4
Education and Training of a Regulatory Science Workforce
Key Messages
• Some successful regulatory science education and training programs offer a menu of educational opportunities that build core competencies while allowing participants to focus on aspects of regulatory science relevant to their specific areas of interest.
• Multiple levels of recognized training could be employed, including professional certificates, master’s degrees, doctoral degrees, and fellowships and rotations that blend work and training.
• A broad range of training and fellowship programs are available at FDA and within other agencies that create opportunities for scientists at all career stages to become more well versed in regulatory science and to have careers in regulatory science.
Defining a workforce that, taken as a whole, fosters the core competencies in regulatory science calls for a concerted effort to educate and train the existing and next generation workforce. The workshop discussions on education and training sought to identify current gaps and specific opportunities, including collaborative approaches, to strengthen the education and training of a regulatory science workforce. The workshop also examined barriers to implementing education and training strategies and potential ways to overcome these barriers.
Carl Peck, UCSF, presented an overview of certain existing training programs in regulatory science. Emma Meagher, Director of Translational Research Education, University of Pennsylvania Perelman School of Medicine, and Annette Mollet, Head of Training and Education, European Center of Pharmaceutical Medicine (ECPM), University of Basel, discussed the necessary components of an effective education and training strategy and how to develop those components. A panel discussion focused on fellowship and exchange programs.
AN OVERVIEW OF EXISTING TRAINING PROGRAMS1
Training Opportunities Within FDA
FDA is the major locus of regulatory research and training in the United States, Peck observed. Peck noted that the so-called “Subpart E Regulation,” passed by Congress in 1989, included a provision that gave FDA authority to conduct regulatory research: “At the discretion of the agency, FDA may undertake focused regulatory research on critical rate-limiting aspects of the preclinical, chemical/manufacturing, and clinical phases of drug development and evaluation.”2 Regulatory research is an inherent component of FDA’s activities, and the agency has contributed to the advancement of the drug development field through its work with sponsors to advance development and evaluation of products more rapidly. FDA advancements span a wide range and include, for example, evaluation of diagnostic biomarkers for HIV/AIDS. FDA has innovated in clinical trial design and in modeling and simulation. FDA’s Critical Path Initiative also has led to the establishment of numerous consortia for advancing the development of predictive safety biomarkers and advanced physiologically based modeling. Over the past 3 years, FDA scientists have published more than 1,000 papers.3
Peck commented that FDA has created a university-like environment that generates both debate and important science. And, as with any good university, FDA has developed strong training programs, according to
________________
1This section is based on the presentation by Carl Peck, Professor of Pharmacology and Medicine, UCSF. The section is intended to offer a broad but brief look at certain regulatory research and training opportunities currently available. It is not necessarily complete or exhaustive.
221 CFR § 312.86, Focused Regulatory Research, available at http://www.gpo.gov/fdsys/pkg/CFR-2011-title21-vol5/pdf/CFR-2011-title21-vol5-sec312-86.pdf (accessed November 28, 2011).
3Vicki Seyfert-Margolis, Senior Advisor for Science Innovation and Policy, Office of the Commissioner, FDA, commented that, as of September 2011, there was a total FDA workforce of 13,800.
Peck. The Center for Drug Evaluation and Research (CDER) Staff College includes over 50 courses covering a wide range of graduate-level subjects. A committee on advanced scientific education certifies these courses and the faculty members who teach them. CDER also has a program that has defined the competencies necessary for regulatory scientists working at FDA who review drug applications and provide counsel to drug developers. In 2011, FDA expanded this latter effort and created the CDER Federated Training Model, which has published a public list of the competencies expected by discipline.4 The agency has developed an outreach program to educate consumers, clinical investigators, and other key constituencies. In addition, FDA has established the Commissioner’s Fellowship Program (CFP), a 2-year program for academics to come to FDA, learn the craft of regulation, and become engaged in a research project.
Additional information about FDA fellowships and training opportunities is provided below.
Training Opportunities Outside FDA
Peck described a number of courses offered outside of FDA, most of which fall within the category of regulatory affairs (rather than innovative regulatory science or regulatory research).5 Specific training programs in regulatory research are rare, said Peck, though many universities have produced new methodologies and good scientists who have contributed to advances in regulatory science. These advances have come largely from pharmaceutical science departments at schools of pharmacy in the United States and Europe, clinical pharmacology research fellowships, clinical investigator fellowships, and some NIH programs (such as the year-long clinical pharmacology course that has a regulatory framework), as well as the University of Liverpool CDSS and the UCSF Center for Drug Development Science, he said.
ECPM has a two-decade history of developing and offering sophisticated courses in drug development science and regulatory science. An offshoot of this effort is the American Course on Drug Development and Regulatory Sciences offered by UCSF, which was launched in 2007 and modeled after the program developed by ECPM. It is offered by the UCSF Department of Bioengineering and Therapeutic Sciences and operates with substantial input from FDA, other universities, and industry. This
________________
4See http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ManualofPoliciesProcedures/UCM263365.pdf (accessed November 28, 2011).
5Both the Regulatory Affairs Association and Drug Information Association have a large catalog of courses on specific issues pertaining to regulatory affairs.
intensive, 2-year course is given at the UCSF Mission Bay campus and in Washington, DC, with the participation of 120 faculty members drawn largely from industry and FDA. The course has been given three times in Washington and twice in San Francisco. The course consists of six “sessions” (each containing 4 days of lectures and case studies), and a 3-hour final exam.
Master’s degree programs offering a research training component include the Regulatory Science Program at the University of Southern California (USC), as well as similar programs at Temple University, the University of Maryland, and the Liverpool CDSS. A participant added that USC School of Pharmacy has developed international M.S., Pharm.D./M.S., and D.R.Sc. (Doctorate in Regulatory Science) programs, with an initial three doctoral students graduating in 2011.
DEVELOPING EDUCATION AND TRAINING PROGRAMS IN REGULATORY SCIENCE6
Meagher described an approach to developing education programs in regulatory science that leverages what has been learned in the development of training programs for translational scientists, and this model for regulatory science program development is being refined and adopted by all of the institutions that are part of the CTSA network.
The target audience for such an education program is broad, and Meagher noted that it is necessary to break out of the mindset that regulatory science resides totally with FDA and that the field’s obligation is to create a workforce that will function within the confines of FDA. Regulatory science is a collaborative effort that goes beyond FDA.
To better identify their audience, the Department of Translational Research Education at the University of Pennsylvania Perelman School of Medicine surveyed the many constituencies that have a stake in regulation and drug development. Meagher noted the following findings from the survey:
• Geographic location should not limit opportunities for educational encounters or for training programs.
• Attrition is a significant problem; the lack of a defined career path and professional recognition are the major reasons for the high rate of attrition the field is experiencing.
• Different groups have differing definitions of regulatory science; curricula need to be flexible and heterogeneous although still integrated.
________________
6This section is based on the presentation by Emma Meagher, Director of Translational Research Education, University of Pennsylvania Perelman School of Medicine.
Critical needs for a regulatory science training program include understanding research and scientific methodology, pharmacology, toxicology, therapeutics, and the science that underpins the regulatory process. Competency areas include
• Biostatistics, decision theory, and information technology
• Fundamentals of pharmacology
• Scientific methodology
• Clinical trial design
• Drug and device discovery and development
• Clinical research
• Monitoring and quality assurance
• Food, drug, and device law and regulation
Effective training programs will incorporate rapidly changing science. As new technologies drive the development of many technological platforms capable of evaluating drugs efficiently, training programs could adapt and evolve to incorporate understanding of these new technologies and how they might be used in a regulatory setting.
Meagher suggested that programs include both professional certificate programs that would be suitable for such professionals as quality assurance specialists, regulatory coordinators, research nurses, project managers, research directors, and lawyers, as well as master’s degree programs that would be suitable for FDA scientists, investigators, research directors, and lawyers. These types of tangible achievement-oriented programs help define a career path for professionals interested in regulatory science. The survey found, too, that most professionals prefer part-time programs that enable them to mix work responsibilities and interests with training opportunities; they prefer to be trained while remaining a part of the workforce.
Incorporation of opportunities to create and participate in internships outside of the university setting is a valuable component of a training program because it offers research training opportunities at FDA and within the pharmaceutical and biotechnology industries.
Evaluation will help determine if the programs are valuable and are meeting the needs of the stakeholders in regulatory science. Metrics would assess whether training increases the ability of the research workforce to meet the needs of the regulatory science initiative, whether the programs create a viable career structure, and whether the training improves the quality of research management. At the individual level, metrics can show if a program enables a student to demonstrate knowledge in core concepts and to apply that knowledge through completion of a mentored project designed to enhance the individual’s professional abilities.
MODELS FOR EDUCATION AND TRAINING7
In 1999 Europe established the Bologna Process to harmonize higher education across the continent. Curricula for training developed within the Bologna system include programs for bachelor’s and master’s degrees as well as postgraduate training leading to a diploma of advanced studies, a master’s of advanced studies, and a Ph.D. The Bologna system allows students mobility and flexibility to complete different training modules at different universities. The system includes common quality assurance standards for continuing professional education and training, which provides employers with the means to assess the quality of training prospective employees have received regardless of where they received their degree.
ECPM established a three-tier modular approach through its training curricula that were originally founded in 1991. Over the past 20 years, ECPM’s program has trained more than 1,200 participants from 31 countries, with current enrollment standing at 147 participants. Like the UCSF program, there are multiple modules consisting of 4 days of training—3 days that cover state-of-the-art drug development science and 1 day that addresses current hot topics.
The Innovative Medicines Initiative (IMI) Training Excellence Programme is a large 5-year collaborative program run jointly by the European Union (EU) and the European Federation of Pharmaceutical Industries Association in partnership with academia and regulatory agencies. Funding for the IMI, which totals 2 billion euros (with 1 billion euro contribution from industry and 1 billion euro contribution from the EU), pays for training and collaborative research projects that cover safety, efficacy, education and training, and knowledge management. The following four education and training programs currently receive IMI funding: the European Medicines Research Training Network, the European Modular Education and Training Programme in Safety Sciences for Medicine, the European Programme of Pharmacovigilance and Pharmacoepidemiology, and the Pharmaceutical Medicine Training Programme (PharmaTrain).
PharmaTrain was started in May 2009 with a goal of harmonizing the syllabus for training, teaching, and examination across the European Union. Currently, 25 universities, 13 learned societies, and 15 companies participate in PharmaTrain. PharmaTrain has established a three-tier postgraduate track that builds core competencies through a set of base courses, creates expertise through a series of extension modules, and
________________
7This section is based on the presentation by Annette Mollet, Head of Training and Education, ECPM, University of Basel.
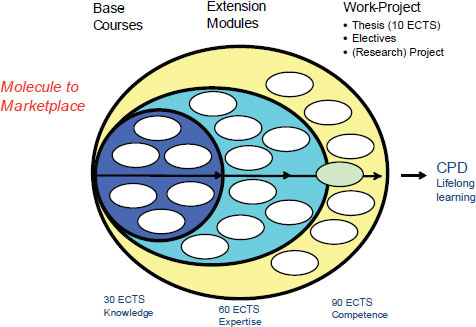
NOTE: CPD, continued professional development; ECTS, European Credit Transfer and Accumulation System.
SOURCE: Mollet, 2011. Presentation at IOM workshop on Strengthening a Workforce for Innovative Regulatory Science in Therapeutics Development.
develops expertise through electives and research experience (Figure 4-1). Students can choose to stop upon completion of each level, and they can return for further modular education and training at a later date. A mechanism for lifelong learning or continued professional development also exists.
FELLOWSHIPS AND EXCHANGE PROGRAMS
Fellowships and Exchange at FDA
Leslie Wheelock, Director, Office of Scientific Professional Development, Office of the Chief Scientist, FDA, described FDA’s CFP. This 2-year training fellowship program, which began in October 2008, has three primary goals: attracting scientists to FDA, training scientists in regulatory science, and retention of those scientists at FDA. FDA has recruited up to 50 fellows annually. The fellows are hired into the Office of the Chief
Scientist and then placed with preceptors across the agency. During the 2-year program, fellows complete about 210 hours of classroom training in four content areas, including content specific to the center and office in which they will carry out their research projects. Each project is identified by the centers as being of critical importance to address a scientific regulatory issue. Wheelock noted that FDA promotes this program widely to academic institutions and trade organizations in recognition that the agency presents a nontraditional career path for scientists calling for broad promotion and efforts to increase understanding and familiarity with science careers at FDA.
Among the first class of fellows, which graduated in fall 2010, all 48 fellows completed the program, and 38 accepted permanent positions at FDA (a retention rate of 79 percent), according to Wheelock. Five of the fellows took positions in industry, putting them in a position to serve as “ambassadors” between industry and FDA. The remaining fellows returned to academia.
Uros Djekic, Senior Regulatory Reviewer/Policy Analyst, CBER, FDA, provided the perspective of someone who participated in the CFP (as a member of the inaugural group of fellows from 2008 to 2010). He described the CFP as a collaborative paradigm for supporting regulatory science. Fellows apply their scientific expertise to a specific project directed by a “sponsor” at FDA. The fellow and sponsoring FDA staff member commit to finish the project during the 2 years in which the fellow continues to receive training. At the moment, he said, there are only about 30 fellows—a number limited by funding, not by opportunity. About half of the projects, he noted, are laboratory based, while the other half are examining issues of regulatory policy. A list of past, current, and proposed projects is available on FDA’s website.8 Example projects include
• Djekic’s project, which looked at policy issues involving over–the-counter HIV tests, particularly concerning the clinical trials that would be necessary for such products to receive regulatory clearance.
• A project to develop the proposed International Consortium of Orthopedic Registries.
Carolyn Wilson, of FDA’s CBER, added that FDA’s staff fellowship program is an umbrella program that incorporates the CFP as well as two
________________
8See http://www.fda.gov/AboutFDA/WorkingatFDA/FellowshipInternshipGraduateFacultyPrograms/CommissionersFellowshipProgram/default.htm (accessed November 28, 2011).
additional tracks: (1) a support-scientist fellowship that starts as a 4-year postdoctoral fellowship, with the possibility of extension of an additional 3 years and opportunity to be considered for conversion to a permanent support scientist; and (2) a senior staff fellowship track that recruits investigators to develop their own independent research, also with the opportunity after a 7-year period to be considered for permanent hiring as a senior investigator. These positions require that the fellows spend about half of their time engaged in regulatory review activities. In that regard, the fellows engage in all of the same activities that full-time reviewers do, including conducting inspections, reviewing submissions, participating in advisory committees, writing guidance documents, and developing policy. FDA also offers more traditional postdoctoral fellowships that can last up to 5 years, which do not include any regulatory duties.
CTP has created a Tobacco Regulatory Science Fellowship Program, which Laurence Deyton, FDA, described. The program, which is CTP’s first initiative to help build its workforce, is designed to incorporate the best practices of the FDA’s CFP, the NCI’s Cancer Prevention Fellowship, and others. The fellowship will involve a core curriculum related to regulatory science and FDA operations. It also will include a research component that will require fellows to take charge of projects. CTP hopes to attract midcareer professionals for the first few of the program’s cycles to develop a cadre of experienced professionals who then can advise CTP on how best to build and expand its efforts while also serving as mentors.
Kate Ahlport, Executive Director, Health Research Alliance (HRA), described two new collaborative models at FDA that grew out of the HRA. The HRA is a national consortium of 48 nonprofit, nongovernmental funders of biomedical research and training that are interested in maximizing the impact of the nation’s investment in biomedical research. The HRA has decided to fund the two new initiatives at FDA to further its interest in and support for regulatory science:
• New Frontiers in Science Distinguished Lectureship Program. The purpose of this program is to bring scientific expertise to the agency in the priority areas identified in FDA’s strategic plan for regulatory science in the form of quarterly guest lecturers. Lecturers will spend 1 to 3 days at FDA giving seminars, meeting with staff, and providing tutorials in their area of scientific expertise. Lecturers will receive an honorarium and reimbursement of travel expenses.
• Distinguished Scholar’s Pilot Program (proposed). A distinguished scholar’s pilot program is in the discussion and planning stage. This program would be similar to the FDA’s CFP but focused on senior-level scholars who would be selected competitively to
spend up to a year at FDA and work on special regulatory science projects that cut across disease areas. It is expected that benefits will flow both directly to FDA (in the exchanges with scholars) and to the broader scientific community (as scholars bring back to their home institutions or companies a new level of knowledge and understanding of FDA).
Fellowships and Exchange Sponsored by NIH
Juan Lertora, Director, Clinical Pharmacology Program, NIH Clinical Center, described the NIH Clinical Center’s Rotations for Clinical Research Fellows at FDA. This program identifies scientists from the NIH Clinical Center for placement in short-term rotations in FDA’s Office of Clinical Pharmacology or the Office of New Drugs. The rotations last a minimum of 8 weeks and provide learning experiences focused on the issues that would enable fellows to file an IND application. Fellows are assigned to mentors at FDA in their areas of interest, and they participate in the review of preclinical and clinical data on investigational drugs. They also attend specialized therapeutic team meetings, participate in IND 30-day safety review approvals and in meetings with sponsors, and enroll in a curriculum of educational modules and courses at FDA. Since the program’s inception in 2008, 10 fellows from a number of NIH institutes and the Clinical Center have completed rotations. Three of the initial 10 rotating fellows have since joined the Office of New Drugs as medical review officers, said Lertora.
Jonathan Wiest, Director for Training and Education, NCI, NIH, described a postdoctoral training program that emerged from work of the FDA-NCI Interagency Oncology Task Force (IOTF). The IOTF Joint Fellowship Program, which is a direct collaboration between the NCI Director’s Office and the FDA Commissioner’s Office, aims to increase the number of reviewers capable of handling the large number of cancer drugs that are moving through the development pipeline by recruiting individuals trained in cancer biology into the program. A second program goal is for some fellows to go to industry, where they can build awareness of regulatory requirements into the early stages of the product development process and improve planning throughout the research and regulatory review process.
Four types of fellowships are included in the program.9 NCI funds the program and develops a training plan for each fellow. FDA provides the mentors and the regulatory training opportunities. Fellows are required to take courses in drug law, reviewer training, statistics, and clinical trial design. Depending on background and experience, fellows also take classes on risk assessment and risk management, good manufacturing practice and good laboratory practice, technical writing, presentation skills, IND regulations, and NDA regulations. Fellows also participate in the review process, with at least 50 percent of a fellow’s time being spent on research under the supervision of a mentor. Mentors also must meet certain requirements, including having an active regulatory research program, evidence of productivity, and a record of outstanding mentoring.
Currently, there are 12 fellows, with 4 getting ready to transition out, said Wiest. Two of these fellows will be staying at FDA, and one will be joining a biotechnology company. Twelve past fellows work at FDA, four have joined pharmaceutical companies, one joined NCI, one went into consulting, and one works in the health care industry.
________________
9The first two programs are targeted to cancer researchers with an M.D. or M.D./Ph.D.; one offers a medical oncology residency (for up to 3 years) and the other is for board-certified or board-eligible oncologists (for up to 1 year). The second two are targeted to M.D. scientists; one is for basic scientists and molecular biologists (for up to 2 years) and the other is a cancer prevention fellowship (for up to 3 years).
This page intentionally left blank.












