Modeling and Simulation of Small Particles
FROM ATOMISTIC SIMULATIONS TO HEALTH EFFECTS
Epidemiological studies, said Angela Violi of the University of Michigan, have demonstrated a correlation between exposure to an elevated concentration of particles and the development of fibrosis and asthma. A variety of studies has shown that smaller particles are able to penetrate and accumulate deep within the alveolar regions of the lung. Exposure is not limited, however, to the lungs, because research has shown that particles can translocate out of the lungs and circulate throughout the body, even crossing the blood-brain barrier and entering the brain.
According to data from the Environmental Protection Agency (EPA), most particulate matter (PM) in urban environments is carbonaceous (Figure 5-1), and most enters the urban atmosphere as part of diesel exhaust (EPA, 2010). Violi noted that particles from 1 to 50 nanometers in diameter represent about 1 percent of the particle mass emitted from a diesel engine, but from 35 to 97 percent of the number of particles. Current EPA particle emissions standards apply only to particles of 2.5 microns or larger, which will have no impact on the particles that are most worrisome. She added that exhaust particles are not pure carbon; they carry carcinogenic compounds such as benzopyrene and other polyaromatic hydrocarbons and thus can serve as effective delivery vehicles for dangerous compounds.
Carbon particles form during combustion, whether it is in an engine cylinder or in a candle flame. The process starts with fuel molecules that are not oxidized completely and instead react with one another to form precursor molecules, which in turn react with one another to form polyaromatic hydrocarbons over a timeframe of 10 milliseconds or so. These reactions continue, and by 50 milliseconds the molecules grow to a point that chains and agglomerates begin to form. It is this time period, when gas-phase carcinogenic species form, but before soot forms, that Violi is working to model, with the eventual goal of examining how nanoparticles interact with biological systems.
“The problem with this kind of process is the timescale,” she explained. While particle formation takes place on a millisecond timescale, other chemistry that may take place on the surface of a growing particle, such as intramolecular rearrangements, can happen on the nanosecond to microsecond timescale. Modeling across both timescales is challenging.
Molecular dynamics modeling works very well for fast events, but even the biggest computers can only handle nanosecond timeframes for the complex processes involved in combustion particle formation. Continuum modeling could span the necessary timescale, but at the expense of excluding most of the chemical details. Instead, Violi’s group developed a computational code that combines aspects of molecular dynamics with a kinetic Monte Carlo approach. The resulting stochastic code, which she named Atomistic Model for Particle Interception (AMPI), follows the growth of particle formation over a long timescale and large length scale and retains information on the chemical and physical properties of the system (Violi and Venkatnathan, 2006, Chung and Violi, 2007).
Using this model, Violi and her colleagues can follow particle formation as a function of fuel and have shown that particle morphology and chemical composition change dramatically even though the number of carbon atoms in the combustion products remains the same. The model, which can grow particles up to 20 nanometers in diameter, also reveals that molecular morphology affects particle morphology, which in turn affects how the particles agglomerate and whether they form block-like structures or more flaky structures.
Violi explained that AMPI can provide information not only on particle morphology, but also on hydrogen-to-carbon
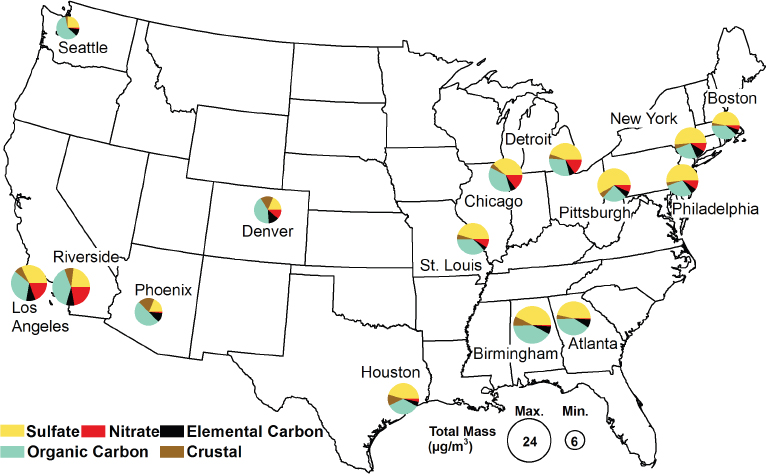
FIGURE 5-1 Four-season average of PM2.5 composition for 15 U.S. cities, 2008.
SOURCE: EPA, 2010.
ratio, free radical concentration, surface reactivity, optical properties, functional group distribution, porosity, surface area, pore size distribution, surface-averaged energy distribution, and particle density. “The whole point of this modeling approach is that the transition from gas phase to particles is very fast, and there are very few techniques—I think almost none—that can deal with the 1-to-50-nanometers range,” she said. “So modeling can basically help to fill the gap in these regimes that we’re interested in.”
Interactions with Biology
The AMPI calculations generate a list of possible chemical structures that would be present in a carbonaceous material in the environment, but the question then becomes, how do these chemicals and particles interact with biological systems? The answer, explained Violi, is the province of nanotoxicology at the level of the individual cell, the smallest unit in a living organism, and the point of interaction between a carbonaceous nanoparticle and a cell is the cell membrane.
The cell membrane is a lipid bilayer composed of lipids, cholesterol, and proteins surrounded by water. It is a fluid system, one in which the lipids are always moving and through which diffusion can occur. Using a molecular dynamics approach, Violi’s team has been able to model how carbonaceous particles of varying morphologies impact the natural diffusion of lipids that is constantly taking place in the cell membrane. Indeed, model calculations show that carbonaceous nanoparticles immediately alter lipid diffusion and that the alterations depend on the morphology of the particles (Fiedler and Violi, 2010). Because the proper functioning of the lipid membrane depends on lipid diffusion, this model suggests at least one mechanism by which these particles could cause toxicity.
Violi and her team have since used this model to test whether the decision by an international consortium of regulatory agencies, including EPA, to use C60 fullerenes as the only standard for toxicological testing protocols of nanoparticles in the range of 0.5-1.5 nanometers is scientifically sound. The team ran the model using the C60 nanoparticle to see if the results reproduced all of the characteristics seen in model runs that used carbonaceous material from combustion. The answer was no; the reason appears to be that an important parameter is surface area, and the surface area of C60 is small compared to that of many other carbonaceous nanoparticles.
Violi’s team also modeled the interaction between carbonaceous particles and lung surfactant, a lipid-based material. They demonstrated that the particles eventually become wrapped in surfactant lipids. “The point is, even if you start with a carbonaceous material, by the time it translocates into the body it’s a totally different animal,” said Violi.
Discussion
In response to a question from Patricia Thiel of Iowa State University about the existence of experimental data to test the results of these modeling activities, Violi said that her team has used a differential mobility analyzer, which provides information of particle size to sample particles in actual flames. The data from those experiments validate the model in terms of particle size distribution. Currently, they do not have data on the chemical makeup of those particles. Regarding the C60 modeling results, her team is in the process of creating synthetic lipid bilayers and will generate diffusion data using commercially available samples of C60 to test their model results.
SCALING SIMULATIONS TO MODEL ENVIRONMENTAL IMPACTS
Douglas Tobias and his colleagues at the University of California at Irvine are trying to understand how the surface of a particle differs at the atomic level from the bulk of a particle and how the interface between that surface and its environment affects the chemistry of a particle. His group also is starting to explore the more coarse-grained interactions between particles and biological systems, particularly the lipid bilayer that makes up the cell membrane.
To Tobias, atomistic modeling means putting every atom of the system into the model and using molecular dynamics to solve the F = ma equation for every particle in the system using a ball-and-spring model to represent atoms and bonds. The calculations continually update the positions and velocities of each atom and generate their trajectories.
The aerosol particles Tobias is interested in are typically on the order of 100 nm in size and contains too many atoms to simulate in its entirety. Because his interest lies primarily in the interface between a particle and its environment, Tobias’ approach is to carve out a little chunk of the particle to create what he calls slab models (Figure 5-2). These slabs can be decorated with organics and include a variety of molecules at the interfaces.
Another type of model, used if more detail is required, replaces the ball-and-spring representation of the atoms and bonds with nuclei and electrons, and solves the electronic structure problem, producing a wave function that generates the force needed to perform ordinary molecular dynamics calculations. This type of model shows that interactions at an interface are very dynamic and occur on a timescale of a few picoseconds. Current applications for this type of model include acid-base chemistry and oxidation of halides at the air-water interface and in bulk aqueous solutions.
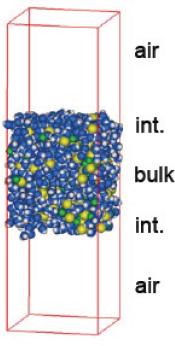
FIGURE 5-2 A “slab” model of aqueous aerosol surfaces.
SOURCE: Tobias, 2010.
Modeling larger systems, albeit with less detail, is possible using a technique known as coarse-graining, which involves lumping together certain groups of atoms that are chemically similar into so-called coarse grain beads. This method can drastically reduce the number of particles being simulated. Tobias explained how this approach was used to model a membrane lipid by reducing 138 atoms to 15 beads (Marrink et al., 2007). Coarse graining, which involves defining different types of interactions, such as polar, mildly polar, and charged, can handle mesoscopic systems over microsecond timescales with good molecular detail.
Understanding Sea Salt Aerosols
Using these modeling approaches, Tobias’s group has been studying the chemistry of sea salt aerosols. Sea salt aerosol is produced when bursting bubbles shoot 100-nanometer to 100-micron droplets of sea water into the atmosphere. The droplets are primarily concentrated salt solutions consisting mostly of sodium chloride with small amounts of bromide. The latter is important because bromide is more reactive than chloride. Various field and laboratory measurements have established that these halides in sea salt can be oxidized to produce very reactive molecular halogen species (Figure 5-3). “Because of the importance of molecular halogens in the atmosphere, this chemistry is important to understand in terms of mechanisms and kinetics in order to find out if these compounds are going to be atmospherically relevant,” he explained.
Tobias became involved in this research because existing kinetic models failed to reproduce the actual production of molecular chlorine from sea salt that had been measured both in the laboratory and field studies. Missing from these models, Tobias said, was surface chemistry that would make the process heterogeneous rather than homogenous.
Using a molecular dynamic simulation of a concentrated sodium chloride solution, Tobias’s modeling studies surprisingly revealed that chloride anions rose to the surface of the salt solution while sodium tended to stay beneath the surface. Following the extent of surface exposure of the chloride anions in time showed that 12 percent of the droplet surface was covered by chloride anions, a finding that contradicted more than a century’s worth of conventional wisdom about the behavior of halide ions at an air-water interface; that is, the ions should stay inside the solution. What appears to happen, however, is that sodium and fluoride ions are repelled from the interface while chloride ions adsorb to the interface. Bromide and iodine ions, meanwhile, also concentrate at the surface and act as surfactants (Jungwirth and Tobias, 2001).
“What we’ve seen is that anion adsorption increases with ion size and polarizability,” said Tobias. “These are actually important concepts for understanding the behavior of ions near interfaces.” And, in fact, this basic picture of increasing anion adsorption with the halide mass has recently been confirmed by x-ray photoelectron spectroscopy (Ghosal et al., 2005).
The next step in Tobias’s modeling effort was to insert hydroxyl radical. The model shows that hydroxyl radicals also accumulate at the air-water interface and frequently encounter chloride ions. Quantum chemical electronic structure calculations and molecular dynamics simulations suggest that a mechanism based on the formation of a hydroxyl radical-chloride complex is plausible. When this mechanism is then used to refine the original model, it accurately reproduced the observed production of chlorine from sea salt (Knipping et al., 2000).
These models are not just of theoretical use, noted Tobias. When the sea salt reaction is included in airshed models of the South Coast Air Basin of California, which are used to calculate regional ozone levels in the Los Angeles Basin, the adjusted models predict that ozone levels will increase over most of the basin, and that some regions will experience quite significant increases at certain times of the day (Knipping and Dabdub, 2003).
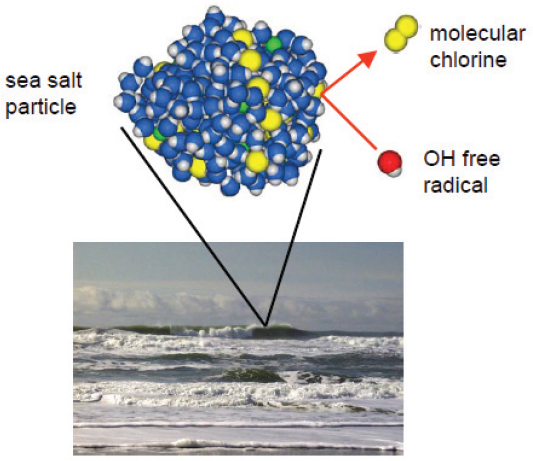
FIGURE 5-3 Sea salt particles are primarily concentrated sodium chloride, but also contain reactive species such as molecular chlorine.
SOURCE: Tobias, 2010.
Small Molecule Interactions with Biological Structures
Recently, Tobias has become interested in modeling how particles traverse membranes as an approach to understanding how particles may cross the blood-brain barrier via a passive transport process. Preliminary work using coarse-grained molecular dynamics modeling showed that a spherical, nonpolar nanoparticle can easily pass through the lipid bilayer of a larger vesicle. In this simple model, the highly curved surface of the vesicle likely plays an important role in facilitating the easy passage of the inert nanoparticle through the membrane.
His group is now working on more systematic studies to determine which particle properties actually determine its ability to cross membranes. This effort has so far shown that particle polarity is one feature that exerts a strong influence on a particle’s ability to enter and cross membranes. Tobias plans to explore how variations in size, shape, and surface chemistry of a particle impact membrane permeability. He also plans to examine the effect on passive transport that results from changing membrane composition and adding lipid-embedded proteins to the lipid bilayer. As a closing comment, he briefly described atomistic modeling work performed by another group that showed that C60 fullerenes are capable of entering the pore of a potassium channel and becoming lodged there, blocking the channel and shutting off potassium ion flow (Kraszewski et al., 2010).
Discussion
In response to a comment by Steve Schwartz about the presence of organic material on the surface and the fact that this material can form a concentrated film on the surface of sea salt particles, Tobias said that his group has modeled particles that include a surfactant layer (Figure 5-4). The calculations show that gases can get stuck in the surfactant layer and therefore undergo additional collisions with the reactive species in solution. In that sense, the surfactant layer can act as a barrier to exclude gases from the initial sea water droplet, but it can also enhance reactivity by trapping those gas molecules that do enter the droplet. Molecular dynamic simulations suggest that these two competing processes actually balance each other out, with the collision rate between reactive trace gases and ions in solution being very similar with and without the surfactant layer. In response to a second question from Schwartz, Tobias stated that the dynamic simulations include evaporation.
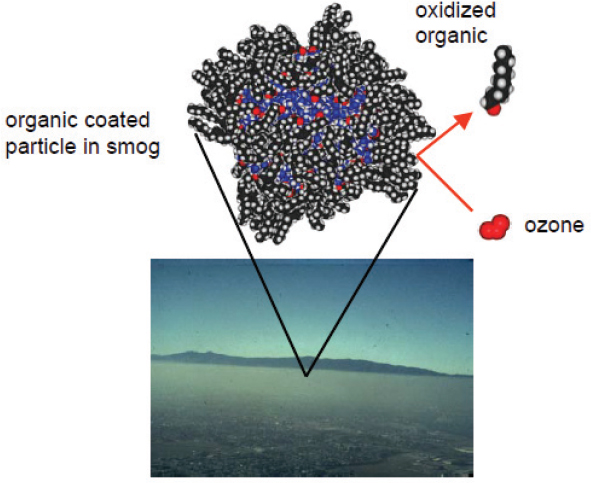
FIGURE 5-4 Sea salt particles coated with organic material in smog can lead to an increase in ozone levels.
SOURCE: Tobias, 2010.
In response to a question from Barbara Finlayson-Pitts about whether the soot models account for the complex organic functionality found on the surface of soot particles, Violi said that the modeling code she described tracks all of the surface chemistry and all of the functional groups and reactive sites on a particle’s surface. The models also reproduce the finding that radical species exist on the surfaces of soot particles.
Mort Lippmann commented that he was encouraged that modeling efforts are being directed at aqueous droplets such as hygroscopic sea salt because liquid microdroplets are very important from a health perspective. He added, however, that he would like to see more work on droplets as well as on particles other than carbon because metal-containing nanoparticles are probably a bigger issue for human health. Rhonda Stroud remarked that looking at liquid particles is a challenge because many of the tools available today are geared toward studying solid materials. However, efforts are under way to design microfluidic cells for electron microscopy instruments that may be useful for studying soft materials such as droplets.
Steve Schwartz suggested that the modeling community might want to pursue knowledge of the ice crystal habitat in clouds given the importance of ice crystals to climate. Violi agreed with Schwartz’s subsequent comment that it could prove fruitful to examine the kinetics of ice crystal growth under varying conditions, and Tobias noted that the atomistic simulation community has undertaken a large effort to model ice crystal formation. Stroud added that NASA Ames has a transmission electron microscope specifically designed to take in situ measurements of ice that may provide the kind of data needed to inform and validate modeling efforts.
Schwartz reiterated his earlier comment about the importance of validating model results with experimental data, particularly those on the biological impacts of nanomaterials. Violi remarked that her team always tries to conduct its modeling work in collaboration with experimentalists. The work on lipid bilayer modeling, however, is a relatively new endeavor, and efforts are under way to develop experimental benchmarks for these models. Tobias agreed with both sets of comments and suggested some experiments that could prove useful. For example, x-ray diffraction studies on stacks of lipid bilayers could look for changes in the density distributions with and without particles. If the particles were deuterium labeled, then it would be possible to use neutron diffraction to determine the exact location of the particles and how they are distributed in the bilayer.






