Improving Public Participation in Clinical Trials
In previous Forum workshops in the clinical trials series (IOM, 2010a, 2012a), it has been repeatedly observed that increased public understanding of, and commitment to, the importance of clinical trials is a key component of enhancing the CTE and achieving a learning health system. The participation of both clinicians and patients and healthy volunteers has been considered in these discussions. The workshop’s second session, “Developing a Robust Clinical Trials Workforce,” and the following keynote session explored ways to identify and meet workforce needs and to enhance public engagement in the CTE. Many speakers noted that there is an insufficient supply of highly trained researchers to lead, conduct, and analyze clinical trials and proposed how a shortfall of these professionals might be overcome. Building on discussions from a previous IOM workshop (IOM, 2012a), participants further elaborated on ways to expand people’s support for, and involvement in, clinical trials.
DEVELOPING A ROBUST CLINICAL TRIALS WORKFORCE
Should we go so far as to expect, not just encourage, participation in research?
—Ann Bonham, Association of American Medical Colleges;
Robert Califf, Duke University Medical Center;
Elaine Gallin, QE Philanthropic Advisors; and
Michael Lauer, National Heart, Lung, and Blood Institute (NHLBI)
A Five-Tiered Structure1
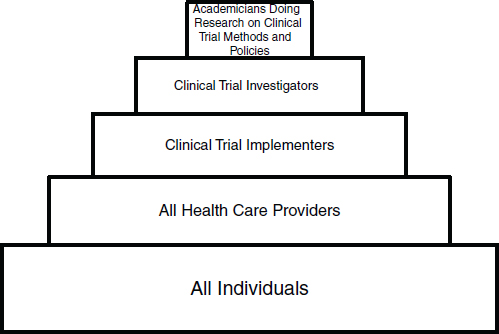
A clinical trials workforce, organized in several dimensions that reflect the broad mission of the CTE, the specific disciplines involved, and the levels of desirable expertise, would consist of several overlapping groups. A construct of five workforce groups was described in a Discussion Paper prepared by Ann Bonham, Chief Scientific Officer, Association of American Medical Colleges; Robert Califf, Duke University Medical Center; Elaine Gallin, Principal, QE Philanthropic Advisors; and Michael Lauer, Director, Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute (NHLBI), NIH. (See “Developing a Robust Clinical Trials Workforce” in Appendix E.) The co-authors suggested that the structure of the workforce groups necessary to meet the demands of the CTE might resemble a pyramid with five tiers (see Figure 3-1):
• Public—the broadest base of the pyramid consists of patients, families, and citizens who, in the final analysis, have the greatest stake in research results. This tier consists of engaged citizens who support development of the CTE as a national resource or public good and who enroll in trials on a volunteer or basis.
• Community practitioners—health professionals who participate in trials as part of their clinical practices, or at least help enroll their patients as participants. This group will include physicians, nurses, pharmacists, social workers, physical therapists, respiratory therapists, and other health professionals.
• Implementers—individuals who devote specified portions of their professional efforts to serving as principal investigators or collaborating co-investigators, with primary responsibility for implementing clinical trials at a hospital or research site. This workforce group would include physician-scientists, nurse-investigators, clinical pharmacologists, research-oriented social workers, operations specialists, data managers, computer specialists, clinical research coordinators, and research site managers.
• Investigators—leaders and designers of clinical trials and scientific experts who develop tools and innovative approaches for conducting
______________________
1 This section is based on the presentations and Discussion Paper by Ann Bonham, Chief Scientific Officer, Association of American Medical Colleges (unable to attend workshop); Robert Califf, Vice Chancellor for Clinical Research, Director of the Duke Translational Medicine Institute, and Professor of Medicine in the Division of Cardiology at the Duke University Medical Center; Elaine Gallin, Principal, QE Philanthropic Advisors; and Michael Lauer, Director, Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute (NHLBI), NIH. (See Appendix E for the Discussion Paper “Developing a Robust Clinical Trials Workforce.”)
SOURCE: Bonham et al, 2012. Developing a Robust Clinical Trials Workforce. Discussion Paper, Institute of Medicine. (See Appendix E.)
trials. This group will include biostatisticians and informaticists, among others.
• Methodologists—the apex of the pyramid consists of research-minded experts who explore the methodologies of conducting clinical trials and design the analysis portions of studies. This group of academicians will include clinical investigators, biostatisticians, epidemiologists, and health services researchers.
The co-authors postulated that a great deal of mobility and teamwork would occur among and across these groups. Each tier, in this vision, would be associated with appropriate education or training, geared to individuals’ interests and capabilities. This would include training in research cooperation, or teamwork, across disciplines and among workforce groups. Table 3-1 suggests how other training essentials might match up with the five described tiers. In general, according to the co-authors, the size of each tier is currently inadequate to contribute enough knowledge to achieve transformation of the CTE. Expanding the size of the three top tiers (methodologists, investigators, and implementers) could require development of more rewarding career paths.
TABLE 3-1 Education and Training Needs of Groups in the Clinical Trials Workforce
| Workforce Groupa | Education and Training Needs |
| Public | Awareness of how clinical research can lead to higher quality care; information about available trials and concept of equipoiseb; relevant informed consent policies. Marketing techniques might be used to reach this group. |
| Community Practitioners | Introduction to clinical trials; core competencies in translational and clinical research and public health |
| Implementers | Pragmatic training in management of research projects; discipline-specific education and training |
| Investigators | Education and training in clinical subspecialties (such as cardiology and infectious diseases), biomedical research, biostatistics, health services research, regulatory science, epidemiology, computational biology, genomics, and new technologies (such as imaging, cell and tissue engineering, and nanotechnology) |
| Academicians or Methodologists | Doctoral-level education in health research disciplines |
a See Figure 3-1.
b Equipoise is the point at which a rational, informed person has no preference between two (or more) available treatments (Lilford and Jackson, 1995). In clinical research, the ethical concept of equipoise is satisfied when genuine uncertainty exists as to the comparative therapeutic benefits of the therapies in each arm of a clinical trial.
SOURCE: Bonham et al., 2012. Developing a Robust Clinical Trials Workforce. Discussion Paper, Institute of Medicine. (See Appendix E.)
Across the CTE workforce, increased demographic diversity—namely, greater levels of participation by racial and ethnic minorities—could help ensure that research conduct is relevant to, and research results serve the needs of, largely underserved communities. Those who experience health disparities often do not participate in trials at rates high enough to constitute subpopulations from which statistically significant findings are produced (e.g., Pollack, 2011). Inasmuch as current U.S. Census projections suggest that by 2020 nearly one in three Americans will be African American or Hispanic, improving the health of these populations is essential to maintaining and improving the health of the country as a whole. Similarly, higher rates of participation in clinical trials by people over age 65, and especially over age 85, could make trial results more relevant to the very large and expanding elderly population, given projections that 1 in 5 U.S. residents will be 65 or older by 2030 (Vincent and Velkoff, 2010). Clinical trials participation by overweight or obese people, people with chronic diseases, and residents of urban communities also will have to
increase in order for research to be representative of the total population. Women and racial minorities remain underrepresented in the ranks of principal investigators. One study found that 16.9 percent of White physicians participate as principal investigators in clinical trials compared to 14 percent of African American physicians, 10.8 percent of Hispanic, and 9.6 percent of Asian physicians (Tufts Center for the Study of Drug Development, 2007). The study also found that 10.9 percent of female physicians participate as principal investigators compared to 16.9 percent of male physicians. The co-authors noted that, even if demographic parity is not achieved in all areas of the workforce, the CTE could benefit if researchers are skilled in engaging the community in clinical trials and able to accommodate demographic trends in designing, implementing, and analyzing clinical trials.
OPPORTUNITIES TO CREATE A SUSTAINABLE WORKFORCE FOR THE CLINICAL TRIALS ENTERPRISE
In a panel discussion, the session chair, panel presenters, Discussion Paper co-authors, and audience members reacted to and built upon the ideas contained in the Discussion Paper. Participants included session chair Sherine Gabriel, William J. and Charles H. Mayo Professor of Medicine and Epidemiology, Mayo Medical School; and panelists Briggs Morrison, Senior Vice President and Head of Worldwide Medical Excellence, Pfizer Inc.; and Rebecca Jackson, Associate Dean for Clinical Research, Professor of Medicine and Director of the Center for Women’s Health at The Ohio State University. This section provides an integrated summary of their remarks and should not be construed as reflecting consensus or endorsement by the workshop participants, the planning committee, the Forum, or the National Academies.
The Public
The clinical trials “workforce” of the future may consist largely of patients themselves. Public participation in clinical trials could increase if biomedical knowledge is widely accepted as a “public good,” benefiting everyone, so that people perceive a duty to contribute (Schaefer et al., 2009). Already, many patients and patient advocates, including disease-specific organizations, have exhibited a superior understanding of clinical trials. For example, the National Marfan Foundation says on its website that the organization “does not recommend switching from a beta blocker to losartan,” pending results of a clinical trial of losartan still in progress (National Marfan Foundation, 2011). The National Foundation for Infantile Paralysis pioneered public support for clinical research in the early
20th century, partly through the March of Dimes, and this tradition continues with efforts such as the Love/Avon Army of Women, which enlists participants in breast cancer trials in collaboration with the American Association for Cancer Research and the National Breast Cancer Coalition. In the rare diseases clinical research network involving 93 diseases, patient advocacy groups participate in research through such activities as protocol development, study steering committee membership, and review of informed consent policies.
Formal credentialing of individuals also can facilitate broader public participation. In Cleveland, efforts are under way to provide certification in clinical research to community leaders, who then help develop specific research projects.
The low level of health literacy in the U.S. population is well documented (IOM, 2004, 2011c; Rudd et al., 2007). Teaching a basic medical vocabulary and the principles of clinical research in high schools could better prepare people to participate as volunteers in clinical trials, while also introducing more students, including minority students, to the possibility of careers in clinical research. Incorporating clinical research into the high school curriculum could lead to students’ assisting their parents with participation in clinical research studies, including clinical trials. Several workshop participants noted that, given the pace of change in both medical science and health care delivery, the ability to be an “informed consumer” is essential to maintaining health and maximizing benefit from the health care system.
Community Practitioners
Negative physician attitudes toward research, often manifested through limited communication with patients about opportunities to participate in clinical trials, was mentioned by some participants as a major reason why clinical trials today struggle to recruit and retain patients. Only about 7 percent of adult Americans report that a physician has ever suggested to them that they participate in a clinical research study, although three-fourths say they would be very likely or somewhat likely to participate if asked (Research!America, 2007).
Physician behavior is a critical factor in applying research results to health care. Lauer noted that in the Occluded Artery Trial (OAT), no benefit was found to the common U.S. practice of using stents or balloon angioplasty to open occluded arteries that had not been opened within 12 hours following acute myocardial infarctions (AMIs) (Hochman et al., 2006). Clinical guidelines were revised in accordance with these findings, yet practices generally did not change in the several years immediately following release of the revised guidelines (Deyell et al., 2011). Moreover,
according to Lauer, the study researchers experienced extraordinary difficulty recruiting U.S. physicians to take part in the study and this recruitment difficulty is attributed largely to the fact that the study challenged the conventional wisdom about the benefits of opening persistently occluded arteries. Another workshop participant added that physician recruitment in OAT was further challenged by financial disincentives to the extent that a clinician’s participation in the trial limited the number of artery-opening procedures she or he would be able to perform in compliance with the trial protocol. Another financial disincentive was that if the trial revealed the procedure to be unsafe or ineffective, physician caseloads and revenue would decrease.
According to some participants, the culture of research in North American medical schools appears to be diminishing. Part of physicians’ reluctance to participate in, or apply findings from, clinical trials may stem from attitudes rooted in the Flexner reforms of medical education dating back a century ago, noted Califf. Under the Flexner model, the basis of medical practice is knowledge of human biology. Today it may make more sense for the basis of medical practice to consist of clinical evidence (albeit with an understanding of biology). Another partial explanation for inadequate attention to research in medical schools may be faculty members’ relative lack of knowledge or comfort with research science.
According to Jackson, empiricism could be inculcated into undergraduate students, medical students, residents, and other future health professionals, such as dietitians, by teaching about the scientific investigations process and its relationship to health care, and by integrating clinical research into other coursework. In this way, students would learn to think of a working diagnosis as a hypothesis, and to view medical examinations and tests as an experiment to test the diagnostic hypothesis. It would be helpful for education to include use of research results and interpretation of statistical findings. It was suggested that, to introduce and take advantage of these changes, external pressures may be needed. Individual workshop participants highlighted several possible strategies for improving the education of community practitioners about research to help enhance their future participation in clinical trials:
• Revision of curricula in some medical schools, which may provide opportunities to introduce research problems into each educational module;
• Inclusion of questions about research in national medical board and nursing examinations; and
• Strengthening of medical school accreditation standards and requirements related to the teaching of research methods with the goal of increasing support for a culture of research.
An additional frontier of learning involves management of trials. Although some practitioners are very adept at managing logistics—a key skill in clinical research—others are not, and the teaching of management skills is not yet included in most health professions’ educational curricula.
Implementers
Implementers are the people who conduct trials as a primary job function but are not engaged in the academic study of trial design methods. They (and others) will benefit from being taught to think in innovative ways and being trained to look at research problems from the perspective of patients and clinicians. It would be beneficial if education programs were refocused in order to meet not only gaps in knowledge but also gaps in methodology, implementation, and application, and to create cultural change. To this end, the NIH Clinical and Translational Science Awards (CTSAs) are striving to improve and increase training through a network of 60 medical research institutions. The program’s training component includes a mentored clinical research scholar program at the postdoctoral level and tailored clinical research training for predoctoral students.
One core constituency of the implementers group is clinical research coordinators. Unfortunately, clinical research coordinators have been found to leave the field within 3 years, a waste of training and talent that apparently results from inadequate career paths. Indeed, retention of clinical research coordinators is now often considered a greater problem than attracting applicants to coordinator positions. Partly to promote the field and imbue it with the advantages of a recognized profession, efforts to develop an accreditation program for these professionals have begun at the University of Pennsylvania, and a Society of Clinical Research Associates has been created. With expertise in both management and clinical sciences, clinical research coordinators conceivably could be educated through master’s degree programs jointly developed by medical and business schools.
Investigators
Investigators are the personnel charged with leading and designing clinical trials. This group would be composed of M.D.-, M.D./Ph.D.-, and Ph.D.-level investigators from a range of disciplines with the opportunity to supplement their expertise with specific training in translational and clinical research offered by the CTSAs. Paper co-authors suggested that the future transformation of AHSSs into IHSs will bridge translational gaps between discovery science and health care delivery and will require more of this highly trained cadre of clinical investigators.
Several workshop participants suggested that a sustainable and interesting career path is at least as important as, if not more important than, instituting formal training in the medical school curriculum. The rise of medical informatics may be instructive. Medical informatics is emerging as a board-certified specialty due to demand on the part of young physicians attracted to a field that hospitals and delivery systems find essential. Likewise, clinical research could become attractive if many more job positions were created.
In addition to bench researchers, it also is important to prepare people for positions in regulatory science,2 notably within FDA. FDA regulations ensure the safety, efficacy, quality, and performance of products that represent about 25 cents of every dollar spent by American consumers. In the face of rapidly changing technology and increasingly complex products, FDA and other regulatory agencies must maintain their staff’s scientific and technical expertise while responding as rapidly and effectively as possible to patients’ needs and to public health emergencies.
A 2012 IOM report suggested the wide range of core competencies needed for professionals working in the regulatory sciences. In medicine, these competencies might include bioengineering, bioethics, clinical pharmacology, epidemiology, genetics, nutrition, public health, toxicology, and many others, going well beyond the disciplines traditionally associated with regulation, such as statistics and clinical research. In some cases, needed competencies may even be found outside the biomedical sciences in disciplines such as economics and sociology (IOM, 2012b).
Academicians and Methodologists
Academicians and methodologists are those who conduct research in new clinical trial methods and policies. This group of clinical investigators would consist of biostatisticians, epidemiologists, and health services researchers tasked with advancing and improving upon clinical trial methodologies. The workforce group would reside primarily in AHSSs, government, research institutes, and some larger industry groups that could provide protected time for research.
Investigators and academicians and methodologists are not entirely distinct from each other in that both design clinical trials. Ideally, these workforce groups will be taught to focus on what clinicians and patients need and will become proficient in behavioral skills and teamwork and even will become “design thinkers.” According to several workshop
______________________
2 FDA has defined regulatory science as the “science of developing new tools, standards, and approaches to assess the safety, efficacy, quality, and performance of FDA-regulated products” (FDA, 2010).
participants, the CTSA program and other initiatives have spurred the development of innovative research teams that tackle complex health and research challenges to identify ways to turn their discoveries into practical solutions to problems in patient care. Even at this high level, lifelong learning can help maintain and improve competencies, especially as scientists move from one career challenge to another in both private and public sectors.
SUSTAINING INSTITUTIONAL SUPPORT AND PATIENT ENGAGEMENT IN CLINICAL TRIALS
Clinical research gives the public hope. And you
cannot underestimate the power of that word.
—John Gallin, NIH Clinical Center
Models and Messages from the NIH Clinical Center3
An innovative and comprehensive approach to facilitating the conduct of clinical research has been developed by the NIH Clinical Center, a 240-bed facility located on the NIH campus in Bethesda, Maryland. The Clinical Center, which calls itself “America’s Research Hospital,” conducts a robust program of clinical research aimed at improving the treatment of both common and rare diseases and conditions. The approach was detailed in a keynote address by John Gallin, Director, NIH Clinical Center. Every patient at the Clinical Center is enrolled in a clinical study protocol and receives care free of charge (see Glossary in Appendix K for definition of protocol). The center’s research portfolio consists mostly of Phase I and II investigations, rather than later-stage, confirmatory research. Studies are divided roughly in half between natural history studies of disease pathogenesis in patients with rare diseases and clinical trials.
NIH intramural clinical research benefits from the agency’s adherences to seven “principles and processes” that it provides researchers: clinical informatics, data management, and protocol tracking; biostatistics support; quality assurance and quality improvement; protocol review; human resources and physical plant; training and education; and research participants, or research partners, as the term used at the Clinical Center. The Clinical Center has established systems to meet these needs for researchers, thereby substantially lightening investigators’ administrative burdens (see Box 3-1). Some of the resources that NIH has developed to support its own intramural clinical trials program are increasingly being
______________________
3 This section is based on the keynote address by John Gallin, Director, NIH Clinical Center.
BOX 3-1a
NIH Clinical Center Key Services for Researchers and Patients
• Services of protocol navigators, writers, and translators
• Office of Protocol Services support as a repository of NIH protocols that also prepares all reports to Congress and provides quality review of protocol actions, such as informed consent documents
• ProtoType, a web-based protocol writing tool that can be used, for example, in developing cost estimates and assessing regulatory compliance, and a “wizard” tool to help determine whether an investigator must submit an Investigational New Drug (IND) Application to FDA
• Biomedical Translational Information System, a web-based integrator of clinical and research data that offers investigators access to deidentified patient data, integrates data sources from multiple institutes, and provides an ontology for representation queries
• Assistance with recruitment and retention of study participants and with external communications, including media relations, use of social media, and outreach efforts, such as vaccine mobile clinics to facilitate vaccination studies
• Volunteer survey research on people’s satisfaction with participation in studies; study participants are viewed as partners in clinical research
• Specialized services, including phenotyping, which is especially useful to researchers in rare diseases, as well as superior imaging services and pharmacy services that can formulate specialized products
• Training services, including NIH Curriculum on Clinical Research (introduction to the principles and practices of clinical research, principles of clinical pharmacology, and ethical and regulatory aspects of clinical research), an online course for clinical investigators on standards in clinical research, and a six-module clinical researcher sabbatical program lasting an average of three and a half months
• Community outreach activities, including a van that travels around the Washington metro area to make it easy for individuals who want to participate in a vaccine clinical trial to get examined and provide blood for purposes of the clinical trial made available to external researchers. These resources are particularly useful for researchers who need more support than what their home institutions can offer. Making them more widely available also helps the Clinical Center fulfill its mandate to open its doors to extramural investigators.
• Annual survey (focus group) of participants’ perceptions of their research experiences. Aggregate survey data will identify best practices and drive improvements at the Clinical Center, and beyond (Kost et al., 2011)
_____________________
aThis box is based on the presentation by John Gallin, Director, NIH Clinical Center.
made available to external researchers. These resources are particularly useful for researchers who need more support than what their home institutions can offer. Making them more widely available also helps the Clinical Center fulfill its mandate to open its doors to extramural investigators.
Protocol navigators are a special feature of the Clinical Center’s approach. Although their specific functions vary from one NIH Institute or Center to another, their role is to interface with components of NIH and to help overcome regulatory barriers. Figure 3-2 displays the National Institute of Allergy and Infectious Diseases (NIAID) navigators’ functions. From 2009 to 2011, the Protocol Navigator Program at NIAID has conducted 29 initial protocol reviews, 10 protocol amendments, and 7 international studies serving 29 investigators from 10 different NIAID labs. The annual cost of the NIAID Protocol Navigator Program is $600,000 and staffs 1 navigation manager, 2 protocol navigators and 3 medical writers. To generate a larger pool of protocol navigators for intramural and extramural research support, the Clinical Center is developing a one-year training program intended as an alternative career path for research nurses, scientific writers, and IRB professionals to become versed in protocol navigation.
The Clinical Center’s new vision statement reads: “The role of the NIH Clinical Center should be to serve as a state-of-the-art national resource, with resources optimally managed to enable both internal and external investigator use” (NIH, Scientific Management Review Board, 2010). The expansion from strictly intramural to joint intra- and extramural research activity is being introduced through a project to create partnerships between extramural and intramural researchers. A partnership program between basic scientists and clinical investigators also is being maintained, for the duration of current funding capacity.
Other Institutional Supports and Patient Engagement
This section is based on a panel discussion with John Gallin; Janet Tobias, CEO, Ikana Health; Annetine Gelijns, Co-chair, Department of Health Evidence and Policy, Mount Sinai School of Medicine; and Heather Snyder, Senior Associate Director for Scientific Grants, Alzheimer’s Association International Research Grant Program. In the discussion, individual panelists and audience members identified challenges and opportunities to improve engagement in, and support of, clinical trials at the institutional and patient levels. This section provides an integrated summary of their remarks and discussions.
Public engagement is essential to the CTE but may constitute a weak link. Clinical trial recruitment and retention rates vary across disease area and provider, with AHSSs showing high variability.
Some barriers to participation by the public in clinical trials include
• lack of awareness of the benefits of engaging in clinical trials and the availability of relevant trials in which to participate (IOM, 2012a);
• clinical trials designed without patient input and therefore lacking acceptability to the patients expected to participate in these studies;
• patient preferences for one treatment over another or the availability of a treatment outside the clinical trial setting;
• logistic hassles in participation (such as requirements of frequent patient visits, lab tests, and questionnaires); and
• inadequate reimbursement (for example, Medicare has paid for routine care in clinical trials since about 2000, but many private payers do not) which might lead to out-of-pocket expenses for trial participants.
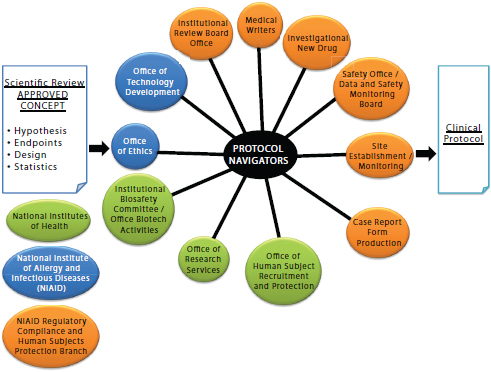
FIGURE 3-2 The protocol navigator interface at the National Institute of Allergy and Infectious Diseases (NIAID).
SOURCE: Gallin, J. 2011. Presentation at IOM workshop on Envisioning a Transformed Clinical Trials Enterprise in the United States: Establishing an Agenda for 2020. Figure created by H. Clifford Lane, NIAID, and publicly available.
Several strategies might be used to reduce these barriers. For example, Gelijns noted that a national coalition of patients, providers, and political leaders might promote change. On a more limited basis, an opportunity may exist to broaden the existing clinical trial networks that
have been established by NIH to increase efficiency in research. These networks could include advocacy groups, community practitioners, and other stakeholders who could enrich trial design, logistics, recruitment, payment, and other aspects of studies. Networks might revolve around academic health and science center hubs and include community practitioners, whose patients could participate in trials through visits to their usual practitioner’s office rather than to a distant research site. Engaging patients in the development of novel clinical trial designs and the determination of clinical end points may help in raising enrollment rates. Routine, effective communication with trial participants about study results also could improve people’s sense of the value of the research effort. Collaboration between payers and the CTE, such as new FDA collaboration with CMS on innovative medical device use, could prove fruitful in ensuring both reimbursement and the application of research results to practice.
Clinical research gives people hope, noted John Gallin. To illustrate, parents enroll children in cancer studies at very high rates, as much as 90 percent. Advocacy groups have proved instrumental in promoting engagement in research on breast cancer, cystic fibrosis (CF), Alzheimer’s disease, and other illnesses, especially when they have been viewed as impartial rather than favoring one treatment or trial over another. It might be useful to devote more resources to public engagement at earlier stages of research efforts, and to employ greater expertise in these communication efforts. Tobias noted that people are more willing to enroll in studies involving diseases perceived as life-threatening, such as cancer, and less willing to enroll in studies involving diseases, such as diabetes or Alzheimer’s disease, that, even though they too are life-threatening and highly prevalent, are not perceived as such. A workshop participant also noted that beyond issues of disease severity, or perception of severity, a patient’s interest in participating in a clinical trial is related to the availability of effective, acceptable treatments. A current trend in Alzheimer’s disease research, according to Snyder, is to seek ways to identify patients as early as possible and to view the disease as a continuum.
There may be various education and communication strategies that could prove useful. Scientific education in elementary, middle, and high schools could enhance people’s scientific literacy and improve physician– patient interactions. As mentioned in Chapter 2, the clinical trials workforce could also become skilled at incorporating the patient perspective into the design of clinical trials so as to make participation more attractive and acceptable to the public. Individually matching a patient with a clinical trial is another approach. When a 5-city survey found that a large proportion of primary care physicians were unaware of local trials and lacked time to find out about them, the Alzheimer’s Association developed a program that has matched 3,300 patients with trials so far, said Snyder.
Enhancing public awareness might only be part of a larger remedy for improving participation in clinical research. In large part, recruitment is a matter of using the right language and communication techniques. To illustrate, mere education about clinical research and randomization may not suffice to increase recruitment rates, especially in the face of the heavy burden of participation. An evaluation of the “Get Randomised” media campaign in Scotland revealed that, although the campaign increased public awareness about clinical research, it did not increase the willingness of individuals to participate in clinical studies (Mackenzie et al., 2010).
This page intentionally left blank.
















