This chapter describes the neurobiology of posttraumatic stress disorder (PTSD) and provides a setting for discussing the optimal treatment for PTSD (see Chapter 7). It begins with a discussion of adaptive versus maladaptive stress responses and describes fear conditioning and fear extinction. Models for the development of PTSD are then presented with an overview of the modulators that affect PTSD expression. In particular, in response to the committee’s statement of task, physiological markers for PTSD are described (see section on biomarkers) as are brain imaging studies (see section on studies using human subjects) and studies correlating brain region physiology and the diagnosis of PTSD (see section on implications for PTSD prevention, diagnosis, and treatment). The chapter concludes with a discussion of the implications of the neurobiology of PTSD for its prevention, diagnosis, and treatment.
Although some research on the neurobiology of PTSD is funded by the National Institutes of Health, other research on this topic is also sponsored by the Department of Defense (DoD) and the Department of Veterans Affairs (VA) (see Chapter 4). For example, the DoD is funding a study on multimodal neurodiagnostic imaging of traumatic brain imaging (TBI) and PTSD and a study on the neurobiology of tinnitus with PTSD as a secondary outcome, but these studies are ongoing and results are not available. The VA is also funding studies on the neurobiology of PTSD, including examinations of memory and the hippocampus in twins, brain imaging of psychotherapy for PTSD, and neural correlates of cognitive rehabilitation in PTSD.
The etiology of PTSD is linked to a known incident or repeated incidences
in which an individual is exposed to a life-threatening event that causes the development of PTSD symptoms. Stimuli present at the time of trauma exposure often become associated with the traumatic event such that subsequent exposure to one or more of those stimuli triggers fear and anxiety. PTSD patients often develop strategies to avoid trauma-associated contexts or cues and develop multiple symptoms, including cognitive and memory impairments and sleep disturbances.
The advent of neuroimaging tools during the past two decades along with the advancement of preclinical research has provided a platform upon which to begin to examine the neurobiology of PTSD, from predisposing factors leading to its development to developing novel strategies to treat the disorder. This chapter reviews some of the models and experimental approaches used in this domain. The committee must emphasize that a comprehensive review of the literature in this area of research is beyond the scope of this report. Moreover, the committee emphasizes there is not a single experimental model that can or will be able to capture every aspect of this complex disorder, and every experimental model (clinical or preclinical) has its advantages and disadvantages (see review by Brewin and Holmes, 2003). With this perspective in mind, the committee uses many references that rely on one or more experimental models or approaches to examine the neurobiology of PTSD, with an objective to build a wealth of information from different approaches that may lead to a comprehensive understanding of the disorder.
ADAPTIVE AND MALADAPTIVE STRESS RESPONSES
The diagnosis of PTSD requires that a person have “experienced, witnessed, or [been] confronted with an event or events that involved actual or threatened death or serious injury, or a threat to the physical integrity of self or others” and that “the person’s response involved fear, helplessness or horror” (APA, 2000); see Chapter 2 for the complete diagnostic criteria for PTSD. Research suggests that the term stress in relation to disease should be “restricted to conditions where an environmental demand exceeds the natural regulatory capacity of an organism, in particular situations that include unpredictability and uncontrollability” (Koolhaas et al., 2011). Examples of unpredictable and uncontrollable situations include rape, childhood abuse, and military combat (Breslau et al., 1991, 1998; Kessler et al., 1995). Research on PTSD has concentrated on two systems, the sympathetic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis, but there are other neurobiologic systems such as the serotonin system, the opiate system, and sex steroidal systems that have been implicated in pathologic and protective responses to stress (IOM, 2008).
The HPA axis is a neuroendocrine system from which there is successive
release of several hormones that leads to the release of cortisol from the adrenal glands. Cortisol circulates to other tissues where it causes an elevation in circulating glucose and, if appropriate, activates immune-cell migration to injured or infected areas of the body. There is some evidence that chronically elevated cortisol concentrations may impair many forms of memory (memory that is dependent on the hippocampus or prefrontal cortex), but such elevated concentrations favor memories that trigger fear (memory that is dependent on the amygdala) (see reviews by McEwen, 2005, 2006; Vanitallie, 2002). This is an important distinction because fear memories depend on the amygdala and extinction memories depend on the hippocampus and prefrontal cortex.
Events that are perceived as uncontrollable and threatening cause a series of reactions via the HPA axis, the locus coeruleus, and the nor-adrenergic system (Koenen et al., 2009a). These systems have reciprocal connections with limbic structures that mediate fear conditioning and memory consolidation (the amygdala and hippocampus) and prefrontal brain structures that mediate the extinction of fear memories. Structures in the brain initially respond to stress caused by an acute threat through adaptive mechanisms such as energy mobilization, increased vigilance and focus, and the facilitation of memory formation (Charney, 2004). When the body determines there is no longer an acute threat, it attempts to return to homeostasis through an elaborate negative feedback system. Figure 3-1 shows the major pathways that are triggered during a response to stress.
There are some cases where the adaptive response described above becomes persistent and pathologic (Koenen et al., 2009a). When a person is exposed to a traumatic event, sensory stimuli present at the time of exposure become associated with it. Later exposure to one or more of the now-conditioned sensory cues can lead to reactivation of the traumatic memories.
The initial re-exposure to the conditioned cues leads to the expression of intense fear and anxiety in most people. Repeated exposure to those cues in the absence of additional negative reinforcement (that is, without additional traumatic events) will lead to the gradual reduction of emotion associated with the traumatic event (that is, extinction). As stated by Mao et al. (2006), “much evidence indicates that extinction training does not erase memory traces but instead forms inhibitory learning that prevents the expression of the original memory” (see also Quirk, 2002). Reinstatement, renewal, and spontaneous recovery are phenomena that provide direct evidence that fear extinction is new inhibitory learning rather than erasure or forgetting (Archbold et al., 2010; Bouton, 2004; Myers and Davis, 2007). Reinstatement is a phenomenon in which a person or animal undergoes extinction training and is then exposed to an unsignaled unconditioned stimulus (see Box 3-1 for definition), resulting in the reappearance of an extinguished
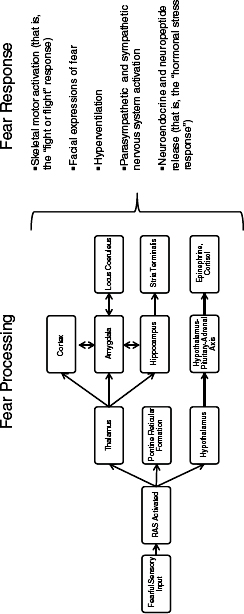
FIGURE 3-1 Depiction of the major pathways triggered during a response to stress. During a normal stress response, the sympathetic nervous system and the reticular activating system (RAS) are activated. The sympathetic nervous system controls the response of internal organs (for example, increasing the heart rate, decreasing digestion activities, and mobilizing energy stores from the liver). The RAS, which is required for a stress response, activates the pontine reticular formation (which induces the startle response), activates the thalamus to stimulate the cortex, and communicates with forebrain structures to activate the hypothalamus, which triggers the HPA axis and the release of epinephrine (IOM, 2008).
fear response. Renewal occurs when an extinguished conditioned response (see Box 3-1 for definition) reappears in a person or animal in a context that is different from the context in which the extinction training took place. Spontaneous recovery is the term used when the extinguished conditioned response reappears after nothing but the passage of time following extinction training.
It has been hypothesized that PTSD may result from a failure to recover from a traumatic experience, which leads to an inability to extinguish the fear and anxiety associated with conditioned sensory cues. For example, a service member might witness a friend being killed while a helicopter is hovering
BOX 3-1
Definitions of Selected Terms Associated with
Fear Conditioning and Fear Extinction
Classical conditioning—A process by which previously neutral stimuli acquire meaning to the organism
Unconditioned stimulus (US)—A trigger that produces an automatic, unlearned response
Unconditioned response (UR)—A naturally occurring reaction to a US
Conditioned stimulus (CS)—A neutral trigger that, through classical conditioning, is able to produce a conditioned response
Conditioned response (CR)—The learned reaction and instrumentational actions to a CS
Acquisition—The initial stage of learning, where a neutral stimulus (CS) is associated with a meaningful stimulus (US) and obtains the capacity to elicit a similar response (CR)
Short-term memory—Memory that is held for a short period of time
Long-term memory—Memory that lasts over a long period of time
Consolidation—The process by which short-term memory is converted into long-term memory
Retrieval—Reactivation of the memory trace or expression of a fear memory
Reconsolidation—A process by which a previously consolidated memory, which has been retrieved and becomes labile, undergoes another consolidation
Extinction—The process by which a CS loses the ability to elicit a CR
SOURCE: Adapted from Garakani et al., 2006; reproduced with permission of John Wiley & Sons, Inc.
overhead. The service member associates the sounds of the helicopter with the friend’s death. Later, when the service member hears a helicopter, he or she may experience the same fear and anxiety that were experienced during the traumatic event. This is a prototypic example of Pavlovian conditioning whereby sounds of the helicopter trigger the service member to prepare for an attack. This reaction would be adaptive in a combat situation but is maladaptive outside of it.
Conditioned fear may also lead to instrumental behaviors that contribute to the development and maintenance of PTSD. Instrumental behaviors often take the form of active and passive avoidance. Such behaviors are exhibited when the organism has control over threats and can minimize exposure to threats and traumas (conditioned and unconditioned stimuli) by virtue of how it responds. For example, a person diagnosed with PTSD may actively avoid reminders of the traumatic event, such as avoiding members of his or her unit because they are reminders of the traumatic experience of combat. A person diagnosed with PTSD may also passively (or involuntarily) avoid reminders of a traumatic situation, such as becoming disengaged. Fear extinction is therefore about learning that the conditioned stimulus no longer predicts the unconditioned stimulus, thus eliminating the need for all defensive responding (such as fear reactions and instrumental responses). Both active and passive avoidance strategies prevent fear reactions and subjective feelings of fear, and they can be adaptive provided they do not interfere with normal activities. Active avoidance mechanisms may even have relevance to active coping strategies that effectively deal with traumatic fear (Cain and LeDoux, 2007).
Fear and anxiety are a normal response to trauma. For the majority of exposed individuals, this fear and anxiety extinguish over time. For a significant minority, they do not. Therefore, it has been hypothesized that PTSD is a disorder of fear extinction (Cohen and Richter-Levin, 2009; Herry et al., 2010; Lang et al., 2000; Rasmusson and Charney, 1997; Rothbaum and Davis, 2003; Siegmund and Wotjak, 2006). The committee recognizes that other models have been proposed, including models for learning and for processing stress, information, memory, and emotion (see reviews by Brewin and Holmes, 2003; Cahill and Foa, 2007; and Ursano et al., 2008). However, it is difficult for one model to capture all aspects of PTSD phenomenology, especially associated symptoms such as shame and guilt, the latter two symptoms are not included in the Diagnostic and Statistical Maual of Mental Disorders, Fourth Edition (DSM-IV) list of symptoms for the diagnosis of PTSD (APA, 2000).
Box 3-1 defines some terms commonly used in association with fear conditioning and fear extinction. The fear-conditioning model was first described by Pavlov (1927) in a study with dogs. As described by Pitman and Delahanty (2005), a “traumatic event (unconditioned stimulus) overstimulates
endogenous stress hormones (unconditioned response); these mediate an overconsolidation of the event’s memory trace; recall of the event in response to reminders (conditioned stimulus) releases further stress hormones (conditioned response); these cause further overconsolidation; and the overconsolidated memory generates PTSD symptoms. Noradren-ergic hyperactivity in the basolateral amygdala is hypothesized to mediate this cycle.” The three main clusters of PTSD symptoms that result from a persistent pathologic response to uncontrollable stress are re-experiencing or reliving the traumatic event, avoiding reminders of the traumatic event (which prevents extinction of the fear memory) and emotional numbing, and generalized state of hyperarousal or hypervigilance (Koenen et al., 2009a).
In the model of fear extinction, the animal is able to adapt to its environment by dissociating the acquired conditioned fear response from the conditioned stimulus (Cohen and Richter-Levin, 2009). The inability to extinguish a conditioned fear response may play a role in the persistence of PTSD symptoms (Pitman, 1988; Rauch et al., 1998). Of particular interest is whether fear extinction is the result of learning a new response when presented with a conditioned stimulus, unlearning the original fear response when presented with a conditioned stimulus, habituation of the fear response, or a combination of these. As discussed earlier in this section, several reviews point toward the concept that fear extinction is a process more of relearning rather than of removal of a previous memory (Bouton et al., 2011; Cohen and Richter-Levin, 2009; Herry et al., 2010; Maren, 2011; Milad and Quirk, 2012).
Animal studies have shown that the recollection of previously consolidated conditioned fear memories can bring them into a labile state, and these memories then need to go through another phase of reconsolidation. Interrupting this second wave of reconsolidation by using, for example, protein synthesis inhibitors such as anisomycin, has the potential to prevent the restorage of memory (Nader et al., 2000). Reconsolidation blockade has also been shown in humans, and it has been suggested that extinction training may substitute the use of pharmacologic agents to block memory reconsolidation (Schiller et al., 2010). There is, however, some controversy surrounding the relationship between fear extinction and its relationship to fear reconsolidation (that is, memories that have been consolidated have the ability to be altered through retrieval or reactivation) (Myers and Davis, 2007). Differences may be caused by the methods used during studies where deficits were observed in reconsolidation or extinction. These factors may include the amount of time between re-exposure and the previously conditioned cue, protein synthesis in the amygdala and the medial prefrontal cortex, the strength of the conditioned fear memory, and the possibility that
the “extinction memory, once firmly established, itself undergoes reconsoli-dation” (Myers and Davis, 2007).
Neurobiology that supports the animal model of fear conditioning has been much studied. The process is thought to rely heavily on neuronal circuits in the amygdala, prefrontal cortex, hippocampus, and brain stem (Herry et al., 2010; Milad and Quirk, 2012; Ressler, 2010). The neurobiologic processes underlying fear conditioning in animal models and human correlation studies of PTSD are well characterized, and the fear-conditioning model is a guide for the study of the neurobiology of PTSD (Amstadter et al., 2009a; Jovanovic and Ressler, 2010; Lonsdorf and Kalisch, 2011). However, it is important to note that the fear-conditioning model does not capture all features associated with PTSD and is sometimes criticized as too simplistic to explain its pathophysiology. A number of other experimental approaches have been used in both rodents and humans, including symptom-provocation studies, testing of brain responses to the explicit and implicit presentation of fear stimuli in humans, and prolonged-stress exposure models in rodents.
MODELS FOR THE DEVELOPMENT OF PTSD
Numerous experiments have been undertaken in animals and humans to gain a better understanding of the pathophysiology underlying PTSD. The experimental models have benefits and limitations, as will be discussed in the following sections.
Animal Models
Animal models of fear conditioning and extinction have been critical in improving understanding of the neurobiology of PTSD. They are useful because they allow the researcher to manipulate stressors and to control for other variable factors in an experiment. Such models start with a stressor, and the intensity of the stressor (and other determinants) predicts the PTSD. The usual stressors in animals include such conditions as restraint stress, exposure to predators, and underwater foot or tail shocks. Because human PTSD occurs chiefly in the context of life-threatening stressors, the intensity of the stressors used to develop valid animal models need especially careful consideration.
In animal PTSD models, there are complex outcomes that are akin to the variety and severity of PTSD symptoms observed in humans (Kehne and Cain, 2010; Ursano et al., 2009). Animal outcomes include stress, the complementary outcome of fear, and anxiety. Those are sometimes dissociable and indistinct, but all have been characterized in animals (Graham and Milad, 2011; Ressler, 2010; Shin and Liberzon, 2010). Both unconditioned-fear models (for example, experimental models that include an ethologically
relevant fear stimulus, elevated plus maze, light–dark test, social interaction, light-enhanced startle, or distress vocalizations) and conditioned-fear models (for example, experimental models that include a conditioned-fear paradigm, conditioned freezing, or fear-potentiated startle) have been developed in animals.
Through the extensive study of animal models of emotional learning and memory (LeDoux, 2000; Maren, 2001), brain regions of interest for PTSD pathology have been described, fear circuits have been defined, and specific molecular outcomes of emotional learning and memory have been identified (Sotres-Bayon et al., 2009). These types of animal models, although they may be incomplete with respect to a full PTSD phenotype, suggest a process for the acquisition and storage of memories, plasticity processes (that is, the ability of pathways in the brain to reorganize structurally and functionally), and molecular markers that might be exploited during the investigation of PTSD resilience, diagnosis, and treatment.
It is important to keep in mind the limitations when evaluating an animal model and its implications for humans. For example, diagnosis of PTSD in humans usually requires a person communicate his or her experiences, thoughts, dreams, and emotions, whereas animal models rely on the observation of behavior. Some of the PTSD phenotypes observed in humans may not be present in animals. Also, the traumatic event that triggers PTSD symptoms in humans may be perceived as life threatening; this perception may cause a type or severity of stress response different from the stress response in an animal after it is restrained or receives a foot shock (Cohen and Richter-Levin, 2009). There are also differences in timing that limit the translation of animal models to human applications. For example, service members may have much longer exposures to traumatic events than animals, and because the average life span of animal models is relatively short, there may not be enough time for PTSD symptoms to develop.
Although no single animal model can capture the complex clinical features of PTSD, a number of models have provided a wealth of information regarding the neural circuits of emotional learning and memory. Examples of animal models for the study of PTSD and PTSD-related phenomena include fear conditioning and extinction models (Cohen and Richter-Levin, 2009), stress-based models (Cohen and Richter-Levin, 2009; Khan and Liberzon, 2004; Yamamoto et al., 2009), fear-potentiated startle models (Davis, 1986; Lang et al., 2000), and learned-helplessness models (Rasmusson and Charney, 1997).
Homologous Brain Structures
Several brain structures and circuits relevant to the fear-learning and fear-extinction processes have been identified in animal models that are homologous to neurologic structures and circuits in humans. For example,
the rodent prelimbic cortex increases fear expression and opposes extinction (Vidal-Gonzalez et al., 2006). The human homologue of the rodent prelimbic cortex is the dorsal anterior cingulate cortex (Etkin et al., 2011; Graham and Milad, 2011). The dorsal anterior cingulate cortex has been described as the center for processing cognitive stimuli, error processing and detection, and fear expression (Etkin et al., 2011; Vogt, 2005). The rodent infralimbic subregion plays a key role in inhibiting fear expression and promoting extinction (Milad and Quirk, 2002, 2012; Quirk and Mueller, 2008). The human homologue of the infralimbic subregion appears to be the ventromedial prefrontal cortex, and recent studies show that its structure (Hartley et al., 2011; Milad et al., 2005) and function (Kalisch et al., 2006; Milad et al., 2007; Phelps et al., 2004) correlate with the magnitude of fear extinction. Evidence of the role of the ventromedial prefrontal cortex in the pathophysiology of PTSD comes from a number of recent conditioning and extinction studies in humans (Bremner et al., 2005; Linnman et al., 2012; Milad et al., 2009a).
Studies Using Human Subjects
In humans, the key region involved in fear learning and extinction is the amygdala, which is in the medial temporal lobe (Lang et al., 2000; Rauch et al., 2006). Some evidence suggests that regions of the lateral prefrontal cortex involved in the regulation of cognitive emotion may influence the amygdala (Delgado et al., 2008; Phan et al., 2005; Somerville et al., 2012). Of the 13 amygdala nuclei, 3 (the basal amygdala, lateral amygdala, and central nuclei) are implicated in the brain’s response to fear (Amorapanth et al., 2000; Cain and LeDoux, 2008; Garakani et al., 2006; Sah et al., 2003). Functional magnetic resonance imaging (MRI) has limited spatial resolution, and human studies have not yet confirmed the importance of these specific anygdala subnuclei. Other neurologic areas of particular interest include subregions of the medial prefrontal cortex, hippocampus, and the insula. The involvement of these regions has been reported on the basis of resting activity studies that use positron emission tomography, functional MRI studies of patients who were performing a variety of emotional tasks or viewing emotional stimuli, and several structural MRI studies. Some of those findings are reviewed below.
Neuroimaging Studies Using Symptom Provocation
The initial neuroimaging studies of PTSD focused on a paradigm known as symptom provocation. In this paradigm, patients are reminded of their traumatic events while their brains are being scanned. The brain scans are then analyzed for increases and decreases in blood flow in particular
regions of the brain. For example, one study reported decreases in medial frontal gyrus blood flow in PTSD participants exposed to reminders of traumatic events compared with trauma-exposed controls who did not have PTSD, and medial frontal gyrus blood flow was inversely correlated with changes in amygdala blood flow (Bremner et al., 1999; Shin et al., 2004). Shin et al. (2004) also reported a positive correlation between changes in amygdala blood flow and symptom severity and a negative correlation between changes in medial frontal gyrus blood flow and symptom severity. Heightened amygdala activity (Rauch et al., 2000; Shin et al., 2004) and diminished ventromedial prefrontal cortex activity (Shin et al., 2005) have also been reported in PTSD subjects who viewed fearful faces during functional MRI compared to trauma-exposed controls who did not have PTSD. The results of those studies reveal critical areas of the brain that play a role in the pathophysiology of PTSD.
Recent studies have also shown that the function of the ventromedial prefrontal cortex and amygdala in patients with PTSD appears to be impaired even in response to the presentation of nontrauma-related stressful cues (Gold et al., 2011; Phan et al., 2006). In addition, functional abnormalities (both resting state and functional reactivation) in the rostral and more dorsal areas of the anterior cingulate cortex have been reported when PTSD patients undergo cognitive tasks (Shin et al., 2009, 2011). Another area that plays a key role in the pathophysiology of PTSD include the insular cortex. This brain region is involved in interoception and the monitoring of internal states and appears to also predict autonomic responses during fear learning (Linnman et al., 2012). People diagnosed with PTSD exhibit exaggerated insula activation in a number of different paradigms, such as during the responses to the presentation of fearful faces, painful stimuli, and traumatic memories (Simmons et al., 2008; Strigo et al., 2010).
Neural Connectivity Studies
More recent studies have used imaging techniques to measure the strength of connectivity between the ventromedial prefrontal cortex and the amygdala and to correlate it with traits that indicate anxiety. For example, when diffusion tensor imaging was used, the strength of the connections between the amygdala and the prefrontal cortex predicted the intensity of a person’s anxiety; the weaker the pathway, the greater the intensity of the traits associated with anxiety (Kim and Whalen, 2009). Another study reported that the resting state activity of the amygdala was positively coupled to ventromedial prefrontal cortex activity in subjects who had low levels of anxiety and negatively coupled to ventromedial prefrontal cortex activity in subjects who had high levels of anxiety (Kim et al., 2011a). Together, these studies suggest that a dysfunction in the connection between the ventromedial
prefrontal cortex and the amygdala may mediate susceptibility to the anxiety symptoms observed in PTSD. In support of this, recent neuroimag-ing studies examined the functional connectivity during the resting state in PTSD patients and found dysfunctional connectivity between different nodes of the fear network, including the thalamus, amygdala, insular cortex, hippocampus, and different subregions of the anterior cingulate cortex (Bluhm et al., 2009; Rabinak et al., 2011; Sripada et al., 2012; Yin et al., 2011).
Psychophysiologic and Behavioral Studies
Fear extinction has two components—a learning component (that is, learning to extinguish the fear) and a memory component (that is, recalling a safety memory to block the fear response). One of the hypotheses regarding the psychopathology of PTSD is that people who have PTSD show intact fear learning but impairment in the fear-extinction process. Several studies have directly measured the ability to extinguish fear in anxious populations by using laboratory extinction tasks. In support of that hypothesis, enhanced resistance to extinction has consistently been reported in PTSD populations compared with trauma-exposed controls who did not have PTSD or control groups who did not have PTSD and who had not been exposed to trauma, as indexed by larger differential skin conductive responses to the presence of a conditioned stimulus (Orr et al., 2000), greater heart rate responses (Peri et al., 2000), and stronger online valence and expectancy ratings (Blechert et al., 2007).
Recent studies also show that although learning to extinguish fear may be intact in PTSD, recalling the safety memory (extinction memory) is deficient in PTSD (Milad et al., 2008, 2009a). One of those studies also reported a negative correlation between symptom severity in PTSD and extinction recall (Milad et al., 2009a). In another, enhanced fear conditioning combined with impairments in fear extinction were reported in PTSD subjects compared with trauma-exposed controls who did not have PTSD, and there was a positive correlation between symptom severity and both the enhanced conditioning and the impairment in extinction (Norrholm et al., 2011). Impairment in fear extinction was also reported in PTSD subjects when a model of inhibition that isolates the inhibitory component of extinction was used (Jovanovic et al., 2009); this effect was not detected in a cohort of people who had a diagnosis of depression (Jovanovic and Ressler, 2010).
Neuroimaging Studies of Treatment Outcomes
Another way to examine the relationship between functional brain circuitry and the pathophysiology of a given disorder is to determine whether
successful recovery from an adverse health outcome (such as anxiety) correlates with changes in the neural circuitry. Some studies using this approach in PTSD and other anxiety disorders are emerging. For example, one session of intensive exposure therapy has been shown to reduce amygdala, dorsal anterior cingulate cortex, and insula hyperactivation in response to viewing phobia-relevant stimuli in people who have arachnophobia, as measured 2 weeks after exposure (Goossens et al., 2007). Another study reported reduced hyperactivity in the anterior cingulate cortex and insula after cognitive behavioral therapy (CBT) treatment for arachnophobia in comparison with a wait list control group (Straube et al., 2006). Decreases in anterior cingulate cortex blood flow and increases in ventromedial prefrontal cortex blood flow have been reported after CBT for panic disorders (Sakai et al., 2006). Those effects do not appear to be restricted to CBT, as similar neural changes have also been reported after pharmacologic treatment for social phobia (Furmark et al., 2002). The latter study reported a comparable decrease in regional cerebral blood flow in the amygdala and hippocampus after successful treatment with citalopram or CBT. Although those studies are not specific to PTSD, they suggest that successful pharmacologic and psychologic treatments in some cases target the same dysfunction in the neural circuitry that underlies the regulation of emotion.
In a number of recent neuroimaging studies, meditation has appeared to change the structural and functional integrity of several brain regions, including the insula, amygdala, prefrontal cortex, and other regions involved in emotion regulation and empathy (Holzel et al., 2008; Kilpatrick et al., 2011). This could have important implications for PTSD treatment and possibly prevention.
Structural Imaging Studies in PTSD
In addition to function, neuroimaging tools have been used to examine the structural integrity of brain regions implicated in PTSD, with particular emphasis on the hippocampus and prefrontal cortex. Reduced volume in the hippocampus has been reported in a number of studies (Bremner et al., 1995; Kitayama et al., 2005; Stein et al., 1997; Wang et al., 2010a; Woon and Hedges, 2011), and has been proposed to be a predisposing factor for developing PTSD (Gilbertson et al., 2002). However, other studies, including some conducted in children, have failed to replicate the hippocampal reduction in PTSD patients (Bonne et al., 2001; Bremner, 2001; De Bellis et al., 2001; Fennema-Notestine et al., 2002). The cause for this apparent discrepancy regarding hippocampal volume in PTSD is not clear, but it may be related to a number of factors including symptom severity and the chronicity of diagnosis. Structural abnormalities have also been reported in different subregions of the prefrontal cortex (Kasai et al., 2008; Kitayama et al., 2006; Rauch et al., 2003). Unlike the hippocampus, it is proposed
that the structural deficits observed in PTSD patients may be the result of having PTSD rather than as a predisposing factor (Kasai et al., 2008).
FACTORS THAT INFLUENCE THE DEVELOPMENT OF PTSD
Exposure to traumatic events is common, particularly for military personnel who may experience multiple deployments. Although exposure to trauma appears to be the rule, the development of PTSD is not. In fact, the majority of service members recover well after trauma without showing signs or symptoms of PTSD (Hoge et al., 2004, 2006; Koenen et al., 2008; Kulka et al., 1990). Those facts have led to the generation of a number of questions: Why is it that some people develop PTSD and others do not? Are there factors that make some people more resilient and others more susceptible to developing PTSD? Could resilience be developed or enhanced, and could the development of PTSD be prevented? Current research efforts in neuroscience, psychology, and psychiatry are trying to answer those questions. In the following sections, the committee summarizes some social and biologic factors that could influence PTSD development and prevention (PTSD prevention is the subject of Chapter 5).
Sex Differences
Emerging evidence from rodent and human imaging studies suggests differences in emotional learning and memory processing between males and females. For example, the network of brain regions that are known to process emotional memory, fear learning, and fear extinction—the amygdala, hippocampus, and prefrontal cortex—is sexually dimorphic (Goldstein et al., 2010). A number of studies show that activation of the amygdala during memory encoding differs between men and women (reviewed in Andreano and Cahill, 2009) and damage to the human ventromedial pre-frontal cortex, which is critically involved in fear extinction, differentially affects men and women. Specifically, a unilateral ventromedial prefrontal cortex lesion in the right hemisphere produces severe emotional defects in men, but lesions in the left hemisphere of the same brain region produce no effect (Tranel et al., 2005). The opposite is true in women; a unilateral lesion in the left hemisphere produces severe defects in women, but the same lesion in the right hemisphere has no effects. A study by Bryant and Harvey (2003) of motor vehicle collision survivors examined whether acute stress disorder predicts the development of PTSD (Bryant and Harvey, 2003). The study was based on the idea that “dissociation at the time of trauma results in fragmented encoding of the event, which impedes subsequent emotional processing of the experience and purportedly leads to longer-term psychopathology” (Bryant and Harvey, 2003). Their sample was
small, but the authors found that a diagnosis of acute stress disorder was more of a predictor of the development of PTSD in females than in males. Differences in sex can also result in differences in response to PTSD treatments. For example, in a study of PTSD subjects on the effects of treatment with tiagabine, fluoxetine, sertraline alone, and sertraline with CBT, females showed a significantly better response than males (Davidson et al., 2005).
Emerging evidence from experimental human and animal studies indicates that these differences may be influenced by sex hormones. For example, viewing emotionally salient stimuli has been shown to activate the amygdala, the ventromedial prefrontal cortex, and other brain regions involved in the stress-response circuitry differently when a subject is in a high-estrogen state compared to when that subject in a low-estrogen state (Goldstein et al., 2010). Stronger activation of the prefrontal cortex was observed in a go or no-go emotional task in women during the luteal phase (higher estrogen) than during the follicular phase (lower estrogen) (Protopopescu et al., 2005). Activation of the ventromedial prefrontal cortex, the amygdala, and the hippocampus is also increased and associated with facilitated fear inhibition in women in a high-estrogen state (Zeidan et al., 2011). The data suggest that sex hormones (such as estrogen) may be involved in modulating memory formation in women in a way that impacts the control of fear.
Rodent studies have shown direct evidence linking sex hormones (such as estrogen) to synaptic plasticity, activation of molecular machinery involved in learning, and long-term potentiation (for review, see Gillies and McArthur, 2010). Sex hormones also modulate dendritic spine density in the prefrontal cortex (Hao et al., 2006) and modulate the mechanisms by which stress influences the function of the ventromedial prefrontal cortex and the hippocampus (Maeng et al., 2010; Shansky et al., 2010). Recent studies show that in rodents, exogenous estradiol administration facilitates fear inhibition (Chang et al., 2009; Milad et al., 2009b, 2010; Zeidan et al., 2011).
Another important matter to consider in this line of research is oral contraceptives. It has been estimated that 34% of women 18–29 years old in the military are using oral contraceptives (Enewold et al., 2010). If cycling hormones could influence emotional learning and memory, oral contraceptives may influence, modulate, or interfere with these processes. It is known, for example, that most oral contraceptives have an overall effect of reducing cycling estrogens and progesterone in women. A recent study showed that memory formation differed between naturally cycling women and those using oral contraceptives (Nielsen et al., 2011). Thus, future research could be informative in describing the mechanistic differences between the brains of males and females in processing fear extinction. Such research could potentially contribute to an understanding of the relationship
between sex and the prevalence of PTSD and to the development of sex-specific treatments for PTSD and other mood and anxiety disorders.
Age
Although trauma exposure of military personnel occurs predominantly during adulthood, it is important to understand how variance in age may influence the formation, consolidation, and retrieval of emotional memories, especially as they are related to fear inhibition in general and to PTSD in particular. A number of studies of fear extinction, for example, have shown that fear-learning and fear-extinction processes differ during the life span. A review by Kim and Richardson (2010) indicates that extinction in preweanling rats is not context dependent—that is, a fear-extinction memory can be expressed in contexts other than the one in which the extinction learning took place. That is a process that does not take place in the adult rat. Moreover, extinction learning and its consolidation in preweanling rats do not require n-methyl-d-aspartate (NMDA) receptors (which are important for synaptic plasticity) and do not require the ven-tromedial prefrontal cortex (Kim and Richardson, 2010). Fear extinction in the adult rat activates the ventromedial prefrontal cortex to inhibit the amygdala, whereas extinction training in early age appears to erase the conditioned fear associations in the amygdala (Gogolla et al., 2009; Kim and Richardson, 2008). During adolescence, extinction learning requires many more training trials (Esmorís-Arranz et al., 2008; Kim et al., 2011b). In the aged rat, fear extinction appears to be impaired and is associated with a shift of excitability from one prefrontal region to another (Kaczorowski et al., 2011). Conclusions from studies of rats imply that fear training in adult rats causes a suppression in fear behavior, whereas in juvenile rats the fear memory is either unlearned or erased (Herry et al., 2010).
A number of neuroimaging studies of humans have shown changes in the amygdala with aging. For example, the response in the amygdala and hippocampus to faces that show emotion compared to faces that have a neutral expression appears to change with age (Iidaka et al., 2002; Wright et al., 2003). A recent study reported that during the encoding of emotionally negative stimuli, the insular and prefrontal cortices are activated to a larger extent in older adults (average age 74.3 years old) than in young adults (average age 24.7 years old). In contrast, greater activation of the amygdala and hippocampus is observed more often in young adults (Fischer et al., 2010). Studies specifically of the neurobiology of fear inhibition in aging have not been conducted. One study, however, reported that older subjects (66–80 years old) exhibit decreased awareness of the association between a neutral cue and an aversive unconditioned stimulus (Labar et al., 2004). Thus, understanding the interaction between age and the function
of the network that mediates emotional learning and memory could further the understanding of the etiology of PTSD at different ages and possibly of treatment for PTSD. That is especially important given that recent studies (reviewed above) suggest that such treatments as CBT potentially could change the neural plasticity of the brain regions that mediate fear learning and its extinction.
Cognitive Reserve
Cognitive reserve is related to differences in brain structure (for example, density of neuronal synapses) and function (for example, processing efficiency), and it has been proposed as an important etiologic factor in the development and severity of neuropsychiatric disorders (Barnett et al., 2006; Koenen et al., 2009b). As discussed in Chapter 2, there is evidence from the field of cognitive epidemiology (Deary and Batty, 2007) indicating that intelligence quotient (IQ), a marker of cognitive reserve, is inversely related to a person’s risk for being diagnosed with a psychiatric illness (Batty et al., 2005; Walker et al., 2002).
The mechanism of the association between IQ and PTSD is not well understood. Emerging evidence suggests the importance of cognitive processes for the extinction of fear memories. Hoffman and Mathew (2008) argued that the comparable effectiveness of cognitive and exposure therapy for the treatment of fear disorders supports the importance of higher-level cognitive processes in extinction. One hypothesis is that persons with greater cognitive ability are more effective and efficient in engaging in such higher-level cognitive processing and thus more effective in extinguishing fear memories. Given the robust inverse association between cognitive ability and risk of PTSD, a better understanding of the mechanisms underlying this association may be helpful in prevention.
Genetic Factors
Research on the molecular genetics of PTSD is still in its early stages. The first molecular genetic study of PTSD was published in 1991, so this is still a relatively new area of investigation. Chapter 2 discussed family and twin studies that show that PTSD may be heritable and that there is some overlap between the genetic influences on PTSD and genetic influences on other mental disorders (Koenen et al., 2009a). Although there is an extensive literature on PTSD in twins (for example, see Gilbertson et al., 2002; Shin and Liberzon, 2010; Shin et al., 2011), this section focuses on molecular-genetic studies. Recent studies have moved beyond documenting genotype–phenotype associations to identifying epigenetic signatures associated with the disorder (Smith et al., 2011; Uddin et al., 2010) and examining
how individual differences in epigenetic programming may modify risk of PTSD in association with exposure to trauma (Koenen et al., 2011). This section provides an overview, not a comprehensive review.
One type of molecular-genetic investigation examines variation in polymorphisms to identify specific genetic variants that may be associated with an increased risk or resilience to the development of a particular phenotype (Amstadter et al., 2009a). By identifying such genes, it may be possible to gain an understanding of the neurobiologic factors that contribute to the development of PTSD or the factors that could be a target for the treatment of the disorder (Amstadter et al., 2009a). Although molecular-genetics research could potentially provide very useful knowledge about the etiology of disorders such as PTSD, it does have limitations, such as the interpretation of research findings or the determination of functional genetic variants (Amstadter et al., 2009a). The committee notes that at the time of this writing, such studies have relied completely on the candidate gene approach. That is, they have selected specific genes posited to be related to PTSD and analyzed variations in those genes in relation to the phenotype. The limitations of this approach and of the field of the genetics of PTSD more broadly have been outlined in detail elsewhere. Specifically, investigators have identified the need for genome-wide association studies of PTSD (Cornelis et al., 2010).
Findings from candidate-gene association studies of PTSD support the observation that many genes associated with PTSD are also related to depression and other anxiety disorders. Table 3-1 is a summary of the results of a systematic literature search for candidate genes for PTSD. The table is not exhaustive but provides an overview of candidate genes that have been the focus of at least one published study in humans (only studies in English were considered). Until recently, most molecular-genetics studies of PTSD focused on the dopaminergic and serotonergic systems, but evidence that PTSD involves dysregulation of other systems has led to increasing interest in genes that play a role in the function of the HPA axis, the locus coeruleus and noradrenergic systems, and neurotrophins. The current evidence of a specific genetic variant that increases vulnerability or resilience to PTSD is not robust. The lack of consistency of associations between specific genetic variants and PTSD may be due to study limitations such as small sample size or substantive differences between studies such as modification of genetic effects by environmental factors that are not accounted for consistently among studies. For example, several candidate-gene studies found significant effects of specific genetic variants only under conditions of extreme traumatic stress (Binder et al., 2008; Kilpatrick et al., 2007).
The environment may also modify genetic effects through molecular mechanisms. DNA methylation is one of the major mechanisms of epigen-etic regulation (Bernstein et al., 2007). It involves chemical modifications
that regulate DNA accessibility, which in turn alters the transcriptional activity of the surrounding loci. In many cases, increased methylation in specific gene regions (such as the promoter region) is associated with reduced transcriptional activity and therefore with reduced gene expression. Two recent studies of human samples found that those with PTSD were distinguished by methylation profiles that suggest upregulation of immune-system–related genes and relative downregulation of genes involved in neurogenesis and the startle response (Uddin et al., 2010). The upregulation of these genes is indicated by higher concentrations of biomarkers (cyto-megalovirus, interlukin-2, interleukin-4, and tumor-necrosis factor-alpha) that are associated with immune system reactivity in participants who have PTSD. Another study showed that SLC6A4 methylation modified the effect that traumatic events had on the development of PTSD when the SLC6A4 genotype was controlled for (Koenen et al., 2011). There was an association between persons who experienced events that were of a more traumatic nature and increased risk for PTSD, but only when lower meth-ylation levels were observed. Persons who experienced events that were of a more traumatic nature appeared to be protected from the development of PTSD when methylation levels were higher. The same interaction was observed regardless of the outcome (PTSD diagnosis, symptom severity, or number of symptoms). The findings described above suggest that gene-specific methylation patterns may be associated with increased risk of and resilience to PTSD (Koenen et al., 2011). The human studies did not, however, demonstrate that the reported PTSD-associated epigenetic differences were associated with downstream differences in gene expression.
A small but growing literature has provided evidence of gene expression patterns that distinguish between those who do and those who do not have PTSD. The majority of these microarray-based studies have assessed gene expression changes in RNA derived from peripheral blood mono-nuclear cells or whole blood. The earliest work assessed PTSD-associated gene expression signatures in trauma survivors admitted to an emergency room immediately after a traumatic event (Segman et al., 2005). Bioinfor-matic functional analyses of transcripts that were differentially expressed between those who met DSM-IV diagnostic criteria for PTSD at 1 and 4 months, and those who met no PTSD criterion showed reduced expression of transcriptional enhancers, distinct expression signatures of transcripts involved in immune activation, and substantial enrichment of genes that encode neural and endocrine proteins (Segman et al., 2005).
One study used a custom-made “stress/immune” complementary DNA microarray to assess the expression of 384 genes in RNA obtained from whole blood of PTSD-affected and PTSD-unaffected people (Zieker et al., 2007). All the PTSD-affected people had been exposed to the same traumatic event almost 20 years before testing—the Ramstein air show
TABLE 3-1 Overview of Candidate Genes Studied in Relation to Posttraumatic Stress Disordera
| Gene | Common naime(s) | Location | Published Reports | Significant Findings | Null Findings |
| RD2 (D2R, D2DR) | Dopamine receptor DR |
11q23 | 6 | 4 (Comings et al., 1991, 1996; Voisey et al., 2009; Young et al., 2002) |
2 (Bailey etal., 2011; Gelernter et al., 1999) |
| DRD4 (D4DR) | Dopamine receptor D4 |
11p15.5 | 1 | 1 (Dragan and Oniszczenko, 2009) |
0 |
| SLC6A3 (DAT1) | Dopamine transporter | 5p15.3 | 4 | 2 (Drury et al., 2009; Segman et al., 2002; Valente et al., 2011) |
2 (Bailey etal., 2011) |
| DBH | Dopamine beta-hydroxylase | 9q34 | 1 | 0 | 1 (Mustapic et al., 2007) |
| SLC6A4 (HTT, 5HTT,Serotonin transporter SERT, 5-HTTLPR) | 17q11 | 13 | 10 (Grabe et al., 2009; Kilpatrick et al., 2007; Koenen et al., 2009c; Kolassa etal., 2010a; Lee et al., 2005; Mercer etal., 2011; Morey etal., 2011; Thakur et al., 2009; Wang et al., 2011; Xie et al., 2009) |
3 (Mellman et al., 2009; Sayin etal., 2010; Valente etal., 2011) |
|
| HTR2 (5-HT2A) | 5-Hydroxytryptamine (serotonin) receptor 2A | 13q14-q21 | 1 | 1 (Lee et al., 2007) |
0 |
| FKBPS | FK506 binding protein 5 | 6p21 | 4 | 4 (Binder et al., 2008; Boscarino et al., 2011; Koenen et al., 2005; Xieetal., 2010) |
0 |
| GCCR (NR3C1) | Glucocorticoid receptor | 5q31.3 | 1 | 0 | 1 (Bachmann et al., 2005) |
| CRHR1 | Corticotropin-releasing hormone receptor 1 | 17q 12-22 | 1 | 1 (Amstadter et al., 2011) |
0 |
| RGS2 | Regulator of G-protein signaling 2 | lq31 | 1 | 1 (Amstadter et al., 2009b) |
0 |
| CNR1 (CB1, CNR) | Cannabinoid receptor 1 (brain) | 6ql4-ql5 | 1 | 0 | 1 (Lu etal., 2008) |
| APOE | Apolopoprotein E | 19ql3 | 1 | 1 (Freeman etal., 2005) |
0 |
| BDNF | Brain-derived neurotrophic factor | Upl3 | 3 | 0 | 3 (Lee et al., 2006; Mustapic et al., 2007; Valente etal., 2011) |
| NPY | Neuropeptide Y | 7pl5.1 | 1 | 0 | 1 (Lappalainen et ah, 2002) |
| Gene | Common name(s) | Location | Published Reports | Signigifcant Findings | Null Findings |
| GABRA2 | GABAA | 4pl2 | 1 | 1 (Nelson et al., 2009) |
0 |
| COMT | Catechol-O-methyltransferase | 22qll | 2 | 2 (Boscarino et al., 2011; Kolassa et al., 2010b) |
0 |
| ADCYAP1R1 | Receptor for adenylate cyclase-activating polypeptide 1 | 7pl4 | 2 | 1 (Ressler et al., 2011) |
1 {Chang eta!., 2012) |
| DTNBP1 | Dystrobrevin-binding protein 1 | 6p22 | 1 | 1 (Voisey et al.. 2010) |
0 |
| CRKAS | Cholinergic receptor, neuronal nicotinic, alpha polypeptide 5 | 15q25.1 | 1 | 1 (Boscarino et al., 2011) |
0 |
aThis list has been updated from Cornelis et al., 2010. Although a systematic literature review was conducted, this list is not exhaustive and includes only human studies published in English.
catastrophe of 1989—and typical PTSD symptoms persisted in this group. Analyses showed a total of 19 differentially expressed transcripts, five and 14 of which were upregulated and downregulated, respectively (Zieker et al., 2007). Most of the downregulated transcripts (which were the focus of the study) were associated with immune functions or with reactive oxygen species.
Most recently, Yehuda et al. (2009) reported levels of expression of whole-blood–derived genes of PTSD-affected and PTSD-unaffected people who were exposed to the attack on New York City on September 11, 2001. Differential expression was detected in 16 genes, several of which are involved in signal transduction, brain and immune cell function, and HPA-axis activity. Although several genes in the study, such as FKBP5 (Binder et al., 2008) and major histone compatibility Class II (Chauhan et al., 2003), had previously been linked to PTSD or other stress-related outcomes, the gene showing the largest difference in expression was mannosidase, alpha, class 2C, member 1 (MAN2C1), a locus that had not previously been linked to PTSD. MAN2C1 distinguishes between those who have and those who do not have PTSD on the basis not only of gene expression (Yehuda et al., 2009) but also of methylation (Uddin et al., 2011).
Collectively, those studies suggest that genotype, methylation, and gene expression differences are promising areas for future research aimed at understanding the etiology of PTSD. However, the committee is not aware of any study that has incorporated all three forms of genetic information into one study, nor are there any definitive findings of any single gene or gene system in the etiology of PTSD.
Sleep
Sleep quality and quantity have been implicated as major factors in the consolidation—that is the conversion of a short-term memory into a long-term memory—of fear-extinction learning. Insomnia and sleep disruptions that affect rapid-eye-movement (REM) sleep are often found in patients who have PTSD, and many studies have investigated the effect of diminished sleep on learning and memory processes. Research has found that the treatment of insomnia may have favorable effects on other pathologic conditions, such as the reduction of co-occurring depressive symptoms (Isaac, 2011). Restoring restful sleep patterns may have the beneficial side effect of reducing the depression symptoms common in PTSD.
Restoring healthy sleep patterns may also preserve REM sleep that contributes to the consolidation of memories associated with fear conditioning and fear extinction. Mohammed et al. (2011) found that REM-sleep deprivation increased the concentration of the neurotransmitters glutamate, glycine, and taurine in the cortex of the rat brain and increased concentrations
of glutamate, aspartate, glutamine, and glycine in the hippocampus. Those neurotransmitters affect brain performance, and increased concentrations suggest that REM-sleep deprivation can affect the parts of the brain that are crucial in memory consolidation and motor, sensory, and associative function.
Fear conditioning can decrease REM sleep, but as fear extinction is learned, healthy REM-sleep levels can be restored (Deschaux et al., 2010). Spoormaker et al. (2010) studied REM-sleep disturbances in humans by using functional MRI and skin conductance response measurements. They found that after fear conditioning, REM-deprived participants had “significantly slower decline of [skin-conductance response] and neural activity of the laterodorsal tegmentum.” REM sleep not only is implicated as a factor that is fostered by fear learning and supports an appropriate fear response, but when restored in people who have co-occurring depression, it may alleviate depressive symptoms. In contrast, REM-sleep deprivation, which affects concentrations of neurotransmitters in the brain, retards the fear-extinction process.
A recent study reported additional effects of sleep on fear extinction (Pace-Schott et al., 2009). Volunteers underwent a protocol of habituation, conditioning, extinction, and extinction recall. Those phases were carried out in a group that was allowed to get a full night of sleep and a group that had to stay awake during the 12-hour testing period. The authors found that “adequate sleep may promote generalization of extinction memory from specific stimuli treated during exposure therapy to similar stimuli later encountered.” Those results are clinically relevant because PTSD patients tend to generalize fear responses to different stimuli. The implication of these results is that adequate sleep could potentially allow generalization of the beneficial effects of therapy to a number of fear-inducing stimuli in PTSD patients.
Chronic Stressors
Chronic stressors can play a role in the development of PTSD by altering brain hormones and receptors that are responsible for fear learning, fear extinction, decision and risk assessment, and mood, thereby increasing vulnerability to fear conditioning. The continual stress faced by service members (such as dangerous environments, fear of attack, intrusive noise and smells, and family distance) leading up to the trigger exposure may also contribute to treatment resistance and overreaction to later fearful stressors. In animal models, Rau et al. (2005) found that “pre-exposure to shock sensitizes conditional fear [in response] to similar less intense stressors,” which could explain why the fear response to subtle cues in people who have PTSD may result in more severe fearful responses than would otherwise be
merited. They also found the subjects were not as responsive to treatment with NMDA antagonists after sensitization from repeated fear exposures.
Gourley et al. (2009) investigated a cellular mechanism by which chronic stress, in the form of chronic corticosterone exposure, can play a role in the development of PTSD or other psychologic disorders. Exposure to corticosterone over a long period of time, as might be experienced by service members, can lead to a decrease in endogenous corticosterone response to the re-exposure of the fearful context, impair extinction of fear learning, and decrease sucrose preference that is associated with emotional numbing. Chronic corticosterone exposure also decreases the quantity of receptors in the cortex, changing the functioning of the ventromedial prefrontal cortex responsible for processing fear, decision making, and risk taking.
Chronic stress may even affect the long-term genetic expression within the brain that can affect its ability to rebound from fear conditioning. Ponomarev et al. (2010) found that when stress-enhanced fear-learning rats received 15 foot shocks, they “produced robust long-lasting effects on amygdalar transcriptome, which reflected functional and structural changes in neurons and astroglia, which are necessary for plasticity and [stress-enhanced fear-learning].”
IMPLICATIONS FOR PTSD PREVENTION, DIAGNOSIS, AND TREATMENT
Enhancing Resilience
Resilience can be described as a person’s ability to “recover or bounce back” (Davidson et al., 2005) from an injury, trauma, insult, or disease (see Chapter 5). There is evidence that some phenotypes or personality attributes increase resilience to stressful situations. They include a person’s ability to use coping strategies to deal with stressful situations, the presence of a positive disposition, the ability to reframe a stressful situation in a more positive light, the ability to obtain support from family and friends, and the presence of a moral compass and spirituality in a person’s life (Feder et al., 2009). Some of those attributes can be enhanced through psychotherapy or pharmacologic agents.
Resilience in the context of PTSD has been studied relatively little, although the number of studies in the published literature is increasing. Many of the studies have used self-rating scales, such as the Connor-Davidson Resilience Scale, to investigate improvements in resilience after pharmacologic treatment or other experimental protocols (Davidson et al., 2005, 2008; Lavretsky et al., 2010). In addition, although many studies have focused on the adverse effects of stressful situations, Dolbier et al. (2010) suggest that favorable changes can also occur after exposure to a traumatic situation or
event (Dolbier et al., 2010; Steinhardt and Dolbier, 2008). These resilience studies and others are discussed in further detail in Chapter 5. These studies are informative, but a better understanding of the neural circuits underlying fear inhibition and extinction is necessary to elucidate the role of resilience in preventing the onset or severity of PTSD symptoms.
Potential Targets for Pharmacologic Treatment of PTSD
Numerous neurotransmitter and neurohormonal systems are involved in the regulation of stress and the dysregulation that characterizes PTSD. Understanding the mechanisms that facilitate the symptoms of PTSD by blocking fear inhibition and extinction is important for the development of pharmacologic targets. Furthermore, having a better understanding of fear reconsolidation may help to identify novel PTSD treatments. Several targets have been identified and are discussed below, but it should be noted that people vary in how they respond to pharmacologic treatments (Narasimhan et al., 2011; Shi et al., 2011; Uher, 2011; Xu et al., 2011a, b).
Glucocorticoids
Glucocorticoids and associated receptors are widely distributed in the central and peripheral nervous systems and play an important role in the body’s response to stress (Horvath, 2011). As part of the HPA axis, gluco-corticoids play a role in feedback loops of the hippocampal and hypotha-lamic paraventricular nucleus, in the release of adrenocorticotropin, and in binding to glucocorticoid and mineralocorticoid receptors (Heim and Nemeroff, 2009).
As previously discussed, cortisol is one key glucocorticoid that is involved in the stress response. The mechanisms underlying the function of cortisol are not well known. However, it is thought that low concentrations of cortisol may result in repeated recollections of traumatic memories. Schelling et al. (2004) suggested that administering hydrocortisone reduces the incidence and intensity of traumatic recollections, thereby reducing PTSD symptoms. Putman and Roelofs (2011) reviewed studies that investigated the effects associated with the administration of a single dose of cortisol and concluded that high concentrations of cortisol immediately after a traumatic event may facilitate coping with stress in some individuals. This is supported by a clinical study (using 17 subjects) and a preclinical study (using rats) that were recently undertaken in parallel to investigate the therapeutic effect of hydrocortisone in acutely traumatized participants and the morphologic and molecular alterations in the brain (Zohar et al., 2011). The results suggest that administering a high-dose of hydrocortisone
immediately after a traumatic event “may be protective against the subsequent development of PTSD after a traumatic event.”
Catecholamines
Catecholamines play an important role in the body’s response to stressful situations. Concentrations of these molecules in the blood have been used as markers of stress, and high concentrations indicate that the body is reacting to a stressful situation (Bowirrat et al., 2010). Neurotransmitters that are part of the catecholamine family include epinephrine (adrenaline), norepinephrine (noradrenaline), and dopamine, which are derived from the amino acid tyrosine. In noradrenergic neurons, dopamine is converted into norepinephrine, which then acts as a principal mediator of the central nervous system and autonomic stress responses (Heim and Nemeroff, 2009). Norepinephrine and epinephrine bind to ?- and ?-adrenergic receptors (Strawn and Geracioti, 2008), and it has been hypothesized that noradrenergic activity increases during traumatic events and results in the enhancement of the coding of a traumatic memory (Debiec et al., 2011). This system has been a target site for several possible therapies for PTSD symptoms.
A drug that targets the adrenergic receptor is yohimbine. Some studies have shown that yohimbine facilitates not only the formation and recall of aversive memories in humans (Cain et al., 2004; Mueller et al., 2009), but also the extinction of cue and contextual fear in rats and mice (Mueller et al., 2009). For example, Cain et al. (2004) administered yohimbine to mice before extinction sessions in several experimental protocols and found that it facilitated long-term extinction learning and extinguished fear faster than controls when tested on the second day of the experimental protocol. The authors suggested that yohimbine might be a useful adjunct to some types of behavior therapy. Morris and Bouton (2007) investigated yohimbine in rats in the context of six different experiments and found that it reduced short-term and long-term freezing caused by the extinguished conditioned stimulus. In contrast, although yohimbine may decrease freezing in rats, there is evidence that it does not strengthen the retention of fear extinction. Mueller et al. (2009) administered yohimbine to rats before extinction training and then tested them the next day without any drugs. They observed a decrease in freezing as described by Cain et al. (2004) and Morris and Bouton (2007), but a second measure of fear expression indicated that “yohimbine failed to enhance long-term retention of extinction” (Mueller et al., 2009). These negative results should be taken with caution because the protocol they used examined fear behavior in conflict with appetitive behavior in hungry rats, which is different than other yohimbine protocols that evaluated fear behavior in isolation.
In humans, there is not yet concrete evidence as to the efficacy and evidence of yohimbine. Based on the animal studies described above and others, Powers et al. (2009) undertook a randomized placebo-controlled clinical trial to test the efficacy of yohimbine to enhance the effects of exposure therapy. Although the study was small (12 participants given the placebo and 12 participants given yohimbine), was composed mostly of students, and was conducted in claustrophobic individuals rather than individuals diagnosed with PTSD, the authors concluded there is some initial evidence in humans that a single dose of yohimbine can increase overall fear reduction, thereby enhancing the clinical outcomes when given to individuals who are undergoing exposure therapy. Based on animal studies, the authors suggested the reduction in fear occurs through the enhancement of extinction memories (Powers et al., 2009). A more recent study in humans failed to detect a yohimbine benefit using extinction-based exposure therapy (Meyerbroeker et al., 2012). In this randomized placebo-controlled trial, 48 participants with a fear of flying completed treatment. These participants were randomized to four sessions of virtual reality exposure therapy combined with yohimbine or four sessions of virtual reality exposure with a placebo. Results indicated that noradrenalin levels were manipulated with yohimbine, but treatment with yohimbine did not appear to enhance exposure therapy. In this study, both the drug and placebo groups showed significant long-term therapy effects, and it is possible that yohimbine may only enhance weak extinction (Cain et al., 2004).
Other adrenergic-blocker drugs that have been investigated include prazosin and propranolol. Several clinical trials have investigated prazosin (Raskind et al., 2003, 2007; Taylor et al., 2006), and results show that treatment with prazosin is more effective than treatment with a placebo. Based on animal studies that show propranolol reduces the consolidation of aversive memories (for example, Rodriguez-Romaguera et al., 2009), research has been conducted on the effectiveness of this drug to prevent PTSD in humans. Most of the results have been negative (Hoge et al., 2012; Pitman et al., 2002; Stein et al., 2007), but there is some evidence that treatment with propranolol may block memory reconsolidation through fear extinction (Schiller et al., 2010). These drugs are discussed in further detail in Chapter 7.
Selective Serotonin Reuptake Inhibitors
Serotonin (5-hydroxytyptamine [5-HT]) is a neurotransmitter that is synthesized from the amino acid tryptophan. It is involved in anxiety, arousal, vigilance, aggression, mood, and impulsivity processes and has been implicated in the pathophysiology of stress and mood disorders (Vermetten and Bremner, 2002). It has been hypothesized that serotonin acts by binding
to 5-HT2 receptors to increase the stress response and by binding to 5-HT1A receptors to facilitate fear extinction (Heim and Nemeroff, 2009). It also affects the stress response by interacting with norepinephrine and cortico-trophin-releasing factor. Selective serotonin reuptake inhibitors (SSRIs) are recognized as the primary pharmacologic treatment for PTSD (Norrholm and Jovanovic, 2010). Preventing the reuptake of serotonin results in an increased concentration in the synaptic cleft. SSRIs are discussed further in Chapter 7.
N-methyl-d-aspartate Receptor Agonists and Antagonists
The NMDA receptor binds glutamate and is thought to play a role in memory and learning through synaptic plasticity (Heim and Nemeroff, 2009) and enhanced extinction of the fear response (Graham and Milad, 2011). It is hypothesized that targeting the NMDA receptor can modify or extinguish memories of traumatic events. D-Cycloserine (DCS) is a partial agonist of the NMDA receptor and has been shown to improve the extinction of fear in rodents (Heim and Nemeroff, 2009; Ledgerwood et al., 2005; Myers et al., 2011) when administered in the basolateral amygdala (Norrholm and Jovanovic, 2010). For example, Ledgerwood et al. (2005) investigated the extinction of fear in male rats and the reinstatement of that fear in other contexts. They found that if DCS was administered to a rat immediately after an extinction training exercise and the rat was then re-exposed to the unconditioned stimulus, the conditioned fear would not be reinstated and the rat would not show a fear response. Woods and Bouton (2006) also looked at fear extinction and the renewal of the extinguished fear after administering DCS in female instead of male rats. They reported the fear was extinguished in four trials of DCS dosing, which is confirmed by previous results in animals; however, the facilitated extinction did not have any association with the strength of the renewal effect, so there may still be a potential for relapse. A meta-analysis of English-language journal articles from 1998 to 2007 led to the conclusion that DCS has the ability to enhance fear extinction and exposure therapy if administered immediately before or after extinction training in animals or exposure therapy in humans (Norberg et al., 2008). Limitations of the treatment include nonspecificity to conditioned stimuli and increased tolerance after repeated DCS dosing (Parnas et al., 2005).
Ketamine is hypothesized to ameliorate symptoms of mood and anxiety disorders, including PTSD, by acting as an antagonist at the NMDA receptor (Chambers et al., 1999). It may also reduce the PTSD incidence rate in certain circumstances, although some researchers caution that this association is weak (McGhee et al., 2008). Other modulators of the NMDA receptor are the naturally occurring polyamines spermidine and spermine.
A study in rats showed that intrahippocampal administration of spermidine facilitated the extinction of conditioned fear, probably acting through a subunit of the NMDA receptor (Gomes et al., 2010).
Brain-Derived Neurotrophic Factor
Neurotrophins are involved in the regulation of cell growth and survival, differentiation, apoptosis, and restructuring. Much research has centered around one particular neurotrophin; brain-derived neurotrophic factor (BDNF). Studies of BDNF have shown that it increases the survival of neurons, increases synaptic transmission and plasticity (Kaplan et al., 2010), and blocks or reverses atrophy and neuronal loss caused by stress (Duman and Monteggia, 2006). In a review of the scientific literature, evidence indicates that BDNF is dysregulated in PTSD (Kaplan et al., 2010). The authors also observed that prolonged exposure to glucocorticoids due to chronic stress may reduce BDNF concentrations and may result in retraction, restructuring, and disconnection of dendrites in the hippocampus and ventromedial prefrontal cortex. In another review, Duman and Monteggia (2006) found that hippocampal BDNF was decreased in 11 of 12 animal studies that investigated at stress, but there is limited evidence in humans in the context of PTSD (Duman and Monteggia, 2006; Kaplan et al., 2010). One exception is a study that looked at BDNF plasma concentrations in 18 subjects who had a diagnosis of PTSD and 18 healthy controls (Dell’osso et al., 2009). Results indicated significantly lower blood concentrations in the PTSD patients than in controls, although these results need to be validated by further research. Limitations of the study include the small sample and the lack of a full understanding of how concentrations of BDNF in the blood correlate with concentrations in the brain.
BDNF may play a role in PTSD treatment by promoting neuroplasti-city and adaptation to physiologic processes caused by chronic stress. For example, Radecki et al. (2005) performed hippocampal infusions of BDNF in male rats for 14 days. On day 7, the rats were exposed to chronic immobilization stress 2 hours/day for 7 days. The group of 20 rats that received infusion of BDNF and were put under stress was compared with five control groups of 20 rats each: stress treatment and saline infusions, stress treatment with no infusions, saline infusions without stress treatment, non-stressed with no infusions, and BDNF infusions without stress treatments. The authors found that BDNF infusions led to the reversal of stress-induced impairments in spatial learning and memory. Peters et al. (2010) carried out several studies in rats in an effort to investigate the molecular mechanisms underlying extinction-related plasticity. Rats were subjected to auditory fear conditioning and then given infusions of BDNF protein. The results showed
that conditioned fear was reduced for up to 48 hours after BDNF infusion even in the absence of extinction training.
In addition to the above studies, treatment with histone deacetylase inhibitors has resulted in an increase in the expression of BDNF in rats and an expansion in the cell populations in the subventricular zone and the hippocampal dentate gyrus (Kim et al., 2009). In a mouse model, treatment with the histone deacetylase inhibitor valproate has been shown to enhance long-term memory for extinction (Bredy et al., 2007). BDNF concentrations and associated proteins necessary for synaptic function have also been found to be increased by exercise. Surgery was performed on 89 male rats to cause lateral fluid-percussion injury (Griesbach et al., 2004). The experimental group and a sham injury group of 72 rats were housed in a cage with or without access to a running wheel at different times after the surgery. The authors saw an increase in endogenous BDNF and enhanced recovery if rats exercised 14–20 days after surgery, but exercise 0–6 days after surgery appeared to disrupt and possibly even delay recovery. In contrast, some negative studies have been associated with BDNF treatment. Rats that had traumatic brain injuries in the parietal cortex were treated with BDNF infusions for 2 weeks and showed no improvements in the measured outcomes of neurologic function, learning, memory, or neuronal loss (Blaha et al., 2000).
BDNF upregulation has been observed with several classes of anti-depressant drug treatments (Duman and Monteggia, 2006). Nibuya et al. (1995) investigated the influence of the antidepressant tranylcypromine (a monoamine oxidase inhibitor), desipramine (a tricyclic antidepressant), sertraline (an SSRI), and mianserin (an atypical antidepressant) on the expression of BDNF and its receptor trkB. The authors put male rats through a stress test and compared the antidepressants listed above with several psychotropic drugs (morphine, cocaine, and haloperidol) and saline. The rats were given the pharmacologic agents intraperitoneally in acute and chronic settings. The acute experiment was one dose, and the chronic experiment included dosing once a day for 21 days (except that morphine was administered in a pellet once a day for 5 days). The authors found that BDNF messenger RNA expression was significantly upregulated in the frontal cortex after chronic dosing with tranylcypromine and that BDNF and trkB messenger RNA expression was significantly upregulated in the hippocampus after chronic dosing with all of the antidepressants.
In humans, postmortem samples of the anterior hippocampus were obtained from people who had a diagnosis of major depressive disorder (n = 12), bipolar disorder (n = 11), or schizophrenia (n = 12), and from controls (n = 15) (Chen et al., 2002). The authors used BDNF immunohis-tochemistry to determine levels of BDNF expression. Although the samples were small, the authors found there were no statistical differences in BDNF
expression among the different diagnoses, but BDNF expression in the dentate gyrus, hilus, and supragranular regions of the hippocampus was significantly higher in people who had been treated with antidepressants at the time of death than in those who had not been. Those results, in addition to information in a literature review by D’Sa and Duman (2002), indicate that antidepressant treatments could play a role in treatment of PTSD by promoting neuronal survival and by protecting neurons from stress-induced damage.
Other neurotrophic factors have been linked to the pathophysiology of PTSD, including fibroblast growth factor 2 (FGF2). FGF2 is similar to BDNF in being sensitive to acute stress, particularly in the hippocampal region. Several preliminary studies in rats have shown increases in the expression of FGF2 mRNA in the hippocampus and medial prefrontal cortex after exposure to stress that was uncontrollable (inescapable tail shock) and behaviorally controllable (escapable tail shock) (Bland et al., 2006, 2007). This is a growing field of research that could contribute to the understanding of the pathophysiology of PTSD.
One goal of better understanding the neurobiology of PTSD is to identify biomarkers of the disorder. At least four kinds of biomarkers are of interest. The first are biomarkers that could reliably predict who is at risk for the development of PTSD; such markers may be especially useful in persons—such as service members—who are at high risk for trauma exposure or who have recently been exposed to trauma. The second are biomarkers that could contribute to PTSD diagnosis. Currently, PTSD diagnosis depends completely on self-reporting of symptoms. That is sometimes problematic in military populations because of concerns about stigma, which can lead to an underreporting of symptoms (Corrigan, 2004; Warner et al., 2011), or seeking compensation, which can lead to exaggeration of symptoms (Frueh et al., 1997; IOM, 2007). The third are biomarkers that would be used to match service members to specific evidence-based treatments. The fourth are biomarkers that would be able to predict who is at risk for relapse. These are areas of consideration for moving forward with the prevention, diagnosis, and treatment of PTSD.
It would be beneficial if biomarkers could be used to screen for or diagnose PTSD. For example, peripheral markers, which are found in blood and other bodily fluids, could potentially be used to screen for PTSD. These markers include catecholamine (Yehuda et al., 1992), cortisol (Bremner et al., 2007; de Kloet et al., 2006; Flory et al., 2009; Luo et al., 2012; Vythilingam et al., 2010; Wang et al., 2010b; Yehuda, 2009; Yehuda et al., 2002), dehydroepiandrosterone (Olff et al., 2007; Yehuda et al., 2010),
BDNF (Dell’osso et al., 2009; Frielingsdorf et al., 2010), interleukin-8 (Song et al., 2007), T-cell markers (Lemieux et al., 2008), and peripheral blood mononuclear cells (Segman et al., 2005; Su et al., 2009). Neurologic markers may also be candidates for screening for PTSD. Some that have recently been studied include the difference in activity between certain areas of the brain, such as the dorsolateral prefrontal cortex (Shin et al., 2004, 2006; Pissiota et al., 2002), amygdala (Pissiota et al., 2002; Shin et al., 2004, 2006; Vermetten and Bremner, 2002), and HPA axis (Southwick et al., 1994; Yehuda et al., 1991). Resting metabolism in the brain could potentially also be used to predict autonomic and neuronal responses that are associated with PTSD (Linnman et al., 2012).
PTSD has numerous biologic correlates. In most biologically oriented PTSD research, traditional case–control designs are used to assess whether a given biologic marker is more prevalent in people who have PTSD than in trauma-exposed control subjects that did not develop PTSD. Such studies show whether a biologic factor is a correlate of PTSD; however, research beyond the traditional case–control design is needed to determine whether the biologic factor can be used as a biomarker. To be considered a bio-marker of value in predicting risk of PTSD, there must be evidence that the biologic factor was present before trauma exposure, is associated with risk of PTSD, and has predictive validity. To be considered a biomarker of value in diagnosis, a biologic correlate would have to have excellent sensitivity and specificity in distinguishing persons who have and do not have PTSD. Similarly, to be used to match persons who have PTSD with specific treatments, a biomarker would have to be shown to differentiate between those who do and do not respond to specific treatments.
Despite the extensive knowledge of the neurobiology of PTSD, no biologic factors have enough predictive validity to be used at the individual level for any of the purposes described above. For example, C-reactive protein (a marker of inflammation) can be used to identify persons at risk of developing heart disease and distinguish between those who did and did not have a myocardial infarction (Ridker, 2003). No similar biomarkers exist for PTSD. It should also be noted that even if a specific biologic marker of PTSD were identified, such considerations as acceptability, costs, and time would have to be weighed.
The understanding of the neurobiology of PTSD continues to grow, but there is still much to be learned. A large part of this chapter has focused on studies, both in animals and in humans, that show correlations between a stressor or a risk factor and PTSD symptoms. This work is very important; however, causal studies are necessary to firmly implicate any neurobiologic
mechanisms for PTSD risk or resilience, and at present, these studies are lacking. Because many of the required invasive techniques cannot be undertaken in humans, these studies will require animal research on learning and stress systems. Another area of science that could be improved is the understanding of how environmental and biologic factors (such as genetics) contribute to the onset and severity of PTSD symptoms. This research will have important implications for PTSD prevention and treatment. Furthermore, the identification of biomarkers and brain-imaging models for the diagnosis of PTSD would help to reduce the dependence on self-reported symptoms. For the treatment of PTSD, it is important that pharmacologic agents that have the potential to enhance therapy-related learning be investigated further.
This chapter and Chapter 2, which described epidemiologic studies, are intended to set the stage for understanding how science can inform clinical practices in PTSD prevention (Chapter 5), diagnosis (Chapter 6), and treatment (Chapter 7), and for understanding the effects that comorbidities can have on the pathobiology of PTSD (Chapter 8).
Amorapanth, P., J. E. LeDoux, and K. Nader. 2000. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nature Neuroscience 3:74-79.
Amstadter, A., N. Nugent, and K. Koenen. 2009a. Genetics of PTSD: Fear conditioning as a model for future research. Psychiatric Annals 39(6):358-367.
Amstadter, A. B., K. C. Koenen, K. J. Ruggiero, R. Acierno, S. Galea, D. G. Kilpatrick, and J. Gelernter. 2009b. Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. Journal of Anxiety Disorders 23(3):369-373.
Amstadter, A. B., N. R. Nugent, B. Z. Yang, A. Miller, R. Siburian, P. Moorjani, S. Haddad, A. Basu, J. Fagerness, G. Saxe, J. W. Smoller, and K. C. Koenen. 2011. Corticotrophin-releasing hormone type 1 receptor gene (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Disease Markers 30(2-3):89-99.
Andreano, J. M., and L. Cahill. 2009. Sex influences on the neurobiology of learning and memory. Learning and Memory 16(4):248-266.
APA (American Psychiatric Association). 2000. Diagnostic and statistical manual of mental disorders, fourth edition, text revision (DSM-IV-TR). Washington, DC: American Psychiatric Association.
Archbold, G. E., M. E. Bouton, and K. Nader. 2010. Evidence for the persistence of contextual fear memories following immediate extinction. European Journal of Neuroscience 31(7):1303-1311.
Bachmann, A. W., T. L. Sedgley, R. V. Jackson, J. N. Gibson, R. M. Young, and D. J. Torpy. 2005. Glucocorticoid receptor polymorphisms and post-traumatic stress disorder. Psy-choneuroendocrinology 30(3):297-306.
Bailey, J. N., A. K. Goenjian, E. P. Noble, D. P. Walling, T. Ritchie, and H. A. Goenjian. 2011. PTSD and dopaminergic genes, DRD2 and DAT, in multigenerational families exposed to the Spitak earthquake. Psychiatry Research 178(3):507-510.
Barnett, J. H., C. H. Salmond, P. B. Jones, and B. J. Sahakian. 2006. Cognitive reserve in neuropsychiatry. Psychological Medicine 36(8):1053-1064.
Batty, G. D., E. L. Mortensen, and M. Osler. 2005. Childhood IQ in relation to later psychiatric disorder: Evidence from a Danish birth cohort study. British Journal of Psychiatry 187:180-181.
Bernstein, B. E., A. Meissner, and E. S. Lander. 2007. The mammalian epigenome. Cell 128(4):669-681.
Binder, E. B., R. G. Bradley, W. Liu, M. P. Epstein, T. C. Deveau, K. B. Mercer, Y. Tang, C. F. Gillespie, C. M. Heim, C. B. Nemeroff, A. C. Schwartz, J. F. Cubells, and K. J. Ressler. 2008. Association of FKBP5 polymorphisms and childhood abuse with risk of posttrau-matic stress disorder symptoms in adults. Journal of the American Medical Association 299(11):1291-1305.
Blaha, G. R., R. Raghupathi, K. E. Saatman, and T. K. McIntosh. 2000. Brain-derived neu-rotrophic factor administration after traumatic brain injury in the rat does not protect against behavioral or histological deficits. Neuroscience 99(3):483-493.
Bland, S. T., M. J. Schmid, B. N. Greenwood, L. R. Watkins, and S. F. Maier. 2006. Behavioral control of the stressor modulates stress-induced changes in neurogenesis and fibroblast growth factor-2. Neuroreport 17(6):593-597.
Bland, S. T., J. P. Tamlyn, R. M. Barrientos, B. N. Greenwood, L. R. Watkins, S. Campeau, H. E. Day, and S. F. Maier. 2007. Expression of fibroblast growth factor-2 and brain-derived neurotrophic factor mRNA in the medial prefrontal cortex and hippocampus after uncontrollable or controllable stress. Neuroscience 144(4):1219-1228.
Blechert, J., T. Michael, N. Vriends, J. Margraf, and F. H. Wilhelm. 2007. Fear conditioning in posttraumatic stress disorder: Evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behaviour Research and Therapy 45(9):2019-2033.
Bluhm, R. L., R. P. Williamson, E. A. Osuch, P. A. Frewen, T. K. Stevens, K. Boksman, R. W. Neurfeld, J. Théberge, and R. A. Lanius. 2009. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. Journal of Psychiatry and Neuroscience 34(3):187-194.
Bonne, O., D. Brandes, A. Gilboa, J. M. Gomori, M. E. Shenton, R. K. Pitman, and A. Y. Shalev. 2001. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. American Journal of Psychiatry 158(8):1284-1251.
Boscarino, J. A., P. M. Erlich, S. N. Hoffman, M. Rukstalis, and W. F. Stewart. 2011. Association of FKBP5, COMT, and CHRNA5 polymorphisms with PTSD among outpatients at risk for PTSD. Psychiatry Research 188(1):173-174.
Bouton, M. E. 2004. Context and behavioral processes in extinction. Learning and Memory 11(5):485-494.
Bouton, M., T. Todd, D. Vurbic, and N. Winterbauer. 2011. Renewal after the extinction of free operant behavior. Learning & Behavior 39(1):57-67.
Bowirrat, A., T. J. Chen, K. Blum, M. Madigan, J. A. Bailey, A. L. Chuan Chen, B. W. Downs, E. R. Braverman, S. Radi, R. L. Waite, M. Kerner, J. Giordano, S. Morse, M. Oscar-Berman, and M. Gold. 2010. Neuro-psychopharmacogenetics and neurological antecedents of posttraumatic stress disorder: Unlocking the mysteries of resilience and vulnerability. Current Neuropharmacology 8(4):335-358.
Bredy, T. W., H. Wu, C. Crego, J. Zellhoefer, Y. E. Sun, and M. Barad. 2007. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learning and Memory 14:267-276.
Bremner, J. D. 2001. Hypotheses and controversies related to effects of stress on the hippocampus: an argument for stress-induced damage to the hippocampus in patients with posttraumatic stress disorder. Hippocampus 11(2):75-81.
Bremner, J. D., P. Randall, T. M. Scott, R. A. Bronen, J. P. Seibyl, S. M. Southwick, R. C. Delaney, G. McCarthy, D. S. Charney, and R. B. Innis. 1995. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. American Journal of Psychiatry 152(7):973-981.
Bremner, J. D., L. H. Staib, D. Kaloupek, S. M. Southwick, R. Soufer, and D. S. Charney. 1999. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biological Psychiatry 45(7):806-816.
Bremner, D., E. Vermetten, and M. E. Kelley. 2007. Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related post-traumatic stress disorder. Journal of Nervous and Mental Disease 195(11):919-927.
Breslau, N., G. C. Davis, P. Andreski, and E. Peterson. 1991. Traumatic events and post-traumatic stress disorder in an urban population of young adults. Archives of General Psychiatry 48:216-222.
Breslau, N., R. Kessler, H. D. Chilcoat, L. R. Schultz, G. C. Davis, and P. Andreski. 1998. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit area survey of trauma. Archives of General Psychiatry 55:626-632.
Brewin, C. R., and E. A. Holmes. 2003. Psychological theories of postraumatic stress disorder. Clinical Psychology Review 23:339-376.
Bryant, R. A., and A. G. Harvey. 2003. Gender differences in the relationship between acute stress disorder and posttraumatic stress disorder following motor vehicle accidents. Australian and New Zealand Journal of Psychiatry 37(2):226-229.
Cahill, S. P., and E. B. Foa. 2007. Chapter 4: Psychological theories of PTSD. In Handbook of PTSD: Science and Practice, edited by M. J. Friedman, T. M. Keane, and P. A. Resnick. New York: Guilford Press.
Cain, C. K., and J. E. LeDoux. 2007. Escape from fear: A detailed behavioral analysis of two atypical responses reinforced by CS termination. Journal of Experimental Psychology. Animal Behavioural Processes 33(4):451-463.
Cain, C. K., and J. E. Ledoux. 2008. Emotional processing and motivation: In search of brain mechanisms. In Handbook of Approach and Avoidance Motivation, edited by A. J. Elliot. New York: Psychology Press. Pp. 17-34.
Cain, C. K., A. M. Blouin, and M. Barad. 2004. Adrenergic transmission facilitates extinction of conditional fear in mice. Learning & Memory 11(2):179-187.
Chambers, R. A., J. D. Bremner, B. O. Moghaddam, S. M. Southwick, D. S. Charney, and J. H. Krystal. 1999. Glutamate and post-traumatic stress disorder: Toward a psychobiology of dissociation. Seminars in Clinical Neuropsychiatry 4:274-281.
Chang, S. C., P. Xie, R. F. Anton, I. De Vivo, L. A. Farrer, H. R. Kranzler, D. Oslin, S. M. Purcell, A. L. Roberts, J. W. Smoller, M. Uddin, J. Gelernter, and K. C. Koenen. 2012. No association between ADCYAP1R1 and post-traumatic stress disorder in two independent samples. Molecular Psychiatry 17(3):239-241.
Chang., Y. J., C. H. Yang, Y. C. Liang, C. M. Yeh, C. C. Huang, and K. S. Hsu. 2009. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus 19(11):1142-1150.
Charney, D. S. 2004. Psychobiological mechanisms of resilience and vulnerability: Implications for successful adaptation to extreme stress. American Journal of Psychiatry 161(2):195-216.
Chauhan, S., C. H. Leach, S. Kunz, J. W. Bloom, and R. L. Miesfeld. 2003. Glucocorticoid regulation of human eosinophil gene expression. Journal of Steroid Biochemical Molecular Biology 84(4):441-452.
Chen, Y., R. P. Sharma, R. H. Costa, E. Costa, and D. R. Grayson. 2002. On the epigenetic regulation of the human reelin promoter. Nucleic Acids Research 30(13):2930-2939.
Cohen, H., and G. Richter-Levin. 2009. Toward animal models of post-traumatic stress disorder. In Post-traumatic stress disorder: Basic science and clinical practice. New York: Humana Press.
Comings, D. E., B. G. Comings, D. Muhleman, G. Dietz, B. Shabbahrami, and D. Tast. 1991. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorder. Journal of the American Medical Association 266:1793-1800.
Comings, D. E., D. Muhleman, and R. Gysin. 1996. Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: A study and replication. Biological Psychiatry 40:368-372.
Cornelis, M. C., N. R. Nugent, A. B. Amstadter, and K. C. Koenen. 2010. Genetics of post-traumatic stress disorder: Review and recommendations for genome-wide association studies. Current Psychiatry Report 12(4):313-326.
Corrigan, P. 2004. How stigma interferes with mental health care. American Psychologist 59(7):614-625.
Davidson, J. R., V. M. Payne, K. M. Connor, E. B. Foa, B. O. Rothbaum, M. A. Hertzberg, and R. H. Weisler. 2005. Trauma, resilience and saliostasis: Effects of treatment in post-traumatic stress disorder. International Clinical Psychopharmacology 20(1):43-48.
Davidson, J., D. S. Baldwin, D. J. Stein, R. Pedersen, S. Ahmed, J. Musgnung, I. Benattia, and B. O. Rothbaum. 2008. Effects of venlafaxine extended release on resilience in post-traumatic stress disorder: An item analysis of the Connor-Davidson Resilience Scale. International Clinical Psychopharmacology 23(5):299-303.
Davis, M. 1986. Pharmacological and anatomical analysis of fear conditioning using the fear-potentiated startle paradigm. Behavioral Neuroscience 100(6):814-824.
De Bellis, M. D., J. Hall, A. M. Boring, K. Frustaci, and G. Moritz. 2001. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biological Psychiatry 50(4):305-309.
de Kloet, C. S., E. Vermetten, E. Geuze, A. Kavelaars, C. J. Heijnen, and H. G. M. Westenberg. 2006. Assessment of HPA-axis function in posttraumatic stress disorder: Pharmacological and non-pharmacological challenge tests, a review. Journal of Psychiatric Research 40(6):550-567.
Deary, I. J., and G. D. Batty. 2007. Cognitive epidemiology. Journal of Epidemiology and Community Health 61(5):378-384.
Debiec, J., D. E. A. Bush, and J. E. LeDoux. 2011. Noradrenergic enhancement of recon-solidation in the amygdala impairs extinction of conditioned fear in rats—a possible mechanism for the persistence of traumatic memories in PTSD. Depression and Anxiety 28(3):186-193.
Delgado, M. R., K. I. Nearing, J. E. LeDoux, and E. A. Phelps. 2008. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 59(5):829-838.
Dell’osso, L., C. Carmassi, A. Del Debbio, M. C. Dell’osso, C. Bianchi, E. da Pozzo, N. Origlia, L. Domenici, G. Massimetti, D. Marazziti, and A. Piccinni. 2009. Brain-derived neurotrophic factor plasma levels in patients suffering from post-traumatic stress disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry 33(5):899-902.
Deschaux, O., A. Thevenet, G. Spennato, C. Arnaud, J. L. Moreau, and R. Garcia. 2010. Low-frequency stimulation of the hippocampus following fear extinction impairs both restoration of rapid eye movement sleep and retrieval of extinction memory. Neurosci-ence 170(1):92-98.
Dolbier, C. L., S. S. Jaggars, and M. A. Steinhardt. 2010. Stress-related growth: Pre-intervention correlates and change following a resilience intervention. Stress and Health 26(2):135-147.
Dragan, W. L., and W. Ç. Oniszczenko. 2009. The association between dopamine D4 receptor exon III polymorphism and intensity of PTSD symptoms among flood survivors. Anxiety, Stress & Coping: An International Journal 22(5):483-495.
Drury, S. S., K. P. Theall, B. J. Keats, and M. Scheeringa. 2009. The role of the dopamine transporter (DAT) in the development of PTSD in preschool children. Journal of Traumatic Stress 22(6):534-539.
D’Sa, C., and R. S. Duman. 2002. Antidepressants and neuroplasticity. Bipolar Disorders 4(3):183-194.
Duman, R. S., and L. M. Monteggia. 2006. A neurotrophic model for stress-related mood disorders. Biological Psychiatry 59(12):1116-1127.
Enewold, L., L. A. Brinton, K. A. McGlynn, S. H. Zahm, J. F. Potter, and K. Zhu. 2010. Oral contraceptive use among women in the military and the general U.S. population. Journal of Women’s Health 19(5):839-845.
Esmorís-Arranz, F. J., C. Méndez, and N. W. Spear. 2008. Contextual fear conditioning differs for infant, adolescent, and adult rats. Behavioural Processes 78(3):340-350.
Etkin, A., T. Egner, and R. Kalisch. 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences 15(2):85-93.
Feder, A., E. J. Nestler, and D. S. Charney. 2009. Psychobiology and molecular genetics of resilience. Nature Reviews Neuroscience 10(6):446-457.
Fennema-Notestine, C., M. B. Stein, C. M. Kennedy, S. L. Archibald, and T. L. Jernigan. 2002. Brain morphometry in female victims of intimate partner violence with and without post-traumatic stress disorder. Biological Psychiatry 52(11):1089-1101.
Fischer, H., L. Nyberg, and L. Backman. 2010. Age-related differences in brain regions supporting successful encoding of emotional faces. Cortex 46(4):490-497.
Flory, J. D., R. Yehuda, R. Grossman, A. S. New, V. Mitropoulou, and L. J. Siever. 2009. Childhood trauma and basal cortisol in people with personality disorders. Comprehensive Psychiatry 50(1):34-37.
Freeman, T., V. Roca, F. Guggenheim, T. Kimbrell, and W. S. Griffin. 2005. Neuropsychiatric associations of apolipoprotein E alleles in subjects with combat-related posttraumatic stress disorder. Journal of Neuropsychiatry and Clinical Neurosciences 17(4):541-543.
Frielingsdorf, H., K. G. Bath, F. Soliman, J. Difede, B. J. Casey, and F. S. Lee. 2010. Variant brain-derived neurotrophic factor VAL66MET endophenotypes: Implications for post-traumatic stress disorder. Annals of the New York Academy of Sciences 1208:150-157.
Frueh, B. C., P. B. Gold, and M. A. de Arellano. 1997. Symptom overreporting in combat veterans evaluated for PTSD: Differentiation on the basis of compensation seeking status. Journal of Personality Assessment 68(2):369-384.
Furmark, T., M. Tillfors, I. Marteinsdottir, H. Fischer, A. Pissiota, B. Langstrom, and M. Fredrikson. 2002. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Archive of General Psychiatry 59(5):425-433.
Garakani, A., S. J. Mathew, and D. S. Charney. 2006. Neurobiology of anxiety disorders and implications for treatment. Mount Sinai Journal of Medicine 73(7):941-949.
Gelernter, J., S. Southwick, S. Goodson, A. Morgan, L. Nagy, and D. S. Charney. 1999. No association between D2 dopamine receptor (DRD2) ‘A’ system alleles, or DRD2 haplo-types, and posttraumatic stress disorder. Biological Psychiatry 45:620-625.
Gilbertson, M. W., M. E. Shenton, A. Ciszewski, K. Kasai, N. B. Lasko, S. P. Orr, and R. K. Pitman. 2002. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience 5:1242-1247.
Gillies, G. E., and S. McArthur. 2010. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacologic Reviews 62(2):155-198.
Gogolla, N., P. Caroni, A. Lüthi, and C. Herry. 2009. Perineuronal nets protect fear memories from erasure. Science 325(5945)1258-1261.
Gold, A. L., L. M. Shin, S. P. Orr, M. A. Carson, S. L. Rauch, M. L. Macklin, N. B. Lasko, L. J. Metzger, D. D. Dougherty, N. M. Alpert, A. J. Fischman, and R. K. Pitman. 2011. Decreased regional cerebral blood flow in medial prefrontal cortex during trauma-unrelated stressful imagery in Vietnam veterans with post-traumatic stress disorder. Psychological Medicine 41(12):2563-2572.
Goldstein, J. M., M. Jerram, B. Abbs, S. Whitfield-Gabrieli, and N. Makris. 2010. Sex differences in stress response circuitry activation dependent on female hormonal cycle. Journal of Neuroscience 30(2):431-438.
Gomes, G. M., C. F. Mellow, M. M. da Rosa, G. V. Bochi, J. Ferreira, S. Barron, and M. A. Rubin. 2010. Polyaminergic agents modulate contextual fear extinction in rats. Neuro-biology of Learning and Memory 93(4):589-595.
Goossens, L., S. Sunaert, R. Peeters, E. J. L. Griez, and K. R. J. Schruers. 2007. Amygdala hyperfunction in phobic fear normalizes after exposure. Biological Psychiatry 62(10): 1119-1125.
Gourley, S. L., A. T. Kedves, P. Olausson, and J. R. Taylor. 2009. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GLUR2/3, and BDNF. Neuropsy-chopharmacology 34(3):707-716.
Grabe, H. J., C. Spitzer, C. Schwahn, A. Marcinek, A. Frahnow, S. Barnow, M. Lucht, H. J. Freyberger, U. John, H. Wallaschofski, H. Volzke, and D. Rosskopf. 2009. Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to post-traumatic stress disorder in the general population. American Journal of Psychiatry 166(8):926-933.
Graham, B. M., and M. R. Milad. 2011. Translational research in the neuroscience of fear extinction: Implications for anxiety disorders. American Journal of Psychiatry 168(12): 1255-1265.
Griesbach, G. S., D. A. Hovda, R. Molteni, A. Wu, and F. Gomez-Pinilla. 2004. Voluntary exercise following traumatic brain injury: Brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience 125(1):129-139.
Hao, J., P. R. Rapp, A. E. Leffler, S. R. Leffler, W. G. Janssen, W. Lou, H. McKay, J. A. Roberts, S. L. Wearne, P. R. Hof, and J. H. Morrison. 2006. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus moneys. Journal of Neuroscience 26(9):2571-2578.
Hartley, C. A., B. Fischl, and E. A. Phelps. 2011. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cereberal Cortex 21(9):1954-1962.
Heim, C., and C. B. Nemeroff. 2009. Neurobiology of posttraumatic stress disorder. CNS Spectrums 14(1):13-24.
Herry, C., F. Ferraguti, N. Singewald, J. J. Letzkus, I. Ehrlich, and A. Luthi. 2010. Neuronal circuits of fear extinction. European Journal of Neuroscience 31(4):599-612.
Hoffman, E. J., and S. J. Mathew. 2008. Anxiety disorders: A comprehensive review of phar-macotherapies. Mount Sinai Journal of Medicine 75(3):248-262.
Hoge, C. W., C. A. Castro, S. C. Messer, D. McGurk, D. I. Cotting, and R. L. Koffman. 2004. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine 351(1):13-22.
Hoge, C. W., J. L. Auchterlonie, and C. S. Milliken. 2006. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. Journal of the American Medical Association 295(9):1023-1032.
Hoge, E. A., J. J. Worthington, J. T. Nagurney, Y. Chang, E. B. Kay, C. M. Geterowski, A. R. Katzman, J. M. Goetz, M. L. Rosasco, N. B. Lasko, R. M. Zusman, M. H. Pollack, S. P. Orr, and R. K. Pitman. 2012. Effect of acute posttrauma propranolol on PTSD outcome and physiological responses during script-driven imagery. CNS Neuroscience and Therapeutics 18(1):21-27.
Holzel, B. K., U. Ott, T. Gard, H. Hempel, M. Weygandt, K. Morgen, and D. Vaitl. 2008. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Social Cognitive and Affective Neuroscience 3(1):55-61.
Horvath, G. 2011. Neurochemistry of endogenous antinociception. Advances in Neurobiol-ogy 1:118.
Iidaka, T., T. Okada, T. Murata, M. Omori, H. Kosaka, N. Sadato, and Y. Yonekura. 2002. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus 12(3):352-362.
IOM (Institute of Medicine). 2007. PTSD compensation and military service. Washington, DC: The National Academies Press.
IOM. 2008. Gulf War and health: Physiologic, psychologic, and psychosocial effects of deployment-related stress. Washington, DC: The National Academies Press.
Isaac, F. 2011. The relationship between insomnia and depressive symptoms: Genuine or artifact? Neuropsychiatric Disease and Treatment 7:57-63.
Jovanovic, T., and K. J. Ressler. 2010. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. American Journal of Psychiatry 167(6):648-662.
Jovanovic, T., S. D. Norrholm, J. E. Fennell, M. Keyes, A. M. Fiallos, K. M. Myers, M. Davis, and E. J. Duncan. 2009. Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Research 167(1-2):151-160.
Kaczorowski, C. C., S. J. Davis, and J. R. Moyer, Jr. 2011. Aging redistributes medial prefron-tal neuronal excitability and impedes extinction of trace fear conditioning. Neurobiology of Aging April 30 [Epub ahead of print].
Kalisch, R., E. Korenfeld, K. E. Stephan, N. Weiskopf, B. Seymour, and R. J. Dolan. 2006. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. Journal of Neuroscience 26(37):9503-9511.
Kaplan, G. B., J. J. Vasterling, and P. C. Vedak. 2010. Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: Role in pathogenesis and treatment. Behavioural Pharmacology 21(5-6):427-437.
Kasai, K., H. Yamasue, M. W. Gilbertson, M. E. Shenton, S. L. Rauch, and R. K. Pitman. 2008. Evidence for acquired pregenual anterior vingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biological Psychiatry 63(6):550-556.
Kehne, J. H., and C. K. Cain. 2010. Therapeutic utility of non-peptidic CRF1 receptor antagonists in anxiety, depression, and stress-related disorders: Evidence from animal models. Pharmacology & Therapeutics 128(3):460-487.
Kessler, R. C., A. Sonnega, E. Bromet, M. Hughes, and C. B. Nelson. 1995. Posttrau-matic stress disorder in the national comorbidity survey. Archives of General Psychiatry 52:1048-1060.
Khan, S., and I. Liberzon. 2004. Topiramate attenuates exaggerated acoustic startle in an animal model of PTSD. Psychopharmacology 172(2):225-229.
Kilpatrick, D. G., K. C. Koenen, K. J. Ruggiero, R. Acierno, S. Galea, H. S. Resnick, J. Roitzsch, J. Boyle, and J. Gelernter. 2007. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. American Journal of Psychiatry 164(11):1693-1699.
Kilpatrick, L. A., B. Y. Suyenobu, S. R. Smith, J. A. Bueller, T. Goodman, J. D. Creswell, K. Tillisch, E. A. Mayer, and B. D. Naliboff. 2011. Impact of mindfulness-based stress reduction training on intrinsic brain connectivity. Neuroimage 56(1):290-298.
Kim, H. J., P. Leeds, and D. M. Chuang. 2009. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. Journal of Neurochemistry 110(4):1226-1240.
Kim, J. H., S. Li, and R. Richardson. 2011b. Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cerebral Cortex 23(3):530-538.
Kim, J. H., and R. Richardson. 2008. The effect of temporary amgydala inactivation on extinction and reextinction of fear in the developing rat: Unlearning as a potential mechanism for extinction early in development. Journal of Neuroscience 28(6):1282-1290.
Kim, J. H., and R. Richardson. 2010. Extinction in preweanling rats does not involve NMDA receptors. Neurobiology of Learning and Memory 94(2):176-182.
Kim, M. J., and P. J. Whalen. 2009. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience 29(37):11614-11618.
Kim, M. J., D. G. Gee, R. A. Loucks, F. C. Davis, and P. J. Whalen. 2011a. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral Cortex 21(7):1667-1673.
Kitayama, N., V. Vaccarino, M. Kutner, P. Weiss, and J. D. Bremner. 2005. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: A meta-analysis. Journal of Affective Disorders 88(1):79-86.
Kitayama, N. S. Quinn, and J. D. Bremner. 2006. Smaller volume of anterior cingulate cortex in abuse-related posstraumatic stress disorder. Journal of Affective Disorders 90(2-3):171-174.
Koenen, K. C., G. Saxe, S. Purcell, J. W. Smoller, D. Bartholomew, A. Miller, E. Hall, J. Kaplow, M. Bosquet, S. Moulton, and C. Baldwin. 2005. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Molecular Psychiatry 10(12):1058-1059.
Koenen, K. C., S. D. Stellman, J. F. Sommer, Jr., and J. M. Stellman. 2008. Persisting posttrau-matic stress disorder symptoms and their relationship to functioning in Vietnam veterans: A 14-year follow-up. Journal of Traumatic Stress 21(1):49-57.
Koenen, K. C., I. De Vivo, J. Rich-Edwards, J. W. Smoller, R. J. Wright, and S. M. Purcell. 2009a. Protocol for investigating genetic determinants of posttraumatic stress disorder in women from the Nurses’ Health Study II. BMC Pyschiatry 9:29.
Koenen, K. C., T. E. Moffitt, A. L. Roberts, L. T. Martin, L. Kubzansky, H. Harrington, R. Poulton, and A. Caspi. 2009b. Childhood IQ and adult mental disorders: A test of the cognitive reserve hypothesis. American Journal of Psychiatry 166(1):50-57.
Koenen, K. C., A. Aiello, E. Bakshis, A. B. Amstadter, K. J. Ruggiero, R. Acierno, D. G. Kilpatrick, J. Gelernter, and S. Galea. 2009c. County-level social environment modifies the association between serotonin transporter genotype and risk of post-traumatic stress disorder in adults. American Journal of Epidemiology 169(6):704-711.
Koenen, K. C., M. Uddin, S. C. Chang, A. E. Aiello, D. E. Wildman, E. Goldmann, and S. Galea. 2011. Slc6a4 methylation modifies the effect of the number of traumatic events on risk for posttraumatic stress disorder. Depression and Anxiety 28(8):639-647.
Kolassa, I. T., V. Ertl, C. Eckart, F. Glockner, S. Kolassa, A. Papassotiropoulos, D. J. de Quervain, and T. Elbert. 2010a. Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: Evidence from survivors of the Rwandan genocide. Journal of Clinical Psychiatry 71(5):543-547.
Kolassa, I. T., S. Kolassa, V. Ertl, A. Papassotiropoulos, and D. J. De Quervain. 2010b. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-O-methyltransferase val(158)met polymorphism. Biological Psychiatry 67(4): 304-308.
Koolhaas, J. M., A. Bartolomucci, B. Buwalda, S. F. de Boer, G. Flugge, S. M. Korte, P. Meerlo, R. Murison, B. Olivier, P. Palanza, G. Richter-Levin, A. Sgoifo, T. Steimer, O. Stiedl, G. van Dijk, M. Wohr, and E. Fuchs. 2011. Stress revisited: A critical evaluation of the stress concept. Neuroscience and Biobehavioral Reviews 35(5):1291-1301.
Kulka, R. A., W. E. Schlenger, J. A. Fairbank, R. L. Hough, B. K. Jordan, C. R. Marmar, and D. S. Weiss. 1990. Trauma and the Vietnam War generation: Report of findings from the National Vietnam Veterans Readjustment Study. New York: Bruner/Mazel.
Labar, K. S., C. A. Cook, D. C. Torpey, and K. A. Welsh-Bohmer. 2004. Impact of healthy aging on awareness and fear conditioning. Behavioural Neuroscience 118(5):905-915.
Lang, P. J., M. Davis, and A. Ohman. 2000. Fear and anxiety: Animal models and human cognitive psychophysiology. Journal of Affective Disorders 61(3):137-159.
Lappalainen, J., H. R. Kranzler, R. Malison, L. H. Price, D. Van Dyck, J. H. Krystal, and J. Gelernter. 2002. A functional neuropeptide Y leu7pro polymorphism associated with alcohol dependence in a large population sample from the United States. Archives of General Psychiatry 59:825-831.
Lavretsky, H., P. Siddarth, and M. R. Irwin. 2010. Improving depression and enhancing resilience in family dementia caregivers: A pilot randomized placebo-controlled trial of escitalopram. American Journal of Geriatric Psychiatry 18(2):154-162.
Ledgerwood, L., R. Richardson, and J. Cranney. 2005. D-cycloserine facilitates extinction of learned fear: Effects on reacquisition and generalized extinction. Biological Psychiatry 57(8):841-847.
LeDoux, J. E. 2000. Emotion circuits in the brain. Annual Review of Neuroscience 23:155-184.
Lee, H. J., M. S. Lee, R. H. Kang, H. Kim, S. D. Kim, B. S. Kee, Y. H. Kim, Y. K. Kim, J. B. Kim, B. K. Yeon, K. S. Oh, B. H. Oh, J. S. Yoon, C. Lee, H. Y. Jung, I. S. Chee, and I. H. Paik. 2005. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depression and Anxiety 21(3):135-139.
Lee, H., R. Kang, S. Lim, J. Paik, M. Coi, and M. Lee. 2006. No association between the brain-derived nurotrophic factor gene val66met polymorphism and post-traumatic stress disorder. Stress and Health 22(2):115-119.
Lee, H., S. Kwak, J. Paik, R. Kang, and M. Lee. 2007. Association between serotonin 2a receptor gene polymorphism and posttraumatic stress disorder. Psychiatry Investigations 4(2):104-108.
Lemieux, A., C. L. Coe, and M. Carnes. 2008. Symptom severity predicts degree of T cell activation in adult women following childhood maltreatment. Brain, Behavior, and Immunity 22(6):994-1003.
Linnman, C., M. A. Zeidan, R. K. Pitman, and M. R. Milad. 2012. Resting cerebral metabolism correlates with skin conductance and functional brain activation during fear conditioning. Biological Psychology 89(2):450-459.
Lonsdorf, T. B., and R. Kalisch. 2011. A review on experimental and clinical genetic association studies on fear contitioning, extinction and cognitive-behavioral treatment. Trans-lational Psychiatric 1(9):e41.
Lu, A. T., M. N. Ogdie, M. Jarvelin, I. K. Moilanen, S. K. Loo, J. T. McCracken, J. J. McGough, M. H. Yang, L. Peltonen, S. F. Nelson, R. M. Cantor, and S. L. Smalley. 2008. Association of the cannabinoid receptor gene (CNR1) with ADHD and post-traumatic stress disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 147B(8):1488-1494.
Luo, H., X. Hu, X. Liu, X. Ma, W. Guo, C. Qiu, Y. Wang, Q. Wang, X. Zhang, W. Zhang, G. Hannum, K. Zhang, X. Liu, and T. Li. 2012. Hair cortisol level as a biomarker for altered hypothalamic-pituitary-adrenal activity in female adolescents with posttraumatic stress disorder after the 2008 Wenchuan earthquake. Biological Psychiatry Feb 1 [Epub ahead of print].
Maeng, L. Y., J. Waddell, and T. J. Shors. 2010. The prefrontal corex communicates with the amygdala to impair learning after acute stress in females but not in males. Journal of Neuroscience 30(48):16188-16196.
Mao, S. C., Y. H. Hsiao, and P. W. Gean. 2006. Extinction training in conjunction with a partial agonist of the glycine site on the NMDA receptor erases memory trace. Journal of Neuroscience 26(35):8892-8899.
Maren, S. 2001. Neurobiology of Pavlovian fear conditioning. Annual Review of Neurosci-ence 24:897-931.
Maren, S. 2011. Seeking a spotless mind: Extinction, deconsolidation, and erasure of fear memory. Neuron 70(5):830-845.
McEwen, B. S. 2005. Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism Clinical and Experimental 54(Suppl 1):20-23.
McEwen, B. S. 2006. Protective and damaging effects of stress mediators: Central role of the brain. Dialogues in Clinical Neuroscience 8(4):367-381.
McGhee, L. L., C. V. Maani, T. H. Garza, K. M. Gaylord, and I. H. Black. 2008. The correlation between ketamine and posttraumatic stress disorder in burned service members. Journal of Trauma 64:S195-S198; Discussion S197-S198.
Mellman, T. A., T. Alim, D. D. Brown, E. Gorodetsky, B. Buzas, W. B. Lawson, D. Goldman, and D. S. Charney. 2009. Serotonin polymorphisms and posttraumatic stress disorder in a trauma exposed African American population. Depression and Anxiety 26(11):993-997.
Mercer, K. B., H. K. Orcutt, J. F. Quinn, C. A. Fitzgerald, K. N. Conneely, R. T. Barfield, C. F. Gillespie, and K. J. Ressler. 2011. Acute and posttraumatic stress symptoms in a prospective gene X environment study of a university campus shooting. Archives of General Psychiatry 69(1):89-97.
Meyerbroeker, K., M. B. Powers, A. van Stegeren, and P. M. Emmelkamp. 2012. Does yohim-bine hydrochloride facilitate fear extinction in virtual reality treatment of fear of flying? A randomized placebo-controlled trial. Psychotherapy and Psychosomantics 81(1):29-37.
Milad, M. R., and G. J. Quirk. 2002. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420(6911):70-74.
Milad, M. R., and G. J. Quirk. 2012. Fear extinction as a model for translational neuroscience: Ten years of progress. Annual Review of Psychology 63:129-151.
Milad, M. R., S. P. Orr, R. K. Pitman, and S. L. Rauch. 2005. Context modulation of memory for fear extinction in humans. Psychophysiology 42(4):456-464.
Milad, M. R., C. I. Wright, S. P. Orr, R. K. Pitman, G. J. Quirk, and S. L. Rauch. 2007. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry 62(5):446-454.
Milad, M. R., S. P. Orr, N. B. Lasko, Y. C. Chang, S. L. Rauch, and R. K. Pitman. 2008. Presence and acquired origin of reduced recall for fear extinction in PTSD: Results of a twin study. Journal of Psychiatric Research 42(7):515-520.
Milad, M. R., S. A. Igoe, K. Lebron-Milad, and J. E. Novales. 2009a. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 164(3):887-895.
Milad, M. R., R. K. Pitman, C. B. Ellis, A. L. Gold, L. M. Shin, N. B. Lasko, M. A. Zeidan, K. Handwerger, S. P. Orr, and S. L. Rauch. 2009b. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry 66(12): 1075-1082.
Milad, M. R., M. A. Zeidan, A. Contero, R. K. Pitman, A. Klibanski, S. L. Rauch, and J. M. Goldstein. 2010. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience 168(3):652-658.
Mohammed, H. S., H. S. A. Ezz, Y. A. Khadrawy, and N. A. Noor. 2011. Neurochemical and electrophysiological changes induced by paradoxical sleep deprivation in rats. Behavioural Brain Research 225(1):39-46.
Morey, R. A., A. R. Hariri, A. L. Gold, M. A. Hauser, H. J. Munger, F. Dolcos, and G. McCarthy. 2011. Serotonin transporter gene polymorphisms and brain function during emotional distraction from cognitive processing in posttraumatic stress disorder. BioMed Central Psychiatry 11:76.
Morris, R. W., and M. E. Bouton. 2007. The effect of yohimbine on the extinction of conditioned fear: A role for context. Behavioral Neuroscience 121(3):501-514.
Mueller, D., L. A. Olivera-Figueroa, D. S. Pine, and G. J. Quirk. 2009. The effects of yohim-bine and amphetamine on fear expression and extinction in rats. Psychopharmacology 204(4):599-606.
Mustapic, M., N. Pivac, D. Kozaric-Kovacic, M. Dezeljin, J. F. Cubells, and D. Muck-Seler. 2007. Dopamine beta-hydroxylase (DBH) activity and -1021c/t polymorphism of DBH gene in combat-related posttraumatic stress disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 144B:1087-1089.
Myers, K. M., and M. Davis. 2007. Mechanisms of fear extinction. Molecular Psychiatry 12(2):120-150.
Myers, K. M., W. A. Carlezon, Jr., and M. Davis. 2011. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology 36(1):274-293.
Nader, K., G. E. Schafe, and J. E. LeDoux. 2000. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406(6797):722-726.
Narasimhan, S., T. D. Aquino, R. Hodge, K. Rickels, and F. W. Lohoff. 2011. Association analysis between the val66met polymorphism in the brain-derived neurotrophic factor (BDNF) gene and treatment response to venlafaxine xr in generalized anxiety disorder. Neuroscience Letters 503(3):200-202.
Nelson, E. C., A. Agrawal, M. L. Pergadia, M. T. Lynskey, A. A. Todorov, J. C. Wang, R. D. Todd, N. G. Martin, A. C. Heath, A. M. Goate, G. W. Montgomery, and P. A. F. Madden. 2009. Association of childhood trauma exposure and GABRA2 polymorphisms with risk of posttraumatic stress disorder in adults. Molecular Psychiatry 14:234-238.
Nibuya, M., S. Morinobu, and R. S. Duman. 1995. Regulation of BDNF and TRKB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. Journal of Neuroscience 15(11):7539-7547.
Nielsen, S. E., N. Ertman, Y. S. Lakhani, and L. Cahill. 2011. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiology of Learning and Memory 96(2):378-384.
Norberg, M. M., J. H. Krystal, and D. F. Tolin. 2008. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biological Psychiatry 63(12): 1118-1126.
Norrholm, S. D., and T. Jovanovic. 2010. Tailoring therapeutic strategies for treating post-traumatic stress disorder symptom clusters. Neuropsychiatric Disease and Treatment 6:517-532.
Norrholm, S. D., T. Jovanovic, I. W. Olin, L. A. Sands, I. Karapanou, B. Bradley, and K. J. Ressler. 2011. Fear extinction in traumatized civilians with posttraumatic stress disorder: Relation to symptom severity. Biological Psychiatry 69(6):556-563.
Olff, M., G. J. de Vries, Y. Guzelcan, J. Assies, and B. P. R. Gersons. 2007. Changes in cortisol and DHEA plasma levels after psychotherapy for PTSD. Psychoneuroendocrinology 32(6):619-626.
Orr, S. P., L. J. Metzger, N. B. Lasko, M. L. Macklin, T. Peri, and R. K. Pitman. 2000. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology 109(2):290-298.
Pace-Schott, E. F., M. R. Milad, S. P. Orr, S. L. Rauch, R. Stickgold, and R. K. Pitman. 2009. Sleep promotes generalization of extinction of conditioned fear. Sleep 32(1):19-26.
Parnas, A. S., M. Weber, and R. Richardson. 2005. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiology of Learning and Memory 83(3):224-231.
Pavlov, I. P. 1927. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. London, UK: Oxford University Press.
Peri, T., G. Ben-Shakhar, S. P. Orr, and A. Y. Shalev. 2000. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biological Psychiatry 47(6): 512-519.
Peters, J., L. M. Dieppa-Perea, L. M. Melendez, and G. J. Quirk. 2010. Induction of fear extinction with hippocampal-infralimbic BDNF. Science 328(5983):1288-1290.
Phan, K. L., D. A. Fitzgerald, P. J. Nathan, G. J. Moore, T. W. Uhde, and M. E. Tancer. 2005. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry 57(3):210-219.
Phan, K. L., J. C. Britton, S. F. Taylor, L. M. Fig, and I. Liberzon. 2006. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Archives of General Psychiatry 63(2):184-192.
Phelps, E. A., M. R. Delgado, K. I. Nearing, and J. E. LeDoux. 2004. Extinction learning in humans: Role of the amygdala and VMPFC. Neuron 43(6):897-905.
Pissiota, A., O. Frans, M. Fernandez, L. von Knorring, H. Fischer, and M. Fredrikson. 2002. Neurofunctional correlates of posttraumatic stress disorder: A PET symptom provocation study. European Archives of Psychiatry and Clinical Neuroscience 252(2):68-75.
Pitman, R. K. 1988. Post-traumatic stress disorder, conditioning, and network theory. Psychiatric Annals 18(3):182-189.
Pitman, R. K., and D. L. Delahanty. 2005. Conceptually driven pharmacologic approaches to acute trauma. CNS Spectrums 10(2):99-106.
Pitman, R. K., K. M. Sanders, R. M. Zusman, A. R. Healy, F. Cheema, N. B. Lasko, L. Cahill, and S. P. Orr. 2002. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biological Psychiatry 51(2):189-192.
Ponomarev, I., V. Rau, E. I. Eger, R. A. Harris, and M. S. Fanselow. 2010. Amygdala transcrip-tome and cellular mechanisms underlying stress-enhanced fear learning in a rat model of posttraumatic stress disorder. Neuropsychopharmacology 35(6):1402-1411.
Powers, M. B., J. A. Smits, M. W. Otto, C. Sanders, and P. M. Emmelkamp. 2009. Facilitation of fear extinction in phobic participants with a novel cognitive enhancer: A randomized placebo controlled trial of yohimbine augmentation. Journal of Anxiety Disorders 23(3):350-356.
Protopopescu, X., H. Pan, M. Altemus, O. Tuescher, M. Polanecsky, B. McEwen, D. Silbersweig, and E. Stern. 2005. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proceedings of the National Academy of Sciences of the United States of America 102(44):16060-16065.
Putman, P., and K. Roelofs. 2011. Effects of single cortisol administrations on human affect reviewed: Coping with stress through adaptive regulatoin of automatic cognitive processing. Psychoneuroendocrinology 36(4):439-448.
Quirk, G. J. 2002. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learning & Memory 9(6):402-407.
Quirk, G. J., and D. Mueller. 2008. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33(1):56-72.
Rabinak, C. A., M. Angstadt, R. C. Welsh, A. E. Kenndy, M. Lyubkin, B. Martis, and K. L. Phan. 2011. Altered amygdala resting-state function connectivity in post-traumatic stress disorder. Frontiers in Psychiatry 2:62.
Radecki, D. T., L. M. Brown, J. Martinez, and T. J. Teyler. 2005. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus 15(2):246-253.
Raskind, M. A., E. R. Peskind, E. D. Kanter, E. C. Petrie, A. Radant, C. E. Thompson, D. J. Dobie, D. Hoff, R. J. Rein, K. Straits-Troster, R. G. Thomas, and M. M. McFall. 2003. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: A placebo-controlled study. American Journal of Psychiatry 160(2):371-373.
Raskind, M. A., E. R. Peskind, D. J. Hoff, K. L. Hart, H. A. Holmes, D. Warren, J. Shofer, J. O’Connell, F. Taylor, C. Gross, K. Rohde, and M. E. McFall. 2007. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biological Psychiatry 61(8):928-934.
Rasmusson, A. M., and D. S. Charney. 1997. Animal models of relevance to PTSD. Annals of the New York Academy of Science 821:332-351.
Rau, V., J. P. DeCola, and M. S. Fanselow. 2005. Stress-induced enhancement of fear learning: An animal model of posttraumatic stress disorder. Neuroscience and Biobehavioral Reviews 29(8):1207-1223.
Rauch, S., L. M. Shin, P. J. Whalen, and R. K. Pitman. 1998. Neuroimaging and the neuro-anatomy of PTSD. CNS Spectrums 3(Suppl. 2):30-41.
Rauch, S. L., P. J. Whalen, L. M. Shin, S. C. McInerney, M. L. Macklin, N. B. Lasko, S. P. Orr, and R. K. Pitman. 2000. Exaggerated amygdala response to masked facial stimuli in post-traumatic stress disorder: A functional MRI study. Biological Psychiatry 47(9):769-776.
Rauch, S. L., L. M. Shin, E. Segal, R. K. Pitman, M. A. Carson, K. McMullin, P. J. Whalen, and N. Makris. 2003. Selectively reduced regional cortical volumes inpost-traumatic stress disorder. Neuroreport 14(7):913-916.
Rauch, S. L., L. M. Shin, and E. A. Phelps. 2006. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—past, present, and future. Biological Psychiatry 60(4):376-382.
Ressler, K. J. 2010. Amygdala activity, fear, and anxiety: Modulation by stress. Biological Psychiatry 67(12):1117-1119.
Ressler, K. J., K. B. Mercer, B. Bradley, T. Jovanovic, A. Mahan, K. Kerley, S. D. Norrholm, V. Kilaru, A. K. Smith, A. J. Myers, M. Ramirez, A. Engel, S. E. Hammack, D. Toufexis, K. M. Braas, E. B. Binder, and V. May. 2011. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470(7335):492-497.
Ridker, P. M. 2003. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 107(3):363-369.
Rodriguez-Romaguera, J., F. Sotres-Bayon, D. Mueller, and G. J. Quirk. 2009. Systemic pro-pranolol acts centrally to reduce conditioned fear in rats without impairing extinction. Biological Psychiatry 65(10):887-892.
Rothbaum, B. O., and M. Davis. 2003. Applying learning principles to the treatment of posttrauma reactions. Annals of the New York Academy of Sciences 1008:112-121.
Sah, P., E. S. Faber, M. Lopez De Armentia, and J. Power. 2003. The amygdaloid complex: Anatomy and physiology. Physiological Reviews 83(3):803-834.
Sakai, Y., H. Kumano, M. Nishikawa, Y. Sakano, H. Kaiya, E. Imabayashi, T. Ohnishi, H. Matsuda, A. Yasuda, A. Sato, M. Diksic, and T. Kuboki. 2006. Changes in cerebral glucose utilization in patients with panic disorder treated with cognitive-behavioral therapy. Neuroimage 33(1):218-226.
Sayin, A., S. Kucukyildirim, T. Akar, Z. Bakkaloglu, A. Demircan, G. Kurtoglu, B. Demirel, S. Candansayar, and H. Mergen. 2010. A prospective study of serotonin transporter gene promoter (5-HTT gene linked polymorphic region) and intron 2 (variable number of tandem repeats) polymorphisms as predictors of trauma response to mild physical injury. DNA and Cell Biology 29(2):71-77.
Schelling, G., E. Kilger, B. Roozendaal, D. J. de Quervain, J. Briegel, A. Dagge, H. B. Rothenhausler, T. Krauseneck, G. Nollert, and H. P. Kapfhammer. 2004. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: A randomized study. Biological Psychiatry 55(6):627-633.
Schiller, D., M. H. Monfils, C. M. Raio, D. C. Johnson, J. E. Ledoux, and E. A. Phelps. 2010. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463:49-53.
Segman, R. H., R. Cooper-Kazaz, F. Macciardi, T. Goltser, Y. Halfon, T. Dobroborski, and A. Y. Shalev. 2002. Association between the dopamine transporter gene and posttrau-matic stress disorder. Molecular Psychiatry 7:903-907.
Segman, R. H., N. Shefi, T. Goltser-Dubner, N. Friedman, N. Kaminski, and A. Y. Shalev. 2005. Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Molecular Psychiatry 10(5):500-513, 425.
Shansky, R. M., C. Hamo, P. R. Hof, W. Lou, B. S. McEwen, and J. H. Morrison. 2010. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cerebral Cortex 20(11):2560-2567.
Shi, Y., Y. Yuan, Z. Xu, M. Pu, C. Wang, Y. Zhang, Z. Liu, L. Li, and Z. Zhang. 2011. Genetic variation in the calcium/calmodulin-dependent protein kinase (CAMK) pathway is associated with antidepressant response in females. Journal of Affective Disorders 136(3):558-566.
Shin, L. M., and I. Liberzon. 2010. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35(1):169-191.
Shin, L. M., S. P. Orr, M. A. Carson, S. L. Rauch, M. L. Macklin, N. B. Lasko, P. M. Peters, L. J. Metzger, D. D. Dougherty, P. A. Cannistraro, N. M. Alpert, A. J. Fischman, and R. K. Pitman. 2004. Regional cerebral blood flow in the amygdala and medial prefron-tal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry 61(2):168-176.
Shin, L. M., C. I. Wright, P. A. Cannistraro, M. M. Wedig, K. McMullin, B. Martis, M. L. Macklin, N. B. Lasko, S. R. Cavanagh, T. S. Krangel, S. P. Orr, R. K. Pitman, P. J. Whalen, and S. L. Rauch. 2005. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry 62(3):273-281.
Shin, L. M., S. L. Rauch, and R. K. Pitman. 2006. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Psychobiology of Posttraumatic Stress Disorder: A Decade of Progress 1071:67-79.
Shin, K. M., N. B. Lasko, M. L. Macklin, R. D. Karpf, M. R. Milad, S. P. Orr, J. M. Goetz, A. J. Fischman, S. L. Rauch, and R. K. Pitman. 2009. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Archives of General Psychiatry 66(10):1099-1107.
Shin, L. M., G. Bush, M. R. Milad, N. B. Lasko, K. H. Brohawn, K. C. Hughes, M. L. Macklin, A. L. Gold, R. D. Karpf, S. P. Orr, S. L. Rauch, and R. K. Pitman. 2011. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: A monozygotic twin study of posttraumatic stress disorder. American Journal of Psychiatry 168(9):979-985.
Siegmund, A., and C. T. Wotjak. 2006. Toward an animal model of posttraumatic stress disorder. Annals of the New York Academy of Sciences 1071:324-334.
Simmons, A. N., M. P. Paulus, S. R. Thorp, S. C. Matthews, S. B. Norman, and M. B. Stein. 2008. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biological Psychiatry 64(8):681-690.
Smith, A. K., K. N. Conneely, V. Kilaru, K. B. Mercer, T. E. Weiss, B. Bradley, Y. Tang, C. F. Gillespie, J. F. Cubells, and K. J. Ressler. 2011. Differential immune system DNA meth-ylation and cytokine regulation in post-traumatic stress disorder. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics 156(6):700-708.
Somerville, L. H., D. D. Wagner, G. S. Wig, J. M. Moran, P. J. Whalen, and W. M. Kelley. 2012. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cerebral Cortex Jan 16 [Epub ahead of print].
Song, Y., D. Zhou, Z. Guan, and X. Wang. 2007. Disturbance of serum interleukin-2 and interleukin-8 levels in posttraumatic and non-posttraumatic stress disorder earthquake survivors in northern China. Neuroimmunomodulation 14(5):248-254.
Sotres-Bayon, F., L. Diaz-Mataix, D. E. Bush, and J. E. LeDoux. 2009. Dissociable roles for the ventromedial prefrontal cortex and amygdala in fear extinction: NR2B contribution. Cerebral Cortex 19(2):474-482.
Southwick, S. M., D. Bremner, J. H. Krystal, and D. S. Charney. 1994. Psychobiologic research in post-traumatic stress disorder. Psychiatric Clinics of North America 17(2):251-264.
Spoormaker, V. I., A. Sturm, K. C. Andrade, M. S. Schroter, R. Goya-Maldonado, F. Holsboer, T. C. Wetter, P. C. Samann, and M. Czisch. 2010. The neural correlates and temporal sequence of the relationship between shock exposure, disturbed sleep and impaired consolidation of fear extinction. Journal of Psychiatric Research 44(16):1121-1128.
Sripada, R. K., A. P. King, S. N. Garfinkel, X. Wang, C. S. Sripada, R. C. Welsh, and I. Liberzon. 2012. Altered resting-state amygdala functional connectivity in men with post-traumatic stress disorder. Journal of Psychiatry and Neuroscience 37(2):110069 [Epub ahead of print].
Stein, M. B., C. Koverola, C. Hanna, M. G. Torchia, and B. McClarty. 1997. Hippocam-pal volume in women victimized by childhood sexual abuse. Psychological Medicine 27(4):951-959.
Stein, M. B., C. Kerridge, J. E. Dimsdale, and D. B. Hoyt. 2007. Pharmacotherapy to prevent PTSD: Results from a randomized controlled proof-of-concept trial in physically injured patients. Journal of Traumatic Stress 20(6):923-932.
Steinhardt, M., and C. Dolbier. 2008. Evaluation of a resilience intervention to enhance coping strategies and protective factors and decrease symptomatology. Journal of American College Health 56(4):445-453.
Straube, T., H. J. Mentzel, and W. H. R. Miltner. 2006. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biological Psychiatry 59(2):162-170.
Strawn, J. R., and T. D. Geracioti, Jr. 2008. Noradrenergic dysfunction and the psychophar-macology of posttraumatic stress disorder. Depression & Anxiety 25(3):260-271.
Strigo, I. A., A. N. Simmons, S. C. Matthews, E. M. Grimes, C. B. Allard, L. E. Reinhardt, M. P. Paulus, and M. B. Stein. 2010. Neural correlates of altered pain response in women with posstraumatic stress disorder from intimate parner violence. Biological Psychiatry 65(5):442-450.
Su, T. P., L. Zhang, M. Y. Chung, Y. S. Chen, Y. M. Bi, Y. H. Chou, J. L. Barker, J. E. Barrett, D. Maric, X. X. Li, H. Li, M. J. Webster, D. Benedek, J. R. Carlton, and R. Ursano. 2009. Levels of the potential biomarker p11 in peripheral blood cells distinguish patients with PTSD from those with other major psychiatric disorders. Journal of Psychiatric Research 43(13):1078-1085.
Taylor, F. B., K. Lowe, C. Thompson, M. M. McFall, E. R. Peskind, E. D. Kanter, N. Allison, J. Williams, P. Martin, and M. A. Raskind. 2006. Daytime prazosin reduces psychological distress to trauma-specific cues in civilian trauma posttraumatic stress disorder. Biological Psychiatry 59(7):577-581.
Thakur, G. A., R. Joober, and A. Brunet. 2009. Development and persistence of posttrau-matic stress disorder and the 5-HTTLPR polymorphism. Journal of Traumatic Stress 22(3):240-243.
Tranel, D., H. Damasio, N. L. Denburg, and A. Bechara. 2005. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain: A Journal of Neurology 128(Pt 12):2872-2881.
Uddin, M., A. E. Aiello, D. E. Wildman, K. C. Koenen, G. Pawelec, R. de Los Santos, E. Goldmann, and S. Galea. 2010. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America 107(20):9470-9475.
Uddin, M., S. Galea, S. C. Chang, A. E. Aiello, D. E. Wildman, R. de los Santos, and K. C. Koenen. 2011. Gene expression and methylation signatures of MAN2C1 are associated with PTSD. Disease Markers 30(2-3):111-121.
Uher, R. 2011. Genes, environment, and individual differences in responding to treatment for depression. Harvard Review of Psychiatry 19(3):109-124.
Ursano, R. J., L. H. Zhang, C. J. Hough, C. S. Fullerton, D. M. Benedek, T. A. Grieger, and H. C. Holloway. 2008. Models of PTSD and traumatic stress: the importance of research “from bedside to bench to bedside.” Progress in Brain Research 167:203-215.
Ursano, R. J., L. Zhang, H. Li, L. Johnson, J. Carlton, C. S. Fullerton, and D. M. Benedek. 2009. PTSD and traumatic stress from gene to community and bench to bedside. Brain Research 1293:2-12.
Valente, N. L., H. Vallada, Q. Cordeiro, K. Miguita, R. A. Bressan, S. B. Andreoli, J. J. Mari, and M. F. Mello. 2011. Candidate-gene approach in posttraumatic stress disorder after urban violence: Association analysis of the genes encoding serotonin transporter, dopa-mine transporter, and BDNF. Journal of Molecular Neuroscience 44(1):59-67.
Vanitallie, T. B. 2002. Stress: A risk factor for serious illness. Metabolism 51(6 Suppl 1):40-45.
Vermetten, E., and J. D. Bremner. 2002. Circuits and systems in stress. II. Applications to neurobiology and treatment in posttraumatic stress disorder. Depression and Anxiety 16(1):14-38.
Vidal-Gonzalez, I., B. Vidal-Gonzalez, S. L. Rauch, and G. J. Quirk. 2006. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learning & Memory 13(6):728-733.
Vogt, B. A. 2005. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience 6(7):533-544.
Voisey, J., C. D. Swagell, I. P. Hughes, P. Morris, A. van Daal, E. P. Nobel, B. Kann, K. A. Heslop, R. M. Young, and B. R. Lawford. 2009. The DRD2 gene 957c![]() t polymorphism is associated with posttraumatic stress disorder in war veterans. Depression and Anxiety 26(1):28-33.
t polymorphism is associated with posttraumatic stress disorder in war veterans. Depression and Anxiety 26(1):28-33.
Voisey, J., C. D. Swagell, I. P. Hughes, J. P. Connor, B. R. Lawford, R. M. Young, and C. P. Morris. 2010. A polymorphism in the dysbindin gene (DTNBP1) associated with multiple psychiatric disorders including schizophrenia. Behavioral and Brain Functions 6:41.
Vythilingam, M., J. M. Gill, D. A. Luckenbaugh, P. W. Gold, C. Collin, O. Bonne, K. Plumb, E. Polignano, K. West, and D. Charney. 2010. Low early morning plasma cortisol in post-traumatic stress disorder is associated with co-morbid depression but not with enhanced glucocorticoid feedback inhibition. Psychoneuroendocrinology 35(3):442-450.
Walker, N., P. McConville, D. Hunter, I. J. Deary, and L. J. Whalley. 2002. Childhood mental ability and lifetime psychiatric contact: A 66-year follow-up study of the 1932 Scottish mental ability survey. Intelligence 30:233-245.
Wang, Z., T. C. Neylan, S. G. Mueller, M. Lenoci, D. Truran, C. R. Marmar, M. W. Weiner, and N. Schuff. 2010a. Magnetic resonance imaging of hippocampal subfields in post-traumatic stress disorder. Archives of General Psychiatry 67(3):296-303.
Wang, H. H., Z. J. Zhang, Q. R. Tan, H. Yin, Y. C. Chen, H. N. Wang, R. G. Zhang, Z. Z. Wang, L. Guo, L. H. Tang, and L. J. Li. 2010b. Psychopathological, biological, and neuroimaging characterization of posttraumatic stress disorder in survivors of a severe coalmining disaster in China. Journal of Psychiatric Research 44(6):385-392.
Wang, Z., D. G. Baker, J. Harrer, M. Hamner, M. Price, and A. Amstadter. 2011. The relationship between combat-related posttraumatic stress disorder and the 5-HTTLPR/RS25531 polymorphism. Depression and Anxiety 28(12):1067-1073.
Warner, C. H., G. N. Appenzeller, T. A. Grieger, S. Belenkly, J. Breitback, J. Parker, C. M. Warner, and C. W. Hoge. 2011. Importance of anonymity to encourage honest reporting in mental health screening after combat deployment. Archives of General Psychiatry 68:1065-1071.
Woods, A. M., and M. E. Bouton. 2006. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behavioral Neuroscience 120(5): 1159-1162.
Woon, F., and D. W. Hedges. 2011. Gender does not moderate hippocampal volume deficits in adults with posttraumatic stress disorder: A meta-analysis. Hippocampus 31(3):243-252.
Wright, C. I., B. Martis, K. McMullin, L. M. Shin, and S. L. Rauch. 2003. Amygdala and insular responses to emotionally valenced human faces in small animal-specific phobia. Biological Psychiatry 54(10):1067-1076.
Xie, P., H. R. Kranzler, J. Poling, M. B. Stein, R. F. Anton, K. Brady, R. D. Weiss, L. Farrer, and J. Gelernter. 2009. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Archives of General Psychiatry 66(11):1201-1209.
Xie, P., H. R. Kranzler, J. Poling, M. B. Stein, R. F. Anton, L. A. Farrer, and J. Gelernter. 2010. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology 35(8):1684-1692.
Xu, Z., Z. Zhang, Y. Shi, M. Pu, Y. Yuan, X. Zhang, and L. Li. 2011a. Influence and interaction of genetic polymorphisms in catecholamine neurotransmitter systems and early life stress on antidepressant drug response. Journal of Affective Disorders 133(1-2):165-173.
Xu, Z., Z. Zhang, Y. Shi, M. Pu, Y. Yuan, X. Zhang, L. Li, and G. P. Reynolds. 2011b. Influence and interaction of genetic polymorphisms in the serotonin system and life stress on antidepressant drug response. Journal of Psychopharmacology 133(1-2):165-173.
Yamamoto, S., S. Morinobu, S. Takei, M. Fuchikami, A. Matsuki, S. Yamawaki, and I. Liberzon. 2009. Single prolonged stress: Toward an animal model of posttraumatic stress disorder. Depression & Anxiety 26(12):1110-1117.
Yehuda, R. 2009. Status of glucocorticoid alterations in post-traumatic stress disorder. Gluco-corticoids and Mood Clinical Manifestations, Risk Factors, and Molecular Mechanisms 1179:56-69.
Yehuda, R., E. L. Giller, S. M. Southwick, M. T. Lowy, and J. W. Mason. 1991. Hypotha-lamic pituitary-adrenal dysfunction in posttraumatic-stress-disorder. Biological Psychiatry 30(10):1031-1048.
Yehuda, R., S. Southwick, E. L. Giller, X. Ma, and J. W. Mason. 1992. Urinary catecholamine excretion and severity of PTSD symptoms in Vietnam combat veterans. Journal of Nervous and Mental Disease 180(5):321-325.
Yehuda, R., S. L. Halligan, R. Grossman, J. A. Golier, and C. Wong. 2002. The cortisol and glucocorticoid receptor response to low dose dexamethasone administration in aging combat veterans and holocaust survivors with and without posttraumatic stress disorder. Biological Psychiatry 52(5):393-403.
Yehuda, R., G. Cai, J. A. Golier, C. Sarapas, S. Galea, M. Ising, T. Rein, J. Schmeidler, B. Muller-Myhsok, F. Holsboer, and J. D. Buxbaum. 2009. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biological Psychiatry 66(7):708-711.
Yehuda, R., L. M. Bierer, L. C. Pratchett, and M. Pelcovitz. 2010. Using biological markers to inform a clinically meaningful treatment response. Annals of the New York Academy of Sciences 1208:158-163.
Yin, Y., L. Li, C. Jin, X. Hu, L. Duan, L. T. Eyler, Q. Gong, M. Song, T. Jiang, M. Liao, Y. Zhang, and W. Li. 2011. Abnormal baseline brain activity in posttraumatic stress disorder: a resting-state functional magnetic resonance imaging study. Neuroscience Letters 498(3):185-189.
Young, B. R., B. R. Lawford, E. P. Noble, B. Kanin, A. Wilkie, T. Ritchie, L. Arnold, and S. Shadforth. 2002. Harmful drinking in military veterans with posttraumatic stress disorder: Association with the D2 dopamine receptor a1 allele. Alcohol and Alcoholism 37(5):451-456.
Zeidan, M. A., S. A. Igoe, C. Linnman, A. Vitalo, J. B. Levine, A. Klibanski, J. M. Goldstein, and M. R. Milad. 2011. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological Psychiatry 70(10): 920-927.
Zieker, J., D. Zieker, A. Jatzko, J. Dietzsch, K. Nieselt, A. Schmitt, T. Bertsch, K. Fassbender, R. Spanagel, H. Northoff, and P. J. Gebicke-Haerter. 2007. Differential gene expression in peripheral blood of patients suffering from post-traumatic stress disorder. Molecular Psychiatry 12(2):116-118.
Zohar, J., H. Yahalom, N. Kozlovsky, S. Cwikel-Hamzany, M. A. Matar, Z. Kaplan, R. Yehuda, and H. Cohen. 2011. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: Interplay between clinical and animal studies. European Neuropsychopharmacology 21(11):796-809.




















































