From March to September 2011, the committee heard from various stakeholders in the United States and abroad. In the foreign workshops the travel delegations met government regulators from a dozen different low- and middle-income countries (see Appendix E). It also met with representatives of multinational and national food and medical companies, nongovernmental organizations (NGOs), regional economic organizations, donor organizations, and universities (see Appendix E). In its deliberations the committee synthesized what it learned in these workshops, identifying nine common problems that cut across countries and industries. These are the nine main problems on which the committee focused its discussions. This input and background research informed its analysis of the main issues developing country regulators face.
The committee found that regulators abroad face problems with: adhering to international standards, controlling supply chains, infrastructure, their laws, their workforce, institutional fragmentation, surveillance, communication, and political will. A detailed analysis of each of these gaps follows.
ADHERENCE TO INTERNATIONAL STANDARDS
One of the main responsibilities of a regulator is to ensure the food and medical product supply meets agreed upon standards for safety and quality. National regulatory authorities are entitled to set their own standards, but established international norms are expedient to use; they also facilitate trade. Some standards are set into a country’s legal code, others are set by private organizations or corporations (Giovannucci and Purcell, 2008).
Standard setting is one of the regulatory authority’s main responsibilities, separate from its responsibilities to enforce standards. For the purposes of this section, standards means “established norms or codified requirements for a product, such as material specifications or technical standards for performance. Standards may be developed by regulatory agencies, public organizations, or industry associations” (Marucheck et al., 2011, p. 714). Tables 3-1 and 3-2 list some important organizations and describe their work in standard setting.
Proponents of standards maintain that their use helps traceability through the supply chain, eliminates redundant audits, and when, harmonized across markets, decreases bureaucracy. Others see standards as little more than fines on poor countries because of the high costs of compliance (Marucheck et al., 2011). A debate on this topic is outside the scope of this report. Regardless of the reasons these standards exist, quality assurance and adherence to international norms are essential as developing countries introduce regulated goods into the global marketplace.
Adherence to Food Standards
Adherence to international standards is a problem in the agri-food industry in many low- and middle-income countries. In these countries there is a large domestic market for products that stringent regulatory authorities would reject. People in developing countries often do not demand, for example, process certification or assurance of minimal pesticide residues. This may be because they are often not aware of the public health risks international standards aim to protect against. They may also assume, sometimes incorrectly, that it is possible to assess the producer’s quality practices at point of purchase when the market has few middle men. More importantly, these countries still struggle to feed their citizens; concerns about trace pesticide residues seem frivolous in comparison to hunger. The threat of death from starvation in the next month will dwarf theoretical cancer risks in 50 years.
In China, for example, food safety has only been an official priority for the past 12 years (Gale and Buzby, 2009). It is especially difficult in such a large country to keep the estimated 200 million farmers working plots of 2 acres or less abreast of good agricultural practices (Gale and Buzby, 2009). China’s roughly 400,000 cottage industry food processers face similar challenges (Gale and Buzby, 2009).
The involvement of the least developed countries and their institutions in international standard setting organizations such as Codex is often nominal. The Codex Trust Fund aims to correct this by supporting scientists from the least developed countries and small island nations to participate better at Codex (WHO, 2011a).
TABLE 3-1
International Standard Setting Organizations for Food
| Organization | Year Established |
What they do | What they set standards in | ||||||
|
United States Pharmacopeial Convention (USP) |
|
1820 |
|
“USP establishes documentary and reference standards to ensure the quality and consistency of medicines, dietary supplements, and food ingredients” (USP, 2008). |
|
• Purity and identity of food ingredients (USP, 2011a) |
|||
|
AOAC International |
|
1884 |
|
AOAC International provides science-based expertise to de-velop voluntary consensus standards or technical standards through stakeholder consensus and working groups (AOAC, 2011). |
|
• Single laboratory validation for botanicals • Study validation • Food microbiology • Characterization of antibodies used in immunochemical methods of analysis for mycotoxins and phycotoxins (AOAC, 2009) |
|||
|
International Organization for Standardization (ISO) |
|
1947 |
|
A network of national standards institutes from 162 coun-tries (ISO, 2011a) that sets trade standards and fosters standardization activities (Giovannucci and Purcell, 2008b). |
|
• Food Products • Good Management Practices • Management systems for food safety |
|||
|
International Plant Protection Convention (IPPC) |
|
1952 |
|
“The IPPC provides an international framework for plant protection that includes developing international standards for phytosanitary measures for safeguarding plant resources” (IPPC, 2011). |
|
• Procedures and references • Pest surveillance, survey, and monitoring • Import regulations and pest risk analysis • Compliance procedures and phytosanitary inspection methodologies • Pest management • Post entry quarantine • Exotic pest emergency response, control, and eradication • Export certifi cation (IPPC, 2011) |
|||
|
International Commission on Microbiological Specifi cations for Food (ICMSF) |
|
1962 |
|
ICMSF provides science-based guidance to both government and industry on evaluating and controlling the microbiological safety of foods (ICMSF, 2011). ICMF is not a standard setting organization. It is included here because of its valuable advisory role. |
|
• Microbiological limits and criteria in food (ICMSF, 2011) |
|||
|
Codex Alimentarius Commission |
|
1963 |
|
Codex is responsible for developing “food standards, guidelines and related texts such as codes of practice under the Joint FAO/WHO Food Standards Program” (CAC, 2011). “The purpose of the program is to protect the health of consumers, ensure fair trade practices in food trade, and promote the coordination of all food standards work undertaken by international governmental and non-governmental organizations” (CAC, 2011). |
|
• Food quality and safety • Codes of hygienic or technological practice • Pesticide and food additive evaluation • Limits for pesticide residue • Guidelines for contaminants (Henson and Humphrey, 2009) |
TABLE 3-2
International Standard Setting Organizations for Medical Products
| Organization | Year Established |
What they do | What they set standards in | ||||||
|
United States Pharmacopeial Convention (USP) |
|
1820 |
|
“USP establishes documentary and reference standards to ensure the quality and consistency of medicines, dietary supplements, and food ingredients” (USP, 2008). |
|
• Identifi cation testing • Limit testing for impurities or related compounds • Assays for drug substances and formulations • System suitability testing (USP, 2011c) • Product quality verifi cation services for drug substances and excipients used to make over-the-counter and prescription pharmaceuticals (USP, 2011d) |
|||
|
International Organization for Standardization (ISO) |
|
1947 |
|
A network of national standards institutes from 162 coun-tries (ISO, 2011a) that sets trade standards and fosters stan-dardization activities (Giovannucci and Purcell, 2008b). |
|
• Requirements and testing methods for medical devices (ISO, 2011b) |
|||
|
International Conference on Harmonization (ICH) |
|
1990 |
|
The ICH “makes recommendations toward achieving greater harmonization in the interpretation and application of technical guidelines and requirements for pharmaceutical product registration” (ICH, 2011). |
|
• Good Clinical Practices • Good Manufacturing Practices of medicines, biologics, and vaccines • Standards for the transfer of regulatory information |
|||
|
The Global Harmonization Task Force (GHTF) |
|
1992 |
|
The GHTF encourages the harmonization of regulatory prac-tices related to ensuring the safety, e. ectiveness, perfor-mance, and quality of medical devices, promoting advance-ments in technology, and assisting international trade. This is accomplished through the publication and dissemination of harmonized guidance documents on basic regulatory practices (GHTF, 2007). • Medical device safety, e. ectiveness, performance, and quality (GHTF, 2007) |
|||||
|
European Directorate for the Quality of Medicines and Healthcare (EDQM) |
|
1996 |
|
The EDQM “protects public health by enabling the development, supporting the implementation, and monitoring the application of quality standards for safe medicines and their safe use (EDQM, 2011).” |
|
• Quality and safety of medical products (EDQM, 2011) |
|||
Still, the poorest countries do not have representatives with sufficient expertise to participate meaningfully in standard setting meetings (African Union Interafrican Bureau for Animal Resources, 2011). Sometimes logistical constraints complicate participation in these meetings. International travel is too expensive for regulatory agencies to fund (World Bank, 2008).
There is also evidence that, especially in Sub-Saharan Africa, the food producers have no way to give input into standard development (World Bank, 2003). This means that small- and medium-size enterprises, and even some larger firms, rely on their importing agents or their national regulatory authority to make information available. There are also too few scientists qualified to analyze the standards. Without advocates these countries become “standards-takers” rather than active participants in the dialogue (World Bank, 2003).
It is expensive to adhere to international standards. At the very least it requires a supplier to be able to trace products through the supply chain and show proof of adherence to best practices at all stages of production. This proof usually takes the form of a certificate of inspection, audit, or accreditation. Producers pay for inspections and certification, and for small producers these costs are prohibitively high (Giovannucci and Purcell, 2008). Some agri-food standards, those on pesticide residues for example, rely on technical skills and laboratory equipment that are essentially missing in many developing countries (Jaffee and Henson, 2004; World Bank, 2003). For all these reasons, the World Trade Organization (WTO) called for donor aid to improve developing countries adherence to standards in their Sanitary and Phytosanitary (SPS) agreement. Bilateral and multilateral agencies spent between $65-$75 million a year in the years after the agreement on building capacity for agri-food health management (Jaffee and Henson, 2004). The full benefit of these investments is yet hard to measure. There is a learning curve when new technology is introduced to a sector, as well as a time lag when new staff are trained to use it (World Bank, 2003).
In the meantime, the inability to adhere to standards deepens inequalities in market access between counties (Belton et al., 2010). Only eight countries, most of them in Latin American, account for two-thirds of all fruit and vegetable exports from emerging economies (Stcichele et al., 2006). Even these Latin American countries, with relatively advanced systems for maintaining standards, can be subject to border rejections, and rejections cost middle-income countries about $1.8 billion in 2001 (Jaffee and Henson, 2004). Border rejections are only a fraction of the income lost. Econometric analysis indicates that China alone lost an estimated $8 billion in export income in 2002 because of failure to meet standards (Lu, 2005). The individual financial losses are also heavy. Vietnamese farmers who can
comply with supermarket standards earn about 400 percent higher profits than those who cannot (M4P, 2006).
Access to export markets could improve the economies in some of the least developed countries, and the health and social benefits of adhering to standards cannot be understated. Aflatoxin, a food contaminant, accounts for an estimated 25,200-155,000 cases of liver cancer a year, overwhelmingly in countries without strict food standards (Liu and Wu, 2010). Even in the United States roughly 3,000 people die every year from foodborne illness (CDC, 2011). The Centers for Disease Control and Prevention (CDC) estimates that food imported to the United States caused 2,348 illnesses between 2005 and 2010 (CDC, 2012). Half of these outbreaks happened in 2009 or 2010, and about 45 percent of them have a probable source in Asia (CDC, 2012).
Globally, there are an estimated 155,000 deaths each year from foodborne Salmonella infections alone (Majowicz et al., 2010). Adherence to manufacturing and agriculture standards would improve working conditions and protect the environment in many countries. Farming in accordance with good agricultural practices, for example, improves soil quality and prevents erosion (Poisot et al., 2004).
Adherence to Medical Product Standards
In many ways, problems in adhering to international standards in the medical products industry are similar to those in agriculture and food. Regulators in low- and middle-income countries depend on standards developed abroad; they often have minimal input into the standard setting process. Even more so than with agri-food standards, adhering to drug, biologics, and device standards demands sophisticated testing laboratories and control of complicated supply chains.
The International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) is a standard setting organization for drugs; it has membership from European, Japanese, and U.S. pharmaceutical industry associations, and the drug regulatory agencies of 17 countries (Abraham, 2002; ICH, 2011a). ICH activities generally focus on its member countries, but it is increasingly working to improve good manufacturing practices around the world. It held a training on the same for southern African regulators in Tanzania in June of 2011, for example (ICH, 2011b).
As in the agri-food sector, developing countries are standard-takers rather than standard-makers. This can cause problems. Until recently the ICH guidelines on medicine shelf life, for example, failed to account for stability in hot, humid climates (Kopp, 2006). A working group of Southeast Asian nations remedied this and brought attention to the problems of
accepting ICH guidelines outside of ICH regions (Kopp, 2006). In other cases, the solutions for the standard-takers are not as clear. Far more rich countries than poor ones regulate diagnostics; of those that do only 68 percent require regulatory review of clinical trial data, and trial data with as few as 15 subjects are often acceptable (Peeling et al., 2010). When clinicians in developing countries use diagnostics developed abroad they base their understanding of the tests’ predictive value on product inserts, values that are not accurate if the disease prevalence in the trial population differs from that in the population tested.
Even when international standards are available to regulators and are appropriate, there are problems in adhering to quality standards if the medical regulatory authority has insufficient funding or trained staff or both. For example, one essential function of drug and biologic standards is to answer the questions, “Is this drug what is says it is, in the stated strength, and is it free of contaminants?” (Kenyon et al., 1994, p. 615). Quality-control laboratories answer these questions, but many countries cannot afford to set up and staff these laboratories (Leng and Matsoso, 2008). Outsourcing quality control is one way around this; private companies can do quality control for a national drug supply, as is the case in the United Kingdom. Leng and colleagues recounted hesitation to use private laboratories in both South Africa and Algeria though because of concerns about conflicts of interests given that the quality-control laboratories in question worked for both government and industry (Leng and Matsoso, 2008).
Developing countries also face challenges in implementing good manufacturing practices; the standards that ensure all manufacturing steps can be reproduced and result in the desired products. These are of critical importance in the production of vaccines and other biological products, given the inherent variability in testing a biologically active product (Milstien et al., 2009). The World Health Organization (WHO) Prequalification of Medicines and Vaccines Program facilitates access to quality medicines and vaccines for treating priority diseases. As Chapter 2 describes, this program evaluates product safety, quality, and efficacy and serves as the grounds for donor procurement. The vaccine and medicine companies that pass the evaluation must meet good manufacturing practices and be overseen by a competent national regulatory authority; it is the government regulator’s responsibility to enforce manufacturing standards (Brhlikova et al., 2007). In 2009 the WHO announced that it would withhold new prequalification evaluations from Indian companies barring improvements to their national regulatory authority (Milstien et al., 2009).
In the same way, some consumers see WHO prequalification as an international vote of confidence in the national regulatory authority. On March 1, 2011, the WHO recognized the Chinese State Food and Drug Administration as compliant with international regulatory standards, a
decision that will allow for the eventual introduction of Chinese vaccines into international use (Jia and Carey, 2011). This may help restore public confidence in Chinese vaccine companies after a year of scandals. Substandard rabies and hepatitis B vaccines were rumored to have killed or sickened about 100 babies in Shanxi province in 2010; shortly afterward a company in Jiangsu province also produced substandard rabies vaccines (Jia and Carey, 2011).
WHO prequalification drives compliance with international good manufacturing practices and gives incentive to improve government regulation. Economies of scale keep small countries out of the vaccine prequalification system. Similarly, WHO drug prequalification encourages adherence to international standards, at least in emerging economies large enough to support a manufacturing sector. Smaller countries depend on prequalification in their drug procurement.
Pharmaceutical manufacture in most emerging economies was designed for generics, and their drug innovation system suffers. Some developing countries do not regulate human subjects’ protection in trials or require peer review of human subject protocols by institutional review boards, perhaps because governments see trials as a way for some of their citizens to get medical care (Kelleher, 2004). Still, the richest countries are home to 15 percent of the world’s population and 75 percent of drug trial participants (Herring, 2011). Consistent adherence to international research standards could change this and would give depth to the results of drug trials, increase understanding of drug development, benefit patients in the developing world, and improve the economies of least developed countries (Herring, 2011).
CONTROLLING SUPPLY CHAINS
Food and medical product supply chains are complex and far-reaching. In the United States, the 2002 Bioterrorism Act requires all parties in the food supply chain to identify the immediate previous source of their products and the immediate recipient; known as one-up, one-back traceability (Gessner et al., 2007). When every actor is responsible for one-up, one-back reporting, it is possible to re-create the entire supply chain, even if no one party has a complete picture of it. Traceability requirements are less clear in medical products supply chains. During the 2008 heparin crisis neither the U.S. Food and Drug Administration (FDA) nor Baxter was able to re-create the heparin supply chain quickly; it took weeks to even get close to the source. The exact identification of the responsible actors was never possible (Pew Health Group, 2011). Multinational companies are exploring radio frequency identification tags and two dimensional bar codes to trace
products through their supply chains (McMeekin et al., 2006). In developing countries, controlling supply chains is even more of a problem.
The Food Supply Chain
Large multinational corporations such as Wal-Mart, Archer Daniels Midland, ConAgra, Nestle, Cargill, and Unilever control a great deal of the international food market. These companies have close relationships with their suppliers; they can trace their supply chains in developing countries, a considerable accomplishment considering that a granola bar contains ingredients from half a dozen different countries (Figure 3-1) (Carey, 2007). These companies monitor their supply chains using the principles of Hazard Analysis and Critical Control Points (HACCP), described in Chapter 2. There are five main links in the food supply chain: the farm, the packing house, the transportation, the market, and the consumer (UC Davis Department of Plant Sciences, 2011).
Over the past decade there has been a rapid growth in production of high-value agriculture, premium products such as vegetables, fruits, and animal products. Much of Africa’s high-value exports are grown in countries with high altitudes and year-round growing seasons and exported to Europe (Okello et al., 2007). There are usually separate supply chains feeding the export and domestic markets, with relatively little crossover. High-value agricultural products are highly perishable; logistics, in particular the availability of airfreight space, play a significant role in their trade
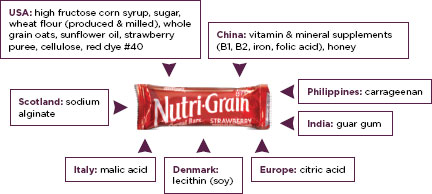
FIGURE 3-1
Global sourcing of food ingredients.
SOURCE: Roth et al., 2008.
(Okello et al., 2007). Orders from retailers come in late at night once the European markets are closed, but the crops are picked earlier in the day. When the export order does not match what the supplier packed, the order may end up in the local market, though usually the exporters cannot get the same price that they would have in the European market (Henson and Humphrey, 2009).
With the notable exception of one participant from Uruguay, the guests at the various site visits for this study explained that there are two supply chains in their countries: one for export and one for local consumption. Standards are generally lower for the domestic market (Broughton and Walker, 2010; Llana, 2010). At the New Delhi meetings for this report, Indian participants mentioned that having two food safety standards does not trouble them; some stressed that Indians take care to avoid food spoilage at home by marketing daily and boiling their milk every hour. Others believe that Indians have higher innate immunity to foodborne disease than Westerners. Similar misconceptions are common in China (Roth et al., 2008).
Spoilage is one of the main problems in the domestic supply chain of

Workers in Honduras wash thousands of bananas a day, preparing them for evaluation. Bananas that are exactly the right weight, length, and color are packaged and shipped to the United States; those that are not acceptable are sold in Honduras.
SOURCE: © 2007 Sarah Axelson, Courtesy of Photoshare.
developing countries. Often it takes too long for products to get to market over poor roads and without refrigeration. There are, for example, 280,000 refrigerated trucks transporting food in the United States, while China, with its vastly larger population, has only 30,000 (Barboza, 2007). As much as 35 to 40 percent of fresh produce in India spoils because of lack of refrigeration in the wholesale or retail markets (Godfray et al., 2010; Kader, 2010). Some experts predict that this will change. The Indian grape business has had recent success by bringing small grape farmers together in Mahagrape, an association of grape growing collectives (Roy and Thorat, 2008). These for-profit collectives give farmers access to cooling and storage infrastructure. The Indian agricultural cold chain business has an estimated net value of $2.6 billion, expected to more than quadruple by 2015 (Narula, 2011).
High-value agricultural products, such as tomatoes and green beans, need to be kept at chill temperatures; they can spoil quickly in heat or cold. Grains have a longer shelf life, but rats will eat them if they are not stored in silos or grain safes; one-third of the grain stores in Southeast Asia are lost to pests (Godfray et al., 2010). According to an expert at the International Fund for Agriculture and Development, these losses could be reduced by half with proper refrigeration and post-harvest storage (Waste not, want not, 2011). Figure 3-2 shows the relative food lost between the farm and fork in different regions of the world. Notably, household waste
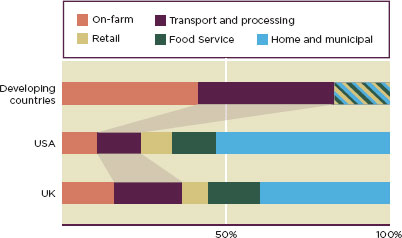
FIGURE 3-2
Makeup of total food waste in developed and developing countries. Retail, food service, and home and municipal categories are lumped together for developing countries.
SOURCE: Godfray et al., 2010.
is a small fraction of the food lost in most regions (Figure 3-2). Reductions in post-harvest losses would be of tremendous value to the poor in developing countries. Less than 5 percent of agricultural research funding goes to post-harvest losses (Kitinoja et al., 2011).
Protecting the transportation and storage steps of the supply chain becomes increasingly important as the population in developing countries becomes more urban. Supermarkets, which generally have high quality standards and interest in their branding, are increasingly the food markets of choice in middle-income countries, such as Vietnam, and middle-class shoppers in low-income countries, such as India (M4P, 2006). Small farmers struggle to meet supermarket standards; supermarkets will reject produce only for cosmetic reasons that have nothing to do with safety or nutritional value (Gustavasson et al., 2011). Cities in the least developed countries have fewer supermarkets and more wholesale and street markets that “are often small, overcrowded, unsanitary, and lacking in cooling equipment” (Gustavasson et al., 2011, p. 13). Food spoils quickly in these markets, but poor shoppers have little choice but to buy it anyway. This is offset, in part, by daily marketing, a common practice in developing countries.
Disorganized retail supply chains hurt farmers as well. Desperation often drives poor farmers to sell under-ripe crops during the pre-harvest hungry season, sabotaging their income and the nutritional value of the food (Gustavasson et al., 2011). In Rajasthan, a large onion-growing state in west India, farmers routinely dump part of their crop along the highway, because their revenues do not even cover the costs to bring the crop to market (Maheshwar and Chanakwa, 2006). The use of relatively simple technologies could increase small farmers’ incomes and reduce waste in developing countries. Drying and juice making near the farm could preserve expensive fruits and vegetables, for example, provided there is equipment to pasteurize and package the food.
The Medical Products Supply Chain
A typical pharmaceutical supply chain consists of the primary manufacture of chemicals from their raw state; several steps of secondary manufacture from processed products; market warehouses and distribution centers; wholesalers; retailers; hospitals, clinics, or pharmacies; and, finally, patients (Yu et al., 2010). Drug regulatory authorities in developing countries often lack the ability to monitor the steps on this supply chain. These drug regulatory authorities are often supported partly from the government and the rest from user fees (Yadav, 2009). They are focused on the most pressing tasks: licensing and registering products and giving marketing approvals (Yadav, 2009). There is little attention to factory inspections; quality-control tests at retail or wholesale points are almost unknown. As mentioned in the section
on standards, there is little postmarketing surveillance. It is also difficult to control imports, especially in parts of the world where there are many small, neighboring, landlocked countries. These factors make for a porous pharmaceutical supply chain. Fake drugs are a common problem.
A full analysis of the problem of counterfeit, falsified, and substandard drugs is outside the scope of this report, but medicines regulators in the countries visited for this study repeatedly raised it as a concern (Box 3-1). In September 2011 the Food and Drug Administration (FDA) commissioned the Institute of Medicine (IOM) to convene a consensus study entitled Understanding the Global Public Health Implications of Substandard, Falsified, and Counterfeit Medical Products. This report, which will be released in 2013, will aim to clarify the terms used to discuss pharmaceutical fraud, describe the scope of the problem, and recommend action to reduce the public health consequences of fake drugs in developing countries.
By WHO estimates, between 20 and 90 percent of antimalarials in Sub-Saharan Africa and 38-53 percent of the same drugs in Southeast Asia fail quality testing (Newton et al., 2010; WHO, 2005). Fraud also affects medical devices and in vitro diagnostics, a topic reported on in the Asian press (Mori et al., 2011). Tampering with expiry dates on in vitro diagnostics in Vietnam was the subject of Lancet correspondence (Day et al., 2004; Watt, 2004). There are a variety of sophisticated techniques that can prevent this fraud, but many are expensive and impractical in developing countries (Newton et al., 2010). Organizations such as Sproxil have made some progress recently with using mobile phones and paper watermarking to authenticate bar codes (Sharma et al., 2008; Sproxil, 2011). There is a need for more inexpensive ways to secure medical products supply chains in developing countries, however.
As in the food supply chain, some of the problems with medical products supply chains are related to infrastructure. There is a lack of hard data on where in the pharmaceutical supply chain bottlenecks exist (Oluka et al., 2010). In an assessment of the pharmaceutical sector in East Timor, Norris and colleagues described small warehouses and medicines being kept in tropical heat and humidity at every point between entering the country and the patients’ hands (Norris et al., 2007). In 2008, the Global Fund identified similar problems with medicine storage and inventory control in an audit of its Indian grant programs (Global Fund, 2008).
Vaccines are particularly vulnerable to spoilage in developing countries. An incomplete cold chain was the probable cause of a polio outbreak in South Africa in the mid-1990s (Schoub and Cameron, 1996; Setia et al., 2002). The problems are not confined to tropical climates: Lugosi and colleagues found that cold weather damaged 38 percent of vaccines sampled in Hungary (Lugosi and Battersby, 1990). By 2019 another dozen vaccines may be introduced in developing countries, but without fast-moving,
BOX 3-1
Counterfeit, Falsified, and Substandard Drugs
There are no universally accepted definitions for counterfeit, falsified, and substandard drugs (Clift, 2010a, 2010b). A single product can be simultaneously counterfeit, falsified, and substandard, or some combination of the three (Oxfam International, 2011).
The World Health Organization defines counterfeit drugs as “deliberately and fraudulently mislabeled with respect to identity or source” (WHO, 2011b). Counterfeit applies to “both branded and generic products [and] may include products with the correct ingredients or with the wrong ingredients, without active ingredients, with insufficient active ingredients or with fake packaging” (WHO, 2011b). This definition has been a source of ongoing controversy. It conflates the definition of counter-feit, which has a specific legal meaning in the context of intellectual property, with the drug quality and safety (Clift, 2010a). According to the WHO, however, whether “a good is considered counterfeit from a public health perspective is independent of whether the product infringes on intellectual property rights” (WHO, 2010b, p. 3). A counter-feit medicine, following the WHO definition, may or may not violate intellectual property rights.
The term falsified evolved, primarily in Europe and Latin America, as a way of distinguishing between intellectual property or trademark violations and fake drugs (Clift, 2010a). It refers to drugs “falsified in relation to their identity, history or source. Those products usually contain sub-standard or falsified ingredients, or no ingredients or ingredients in the wrong dosage, including active ingredients, thus posing an important threat to public health” (EU, 2011).
The definition of substandard is generally agreed upon as drugs that fail to meet quality specifications established by WHO standards (Clift, 2010a; Oxfam International, 2011). What is not agreed upon, however, is whether or not the category of substandard drugs includes counterfeit and falsified medicines. In 2003, the WHO stated that substandard medical products may be a “result of negligence, human error, insufficient human and financial resources, or counterfeiting. Counterfeit medicines are part of the broader phenomenon of substandard pharmaceuticals. The difference is that they are deliberately and fraudulently mislabeled with respect to identity or source” (WHO, 2003b). In 2009, however, it revised this definition to specifically exclude counterfeiting (Clift, 2010a). The revised definition defines substandard drugs as drugs that do not meet quality specifications, but that are produced by manufacturers authorized by a given national medical regulatory authority.
temperature controlled supply chains, these vaccines will not be effective (Kauffmann et al., 2011). By some estimates, the demands on the vaccine cold chain will increase 20-fold during this time (see Figure 3-3) (Sabot et al., 2011). Box 3-2 describes the vaccine supply chain in developing countries.
Even considering only routine immunization using the currently available vaccines, the vaccine cold chain capacity is insufficient, outdated, and broken—a serious bottleneck in increasing immunization rates. The poor cold chain compromises vaccine efficacy and, in some cases, vaccine safety as well. The projected expansion of the immunization program will surely aggravate this problem (Sabot et al., 2011). In 2007 PATH and the WHO launched the Optimize Project, with funding from the Bill and Melinda Gates Foundation (PATH, 2012a). Optimize aims to identify sustainable solutions for building cold chain capacity for future vaccines (PATH, 2012b).
There are also promising improvements in the heat stability of vaccines. A high throughput screening process for identifying thermostable formulations promises to improve the stability of a number of new and existing vaccines, while developments in controlled-temperature vaccines can mitigate the problems of cold chain breaks (Chen and Kristensen, 2009; Schlehuber et al., 2011). Other simple technologies have the potential to improve the strength of the vaccine cold chain. Temperature-sensitive labels, for example, that change color to indicate when a vaccine has been exposed to damaging temperatures are currently being procured by the United Nations Children’s Fund (UNICEF) (PATH, 2012c). New investments in cold chain capacity, coupled with new technological advances such as thermostable vaccines, will be invaluable tools to meet increased demands on the vaccine cold chain over the next decade (Chen and Kristensen, 2009; Sabot et al., 2011). Developing country regulatory authorities need to be kept informed of these developments.
There are also problems with the points on the medicine supply chain closest to the patient. In 2002 the consulting firm A.T. Kearney estimated that half of the medicine shortages in Mexico were because of poor inventory management and demand planning (Box 3-3) (A.T. Kearney, 2004a; Sarley et al., 2006). Hospital administrators or pharmacists can estimate their demand for medicines either by modifying previous years’ records or by calculating the number of patients presenting with a given condition from national morbidity data (A.T. Kearney, 2004b). Either way, supply chain planning requires reliable surveillance and some managerial proficiency in the health care workforce, common shortcomings that will be discussed later in this chapter.
Once the hospital or pharmacy has an estimated medicines projection, it should communicate its need to the warehouse, distribution center,
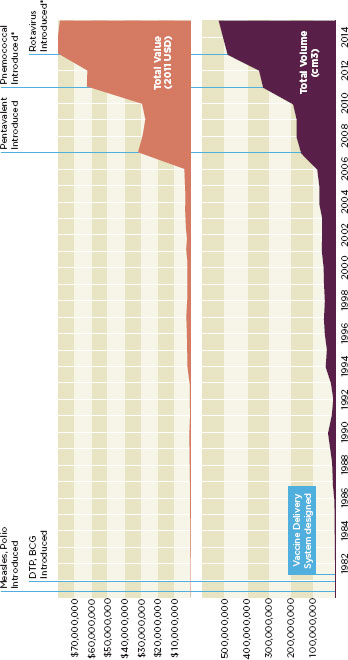
FIGURE 3-3
Demands on vaccine delivery systems are rising dramatically.
(Cumulative value and volume of vaccines used in routine childhood immunization: Ethiopia).
* Planned introduction date.
SOURCE: Sabot et al., 2011.
BOX 3-2
Vaccine Supply Chains in Low- and Middle-Income Countries
Vaccines usually need to be stored between 2° and 8°C (Chen and Kristensen, 2009). Some are heat-sensitive, rendered inactive at high temperatures; others are cold sensitive, rendered inactive by freezing. Maintaining temperature control in places without electricity is challenging and gets more complicated when health workers carry the vaccine for miles to give immunizations in remote villages. Vaccines also have a short shelf life that leaves little room for forecasting errors, inefficient management, or slow distribution. Many countries waste as much vaccine as they use. This will have to change over the next decade when more and costlier vaccines come into use. Trained logisticians and supply chain managers will be invaluable to this effort, but they are hard to find in the places that need them most.
There are two vaccine supply chains in developing countries: one that carries the vaccines from the factory to the developing country port of entry and one that carries the vaccines from the port of entry to the patient. The supply chain that carries the product from the supplier to the port of entry is generally strong, thanks to UNICEF and the shipping companies they contract with. Within the recipient country, immunization program managers decide how and where to store the shipments and when to release them to regional or provincial storehouses.

A nurse vaccinates a 4-month-old baby outside her home in Nueva Segovia, Nicaragua.
SOURCE: © 2008 Adrian Brooks, Courtesy of Photoshare.
In their analysis of vaccine supply chains Kauffman and colleagues stress the importance of moving vaccines to patients and discourage the common practice of holding large inventories in case of emergency. To this end, they suggested removing unnecessary storage levels. Warehouse management is complicated and introduces opportunities for the supply chain to breakdown. The Thai government reorganized its vaccine supply chain in 2009, removing three levels of store housing and began shipping directly from the central warehouse to health centers.
Donors could also help by not insisting on separate shipments, storage, and handling for donated vaccines. Kauffmann and colleagues describe a Kenyan health center that takes shipment from five warehouses, 13 procurement agencies, and 18 donor organizations. Such redundancy hinders the development of integrated, efficient supply chains.
SOURCE: Kauffmann et al., 2011
or whole seller. Sometimes the ordering system slows down this process. In both Tanzania and South Africa, for example, the Ministry of Health requires health workers to report detailed patient summaries to a central pharmacy when ordering the essential medicine acyclovir (Corbell et al., 2010). This extra step slows procurement and leads to frequent stock-outs (Corbell et al., 2010). Communication with central distribution is a common bottleneck, one that modern information technology and supply chain management could do much to unblock (Oluka et al., 2010). Figure 3-4 highlights other supply chain gaps.
There is also often an erratic lead-time between placing the order and having it delivered (Jahre et al., 2010). When dispensary managers cannot predict how long it will take to refill their drug supply, they stockpile drugs. Stockpiling in turn encourages other supply chain problems, such as using drugs past their expiry date. Stockpiling in one dispensary often causes shortages in another (Corbell et al., 2010).
The expanded use of anti-retroviral drugs in Sub-Saharan Africa has brought attention to the problems of supply chain management. The National University of Rwanda’s pharmacy department includes pharmaceutical management in its pre-service curriculum. Makerere University in Uganda and Muhimbili University of Health and Allied Sciences in Tanzania both have plans to develop master’s programs in pharmaceutical supply chain management (Matowe et al., 2008). The United States
BOX 3-3
Supply Chain Management in Mexico
Frequent stock-outs were a problem at Mexican pharmacies and health centers in 2002 when President Vincente Fox enlisted the help of the management consulting firm A.T. Kearney to improve the Mexican pharmaceutical supply chain (A.T. Kearney, 2004a). Working with the Mexican government, A.T. Kearney developed solutions that relieved the pressure of health care budgets, reduced the cost of medicine, and improved the efficiency of the drug supply chain (A.T. Kearney, 2004b). This included adopting a consistent demand-planning methodology, streamlining the drug procurement process, and improving inventory management (A.T. Kearney, 2004a).
The consultants found that more than half of the medicine shortage in Mexico was caused by poor inventory management. They recommended calculating drug demand using morbidity data (A.T. Kearney, 2004a). That is, health center staff estimated the number of patients they would treat for a given disease and combined the estimated number of patients with the approximate amount of medicine required to treat them. The forecasts were adjustable, to account for local differences in morbidity and local treatment preferences. The adoption of this method resulted in an 80 percent accuracy rate in Mexico’s drug forecasting (A.T. Kearney, 2004a).
Long delays in drug procurement were still a problem, however. The procurement process took 4 months, causing a drug shortage in the first quarter of every year (A.T. Kearney, 2004a). Poor communication among many small hospitals and clinics prevented them from pooling their drug orders. Working together and using a standardized, public bidding process, these institutions switched to a system of large drug orders placed less frequently (A.T. Kearney, 2004a). They also switched to a pull system* where hospitals and health centers could order their own medicines. By adopting this system, health officials were able to improve the management of their drug inventory and reduce costs (A.T. Kearney, 2004a).
In 2002, much of the Mexican drug legislation was outdated and poorly understood, even by health professionals (A.T. Kearney, 2004a). This led to confusion and an overall frustration with the system as a whole. A.T. Kearney worked with officials to eliminate unnecessary rules that hampered the purchasing of drugs from suppliers.
Within 2 years of making these simple changes and restructuring the value chain, the percentage of Mexicans receiving full prescriptions rose from 70 percent to more than 90 percent, with no added costs to the consumer or manufacturer (A.T. Kearney, 2004a).
![]()
* In a pull system, each level of the supply chain determines its drug needs using a formula that takes costs, demand, distribution, and the level of inventory into consideration. Orders of medicines are based on real consumption data. (A.T. Kearney, 2004a).
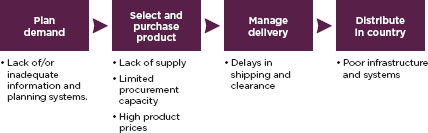
FIGURE 3-4
Challenges and bottlenecks in a drug supply chain.
SOURCE: Oluka et al., 2010.
Agency for International Development’s (USAID’s) Supply Chain Management program is also working in President’s Emergency Plan for AIDS Relief (PEPFAR) countries to build on existing drug supply chain to better handle the increase brought on by anti-retroviral drugs (USAID, 2011).
INFRASTRUCTURE
It is difficult to separate problems controlling supply chains from problems with infrastructure. Inadequate storage for foods, medicines, and vaccines are infrastructure deficits. The vaccine supply chain described in Box 3-2, for example, aims to move vaccines swiftly from the airport to the patient; it depends on reliable electricity for temperature control, strong telecommunications systems to facilitate timely orders, and decent roads, all common infrastructure gaps in poor countries. A strong food and medical products regulatory system is itself a key piece of the public health infrastructure. Similarly, a surveillance system is part of the regulatory infrastructure. Without surveillance and staff trained in management and causal inferences, countries are vulnerable to vaccine safety scares, for example (Black et al., 2010). But for the purposes of organizing this report, the infrastructure gaps the committee identified in developing countries fall into the categories of laboratory, manufacturing, and market infrastructure, and information and communication infrastructure.
Laboratory, Manufacturing, and Market Infrastructure
Food and medical product regulators in poor countries do not have the quality control and reference laboratories that their counterparts in rich countries take for granted. In India, for example, the site visitors heard repeatedly that Indian food production was totally compliant with the Inter-
national Standards Organization, but there was little evidence of a sufficient testing infrastructure to confirm this. A World Bank analysis confirmed that India’s 4 national and 79 state food safety laboratories had neither the equipment nor the personnel to properly collect and analyze food samples (World Bank, 2009). The same assessment found that of the 19 drug testing laboratories in India, only 7 had the ability to run a full range of assays (World Bank, 2009). Some countries work around their infrastructure shortages. In South Africa, the drug regulatory authority contracts universities to do quality control testing for biologics and drugs (Essack et al., 2011). But in some of the poorest countries there are no accredited safety testing laboratories (Abegaz, 2006). Some countries, such as Pakistan, need to rely on regional analytic labs, and sending samples regularly to distant labs is time consuming, expensive, and slow (Hao, 2012). There are only five WHO-prequalified medicine quality control laboratories in Sub-Saharan Africa, two in India, two in Singapore, and one in Vietnam (WHO, 2011h). Building laboratory capacity is a priority for the Asia Pacific Economic Cooperation’s Food Safety program (APEC, 2008). Box 3-4 describes recent success in laboratory capacity building in Southeast Asia.
In his March presentation to the committee, Paul Young, Director of Chemical Analysis Operations at Waters Corporation, described visiting food safety laboratories in a number of developing countries while working as a food regulator in Europe and finding donated equipment stored under plastic sheets, because no one had been trained in its use, the people trained to use it no longer worked at the lab, or because basic infrastructure to run the equipment was inadequate. Tropical climates and power surges are hard on sensitive electronics. In many ways the challenge of supporting laboratory infrastructure is complicated by the more basic deficits of sanitation and a stable power supply.
Shortages of laboratory infrastructure in turn encourage other gaps in regulatory systems. At the Pretoria visit for this study, the Tanzanian Food and Drug Authority’s Raymond Wigenge explained that Sub-Saharan African countries’ limitations in laboratory science cause their poor participation in Codex and other standard setting meetings. He explained that if African scientists were better able to do exposure assessments they would bring data on the accurate maximum exposure for mycotoxins to Codex and contribute to setting the Codex mycotoxin standard.
There are clear ties between problems with water sanitation infrastructure and ensuring safety in food production. Good agricultural practices require deep pit latrines and the separation of defecation and farming fields (Agribusiness and Allied Kenya Ltd et al., 2006). Grains and spices need to be properly dried to reduce risk of mycotoxin contamination. As mentioned in the discussion of supply chain problems, rural roads are poor and transportation is expensive (Hazell and Wood, 2008). Farmers and
BOX 3-4
Strengthening Laboratory Capacity in Southeast Asia
A solid laboratory system is essential for medicines regulation, but is missing even in many middle-income manufacturing counties. U.S. Pharmacopeia, USAID, and Asian universities are all working to improve regulatory and laboratory capacity. Their efforts are improving reference laboratories and supporting pharmacists in good clinical practice and good pharmacy practice.
The Southeast Asia Infectious Disease Clinical Research Network (SEAICRN) is increasing laboratory capacity through collaborative partnership. The network brings together hospitals, universities, and other research organizations from Thailand, Vietnam, Indonesia, and Singapore to improve laboratories, equip them well, train scientists, and ensure quality laboratory management. Through the integrated, collaborative model, countries in the network are responding more rapidly to emerging disease issues, such as the assessment of oseltamivir resistance in A/H1N1 in 2008 (-Wertheim et al., 2010).
U.S. Pharmacopeia and USAID’s Promoting the Quality of Medicines program is also active in laboratory capacity building. Promoting the Quality of Medicines works to improve post-market surveillance for product quality and safety (Lukulay, 2011). Southeast Asian police have drawn on the program’s data and closed more than 100 illicit drug vendors in the region (USP, 2011b). The map on the right shows sentinel surveillance sites in the Mekong Delta region as of 2008 that are staffed by two scientists each and use portable mini-laboratories to test medicine quality (Global Pharma Health Fund, 2011; Lukulay, 2011). Some sites also monitor the efficacy of malaria treatments.
SOURCE: Promoting the Quality of Medicines (USAID and USP Cooperative Agreement).

A woman and child prepare a vegetable harvest for transport in western China.
SOURCE: © 2008 Xiaobo Zhang, Courtesy of Photoshare.
distributors have higher vehicle operating costs from damages caused by unpaved roads (Donnges et al., 2007). A distribution system that moves foods more quickly from the farm to the market could do much to promote food safety (Kader, 2010).
Market infrastructure is also lacking in the growing cities of Africa and Asia. Only 20 percent of markets in the Indian state of Maharashtra have cold storage, compared to 5 percent in Tamil Nadu, and none in Orissa or Uttar Pradesh (Umali-Deininger and Sur, 2007). The majority of the same markets surveyed have no system for pest control (Umali-Deininger and Sur, 2007). Pest infestation in markets is a clear disease risk and can introduce other contaminants, such as heavy metals, to food (Sharma et al., 2009).
Local manufacture and sale of processed foods is part of life around the world, but the manufacture of medical products is more controversial. In 2005 some experts discouraged local medicines production in the poorest countries, believing the energy and raw materials costs of domestic manufacture to be prohibitively high for them (Attridge and Preker, 2005). Local manufacture is sometimes thought to put economic and industrial development before public health in the name of self-sufficiency (Anderson, 2010).
Others maintain that as long as one-third of the world, mostly in Africa and Asia, does not have access to essential medicines, local drug manufacture can build crucial industrial infrastructure, and that the least developed countries have a brief window to do so before the TRIPS agreement binds them to observe pharmaceutical patents (Anderson, 2010; Chaudhuri et al., 2010; Losse et al., 2007). Local manufacture of essential medicines could also guarantee a more reliable local medicine source in countries otherwise dependent on trade or foreign aid. A full analysis of this dynamic controversy is outside the scope of this report. But in 2010 the WHO prequalified artemisinin combination therapy manufacturers in Uganda and a Kenyan anti-retroviral manufacturer (Manson, 2011; WHO, 2011g). Nevertheless, local production of medical products depends on having decent industrial infrastructure and factories that are designed to facilitate meeting international manufacturing standards (Milstien et al., 2009). When the manufacturing infrastructure lags behind, regulators in the developing countries face a harder job enforcing safety controls.
Information and Communication Technology
Low- and middle-income countries do not have the technology necessary to track and trace products through their supply chains. This is not surprising, as traceability in the food and pharmaceutical industries is difficult even for immensely profitable multinational conglomerates with a stake in protecting their brand names. Food and medicines are made from ingredients that are processed and aggregated at different steps in manufacture, often in different countries (Roth et al., 2008). Guy Blissett, the head of consumer products at the IBM Institute for Business Value, has described traceability as “a global information management problem” (Roth et al., 2008, p. 32).
In India, the Agricultural and Processed Food Export Development Authority has invested in traceability systems when there is a clear commercial benefit to doing so, such as tracing grapes for the European market. The pressure to trace foods through the domestic market is not strong, however. Some speculate that nothing will change until domestic consumers show interest and willingness to pay for traceability (Roth et al., 2008; Umali-Deininger and Sur, 2007). Even if emerging economies had traceability systems in place, they do not have the ability to issue rapid recalls. Recalls depend as much on transportation and communication infrastructure as they do on product tracing.
Farmers in poor countries are usually obliged to sell their crops at harvest, when the market is glutted and prices are lowest, because the spoilage process starts quickly, as mentioned above in the discussion of supply chains. Investments in silos and temperature controlled storage are one way around
this, as is preserving the perishable foods. Information technology can also help farmers manage their inventory. For example, the Indian agricultural commodities firm ITC Ltd. trained soybean farmers to use the Internet to monitor the weather forecast, to learn about best agricultural practices, and to track soy prices and the Chicago Board of Trade 10-day global market outlook. Armed with better information, farmers could schedule their marketing to coincide with periods of demand (Upton and Fuller, 2004). There are transferable lessons in ITC’s experience for food regulators interested in monitoring the food supply from farm to table. Most Indian farmers still rely on their own or their friends’ observations for their information about crop prices (Umali-Deininger and Sur, 2007). Using simple information technology to monitor commodity prices is a way to involve farmers in the agricultural extension system. The ITC trainers found that by making information technology available they built trust with the soybean farmers and had a strong foundation on which to build future collaborations (Upton and Fuller, 2004). The use of information technology is a simple way to build trust with rural suppliers and encourage ownership in food safety technology.
In the ITC model, farmers connected to the Internet though landlines or very small aperture terminals (Upton and Fuller, 2004). The bandwidth available was not high, but was sufficient for the project. Poor bandwidth limits more ambitious use of information technology in developing countries. Food and drug safety information is available online, but still not accessible to developing country regulators. Even universities, whose informatics infrastructure is often better than the government’s, are “digitally isolated from the rest of the world. [Their Internet capacity is] equivalent to 30,000 people trying to use a single connection. Bandwidth can be exorbitantly expensive, and services are often unreliable. The result is that faculty and students rarely have access to the latest knowledge, and universities cannot form effective partnerships with academics and institutions in other countries. High-speed access to the Internet—at a minimum of 1 gigabyte per second—would serve as a lifeline for universities and help to drive a country’s economic renewal” (Juma, 2008, p. 17). Without Internet access, the WHO’s vast and useful library of handbooks are beyond the reach of regulators in the poorest countries, the people who need them most.
LAWS
Relevant and enforceable laws are the foundation of food and medical production regulation (FAO/WHO; WHO, 2007). Governments pass food and medical product laws to protect public health, prevent fraud, and promote fair trade (WHO, 2003a). The laws governing food and medical products invariably reflect a country’s political, economic, and cultural
BOX 3-5
Historical and Political Influences on Indian Drug Legislation
At the time of Indian independence, Western multinational corporations controlled 80-90 percent of the Indian pharmaceutical market (Greene, 2007). In an effort to foster self-sufficiency and create an independent supply of pharmaceutical products, the Indian government enacted high tariffs and import restrictions to encourage domestic production. As part of this program, the 1970 Patent Act ended Indian recognition of product patent protection. The Patent Act allowed Indian drug companies to reverse-engineer expensive, patented drugs without paying licensing fees. In the absence of legal patent protection, most foreign manufacturers left India. As of 2005, foreign companies held less than 20 percent of the Indian drug market (Greene, 2007).
Indian government policy long encouraged small- and medium-sized drug companies to enter the market. Consequently, today’s market in India is fragmented and competitive—there are more than 20,000 drug manufacturers (KPMG International, 2006). Roughly 300 of these account for 70 percent of the market; the top 10 firms account for 30 percent (KPMG International, 2006).
The industry changed in 2005 when the Indian government amended the Patent Act to comply with the TRIPS agreement and Indian pharmaceutical companies could no longer reverse-engineer patented drugs. Indian firms sought to replace lost revenues in several ways. First, they increased generic exports. As of 2007, generics accounted for 60-100 percent of sales in India’s top 10 firms (Greene, 2007). In addition, most have entered into contract research and manufacturing agreements with foreign drug companies. Indian companies have costs far below those of Western ones—one-eighth for research and development and one-fifth for manufacturing (Nauriyal, 2006). Low costs, both in labor and capital, coupled with India’s recognition of foreign patent laws, have made India an attractive destination for clinical trials and drug discovery and research. Indian companies are now building more and better factories and working to comply with international manufacturing standards in an effort to secure manufacturing contracts from multi-national pharmaceutical corporations (Nauriyal, 2006).
history. Muslim countries may include halal criteria in their national food law, for example. Box 3-5 describes the political and historical influences on Indian intellectual property and drug legislation.
Some developing countries have no laws governing food or drug safety; others have a surfeit of confusing and contradictory ones (Vapnek and Spreij, 2005; WHO, 2005). Participants at the São Paulo and Pretoria meetings for this study explained that in many of their countries the regulatory
legislation dates from the turn of the last century and is not suitable for the modern world. Governments should periodically revisit their laws governing product safety to ensure they are up-to-date and cogent (WHO, 2003a). Poorly coordinated legislation can also create fragmentation by assigning the same responsibilities to several agencies. The subsequent section on fragmentation discusses this problem in more detail.
Enforcement of Existing Regulations
One of the main problems developing country regulators have with their laws is with implementing punitive measure for violators. Participants at the São Paulo, Delhi, and Beijing workshops all noted that small producers can easily close their operations and re-open under a different name to avoid penalty. A 2010 Government Accountability Office (GAO) report described the FDA’s helplessness to the same problem (GAO, 2010). An FDA effort to verify foreign producers in 2010 found that of 43 drug manufacturers visited, 7 did not exist at the address in FDA’s database (GAO, 2010).
There was consensus in the Delhi and Beijing workshops that both India and China have a thorough legal regulatory framework in place. In these countries, as in many other emerging economies, regulatory authorities face more problems enforcing their laws than creating them. It is difficult to strengthen law enforcement in the face of poor staffing, inadequate infrastructure, and lack of political will (Bollyky, 2009). A World Bank appraisal of food and drug regulatory oversight identified weak enforcement of existing regulations as one of India’s four main problems in both food and drug safety (World Bank, 2009). They found the food system had “traditionally … depended on spot checks of manufacturing conditions and random sampling of final products. Even this system was not evenly enforced “(World Bank, 2009, p. 1). Of the drug system, they concluded, “enforcement of good manufacturing practice was highly variable. The quality of training for drug inspectors was uneven … [and there was] anecdotal evidence of lack of transparency in granting licenses”(World Bank, 2009, p. 2).
In an analysis of food safety law enforcement in China, Ni and Zeng compared China’s food safety laws to its environmental laws. The laws increase in number as the environment degrades and the government focuses on punishing offenders (2009). This is consistent with the committee’s observation that the Chinese government prefers to enforce its laws by punishing offenders and is less interested in rewarding compliance. This tactic is itself a limiting factor in a country as large as China with so few inspectors (see section on Workforce). Competing societal forces will also undermine the government’s best efforts at punishment. Global business is
increasingly the purview of large corporations operating on narrow profit margins, especially in the food sector (Garrett and Huang, 2011). Their suppliers are under pressure to cut costs; too often they do so by using unsafe ingredients and cutting corners on good practices (Garrett and Huang, 2011). Sometimes industry’s interest in protecting their brand and a fear of liability laws are enough to prevent fraud and adulteration, but in many low- and middle-income countries it is not so.
Civil Liability
Appendix B, “A Review of Tort Liability’s Role in Food and Medical Product Regulation,” describes the role of product liability in regulatory systems and provides an overview of the different systems in place in South Africa, Brazil, China, and India.
WORKFORCE
During the public meetings for this study the committee repeatedly heard that regulatory authorities in developing countries have too few staff, insufficient technical training for staff, and an inability to retain staff. They cannot offer private-sector salaries, and, perhaps more importantly, there is little espirit de corps among regulators. Some are sacked for political reasons; others grow frustrated and quit. While they are serious concerns for government regulators, these workforce problems reverberate in the public sector.
Too Few Staff in the Regulatory Authority
In an interview for this study, FDA staff in China explained that at first glance China has an army of food and drug inspectors, 400,000 by some estimates (Becker, 2008), but that most of them work part time, and many perform an average of one inspection a year. Chinese Minister of Health Chen Zhu gives a much lower estimate of the number of food safety inspectors in China: approximately 133,000, or fewer than 1 for every 10,000 people (LaFraniere, 2011). In a 2010 assessment of medicines regulatory authorities in Sub-Saharan Africa, the WHO found that all 26 of the countries evaluated reported a shortage of qualified inspectors (WHO, 2010a). Indian drug regulatory authorities, especially those at the state level responsible for most inspections, have far too few staff to enforce their laws (Langer, 2008).
The inspectorate is only one arm of the workforce in a regulatory authority. Regulatory science and its constituent fields are new areas of study in most of the world. Ahuja and Sharma summed up the problem in India
with one example, “the supply-demand situation for skilled manpower is highly skewed in favor of the demand, as this field [e.g., pharmacovigilance] is new in India and elsewhere” (Sharma and Ahuja, 2010, p. 1). Despite having more than half a million physicians, India has less than 200 investigators trained in good clinical practices (Prakash, 2009).
If China and India, with their massive populations, cannot staff a regulatory authority, the problem is even more serious in smaller countries. In many low- and middle-income countries environmental health inspectors often do the job of food safety inspectors, and analytical positions in both food and medical product quality control laboratories often go unfilled (FAO/WHO, 2003; WHO, 2010a). A 2002 comparative analysis of 10 different drug regulatory authorities found the shortage of qualified staff to be the main problem facing medicines regulatory authorities around the world (Ratanawijitrasin and Wondemagegnehu, 2002).
Insufficient Technical Training for Staff
The problem of too few staff at regulatory agencies is closely related to the problem of staff competency. In China, for example, many of the inspectors have only a middle-school education; they lack the scientific background to do more than a superficial inspection, a problem more pronounced in the central and western part of the country (UN, 2008). The technical proficiency of the Chinese inspectorate is concern enough that both the FDA and GIZ, the German government’s aid agency, train inspectors or train trainers. The WHO has also encouraged the Chinese government to develop a central training institute for food safety, but the government has balked at this suggestion because of difficulties in implementing such a large project.
These problems are by no means unique to China. A study of food inspectors in Andhra Pradesh, India found limited knowledge of food microbiology in the inspectorate, a weakness attributed to lack of in-service training (Sudershan et al., 2008). A joint Food and Agriculture Organization of the United Nations (FAO) and WHO report recommended offering in-service training for regulatory staff as a way to strengthen food safety systems (FAO/WHO, 2003). The FAO has also recommended a central food safety training center for South America, and participants at the IOM meeting in São Paulo were eager to see such a center open because it would enrich training for people from small countries. Such institutions would be most useful if their curricula were designed specifically for the school’s region. In a systematic review of problems facing the pharmacy workforce, Hawthorne and Anderson reported that curricula developed in North America or Europe are used in developing countries with the best intentions, but this practice contributes to job dissatisfaction as pharmacists trained on a foreign curriculum
are not prepared for the reality of work in developing countries (Hawthorne and Anderson, 2009).
The need for a properly trained regulatory staff will increase in the next decade. In the past, the review process for new chemical entities took place mostly in industrialized countries; low- and middle-income countries only had to register or give market approval to a drug tested abroad (Moran et al., 2011). Now there is more interest in developing treatments for neglected diseases; in 2007 over $2.5 billion was invested globally in research on neglected tropical disease (Moran et al., 2009). These products are now coming up for regulatory review in Asia, Sub-Saharan Africa, and parts of Latin America, and it is imperative that the regulatory workforce has the depth to register and review these new products.
Therefore, education in regulatory science is a particular need. Regulatory science is a relatively new field that includes training in basic sciences that relate to the regulatory system; the development and validation of regulatory tests; screening and compliance testing; investigation of test results; and submission of dossiers for government or in-house review (Irwin et al., 1997). Increasingly, any food production house or medical manufacturer needs to have a regulatory affairs specialist on staff. Until recently, developed countries generally relied on on-the-job training for regulatory affairs personnel, but this created important blind spots, such as poor understanding of how international organizations work to harmonize standards (Gundersen, 2001). There are now a few, but only a few, universities that train in regulatory science, some of which also offer distance-education classes (Gundersen, 2001). Improved education in regulatory science is a need around the world, and there is increasing attention to its international importance (Hamburg, 2011).
The problem of inadequate training extends to the workforce as a whole, not just to government regulators. In 2011 an African business newsletter reported that staff at African food companies often fail to follow proper food safety protocols because they have never been trained in them (Bester, 2011). Similarly, the non-profit organization Engineering and World Health identified lack of trained staff as a serious barrier to the use of high-tech medical devices in the poorest countries, explaining, “In countries where the literacy rate can be 50 percent, eligible workers can be difficult to find” (Malkin, 2007, p. 579). Chinese participants at the public meeting for this report agreed that while much adulteration in China is frank criminal behavior, some is attributable only to worker ignorance, which can have disastrous consequences. In 2011 Chinese farmers used the chemical forcholfenuron to speed the growth of melons and caused the entire crop to explode in the fields (Watts, 2011).
Donor organizations can fill training gaps, but donor training is sporadic and short term. A World Bank analysis of capacity for food safety
in Zambia found that beyond a few workshops for street vendors, donors were not interested in food safety in Zambia (Abegaz, 2006). In larger countries, and in countries that export foods, there is much more donor involvement. Last year in China, for example, the World Bank committed $100 million to increasing and improving safety in a single Chinese province. The $100 million was accompanied by matching grants for producers to set up 200-300 training sites for good agricultural practices (World Bank, 2010). Additionally, the WHO has a permanent food safety presence in China. With funding from the Asian Development Bank, it advises the State Food and Drug Administration on food safety management, policies, and international standards (WHO, 2008b).
At the Pretoria workshop for this study, the participants agreed that donor trainings, no matter how technically rigorous, are not helpful unless donors coordinate their plans with the appropriate central government agencies. There have also been calls for donors to coordinate at the international level. The lack of a clear international consensus on how to best support the poorest countries holds back biotechnology development, and the same can be said of general regulatory systems development (Byerlee and Fischer, 2002).
Donor trainings are also vulnerable to problems in recruiting the proper audience. Opportunities to travel and collect per diem, i.e. donor trainings, are too often a reward to senior staff for their years of service. More junior implementing staff are harder to reach. Reaching and training the proper staff for a variety of jobs in the food and drug regulatory authority are of special concern to this report. At all the international workshops for this study, participants mentioned a need for training, specifically, training in risk analysis to inform their regulatory work. The importance of more rigorous training for regulatory staff cannot be understated. If regulators had similarly rigorous training they would develop comparable systems. Ongoing professional development is itself an incentive that could be used to keep technical expertise in government service. This is one reason American government agencies and universities are able to keep their staff despite the higher salaries offered in industry.
A more sustainable solution to the training problem in developing countries depends on academia (Lupien, 2007). At their workshops abroad, the site visitors heard many times that academia does not contribute to food or medical product safety: they neither research public health problems nor emphasize real-world experience in their teaching. Part of this convention may come from the way people think about education, especially in Asia. In China, for example, anything seen to distract students from their studies is frowned upon (A tale of two expats: Business in China and the west, 2011); this extends even to professional internships. Technical internships are lacking in India as well; an Asian Development
Bank publication reported that only one-quarter of Indian engineering graduates had the skills they needed to find work without further training (Xiaoguang and Fengqiao, 2010). Professors can hardly be expected to train students for careers they have had no exposure to themselves. Except for the elite Indian Institutes of Technology, Indian universities do little research and development work (World Bank, 2007). Some of the problem may stem from a “passive national learning system” in post-colonial countries, where policy has encouraged copying technology developed abroad and failed to foster home-grown innovation (Morel et al., 2007, p. 180; Viotti, 2002).
The links between industry, government, and academia appear to be stronger in Latin America (Juma, 2008; Sutz, 2000). At the São Paulo workshop for this study, Rosane Cuber Guimarães, Good Practices Manager at Biomanguinhos, the technical and scientific unit of vaccine production at Fiocruz, Brazil’s national public health institute, discussed her institute’s training program. She explained that it has a rigorous training program and enrolled about 30 master’s and 2 doctoral students in 2011. The problem at Biomanguinhos, and at many public institutions, is retaining their graduates in public service.
Problems Retaining Staff
Government jobs in food and medical product regulation do not pay as well as positions of comparable seniority and scientific expertise in the private sector. This is true in rich countries as well, although in 2007 Congress authorized incentive pay for government scientists in an effort to close this pay gap (Bridges, 2007). The WHO assessment of medicines regulation in Sub-Saharan Africa found a universal lack of sustainable funding for staff salaries; only 8 percent had a staff development plan (WHO, 2010a). Almost without exception the government regulators who took part in workshops for this study mentioned an internal brain drain, where talented staff leave government service. In countries with a robust private sector, such as China, India, and Brazil, they commonly leave for positions in industry, while in the least developed countries they are more likely to find work on donor projects or with NGOs.
At the São Paulo workshop for this study, participants raised the concern that government regulators can lose their jobs for political reasons, when a newly elected politician wants to slim down the government payroll, for example. Valuable training is wasted when scientific staff are dismissed arbitrarily. It also impairs institutional memory when entire cohorts of senior staff leave an organization in unison. Anvisa, the Brazilian equivalent of the FDA, has put systems into place that insulate its staff from political patronage (Box 3-6) (Prado, 2006). Government agencies in the countries
BOX 3-6
Brazilian Regulatory Restructuring
Independent regulatory agencies are a relatively new phenomenon in Latin America. In a regulatory authority, independence means that there are systems in place to protect scientific decision making from political actors (Prado, 2008). This includes having certain safeguards in place. The president of the country should not be able to remove the agencies’ leaders, and the senate should approve the commissioner. Decisions should be made by a board and agency commissioners should have pre-defined terms of office and these terms should be staggered, so that all leadership does not retire at the same time. Finally, independent agencies should have independent funding (Prado, 2008).
In the 1990s, Brazil established nine independent regulatory agencies, adopting many institutional formulas from the United States (Prado, 2008). At the time, Brazil was in a process of privatizing state-owned companies and changing regulatory oversight, in part to be more attractive to investors. The government understood that a stable, independent regulatory authority was important to economic development: investors are reluctant to fund industry if the regulations governing it change with every election.
The National Health Surveillance Agency (Anvisa) was created in 1999 as part of Brazil’s regulatory restructuring; its bylaws were approved in 2000. Anvisa’s mission is to protect the health of the Brazilian people by exercising sanitary control over production and marketing of products and services subject to sanitary surveillance (Anvisa, 2003; Aragão, 2010). Structured within the Brazilian federal public administration and linked to the Ministry of Health, the agency is managed by a collegiate board of directors, comprised of five members with staggered 3-year terms (Anvisa, 2003). It is an independent, financially autonomous regulatory agency (Anvisa, 2003; Aragão, 2010).
visited for this study were slow to adopt modern management principles or implement succession plans, however.
At the public workshops in India and South Africa, participants hinted at concerns with corruption, a reality of work in many countries that can push professionals to look for other jobs. In China, a senior Ministry of Health official, spoke with great candor about a desire to develop a professional ethic in both the inspectorate and in industry. In India, the 2003 Mashekellar report on spurious drugs cited government corruption as a factor encouraging the trade in fake drugs (Government of India, 2003). Corruption is a sensitive topic, one that industry, government, and academia are all understandably hesitant to discuss. Corruption is hard to measure
but is prevalent in places “without robust institutional checks, [where] government regulators can make discretionary decisions rather than decisions based on uniform criteria” (Cohen, 2005, p. 78). A culture of accepting bribes does not encourage trust in government or respect of civil servants; staff who are not respected or have no pride in their agency have little reason to refuse bribes. Working in a vicious cycle, corruption is both a cause and an effect of the staff retention problems at many regulatory agencies.
FRAGMENTATION
Regulatory systems in both developed and developing countries often suffer from fragmentation, i.e., “the assign[ing] of different responsibilities to different regulatory bodies” (Ratanawijitrasin and Wondemagegnehu, 2002, p. 2). There is also sometimes a similar problem of assigning the same responsibilities to different regulatory bodies. Even in the United States, a dozen different federal agencies enforce 35 different food safety laws (Martin, 2007). This is a commonly cited complaint about the U.S. food safety and, to a lesser extent, drug safety systems (CSPI, 2007; Genetics and Public Policy Center, 2010). Fragmentation is often a consequence of historical compromises. This kind of fragmentation is common in developed and developing countries alike. The FDA, for example, began as an office within the U.S. Department of Agriculture (USDA), the differentiation of their responsibilities continues to evolve today. The strongest regulatory systems can evolve to match changing production practices. For example, in 2011 Commissioner Hamburg reorganized the FDA’s reporting chain and added an Office of Operations and a Deputy Commissioner for Global Regulatory Operations and Policy, in order to help the agency respond to the challenges of globalization (May, 2011).
In its overseas workshops, however, the committee identified a different fragmentation in the regulatory systems of emerging economies, a fragmentation without a clear assignment of responsibilities, an established protocol for enforcement, or an articulated chain of command. This confuses already complicated systems. Appendix C shows the sprawling organization of food and drug safety systems in South Africa, Brazil, India, and China.
Fragmentation is even more serious a problem in the poorest countries. In its analysis of medicines regulatory authorities in Sub-Saharan Africa, the WHO found that an organizational chart was missing in four countries; nine had unclear or missing job descriptions for key positions (WHO, 2010a). Though, as the figures in Appendix C show, drug safety laws are scattered among fewer agencies than food safety laws.1 In many of the countries visited, the lack of established communication channels
![]()
1 See Appendix D for more detailed notes on the Chinese food safety system.
between and within agencies confounds the fragmentation problem. This is discussed in more detail below.
Many countries, the United States included, have recently attempted to unify their food or medical product safety regulation. The Brazilian government’s work on Anvisa is an example of one such successful project (Box 3-6). Ambitious legal restructuring of food and drugs regulation is not usually an option, however. When the Brazilian government created Anvisa it was part of a larger restructuring and privatization movement happening across Latin America (Prado, 2008). Rarely do governments have the money or the political capital to support such a large effort. In the United States, for example, the division of responsibility between the USDA and the FDA is a patchwork of historical compromises. The system works, however, because the delegation of responsibility between the agencies is clear and because they work together and with their counterparts in other government agencies. Both agencies also have clearly articulated chains of command; they coordinate their efforts to avoid duplicating each other’s work.
In their analysis of drug regulatory systems in 10 different countries, Ratanawijitrasin and Wondemagegnehu (2002) concluded that fragmentation is either a problem of delegation with no authority to enforce the laws or delegation with full authority but no coordination with other regulatory agencies. Large countries tend to face the former problem: their provincial and local governments staff local regulatory authorities with limited authority to enforce federal regulations.
In both China and India, every regulatory authority has offices at multiple levels of government. That is, the national drug regulatory authority has provincial and municipal levels as well. There are different regulations at the federal, provincial, and municipal levels; sometimes these regulations overlap, other times they conflict. For example the Drugs Controller General of India, a federal office, approves all drugs sold in the country, but state authorities, whose standards vary widely, issue manufacturing licenses (Jeffery and Santhosh, 2009). Once a drug is licensed in one state, it is automatically approved for sale throughout the country. There are more than 70,000 drugs for sale on the Indian market, many of them sold in irrational fixed-dose combinations, stressing the already bursting quality control laboratories (Jeffery and Santhosh, 2009). If every agency had a clearer delineation of its responsibilities, then there would be more efficient use of the government’s limited staff (Lu and Kjeldsen-Kragh, 2008).
Routine reporting from local to provincial and state governments is of the utmost importance in larger countries. In Malaysia, for example, the Deputy Director of Health in each state reports directly to the Pharmaceutical Services within the National Pharmaceutical Control Bureau.
This ensures communication between the state and federal level within the government hierarchy (Ratanawijitrasin and Wondemagegnehu, 2002).
In the public workshops for this report, the committee learned that different agencies often repeat each other’s testing. In South Africa, for example, the Departments of Health and Agriculture are both required to test unprocessed foods, though both agencies have insufficient laboratory capacity to do so (Chanda et al., 2010). Different agencies regulating food and medical products frequently lack an established protocol to share data. This makes surveillance as much a political task as a scientific one.
Small countries more commonly face the problem of delegation of responsibilities with full authority, but poor coordination. At the São Paulo meeting for this report, Claudio Poblete, a consultant retired from the Chilean agricultural service, explained that he had seen some uncoordinated delegation in the Chilean food safety systems, particularly in that the ministries of health and agriculture are meant to enforce food safety laws codified in the Animal Health and Protection Code, a decree released by the Ministry of Finance in 1963 as part of land reform.
Pakistan is another country where uncoordinated delegation is a problem. In January 2012 over 100 heart patients died from adulterated medicine at the Punjab Institute of Cardiology in Lahore (Guerin, 2012). There is a greater risk for such disasters since July 2011, when the government devolved the country’s drug regulations to the provinces (DRA establishment at federal level demanded, 2012). With no central drug regulatory agency, there is no way to coordinate provincial regulatory work (Abudhoo, 2012; DRA establishment at federal level demanded, 2012).
SURVEILLANCE
Surveillance is one of the essential functions of the regulatory and public health systems. The WHO’s International Health Regulations-2005 define surveillance as “systematic ongoing collection, collation and analysis of public health information [and] … assessment and public health response as necessary” (WHO, 2008a, p. 10). Surveillance depends on a strong health infrastructure, established methods for data collection, and epidemiologists and statisticians to analyze and interpret data and disseminate their findings. If data quality is not good, then the analysis and interpretation are doomed, but rigorous data collection is difficult in low- and middle-income countries, especially in remote places. Regulators in developing countries struggle to maintain affordable surveillance systems that produce reliable data. Too often data collection is difficult and data quality uneven, calling into question the returns on the investments developing countries make in surveillance systems (Frerichs, 1991). In the era of global supply chains, anti-microbial resistance, and international epidemics, “all countries …
have a stake in the success or failure of surveillance and response capacity development in any one country” (Kimball et al., 2008, p. 1464). This realization has driven growth in global and regional surveillance networks during the past 10 years (Castillo-Salgado, 2010). At every overseas workshop for this study, the participants told the committee that their regulatory authorities have problems conducting risk assessment. Risk assessment identifies hazards and their sources, the characteristics of theses hazards and their health risks, and projects the impacts of different ways to control hazard (Todd and Narrod, 2006; WHO, 2012). Some of the problems regulators face in conducting risk analysis stem from insufficient training for their staff, a gap discussed earlier in this chapter. Poor surveillance also limits risk assessment. Although training regulators abroad in risk assessment is an excellent goal, this training should accompany improvements to national food and medical product surveillance systems.
Food Safety Surveillance
Food safety surveillance depends on the reporting of cases of foodborne disease to a central data repository; the epidemiological investigation of foodborne disease; laboratory identification of the pathogen that differentiates it from similar agents; trace-back capability to the source of the contamination; and the recall of contaminated products from the market when necessary. Although the health surveillance system is seldom part of the regulatory agency (the CDC is a separate agency from the USDA’s Food Safety Inspection Service, for example) surveillance is still part of the regulatory system.
It is difficult to trace back through a supply chain that includes many anonymous transactions (Todd and Narrod, 2006). In its outline for the food safety system for India, the International Life Sciences Institute emphasized that monitoring contaminants in food and water is a prerequisite for monitoring disease in the population (International Life Sciences Institute-India, 2007).
Foodborne disease is extremely common in developing countries. By WHO estimates, 2.2 million people, more than half of them children, die from diarrheal disease every year, primarily caused by contaminated food and water (UNICEF, 2011; UNICEF and WHO, 2009). Disease surveillance is poor in most countries, especially for mundane diseases like diarrhea, so these estimates are almost certainly too low (Todd and Narrod, 2006).
In passive surveillance systems, health workers or patients identify what they suspect to be an adverse event due to a drug and then report it to the regulatory authority. Passive disease surveillance cannot effectively detect foodborne outbreaks: diarrhea is not often treated in clinics, biospecimens are collected for microbiological analysis, and health workers
do not report serious cases or deaths into a central repository (Zaidi et al., 2008). It is difficult to identify clusters of similar cases in an area over a short time when many cases are out of reach of the health system. It is also difficult to report a spike in illness without first knowing the baseline disease prevalence. Foodborne listeriosis, for example, is closely monitored in the United States, but its prevalence is unknown in developing countries, even in the Middle East where experts assume it to be a public health problem given the popularity of cold, cooked meats and soft cheeses (Todd and Notermans, 2011).
Twenty years ago, foodborne outbreaks often came from improper food handling in the kitchen (Swaminathan et al., 2001). An outbreak at a restaurant or party was easy to identify. Nowadays, when food is contaminated at or near the farm or in processing, the global food supply chain can quickly spread the pathogen. Modern outbreaks can be far removed from their triggers in time and place. From May to July 2011, an epidemic of bloody diarrhea and hemolytic-uremic syndrome sickened over 4,000 people and killed 50, mostly in Germany, but also in Sweden and other parts of Europe (Blaser, 2011; Reuters, 2010). Epidemiologists eventually traced the epidemic to Egyptian fenugreek seed contaminated by human or animal feces either in storage or transport, possibly as early as 2009 (Blaser, 2011). Because contaminated food can look, taste, and smell normal, identifying contaminants requires sophisticated microbiological assays. DNA fingerprinting and molecular subtyping are part of the modern epidemiologic investigation of a foodborne outbreak (Swaminathan et al., 2001). The CDC’s gold-standard technique, pulsed field gel electrophoresis, depends on laboratory infrastructure that is often missing in developing countries. The WHO’s Global Foodborne Infections Network is working to improve laboratory serotyping of enteric pathogens in its member countries (WHO, 2011e). Continued extension of PulseNet, the CDC’s molecular subtyping network, would do much to advance the science of molecular epidemiology in developing countries and could improve the speedy investigation of outbreaks around the world (Swaminathan et al., 2006).
In countries that have the capacity to do molecular epidemiological investigation of outbreaks, the information gained from them is invaluable. Serotyping and pulsed field gel electrophoresis of Salmonella in Thailand identified a geographically disparate cluster of the same pathogen in Thailand and the United States (Pornruangwong et al., 2011). Mexico also has an integrated food chain surveillance system; it has identified Salmonella clusters and their animal reservoirs, the baseline Salmonella contamination in retail meats, and the prevalence of asymptomatic Salmonella infection in different parts of the country (Zaidi et al., 2008).
There are also surveillance techniques that do not depend on the laboratory. The emerging field of public health informatics analyzes search pat-
terns on the Internet to give early warning of outbreaks (Castillo-Salgado, 2010). Private companies such as Voxiva use mobile phones for electronic surveillance in remote places (Castillo-Salgado, 2010; Voxiva, 2011). These novel surveillance methods can be especially useful in low- and middle-income countries.
Improvements to food safety surveillance systems within developing countries can be motivated by the demands of foreign importers or by the rising expectations of local consumers. In recent years, increasing concern with bioterrorism and imported zoonotic diseases is an example of how the concerns of rich countries may, as a consequence, drive improvements that also benefit poor ones. Some of these improvements, like food chain security and information sharing, can also lead to mutually beneficial effects on intentional and accidental food contamination (Alpas and Smith, 2011). Related improvements in global disease monitoring can also assist with the control of antimicrobial resistance.
Drug and Device Surveillance
Medical products go through safety and efficacy evaluations before they come to market. Even large-scale trials, however, cannot identify rare or latent problems with the product, nor do trials have the power to assess product safety in small sub-populations. Through postmarket surveillance, regulators monitor for known side-effects and detect and investigate new signals. The science of detecting, assessing, and preventing adverse of effects of medicines, and by extension, all medical products, is called pharmacovigilance (WHO, 2011f). Ensuring the safety of all drugs sold in a country is the responsibility of the drug regulatory authority. The regulatory authority should cooperate with health workers to monitor for drug safety signals. After identifying a possible signal, the regulatory authority should be able to evaluate the relationship between the drug and the adverse event. Finally, it should be able to take action if it verifies a problem. That action might be changing the drug label, issuing warnings, or, in rare cases, withdrawing it from the market (Bandekar et al., 2010; Kshirsagar et al., 2010). Pharmacovigilance is a common problem in poor and middle-income countries (Bakare et al., 2011; Olsson et al., 2010; Pirmohamed et al., 2007).
The Uppsala Monitoring Center (UMC) is the WHO’s international pharmacovigilance center. As of the summer of 2011, 106 countries had joined the WHO drug monitoring program and 34 other countries were waiting for full membership (UMC, 2011). Drug regulators from participating countries report possible adverse drug reactions to Uppsala, where the information is pooled and analyzed (Bandekar et al., 2010; Kshirsagar et al., 2010). To ensure that all the data can be pooled and analyzed, drug regulators need to collect and transmit data in a standard way. Bandekar
and colleagues identified serious inconsistencies in the adverse event reporting forms used in 10 different countries, finding for example, that many forms failed to account for the patient’s pregnancy status or known allergies (Bandekar et al., 2010). This problem is not limited to developing countries, however. By their scoring, Malaysia had the most thorough spontaneous reporting form reviewed, exceeding that of the United States or Britain (Bandekar et al., 2010).
Less than 27 percent of lower-middle-income and low-income countries have pharmacovigilance systems in place, compared to 96 percent of the wealthier countries in the Organization for Economic Cooperation and Development (Pirmohamed et al., 2007). The poorest countries generally do not have drug regulatory authorities with sufficient pharmacovigilance systems to become full members of Uppsala. Table 3-3 describes drug safety surveillance systems in different low- and middle-income countries.
The most frequent approach to surveillance for adverse effects to medicines in developing countries is passive or spontaneous adverse event reporting. Passive reporting systems rely on patients or health workers to report adverse events. Spontaneous reporting systems have important limitations. Most obviously, they cannot capture events that happen outside of the formal health care system, or even events within the system if the health workers do not report them (UNAIDS and WHO, 2011). It is also impossible to calculate the rates of the event without knowing the number of people in the same population using the drug without problems, i.e., the denominator. Spontaneous reporting is time consuming and adds to the workload of already overburdened health professionals (Bakare et al., 2011).
Active surveillance can add depth to the background passive surveillance systems. Cohort event monitoring is a useful surveillance technique in developing countries. This type of surveillance enrolls a group of people taking a medication in a prospective cohort study and systematically records data on all adverse events that happen to the patients in the cohort (UNAIDS and WHO, 2011). Although this is often impractical for large-scale surveillance, active surveillance can let regulators detect signals early and keep missing data and reporting bias to a minimum (Bakare et al., 2011).
Because they monitor a complete sample, active surveillance methods allow for the calculation of true event rates. Active surveillance also allows for risk factor analysis and generally a fuller picture of the drug effects. Sentinel surveillance programs, i.e., surveillance by a few select sites, usually hospitals or universities, can also provide a depth of data from a small population and has the added benefit of logistical ease (SPS, 2010b). Sentinel surveillance can use active or passive surveillance methods.
Ideally, active surveillance following drug or device safety lapses or the introduction of a new product would complement the background pas-
TABLE 3-3
Comparison of Postmarketing Drug Safety Surveillance or Pharmacovigilance Status Among Selected Countries
| Countries | Regulatory Agency |
Pharmaco- vigilance Center/ADR Reporting Center |
Number of ADR Reports Received |
Regulatory Guidelines |
Electronic ADRs Reporting Possible? |
ADR Reports by Market Authorization Holders |
Dedicated Safety Staff Present? |
Risk Management Strategies |
Communication to the Public by Bulletins, Drug Alerts |
|
Low-income countries |
|||||||||
|
Tanzania |
Tanzania Food and Drugs Authority |
National ADR Monitoring Center |
From 117 (in 2002) to 79 (in 2004-2005) |
Yes |
Yes |
No reports |
No |
None |
Information not available |
|
Zambia |
Pharmaceuti-cal Regulatory Authority |
Plan to set up Zambia phar-macovigilance Center |
N/A |
No |
No |
Not yet done |
N/A |
None |
N/A |
|
India |
Central Drugs Standard Control Organization |
National Pharmacovigilance center |
9,964 (in 2005-2006) |
Yes |
Yes |
Mandatory |
No |
None |
Yes |
|
Lower-middle-income countries |
|||||||||
|
Cuba |
Health Ministry |
National Coor-dinating Unit of Pharmaco-vigilance |
From 16,500 (in 2001) to 7,100 (in 2005) |
No |
Yes |
Voluntary |
No |
None |
N/A |
|
Ukraine |
Ministry of Health |
Ukrainian Pharmacovigi-lance Center |
From 122 (in 1996) to 667 (in 2002) |
No |
N/A |
N/A |
N/A |
None |
N/A |
|
China |
State Food and Drug Administration |
National Center for Adverse Drug Reaction Monitoring |
From 4,700 (in 1988-1999) to 173,000 (in 2005) |
Yes |
Yes |
Mandatory |
No |
None |
Yes |
|
Upper-middle-income countries |
|||||||||
|
South Africa |
Ministry of Health |
National Adverse Drug Event Monitoring Center |
Approx. 200 (in 1997) to more than 500 (in 1999) |
Yes |
Yes |
Voluntary |
No |
None |
Yes |
|
Malaysia |
Drug Control Authority |
Malaysian Adverse Drug Reaction Advisory Committee |
From 787 (in 2001) to 2,363 (in 2005) |
Yes |
Yes |
N/A |
Yes |
None |
Yes |
|
Chile |
Health Authority |
National Drug Information and Pharma-covigilance Center |
From 424 (in 2000) to 771 (in 2002) |
No |
N/A |
N/A |
N/A |
None |
N/A |
SOURCE: Vaidya et al., 2010.
sive surveillance system. However, at the USAID conference on national pharmacovigilance systems in Nairobi in 2010, participants agreed that establishing a minimal functional pharmacovigilance system is a good goal for most countries and that active surveillance may be beyond the minimum requirements (SPS, 2010a). A study in South Africa found that most health professionals, including pharmacists, do not understand what to report as an adverse event (Suleman, 2010). Less than half of the 88 national pharmacovigilance centers surveyed by Uppsala require health workers to report adverse events (Benabdallah et al., 2011).
Less than half of the 55 low- and middle-income countries responding to a 2008 survey had budget support for pharmacovigilance; 13 percent2 had no pharmacovigilance system at all (Olsson et al., 2010). Of the countries that did have a pharmacovigilance center, 69 percent were a part of the drug regulatory authority, 20 percent were part of the Ministry of Health, and 9 percent were part of a university or scientific center (Olsson et al., 2010). It is not surprising that the poorest countries have weak pharmacovigilance systems, but it is a more striking regulatory gap in emerging manufacturing powers. Both the Indian and Chinese drug industries were developed for production of generic drugs. Because generic drug companies do not run clinical trials or develop drugs, they tend to have weaker overall systems for safety surveillance. Since 2005 the World Bank has sponsored a national pharmacovigilance program in India with more than 26 peripheral reporting centers around the country (Kumar, 2011). This program has fostered a reporting culture among Indian health workers and built up the woefully small cadre of pharmaco-epidemiologists who can advise the drug regulatory authority in the future (Kumar, 2011). This will become a more important need as India becomes more active as a larger clinical research hub for neglected tropical diseases.
The WHO and the Global Fund describe the minimum requirements for a national pharmacovigilance system and lay out clear steps regulators can take to ensure medicines safety (WHO and Global Fund, 2010). These are having a national pharmacovigilance center, a spontaneous reporting system, a central database, an pharmacovigilance advisory committee, and a strategy to communicate with the public (WHO and Global Fund, 2010).
The postmarket surveillance for medical products is complicated, and it is a wide gap in developing countries. Medical device surveillance is not optimal even in the United States (Rumsfeld and Peterson, 2010). Although the FDA keeps records of device failures, it does not have adequate data on total device usage that would allow it to calculate a failure rate (Rumsfeld and Peterson, 2010). Many developing country regulatory authorities will authorize use of a device if it is registered for use by a stringent regulatory
![]()
2 Bangladesh, East Timor, Ecuador, Liberia, Malawi, Mauritius, and United Arab Emirates.
authority. Device surveillance is not a priority, and device recalls depend entirely on the manufacturer. When 21 Croatian kidney patients died immediately after dialysis in 2001, the Ministry of Health suspected a problem, but the faulty blood filters that caused the deaths were not removed from the market until Baxter International recalled them several months later (Reuters, 2001). Venezuela, Uruguay, and Costa Rica also have problems with medical device surveillance, and none has the legal ability to recall a device (Morroney et al., 2010).
Vaccine Surveillance and Public Trust
Postmarketing surveillance of vaccines is important because vaccines are biologically active. They are given to large numbers of healthy people, often children. There is a likelihood of a reaction at the injection site. Live attenuated vaccines may elicit a mild form of the disease, or, in rare instances, a full-blown case (GACVS and WHO Secretariat, 2009). More commonly, vaccines are the target of emotional scare campaigns. In places where childhood diseases are well controlled, rumors fly that immunization causes autism (Cheng, 2010; Freed et al., 2010). In India, Nicaragua, the Philippines, and Nigeria, conspiracy theorists have speculated that vaccines are part of government plot to sterilize children or spread HIV (Larson et al., 2011). Regulators need to respond to these rumors quickly and with accurate information, or they risk losing the public’s trust and jeopardizing immunization programs. They cannot respond without data collected from safety surveillance.
The WHO’s Developing Country Vaccine Regulator’s Network is a useful forum for representatives of drug regulatory agencies of Brazil, China, Cuba, South Korea, India, Indonesia, South Africa, and Thailand to come together to share their knowledge and experience (Chocarro et al., 2011). The WHO also runs the Global Advisory Committee on Vaccine Safety to advise on vaccine safety and to analyze and interpret vaccine surveillance data. The committee also advises the WHO on how to strengthen vaccine monitoring in developing countries (GACVS and WHO Secretariat, 2009; WHO, 2011c). The advisory committee’s analysis of vaccine safety can be limited by the quality of the data collected in the poorest countries, few of which have proper vaccine safety—monitoring systems (Brighton Collaboration, 2010). Furthermore, detecting rare events after immunization also requires sample sizes in excess of 100 million, pooled from comparable sources from different countries. The Global Vaccine Safety Blueprint Project, funded by the Bill and Melinda Gates Foundation, aims to improve vaccine surveillance in low- and middle-income countries and to support international information exchange (WHO, 2011d). The Brighton Collaboration, a global research network on vaccine safety, together with the
BOX 3-7
SANEVA: A Model of International Collaboration for Vaccine Safety Monitoring
In 2006, with the goal of improving vaccine safety, Argentina, Brazil, Mexico, Panama, and Venezuela formed SANEVA, a collaborative pharmaco-vigilance program. SANEVA is working both to strengthen existing passive surveillance systems and to develop a regional active surveillance system (GACVS and WHO Secretariat, 2009). To meet its objectives, the network is tasked with:
• monitoring adverse events attributed to the introduction of rotavirus vaccines;
• maintaining a system capable of both early identification of potential risks and efficient information sharing;
• developing a rapid alert system for reporting severe and unanticipated adverse events;
• recommending corrections;
• supporting other countries that introduce vaccines that SANEVA is monitoring; and
• boosting national, regional, and global surveillance capacities (PAHO, 2011).
Because of SANEVA’s efforts, better data on adverse events after vaccination are now available, allowing Latin American scientists to distinguish more accurately between real reactions to vaccines and problems that coincidentally followed vaccination. An example of this improvement is the monitoring of the RotaShield vaccine in Mexico and Brazil. In 1999, RotaShield was withdrawn from U.S. markets because of associations with intussusception. Mexican and Brazilian regulators were therefore cautious when introducing the vaccine into their countries in 2006 and 2007. Through improved surveillance capabilities, reliable data were collected rapidly and across a broad base following the vaccine’s introduction. The data showed that the number of deaths and hospitalizations averted because of the vaccine far exceeded the number of intussusception cases potentially associated with the vaccination (Patel et al., 2011).
As exemplified by the surveillance of RotaShield, SANEVA’s approach is improving vaccine safety monitoring in Latin America. To further increase the effectiveness of this program, SANEVA plans to expand beyond its original five members to include more Latin American countries (GACVS and WHO Secretariat, 2009).
WHO and the Bill and Melinda Gates Foundation, works to improve the ability to collect safety data in the poorest countries. It also promotes electronic data sharing and the linking of exposure and event data (Brighton Collaboration, 2010).
Brazil has a strong system for reporting adverse events after immunization (Martins et al., 2010). Brazil also develops vaccines for tropical diseases at Bio-Manguinhos Fiocruz, the national institute for vaccine production and development, and is active in regional vaccine surveillance as part of SANEVA (Box 3-7). Other emerging economies are not as strong in vaccine safety surveillance. An anonymous executive at one of China’s largest vaccine companies told Nature Biotechnology that Chinese vaccine companies fear reporting side effects, and they do not collect or report data on adverse events (Jia and Carey, 2011). The Indian government also has problems responding to distrust of vaccine trials. In 2010 the Ministry of Health and Family Welfare suspended a demonstration project, a kind of bridging trial required to introduce a vaccine tested abroad into a different country, of human papillomavirus (HPV) vaccine in response to claims that the vaccine had killed four girls (Larson et al., 2010). Later investigations revealed that the deaths were unrelated to the trial (Larson et al., 2010). An open discussion of the surveillance data and investigation into the deaths might have assuaged public fears and hastened the use of a lifesaving vaccine in India. The Indian HPV scandal is a reminder that even if developing country regulators improve their capacity for pharmacovigilance, it will be of little value without complementary improvements in communication.
COMMUNICATION
For food and medical product regulators, who are privy to trade secrets and confidential information on product development, trial results, and inspections, the balance between sharing information and building trust in confidentiality can be hard to strike. Even the FDA, widely considered a gold-standard regulatory agency, has been criticized for lack of openness (Hampton, 2011) and “an internal culture that stifles dissent” (Okie, 2010, p. 1493).
Communication is a broad term. In this report it refers to the need to share information, the strategy to do so appropriately, and the culture that supports open dialogue. Unlike many other components of a regulatory system, communication is less linked to international standards and more linked to local customs and policy. In liberal democracies governments report to their citizens; they have established protocols for communicating with the public and for making government business public. This tradition is less entrenched in some post-colonial (even those that are liberal democracies,
such as South Africa and India) and former communist countries, leading to a communication gap.
The communication problems developing country regulators face fall into four broad categories: communication within a regulatory authority and across government agencies that share regulatory responsibility; communication between regulatory authorities and those they regulate; communication to the public; and communication with counterpart regulatory agencies abroad.
Communication Within a Regulatory Authority and Across Government Agencies That Share Regulatory Responsibility
When multiple agencies within a country share oversight and responsibility for regulation, the need for communication among all the agencies is paramount. The foreign regulators who met with the committee for this study were often unaware of the need for information sharing between government agencies. For others, the political environment of their agency is not one that fosters dialogue. Even if staff may realize the need for communication across sectors, changing communication patterns can only happen from the top. This influences the ability of a government to address major safety problems; it also inhibits the prospective development of risk mitigation plans. Especially in China, the cultural imperative to be indirect is at odds with modern management principles that stress the need to acknowledge problems and respond to them promptly (Roth et al., 2008).
Communication Between Regulatory Authorities and Regulated Industry
At the overseas workshops for this report, representatives from regulated industry raised concerns that there are few, if any, forums for regulators and industry to share information on general product policy and the development of regulations. At an Asian Productivity Organization conference on reducing post-harvest waste, Iranian participants saw the lack of two-way communication between the government and other stakeholders as the main problem in their horticulture supply chain (Asian Productivity Organization, 2006). In most cases, there is not a good venue for dialogue between industry and regulators. Regulation is more effective when industry has a voice in the development of the regulations. For example, the FDA and PhRMA, the Pharmaceutical Research and Manufacturers of America, have regular conferences on drug development policy. Recently, their conference dealt expressly with improving communication between sponsors during drug development (FDA, 2011).
The communication gap that exists in poor countries between industry and regulators can be blamed to some extent on weak or non-existent
industry associations in developing countries. Industry associations allow companies to collaborate on meeting regulations that affect them all. They also allow regulators to hear input from the people who implement their regulations. Industry associations also have a useful, if unintended, effect of encouraging adherence to standards through subtle peer pressure. Strong industry associations can support regulators. In places were these groups are discouraged, and in the least developed countries where there is little industry, the lack of robust associations hinders communication.
Communication Between the Regulatory Authority and the Public
The regulatory authority has a duty to communicate promptly and clearly with the public during an emergency. This is a difficult task, even in developed countries with a tradition of openness. In developing countries, it is more complicated. Sometimes a culture of saving face prevents admission of problems; other times the regulatory authority may question the utility of taking public action, such as issuing a recall, if it lacks the muscle to implement it. Most commonly, the regulatory agencies simply do not have accurate data to know when a crisis is brewing. A culture of keeping safety threats quiet is a clear failure of communication between regulators and the public. In 2010 the agriculture bureau of Hubei province in south China destroyed 3.5 tons of black-eyed peas found contaminated with a toxic pesticide and issued a national warning about the same (Wong, 2010). Contaminated peas were eventually discovered in three other Chinese provinces, but the provincial regulators were not rewarded for whistle blowing. On the contrary, they were criticized for “breaking an ‘unspoken rule’ that officials in different cities and provinces report problems to one another rather than telling the public” (Wong, 2010).
In China, the political structure discourages any but the most senior government officials from communicating with the public. A change in organizational thinking might be needed so that admission of safety problems would be seen as accountability rather than humiliation. Government regulators might also be understandably reluctant to admit safety lapses to a public that does not have sufficient understanding of food and drug safety to process the information. The World Bank’s 2003-2009 food and drug capacity building project in India cited lack of public awareness as a major safety barrier (World Bank, 2009).
The public also needs to have a system to communicate with government regulators. There is a lack of active consumer organizations in the countries the committee visited for this report. In general, Sub-Saharan African countries “do not have culture of consumer complaints” (Bester, 2011). This is true in all the least developed countries because poor education is one of the main barriers to consumer communication with govern-
ment (Affin, 1993). Consumers in middle-income countries may be more vocal, but grassroots consumer advocacy is still in its early stages. At the Delhi meetings for this report, Bejon Misra, founder of Partnership for Safe Medicines, India, estimated that of the roughly 2,500 consumer groups in India, only about 4 have the staff and management structure to effectively advocate for public safety. Regulators in developing countries often do not see consumer participation as a priority; consumer education is a sliver of the food safety budget in Asia and Pacific countries (Affin, 1993).
Communication with Counterpart Regulatory Agencies Abroad
Regulators need to be able to share information with their counterparts abroad, especially during international emergencies. International communication is especially difficult; it depends on trust and willingness to share such information and on geopolitical factors beyond the regulatory authority’s control. Governments may fear “expos[ing] their data to interpretations other than those published by their official statisticians” (Pisani and AbouZahr, 2010, p. 463). In post-colonial countries, there is a suspicion that pharmaceutical companies could exploit health surveillance data to their profit (Pisani and AbouZahr, 2010).
At this study’s international workshops in São Paulo and Pretoria participants repeatedly mentioned that the Institute of Medicine meeting brought them together with people whom they had never met, despite working in the same field in neighboring countries. There are international forums that bring regulators together, however. The WHO’s International Food Safety Authorities Network and its International Conference of Drug Regulatory Authorities bring together regulators to strengthen international collaboration. Some changes in the education of the workforce would help stress that communication is a key part of a modern regulatory system.
One factor that will continue to hold back international communication is the need for trust and confidentiality. In 2011 the FDA and the European Medicines Agency (EMA) completed a pilot study of sharing inspections for good clinical practices inside the European Union. Both agencies found this collaboration useful, allowing them to “identify the gaps in each agency’s inspection processes and to fill in those gaps” (EMA and FDA, 2011, p. 17). The agencies’ future plan includes expanding the program to sites outside of the United States and Europe (EMA and FDA, 2011). The FDA, EMA, and Australia’s Therapeutic Goods Commission are also in the early stages of collaboration on inspecting factories producing active pharmaceutical ingredients (EMA, 2011). Such exemplary communication between regulatory authorities is the result of confidentiality agreements and trust. This can be difficult to cultivate, even among developed countries with established historical alliances and comparable regulatory standards.
POLITICAL WILL
Compared to most emerging economies, the United States has roughly a 100-year head start on enforcing food and medical product safety laws and a large, educated, vocal middle class to demand as much. Even so, American support for government regulation has waned since the 2008-2009 economic crisis (Newport, 2010). During a recession public opinion tends toward encouraging business and reducing unemployment, not asking industry to spend money complying with regulations. A 2010 Gallup poll found that 57 percent of Americans fear too much government regulation; only 37 percent fear too little (Newport, 2010). Public opinion will change promptly during a crisis, however. Even during the recession, public outcry over Salmonella-contaminated peanut butter and eggs contributed to Congress passing the Food Safety Modernization Act (Harris and Neuman, 2010).
Public opinion can drive political will, especially in a democracy. This is why a press that is free to publish investigative journalism is an important part of the political process. Independent consumer groups serve a similar purpose. Both are essential for an accountable product safety system. In its deliberations, the committee identified problems with political will, acknowledging that sometimes it is difficult to separate from the public opinion that drives political will.
Competing Priorities, Limited Budgets
In developing countries, even more than in the United States, food and medical product safety often takes a backseat to economic development. In China, for example, 70 percent of local government officials’ annual review comes from the gross national product (GDP) growth in their districts (Roth et al., 2008). Enforcing product safety regulations would shut down many companies and hurt local politicians (Box 3-8). Furthermore, in some developing countries, the regulatory authorities have non-regulatory responsibilities (WHO, 2007). In Cuba, the drug regulatory authority is also in charge of manufacturing drugs; in India the drug regulatory authority runs the Pharmacopeia Commission. In some emerging economies there is reluctance to enforce product safety regulations that might stifle economic growth (Bamberger and Guzman, 2008).
In some large developing countries food and medical product safety programs are the purview of the same agencies responsible for either promoting commerce or assuring drug availability through price controls. For example, the Ministry of Commerce, whose principal goal is to increase exports, is responsible for food safety in India. In China, final responsibility for product safety resides with the local Communist party, whose primary
BOX 3-8
Enforcing Drug Regulation in China
State control of the pharmaceutical industry has receded in China since the 1980s. At the same time, decentralization has encouraged local governments to develop their own economies. The 1984 China Pharmaceutical Administration Law gave drug approval and production licensing power to local governments (Liu, 2010). During the same time, government funding to hospitals decreased, and drug sales were a way for clinics to cover costs. To survive clinics needed to minimize cost and maximize profit. Selling many inexpensive drugs was a way to do that (Liu, 2010). The Chinese government is trying to change this, but hospitals still get 25-60 percent of their revenue from drug sales (U.S. Commercial Service, 2002).
In 1998, the State Drug Administration, later reorganized into the State Food and Drug Administration (SFDA), was created as an inde-pendent medicines regulator (Zhen, 2004). The SFDA is in charge of “the administrative and technical supervision of the research, production, distribution, and utilization of drugs, bulk chemicals, medical devices, medical dressings, and pharmaceutical packaging materials” (Zhen, 2004, p. 347).
In recent years, the Chinese government has launched a series of crackdowns on shoddy drug regulation. Its 2007 anti-counterfeiting campaign shut down 300 hundred drug and medical device manufacturers and rescinded 150 certificates of good manufacturing practices (Bate and Porter, 2009). Shortly thereafter, Zheng Xiaoyu, former head of the SFDA, was executed for accepting bribes from drug and medical equipment companies (Bate and Porter, 2009). After Xiaoyu’s sentence, the Ministry of Health took over the SFDA, and SFDA employees were required to divest of pharmaceutical stock (Liu, 2010).
Despite publicized campaigns, the SFDA has a pervasive problem enforcing regulations. To begin with, the counterfeit drug business employs 3 to 5 million people and brings in an estimated $40-$80 billion annually (Clark, 2003). In some estimations “counterfeiting is now so huge [that] radical action would crash the economy overnight and even destabilize a government where counterfeit factories and warehouses are often owned by local military and political grandees” (Bate and Porter, 2009, p. 3). The low penalties for counterfeiting, a fine of about $15-$580, suggest that SFDA penalties do not reflect the magnitude of public outrage over the crime (Bate and Porter, 2009).
goal is economic development. Both India and Brazil maintain price-control agencies with the laudable goals of assuring drug availability throughout society. In India these agencies also assure the productivity of small drug firms. These well-intentioned systems may put economic growth at odds with medical product and food safety actions. This is especially true at municipal government levels, where the very existence of the food safety system depends on funding that comes from commerce. Similarly, in the attempt to assure drug availability for the poorest, governments can cut prices to point cutting quality out of the system. Both of these scenarios also encourage bypassing proper distribution channels, inadvertently promoting substandard products.
The conflict of interest in promoting exports and regulating export safety may trouble India and China’s trading partners. Their own citizens have different concerns. In most countries, the Ministry of Health has a great deal of medical product regulatory responsibility; it often has food regulatory responsibility as well. Ministers of Health in these countries have many problems: malnutrition, child mortality, infectious disease, water shortages, health financing, and poor sanitation, to name a few. With so many demands on their attention, they do not always see food and medical product safety as a high priority. Even when they do, the heads of government may be more concerned with agriculture, labor, or commerce.
Donors, on the other hand, are enthusiastic funders of the health sector; health aid is an important piece of many countries’ foreign policy. Development aid for health grew more than $16 billion between 1990 and 2007 (Who runs global health?, 2009). In the same time, government spending on health dropped from 24.3 to 20.9 percent in low-income countries, and from 52.2 to 48.6 percent in middle-income countries (Farag et al., 2009). By some analyses, every dollar donors spend on health accompanies a $0.46 decrease in government health spending (Lu et al., 2010). The substitution of donor aid for government spending is also common in agriculture, although less so in infrastructure building and education (Farag et al., 2009).
Reallocating money from health and agriculture to other sectors is an understandable choice for low- and middle-income countries facing a rare windfall of donor generosity. Heads of government can justify their budgets, explaining that national projects on HIV, malaria, and tuberculosis are still better off than they would have been on public money. Health and medical product regulatory systems are a clear loser in this equation, however. With notable exceptions such as USAID’s Strengthening Pharmaceutical Systems Program, the African Medicines Regulatory Harmonization Initiative (a joint effort including the WHO, World Bank, NEPAD, the Clinton Health Access Initiative and the Gates Foundation), and the FAO’s Food Quality and Standards Service, donors are not interested in building
regulatory systems. Smaller budgets for health and agriculture mean less support for food and medical product safety.
The U.S. government has recognized a need to shift from health aid for specific diseases, also called vertical programs, to broader-based health systems aid, or horizontal programs. The 2008 Global Health Initiative Strategy Document describes the Obama administration’s commitment to strengthening health systems, in particular the goal of “improved research and regulatory capacity to support clinical trials, bring new, high-quality innovations to partner country markets; and monitor the quality, safety, and efficacy of the supply chain” (GHI, 2012, p. 21).
Corruption and Accountability
The previous section on gaps in the regulatory workforce describes the ways in which corruption contributes to staff retention problems in the government. Similarly, corruption saps political will to enforce regulations. Mexico, China, and Thailand are the second, third, and fourth largest food suppliers to the United States (Muchmore, 2010). Transparency International rates all three as having serious corruption problems (Muchmore, 2010). It is difficult for a regulatory authority to prevent its inspectors from taking bribes in these countries, especially when the bribe is accompanied with a threat (Muchmore, 2010, p. 403).
There are reasons for optimism, however. The middle class is growing in India, China, Mexico, Brazil, South Africa, Thailand, Indonesia, and many other countries. According to the Asian and African development banks the middle class now accounts for roughly one-third of the population of Africa, one-quarter of India, three-quarters of Latin America, and 90 percent of China3 (Politics in emerging markets: The new middle classes rise up, 2011; Chun, 2010). Middle class voters tend to be disproportionately urban; they are concerned with health care and generally distanced from farming (ADB, 2010). They buy medicines in pharmacies or dispensaries and buy food in markets. Public health and product safety are more important to the middle class than to the poor. In August 2011, China saw its largest popular uprising since Tiananmen Square over a toxic chemical plant in the wealthy city of Dalian (Politics in emerging markets: The new middle classes rise up, 2011).
Eliminating corruption is also important to middle class voters. In 2011 Brazilian president Dilma Rousseff sacked numerous corrupt officials,
![]()
3 The development banks define the middle class as those earning from $2 to $20 a day, a definition that includes people only barely out of poverty. It is a particularly rough measure in China, where the value of the currency is set at 7 Yuan to 1 dollar. By more modest estimates, there are only 80 million middle class consumers in China (Hodgson, 2007).

Indian customers at a Big Apple convenience store in New Delhi.
SOURCE: AFP/Getty Images.
including the second in command at the Ministry of Agriculture, in a house cleaning The Economist attributes in part to her country’s changing demographics and priorities (Dilma tries to drain the swamp, 2011; Politics in emerging markets: The new middle classes rise up, 2011). In August 2011, Indian activist Anna Hazare’s 4-day hunger strike protested government corruption with support from the middle class (Denyer and Lakshmi, 2011).
The momentum against corruption and in support of food and medical product safety is likely to grow as communication gets easier. In China, blogging services such as Sino Weibo have 140 million users; China has half a billion cell phone users (Politics in emerging markets: The new middle classes rise up, 2011; Freeman, 2009). The priorities of a growing and technologically active middle class are driving change.
CONCLUSION
In its deliberations the committee identified nine main critical issues facing developing country regulators. Problems adhering to international standards endanger product safety in low- and middle-income countries. It also hedges them out of lucrative export markets. International standards assume control of supply chains. In places where there are many, sometimes anonymous, transactions that occur over long supply chains this is particularly difficult. Poor roads, unreliable power, water shortages, poor sanitation, and other infrastructure problems make supply chain management difficult. Companies in low- and middle-income countries cannot track
and trace their products without improvements in information technology infrastructure.
Laws are at the foundation of food and medical product regulation, and many countries lack the ability to enforce their product safety laws. Law enforcement might be easier if regulatory authorities had more and better trained staff. It is hard to keep trained scientists in government service in low- and middle-income countries. Better salaries on projects and in industry draw many away. Others grow frustrated and quit.
The job of a government regulator in a developing country is often frustrating, in part because regulatory responsibilities are usually scattered among many different government agencies. And, while this is often true in rich countries as well, fragmented responsibilities can cripple an agency without strong communication systems. Lack of communication with the public and with regulated industry is also a common weakness in low- and middle-income countries. During emergencies prompt and accurate communication is essential. Regulatory authorities need to draw on accurate data to inform their message. Poor surveillance systems prevent them from forming clear and accurate messages to share with the public. Poor surveillance also impedes an important part of the regulators’ jobs. Modern regulatory systems use risk assessment to inform their decisions, and regulators in emerging economies have neither the reliable data to inform risk analysis or personnel with the mathematics and epidemiology training to do it.
In some cases, the very structure of the regulatory system encourages these problems. Regulatory systems in emerging economies assign to their staff conflicting responsibilities; a regulator given both product safety and commerce promotion jobs can do neither job well. More commonly, food and medical product safety slip through the many cracks in nascent health systems. Ironically, the past decade of donor support for health programming has encouraged governments to spend their money in other sectors. Food and medical product regulation have suffered because of this. There is reason to believe that this will change in the future, as the middle class continues to grow in the world’s emerging manufacturing nations.
There are many root causes of the nine main gaps discussed in this chapter. Poverty and a lack of a strong government infrastructure are two of the main ones. The regulatory systems in Latin America, for example, benefit from greater wealth and good school system. These are advantages that cannot be immediately replicated in South Asia or Sub-Saharan Africa. The committee’s recommendations are for improvements that could elicit a meaningful change across a wide range of countries at different levels of development.
REFERENCES
A tale of two expats: Business in China and the west. 2011. Economist, January 1.
A.T. Kearney. 2004a. Healing Mexico’s health-care system. Chicago, IL: A.T. Kearney.
———. 2004b. An imperative for public health care: Improving the medicine supply chain. Chicago, IL: A.T. Kearney.
Abegaz, M. 2006. Working paper on: Assessment of the capacity of food safety and quality in Zambia. Washington, DC: The World Bank.
Abraham, J. 2002. The pharmaceutical industry as a political player. Lancet 360(9344):1498-1502.
Abudhoo, S. 2012. Devolution of drug regulations to raise manufacturing cost. The Nation, January 2.
ADB (Asian Development Bank). 2010. The rise of Asia’s middle class. Mandaluyong City, Philippines: Asian Development Bank.
Affin, A. B. 1993. Food control and consumer affairs in developing countries. Food, Nutrition and Agriculture 8/9.
African Union Interafrican Bureau for Animal Resources. 2011. Participation of African nations in sanitary and phytosanitary standard-setting organisations. http://www.au-ibar.org/index.php?option=com_flexicontent&view=items&cid=67:projects&id=83:pan-spso&Itemid=39 (accessed October 4, 2011).
Agribusiness and Allied Kenya Ltd, Standards and Solutions Consulting Ltd, and the International Centre of Insect Physiology. 2006. Generic manual on quality management system for smallholder horticultural farmer groups in Kenya for certification to EUREPGAP option 2. Nairobi, Kenya.
Anderson, T. 2010. Tide turns for drug manufacturing in Africa. Lancet 375(9726):1597-1598.
Anvisa. 2003. Overview. http://www.anvisa.gov.br/eng/institution/introduction.htm (accessed October 21, 2011).
AOAC (Association of Analytical Communities International). AOAC capabilities. http://www.aoac.org/about/aoac_capabilities.pdf. (accessed September 20, 2011).
———. 2009. Validation guidelines. http://www.aoac.org/vmeth/Validation_Guidelines.htm (accessed September 23, 2011).
APEC (Asia Pacific Economic Cooperation). 2008. Draft implementation plan for strengthening food safety standards practices in APEC economies for 2008-2011 (agenda item 4[5]). Paper presented at First Sub-Committee on Standards and Conformance Meeting, Lima, Peru, February 25-26.
Aragão, A. 2010. As agências reguladoras independentes brasileiras: O caso da agência nacional de vigilância sanitária. Revista de Direito Sanitário 10(3):77-89.
Asian Productivity Organization. 2006. Postharvest management of fruit and vegetables in the Asia-Pacific region. Tokyo, Japan: Asian Productivity Organization.
Attridge, C. J., and A. S. Preker. 2005. Improving access to medicines in developing countries: Application of new institutional economics to the analysis of manufacturing and distribution issues. Washington, DC: The World Bank’s Human Development Network.
Bakare, N., I. R. Edwards, A. Stergachis, S. Pal, C. B. Holmes, M. Lindquist, C. Duncombe, A. Dodoo, J. Novendstern, J. Nwokike, R. Kuchenbecker, J. A. Aberg, V. Miller, and J. Strobos. 2011. Global pharmacovigilance for antiretroviral drugs: Overcoming contrasting priorities. PLoS Med 8(7):e1001054.
Bamberger, K. A., and A. T. Guzman. 2008. Keeping imports safe: A proposal for discriminatory regulation of international trade. California Law Review 96(6):1405-1447.
Bandekar, M. S., S. R. Anwikar, and N. A. Kshirsagar. 2010. Quality check of spontaneous adverse drug reaction reporting forms of different countries. Pharmacoepidemiology and Drug Safety 19(11):1181-1185.
Barboza, D. 2007. Food-safety crackdown in China. New York Times, June 28.
Bate, R., and K. Porter. 2009. The problems and potential of China’s pharmaceutical industry. AEI Health Policy Outlook 3(April):1-10.
Becker, G. S. 2008. Food and agricultural imports from China. Washington, DC: Congressional Research Service.
Belton, B., M. M. Haque, D. C. Little, and L. X. Sinh. 2010. Certifying catfish in Vietnam and Bangladesh: Who will make the grade and will it matter? Food Policy 36(2):289-299.
Benabdallah, G., R. Benkirane, A. Khattabi, I. R. Edwards, and R. S. Bencheikh. 2011. The involvement of pharmacovigilance centres in medication errors detection: A questionnaire-based analysis. The International Journal of Risk and Safety in Medicine 23(1):17-29.
Bester, A. 2011. The importance of food safety in Africa. http://www.howwemadeitinafrica.com/the-importance-of-food-safety-in-africa/5043/ (accessed June 14).
Black, S., K. Mulholland, and N. A. Halsey. 2010. Developing sustainable funding for vaccine safety infrastructure. Vaccine 28(5):1133-1134.
Blaser, M. J. 2011. Deconstructing a lethal foodborne epidemic. New England Journal of Medicine 365(19):1835-1836.
Bollyky, T. J. 2009. Global health interventions for U.S. food and drug safety. Washington, DC: CSIS Global Health Policy Center.
Brhlikova, P., I. Harper, and A. Pollock. 2007. Good manufacturing practice in the pharmaceutical industry. Working Paper 3. Paper presented at Workshop on “Tracing Pharmaceuticals in South Asia,” University of Edinburgh, Scotland, UK, July 2-3.
Bridges, A. 2007. FDA bonuses spending to draw scrutiny. USA Today, July 17.
Brighton Collaboration. 2010. What we do: Capacity building. https://brightoncollaboration.org/public/what-we-do/capacity.html (accessed November 3, 2011).
Broughton, E. I., and D. G. Walker. 2010. Policies and practices for aquaculture food safety in China. Food Policy 35(5):471-478.
Byerlee, D., and K. Fischer. 2002. Accessing modern science: Policy and institutional options for agricultural biotechnology in developing countries. World Development 30(6):931-948.
CAC (Codex Alimentarius Commission). 2011. Home page. http://www.codexalimentarius.net/web/index_en.jsp (accessed June 14, 2011).
Carey, J. 2007. Not made in China. Business Week, July 30, 41-43.
Castillo-Salgado, C. 2010. Trends and directions of global public health surveillance. Epidemiologic Reviews 32(1):93-109.
CDC (Centers for Disease Control and Prevention). 2011. CDC estimates of foodborne illness in the United States. Atlanta, GA: National Center for Emerging & Zoonotic Infectious Diseases, Division of Foodborne, Waterborne, and Environmental Diseases.
———. 2012. CDC research shows outbreaks linked to imported foods increasing. http://www.cdc.gov/media/releases/2012/p0314_foodborne.html (accessed March 16, 2012).
Chanda, R. R., R. J. Finchman, and P. Venter. 2010. A review of the South African food control system: Challenges of fragmentation. Food Control 21(6):816-824.
Chaudhuri, S., M. Mackintosh, and P. G. M. Mujinja. 2010. Indian generics producers, access to essential medicines and local production in Africa: An argument with reference to Tanzania. European Journal of Development Research 22(4):451-468.
Chen, D., and D. Kristensen. 2009. Opportunities and challenges of developing thermostable vaccines. Expert Review of Vaccines 8(5):547-557.
Cheng, M. 2010. Britain bans doctor who linked autism to vaccine. Associated Press, May 25.
Chocarro, L., P. Duclos, K. Senouci, and J. Southern. 2011. Consultation on interactions between national regulatory authorities and national immunization technical advisory groups. Expert Review of Vaccines 10(9):1265-1270.
Chun, N. 2010. Middle class size in the past, present, and future: A description of trends in Asia. Mandaluyong City, Philippines: The Asian Development Bank.
Clark, D. J. 2003. Product counterfeiting in China and one American company’s response. Washington, DC: National Defense University.
Clift, C. 2010a. Combating counterfeit, falsified, and substandard medicines: Defining the way forward? London, UK: Chatham House.
———. 2010b. Meeting summary: Combating counterfeit, falsified, and substandard medicines. London, UK: Chatham House.
Cohen, J. C. 2005. Corruption in the pharmaceutical sector. In Corruption in health. Boston, MA: Boston University. Pp. 76-102.
Corbell, C., A. Stergachis, F. Ndowa, P. Ndase, L. Barnes, and C. Celum. 2010. Genital ulcer disease treatment policies and access to acylovir in eight Sub-Saharan African countries. Sexually Transmitted Diseases 37(8):1-6.
CSPI (Center for Science in the Public Interest). 2007. Combine all U.S. food safety functions into a single agency, the Food Safety Administration: Support S. 654, H.R. 1148—the Safe Food Act. Washington, DC: Center for Science in the Public Interest.
Day, J. N., T. T. Hien, and J. Farrar. 2004. Expiry-date tampering. Lancet 363(9403):172.
Denyer, S., and R. Lakshmi. 2011. Anna Hazare inspires young, middle-class awakening in India. Washington Post, August 19.
Dilma tries to drain the swamp. 2011. Economist, August 20.
Donnges, C., G. Edmonds, and B. Johannessen. 2007. Rural road maintenance: Sustaining the benefits of improved access. Geneva, Switzerland: International Labour Organization.
DRA establishment at federal level demanded. 2012. Daily Times, January 27.
EDQM (European Directorate for the Quality of Medicines and HealthCare). Vision, mission & values. http://www.edqm.eu/en/Vision-Mission-amp-Values-604.html (accessed September 21, 2011).
EMA (European Medicines Agency). 2011. Final report on the international API inspection pilot programme. London, UK: EMA, Australian Government, and FDA.
EMA and FDA (U.S. Food and Drug Administration). 2011. Report on the pilot EMA-FDA GCP initiative: September 2009-March 2011. London, UK: EMA and FDA.
Essack, S. Y., N. Shchellack, T. Pople, L. v. d. Merwe, F. Suleman, J. C. Meyer, A. G. S. Gous, and D. Benjamin. 2011. Antibiotic supply chain and management in human health. South African Medical Journal 101(8). FAO and WHO (Food and Agriculture Organization of the United Nations and the World Health Organization). 2003. Assuring food safety and quality: Guidelines for strengthening national food control systems. Rome, Italy: FAO and WHO.
Farag, M., A. K. Nandakumar, S. S. Wallack, G. Gaumer, and D. Hodgkin. 2009. Does funding from donors displace government spending for health in developing countries? Health Affairs 28(4):1045-1055.
FDA (Food and Drug Administration). 2011. FDA-industry PDUFA V reauthorization meeting. Teleconference. Silver Spring, MD.
Freed, G. L., S. J. Clark, A. T. Butchart, D. C. Singer, and M. M. Davis. 2010. Parental vaccine safety concerns in 2009. Pediatrics 125(4):654-659.
Freeman, C. W. 2009. China’s capacity to manage infectious diseases: Global implications. Washington, DC: Center for Strategic and International Studies.
Frerichs, R. R. 1991. Epidemiologic surveillance in developing countries. Annual Review of Public Health 12(1):257-280.
GACVS (Global Advisory Committee on Vaccine Safety) and WHO Secretariat. 2009. Global safety of vaccines: Strengthening systems for monitoring, management and the role of GACVS. Expert Reviews Vaccines 8(6):705-716.
Gale, F., and C. J. Buzby. 2009. Imports from China and food safety issues. Economic Information Bulletin 52(January 14). http://www.ers.usda.gov/publications/eib52/.
GAO (Government Accountability Office). 2010. Drug safety: FDA has conducted more foreign inspections and begun to improve its information on foreign establishments but more progess is needed. GAO-10-961. Washington, DC: GAO.
Garrett, L., and Y. Huang. 2011. Food and drugs: Can safety be ensured in a time of increased globalization? Washington, DC: Council on Foreign Relations.
Genetics and Public Policy Center. 2010. Reproductive genetic testing: A regulatory patchwork. http://www.dnapolicy.org/policy.international.php?action=detail&laws_id=63 (accessed October 25, 2010).
Gessner, G. H., L. Volonino, and L. A. Fish. 2007. One-up, one-back ERM in the food supply chain. Information Systems Management 24(3):213-222.
GHI (Global Health Initiative). The United States government global health initiative: Strategy document. http://www.pepfar.gov/documents/organization/136504.pdf (accessed February 24, 2012).
GHTF (Global Harmonization Task Force). 2007. Overview and mission. http://www.ghtf.org/about/overview.html (accessed September 23, 2011).
Giovannucci, D., and T. Purcell. 2008. Standards and agricultural trade in Asia. Tokyo, Japan: Asian Development Bank Institute.
Global Fund. 2008. Procurement, supply chain management and service delivery of the Global Fund grants to the government of India. Geneva, Switzerland: The Global Fund.
Global Pharma Health Fund. The GPHF-Minilab®—Protection against counterfeit medicines. http://www.gphf.org/web/en/minilab (accessed November 9, 2011).
Godfray, H. C. J., J. R. Beddington, I. R. Crute, L. Haddad, D. Lawrence, J. F. Muir, J. Pretty, S. Robinson, S. M. Thomas, and C. Toulmin. 2010. Food security: The challenge of feeding 9 billion people. Science 327(5967):812-818.
Government of India. 2003. Report of the expert committee on a comprehensive examination of drug regulatory issues, including the problem of spurious drugs. New Delhi: Government of India.
Greene, W. 2007. The emergence of India’s pharmaceutical industry and implications for the U.S. generic drug market. Washington, DC: Office of Economics, U.S. International Trade Comission.
Guerin, O. 2012. Lahore grieves over heart pill deaths. BBC News, January 29.
Gundersen, L. E. 2001. Training needs in regulatory science for the biopharmaceutical industry. Nature Biotechnology 19(12):1187-1188.
Gustavasson, J., C. Cederberg, U. Sonesson, R. v. Otterdijk, and A. Meybeck. 2011. Global food losses and food waste: Extent, causes, and prevention. Dusseldorf, Germany: Food and Agriculture Organization.
Hamburg, M. A. 2011. Advancing regulatory science. Science 331(6020):987-987.
Hampton, T. 2011. European drug agency under fire: Critics charge that trial data are too inaccessible. Journal of the American Medical Association 306(6):593-595.
Hao, Z. 2012. Substandard cardiac drugs claim over 100 lives in Pakistan. http://english.cntv.cn/20120206/113321.shtml (accessed Febuary 12, 2012).
Harris, G., and W. Neuman. 2010. Senate passes sweeping law on food safety. New York Times, November 30.
Hawthorne, N., and C. Anderson. 2009. The global pharmacy workforce: A systematic review of the literature. Human Resources for Health 7.
Hazell, P., and S. Wood. 2008. Drivers of change in global agriculture. Philosophical Transactions of the Royal Society B: Biological Sciences 363(1491):495-515.
Henson, S., and J. Humphrey. 2009. The impacts of private food safety standards on the food chain and on public standard-setting processes: Paper prepared for FAO/WHO. Rome, Italy: FAO and WHO.
Herring, J. 2011. Consistent clinical research standards benefit patients around the world. Nature Medicine 17(9):1036.
Hodgson, A. 2007. China’s middle class reaches 80 million. Euromonitor International, July 25. http://blog.euromonitor.com/2007/07/chinas-middle-class-reaches-80-million.html.
ICH (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use). 2011a. ICH harmonisation for better health: Official website. http://www.ich.org/ (accessed October 4, 2011).
———. 2011b. Southern African Development Community International Conference on Harmonisation Quality Guidelines No 7 (ICH Q7) Training Venue, Arusha, United Republic of Tanzania, June 27-30. http://www.ich.org/fileadmin/Public_Web_Site/Training/SADC_Q7_workshop_June_2011/ICH_Q7_training_20June2011.pdf (accessed September 30, 2011).
———. 2011c. Vision. http://www.ich.org/about/vision.html (accessed September 21, 2011).
ICMSF (International Commission on Microbiological Specifications for Foods). Purpose. http://www.icmsf.iit.edu/main/home.html (accessed September 20, 2011).
International Life Sciences Institute—India. 2007. A surveillance and monitoring system for food safety in India (under Food Safety and Standards Act—2005). New Delhi, India: International Life Sciences Institute.
IPPC (International Plant Protection Convention). 2011. What we do. https://www.ippc.int/index.php?id=what&no_cache=1&L=0 (accessed September 23, 2011).
Irwin, A., H. Rothstein, S. Yearley, and E. McCarthy. 1997. Regulatory science—Towards a sociological framework. Futures 29(1):17-31.
ISO (International Standards Organization). 2011a. About ISO. http://www.iso.org/iso/about.htm (accessed September 22, 2011).
———. 2011b. ISO 28620:2010. http://www.iso.org/iso/iso_catalogue/catalogue_tc/catalogue_detail.htm?csnumber=44810 (accessed September 26, 2011).
Jaffee, S., and S. Henson. 2004. Standards and agro-food exports from developing countries: Rebalancing the debate. World Bank Policy Research Working Paper No. 3348. Washington, DC: The World Bank.
Jahre, M., L. Dumoulin, L. B. Greenhalgh, C. Hudspeth, P. Limlim, and A. Spindler. 2010. Improving health systems in developing countries by reducing the complexity of drug supply chains. Paper presented at the 8th International Conference on Logistics and SCM Research, Bordeaux, France, September 30-October 1.
Jeffery, R., and M. R. Santhosh. 2009. Architecture of drug regulation in India: What are the barriers to regulatory reform? Journal of Health Studies II:13-32.
Jia, H., and K. Carey. 2011. Chinese vaccine developers gain WHO imprimatur. Nature Biotechnology 29(6):471-472.
Juma, C. 2008. Learn to earn. Nature 456(n1s):15-17.
Kader, A. A. 2010. Handling of horticultural perishables in developing vs. developed countries. University of California, Davis.
Kauffmann, J. R., R. Miller, and J. Cheyne. 2011. Vaccine supply chains need to be better funded and strengthened, or lives will be at risk. Health Affairs 30(6):1113-1121.
Kelleher, F. 2004. The pharmaceutical industry’s responsibility for protecting human subjects of clinical trials in developing nations. Columbia Journal of Law and Social Problems 38(1):67-106.
Kenyon, T. A., A. S. Kenyon, and T. Sibiya. 1994. Drug quality screening in developing countries: Establishment of an appropriate laboratory in Swaziland. Bulletin of the World Health Organization 72(4):615.
Kimball, A. M., M. Moore, H. M. French, Y. Arima, K. Ungchusak, S. Wibulpolprasert, T. Taylor, S. Touch, and A. Leventhal. 2008. Regional infectious disease surveillance networks and their potential to facilitate the implementation of the International Health Regulations. Medical Clinics of North America 92(6):1459-1471.
Kitinoja, L., S. Saran, S. K. Roy, and A. A. Kader. 2011. Postharvest technology for developing countries: Challenges and opportunities in research, outreach and advocacy. Journal of the Science of Food and Agriculture 91(4):597-603.
Kopp, S. 2006. Stability testing of pharmaceutical products in a global environment. The Regulatory Affairs Journal 4.
KPMG International. 2006. The Indian pharmaceutical industry: Collaboration for growth. The Netherlands: KPMG International.
Kshirsagar, N. A., S. Olsson, and R. E. Ferner. 2010. Consideration of the desirable features and possible forms of practical indicators of the performance of pharmacovigilance centres. The International Journal of Risk and Safety in Medicine 22(2):59-66.
Kumar, A. 2011. Past, present and future of pharmacovigilance in India. Systematic Reviews in Pharmacy 2(1):55-58.
LaFraniere, S. 2011. In China, fear of fake eggs and “recycled” buns. New York Times, May 7.
Langer, E. S. 2008. Biologics regulation in India. BioPharm Internatiaonal 21(3).
Larson, H. J., P. Brocard, and G. Garnett. 2010. The India HPV-vaccine suspension. Lancet 376(9741):572-573.
Larson, H. J., L. Z. Cooper, J. Eskola, S. L. Katz, and S. Ratzan. 2011. Addressing the vaccine confidence gap. Lancet 378(9790):526-535.
Leng, H. M. J., and M. P. Matsoso. 2008. Pharmaceutical and analytical quality control services: Ownership or outsourcing—a South African case study. Drug Information Journal 42(2):161-173.
Liu, P. 2010. From decentralised developmental state towards authoritarian regulatory state: A case study on drug safety regulation in China. China: An International Journal 8(1):110-137.
Liu, Y., and F. Wu. 2010. Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environmental Health Perspectives 118(6).
Llana, S. M. 2010. Food safety: From Mexican farm, to Costco, to your plate. Christian Science Monitor, October 23.
Losse, K., E. Schneider, and C. Spennemann. 2007. The viability of local pharmaceutical production in Tanzania. Eschborn, Germany: Deutsche Gesellschaft für Technische Zusammenarbeit.
Lu, C., M. T. Schneider, P. Gubbins, K. Leach-Kemon, D. Jamison, and C. J. L. Murray. 2010. Public financing of health in developing countries: A cross-national systematic analysis. Lancet 375(9723):1375-1387.
Lu, W. 2005. Trade and environment dimensions in the food and food processing industries in Asia and the Pacific: A country case study of China. Hangzhou, China: Department of Agricultural Economics, Zhejiang University.
Lu, W., and S. Kjeldsen-Kragh. 2008. International food safety standards: Catalysts for increased Chinese food quality? Copenhagen Journal of Asian Studies 26(1):70-90.
Lugosi, L., and Battersby. 1990. Transport and storage of vaccines in Hungary: The first cold chain monitor study in Europe. Bulletin of the World Health Organization 68(4):431-439.
Lukulay, P. 2011. Promoting the quality of medicines (PQM) program. Paper presented at Strengthening Core Elements of Regulatory Systems in Developing Countries: Meeting One, Washington, DC, March 3.
Lupien, J. R. 2007. Prevention and control of food safety risks: The role of governments, food producers, marketers, and academia. Asia Pacific Journal of Clinical Nutrition 16(Suppl 1):74-79.
M4P. 2006. The participation of the poor in supermarkets and other distribution value chains. Hanoi, Vietnam: The Asian Development Bank.
Maheshwar, C., and T. S. Chanakwa. 2006. Post harvest losses due to gaps in cold chain in India. IHS Acta Horticulturae 712:777-784.
Majowicz, S. E., J. Musto, E. Scallan, F. J. Angulo, M. Kirk, S. J. O’Brien, T. F. Jones, A. Fazil, R. M. Hoekstra, for the International Collaboration on Enteric Disease “Burden of Illness” Studies. 2010. The global burden of nontyphoidal salmonella gastroenteritis. Clinical Infectious Diseases 50(6):882-889.
Malkin, R. A. 2007. Design of health care technologies for the developing world. Duke University, Durham, NC.
Manson, K. 2011. ACT production: “The biggest challenge is access to the market.” Financial Times, April 21.
Martin, D. S. 2007. Lawmakers push for change in food safety oversight. CNN.com, May 17.
Martins, R. d. M., M. d. L. d. S. Maia, E. M. d. Santos, R. L. d. S. Cruz, P. R. G. dos Santos, S. M. D. Carvalho, H. K. Sato, M. T. Schermann, R. Mohrdieck, M. d. L. F. Leal, and A. Homma. 2010. Yellow fever vaccine post-marketing surveillance in Brazil. Procedia in Vaccinology 2(2):178-183.
Marucheck, A., N. Greis, C. Mena, and L. Cai. 2011. Product safety and security in the global supply chain: Issues, challenges and research opportunities. Journal of Operations Management 29(7-8):707-720.
Matowe, L., P. Waako, R. Adome, I. Kibwage, O. Minzi, and E. Bienvenu. 2008. A strategy to improve skills in pharmaceutical supply management in East Africa: The regional technical resource collaboration for pharmaceutical management. Human Resources for Health 6(1):30.
May, M. 2011. FDA reorganization inspires hope for better coordination. Nature Medicine 17(9):1024-1024.
McMeekin, T. A., J. Baranyi, J. Bowman, P. Dalgaard, M. Kirk, T. Ross, S. Schmid, and M. H. Zwietering. 2006. Information systems in food safety management. International Journal of Food Microbiology 112(3):181-194.
Milstien, J., A. Costa, S. Jadhav, and R. Dhere. 2009. Reaching international GMP standards for vaccine production: Challenges for developing countries. Expert Review of Vaccines 8(5):559-566.
Moran, M., J. Guzman, A.-L. Ropars, A. McDonald, N. Jameson, B. Omune, S. Ryan, and L. Wu. 2009. Neglected disease research and development: How much are we really spending? PLoS Med 6(2):e1000030.
Moran, M., N. Strub-Wourgaft, J. Guzman, P. Boulet, L. Wu, and B. Pecoul. 2011. Registering new drugs for low-income countries: The African challenge. PLoS Medicine 8(2):e1000411.
Morel, C. M., J. R. Carvalheiro, C. N. P. Romero, E. A. Costa, and P. M. Buss. 2007. The road to recovery. Nature 449(7159):180-182.
Mori, M., R. Ravinetto, and J. Jacobs. 2011. Quality of medical devices and in vitro diagnostics in resource-limited settings. Tropical Medicine and International Health 16(11):1439-1449.
Morroney, R., J. Arrieta, A. Belza, M. Biere, G. Castaneda, L. Funari, S. Gilbert, B. Marinaro, and Y. Obando. 2010. Medical device regulation in Latin America. Rockville, MD: Regulatory Affairs Professional Society.
Muchmore, A. I. 2010. Private regulation and foreign conduct. San Diego Law Review 47(2).
Narula, S. A. 2011. Reinventing cold chain industry in India: Need of the hour. Interview with Mr. Sanjay Aggarway. Journal of Agribusiness in Developing and Emerging Economies 1(2).
Nauriyal, D. K. 2006. TRIPS-compliant new patents act and Indian pharmaceutical sector: Directions and strategy in R&D. Indian Journal of Economics and Business, Special Issue, China & India 1:2-20.
Newport, F. 2010. Americans leery of too much gov’t regulation of business. Princeton, NJ: Gallup.
Newton, P. N., M. D. Green, and F. M. Fernández. 2010. Impact of poor-quality medicines in the “developing” world. Trends in Pharmacological Sciences 31(3):99-101.
Ni, H.-G., and H. Zeng. 2009. Law enforcement is key to China’s food safety. Environmental Pollution 157(7):1990-1992.
Norris, P., R. B. Dos Santos, D. Woods, and W. Tobata. 2007. Delivering medicines in a challenging environment: The pharmaceutical sector in East Timor (a descriptive study). Pharmacy Practice 5(4):145-150.
Okello, J. J., C. Narrod, and D. Roy. 2007. Food safety requirements in African green bean exports and their impact on small farmers. Washington, DC: International Food Policy and Research Institute.
Okie, S. 2010. Reviving the FDA. New England Journal of Medicine 363(16):1492-1494.
Olsson, S., S. N. Pal, A. Stergachis, and M. Couper. 2010. Pharmacovigilance activities in 55 low- and middle-income countries: A questionnaire-based analysis. Drug Safety 33(8):689-703.
Oluka, N., F. Ssennoga, and S. Kambaza. 2010. Tackling supply chain bottlenecks of essential drugs: A case of Uganda local government health units. Paper presented at 4th International Procurement Conference, Seoul, South Korea, August 26-28.
Oxfam International. 2011. Eye on the ball: Medicine regulation—not IP enforcement—can best deliver quality medicines. http://www.oxfam.org/sites/www.oxfam.org/files/eye-on-the-ball-medicine-regulation-020211-en.pdf. PAHO (Pan American Health Organization). 2011. SANEVA—Adverse Event Sentinel Surveillance Network for new vaccines. http://66.101.212.220/hq/index.php?option=com_content&task=view&id=1033&Itemid=243 (accessed November 8, 2011).
Patel, M. M., V. R. López-Collada, M. M. Bulhões, L. H. De Oliveira, A. B. Márquez, B. Flannery, M. Esparza-Aguilar, E. I. Montenegro Renoiner, M. E. Luna-Cruz, H. K. Sato, L. d. C. Hernández-Hernández, G. Toledo-Cortina, M. Cerón-Rodríguez, N. Osnaya-Romero, M. Martínez-Alcazar, R. G. Aguinaga-Villasenor, A. Plascencia-Hernández, F. Fojaco-González, G. Hernández-Peredo Rezk, S. F. Gutierrez-Ramírez, R. Dorame-Castillo, R. Tinajero-Pizano, B. Mercado-Villegas, M. R. Barbosa, E. M. C. Maluf, L. B. Ferreira, F. M. de Carvalho, A. R. dos Santos, E. D. Cesar, M. E. P. de Oliveira, C. L. Osterno Silva, M. de los Angeles Cortes, C. Ruiz Matus, J. Tate, P. Gargiullo, and U. D. Parashar. 2011. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. New England Journal of Medicine 364(24):2283-2292.
PATH. 2012a. Project Optimize: At a glance. http://www.path.org/projects/project-optimize-ataglance.php (accessed January 31, 2012).
———. 2012b. Rethinking the vaccine supply chain. http://www.path.org/projects/project-optimize.php (accessed January 31, 2012).
———. 2012c. World’s smartest sticker. http://www.path.org/projects/vaccine_vial_monitor.php (accessed January 31, 2012).
Peeling, R. W., P. G. Smith, and P. M. M. Bossuyt. 2010. A guide for diagnostic evaluations. Nature Reviews Microbiology. http://www.nature.com/nrmicro/journal/v8/n12_supp/pdf/nrmicro1522.pdf.
Pew Health Group. 2011. After heparin: Protecting consumers from the risks of substandard and counterfeit drugs. Washington, DC: Pew Health Group.
Pirmohamed, M., K. N. Atuah, A. N. O. Dodoo, and P. Winstanley. 2007. Pharmacovigilance in developing countries. BMJ 335.
Pisani, E., and C. AbouZahr. 2010. Sharing health data: Good intentions are not enough. Bulletin of the World Health Organization 88(6):462-466.
Poisot, A.-S., A. Speedy, and E. Kueneman. 2004. Good agricultural practices—a working concept: Background paper for the FAO internal workshop on good agricultural practices. Rome, Italy: FAO.
Politics in emerging markets: The new middle classes rise up. 2011. Economist, September 3.
Pornruangwong, S., R. S. Hendriksen, C. Pulrikarn, A. Bangstrakulnonth, M. Mikoleit, R. H. Davies, F. M. Aarestrup, and L. Garcia-Migura. 2011. Epidemiological investigation of Salmonella enterica Serovar Kedougou in Thailand. Foodborne Pathogens and Disease 8(2):203-211.
Prado, M. M. 2006. Independent regulatory agencies, patronage and clientelism lessons from Brazil. Paper presented at Primera Conferencia Internacional sobre Corrupción y Transpacencia: Debatiendo las Fronteras entre Estado, Mercado y Sociedad, Mexico City, March 23-25.
———. 2008. The challenges and risks of creating independent regulatory agencies: A cautionary tale from Brazil. Vanderbilt Journal of Transnational Law 41(2):435-503.
Prakash, S. 2009. Evolution of regulatory system in India. India Journal of Pharmacology 41(5):207.
Ratanawijitrasin, S., and E. Wondemagegnehu. 2002. Effective drug regulation: A multicountry study. Geneva, Switzerland: The World Health Organization.
Reuters. 2001. Baxter recalls kidney filters after deaths. USA Today, October 18.
———. 2010. E coli outbreak blamed on Egyptian fenugreek seeds. Guardian, July 5.
Roth, A. V., A. A. Tsay, M. E. Pullman, and J. V. Gray. 2008. Unraveling the food supply chain: Strategic insights from China and the 2007 recalls. Journal of Supply Chain Management 44(1):22-39.
Roy, D., and A. Thorat. 2008. Success in high value horticultural export markets for the small farmers: The case of mahagrapes in India. World Development 36(10):1874-1890.
Rumsfeld, J. S., and E. D. Peterson. 2010. Achieving meaningful device surveillance. Journal of the American Medical Association 304(18):2065-2066.
Sabot, O., P. Yadav, and M. Zaffran. 2011. Maximizing every dose and dollar: The imperative of efficiency in vaccine delivery. Seattle, WA: The National Bureau of Asian Research.
Sarley, D., V. Dayaratna, W. Abramson, J. Gribble, N. Quesada, N. Olson, and V. S. Betancourt. 2006. Options for contraceptive procurement: Lessons learned from Latin America and the Caribbean. Arlington, VA, and Washington, DC: DELIVER, USAID—Health Policy Initiative for the U.S. Agency for International Development.
Schlehuber, L. D., I. J. McFadyen, Y. Shu, J. Carignan, W. P. Duprex, W. R. Forsyth, J. H. Ho, C. M. Kitsos, G. Y. Lee, D. A. Levinson, S. C. Lucier, C. B. Moore, N. T. Nguyen, J. Ramos, B. A. Weinstock, J. Zhang, J. A. Monagle, C. R. Gardner, and J. C. Alvarez. 2011. Towards ambient temperature-stable vaccines: The identification of thermally stabilizing liquid formulations for measles virus using an innovative high-throughput infectivity assay. Vaccine 29(31):5031-5039.
Schoub, B. D., and N. A. Cameron. 1996. Problems encountered in the delivery and storage of OPV in an African country. Developments in biological standardization 87:27-32.
Setia, S., H. Mainzer, M. L. Washington, G. Coil, R. Snyder, and B. G. Weniger. 2002. Frequency and causes of vaccine wastage. Vaccine 20(7-8):1148-1156.
Sharma, A., L. Subramanian, and E. A. Brewer. 2008. Secure rural supply chain management using low cost paper watermarking. NSDR ’08: Proceedings of the second ACM SIGCOMM workshop on networked systems for developing regions, New York.
Sharma, R. K., M. Agrawal, and F. M. Marshall. 2009. Heavy metals in vegetables collected from production and market sites of a tropical urban area of India. Food and Chemical Toxicology 47(3):583-591.
Sharma, V., and V. Ahuja. 2010. Training in post-authorization pharmacovigilance. Perspectives in Clinical Research 1(2):70-75.
Sproxil. 2011. Sproxil—world-class brand protection for emerging markets. http://sproxil.com/ (accessed October 19, 2011).
SPS (Strengthening Pharmaceutical Systems). 2010a. National pharmacovigilance systems: Ensuring the safe use of medicines. Submitted to the U.S. Agency for International Development by the Strengthening Pharmaceutical Systems (SPS) Program, Nairobi, Kenya, August 16-18.
———. 2010b. Sentinel site-based pilot active surveillance pharmacovigilance in the Vietnam ART program. Arlington, VA: USAID.
Stcichele, M. V., S. v. d. Wal, and J. Oldenziel. 2006. Who reaps the fruit? Critical issues in the fresh fruit and vegetable supply chain (update). Amsterdam: Centre for Research on Multinational Corporations.
Sudershan, R. V., P. Rao, K. Polasa, G. M. Subba Rao, and M. Vishnu Vardhana Rao. 2008. Knowledge and practices of food safety regulators in southern India. Nutrition and Food Science 38(2):110-120.
Suleman, F. 2010. Pharmacovigilance: Who is responsible and why should we care? South African Pharmaceutical Journal 77(9).
Sutz, J. 2000. The university—industry—government relations in Latin America. Research Policy 29(2):279-290.
Swaminathan, B., T. J. Barrett, S. B. Hunter, R. V. Tauxe, and CDC Pulsenet Taskforce. 2001. Puslenet: The molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerging Infectious Diseases 7(3):382-389.
Swaminathan, B., P. Gerner-Smidt, L.-K. NG, S. Lukinmaa, K.-M. Kam, S. Rolando, E. P. Gutierrez, and N. Binsztein. 2006. Building PulseNet international: An interconnected system of laboratory networks to faciliatate timely public health recognition and response to foodborne disesase outbreaks and emerging foodborne diseases. Foodborne Pathogens and Disease 3(1):36-50.
Todd, E. C. D., and C. Narrod. 2006. Agriculture, food safety and foodborne disease. Washington, DC: International Food Policy Research Institute.
Todd, E. C. D., and S. Notermans. 2011. Surveillance of listeriosis and its causative pathogen, listeria monocytogenes. Food Control 22(9):1484-1490.
UC Davis Department of Plant Sciences. 2011. Postharvest technology. http://www.plantsciences.ucdavis.edu/plantsciences/outreach/postharvest_tech.htm (accessed January 12, 2011).
Umali-Deininger, D., and M. Sur. 2007. Food safety in a globalizing world: Opportunities and challenges for India. Agricultural Economics 37:135-147.
UMC (Uppsala Monitoring Centre). The WHO programme. http://www.who-umc.org/DynPage.aspx?id=98078&mn1=7347&mn2=7252&mn3=7322 (accessed November 4, 2011).
———. 2011. WHO programme members. http://www.who-umc.org/DynPage.aspx?id=100653&mn1=7347&mn2=7252&mn3=7322&mn4=7442 (accessed November 28, 2011).
UN (United Nations). 2008. Advancing food safety in China. Office of the United Nations Resident Coordinator in China.
UNAIDS (Joint United Nations Programme on HIV/AIDS) and WHO (World Health Organization). 2011. Pharmacovigilance for antiretroviral drugs: Rationale for including pharmacovigilance in the proposal. Geneva, Switzerland: UNAIDS and WHO.
UNICEF (United Nations Children’s Fund). Child survival fact sheet: Water and sanitation. http://www.unicef.org/media/media_21423.html (accessed November 4, 2011).
UNICEF and WHO. 2009. Diarrhoea: Why children are still dying and what can be done. Geneva, Switzerland: UNICEF and WHO.
Upton, D. M., and V. A. Fuller. 2004. The ITC eChoupal initiative. Harvard Business Review 604(016):20.
U.S. Commercial Service. 2002. Contact China: A resource guide for doing business in the People’s Republic of China. Beijing, China.
USAID (U.S. Agency for International Development). 2011. Supply chain management: Providing quality medicines for people living with and affected by HIV/AIDS. http://www.usaid.gov/our_work/global_health/aids/TechAreas/treatment/scms.html (accessed November 29, 2011).
USP (U.S. Pharmacopeia). 2008. USP standards development. http://us.vocuspr.com/Newsroom/ViewAttachment.aspx?SiteName=USPharm&Entity=PRAsset&AttachmentType=?F&EntityID=97277&AttachmentID=6026e674-9b0f-4b23-aced-85f8853d7da4 (accessed September 23, 2011).
———. 2011a. Food Chemicals Codex—an overview. http://www.usp.org/fcc/ (accessed September 26, 2011).
———. 2011b. PQM in Asia. http://www.usp.org/worldwide/dqi/regions/asia.html (accessed November 9, 2011).
———. 2011c. USP reference standards—an overview. http://www.usp.org/referenceStandards/ (accessed September 24, 2011).
———. 2011d. USP verified. http://www.usp.org/USPVerified/ (accessed September 26, 2011).
Vaidya, S. S., J. J. Guo, P. C. Heaton, and M. Steinbuch. 2010. Overview and comparison of postmarketing drug safety surveillance in selected developing and well-developed countries. Drug Information Journal 44(5):519-533.
Vapnek, J., and M. Spreij. 2005. Perspectives and guidelines on food legislation, with a new model food law. Rome, Italy: Food and Agriculture Organization of the United Nations.
Viotti, E. B. 2002. National learning systems: A new approach to technological change in late industrializing economies and evidences from the cases of Brazil and South Korea. Technological Forecasing and Social Change 69:653-680.
Voxiva. 2011. Voxiva: About. http://voxiva.com/company/about.html (accessed November 4, 2011).
Waste not, want not. 2011. Economist, February 26.
Watt, G. 2004. Expiry-date tampering. Lancet 363(9403):172.
Watts, J. 2011. Exploding watermelons put spotlight on Chinese farming practices. Guardian, May 17.
Wertheim, H. F. L., P. Puthavathana, N. Ngoc My, H. R. van Doorn, N. Trung Vu, P. Hung Viet, D. Subekti, S. Harun, S. Malik, J. Robinson, M. Rahman, W. Taylor, N. Lindegardh, S. Wignall, J. J. Farrar, and M. D. de Jong. 2010. Laboratory capacity building in Asia for infectious disease research: Experiences from the South East Asia Infectious Disease Clinical Research Network (SEAICRN). PLoS Medicine 7(4):1-5.
WHO (World Health Organization). 2003a. Effective medicines regulation: Ensuring safety, efficacy and quality. WHO Policy Perspectives on Medicines 007:1-6.
———. 2003b. Substandard and counterfeit medicines. http://www.who.int/mediacentre/factsheets/2003/fs275/en/ (accessed November 9, 2011).
———. 2005. African Medicines Regulatory Harmonization Initiative (AMRHI): A WHO initiative. Geneva, Switzerland: WHO.
———. 2007. Practical guidance for conducting a review. Geneva, Switzerland: WHO.
———. 2008a. International health regulations (2005). Geneva, Switzerland: WHO.
———. 2008b. What has been achieved so far. http://www.wpro.who.int/china/sites/fos/achievements.htm (accessed October 4, 2011).
———. 2010a. Assessment of medicines regulatory systems in sub-Saharan African countries. Geneva, Switzerland: WHO.
———. 2010b. Counterfeit medical products: International medical products anti-counterfeiting task force: Report by the secretariat. Geneva, Switzerland: WHO.
———. 2011a. Codex trust fund: News. http://www.who.int/foodsafety/codex/trustfund/en/ (accessed November 21, 2011).
———. 2011b. General information on counterfeit medicines. http://www.who.int/medicines/services/counterfeit/overview/en/ (accessed November 9, 2011).
———. 2011c. Global advisory committee on vaccine safety: About us. http://www.who.int/vaccine_safety/about/indepth/en/index.html (accessed November 3, 2011).
———. 2011d. Global vaccine safety blueprint: Draft for discussion. Geneva, Switzerland: WHO.
———. 2011e. An integrated approach to food safety and zoonoses: Building capacity to detect, control and prevent foodborne infections. Geneva, Switzerland: WHO.
———. 2011f. Pharmacovigilance. http://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/index.html (accessed November 28, 2011).
———. 2011g. WHO list of prequalified medicinal products. Geneva, Switzerland: WHO
———. 2011h. WHO list of prequalified quality control laboratories. Geneva, Switzerland: WHO.
———. 2012. Risk assessment. http://www.who.int/foodsafety/micro/riskassessment/en/index.html (accessed January 18, 2012).
WHO and the Global Fund. 2010. Minimum requirements for a functional pharmacovigilance system. Geneva, Switzerland: WHO and the Global Fund.
Who runs global health? 2009. Lancet 373(9681):2083.
Wong, E. 2010. Officials in China at odds over food scandal. New York Times, March 2.
World Bank. 2003. Standards and global trade: A voice for Africa. Washington, DC: The World Bank.
———. 2007. Unleashing India’s innovation. Washington, DC: The World Bank.
———. 2008. Training regulators in Africa. Washington, DC: The World Bank.
———. 2009. Implementation, completion and results report on a credit in the amount of SDR 39.7 million (US$54 million equivalent) to India for food and drugs capacity building project. Washington, DC: The World Bank.
———. 2010. World Bank to help improve food safety in China. http://www.worldbank.org/en/news/2010/05/13/world-bank-help-improve-food-safety-china (accessed November 21, 2011).
Xiaoguang, S., and Y. Fengqiao. 2010. Higher and professional education in India. Manila, Philippines: The Asian Development Bank.
Yadav, P. 2009. Counterfeit drug resistance in the developing world: An assessment of incentives across the value chain and recommendations of policy interventions. Washington, DC: Center for Global Development.
Yu, X., C. Li, Y. Shi, and M. Yu. 2010. Pharmaceutical supply chain in China: Current issues and implications for health system reform. Health Policy 97(1):8-15.
Zaidi, M. B., J. J. Calva, M. T. Estrada-Garcia, V. Leon, G. Vazquez, G. Figueroa, E. Lopez, J. Contreras, J. Abbott, S. Zhao, P. McDermott, and L. Tollefson. 2008. Integrated food chain surveillance system for salmoella spp. in Mexico. Emerging Infectious Diseases 14(3):429-435.
Zhen, L. H. 2004. Drug control authorities in China. Annals of Pharmacotherapy 38(2):346-350.




































































