5
Career Paths Within Academia and Industry
Key Messages
• The lack of defined funding and other career support mechanisms for regula-tory scientists presents challenges to attracting qualified candidates to the field.
• Though regulatory science is a multidisciplinary field with a broad set of core competencies, it may be a more effective career path for a scientist-investigator to associate with a particular established discipline, which can provide the means for obtaining funding, publication, and recognition needed for a suc-cessful academic career.
• Regulatory scientists can fill a gap in expertise at their home academic insti-tution, offering the opportunity to demonstrate the importance of regulatory science and build institutional support for the field.
Attracting talent to the field of regulatory science requires that there be solid career paths for regulatory scientists. The workshop examined career paths and career development opportunities, both within and outside of academia, that are currently available—or that would need to be available—to strengthen and support regulatory science in therapeutics development. William Chin, Executive Dean for Research, Harvard Medical School; David DeMets, Professor, Department of Biostatistics and Medical Informatics, University of Wisconsin-Madison; and Kathy
Giacomini, Professor and Co-Chair, Department of Bioengineering and Therapeutic Sciences, UCSF, presented their ideas on academic career paths for regulatory scientists. Henrietta Ukwu, Senior Vice President for Global Regulatory Affairs, PPD, Inc., then offered comments on regulatory science career paths in industry.
Barriers and Opportunities in Academia1
Given the lack of established programs in regulatory science in academia, this gap could be addressed by increasing opportunities for exchanges among academia, industry, and government, as described in this chapter and in Chapter 7. Such collaborative approaches would support an ecosystem that will foster the development of career paths within all three sectors. Chin listed questions for consideration in defining a regulatory science career path:
• Is there a clear definition of the field?
• Are tools and technologies available to answer research questions?
• Are multiple training options available that involve innovative research?
• Who are the role models?
• Is the career track clear, and is there a clear path for professional development and promotion in an academic home?
• What is the availability and sustainability of research funding?
• Are academic societies and publications available that provide opportunities for impact and recognition?
• Are alternative career pathways available?
The biggest barrier to the development of an academic discipline is that the nature of academia does not lend itself to a regulatory mindset, said Chin. Furthermore, the unsupportive funding climate makes it unlikely that many universities would commit the resources needed to create the necessary educational and research programs that would cross disciplinary boundaries.
There are, however, opportunities to associate regulatory science with areas that are getting support, such as translational science and therapeutics, or with rapidly developing fields whose progress eventually will depend on good regulatory science, including personalized medi-
________________
1This section is based on the presentation by William Chin, Executive Dean for Research, Harvard Medical School.
cine, regenerative medicine, and gene therapy. For example, biologically inspired engineering is an emerging discipline that applies biological principles to develop new engineering solutions that meet real-world needs, with potential to produce tissues-on-a-chip that accelerate drug development and replace animal tissues. Regulatory science advances are needed to advance development of such devices as drug development tools. Moreover, associating regulatory science with such scientific fields can help raise the visibility of the discipline and ultimately overcome the barriers, such as lack of acceptance and credibility, impeding the development of regulatory science as an academic discipline.
Issues Confronting Academic Regulatory Scientists2
DeMets offered remarks geared toward establishing credibility for expertise in regulatory science in academic institutions. Because regulatory science is inherently multidisciplinary, it is unreasonable for any one person to be well versed in all the involved fields of science. Given that the structure of academic institutions is still based overwhelmingly on single disciplines, investigators with an interest in regulatory science should structure their approach to research in such a way as to put an established discipline, such as biostatistics, at the center of their work. A key to this approach is to find problems in a given field that tie directly into regulatory science. As an example, DeMets discussed several important and interesting gaps in biostatistics, such as the need for tools for comparative effectiveness research or for assessing composite and surrogate outcomes.
DeMets also noted that most universities have a real need for expertise in regulatory science even if they do not acknowledge it. Few universities have faculty who are well versed in regulatory requirements and guidelines. Being the expert who joins research teams can be one way to build support for regulatory science in academic institutions. Demonstrating the value of such expertise can then open the door to creating training opportunities for other members of a multidisciplinary research team, which in turn can help start the process of institutionalizing regulatory science.
Building a Home for Regulatory Science in Academia3
Giacomini provided observations about the relationship between the disciplines of regulatory science and translational medicine and thera-
________________
2This section is based on the presentation by David DeMets, Professor, Department of Biostatistics and Medical Informatics, University of Wisconsin-Madison.
3This section is based on the presentation by Kathy Giacomini, Professor and Co-Chair, Department of Bioengineering and Therapeutic Sciences, UCSF.
peutic sciences and, in that context, presented a case study from her own institution illustrating the challenges of recruiting and retaining regulatory scientists. She commented that the already-defined core competencies for translational medicine and therapeutic sciences can provide a framework in which would reside a subset of competencies needed for the regulatory sciences. As with any inherently broad-based field, such as systems pharmacology or pharmacogenomics, the field comprises multiple core competencies, but each regulatory scientist would acquire a deeper understanding in a defined, smaller area, and that discipline would serve as the base for developing an academic career.
Giacomini cited an example from her home department, the UCSF Department of Bioengineering and Therapeutic Sciences, a multidisciplinary department that came about through the merger of three departments spanning pharmacy, biopharmaceutical sciences, and bioengineering. The department confronted challenges recruiting and retaining a regulatory scientist for an identified position. In recruiting they found that the pipeline for training regulatory scientists for academic research careers is sparse. Moreover, concerns arose relating to academic sustainability, both in terms of grant support and opportunities for publication and recognition that are essential to building an academic career. UCSF at one time had a core group of pharmaceutical scientists doing research in physiologically based pharmacokinetics and drug delivery. Seven of these individuals left academia not because they were unsuccessful but because there was no NIH support for creative research in these fields, she said, adding that, to be successful, regulatory science as a discipline needs to encourage funding from NIH, FDA, and other parties to make this a sustainable academic career track. Giacomini also cited the lack of departmental homes for regulatory scientists as a key barrier to development of a workforce in the field.
Advancing Academic Regulatory Science
It was emphasized by several of the panelists that regulatory science workforce development is dependent on career advancement opportunities and visibility and credibility of the work. Otherwise, training programs are for naught. Moreover, to ensure that teaching and training is current, training opportunities and programs could have mechanisms to evolve in parallel with the anticipated growth in regulatory science and research. Workshop discussants noted that to seed the practice of regulatory science, support the advancement and credibility of the discipline, and provide clear, discernible career paths, it is important to identify, fund, and pursue the “big questions” in regulatory science. Chin characterized these as the problems or questions that are of ultimate impor-
BOX 5-1
A Nonexhaustive List of the “Big Questions” Identified by Participants
• Need for appropriate science experiments that will define risk and benefit in better ways.
• Evaluate and better understand the preclinical to clinical transition.
• Need for better predictions of human clinical outcomes. Need for better selection of animal models and improved correlation with whole animal studies to human disease outcomes.
• How to develop and regulate drug combinations.
• How to address drug-drug interactions. Developing in vitro methodologies for predicting drug-drug interactions is suited to academic research, for example.
• Need for a collaboratively developed national research agenda in regulatory science.
• Develop better understanding of methods to analyze huge volumes of data and use big databases to answer questions in regulatory science.
• Need for a science-based process to identify and qualify biomarkers.
• Develop and refine novel approaches to clinical trial design.
tance for the innovation ecosystem. Discussion at the workshop collected a nonexhaustive list of those potential big questions, which are compiled in Box 5-1 as an integrated summary of speakers’ and participants’ remarks and discussions, and which should not be construed as reflecting consensus or endorsement by the participants, planning committee, the Forum, or the National Academies. Several participants noted that further work could be done to compile and catalog these “big questions” to help advance the discipline.
Ukwu identified the locus of the emergence of regulatory science in the “perfect storm” of 2006, in which industry saw a decline in productivity and rise in product failures, which highlighted that the current paradigm for drug development was unsustainable (Figure 5-1). She stated that the challenges plaguing industry forced introspection, leading to identification of a number of emerging regulatory trends, such as the use of adaptive trial designs and the ability to collaborate more closely with regulatory agencies during the development process.
________________
4This section is based on the presentation by Henrietta Ukwu, Senior Vice President for Global Regulatory Affairs, PPD, Inc.
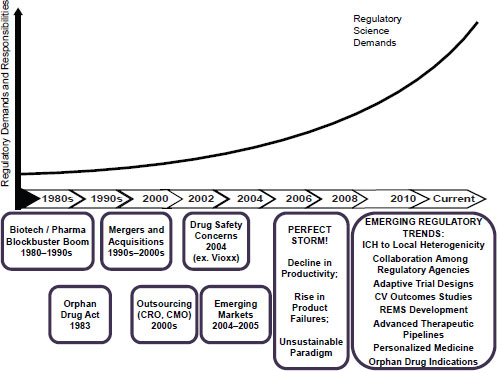
NOTE: CMO, Contract Manufacturing Organization; CRO, Contract Research Organization; CV, cardiovascular; ICH, The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; REMS, Risk Evaluation and Mitigation Strategies.
SOURCE: Ukwu, 2011. Presentation at IOM workshop on Strengthening a Workforce for Innovative Regulatory Science in Therapeutics Development.
Regulatory science could lead to better incorporation of scientific, translational, and clinical knowledge into regulatory development planning for industry. Regulatory scientists could liaise between multidisciplinary groups and could bring clinical reasoning and scientific methodology to a process-driven field. The need for precision, prediction, and intelligence in adapting the regulatory process to product development then could introduce proactive approaches to drug development and lead to the incorporation of better analytical processes. Regulatory scientists also could contribute to the business development process by providing input to licensing and outsourcing strategies and to partnerships in contract research organizations (CROs).
Regulatory scientists working in industry typically have a terminal
clinical or scientific degree, with a background in translational research or medicine and expertise in a therapeutic area. A regulatory scientist should also have experience in data review and actively participate in relevant professional organizations. A regulatory scientist’s responsibilities would combine strategic and operational excellence in program development planning, strategic regulatory intelligence, regulatory meetings, clinical trial design using advanced methodologies, global regulatory issues, and supervising in a matrix-organization environment. In this regard, Ukwu observed, industry highly values regulatory agency fellowship programs because they turn out regulatory scientists who meet these needs.
Ukwu also described career development paths for regulatory scientists in a CRO. Because CROs provide advice and guidance to industry, there is a clear need for regulatory scientists who can help a client identify gaps in a development plan and strengthen the position of products early in the development process. These efforts increase the odds of the client’s product succeeding both with regulators and in the marketplace.
This page intentionally left blank.








