Over the past 30 years, international trade, outsourcing, and improvements in telecommunication have created a more unified world economic system. This system presents new challenges. No country can rely solely on its own national regulatory authority to ensure food and medical product safety. Regulators today depend on their counterparts abroad in ways that no one could have foreseen in the 19th and early 20th centuries when many food and medical product regulatory systems were designed.
Changes in trade and manufacturing patterns call for changes in the ways countries work together for safety. The committee recommends that governments, industries, and academia work more consciously across borders to their mutual benefit. This chapter recommends specific areas for international cooperation, namely: increasing international investments in regulatory systems; encouraging open dialogue among government, industry, and academia; working toward voluntary sharing of inspection reports; and supporting surveillance.
INCREASING INTERNATIONAL INVESTMENTS
There is a common ground where food and medical product safety, global health, international trade, and development are mutually reinforcing (Henson and Jaffee, 2008; Horton and Wright, 2008; Maertens and Swinnen, 2009). Chapter 4 describes the nexus of these topics, and this report suggests actions that will advance their common goals. Both the health and the economy in developing countries would benefit from investments in their regulatory systems; these investments are also tools for international
trade. Intergovernmental institutions and international donor agencies have not yet recognized this or, if they have, their investments do not reflect it.
Recommendation 5-1: In the next 3 to 5 years, international and intergovernmental organizations should invest more in strengthening the capacity of regulatory systems in developing countries. The United States should work with interested countries to add it to the G20 agenda. Investments in international food and medical product safety should be a significant and explicitly tracked priority at development banks, regional economic communities, and public health institutions. International organizations should provide assistance to achieve meaningful participation of developing country representatives at international harmonization and standardization meetings.
One measure of this recommendation will be the extent to which the 2012 Group of 20 (G20) meeting in Mexico includes food and medical product safety on its agenda. The amount of discussion at the Mexico meeting and at subsequent G20 meetings will be a further measure. Actions from the G20 meeting and increased allocations to regulatory systems can also measure this recommendation. An increased attendance of scientists from developing countries at standard setting meetings and the development of programs that improve their participation would also be measures.
Putting this topic on the agenda at the 2012 G20 meeting can be accomplished in the next year. Increasing investments in building regulatory systems and tracking these investments could take longer; this should begin in the next 3 to 5 years and continue.
Advancing Safety Standards Through Trade
Safety standards serve many purposes. They protect health by reducing the likelihood of harmful products circulating in the market. They also facilitate trade: countries with disparate product safety regulations use common standards in the international market (Maertens and Swinnen, 2009). International or harmonized standards and certification regimes are useful to both exporters and importers. They lend predictability to regulatory decisions and protect against accusations of arbitrary barriers to trade. International standards also simplify the requirements for export to multiple markets. The committee commends the Global Food Safety Fund, supported by Waters Corporation, Mars Inc., and the U.S. Agency for International Development (USAID), for its pilot training program in the Asia Pacific Economic Cooperation (APEC) countries. This program draws on funding and expertise from government and the private sector to establish training programs and improve testing in developing countries (Goetz, 2011).
Complying with international safety standards can be expensive, especially when starting from scratch. However, once a producer has invested in meeting standards for one market, his or her marginal compliance costs decrease as the size of the market increases. These economies of scale could drive better safety standards in emerging economies (Henson and Jaffee, 2008; Horton and Wright, 2008). International trade negotiations and agreements such as the World Trade Organization (WTO) Agreements on Application of Sanitary and Phytosanitary Measures (SPS) and the Technical Barriers to Trade (TBT) promote harmonization and encourage the use of international, science-based food and drug standards.1,2 Developing countries can obtain guidance on compliance from Codex, the World Organization for Animal Health, the World Health Organization (WHO), and other international organizations.
Ideally, low- and middle-income countries would use international standards in their own regulatory systems. This would protect health in places where foodborne disease and substandard drugs kill many. It would also promote the competitiveness of exports from low- and middle-income countries in hard-currency markets (Henson, 2003; Maertens and Swinnen, 2009; World Bank, 2005). Failure to comply with food and drug safety standards can lead to product border detentions, import bans, and contractual penalties. The costs of failing to meet standards are substantial, especially for producers in low- and middle-income countries (Henson, 2003). Therefore, even developing countries with little public health infrastructure have reason to invest in oversight of food and drug exports.
The prospect of increased trade can motivate developing countries to invest in safety standards and regulatory oversight. Demonstrated ability to adhere to standards can improve their ability to export to tightly regulated markets (Maertens and Swinnen, 2009). These improvements in turn encourage foreign direct investment in local food and drug processing, exporting, and retailing (Henson and Jaffee, 2008). This arrangement can have spillover benefits for local populations: they can count on safe food and medicine, and their economies thrive (Unnevehr et al., 2003). Investors and multinational companies spread the use of high standards in developing countries to reduce transaction costs in regional distribution and supply chains, and to harmonize production and processing
![]()
1 Agreement on the Application of Sanitary and Phytosanitary Measures, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1B, THE LEGAL TEXTS: THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS 121 (1999), 1867 U.N.T.S. 493 (1994), art. 2, 4
2 Agreement on Technical Barriers to Trade, Apr. 15, 1994, Marrakesh Agreement Establishing the World Trade Organization, Annex 1B, THE LEGAL TEXTS: THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS 59 (1999), 1868 U.N.T.S. 120 (1994), art. 2.4.
standards across subsidiaries (Henson and Jaffee, 2008; Maertens and Swinnen, 2009).
The mechanism of this spillover benefit is complicated. The committee recognizes that many countries do support higher safety controls on exported products and neglect to implement best practices for their domestic markets. There is reason this could change, and the committee believes the changes will be more quickly realized in the medical products industry than in the food industry. Medical products are complicated to manufacture; cottage industry production of drug ingredients, while not unheard of, is rare.
The spillover benefits of better product standards will take time, probably at least 10 years, and will happen at the company and industry levels and at the regulatory system level. First of all, at the company level, there are common manufacturing processes for both the export and domestic markets. The process a company goes through to meet international standards creates greater knowledge, awareness, and experience with the standards throughout the company and industry. Staff who train against international best practices also bring their skills to other firms as they progress in their careers. There is room for cross-fertilization of ideas within companies and, because of job turnover, within industries.
The committee also sees room for spillover at the national level. As regulators inspect companies against domestic standards, they will be exposed to records, book-keeping, and audit reports reflecting international standards. Regulators will become better acquainted with international standards. Furthermore, regulators in low- and middle-income countries are already keen to enforce international standards; the international meetings for this study convinced the committee of this. However, lobbying forces in the domestic industry work to prevent this. The committee believes that pressure at the ministry levels of government could override the lobbying from industry and create momentum for higher domestic standards.
Much of this depends on the demands people in developing countries put on their governments. Chapter 3 explains how increased prosperity in parts of Asia, Africa, and Latin America drives increased attention to food and drug safety. Middle-class consumers will pay more for safer food (Morehouse and Moriarty, 2007). Studies in the United States and Europe indicate that consumers will pay more for higher quality food (Enneking, 2004; Lusk et al., 2003). Improving the ability of producers in low- and middle-income countries to adhere to international standards is to the shared advantage of consumers around the world. As demand for animal-source foods increases and access to medicines improves in low- and middle-income countries, the interests of consumers in developed and developing countries will overlap more (Unnevehr et al., 2003).
Many low- and middle-income countries have a two-tier regulatory model in which products for export are fairly well regulated, but those for
domestic consumption are not (World Bank, 2006). Consumers in these countries could come to resent being subjected to lower standards. There is also invariably intermixing of domestic and export product lines. This undermines developed country regulators’ confidence in the products they import.
International food and drug safety standards promote trade and global health, but the proliferation of overlapping, often inconsistent national and private standards do not. Adoption of international food standards has been slow and, in high-income countries such as the United States, poor (Roberts and Josling, 2011). The United States puts great effort into ensuring its standards are close to the Codex ones, but, because of an apparent disconnect between international and domestic priorities, these standards are often not adopted. When large, hard-currency economies disagree on standards, they undermine the efforts to enforce them in low- and middle-income countries (Horton and Wright, 2008). Inconsistent national standards impede market access and breed trade disputes, which in turn undermine developing country support for future multilateral trade agreements (Henson and Jaffee, 2008). The committee advocates for greater consistency in the use of standards on the part of the developed countries. Although the SPS Agreement allows signatories to adopt more stringent standards than Codex calls for if they have scientific reason, the committee sees this practice as unnecessarily harmful to emerging economies.
For example, high-value agricultural products are a promising business for farmers in the Horn of Africa. In order to sell on the European market these farmers need to meet requirements for pesticide residues, field and pack house operations, and traceability (Okello et al., 2007). Businesses increasingly rely on private organization standards, voluntary third-party certifications, and their own safety and quality management systems to regulate their suppliers.
This is not an efficient system. It increases the cost of compliance for producers and can lock small- and medium-sized holders out of the market (Maskus et al., 2005). This is a particular problem in low- and middle-income countries, where small- and medium-sized businesses dominate much of the pharmaceutical (and vastly more of the food) supply chain. Public—private partnerships can play a key role in satisfying market demands for food safety while retaining smallholders in the supply chain (Narrod et al., 2009).
Priorities for Future Multilateral and International Engagement
The United States should work with like-minded governments to pursue food and drug safety at intergovernmental trade, development, and global health forums. A global approach to food and drug safety would
lessen claims of double standards and reflect the shared interests of developed and developing countries. Intergovernmental venues are important because food and drug regulation in one state is increasingly dependent on the adequacy of regulation in other states. Linking global health, trade, and economic development objectives can help engage a wide variety of stakeholders. The committee recognizes that many international organizations work to build food and medical product regulatory capacity around the world. Tables 2-2 and 2-3 give examples of some prominent capacity building projects in food and drug safety.
Action for the Group of Twenty (G20)
The G20 brings together leaders from industrialized and emerging economies to work together for global economic stability and development. The support of the G20 would do much to advance the international food and drug safety agenda. As a leaders’ summit of the largest economies in the world, the G20 has the political and economic influence to advance food and medical product safety internationally (Kharas, 2011). The G20 membership includes many important stakeholders—representatives of emerging manufacturing economies including India, China, South Africa, Mexico, Thailand, and Brazil, as well as the developed countries in a position to offer technical assistance like the United States and members of the European Union (G20, 2011). The G20 countries need to commit to improving regulatory systems; without their support, change is unlikely (Drezner, 2007). If food and medical product regulatory systems received prominent attention at a G20 meeting it could spur investment from other donors, intergovernmental institutions, and national governments.
Global food and drug safety is well suited for the G20 agenda. The G20 development priorities include international trade, food security, investment, and job creation in low- and middle-income countries (G20, 2010). The multi-year G20 development agenda already includes many programs that promote food and medical product safety: identifying practical ways to support trade integration; harnessing agriculture to reduce poverty; increasing private-sector participation in development; and promoting small business’ access to international markets (G20, 2010). The G20 has recognized international development as integral to its “mandate of global economic cooperation” and a critical component of the G20’s goal is “strengthening the relationships among high-, middle-, and low-income countries” (G20, 2010, p. 1). The G20 development priorities include matters dependent on improved food and drug safety: international trade, food security, and investment and job creation in low- and middle-income countries (G20, 2010).
The G20 should take food and drug safety seriously because it has significant implications for public health, trade, and economic develop-
ment in G20 countries. The means to address this issue—improved regulatory cooperation, information sharing, and adoption of international standards—are also all areas in which consensus among G20 countries is possible despite the diversity of their economies. This should make it easier for G20 members to act (Kharas, 2011).
Mexico will host the 2012 G20 meeting. As a middle-income country with a vigorous export economy, Mexico would be an ideal country to lead an initiative on global food and drug safety. This initiative might include increasing information sharing among stringent regulatory authorities to reduce redundant audits. Another valuable action would be enhancing the meaningful participation of developing country scientists in international standard setting. They might also emphasize supporting small- and medium-sized developing country producers in complying with international standards. The United States, working with other G20 member states, should encourage and support Mexico before, during, and after the 2012 G20 meeting.
Actions for the WTO, Development Banks, and Regional Economic Institutions
The development banks, regional economic communities, and public health institutions need to invest more in food and medical product safety; this includes investments in the systems and processes that ensure product safety. A 2005 World Bank report stressed that capacity building, especially as it relates to product safety standards, should avoid isolated interventions and work to increase broader market competitiveness (World Bank, 2005). The World Bank is gradually increasing such loans. In 2005 it lent Colombia $30 million to improve the competitiveness of its meat and milk exports (Nuthall, 2005), and a 2010 program invested $100 million in China’s adherence to good agricultural practices (World Bank, 2010). Some of the regional banks are working on the same issues. A 2009 $95 million Asian Development Bank loan to Vietnam aims to improve the quality of commercial agriculture (ADB, 2009). These loans are a step in the right direction, but they need to reach more countries.
Standard Setting
As Chapter 3 discusses, scientists from developing countries are often not prepared to make meaningful contributions at international standard setting meetings. Their countries clearly suffer as a result, becoming standard-takers, not equal standard-setters. Their silence also undermines the legitimacy of the standard setting process. And, in the end, they are unable to comply with the standards produced (Horton and Wright, 2008).
The committee recognized that the three international food standard setting organizations recognized by the WTO—Codex Alimentarius, the International Plant Protection Convention (IPPC), and the World Organization for Animal Health (OIE)—are valuable sources of information and training for regulators in developing countries. Nevertheless, more must be done to mobilize resources and provide technical support that can encourage the participation of developing countries. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) should likewise do more to encourage meaningful engagement by low- and middle-income countries, consistent with trends in the global production and consumption of medicines. These organizations should work closely with regional economic and public health institutions such as APEC, the Pan American Health Organization, the Association of Southeast Asian Nations, the American Institute for Cooperation on Agriculture, and the African Union Interafrican Bureau for Animal Resources to engage low- and middle-income countries.
ENCOURAGING OPEN DIALOGUE AMONG GOVERNMENT, INDUSTRY, AND ACADEMIA IN EMERGING ECONOMIES
A robust regulatory system depends on input from industry and academia; government simply cannot shoulder the burden alone. Most developed country regulators describe their system as a stool supported by three legs: industry, government, and academia. The shared responsibility makes for a stronger system with much wider ownership. In low- and middle-income countries, especially in Asia and Africa, it is not so.
It is important for consumer groups and industry to have a chance to comment on regulations before they are made. It is also important that all parties should be able to modify laws if they have scientific evidence to support a change.
Recommendation 5-2: In emerging economies, national regulatory authorities, regulated industry, and industry associations should engage in open and regular dialogue to exchange scientific and technical information before policies are written and after they are implemented. Starting in the next 3 to 5 years, these regulatory authorities should identify third parties, such as science academies, to convene the three pillars of a regulatory system—government, industry, and academia—in ongoing discussion to advance regulatory science, policy, and training.
The number of meetings among industry, academia, and government regulators in low- and middle-income countries will be one measure of this recommendation. Another important measure will be the policy outcomes
of the meetings. However, in measuring the impact of open dialogue the process is as important, if not more important, than the outcome. Openness in involving all stakeholders and actively seeking neutral forums for discourse are the most important outcomes of this recommendation.
Communication and Transparency
Lack of communication and transparency are major gaps found within and between regulatory systems around the world (IOM, 2009; IUF, 2009). A lack of openness aggravates the problems, as does poor communication between industry and consumer groups. It is therefore not surprising that there are problems with both transparency and communication across borders. Regulatory systems in low- and middle-income countries have this problem, and similar communication gaps exist in the United States. However, the United States has public reporting requirements, external advisory boards, independent national association meetings, and study panels, such as those convened by the Institute of Medicine, that provide avenues for communication. This kind of open dialogue is essential for progress. These opportunities for open dialogue are often lacking in developing countries.
In low- and middle-income countries, the line between scientific and policy decisions is often blurred. Both often require political clearance at the highest government levels. This hampers efforts to promptly transmit the technical data crucial for assuring product safety. In some countries, so-called independent organizations act as go-betweens for government and industry, but in reality their independence is nominal. In other cases, the lack of suitable venues or personnel to transmit technical data, such as the testing requirements for a new regulation, holds back communication. This gap is especially evident as one goes from national to state and provincial authorities. This problem is complicated by the dearth of scientific staff at the more local levels. Similarly, the regulated industry has little ability to provide input into new regulations or get technical guidance on compliance. Academia can help bridge regulatory agencies and industry, but its involvement is generally minimal.
Avenues to More Effective Dialogue
There are examples in North America, Europe, and Australia where the three independent pillars of well functioning stringent regulatory systems (government, industry, and academia) are brought together to discuss issues of mutual concern (Australian Government, 2011; Global Harmonization Initiative, 2011; Health Canada, 2009; IOM, 2011). For example, the U.S. Department of Agriculture (USDA) and the U.S. Food and Drug Administration (FDA) worked with the Department of Homeland Security and the
Federal Bureau of Investigation to jointly engage states and private industry in food defense (USDA, 2005). These partnerships lead to better protection of the food supply from farm to table.
These partnerships present a number of opportunities. First, stakeholders can discuss technical issues related to standard setting, testing, and approaches to implementation. Second, they provide a mechanism for information dissemination, especially on adopting new regulations. Third, international experts could teach satellite courses to educate provincial or municipal staff on implementing regulations from the national regulatory authority. These recommendations are generally concerned with opportunities for training and training trainers, and training sessions are excellent venues for open dialogue.
In emerging economies, existing regional bodies will be an important venues for communication among neighboring countries facing similar problems. The FDA could help facilitate such meetings by sending its overseas staff when appropriate opportunities arise.
Convening Three Pillars of a Regulatory System
Professional associations and academic institutions are often good places to bring together stakeholders for balanced and open dialogue on regulatory policy (ASM, 2011; IFT, 2011). Although such venues cannot and should not assume decision-making functions of government agencies, they do open lines of communication among regulators, sister agencies, academic experts, and multiple levels of regulated industry.
There are notable glimmers of improving communication in some places. In November 2011, the Indian food regulatory authority took public input from food industry associations in designing its product recall plan (FSSAI, 2011). Even in this case, however, academia was notably uninvolved. This is a problem as gaps in basic and regulatory sciences prevent a regulatory authority from doing its job (Mattes et al., 2010).
Regulatory science is a new field (Gundersen, 2001). It is multidisciplinary and includes elements of basic science, epidemiology, statistics, social science, business management, public policy, and communication. A field of such breadth needs instructors with practical experience. The emergence of such cross-cutting disciplines requires collaboration between universities, industry, and government. The first step to advance regulatory science in emerging economies is to bring these stakeholders together.
National Science Academies
As noted previously, a number of organizations have at various times convened government, industry, and academia on product safety. Science
academies are uniquely positioned to serve in this area. Science academies have expertise to draw from among their members, who are elected by their peers. Election to a national academy is an honor. It is also a chance for elite scientists to serve their country, and it gives countries a trusted and independent advisory body. Science academies are, in many countries, a neutral space that can bring together stakeholders from various disciplines. Their focus on evidence-based decision making provides the neutral setting needed to bring together academia, government, and industry.
Public Health Forums
According to the Institute of Medicine’s report on the Future of Public Health, “[p]ublic health is what we, as a society, do collectively to assure the conditions for people to be healthy” (IOM, 1988, p. 20). Public health agencies work at the intersection of science, government, business, and civil society. They oversee the implementation of health policies and regulations. However, state health agencies in many developing countries lack the infrastructure to carry out standard public health functions such as surveillance (Mok et al., 2010). The Bill and Melinda Gates Foundation funded the International Association of National Public Health Institutes in 2006 to build public health institutes in less-developed countries (IANPHI, 2011b). A particular focus of its work has been on improving public health functions like disease surveillance, outbreak investigation and response, and operations research (IANPHI, 2011a).
The Association of Food and Drug Officials (AFDO), established in 1896, works toward public health safety and consumer protection in regulatory areas concerned with food, drugs, devices, cosmetics, and consumer products (AFDO, 2010). Along with promoting education and dialogue among government, industry, and consumers, AFDO also provides “guidance and training programs for regulatory officials and the regulated industry, to promote nationally and internationally uniform inspections, analyses, interpretations and investigations” (AFDO, 2010). Other international organizations such as the WHO, the Food and Agriculture Organization of the United Nations (FAO), and WTO have provided similar forums and support for discussions on food and medical product regulation (FAO/WHO, 2005; GIFSL, 2010; WHO, 2011; WTO, 2011). In addition, the International Biopharmaceutical Association brings together biopharmaceutical and clinical research institutions and organizations from different countries. All of these organizations could convene and educate students, possibly through their online discussion groups.
WORKING TOWARD SHARED INSPECTIONS
Sharing inspection reports is a first step to international regulatory harmonization. It is also a simple change that could reduce a great deal of waste; there is no need for American and European inspectors to duplicate each other’s work, especially when a vast number of facilities go uninspected. Eventually, regulatory authorities in emerging economies would also be able to share inspections.
Recommendation 5-3: Countries with stringent regulatory agencies3 should, within the next 18 months, convene a technical working group on sharing inspection reports with the longer-term goal of establishing a system for mutual recognition of inspection reports.
This recommendation can be measured by looking at the number of inspections the United States, Canada, Australia, New Zealand, Japan, and European Union countries share and the steps they take toward mutual recognition of inspections. The objectives of these working groups will depend on the relationships between the regulatory authorities and the baseline similarity of their systems.
In the longer term, that is, over the next decade, this recommendation will be measured by monitoring the same involvement from emerging economies.
Collaboration Among National Regulatory Authorities
The FDA and other stringent regulatory authorities need to respond to globalization by formally recognizing their dependence on each other. No single regulatory agency can conduct the bulk of the world’s food and drug facility inspections. The most technologically advanced regulatory agencies could coordinate on planning inspections and share the results of inspections (GAO, 2010). It is extremely complicated for the FDA to inspect the vast number of food producers and medical product manufacturers outside the United States. Among other things, the FDA’s records on foreign manufacturers are often incomplete and inaccurate (GAO, 2008, 2011). All parties could vastly increase the accuracy and breadth of their information with relatively simple collaborations. In the longer term, including developing country regulatory authorities in these collaborations would be a valuable opportunity for sharing knowledge.
![]()
3 By the ICH definition countries with stringent regulatory agencies include the United States, European Union member states, and Japan. For the purposes of their recommendations the committee includes ICH Observers and Associates: Australia, New Zealand, Norway, Iceland, Switzerland, and Canada, in this group.
The International API Inspection Pilot Programme is an exceptionally promising collaboration among medicines regulatory authorities of the EU countries most active in active pharmaceutical ingredient (API) inspections (France, Germany, Ireland, Italy, and the United Kingdom), the Australian Therapeutic Goods Administration, and the FDA (EMA, 2011). Starting in 2008, all participating regulatory authorities shared their inspection plans using a common template (EMA, 2011). They also shared their retrospective data from 2005, identifying 85 duplicate inspections in 3 years (EMA, 2011). During the study, participating agencies developed an API facility master list that all agencies would use to plan future inspections and to share the results of each (EMA, 2011). They conducted nine joint inspections and have made a plan to coordinate and share their inspections in the future (EMA, 2011). This is an exemplary step toward efficient international cooperation.
The FDA and European Medicines Agency (EMA) have also worked together on sharing inspections for compliance with good clinical practice. The report on this pilot program concluded that sharing inspections is less time-consuming and more efficient than conducting separate inspections (EMA and FDA, 2011). It also stressed an unforeseen benefit of the pilot program: it allowed regulators to identify and fill gaps in their inspection processes (EMA and FDA, 2011). In joint reports, the FDA and the EMA praised the pilot programs as efficient and valuable collaborations that have great promise for better future operations (EMA, 2011; EMA and FDA, 2011).
The FDA has confidentiality agreements with Health Canada, the Swiss Medic, Anvisa, and many other regulatory agencies abroad (FDA, 1973, 2003, 2010). A confidentiality agreement is the legal first step in sharing sensitive data, such as inspection reports.
The committee recommends that the FDA, USDA, EMA, and other technologically advanced regulatory authorities do a similar pilot study on sharing inspections of farms and food producers. The Food Safety Modernization Act increased the number of overseas inspections required of the FDA to 600 in 2011, doubling every year after that until 2016 (FDA, 2011). Under such high demands, the agency and its counterpart agencies in developed countries need to share inspections. In a joint report, the PEW Health Group and the Center for Science in the Public Interest encouraged the FDA to accept inspection reports from trusted foreign governments with similar regulatory rigor (CSPI, 2011). Sharing inspection reports and conducting joint inspections increases efficiency and helps all parties see ways to improve their systems (NRC, 2011).
The continuation of the International API Inspection Program is an invaluable step in the right direction toward better information sharing among regulatory agencies. The committee feels that the larger goals of
the program could be well served by forming a standing technical working group on mutual recognition. In the next decade this working group could expand, assuming all confidentially agreements were met, to include low- and middle-income countries. The gradual inclusion of low- and middle-income countries in the working group would be an opportunity for regulators from these countries to learn more about international inspections and best practices. It would also allow them to see close-up how technically advanced regulatory agencies operate. This working group would likely have the unplanned secondary benefit of encouraging cross-fertilization of ideas. The WHO emphasizes a similar idea in the WHO Prequalification Program’s guidelines on collaborative inspections. Participating in the WHO prequalification inspections is a learning experience for the inspectors nominated by their national regulatory authorities. It also eases the inspection burden on the national regulatory authority (WHO, 2010).
Information Sharing Challenges and Incentives
In order for regulatory agencies to share inspections and work toward mutual recognition, they need to first set up systems for collecting the same data. The API inspection pilot gave great attention to the design of a common data collection template (EMA, 2011). In the early stages of their work, the regulatory agencies can streamline their data collection tools. The use of handheld computers could make the inspectors’ job simpler and protect the reports from careless mistakes. Paper and pencil data collection systems are still shockingly common, however. The 2002 Bioterrorism Act has forced the American food industry and government agencies to use electronic data systems, but this is not so in other parts of the world, even in developed countries (Rosenberg, 2006). At the 2010 International Conference of Drug Regulatory Authorities, drug regulators stressed their need for a protected electronic system that would allow them to safely share confidential information (ICDRA, 2010).
The committee realizes that one of the first main steps to sharing inspections is negotiating a system by which countries can share confidential information. While the different regulatory laws that govern the stringent regulatory authorities will make this challenging, it is possible to agree to a set of harmonized rules for making information confidential.
The committee also recognizes that, in the long run, in order to share inspection duties with other advanced regulatory authorities, the FDA will need to ask Congress to revise the terms of the inspections it mandates. Currently, the FDA is legally obligated to inspect a certain number of foreign producers, but it would be more efficient for Congress to encourage inspection sharing with trusted nations. Other advanced regulatory
authorities might have similar legal mandates. In the short term, all parties can increase their efficiency by planning inspections together so as to avoid duplicating work. Furthermore, the committee sees no legal barriers to joint inspections, which are useful for all parties and pave the way for future mutual recognition.
SHARING INSPECTION RESULTS VOLUNTARILY
As Recommendation 5-3 describes, regulatory authorities should cooperate better in inspections and work toward mutual recognition. Government collaborations can only advance product safety so far, however. Manufacturers and producers have the most thorough knowledge of their supply chains; they need to share information as well.
Regulated industry has a wealth of information in its internal inspection reports. Once a manufacturer has identified a risk in its system, this knowledge could be made available to others in the industry as a way to avoid repeating the same problem. Industry associations such as the Pharmaceutical Research and Manufacturers Association, the Biotechnology Industry Organization, the Generic Pharmaceutical Association, the Medical Device Manufacturers Association, Food Industry Association Executives, the Grocery Manufacturers Association, and others could work toward making inspection and audit reports available to other association members.
Recommendation 5-4: Industry associations should, over the next 3 years, define an acceptable protocol for sharing of internal inspection results among their members. After agreeing on the methods, they should regularly share their results among their members.
The number of inspection results shared and number of associations working on voluntary sharing programs will be the best measures of this recommendation.
The committee recognizes that it will take food and medical product industry associations 3 years to define a trusted, nonthreatening way for their members to share internal inspection results. Once there is a system in place, the analysis of anonymous reports should be shared in newsletters on an ongoing basis.
Reluctance to Share Information
Industry is often reluctant to share its internal data and inspection reports with anyone. This reticence is appropriate: industries have a responsibility to their shareholders to protect proprietary information and
avoid harming the brand with rumors. Furthermore, some internal audit reports identify problems, and if it became apparent that an executive had ignored warnings and released a product anyway, then the company could face monumental negligence litigation. Economic incentives and limiting liability may encourage greater information sharing, though much depends upon the data that industry is asked to share.
Industry associations are well positioned to work toward voluntary information sharing among their members. Associations have established relationships with their members, and member dues support their operations. They are also responsible for initiating collaborations between companies that advance their mutual goals. When sharing inspection reports, trust in confidentiality will be critical. This is why industry associations are the ideal leaders: they have established good relationships with their member companies and have an interest in protecting the industry from damaging rumors. A trusted industry association could serve as an information clearinghouse. Association staff could analyze blinded, de-identified data from across the supply chain and disseminate their results at meetings and in association newsletters.
The committee realizes that this raw data will not be accessible to the FDA or any regulatory agency, but it believes in the value of regulated industry sharing information and learning from formal analysis of a wide cross-section of data. Private-sector supply chains, especially in the branded food industry, are often excellent. There is a need to draw on industry’s knowledge of supply chain management. The conclusions that industry draws from analysis of de-identified inspection reports would be invaluable to government and academic stakeholders, as well as to the industry and the suppliers.
It will not be possible to improve product safety without taking advantage of industry’s expertise. As Chapter 3 explains, there is no tradition of collaboration between regulatory authorities, industry, and academia in most developing countries. The committee also sees much room for improvement in developed countries when it comes to sharing information and learning from the depth of experience in industry. Industry associations around the world can help fill this gap by sharing the lessons learned from aggregate inspection reports.
Examples of Collaboration
Although voluntary sharing of inspection results within industry is not common, there is precedent for such collaboration in both the food and pharmaceutical industries. The non-profit organization Rx-360 is an industry consortium that brings together regulators and pharmaceutical and supplier executives to improve security in the drug supply chain (Rx-360, 2009).
Martin Van Trieste, the former president of Rx-360, described the collaborative’s joint audit program in his 2011 Senate testimony (VanTrieste, 2011). This program grew out of a response to the 2008 heparin crisis and allows participating companies to share redacted audit reports via a common database (VanTrieste, 2011). In his testimony, Van Trieste also recommended that excipient and API brokers disclose to manufacturers the exact origin of all their products, something not currently required (VanTrieste, 2011).
Much as the Rx-360 consortium grew out of a response to the heparin crisis, the beef industry has responded to the virulent E.coli O157:H7 outbreaks with regular summits that include cattlemen, butchers, retailers, government, and academics (Cattlemen’s Beef Board and National Cattlemen’s Beef Association, 2003, 2009, 2010, 2011). In January 2003, summit participants developed a plan to control E. coli O157:H7 throughout the supply chain, emphasizing the need for “industry to maintain open communication and to share data regarding pre-harvest interventions and good management practices” (Cattlemen’s Beef Board and National Cattlemen’s Beef Association, 2003).
Clearly, industry stakeholders have an interest in sharing their best practices. Product recalls are costly and logistically complicated. Companies need to protect their brands. Sharing information across the supply chain can help them avoid product safety lapses and thereby strengthen their brands.
STRENGTHENING SURVEILLANCE SYSTEMS
Surveillance is one of the main responsibilities of food and medical products regulatory authorities, and, as Chapter 3 describes, it is a major gap in regulatory systems in emerging economies. Trade and international travel make this a problem for people all over the world. Foodborne pathogens can spread quickly through the supply chain. Similarly, adverse drug events, often a signal of an adulteration, threaten disparate populations.
The USAID, FDA, CDC, EMA, and the WHO Prequalification Programme all have technical depth and training capabilities in surveillance. The committee aims to mobilize their expertise to support surveillance systems in low- and middle-income countries. The committee recognizes that regulatory agencies do not generally have the budget or mandate to support intensive capacity building projects. Therefore, other agencies and other organizations will need to support surveillance as well.
Recommendation 5-5: Starting in the next 5 years USAID, FDA, CDC, and USDA should provide (both directly and through WHO and FAO) technical support for strengthening surveillance systems in developing countries. This technical support could include development and sha-
ring of surveillance tools, protocols for foodborne disease surveillance and post market surveillance of medical products, and training of national regulatory authority staff and national experts.
The most direct measure of this recommendation will be the number of programs these agencies initiate to improve foodborne disease and postmarket surveillance systems in developing countries. Over time, a change in the number of surveillance staff at regulatory agencies in low- and middle-income countries will be another measure of this recommendation.
In addition to measuring these process indicators, the functioning of the surveillance tools developed will be measured using sensitivity and specificity criteria specific to each tool. The scientists developing these tools will need to articulate the minimum threshold at which the tool is functioning properly.
Building a cadre of trained epidemiologists will take time. This important step of strengthening surveillance systems may take 10 years or longer to develop. In the next 3 years, USAID, FDA, CDC, and USDA can work with their host country counterparts to develop and strengthen manageable systems for postmarket surveillance of medical products. Developing a foodborne disease surveillance system will require improvements in laboratory infrastructure and will therefore take longer, but the committee believes meaningful improvements, such as the expansion of the CDC PulseNet program, can begin in the next 5 years.
Surveillance Tools
The most frequent approach to postmarket surveillance of medical products in developing countries is spontaneous or passive reporting by health workers. Spontaneous reporting systems have important limitations. Because they rely on overworked doctors and nurses or, even worse, on patient initiative, spontaneous reporting is synonymous with underreporting. Spontaneous reporting systems can generate useful data and give early signals of medical product safety problems, but in the poorest countries even passive reporting systems are not functional (Kuemmerle et al., 2011).
Active surveillance complements spontaneous reporting systems. Active surveillance involves methodically searching for exposures of interest or adverse events at sentinel surveillance sites. These sites, sometimes hospitals or clinics, collect enough data to allow analysts to calculate event rates with an accurate denominator. Sentinel surveillance sites at hospitals and health centers in developing countries need to be improved. These improvements should accompany the development of active surveillance when necessary. For example, drug regulators can engage active surveillance systems after passive event reporting or sentinel sites identify a signal. The WHO and

The CDC’s Global Disease Detection network builds capacity for active surveillance in developing countries. This researcher in Kibera, Kenya uses a handheld computer to track disease symptoms.
© 2008 Dana Pitts, Courtesy of Photoshare.
Global Fund have proposed the essential elements of a national pharmacovigilance system (Xueref, 2010). Trainers should work to align their technical support to these systems.
Independent laboratories are also essential for functional surveillance systems. Food safety surveillance in particular depends on laboratories for molecular subtyping of pathogens. This is challenging in developing countries, and the committee sees expanding laboratory capacity as a key piece of the technical support U.S. and international organizations should give. The CDC’s PulseNet program has given valuable technical support
in developing clinical, reference, and food safety laboratories in Asia and Latin America (Swaminathan et al., 2006). The committee encourages this expansion of PulseNet over the next 5 years and believes every part of the world could benefit from the PulseNet system.
Fortunately, information technology has created a wealth of surveillance tools more easily adapted to middle-income countries. These methods are often described as event-based. That is, they rely on patterns of events: Google searches on symptom clusters, news reports, and discussion threads on blogs and in Internet chat rooms. Figure 5-1 describes how these events can be alerts of an epidemic that is still in the early stages. Twitter and other Internet-based surveillance tools have been useful in tracking the incidence of dengue fever in Brazil (Gomide et al., 2011). In the poorest countries, the lack of Internet access will prevent the reliable use of Internet-based surveillance, but the conceptually similar mobile phone surveillance shows promise (Breiman et al., 2008). The CDC’s BioSense system is an example of a surveillance tool that uses Internet technology to create an online surveillance community (Box 5-1).
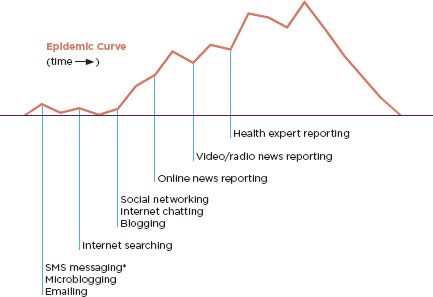
FIGURE 5-1
Hypothetical timing of informal electronic sources available during an outbreak.
* SMS, short message service.
SOURCE: Keller et al., 2009.
BOX 5-1
The BioSense Redesign
Following the anthrax attacks of 2001, the U.S. government recognized the need for a more informed and better equipped public health sector to deal with potential and actual bioterrorism threats (SEMP, 2008). In 2002, Congress passed the Public Health Security and Bioterrorism Preparedness and Response Act (FDA, 2002). This act mandated the formation of BioSense, a program housed in the CDC. BioSense is a data collection and analysis program that helps public health officials throughout the United States plan for, detect, and respond to disease outbreaks that may be related to bioterrorism. BioSense is used for both prevention and response, making it a multifaceted tool for the preservation and advancement of national health (CDC, 2012).
After operating for several years as a program focused primarily on bioterror threat detection, in 2010 the CDC began redesigning BioSense to better meet the needs of the public health sector (CDC, 2012). Some users objected to BioSense’s narrow focus and insufficient integration with other, similar programs already in use (RTI International, 2011). Guided by the suggestions of public health officials at municipal, state, and national levels, the CDC restructured BioSense to respond to a wider range of health threats. This revamped program, BioSense 2.0, facilitates collaboration within and between the levels of the public health infrastructure, and provides users with the information, analysis, and tools they need to best respond to health threats (BioSense Redesign, 2011; CDC, 2012). In essence, BioSense 2.0 creates a “public health surveillance community” comprised of public health professionals across disciplines, borders, and organizations (BioSense Redesign, 2011). The redesign project will conclude in June 2013 (RTI International, 2011).
The CDC solicited input from a range of stakeholders during the BioSense redesign. A key element of this mission was the BioSense Redesign Collaboration Site, a website that solicited suggestions and asked for feedback on the project’s process (CDC, 2012). Through the website, all stakeholders were involved in the work, and their needs were incorporated into the BioSense revisions (RTI International, 2011).
Foodborne disease lends itself to event-based surveillance. The Global Public Health Information Network relies on information from all pertinent news streams. This web-mining surveillance system was instrumental in containing SARS (Brownstein et al., 2009; Keller et al., 2009). Box 5-2 describes how the state of North Carolina uses an early warning system that integrates many types of signals for better food safety. The committee sees web-mining and event-based surveillance as potentially valuable tools for developing country regulators and believes all technical support should draw on this valuable new technology.
BOX 5-2
North Carolina Foodborne Events
Data Integration and Analysis Tool
Researchers from the University of North Carolina’s Center for Logistics and Digital Strategy at the Kenan-Flagler Business School and the North Carolina Center for Public Health Preparedness in the Gillings School of Global Public Health have developed the North Carolina Foodborne Events Data Integration and Analysis (NCFEDA) tool to bridge gaps in North Carolina’s food safety system (Greis et al., 2011).
The public and private sectors and consumers must all work together for food safety. There are information delays in a standard food surveillance system that can allow months to pass between the time of suspected contamination and the removal of affected products from grocery shelves. New, more timely, and more informative data sources can reduce the latent time between contamination and removal. NCFEDA reduces these latencies by making real-time information—from consumer complaints and hospital emergency room visits to social media, FDA recall information, and private-sector data—available to public health officials (Greis et al., 2011).
Public health officials in North Carolina collaborated with Kenan-Flager School faculty in designing the NCFEDA tool. The tool integrates four essential capabilities that contribute to improved situational awareness. First, it integrates data from many different types of signals, such as consumer complaints and emergency room visits. It also relies on analytical tools that help make connections across these signals to better recognize disease or contamination patterns. The tool includes a visualization piece that allows mapping and other graphic data display. Finally, the tool works in real time; all stakeholders work together on a coordinated response (Greis et al., 2011).
New information systems like NCFEDA can help assure better food safety and minimize the impact of food contamination events—especially for products that originate abroad. NCFEDA is a first step toward integrating a diverse set of stakeholders across North Carolina food safety systems. NCFEDA aligns with current national strategic plans for food safety, such as those outlined in the Food Safety Modernization Act. Other states and countries can use this system as a model.
The committee believes that Internet-based surveillance tools might be useful in emerging economies with reasonably sophisticated technology infrastructures, such as China, India, Brazil, South Africa, Mexico, and Thailand. In less-developed countries, mobile phone technology might be used to the same end: building a novel foodborne disease and drug postmarket surveillance system.
Surveillance Experts
Web-mining is a promising piece of surveillance development in low- and middle-income countries, but it is not the only remedy the committee suggests. In an interview with Nature, the head of animal health at the French food safety agency cautioned against seeing Internet-based surveillance systems as an alternative to building a cadre of local epidemiologists (Butler, 2006). In the same article, Peter Roeder, a consultant with the FAO, explained, “No amount of setting international guidelines and publishing global action plans is going to help when you have an organization within the country that doesn’t know what to do” (Butler, 2006, p. 6). The committee agrees that training in-country staff in epidemiology and modern surveillance methods should be central to any and all surveillance programs. Similarly, building modern surveillance systems will include building a culture of reporting adverse effects among health workers and advertising the proper pathways for reporting.
CONCLUSIONS
The committee’s strategy for building regulatory systems in developing countries emphasizes international cooperation. The unified world market has united countries in many positive ways, but has also introduced new liabilities. No country’s regulatory authority can vouch for the safety of all foods and medical products in its market. The committee identified five areas where stakeholders around the world could act to improve food and medical product safety.
First, the development banks, regional economic communities, and public health institutes should ensure that scientists from the least developed countries are better prepared to participate in international standard setting. The G20 is also an excellent forum to discuss how to increase investments in regulatory systems. The United States and other G20 members should support Mexico, the 2012 G20 host, in sponsoring a global initiative on building food and medical product regulatory systems. In the next 3 to 5 years, increased investment in strengthening regulatory systems capacity should be explicitly tracked at international organizations.
The committee was struck by the isolation that many developing country regulators work in. They lack the involved support of industry and academia. National regulatory authorities in emerging economies should work to change this in the next 3 to 5 years and foster an open discussion on science and policy with all stakeholders. To this end, they may need to ask their national science academies to convene a stakeholder meeting.
More open communication about policy will benefit all parties, but the changes should not stop there. Stringent regulatory authorities should im-
mediately work toward sharing inspection reports; they should also coordinate their inspections in emerging economies and conduct joint inspections when possible. This collaboration will encourage cross-fertilization of ideas and, more importantly, will prevent duplicating inspections, something nobody can afford. In the next decade, they could work toward a system of mutual recognition of inspection reports, a system developing countries might also join. Industry also has a wealth of information in its internal inspection reports. In the next 3 years, industry associations should develop ways to share this information that are acceptable to their members.
Finally, U.S. agencies and multilaterals with appropriate expertise should support surveillance systems in developing countries. Without reliable data on postmarket surveillance of medical products and foodborne disease, risk assessment is meaningless, and risk assessment is the cornerstone of any modern regulatory agency. In the next 3 years, it will be possible to develop a system for the postmarket surveillance of medical products, and the expansion of the CDC’s PulseNet program to more developing countries can start in 5 years. Over the next decade, the training of a cadre of developing country epidemiologists can complement this surveillance development.
REFERENCES
ADB (Asian Development Bank). 2009. Viet Nam’s drive to improve food safety receives $95M boost from ADB. http://beta.adb.org/news/viet-nams-drive-improve-food-safety-receives-95m-boost-adb (accessed November 20, 2011).
AFDO (Association of Food and Drug Officials). 2010. The AFDO mission. http://www.afdo.org/afdo/MissionStatement.cfm (accessed November 22, 2011).
ASM (American Society for Microbiology). 2011. ICAAC final program. 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, September 17-20.
Australian Government. 2011. International Medical Device Regulators’ Forum (IMDRF). http://www.tga.gov.au/about/international-imdrf-111105.htm (accessed November 22, 2011).
BioSense Redesign. 2011. BioSense redesign fact sheet. https://sites.google.com/site/biosenseredesign/file-cabinet (accessed February 9, 2012).
Breiman, R. F., M. K. Njenga, S. Cleaveland, S. Sharif, M. Mbabu, and L. King. 2008. Lessons from the 2006-2007 Rift Valley fever outbreak in East Africa: Implications for prevention of emerging infectious diseases. Future Virology 3(5):411-417.
Brownstein, J. S., C. C. Freifeld, and L. C. Madoff. 2009. Digital disease detection—harnessing the web for public health surveillance. New England Journal of Medicine 360(21):2153-2157.
Butler, D. 2006. Disease surveillance needs a revolution. Nature 440(7080):6-7.
Cattlemen’s Beef Board and National Cattlemen’s Beef Association. 2003. E.Coli O157:H7 solutions: The farm to table continuum (executive summary). San Antonio, TX: Beef Industry E.coli Summit Meeting.
———. 2009. Beef industry safety summit: Executive summary. Paper read at Beef Industry Safety Summit, San Diego, California, March 4-6.
———. 2010. 2010 beef industry safety summit: Executive summary. Paper read at 2010 Beef Industry Safety Summit, Dallas, TX, March 3-5.
———. 2011. 2011 beef safety summit executive summary. Paper read at Beef Safety Summit, Dallas, TX, March 2-4.
CDC (Centers for Disease Control and Prevention). 2012. BioSense. http://www.cdc.gov/biosense/ (accessed February 9, 2012).
CSPI (Center for Science in the Public Interest). 2011. Focus on: Food import safety. Washington, DC: Pew Health Group and the Center for Science in the Public Interest.
Drezner, D. W. 2007. All politics is global: Explaining international regulatory regimes. Princeton, NJ: Princeton University Press.
EMA (European Medicines Agency). 2011. Final report on the international API inspection pilot programme. London: EMA, Australian Government, and FDA.
EMA and FDA (Food and Drug Administration). 2011. Report on the pilot EMA-FDA GCP initiative: September 2009-March 2011. London: EMA and FDA.
Enneking, U. 2004. Willingness-to-pay for safety improvements in the German meat sector: The case of the Q&S label. European Review of Agricultural Economics 31(2):205-223.
FAO (Food and Agriculture Organization of the United Nations) and WHO (World Health Organization). 2005. Importance of stakeholder collaboration in Canada’s food safety system. Paper presented at FAO/WHO Regional Conference on Food Safety for the Americas and the Caribbean, San Jose, Costa Rica, December 6-9.
FDA (Food and Drug Administration). 1973. FDA—Canadian Department of National Health and Welfare agreement of cooperation between the Canadian Department of National Health and Welfare and the Food and Drug Administration. Silver Spring, MD: FDA.
———. 2002. Regulatory information: Bioterrorism Act of 2002. http://www.fda.gov/regulatoryinformation/legislation/ucm148797.htm (accessed February 16, 2012).
———. 2003. Confidentiality commitment statement of legal authority and commitment from Swissmedic not to publicly disclose non-public information shared by the United States Food and Drug Administration. Silver Spring, MD: FDA.
———. 2010. Statement of authority and confidentiality commitment from the United States Food and Drug Administration not to publicly disclose non-public information shared by the Agência Nacional de Vigilância Sanitária of Brazil. Silver Spring, MD: FDA
———. 2011. Pathway to global product safety and quality. Silver Spring, MD: FDA.
FSSAI (Food Safety and Standards Authority of India). 2011. Product recall pilot by FSSAI. http://www.fssai.gov.in/Product_Recall.aspx (accessed December 15, 2011).
G20. 2010. G20 Seoul development consensus for shared growth (annex 1). Paper presented at the G20 Seoul Summit, Seoul, Korea, November 11-12.
———. 2011. Members. http://www.g20.org/en/g20/members (accessed February 8, 2012).
GAO (Government Accountability Office). 2008. Drug safety: Preliminary findings suggest recent FDA initiatives have potential, but do not fully address weaknesses in its foreign drug inspection program. GAO-08-701T. Washington, DC: GAO.
———. 2010. Drug safety: FDA has conducted more foreign inspections and begun to improve its information on foreign establishements but more progress is needed. GAO-10-961. Washington, DC: GAO.
———. 2011. Drug safety: FDA faces challenges overseeing the foreign drug manufacturing supply chain. GAO-11-936T. Washington, DC: GAO.
GIFSL (Global Initiative for Food Systems Leadership). 2010. International food safety administration programme for senior officials (IFSA). http://foodsystemsleadership.org/Programs/program.aspx?proID=2 (accessed November 22, 2011).
Global Harmonization Initiative. 2011. About GHI. http://www.globalharmonization.net/background (accessed November 22, 2011).
Goetz, G. 2011. World food safety fund launched at APEC. http://www.foodsafetynews.com/2011/11/world-food-safety-fund-launched/ (accessed November 15, 2011).
Gomide, J., A. Veloso, W. Meira, F. Benuvenuto, V. Almeida, F. Ferraz, and M. Teixeira. 2011. Dengue surveillance based on a computational model of spatio-temporal locality of Twitter. Proceedings of the ACM WebSci’11, June 14-17:1-8.
Greis, N. P., M. Nogueira, P. MacDonald, and R. Wilfert. 2011. NCFEDA North Carolina foodborne events data integration and analysis tool: A new informatics tool for food safety in North Carolina. Research Triangle Park, NC: Prepared by RTI International—Institute for Homeland Security Solutions under contract HSHQDC-08-C-00100.
Gundersen, L. E. 2001. Training needs in regulatory science for the biopharmaceutical industry. Nature Biotechnology 19(12):1187-1188.
Health Canada. 2009. Report on the Health Canada/US-FDA and industry BPA value chain meeting. http://www.hc-sc.gc.ca/fn-an/securit/packag-emball/bpa/bpa_meet-reunion_20090130-eng.php (accessed November 22, 2011).
Henson, S. 2003. Food safety issues in international trade. Washington, DC: International Food Policy Research.
Henson, S., and S. Jaffee. 2008. Understanding developing country strategic responses to the enhancement of food safety standards. World Economy 31(4):548-568.
Horton, L. R., and E. Wright. 2008. Reconciling food safety with import facilitation objectives: Helping developing country producers meet U.S. and EU food requirements through transatlantic cooperation. Washington, DC: International Food and Agricultural Trade Policy Council.
IANPHI (International Association of National Public Health Institutes). 2011a. Long-term development projects: Saving lives through NPHIs. Atlanta, GA: IANPHI.
———. 2011b. Who we are. http://www.ianphi.org/who-we-are/ (accessed December 14, 2011).
ICDRA (International Conference of Drug Regulatory Authorities). 2010. 14 ICDRA recommendations. Paper presented at the Fourteenth International Conference of Drug Regulatory Authorities, Singapore, November 30-December 3.
IFT (Institute of Food Technologists). 2011. IFT 12: Annual meeting and food expo. http://www.am-fe.ift.org/cms/ (accessed November 21, 2011).
IOM (Institute of Medicine). 1988. The future of public health. Washington, DC: National Academy Press.
———. 2009. Managing food safety practices from farm to table: Workshop summary. Washington, DC: The National Academies Press.
———. 2011. Food forum. http://www.iom.edu/Activities/Nutrition/FoodForum.aspx (accessed November 22, 2011).
IUF (International Union of Food, Agricultural, Hotel, Restaurant, Catering, Tobacco, and Allied Workers’ Associations). 2009. Melamine poisoning and the death penalty in China: Scapegoats punished to avoid the real scandal. http://asianfoodworker.net/?p=409 (accessed November 22, 2011).
Keller, M., M. Blench, H. Tolentino, C. C. Freifeld, K. D. Mandl, A. Mawudeku, G. Eysenbach, and J. S. Brownstein. 2009. Use of unstructured event-based reports for global infectious disease surveillance. Emerging Infectious Diseases 15(5):689-695.
Kharas, H. 2011. The G-20’s development agenda. Washington, DC: The Brookings Institute.
Kuemmerle, A., A. Dodoo, S. Olsson, J. Van Erps, C. Burri, and P. Lalvani. 2011. Assessment of global reporting of adverse drug reactions for anti-malarials, including artemisinin-based combination therapy, to the WHO programme for international drug monitoring. Malaria Journal 10(1):57.
Lusk, J. L., J. Roosen, and J. A. Fox. 2003. Demand for beef from cattle administered growth hormones or fed genetically modified corn: A comparison of consumers in France, Germany, the United Kingdom, and the United States. American Journal of Agricultural Economics 85(1):16-29.
Maertens, M., and J. Swinnen. 2009. Food standards, trade and development. Review of Business and Economics LIV(3):313-326.
Maskus, K. E., T. Otsuki, and J. S. Wilson. 2005. The cost of compliance with product standards for firms in developing countries: An econometric study. Washington, DC: The World Bank.
Mattes, W. B., E. G. Walker, E. Abadie, F. D. Sistare, J. Vonderscher, J. Woodcock, and R. L. Woosley. 2010. Research at the interface of industry, academia and regulatory science. Nature Biotechnology 28(5):432-433.
Mok, E. A., L. O. Gostin, M. D. Gupta, and M. Levin. 2010. Implementing public health regulations in developing countries: Lessons from the OECD countries. Journal of Law, Medicine and Ethics 38(3):508-519.
Morehouse, J., and M. Moriarty. 2007. Food safety in China: What it means for global companies. Chicago, IL: A.T. Kearney.
Narrod, C., D. Roy, J. Okello, B. Avendaño, K. Rich, and A. Thorat. 2009. Public-private partnerships and collective action in high value fruit and vegetable supply chains. Food Policy 34(1):8-15.
NRC (National Research Council). 2011. The potential consequences of public release of food safety and inspection service establishment-specific data. Washington, DC: The National Academies Press.
Nuthall, K. 2005. Columbia: World Bank to make food production more competitive. Just Food, July 11.
Okello, J. J., C. Narrod, and D. Roy. 2007. Food safety requirements in African green bean exports and their impact on small farmers. Washington, DC: International Food Policy and Research Institute.
Roberts, D., and T. Josling. 2011. Tracking the implementation of internationally agreed standards in food and agricultural production. Washington, DC: International Food and Agricultural Trade Policy Council.
Rosenberg, S. 2006. Meeting the 2005 requirements for the Bioterrorism Act. Lawrenceville, NJ: Voluntary Interindustry Commerce Solutions.
RTI (Research Triangle Institute) International. 2011. BioSense strategic plan. https://sites.google.com/site/biosenseredesign/file-cabinet (accessed February 9, 2012).
Rx-360. 2009. About Rx-360. http://www.rx-360.org/AboutRx360/tabid/55/Default.aspx (accessed November 22, 2011).
SEMP (Suburban Emergency Management Project). 2008. CDC’s BioSense biosurveillance program: Performance update. http://www.semp.us/publications/biot_reader.php?BiotID=570 (accessed February 9, 2012).
Swaminathan, B., P. Gerner-Smidt, L.-K. Ng, S. Lukinmaa, K.-M. Kam, S. Rolando, E. P. Gutierrez, and N. Binsztein. 2006. Building PulseNet international: An interconnected system of laboratory networks to facilitate timely public health recognition and response to foodborne disesase outbreaks and emerging foodborne diseases. Foodborne Pathogens and Disease 3(1):36-50.
Unnevehr, L., L. Haddad, and C. Delgado. 2003. Food safety policy issues for developing countries. Washington, DC: International Food Policy Research Institute.
USDA (U.S. Department of Agriculture). 2005. USDA, FDA, DHS and FBI join states and private industry to protect nation’s food and agriculture supply from agroterrorism. http://www.usda.gov/wps/portal/usda/usdahome?contentidonly=true&contentid=2005/07/0279.xml (accessed November 22, 2011).
VanTrieste, M. 2011. Testimony before the Senate Health, Education, Labor, and Pensions Committee: Securing the pharmaceutical supply chain. Washington, DC.
WHO (World Health Organization). 2010. Guidance: Collaborative procedure between World Health Organization Prequalification of Medicines Programme (WHO-PQ) and selected National Medicines Regulatory Authorities (NMRAs) in inspection activities. Geneva, Switzerland: WHO.
———. 2011. International conference of drug regulatory authorities. http://www.who.int/medicines/areas/quality_safety/regulation_legislation/icdra/en/index.html (accessed October 5, 2011).
World Bank. 2005. Food safety and agricultural health standards: Challenges and opportunities for developing country exports. Washington, DC: The World Bank.
———. 2006. China’s compliance with food safety requirements for fruits and vegetables: Promoting food safety, competitiveness, and poverty reduction. Washington, DC: The World Bank.
———. 2010. World Bank to help improve food safety in China. http://www.worldbank.org/en/news/2010/05/13/world-bank-help-improve-food-safety-china (accessed November 21, 2011).
WTO (World Trade Organization). 2011. WTO public forum 2011: Topics for discussion. Geneva, Switzerland: WTO.
Xueref, S. 2010. Towards a global strategy on pharmacovigilance. Paper presented at Global Surveillance of Anitretroviral Drug Safety meeting, Washington, DC, June 2010.




























