Discussion Paper1
Canadian Strategy on Patient-Oriented Research
Jean L. Rouleau, Institute of Circulatory and Respiratory Health, Canadian Institutes of Health and Research; Penny Moody-Corbett, Strategy on Patient-Oriented Research, Canadian Institutes of Health and Research2
In Canada, as elsewhere, the translation of health research outcomes to development of products and services for health care and final implementation in patients does not progress as rapidly, efficiently, or successfully as it should. In order to address this problem, Canada has embarked on a bold new strategy to improve health care and health care delivery based on evidence from research outcomes and integration of knowledge to the policy makers and users. The Strategy on Patient-Oriented Research (SPOR) is a pan-Canadian program involving health researchers and professionals, policy makers, and patients. It is a partnership led by a national steering committee which is co-chaired by the president of the Canadian Institutes of Health Research, the leading funder of health research in Canada, and the president and chief scientific officer of the University Health Network, one of Canada’s leading hospitals and research institutes, and includes membership from all levels of government, the private
______________________
1 The views expressed in this discussion paper are those of the authors and not necessarily of the authors’ organizations or of the Institute of Medicine. The paper is intended to help inform and stimulate discussion. It has not been subjected to the review procedures of the Institute of Medicine and is not a report of the Institute of Medicine or of the National Research Council.
2 Participants in the activities of the IOM Forum on Drug Discovery, Development, and Translation. This discussion paper is based on a submission to the Forum’s November 2011 workshop, Envisioning a Transformed Clinical Trials Enterprise in the United States: Establishing an Agenda for 2020, to inform the workshop discussions surrounding international case studies in the area of clinical research transformation.
sector, health charities, health science networks, universities, and patient advocacy. The goal of the Canadian patient-oriented research strategy is to improve health outcomes and enhance patient’s health care experience based on translation of research outcomes into the health care system. The Strategy consists of four major objectives:
1. Improvement of the human health research environment and infrastructure.
2. Establishment of mechanisms to better train and mentor health professionals and non-clinicians in health research.
3. Improvement of organizational, regulatory, and financial support for multisite studies.
4. Integration of best practices into the health care system.
In order to improve the human research environment and infrastructure in Canada, SPOR will help develop specialized Patient-Oriented Research Networks (hereafter, Networks) and specialized research service centers referred to as Support for People and Patient-Oriented Research and Trials (SUPPORT) units. The Networks will be led by a team of principal investigators with internationally recognized research credentials and health systems/delivery expertise. The Networks will be national in scope and will bring together a unified group to build a critical mass of technical and scientific expertise to provide research leadership in an effort to improve the delivery of care through the development and validation of health care interventions. The Networks will be focused in specialty areas of national importance (e.g., mental health, community-based primary health care, etc.) as judged by the national steering committee, and the Networks will work with international partners to generate evidence on best practices for patient care in the community. Through mentoring and training, the Networks will provide the tools for the next generation of health researchers to better integrate research with improved health care and health care delivery.
In order to ensure nationally distributed health research services and expertise, SUPPORT units will be developed and housed in each of the provinces or regions of the country. The SUPPORT units will provide the personnel and infrastructure to support large-scale multi-site national and international studies involving patients and/or patient records. The units will themselves be centres of research excellence and opportunity, provided through senior research chairs with specializations in areas such as health research methodologies, health systems research, and knowledge translation. The SUPPORT units will include people with expertise to design, analyze, and manage large-scale, multisite studies (e.g., bio statisticians, epidemiologists, health economists, etc.). The SUPPORT
units will offer consultation services for the research community which will be provided by qualified personnel and trainees who will gain practical experience in this work environment while providing support to the research community. Expertise from the SUPPORT units will be available for local and regional health initiatives. Nationally, the SPOR initiative will ensure common approaches for SUPPORT units across the country and thereby enhance the ability of Canadian researchers to engage in large national studies, such as those initiated by the SPOR Networks (see Figure 1). It is anticipated that the SUPPORT units will be recognized internationally as an access point to the Canadian patient-centered research community.
Health researchers and policy developers recognize the advantages as well as the challenges of doing research in Canada. As in other international jurisdictions, it is acknowledged that complex organizational, regulatory, and financial support for clinical and health research can act as barriers to effective research or implementation of research outcomes. In order to address these areas, SPOR has established external advisory committees that will provide specific direction or guidance for improved or coordinated national activities. For example, over the past 6 months, Canada has embarked on a common contracts pilot program facilitated by the Canadian Institutes of Health Research, R&D (the umbrella organization for the Canadian pharmaceutical industry) and the Association of Canadian Academic Healthcare Organizations. External advisory committees have been established to address topics that will simplify and focus clinical research reporting and develop more flexible and adaptive protocols; streamline the process of research ethics review, in particular for multisite studies, and provide appropriate infrastructure and access for the rich source of population and administrative data and electronic records in Canada. In addition, an external advisory committee exists to develop a strategy for students and young scholars, both clinical and non-clinical, wishing to pursue careers in the field of patient-oriented research.
The success of initiatives like SPOR in resolving the multiple challenges facing patient-oriented research in Canada relies upon many factors, some of which are specific to Canada. SPOR has three characteristics that, although present in other national and international initiatives, have perhaps received greater attention in Canada’s initiative. First, all programs require partnerships and buy-in from the community in order to assure alignment of all stakeholders and to optimize coherence and impact. Partners include federal and provincial governments, academic institutions such as academic health science networks, universities and institutes, life science industries, charities, funding agencies and, indeed, all health research–related stakeholders. Second, in order to optimize coherence and impact, the SPOR program is a comprehensive one that
attempts to cluster the multiple challenges and solutions facing patient-oriented research under one umbrella. And third, SPOR proposes a major focus on developing clinical research efforts addressing the needs for improving the quality and efficiency of our health care system.
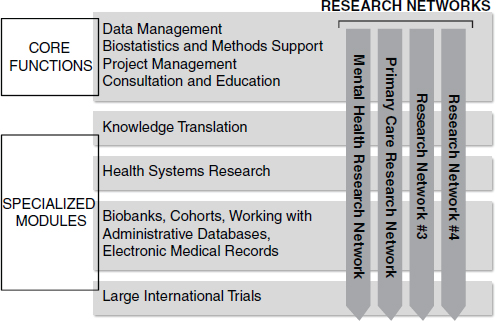
FIGURE 1 Relationship between SUPPORT units and Research Networks: When combined, SUPPORT units will provide the infrastructure and skills for highly specialized Research Networks to identify and tackle key clinical questions. The primary focus of Research Networks #3 and #4 has not yet been decided by the national steering committee.




