Codevelopment of Therapies for HIV as a Model
Two speakers gave presentations on how combination therapies for human immunodeficiency virus (HIV) were developed, and how the lessons learned from that experience are applicable to developing investigational combination cancer therapies. Dr. Carl Dieffenbach, director of the Division of Acquired Immunodeficiency Syndrome (AIDS) at the National Institute of Allergy and Infectious Diseases (NIAID) pointed out that HIV, like cancer, is extremely heterogeneous and because of the numerous different strains and the ability of the virus to rapidly mutate to elude the immune system, combination therapies have been key to keeping the virus in check in infected patients. “The problem with HIV is we literally have almost an infinite number of viruses,” said Dr. Gary Nabel, director of the Vaccine Research Center at NIAID. “Within a single individual shortly after infection, there are essentially millions of variants, so like for cancer therapy, where the cancer cells are constantly mutating, combinations for us are very important.”
In 1996, three agents were successfully combined to treat HIV after they were shown to induce rapid reduction of viral loads and led to sustainable undetectable levels of virus in the blood. Since then, the entire field of therapeutics has focused on optimizing these combinations for safety, tolerability, and dosing. Currently, more than 30 compounds are approved for treating HIV and six fixed-dose combinations are available.
Dr. Dieffenbach pointed out that since 1998, NIH’s main role in HIV drug development has been to focus on strategy trials—that is, once a single agent is approved, showing how to combine it in appropriate ways
with other agents. For these studies “the industry has been really good to us, in terms of donating drugs for trials,” Dr. Dieffenbach noted. His NIH division just completed a series of prevention trials using antivirals, and all of those drugs were donated, with the total cost of the drugs being approximately $50 or $60 million. “It’s been a very productive partnership because we built an industry, in terms of training a series of clinicians, putting together the infrastructure to run these kinds of drug trials. That has then largely become industry supported,” Dr. Dieffenbach said.
One lesson learned from this experience is the need to target two steps in the life cycle of the virus, according to Dr. Dieffenbach. Combinations that target a single step tend to have overlapping toxicities and run the risk of pharmacological and potentially virological interference, he said. He added that potency matters in terms of the dosing and the impact. “Unlike cancer chemotherapy, these are drugs that are designed to be taken every day for the rest of the patients’ lives so what we want is a safe drug,” Dr. Dieffenbach stressed.
THE IMPORTANCE OF SURROGATE MARKERS
HIV drug development benefited immensely from the ability to use viral load as a validated surrogate for response to therapy, Dr. Dieffenbach stressed. Dr. Nabel added that “the fundamental difference scientifically between HIV and cancer is that we really have a crystal clear biomarker—viral load—that makes it so much easier for everybody, because you really can rally to one thing. That’s how we ended up getting six targets of different classes because you were all aligned to that one thing,” he said. Dr. Nabel suggested that it would help the combination cancer therapy field if advocacy groups and scientists joined together to formulate a plan for showing the utility of biomarkers. “There has to be some kind of coalescence, and what may be hurting your efforts in advocacy and the scientific efforts is the lack of focus and the ambiguity and the biomarkers,” he said. He added that the use of HIV neutralizing antibody as a biomarker for vaccine effectiveness has also helped the development of HIV vaccines.
It is also helpful to have a system for measuring the comparative effectiveness of various combinations, Dr. Dieffenbach pointed out. He showed a recent effort to do this in HIV by Shen and colleagues (2008, 2009). These researchers, through simple mathematical manipulations, were able to rate combination therapies on their ability to inhibit virus replication and graphically represent which ones were the best treatments (see Figure 8-1). He raised the question of whether a similar approach could be used to compare the effectiveness of combination cancer chemotherapies.
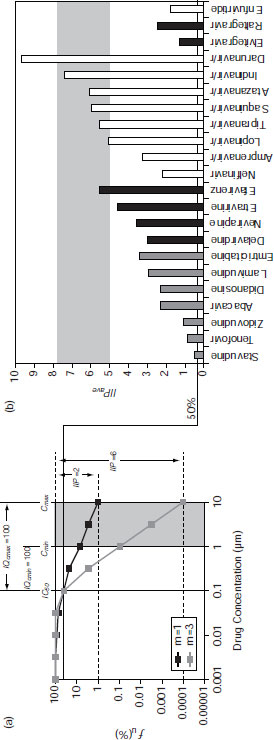
SOURCE: Dieffenbach presentation (June 14, 2011). Reprinted from Shen, L., S. Rabi, and R. Siliciano. 2009. A novel method for determining the inhibitory potential of anti-HIV drugs. Trends in Pharmacological Science 30(12):610–616, with permission from Elsevier.
VACCINES IN COMBINATION THERAPIES
Current areas of exploration and codevelopment in the HIV arena include vaccine-drug combinations and combination vaccines that might ultimately liberate patients from the need to continue on anti-HIV drug therapy for the rest of their lives. One approach is to use standard antiviral therapy to reduce viral levels and then use vaccines to provide a new level of immunity that gives patients the ability to control the virus once the drugs are stopped. In the United States there are 7 to 10 therapeutic HIV vaccines currently in trial, according to Dr. Dieffenbach.
Dr. Nabel noted that combination vaccines have a long history beginning with the polio vaccine. This vaccine was successful because it prompted immunity to all three strains of polio. “There’s a historic precedent for a combination therapy that essentially wiped out a devastating human disease and wouldn’t have happened in any other way,” he said.
Three types of combinations for vaccines are being explored for HIV. One is combinations of different vectors or delivery platforms for the vaccines that stimulate qualitatively different immune responses. Another type combines different inserts in the vaccines. This approach increases the breadth of response that improves protection against diverse viral strains. The third type of combination joins drug and antibody treatments with immune stimulants. Such combinations show efficacy not seen with either one alone, according to Dr. Nabel.
One cancer-related example Dr. Nabel gave for that third type of combination treatment is standard chemotherapy combined with a vaccine aimed at boosting immunity to tumor-promoting proteins generated by human papillomavirus (HPV). He said this combination completely suppresses the growth of cervical tumors in a mouse model, whereas neither treatment alone is effective. He emphasized that “if you were to require that each agent in the combination be tested alone and approved based on a marginal degree of efficacy, you would not have approved this drug or vaccine, and you would never have gotten to test the combination. Going forward, it’s really important to recognize that this criteria of having even marginal—10 percent—effects is one that’s going to limit the opportunities for finding new and effective drug and immunotherapies,” he stressed.
THE KEY ROLE OF PATIENT ADVOCATES
Another lesson learned from HIV combination therapy development that may be applicable to the development of cancer combination therapies is the key role patient advocates played in fostering collaborations, Drs. Dieffenbach and Nabel pointed out. “A highly educated patient population has pushed NIH and industry to do these combinations. This
productive working relationship with the activist community really has driven this type of integrated drug development,” said Dr. Dieffenbach. Dr. Nabel added that there were specific granting mechanisms that promoted development in certain areas, including the NCDDG Program (National Cooperative Drug Discovery Groups),1 which were cooperative grants between scientists and industry. “It really put together the best of basic scientists exploring specific targets with industry in a way that allowed both to focus on drug development and on scientific discovery of new targets. That really helped to spread the effort onto particular targets and made progress quick in those areas,” Dr. Nabel said. Dr. Dieffenbach agreed, noting that “these grants turned out to be quite catalytic, because what happened over time is the industry got to know the leaders scientifically, and these natural bonds have continued over time.”
Several participants were struck by the success of combination therapy development for HIV. “We need to, having the HIV experience as the lead, think about how to get from AZT to 32 drugs on the market, and how to use them in combination wisely,” said Dr. Flaherty. Dr. Perlmutter added, “Thirty years ago, AIDS was not even really acknowledged and went from being the most lethal and scary disease to one that is relatively manageable with a cocktail. There are lots of cancer patients that are out there ready to take their cocktail.”
Dr. Sharon Murphy stressed the powerful role of advocacy groups that put pressure on NIH and pharmaceutical companies. Such a loud voice given to combination therapy by advocates joining together and focusing on the same specific goals has not happened within the cancer arena, she pointed out. “We need to learn from this,” she said. Dr. Perlmutter agreed, noting that the AIDS community “had crystal clear goals.” She suggested researchers invite advocates to put pressure on the appropriate drug companies to have them collaborate more. “You know where it might be profitable to work early and you can invite advocates to [put pressure on] them,” she said. But she added that there will not be millions of patient advocates coming together without any clear strategy, which researchers can help provide. Dr. Nabel added that mutual education between advocates and scientists was helpful in the AIDS arena. “The scientists were somewhat disconnected from what the real people with the disease were feeling and vice versa—the advocates didn’t understand some of the scientific problems. When people could bridge that [educational] divide and make those connections, that’s when things started to happen,” he said.
![]()
1 See http://dtp.nci.nih.gov/branches/gcob/gcob_web3.html (accessed December 14, 2011).
This page intentionally left blank.






