Joan Stachnik and Michael Gabay*
Biologics have a long history of use as therapeutic agents in the United States (FDA, 2002). Vaccines, primarily derived from animal sources, were among the first biologics developed. The smallpox vaccine was introduced in 1800 (Barquet and Domingo, 1997), followed by other vaccines, such as the rabies and diphtheria vaccines (Junod, 2002). These vaccines were widely used but had little regulatory oversight. This changed in 1902 with the passage of the Biologics Control Act of 1902, which established regulations for vaccine production and licensing, following the deaths of 22 children in separate incidents involving contaminated diphtheria antitoxin and contaminated smallpox vaccine (Junod, 2002).
Since the time that these early biological products began to be regulated, advances in science and technology have allowed more purified and complex biologics, including those derived from human blood components or produced using recombinant technology1 (Roque et al., 2004; Burnouf, 2011). Biologics are now used not only to prevent infectious conditions but also to treat a wide array of diseases, such as rheumatoid arthritis, cancers, and other
![]()
* Joan Stachnik, M.Ed., Pharm.D., B.C.P.S., is clinical associate professor in the Drug Information Group, Department of Pharmacy Practice, College of Pharmacy, University of Illinois at Chicago. Michael Gabay, Pharm.D., J.D., B.C.P.S., is director and clinical associate professor in the Drug Information Group, Department of Pharmacy Practice, College of Pharmacy, University of Illinois at Chicago.
1 Recombinant technology involves the combining of DNA sequences responsible for expression of specific proteins or the fusion of target regions of antibodies, antibody fragments, or proteins.
immune-mediated conditions. Although some of these diseases are diagnosed in the pediatric population, research with these age groups is limited.
Since 1972, the Food and Drug Administration (FDA) has been responsible for the regulation of biologics. FDA licenses biological products under the Public Health Service Act licensing provisions and approves drugs under the federal Food, Drug, and Cosmetic (FDC) Act approval provisions. Under the FDC Act, certain old, relatively simple, biologically based products (e.g., insulin and human growth hormone) have long been regulated by the Center for Drug Evaluation and Research (CDER) through the New Drug Application process rather than through the Biologics License Application process of the Public Health Service Act (FDA, 2009c). In 2003, CDER also assumed responsibility for certain biologics. These are sometimes referred to as “therapeutic biologics,” although responsibility for regulation of other therapeutic biologics, such as intravenous immune globulins, remained with the Center for Biologics Evaluation and Research (CBER). CDER-regulated biologics include monoclonal antibodies for in vivo use, cytokines, growth factors, enzymes, immunomodulators, thrombolytics, certain therapeutic proteins, and nonvaccine immunotherapies (FDA, 2009d, 2010). Regulation of allergenics, blood and blood components (including recombinant proteins of blood components), gene therapy products, certain human cellular and tissue-based products (including stem cells and tissues for implantation or transplantation), vaccines, and nonhuman cells or tissues for transplantation remains under the authority of CBER (FDA, 2009a). This paper focuses on the biologics regulated by CDER and the CBER-regulated biologics that are derived from blood and blood components, with the exception of vaccines.
DEFINITION AND REGULATION OF BIOLOGICS
Generally described, biologics are “isolated from a variety of natural sources—human, animal, or microorganism—and may be produced by biotechnology methods and other cutting-edge technologies” (FDA, 2009e, unpaged). The regulatory definition provided in the Public Health Service Act (as amended in 2010) states that a biologic is “a virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, allergenic product, protein (except any chemically synthesized polypeptide), or analogous product, or arsphenamine or derivative of arsphenamine (or any other trivalent organic arsenic compound) applicable to the prevention, treatment, or cure of a disease or condition of human beings” (42 USC 262(i)).
Biologics differ from conventional drugs in complexity and source. Unlike small-molecule drugs, which are produced by chemical reactions and
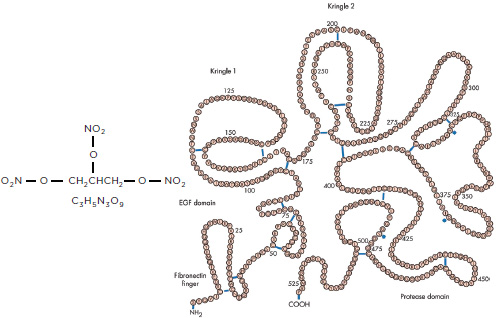
NOTE: EGF = epidermal growth factor.
SOURCE: For alteplase, reproduced from Heart, T. K. Nordt and C. Bode, 89(11): 1358-1362, 2003 with permission from BMJ Publishing Group Ltd.
have a known structure, biologics can be derived from human, microbiological, or animal sources and have complex structures consisting of amino acids, sugars, and nucleic acids (Figure C-1 shows an approximation of the difference in scale and complexity). Because of their higher complexity, stability is usually a greater issue with biologics than drugs (FDA, 2009e).
OVERVIEW OF CHARACTERISTICS OF SELECTED BIOLOGICS
Some biologics are derived from blood, primarily plasma proteins (Table C-1) or are produced via recombinant technology. Plasma, either recovered from blood or donated directly, undergoes a fractionation process, which was first developed in the 1940s, to isolate proteins that can be used therapeutically (Burnouf, 2007). Isolation of a different protein occurs at each step of the fractionation process. For example, the first precipitate of the process—cryoprecipitate—is a rich source of coagulation proteins or factors (e.g., factor VIII and fibrinogen). Later in the fractionation process, other proteins such as albumin and immunoglobulins are separated out of the plasma after exposure to different ethanol concentrations and pHs. The safety of plasma-derived proteins is increased through the use of various
TABLE C-1 Plasma-Derived Therapeutic Proteins
|
|
||
| Plasma-Derived Protein | General Uses | |
|
|
||
| Coagulation factors (single factors and prothrombin complex) | Treatment or prevention of bleeding in patients with factor deficiency | |
| Fibrinogen | Control of acute bleeding in patients with congenital fibrinogen deficiency | |
| von Willebrand factor | Treatment or prevention of bleeding in patients with von Willebrand disease | |
| Thrombin (human and bovine) | Achievement of hemostasis during surgery | |
| Antithrombin | Treatment or prevention of thromboembolism in patients with antithrombin deficiency | |
| α1-Antitrypsin (α1-protease inhibitor | Replacement therapy for patients with congenital α1-)antitrypsin deficiency and emphysema | |
| C1-esterase inhibitor | Prevention of angioedema in patients with hereditary angioedema | |
| Immunoglobulins | Treatment of primary immunodeficiency diseases and immune thrombocytopenic purpura | |
| Albumin | Treatment of fluid resuscitation and shock | |
|
|
||
SOURCES: Burnouf (2007), McEvoy (2011).
methods to reduce the risk of transmission of human immunodeficiency virus, hepatitis viruses, and other viruses. These methods include chromatography (ion-exchange, affinity, and size-exclusion chromatography), filtration, solvent-detergent treatment, pasteurization, and heat treatment.
Beginning in the early 1980s, advances in genetic engineering and cell expression systems allowed production of recombinant forms of some human plasma proteins and the development of new biologics with specific cellular targets (Burnouf, 2011). Recombinant therapeutics generally include monoclonal antibodies, fusion proteins, and recombinant versions of human proteins (e.g., recombinant-derived coagulation factors). In addition to the different methods of production, recombinant therapeutics can differ in action, with some blocking or preventing release of cytokines and others acting as replacement proteins for deficient endogenous human proteins (Grabenstein, 2011).
Monoclonal antibodies represent the largest class of recombinant-derived therapeutics (An, 2010). Monoclonal antibodies have structures similar to those of immunoglobulins but are modified by recombinant technology to have a high specificity and affinity for a particular target, such as cytokines, cell markers, or their receptors, to prevent subsequent effects or production of inflammatory mediators (Table C-2) (An, 2010;
TABLE C-2 Therapeutic Monoclonal Antibodies and Fusion Proteins
|
|
||
| Biologic | Target | Sourcea |
|
|
||
| Monoclonal antibodies | ||
|
|
||
| Abciximab (ReoPro) | Glycoprotein IIb/IIIa receptor | Chimeric |
| Adalimumab (Humira) | Human tumor necrosis factor alpha | Human |
| Certolizumab (Cimzia) | Humanized | |
| Golimumab (Simponi) | Humanized | |
| Infliximab (Remicade) | Chimeric | |
| Alemtuzumab (Campath) | CD52 surface antigen on B and T lymphocytes; most monocytes, macrophages, and natural killer cells; and some granulocytes | Humanized |
| Basiliximab (Simulect) Daclizumab (Zenapax) |
Interleukin-2 receptor (CD25 surface antigen) on activated lymphocytes | Humanized Humanized |
| Bevacizumab (Avastin) Ranibizumab (Lucentis) |
Human vascular endothelial growth factor-A receptor | Humanized Humanized |
| Canakinumab (Ilaris) | Interleukin-1β | Humanized |
| Capromab (ProstaScint) | Prostate-specific membrane antigen | Murine |
| Cetuximab (Erbitux) Panitumumab (Vectibix) |
Human epidermal growth factor receptor expressed on normal and tumor cells | Chimeric Humanized |
| Denosumab (Prolia/Xgeva) | Human receptor activator for nuclear factor-kappa B ligand | Humanized |
| Eculizumab (Soliris) | Complement protein C5 | Humanized |
| Ibritumomab tiuxetan (Zevalin) | CD20 surface antigen on B lymphocytes | Murine |
| Ofatumumab (Arzerra) Rituximab (Rituxan) Tositumomab, Iodine I 131 tositumomab (Bexxar) |
Humanized Chimeric Murine |
|
| Muronomab (Orthoclone OKT3) | CD3 surface antigen of T cells | Humanized |
| Natalizumab (Tysabri) | α4-Integrin on the surface of all leukocytes except neutrophils | Humanized |
| Omalizumab (Xolair) | Human immunoglobulin E | Humanized |
| Palivizumab (Synagis) | The A antigenic site of F protein of respiratory syncytial virus | Humanized |
| Tocilizumab (Actemra) | Interleukin-6 receptor | Humanized |
| Trastuzumab (Herceptin) | Human epithelial growth factor receptor-2 protein | Humanized |
| Ustekinumab (Stelara) | p40 subunits of interleukin-12 and interleukin-23 | Humanized |
|
|
||
TABLE C-2 Continued
|
|
||
| Biologic | Target | |
|
|
||
| Fusion proteins | ||
|
|
||
| Abatacept (Orencia) | CD80 and CD86 surface antigens on T cells | |
| Alefacept (Amevive) | CD2 surface antigens on T cells | |
| Etanercept (Enbrel) | Human tumor necrosis factor | |
| Rilonacept (Arcalyst) | Interleukin-1 receptor | |
| Denileukin (Ontak) | Interleukin-2 receptor | |
| Romiplostim (Nplate) | Thrombopoietin receptor | |
|
|
||
a Sources of fragments used for monoclonal antibody production include human and nonhuman species. A portion of chimeric monoclonal antibodies (25 percent) are murine derived, humanized monoclonal antibodies are 5 percent murine derived, and human monoclonal antibodies are fully human. Immunogenicity is decreased with more human monoclonal antibodies.
SOURCES: An (2010), Lee and Ballow (2010), Burnouf (2011), Grabenstein (2011), McEvoy (2011), Wickersham (2011).
Burnouf, 2011; Grabenstein, 2011). In addition to monoclonal antibodies, fusion proteins bind to cytokines or receptor sites to block the effects or production of cytokines (Table C-2). Fusion proteins consist of a portion of a native protein (e.g., a cell surface receptor) fused to another molecule, often via a portion of human immunoglobulin (Lee and Ballow, 2010).
Finally, recombinant versions of human plasma proteins, as well as enzymes, have been developed for treatment of disorders resulting from qualitative or quantitative deficiencies of these substances. These products are listed in Table C-3 (Rohrbach and Clarke, 2007; Brooker, 2008; Wickersham, 2011).
The biologics described in Tables C-1 to C-3 are used for the treatment of a wide array of diseases and disorders (An, 2010; Burnouf, 2011). Because of their mechanisms of action, many of the monoclonal antibodies and fusion proteins are used for treatment of immune-mediated diseases, such as rheumatoid arthritis, Crohn’s disease, multiple sclerosis, cancers, and psoriasis. Most are classified as antineoplastics, disease-modifying antirheumatic drugs, biologic response modifiers, or immunosuppressive agents (McEvoy, 2011). The activities of recombinant-based versions of human plasma proteins (e.g., epoetin, pegfilgrastim, antihemophilic factor, palifermin, and drotrecogin alfa) and enzymes (e.g., rasburicase, laronidase, naglazyme, and alglucosidase alfa) as well as plasma-derived proteins (e.g., immunoglobulins, albumin, von Willebrand factor, and C1-esterase) generally mimic the activity of the endogenous protein or enzyme to achieve a therapeutic effect.
TABLE C-3 Additional Therapeutic Recombinant Human Proteins
|
|
|
| Biologic | Description |
|
|
|
| Agalsidase beta (Fabrazyme) | Recombinant human form of α-galactosidase |
| Alglucosidase alfa (Myozyme/Lumizyme) | Recombinant human lysosomal glucogen-specific enzyme (α-glucosidase) |
| Alteplase (Activase) | Recombinant human tissue-type plasminogen activator |
| Anakinra (Kineret) | Nonglycosylated interleukin-1 receptor antagonist |
| Antithrombin alfa (ATryn) | Recombinant human antithrombin III |
| Becaplerin (Regranex) | Recombinant human platelet-derived growth factor |
| Darbepoetin alfa (Aranesp) | Recombinant human erythropoietin (modifed by the addition of two carbohydrate chains) |
| Drotrecogin alfa (Xigris) | Recombinant activated human protein C |
| Ecallantide (Kalbitor) | Recombinant human reversible inhibitor of plasma kallikrein |
| Epoetin (Epogen) | Recombinant human erythropoietin |
| Factor IX (Benefix) | Recombinant human coagulation factor IX |
| Factor VIIa (NovoSeven-RT) | Activated recombinant human coagulation factor VII |
| Factor VIII, B domain deleted (Xyntha) | Recombinant human coagulation factor VIII with deletion of the B domain |
| Factor VIII, full length (Recombinate, Helixate, Kogenate, Advate) | Recombinant human coagulation factor VIII (antihemophilic factor) |
| Idursulfase (Elaprase) | Recombinant human iduronate-2-sulfatase |
| Interferon alfacon-1 (Infergen) | Recombinant hybrid of human interferon alpha |
| Interferon gamma 1B (Actimmune) | Recombinant human interferon gamma |
| Interferon beta (Betaseron, beta-1b; Avonex, Rebif, beta-1a) | Recombinant human interferon beta |
| Laronidase (Aldurazyme) | Recombinant human lysosomal glucogen-specific enzyme (l-iduronidase) |
| Naglazyme (Galsulfase) | Recombinant human lysosomal enzyme (N-acetylgalactosamine-4-sulfatase) |
| Oprelvekin (Neumega) | Recombinant human interleukin-11 (thrombopoietic growth factor) |
| Palifermin (Kepivance) | Recombinant analog of human keratinocyte growth factor |
| Pegfilgrastim (Neulasta) | Covalent conjugate of filgrastim and monomethoxypolyethylene glycol |
|
|
|
TABLE C-3 Continued
|
|
|
| Biologic | Description |
|
|
|
| Peginterferon alfa (Pegasys [alfa-2a]; PegIntron [alfa-2b]) | Recombinant human interferon alpha covalently bound to polyethylene glycol monomethoxy ether |
| Pegloticase (Krystexxa) | Pegylated recombinant human uric acid-specific enzyme |
| Rasburicase (Elitek) | Recombinant human of urate oxidase |
| Reteplase (Retavase) | Recombinant human tissue-type plasminogen activator |
| Tenectaplase (TNKase) | Recombinant human tissue-type plasminogen activator |
| Thrombin alfa (Recothrom) | Recombinant human thrombin |
|
|
|
SOURCES: Burnouf (2011), McEvoy (2011), Wickersham (2011).
CLINICAL PHARMACOLOGY OF BIOLOGICS
Well-established pharmacokinetic data for many drugs and biologics for the pediatric population are lacking. FDA has recognized the paucity of pediatric pharmacokinetic data and in response published draft guidance for industry in 1998 (FDA, 1998). The focus of the guidance was to elaborate on the pharmacokinetic information needed to determine appropriate medication doses in the pediatric population across all age groups, from neonates to adolescents. This determination is of particular concern in pediatrics because of growth and developmental changes that influence the absorption, distribution, metabolism, and excretion of drugs and biologics. Within the guidance, FDA recommended that pediatric pharmacokinetic studies evaluate how dosage regimens should be adjusted to attain “approximately the same level of systemic exposure that is safe and effective in adults” (FDA, 1998, p. 4).
If pediatric pharmacokinetic data are lacking for traditional drugs, these data are even scarcer for biologics, including monoclonal antibodies, although published data continue to expand (Dirks and Meibohm, 2010; Keizer et al., 2010). Monoclonal antibodies are immunoglobulins, which are used to treat a wide range of illnesses. Although there are five separate types of immunoglobulins in humans: immunoglobulin A (IgA), IgD, IgE, IgG, and IgM. An estimated 80 percent of all antibodies in humans are of the IgG family; all approved therapeutic monoclonal antibodies are of this family as well (Keizer et al., 2010).
The primary route of administration for approved monoclonal antibodies is intravenous (IV); however, some agents may be administered via
the subcutaneous (SC) or intramuscular (IM) route (Keizer et al., 2010). Absorption via these secondary routes is facilitated by the lymphatic system, which often results in low to intermediate bioavailability. Peak concentrations in serum generally do not occur until a few days after SC or IM administration because of slow absorption into the systemic circulation. Effective systemic therapy with monoclonal antibodies via the oral route is not currently possible because of their size, polarity, and the occurrence of gastrointestinal degradation. Monoclonal antibodies generally have low volumes of distribution primarily because of their large size and hydrophilic nature. Also, their bulky molecular size does not allow urinary excretion. Rather, monoclonal antibodies are metabolized to peptides and amino acids that are then either reused by the body or excreted by the kidney. The specific mechanisms of elimination of monoclonal antibodies are not well understood. In pediatric populations, specific pharmacokinetic parameters for monoclonal antibodies are not well studied.
The clearance of monoclonal antibodies from the body may be lengthened through a process called pegylation (i.e., the attachment of polyethylene glycol polymer chains to another molecule like a drug or therapeutic protein). Prolonging the half-life may allow reduced dosing or less frequent administration; however, this manipulation may also cause increased toxicities, such as a greater risk of allergic reactions. The formation of antibodies against monoclonal antibodies can have a significant impact on their efficacy in pediatric populations through effects on pharmacokinetics. The development of anti-monoclonal antibodies has been linked to a reduction in levels in serum and an increase in antibody clearance correlating to a reduced clinical response (Keizer et al., 2010).
For plasma-derived therapeutics, such as hemophilia factor concentrates and immune globulin intravenous (IGIV),2 more specific, yet limited, pediatric pharmacokinetic data are available. In the pediatric population, both the clearance and volume of distribution of factor concentrates appear to increase with age and body weight (Bjorkman and Berntrop, 2001). In neonates administered IGIV for prevention of infection, the estimated elimination of IGIV was found to be quite prolonged: 16 to 36 days across various studies (Koleba and Ensom, 2006). In 2008, the FDA published guidance regarding safety, efficacy, and pharmacokinetic studies to support marketing of IGIV as replacement therapy for primary humoral immunodeficiency (FDA, 2008). Within this guidance, the FDA recommended that “if possible and needed, the pharmacokinetic study of an IGIV product should be conducted across all pediatric age groups” (p. 10).
![]()
2 Although immune globulin intravenous (IGIV) is the official name of these products, many clinicians continue to refer to these plasma-derived therapeutics as intravenous immune globulin (IVIG).
SAFETY CONCERNS IN PEDIATRIC POPULATIONS
Plasma-derived proteins such as coagulation factors and IGIV are commonly used to treat hemophilia and immune deficiency disorders in children, respectively. Historically, the major safety concern with these proteins was the risk of blood-borne infections; however, donor screening, improved testing methods (e.g., nucleic acid amplification), and viral inactivation procedures in the manufacturing process have made the potential for infection less of a concern (Tarantino et al., 2007; Radosevich and Burnouf, 2010). Today, there are different safety concerns with each of these products.
For pediatric patients with hemophilia, inhibitor development may be a serious roadblock to successful therapy. An inhibitor is a type of antibody, and in the case of hemophiliacs, these antibodies attach to coagulation factor VIII or factor IX and inhibit the ability of the factor to stop bleeding (DiMichele, 2008). As opposed to patients without inhibitors, hemophiliacs who develop inhibitors to factor products experience orthopedic and life-threatening bleeding complications more frequently because of the difficulties with the treatment of such patients (DiMichele, 2008). In addition, these individuals experience more disability in their everyday activities (DiMichele, 2008).
A variety of potential safety concerns arise with the administration of IGIV, with infusion-related reactions (arising from the triggering of an inflammatory response by components within an IGIV preparation) of various severities being the most common (Duhem et al., 1994; Nydegger and Sturzenegger, 1999). These reactions are often mild, self-limiting, and more common in IGIV-naïve patients and generally occur within 30 to 60 minutes after the start of an infusion. This reaction may manifest itself clinically as a low-grade fever, chills, mild headache, myalgias, and backache. Anaphylactic reactions occur rarely (<5 percent of IGIV recipients) and are most commonly observed in patients with IgA deficiency. The use of products that contain large amounts of IgA should be avoided in these patients (Nydegger and Sturzenegger, 1999).
Other rare, but serious, adverse events that can occur with IGIV administration include renal failure, aseptic meningitis, hemolysis, transfusion-related acute lung injury, and thrombotic events. Renal failure most commonly occurs with the use of sucrose-containing IGIV products (Epstein and Zoon, 1999).
Long-term safety concerns for certain biologics—in particular, the chronic administration of human tumor necrosis factor (TNF) inhibitors such as adalimumab, etanercept, and infliximab—may be quite serious (Hashkes et al., 2010). These concerns, which are controversial, include the possible occurrence of malignancies; an increased risk of serious infections; and the development of autoimmune phenomena such as demyelinating
disease, autoantibodies, uveitis, lupus-like syndrome, inflammatory bowel disease, and psoriasis. A search of FDA’s Adverse Event Reporting System (through April 29, 2008) revealed 48 cases of malignancy among pediatric patients prescribed TNF inhibitors, primarily for inflammatory bowel disease (Diak et al., 2010). Although the reported malignancy rates among children who received infliximab and etanercept were found to be higher than the background rates in the general pediatric population, a clear causal connection could not be established due to confounding factors such as concurrent immunosuppressant therapy and the potential risk of malignancy associated with underlying illnesses.
Administration of TNF inhibitors had been associated with an increase in granulomatous infections, particularly tuberculosis, prior to the widespread implementation of pretreatment screening and administration of appropriate prophylactic medications (Keane et al., 2001; Wallis et al., 2004; Hashkes et al., 2010). Reports of such infections in children administered these agents have subsequently decreased since 2000, with only a few case reports demonstrating development of tuberculosis (Myers et al., 2002; Armbrust et al., 2004) and histoplasmosis (Lee et al., 2002) being published.
Because of the complex effects of TNF in the immune system, inhibition may lead to autoimmune phenomena, including the development of autoimmune disorders for which TNF inhibitors are standard treatments, though a definitive association of autoimmune disorders with TNF inhibitors has not been shown. Published case reports have documented the occurrence of a variety of these phenomena in children prescribed TNF inhibitors, including psoriasis (Peek et al., 2006), demyelination (Mohan et al., 2001), uveitis (Hashkes and Shajrawi, 2003), autoantibody development (Kanakoudi-Tsakalidou et al., 2008), diabetes mellitus (Bloom, 2000), systemic lupus erythematosus (Lepore et al., 2003; Bout-Tabaku et al., 2007), autoimmune hepatitis (Fathalla et al., 2008), and Crohn’s disease (Ruemmele et al., 2004; Wiegering et al., 2010).
Infusion or injection-site reactions are common with administration of TNF inhibitors and other biologics such as interleukin-1 receptor antagonists (i.e., anakinra) and fusion proteins (Hashkes et al., 2010). Injection-site reactions (erythema, pruritus, pain, edema) occur frequently with the TNF inhibitors etanercept and adalimumab (28 to 39 percent) but do not often result in discontinuation of therapy. In contrast, infusion-related reactions with infliximab (fever, chills, dyspnea, urticaria, and hypotension, which may be due to anaphylaxis or the development of antibodies to infliximab) have been reported to result in cessation of therapy in approximately 20 percent of pediatric patients with juvenile idiopathic arthritis in a long-term prospective study (Gerloni et al., 2008).
BIOLOGICS AND DISEASES AFFECTING CHILDREN
Although pediatric diseases are often acute and self-limiting (e.g., otitis media, respiratory infections, and gastrointestinal illnesses), some children develop chronic health problems such as asthma, diabetes, cystic fibrosis, obesity, malnutrition, cerebral palsy, behavioral disorders such as attention deficit hyperactivity disorder and autism, mental illnesses such as depression, and consequences of low birth weight and prematurity (i.e., retinopathy, chronic lung disease, and developmental delays) (Torpy et al., 2010).
For the majority of these conditions, biologics are not currently employed as treatment options. However, there are exceptions, including insulin—a product not regulated by FDA as a biologic but originally derived from animal sources and now through recombinant methods—for the treatment of diabetes and omalizumab (Xolair), a monoclonal antibody, approved for use as an adjunctive therapy in moderate to severe persistent asthma in patients 12 years of age and older (National Heart, Lung, and Blood Institute, 2007). Other biologics are being studied for the treatment of some of the conditions listed above, for example, forms of botulinum toxin for spasticity in cerebral palsy (see Appendix D).
The following sections review data on the use of biologics for the treatment of selected conditions in the pediatric population. The section on treatment of choice briefly reviews pediatric disorders in which a biological agent is the primary or preferred treatment option. The section on potential therapeutic options covers a variety of disease states for which biologics are either approved alternative agents or for which data are fairly limited. The goal of the latter section is not only to identify current pediatric biologic-related data in major branches of medicine (rheumatology, dermatology, gastroenterology, oncology, and endocrinology) but also to discuss gaps in our current clinical knowledge and use of these agents in pediatrics.
Biologics as the Treatment of Choice for Certain Disorders
Some diseases, although not widespread among children, commonly employ biologics, particularly plasma-derived or recombinant products, as treatment options. Examples of these diseases include hemophilia/bleeding disorders, immune deficiency syndromes, Kawasaki disease, and immune thrombocytopenic purpura (ITP).
Hemophilia
Hemophilia is a chromosome X-linked congenital bleeding disorder (World Federation of Hemophilia Guidelines, 2005). Globally, the number of affected individuals is approximately 400,000. In the United States, the
Centers for Disease Control and Prevention (CDC) reports that hemophilia occurs in about 1 in 5,000 male births and that about 400 infants are born with the condition annually. Currently, the CDC estimates that about 20,000 people in the United States have hemophilia (Soucie et al., 1998).
The disease is caused by a deficiency in coagulation factors, specifically, factor VIII deficiency (hemophilia A) or factor IX deficiency (hemophilia B), with hemophilia A accounting for about 80 to 85 percent of all diagnoses (World Federation of Hemophilia Guidelines, 2005). Prevention and treatment of bleeding in individuals with hemophilia are accomplished through the administration of plasma-derived or recombinant products that supply these deficient factors. Although hemophilia qualifies as a rare disease under the Orphan Drug Act and many antihemophilic biologics have orphan drug designations and are exempt from the Pediatric Research Equity Act, all of the products that are listed in Appendix D are labeled for pediatric use for at least one indication.
The World Federation of Hemophilia guidelines state that two issues deserve special consideration when a choice regarding factor replacement therapy is made for patients with hemophilia: product purity and viral inactivation/elimination (World Federation of Hemophilia Guidelines, 2005). Although no classification of factor products based on purity is universally agreed upon, high-purity factor IX products are preferable for the treatment of hemophilia B because of a reduced risk of thromboembolic complications compared with the risk associated with the use of other plasma-derived products, such as prothrombin complex concentrates. The purity of the factor VIII product does not appear to enhance safety for patients with hemophilia A; therefore, this product characteristic does not affect factor VIII product selection.
With regard to viral inactivation/elimination, the World Federation of Hemophilia simply states that plasma quality and testing of the factor concentrate should definitely be considered but does not firmly recommend a particular coagulation factor product as being a safer option. In addition, the federation “does not express a preference for recombinant over plasma-derived concentrates and the eventual choice between these classes of product will be made according to local criteria” (World Federation of Hemophilia Guidelines, 2005, p. 31). This is in contrast to the United Kingdom Hemophilia Center Doctors’ Organization (UKHCDO) guideline on the selection and use of therapeutic products to treat hemophilia and other hereditary bleeding disorders (Keeling et al., 2008). The UKHCDO document specifically recommends recombinant factor VIII and factor IX as the treatments of choice for hemophilia A and B, respectively. This recommendation is due to a theoretical reduced risk of infectious agent transmission with recombinant products.
Primary Immune Deficiency Syndromes
Primary immune deficiency syndromes are inherited disorders that can result in an increased rate and severity of infection, immune dysregulation with autoimmune disease, and malignancy (Bonilla et al., 2005). More than 100 different genetic disorders that affect immune function have been identified, occurring in as many as 1 in 2,000 live births. For certain immune deficiencies, such as severe combined immunodeficiency or complement/phagocyte defects, bone marrow transplantation is the primary treatment option.
For other primary syndromes, such as common variable immunodeficiency, chromosome X-linked agammaglobulinemia, or autosomal recessive agammaglobulinemia, administration of IGIV or subcutaneous immunoglobulin is the treatment of choice. These products may also be used as adjunctive therapies in other situations (Bonilla et al., 2005; Roifman et al., 2008). A variety of IGIV products for treatment of primary immune deficiency syndrome are currently commercially available. Although they are often used interchangeably, the components of some IGIV products may be contraindicated in patients with certain medical conditions. For example, some IGIV formulations contain sucrose, which may contribute to the development or progression of renal insufficiency or failure (Siegel, 2010). In neonates, additional concerns exist regarding minimizing fluid volume and the pH and osmolarity of the IGIV solution. All of these factors must be taken into consideration when an appropriate IGIV product is chosen.
Kawasaki Disease
Kawasaki disease is an acute, self-limiting vasculitis of childhood that is most prevalent in Japan and among children of East Asian ancestry (Newburger et al., 2004). Approximately 4,250 Kawasaki disease-related hospitalizations occurred in the United States in 2000, with the median age at the time of diagnosis being 2 years. Symptoms of the disease include fever, bilateral nonexudative conjunctivitis, erythema of the lips and oral mucosa, changes in the extremities, rash, and cervical lymphadenopathy. An estimated 15 to 25 percent of untreated children develop coronary artery aneurysms or ectasia, which may subsequently lead to ischemic heart disease or sudden death.
The American Academy of Pediatrics (AAP) and American Heart Association (AHA) recommend IGIV, in combination with aspirin, as the first-line treatment for children with Kawasaki disease (Newburger et al., 2004). The AAP/AHA statement on management of Kawasaki disease specifically states that “the results of clinical studies comparing the efficacy of immune globulin products have conflicted, with most studies failing to find
a significant difference between brands” (Newburger et al., 2004, p. 1720). The choice of IGIV product basically comes down to patient and product characteristics, similar to IGIV selection for primary immune deficiency syndrome.
Immune Thrombocytopenic Purpura
Immune thrombocytopenic purpura (ITP) is an autoimmune disorder characterized by a low platelet count. The disease is often classified on the basis of the age of the patient (child versus adult), illness duration (acute versus chronic), and underlying disorder (primary versus secondary) (Blanchette and Bolton-Maggs, 2010). In children, the diagnosis of ITP is often one of exclusion (Provan et al., 2010). With acute ITP (low platelet counts for ages <6 months), children present with sudden onset of bruising or petechial rash, often preceded by an acute infectious illness such as an upper respiratory infection (Blanchette and Bolton-Maggs, 2010). Approximately 20 to 25 percent of these children will continue on to chronic ITP (i.e., low platelet counts for longer than 6 months after the initial diagnosis). Clinically significant symptoms of ITP are not common but may include severe epistaxis, gastrointestinal bleeding, and intracerebral hemorrhage (Provan et al., 2010). A recent international consensus report on the management of primary ITP recommended plasma-derived therapeutics, IGIV, and IV anti-D immunoglobulin as first-line treatment options in children when therapy is warranted (Provan et al., 2010).
Potential Therapeutic Options for Other Selected Pediatric Disorders
Rheumatology: Juvenile Idiopathic Arthritis
Juvenile idiopathic arthritis (JIA) is defined by the International League of Associations for Rheumatology as “arthritis of unknown etiology that begins before the 16th birthday and persists for at least 6 weeks” (Petty et al., 2004, p. 390). JIA encompasses a group of heterogeneous arthritic conditions, including systemic arthritis, oligoarthritis, polyarthritis, psoriatic arthritis, enthesitis-related arthritis, and undifferentiated arthritis. In Europe and North America, the incidence of JIA is estimated to be approximately 10 to 19 cases per year for every 100,000 children (Gare, 1999). Globally, prevalence rates for juvenile arthritis vary widely, from 0.07 to 4.01 per 1,000 children, because of various factors, including differing case definitions and development of new diagnostic criteria (Manners and Bower, 2002).
Four biological agents have been approved by the FDA for treatment of moderate to severe JIA. These include
• Etanercept (Enbrel)—approved for polyarticular JIA in patients 2 years of age or older (Amgen, 2011)
• Adalimumab (Humira)—approved for treatment of polyarticular JIA in patients 4 years of age and older (Abbott Laboratories, 2011)
• Abatacept (Orencia)—approved for treatment of polyarticular JIA in patients 6 years of age and older as monotherapy or in combination with methotrexate (Bristol-Myers Squibb, 2009)
• Tocilizumab (Actemra)—approved for treatment of systemic JIA in patients 2 years of age and older (Genentech, 2011)
Etanercept was the initial biologic to receive approval for treatment of polyarticular JIA in children on the basis of results from a randomized, double-blind, multicenter study involving 69 patients (ages 4 to 17 years) (Lovell et al., 2000). The study design involved up to 3 months of open-label etanercept therapy, followed by a double-blind period where patients were randomly assigned to receive either etanercept or placebo for 4 months or until disease flare occurred. Response to therapy during the open-label period was defined as an improvement of 30 percent or greater in at least three of six disease activity indicators, with no greater than one indicator worsening by more than 30 percent. Of the 69 pediatric patients enrolled in the open-label phase, 51 (74 percent) achieved a response to etanercept therapy and were then randomized to receive etanercept or placebo during the double-blind period. Results from the double-blind phase revealed that significantly more patients administered placebo than those receiving etanercept withdrew because of a disease flare (81 versus 28 percent). In addition, etanercept therapy was associated with a significantly longer median time to disease flare (116 versus 28 days). No significant differences in the frequency of adverse effects were observed between etanercept and placebo. In a long-term follow-up of patients from the original trial who continued on open-label etanercept, the efficacy and safety of etanercept were maintained for up to 8 years (Lovell et al., 2008a). No cases of serious adverse events such as lupus, demyelinating disorders, malignancies, or lymphomas were reported; nine medically important infections were seen over this time period, translating to an exposure-adjusted rate of 0.03 events per patient-year.
The efficacy and safety of adalimumab for JIA were established through the results of a randomized, double-blind, stratified, placebo-controlled study enrolling 171 pediatric patients (ages 4 to 17 years) (Lovell et al., 2008b). The study design consisted of a 16-week open-label lead-in phase (during which patients were stratified according to methotrexate use and all received adalimumab therapy), followed by a 32-week double-blind withdrawal phase and then an open-label extension. After the lead-in phase,
133 patients fulfilling the American College of Rheumatology (ACR) Pediatric (Pedi) 30 response criteria were randomly assigned to receive either adalimumab or placebo for 32 weeks. The primary outcome measure was disease flare occurrence during the double-blind period in the group of patients not receiving methotrexate. Results revealed disease flares to be less common among patients receiving adalimumab regardless of concurrent methotrexate use (43 versus 71 percent in patients not receiving methotrexate and 37 versus 65 percent in patients administered methotrexate). The number of pediatric patients who had ACR Pedi 30, 50, 70, or 90 responses was significantly greater for those receiving adalimumab in combination with methotrexate than those receiving methotrexate therapy alone. The differences between patients who received adalimumab but not treated with methotrexate and those who received placebo were not significant. Response rates were sustained even after 104 weeks of open-label extension therapy, with 40 percent of children experiencing an ACR Pedi 100 response. Fourteen serious adverse events were determined to be possibly related to adalimumab, including seven serious infections (e.g., bronchopneumonia, herpes simplex, pharyngitis, pneumonia, and herpes zoster).
Abatacept was found to be an effective and safe treatment for JIA in a double-blind, randomized, controlled, withdrawal, multicenter trial enrolling 190 pediatric patients (ages 6 to 17 years) with a similar design to the previous studies (Ruperto et al., 2008). After the lead-in phase, 122 children who achieved an ACR Pedi 30 response were
randomly assigned to receive abatacept or placebo for 6 months or until a flare of arthritis occurred. Results revealed that arthritic flares occurred more frequently with placebo than abatacept therapy (53 versus 20 percent). In addition, the risk of disease flare during the double-blind period for patients administered abatacept was less than a third of that for controls. The frequency of adverse events was similar between the groups, with only two serious adverse events being reported, and both of these occurred in the placebo group.
Tocilizumab is the most recent biological agent to receive approval by the FDA for JIA and was approved specifically for the subset of patients with systemic JIA. This approval was based upon results from a double-blind, placebo-controlled trial enrolling 112 children ages 2 to 17 years with systemic JIA who had an inadequate response or who were unable to take nonsteroidal anti-inflammatory drugs and corticosteroids. Eighty-five percent of patients receiving tocilizumab but only 24 percent of patients receiving placebo experienced at least an ACR Pedi 30 response along with absence of fever in the preceding 7 days (Genentech, 2011). The most commonly reported serious infections included pneumonia, gastroenteritis, varicella, and otitis media. This trial was supported by a randomized withdrawal trial enrolling 56 children (ages 2 to 19 years) with disease refractory to conventional treatments (Yokota et al., 2008). Patients were randomly assigned to tocilizumab or placebo for 12 weeks or until withdrawal for rescue medication, following a 6-week open-label phase to determine responders. The primary outcome measure of the double-blind phase was an ACR Pedi 30 response and C-reactive protein concentrations of <15 mg/L. Results for the 43 patients in the double-blind phase revealed that significantly more patients receiving tocilizumab than patients receiving placebo met the primary endpoint (80 versus 17 percent). Continued efficacy of tocilizumab was noted through week 48 of the open-label extension phase. Gastroenteritis, bronchitis, and anaphylactoid reaction were among the serious adverse events reported in the study.
Beyond the approved agents, studies have been conducted with other biologics, including infliximab (Ruperto et al., 2007) and anakinra (Quartier et al., 2011). Ruperto and colleagues (2007) concluded that the combination of various doses of infliximab and methotrexate produced a rapid, durable response in children with polyarticular JIA at 1 year; however, the primary endpoint of the study (ACR Pedi 30 at week 14) did not reveal a significant difference between infliximab and placebo (Ruperto et al., 2007). Less positive results were also seen with anakinra therapy for systemic JIA, which has an extremely difficult to treat systemic inflammatory component compared with polyarticular JIA (Quartier et al., 2011). After 1 month of treatment, a significantly higher proportion of responders was found among those receiving anakinra therapy than those receiving placebo; however, a loss of response was seen with most patients over time.
In contrast to many other pediatric diseases, several biologics are approved for use by pediatric patients with JIA; however, various unanswered, challenging questions that can be addressed only through rigorous clinical trials remain. These issues include the following (Pain and McCann, 2009):
• Which biologic is most beneficial in which JIA subgroup?
• What is the benefit, if any, of changing biologics if a TNF inhibitor fails?
• What is the duration of biologic therapy for children with JIA? Would children benefit from gradual dose reduction or frequency of administration if they were on long-term therapy?
• Should the biologic with the most efficacy and safety data, etanercept, be used only for refractory disease, or should it be considered for use as an initial treatment?
• Since no head-to-head trials of biologics in patients with JIA have been conducted, should such a trial be completed?
Dermatology: Atopic Dermatitis
Atopic dermatitis is a chronic, relapsing, eczematous skin disease generally characterized by pruritus and inflammation (Saeki et al., 2009). It is
one of the most common skin disorders among children, with a prevalence of 10 to 20 percent in the United States (Spergel, 2010). Most children appear to develop symptoms of the disease early in life (age <2 years). Historically, atopic dermatitis was thought to be a disease that spontaneously resolved; however, more recent studies have found that 50 percent of patients continue to have intermittent symptoms until 7 years of age and others will continue to manifest symptoms into adulthood. In addition, children with atopic dermatitis are more likely to be diagnosed with other atopic diseases such as asthma or allergic rhinitis.
Treatment of mild to moderate atopic dermatitis generally involves the use of emollients alone or in combination with other topical therapies (Bremmer et al., 2009; Saeki et al., 2009); however, severe, persistent disease often requires systemic treatments or phototherapy. No systemic agents, including biologics, have been approved for the treatment of atopic dermatitis in children. Many of the currently available systemic therapies for severe disease have potential toxicities and modest efficacy; therefore, biologics may be another option for children with severe atopic dermatitis, though none have been approved by the FDA as safe and effective treatments (Bremmer et al., 2009).
Clinical data about the use of biologics for treatment of atopic dermatitis are limited to case reports and small open-label pilot studies for both pediatric and adult populations (Buka et al., 2005; Jacobi et al., 2005; Krathen and Hsu, 2005; Lane et al., 2006; Vigo et al., 2006; Weinberg and Siegfried, 2006; Hassan et al., 2007; Siegfried, 2007; Takiguchi et al., 2007; Moul et al., 2008; Simon et al., 2008). In the pediatric-specific reports, results with biologic therapy (i.e., efalizumab, omalizumab, and etanercept) have varied (Buka et al., 2005; Vigo et al., 2006; Weinberg and Siegfried, 2006).
Dermatology: Psoriasis
Psoriasis is a common, chronic disease that predominantly affects the skin and joints; approximately 7.5 million people in the United States are affected (Menter et al., 2008; National Psoriasis Foundation, 2011). Many different types of psoriasis exist, including plaque (occurring in approximately 80 to 90 percent of people with psoriasis), inverse, erythrodermic, pustular, and guttate (Menter et al., 2008). The primary clinical manifestation of psoriasis is disfiguring, scaling, and erythematous skin plaques that may be painful or pruritic. Plaques may occur anywhere on the body but are most commonly seen on the elbows, knees, scalp, buttocks, and lower back. The disease can range in severity from mild to severe, with symptoms improving or worsening over time. An estimated 80 percent of individuals with psoriasis have mild to moderate disease. This form of the disease is often effectively managed with localized topical therapies. The remaining
20 percent have moderate to severe disease, which may often require the use of biologic therapy.
Although psoriasis can occur at any age, the disease is more common in individuals between 15 and 30 years of age and then later in life between the ages of 50 and 60 years (Levine and Gottlieb, 2009). Data on the incidence of psoriasis among children are limited; however, a recent population-based retrospective study found the overall age-and sex-adjusted annual incidence of pediatric psoriasis to be 40.8 per 100,000 (Tollefson et al., 2010). In addition, the incidence of psoriasis among children was found to increase significantly over time: 29.6 per 100,000 (1970 to 1974) to 62.7 per 100,000 (1995 to 1999). The most common type of psoriasis reported was plaque psoriasis (74.7 percent), followed by guttate psoriasis (14 percent). Although the exact incidence of moderate to severe psoriasis among the pediatric population is unknown, it has been reported that approximately 8 percent of pediatric patients with psoriasis require phototherapy or systemic medications (Sukhatme and Gottlieb, 2009). The onset of psoriasis in childhood does not always lead to persistence into adulthood and is not correlated with severity of disease in adult life.
In addition to the cutaneous manifestations, psoriasis has been associated with several nondermatological comorbidities, including arthritis, cardiovascular disease, diabetes, obesity, Crohn’s disease, and depression (Sukhatme and Gottlieb, 2009; Marji et al., 2010). For pediatric patients with moderate to severe psoriasis, the emotional and psychological impact of this chronic disease cannot be overestimated. Currently, none of the available biologics is approved for use by children with moderate to severe psoriasis. In 2008, an FDA advisory panel voted to recommend approval of etanercept for treatment of moderate to severe plaque psoriasis in children and adolescents unresponsive to other therapies. However, the manufacturer (Amgen) declined to continue with the approval process.
The advisory panel recommendation was based only on data from a multicenter study involving 211 patients (ages 4 to 17 years) with moderate to severe plaque psoriasis (Paller et al., 2008). The study design included three phases: an initial 12-week randomized, double-blind, placebo-controlled treatment period, followed by a 24-week open-label treatment period and, finally, another 12-week randomized, double-blind, withdrawal-retreatment period. The primary endpoint was a composite of 75 percent or greater improvement from baseline in the psoriasis area and severity index (PASI 75) at week 12. Results revealed that significantly more patients receiving etanercept than patients receiving placebo achieved PASI 75 (57 versus 11 percent; p < 0.001) at week 12. During the open-label treatment period, 62 percent of patients who were originally administered placebo and 69 percent who continued to receive etanercept from study initiation achieved PASI 75 at week 24. This level of response was maintained
through week 36. During the final 12-week withdrawal-retreatment phase, response was lost in 29 (42 percent) of the 69 patients randomly assigned to placebo. These pediatric patients were then retreated with etanercept, with response rates being similar to those for patients initially treated with the biologic. Evaluation of safety concerns showed that the rates of infectious and noninfectious adverse events were similar between etanercept and placebo. Only a few serious adverse infectious events in patients on etanercept were noted: a 7-year-old with a history of asthma was treated with IV antibiotics for left basilar pneumonia, and a 9-year-old experienced concurrent serious episodes of gastroenteritis. No cancers, opportunistic infections, tuberculosis, or demyelination events were reported, though subjects were monitored for only 48 weeks.
The remaining published clinical data involving etanercept for the treatment of pediatric psoriasis are from single case reports or case series (Hawrot et al., 2006; Kress, 2006; Papoutsaki et al., 2006; Safa et al., 2007; Floristan et al., 2011). The outcomes of these reports have primarily been favorable, with improvement of severe disease and only minor adverse events being reported. Data are even more limited or nonexistent for other biologics. Currently, only two case reports of infliximab administration in pediatric patients with psoriasis have been published (Menter and Cush, 2004; Farnsworth et al., 2005). One of these reports details the successful use of infliximab following a failed treatment course of etanercept (Farnsworth et al., 2005).
Of the dermatologic conditions, no controlled trials involving biologic therapies for severe atopic dermatitis have been published. Published data primarily involve case reports with various outcomes with the biologic administered. More data are available for biologic administration for psoriasis; however, basic gaps in our understanding remain, including the place in therapy for biologics (i.e., should these agents be used only after other systemic treatments); appropriate dosing regimens; and long-term safety concerns, including the potential increased risk of lymphoma and other cancers in children and adolescents administered TNF inhibitors (FDA, 2009b).
Gastroenterology: Inflammatory Bowel Disease
Crohn’s disease and ulcerative colitis, the two major types of inflammatory bowel diseases (IBDs), are both chronic conditions manifesting with exacerbation and remission of severe diarrhea, rectal bleeding, and abdominal pain (Shikhare and Kugathasan, 2010). The prevalence of IBD among children and adolescents in the United States was reported by Kappelman and colleagues and was based on a 2007 survey that collected data from 87 health plans in 33 states (Kappelman et al., 2007). For Crohn’s disease, the rates for children ages 2 to <5 years and 5 to <10 years were 2.3 and
9.4 per 100,000, respectively. For ulcerative colitis, the corresponding rates were 5.4 and 8.5 per 100,000, respectively. A considerable increase in the prevalence of these IBDs was noted after the age of 10 years, at 45 and 22 per 100,000 for Crohn’s disease and ulcerative colitis, respectively, with a further increase to 85 and 58 per 100,000, respectively, for individuals ages 15 to 20 years. In 2010, Abramson and colleagues published the results of an 11-year study, reporting an increase in the incidence of both Crohn’s disease and ulcerative colitis among pediatric patients enrolled in a community-based health care system (Abramson et al., 2010). The incidence of Crohn’s disease rose from 2.2 to 4.3 per 100,000, and that of ulcerative colitis rose from 1.8 to 4.9 per 100,000.
Both pediatric-onset Crohn’s disease and pediatric-onset ulcerative colitis have characteristics different from those of adult-onset disease. For Crohn’s disease, disease at onset tends to be more severe in children than adults, necessitating a higher frequency of immunosuppressant use (Pigneur et al., 2010). In addition, the location of the disease at presentation differs from that for adult-onset disease for both Crohn’s disease and ulcerative colitis (Sauer and Kugasthasan, 2010). However, as with adult-onset IBD, pediatric-onset IBD is associated with gastrointestinal symptoms, extraintestinal manifestations, and negative long-term health outcomes. The development of chronic conditions, such as joint and biliary duct diseases, as well as adverse effects on growth and bone health, all contribute to a decreased quality of life for pediatric patients, which can persist into adulthood (Sawczenko et al., 2006; Pfefferkorn et al., 2009; Turunen et al., 2009; Dotson et al., 2010; Pappa et al., 2011).
Given the significant impact of IBD on pediatric patients, effective treatment to control exacerbations is essential, as is minimizing the need for corticosteroids, which can further impede normal growth and development (Griffiths, 2009). Biologics have been shown to be an effective treatment for IBD in adults. A recent meta-analysis evaluating 27 double-blind trials (4,526 adult patients) on the use of biologics in the treatment of IBD found these agents to be effective in inducing and maintaining remission in luminal Crohn’s disease and in inducing remission in ulcerative colitis (Ford et al., 2011).
However, of the biologics approved for the treatment of IBD in adults, only one is indicated for treatment of IBD (both Crohn’s disease and ulcerative colitis) in pediatric patients: infliximab (Centocor Ortho Biotech, Inc., 2011). Data for the indication for Crohn’s disease are primarily from the REACH trial, an open-label study enrolling 112 pediatric patients with moderate to severe, active Crohn’s disease (Hyams et al., 2007; Centocor Ortho Biotech, Inc., 2011). Outcomes with long-term use of infliximab were also reported by the Pediatric Inflammatory Bowel Disease Collaborative Research Group (Hyams et al., 2009). Sustained clinical response and
remission rates ranged from 64 to 83 percent and from 26 to 44 percent, respectively, during each year of follow-up. For ulcerative colitis, labeling for infliximab indicates that the efficacy of the biologic for pediatric patients is based on the results of adult clinical trials, with safety and pharmacokinetic data obtained from a study enrolling 60 pediatric patients (Centocor Ortho Biotech, Inc., 2011).
In addition to disease remission, use of infliximab was associated with improvement in markers of bone turnover, on the basis of results from an open-label extension of the REACH trial (Thayu et al., 2008). Walters and colleagues reported infliximab to increase height velocity and stature in a small group of children treated for refractory Crohn’s disease (Walters et al., 2007).
The other biologic that has been evaluated in children is adalimumab, although data are limited. A retrospective review (the RESEAT trial) of data from the Pediatric Inflammatory Bowel Disease Collaborative Research Group included 115 patients who were given at least one dose of adalimumab (Rosh et al., 2009). Clinical response rates with adalimumab at 3, 6, and 12 months were 65, 71, and 70 percent, respectively. Remission rates for the same time points ranged from 32 to 49 percent. Similar findings were reported for a small prospective study enrolling 23 patients, 14 of whom had previously been treated with infliximab (Viola et al., 2009). The 12-, 24-, and 48-week remission rates were 30.5, 50, and 65 percent, respectively. Corresponding response rates were 79, 86, and 91 percent.
Although data on the use of infliximab and, to a limited extent, adalimumab in pediatric patients are available, the role of biologics in the treatment of IBD in children remains unclear. Several advantages of biologics (primarily infliximab) have been observed, including a corticosteroid-sparing effect and improvement in growth. However, the long-term effects of these agents remain unknown, and prospective studies have been called for to evaluate the infection-and malignancy-related risks of these agents in children when given for prolonged periods of time (Rosh, 2009).
Recent guidelines on the treatment of pediatric IBD are available from the IBD Working Group of the British Society of Paediatric Gastroenterology, Hepatology, and Nutrition. These guidelines follow the typical step-up approach to therapy, placing infliximab as third-line therapy (the same level as surgery) for Crohn’s disease, following failure of other agents such as corticosteroids and immunosuppressants (Sandhu et al., 2010). This approach, however, has been questioned. A top-down therapy with biologics early in the course of the disease has been suggested to reduce corticosteroid use, promote mucosal healing, avoid delays in growth, and improve quality of life in children (Cucchiara and Morelty-Fletchter, 2007; de Zoeten and Mamula, 2008).
The possible use of combination therapy with a biologic and immu-
nosuppressants (e.g., azathioprine or 6-mercaptopurine) has also been considered. In addition to improved efficacy, concomitant use of immunosuppressants might decrease the immunogenicity of biologics (Rosh, 2009). However, adverse outcomes, including fatal hepatosplenic T-cell lymphoma, have been reported with the combination of a biologic and an immunosuppressant in young patients with IBD, making assessment of the risks and benefits of combination treatment critical (Mackey et al., 2009).
All of these concerns on the use of biologics in pediatric-onset IBD can be addressed only with well-designed clinical trials with proper attention to dose finding and understanding of the issues of extrapolation of efficacy in adults to that in children.
Oncology: Childhood Cancers
Cancer is the leading medical cause of death among children and adolescents, with a reported mortality rate of 2.3 per 100,000 (Jemal et al., 2010). Leukemias account for approximately 30 percent of newly diagnosed childhood cancers, followed by brain/central nervous system (CNS) cancers at 21 percent. The survival rate for childhood cancers has increased over the past 25 years, from 58 percent in 1975 to 81 percent in 2005. However, despite this increase in survival, research on therapy specific to pediatrics is limited. Since children and adolescents account for only a small percentage (~1 percent) of all cancer diagnoses each year, most cancer research (including research on biologics) focuses on treatment of cancers commonly found in adults, such as breast, lung, colon, and prostate cancers (Jemal et al., 2010; Morgan, 2011).
Data from clinical trials in oncology usually cannot be extrapolated to the pediatric population because both the types and underlying biology of childhood cancers differ from those that occur in adults. The cytotoxic effects of conventional cancer therapies (e.g., alkylating agents, anthracyclines, and radiation) may have a greater impact on pediatric patients than adults and may manifest as chronic conditions (also referred to as “late effects”) in adulthood (Oeffinger et al., 2006). Oeffinger and colleagues conducted a retrospective study using a cohort of 10,397 adult childhood cancer survivors and 3,034 siblings without a history of cancer to determine the incidence and severity of chronic health conditions in these groups. Among adults who had survived a childhood cancer, 62 percent had at least one chronic health condition, whereas only 36 percent of siblings did. The chronic health condition was severe, life-threatening, or disabling in 27 percent of survivors, with a relative risk of 8.2 when the incidence was compared with that in the siblings. These conditions included major joint replacement, heart failure, second malignancy, cognitive dysfunction, coronary artery disease, renal failure or need for dialysis, noncorrectable hearing or vision impairments, and ovarian failure.
Biologics are among the most advanced treatments for cancers; however, the available biologics approved for use in cancer are primarily indicated for adult solid tumor cancers rather than the malignancies most common in pediatric patients (Wickersham, 2011). Although some of these agents may be used in clinical practice, data from controlled trials on use of these agents in pediatrics are limited; some biologics have been evaluated in small, usually Phase I or II studies.
Meinhardt and colleagues evaluated the efficacy of rituximab in addition to standard chemotherapy in the treatment of new-onset mature B-cell non-Hodgkin’s lymphoma or Burkitt leukemia in children and adolescents (Meinhardt et al., 2010). The primary outcome of the study was the response rate, with tolerability being a secondary outcome. A response (complete or partial) was seen in 41.4 percent of these patients. The frequent toxicities attributed to rituximab included rigors/chills, fatigue, hypotension, hematologic toxicities, infection, and nausea and vomiting. In addition, seven patients experienced an allergic reaction considered probably related to rituximab. The Children’s Oncology Group also evaluated rituximab in the pediatric population for treatment of recurrent or refractory B-cell non-Hodgkin’s lymphoma and mature B-cell acute lymphoblastic leukemia (Griffin et al., 2009). Sixty percent of patients had a response (complete or partial), a rate higher than the historical rate (51 percent) for usual chemotherapy without rituximab. Follow-up data reported that five patients were alive at 14 to 30 months following stem cell transplant, and four of these five patients were free of disease.
Bevacizumab has been studied in two trials for treatment of refractory solid tumors, including recurrent malignant gliomas (Glade-Bender et al., 2008; Gururangan et al., 2010). A Phase I trial enrolled 21 patients (median age, 13 years) with refractory solid tumors (excluding lymphomas or brain tumors) to evaluate the pharmacokinetics and safety of the agent (Glade-Bender et al., 2008). Although none of the treated patients experienced a response (either complete or partial), dose-limiting toxicities were not seen in any patients and treatment was generally well tolerated at doses of up to 15 mg/kg every 2 weeks. In the second trial, bevacizumab was ineffective for the treatment of recurrent malignant or diffuse brain stem gliomas (Gururangan et al., 2010).
Finally, Trippett and colleagues conducted a Phase I trial of cetuximab in children with refractory solid tumors, including CNS tumors (Trippett et al., 2009). Diarrhea and neutropenia were dose-limiting toxicities, and both were considered to be a result of the irinotecan used concomitantly. The maximum tolerated dose of cetuximab was found to be 250 mg/m2. Although not an objective of the study, an overall clinical benefit rate of 46.2 percent was seen among 26 patients with primary CNS tumors. The rate of anticetuximab antibody formation was also reported by the authors and was 4 percent, similar to findings for adults.
Targeted therapies with biologics have the potential to improve the prognosis of childhood cancers with historically poor outcomes (Bernstein, 2011). However, there are many unknowns regarding the use of biologics in childhood cancers. As noted above, cancers in children differ from those in adults, and these differences can alter the effects of biologics, in terms of both efficacy and adverse events. Additionally, exposure to conventional chemotherapy has long-term effects in adult survivors of childhood cancer. An important question for long-term investigation is whether exposure to biologics during childhood predisposes pediatric patients to adult-onset chronic conditions or to other cancers to a similar degree. In addition, the impact of biologics on the growth and development of children is unknown.
Endocrinology: Diabetes
Both type 1 and type 2 diabetes can have a significant impact on quality of life in children (American Diabetes Association, 2011). Although the incidence of type 2 diabetes in children is increasing, in part because of the rise in the incidence of obesity among children, the onset is more common in adulthood. In contrast, the onset of type 1 diabetes is frequently seen during childhood. One epidemiologic study reported that approximately 26 percent of cases of type 1 diabetes presented in children less than 4 years of age and 37 percent presented at 5 to 14 years of age (Harjutsalo et al., 2008). However, the frequency of diabetic ketoacidosis at onset of the disease is higher in younger children (40 to 50 percent for ages 0 to 4 years) than in older adolescents (12 to 15 percent for ages 15 to 21 years) (Daneman, 2006). Type 1 diabetes accounts for only 5 to 10 percent of all cases of diabetes; but its early onset, faster and more intense destruction of pancreatic β cells (compared with type 2 diabetes), and association with short-and long-term complications make it a serious, chronic disorder of importance among children.
Type 1 diabetes results from destruction of pancreatic β cells resulting from a cell-mediated autoimmune reaction (Daneman, 2006). This then causes a progressive loss of insulin production; patients eventually have an absolute insulin deficiency, requiring exogenous insulin to maintain glucose hemostasis. Although insulin is an effective treatment and the new analog insulins allow greater physiologic control of glucose, complications from treatment can still frequently occur. In the short term, hypoglycemia is likely the most important complication of type 1 diabetes, which can be life-threatening and can interfere with effective glucose control. Side effects of insulin in both adults and children can include hypersensitivity reactions, lipohypertrophy or -atrophy, and pain at the injection site (Bangstad et al., 2007). Long-term diabetes is associated with micro-and macrovascular complications, including nephropathy, retinopathy, and cardiovascular dis-
ease (Daneman, 2006). Some of these complications, such as retinopathy, may be seen early in the course of the disease (Maguire et al., 2005).
Given the role of the immune system in the development of type 1 diabetes, studies have looked at the effects of monoclonal antibodies—primarily CD3-specific antibodies—on the preservation of β-cell function (Kaufman and Herold, 2009). Otelixizumab, an investigational CD3 surface antigen antibody, was evaluated for its effects on new-onset type 1 diabetes (Keymeulen et al., 2005). The CD3 surface antigen was targeted because of the T-cell-mediated autoimmune mechanism of type 1 diabetes. Residual β-cell function (as measured by C-peptide release) was maintained among patients given otelixizumab and returned to baseline at 18 months after treatment. Patients given placebo had reductions in β-cell function of just over 30 percent during the same time period. In addition, treatment with the monoclonal antibody had a greater effect in patients with higher residual β-cell function at baseline (≥50th percentile). Adverse effects of treatment were transient but significant, with nearly all treated patients experiencing fever, headache, gastrointestinal events, arthralgia, myalgia, rash, and an acute mononucleosis-like syndrome.
A second investigational anti-CD3 monoclonal antibody, teplizumab, was evaluated in 24 patients with a diagnosis of type 1 diabetes of 6 weeks or less (Herold et al., 2002). Teplizumab or placebo was given as a 14-day course of treatment, and patients were assessed after 1 year. The monoclonal antibody significantly attenuated the decline in C-peptide response compared with placebo. A decline in both glycosylated hemoglobin (A1C) levels and insulin dose were also seen with teplizumab. Similar results were reported in a 2-year follow-up; the effects of teplizumab were maintained (Herold et al., 2005).
A second trial of teplizumab was initiated with patients with recent-onset type 1 diabetes (Herold et al., 2009). This study, however, was stopped after enrollment of 10 patients (6 given teplizumab) due to a substantially higher rate of adverse events than previously seen, despite use of the same dosage regimen (Herold et al., 2002, 2005). It was later determined that a change in the manufacturing of teplizumab—use of a stoppered vial instead of a glass ampoule—resulted in a 40 percent increase in the dose of teplizumab over previous trials and a subsequent increase in adverse events (Herold et al., 2009). During preparation for administration, the contents of the glass ampoule were filtered, whereas a filter was not used when the agent was packaged in a stoppered vial. An extended follow-up of patients given teplizumab was conducted. At 60 months, the mean loss of baseline function (based on C-peptide response) was 63.8 percent, indicating that the monoclonal antibody had a prolonged effect.
A more recent, larger study of teplizumab enrolled 516 patients (ages 8 to 35 years) with type 1 diabetes within 12 weeks from diagnosis (Sherry
et al., 2011). Results of the trial did not show an effect on β-cell preservation at 1 year. However, an exploratory analysis on the effect of teplizumab in the children suggested a better C-peptide response, findings that need to be confirmed.
In addition to CD3-specific antibodies, rituximab, an anti-CD20 monoclonal antibody, has been evaluated for preservation of β-cell function (Pescovitz et al., 2009). At 1 year after treatment, a significantly lesser decline in the level of C peptide (as a marker of β-cell function) from baseline was seen with rituximab than placebo, and the decline was accompanied by reductions in both A1C and total insulin use. Adverse events occurred significantly more often with the use of rituximab than placebo, including fever, rash, hypotension, nausea, fever, and tachycardia.
Overall, immunotherapy seems to be a promising area for research. As a life-long disease, the safety of biologics in the treatment of type 1 diabetes in children is of utmost importance. On the basis of the available data, treatment must be initiated shortly after diagnosis (before extensive loss of β-cell function) to preserve endogenous insulin production. However, the effects of biologics on growth and development of young children are largely unknown. Additionally, since a single course of therapy with a biologic may have a prolonged effect on β-cell preservation, the optimal frequency of treatment needs to be established. Finally, another critical question for evaluation is whether the risks associated with biologics outweigh the benefits of delaying or minimizing the long-term complications of type 1 diabetes.
CONCLUSION
For many disease states, biologics represent the most advanced therapeutic approach. The use of biologics for chronic conditions such as rheumatoid arthritis, psoriasis, and IBD has been established in adults. These agents have improved the quality of life of adult patients with these and similar immune-mediated diseases and induce a remission of symptoms for some diseases. However, the role of biologics (excluding plasma-derived or recombinant factor proteins) in many pediatric disease states is less clear. Most data on biologics appear to be for JIA, with some biologics approved for children as young as 2 years of age. IBD, atopic dermatitis, psoriasis, childhood cancers, and type 1 diabetes—the conditions discussed in this paper—all have a significant impact on the quality of life of children, which in many cases extends to adulthood. Taking prevalence, burden of disease, and life expectancy as well as a lack of pediatric studies into account, the two areas in which research in biologics may be the most needed are childhood cancers and type 1 diabetes.
For childhood cancers, use of many therapies is extrapolated from data
for adults because of the limited availability of data for the pediatric population. Although childhood cancers represent only about 1 percent of all cancers, they are the leading medical cause of death among children, making improvements to the survival of these patients a priority. Additionally, the cure of a childhood cancer prolongs life not by 10 or 20 years, as in adults, but potentially by 60 or 70 years, balancing any higher therapeutic costs with a substantial gain in life-years.
Also important is type 1 diabetes. Although type 1 diabetes accounts for only 5 to 10 percent of cases of diabetes, nearly half of these cases are diagnosed in childhood. The only effective therapy is insulin, and despite appropriate treatment, type 1 diabetes is associated with significant morbidity and mortality from micro-and macrovascular complications. Preliminary data suggest that early intervention with biologics has the potential to preserve β-cell function and endogenous insulin secretion (Herold et al., 2005; Keymeulen et al., 2005; Kaufman and Herold, 2009; Pescovitz et al., 2009). This could potentially prevent or limit the long-term complications of the disease and greatly improve the quality of life of patients with type 1 diabetes. Although biologic therapy is likely to be more costly than current insulin therapies, the cost of biologic therapy in childhood may be offset by the benefits of decreased morbidity in adulthood.
A major concern about which little is known is the effect, if any, that biologics can have on childhood development and growth or if negative effects of treatment may be seen in adulthood. As noted above, some established treatments used with children may potentially increase the risk of subsequent malignancies. In addition to well-designed clinical trials, establishment and continued use of registry data are important for investigation of the long-term effects of biologics.
REFERENCES
Abbott Laboratories. Humira package insert. North Chicago, IL: Abbott Laboratories; 2011.
Abramson O, Durant M, Mow W, et al. Incidence, prevalence, and time trends of pediatric inflammatory bowel disease in Northern California, 1996 to 2006. J Pediatr. 2010;157(2):233-239.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(suppl 1):S62-S69.
Amgen. Enbrel package insert. Thousand Oaks, CA: Amgen; 2011.
An Z. Monoclonal antibodies—a proven and rapidly expanding therapeutic modality for human diseases. Protein Cell. 2010;1(4):319-330.
Armbrust W, Kamphuis SSM, Wolfs TWF, et al. Tuberculosis in a nine-year-old girl treated with infliximab for systemic juvenile idiopathic arthritis. Rheumatology (Oxford). 2004; 43(4):527-529.
Bangstad HJ, Danne T, Deeb LC, Jarosz-Chobot P, Urakami T, Hanas R. ISPAD Clinical Practice Consensus Guidelines 2006-2007. Insulin treatment. Pediatric Diabetes. 2007;8(2):88-102.
Barquet N, Domingo P. Smallpox: the triumph over the most terrible of the ministers of death. Ann Intern Med. 1997;127(8):635-642.
Bernstein ML. Targeted therapy in pediatric and adolescent oncology. Cancer. 2011;117(10 suppl):2268-2274.
Bjorkman S, Berntrop E. Pharmacokinetics of coagulation factors. Clinical relevance for patients with hemophilia. Clin Pharmacokinet. 2001;40(11):815-832.
Blanchette V, Bolton-Maggs P. Childhood immune thrombocytopenic purpura: diagnosis and management. Hematol Oncol Clin N Am. 2010;24(1):249-273.
Bloom BJ. Development of diabetes mellitus during etanercept therapy in a child with systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2000;43(11):2606-2608.
Bonilla FA, Bernstein IL, Khan DA, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94(5 suppl 1):S1-S63.
Bout-Tabaku S, Rivas-Chacon R, Restrepo R. Systemic lupus erythematosus in a patient treated with etanercept for polyarticular juvenile rheumatoid arthritis. J Rheumatol. 2007;34(12):2503-2504.
Bremmer MS, Bremmer SF, Baig-Lewis S, Simpson EL. Are biologics safe in the treatment of atopic dermatitis? A review with a focus on immediate hypersensitivity reactions. J Am Acad Dermatol. 2009;61(4):666-676.
Bristol-Myers Squibb. Orencia package insert. Princeton, NJ: Bristol-Myers Squibb; 2009.
Brooker M. Registry of clotting factor concentrations. Montreal, Quebec, Canada: World Federation of Hemophilia; 2008. http://www.wfh.org/2/docs/Publications/Treatment_Products/Monographs/FF6_Registry_8th_2008.pdf. Accessed July 24, 2011.
Buka RL, Resh B, Roberts B, Cunningham BB, Friedlander S. Etanercept is minimally effective in 2 children with atopic dermatitis. J Am Acad Dermatol. 2005;53(2):358-359.
Burnouf T. Modern plasma fractionation. Transfus Med Rev. 2007;21(2):101-117.
Burnouf T. Recombinant plasma proteins. Vox Sang. 2011;100(1):68-83.
Centocor Ortho Biotech, Inc. Remicade package insert. Malvern, PA: Centocor Ortho Biotech, Inc; 2011.
Cucchiara S, Morelty-Fletcher A. “New drugs: kids come first”: children should be included in trials of new biological treatments. Inflamm Bowel Dis. 2007;13(9):1165-1169.
Daneman D. Type 1 diabetes. Lancet. 2006;367(9513):847-858.
de Zoeten E, Mamula P. What are the guidelines for use of biologics in pediatric patients? Inflamm Bowel Dis. 2008;14(suppl 2):S259-S261.
Diak P, Siegel J, La Grenade L, Choi L, Lemery S, McMahon A. Tumor necrosis factor α blockers and malignancy in children. Arthritis Rheum. 2010;62(8):2517-2524.
DiMichele DM. Inhibitors in hemophilia: a primer. Montreal, Quebec, Canada: World Federation of Hemophilia; 2008. http://www.wfh.org/2/docs/Publications/Inhibitors/TOH-7%20Inhibitor-Primer-Revised2008.pdf. Accessed July 14, 2011.
Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(10):633-659.
Dotson JL, Hyams JS, Markowitz J, et al. Extraintestinal manifestations of pediatric inflammatory bowel disease and their relation to disease type and severity. J Pediatr Gastroenterol Nutr. 2010;51(2):140-145.
Duhem C, Dicato MA, Ries F. Side-effects of intravenous immune globulins. Clin Exp Immunol. 1994;97(suppl 1):79-83.
Epstein JS, Zoon KC. Acute renal failure associated with immune globulin intravenous (human). Bethesda, MD: Centers for Biologics Evaluation and Research, FDA. September 24, 1999.
Farnsworth NN, George SJ, Hsu S. Successful use of infliximab following a failed course of etanercept in a pediatric patient. Dermatol Online J. 2005;11(3):11.
Fathalla BM, Goldsmith DP, Pascasio JMC, Baldridge A. Development of autoimmune hepatitis in a child with systemic-onset juvenile idiopathic arthritis during therapy with etanercept. J Clin Rheumatol. 2008;14(5):297-298.
FDA (Food and Drug Administration). Guidance for industry. General considerations for pediatric pharmacokinetic studies for drugs and biological products. Rockville, MD: FDA; 1998. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072114.pdf. Accessed July 11, 2011.
FDA. Science and the regulation of biological products. Rockville, MD: FDA; 2002. http://www.fda.gov/downloads/AboutFDA/WhatWeDo/History/ProductRegulation/100YearsofBiologicsRegulation/UCM070313.pdf. Accessed July 20, 2011.
FDA. Guidance for industry. Safety, efficacy, and pharmacokinetic studies to support marketing of immune globulin intravenous (human) as replacement therapy for primary humoral immunodeficiency. Rockville, MD: FDA; June 2008. http://www.fda.gov/down loads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/ucm078526.pdf. Accessed July 11, 2011.
FDA. Biologics regulated products. Rockville, MD: FDA; 2009a. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CBER/ucm123205.htm. Accessed July 20, 2011.
FDA. Follow-up to the June 4, 2008 early communication about the ongoing safety review of tumor necrosis factor (TNF) blockers (marketed as Remicade, Enbrel, Humira, Cimzia, and Simponi). Rockville, MD: FDA; 2009b. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHealthcareProfessionals/ucm174449.htm. August 4, 2009. Accessed November 28, 2011.
FDA. Regulatory information. Federal Food, Drug, and Cosmetic Act (FD&C Act). SEC. 201. [21 U.S.C. ![]() 321] Chapter II—Definitions 1. Rockville, MD: FDA; 2009c. http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/FDCActChaptersIandIIShortTitleandDefinitions/ucm086297.htm. Accessed July 20, 2011.
321] Chapter II—Definitions 1. Rockville, MD: FDA; 2009c. http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/FDCActChaptersIandIIShortTitleandDefinitions/ucm086297.htm. Accessed July 20, 2011.
FDA. Therapeutic biological applications (BLA). Rockville, MD: FDA; 2009d. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/default.htm. Accessed July 20, 2011.
FDA. What are “biologics” questions and answers. Washington, DC: FDA; 2009e. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CBER/ucm133077.htm. Accessed July 20, 2011.
FDA. Transfer of therapeutic products to the Center for Drug Evaluation and Research. Washington, DC: FDA; 2010. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CBER/ucm133463.htm. Accessed July 20, 2011.
Floristan U, Feltes R, Ramirez P, Alonso ML, de Lucas R. Recalcitrant palmoplantar pustular psoriasis treated with etanercept. Pediatr Dermatol. 2011;28(3):349-350.
Ford A, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):644-659.
Gare BA. Juvenile arthritis—who gets it, where and when? A review of current data on incidence and prevalence. Clin Exp Rheumatol. 1999;17(3):367-374.
Genentech. Actemra package insert. South San Francisco, CA: Genentech; 2011.
Gerloni V, Pontikaki I, Gattinara M, Fantini F. Focus on adverse events of tumour necrosis factor α blockade in juvenile idiopathic arthritis in an open monocentric long-term prospective study of 163 patients. Ann Rheum Dis. 2008;67(8):1145-1152.
Glade-Bender JL, Adamson PC, Reid JM, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol. 2008;26(3):399-405.
Grabenstein JD, editor. Immunofacts 2011. St. Louis, MO: Wolters Kluwer Health; 2011.
Griffin TC, Weitzman S, Weinstein H, et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2009;52(2):177-181.
Griffiths A. Growth retardation in early-onset inflammatory bowel disease: should we monitor and treat these patients differently? Dig Dis. 2009;27(3):404-411.
Gururangan S, Chi SN, Young Poussaint T, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28(18):3069-3075.
Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371(9626):1777-1782.
Hashkes PJ, Shajrawi I. Sarcoid-related uveitis occurring during etanercept therapy. Clin Exp Rheumatol. 2003;21(5):645-646.
Hashkes PJ, Uziel Y, Laxer RM. The safety profile of biologic therapies for juvenile idiopathic arthritis. Nat Rev Rheumatol. 2010;6(10):561-571.
Hassan AD, Kaelin U, Braathen LR, Yawalkar N. Clinical and immunopathologic findings during treatment of recalcitrant atopic eczema with efalizumab. J Am Acad Dermatol. 2007;56(2):217-221.
Hawrot AC, Metry DW, Theos AJ, Levy ML. Etanercept for psoriasis in the pediatric population: experience in nine patients. Pediatr Dermatol. 2006;23(1):67-71.
Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1692-1698.
Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005; 54(6):1763-1769.
Herold KC, Gitelman S, Greenbaum C, et al. Treatment of patients with new onset type 1 diabetes with a single course of anti-CD3 mAb teplizumab preserves insulin production for up to 5 years. Clin Immunol. 2009;132(2):166-173.
Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology. 2007;132(3):863-873.
Hyams JS, Lerer T, Griffiths A, et al. Long-term outcome of maintenance infliximab therapy in children with Crohn’s disease. Inflamm Bowel Dis. 2009;15(6):816-822.
Jacobi A, Antoni C, Manger B, Schuler G, Hertl M. Infliximab in the treatment of moderate to severe atopic dermatitis. J Am Acad Dermatol. 2005;52(3):522-526.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
Junod SW. Biologics centennial: 100 years of biologics regulation. Update. Silver Spring, MD: Food and Drug Administration; November-December 2002. Available at http://www.fda.gov/aboutfda/whatwedo/history/productregulation/selectionsfromfdliupdateseriesonfdahistory/ucm091754.htm Accessed July 20, 2011.
Kanakoudi-Tsakalidou F, Tzimouli V, Pratsidou-Gertsi P, Chronopoulou E, Trachana M. The significance of persistent newly developed autoantibodies in JIA patients under long-term anti-TNF treatment. Cytokine. 2008;42(3):293-297.
Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5(12):1424-1429.
Kaufman A, Herold KC. Anti-CD3 mAbs for treatment of type 1 diabetes. Diabetes Metab Res Rev. 2009;25(4):302-306.
Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N Engl J Med. 2001;345(15):1098-1104.
Keeling D, Tait C, Makris M. Guideline on the selection and use of therapeutic products to treat hemophilia and other hereditary disorders. Haemophilia. 2008;14(4):671-684.
Keizer RJ, Huitema ADR, Schelllens JHM, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493-507.
Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352(52):2598-2608.
Koleba T, Ensom MHH. Pharmacokinetics of intravenous immunoglobulin: a systematic review. Pharmacotherapy. 2006;26(6):813-827.
Krathen RA, Hsu S. Failure of omalizumab for treatment of severe adult atopic dermatitis. J Am Acad Dermatol. 2005;53(2):338-340.
Kress DW. Etanercept therapy improves symptoms and allows tapering of other medications in children and adolescents with moderate to severe psoriasis. J Am Acad Dermatol. 2006;54(3):S126-S128.
Lane JE, Cheyney JM, Lane TN, Kent DE, Cohen DJ. Treatment of recalcitrant atopic dermatitis with omalizumab. J Am Acad Dermatol. 2006;54(1):68-72.
Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor α antagonists infliximab and etanercept. Arthritis Rheum. 2002;46(10):2565-2570.
Lee S, Ballow M. Monoclonal antibodies and fusion proteins and their complications: targeting B cells in autoimmune diseases. J Allergy Clin Immunol. 2010;125(4):814-820.
Lepore L, Leone V, Marchetti F, Ventura A, Facchini S. Drug-induced systemic lupus erythematosus associated with etanercept therapy in a child with juvenile idiopathic arthritis. Clin Exp Rheumatol. 2003;21(2):276-277.
Levine D, Gottlieb A. Evaluation and management of psoriasis: an internist’s guide. Med Clin N Am. 2009;93(6):1291-1303.
Lovell DJ, Giannini EH, Reiff A, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. N Engl J Med. 2000;342(11):763-769.
Lovell DJ, Reiff A, Ilowite NT, et al. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis Rheum. 2008a;58(5):1496-1504.
Lovell DJ, Ruperto N, Goodman S, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008b;359(8):810-820.
Mackey A, Green L, Leptak C, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease: update. J Pediatr Gastroenterol Nutr. 2009;48(3):386-388.
Maguire A, Craig M, Chan A, et al. The case for biennial retinopathy screening in children and adolescents. Diabetes Care. 2005;28(3):509-513.
Manners PJ, Bower C. Worldwide prevalence of juvenile arthritis—why does it vary so much? J Rheumatol. 2002;29(7):1520-1530.
Marji JS, Marcus R, Moennich J, et al. Use of biologic agents in pediatric psoriasis. J Drugs Dermatol. 2010;9(8):975-986.
McEvoy GK, editor. AHFS Drug Information 2011. In STAT!Ref Online Electronic Medical Library [online database]. Jackson, WY: Teton Data Systems; 2011. http://online.statref.com.proxy.cc.uic.edu/Document/Document.aspx?docAddress=pDsr3ldZEAdtFTdz9Db3Dw%3d%3d&offset=81&SessionId=15854A2CXDYSQQRR. Accessed July 24, 2011.
Meinhardt A, Burkhardt B, Zimmermann M, et al. Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin’s lymphoma and Burkitt leukemia. J Clin Oncol. 2010;28(19):3115-3121.
Menter M, Cush JM. Successful treatment of pediatric psoriasis with infliximab. Pediatr Dermatol. 2004;21(1):87-88.
Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826-850.
Mohan N, Edwards ET, Cupps TR, et al. Demyelination occurring during anti-tumor necrosis factor α therapy for inflammatory arthritides. Arthritis Rheum. 2001;44(12):2862-2869.
Morgan B. The search for child cancer drugs grows up. Nat Med. 2011;17(3):267.
Moul DK, Routhouska SB, Robinson MR, Korman NJ. Alefacept for moderate to severe atopic dermatitis: a pilot study in adults. J Am Acad Dermatol. 2008;58(6):984-989.
Myers A, Clark J, Foster H. Tuberculosis and treatment with infliximab. N Engl J Med. 2002;346(8):625.
National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program Expert Panel Report 3. Guidelines for the diagnosis and management of asthma. Summary report. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. http://www.nhlbi.nih.gov/guidelines/asthma/asthsumm.pdf. Accessed July 14, 2011.
National Psoriasis Foundation. About psoriasis. Statistics. Portland, OR: National Psoriasis Foundation. http://www.psoriasis.org/learn_statistics. Accessed July 16, 2011.
Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114(6):1708-1733.
Nordt TK, Bode C. Thrombolysis: newer thrombolytic agents and their role in clinical medicine. Heart. 2003;89(11):1358-1362.
Nydegger UE, Sturzenegger M. Adverse effects of intravenous immunoglobulin therapy. Drug Saf. 1999;21(3):171-185.
Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572-1582.
Pain CE, McCann LJ. Challenges in the management of juvenile idiopathic arthritis with etanercept. Biologics. 2009;3:127-139.
Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358(3):241-251.
Papoutsaki M, Costanzo A, Mazzotta A, Gramiccia T, Soda R, Chimenti S. Etanercept for the treatment of severe childhood psoriasis. Br J Dermatol. 2006;154(1):181-183.
Pappa H, Thayu M, Sylvester F, Leonard M, Zemal B, Gordon C. Skeletal health of children and adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2011;53(1):11-25.
Peek R, Scott-Jupp R, Strike H, Clinch J, Ramanan AV. Psoriasis after treatment of juvenile idiopathic arthritis with etanercept. Ann Rheum Dis. 2006;65(9):1259.
Peskovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143-2152.
Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390-392.
Pfefferkorn M, Burke G, Griffiths A, et al. Growth abnormalities persist in newly diagnosed children with Crohn’s disease despite current treatment paradigms. J Pediatr Gastroenterol Nutr. 2009;48(2):168-174.
Pigneur B, Seksik P, Viola S, et al. Natural history of Crohn’s disease: comparison between childhood-and adult-onset disease. Inflamm Bowel Dis. 2010;16(6):953-961.
Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168-186.
Quartier P, Allantaz F, Cimaz R, et al. A multicenter, randomized, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial). Ann Rheum Dis. 2011;70(5): 747-754.
Radosevich M, Burnouf T. Intravenous immunoglobulin G: trends in production methods, quality control, and quality assurance. Vox Sang. 2010;98(1):12-28.
Rohrbach M, Clarke JTR. Treatment of lysosomal storage disorders. Drugs. 2007;67(18):2697-2716.
Roifman CM, Berger M, Notarangelo LD. Management of primary antibody deficiency with replacement therapy: summary of guidelines. Immunol Allergy Clin N Am. 2008;28(4):875-876.
Roque AC, Lowe CR, Taipa MA. Antibodies and genetically engineered related molecules: production and purification. Biotechnol Prog. 2004;20(3):639-654.
Rosh JR. Use of biologic agents in pediatric inflammatory bowel disease. Curr Opin Pediatr. 2009;21(5):646-650.
Rosh JR, Lerer T, Markowitz J, et al. Retrospective evaluation of the safety and effect of adalimumab therapy (RESEAT) in pediatric Crohn’s disease. Am J Gastroenterol. 2009;104(12):3042-3049.
Ruemmele FM, Prieur AM, Talbotec C, Goulet O, Schmitz J. Development of Crohn disease during anti-TNF-α therapy in a child with juvenile idiopathic arthritis. J Pediatr Gastroenterol Nutr. 2004;39(2):203-206.
Ruperto N, Lovell DJ, Cuttica R, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56(9):3096-3106.
Ruperto N, Lovell DJ, Quartier P, et al. Abatacept in children with juvenile idiopathic arthritis: a randomized, double-blind, placebo-controlled withdrawal trial. Lancet. 2008; 372(9636):383-391.
Saeki H, Furue M, Furukawa F, et al. Guidelines for management of atopic dermatitis. J Dermatol. 2009;36(10):563-577.
Safa G, Loppin M, Bousser AM, Barbarot S. Etanercept in a 7-year-old boy with severe and recalcitrant psoriasis. J Am Acad Dermatol. 2007;56(2 suppl):S19-S20.
Sandhu BK, Fell JME, Beattie M, et al. Guidelines for the management of inflammatory bowel disease in children in the United Kingdom. J Pediatr Gastroenterol Nutr. 2010;50(suppl 1):S1-S13.
Sauer CG, Kugathasan S. Pediatric inflammatory bowel disease: highlighting pediatric differences in IBD. Med Clin N Am. 2010;94(1):35-52.
Sawczenko A, Ballinger AB, Savage MO, Sanderson IR. Clinical features affecting final adult height in patients with pediatric-onset Crohn’s disease. Pediatrics. 2006;118:124.
Sherry N, Hagopian W, Ludvigsson H, et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378 (9780):487-497.
Shikhare G, Kugathasan S. Inflammatory bowel disease in children: current trends. J Gastroenterol. 2010;45(7):673-682.
Siegel J. IVIG medication safety: a stepwise guide to product selection and use. Pharmacy Practice News. 2010;1-8.
Siegfried EC. Long-term follow-up of a child treated with efalizumab for atopic dermatitis. Arch Dermatol. 2007;143(8):1077-1078.
Simon D, Hosli S, Kostylina G, Yawalker N, Simon HU. Anti-CD20 (rituximab) treatment improves atopic eczema. J Allergy Clin Immunol. 2008;121(1):122-128.
Soucie JM, Evatt B, Jackson D, and the Hemophilia Surveillance System Project Investigators. Occurrence of hemophilia in the United States. Am J Hematol. 1998;59(4):288-294.
Spergel JM. Epidemiology of atopic dermatitis and atopic march in children. Immunol Allergy Clin N Am. 2010;30(3):269-280.
Sukhatme SV, Gottlieb AB. Pediatric psoriasis: updates in biologic therapies. Dermatol Ther. 2009;22(1):34-39.
Takiguchi R, Tofte S, Simpson B, et al. Efalizumab for severe atopic dermatitis: a pilot study in adults. J Am Acad Dermatol. 2007;56(2):222-226.
Tarantino M, Ma A, Aledort L. Safety of human plasma-derived clotting factor products and their role in hemostasis in patients with hemophilia: meeting report. Haemophilia. 2007;13(5):663-669.
Thayu M, Leonard MB, Hyams JS, et al. Improvement in biomarkers of bone formation during infliximab therapy in pediatric Crohn’s disease: results of the REACH study. Clin Gastroenterol Hepatol. 2008;6(12):1378-1384.
Tollefson MM, Crowson CS, McEvoy MT, Kremers HM. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol. 2010;62(6):979-987.
Torpy JM, Campbell A, Glass RM. Chronic diseases of children. JAMA. 2010;303(7):682.
Trippett TM, Herzog C, Whitlock JA, et al. Phase I and pharmacokinetic study of cetuximab and irinotecan in children with refractory solid tumors: a study of the pediatric oncology experimental therapeutic investigators’ consortium. J Clin Oncol. 2009; 27(30):5102-5108.
Turunen P, Ashorn M, Auvinen A, Iltanen S, Huhtala H, Kolho K. Long-term health outcomes in pediatric inflammatory bowel disease: a population-based study. Inflamm Bowel Dis. 2009;15(1):56-62.
Vigo PG, Girgis KR, Pfuetze BL, Critchlow ME, Fisher J, Hussain I. Efficacy of anti-IgE therapy in patients with atopic dermatitis. J Am Acad Dermatol. 2006;55(1):168-170.
Viola F, Civitelli F, DiNardo G, et al. Efficacy of adalimumab in moderate-to-severe pediatric Crohn’s disease. Am J Gastroenterol. 2009;104(10):2566-2571.
Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious disease associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38(9):1261-1265.
Walters TD, Gilman AR, Griffiths AM. Linear growth improves during infliximab therapy in children with chronically active severe Crohn’s disease. Inflamm Bowel Dis. 2007;13(4):424-430.
Weinberg JM, Siegfried EC. Successful treatment of severe atopic dermatitis in a child and an adult with the T-cell modulator efalizumab. Arch Dermatol. 2006;142(5):555-558.
Wickersham RM, editor. Drug Facts and Comparisons. St. Louis, MO: Wolters Kluwer Health; 2011.
Wiegering V, Morbach H, Dick A, Girschick HJ. Crohn’s disease during etanercept therapy in juvenile idiopathic arthritis: a case report and review of the literature. Rheumatol Int. 2010:30(6):801-804.
World Federation of Hemophilia. Guidelines for the management of hemophilia. Montreal, Quebec, Canada: World Federation of Hemophilia; 2005. http://www.wfh.org/2/docs/Publications/Diagnosis_and_Treatment/Guidelines_Mng_Hemophilia.pdf. Accessed July 14, 2011.
Yokota S, Imagawa T, Mori M, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomized, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371(9617):998-1006.




































