HIGH-CONTAINMENT MICROBIOLOGY LABORATORIES IN EUROPE
Ingegerd Kallings,a M.D. and Kathrin Summermatter,b Ph.D.
aSwedish Institute for Communicable Disease Control, Stockholm, Sweden
bInstitute for Virology and Immunoprophylaxis, Mittelhaeusern, Switzerland
This report is partly based on the final report from the Consortium project BIOSAFETY-EUROPE, funded by the 6th Framework Program of the European Commission: Co-ordination, harmonization and exchange of biosafety and biosecurity practices within a pan-European network.1
BIOSAFETY-EUROPE, a coordination action within the European Commission 6th Framework, had the aims to explore harmonization and exchange of biosafety and biosecurity practices within a pan-European network. The consortium included expertise in biosafety, biosecurity, risk assessment and control, containment measures, and on the underlying legal frameworks of the European Union and its Member States (MS). The consortium was comprised of 18 partners from 10 European countries representing industry, academia, and government agencies. It commenced in April 2006 and lasted until the end of 2008. Through detailed questionnaires, information was gathered on the implementation of European biosafety legislation on the national level and on physical containment, practices, and procedures at containment levels 3 and 4. Laboratory biosecurity was addressed as well.
High-containment biological facilities in Europe
A first questionnaire (Q1) was sent to 319 containment level (CL) 3 and 4 laboratories in the 27 EU MS identified through national regulatory agencies and through personal knowledge by consortium partners (see Figure E2-1). Responses were received from 98 laboratories (13 CL 4; 85 CL 3) in 18 countries. Questions were asked on lab type (government, private, academia, or industry), lab activities (clinical, public health, research, human and/or animal, food, defense), the national regulatory framework and the implementation of EU legislation, the implementation of biosafety and biosecurity management and associated controls, and inspection regimes.
EU and national governance of high-containment biological facilities
At the highest EU level, many departments (DGs) deal with laboratory biosafety and biosecurity e.g., DG Health and Consumers, DG Research and Innovation, DG Home Affairs, DG Environment, DG Mobility and Transport, DG Employment, and Social Affairs and Inclusion. More directly, safety at work with biological agents is the responsibility of the EU Occupational Health and Safety Agency (EU-OSHA) that developed the EU Directive 2000/54/EC on the protection of workers from risks related to exposure to biological agents at work.2
EU MS are obliged to implement EU Directives, and all 27 MS have reported to the EU they have adopted and implemented Directive 2000/54/EC. MS must not delete any requirements from EU legislation when adopting it into national legislation, but some countries have additional requirements.
![]()
2http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2000:262:0021:0045:EN:PDF.
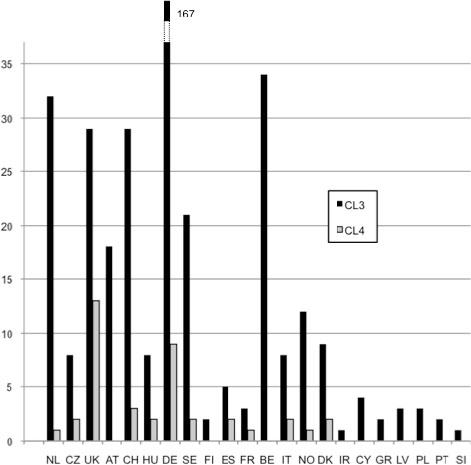
Figure E2-1 Country distribution of CL3 and CL4 laboratories that received the first questionnaire (Q1). The CL3 labs follow a variety of different standards. Additionally, the term “CL4” is not used consistently throughout Europe. CL4 labs may be glove box labs or suit labs. In some countries, a lab (e.g., a veterinary lab) that works with foot and mouth disease virus is automatically considered CL4. The numbers reflect all laboratories to which the questionnaire was sent, including some that were in the planning phase at the time.
SOURCE: BIOSAFETY-EUROPE
NL: Netherlands; CZ: Czech Republic; UK: United Kingdom; CH: Switzerland; HU: Hungary; DE: Germany; SE: Sweden; FI: Finland; FR: France; BE: Belgium; IT: Italy; NO: Norway; DK: Denmark; IR: Ireland; CY: Cyprus; GR: Greece; LV: Latvia; PL: Poland; PT: Portugal; SI: Slovenia
In many countries, several ministries, agencies, and organizations are involved in oversight of biosafety and the regulatory framework for biosafety (e.g., Ministry of Health, Ministry of Agriculture, Ministry of Environment, Ministry of Internal Affairs/Occupational Health) whereas the Ministry of Defense and the Ministry of Foreign Affairs usually are involved in biosecurity in a wider sense. In some countries, there is a department within the Ministry of Health (occasionally the Ministry of Defense) whereas other countries have an independent government agency as the main authority for biosafety matters as in Sweden where this responsibility lies with the Swedish Work Environment Agency. It was clearly shown by the
BIOSAFETY-EUROPE findings that the interpretation and implementation at the actual laboratory level of the common EU legislation varied greatly between MS.
A recent inventory made by the European Biosafety Association (EBSA) found that 20 of 27 countries have a body (agency, commission, or committee) regulating or providing advice on the contained use of Genetically Modified Microorganisms (GMM). For the remaining seven countries, no information was available.
In addition, there are a number of agencies at the EU level dealing with the implementation of biosafety and biosecurity regulations and guidelines: the European Agency for Safety and Health at Work (EU-OHSA), the European Centre for Disease Control and Prevention (ECDC), the European Food Safety Authority (EFSA), the European Food Information Council (EUFIC), the European Commission’s Joint Research Centre (JRC), the European Medicines Agency/European Agency for the Evaluation of Medicinal Products (EMEA), and the European Molecular Biology Laboratory (EMBL). Several nongovernmental organizations are involved: the European Federation of Biotechnology (EFB); the Federation of European Microbiological Societies (FEMS); the Confederation of the Food and Drink Industries of the EU (CIIA); the International Centre for Genetic Engineering and Biotechnology (ICGEB); the European Association for Bio industries (EuropaBio); EU News, Policy Positions & EU Actors online (EurActiv.com); and the European Society of Gene Therapy (ESGT).
Internationally, the World Health Organisation (WHO), the World Organisation for Animal Health (OIE), and the Food and Agriculture Organisation (FAO) issue guidelines in biosafety and biosecurity. These guidelines have recommendatory character, but they often influence the development of EU-wide and national regulatory frameworks.
BIOSAFETY-EUROPE - Biosafety Findings
National biosafety regulations and practices derived from EU Directives 2000/54/EC1 and 98/81/EC2 varied from country to country. In many countries, the regulatory framework for genetically modified microorganisms (GMMs) was more strongly enforced than that for biological agents in general. There is often no specific biosafety regulation for epizootics except for those microorganisms regulated under the two guidelines mentioned above.
Facilities and practices in containment level 3 laboratories throughout the EU are not of a comparable standard, e.g. a large range of different terminologies for “containment level (CL)” were used within the MS. Many laboratories referred to the WHO term ‘biosafety level (BSL). It was concluded that EU Directives 2000/54/EC3 and 98/81/EC require revision and updating to reflect the current state-of-the-art including continuous review of the classification list of microorganisms and the definition of harmonized best practices. (The Directive 98/81/EC has since been revised and replaced by 2009/41/EC,4but many inconsistencies remain).
Moreover, biosafety responsibilities appear often to be attributed to staff in management positions with functional roles that could be in conflict with strict biosafety considerations. Less than half of the respondents were subject to oversight by a biosafety committee.
EC legislation on biological agents and GMMs is often not specific enough to ensure harmonization of the implementation on the national level. There is a lack of European-wide harmonized practical guidance on how to implement the European Directives on biological agents and GMMs. A few EU Member States have developed their own national guidance based on the EC Directives. In other cases, these gaps are filled by e.g., U.S. Biosafety in Microbiological and Biomedical Laboratories (BMBL) and Canadian guidelines. The varying interpretation of the EU Directives allows different approaches to biosafety and
![]()
1 Op. cit. see note 2
2http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:1998:330:0013:0031:EN:PDF
3 Op. cit. see note 2
4http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:125:0075:0097:EN:PDF
laboratory biosecurity. This and differences in terminology make the exchange of scientists between member states problematic.
BIOSAFETY-EUROPE - Biosecurity Findings
Laboratory biosecurity is a relatively new concept that is still developing, and during the project period there was little consensus across Europe as to what biosecurity means, even within the laboratory environment. BIOSAFETY-EUROPE used the term “Laboratory Biosecurity” to describe protection against, control of, and accountability for biological agents and toxins within laboratories, in order to prevent their loss, theft, misuse, diversion, unauthorized access, or intentional unauthorized release (adapted from WHO5).
In contrast to this more laboratory-focused definition, it has to be mentioned that the term “livestock biosecurity” has long existed in the veterinary field and describes the prevention of disease-causing agents entering or leaving any place where farm animals are present.
No EU level legislation exists that has been specifically developed to address the protection of biological agents in the laboratory from loss or willful misuse. However, due to the many synergies between biosafety and biosecurity, the EU Directives developed to protect workers from exposure to biological agents or GMMs address most of the issues related to laboratory biosecurity. Only a few Member States are known to have introduced special laboratory biosecurity legislation i.e. England and Denmark. Many facilities implement some biosecurity controls, but these are often focused on physical security and often are not based on a specific risk assessment. Less attention is paid to information security or organizational security issues, despite the fact that internal threats from individuals with authorized access to the laboratory must be recognized.
The recently adopted Minimum standards for laboratories working with FMDV in vitro/in vivo (Council Directive 2003/85/EC6) is the only binding European document including some aspects of biosecurity. The implementation of this standard is mandatory for laboratories handling live foot and mouth disease virus (FMDV).
Criteria for establishing containment facilities
There are currently no common criteria on the European level for the establishment of containment facilities with regard to rationale, work to be performed, and placement of the facility. However, the EU Directive 2000/54/EC7 classifies microorganisms into 4 risk groups, based on the WHO criteria, and states that the application of Directive requirements will follow a risk assessment. Some countries may have national regulations, rules, or guidelines.
As indicated above, the differences between MS are huge in biosafety and laboratory biosecurity regulatory framework implementation. Most countries have a National Board of Health, a Central Public Health Institute, and a National Veterinary Institute providing expert advice on the handling of biological agents and toxins.
Standards
There are several ISO/EN standards available in the EU that can be applied for containment laboratory planning, construction, and operation e.g., ISO/EN 15189:2003 Medical laboratories—Particular requirements for quality and competence, CEN/CR 12739:1998 Biotechnology—Laboratories for research, development and analysis—Report on the selection of equipment needed for biotechnology laboratories according to the degree of hazard. In addition there are CEN standards developed for biosafety equipment e.g., autoclaves, biosafety cabinets, and personal protective equipment. National standards may cover the construction and the licensing of containment facilities, but there is no harmonization of licensing across Europe. Regular oversight and re-certification mainly depends on
![]()
5http://www.who.int/csr/resources/publications/biosafety/WHO_CDS_EPR_2006_6.pdf.
6http://www.fao.org/ag/againfo/commissions/docs/genses38/Appendix_10.pdf.
7 Op. cit. see note 2
national specifications. Member States have a national standardization institute making CEN standards available in the country.
Laboratory-associated Infections (LAI)
No harmonized system for the reporting of laboratory incidents and accidents is in place in the EU. Few laboratory-associated infections (LAI) and laboratory incidents/accidents have been reported in the literature from Europe during the recent decade (see Box E2-1). There is reason to believe that a serious underreporting is at hand. National reporting systems are not easily accessible. Historically, Northern European countries report higher numbers of LAI than other parts of Europe, presumably reflecting failure to recognize LAI as such as well as reporting inefficiencies. There is a variation between MS regarding to whom and when to report, who investigates the incident, and if there is a possibility for legal actions. Many MS are taking steps to improve the status of biosafety in their laboratories, and awareness of biosecurity issues is increasing.
Box E2-1
During 2000-2010, only 5 incidents of release and/or exposure to infectious agents in risk class 3-4 were reported in the literature from EU Member States:
• In 2000, a broken centrifuge tube caused 12 cases of brucellosis in Italy;1
• In 2003, work with genetically modified vaccinia strains in Germany caused vaccinia infection;2
• In 2005, a survey of 1,240 laboratory staff in Spain revealed 75 cases of brucellosis (43 microbiologists and 32 technicians);3
• In 2007, in the UK, inefficient decontamination of industrial material/leakage in the sewer system caused a release of FMDV causing infections in cattle and sheep, and huge costs to the Government and the British livestock industry;4
• In 2009, in Germany, a needle stick injury with Ebola led to no signs of infection after administration of an experimental vaccine.5
1Fiori P, Mastrandrea S, Rappelli P, Capuccinelli P. Brucella abortus infection acquired in microbiology laboratories. Journal of Clinical Microbiology 2000; 38: 2005-2006.
2Mempel M, Isa G, Klugbauer N, et al. Laboratory acquired infection with recombinant vaccinia virus containing an immunomodulating construct. J Invest Dermatol 2003; 120: 356-358.
3Bouza E, Sanchez-Carrillo C, Hernangomez S, Gonzalez MJ. Laboratory-acquired brucellosis: a Spanish national survey. J Hosp Infect 2005; 61: 80-83.
4Spratt, B Independent Review of the safety of UK facilities handling foot-and-mouth disease virus, Presented to the Secretary of State for Environment, Food and Rural Affairs and the Chief Veterinary Officer 31 August 2007
5Tuffs A. Experimental vaccine may have saved Hamburg scientist from Ebola fever. Bmj 2009; 338: 1223.
Future outlook - EU CBRN Action Plan
On June 24, 2009, the European Commission adopted its communication on strengthening chemical, biological, radiological, and nuclear (CBRN) security in the European Union—as an EU CBRN Action Plan.8 The overall goal is an all-hazard approach to reduce the threat of and damage from CBRN incidents of accidental, natural, or intentional origin, including acts of terrorism. The Action Plan has set up a number of goals within three areas: prevention, detection, and preparedness and response:
Prevention
• Develop EU lists of high risk CBRN materials and risk-based approaches to security;
• Enhance the security of high risk CBRN facilities;
![]()
8http://register.consilium.europa.eu/pdf/en/09/st15/st15505-re01co02.en09.pdf.
• Enhance control over high risk CBRN materials;
• Contribute to the development of a high security culture among staff;
• Improve the identification and reporting of suspicious transactions and behavior;
• Enhance the security of transport;
• Improve information exchange;
• Strengthen the import/export regime; and
• Strengthen cooperation on the security of nuclear materials.
Detection
• Establish a scenario-based modeling approach to identifying work priorities in the detection field;
• Establish trialing, testing, and certification schemes for CBRN detection in the EU;
• Develop minimum detection standards;
• Identify good practices related to the detection of CBRN materials, awareness-raising, and training; and
• Improve the exchange of information.
Preparedness and response
• Improve emergency planning;
• Strengthen countermeasure capacity;
• Improve domestic and international information flows regarding CBRN emergencies;
• Develop improved modeling tools and strengthen decontamination and remediation capacity; and
• Improve the capacity to conduct criminal investigations.
Actions encompassing all CBRN fields are listed as 67 horizontal actions whereas there are 14 B-specific actions listed, among them:
• To assist the MS in the proper implementation of applicable procedures at “the laboratory bench level” and in developing mechanisms for assessing and monitoring its correct implementation;
• The MS should establish:
![]() a registry of facilities possessing any of the substances on the EU list of high risk biological agents and toxins;
a registry of facilities possessing any of the substances on the EU list of high risk biological agents and toxins;
![]() a process to verify whether security arrangements of facilities are adequate, including diagnostic laboratories handling and possessing any of the EU list of high risk biological agents and toxins;
a process to verify whether security arrangements of facilities are adequate, including diagnostic laboratories handling and possessing any of the EU list of high risk biological agents and toxins;
![]() a mechanism within facilities storing biological agents and toxins on the EU list of high risk biological agents and toxins to regularly review the need for such biological agents and toxins;
a mechanism within facilities storing biological agents and toxins on the EU list of high risk biological agents and toxins to regularly review the need for such biological agents and toxins;
• The Commission together with the MS should take relevant steps so that:
![]() a comprehensive overview of the relevant regulations or standards at hand and their relevance to biosecurity and biosafety is achieved;
a comprehensive overview of the relevant regulations or standards at hand and their relevance to biosecurity and biosafety is achieved;
![]() facilities possessing substances on the EU list of high risk biological agents and toxins consider as appropriate the implementation of the CEN Workshop Agreement (CWA 157939), WHO Laboratory Biosecurity Guidance, or their national equivalent standards -unless equal or more stringent national regulations have to be considered;
facilities possessing substances on the EU list of high risk biological agents and toxins consider as appropriate the implementation of the CEN Workshop Agreement (CWA 157939), WHO Laboratory Biosecurity Guidance, or their national equivalent standards -unless equal or more stringent national regulations have to be considered;
![]() appropriate national regulations or standards are met as part of a national authorization or accreditation process or as a condition for issuing licenses for work with substances on the EU list of high risk biological agents and toxins.
appropriate national regulations or standards are met as part of a national authorization or accreditation process or as a condition for issuing licenses for work with substances on the EU list of high risk biological agents and toxins.
• The Commission together with the MS should encourage professional and other relevant associations working on bio-issues to develop and adopt codes of conduct for their members;
• The Member States together with the Commission should define requirements for biosafety officers (roles, competences, and training).
![]()
Other areas addressed in the B-specific list of actions are: transport, detection, and validation of methods, establishment of reference material for quality assurance, international cooperation and networking, research, implementation of good practices, and improved cooperation between relevant agencies in crisis situations.
It is the responsibility of each Member State to protect its population against CBRN incidents and to implement the action plan, whereas the European Union can provide added value and support projects across the EU, to ensure a coherent and consistent approach to cooperation on this issue between the Member States.
The implementation of the EU CBRN Action Plan is now in its second year. Meetings with MS experts are frequently arranged by the Department of Home Affairs (former JLS).
This page intentionally left blank.








