ATTACHMENT B
WORK PLAN
SITE-SPECIFIC RISK ASSESSMENT FOR THE MEDICAL COUNTERMEASURES TESTING AND EVALUATION FACILITY AT FORT DETRICK IN FREDERICK COUNTY, MARYLAND
Final | 15 July 2011
Prepared by: BSA Environmental Services, Inc.
This page intentionally left blank.
Table of Contents
Project Description (Planning and Scoping Stage)
Approach for Qualitative Risk Assessment
Approach for Quantitative Risk Assessment
Appendix 1. Additional Planning by Agent based on Figure 3-2 of Science and Decisions (NRC 2008)
List of Figures
Figure 1. Illustration of the scope of the MCMT&EF SSRA
Figure 2. Typical probabilistic risk assessment task flow
List of Tables
Table 2. Major Elements of Analysis Plan (Box 3-4, NRC 2008)
This page intentionally left blank.
Project Description (Planning and Scoping Stage)
The project goal for the U.S. Army Medical Research and Materiel Command (USAMRMC) is to develop a site-specific risk assessment (SSRA) for the Medical Countermeasures Testing and Evaluation Facility (MCMT&EF) at Fort Detrick, MD. The risk assessment aims to document the likelihood, adverse consequences, and uncertainty of reasonably foreseeable events that can affect the health of people working in and around the laboratory as well as members of the community. Environmental impacts will be identified and characterized in the Environmental Impact Statement (EIS) that will include the SSRA as an appendix that addresses human health risks.
USAMRMC will be conducting vaccine and drug research for agents in the medical countermeasures portfolio. The SSRA will provide decision support for USAMRMC to address the adequacy of current controls and interventions protecting workers and preventing accidental releases that could cause human illness in the surrounding community. The SSRA will only address risk associated with acute health issues particular to the laboratory work conducted at MCMT&EF. Examples of possible intentional release scenarios will be consider within the constraints of the current Biosurety Program, regulations, and barriers for containment.
The risk assessment approach described below represents a tiered assessment consistent with current knowledge of disease for portfolio agents and key risk references (National Research Council [NRC], 2008 Science and Decisions; the International Life Sciences Institute framework for microbial risk assessment (International Life Sciences Institute [ILSI], 2000); and National Academy of Sciences [NAS] letter reports (2011 and others). The major objectives of the tiered assessment are:
- Compile and structure available scientific evidence on conditions necessary to cause disease (sources, stressors, populations, routes, pathways, endpoints; Figure 1) and provide transparency regarding knowledge and gaps for portfolio agents listed below:
- Bacillus anthracis (anthrax)
- Brucella spp. (brucellosis)
- Ebola/Marburg viruses (viral hemorrhagic fevers)
- Francisella tularensis (tularemia)
- Venezuelan Equine Encephalitis (VEE)/ Eastern Equine Encephalitis (EEE)/ Western Equine Encephalitis (WEE) viruses (febrile disease, encephalitis)
- Yersinia pestis (plague)
- Conduct a comprehensive review of contemporary scientific literature and publically available information from national surveys to identify risks, hazards and mitigation processes associated with laboratory acquired illnesses for above listed pathogens, transmission to the community, intentional and accidental release, transportation release, work with animal species anticipated at MCMT&E, and the development and testing of vaccines and countermeasures
- Construct reasonably foreseeable scenarios (possible scenarios hereafter) consistent with mechanisms of disease and knowledge of dose-response
-
relationships for likelihood and severity of disease given exposure, considering source, stressors (agents), populations, route, pathway, and endpoint (see Figure 1, including illustrative examples for tularemia)
- Develop conceptual model for analysis (see Figure 2)
- Characterize human health risk qualitatively (unlikely or possible)
- Identify possible scenarios amenable to quantitative analysis
- Develop and run exposure assessment and dose-response assessment models to characterize human health risks, with attendant uncertainty (qualitative narrative)
- Prepare risk communication materials from qualitative and quantitative results
- Document future expansions for consideration as new data become available
As previously stated (March 21st presentation to the NAS panel), agent-specific evidence for disease mechanisms will be considered for defining plausible agent and route combinations. If the qualitative risk assessment (QualRA) results in ‘unlikely’ determination for either the exposure assessment or the dose-response assessment, the pathway or hypothetical scenario may be implausible. In light of the high level of community interest for the MCMT&EF, our strategy is to meticulously communicate what is known (and what is unknown) to preclude misleading the public, particularly when feared scenarios are implausible. For example, available evidence supports quantitative modeling of the exposure pathways for Ebola by the dermal/percutaneous route for laboratory workers, not ingestion and mucosal/ocular routes (see Appendix Figure A-3). Pathways determined unlikely in the QualRA for each agent would not be modeled due to inconsistency with current knowledge of mechanisms of disease, as scientific rigor may be insufficient to support modeling for the possible inhalation route for this agent.
Scientific evidence will be structured to support both tiers (qualitative and quantitative) risk assessment. Structural evidence will be used to estimate unlikely and possible scenarios for QualRA and frequency and consequences of possible scenarios. Both approaches will address confidence measures representing uncertainties. Quantitative risk assessment (QuantRA) will be employed as a second tier of analysis when first-tier qualitative assessments cannot confidently bound scenario risks as ‘unlikely’ and sufficient data are available to support a quantitative assessment that significantly improves risk characterization. Gaps in scientific knowledge and research in progress will be noted as appropriate in uncertainty analyses.
1) Approach for Qualitative Risk Assessment
The approach was developed based on knowledge of microbial risk assessment frameworks (e.g., ILSI, 2000), as well as published and ongoing research informing biothreat risk assessment, supplemented by targeted searches of the literature to identify additional relevant published studies for the pathogens in the current agent portfolio. Sections 2 and 3 of the work plan present our approach with specific examples for tularemia due to the concern of the local community and the recent laboratory
associated tularemia infection. For completeness and transparency, approaches planned for other agents in portfolio are outlined briefly in Appendix 1.
A) Hazard Identification for Tularemia
Tularemia is a zoonotic disease (an animal disease that can be transmitted to humans) caused by the Gram negative coccobacillus Francisella tularensis. This agent is thought to infect up to 250 animal hosts, more than any other known zoonotic pathogen (Dempsey et al., 2006). Contact with the following animals is associated with cases of human tularemia: beavers; cats; crayfish; dogs; dormice; hamsters; hogs and wild boars; mule deer; muskrats; non-human primates (NHPs); pheasants; prairie dogs; wild rabbits and hares; sheep; and squirrels. Tularemia is endemic in the U.S. (including Maryland) and around the world and is thought to persist in nature in enzootic cycles involving wild mammals (largely rodents, rabbits, and hares) and arthropod vectors (ticks, mosquitoes, flies) or amoeba.
Evidence for the disease triangle or triad (pathogen, host, and environment, with interactions) influencing disease likelihood and severity) was compiled for tularemia as outlined below. Human tularemia is characterized by abrupt onset of febrile illness (fever and flu-like symptoms) that is often self-limiting and rarely fatal. Human cases from laboratory acquired infections (LAIs), clusters of sporadic cases, and outbreak cases were considered, as well as clinical studies in humans and NHPs, the most relevant animal models to humans anatomically and physiologically. Key studies include the following: Saslaw et al. 1961; Eigelsbach et al. 1962; Eigelsbach et al. 1968; Dahlstrand et al. 1971; Schricker et al., 1972; Martone and Marshall et al. 1979; Deverill et al. 1996; Feldman et al. 2003; Siret et al. 2006; Twenhafel et al. 2009; and Hauri et al. 2010. Also considered in development of this work plan are a consensus statement published in the medical literature (Dennis et al. 2001) and reviews by Adamovicz et al. (2006), the World Health Organization (WHO) (2007), Lyons and Wu (2007), and Sinclair et al. (2008).
- a) Pathogen
- (i) Major F. tularensis subspecies or biotypes causing human illness include:
- • Subspecies tularensis (Schu S4)
- • Subspecies holarctica (425; attenuated live vaccine strain)
- (ii) Fastidious and slow-growing bacteria requiring cysteine and sulfhydryl compounds; unlikely to grow in the environment outside of hosts and vectors
- b) Host
- (i) Describe
- • Typically occurring in previously healthy adults
- o Workers in laboratory, landscaping, hunting and trapping, agriculture (farmers, hay handlers, herders, ranchers)
- o Butchers, campers, cooks (game meats), sugar factory workers, veterinarians, walkers
- • Little knowledge for more susceptible populations
- o Outbreak data include middle aged adults and some children and elderly adults
-
- o Occasional isolations from hospitalized febrile patients with underlying conditions (neutrophil deficiency; immunosuppression due to organ transplant, cancer, HIV; or the presence of a prosthetic medical device) from endemic areas
- • Human vs. NHP data
- c) Environment
- (i) Consider factors and pathways influencing viability, infectivity, and persistence of strains in various environments
- • Factors include humidity, temperature, ultraviolet radiation exposure
- (ii) Consider representativeness of experimental conditions to hypothetical releases
- • Pathways by route
- d) Interactions (conditions necessary to cause cases or disease outbreaks)
- (i) Tularemia is endemic worldwide, and human outbreaks are often associated with outbreaks in wild animal populations from direct contact with infected or dead animals or contaminated fecal material in air or water. Tularemia is perpetuated in complex enzootic cycles between wild mammals (predominantly rodents, rabbits, and hares) and invertebrates (~50 species of arthropods including ticks, mosquitoes, flies) and amoeba
- (ii) Present state of knowledge for mechanisms of host-pathogen interactions as illustrated in Figure 1. Temporal and spatial patterns of disease and disease progression for human and zoonotic disease will be addressed.
- • Incubation period before onset of disease; time to detection in lymph nodes, blood, lungs and pleura, spleen, liver, and kidneys; influence of innate and adaptive immunity, with and without vaccination; time to death; global distribution and severity
- • Human clinical disease forms
- o Outbreaks or clustered exposures commonly ulceroglandular (frequently by tick or mosquito vectors or contact with infected animals or die-offs (e.g., voles, mice, rabbits, muskrats)
- o Less commonly oropharyngeal following ingestion of food or water contaminated by infected or dead animals or feces
- o Rare ocular associated with direct contact with infected pets or other animals or by transmittal on fingertips after handling an infected or dead animal
- o Rare pneumonic disease outbreaks from contaminated agricultural dusts (landscapers in Martha’s vineyard, Swedish agricultural workers), contaminated aerosols from infected hares among participants in a hunt in Germany, and uncertain aerosol source infecting vacationers at a renovated mill in France
- (iii) Routes of human exposure
- • Primary (inhalation; ingestion; dermal/percutaneous; ocular/mucosal)
- • Secondary (no evidence for person-to-person or monkey-to-monkey transmission)
- (iv) Describe estimated occupational exposures (for laboratory workers, hunters, landscapers, agricultural workers) and estimated exposures to community members
- • Describe bounds for exposures, e.g., U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) maximum production volume of culture slurries (20 mL per batch culture, USAMRIID 2008. Agent Information Sheet for ciprofloxacin-resistant F. tularensis)
- (v) Summarize sources of dose-response data and tabulate results by stressor (pathogen strain), population (and host), route, pathway, and endpoint causing mortality, illness, and no illness (Figure 1 for illustrative examples regarding tularemia; Appendix 1 for other agents in portfolio)
- (vi) Identify unlikely and possible scenarios
B) Problem Formulation
- a) Develop conceptual models by agent, as illustrated in Figure 2, incorporating data collection and analysis from hazard identification and other information as needed
- b) Define objectives and key variables for inclusion
- (i) LAIs
- • Rate declining. For unspecified facilities, USAMRIID reported 225 cases prior to 1976, 2 deaths; for unspecified laboratories, WHO reported declining rates of LAIs from 5.7 cases per 1,000 workers in the 1950s to 0.3 cases per 1,000 workers in the 1960s
- • Risk mitigations for workers and community (e.g., training materials identify high risk activities; personal protective equipment; laboratory containment equipment and design specifications (e.g., negative pressure); autoclaving)
- • Recent LAIs generated by uncertain errors or deviations from protocols without transmission in community
- (ii) Define stability limits in air and water
- • Short-distance aerosol pathways (plumes/puffs) may be possible (pneumonic tularemia is rare, despite endemic presence and high experimental infectivity in humans and NHPs)
- • Short-distance water-borne pathways may be possible
- • Long-term exposures unlikely
- (iii) Define boundaries for selected accidental and intentional releases as possible scenarios that are reasonably foreseeable events during operation of MCMT&EF
- c) Describe key variables (populations, routes, pathways) for exclusion and rationale
- (i) Indoor air for community
- (ii) Outdoor air for workers
- (iii) Ingestion for workers
- (iv) Dermal/percutaneous for community
- (v) Mucosal/ocular transmission for workers (in personal protective equipment [PPE]) and community
- (vi) Secondary transmission
- (vii)Vector transmission
- d) Describe inputs, outputs, data sources, data quality and quantity, methods of analysis, data gaps, and inferences, assumptions or judgments
- (i) Describe relationships in common language and in mathematical terms
C) Technical Analysis (qualitative)
- a) Conduct exposure analysis
- (i) Describe evidence on exposure routes and pathways and discuss unlikely and possible scenarios for human tularemia cases in workers and the community (populations)
- (ii) Survival and decline by pathway
- (iii) Derive boundaries for magnitude, frequency, and duration of human exposures for possible scenarios
- (iv) Provide rationale for possible and unlikely scenarios
- (v) Identify data gaps
- (vi) Other issues TBD
- b) Conduct dose-response analysis
- (i) Describe evidence for dose-dependencies, for populations, routes, pathways, and endpoints (what we know and what we don’t know about human tularemia dose-response relationships)
- • Address resistance to illness (asymptomatic illness)
- • Address susceptibility to illness (mild/moderate/severe/fatal illness); reported quantitative measures include infective doses (IDs) for exposed volunteers and IDs and or lethal doses (LDs) for animals exposed in clinical studies
- o ID50s (inhalation) in humans ~100 and in NHPs ~50
- o ID50s (ingestion) in humans and NHPs >106 and <108 (no illness in NHPs at 104)
- o LD50s (inhalation) in humans unknown and in NHPs ~50 and >106
- • Report incubation periods, duration and severity of illness
- (ii) Identify data gaps
- (iii) Other issues TBD
- c) Conduct risk characterization
- (i) Compare outputs of exposure assessment and dose-response assessment to estimate the likelihood and severity for human morbidity and mortality for possible scenarios
- (ii) Prepare narrative summaries of results
- (iii) Describe sources of uncertainty and impacts on risk estimates
- d) Prepare risk communication materials
2) Approach for Quantitative Risk Assessment
A) Problem Formulation
- a) As mentioned above, QuantRA will be employed as a second tier of analysis for scenarios first-tier qualitative assessments cannot confidently bound scenario risks as “unlikely”, and there is data to support a quantitative assessment that significantly improves the characterization of risk.
- (i) Approaches to QuantRA for complex systems can be subdivided between fine-grained and course-grained methods.
- • Fine-grained methods typically attempt to characterize risk and consequences of a scenario through detailed representations of system state changes. They are computationally intensive, require large amounts of data for parameterization and validation, and are often non-transparent because of their complexity and the platform specific aspects of their implementations.
- • Course-grained methods, sometimes referred to as semi-quantitative methods, typically use simple quantitative models that are more transparent but less exhaustive. We have determined that the course-grained approach is the preferred methodology for most of the current risk assessment for several reasons.
- b) Because of the limitations on the data available for the agents in the scope of this risk assessment and many open scientific questions regarding both the biology and the computational methodologies, we do not expect fine-grained methods to adequately reduce uncertainties in risk characterizations relative to course-grained methods.
- c) Since the primary goal of the scenario analyses will be to document scenarios and pathways with risk and consequences that will need mitigation and management, we feel the more-transparent course-grained methodologies will do the most appropriate for this assessment
B) Technical Analysis and Modeling
- a) Structure and simulate possible exposure scenarios
- (i) Estimate magnitude, frequency, and duration of human exposures based on available evidence
- (ii) Identify the patterns and distribution of health consequences for exposure scenarios
- (iii) Address uncertainties for data and impacts of assumptions
- (iv) Identify data gaps
- (v) Other issues TBD
- b) Model dose-response relationships for likelihood and severity of human and NHP illness based on key studies
- (i) Address uncertainties for extrapolations (pathogen strain, host, endpoint)
- (ii) Identify data gaps
- (iii) Other issues TBD
- c) Conduct risk characterization
-
- (i) Integrate outputs of exposure assessment and dose-response assessment to estimate the likelihood of human health effects (mortality or survival) for possible scenarios
- (ii) Prepare summaries of simulation results
- (iii) Conduct uncertainty and sensitivity analyses
- (iv) Note influential data gaps and assumptions and provide interpretation
- d) Prepare risk communication materials
- (i) Risks will be presented in the context of existing background risks for accidental zoonotic disease transmission and other risks in daily life (community and occupational risks; auto accidents, heart disease…)
- (ii) Three common concerns are often raised in the evaluation of quantitative risk assessments: the completeness/comprehensiveness of the analysis; the scrutability/transparency of the analysis for independent evaluation; and the quantification and communication of uncertainty and sensitivity in risk estimates. While an ideal risk assessment will be comprehensive, transparent, and strongly validated, practical logistic constraints must also be considered to prepare a thorough and timely analysis fit-to-purpose, in this case, appropriate to support USAMRMC decisions. We are aware of competing interests for this project, including interests and concerns of the NAS committee and the community. Our plan includes full consideration of recommendations of both groups in Section 2, as appropriate. For example, extensive literature for dose response data and models is available, only data relevant to the potential exposure will be reviewed. Rather, the team will build on published sources for existing models or key datasets judged most influential and relevant biologically for predicting human disease. Specifically, our rationale is to focus on primate data due to anatomical, physiological, and immunological similarities for deposition and clearance of agents more representative of human systems than rodents, until mechanistic models in development for anthrax (Gutting et al., 2008) and tularemia (McClellan, 2009) are available for more definitive extrapolations. The team will also focus on boundary analysis where competing assumptions are not validated experimentally. In this manner, the tiered analysis will build on what is known scientifically and acknowledge gaps that limit credible predictions
- (iii) The value of QuantRA for a biological laboratory is limited by uncertainties in the importance of many factors. This is not unusual, it has been identified as a problem in risk assessments from the nuclear and space programs since at least the 1970’s. What is unusual is that the laboratory facilities being evaluated have missions that are specifically aimed at reducing these uncertainties. To reduce uncertainty, laboratories are needed which may contribute to the very risk we are seeking to manage. This is not an irreconcilable issue, but a reality needing acknowledgement
Working documents and results of analyses will be available to all team members on project File Transfer Protocol (FTP) site. One team member will draft sections and analyses, and a different team member will review the draft for accuracy and transparency. Final reports will be reviewed by the team prior to other quality control checks overseen by BSA Environmental Services Inc.
A) Quality will be ensured in each step
- a) Citation of scientific metrics (and their limitations) from epidemiologic and clinical literature, with particular emphasis on body of literature from LAIs
- b) Rationale for qualitative and quantitative analyses (inclusions and exclusions)
- c) Clear identification of assumptions and expert opinions where direct scientific evidence is lacking or weak
B) The work plan, intermediate results, and final reports will be provided to USAMRMC as scheduled
4) Milestones and Deliverables
The following work plan summary is proposed for assessing progress toward completion of final report to USAMRMC.
| Stage | Deliverable or Section | Specific Tasks/Activities |
| Planning and Scoping | Work Plan Conceptual Model |
|
| QualRA |
Problem Formulation
Hazard Identification Exposure Analysis Dose-Response Analysis Risk Characterization Interim Report |
|
| Risk Communication |
|
| QuantRA |
Technical Analysis
Exposure Analysis Dose-Response Analysis Risk Characterization Interim Report |
|
| Risk Communication |
|
|
| SSRA | Final Report |
|
| Additional Cycles of Analysis and Deliberation |
|
Table 2. Major Elements of Analysis Plan (Box 3-4, NRC 2008)
| Sources |
Obtaining and analyzing information on the sources in the analysis (e.g., source location, important release parameters)
|
| Agent (Pollutants) |
Confirming agents of interest and estimating potential exposure values Agent list for current portfolio for medical countermeasure T&E
|
| Exposure pathways and routes |
Assessing exposure pathways and ambient exposures
|
| Exposed populations(s) |
Characterizing populations of interest and estimating exposures including temporal and spatial variables
|
|
and spatial boundaries derived from available literature
|
|
| End points (morbidity, mortality) |
Proposed sources of evidence on pathogenicity and virulence of agents and risk metrics
|
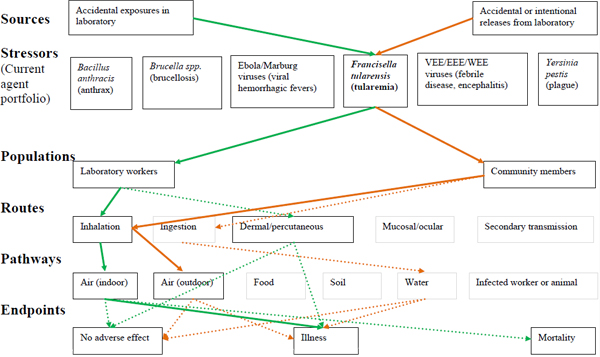
Figure 1. Illustration of the scope of the MCMT&EF SSRA with example pathways for tularemia
Figure 1 legend. The scope of the MCMT&EF SSRA is illustrated, as adapted from Science and Decisions (NRC, 2008; Figure 3-2). Lines linking boxes represent example linkages for tularemia scenarios. Solid lines indicate scenarios for recent observations of LAIs and outbreaks, dashed lines indicate possible scenarios, and boxes without connecting lines indicate unlikely scenarios that are excluded from quantitative analysis. Rationale will be provided in QualRA section of the SSRA report for the scenarios considered and excluded. Sources are indicated by green lines for accidental exposures in laboratories and orange lines for accidental or intentional releases from the laboratory. Stressors are the current agent portfolio. Populations are laboratory workers or community members, as will be discussed in detail in the hazard identification of the SSRA report. Routes are agent specific and include primary (inhalation, ingestion, dermal/percutaneous, mucosal/ocular) and secondary transmission. Pathways are agent-specific and include air, food, soil, water, and an infected worker or animal. Endpoints include no adverse effect, illness, or mortality.
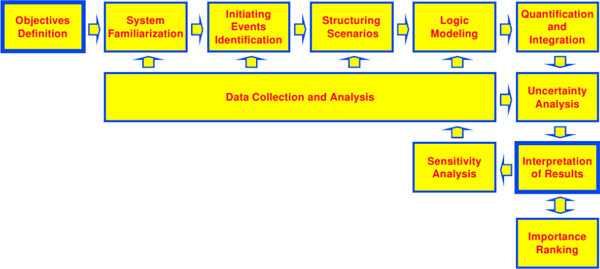
Figure 2. Typical probabilistic risk assessment task flow (Figure 3-13; NASA Probabilistic Risk Assessment Procedures Guide for NASA Managers and Practitioners.) The work flow of a probabilistic risk assessment is a cyclic process. Once the objectives and perspectives of the PRA are defined, the first step is a period of familiarization with the system under study. This familiarization period is needed to assist in the identification of initiating events that will be the risk assessment. For each initiating event, scenarios are structured, and then modeled as sets of logical pathways leading up to a consequential event and determining the consequences that follow. The likelihoods and impacts of these pathways are then quantified and integrated to determine risk under the preferred metrics, and the uncertainty of these risk metrics is documented based on the pathway identification and quantification. All of these steps incorporate data collection and analysis in various forms. The risks determined for each scenario are then interpreted and critiqued. The sensitivity of the results to the model assumptions should be considered, potentially initiating another round of initiating-event identification and scenario analysis. When needed, risks may be rank in terms of importance to assist in action and decision prioritization.
This page intentionally left blank.
Adamovicz J.J., Wargo E.P., Waag D.M. 2006. Chapter 9: Tularemia. In: Biodefense Research Methodology and Animal Models. J.R. Swearengen, ed. Taylor & Francis, New York. 137-162.
Dahlstrand S., Ringertz O., Zetterberg B. 1971. Airborne tularemia in Sweden. Scand J Infect Dis 3:7-16.
Dempsey, M. P., Nietfeldt, J., Ravel, J., Hinrichs, S., Crawford, R., Benson, A.K. 2006.
Paired-end sequence mapping detects extensive genomic rearrangement and translocation during divergence of Francisella tularensis subspecies tularensis and Francisella tularensis subspecies holarctica populations. J Bacteriology 188:5904-14.
Dennis D.T., Inglesby T.V., Henderson D.A., Bartlett J.G., Ascher M.S., Eitzen E., Fine A.D., Friedlander A.M., Hauer J., Layton M., Lillibridge S.R., McDade J.E., Osterholm M.T., O’Toole T., Parker G., Perl T.M., Russell P.K., Tonat K. 2001. Consensus Statement: Tularemia as a Biological Weapon. JAMA 285:2763-73.
Deverill, A.P., Fred, M.R., Groton, W., Anno, G., Sanemitsu, S., McClellan, G.E. 1996.
The effect of tularemia on human performance. Defense Nuclear Agency.
Eigelsbach H.T., Tigertt W.D., Saslaw S., Mccrumb F.R. 1962. Live and Killed Tularemia Vaccines: Evaluation In Animals and Man. Army Biological Labs, Frederick, MD.
Eigelsbach H.T., Saslaw S., Tulis J.J., Hornick R.B. 1968. Tularemia: monkey as a model for man. Use of Nonhuman Primates for Drug Evaluation, Symposium. 230-48.
Feldman K.A., Stiles-Enos D., Julian K., Matyas B.T., Telford S.R., Chu M.C., Petersen L.R., Hayes E.B. 2003. Tularemia on Martha’s Vineyard: Seroprevalence and Occupational Risk. Emerging Infectious Diseases 9:350-4.
Gutting B.W., Channel S.R., Berger A.E., Gearhart J.M., Andrews G.A., Sherwood R.L., Nichols T.L. 2008. Mathematically modeling inhalation anthrax. Microbe 3(2)78-85.
Hauri A.M., Hofstetter I., Seibold E., Kaysser P., Eckert J., Neubauer H., Splettstoesser W.D. 2010. Investigating an airborne tularemia outbreak, Germany. Emerg Infect Dis 16(2):238-243.
International Life Sciences Institute. 2000. Revised Framework for Microbial Risk Assessment, An ILSI Risk Science Institute Workshop Report. ILSI Press, Washington. Lyons C.R., Wu T.R. 2007. Animal models of Francisella tularensis infection. Annals of the New York Academy of Sciences 1105 (Francisella tularensis: Biology, Pathogenicity, Epidemiology, and Biodefense) pp. 238-265.
Martone W.J., Marshall L.W., Kaufmann A.F., Hobbs J.H., Levy M.E. 1979. Tularemia pneumonia in Washington, DC. A report of three cases with possible common-source exposures. JAMA 242:2315-2317.
McClellan G.E. 2009. An Improved Model of Human Response to Aerosol Chemical and Biological Agent Hazards. Chemical and Biological Defense Science & Technology Conference. (available at: http://cbdstconf.sainc.com/pdfs%5CWednesday_12_0930_McClellan.pdf).
National Academy of Sciences Committee Reports.
National Research Council. 2009. Science and Decisions: Advancing Risk Assessment. National Academies Press, Washington, DC. 424 p.
Saslaw S., Eigelsbach H.T., Prior J.A., Wilson H.E., Carhart S. 1961. Tularemia Vaccine Study II. Respiratory Challenge. Archives of Internal Medicine 107:702-14.
Schricker R.L., Eigelsbach H.T., Mitten J.Q., Hall W.C. 1972. Pathogenesis of tularemia in monkeys aerogenically exposed to Francisella tularensis 425. Infection and Immunity 5:734-44.
Sinclair R., Boone S.A., Greenberg D., Keim P., Gerba C.P. 2008. Persistence of Category A Select Agents in the Environment. Appl Environ Microbiol 74(3): 555–563.
Siret V., Barataud D., Prat M., Vaillant V., Ansart S., Le Coustumier A., Vaissaire J., Raffi F., Garre M., Capek I. 2006. An outbreak of airborne tularaemia in France, August 2004. Euro Surveillance. 11(2):58-60.
Twenhafel N.A., Alves D.A., Purcell B.K. 2009. Pathology of inhalational Francisella tularensis spp. tularensis SCHU S4 infection in African green monkeys (Chlorocebus aethiops). Vet Pathol 46(4):698-706.
USAMRIID Agent Information Sheets.
World Health Organization. 2007. Tularemia.
| CDC | Center for Disease Control |
| EEE | Eastern Equine Encephalitis |
| EIS | Environmental Impact Statement |
| FTP | File Transfer Protocol |
| ID | infectious dose |
| ILSI | International Life Sciences Institute |
| LAIs | laboratory acquired infections |
| LD | lethal dose |
| MCMT&EF | Medical Countermeasures Testing and Evaluation Facility |
| NAS | National Academy of Sciences |
| NHP | non-human primates |
| NRC | National Research Council |
| PPE | personal protective equipment |
| QualRA | Qualitative Risk Assessment |
| QuanRA | Quantitative Risk Assessment |
| SSRA | Site-specific Risk Assessment |
| USAMRIID | U.S. Army Medical Research Institute of Infectious Diseases |
| USAMRMC | U.S. Army Medical Research and Materiel Command |
| VEE | Venezuelan Equine Encephalitis |
| WEE | Western Equine Encephalitis |
| WHO | World Health Organization |
This page intentionally left blank.
This page intentionally left blank.
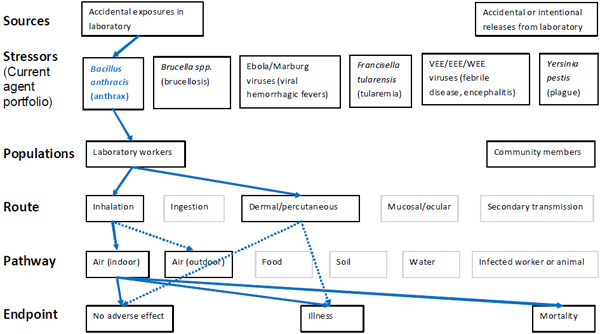
Figure A-1. Scope of microbial risk assessment for current portfolio of agents planned for MCMT&EF. For anthrax from accidental exposures in the laboratory, pathways are identified by solid blue lines for observed exposures in recent laboratory associated infections (LAIs) and by dashed blue lines for possible exposures. Unlikely scenarios are excluded (gray text box borders). Supporting evidence and rationale will be provided for all pathways in the qualitative risk assessment section.
This page intentionally left blank.
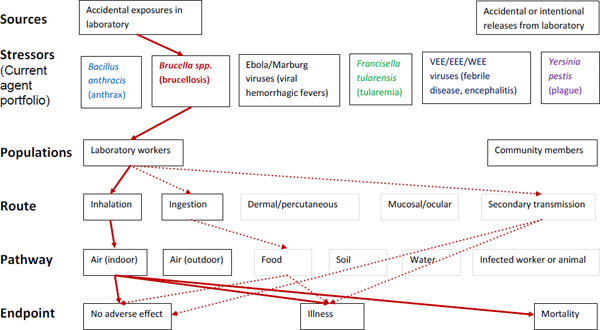
Figure A-2. Scope of microbial risk assessment for current portfolio of agents planned for MCMT&EF. For brucellosis from accidental exposures in the laboratory, pathways are identified by solid red lines for observed exposures in recent laboratory associated infections (LAIs) and by dashed red lines for possible exposures. Unlikely scenarios are excluded (gray text box borders). Supporting evidence and rationale will be provided for all pathways in the qualitative risk assessment section.
This page intentionally left blank.
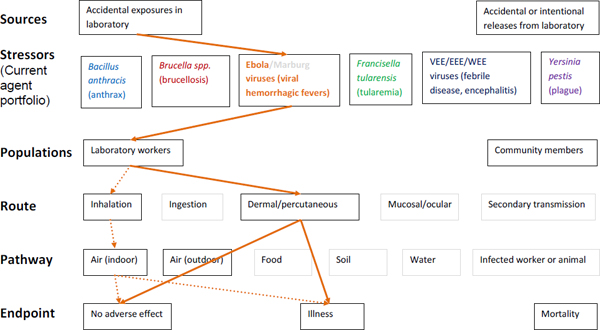
Figure A-3. Scope of microbial risk assessment for current portfolio of agents planned for MCMT&EF. For Ebola infections from accidental exposures in the laboratory, pathways are identified by solid orange lines for observed exposures in recent laboratory associated infections (LAIs) and by dashed orange lines for possible exposures. Unlikely scenarios are excluded (gray text box borders). Supporting evidence and rationale will be provided for all pathways in the qualitative risk assessment section.
This page intentionally left blank.
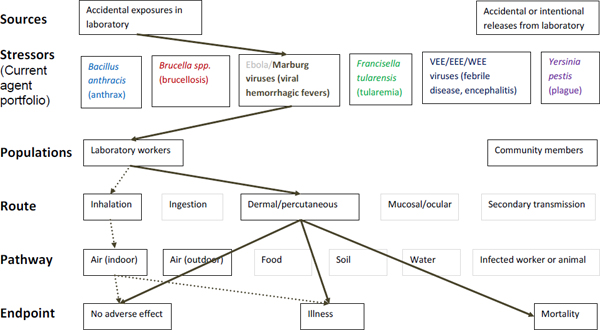
Figure A-4. Scope of microbial risk assessment for current portfolio of agents planned for MCMT&EF. For Marburg infections from accidental exposures in the laboratory, pathways are identified by solid brown lines for observed exposures in recent laboratory associated infections (LAIs) and by dashed brown lines for possible exposures. Unlikely scenarios are excluded (gray text box borders). Supporting evidence and rationale will be provided for all pathways in the qualitative risk assessment section.
This page intentionally left blank.
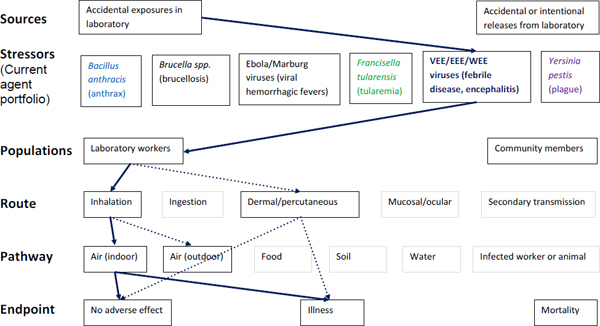
Figure A-5. Scope of microbial risk assessment for current portfolio of agents planned for MCMT&EF. For encephalytic infections from accidental exposures in the laboratory, pathways are identified by solid dark blue lines for observed exposures in recent laboratory associated infections (LAIs) and by dashed dark blue lines for possible exposures. Unlikely scenarios are excluded (gray text box borders). Supporting evidence and rationale will be provided for all pathways in the qualitative risk assessment section.
This page intentionally left blank.
Figure A-6. Scope of microbial risk assessment for current portfolio of agents planned for MCMT&EF. For plague infections from accidental exposures in the laboratory, pathways are identified by solid purple lines for observed exposures in recent laboratory associated infections (LAIs) and by dashed purple lines for possible exposures. Unlikely scenarios are excluded (gray text box borders). Supporting evidence and rationale will be provided for all pathways in the qualitative risk assessment section.






































