Analyzing Nanoparticles in Complex Mixtures
In real-world applications, synthetic nanoparticles are rarely if ever encountered in their native form. Rather, they occur mixed with various inert ingredients to create pills, tablets, and pellets. In the environment, however, nanoparticles interact with other materials, becoming coated with organic matter or clumping together in agglomerates. Research has shown that these and other transformations produce materials with chemical, toxicological, and environmental behavior that differ, sometimes in profound ways, from that of the native nanomaterial. As a result, it can be challenging to predict the consequences of releasing a nanomaterial into the environment. Speakers in this session addressed some of the challenges to measuring and predicting the properties and behavior of complex nanoparticle formulations and discussed the often surprising findings that come from studying nanomaterials as they occur in the real world.
DESIGN AND MANUFACTURE OF DELIVERY FORMS FOR SMALL PARTICLES
James Litster of Purdue University spoke about particles in the micron and submicron size range that are used in industrial applications, with a specific focus on the delivery forms for those particles. For example, catalysts and absorbents are often used in pellet form, detergents in granular form, and drugs in micronized form. Many of the new drug molecules being developed are poorly soluble, so drug formulators are using small particle delivery vehicles to increase the solubility and uptake of these molecules. Dry powder aerosols based on relatively large lactose particles are used to deliver drugs into the lungs, although dispersion of the powder aerosol is often poor. Whatever the route of administration, the goal is to deliver a drug payload in a way that does not change the drug molecule and that does not allow it to aggregate.
Although particulate delivery systems are used widely in many industries to solve specific delivery problems, they are not without their own issues. Dry powders create dust and can be hard to handle. They can flood out of hoppers or not flow at all. If inhaled, they can cause health problems. “We want to take advantage of their properties, but we want to handle them in delivery forms that are suitable for us,” said Litster. To illustrate the versatility of powder processing, he listed many of the reasons for packaging small particles in dry delivery form (Table 4-1).
The processes used to make these delivery forms are many and varied, Litster explained (Figure 4-1). Materials can be compacted by tableting, dry granulation, or compaction. They can be mixed with a liquid binder in a fluidized system to form granules or spray dried from slurries or solutions.
With this variety of methods available for creating small particles, a major area of research involves determining how the choice of method, or a change from one method to another, impacts the structure of the delivery form and its distribution properties. The real goal of research in this field, explained Litster, is to understand both those processes and the properties they impact to facilitate design of a granule that disintegrates and disperses in a particular way, that has a certain resistance to attrition, or that has a certain chemical stability and shelf life. “This is a non-trivial and only partially solved problem,” he said.
Many barriers to progress in this area exist. Researchers lack a quantitative understanding of how microstructure develops during processing of complex, multiphase delivery forms. In addition, predictive and quantitative scaling rules, and process design models that track multidimensional distributions of properties are relatively rare. Finally, robust on-line techniques for measuring the microstructure and distributions of important properties also are rare.
TABLE 4-1 Reasons for Packaging Small Particles in Dry Delivery Form
![]()
| Reason | Typical Application |
| To produce useful structural forms | powder metallurgy |
| To provide a defined quantity for dispensing and metering | agricultural chemical granules, pharmaceutical tablets |
| To eliminate dust handling hazards or losses | briquetting of waste fines |
| To improve product appearance | food products |
| To reduce caking and lump formation | fertilizers |
| To improve flow properties for further processing | pharmaceuticals, ceramics |
| To increase bulk density for storage | detergents |
| To control dispersion and solubility | instant food products |
| To control porosity and surface-to-volume ratio | catalyst supports |
| To improve permeability for further processing | ore smelting |
| To create nonsegregating blends of powder ingredients | ore smelting, agricultural chemicals, pharmaceuticals |
![]()
SOURCE: Litster, 2010.
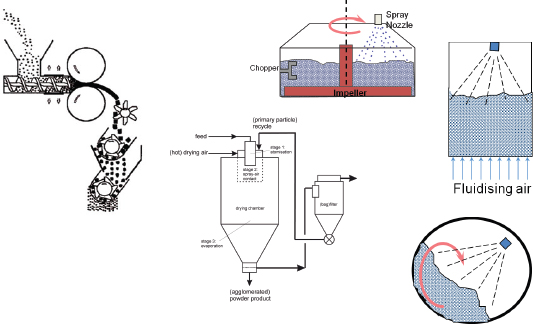
FIGURE 4-1 Approaches to creating delivery forms.
SOURCE: Litster, 2010.
Although these comments apply to powder processing in general, they are especially true when the primary particle size is less than 10 microns. In that size domain, Litster explained, “the surface forces that want the particles to stick together dominate over gravity and inertia of the particle, resulting in particles that are cohesive, flow poorly, and make complex structures with different levels of aggregation at different length scales.”
Fine powders behave very differently because they don’t fluidize properly. Litster demonstrated this fact with images showing the dramatically different behavior of a drop of liquid on a bed of fine powder versus coarse powder (Figure 4-2). The granule structure of fine powder is round, and the drop burrows into the bed and absorbs loose aggregates of the particles to make relatively round granules. Coarse powder does not have a uniform packing structure, and therefore the mechanism by which the liquid interacts with the powder is completely different. As a result, a disk-shaped rather than a spherical granule forms.
Models for Engineering Design
Creating an engineering design requires understanding at many different levels, including of the physics and accompanying models at different length scales. It is also necessary to understand the physical interactions that occur at the single-granule or single-particle level as well as at the bulk flow level. Also needed are macroscopic models that provide the structure of the delivery form or the distribution of properties and product models and account for how the delivery form
FIGURE 4-2 Differences in behavior for (top) fine powder (5 mm), and (bottom) coarse powder (70 mm).
SOURCE: Litster, 2010.
behaves when used as intended, that is, what happens to a medication tablet when it is swallowed or to a detergent granule when it is added to the washing machine. The properties of the particles of interest and the processing equipment used to make the delivery system serve as the input for the models, which span from the molecular to the bulk scale.
Together with his colleagues at the National Science Foundation-funded Engineering Research Center for Structured Organic Particulate Systems (SOPS), Litster is following a multiscale approach to compaction modeling, with the goal of developing function-structure relationships for the design and optimization of delivery systems. As an example of what this approach entails, he discussed a process wherein particles are used to surface coat a larger micron-sized particle, which then becomes part of a cluster of particles or granules and ultimately a tablet. The functions and properties of each of these particles must be characterized at different length scales. Also needed are modeling approaches that address length scales from the single-particle level through the macroscopic level, ultimately providing the tablet’s density and chemical distribution.
Litster discussed two research projects that are being carried out at the Center. The first project involves micron-sized particles of an active pharmaceutical ingredient (API) that are meant to be delivered by an aerosol. The project’s goal is to determine if drugs in this size range can be modified by coating them with smaller particles to turn cohesive powders into free-flowing powders that can be more easily metered and dosed in different delivery forms.
In a set of experiments, SOPS researchers used a mechanofusion approach to distribute a silica nanoparticle coating onto API particles and measured the bulk flowability of the powder versus bulk density. The resulting measurements revealed that the coating increased the bulk density of the API particles, which had the beneficial effect of increasing flowability.
Using atomic force microscopy, the SOPS researchers are currently studying a simpler system—one in which aluminum particles replace the API particles—to study how the roughness and morphology of the core particle affect adhesion forces and the properties of the final coated particle. They use a combination of modeling techniques to predict a distribution of adhesive forces across the core particle’s surface. The experiments have shown that the adhesion force is substantially reduced when the particles are coated, primarily because the coating creates a larger separation between the API particles, which reduces the overall van der Waal’s interaction. The experiments, said Litster, demonstrate that it is now possible to measure single particle properties for real particles that are nonspherical and have rough surfaces. Moreover, the measurements can be used to predict the behavior of the particles and to estimate how much coating is needed to produce the necessary level of disaggregation.
The second project involves making an agglomerate, as opposed to single particles, the delivery form. An intermediate step to making a tablet involves creating and then compacting a ribbon of a formulation, the key properties of which are its bulk density and its distribution. Near-infrared images of the ribbon revealed that variation in the density of the ribbon depends on the formulation used and the conditions under which the ribbon is produced. The ribbon’s density affects the granule and ultimately the tablet properties.
The researchers question whether they can predict density on the basis of particle properties and process condi-
tions. They have created a macroscopic finite element model that predicts the ribbon density distribution using data on the macroscopic bulk properties for the powder going into the ribbon-making machine. Now, the researchers want to be able to predict how the bulk properties of the ribbon would be altered by changes in the properties of the original mixture resulting from, for example, a change in the surface properties of the API.
To explore this idea, the researchers have created a multi-particle finite element model that represents a ribbon under stress. With this model, they can predict the behavior of a particle assembly under shearing or compaction. The surface energy, adhesive properties, roughness, and shape of the constituent particles are model inputs. The model also requires data on the mechanical properties of the particles, such as their elastic moduli and plastic properties.
In conclusion, said Litster, the end performance of small particles depends critically on how they are packaged. However, many problems still must be solved to predict product structure and performance from formulation properties and process variables. However, he added, multiscale approaches to both characterization and modeling hold promise for better engineered products.
Discussion
In response to a question about combination therapy and multiple drug absorption sites, Litster said that research has yet to address the issue of differential drug delivery of this sort, where one drug would be absorbed in the stomach and the other in the intestines. One possibility is to use some of the techniques for dealing with low-solubility drugs. In that case, making the drug particles tiny and delivering them in amorphous form can produce locally high drug concentrations in the stomach, where they will be absorbed faster than expected. It might then be possible to create larger, metastable particles that flow through the stomach and reach the intestines, where they will be absorbed.
Research is being conducted in these areas, and one approach to tackling this problem would be to measure primary properties and rate constants under well-defined conditions. Computer simulations could then predict what combinations of particle size, dissolution rate, and additional nucleation-inhibiting polymer would produce the best system.
In response to a question from Schwartz, Litster stated that the roles that thermodynamics and kinetics play in determining how particle size affects solubility remain controversial. Kinetics likely plays a role, but so, too, will thermodynamics because the concentration of drug in solution is significantly higher than the solubility of the most stable crystalline phase. Litster agreed with Schwartz’s comment that a metastable phase will yield a higher solubility, and he added that the pharmaceutical industry is showing a great deal of interest in supplying drugs in a stabilized amorphous state. The downside of such an approach is likely to be reduced stability and shorter shelf life.
Pedro Alvarez of Rice University said there is a general consensus in the environmental engineering community that engineered nanoparticles are being used and introduced into commercial products at a rate that is much faster than the rate at which we are acquiring the information needed to ensure that these materials are compatible with the environment, that we can handle them responsibly, and that we can dispose of them properly. It is his belief “we are at that point in history where we can actually steward nanotechnology as a tool for sustainability as opposed to it becoming a future environmental liability, but this requires taking a proactive approach to risk assessment, and I would like to argue that we are not doing enough in that regard.”
To make his point, he noted that the number of research publications on nanotechnology are doubling every 3 years, but the number of publications relating to environmental nanotechnology account for only 5 percent of that total. And in fact, the papers that actually make important contributions to understanding the fate, transport, reactivity, and bioavailability of nanoparticles in the environment account for only 0.25 percent of the total nanotechnology literature.
Alvarez’s group’s contribution to the field is in the area of how nanoparticles interact with microorganisms in the environment. Bacteria are the foundation of all ecosystems, but they also are very convenient models for studying cytotoxicity and therefore can serve as a useful model system for studying possible environmental impacts of nanotechnology. In other words, if a microbe is adversely affected by exposure to a substance, it indicates a need to worry about what the substance may do to higher order organisms.
After 7 years in the field, Alvarez has learned that it is difficult to generalize about the potential impact of nanomaterials because of the many ways in which they can interact with the microbial ecosystem. For example, some engineered nanomaterials, such as the fullerenes or ceria, require direct contact with the cell to exert a toxic effect. For those materials, anything that happens in the environment that hinders their bioavailability—becoming coated with organic matter, for example—could mitigate any potential toxicities. Other materials, such as quantum dots, can release toxic ions that kill microorganisms. Titanium dioxide nanoparticles and aminofullerenes can generate reactive oxygen species that kill microbes.
Understanding how these materials impact the microbial ecosystem is challenging because much of the work conducted today uses pure and very well-characterized nanoparticles, which Alvarez referred to as “virgin nanoparticles.” However, nanoparticles can undergo a wide vari-
ety of modifications and transformations when they are in the environment (Figure 4-3). “They are going to agglomerate, they are going to be chemically or biologically transformed,” he said. “They may aggregate and precipitate, and at the very least, they are going to lose coatings or acquire coatings.”
Each of these modifications will affect a nanoparticle’s mobility, reactivity, bioavailability, and toxicity. As a result, said Alvarez, the field has a real need for more analytical methods that allow for dynamic characterization of nanoparticles as they interact with bacteria in conditions that resemble their environment. For example, the idea of dose is not well understood because it is unclear how much of a material actually gets into a bacteria and whether it agglomerates or partitions inside a cell.
What is known, Alvarez said, is that nanoparticles tend to aggregate. Salts in the environment promote coagulation and precipitation, which reduce the potential toxicity of a nanomaterial. Aggregation reduces the specific surface area of a particle, which, in turn, decreases toxicity. Changes in surface chemistry and reactivity can also reduce the toxicity of aggregated particles compared to individual particles.
It is also clear that nanomaterials almost certainly end up in wastewater streams and ultimately in sludge. Therefore, it is important to consider the impact that nanoparticles in sludge might have on the terrestrial food chain. Using carbon-14 labeling, Alvarez’s group has shown, for example, that fullerenes accumulate in earthworms (Li et al., 2010).
Commonalities
Alvarez made two points about commonalities. First, as the concentration of nanoparticles increases to the point that it exceeds the absorption capacity of the soil, the nanoparticle no longer exists in its original structure. Instead, the nanoparticle forms large aggregates and precipitates that are more difficult to bioaccumulate. To illustrate why this is important, Alvarez compared the bioaccumulation of C60 to that of the polynuclear aromatic hydrocarbon phenanthrene. In theory, phenanthrene is less hydrophobic than C60 and, therefore, should be less bioavailable. However, bioaccumulation of C60 is 100 times lower than that of phenanthrene because its molecular size makes it more difficult to transport across the cell membrane. “The take-home message here is that the traditional risk assessment protocol using thermodynamically based partitioning coefficients to predict fate in the environment does not work,” said Alvarez.
The second commonality is found in the interaction of nanoparticles with natural organic matter (NOM). In the case of C60, NOM can serve as a sponge that traps this compound, hindering direct contact and bioavailability, and therefore toxicity. In one set of experiments, Alvarez’s team showed that adding just a few of milligrams of C60 to a bacterial culture caused the organisms to stop producing carbon dioxide, a sign of metabolic activity. Adding clean sand to the culture along with the C60 had no protective effect, but adding even
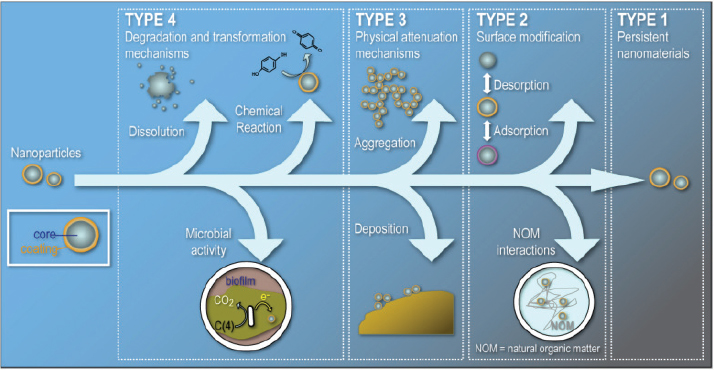
FIGURE 4-3 Processes that can possibly transform manufactured nanoparticles or modify their surfaces and aggregation states in the environment.
SOURCE: Alvarez et al., 2009.
a little bit of soil containing only 0.2 percent organic matter had a marked effect on bioavailability and restored metabolic activity (Li et al., 2008). They found that they could produce this protective effect by adding as little as 0.1 milligrams/liter (mg/L) of humic acid to the culture medium. Because of these results, and the fact that NOM levels in water in the environment are typically on the order of 3-10 mg/L or higher, Alvarez said he is much less worried about the acute ecotoxicity of C60.
Particle coating can also influence nanomaterial toxicity, explained Alvarez. His group has shown, for example, that iron nanoparticles coated with polyaspartate do not stick to bacteria, eliminating toxicity. The caveat to this observation is that coated nanoparticles are also more stable in the environment and more likely to bind to NOM in water, which may increase transport in the environment. Therefore, there may be a tradeoff in terms of reducing the possibility of acute toxicity versus increasing transport and long-term accumulation.
The bottom line, said Alvarez, is that no matter how toxic a material might be, if there is no exposure, then there is no risk. Preventing exposure by intercepting nanomaterials before they enter the environment might be the most effective means of limiting potential environmental damage. If eliminating exposure is not feasible, then another approach is to engineer away the properties that make a nanomaterial hazardous without compromising the properties that make it useful. This approach was taken when hydrofluorocarbons were engineered to replace chlorofluorocarbons when the latter were found to be environmental hazards.
Nanoparticles Are Abundant in Nature
Alvarez reiterated the ideas that the environment is filled with nanoparticles and that, given the large number of naturally occurring nanoparticles compared to the number of engineered nanoparticles, it may be difficult to discern the risk from exposure to the engineered nanoparticles. He believes that the size-dependent properties of engineered nanoparticles are more accentuated than those of many of the naturally occurring nanoparticles that have been in contact with nature for millions of years.
To truly understand exposure and risk, there is a need to obtain more information about the sources of nanoparticles. To do so, three questions need to be answered:
- What are the main entry points of engineered nanoparticles and the scale of discharge into the various environmental compartments?
- What forms of an engineered nanoparticle are being discharged?
- What are the realistic, environmentally relevant exposures to those forms?
He said the matter of inventorying nanoparticle production, use, and release within well-defined spatial domains must be settled. There is still debate as to whether inventorying should be mandatory or voluntary, and whether products should have labels informing consumers about the presence of certain nanomaterials. Some in the scientific community have suggested that nanoparticles be labeled (for example, to be fluorescent, or with a radioisotope) so they can be traced in the environment.
Alvarez noted that in March 2009 Rice University held an international workshop on the eco-responsible design and disposal of engineered nanomaterials at which about 50 environmental engineers and scientists were asked to address the question, “What critical knowledge gaps and opportunities exist to inform and advance the design of environmentally benign engineered nanomaterials and the management of wastes containing them?” Figure 4-4 summarizes their consensus (Alvarez et al., 2009).
Alvarez concluded by saying that there is much to learn about nanomaterial bioavailability, potential toxicity, and mechanisms of action by looking at how these materials interact with bacteria and other microorganisms, with the implication that materials that impact microbial processes can seriously damage an ecosystem’s health. The good news, he added, is that many things in the natural environment interact with nanomaterials in a way that reduces their bioavailability and therefore their potential to cause harm. Although nanotechnology-triggered microbial toxicity represents a worry, nanotechnology also offers many new approaches to addressing environmental problems in a more benign way. For example, nanomaterials may enable new approaches to disinfecting water that does not require the use of chlorine.
Discussion
Barbara Finlayson-Pitts commented that one message she got from Alvarez’s presentation was that equilibrium modeling is not going to work, just as it is not working with atmospheric nanoparticles. Alvarez agreed with this assessment, noting that nanoparticles constantly change in the environment as they acquire and lose surface features and undergo reactions with materials in the environment. Therefore, modeling their fate in the environment is very challenging; models for predicting the behavior of nanomaterials are not going to be as accurate as are models for dealing with pure chemicals.
Finlayson-Pitts also asked Alvarez to discuss how the shape and dimension of a nanoparticle affects its health impacts. Alvarez replied that it is important to consider that there are two categories of properties that matter for determining health impacts. The first is how the particle moves to a receptor, and the second is the properties of the original particle (such as shape or dimension). In terms of impact
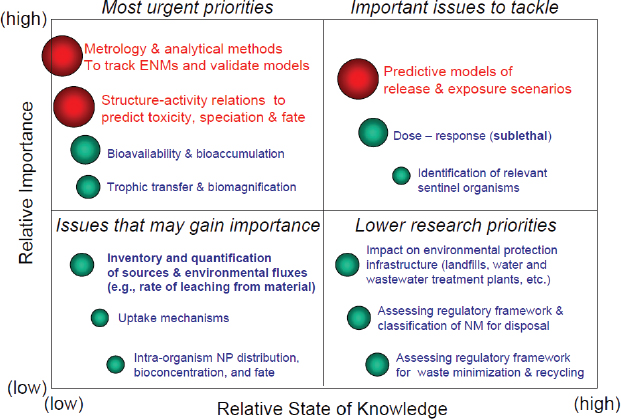
FIGURE 4-4 Toward eco-responsible nanotechnology.
SOURCE: Alvarez et al., 2009.
or reactivity, it may be that the properties of the original nanoparticle will end up being the most important, but that is still unknown.
Andrea Violi of the University of Michigan asked if there is more exposure to engineered C60 or to C60 particles emitted during combustion or volcanic eruption. Alvarez did not know the answer but noted that C60 reportedly can be produced by volcanic eruptions and that it might exist at trace levels in nature. He added, however, that when it comes to human health, the leading source of risk is likely to be associated with respiratory uptake of particles in the work environment.
SURFACE CHEMISTRY, TRANSFORMATIONS, AND GLOBAL IMPACTS
Vicki Grassian of the University of Iowa focused her remarks on transformations and surface chemistry of mineral dust, which makes up a large fraction of the aerosol mass. Mineral dust particles measure from about 0.1 microns (or 100 nanometers) to much larger particles. Particles at the smaller end of this range stay aloft for 1 to 2 weeks and are transported great distances by the atmosphere. During that 1- to 2-week time period, a lot of interesting chemistry takes place on the particle’s surface, and the particle transforms as it undergoes surface reactions with other chemicals that enter the environment.
Mineral dust is composed of oxides and carbonates, and, as the catalyst community has demonstrated, particulate oxides are reactive materials. As a result, a large amount of rich chemistry occurs on the surface of mineral dust particles in the atmosphere. Grassian described six different types of surface reactions and mechanisms that might be operating. “What we try to do is design laboratory studies to better understand this surface chemistry,” she said, with the goal of understanding the global impacts of small particles.
Grassian’s research approach involves examining the individual components of mineral dust to determine how chemistry differs among the different clays, oxides, and carbonates that occur in mineral dust. For example, her group has been studying the effect of relative humidity on the chemistry that takes place on the surface of various mineral particles. Although a great deal of research has been conducted on particle surface chemistry, she said little is known about what happens in the environment that lies between the two extremes of dry and wet, a domain in which surface-absorbed water is likely to play an important role.
In one set of experiments, for example, Grassian’s group looked at the reactions of nitric acid on calcium carbonate particles at various levels of relative humidity (RH), all greater than 10 percent RH. The study showed that nitrate forms on the surface of the particles at the same time that water is absorbed on the particles, but nitrate forms only in
the presence of water vapor. Microscopic images clearly show that, during the process, the particles undergo a marked change in morphology (Figure 4-5) and transform from solid to liquid. This transformation likely occurs in the atmosphere, Grassian said.
When these transformations take place in the atmosphere, they can alter the particles’ impact on climate. Grassian’s team has shown that as calcium carbonate particles react with nitric acid and transform into calcium nitrate particles, their absorption of water increases dramatically and the particles nearly double in size. These transformed particles are not only 100 times better at serving as cloud condensation nuclei than were the original particles, but also they become photochemically active. This increased photochemical increases the possibility that additional chemistry will occur at the surface of the transformed particles, leading to further changes that might influence climate in ways that are not yet understood.
Another type of chemistry that can occur on the surface of calcium carbonate particles starts with the absorption of sulfur dioxide. In the absence of water vapor, little happens when calcium carbonate particles mix with sulfur dioxide, even in the presence of oxygen. The surface absorbs sulfur dioxide, but little chemistry occurs. In the presence of water vapor, however, calcium sulfite crystals start to grow on the particles. Water vapor clearly triggers chemistry to take place on the particle surface. Grassian noted that molecular dynamics simulations of ion mobility over the surface of these particles would provide useful insights into these processes.
The results of these types of studies are now being used to help interpret measurements being made in the field. For example, field measurements in Israel and China have shown that calcium carbonate particles react with sulfur dioxide in the environment and that sulfite forms in an intermediate step that eventually leads to the production of sulfate (Laskin et al., 2005, Sullivan et al., 2006), which atmospheric chemists had not anticipated.
Complex Mineral Dusts
Grassian is working to forge even stronger links between her group’s experimental work and atmospheric chemistry. Recently, she and her collaborators have begun studying complex authentic mineral dust particles from different sources. As a first step, her team conducted elemental analysis on bulk samples collected from China and Saudi Arabia. All of the dusts contain silicon, aluminum, calcium, iron, magnesium, and a few other elements, but the relative levels of these elements vary across samples.
This finding raised the question of how best to think about those particles. “Maybe we want to measure elemental compositions of single particles and then look at the particles that contain 15 percent calcium and compare them to particles with less than 15 percent calcium,” Grassian said. “But what if all the calcium is on the inside of a particle that’s coated by a silica shell? Then perhaps what we really want to measure is single particle surface chemistry.”
In a series of experiments, Grassian took calcium-containing dust particles from several dust sources and mixed them with gaseous nitric acid. As expected from her team’s earlier work, the particles reacted with nitric acid, but the reaction was not uniform when viewed using scanning electron microscopy (SEM). SEM images showed that the reaction occurred only at places on the particle surface that associated with local composition of the particle, and hydroscopic growth was complex as a result.
Natural particles are more complex not only chemically, but also morphologically. They are not all spheres, which will impact remote sensing data from which aerosol concentra-

FIGURE 4-5 Calcium carbonate particles react with nitric acid in the presence of water vapor.
SOURCE: Grassian, 2010.
tions are calculated. Research is needed, Grassian said, to better understand how the complex shape distribution of natural dust particles influences optical and chemical behavior in the atmosphere.
Particle size is also heterogeneous in natural dusts, and the behaviors of particles in the atmosphere vary with their size. Particles can aggregate in the atmosphere, which impacts the particles’ size, shape, and density and their available surface area, which in turn impacts their surface chemistry and their dissolution in water droplets. Dissolution will then affect aggregation.
Grassian’s team has started to examine aggregation and dissolution of differently shaped and sized particles. In one study of iron nanorods and microrods, they found that isolated nanorods displayed enhanced dissolution compared to the microrods. However, in the aggregated state, nanorods were stable against dissolution, such that microrod dissolution was significantly suppressed (Rubasinghege et al., 2010). Aggregation affected not only the size of the particle, but also its chemistry.
To conclude, Grassian stated that it is important to recognize that particles are not stable entities in the atmosphere; rather they can undergo chemical and physical transformations that will impact their “climate” properties. Laboratory studies can provide insights into these transformations, but the complexity of real atmospheric dusts means that the insights may not reflect what really happens in the environment.
Discussion
In response to a comment from Abhaya Datye about the possibility that metals from catalytic converters may be getting into road dust, Grassian responded that there is evidence of those metals, including nanosized platinum particles, in road dust. She added that research is starting to look at the health effects of those metal dusts.
When asked what characterization tools she would like to have to advance her work, Grassian said she would like to perform single particle surface chemistry routinely, particularly of single particles that are components in a complex mixture. Alvarez seconded this wish, adding that he would like access to a technology capable of discerning surface properties or surface chemistries in complex matrices.
This page is Blank










