DESCRIPTION OF TOUR OF PENDIK VETERINARY CONTROL AND RESEARCH INSTITUTE
Summary prepared by Barbara Johnson (Biosafety Biosecurity International, United States)
Following the conclusion of the workshop, many of the participants visited the nearby Pendik Veterinary Control and Research Institute (PVCRI) and toured its BSL-3 lab, which was undergoing the final stages of construction. PVCRI, which is located in the Asian part of Istanbul, Turkey, was founded in 1894 and moved to its present location in 1912. PVCRI produces veterinary vaccines, serums, antigens, and biological materials and serves as a national reference lab for the Turkish Ministry of Agriculture and Rural Affairs (MARA). The lab’s mission includes research, diagnostics, and community education.
As one of three labs in Turkey with the ability to both isolate and identify avian influenza virus, PVCRI played a key role in recent efforts to detect and combat avian influenza in Turkey. In 2006, MARA and the Turkish Ministry of Health produced a new coordinated preparedness and response plan for avian influenza that called for PVCRI and two other labs to expand their capabilities and begin performing virus pathogenicity characterization on-site. At the time of the decision, none of the three labs met the safety recommendations for the proposed work, so the General Directorate of Protection and Control decided to upgrade them to BSL-3 capabilities
For the BSL-3 lab at PVCRI, MARA chose to renovate a portion of the existing facility. Initial construction began in 2008, and the current construction company, HTL, took over from the previous firm in 2010. HTL is a Turkish company with experience constructing not only clean rooms but also containment facilities.
As shown by the floor plan (Figure D-1) personnel enter through a clean corridor. They then pass through a change room where a complete change of clothing is provided prior to transiting through the shower and dirty side change room into a set of corridors that allow entry to bacteriology, virology, necropsy, procedure, and diagnostic laboratories. Sample accessioning is accomplished in a clean room outside the containment zone, and samples enter the BSL-3 via a pass-through box to the adjacent procedure lab. A decontamination airlock is available for decontamination and movement of large pieces of equipment, and waste is passed through a double door bioseal autoclave prior to disposal. For safety, an emergency egress door is provided at the far end of the containment corridor. To facilitate sealing the decontamination airlock and as an added measure of safety above that required for BSL-3, gasketed pneumatic airtight doors are installed on both sides of the decontamination vestibule, the emergency exit, and at the containment boundary from each clean change room to the shower. Viewing windows are provided to the labs and the containment office space.
The floors, walls, and ceilings in the containment area are coved, were constructed to facilitate cleaning, and employ materials resistant to commonly used decontaminating agents (Figure D-2). The entire lab can be sterilized using gas, and each room can also be sterilized separately. Class II biological safety cabinets (BSCs) are used for all manipulations of infectious materials, and procedure rooms are equipped with HEPA-filtered poultry isolators with pass-through chambers for the movement of supplies, animals, and waste. Hands-free sinks and eyewashes are located near the exit of the labs. Doors are self-closing and equipped with locks for biosafety as well as biosecurity. In keeping with international best practices, additional biosecurity features have also been installed.
The exhaust air passes through welded, stainless steel ducts to two HEPA filters in series in the mechanical room that is accessible from outside the containment area (Figure D-
3) The HVAC system is supported by an emergency generator and has a redundant exhaust fan as well as redundant HEPA filter banks, as PVCRI’s mission requires it be operational every day with the exception of scheduled maintenance shut downs. Potentially contaminated liquid effluent is gravity fed to an Actini continuous flow liquid sterilization system, which also features a second system for N+1 redundancy.
Ayse Selma Iyisan (PVCRI) hosted the tour and was assisted by Aysen Gargili (Marmara University), Naci Sivri (HTL), and Sevil Erdenlig (PVCRI).
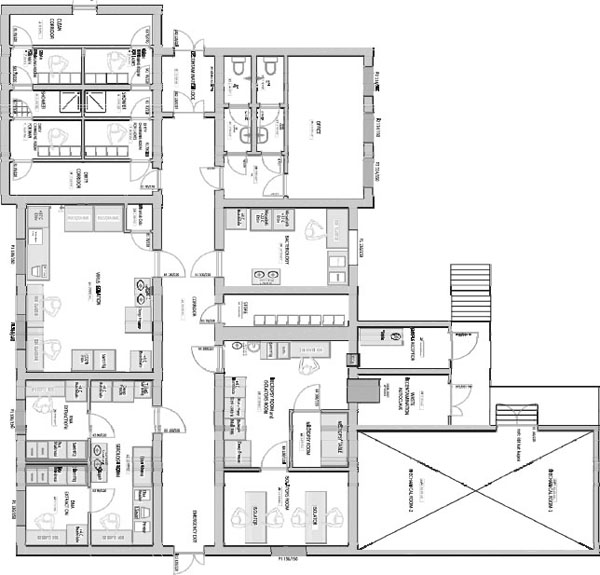
Figure D-1 Floor plan of PVCRI BSL-3 laboratory.
SOURCE: HTL Gebze Plastic Manufacturer Organization.

Figure D-2 Interior corridor of PVCRI BSL-3 lab.
SOURCE: Barbara Johnson, committee member.

Figure D-3 PVCRI HEPA filter bank.
SOURCE: Barbara Johnson, committee member.
This page intentionally left blank.




