Safety and Efficacy Assessments
in Studies Conducted Under
BPCA and PREA
The goal of the Best Pharmaceuticals for Children Act (BPCA) and the Pediatric Research Equity Act (PREA) is to improve pediatric therapeutics through preclinical and clinical studies of drugs and biologics that are prescribed for children or that have the potential to benefit children. Ideally, such studies lead to the addition of useful information to the labeling of these products and then to the effective dissemination and application of that information to improve clinical care and child health.
BPCA and PREA are components of a complex system for ensuring the drugs for children and adults are safe and effective. The Food and Drug Administration (FDA) and its statutory and regulatory foundations are central elements of this system. As summarized in Chapters 3 and 4, FDA not only assesses and monitors the safety and effectiveness of drugs but also requires protections for adults and children who participate in the trials that are the basis for agency assessments. The agency’s effectiveness in its multiple roles depends on science-based decision making, credible leadership, committed and well-trained staff, adequate financial resources, and timely and trustworthy communication to professionals and the public (FDA Science Board, 2007; IOM, 2007).
Beyond FDA, the system for ensuring safety and efficacy extends to the organizations and individuals responsible for conducting drug studies and protecting research participants and research integrity. It thus includes commercial and other sponsors of research, clinical investigators, and institutional review boards (IRBs), as well as health services researchers and others who analyze medication use in clinical practice in an effort to improve the quality, effectiveness, and efficiency of health care. The system
also encompasses clinicians who consider available evidence about drug safety and effectiveness as they care for children. Parents have a role, too, including in drug research when they administer test drugs or placebos at home and keep diaries or other records necessary for the assessment of safety and efficacy outcomes.
This chapter discusses selected aspects of FDA’s assessments of the safety and efficacy of drugs and biologics based on data from pediatric studies requested under BPCA or required under PREA. For safety, these aspects include reviewer conclusions about overall safety signals, risk-benefit assessments, and extrapolation of safety and findings of the 1-year safety reviews first required in BPCA of 2002. For efficacy, the discussion focuses on the use of alternative endpoints and extrapolation.
SOURCES OF INFORMATION ABOUT SAFETY AND
EFFICACY RESULTS IN PEDIATRIC DRUG STUDIES
The most comprehensive perspective on the pediatric study data submitted by sponsors and evaluated by FDA is provided in the clinical reviews prepared by staff of the Center for Drug Evaluation and Research (CDER) or the Center for Biologics Evaluation and Research (CBER). For this report, these reviews were the primary source of information on the characteristics and findings of pediatric studies conducted under BPCA or PREA. The committee also consulted clinical pharmacology and statistical reviews (if any), product labeling, and letters describing FDA’s approval action and any further requirements (e.g., further pediatric studies). FDA managers may prepare memoranda that provide additional context for decisions or explain why a reviewer’s recommendations were not accepted. For some labeling changes, the committee consulted minutes from FDA advisory committee meetings.
Following congressional directives described in Chapter 3, CDER and CBER now post the reviews for products approved on or after September 27, 2007.1 For products approved earlier, clinical and other reviews are posted for a few products, but the committee had to request that FDA make public the reviews for most products approved before September 2007. (Appendix A describes how the committee selected the sample of requests, studies, and labeling changes assessed in this report.)
As described by CDER, the clinical review (sometimes called the medical review) is a “comprehensive summary and analysis of the clinical data
![]()
1 For CDER and CBER respectively, the reviews posted after September 26, 2007, are at http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm049872.htm and http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CBER/ucm122938.htm.
submitted in support of a marketing application … [that] also includes the clinical reviewer’s assessment of and conclusions about: (1) the evidence of effectiveness and safety under the proposed conditions of use; (2) the adequacy of the directions for use; and (3) recommendations on regulatory action based on the clinical data submitted by an applicant” (CDER, 2010, p. 3). Clinical reviews may summarize findings from other areas of scientific review (e.g., toxicology and microbiology), and reviewers may also cite their own literature searches.
In the years since BPCA and PREA and their predecessor policies went into effect, FDA has improved the organization and completeness of the clinical reviews. In 2004, CDER added to its policy manual a standardized template for clinical reviews, although some reviewers had been using a similar format for some time. Box 5-1 shows the major headings of the CDER template as revised in 2010. (Details of the safety and efficacy sections of the template are presented later in this chapter.) CDER has also created templates for clinical pharmacology and biopharmaceutics reviews and for statistical reviews. In addition, CDER has created a 65-page desk reference guide that provides staff with an accessible resource of principles and procedures (CDER, 2011a). The guide also describes the roles of review team members, including those with specialized expertise (e.g., pediatrics) who may be included as needed.
As described in the desk reference guide, the primary audience for the clinical review includes the review team (i.e., those with responsibility for
BOX 5-1
CDER Template for Clinical Reviews (2010)
1. Recommendations/Risk-Benefit Analysis
2. Introduction and Regulatory Background
3. Ethics and Good Clinical Practices
4. Significant Efficacy/Safety Issues Related to Other Review Disciplines
5. Sources of Clinical Data
6. Review of Efficacy
7. Review of Safety
8. Postmarketing Experience
9. Appendices
9.1 Literature Review/References
9.2 Labeling Recommendations
9.3 Advisory Committee Meeting
![]()
SOURCE: CDER Manual of Policies and Procedures, 6010.3R (issued December 14, 2010).
various aspects of the overall review), division staff, and CDER managers. The guide notes that reviewers should anticipate “the availability of the document to a public audience” (CDER, 2010, p. A-1).
The Center for Biologics Evaluation and Research (CBER) has not completed work on a standard format for reviews (personal communication, Catherine Lee, Office of Pediatric Therapeutics, FDA, August 8, 2011). Some CBER reviewers have, however, used an outline format similar to that used in CDER reviews. In general, the committee found that CBER reviews were more variable than CDER reviews.
Overall, the committee found that the FDA reviews from recent years tended to be more systematic and focused than earlier reviews. The recent reviews were more likely to highlight key conclusions about safety and efficacy, although they did not invariably follow the template. (Reviews may not follow the template for submissions that involve only pharmacokinetic and limited safety data, as requested by FDA.) Recent reviews also tended to provide more regulatory and other context about the origins and rationales for studies. Occasionally, the reviews summarize interactions between the FDA and sponsors and provide insights into how and why studies changed over time.
ASSESSING AND MONITORING SAFETY IN
PEDIATRIC DRUG STUDIES: SELECTED ISSUES
A sponsor’s submission of a new drug application (NDA) or biologics license application (BLA) will generally report safety data from preclinical and clinical studies and offer the sponsor’s assessments of these data. Submissions may also include data from adult pharmacokinetic and other studies, a review of relevant literature, and postmarket safety reports for already marketed drugs. As noted in Chapter 7, almost 10 percent of labeling changes attributed to studies requested under BPCA or required under PREA involved no information from new pediatric studies.
During the course of a clinical trial, the sponsor is responsible for trial monitoring. Depending on the anticipated risks in a trial (usually a Phase III trial), the sponsor may appoint a data monitoring committee (DMC; sometimes called a data safety monitoring board or data and safety monitoring committee) to evaluate safety data as it accumulates.2 If a DMC identifies serious safety concerns in interim assessments of trial data, it can recom-
![]()
2 As described in FDA guidance, a DMC is “a group of individuals with pertinent expertise that reviews on a regular basis accumulating data from one or more ongoing clinical trials. The DMC advises the sponsor regarding the continuing safety of trial subjects and those yet to be recruited to the trial, as well as the continuing validity and scientific merit of the trial” (CDER/CBER/CDRH, 2006, p. 1).
mend modification or early termination of a trial. It is the sponsor’s responsibility to report serious adverse events and DMC recommendations related to such events to FDA. Unlike the National Institutes of Health (NIH), FDA regulations do not require the appointment of a DMC except in rare circumstances (CDER/CBER/CDRH, 2006). However, CDER’s template for written requests includes the option for the agency to require a DMC under other circumstances (CDER, 2011c).3 The Pediatric Review Committee (PeRC, described in Chapter 3) discusses whether a DMC should be required, and FDA may place a clinical hold on a protocol if it concludes that the absence of a DMC puts research participants at unreasonable and significant risk (personal communication, Robert Nelson, Office of Pediatric Therapeutics, FDA, January 16, 2012). An assessment of the use of DMCs in pediatric clinical trials was beyond the task for the committee but may warrant future examination.
CDER Template for Review of Safety in Drug Studies
The CDER template for clinical reviews outlines a comprehensive evaluation and discussion of safety that covers key topics and data sources in a systematic order (Box 5-2). One subsection of the template provides for a discussion (if relevant) of pediatrics and assessment of effects on growth. In practice, reviewers may tailor the format of their assessments to take into account the specifics of a particular submission, for example, whether it presents only a pharmacokinetic and pharmacodynamic study, as requested by FDA. Similarly, although the agency prefers that analyses pool data across studies, some sponsor submissions may not support this strategy.
New Rules to Improve Reporting of Adverse Events
and Analysis of Safety Data from Clinical Trials
Central to the assessment of drug safety are the identification and evaluation of adverse events both during clinical trials and after marketing approval. FDA regulations define an adverse event as “any untoward medical occurrence associated with the use of a drug in humans, whether or not considered drug related” (21 CFR 312.32(a)). Such an event can involve, for example, a laboratory or other test result, a symptom, a hospitaliza-
![]()
3 For example, in 2005, FDA requested a study of griseofulvin (an off-patent drug approved for treatment of tinea capitis in children 2 years of age or older) to provide more data on pharmacokinetics, safety, and efficacy related to different dosing recommendations. FDA stated that a “Data Monitoring Committee with pertinent expertise must be used to provide ongoing oversight of patient safety” (Beitz, 2005, p. 4).
BOX 5-2
Safety Review Section of CDER Clinical
Review Template (2010)
Safety Summary
Methods
Studies/Clinical Trials Used to Evaluate Safety
Categorization of Adverse Events
Pooling of Data Across Studies/Clinical Trials to Estimate and Compare Incidence
Adequacy of Safety Assessments
Overall Exposure at Appropriate Doses/Durations and Demographics of Target Populations
Explorations for Dose Response
Special Animal and/or In Vitro Testing
Routine Clinical Testing
Metabolic, Clearance, and Interaction Workup
Evaluation for Potential Adverse Events for Similar Drugs in Drug Class
Major Safety Results
Deaths
Nonfatal Serious Adverse Events
Dropouts and/or Discontinuations
Significant Adverse Events
Submission-Specific Primary Safety Concerns
Supportive Safety Results
Common Adverse Events
Laboratory Findings
Vital Signs
Electrocardiograms (ECGs)
Special Safety Studies/Clinical Trials
Immunogenicity
Other Safety Explorations
Dose Dependency for Adverse Events
Time Dependency for Adverse Events
Drug-Demographic Interactions
Drug-Disease Interactions
Drug-Drug Interactions
Additional Safety Explorations
Human Carcinogenicity
Human Reproduction and Pregnancy Data
Pediatrics and Assessment of Effects on Growth
Overdose, Drug Abuse Potential, Withdrawal, and Rebound
Additional Submissions/Safety Issues
![]()
SOURCE: CDER Manual of Policies and Procedures (Section 7 of Clinical Review Template), 6010.3R (issued December 14, 2010).
tion, or a death. An adverse reaction is an adverse event that is attributed to use of the drug.
In 2010, FDA issued new regulations and guidance on safety reporting for clinical trials (CDER/CBER, 2010a). The goal was to “increase the interpretability of and usefulness of safety data available to the clinical investigators, IRBs, and the FDA” (Sherman et al., 2011, p. 5). The rules require clinical investigators to report all serious adverse events to trial sponsors. They shift the responsibility for assessing whether an isolated adverse event is likely to be drug related from individual investigators to sponsors. As a result, sponsors should have a larger and more complete pool of data to support assessments of causality. These assessments should improve the relevance of their reports to FDA.
The 2010 rules also offered several examples of the kinds of events on which sponsors should focus. They include the following:
• A single occurrence of an event that is uncommon and known to be strongly associated with drug exposure (e.g., angioedema, hepatic injury, and Stevens-Johnson syndrome);
• One or more occurrences of an event that is not commonly associated with drug exposure but that is otherwise uncommon in the population exposed to the drug (e.g., tendon rupture); and
• An aggregate analysis of specific events observed in a clinical trial (such as known consequences of the underlying disease or condition under investigation or other events that commonly occur in the study population independent of drug therapy) that indicates that those events occur more frequently in the drug treatment group than in a concurrent or historical control group. (CDER/CBER, 2010b, p. 4)
IOM Review of Safety Assessments in Pediatric Drug Studies
As explained in Chapter 1, the Institute of Medicine (IOM) was asked to assess “the number and type of pediatric adverse events” in a sample of studies conducted under PREA or precursor regulations. The committee also included a sample of studies stemming from requests under BPCA. This broader scope provided additional context for understanding FDA’s evaluation of safety findings in pediatric drug studies.
Unfortunately, the FDA clinical reviews examined by the committee were completed before FDA’s shift to the new, more targeted strategy for reporting adverse events. The typical clinical review included numerous, sometimes lengthy tables and reports of various categories of adverse events that correspond to topics in the review template. The reviews focused on
serious and unexpected adverse events reported in clinical trials, but they also discussed less serious events. The sponsor and FDA reviewers judged many adverse events described in the clinical reviews not to be related to the test product.
Given the thoroughness of most reviews and the usual judgment that a substantial proportion of reported adverse events were not related to the test drug, the IOM committee decided that it would not be productive to review and assess the numbers and types of these events. Instead of counting and categorizing individual adverse events, the committee focused on the clinical reviewer’s more general and relevant conclusions about a product’s safety profile. For example, for products that had been studied in adults, did the FDA reviewer conclude that pediatric studies of a drug or biologic showed a safety profile that was similar to that reported for adults? Alternatively, did the profile for children differ from that for adults in ways that, at a minimum, warranted discussion in the product’s labeling? If the FDA reviewer did not compare pediatric safety findings to adult safety findings, did he or she make other appropriate comparisons (e.g., with findings for a control group or with safety findings in other pediatric studies of similar drugs for the same condition)?
Because safety is relative, FDA must weigh findings about the risks of a product against expected benefits and judge whether the expected benefits sufficiently outweigh expected harms to justify approval for marketing. (FDA may disapprove the labeling of a product for pediatric use but provide for the addition of safety or other information from pediatric studies to the product labeling for already marketed products.) In assessing clinical reviews, the committee looked for a risk-benefit assessment (to use FDA’s language), that is, an explicit overall judgment about risks in relation to expected benefits. In some cases, the committee found that a reviewer’s discussion of the risk-benefit assessment was redacted without explanation. A memo from a division director or review team leader sometimes indicated that agency management reached different conclusions from the primary reviewer.
The committee initially intended to assess the extent to which labeling changes were consistent with the reviewer’s conclusions about safety signals or significant adverse events. However, after discovering that FDA generally redacted all or much of the discussion of labeling in clinical reviews, the committee decided that it could not be confident in making such assessments. As discussed in Chapter 3, the sponsor owns the label, and new labeling or changes in labeling usually result from a process involving negotiation between the sponsor and FDA about the sponsor’s proposed wording.
Analysis of Safety Profile
For products that had also been studied in adults, most clinical reviews that the committee examined offered relatively straightforward and easily understood conclusions about whether the safety findings from pediatric studies showed results similar to those found from adult studies. The majority of the reviews that included comparisons of the results for children with the results for adults (or with the known safety profile of the product) concluded that the safety profile was similar for children. For example, in the assessment of leflunomide (Arava) for the treatment of juvenile rheumatoid arthritis, the reviewer’s summary conclusion was that the “overall profile of adverse events was consistent with the underlying disease and known serious adverse events of leflunomide” (Yancey, 2004, p. 68). The summary also notes hepatotoxicity to be a known risk of the drug. To cite another example, the clinical reviewer for tenofovir disoproxil fumarate (Viread) noted that “[o]verall, the safety issues identified in the adolescent study are similar to those previously identified in the adult clinical trials and are included in the current product label” (Levorson, 2010, p. 40). The reviewer then described several specific safety issues, including reductions in bone mass density, renal toxicity, and gastrointestinal events. For one product (eletriptan hydrobromide [Relpax]), the labeling—but not the redacted clinical review—stated that the profile of adverse events in a pediatric study was similar to that reported in studies with adults.
Because one objective of FDA’s evaluation of adverse events in pediatric studies is to determine whether a product’s labeling needs to be revised, reviewers sometimes explicitly noted whether the findings about treatment-related adverse events in children were reflected in the existing labeling (for previously approved products) or whether some revisions were needed. As already noted, reviewers’ specific discussions of the text of proposed labeling were mostly or entirely redacted.
For some products, reviewers found different safety signals, usually in the form of events that, although expected, were more common in children than in adults. In a few instances, the findings were unexpected on the basis of the data for adults. Box 5-3 provides examples of these kinds of reports.
Some drugs were studied in populations and for indications that did not lend themselves to comparisons with the findings of studies with adults. An example is nitric oxide (INOmax) for the treatment of neonates with bronchopulmonary dysplasia, a condition not diagnosed in adults. Even when comparisons with adult safety findings were possible, some reviewers chose to make other informative comparisons. To cite an example, in the clinical review of a combination salmeterol xinafoate and fluticasone propionate product (Advair Diskus), the comparison was with the safety
Adalimumab (Humira) for treatment of juvenile idiopathic arthritis. “Safety was similar to that seen in adults but there were several safety signals not observed in the adults, including elevations of creatine phosphokinase (CPK). In addition, a higher rate of immunogenicity was observed in children as compared to adults as well as a higher rate of non-serious hypersensitivity reactions. There was disagreement between the primary clinical reviewer and the secondary reviewer on the specific details of the post-marketing registry that should be conducted” (Siegel, 2008b, p. 3).
Aripiprazole (Abilify) for treatment of schizophrenia. “Based on a comparison of the results of five short-term adult studies in schizophrenia with the results of this pediatric schizophrenia study, the safety profile of aripiprazole in adolescents with the diagnosis of schizophrenia is comparable to the adult schizophrenia population, with the exception of dose-related occurrence of higher frequency of somnolence and extrapyramidal symptoms observed in the pediatric population” (Zhang, 2008, p. 35).
Desflurane (Suprane) for induction or maintenance of anesthesia. “The clinical data submitted in this supplement demonstrated a marked increase in the incidence of both major (associated with significant oxygen desaturation) and minor respiratory events including laryngospasm, airway obstruction, secretions, and breath holding in non-intubated pediatric patients who underwent maintenance anesthesia with desflurane compared to a cohort of children treated similarly with isoflurane. The incidence of these respiratory events appeared to be related to the inspired concentration of desflurane. These data do not support the use of desflurane for induction (which was a prior finding) or maintenance of anesthesia in non-intubated children” (Shibuya, 2006, p. 4).
Olmesartan (Benicar) for treatment of hypertension. “In this whole study program, transient minor to moderate headache was the major adverse event with this product in pediatric population. Other than that, there does not appear to be any other unexpected adverse events in children compared to adults” (Xiao, 2009, p. 10).
Omalizumab (Xolair) for treatment of asthma. “[I]n patients 6–11 years of age with IgE [immunoglobulin E] levels above 500 IU/mL, circulating trough levels of omalizumab and omalizumab-IgE complexes are higher than those achieved in patients 12 years of age and older with IgE levels up to 700 IU/mL. These complexes take months to clear after termination of Xolair treatment. Although no urinary abnormalities or evidence of serum sickness was noted in the safety database, the clinical meaning of higher circulating immune complex exposure, particularly over many years of chronic exposure, is unknown. Thus, lack of evidence supporting the long-term safety of a dosing regimen associated with circulating immune complex levels that are higher in children higher [sic] than those studied and approved in adults is a safety concern with this application” (Starke, 2009, p. 12).
profiles for the individual components of the product, which were similar to those for the combination product (Johnson, 2000). For some products, the comparison was with previously studied products or formulations. In the review of mometasone furoate (Asmanex) for treatment of asthma, for example, the reviewer noted that the adverse events identified were common and consistent with those found in other trials of similar drugs in pediatric patients “and do not suggest a new safety signal” (Karimi-Shah, 2007, p. 11).
In reaching overall conclusions about safety, some reviewers did not make comparisons with other populations or products. For example, the reviewer for alendronate (Fosamax) for osteogenesis imperfecta stated that “the safety and tolerability profile of alendronate in this population were acceptable, with few serious adverse events (only three of which were possibly related to alendronate) and no deaths” (Schneider, 2003, p. 3). The reviewer also noted one case of leukopenia—a condition not identified to be a risk for adults—and suggested that that this type of event be monitored as a safety issue, regardless of whether FDA approved the drug for treatment of the studied indication.
Some reviews stated only that no unexpected adverse events had been noted. In context, such statements probably can be interpreted as suggesting that the safety profile was similar to that for adults if the product had been previously studied in adults. For example, in an assessment of irinotecan hydrochloride (Camptosar) for refractory solid tumors, a clinical reviewer concluded that the pediatric studies provided no meaningful new safety information (Ibrahim, 2003).
Although reviewers differed in how they summarized and presented the information, the reviews typically supplemented the overall assessment of safety with a summary of serious adverse events that are considered to be related to the drug and a summary of common treatment-related adverse events. One example of a clear, relatively brief summary of such adverse events is provided in the clinical review of a sponsor submission involving almotriptan (Axert) for the treatment of migraine in adolescents (Harris, 2009). In three short paragraphs, the reviewer notes that 67 percent of the study participants had some kind of adverse event (all causality), that 8 percent had an adverse event that was judged to be related to the product, that these events were most often nausea and somnolence (each reported by 1.4 percent of participants), and that 2 percent of participants experienced a serious adverse event, none of which was judged to be treatment-related.
Risk-Benefit Assessment
Explicit statements of risks in relation to benefits usefully underscore the reality that the use of drugs involves the potential for harm as well as
for benefit. Few (7 of 46) of the clinical reviews in the committee’s sample included fairly explicit summary risk-benefit statements.4 An example of an explicit positive assessment is found in the review of tenofovir disoproxil fumarate (TDF; Viread): “The identification of the same potential safety risks in adolescents as in adults on TDF did not outweigh the benefit of TDF as a treatment option for either treatment-experienced or treatment-naïve, HIV-infected patients with HIV-1 virus sensitive to TDF” (Levorson, 2010, p. 8). All explicit statements were in reviews dated 2008 or later.
Most reviews (32 of 46) included no direct statement about the risk-benefit balance. Some of these reviews, however, organized clear but separate summary statements about efficacy and safety close enough in proximity that the overall judgment about the balance was reasonably evident.
FDA reviewers occasionally conclude that study results are not interpretable. For example, in the case of sotalol hydrochloride (Betapace) for arrhythmia, FDA had issued a written request for pharmacokinetic and pharmacodynamic data to guide use of the drug in prepubertal children. Although the FDA reviewer reached some conclusions about dosing, the overall conclusion was that neither the requested studies nor the other pediatric data submitted could “be interpreted with respect to establishing either the safety or the efficacy of sotalol in the pediatric population” (Karkowsky, 2000, p. 3). (See also the entry for etodolac [Lodine] in Box 7-3.)
Two reviews (for esomeprazole magnesium [Nexium] and gatifloxacin ophthalmic [Zymar]) were not classified because the risk-benefit section of the review was significantly redacted. In another review (for omalizumab injection [Xolair]), most of the discussion in the risk-benefit section was redacted, but the review later included this explicit information: “[the Pulmonary-Allergy] Advisory Committee voted against (4 yes, 10 no, 0 abstain) the risk/benefit favoring approval of Xolair, i.e., whether the safety and efficacy data provide substantial and convincing evidence to support
![]()
4 Sorting out risk-benefit assessments could be complicated. One clinical pharmacology review of guanfacine (Intuniv) for attention deficit hyperactivity disorder was explicit but mixed. “The drug has not demonstrated additional benefit over placebo in patients who are 13 years or older (who tend to be heavier), the risk outweighs the benefit in this age group. In patients who are 6-12 years of age, the benefit-risk ratio is probably greater than unity” (Mishina, 2007, p. 17). The clinical reviewer was, however, implicitly positive. The product was labeled for use in both age groups, as explained in the division director’s memo. “An age analysis clearly suggests that the benefits of SPD503 were not demonstrated in adolescents, even though the studies were positive overall. I still think it is reasonable to permit a general claim of efficacy in this broad age range (6-17), along with a mention of this finding in labeling. With mg/kg dosing, I think adolescent patients can be effectively treated. The sponsor’s proposed explanation based on likely inadequate exposure due to higher body weights in adolescents seems entirely reasonable to me. The sponsor has agreed to address this discrepancy in the efficacy findings as a phase 4 commitment. The sponsor has also committed to conducting a maintenance study post-approval” (Laughren, 2009c, p. 3).
approval of Xolair in this age group” (Starke, 2009, p. 96).5 Three reviews involved submissions that did not include efficacy studies.
The reviews that the committee examined did not explain or cite any underlying methodology for weighing safety and efficacy findings. A 2007 IOM report on FDA’s drug safety system noted that “the risk-benefit analysis that currently goes into regulatory decisions appears to be ad hoc, informal, and qualitative” and recommended that FDA “develop and continually improve a systematic approach” to such analyses (IOM, 2007, pp. 123 and 125). In a 2009 summary of responses to that report, the agency reported that it was continuing to explore best practices in risk-benefit assessments, including identifying and developing the information technology and analytic infrastructure to support such assessments (FDA, 2009c). In 2010, FDA announced that in early 2013 it intended to publish for comment a structured benefit-risk assessment framework (FDA, 2010c). A framework based on sound regulatory science could make an important contribution to FDA’s assessments of pediatric drug studies.
Extrapolation of Safety
As discussed later in this chapter, the Food and Drug Administration Amendments Act of 2007 (FDAAA; PL 110-85) and earlier laws and regulations permit the extrapolation of “pediatric effectiveness” on the basis of data from studies with adults (or data from studies with another pediatric age group), usually with additional supplementary information on pharmacokinetics and safety. The extrapolation of safety is not mentioned. In discussions with the committee and staff, FDA representatives said that the agency generally does not accept the extrapolation of safety.
Arguably, the agency does, in some cases, allow the extrapolation of safety. For example, for pancrelipase (Creon), which is used to treat exocrine pancreatic insufficiency due to cystic fibrosis or other conditions, the Pediatric Review Committee (PeRC) made the following recommendation:
On consideration of available information, including studies of the TbMP [to-be-marketed product] in patients with CF [cystic fibrosis]-related EPI [exocrine pancreatic insufficiency] 12 years and older, an extensive literature base describing a favorable risk:benefit balance for long-term
![]()
5 The review, which was categorized as providing an explicit assessment, also noted that the majority of the advisory committee held that safety had not been adequately investigated and that the group split evenly on the evidence of efficacy. On efficacy, those who expressed concerns believed that the drug had not been studied in the patients for whom it was intended (pediatric study subjects had normal results for tests of forced expiratory volume in 1 second [FEV1] tests, whereas adults had severe asthma that was not responsive to other treatments). On safety, one of the primary concerns was “the lack of dose ranging,” particularly the option of a lower dose (p. 95).
use of non-TBMP PEPs [pancreatic enzyme replacement products] in adult and pediatric patients with CF-and chronic pancreatitis-related EPI, and widely implemented dose guidelines (the Cystic Fibrosis Foundation Guidelines) for patients with CF-related EPI based on studies performed with other PEPs, the Pediatric Review Committee (PeRC) recommended to the Division of Gastroenterology Products (DGP) that safety and efficacy in children could be extrapolated to include an indication to treat EPI in children of all ages. (Ku and Hausman, 2009) (emphasis added)
For another product, antihemophilic factor (recombinant) FS (Kogenate), the approval letter stated that the pediatric study requirements had been fulfilled for all age groups (Golding, 2008b). The clinical reviewer, who assessed data submitted for children ages birth up to 2.5 years, stated that PeRC had recommended that the study requirements be considered completed rather than be waived and had judged that the benefits of prophylactic treatment could be “extended to all pediatric age groups provided the patient presents with no existing joint damage” (Jain, 2008, p. 2). The review cited no pharmacokinetic or safety studies for older children and thus implies the extrapolation of safety as well as efficacy. Approvals of contraceptives for use by women past the age of menarche but under age 18 years are routinely granted on the basis of findings from efficacy and safety studies with adult women with no product-specific safety or other studies for younger women (see, e.g., Beitz, 2010).
Extrapolation of safety as well as efficacy for a particular product may be appropriate in special circumstances. In these circumstances, it would be informative for FDA to provide the public with an explicit justification for such extrapolation. If the agency’s position is that decisions such as those just cited do not involve the extrapolation of safety, then it would likewise be desirable for the rationale for decisions to be clarified.
Long-Term or Other Studies or Safety Reporting
After a Pediatric Labeling Change
As it reviewed the safety findings in its sample, the committee identified concerns about long-term product-related adverse events—including neurological and growth-related events—that would not be evident in the submitted studies. As discussed in Chapter 2, results of medication use in actual practice may differ from results in carefully controlled clinical trials that involve selected populations and strict protocols for product use and monitoring. Results may, in particular, differ for products that have been labeled on the basis of short-term studies but that are used on a long-term basis—potentially over a decades-long life span in children—for the treatment of chronic conditions, such as asthma or diabetes. Even when use is
more limited (days, weeks, or months), long-term neurological and other consequences may be a worry for certain products.
Congress and FDA clearly recognize the problem and have taken some steps to address it. The 1-year safety reviews described below provide examples specific to products with labeling changes resulting from studies conducted under BPCA or PREA. In addition, the recent expansion of FDA’s authority to require (non-PREA) studies after marketing approval offers potential safeguards for both children and adults. (See Chapter 3 for further discussion of this authority as well as the Adverse Event Reporting System.)
FDA may also support selective research to assess safety risks to children. For example, the Agency for Healthcare Research and Quality (AHRQ) and FDA recently supported a retrospective cohort study to assess cardiovascular risks of drugs used to treat attention deficit hyperactivity disorder (ADHD). Using data from four U.S. health plans, investigators concluded that the data did not suggest a significant risk of serious cardiovascular events in children or young adults using one of several different classes of drugs for ADHD or using methylphenidate specifically (Cooper et al., 2011). The mean duration of follow-up ranged from 1.5 to 3.9 years. Pharmacoepidemiologic studies of this and other kinds can expand the understanding of long-term safety outcomes.
One-Year Safety Reviews
As a partial response to concerns about long-term safety, Congress now requires the Pediatric Advisory Committee (PAC) to evaluate safety information reported in the year following a labeling change resulting from studies conducted under BPCA or PREA. Such reviews were first required in 2002 for products studied under BPCA. In 2007, Congress extended the review to products studied under PREA. FDA posts the slides for the staff presentations to the advisory committee. These presentations not only may provide information about adverse events (from the Adverse Event Report System database described in Chapter 3) but may also offer brief synopses of the original trials and labeling, any subsequent changes in the safety labeling, and trends in pediatric and adult use. Presentations are abbreviated for products that are not being marketed in the United States, that are not widely used by children, or for which few or no pediatric deaths or serious adverse events have been reported (Murphy, 2011).
Table 5-1 summarizes the results of the 1-year safety reviews. Of the 147 products considered from the initiation of the review process in 2003 through June 2011, 100 stemmed from BPCA-related actions (dating from 2002) and the rest stemmed from PREA-related actions (dating from 2007).
Following the presentation of the 1-year safety reports to PAC, the
|
|
||
| Number of Actions | Type of Action | |
|
|
||
| 98 | Recommended return to routine review | |
| 7 | Requested additional information and then recommended return to routine review | |
| 8 | Requested further review; follow-up has not yet been reported | |
| 16 | Recommended labeling change; labeling change made | |
| 10 | Recommended labeling change; labeling change not yet made | |
| 10 | Recommended other actions for specific drug classes (e.g., proton pump inhibitors and antipsychotics) | |
| 11 | Recommended other actions | |
|
|
||
NOTE: N= 160, excluding one product not marketed in the United States.
SOURCE: Compiled from safety reporting information posted at http://www.fda.gov/ScienceResearch/SpecialTopics/PediatricTherapeuticsResearch/ucm123229.htm.
most common recommendation (61 percent of reviews) has been for a return to routine safety monitoring. Of the 36 recommendations for labeling changes, 16 revisions had occurred as of June 2011; other labeling changes may be made in the future.
The posted summaries of PAC meetings have reported extensive discussions over certain classes of products, including selective serotonin reuptake inhibitors, proton pump inhibitors, and atypical antipsychotics. In September 2011, for example, FDA provided an update on a study to further investigate concerns about pediatric use of second-generation antipsychotics and metabolic effects (Gerhard, 2011). The study (undertaken in collaboration with AHRQ) looked specifically at the risk of type 2 diabetes.
Required Postmarketing Safety Studies
As described in Chapter 3, in approving an NDA or BLA, FDA may require sponsors to undertake additional studies beyond those required under PREA. In its sample of 45 labeling changes, the committee found that nine approval letters included postmarket study requirements not required under PREA. The required studies included
• an analysis of already collected data (example: for salmeterol xinafoate and fluticasone propionate [Advair], a summary of existing pharmacokinetic and pharmacodynamic data for possible gender effects of the drug, with clinical study to be undertaken if this summary was inadequate to identify such effects [Meyer, 2000]);
• a study in animals (example: for almotriptan [Axert], a toxicology study in juvenile rats to identify unexpected and serious adverse effects on postnatal growth and development [Katz, 2009b]);
• a carcinogenicity study (example: for hydrocortisone butyrate [Locoid lotion], a 2-year dermal carcinogenicity study [Kukich and Walker, 2007]);
• a controlled trial to examine effects on bone mineral density (example: for tenofovir disoproxil fumarate [Viread] [Birnkrant, 2010]); and
• a 10-year observational study (example: for the use of adalimumab [Humira] in 800 pediatric patients with polyarticular juvenile idiopathic arthritis [Roca, 2008]).
The long-term (10-year) observational study cited above was the only such study in the committee’s sample, although the committee is aware of a similar study design requirement for at least one other product. In that case, when FDA approved pegylated interferon alfa 2b (PegIntron) in combination with ribavirin (Rebetol) for treatment of chronic hepatitis C virus in children ages 3 to 17 years, it required the completion of a 5-year follow-up observational study to assess the durability of the treatment response, long-term or delayed toxicity, and long-term effects on height and weight (Birnkrant, 2008).
FDA may also encourage rather than require follow-up studies. For example, in approving a supplemental NDA to add information from requested studies of the anticancer drug irinotecan hydrochloride (Camptosar), FDA recommended but did not require a follow-up pharmacokinetic study to characterize the exposure toxicity relationship for the drug (Pazdur, 2004). Similarly, written requests under BPCA may encourage but not formally specify long-term studies. For example, for studies of aripiprazole (Abilify) for pediatric schizophrenia and mania in biopolar disorder, the written request identified the effects of the drug on growth and development to be an important concern, but FDA only “encourage[d]” the sponsor “to consider longer-term studies of a year or more to address this question if the acute studies demonstrate efficacy” (Behrman, 2003, p. 5). The submitted studies involved a 6-week placebo-controlled trial for each indication and a 6-month, open-label, follow-on study that included children from either of the controlled studies.
ASSESSING AND REPORTING EFFICACY IN
PEDIATRIC DRUG STUDIES: SELECTED ISSUES
Efficacy refers to the achievement of desired results in controlled clinical studies. In its statement of task, IOM was specifically asked to assess the use of alternative endpoints and the use of extrapolation for pediatric
subpopulations, both of which are relevant to assessments of efficacy in pediatric drug studies. As was the case for the assessments of safety, the committee primarily relied on FDA clinical reviews for the assessments of efficacy.
Not all studies requested under BPCA or required under PREA specify that studies of efficacy be performed or that determinations of efficacy be a primary objective. For example, when the FDA issued a written request for the controlled study of desflurane (Suprane), the primary objective was to evaluate the safety of the product for the maintenance of anesthesia in nonintubated children (Jenkins, 2001).6 Likewise, some of the neonatal studies discussed in Chapter 6 specified pharmacokinetic and safety studies but not efficacy studies.
CDER Template for Review of Efficacy in Drug Studies
Box 5-4 presents the efficacy review section of CDER’s clinical review template. As is the case for the safety review, this section of a review may include a discussion of sponsor-or reviewer-conducted literature searches and may also cite findings from clinical trials involving adults, in addition to results from trials involving children. The introduction, particularly for recent reviews, usually includes a concise summary of the reviewer’s conclusions about efficacy.
The guidance for use of the template advises that “[c]onsultation with the biostatistical reviewer is invaluable when formulating the review of efficacy” (CDER, 2010, p. A-15). Most but not all clinical reviews of efficacy are accompanied by a statistical review. A statistical review may not be prepared for a variety of reasons, for example, if a study of safety and efficacy enrolls too few children to allow any definitive conclusions about efficacy.
Use of Alternative Endpoints
Definition and Rationales for Use of Alternative Endpoints
For the purposes of this report, alternative endpoints in pediatric studies are defined to be measures of efficacy that take pediatric development into account and thus differ from endpoints that were used in adult studies
![]()
6 The original request specified two studies: one with children ages 2 to 16 years and a second one with children ages 1 month up to 2 years that was to be conducted depending on the findings of the safety analysis conducted in the first study. After the first study raised safety concerns, the second study was dropped in an amended written request (without explicit mention or explanation) (Meyer, 2006).
BOX 5-4
Efficacy Review Section of CDER Clinical Review Template
Efficacy Summary
Indication
Methods
Demographics
Subject Disposition
Analysis of Primary Endpoint(s)
Analysis of Secondary Endpoint(s)
Other Endpoints
Subpopulations
Analysis of Clinical Information Relevant to Dosing Recommendations
Discussion of Persistence of Efficacy and/or Tolerance Effects
Additional Efficacy Issues/Analyses
![]()
SOURCE: CDER Manual of Policies and Procedures (Section 6 of Clinical Review Template), 6010.3R (issued December 14, 2010).
for the condition being investigated. For example, a measure of pain based on a parent’s assessment of a young child’s physical movements or facial expressions is an alternative endpoint if studies with adults relied on direct self-reporting by the research participant. If multiple primary efficacy endpoints are specified for pediatric studies, one endpoint may the same as that used in studies with adults and another may be an alternative endpoint. In addition, if separate efficacy studies with individuals in different age groups are included in the same NDA or BLA, the efficacy endpoints may vary for older and younger age groups.
For a condition that is found solely in children, the pediatric endpoint may be unique. For example, bronchopulmonary dysplasia is a lung disease of neonates that may occur in premature infants who require mechanical ventilation. Thus, a clinical trial endpoint based on the frequency of the condition in ventilated infants after treatment with a test drug or placebo cannot be characterized as an alternative to an endpoint for an adult study. For conditions such as attention deficit hyperactivity disorder and irritability associated with autism that may be first identified and studied with children but are subsequently diagnosed in adults, efficacy endpoints for pediatric drug studies are not considered alternative if they are defined prior to studies with adults.
An alternative endpoint may also be a surrogate endpoint, that is, an
endpoint such as bone mass density that is used in place of an endpoint or outcome that is more directly meaningful to patients, such as a bone fracture.7 In studies of drugs to treat osteoporosis in adults, the rate of fractures is the primary efficacy measure. In pediatric studies of the same drugs to treat low bone mass in osteogenesis imperfecta, FDA has specified change in bone density (a surrogate measure) to be the primary endpoint; fracture rate is one of several secondary endpoints (see, e.g., Schneider, 2003). (In studies focusing on prevention rather than the treatment of osteoporosis in adults, a bone density measure has been a primary endpoint.)
Consultants from CDER’s Study Endpoint and Labeling Development Group may be involved in consultations about pediatric endpoints without being cited in clinical reviews. The group is also involved in the process that FDA created to evaluate and qualify biomarkers, patient-reported outcome tools, and other measures that sponsors may use in specific drug development efforts so that the appropriateness of each such use does not have to be individually evaluated (CDER, 2010).
In addition, FDA may support analyses of alternative or other endpoints in various contexts. For example, in the context of an advisory committee discussion of modifications to a 2001 written request for the study of sildenafil (Revatio) for the treatment of pediatric hypertension, a staff member from CDER’s Office of Biopharmacometrics discussed data on the use of a hemodynamic measure (the pulmonary vascular resistance index) as an alternative to the 6-minute walk test used for adults (Brar, 2010; CRDAC, 2010).
As noted in Chapter 2, alternative endpoints may be used in pediatric studies in several circumstances. These include when
• the use of the adult endpoint is impossible, for example, when that endpoint depends on a pulmonary function test that cannot be reliably performed by young children or when it requires self-reporting of symptoms and the children to be studied are preverbal;
• the use of the adult endpoint is too risky given the circumstances, for example, when a measurement process used only for research purposes (such as an evaluation by magnetic resonance imaging that has no prospect of benefit) requires a research participant to remain still and would require sedation for children in the age group to be studied;
![]()
7 As defined elsewhere by an NIH working group, a surrogate measure is a “biomarker that is intended to substitute for a clinical endpoint. A surrogate endpoint is expected to predict clinical benefit (or harm or lack of benefit or harm) based on epidemiologic, therapeutic, pathophysiologic, or other scientific evidence” (Biomarkers Definitions Working Group, 2001, p. 91).
• the condition being studied has somewhat different manifestations in children (e.g., juvenile rheumatoid arthritis versus adult rheumatoid arthritis);
• the adult endpoint involves measures of common social interactions or functioning (e.g., at work) that do not reflect children’s situations; and
• a before-and-after treatment measure could be affected by children’s development as well as treatment-related change (e.g., change in bone mass density).
Results of Committee Assessments
The clinical and other reviews and the written requests that the committee examined usually did not note whether the endpoints used for pediatric studies were different from the endpoints used for adult studies. They likewise typically did not discuss the rationale for the endpoints. In some cases, the committee consulted descriptions of studies with adults to determine whether different endpoints were used in the pediatric studies.
For the sample of requested or required pediatric studies and labeling changes that the committee examined, almost half (23 of 49) used primary efficacy endpoints that were the same as those used in adult studies. Roughly one-fifth (11 of 49) involved alternative endpoints. For one product for which two primary endpoints were specified, one of the endpoints was also used in studies with adults and the other was an alternative endpoint. In most of the remaining cases, the studied indications were primarily or entirely found in the pediatric population (seven cases) or primary efficacy endpoints were not required or requested (six cases). Three of the 49 product assessments involved efficacy studies that had different primary efficacy endpoints for different age groups. For one efficacy study (for moxifloxacin ophthalmic [Vigamox] for the treatment of bacterial conjunctivitis in neonates), the section of the clinical review that presumably described the endpoint and results was redacted.
Box 5-5 presents examples of the different categories of endpoints reported in the clinical reviews that the committee examined. More than one indication could be evaluated for a single product, and different efficacy endpoints could be used for different age groups.
For the most part, FDA reviewers did not raise concerns about the use of alternative endpoints as such. Some reviewers noted that the endpoints were based on measures validated for the indication and age group studied (see, e.g., Siegel, 2008b). In general, it would be desirable for specification of alternative endpoints to be accompanied by some discussion of evidence supporting their reliability and validity.
For several studies of asthma drugs, the committee had concerns about
BOX 5-5
Examples of Efficacy Endpoints in Pediatric Studies
Alternative Endpoint
Adalimumab (Humira)
Indication: juvenile rheumatoid arthritis
Primary efficacy endpoint: disease fare measured by a 30 percent worsening in at least three of six juvenile rheumatoid arthritis core set criteria and a minimum of two active joints AND 30 percent improvement in not more than six juvenile rheumatoid arthritis core set criteria specified by American College of Rheumatology (Siegel, 2008b)
Alendronate (Fosamax)
Indication: osteogenesis imperfecta
Primary efficacy endpoint: change in lumbar spine bone mass density (BMD) Z-score (standard deviations from the mean for age-matched healthy controls) from baseline (Schneider, 2003)
Buspirone hydrochloride (Buspar)
Indication: generalized anxiety disorder (ages 6 up to 17 years)
Primary efficacy endpoint: change from baseline in the sum of four scores from C KSADS GAD (Columbia Kiddie Schedule for Affective Disorders and Schizophrenia—General Anxiety Disorder scale) that are specific to anxiety (Laughren, 2000)
Endpoint Also Used in Adult Studies
Aripiprazole (Abilify)
Indication: schizophrenia (ages 13 up to 17 years)
Primary efficacy endpoint: Positive and Negative Syndrome Scale (Zhang, 2007)
Hydrocortisone butyrate (Locoid)
Indication studied: atopic dermatitis (ages 3 months or older)
Primary efficacy endpoint: Physician’s Global Assessment score (Katz, 2007)
Other (Primarily or Entirely a Pediatric Condition)
Methylphenidate (Concerta)
Indication studied: attention deficit hyperactivity disorder (ages 6 up to 12 years)
Primary efficacy endpoint: IOWA Conners Teacher Rating Scale (Inattention/Overactivity Subscale) (Mosholder, 2000)
the endpoint specified for studies in children ages 4 to 11 years. The endpoint, forced expiratory volume in 1 second (FEV1), is widely accepted for use with adults and older children, but it requires physical maneuvers that children under age 6 years cannot reliably perform (see Chapter 2). As a result, for levalbuterol hydrochloride [Xopenex inhalation] for the treatment of asthma, FDA approved labeling for use only in the age group 6 to 11 years old, even though the requested study was supposed to assess drug
safety and efficacy in the age group 4 to 11 years old. Three other products (albuterol sulfate [Ventolin HFA], levalbuterol tartrate [Xopenex HFA], and salmeterol xinafoate [Advair Diskus]) were approved for children in the age group 4 to 11 years old, but on the basis of data that were less than adequate for the youngest children in this group.
At least one requested study of asthma in a younger age group (birth up to 4 years of age for albuterol sulfate [Ventolin HFA]) reported the use of alternative endpoints. These involved asthma symptom scales that used parents’ assessments of symptoms (cough, wheeze, and shortness of breath) in one trial and a clinician assessment using the Modified Tal Asthma Symptoms score, which “included components of respiratory rate, wheezing, cyanosis, and accessory respiratory muscle utilization” (Wang, 2008, p. 14). (In an Internet search, the committee did not find an assessment of the latter instrument.)
For the assessment of studies of pantoprazole sodium (Protonix) for the treatment of gastroesophageal reflux disease (GERD), the clinical review explicitly noted that different symptoms in different age groups required different efficacy endpoints (Chen, 2009). The reviewer also noted concerns, expressed by a consultant from the agency’s study endpoints and labeling development team, about the appropriate description for labeling purposes of measures for infants (vomiting/regurgitation, irritability/fussiness, refusal to feed, choking/gagging, arching back) that were observer (parent) rather than patient based. The consultant also observed that the sponsor did not discuss translation or cultural adaptation of the measures for infants, even though the trial had sites in six countries other than the United States.
On occasion, FDA and a sponsor may not identify a measure suitable for a specific age group, and FDA may waive studies required under PREA for that group. For example, when FDA approved dextromethorphan hydrobromide and quinidine sulfate (Nuedexta) for the treatment of pseudobulbar affect, it waived required studies with children less than 2 years of age. The approval letter explained that the condition “involves exaggerated or contradictory episodes of laughing or crying given the patient’s actual emotional state” and “verbal and non-verbal communication is not adequately developed [in this age group] to allow for accurate appraisal of the patient’s actual emotional state” (Katz, 2010, p. 3).
Use of Extrapolation
Chapter 1 described the FDA initiative in the early 1990s to increase pediatric studies. Among other steps, FDA allowed, under certain circumstances, the extrapolation of efficacy findings from studies with adults to children. Specifically,
a pediatric use statement may also be based on adequate and well controlled studies in adults, provided that the agency concludes that the course of the disease and the drug’s effects are sufficiently similar in the pediatric and adult populations to permit extrapolation from the adult efficacy data to pediatric patients. Where needed, pharmacokinetic data to allow determination of an appropriate pediatric dosage, and additional pediatric safety information must also be submitted. (59 FR 64240 at 64241)
In 2007, FDAAA added that “a study may not be needed in each pediatric age group if data from one age group can be extrapolated to another age group” (21 USC 355C(a)(2)(B()ii)).8
Allowance for the use of extrapolation is intended to make pediatric drug studies less onerous and thereby increase the number of such studies undertaken. Although the allowance in 1994 for extrapolation of efficacy to pediatric age groups had little effect on its own as a stimulus to pediatric studies, it became more significant after Congress created the incentives and requirements for pediatric studies under BPCA and PREA and their predecessor policies.
Decision Tree for Extrapolation Decisions
Working from the regulatory framework described above, FDA has developed a decision tree to guide determinations about when extrapolation can be permitted (Figure 5-1). The determinations can differ by age groups (e.g., with extrapolation accepted for adolescents but not for younger children).
As interpreted by FDA, the extrapolation decision is not a simple “allow” or “do not allow” decision. FDA must also specify the extent to which extrapolation can be relied upon for determinations about efficacy. In guidance issued in 1998, FDA stated that evidence relevant to the determinations about similarity of disease course and disease effect included “evidence of common pathophysiology and natural history of the disease in the adult and pediatric populations, evidence of common drug metabolism and similar concentration-response relationships in each population, and experience with the drug, or other drugs in its therapeutic class, in the disease or condition or related diseases or conditions” (CDER/CBER, 1998b, p. 8).
Occasionally, the written requests or FDA clinical reviews that the
![]()
8 In addition to the FDA provisions for extrapolation that were explicitly directed at pediatric studies, FDA also has more general authority to determine effectiveness based on “data from one adequate and well-controlled clinical investigation and confirmatory evidence (obtained prior to or after such investigation)” (21 USC 355(d)). That is, legislation provides for one form of what FDA terms partial extrapolation to be used by sponsors to support the labeling of products for adult uses.
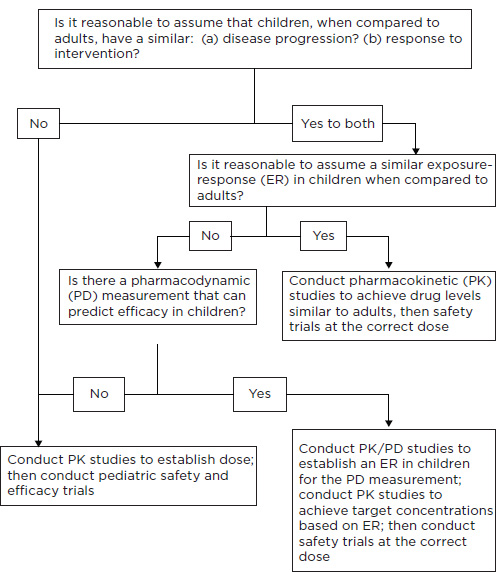
FIGURE 5-1 Use of extrapolation to support pediatric efficacy claims.
SOURCE: Dunne (2010).
committee assessed used the language presented in the decision tree to acknowledge the use of extrapolation. Only rarely did a written request or FDA clinical review provide a more substantive explanation with references to the scientific literature to justify decisions to allow extrapolation. One example of a justification with explicit citation to the literature appears in the written request for a study of aripiprazole (Abilify) for the treatment of schizophrenia in adolescents:
Under FDAMA, 1997, adequate assessment of adolescents (data sufficient to support a labeling claim) might be based on a single study in pediatric patients, together with confirmatory evidence from another source, perhaps adult data for that disorder.… This approach too requires that the adult data be considered reasonably relevant to the course of the disease and the effects of the drug in the pediatric populations. Although we are aware of only two published placebo controlled studies supporting the efficacy of neuroleptics (haloperidol & loxitane) in the treatment of pediatric schizophrenia … we believe that a sufficiently strong case has been made for continuity between adult and adolescent schizophrenia to permit a pediatric claim for a drug already approved in adults to be supported by a single, independent, adequate and well-controlled clinical trial in adolescent schizophrenia. In addition, a pediatric schizophrenia program would need to include pharmacokinetic information and safety information.… Finally, although we are requiring only certain specific studies, you will be expected to maximize the potential of the studies to demonstrate an effect of the drug in adolescents, if there is one. Toward this end, then, we urge you to perform additional studies (see below) in order to ensure that the required studies meet this goal. (Temple, 2003, pp. 6–7)
FDA requests and reviews have become somewhat more consistent in providing justification for extrapolation. Such justifications are often limited in their descriptions and citations of relevant literature. FDAAA specifies only that “a brief documentation of the scientific data” supporting a conclusion about the use of extrapolation be included in agency reviews (21 USC 355c(a)(2)(B)(iii)). Nonetheless, given the significance of the reliance on extrapolation, it would be desirable for requests and reviews to provide the public with a justification somewhat fuller than that now provided in each case in which the agency accepts full or partial extrapolation.
Extent of Use of Extrapolation
Recently, an FDA working group on extrapolation has developed a categorization scheme to label and describe the basic options for the use of extrapolation (Dunne et al., 2011b). The options include
• No extrapolation of efficacy: FDA requires pharmacokinetic data and demonstration of safety and efficacy from two adequate, well-controlled pediatric trials (or from a sequential response and safety trial strategy for oncology products).
• Partial extrapolation of efficacy from studies with adults (or other pediatric age group) with a controlled efficacy trial: FDA requires pharmacokinetic data and confirmation of efficacy and assessment of safety from one adequate and well-controlled pediatric trial.
• Partial extrapolation of efficacy from studies with adults (or other pediatric age group) without a controlled efficacy trial: FDA specifies other acceptable sources of pharmacokinetic, safety, and efficacy or response data.
• Complete extrapolation of efficacy from studies with adults with assessment of safety: FDA requires only safety data or requires safety and pharmacokinetic data to assess age-appropriate dosing.
The working group on extrapolation also analyzed the use of extrapolation studies requested under BPCA based on NDA submissions received between February 1998 and February 2009. As shown in Table 5-2, 29 (17 percent) of 166 submissions of requested studies involved no extrapolation of efficacy, and 24 (14 percent) involved the complete extrapolation of efficacy. The modal submission (67 [40 percent]) included one controlled safety and efficacy trial with additional pharmacokinetic data (which could be obtained during the safety and efficacy trial). For the most part, the fewer the data on efficacy requested by FDA, the more likely it was that a later application for a new or expanded pediatric indication would be approved. The FDA analysis did not examine the use of extrapolation in studies required under PREA.
For its sample, the committee examined FDA’s acceptance of extrapolation to support labeling changes resulting both from studies requested under BPCA and studies required under PREA. Because the use of extrapolation was often not mentioned explicitly in clinical reviews or other documents, the committee had to infer FDA’s reliance on it. For this analysis, as for the one described above, the more extensive that FDA’s acceptance of extrapolation was, the more likely the agency was to approve labeling for a pediatric age group (Table 5-3).
|
|
||||
| No. of Studies with Characteristic/ Total No. of Studies (%) |
||||
|
|
||||
| Extrapolation of Efficacy from Adults or Other Sources | Use for Products with Written Request | New/Expanded Pediatric Indication Achieved | ||
|
|
||||
| No extrapolation (two WCTa) | 29/166 (17) | 10/29 (34) | ||
| Partial extrapolation (one WCT) | 67/166 (40) | 35/67 (52) | ||
| Partial extrapolation (other) | 46/166 (28) | 34/46 (74) | ||
| Complete extrapolation | 24/166 (14) | 15/24 (62) | ||
|
|
||||
a WCT indicates data required from an adequate well-controlled safety and efficacy clinical trial or, for oncology products, from a two-stage trial process to assess response and safety. SOURCE: Dunne et al. (2011b).
For written requests, FDA may reject a sponsor’s proposal for the use of partial extrapolation from adult studies. For example, in the case of the drug buspirone hydrochloride (Buspar), FDA wrote the sponsor, “While we acknowledge your … commitment to conduct two clinical trials for this indication, we do not believe that your new proposal to submit one completed clinical study and one completed pediatric pharmacokinetic study, as a substitute for submitting two completed clinical studies, would be sufficient to support the safety and effectiveness [of the drug] … in the pediatric population and to qualify for pediatric exclusivity” (Temple, 1999, p. 1).
As noted earlier, FDA may allow the use of extrapolation for one age group but not another. In a request for studies of pantoprazole (Protonix) for treatment of erosive esophagitis and nonerosive GERD, FDA concluded that efficacy could be extrapolated from adult data to children 1 to 17 years of age because pathophysiology was similar in the two groups. However, for children younger than age 1 year, the agency concluded that extrapolation was not acceptable because, as described in the clinical review, “the pathophysiology of GERD in infants is believed to be unique” and “symptomatology and prognosis differ between infants and individuals greater than age 1 year” (Griebel, 2009). Nonetheless, the agency did not request two well-controlled safety and efficacy studies for infants. Rather, it requested one such study and another pharmacokinetic, pharmacodynamic, and safety study (Raczkowski, 2001). According to FDA’s current scheme for categorizing determinations, the request allowed for the use of partial
TABLE 5-3 Use of Extrapolation in IOM Sample of BPCA and PREA Labeling Changes
|
|
||||
| No. (%) of Studies | ||||
|
|
||||
| Use of Extrapolation | Extent of Use | Indication Granted, by Extent of Use |
||
|
|
||||
| Extrapolation not accepted (two WCT) | 17/55 (31) | 8/17 (47) | ||
| Partial extrapolation accepted (one WCT) | 26/55 (47) | 15/26 (58) | ||
| Partial extrapolation accepted (other data) | 6/55 (11) | 5/6 (83) | ||
| Complete extrapolation accepted | 1/55 (2) | 1/1 (100) | ||
| Other | 5/55 (9) | 1/5 (20) | ||
|
|
||||
NOTE: Data are for 55 actions, including different decisions for different age groups. WCT indicates data required from an adequate well-controlled safety and efficacy clinical trial or, for oncology products, from a two-stage trial process to assess response and safety; other indicates that the study could involve various combinations of sources of pharamcokinetics, safety and efficacy, response, or activity data. The category “other” includes some submissions for which efficacy was not requested; one for which FDA stated that two WCTs were required but the sponsor only submitted one (which did not show efficacy); and one that included no new pediatric studies.
extrapolation on the basis of one safety and efficacy trial in the age group 1 month up to 1 year old. As it turned out, the studies did not support efficacy in children in this age group (see Chapter 6).
In general, FDA reviewers were careful and thorough in identifying drug-related adverse events, assessing their significance, and reaching conclusions about the safety profile of drugs evaluated in studies with children and the need for any changes in the safety elements of a product’s labeling (if it was already labeled). Summary assessments of a product’s safety profile were generally accompanied by an identification of serious adverse events.
The committee noted variations in the thoroughness of reviews, although recent reviews are generally more thorough and complete. To further improve the quality of reviews, the committee believes that it is time for CBER to adopt formally a systematic, standardized template for clinical and other reviews similar to that used by CDER. The committee also encourages FDA divisions to continue to guide reviewers to follow the safety assessment template, to provide explicit statements about their risk-benefit assessments, and to state clearly their overall conclusions about a product’s safety profile and significant or common adverse events.
If successfully implemented, the agency’s new guidance on safety reporting for clinical trials should improve identification and assessment of treatment-related adverse events and thereby provide a better foundation for conclusions about a drug’s safety profile with pediatric use. Likewise, the structured benefit-risk assessment framework promised by the agency could make an important contribution to FDA’s assessments of pediatric drug studies.
Pediatric studies of drug safety and effectiveness over the long term are important but not commonly requested or required. The 1-year safety reviews mandated by Congress appear to provide a useful opportunity for FDA to examine safety experience and to consider overall safety information after products have had labeling changes based on pediatric studies. In several instances, the reviews have led to revisions of safety information in product labeling or pending recommendations for such changes.
Still, the lack of information about the long-term safety of drugs is a particular worry for developing children—both for drugs that may be used for decades for chronic conditions and for drugs for which short-term use may have adverse consequences months or years later. Given such concerns, FDA might more frequently use its expanded authority to require sponsors to undertake postmarket, follow-up studies of drug safety in pediatric populations.
Although agency staff generally state that the agency does not accept the extrapolation of safety from studies with adult or other pediatric populations, the committee found examples of such extrapolation. This may be appropriate in unusual circumstances, but a public explanation and justification of these circumstances is desirable.
For the most part, FDA’s specification of efficacy endpoints appears to be reasonable, including the use of alternative endpoints when measures used for adults are not appropriate. Written requests and clinical reviews rarely discuss the rationale for endpoints, whether they are alternative or not. For alternative endpoints in particular, FDA should consider providing an explicit discussion of their use, including whether they have been validated in studies with children in the age groups to be studied.
FDA and sponsors rely extensively on extrapolation of efficacy, usually based on requirements for the submission of some efficacy, response, or activity information as well as pharmacokinetic and safety data. The committee found that the justifications were often limited in their descriptions and citations of relevant literature, and Congress requires only brief documentation for the use of extrapolation. Nonetheless, it would be desirable for requests and reviews to provide the public with a justification somewhat fuller than that now provided in each case in which the agency accepts full or partial extrapolation.
The committee recognizes that providing the public with the additional justifications and explanations suggested here adds to the demands on agency staff. In some cases, internal documents (e.g., memoranda for PeRC meetings) or sponsor submissions may already provide much of the basis for such explanations. Overall, the committee believes that the significance of the judgments for which more explicit public rationales or justifications are suggested warrants the additional attention.






























