4
Replacing Critical Materials with Abundant Materials
Replacing critical materials with abundant materials, particularly in applications that use large amounts of catalysts, would have many benefits. Abundant materials are cheaper, less susceptible to supply fluctuations, and more environmentally benign. Cheap and abundant metals also can be less selective, less tolerant of functional groups, and use more expensive ligands than rare and expensive metals, but research gradually is reducing these shortcomings.
A particular application discussed in this chapter is the use of precious metals in automotive catalytic converters. The automotive industry is a major user of platinum, palladium, and rhodium in catalytic converters, which has spurred research on the use of other types of materials as catalysts. Although no good alternatives to the use of these materials yet exist, promising approaches are being investigated.
MOLECULAR ELECTROCATALYSTS FOR ENERGY CONVERSIONS USING ABUNDANT METALS
In many important processes powered by a homogeneous catalyst, the cost of the catalyst’s metal component is a small part of the overall expense. Nonetheless, chemists are developing novel reaction schemes that use homogeneous catalysts made with “cheap metals,” said Morris Bullock, Laboratory Fellow and Director of the Center for Molecular Electrocatalysis at the Pacific Northwest National Laboratory (PNNL). These efforts are centered on using abundant, inexpensive metals—mostly first-row metals, but also molybdenum and tungsten—to replace precious metals.
Even in cases where an expensive metal is a fraction of a catalyst’s total cost, creating efficient catalysts from inexpensive metals is likely to produce significant savings, said Bullock. Platinum, on a per mole basis, is approximately 4,000 times more expensive than nickel and 10,000 times more expensive than iron. Similarly, palladium is 3,000 times more expensive than copper, while ruthenium is 2,000 times more expensive than iron.
Palladium-based homogenous catalysis, in particular, is of critical importance in the pharmaceutical and agricultural industries for forming carbon-carbon bonds. The 2010 Nobel Prize in Chemistry was awarded for palladium-catalyzed cross-coupling reactions, which can be used to make virtually any type of carbon-carbon bond needed. The powerful Buckwald-Hartwig carbon-nitrogen bond-forming reactions are another class of palladium-catalyzed chemistries used widely in the pharmaceutical and agricultural industries (Hartwig, 1998; Wolfe et al., 1998). This latter set of reactions, Bullock noted, uses palladium loadings as low as 10 parts per million (ppm), so the expense of the precious metal in this case is not a significant factor.
It is possible, though, to substitute less expensive metals for palladium. A copper iodide/L-proline catalyst, for example, can be used to form carbon-carbon and carbon-nitrogen bonds (Ma et al., 2003). A nickel catalyst can be used to make carbon-carbon bonds with some stereoselectivity, which enables the assembly of fairly complex organic molecules (Harath and Montgomery, 2008). Chemists also have developed iron catalysts in carbon-carbon bond-forming reactions, although the results are not always what they seem. In one case, researchers made the observation that 98 percent pure iron chloride, compared to 99.99 percent pure material, produced higher yields of the desired product. Further study found that the reaction was actually catalyzed by a 10 ppm copper oxide impurity (Buchwald and Bolm, 2009). “There are plenty of other reactions that do get catalyzed by iron, but it highlights something that you have to be careful about in making sure that you can identify the real catalysts in these reactions,” said Bullock.
The Pros and Cons of Cheap Metals
In addition to the large price advantage that comes with substituting a prevalent, cheap metal for a rare, expensive metal, cheap metals are often environmentally more benign. Losses of metal are more easily tolerated in an industrial process, which can reduce or eliminate the recycling steps that are almost mandatory with expensive metal catalysts. In the pharmaceutical industry, the Food and Drug Administration may or may not allow trace levels of residual catalyst in a final drug product. As Bullock stated, “How much palladium can you have in a pharmaceutical body compared to how much iron?”
The reasons that more cheap metal catalysts are not widely used today are many, and Bullock listed several of them. One reason is that reactions catalyzed by cheap metals have not been widely studied to date, though they are receiving more attention now. Another reason is that the selectivity of cheap metal catalysts is not as good as is obtained with palladium catalysts, and the scope of the reactions is not as broad. Boosting the activity of cheap metal catalysts can mean using more expensive ligands; for example, catalysts based on aryl iodides are more reactive, but more expensive, than aryl chlorides.
Cheap metal catalysts are often less tolerant of functional groups on the reactants. A reaction that works with an ester moiety present may not work when an alcohol or carboxylic acid functional group is present. In contrast, palladium-based catalysts often work with a wide range of modified starting materials. In addition, cheap metals may require a higher catalyst loading than when palladium is used, negating some of the cost advantage. Bullock added, though, that this may be a result of the fact that cheap metal catalysis has not been studied as exhaustively as has palladium-based catalysis, and that additional research is likely to make headway on this problem.
The final problem facing cheap metal catalysts is one of motivation. For a pharmaceutical company making a high-value-added drug at small scale, and for which catalyst cost is not a major factor in the final price of the drug, there is often little motivation to expend research dollars solving a relatively small problem.
To illustrate some of the challenges in developing cheap metal catalysts, Bullock discussed the fact that reaction mechanisms may not be universal, making the search for new catalysts difficult. For example, an important class of chemical reactions hydrogenate carbon-oxygen double bonds. These carbonyl hydrogenation reactions use ruthenium- and rhodium-based catalysts to convert ketones and aldehydes into alcohols. One such ruthenium catalyst, for which the Nobel Prize was awarded, does not operate via the traditional mechanism for ketone hydrogenation. Normally, the reacting ketone would first coordinate with the metal, after which oxygen inserts itself into a metal-hydrogen bond. With this particular ruthenium catalyst, no coordination or insertion is required. Instead, the reaction occurs through a hydride ion on the ruthenium and a proton from the ligand-attached nitrogen coordinated to the ruthenium. The end result is the same, but the mechanism is completely different than expected (Noyori et al., 2001).
“The overall point I want to make is that if you’re trying to develop a new type of catalyst with a different metal, it is going to look a lot different,” said Bullock. “You don’t want to replace platinum or palladium with iron or copper and try to use the same ligand set. The ligands will almost certainly change.” The idea, he explained, is to not try to emulate what precious metals are doing as catalysts. Instead, the intention is to look at the reactivity characteristics of the cheap metals, understand the electronics of the reactions and the energy states, and then build a catalyst around those metals from the ground up using fundamental principles.
As an example, Bullock discussed work done in his laboratory developing a molybdenum-based catalyst for hydrogenating ketones to make alcohols at low temperature and hydrogen pressure and under mild conditions (Bullock and Voges, 2000). This reaction occurs by a different mechanism, one that capitalizes on the reactivity patterns of molybdenum hydrides and involves delivering a proton to the oxygen atom in the ketone first, leaving a metal hydride that then delivers hydride to the carbon atom, creating the saturated alcohol. Fundamental research on the acidity of metal hydrides and both the kinetics and thermodynamics of metal hydride behavior made the development of this catalyst possible.
The same types of basic research studies were done by other researchers to develop an iron-based catalyst that also performs a heterolytic cleavage of hydrogen as the key step in the hydrogenation of carbon-oxygen double bonds (Casey and Guan, 2009). But equally important is the fact that the catalyst is regenerated under low-pressure hydrogen conditions. More recently, another group created an iron-based catalyst that under similarly mild conditions works at very low catalyst loadings of 0.05 mole percent (Langer et al., 2011).
Iron-based catalysts also can be used to hydrogenate carbon-carbon double bonds. Again, this work was based on solid fundamental chemistry research to create redox-active ligands that help drive the reaction. One of these catalysts achieves turnover frequencies of up to 1,800 per hour in the conversion of 1-hexene to hexane (Bart et al., 2004).
High-Volume Applications of Cheap Metal Catalysts
Although the examples cited above show that it is possible to create potent catalysts for the production of the type of low-volume specialty chemicals used in the pharmaceutical and agricultural industries, the impact on the overall demand for expensive and rare metals is not likely to be substantial. An area where a real impact could be had is in the area of
renewable energy production, which would require massive amounts of catalyst, as was discussed in the previous chapter.
“We hope that there is going to be a much higher use of solar energy and other types of renewable energy in the future,” said Bullock. “What we want to do is store that energy in the form of the chemical bonds in a fuel.” Making this conversion, he added, requires “developing electrocatalysts that will convert electrical energy to chemical bonds, largely in the form of molecular hydrogen. Then, when you need the electricity, you can run hydrogen in a fuel cell and get your electricity back.”
Research at PNNL is focusing on developing catalysts that do not require platinum for hydrogen oxidation, which releases electrons, and the reverse reaction, proton reduction, that stores electrons. This work is also germane to the broader topic of oxygen and nitrogen reduction, which are more complex reactions given that oxygen reduction to water is a four-electron and four-proton event and nitrogen reduction to ammonia is a six-proton and six-electron event.
The theoretical framework for this research is based on understanding the first and second coordination spheres of nickel, the region where the electronic properties of the ligands surrounding a metal atom have the biggest influence on the metal’s catalytic properties (Rakowski DuBois and DuBois, 2009). In particular, Bullock and his colleagues are focusing on the role that phosphine ligands bearing pendant amines play in proton relays when these ligands are built into the second coordination sphere around nickel. Research at PNNL has shown that proton transfer into or away from the metal plays a key role in accelerating intra and intermolecular proton transfers and stabilizing binding of hydrogen to the metal. These pendant amine-facilitated proton relays also lower the barrier for heterolytic cleavage of hydrogen (DuBois and Bullock, 2011) and facilitate the coupled proton-electron transfers that are important in the reduction of oxygen and nitrogen.
The inspiration for using pendant amines to design nickel hydrogenation catalysts comes from nature, which does not use rare and precious metals in its catalysts. Protein crystallography studies of the structure of an iron-iron hydrogenase enzyme revealed the presence of pendant amines in the coordination sphere surrounding the metal atoms. But rather than building structural mimics of nature’s catalyst, the PNNL team is designing functional models that re-create the electronic and energy environment of the enzyme’s catalytic center.
Bullock discussed a few early examples of the catalysts developed from this effort. As shown in Figure 4-1, adding two pendant amines to nickel’s coordination sphere produced huge positive changes in the hydrogen oxidation activity of the resulting nickel catalyst by reducing the overpotential of the system, which increases the catalyst’s energy efficiency. Taking this approach one step further, the PNNL team reduced the flexibility of the pendant amines, essentially locking them into place around the nickel atom. This cut the activation energy nearly in half and increased the turnover frequency from less than 0.5 per second to 10 per second.
Developing a clearer understanding of the mechanism involved in catalytic oxidation of hydrogen played an important role in taking these initial results and creating far superior nickel hydrogen oxidation catalysts. These studies, which relied heavily on nuclear magnetic resonance spectroscopy, showed that moving protons onto pendant amines avoids having the reaction pass through a nickel(III)
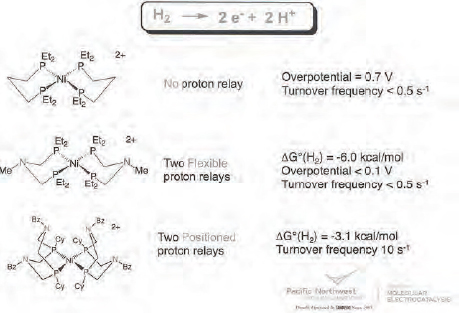
FIGURE 4-1 Nickel catalysts can oxidize hydrogen.
SOURCE: Bullock (2011).
intermediate, which lowers the energy barrier considerably. Based on this mechanistic understanding, the PNNL team created a nickel-based catalyst that produces 50 turnovers per second at one atmosphere of hydrogen (Yang et al., 2010), and another one that was not as good a catalyst for hydrogen oxidation but that was able to mimic the natural hydrogenase enzyme and catalyze both hydrogen oxidation and reduction (Kilgore et al., 2011). This catalyst is the first reported to carry out both the forward and reverse reaction.
One issue that arose in these studies was that protons can become trapped between two pendant amines in the same ligand, reducing catalytic activity. Switching to a ligand that had two phosphorous atoms to coordinate to the nickel core but only one nitrogen, a so-called P2N1 ligand, produced a dramatic increase in catalytic activity and resulted in turnovers of over 100,000 per second, which is more than 10 times faster than the iron-based hydrogenase that served as the inspiration for this work (Helm et al., 2011). However, this catalyst still has a high overpotential that needs to be addressed to improve its energy efficiency.
The PNNL also has used rational design principles to create the first iron-based catalyst for hydrogen oxidation. Turnover rates for this catalyst are only about two per second, but this research is still in its infancy.
In addition, working with a team at the University of California, San Diego, Bullock and his colleagues have created a nickel-based electrochemical catalyst that oxidizes formate. This is the first reported instance of a homogenous formate oxidation catalyst, and it is the first example of a formate oxidation catalyst of any kind that does not use platinum-group metals (Galan et al., 2011). These studies are ongoing.
Bullock concluded his presentation by noting that performing homogenous catalysis without precious metals has many advantages. In particular, iron, nickel, and other abundant metals are much less expensive and are often more environmentally benign. He added that, while an increasing amount of research is being done in this area, more fundamental research is needed to drive catalyst design efforts. The notable successes that the field has achieved in finding replacements for palladium in organic synthesis and for platinum in fuel cell and energy applications were all made possible by fundamental organometallic chemistry research, he observed.
NOVEL METALS AND BASE METALS IN AUTOMOTIVE CATALYST SYSTEMS
The auto industry is a major user of both precious- and base-metal catalysts. In automotive applications, catalysts are used to reduce the amount of regulated pollutants in the exhaust from gasoline and diesel vehicles. These pollutants include hydrocarbons, carbon monoxide, nitrogen oxides (NOx), and particulate matter. Catalysts convert these pollutants into nitrogen gas, carbon dioxide, and water.
The heart of the automotive catalyst system is the catalytic converter, explained Christine Lambert. The catalytic converter is essentially a ceramic honeycomb encased in a metal can attached to the vehicle’s exhaust pipe. Within the ceramic honeycomb is a supported metal catalyst that has been wash-coated onto the ceramic support, as shown in Figure 4-2.
The standard catalytic converter on a gasoline-powered vehicle uses a three-way catalyst engineered over many years to be extremely durable, said Lambert. Current versions are designed to meet emissions standards for 120,000 miles. They operate between 350°C and 650°C, the normal operating range of a gasoline engine, but they are durable to over 1000°C. The catalysts operate in near-stoichiometric exhaust gas, which means that the air and fuel fed into the engine is at the stoichiometric ratio needed to burn that fuel and that there is no excess oxygen. Contact time with the catalyst ranges from 60 to 300 milliseconds (Heck and Farrauto, 2001).
As engine control has improved over the years, the pollutant content of the exhaust has fallen into a tighter range of concentrations. As a result, catalysts are now able to handle all three gaseous pollutants simultaneously using what is called a three-way catalyst. Particulates are an issue only with diesel engines, and they are dealt with separately.
Today’s three-way catalyst is a complex, multicomponent system. The bulk of the catalyst is made of cordierite, a magnesium iron aluminum cyclosilicate that forms the ceramic honeycomb. γ-Alumina forms the support base, and cerium oxide (ceria) and zirconium oxide (zirconia) are oxygen-storage components to help the catalyst when it is in the high-efficiency stoichiometric window.
In addition to the rare earth mineral ceria, two other rare earth minerals, lanthanum oxide and neodymium oxide, maintain the alumina in its gamma form and play an important role in durability. Barium oxide and strontium oxide also serve as stabilizers and can store NOx if the engine is running lean, though that is not their primary purpose. A small amount of nickel oxide suppresses the formation of hydrogen sulfide from the relatively high amount of sulfur that is still present in gasoline. Finally, there is a small amount, about 0.5 percent, of the precious metals platinum, palladium, and rhodium (Gandhi and McCabe, 2004).
Summarizing the 30-plus-year history of gasoline catalyst development, Lambert explained that early fundamental research focused on identifying which elements could be good candidates for further development. Gold and silver were eliminated as candidates because of their limited durability and activity. Ruthenium, iridium, and osmium had suitable activity profiles, but they form volatile oxides at elevated temperatures, ruling them out as automotive catalysts. “At that point, the choices boiled down to platinum and palladium,” said Lambert.
Platinum and palladium were incorporated into the first catalytic converters when regulations mandated reductions
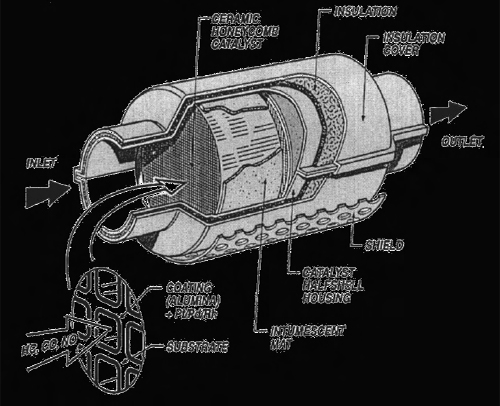
FIGURE 4-2 An automotive catalytic converter contains a catalyst on a ceramic substrate.
SOURCE: Heck and Farrauto (2001).
in hydrocarbon and carbon monoxide emissions. Later, when NOx standards were added to the regulations, rhodium was incorporated into the catalyst mix. Over the years, the stabilizers were added, as were ceria and zirconia as oxygen stabilizers. When lead was removed from gasoline, platinum was eliminated from the catalyst because palladium then could replace all platinum-based activity. Today’s catalytic converters still contain some rhodium because, as Lambert said, there is no acceptable substitute for rhodium when it comes to NOx reduction. Improvements in the physical design of the catalyst, with platinum-group loadings changing from the front to the back of the converter, have reduced the amount of metal needed in the converter (JM, 2011).
Supply and Demand
The automotive catalyst market is not isolated but sits within a supply chain that serves industrial catalyst manufacturers, jewelry makers, and electrical equipment manufacturers. The catalytic converter supply chain starts with the substrate manufacturers, who send their product to the catalyst coaters who purchase the metals. From there, the coated ceramic goes to the canners, who assemble the finished catalytic converter and ship it to the auto manufacturer. Currently, the automotive catalytic converter market consumes more platinum-group metals than it recycles, a situation that needs to be improved, according to Lambert. Unlike the industrial catalyst market, there is no well-developed internal recycling infrastructure in the automotive market.
Most of the world’s platinum comes from South Africa, which supplied over 75 percent of the 6.06 million ounces produced in 2010. Russia supplied just under 14 percent of the total, with the rest coming from other countries. Total demand for platinum in 2010 was 7.88 million ounces, with automobile catalysts requiring 3.125 million ounces, or 40 percent of the total. Recycling was able to make up for the difference between supply and demand.
Russia is the biggest supplier of palladium, producing 3.72 million ounces in 2010, while South Africa produced 2.575 million ounces. Other countries added just under 1 million ounces to the world’s available stores of palladium. Recycling added another 1.845 million ounces, but taken together, the world’s demand for palladium—some 9.625 million ounces—outstripped supplies by nearly a half million ounces. Automotive catalysts accounted for 57 percent of the demand for palladium (JM, 2011). Lambert added that the automotive catalyst market accounts for most of the world demand for rhodium.
Because the automotive industry is a major user, if not the major user, of these metals, the industry is impacted significantly by the price volatility for these metals. Since 1992, platinum, palladium, and rhodium have all seen one or more price spikes. As a result, catalyst manufacturers have developed several designs that use varying amounts
of these three metals in an attempt to mitigate dramatic shifts in costs.
Price volatility also has contributed to the drive to develop catalysts based on less expensive metals such as copper. Though copper prices can spike as well, prices may go from $1 per pound to $4 per pound, compared to a spike from $1,000 per ounce to as high as $12,000 per ounce for rhodium (Kitco, 2011).
However, a comparison of platinum-group metals with other metals shows that the former are hard to beat when it comes to carbon monoxide and hydrocarbon oxidation activity, particularly in the presence of sulfur at temperatures under 500°C (Kummer, 1980). In the early 1990s, researchers at Ford tested a variety of copper and copperchromium combinations in catalytic converters installed in an actual car exhaust system. For NOx conversion, a catalyst comprising 4 weight-percent copper and 2 weight-percent chromium performed the best, but only when operated under rich conditions, which reduced carbon monoxide and hydrocarbon conversion and fuel economy. This study showed, too, that copper catalysts needed to be close-coupled to the engine in order to avoid sulfur poisoning (Theis and Labarge, 1992).
After more than 30 years of research, Lambert concluded, there still are no good options for creating catalysts to treat gasoline engine exhaust that do not use platinum-group metals. The only real advance, she said, was the transition to palladium-rhodium and palladium-only catalysts enabled by the elimination of lead from gasoline.
The Importance of Diesel
As a percentage share of the U.S. market, diesel accounts for about 1 percent of new car sales and under 10 percent of light truck sales. For heavier trucks, such as the Ford F-250 and Dodge Ram, diesel models account for close to 90 percent of new vehicle sales. While diesel car sales are increasing, so, too, are the regulatory demands on diesel emissions. Engine makers have responded by improving catalysts, resulting in a substantial drop in diesel emissions since 1990.
In the mid-1990s, diesel exhaust treatment systems consisted solely of oxidation catalysts. Some diesel exhaust catalysts did not use any platinum-group metals, with ceria and alumina providing just enough catalytic activity to oxidize particulate matter enough to meet emission standards then in force. The introduction of ultra-low-sulfur diesel fuel in 2007 opened new opportunities for diesel exhaust emissions control systems. Particulate filters became standard, as did catalytic converters with high precious-metal content. These new systems operated under lean conditions to control NOx emissions, and some engine manufacturers added NOx traps.
In 2010, engine manufacturers added an extra reductant to the vehicles in the form of aqueous urea and turned to a copper catalyst for NOx control (Figure 4-3). The complete exhaust treatment system now includes a platinum-group metal diesel oxidation catalyst, an aqueous urea tank that is refilled during oil changes, a mixing system that injects the urea into the exhaust stream, a selective catalytic reduction chamber in which a copper-zeolite catalyst converts NOx and urea into nitrogen gas and water, and finally a particulate filter. Approximately every 500 miles, the temperature in the filter is raised to 500°C to burn off the trapped carbon particles, producing carbon dioxide.
Lambert noted that there is a huge exhaust temperature difference between gasoline and diesel engines. Once a diesel engine is at full operating temperature, exhaust temperatures average about 200°C, whereas a gasoline engine’s exhaust runs about 500°C. “It is hard to do catalysis at 200°C under the high space and velocity conditions characteristic of engine exhaust,” she said.
An added complication for diesel engine exhaust is that its oxygen content jumps significantly when the engine decelerates. During deceleration, no fuel goes into the engine, so the exhaust stream is just air with about 20 percent oxygen content, making NOx control under conditions that range from rich to lean challenging. Manufacturers have responded with several systems, including urea-based systems that have a wide temperature window. Other approaches to achieving NOx control under lean conditions include a hydrocarbon selective catalyst reduction (SCR) system that uses platinum but does not require a regenerating filtration system, lean NOx traps that use barium to absorb NOx during lean conditions, and a recently developed system that uses an ethanol-in-silver catalyst that is most useful for off-road diesel engines.
Urea and Zeolite Systems
Urea is a nontoxic commodity chemical produced by fertilizer manufacturers. When heated with water, it produces carbon dioxide and ammonia. It is injected into the exhaust system upstream of the catalyst as an aqueous solution at 32.5 weight-percent, a eutectic mixture with the lowest possible freezing point. In the presence of a suitable catalyst, ammonia reacts with the various nitrogen oxides in the presence of oxygen to produce nitrogen gas and water.
There are a number of non-precious-metal catalysts suitable for NOx reduction. Copper zeolites are best at low temperatures, whereas iron zeolites perform best at high temperatures. Vanadium-based catalysts, which are very inexpensive, are also effective but are not appropriate for U.S. diesels equipped with particulate filters because of the high temperatures at which those filter systems must operate (Cavataio et al., 2007).
Zeolites, Lambert explained, are made of aluminum oxide and silicon oxides with a crystalline structure. They are found in nature but in most cases are synthesized to achieve the high purity needed for industrial purposes. Zeolites are in widespread use as water softeners, absorbents, and desiccants and in oil refining.
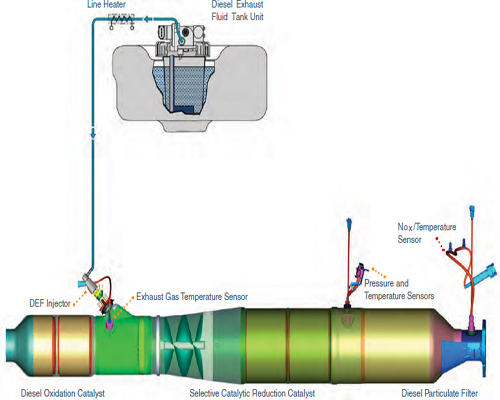
FIGURE 4-3 U.S. diesel exhaust treatment systems have become progressively more sophisticated to reduce emissions.
SOURCE: Ford (2011).
At Ford, Lambert and her colleagues found that a zeolite known as chabazite, which has a very small average pore size, combined with copper is a suitable diesel exhaust catalyst (Kwak et al., 2010; McEwen et al., in press) in an SCR system. Numerous academic studies suggest that copper is located inside the cage of the zeolite structure and that ammonia and NOx enter the cage, where they react in the presence of copper and oxygen to produce nitrogen gas and water.
There were a number of challenges to overcome to commercialize the SCR system that Ford now uses with its diesel engines. First, Lambert and her colleagues had to stabilize the platinum-based oxidation catalyst that sits in front of the SCR system. They accomplished this task by adding palladium to the catalyst mixture. After working with various ratios of platinum to palladium, they found that a 1:4 platinum-to-palladium mixture resulted in the best combination of hydrocarbon oxidation at cold-start temperatures and stability. The latter is important because volatilized platinum interferes with the NOx reduction process by poisoning the copper catalyst in the SCR system (Cavataio et al., 2009). Lambert noted that it is important enough to avoid precious-metal contamination of the copper catalyst that the two components are made in separate buildings. Having even a small amount of platinum on the copper-zeolite catalyst turns the latter into an ammonia oxidation catalyst instead of a NOx reduction catalyst.
Lambert noted that Ford researchers first worked with a beta-type zeolite as the copper support, but that this combination was poisoned by hydrocarbons to some extent. The catalyst could be regenerated by heating it to 500°C, but treatment at this temperature was found to produce melting that destroyed the zeolite’s structure. Moving to chabazite solved this problem. She added that chabazite’s small pore size prevents larger hydrocarbon molecules from reaching the active copper catalyst, preventing the formation of dioxins in diesel exhaust.
Sulfur can negatively impact copper-zeolite catalyst activity, particularly at temperatures below 300°C. Sulfur can be removed from the catalyst at filter regeneration temperatures, however. In fact, research found that at the now-mandated level of sulfur allowed in diesel fuel—15 ppm or less—the copper-chabazite catalyst can tolerate the amount of sulfur absorbed between 500-mile regenerations and still reduce NOx levels enough to meet exhaust standards.
The use of aqueous urea filtration is possible because manufacturers and suppliers worked together under the aus-
pices of the U.S. Council for Automotive Research to define product specifications. Aqueous urea is now sold as Diesel Exhaust Fluid™ (DEF™) in amounts ranging from gallon bottles to drums. The cost of DEF ranges from $2.79 per gallon in bulk to $4.65 per gallon in bottles. The Department of Energy created a website, www.finddef.com, to point users to retail locations that sell DEF.
The use of urea-based SCR conversion systems not only has a direct effect on NOx emissions, but data from the Environmental Protection Agency suggest that it has had an indirect effect on carbon dioxide emissions. Because the SCR systems are performing the bulk of NOx reduction, diesel engines can now run at higher fuel efficiencies, which reduces carbon dioxide emissions. At light and moderate engine loads, engines equipped with urea-based NOx control systems will have a greater than 10 percent advantage over engines equipped with other NOx control systems. Urea-based systems are now used on the majority of diesels sold starting in 2010.
Summarizing the impact of medium-duty diesel vehicles on platinum-group metal utilization, Lambert said that volumes have increased since 2005 because of more stringent emissions standards, but the move in 2010 from an all-platinum catalyst to a palladium-rich catalyst has dropped platinum use close to 2007 levels. Diesel engines still use more platinum than in comparable gasoline-powered medium-duty trucks, but that gap is shrinking.
Looking to the future, Lambert noted that adding an SCR to other lean NOx catalyst components may enable further advances in the development and adoption of lean-burn technologies for gasoline engines that lower or eliminate the need for platinum-group metal catalysts (Xu et al., 2010). However, adopting SCR technologies for use with gasoline engines will require lowering the sulfur content of gasoline from its current 80 ppm to 15 ppm or developing technology that removes sulfur from the exhaust stream prior to the SCR unit.
Research is also aimed at boosting NOx oxidation activity of palladium to continue the trend of replacing platinum with palladium as a cost-reduction strategy. Toward this end, researchers at General Motors have developed a platinum-free perovskite catalyst that rivals the performance of a platinum-based catalyst at reducing NOx levels under lean-burn conditions (Kim et al., 2010).
In response to a question about the role of theory in his work on electrocatalysts, Bullock said that theory and computational models were very helpful in providing insights into how these reactions work and increasing confidence that postulated intermediates in proposed mechanisms really existed. Theory has not yet reached a point where it can predict which molecules to make from the ground up, but theory has been very helpful in understanding energy potentials, reaction intermediates, and step-by-step mechanisms.
With regard to the synthetic complexity and cost of the ligands used with cheap metal catalysts, Bullock acknowledged that these are important issues. He added, though, that the ligands developed at PNNL, despite their apparent complexity, are easy to make in a two-step synthesis from simple starting materials.
He also noted that the cheap metal catalysts developed so far for organic synthesis have not yet achieved turnover rates comparable to palladium-based catalysts. He hoped that industry would now step in and take the catalysts developed in academia and make the improvements needed to create commercially viable catalysts based on cheap, abundant metals.
Finally, Bullock pointed to the importance of being open to unusual and unexpected results. Past examples of such advances should be kept in mind so that novel and important results are not ignored because they are so different from past findings.
Lambert was asked about the high-sulfur marine diesel used in many parts of the world, and she noted that vanadium-based catalysts are highly tolerant of sulfur, but currently they lack the necessary temperature stability. Improving that stability could be a fruitful avenue of research, and in fact a number of catalysts have been developed. She added that there is little commercial pressure for such a catalyst because regions of the world that use high-sulfur diesel fuel do not have stringent standards for particulate matter.
In response to a question about what happens when a vehicle runs out of urea, Lambert replied that the vehicles are designed to run at slower speeds, which frustrates drivers and provides an incentive to refill the urea containers on the vehicles.
Lambert pointed to an increase in research on batteries and electric vehicles at Ford and elsewhere. But she also said that “the internal combustion engine is not dead.” Fundamental research still needs to be done on exhaust gas emissions even as electric vehicle technologies advance.








