells. Although not mutagenic in S. cerevisiae, MEK caused mitotic chromosome loss and aneuploidy in this species. No animal studies involving chronic toxicity and carcinogenicity via the inhalation route were located in the available literature.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism, Disposition, and Kinetics
4.1.1. Metabolism and Disposition
Following a single intraperitoneal injection of 450 mg/kg, male guinea pigs metabolized MEK to 3-hydroxy-2-butanone, 2,3-butanediol, and 2-butanol, the latter a minor metabolite (DiVincenzo et al. 1976). On the basis of these metabolites, the study authors concluded that the metabolism of MEK follows both oxidative and reductive pathways. Reversible reduction of the ketone group yields 2-butanol; microsomal ω-oxidation yields 3-hydroxy-2-butanone, which is reduced to the diol. In this study, the serum half-life of MEK was 270 min, and the clearance time was 12 h. Dietz et al. (1981) detected the same three metabolites in the blood of male Sprague-Dawley rats exposed orally with 1.69 g/kg.
2-Butanol and 2,3-butanediol were measured in the serum or whole blood (Liira et al. 1990b), and 3-hydroxy-2-butanone and 2,3-butanediol (but not 2-butanol) were identified in the urine (Perbellini et al. 1984; Liira et al. 1988a,b) of human subjects exposed to MEK, indicating that the metabolism in humans is similar to that in animals. As excretion of 2,3-butanediol accounted for less than 3% of the inhaled dose and only 5% was exhaled by the lungs as unmetabolized MEK, most of the absorbed MEK apparently enters intermediary metabolism pathways (Liira et al. 1990a; Liira et al. 1991). Urinary excretion of 2,3-butanediol showed great individual variation. Although metabolism is fairly rapid, having an estimated half-life in the blood of 20 to 49 min (Fiserova-Bergerova 1985; Brown et al. 1987), Di Vincenzo et al. (1976) stated that the metabolism of MEK is relatively slow compared with other ketones.
4.1.2. Pharmacokinetic Data
Using human tissues from autopsies, Fiserova-Bergerova and Diaz (1986) determined the tissue-gas partition coefficients of MEK. The fat:gas and blood:gas partition coefficients were 162 and 125, respectively. The gas:tissue partition coefficients for muscle, kidney, lung, and brain ranged from 96 to 107, indicating nearly equal solubility in all tissues. Similar tissue:blood partition coefficients of 0.95 to 1.18 were found by Poulin and Krishnan (1995). Their algorithm utilized information on chemical water solubility and lipid and water content of tissues. Experimentally determined blood:air partition coefficients for an oil and water matrix and rat blood were 159 and 136, respectively (Beliveau and Krishnan 2000). Blood:atmospheric air and blood:alveolar air partition coefficients obtained by Perbellini et al. (1984) were 183 and 104, respectively. A slightly higher value for blood:air of 202 was reported by Sato and Nakajima (1979). At 37°C and using blood from Wistar rats, partition coefficients for wa-
ter and air and olive oil and air were 134 and 131, respectively; during actual exposures of rats to MEK, the thermodynamic partition concentration was calculated to be 103 (Kessler et al. 1989). The capacity of the tissues to hold MEK was considered to be large due to the high blood and tissue solubility of MEK (Liira et al. 1988a). In all of these studies, the solubility of MEK in tissues was similar to that in blood because tissue-to-blood-concentration ratios were all approximately 1.
The kinetics of MEK were studied with human volunteers (Liira et al. 1988a; see also Liira et al. 1988b, 1990a,b). The subjects were nine healthy male volunteers ranging in age from 18 to 34 years. Exposures were at 200 ppm for 4 h; some exposures encompassed several 10-min exercise periods at a level of 100 watts. The same group was exposed to both the sedentary and exercise conditions with a 1-week break between sessions. Concentrations were monitored with an infrared analyzer. Retention by the lungs was 53% and was not influenced by exercise. The estimated pulmonary uptake was 11.38 and 14.30 millimoles (mmol) for sedentary volunteers and volunteers undergoing exercise periods, respectively. Pulmonary excretion of MEK was 0.26 (sedentary) and 0.41 (exercise) mmol. Apparent MEK clearance was 0.44 (sedentary) and 0.33 L/min/kg (exercise). In a similar study, the area under the curve was 23,400 µmol × min/L (Liira et al. 1990b). The blood concentration rose rapidly during the first hour and then steadily during the following 3 h—reaching approximately 95 µmol/L (6.9 µg/mL) in sedentary subjects and 150 µmol/L (10.9 µg/mL) in exercising subjects (Liira et al. 1988a)—and approached steady state. (Blood concentrations in this study and the following studies are summarized in Table 3-6.) Two elimination phases of MEK from blood were observed, having half-lives of 30 and 81 min. Only 2-3% of the absorbed dose was eliminated by exhalation from the lungs. Blood concentrations during and following a 4-h exposure of eight subjects at 200 ppm (Liira et al. 1988b) are graphed in the Figure 3-1.
In a follow-up study, MEK exposures were at 25, 200, or 400 ppm for 4 h (Liira et al. 1990a). The concentrations in venous blood rose rapidly during the first hour and then more slowly during the subsequent 3 h. At the end of 4 h, the concentrations were 5, 93-100, and 229-309 µM, respectively. Using the data from these exposures and a physiologically based simulation model, Liira et al. (1990a) suggested dose-dependent kinetics of MEK during sedentary exposures exceeding 100 ppm. Earlier, using the physiologically based pharmacokinetics model of Johanson and Naslund (1988), Liira et al. (1988a) suggested that saturation kinetics are reached for MEK at a concentration of 200 ppm for 4 h. In a later study conducted at both 200 and 400 ppm in humans, Liira et al. (1990a) suggest that kinetic saturation is reached at blood concentrations above 100 µM (7 µg/mL) in both humans and rats. Pulmonary ventilation is the rate-limiting step of MEK uptake (Liira et al. 1988a).
TABLE 3-6 Blood Concentrations of Methyl Ethyl Ketonea
| Exposure Concentration (ppm) | Blood Concentration (µg/mL) | Exposure Conditions | Reference | |
| Human Subjects | ||||
| Background | 0.007 | Nonoccupational exposures | Ashley et al. 1994 | |
| 25 | 0.36 | 4 h, sedentary subjects | Liira et al. 1988a; | |
| 200 | 6.9 | 4 h, sedentary subjects | Liira et al. 1990a | |
| 200 | 10.9 | 4, h, exercising subjects | Liira et al. 1990a | |
| 400 | 19.4 | 4 h, sedentary subjects | Liira et al. 1990a | |
| 48 | 0.71 | Occupational exposures; simultaneous exposure to other chemicals | Yoshikawa et al. 1995 | |
| ≤100 | 2.6 (range, 0.8-9.6) | Occupational exposures | Brugnone et al. 1980; Perbellini et al. 1984 | |
| 120 | 0.37b | 2 h at rest | Imbriani et al. 1989 | |
| 0.66 | 4 h at rest | |||
| 1.4 | 2 h with exercise | |||
| 100 | 4.3 | 2 h; exercising subjects (50 watts) | Shibata et al. 2002 | |
| 200 | 12.3 | Simultaneous exposure to n-hexane | ||
| 100c | 2.0 | 4 h, sedentary subjects | Brown et al. 1987; Dick et al. 1988, 1992 | |
| 200 | 3.5, 3.7 | 4 h, sedentary subjects | ||
| Ratd | ||||
| 25 | 1.0 | 6 h | Liira et al. 1990a; | |
| 100 | 4.8 | Liira et al. 1991 | ||
| 300 | 25 | |||
| 600 | 75 | |||
aBlood samples were venous blood except for the study of Shibata et al. (2002) in which case the samples were arterial capillary blood.
bBlood samples were collected following exposure, which may be responsible for the low values compared with blood collected during the exposures in the other studies.
cCoexposure to 125 ppm acetone.
dRat whole blood (Liira et al. 1991) was also collected following exposures.
Imbriani et al. (1989) exposed 15 male subjects, ages 25 to 44 years, to various concentrations ranging from 4 to 212 ppm. Individual subjects were exposed to a constant concentration in the following manner: 4, 59, 103, 123, or 212 ppm for 2 h at rest; 15, 36, 54, 91, or 120 ppm for 4 h at rest; or 10, 43, 61, 118, or 168 ppm for 2 h with light physical exercise, 50 watts for 20 min, three times during the 2 h. Uptake averaged 54% regardless of workload. Although findings are based on single subjects, the venous blood concentrations at the end of exposure at approximately 120 ppm increased from 374 µg/L at 2 h to 657 µg at 4 h. Exercise during the 2-h exposure doubled and tripled the values (1,392 µg/L) of the 4- and 2-h exposures at rest.
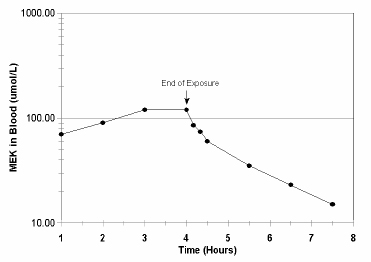
FIGURE 3-1 Methyl ethyl ketone (MEK) concentration (µmol/L) in venous blood during and after a 4-h inhalation exposure of eight subjects at 200 ppm. Source: Adapted from Liira et al. 1988b.
In another kinetics study, a group of 26 healthy males and females (ages 18-32) was exposed to MEK at 200 ppm for 4 h while venous blood and breath concentrations were monitored (Brown et al. 1987; Dick et al. 1988). Blood concentrations reached 3.1 µg/mL at 2 h and 3.5 µg/mL at 4 h. Although the concentration was higher in males (approximately 3.9 µg/mL at 4 h) than females (approximately 3.2 µg/mL at 4 h), the difference did not attain statistical significance. The mean blood concentration was 1.0 µg/mL at 1.5 h postexposure. Exhaled breath concentrations reached equilibrium at 2 h into the exposure, at which time they averaged 11.4 ppm. The exhaled concentration was 0.7 ppm at 1.5 h postexposure and not detectable at 20 h postexposure. MEK was not detected in any pre-exposure blood or breath sample. Results were similar when this study was repeated in 13 males and 11 females (Dick et al. 1992). At 4 h into the exposure, the mean venous blood concentration of MEK was 3.7 ± 1.1 µg/mL (males, 4.0 µg/mL; females, 3.3 µg/mL). At 1.5 h postexposure, the venous blood concentration averaged 1.0 µg/mL. The estimated half-life in this study was 49 min (Brown et al. 1987).
MEK concentrations in arterial capillary blood of four human subjects inhaling 100 or 200 ppm for 2 h, both in combination with n-hexane at 50 ppm, were 60 and 170 µmol/L, respectively, at the end of exposure (Shibata et al. 2002). The subjects performed a workload of 50 watts during the exposures. Neither of the MEK exposures influenced the concentration of n-hexane in the blood compared with the concentration during exposure to n-hexane alone at 50 ppm. However, combined exposure to MEK and n-hexane depressed the metabolism of n-hexane. The effect of n-hexane exposure on MEK metabolism was not reported.
Tada et al. (1972) exposed four subjects to MEK at 300 ppm for 2 h/day for several successive days. Some exposures were for 2 h in the morning and 2 h in the afternoon. Expired air contained MEK at 23 ppm immediately after a 2-h exposure at 300 ppm.
Brugnone et al. (1980; 1983) and Perbellini et al. (1984) studied the uptake and kinetics of MEK during occupational exposures at several factories in Italy. They compared the alveolar concentrations and urinary excretion of MEK to concentrations found in the workplace atmosphere; alveolar concentrations were also compared with blood concentrations. Alveolar and atmospheric concentrations were highly correlated (r = 0.7793). At worker exposures of ≤ 300 µg/L (≤ 100 ppm), the alveolar concentration was 30% of the air concentration (≤ 90 µg/L), indicating an uptake of 70%. The venous blood concentration (842 to 9,573 µg/L; mean, 2,630 µg/L) was 104-116 times the alveolar concentration and 31-35 times the atmospheric concentration. The ratio between blood and alveolar MEK concentration was 104. The correlation between urine 3-hydroxy-2-butanone and atmospheric MEK was good (r = 0.8179). Perbellini et al. (1984) calculated the uptake of MEK in workers exposed at 100 μg/L (33 ppm). They used the following formula: uptake = the environmental concentration (100 µg/L) × alveolar ventilation (15 L/min) × alveolar retention (70%). Using these parameters, lung uptake would be 1.05 mg/min. Brugnone (1985) calculated tissue:blood distribution coefficients in vessel-rich tissue, muscle, and fat of 1.0, 1.2, and 0.88, respectively. Biologic half-lives in vessel-rich tissue, muscle, and fat were 0.8, 21.8, and 23.3 min, respectively. Distribution volumes in the three tissue groups were 6.0, 39.6, and 12.8 L, respectively.
Another monitoring study addressed the relationship between occupational exposure and concentrations in the blood and urine of workers. Yoshikawa et al. (1995) studied a group of 72 workers in a printing factory in Japan. Exposures were to a mixture of solvents including toluene, xylene, isopropyl alcohol, and ethyl acetate. Workers wore personal samplers, and urine and blood samples were taken at the end of the work shift. At atmospheric TWA concentrations of 1.3 to 223.7 ppm (mean, 47.6 ppm), urinary MEK ranged from 0.20 to 8.08 mg/L (mean, 1.19 mg/L), and blood concentrations ranged from 0.01 to 6.68 mg/L (mean, 0.71 mg/L). Correlation coefficients between air and blood, air and urine, and blood and urine were all > 0.8. The correlation coefficient for air and urine concentrations did not improve with correction of urinary values for creatinine. Using the regression equation that described the relationship between air and urinary concentrations, Yoshikawa et al. (1995) calculated the urinary value corresponding to the ACGIH occupational exposure TWA of 200 ppm. This value, referred to as the Biological Exposure Index (BEI), was 5.1 mg/L. The authors then calculated the BEI from other occupational monitoring studies. At mean exposure concentrations of 22.8 ppm (Miyasaka et al. 1982), 34.2 ppm (Perbellini et al. 1984), 200 ppm (Ong et al. 1991), and 137.2 ppm (Jang et al. 1993), the BEIs were 5.3, 2.1, 3.6, and 1.4 mg/L, respectively. Possible reasons for the differing values among the studies and for the deviation from the ACGIH’s recommendation of 2 mg/L (ACGIH 2006) were discussed.
Following accidental ingestion of an unknown amount of MEK, a woman was brought to the hospital with metabolic acidosis (Kopelman and Kalfayan 1983). She became unconscious and was hyperventilating. The blood (plasma) concentration of MEK was 13.2 mmol/L (950 µg/mL). Following treatment of the acidosis, she made a complete recovery.
Following 6 h of exposure to MEK at 25, 100, or 300 ppm, concentrations in the blood of rats were 14, 66, and 348 µM, respectively (Liira et al. 1990a). Rats exposed at 600 ppm for 6 h for 1 day or for 6-10 h/day for 8 days had similar blood concentrations of MEK: 1,041 µmol/L after a single exposure and 1,138 µmol/L after repeated exposure (Liira et al. 1991). MEK caused only marginal effects on microsomal cytochrome P-450 activities of the liver. A comparison of uptake by rats with that by humans indicates that, at similar concentrations, uptake is greater in rats than in humans (Liira et al. 1990a).
Walter et al. (1986) and Kessler et al. (1989) investigated the toxicokinetics of MEK in male Wistar rats. In the first study, saturation kinetics were displayed above 150 ppm, with the maximum rate of metabolism (Vmax) being 600 µmol/h/kg. Below 150 ppm, kinetics were linear. In the second study, metabolism below 180 ppm was not limited by metabolic capacity but by transport to the enzymes. Pulmonary uptake was 40%, and clearance was 53 mL/min.
4.2. Mechanism of Toxicity
MEK is a hydrophilic solvent with actions of CNS depression and irritancy of the nose and eyes, both at relatively high concentrations. Because of its high tissue solubility, low concentrations may be effectively scrubbed by the nasal passages. The mechanism of action of its CNS and anesthetic action is not well understood, although it may involve interaction with cell membranes or changes in membrane-bound receptors (Arlien-Soberg 1991). It is generally accepted that volatile organic chemicals partition into the lipids of myelin sheaths and neuronal membranes and inhibit propagation of action potentials because of their physical presence.
4.3. Structure Activity Relationships
Ketones, such as acetone, MEK, methyl isobutyl ketone, and cyclohexanone, are generally of low acute oral and inhalation toxicity (Morgott et al. 2001). As discussed in Section 4.4.4, ketones metabolized to 2,5-hexanedione cause peripheral neuropathies, generally following repeated exposures. MEK is not metabolized to 2,5-hexanedione by mammalian cells.
4.4. Other Relevant Information
4.4.1. Species Variability
Data on both lethal concentrations and concentrations involving irritancy and signs of toxicity and neurotoxicity for the rat, mouse, and guinea pig were occasionally variable but generally did not indicate great species differences.
The two primary determinants of systemic uptake of volatile chemicals are respiratory rate and cardiac output. Relative to body weight, rodents have a much higher respiratory rate and cardiac output than humans. (The respiratory rate of the mouse may be up to 10 times that of the human [Witschi and Last 2001; Kale et al. 2002].) As a result of the greater respiratory rate and cardiac output, rodents generally receive a greater overall dose than humans. Although exposure durations varied, the concentrations of MEK in blood were higher in rats than in humans when inhaling similar concentrations (Table 3-6). Humans inhaling 25, 100, or 400 ppm for 4 h had venous blood concentrations of 5, 93-100, and 229-309 µM; whereas concentrations in the blood of rats inhaling 25, 100, or 300 ppm were 14, 66, and 348 µM, respectively (Liira et al. 1990a). The human blood samples were taken during exposures, whereas the rat blood samples were taken following exposures, a procedure that may have allowed for some clearance by the rat.
Pharmacodynamic differences between rodents and humans are unknown but presumably would not differ by more than 3-fold. As mentioned, rodents receive greater systemic doses of volatile organic chemicals than humans upon equivalent exposure, and mice are expected to receive the greatest systemic dose. In absorbed dose, rats are expected to be between mice and humans exposed at the same administered air concentration. As a result, the pharmacokinetic component of the interspecies uncertainty factor would actually range from 0.3- to 0.7-fold. In practice, this would offset a potential 3-fold pharmacodynamic interspecies difference.
4.4.2. Susceptible Populations
No studies were located that identified populations that are unusually susceptible to adverse health effects from exposure to MEK. Although individuals may develop a hypersensitivity to particular solvents, results of monitoring studies did not identify a susceptible population. Individuals with sMCS did not appear more sensitive to the odor or potential irritancy of MEK during exposures up to 380 ppm than did individuals who did not report chemical sensitivity (Seeber et al. 2002). These individuals would not necessarily be more susceptible to CNS effects. The very young, the very old, and individuals with existing liver or other diseases may be more susceptible to chemical toxicity (ATSDR 1992).
There is concern that exposure to organic solvents may increase the incidence of children born with CNS defects. According to EPA (2003), there is no evidence from animal studies that MEK induces CNS defects. Developmental studies with rodents identified reduced fetal body weight and delayed ossification as developmental effects.
The primary mechanism of action for “high” concentrations of solvents is CNS depression. Although humans differ in the rate at which they metabolize chemicals, the susceptibility of the general population to CNS anesthetics varies by no more than 2- to 3-fold, as indicated by the minimum alveolar concentration (MAC) (the concentration of an anesthetic that produces immobility in 50% of patients) (Kennedy and Longnecker 1996, p.302; Marshall and Longnecker 1996, p. 307). MEK has anesthetic properties. Studies indicate that children, and particularly infants, are more resistant than adults to the effects of various volatile anesthetics (Gregory et al. 1969; Stevens et al. 1975; Lerman et al. 1983; LeDez and Lerman 1987; Chan et al. 1996; Katoh and Ikeda 1992). The susceptibility of individuals of different ages has been extensively studied in the anesthesia literature, where the concentrations of various anesthetic gases in the lung that produce “anesthesia” (that is, lack of movement) have been measured. MACs for several anesthetic gases have been measured as a function of age. The results consistently show a pattern, there being maximal sensitivity (lowest MAC) in newborns, particularly premature newborns, pregnant women, and the elderly. The least sensitive (highest MAC values) occur in older infants, toddlers, and children compared with normal adults. The total range of sensitivity is 2-3 fold. On the basis of this knowledge, it is not unreasonable to assume that the same 2-3 fold difference in sensitivity among individuals would apply for MEK.
4.4.3. Concentration-Exposure-Duration Relationship
No data were located that provided information on the concentration-exposure-duration relationship for the effect of MEK on the CNS. For the end points of both sensory irritation and depression of the CNS by solvents, there is generally a concentration threshold. During clinical studies involving 200 ppm, uptake was rapid during the first hour, and steady state in the blood was approached by 4 h.
For the end point of death, both concentration and exposure duration may be applicable. However, there are no data relevant to the determination of that relationship. For the end point of death, the concentration-exposure-duration relationship for many irritants and systemically acting vapors and gases may be described by Cn × t = k, where the exponent, n, ranges from 0.8 to 3.5 (ten Berge et al. 1986). Where chemical-specific empirical data are lacking, a conservative approach is to use n = 3 when extrapolating from longer to shorter time points and to use n = 1 when extrapolating from shorter to longer time periods (NRC 2001).
4.4.4. Concurrent Exposure Issues
Solvent exposure in the workplace generally involves mixtures of chemicals. The combined effect of chemicals may be additive, antagonistic, or potentiating. Non-neurotoxic ketones (those that do not induce neuropathies), such as MEK, may potentiate the neurotoxicity and hepatotoxicity of other solvents, including other ketones that are metabolized by the P-450 metabolizing system of the liver (Morgott et al. 2001). Several ketones and related chemicals have been shown to produce a toxic polyneuropathy characterized by multifocal axonal swelling and myelin damage. In humans, symptoms include bilateral symmetrical paresthesia (“pins and needles” feeling) and muscle weakness, primarily in the legs. The neurotoxic agent is thought to be 2,5-hexanedione, a metabolite of several ketones. MEK is not metabolized to 2,5-hexanedione (DiVincenzo et al. 1977).
Data from experimental studies with animals and from clinical data on humans indicate that MEK does not cause neuropathies but potentiates the neurotoxic action of structurally related solvents, such as n-hexane and methyl-nbutyl ketone. Altenkirch et al. (1982a) reported on neuropathies in solvent-sniffing juveniles. Juveniles who chronically inhaled hexane-containing solvents as narcotics suffered a variety of polyneuropathy syndromes and neuromyelopathies. Addition of MEK to the solvents as a denaturant for a period of time resulted in an outbreak of more severe neuropathies. In clinical studies, combined exposure to n-hexane and MEK (200 ppm) did not influence the concentration of n-hexane in the blood (Shibata et al. 2002) but decreased the rate of metabolism of n-hexane to 2,5-hexanedione. Similar results were reported when co-exposures were to n-hexane at 60 ppm and MEK at 200 or 300 ppm (van Engelen et al. 1997).
The potentiation of the neurotoxicity of n-hexane, methyl-n-butyl ketone, ethyl n-butyl ketone, and 2,5-hexanedione by MEK was documented in animal studies (Saida et al. 1976; Altenkirch et al. 1978, 1982a,b; Takeuchi et al. 1983; O’Donoghue et al. 1984; Ralston et al. 1985; Ichihara et al. 1998). In one such study (Altenkirch et al. 1978), male Wistar rats were exposed to MEK at 6,000-10,000 ppm for 8 h/day, 7 days/week, for 15 weeks. These rats did not have neurologic lesions, but rats exposed to n-hexane at 10,000 ppm or MEK plus nhexane at 10,000 ppm (MEK at 1,100 ppm and n-hexane at 8,900 ppm) developed motor neuropathy with swelling of axons in the peripheral and CNS. MEK in the combined exposure shortened the onset and increased the severity of paresis compared with exposure to n-hexane alone.
Exposure to other chemicals may interfere with the metabolism of MEK. Co-exposure to xylene slowed the metabolism of xylene but did not interfere with the metabolism of MEK (Liira et al. 1988b). Blood concentrations of MEK were higher following ethanol ingestion, indicating reduced metabolism, than those following MEK exposure alone (Liira et al. 1990b). In an oral study with rats, exposure to MEK potentiated carbon tetrachloride hepatotoxicity (Traiger and Bruckner 1976).
Exposure of pregnant Wistar rats for 23 h/day, 7 days/week, to a combination of n-hexane at 1,200 ppm and MEK at 300 ppm throughout gestation or during the postnatal period increased the effect of n-hexane alone on birth weight and postnatal weight gain, both of which were reduced (Stoltenburg-Didinger et al. 1990). This combination was neurotoxic to the dams but had no effect on the brain development of the pups.
Liquid MEK applied to the skin of the forearms of human volunteers is rapidly absorbed (Munies and Wurster 1965; Wurster and Munies 1965). Uptake by dermal absorption was evidenced by MEK in expired air. Therefore, unprotected dermal contact may represent a significant exposure route.
5. DATA ANALYSIS FOR AEGL-1
The AEGL-1 concentration may cause notable discomfort or irritation in the general population as well as in susceptible individuals.
5.1. Summary of Human Data Relevant to AEGL-1
MEK is not a respiratory irritant at concentrations less than several thousand parts per million. The clinical studies of Dick et al. (1984; 1988; 1992), Muttray et al. (2002), Seeber et al. (2002), and Shibata et al. (2002) did not report sensory irritation or neurobehavioral deficits at a constant concentration of 200 ppm for 2 or 4 h or at concentrations that ranged between 10 and 380 ppm (average 188 ppm) over 4 h. Twenty-four subjects exposed at 200 ppm for 4 h found the concentration unobjectionable (Dick et al. 1992). In a series of neurobehavioral studies, a 4-h exposure of human subjects at 200 ppm had no significant effect on a variety of behavioral tests (Dick et al. 1984, 1988, 1989). No irritation or subjective symptoms of sensory irritation were reported in four male subjects inhaling MEK at 200 ppm for 2 h (Shibata et al. 2002). The same absence of sensory irritation and neurobehavioral deficits was reported by 19 male subjects inhaling MEK at 00 ppm for 4 h (Muttray et al. 2002). During variable concentrations ranging from 10 ppm to 8-min peaks at 380 ppm, five times over 4 h, subjects rated annoyance and irritation either “hardly at all” or “not at all” (Seeber et al. 2002). Both healthy subjects and subjects with sMCS were tested by Seeber et al. (2002). The primary subjective comment in these studies was a noticeable odor. In the study of Nelson et al. (1943), 10 male and female volunteers exposed to MEK for 3-5 min judged 200 ppm as acceptable for an 8-h exposure and 350 ppm as objectionable for an 8-h exposure. There were no analytic measurements in this early study. Sensory irritation was reported in the Nakaaki (1974) study, but this study used variable concentrations, and neurobehavioral results were difficult to interpret. Additional metabolism studies were conducted at concentrations of 25 to 400 ppm for 4 h, but these studies did not address sensory irritation or neurotoxic effects. Although sensory irritation was not specifically addressed in the metabolism studies of Liira et al (1988a,b,
1990a,b) and Tada et al. (1972), volunteers were routinely exposed to concentrations of 200-400 ppm for 2-4 h without apparent adverse effects.
5.2. Summary of Animal Data Relevant to AEGL-1
No signs of toxicity were observed in rats exposed to MEK at 2,000 ppm for 2 h (Pozzani et al. 1959; Smyth et al. 1962) and guinea pigs exposed at 3,300 for 13.5 h (Patty et al. 1935), although approximately 2,900 ppm was the EC50 for a deficit in schedule-controlled behavior in the mouse (Glowa and Dews 1987).
5.3. Derivation of AEGL-1
Four well-conducted clinical studies indicate that MEK is neither a sensory irritant nor does it induce neurobehavioral changes at a concentration of 200 ppm for 4 h or at concentrations ranging between 10 and 380 ppm (average 188 ppm) over 4 h (Dick et al 1992; Muttray et al 2002; Seeber et al. 2002; Shibata et al. 2002; van Thriel et al. 2003a). Additional metabolism studies were conducted at concentrations of 25 to 400 ppm for 4 h, but these studies did not address sensory irritation or neurotoxic effects. A concentration of 200 ppm was judged unobjectionable for an 8-h exposure (Dick et al. 1992). Subjects with sMCS found concentrations ranging between 10 and 380 ppm over 4 h unobjectionable. Therefore, 200 ppm was selected as a NOAEL for sensory irritation and neurobehavioral deficits. The selection of this value is supported by numerous clinical studies in which volunteers were routinely exposed at 200-400 ppm for up to 4 h (Tada et al. 1972; Dick et al. 1984, 1988, 1989, 1992; Liira et al. 1988a,b, 1990a,b). Because the Dick et al. studies reported no sensory irritation or neurotoxicity at 200 ppm and because metabolism was also addressed by the authors at this concentration, it is unlikely that sensory irritation was experienced in other metabolism studies at similar concentrations. Because effects were not greater at the higher concentration of 380 ppm and because subjects with sMCS, a hypersensitive population, did not report enhanced sensory effects compared with control subjects (Seeber et al. 2002), an intraspecies uncertainty factor of 1 was applied. Because steady-state would be approached within 4 h at this low concentration (Liira et al. 1988a,b; 1990a) and because MEK is rapidly metabolized, the 200 ppm concentration was used across all AEGL-1 exposure durations (Table 3-7). Calculations are in Appendix A. Appendix B contains a category graph of the toxicity data in relation to AEGL values.
TABLE 3-7 AEGL-1 Values for Methyl Ethyl Ketone
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 200 ppm (586 mg/m3) | 200 ppm (586 mg/m3) | 200 ppm (586 mg/m3) | 200 ppm (586 mg/m3) | 200 ppm (586 mg/m3) |
6. DATA ANALYSIS FOR AEGL-2
Concentrations above the AEGL-2 may cause irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
6.1. Summary of Human Data Relevant to AEGL-2
Data on human exposures to concentrations that may cause adverse health effects or an impaired ability to escape are sparse. A concentration of 350 ppm was judged objectionable because it was unpleasant (Nelson et al. 1943), but this concentration would not impair the ability to escape. The lowest concentration reported in the study by Patty et al. (1935), 3,300 ppm, had a moderate-to-strong odor and was moderately irritating to the eyes and nose, but no exposure duration was provided.
6.2. Summary of Animal Data Relevant to AEGL-2
The mean RD50, a concentration that is considered intolerable to humans, was approximately 10,000 ppm in two of three studies with the mouse (De Ceaurriz et al. 1981; Stone et al. 1981). According to Alarie et al (1981), humans would experience “some” sensory irritation during several hours of exposure to MEK at 0.1 × the RD50. This value would be 1,000 ppm. The RD50 could not be attained in a study by Hansen et al. (1992), even when exposures were > 20,000 ppm.
Although MEK is of low inhalation toxicity in animal studies, it has been shown to be slightly fetotoxic in laboratory animals when dams are exposed for half of their gestational period (days 6-15). Results of a series of developmental studies with the rat (Deacon et al. 1981) and the mouse (Mast et al. 1989; Schwetz et al. 1991) determined that 3,000 ppm was toxic to the fetus, as indicated by delayed ossification in rats and reduced fetal body weight in mice. A concentration of 1,000 ppm was a NOAEL. Exposures were for 7 h/day. Maternal toxicity, however, indicated that the fetus is not more sensitive than the dam. The relevance of exposure duration of one-half of the rodent gestational period to a short-term exposure for humans is unknown.
In a repeat-exposure inhalation study (Altenkirch et al. 1978) and a subchronic exposure study (Cavender et al. 1983) of MEK, it was shown that 6,000 and 5,000 ppm, respectively, had little effect on rodents. Only five rats were used in the Altenkirch et al. (1978) study, and the use of controls was not mentioned. The Cavender et al. (1983) study used 15 rats of each sex/group and several exposure concentrations, including a control exposure. In this study, 5000 ppm for 6 h/day for 90 days was a no-effect concentration for neurobehavioral deficits and neuropathy. The absence of CNS effects was reported on the first
day of the study. At 6,000 ppm, mild somnolence was reported in rats, but it was not clear that this effect occurred on the first day of exposure (Altenkirch et al. 1978).
6.3. Derivation of AEGL-2
The sensitive end point of fetotoxicity was not used to derive AEGL-2 values for MEK because the relevance of an exposure of half of the gestational period of a rodent to an acute exposure as short as 10 min for a human is difficult to assess. The end points in the developmental studies of Deacon et al. (1981), Mast et al. (1989), and Schwetz et al. (1991) appear to be related to maternal stress rather than to a direct toxic effect of the chemical.
The AEGL-2 was based on an exposure concentration of MEK that did not result in neurobehavioral effects on the first day of the subchronic exposure study of Cavender et al. (1983). In this study, rats were exposed at 5,000 ppm for 5 days/week for 90 days. No exposure-related lesions were observed. The 5,000-ppm concentration is close to the threshold for neurotoxicity, as evidenced by mild somnolence in another repeat-exposure study in which rats were exposed at 6,000 ppm, 8 h/day, for several weeks (Altenkirch et al. 1978). The Altenkirch et al. (1978) study was not used as the basis of the AEGL-2 because of the small number of animals tested and the apparent failure to include a concurrent control group. Because rodents have a higher respiratory rate and cardiac output than humans, resulting in more rapid and higher uptake of chemicals, an interspecies uncertainty factor of 1 was applied (see discussion in Section 4.4.1 and data of Liira et al. 1990a). Because the threshold for narcosis differs by no more than 2- to 3-fold among the general population (see Section 4.4.2), an intraspecies uncertainty factor of 3 was applied to protect sensitive individuals. Because the threshold for narcosis is concentration-dependent, the resulting 1,700-ppm concentration was applied to the 4- and 8-h exposure durations. The data show that higher exposures can be tolerated for shorter durations (for example, see Hansen et al. 1992). Therefore, the 10- and 30-min and the 1-h values were time-scaled from the 4-h exposure duration with the default n value of 3. AEGL-2 values are listed in Table 3-8. A summary of the calculations can be found in Appendix A. Appendix B is a category graph of toxicity data in relation to AEGL values.
TABLE 3-8 AEGL-2 Values for Methyl Ethyl Ketone
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 4,900 ppma (14,357 mg/m3) | 3,400 ppma (9,962 mg/m3) | 2,700 ppm a (7,911 mg/m3) | 1,700 ppm (4,980 mg/m3) | 1,700 ppm (4,980 mg/m3) |
aThe 10- and 30-min and the 1-h AEGL-2 values are higher than one-tenth of the lower explosive limit (LEL) of methyl ethyl ketone in air (LEL = 18,000 ppm). Therefore, safety considerations against the hazard of explosion must be taken into account.
The values and end point are supported by the study of Altenkirch et al. (1978), in which rats exposed to MEK 10,000 ppm suffered severe irritation; when the concentration was lowered to 6,000 ppm for 8 h/day, the rats were mildly somnolent but arousable.
7. DATA ANALYSIS FOR AEGL-3
At concentrations above the AEGL-3, the general population, including susceptible individuals, could experience life-threatening health effects or death.
7.1. Summary of Human Data Relevant to AEGL-3
No human data relevant to development of AEGL-3 values were located in the available literature. Patty et al. (1935) reported that concentrations >10,000 for an exposure duration of several breaths were almost intolerable to humans due to eye and nose irritation.
7.2. Summary of Animal Data Relevant to AEGL-3
Two of three early studies with rodents reported LC50 values of 11,700 and 8,000 ppm for 4 and 8 h, respectively (La Belle and Brieger 1955; Pozanni et al. 1959), whereas a third study (Patty et al. 1935) reported no deaths of guinea pigs during a 13.5-h exposure at 10,000 ppm. The highest concentration resulting in no deaths of rats during the 4-h exposure period in the La Belle and Brieger (1955) study was 7,850 ppm. No deaths of rodents occurred during short-term exposures, 30 and 45 min, at 92,239 and 50,000 ppm, respectively (Zakhari et al. 1977; Klimisch 1988). Likewise, no deaths occurred in mice exposed at 26,416 ppm for 30 min (Hansen et al. 1992). In the Hansen et al. (1992) study, the concentration that was severely irritating, that is, reduced the respiratory rate by 50%, was projected to be 31,426 ppm.
Fowles et al. (1999) applied the benchmark dose approach to the La Belle and Brieger (1955) rat lethality data to estimate the threshold for lethality. The benchmark dose approach uses several curve fitting models that are applied to data sets. In this case, the log-normal probit and quantal Weibull models were used to estimate the 95% lower confidence limits on the doses producing 1%, 5%, and 10% responses. The results of the models were similar. For the probit model, the benchmark doses corresponding to 1% extra risk (MLE01) and the 95% lower bound on those doses were 7,546 and 5,790 ppm, respectively. The respective values for the Weibull model were 6,579 and 4,193 ppm. The benchmark doses for both models are slightly below the highest exposure concentration of 7,850 ppm, which caused no deaths of rats in the La Belle and Brieger (1955) study.
7.3. Derivation of AEGL-3
The AEGL-3 values for MEK were derived using different studies. The 10- and 30-min time periods were derived using the studies by Klimisch (1988) and Zakhari et al. (1977) with support from Hansen et al. (1992). The 1-, 4-, and 8-h values were derived from the studies by Fowles (1999) using data from La Belle and Brieger (1955). No deaths occurred in rats after a 30-min exposure at 92,239 ppm (Klimisch 1988), and no deaths occurred in mice after a 45-min exposure at 50,000 ppm (Zakhari et al. 1977); a projected value of 32,145 ppm for 30 min would decrease the respiratory rate of mice by 50% (Hansen et al. 1992). The highest tested concentration in the Hansen et al. (1992) study was 26,000 ppm. On the basis of these data, nearly all individuals could be exposed at 10,000 ppm for up to 30 min without developing life-threatening effects. Application of inter- and intraspecies uncertainty factors of 1 and 3 were applied as done for the AEGL-2. Additional studies support the 10,000-ppm value as being nonlethal: 10,000 ppm for 10 or 30 min was narcotic to mice in one study (Glowa and Dews 1987) but not in another (Hansen et al. 1992); 10,000 ppm was tolerated by rats for 8 h/day for several days (Altenkirch et al. 1978), and no deaths occurred in guinea pigs at 10,000 ppm for 13.5 h (Patty et al. 1935).
The longer-term AEGL-3 values were based on the MLE01 of 7,500 ppm calculated by Fowles et al. (1999) from a 4-h study with rats exposed at several concentrations for 4 h (La Belle and Brieger 1955). In this study, the 4-h LC50 was 11,700 ppm, and the highest concentration resulting in no deaths was 7,850 ppm for 4 h. The 7,500-ppm MLE01 concentration was divided by an interspecies uncertainty factor of 1 and an intraspecies uncertainty factor of 3. The resulting value of 2,500 ppm was used for both the 4-h and 8-h AEGL-3 values. The 4-h 2,500 ppm value was time-scaled to the 1-h time, using the default n value of 3 for scaling to shorter time intervals. Calculations are in Appendix A, and values are listed in Table 3-9. Appendix B contains a category graph of toxicity data in relation to AEGL values.
8. SUMMARY OF AEGL VALUES
8.1. AEGL Values and Toxicity End Points
The AEGL values and toxicity end points are listed in Table 3-10. Appendix C contains development summaries for the AEGL values. Information was available on toxicity end points that met the definitions of the three AEGL values. The AEGL-1 was based on four studies with human subjects that were specifically designed to address objectionable odor or irritancy (Dick et al. 1992; Muttray et al. 2002; Seeber et al. 2002; Shibata et al. 2002) and supported by numerous behavioral and metabolism studies in which volunteers were routinely exposed at 200-400 ppm for various periods of time (Tada et al. 1972; Nakaaki 1974; Dick et al. 1984, 1988, 1989; Liira et al. 1988a,b, 1990a,b). Subjects with
sMCS were tested in the study of Seeber et al. (2002) (van Thriel et al. 2003a), and exposures ranged between 10 and 380 ppm (average 188 ppm) over the 4 h. Therefore, 200 ppm was considered safe for the general population, and no uncertainty factor was applied.
TABLE 3-9 AEGL-3 Values for Methyl Ethyl Ketone
| 10 min | 30 min | 1 h | 4 h | 8 h |
| a | a | 4,000 ppmb (11,720 mg/m3) | 2,500 ppmb (7,325 mg/m3) | 2,500 ppmb (7,325 mg/m3) |
aThe 10- and 30-min AEGL-3 value of 10,000 ppm (29,300 mg/m3) is higher than 50% of the LEL of methyl ethyl ketone in air (LEL = 18,000 ppm). Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
bThe 1-, 4-, and 8-h AEGL-3 values are higher than 1/10 of the lower explosive limit (LEL) of methyl ethyl ketone in air (LEL = 18,000 ppm). Therefore, safety considerations against the hazard of explosion must be taken into account.
TABLE 3-10 Summary of AEGL Values for Methyl Ethyl Ketone
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| AEGL-1 (Nondisabling) | 200 ppm (586 mg/m3) | 200 ppm (586 mg/m3) | 200 ppm (586 mg/m3) | 200 ppm (586 mg/m3) | 200 ppm (586 mg/m3) | NOAEL for subjective symptoms in humans (Dick et al. 1992; Muttray et al. 2002; Seeber et al. 2002; Shibata et al. 2002) |
| AEGL-2 (Disabling) | 4,900 ppma (14,357 mg/m3) | 3,400 ppma (9,962 mg/m3) | 2,700 ppma (7,911 mg/m3) | 1,700 ppm (4,980 mg/m3) | 1,700 ppm (4,980 mg/m3) | Threshold for narcosis in rats (Cavender et al. 1983) |
| AEGL-3 (Lethal) | b | b | 4,000 ppma (11,720 mg/m3) | 2,500 ppma (7,325 mg/m3) | 2,500 ppma (7,325 mg/m3) | Threshold for lethality, mouse, rat (La Belle and Brieger 1955; Zakhari et al. 1977; Klimisch 1988; Hansen et al. 1992) |
aThe 10- and 30-min and the 1-h AEGL-2 values and the 1-, 4-, and 8-h AEGL-3 values are higher than one-tenth of the lower explosive limit (LEL) of methyl ethyl ketone in air (LEL = 18,000 ppm). Therefore, safety considerations against the hazard of explosion must be taken into account.
bThe 10- and 30-min AEGL-3 value of 10,000 ppm (29,300 mg/m3) is higher than 50% of the LEL of methyl ethyl ketone in air (LEL = 18,000 ppm). Therefore, extreme safety considerations against the hazard of explosion must be taken into account. Abbreviation: NOAEL, no-observed-adverse-effect level.
The AEGL-2 was based on an exposure concentration that did not result in neurobehavioral deficits on the first day of a subchronic exposure study with rats (Cavender et al. 1983). This concentration of 5,000 ppm is supported by a repeated exposure inhalation study (Altenkirch et al. 1978). A 6,000 ppm concentration was the threshold for narcosis in the rat (Altenkirch et al. 1978). The clinical sign of mild somnolence in this study is consistent with the known effect of solvents on the CNS. The 5,000-ppm NOAEL for CNS depression of the Cavender et al. (1983) study was divided by interspecies and intraspecies uncertainty factors of 1 and 3, respectively. Although CNS depression is concentration-related rather than time-dependent, higher exposures can be tolerated for short durations. Therefore, the 1,700-ppm value was applied to the 4- and 8-h durations and the shorter time values were time-scaled from the 4-h duration.
The AEGL-3 was based on several animal studies. The 10- and 30-min AEGL-3 values were based on several nonlethal rodent values: 92,239 ppm for 30 min (Klimisch 1988), 60,000 ppm for 45 min (Zakhari et al. 1977), and 26,000 ppm for 30 min (Hansen et al. 1992). On the basis of these data, nearly all individuals could be exposed at 10,000 ppm for up to 30 min without developing life-threatening effects. The 10,000 ppm value was applied to both the 10 and 30-min AEGL-3 exposure durations. Values for the longer-term exposure durations were based on the MLE01 of 7,500 ppm calculated from the 4-h rat study of La Belle and Brieger (1955) by Fowles et al. (1999). The resulting 2,500 ppm value was used for both the 4- and 8-h exposure durations, as MEK may approach steady state in the blood during a 4-h exposure. In the absence of empirical data on the relationship between the concentration causing effects and exposure duration, temporal scaling to the 1-h time period was performed using the conservative value of n = 3.
8.2. Comparison with Other Standards and Guidelines
Primarily workplace guidelines have been derived for MEK (Table 3-11). Workplace standards are protective of any adverse health effect during chronic exposures and are most comparable to the AEGL-1. The AEGL-1 is the same as the American Conference of Governmental Industrial Hygienists (ACGIH), Occupational Safety and Health Administration (OSHA), and National Institute for Occupational Safety and Health (NIOSH) recommended workplace exposure limits, as well as the workplace exposure limits derived in Germany and The Netherlands. It is below the NIOSH short-term exposure limit of 300 ppm. The ACGIH based their value on a review of the literature. The NIOSH IDLH is comparable and similar to the 30-min AEGL-2. The IDLH is below the 30-min AEGL-3 of 10,000 ppm. The IDLH of 3,000 ppm is based on early data, which stated that a 2-h exposure of rats to 2,000 ppm caused no deaths, but four of six rats exposed at 4,000 ppm for a 2-h period died (Smyth 1956). NIOSH adjusted the values for a 30-min exposure.
TABLE 3-11 Extant Standards and Guidelines for Methyl Ethyl Ketone
| Guideline | Exposure Duration | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h | |
| AEGL-1 | 200 ppm | 200 ppm | 200 ppm | 200 ppm | 200 ppm |
| AEGL-2 | 4,900 ppma | 3,400 ppma | 2,700 ppma | 1,700 ppm | 1,700 ppm |
| AEGL-3 | b | b | 4,000 ppma | 2,500 ppma | 2,500 ppma |
| IDLH (NIOSH)c | 3,000 ppm | ||||
| TLV-TWA (ACGIH)d | 200 ppm | ||||
| PEL-TWA (OSHA)e | 200 ppm | ||||
| REL-TWA (NIOSH)f | 200 ppm | ||||
| TLV-STEL (ACGIH)g | 300 ppm | ||||
| REL-STEL (NIOSH)h | 300 ppm | ||||
| MAK (Germany)i,j | 200 ppm | ||||
| MAC (The Netherlands)k | 200 ppm, skinl | ||||
aThe 10- and 30-min and 1-h AEGL-2 values and the 1-, 4-, and 8-h AEGL-3 values are higher than 1/10 of the lower explosive limit (LEL) of methyl ethyl ketone in air (LEL = 18,000 ppm). Therefore, safety considerations against the hazard of explosion must be taken into account.
bThe 10- and 30-min AEGL-3 value of 10,000 ppm (29,300 mg/m3) is higher than 50% of the LEL of methyl ethyl ketone in air (LEL = 18,000 ppm). Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
cIDLH (immediately dangerous to life or health, National Institute for Occupational Safety and Health) (NIOSH 1996) represents the maximum concentration from which one could escape within 30 min without any escape-impairing symptoms, or any irreversible health effects.
dTLV-TWA (Threshold Limit Value–time-weighted average, American Conference of Governmental Industrial Hygienists) (ACGIH 2006) is the time-weighted average concentration for a normal 8-h workday and a 40-h workweek to which nearly all workers may be repeatedly exposed, day after day, without adverse effect.
ePEL-TWA (permissible exposure limits–time-weighted average, Occupational Health and Safety Administration) (OSHA) (29 CFR 1910.1000 [1996]) is analogous to the ACGIH TLV-TWA but is for exposures of no more than 10 h/day, 40 h/week.
fREL-TWA (recommended exposure limits–time-weighted average, National Institute for Occupational Safety and Health) (NIOSH 2005) is analogous to the ACGIH TLV-TWA.
gTLV-STEL (Threshold Limit Value–short-term exposure limit, American Conference of Governmental Industrial Hygienists ) (ACGIH 2006) is defined as a 15-min TWA exposure that should not be exceeded at any time during the workday even if the 8-h TWA is within the TLV-TWA. Exposures above the TLV-TWA up to the STEL should not be
longer than 15 min and should not occur more than 4 times per day. There should be at least 60 min between successive exposures in this range.
hREL-STEL (recommended exposure limits–short-term exposure limit, National Institute for Occupational Safety and Health) (NIOSH 2005) is analogous to the ACGIH TLV-TWA.
iMAK (maximale Arbeitsplatzkonzentration [maximum workplace concentration]) (Deutsche Forschungsgemeinschaft [German Research Association] (DFG 2003) is analogous to the ACGIH-TLV-TWA.
jMAK Spitzenbegrenzung (Peak Limit I) (Deutsche Forschungsgemeinschaft [German Research Association] (DFG 2003) is the same as the 8-h MAK.
kMAC (maximaal aanvaarde concentratie [maximum accepted concentration], Dutch Expert Committee for Occupational Standards, The Netherlands) (MSZW 2004) is analogous to the ACGIH-TLV-TWA.
lSkin notation: MEK may be absorbed through the skin.
8.3. Data Adequacy and Research Needs
The database on human studies is extensive and includes controlled clinical and workplace monitoring studies. Human exposures were generally to relatively low concentrations and showed that MEK is not irritating to the eyes and mucous membranes of the upper respiratory tract. Only with unmeasured, presumably higher exposures in association with dermal contact was the CNS involved. Several human studies addressed neurobehavioral effects and metabolism. Animal studies with a variety of species (baboon, rat, mouse, and guinea pig) addressed irritation, neurotoxicity, developmental toxicity, subchronic toxicity, and lethality. Exposure durations ranged from acute to subchronic. Genotoxicity was also addressed. The most notable data deficiency is the lack of a well-defined exposure-response relationship for the AEGL-2 and AEGL-3 end points. However, for many solvents that act as anesthetics, a threshold concentration rather than duration of exposure defines whether an effect will occur.
9. REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 2006. TLVs and BEIs: Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
Alarie, Y., L. Kane, and C. Barrow. 1981. Sensory irritation: The use of an animal model to establish acceptable exposure to airborne chemical irritants. Pp. 48-92 in Toxicology: Principles and Practices, Vol. 1, A.L. Reeves, ed. New York: John Wiley and Sons.
Alderson, M.R., and N.S. Rattan. 1980. Mortality of workers on an isopropyl alcohol plant and two MEK dewaxing plants. Br. J. Ind. Med. 37(1):85-89.
Altenkirch, H., G. Stoltenburg, and H.M. Wagner. 1978. Experimental studies on hydrocarbon neuropathies induced by methyl-ethyl-ketone (MEK). J. Neurol. 219(3): 159-170.
Altenkirch, H., H.M. Wagner, G. Stoltenburg-Didinger, and R. Steppat. 1982a. Potentiation of hexacarbon-neurotoxicity by methyl-ethyl-ketone (MEK) and other substances: Clinical and experimental aspects. Neurobehav. Toxicol. Teratol. 4(6): 623-627.
Altenkirch, H., H.M. Wagner, G. Stoltenburg, and P.S. Spencer. 1982b. Nervous system responses of rats to subchronic inhalation of n-hexane and n-hexane plus methylethyl-ketone mixtures. J. Neurol. Sci. 57(2-3):209-219.
Amoore, J.E., and E. Hautala. 1983. Odor as an aid to chemical safety: Odor threshold compared with the threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J. Appl. Toxicol. 3(6):272-290.
Arlien-Soberg, P. 1991. Solvent Neurotoxicity. Boca Raton: CRC Press.
Ashley, D.L., M.A. Bonin, F.L. Cardinali, J.M. McCraw, and J.V. Wooten. 1994. Blood concentrations of volatile organic compounds in a nonoccupationally exposed U.S. population and in groups with suspected exposure. Clin. Chem. 40(7 Pt 2):1401-1404.
ATSDR (Agency for Toxic Substances and Disease Registry). 1992. Toxicological Profile for 2-Butanone. U.S. Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA. July 1992 [online]. Available: http://www.atsdr.cdc.gov/ToxProfiles/tp29.pdf [accessed Nov. 2, 2010].
Beliveau, M., and K. Krishnan. 2000. Estimation of rat blood:air partition coefficients of volatile organic chemicals using reconstituted mixtures of blood components. Toxicol. Lett. 116(3):183-188.
Billings, C.E., and L.C. Jonas. 1981. Odor thresholds in air as compared to threshold limit values. Am. Ind. Hyg. Assoc. J. 42(6):479-480.
Brown, W.D., J.V. Setzer, R.B. Dick, F.C. Phipps, and L.K. Lowry. 1987. Body burden profiles of single and mixed solvent exposures. J. Occup. Med. 29(11):877-883.
Brugnone, F. 1985. Uptake of solvents from lungs. Br. J. Ind. Med. 42(8):569.
Brugnone, F., L. Perbellini, E. Gaffuri, and P. Apostoli. 1980. Biomonitoring of industrial solvent exposures in workers’ alveolar air. Int. Arch. Occup. Environ. Health 47(3):245-261.
Brugnone, F., L. Perbellini, P. Apostoli, D. Caretta, and V. Cocheo. 1983. Environmental and biological monitoring of occupational methylethyl ketone exposure. Pp. 571-574 in Developments in the Science and Practice of Toxicology, A.W. Hayes, R.C. Schnell, and T.S. Miya, eds. Amsterdam: Elsevier.
Carpenter, C.P., H.F. Smyth, and U.C. Pozanni. 1949. The assay of acute vapor toxicity, the grading and interpretation of results on 96 chemical compounds. J. Ind. Hyg. Toxicol. 31(6):343-346.
Cavender, F.L., H.W. Casey, H. Salem, J.A. Swenberg, and E.J. Gralla. 1983. A 90-day vapor inhalation toxicity study of methyl ethyl ketone. Fundam. Appl. Toxicol. 3(4):264-270.
Chan, M.T., P. Mainland, and T. Gin. 1996. Minimum alveolar concentration of halothane and enflurane are decreased in early pregnancy. Anesthesiology 85(4):782-786.
ChemExpo. 2001. Chemical Profile for Methyl Ethyl Ketone. Chemical Database [online]. Available: http:www.chemexpo.com/news/PROFILE990301.cfm.
Couri, D., L.B. Hetland, M.S. Abdel-Rahman, and H. Weiss. 1977. The influence of inhaled ketone solvent vapors on hepatic microsomal biotransformation activities. Toxicol. Appl. Pharmacol. 41(2):285-289.
Cox, G.E., D.E. Bailey, and K. Morgareidge. 1975. Toxicity Studies in Rats with 2-Butanol including Growth, Reproduction and Teratologic Observations. Report No. 91MR R 1673. Food and Drug Research Laboratories, Inc., Waverly, NY.
Deacon, M.M., M.D. Pilny, J.A. John, B.A. Schwetz, F.J. Murray, H.O. Yakel, and R.A. Kuna. 1981. Embryo- and fetotoxicity of inhaled methyl ethyl ketone in rats. Toxicol. Appl. Pharmacol. 59(3):620-622.
De Ceaurriz, J.C., J.C. Micillino, P. Bonnet, and J.P. Guenier. 1981. Sensory irritation caused by various industrial airborne chemicals. Toxicol. Lett. 9(2):137-143.
De Ceaurriz, J., J.P. Desiles, P. Bonnet, B. Marignac, J. Muller, and J.P. Guenier. 1983. Concentration-dependent behavioral changes in mice following short-term inhalation exposure to various industrial solvents. Toxicol. Appl. Pharmacol. 67(3):383-389.
Devos, M., F. Patte, J. Rouault, P. Laffort, and L.J. Van Gemert, eds. 1990. P. 145 in Standardized Human Olfactory Thresholds. New York: Oxford University Press.
DFG (Deutsche Forschungsgemeinschaft). 2003. List of MAK and BAT Values 2003. Maximum Concentrations and Biological Tolerance Values at the Workplace Report No. 39. Weinheim, Federal Republic of Germany: Wiley VCH.
Dick, R.B., J.V. Setzer, R. Wait, M.B. Hayden, B.J. Taylor, B. Tolos, and V. Putz-Anderson. 1984. Effects of acute exposure of toluene and methyl ethyl ketone on psychomotor performance. Int. Arch. Occup. Environ. Health 54(2):91-109.
Dick, R.B., W.D. Brown, J.V. Setzer, B.J. Taylor, and R. Shukla. 1988. Effects of short duration exposures to acetone and methyl ethyl ketone. Toxicol. Lett. 43(1-3):31-49.
Dick, R.B., J.V. Setzer, B.J. Taylor, and R. Shukla. 1989. Neurobehavioural effects of short duration exposures to acetone and methyl ethyl ketone. Br. J. Ind. Med. 46(2):111-121.
Dick, R.B., E.F. Krieg, Jr., J. Setzer, and B. Taylor. 1992. Neurobehavioral effects from acute exposures to methyl isobutyl ketone and methyl ethyl ketone. Fundam. Appl. Toxicol. 19(3):453-473.
Dietz, F.K., M. Rodriguez-Giaxola, G.J. Traiger, V.J. Stella, and K.J. Himmelstein. 1981. Pharmacokinetics of 2-butanol and its metabolites in the rat. J. Pharmacokinet. Biopharm. 9(5):553-576.
DiVincenzo, G.D., C.J. Kaplan, and J. Dedinas. 1976. Characterization of the metabolites of methyl n-butyl ketone, methyl iso-butyl ketone, and methyl ethyl ketone in guinea pig serum and their clearance. Toxicol. Appl. Pharmacol. 36(3):511-522.
DiVincenzo, G.D., M.L. Hamilton, C.J. Kaplan, and J. Dedinas. 1977. Metabolic fate and disposition of 14C-labeled methyl n-butyl ketone in the rat. Toxicol. Appl. Pharmacol. 41(3):547-560.
Egan, G., P. Spencer, H. Schaumburg, K.J. Murray, M. Bischoff, and R. Scala. 1980. n-Hexane- “free” hexane mixture fails to produce nervous system damage. Neurotoxicology 1(3):515-524.
Enterline, P.E. 1978. Mortality among Workers from Shell Oil Company’s Lubricants’ Dewaxing Unit at Deer Park, Texas. Prepared for Shell Oil Company, by University of Pittsburg. January 25, 1978.
EPA (U.S. Environmental Protection Agency). 2003. Methyl Ethyl Ketone (MEK) (CASRN 78-93-3). Integrated Risk Information System, U.S. Environmental Protection Agency [online]. Available: http://www.epa.gov/ncea/iris/subst/0071.htm [accessed Nov. 5, 2010].
Fiserova-Bergerova, V. 1985. Toxicokinetics of organic solvents. Scand. J. Work Environ. Health 11(Suppl. 1):7-21.
Fiserova-Bergerova, V., and M.L. Diaz. 1986. Determination and prediction of tissue-gas partition coefficients. Int. Arch. Occup. Environ. Health 58(1):75-87.
Fowles, J.R., G.V. Alexeeff, and D. Dodge. 1999. The use of the benchmark dose methodology with acute inhalation lethality data. Regul. Toxicol. Pharmacol. 29(3):262-278.
Geller, I., E. Gause, H. Kaplan, and R.J. Hartmann. 1979. Effects of acetone, methyl ethyl ketone and methyl isobutyl ketone on a match-to-sample task in baboons. Pharmacol. Biochem. Behav. 11(4):401-406.
Ghittori, S., M. Imbriani, G. Pezzagno, and E. Capodaglio. 1987. The urinary concentration of solvents as a biological indicator of exposure: Proposal for the biological exposure limit for nine solvents. Am. Ind. Hyg. Assoc. J. 48(9):786-790.
Glowa, J.R. and P.B. Dews. 1987. Behavioral toxicology of volatile organic solvents. IV. Comparisons of the rate-decreasing effects of acetone, ethyl acetate, methyl ethyl ketone, toluene, and carbon disulfide on schedule-controlled behavior of mice. Int. J. Toxicol. 6(4):461-469.
Gregory, G.A., E.I. Eger, and E.S. Munson. 1969. The relationship between age and halothane requirements in man. Anesthesiology 30(5):488-491.
Greiner, E.O.C., and C. Funada. 2009. Methyl Ethyl Ketone (MEK). P. 675.5000 A in Chemical Economics Handbook. SRI Consulting [online]. Available: http://www.sriconsulting.com/ceh/Public/Reports/675.5000/ [accessed Nov. 2, 2010].
Hansen, L.F., A. Knudsen, and G.D. Nielsen. 1992. Sensory irritation effects of methyl ethyl ketone and its receptor activation mechanism. Pharmacol. Toxicol. 71(3Pt. 1):201-208.
Haumann, K., E. Kiesswetter, C. van Thriel, M. Blaszkewicz, K. Golka, and A. Seeber. 2003. Breathing and heart rate during experimental solvent exposure of young adults with self-reported multiple chemical sensitivity (sMCS). Neurotoxicology 24(2):179-186.
Hellman, T.M., and F.H. Small. 1974. Characterization of the odor properties of 101 petrochemicals using sensory methods. J. Air Pollut. Control Assoc. 24(10):979-982.
Horton, A.W., E.L. Bingham, M.J.G. Burton, and R. Tye. 1965. Carcinogenesis of the skin. III. The contribution of elemental sulfur and organic sulfur compounds. Cancer Res. 25(10):1759-1763.
HSDB (Hazardous Substances Data Bank). 2008. Methyl Ethyl Ketone (CASRN 78-93-3). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB [accessed Nov. 3, 2010].
Ichihara, G., I. Saito, M. Kamijima, X. Yu, E. Shibata, M. Toida, and Y. Takeuchi. 1998. Urinary 2,5-hexanedione increase with potentiation of neurotoxicity in chronic coexposures to n-hexane and methyl ethyl ketone. Int. Arch. Occup. Environ. Health 71(2):100-104.
Imbriani, M., S. Ghittori, G. Pezzagno, and E. Capodaglio. 1989. Methyl ethyl ketone (MEK) exposure in urine as biological index of exposure. Med. Lav. 11(6):255-261.
Jang, J.Y., S.K. Kang, and H.K. Chung. 1993. Biological exposure indices of organic solvents for Korean workers. Int. Arch. Occup. Environ. Health 65(Suppl. 1): S219-S222.
Johanson, G., and P.H. Naslund. 1988. Spreadsheet programming - a new approach in physiologically based modelling of solvent toxicokinetics. Toxicol. Lett. 41(2): 115-127.
Kale, A., S. Praster, W. Thomas, I. Amende, and T.G. Hampton. 2002. Respiratory frequency of mice at rest and after exercise. Mouse Specifics, Inc. [online]. Available: http://www.mousespecifics.com/physio/PFarticle13.html [accessed Nov. 5, 2010].
Katoh, T., and K. Ikeda. 1992. Minimum alveolar concentration of sevoflurane in children. Br. J. Anaesth. 68(2):139-141.
Kennedy, S.K., and D.E. Longnecker. 1996. History and principles of anesthesiology. Pp. 295-306 in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 9th Ed., J.G. Hardman, L.E. Limbird, P.B. Molinoff, and R.W. Ruddon, eds. New York: McGraw-Hill.
Kessler, W., B. Denk, and J.G. Filser. 1989. Species-specific inhalation of 2-nitropropane, methyl ethyl ketone, and n-hexane. Pp. 123-139 in Biologically Based Methods for Cancer Risk Assessment, C.C. Travis, ed. New York: Plenum Press.
Klimisch, H. 1988. The inhalation hazard test: Principle and method. Arch. Toxicol. 61(5):411-416.
Kopelman, P.G., and P.Y. Kalfayan. 1983. Severe metabolic acidosis after ingestion of butanone. Br. Med. J. 286 (6358):21-22.
LaBelle, C.W., and H. Brieger. 1955. The vapor toxicity of a composite solvent and its principal components. AMA Arch. Ind. Health 12(6):623-627.
LeDez, K.M., and J. Lerman. 1987. The minimum alveolar concentration (MAC) of isoflurane in preterm neonates. Anesthesiology 67(3):301-307.
Leonardos, G., D. Kendall, and N. Barnard. 1969. Odor threshold determinations of 53 odorant chemicals. J. Air Pollut. Control Assoc. 19(2):91-95.
Lerman, J., S. Robinson, M.M. Willis, and G.A. Gregory. 1983. Anesthetic requirements for halothane in young children 0-1 months and 1-6 months of age. Anesthesiology 59(5):421-424.
Liira, J., V. Riihimaki, and P. Pfaffli. 1988a. Kinetics of methyl ethyl ketone in man: Absorption, distribution and elimination in inhalation exposure. Int. Arch. Occup. Environ. Health 60(3):195-200.
Liira, J., V. Riihimaki, K. Engstrom, and P. Pfaffli. 1988b. Coexposure of man to mxylene and methyl ethyl ketone: Kinetics and metabolism. Scand. J. Work Environ. Health 14(5):322-327.
Liira, J., G. Johanson, and V. Riihimaki. 1990a. Dose-dependent kinetics of inhaled methylethylketone in man. Toxicol. Lett. 50(2-3):195-201.
Liira, J., V. Riihimaki, and K. Engstrom. 1990b. Effects of ethanol on the kinetics of methyl ethyl ketone in man. Br. J. Ind. Med. 47(5):325-330.
Liira, J., E. Elovaara, H. Raunio, V. Riihimaki, and K. Engstrom. 1991. Metabolic interaction and disposition of methyl ethyl ketone and m-xylene in rats at single and repeated inhalation exposure. Xenobiotica 21(1):53-63.
Marshall, B.E., and D.E. Longnecker. 1996. General anesthetics. Pp. 307-330 in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 9th Ed., J.G. Hardman, L.E. Limbird, P.B. Molinoff, and R.W. Ruddon, eds. New York: McGraw-Hill.
Mast, T.J., J.A. Dill, J.J. Evanoff, R.L. Rommereim, R.J. Weigel, and R.B. Westerberg. 1989. Inhalation Developmental Toxicology Studies: Teratology Study of Methyl Ethyl Ketone in Mice. PNL-6833. NTIS/DE89-009563. Pacific Northwest Laboratory, Richland, WA.
Mitran, E., T. Callender, B. Orha, P. Dragnea, and G. Botezatu. 1997. Neurotoxicity associated with occupational exposure to acetone, methyl ketone, and cyclohexanone. Environ. Res. 73(1-2):181-188.
Miyasaka, M., M. Kumai, A. Koizumi, T. Watanabe, K. Kurasako, K. Sato, and M. Ikeda. 1982. Biological monitoring of occupational exposure to methyl ethyl ketone by means of urinalysis for methyl ethyl ketone itself. Int. Arch. Occup. Environ. Health 50(2):131-137.
Morgott, D.A., D.C. Topping, and J.L. O’Donoghue. 2001. Ketones of four or five carbons. Pp. 117-194 in Patty’s Toxicology, Vol. 6, 5th Ed., E. Bingham, B. Cohrssen, and C.H. Powell, eds. New York: Wiley.
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: 2-Butanon. Den Haag: SDU Uitgevers [online]. Available: http://www.lasrook.net/lasrookNL/maclijst2004.htm [accessed Nov. 3, 2010].
Munies, R., and D.E. Wurster. 1965. Investigation of some factors influencing percutaneous absorption. III. Absorption of methyl ethyl ketone. J. Pharm. Sci. 54(9):1281-1284.
Muttray, A., D. Jung, L. Klimek, and C. Kreiner. 2002. Effects of an external exposure to 200 ppm methyl ethyl ketone on nasal mucosa in healthy volunteers. Int. Arch. Occup. Environ. Health 75(3):197-200.
Nakaaki, K. 1974. An experimental study on the effect of exposure to organic solvent vapor in human subjects. J. Sci. Labour 50:89-96.
Nelson, K.W., J.F. Ege, Jr., M. Ross, L.E. Woodman, and L. Silverman. 1943. Sensory response to certain industrial solvent vapors. J. Ind. Hyg. Toxicol. 25(7):282-285.
NIOSH (National Institute for Occupational Safety and Health). 1996. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLH): NIOSH Chemical Listing and Documentation of Revised IDLH Values (as of 3/1/95)-2-Butanone. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. August 1996 [online]. Available: http://www.cdc.gov/niosh/idlh/78933.html [accessed Nov. 4, 2010].
NIOSH (National Institute for Occupational Safety and Health). 2005. NIOSH Pocket Guide to Chemical Hazards: 2-Butanone. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH. September 2005 [online]. Available: http://www.cdc.gov/niosh/npg/npgd0069.html [accessed Nov. 4, 2010].
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
O’Donoghue, J.L., W.J. Krasavage, G.D. DiVincenzo, and G.V. Katz. 1984. Further studies on ketone neurotoxicity and interactions. Toxicol. Appl. Pharmacol. 72(2):201-209.
O’Neil, M.J., A. Smith, and P.E. Heckelman, eds. 2001. P. 1084 in The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th Ed. Whitehouse Station, NJ: Merck.
Ong, C.N., G.L. Sia, H.Y. Ong, W.H. Phoon, and K.T. Tan. 1991. Biological monitoring of occupational exposure to methyl ethyl ketone. Int. Arch. Occup. Environ. Health 63(5):319-324.
Patty, F.A., H.H. Schrenk, and W.P. Yant. 1935. Acute response of guinea pigs to vapors of some new commercial organic compounds. VIII. Butanone. Public Health Rep. 50(36):1217-1228.
Perbellini, L., F. Brugnone, P. Mozzo, V. Cocheo, and D. Caretta. 1984. Methyl ethyl ketone exposure in industrial workers: Uptake and kinetics. Int. Arch. Occup. Environ. Health 54(1):73-81.
Perocco, P., S. Bolognesi, and W. Alberghini. 1983. Toxic activity of seventeen industrial solvents and halogenated compounds on human lymphocytes cultured in vitro. Toxicol. Lett. 16(1-2):69-75.
Poulin, P., and K. Krishnan. 1995. A biologically-based algorithm for predicting human tissue: Blood partition coefficients of organic chemicals. Hum. Exp. Toxicol. 14(3):273-280.
Pozzani, U.C., C.S. Weil, and C.P. Carpenter. 1959. The toxicological basis of threshold limit values: 5. The experimental inhalation of vapor mixtures by rats, with notes upon the relationship between single dose inhalation and single dose oral data. Am. Ind. Hyg. Assoc. J. 20:364-369.
Ralston, W.H., R.L. Hilderbrand, D.E. Uddin, M.E. Andersen, and R.W. Gardier. 1985. Potentiation of 2,5-hexanedione neurotoxicity by methyl ethyl ketone. Toxicol. Appl. Pharmacol. 81(2):319-327.
Ruth, J.H. 1986. Odor thresholds and irritation levels of several chemical substances: A review. Am. Ind. Hyg. Assoc. J. 47(3):A142-A151.
Saida, K., J.R. Mendell, and H.S. Weiss. 1976. Peripheral nerve changes induced by methyl n-butyl ketone and potentiation by methyl ethyl ketone. J. Neuropathol. Exp. Neurol. 35(3):207-225.
Sato, A., and T. Nakajima. 1979. Partition coefficients of some aromatic hydrocarbons and ketones in water, blood and oil. Br. J. Ind. Med. 36(3):231-234.
Schwetz, B.A., B.K. Leong, and P.J. Gehring. 1974. Embryo- and fetotoxicity of inhaled carbon tetrachloride, 1,1-dichloroethane and methyl ethyl ketone in rats. Toxicol. Appl. Pharmacol. 28(3):452-464.
Schwetz, B.A., T.J. Mast, R.J. Weigel, J.A. Dill, and R.E. Morrissey. 1991. Developmental toxicity of inhaled methyl ethyl ketone in Swiss mice. Fundam. Appl. Toxicol. 16(4):742-748.
Seeber, A., C. van Thriel, K. Haumann, E. Kiesswetter, M. Blaszkewicz, and K. Golka. 2002. Psychological reactions related to chemosensory irritation. Int. Arch. Occup. Environ. Health 75(5):314-325.
Shibata, E., G. Johanson, A. Lof, L. Ernstgard, E. Gullstrand, and K. Sigvardsson. 2002. Changes in n-hexane toxicokinetics in short-term single exposure due to co-exposure to methyl ethyl ketone in volunteers. Int. Arch. Occup. Environ. Health 75(6):399-405.
Smith, A.R., and M.R. Mayers. 1944. Study of poisoning and fire hazards of butanone and acetone. NY State Dept. Labor Ind. Bull. 23:174-176.
Smyth, H.F., Jr. 1956. Improved communication: Hygienic standards for daily inhalation. Am. Ind. Hyg. Assoc. Q. 17(2):129-185.
Smyth, H.F., Jr., C.P. Carpenter, C.S. Weil, U.C. Pozzani, and J.A. Striegel. 1962. Range-finding toxicity data: List VI. Am. Ind. Hyg. Assoc. J. 23:95-107.
Stevens, W.C., W.M. Dolan, R.T. Gibbons, A. White, E.I. Eger, R.D. Miller, R.H. de Jong, and R.M. Elashoff. 1975. Minimum alveolar concentration (MAC) of isoflurane with and without nitrous oxide in patients of various ages. Anesthesiology 42(2):197-200.
Stoltenburg-Didinger, G., H. Altenkirch, and M. Wagner. 1990. Neurotoxicity of organic solvent mixtures: Embryotoxicity and fetotoxicity. Neurotoxicol. Teratol. 12(6):585-589.
Stone, L.C., G.T. Lawhorn, J.C. McKinney, and M.S. McCracken. 1981. Upper respiratory tract sensory responses to volatile chemicals. Toxicologist 1:134 [Abstract 288].
Tada, O., K. Nakaaki, and S. Fukabori. 1972. An experimental study on acetone and methyl ethyl ketone concentrations in urine and expired air after exposure to those vapors. J. Sci. Labour 48:305-336.
Takeuchi, Y., Y. Ono, N. Hisanaga, M. Iwata, M. Aoyama, J. Kitoh, and Y. Sugiura. 1983. An experimental study of the combined effects of n-hexane and methyl ethyl ketone. Br. J. Ind. Med. 40(2):199-203.
ten Berge, W.F., A. Zwart, and L.M. Appleman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Mater. 13(3):301-309.
ToxiGenics, Inc. 1981. 90-Day Vapor Inhalation Toxicity Study of Methyl Ethyl Ketone in Albino Rats. Study 420-0305. EPA 560/1981/CIIT/004. Prepared by ToxiGenics, Inc., Decatur, IL, for the Chemical Industry Institute of Toxicology, Research Triangle Park, NC.
Traiger, G.J., and J.V. Bruckner. 1976. The participation of 2-butanone in 2-butanol-induced potentiation of carbon tetrachloride hepatotoxicity. J. Pharmacol. Exp. Ther. 196(2):493-500.
van Engelen, J.G., W. Rebel-de Haan, J.J. Opdam, and G.J. Mulder. 1997. Effect of co-exposure to methyl ethyl ketone (MEK) on n-hexane toxicokinetics in human volunteers. Toxicol. Appl. Pharmacol. 144(2):385-395.
van Thriel, C., K. Haumann, E. Kiesswetter, M. Blaszkewicz, and A. Seeber. 2002. Time courses of sensory irritations due to 2-butanone and ethyl benzene exposure: Influences of self-reported multiple chemical sensitivity (sMCS). Int. J. Hyg. Environ. Health 204(5-6):367-369.
van Thriel, C., G.A. Wiesmuller, M. Blaszkewicz, K. Golka, E. Kiesswetter, A. Seeber, and C. Bachert. 2003a. Intranasal effects in chemically sensitive volunteers: an experimental exposure study. Environ. Toxicol. Pharmacol. 14(3):129-137.
van Thriel, C., A. Seeber, E. Kiesswetter, M. Blaszkewicz, K. Golka, and G.A. Wiesmuller. 2003b. Physiological and psychological approaches to chemosensory effects of solvents. Toxicol. Lett. 140-141:261-271.
Walter, G., M. Berg, J.G. Filser, and H. Greim. 1986. Toxicokinetics of the inhaled solvents n-hexane, 2-butanone, and toluene in the rat: Alone and in combination. N-S. Arch. Pharmacol. 334 (Suppl.1) R22 [Abstract 87].
Wen, C.P., S.P. Tsai, N.S. Weiss, R.L. Gibson, O. Wong, and W.A. McClellan. 1985. Long-term mortality study of oil refinery workers. IV. Exposure to the lubricating-dewaxing process. J. Natl. Cancer Inst. 74(1):11-18.
WHO (World Health Organization). 1993. Methyl Ethyl Ketone. Environmental Health Criteria 143. Geneva, Switzerland: World Health Organization [online]. Available: http://www.inchem.org/documents/ehc/ehc/ehc143.htm [accessed Nov. 5, 2010].
Wiesmuller, G.A., C. van Thriel, A. Steup, C. Bachert, M. Blaszkewicz, K. Golka, E. Kiesswetter, and A. Seeber. 2002. Nasal function in self-reported chemically intolerant individuals. Arch. Environ. Health 57(3):247-254.
Witschi, H.R., and J.A. Last. 2001. Toxic responses of the respiratory system. P. 519-534 in Casarett & Doull’s Toxicology: The Basic Science of Poisons, C.D. Klaassen, ed. New York: McGraw-Hill.
Wurster, D.E., and R. Munies. 1965. Factors influencing percutaneous absorption II. Absorption of methyl ethyl ketone. J. Pharmaceut. Sci. 54(4):554-556.
Yoshikawa, M., T. Kawamoto, K. Murata, K. Arashidani, T. Katoh, and Y. Kodama. 1995. Biological monitoring of occupational exposure to methyl ethyl ketone in Japanese workers. Arch. Environ. Contam. Toxicol. 29(1):135-139.
Zakhari, S., M. Leibowitz, P. Levy, and D.M. Aviado. 1977. Acute, oral, intraperitoneal, and inhalational toxicity in the mouse. P. 67-69 in Isopropanol and Ketones in the Environment, L. Golberg, ed. Cleveland, OH: CRC Press.
APPENDIX A
DERIVATION OF AEGL VALUES FOR METHYL ETHYL KETONE
| Derivation of AEGL-1 Values | |
| Key studies: | Dick et al. (1992); Muttray et al. 2002; Seeber et al. 2002; Shibata et al. 2002 |
| Toxicity end points: | 200 for 4 h and 380 ppm for several 8-min exposure durations were NOAELs for sensory irritation and CNS effects; the lower number was chosen as the basis for the AEGL-1. |
| Time-scaling: | Not applied |
| Uncertainty factors: | 1 for intraspecies variability |
| Modifying factor: | Not applied |
| Calculations: durations. | The 200-ppm concentration was used for all exposure |
| 10-min AEGL-1: | 200 ppm |
| 30-min AEGL-1: | 200 ppm |
| 1-h AEGL-1: | 200 ppm |
| 4-h AEGL-1: | 200 ppm |
| 8-h AEGL-1: | 200 ppm |
| Derivation of AEGL-2 Values | |
| Key studies: | Altenkirch et al. (1978); Cavender et al. (1983) |
| Toxicity end points: | 5,000 ppm was a NOAEL for neurobehavioral effects (narcosis) on the first and subsequent days of a subchronic study with rats; exposures were for 6 h/day, 5 days/week, for 90 days. |
| Time-scaling: | Default value of n = 3 applied to shorter exposure durations |
| Uncertainty factors: | 1 for interspecies; rodents have higher respiratory rates and cardiac output than humans; metabolism differences will not be significant at high, acute exposures. 3 for intraspecies; differences among humans for CNS effects of anesthetics are not expected to vary greatly. |
| Modifying factor: | Not applied |
| Calculations: | 5,000 ppm/3 = 1,700 ppm |
| C3 × 240 min = k | |
| (1,700)3 × 240 min = 1.179 × 1012 ppm3-min | |
| 10-min AEGL-2: | [(1.179 × 1012 ppm3-min)/10 min]1/3 |
| C = 4,900 ppm | |
| 30-min AEGL-2: | [(1.179 × 1012 ppm3-min)/10 min] 1/3 |
| C = 3400 ppm | |
| 1-h AEGL-2: | [(1.179 × 1012 ppm3-min)/60 min] 1/3 |
| C = 2700 ppm | |
| 4-h AEGL-2: | 1,700 ppm |
| 8-h AEGL-2: | 1,700 ppm |
| Derivation of AEGL-3 Values | |
| Key studies: | 10 and 30 min: Zakhari et al. 1977; |
| Klimisch 1988; Hansen et al. 1992; | |
| 1, 4, and 8 h: La Belle and Brieger 1955 | |
| Toxicity end points: | (1) Threshold for lethality—mouse, rat 30-min exposure of rats at 92,239 ppm (Klimisch 1988) 45-min exposure of mice at 50,000 ppm (Zakhari et al. 1977) |
| Calculated 30-min RD50 of 31,246 ppm—mice (Hansen et al. 1992) | |
| (2) 4-h MLE01 of 7,500 ppm for rat calculated by Fowles et al. (1999) from data of La Belle and Brieger (1955) | |
| Time-scaling | None for 10- and 30-min values |
| None for 4- and 8-h values 1-h value time-scaled from 4-h value using n = 3 (Cn × t = k) |
|
| Uncertainty factors: | 1 for interspecies; rodents have higher respiratory rates and cardiac output than humans; metabolism differences will not be significant at high, acute exposures. |
| 3 for intraspecies; differences among humans for irritancy and CNS effects are not expected to vary greatly. | |
| Modifying factor: | Not applied |
| Calculations: | (1) values adjusted to 10,000 ppm |
| (2) 7,500 ppm/3 = 2,500 ppm | |
| 4-h value: (2,500 ppm)3 × 240 min = 3.75 × 1012 ppm3-min | |
| 10-min AEGL-3: | 10,000 ppm |
| 30-min AEGL-3: | 10,000 ppm |
| 1-h AEGL-3: | C3 × 60 min = 3.75 × 1012 ppm3-min |
| C = 4,000 ppm | |
| 4-h AEGL-3: | 2,500 ppm |
| 8-h AEGL-3: | 2,500 ppm |
APPENDIX B
CATEGORY GRAPH OF TOXICITY DATA AND AEGL VALUES
FIGURE 3-2 Category graph of toxicity data and AEGL values.
TABLE B-1 Data Used in Category Graph
| Source | Species | ppm | Minutes | Categorya |
| NAC/AEGL-1 | 200 | 10 | AEGL | |
| NAC/AEGL-1 | 200 | 30 | AEGL | |
| NAC/AEGL-1 | 200 | 60 | AEGL | |
| NAC/AEGL-1 | 200 | 240 | AEGL | |
| NAC/AEGL-1 | 200 | 480 | AEGL | |
| NAC/AEGL-2 | 4,900 | 10 | AEGL | |
| NAC/AEGL-2 | 3,400 | 30 | AEGL | |
| NAC/AEGL-2 | 2,700 | 60 | AEGL | |
| NAC/AEGL-2 | 1,700 | 240 | AEGL | |
| NAC/AEGL-2 | 1,700 | 480 | AEGL | |
| NAC/AEGL-3 | 10,000 | 10 | AEGL | |
| NAC/AEGL-3 | 10,000 | 30 | AEGL | |
| NAC/AEGL-3 | 4,000 | 60 | AEGL | |
| Source | Species | ppm | Minutes | Categorya |
| NAC/AEGL-3 | 2,500 | 240 | AEGL | |
| NAC/AEGL-3 | 2,500 | 480 | AEGL | |
| Nelson et al. 1943 | Human | 100 | 5 | 0 |
| Human | 200 | 5 | 1 | |
| Human | 350 | 5 | 1 | |
| Shibata et al. 2002 and others | Human | 200 | 120 | 0 |
| Dick et al. 1992 and others | Human | 200 | 240 | 0 |
| Seeber et al. 2002 | Human | 380 | 8 | 0 |
| Patty et al. 1935 | Human | 3,300 | 1 | 2 |
| Seeber et al. 2002 | Human | 10 | 480 | 0 |
| Klimisch 1988 | Rat | 92,239 | 180 | SL |
| Rat | 92,239 | 30 | 2 | |
| Pozzani et al. 1959 | Rat | 8,000 | 480 | SL |
| Smyth et al. 1962 | Rat | 2,000 | 120 | 0 |
| LaBelle and Brieger 1955 | Mouse | 103,000 | 43 | 3 |
| Mouse | 9,090 | 240 | SL | |
| Mouse | 11,700 | 240 | SL | |
| Mouse | 7850 | 240 | 2 | |
| Zakhari et al. 1977 | Mouse | 69,500 | 45 | SL |
| Mouse | 50,000 | 45 | 2 | |
| Patty et al. 1935 | Guinea pig | 100,000 | 45 | 3 |
| Guinea pig | 33,000 | 200 | 3 | |
| Guinea pig | 10,000 | 480 | 2 | |
| Geller et al. 1979 | Baboon | 100 | 480 | 0 |
| Altenkirch et al. 1978 | Rat | 10,000 | 480 | 2 |
| Rat | 6,000 | 480 | 0 | |
| Hansen et al. 1992 | Mouse | 26,000 | 30 | 2 |
| Mouse | 10,000 | 30 | 1 | |
| DeCeaurriz et al. 1981 | Mouse | 10,745 | 5 | 2 |
| Stone et al. 1981 | Mouse | 5,000 | 10 | 1 |
| Mouse | 9,000 | 10 | 2 | |
| Glowa and Dews 1987 | Mouse | 10,000 | 9.5 | 2 |
| Mouse | 5600 | 9.5 | 2 | |
| Mouse | 1,000 | 9.5 | 0 | |
| Mouse | 300 | 9.5 | 0 | |
| Patty et al. 1935 | Guinea pig | 3,300 | 240 | 0 |
| Guinea pig | 10,000 | 40 | 2 | |
aCategories: 0, no effect; 1, discomfort; 2, disabling; SL, some lethality; and 3, lethal.
APPENDIX C
ACUTE EXPOSURE GUIDELINE LEVELS FOR METHYL ETHYL KETONE
Derivation Summary for Methyl Ethyl Ketone
AEGL-1 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 200 ppm | 200 ppm | 200 ppm | 200 ppm | 200 ppm |
| Key references: | ||||
| Dick, R.B., E.F. Krieg, Jr., J. Setzer, and B. Taylor. 1992. Neurobehavioral effects from acute exposures to methyl isobutyl ketone and methyl ethyl ketone. Fundam. | ||||
| Appl. Toxicol. 19(3):453-473. | ||||
| Muttray, A., D. Jung, L. Klimek, and C. Kreiner. 2002. Effects of an external exposure to 200 ppm methyl ethyl ketone on nasal mucosa in healthy volunteers. Int. Arch. Occup. Environ. Health 75(3):197-200. | ||||
| Seeber, A., C. van Thriel, K. Haumann, E. Kiesswetter, M. Blaszkewicz, and K. Golka. 2002. Psychological reactions related to chemosensory irritation. Int. Arch. Occup. Environ. Health 75(5):314-325. | ||||
| Shibata, E., G. Johanson, A. Lof, L. Ernstgard, E. Gullstrand, and K. Sigvardsson. 2002. Changes in n-hexane toxicokinetics in short-term single exposure due to co-exposure to methyl ethyl ketone in volunteers. Int. Arch. Occup. Environ. Health 75(6):399-405. | ||||
| Test species/Strain/Number: | ||||
| Human/24 subjects (Dick et al. 1992); 19 male subjects (Muttray et al. 2002); 24 subjects (Seeber et al. 2002); 4 male subjects (Shibata et al. 2002) | ||||
| Exposure route/Concentrations/Durations: 200 ppm for 4 h (Dick et al. 1992; | ||||
| Muttray et al. 2002); 200 ppm for 2 h (Shibata et al. 2002); 10-380 ppm (average 188 ppm) for over 4 h (Seeber et al. 2002) | ||||
| Effects: At 200 ppm, unobjectionable, no neurobehavioral effects, and no other effects reported in additional studies | ||||
| End point/Concentration/Rationale: NOAEL for irritation and neurobehavioral deficits | ||||
| Uncertainty factors/Rationale: | ||||
| Total uncertainty factor: 1 | ||||
| Interspecies: Not applicable | ||||
| Intraspecies: 1, no susceptible populations were located. The intensity of discomfort associated with 200 ppm is not expected to vary greatly among the general population. | ||||
| Modifying factor: Not applied | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time-scaling: Not applied; a tolerance develops to any irritation | ||||
| Data adequacy: The database on clinical and monitoring studies is extensive. MEK is rapidly metabolized. Short exposures of healthy subjects and subjects with sMCS at 380 ppm without apparent adverse effects supports the concentration of 200 ppm for the general population. | ||||
AEGL-2 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 4,900 ppma | 3,400 ppma | 2,700 ppma | 1,700 ppm | 1,700 ppm |
| Key reference: | ||||
| Cavender, F.L., H.W. Casey, H. Salem, J.A. Swenberg, and E.J. Gralla. 1983. A 90-day vapor inhalation toxicity study of methyl ethyl ketone. Fundam. Appl. Toxicol. 3(4):264-270. | ||||
| Supporting reference: | ||||
| Altenkirch, H., G. Stoltenburg, and H.M. Wagner. 1978a. Experimental studies on hydrocarbon neuropathies induced by methyl-ethyl-ketone (MEK). J. Neurol. 219(3):159-170. | ||||
| Test species/Strain/Number: | ||||
| Rat/Fischer 344/15 males and 15 females (Cavender et al. 1983); Wistar/5 rats (Altenkirch et al. 1978) | ||||
| Exposure route/Concentrations/Durations: (Cavender et al. 1983); 6,000 ppm for 8 h/day, 7 days/week, for several weeks (Altenkirch et al. 1978) | ||||
| Effects: At 5,000 ppm, no irritation, no narcosis, and transient weight loss (Cavender et al. 1983); at 6,000 ppm, hyperexcitability followed by somnolence within 5-10 min and gait disturbance after exposures, no neuropathies, animals died of bronchopneumonia in 7th week (Altenkirch et al. 1978). | ||||
| End point/Concentration/Rationale: NOAEL for neurobehavioral deficits at 5,000 ppm for 6 h | ||||
| Uncertainty factors/Rationale: | ||||
| Total uncertainty factor: 3 | ||||
| Interspecies: 1, uptake is greater and faster in rodents compared with humans. | ||||
| Intraspecies: 3, no susceptible populations identified; metabolism is not expected to vary greatly among individuals; and susceptibility to CNS depression does not vary by more than a factor of 2- to 3-fold among the general population. | ||||
| Modifying factor: Not applicable | ||||
| Animal-to-human dosimetric adjustment: Not applied | ||||
| Time-scaling: The 4- and 8-h exposures were set equal to 1,700 ppm; the 10- and 30- min and 1-h values were time-scaled with the default n value of 3. | ||||
| Data adequacy: Extensive database of human (irritation, neurotoxicity, and metabolism) and animal studies (baboon, rat, mouse, and guinea pig); animal studies addressed irritation, neurotoxicity, developmental toxicity, and subchronic toxicity; the Cavender et al. (1983) study is supported by a study with rats conducted at 6,000 ppm (Altenkirch et al. 1978); the key study utilized groups of 15 male and 15 female rats; and complete histologic examinations were performed at the end of exposure. | ||||
aThe 10- and 30-min and the 1-h AEGL-2 values are higher than one-tenth of the lower explosive limit (LEL) of MEK in air (LEL = 18,000 ppm). Therefore, safety considerations against the hazard of explosion must be taken into account.
AEGL-3 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| a | a | 4,000 ppmb | 2,500 ppmb | 2,500 ppmb |
| Key references: | ||||
| Fowles, J.R., G.V. Alexeeff, and D. Dodge. 1999. The use of the benchmark dose methodology with acute inhalation lethality data. Regul. Toxicol. Pharmacol. 29(3):262-278. | ||||
| Hansen, L.F., A. Knudsen, and G.D. Nielsen. 1992. Sensory irritation effects of methyl ethyl ketone and its receptor activation mechanism. Pharmacol. Toxicol. 71(3 Pt. 1):201-208. | ||||
| Klimisch, H. 1988. The inhalation hazard test; principle and method. Arch. Toxicol. 61(5):411-416. | ||||
| La Belle, C.W., and H. Brieger. 1955. The vapor toxicity of a composite solvent and its principal components. Arch. Ind. Health 12(6):623-627. | ||||
| Zakhari, S., M. Leibowitz, P. Levy, and D.M. Aviado. 1977. Acute, oral, intraperitoneal, and inhalational toxicity in the mouse. P. 67-69 in Isopropanol and Ketones in the Environment, L. Golberg, ed. Cleveland, OH: CRC Press. | ||||
| Test species/Strain/Number: | ||||
| Rat/unspecified/6 rats (Klimisch 1988); mouse/CF-1/10 per group (Zakhari et al. 1977); mouse/CF-1/4 per group (Hansen et al. 1992); rat/strain not given/6 per group (La Belle and Brieger 1955) | ||||
| Exposure route/Concentrations/Durations: | ||||
| Inhalation/92,239 ppm for 30 min or 3 h (Klimisch 1988); 50,000, 60,000, 70,000, 80,000, or 100,000 ppm, for 45 min (Zakhari et al. 1977); 0, 3809, 9136, 12,771, 24,179, or 26,416 ppm, for30 min (Hansen et al. 1992); 7,850, 9,090, 9,260, 12,200, 13,750, 18,100, or 20,200 ppm, for 4 h (La Belle and Brieger 1955) | ||||
| Effects: No deaths at 96,239 ppm for 30 min (Klimisch 1988); no deaths at 50,000 ppm for 45 min (Zakhari et al. 1977); concentration-dependent decrease in respiratory rate and tidal volume; calculated RD50 of 31,246 ppm; no deaths at 26,000 ppm for 30 min (Hansen et al. 1992); and no deaths at 7,850 ppm (La Belle and Brieger 1955) | ||||
| End point/Concentration/Rationale: On the basis of the first three studies, 10,000 ppm would not be life-threatening to humans; at 7,500 ppm for 4-h, maximum likelihood estimate, with a 1% response (MLE01) calculated by Fowles et al. (1999) from data of La Belle and Brieger (1955) | ||||
| Uncertainty factors/Rationale: | ||||
| Total uncertainty factor: 3 Interspecies: 1, uptake is greater and faster in rodents than in humans. |
||||
| Intraspecies: 3, no susceptible populations identified; CNS depression does not vary by more than a factor of 2-3 among the general population. | ||||
| Modifying factor: Not applicable | ||||
| Animal-to-human dosimetric adjustment: Not applied | ||||
| Time-scaling: Cn × t = k. Default value of n = 3 used when scaling from longer to shorter time intervals; both 4- and 8-h values set equal to 2,500 ppm; 1-h value time-scaled from the 4-h value | ||||
| Data adequacy: Extensive database of human (irritation, neurotoxicity, and metabolism) and animal studies (baboon, rat, mouse, and guinea pig); animal studies addressed irritation, neurotoxicity, developmental toxicity, and subchronic toxicity; studies were supported by no deaths in rats exposed at 10,000 ppm for several days (Altenkirch et al. 1978) and no deaths in guinea pigs exposed at 10,000 ppm for 13.5 h (Patty et al. 1935). | ||||
aThe 10- and 30-min AEGL-3 value of 10,000 ppm (29,300 mg/m3) is higher than 50% of the lower explosive limit (LEL) of MEK in air (LEL = 18,000 ppm). Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
bThe 1-, 4-, and 8-h AEGL-3 values are higher than one-tenth of the LEL of MEK in air (LEL = 18,000 ppm). Therefore, safety considerations against the hazard of explosion must be taken into account.


























































