Dissemination of Information
from Pediatric Studies Conducted
Under BPCA and PREA
P. Brian Smith and Matthew M. Laughon*
When the Food and Drug Administration (FDA) approves a sponsor’s application to market a new product or approves a new use or formulation of an existing product, it also arrives at an agreement with the sponsor about the product’s labeling. That label contains prescribing information for clinicians, including information about the approved uses and dosing (including uses, if any, for pediatric populations), pharmacology, safety, and supporting studies. However, the drug label frequently contains little pediatric prescribing information.
The lack of pediatric clinical trials evaluating drug dosing, safety, and efficacy is due in part to the specific challenges in conducting studies with children and, in part, the economic decisions by pharmaceutical sponsors. For most of the 20th century and with the exception of vaccines, most drug development was focused on adults, with perhaps one-quarter of drugs marketed in the United States labeled for pediatric use by the 1990s.1 The FDA Modernization Act in 1997 and the Best Pharmaceuticals for Children Act (BPCA) in 2002 were designed to address this knowledge gap by providing incentives to pharmaceutical sponsors to study on-patent medications and a mechanism to encourage studies of off-patent medications in children. The Pediatric Rule and the Pediatric Research Equity Act (PREA) allowed FDA to require pharmaceutical sponsors to submit pediatric studies
![]()
* P. Brian Smith, M.D., M.P.H., M.H.S., is associate professor of pediatrics, Duke University Medical Center and Duke Clinical Research Institute. Matthew M. Laughon, M.D., M.P.H., is associate professor, Division of Neonatal-Perinatal Medicine, Department of Pediatrics, University of North Carolina at Chapel Hill.
for products that might have substantial use by the pediatric population even when the drug manufacturer was seeking approval only for an adult indication. Since 1998, FDA has approved almost 400 pediatric-specific labeling changes.2
This paper examines what is known about how labeling information, including information about important changes in pediatric labeling, reaches physicians. It describes intermediary resources that include, to various degrees, any information from the FDA label that provides guidance on prescribing medications for children.
PEDIATRIC USE AND PEDIATRIC LABELING
Many medications used by children are not specifically approved by FDA for such use. Often, information on pediatric use of a product is limited to a statement in the label that safety and efficacy in children have not been established. In other instances, the labeling includes brief information from pharmacokinetic (PK) studies as well as short descriptions of studies that did not demonstrate efficacy. Although most information comes from sponsor-supported studies, FDA occasionally seeks labeling changes after analyzing adverse event reports.3 FDA provides, on its website, a list of labeling changes that have occurred under BPCA and PREA with links to the product label.2
In addition, safety reviews and recommendations by FDA’s Pediatric Advisory Committee (PAC) have been available on FDA’s website since 2002.4,5 Safety information for the PAC is obtained from FDA’s voluntary electronic Adverse Event Reporting System (AERS) available to physicians, pharmacists, patients, and parents. BPCA requires the FDA to report to the PAC safety concerns identified in AERS in the 1-year period following the granting of exclusivity. The PAC is able to recommend additional labeling changes, MedGuide production, or continued close surveillance.6 MedGuides are FDA-approved patient information necessary for a patient’s safe and effective use of prescription drugs that pose a serious public health concern. They are given to patients with each prescription. AERS is limited, as it relies on voluntary reports, and because children represent a small percentage of the population receiving drugs for which adverse events are reported to the FDA, pediatric adverse events can get lost among the larger number of reports submitted for adults.
Off-label prescribing is a common cause of drug-related adverse events in children.7 Improper dosing in children leads to higher rates of treatment failures, adverse events, mortality, and long-term morbidities.8,9 Data on drug safety, PKs, pharmacodynamics (PDs), and efficacy for infants are even more limited than data for older children.10–12
Unfortunately, the relationship between drug action and drug exposure in children cannot be completely understood by extrapolating information obtained from studies in adults. Drug clearance is highly variable in children, particularly infants, because processes responsible for drug biotransformation and elimination are under active development. Dosing requirements for children are often substantially different from those for adults, and significant safety discrepancies have been identified6,13,14 (Table B-1). For example, the requirement for fluconazole dosing for the treatment of invasive candidiasis in term and preterm infants is two times higher than that for adults (12 versus 6 mg/kg/day),19 and micafungin dosing requirements for infants are five times higher than those for adults (10 versus 2 mg/kg/day).20,23,24 For these drugs, simple allometric scaling applied in an effort to predict drug clearance across the continuum of development25 would have limited accuracy due to true maturational differences in the pathways responsible for drug clearance.
Although legislative efforts have resulted in a large number of pediatric-specific labeling changes, several limitations to these legislative efforts exist. Pharmaceutical sponsors are not obligated to respond to FDA’s requests for studies, and FDA can require studies only for the indication proposed in a sponsor’s application. Few labeling changes have included infant-specific information. Infants and premature infants represented only 0.2 and 0.01 percent, respectively, of all children studied in trials submitted to FDA through the pediatric exclusivity program from 1998 to 2005.2
Notwithstanding the benefits of the FDA process for approving drugs and authorizing information in the product’s labeling, the question about whether and how this information reaches physicians and how it influences clinical practice remains. The rest of this paper considers the first issue: dissemination of information about labeling changes.
|
|
|||
| Drug | Preferred Adult Dosea (mg/kg/day) |
Pediatric or Infant Dose (mg/kg/day) |
PK Data Available for Infants Born <28 Weeks Gestation |
|
|
|||
| Ampicillin15 | 150–200 | 150–200 | None |
| Ciprofloxacin16 | 17 | 30 | None |
| Daptomycin17 | 4–6 | 12 | None |
| Metronidazole18 | 30 | 7.5–15 | Limited (>7 days of life) |
| Fluconazole19 | 3–6 | 12 | Yes |
| Micafungin20–22 | 2 | 10 | Yes |
|
|
|||
a Calculated by dividing the recommended adult dose by 70 kg.
FDA DISSEMINATION OF INFORMATION
ABOUT LABELING CHANGES
FDA uses several strategies to disseminate information about labeling changes in general. At Drugs@FDA, FDA usually posts at least the letter approving a change and the revised label. For new drugs or new indications, FDA may post other information, including reviews of the information supporting the changes. For the subset of biologics (mainly blood products and vaccines) that are reviewed and approved by FDA’s Center for Biologics Evaluation and Research, FDA posts information on labeling changes by year. To those who sign up, FDA offers e-mail updates on a variety of topics. These include notices of new drug or biologic approvals, new safety warnings, and drug shortages.26
To disseminate information about labeling changes related to pediatric use, FDA also uses formal mechanisms authorized by Congress and cooperates with established sources that physicians who care for children consult for pediatric prescribing guidance. With the reauthorization of BPCA and PREA in the FDA Amendments Act (FDAAA),27 Congress provided for greater public access to information generated from pediatric trials. For labeling changes approved after its date of enactment, FDAAA authorized FDA to provide public access to full medical, statistical, and pharmacological reviews of studies performed in response to FDA requests or requirements.
FDA provides outreach directly to pediatric providers and researchers and to intermediaries who distribute pediatric prescribing information. For example, FDA provides a monthly column for the American Academy of Pediatrics (AAP) Update of the American Academy of Pediatrics on new dosing, safety, and efficacy findings. FDA has also published a number of articles focusing on findings from pediatric trials stimulated by BPCA and PREA.6,13,14,28–32 FDA’s Office of Pediatric Therapeutics has made efforts to work directly with the editors of The Harriet Lane Handbook, commonly used by pediatricians, to update dosing information.
New Meropenem Dosing as Proof of Concept of Identifying
Sources of Disseminating Prescribing Information
As an illustration of how FDA might address efficient and rapid dissemination of labeling changes, we present our experience with a meropenem trial completed under the BPCA off-patent mechanism.33 This PK and safety trial for labeling was performed with 200 critically ill infants. The goal was to establish dosing guidelines for infants <91 days of age. To describe current use and dosing of meropenem in young infants by neonatal care providers and to identify preferred sources of current and new dosing
information, we performed a web-based survey of neonatologists and neonatal nurse practitioners employed by the Pediatrix Medical Group, Inc., in 278 neonatal intensive care units.34 Questions described clinical situations in subgroups of infants according to gestational age where meropenem might be used as the preferred antimicrobial and asked for proper dosing amount/frequency and sources of dosing information. We obtained complete responses from 116 providers. The majority (66 percent) had used meropenem, although meropenem does not have a labeled indication for premature infants. Among providers who used meropenem, 74 percent used a total daily dose of 40 mg/kg for the treatment of sepsis (dosing according to Neofax,35 an online and print formulary for preterm and term infants), 4 percent used a lower dose, 7 percent used a higher dose, and 16 percent did not respond. For the treatment of meningitis, meropenem was dosed by 61 percent of providers at a total daily dose of 120 mg/kg (Neofax35 dosing), 28 percent used a lower dose, 1 percent used a higher dose, and 10 percent did not respond. Neofax was the preferred source of new dosing information (80 percent), followed by pediatric infectious disease specialists, journals, and the hospital formulary (Figure B-1). Thus, the fastest and most efficient way to disseminate new dosing information dose would be to target Neofax and infectious disease specialists. Although the sample size is relatively small, this type of information is critical to target providers who will be prescribing medications.
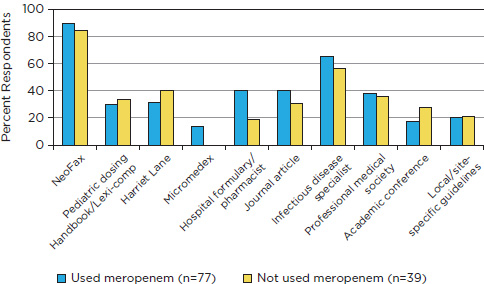
FIGURE B-1 Preferred sources of new dosing information.
SOURCE: Authors’ survey of neonatologists and neonatal nurse practitioners employed by the Pediatrix Medical Group, Inc., in 278 neonatal intensive care units.
SOURCES OF PRESCRIBING INFORMATION USED BY
PHYSICIANS TREATING CHILDREN AND ADOLESCENTS
Clinicians have available a large number of sources that offer prescribing information (Table B-2). They range from local pharmacies to professional societies and from government agencies to publicly traded companies.
Informal communication suggests that clinicians rarely, if ever, consult one available resource: the FDA-authorized drug label. To investigate further, we surveyed 30 pediatric residents and 10 general pediatric attending physicians at the University of North Carolina (UNC) and at Duke University Medical Center about whether they had consulted a drug label for pediatric dosing guidance. None of the 40 clinicians reported that they had read an FDA label or used the FDA label to obtain prescribing information. Most of the respondents reported using The Harriet Lane Handbook. Our informal survey is biased toward inpatient hospital providers. Similarly, in a published survey of 313 practitioners, there were no reports of the use of the drug’s FDA label to guide pediatric dosing.38 Some elements of the drug label are more often recognized than the sections on dosing. For example, FDA black box warnings have a relatively high penetration to outpatient providers (33 to 72 percent), although this may differ by specialty.42–44 Many of the most commonly used medications in pediatrics have little to no pediatric-specific information.39,45
The cost of intermediary resources is an important issue. To keep knowledge up to date, most online sources require a subscription (e.g., UptoDate and MD Consult) and many print editions require purchase of a new book each year (e.g., The Harriet Lane Handbook). The sponsorship and funding of the resources in Table B-2 are opaque. Intermediary resources range from nonprofit professional groups (e.g., AAP) to publicly traded companies. Some sources, particularly those online (e.g., WebMD), are accompanied by drug advertising, and some are provided by pharmaceutical companies to residents (e.g., UNC residents receive free copies of The Harriet Lane Handbook from a pharmaceutical company). Researchers have found associations with higher prescribing frequency, higher costs, or lower prescribing quality when prescribers are provided with information from pharmaceutical companies, but no evidence of improved prescribing practice is available.46 Ideally, the most commonly used sources of prescribing information would have unbiased information free from industry financial influence. To address this issue fully is beyond the scope of this paper. Concerns have also been expressed about industry influence on the content of professional society guidelines and continuing medical education offerings.47
Many of the dosing resources use the FDA label as a source of prescrib-
TABLE B-2 Sources for Prescribing Information for Clinicians
|
|
||||||
| Intermediary Resource |
Publisher(s), Website | Advantages | Disadvantages | |||
|
|
||||||
| The Harriet Lane Handbook36–38 | Johns Hopkins Hospital, Elsevier | Uses FDA label as source | Online version through mdconsult.com | |||
|
|
||||||
| Neofax | Thomson Reuters, http://www.skyscape.com/neofax/ | Uses FDA label as source | Limited to infants | |||
|
|
||||||
| Epocrates38 | Epocrates, www.epocrates.com | Uses FDA label as source, smart phone applications | Advertising might introduce bias | |||
|
|
||||||
| Lexi-Comp38,39 | Lexi-Comp, www.lexi.com | Uses FDA label as source | ||||
|
|
||||||
| Micromedex | Thomson Reuters, www.micromedex.com | Directed toward hospital formularies | ||||
|
|
||||||
| Physicians’ Desk Reference38,39 | PDR Network, www.pdr.net | Uses FDA label as source | ||||
|
|
||||||
| Red Book | American Academy of Pediatrics, www.aap.org | Free to AAP members | ||||
|
|
||||||
| Nelson’s Pocket Book of Pediatric Antimicrobial Therapy40 | American Academy of Pediatrics, www.aap.org | Free to AAP members | ||||
|
|
||||||
| Tarascon Pharmacopoeia41 | Tarascon Publishing, www.tarascon.com | Limited pediatric data | ||||
|
|
||||||
| Medscape/WebMD/emedicine | www.medscape.com, www.eMedicine.com, www.webmd.com | Free, Uses FDA label as source | Publicly traded company, advertising might introduce bias | |||
|
|
||||||
| MD Consult | Elsevier Publishing, www.mdconsult.com | Formulary outsourced to Gold Standard, Inc. | ||||
|
|
||||||
| UpToDate | www.uptodate.com | Formulary linked to another source (Lexi-Comp) | ||||
|
|
||||||
| Drug Facts and Comparisons | Wolters Kluwer Health, www.factsandcomparison.com | Uses FDA label as source | Used mostly by pharmacists | |||
|
|
||||||
| eMPR | Haymarket Media, www.empr.com | Update monthly | Advertising on website | |||
|
|
||||||
TABLE B-2 Continued
|
|
||||||
| AAP News37 | American Academy of Pediatrics, www.aap.org | Unbiased | ||||
|
|
||||||
| Scientific literature38 | Medline, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Cochrane database, etc. | Abstracts usually free | Fully published article might require subscription, findings difficult to interpret | |||
|
|
||||||
| Pharmacy consultation38 | NAa | Fast | Bias | |||
|
|
||||||
| Experience38 | NA | Fast, efficient | Bias | |||
|
|
||||||
| Local pharmacy computer physician order entry pharmacy systems (e.g., Sunrise Clinical Manager38) | NA | Fast | Uncertain how dosing information is derived | |||
|
|
||||||
| Subspecialist guidelines (e.g., pediatric infectious disease, pediatric gastroenterology) | http://www.guideline.gov/, other subspecialty sites | Bias | ||||
|
|
||||||
| Drug label42 | http://dailymed.nlm.nih.gov http://www.nlm.nih.gov/medlineplus/druginfo/meds/a606016.html http://www.accessdata.fda.gov/scripts/cder/drugsatfda/ http://www.fda.gov/BiologicsBloodVaccines/DevelopmentApprovalProcess/BiologicalApprovalsbyYear/default.htm |
Free | Difficult to understand | |||
|
|
||||||
a NA = not applicable.
ing information. Most also have dosing recommendations for off-label use of medications for pediatrics. For example, Neofax has a recommended dose of intravenous immunoglobulin for severe hyperbilirubinemia due to Rh or ABO blood group incompatibility, an indication not approved by FDA for any approved intravenous immunoglobulin product.35 Resources
may rely on expert opinion or review of the medical literature for these indications. It is unclear how experts are chosen, although some are noted to be on the editorial boards of some sources.
Medscape/WebMD/eMedicine is a website covering a variety of health topics, including medications. This website has a section on the FDA, and it should be noted that the FDA and WebMD have a partnership to promote public health.48 Certain articles on WebMD are under editorial control of the FDA and are noted as such.48 The sections on WebMD that review pediatric medications refer to the FDA label and use expert opinion and scientific literature for dosing recommendations. The date of the most recent update is noted on each webpage.
Some intermediary resources are directed specifically toward pediatric providers. The two most commonly used are The Harriet Lane Handbook36 for pediatricians and Neofax for providers working in the neonatal intensive care unit. The Harriet Lane Handbook and Neofax use the FDA labels as a guideline and periodically update (usually every 1 to 2 years) the information provided in the book. The Red Book, an AAP publication that reviews infectious diseases and antimicrobial drugs, is available online and in print and directs users to the FDA website for the product label for antimicrobial agents and related therapy. In addition, Appendix II of the Red Book is devoted to the FDA licensure dates of selected vaccines in the United States. The Red Book also has a section on MedWatch. The Red Book is updated every 3 years, most recently in 2009. AAP also publishes Nelson’s Pocket Book of Pediatric Antimicrobial Therapy, which is updated yearly.40 Both of these resources are limited to antimicrobial therapy.
Other intermediary resources provide both adult and pediatric dosing. Online editions of Lexi-Comp,49 Micromedex,50 the Physicians’ Desk Reference,51 and the Tarascon Pharmacopoeia41 update the information in the FDA label more frequently, approximately every 6 months. The print versions of Lexi-Comp, the Physicians’ Desk Reference, Micromedex, and the Tarascon Pharmacopoeia are updated yearly. Drug Facts and Comparisons52 has online and bound versions and includes appendixes on FDA New Drug Classification and Pregnancy Categories. Drug Facts and Comparisons is used primarily by pharmacists and hospital pharmacy and therapeutic committees.
Epocrates, MD Consult, and UpToDate are online-only resources with adult and pediatric pharmacological information. Epocrates is focused primarily on drug information and has a web-based online version. In addition, applications for each of the major mobile operating systems (e.g., iPhone, BlackBerry, Android, and Windows Mobile) are available.
Epocrates uses the FDA drug label, FDA drug safety alerts, and the primary medical literature for dosing recommendations and is updated once per week. MD Consult and UpToDate are primarily focused on medical
diagnoses and treatment. However, both have some dosing information. MD Consult uses the FDA label and medical literature to update dosing guidelines for pediatric therapeutics and lists the most recent update on each webpage for the drug. UpToDate simply refers to Lexi-Comp directly. eMPR (www.empr.com) and Monthly Prescribing Reference are an online resource and a monthly periodical, respectively, with updated information on dosing. Monthly Prescribing Reference also has a pediatrics edition.
Medical centers and health systems may also provide prescribing resources as well as their own formularies. Both UNC and Duke have proprietary computer order entry systems with a local pediatric formulary on the back end that provides alerts to providers when an order includes a dosage outside the normally accepted range, as established from resources such as those described here combined with local pharmacy input. We found no information on how often these formularies are updated.
Although dosing guidelines are included in these intermediary resources, it remains unclear how or if clinicians follow the recommended dosing guidelines. For example, when clinicians prescribe antibiotics for preterm infants, the rate of compliance with recommendations ranges from 37 to 88 percent.53 In addition, the extent to which the resources referenced in this paper influence practice depends on the content that is available, the way in which information is presented, and other factors, including the economic and organizational context in which clinicians practice. In general, analyses demonstrate a wide variability in the effectiveness of clinical decision support tools.54,55 For example, in a large national health plan, physicians who had access to a handheld electronic formulary (Epocrates) had similar patterns of prescribing nongeneric, nonformulary medications, compared to the prescribing patterns of those physicians without access to such a device.54
EXTENT TO WHICH LABELING CHANGES
ARE REFLECTED IN RESOURCES
A systematic investigation of the extent to which information resources are updated in a timely and accurate way to reflect drug labeling changes was beyond the scope of this paper. However, we did investigate a few recent, significant pediatric labeling changes (Table B-3). Elements of some of these changes are reflected in the most recent editions of intermediary resources. However, some safety findings are not mentioned (e.g., those for topiramate and lamotrigine).36 As noted earlier, information on off-label use is common. For example, dosing information is given for populations in which efficacy is not yet established (e.g., caspofungin) or in which efficacy was studied and not demonstrated (e.g., azithromycin).35 Note that three of the labeling changes involved information based on pediatric studies with
| Information from: | ||||
| Drug | Labeling Change | Date of Labeling Change | The Harriet Lane Handbook | Neofax |
| Topiramate | Lack of efficacy for treatment of seizures for ages 1–24 months Growth retardation lab abnormalities for ages 1–24 months |
12/22/2009 | No dosing information for ages <2 years36 New safety findings not mentioned36 |
No information provided35 |
| Esomeprazole | Lack of efficacy for GERDa for ages <1 year | 6/18/2009 | No dosing information for ages <1 year36 No reference to lack of efficacy for ages <1 year |
No information provided35 |
| Lamotrigine | Lack of efficacy for ages 1–24 months, seizures Associated with increased risk of infectious adverse reactions |
5/8/2009 | No dosing information for ages <2 years36 New safety findings not mentioned36 |
No information provided35 |
| Azithromycin | Efficacy for community-acquired pneumonia not established for ages <6 months Efficacy for sinusitis not established for pediatric population |
10/8/2008 | Dosing for otitis media and community-acquired pneumonia provided for ages ≥6 months36 Dosing for acute sinusitis provided for ages ≥6 months36 |
Dosing provided for infants35 |
| Caspofungin | Safety and efficacy not studied for ages <3 months | 7/29/2008 | Dosing provided for ages <3 months36 | Dosing provided for infants35 |
a GERD = gastroesophageal reflux disease.
negative findings about safety or efficacy or both. Neither The Harriet Lane Handbook nor Neofax routinely notes when dosing is recommended for off-label indications or age groups.35,36,48 Neofax does, however, provide references for its dosing recommendations.35
CONCLUSION
BPCA and PREA have addressed many of the knowledge gaps in pediatric therapeutics, but gaps remain. Many drugs used by children, especially infants, are used off-label for indications that are often not approved by FDA and for which dosing and safety information is not included in the FDA label.
Although FDA rigorously reviews the accuracy and completeness of drug labeling proposed by sponsors and revisions to proposed language are common, this paper suggests that the extent to which providers directly use labels is limited. Instead, clinicians who prescribe medication to children rely upon intermediary resources that come in various printed or online forms. FDA has many competing demands on its resources for investigation and dissemination, but possible shortcomings in the completeness and timeliness of drug information provided by intermediary resources are concerning.
REFERENCES
1. Wilson JT. An update on the therapeutic orphan. Pediatrics 1999;104:585-90.
2. Pediatric Labeling Changes through February 25, 2011. Food and Drug Administration; 2011. (Accessed March 8, 2011, at http://www.fda.gov/downloads/ScienceResearch/SpecialTopics/PediatricTherapeuticsResearch/UCM163159.pdf.)
3. Shirkey HC. Therapeutic orphans—everybody’s business. Ann Pharmacother 2006;40:1174.
4. Best Pharmaceuticals for Children Act. 2002; PL 107-109.
5. Safety Reporting. Food and Drug Administration; 2011. (Accessed at March 14, 2012 at http://www.fda.gov/ScienceResearch/SpecialTopics/PediatricTherapeuticsResearch/ucm123229.htm.)
6. Smith PB, Benjamin DK, Jr, Murphy MD, et al. Safety monitoring of drugs receiving pediatric marketing exclusivity. Pediatrics 2008;122:e628-33.
7. Turner S, Nunn AJ, Fielding K, Choonara I. Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr 1999;88:965-8.
8. Choonara I. Unlicensed and off-label drug use in children: implications for safety. Expert Opin Drug Saf 2004;3:81-3.
9. Roberts R, Rodriguez W, Murphy D, Crescenzi T. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. JAMA 2003;290:905-11.
10. Avenel S, Bomkratz A, Dassieu G, Janaud JC, Danan C. [The incidence of prescriptions without marketing product license in a neonatal intensive care unit]. Arch Pediatr 2000;7:143-7. (In French.)
11. O’Donnell CP, Stone RJ, Morley CJ. Unlicensed and off-label drug use in an Australian neonatal intensive care unit. Pediatrics 2002;110:e52.
12. ’t Jong GW, Vulto AG, de Hoog M, Schimmel KJ, Tibboel D, van den Anker JN. A survey of the use of off-label and unlicensed drugs in a Dutch children’s hospital. Pediatrics 2001;108:1089-93.
13. Benjamin DK, Jr, Smith PB, Sun MJ, et al. Safety and transparency of pediatric drug trials. Arch Pediatr Adolesc Med 2009;163:1080-6.
14. Benjamin DK, Jr, Smith PB, Murphy MD, et al. Peer-reviewed publication of clinical trials completed for pediatric exclusivity. JAMA 2006;296:1266-73.
15. Axline SG, Yaffe SJ, Simon HJ. Clinical pharmacology of antimicrobials in premature infants. II. Ampicillin, methicillin, oxacillin, neomycin, and colistin. Pediatrics 1967;39:97-107.
16. Peltola H, Ukkonen P, Saxen H, Stass H. Single-dose and steady-state pharmacokinetics of a new oral suspension of ciprofloxacin in children. Pediatrics 1998;101:658-62.
17. Abdel-Rahman SM, Benziger DP, Jacobs RF, Jafri HS, Hong EF, Kearns GL. Single-dose pharmacokinetics of daptomycin in children with suspected or proved gram-positive infections. Pediatr Infect Dis J 2008;27:330-4.
18. Upadhyaya P, Bhatnagar V, Basu N. Pharmacokinetics of intravenous metronidazole in neonates. J Pediatr Surg 1988;23:263-5.
19. Wade KC, Wu D, Kaufman DA, et al. Population pharmacokinetics of fluconazole in young infants. Antimicrob Agents Chemother 2008;52:4043-9.
20. Hope WW, Seibel NL, Schwartz CL, et al. Population pharmacokinetics of micafungin in pediatric patients and implications for antifungal dosing. Antimicrob Agents Chemother 2007;51:3714-9.
21. Smith PB, Walsh TJ, Hope W, et al. Pharmacokinetics of an elevated dosage of micafungin in premature neonates. Pediatr Infect Dis J 2009;28:412-5.
22. Benjamin DK, Jr, Smith PB, Arrieta A, et al. Safety and pharmacokinetics of repeat-dose micafungin in young infants. Clin Pharmacol Ther 2010;87:93-9.
23. Cohen-Wolkowiez M, Benjamin DK, Jr, Steinbach WJ, Smith PB. Anidulafungin: a new echinocandin for the treatment of fungal infections. Drugs Today (Barc) 2006;42:533-44.
24. Benjamin DK, Jr, Smith P, Arrieta A, et al. Safety and pharmacokinetics of repeat-dose micafungin in neonates. Program and abstracts of the 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2008; American Society for Microbiology, Washington, DC.
25. Holford N. Dosing in children. Clin Pharmacol Ther 87:367-70.
26. Stay Informed. Food and Drug Administration; 2011. (Accessed September 14, 2011, at http://www.fda.gov/AboutFDA/ContactFDA/StayInformed/GetEmailUpdates/default.htm.)
27. Food and Drug Administration Amendments Act. PL 110-121 Stat 823; 110th Congress; 2007.
28. Publications from the Office of Pediatric Therapeutics and FDA. Food and Drug Administration; 2011. (Accessed July 10, 2011, at http://www.fda.gov/ScienceResearch/SpecialTopics/PediatricTherapeuticsResearch/ucm121586.htm.)
29. Benjamin DK, Jr, Smith PB, Jadhav P, et al. Pediatric antihypertensive trial failures: analysis of end points and dose range. Hypertension 2008;51:834-40.
30. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther 2008;84:315-9.
31. Li JS, Eisenstein EL, Grabowski HG, et al. Economic return of clinical trials performed under the pediatric exclusivity program. JAMA 2007;297:480-8.
32. Smith PB, Li JS, Murphy MD, Califf RM, Benjamin DK, Jr. Safety of placebo controls in pediatric hypertension trials. Hypertension 2008;51:829-33.
33. Smith PB, Capparelli EV, Castro L, et al. Pharmacokinetics and Safety of Meropenem in Young Infants with Intra-Abdominal Infections. Society for Pediatric Research, Vancouver, BC, Canada; 2010.
34. Aliaga S, Smith PB, Clark R, et al. Assessment of Off-Label Prescribing Practices of Meropenem in Young Infants. Society for Pediatric Research, Denver, CO; 2011.
35. Neofax, 24th ed. Thomson Reuters, New York; 2011.
36. Johns Hopkins Hospital, Arcara K, Tschudy M, Lee CKD. The Harriet Lane Handbook: A Manual for Pediatric House Officers, 19th ed. Elsevier, Philadelphia, PA; 2010.
37. Yoon EY, Clark SJ, Gorman R, Nelson S, O’Connor KG, Freed GL. Differences in pediatric drug information sources used by general versus subspecialist pediatricians. Clin Pediatr (Phila) 2010;49:743-9.
38. Barrett JS, Narayan M, Patel D, Zuppa AF, Adamson PC. Prescribing habits and caregiver satisfaction with resources for dosing children: rationale for more informative dosing guidance. BMC Pediatr 2011;11:25.
39. Zuppa AF, Adamson PC, Mondick JT, et al. Drug utilization in the pediatric intensive care unit: monitoring prescribing trends and establishing prioritization of pharmacotherapeutic evaluation of critically ill children. J Clin Pharmacol 2005;45:1305-12.
40. Bradley JS, Nelson JC. Nelson’s Pocket Book of Pediatric Antimicrobial Therapy, 18th ed; 2010-2011 Edition. American Academy of Pediatrics, Elk Grove Village, IL; 2010.
41. Tarascon Pharmacopoeia. Jones and Bartlett Learning, Burlington, MA; 2011.
42. Williams J, Klinepeter K, Palmes G, Pulley A, Meschan Foy J. Behavioral health practices in the midst of black box warnings and mental health reform. Clin Pediatr (Phila) 2007;46:424-30.
43. Cheung A, Sacks D, Dewa CS, Pong J, Levitt A. Pediatric prescribing practices and the FDA black-box warning on antidepressants. J Dev Behav Pediatr 2008;29:213-5.
44. Heneghan A, Garner AS, Storfer-Isser A, Kortepeter K, Stein RE, McCue Horwitz S. Use of selective serotonin reuptake inhibitors by pediatricians: comparing attitudes of primary care pediatricians and child and adolescent psychiatrists. Clin Pediatr (Phila) 2008;47:148-54.
45. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics 2006;117:1979-87.
46. Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med 2010;7:e1000352.
47. Institute of Medicine. Conflict of Interest in Medical Research, Education, and Practice. The National Academies Press, Washington, DC; 2009. (Accessed March 14, 2012 at http://books.nap.edu/openbook.php?record_id=12598.)
48. WebMD. 2011. (Accessed at www.webmd.com.)
49. Lexi-Comp Online. 2011. (Accessed at www.lexi.com.)
50. Micromedex. 2011. (Accessed at www.micromedex.com.)
51. Physicians’ Desk Reference. 2011. (Accessed at www.pdr.net.)
52. Drug Facts and Comparisons. 2011. (Accessed at www.factsandcomparisons.com.)
53. Cheng CL, Yang YH, Lin SJ, Lin CH, Lin YJ. Compliance with dosing recommendations from common references in prescribing antibiotics for preterm neonates. Pharmacoepidemiol Drug Saf 2010;19:51-8.
54. Clauson KA, Marsh WA, Polen HH, Seamon MJ, Ortiz BI. Clinical decision support tools: analysis of online drug information databases. BMC Med Informatics Decision Making 2007;7:7.
55. Lyman JA, Conaway M, Lowenhar S. Formulary access using a PDA-based drug reference tool: does it affect prescribing behavior? AMIA Annual Symposium Proceedings. 2008;1034.














