5
Optoelectronics and Photovoltaics
Beyond their use as catalysts, critical materials have widespread applications in other technologies, including technologies that could undergo rapid expansion in the future. Many critical elements are used in the display and solid-state lighting (SSL) industries. They also are components in many photovoltaic technologies, which are expected to undergo continued rapid growth. Several speakers noted that research to reduce the quantities of these materials needed in new technologies is important, but so is the development of new supplies and improved refining and recycling capabilities.
CRITICAL MATERIALS IN OPTOELECTRONICS
Rare earth elements are used widely in the field of optoelectronics. Europium is a key element in red phosphors, mostly in the form of europium-doped yttrium oxide. Erbium-doped fiber amplifiers are critical components in fiber optics, and erbium, neodymium, and holmium are important dopants in yttrium aluminum garnet (YAG) lasers. White light-emitting diodes (LEDs) will soon be the predominant user of indium in terms of volume, though ITO will continue to find use in the photovoltaic, display, and SSL industries. Chelated heavy metal and rare earth metals, such as palladium, platinum, iridium, and europium, also are used in the display and SSL industries.
Some Lesser Known Facts About Critical Materials
Though it was noted earlier in the workshop that the rare earth elements are not all that rare, what is not widely appreciated, said Joseph Shinar, is that there is only a rough correlation between abundance and price. If the correlation was strong, iridium would be two orders of magnitude more expensive than gold, while in fact it is hardly more expensive at all.
As was shown in Figure 2-5, the rare earth elements are reasonably abundant; they lie between the 25th and 75th percentile in terms of natural abundance, with cerium being the most abundant rare earth and lutetium the least. Quoting Gschneidner (2011), Shinar noted that rare earth elements are found all over the globe, not just in China, which holds about 31 percent of the known reserves. In fact, the United States has one of the historically highest-grade deposits in the world, located at Mountain Pass, California.
In 1970, China possessed 75 percent of the known rare earth reserves, referring to yttrium plus the lanthanides. At that point, China demonstrated a strong presence in the rare earth market. However, in the 40 years since then, China’s share of the world reserves of these elements fell to about 30 percent as new deposits were discovered even though China grew its reserves through discovery by some 290 percent (Gschneidner, 2011).
Recently, said Shinar, China changed its policies, introducing production quotas, export quotas, and export taxes; enforcing environmental legislation; and refusing to grant new rare earth mining licenses. In addition, China announced it will no longer export rare earths because of rapid growth of internal markets and limited reserves, especially the heavy rare earth elements gadolinium through lutetium. As a result, the price for rare earth materials and products containing rare earth has risen to the level at which mining companies and producers outside of China can make a profit. In 2009, estimated non-Chinese production of rare earth oxides was 4 kilotons (Gschneidner, 2011). Also, the smuggling of rare earth elements from China appears to be an important source of these metals, Shinar said.
Another unappreciated fact about the rare earth elements is only about 57 percent of the cerium produced today is used. The reason for this is that cerium must first be removed from rare earth flow streams before the other elements can be
isolated via a countercurrent liquid-liquid extraction process. As a result, cerium is overproduced by about 50,000 tons per year and must be stored at a real cost (Gschneidner, 2011). As expected, the rare earth industry would welcome new large cerium applications, but so, too, would all other users of rare earth elements because a balanced cerium market would significantly reduce the prices of the other rare earth elements.
Shinar observed that rare earths can be separated into three categories—surplus, balanced, and tight. Only neodymium, terbium, and dysprosium fall into the tight-supply category. Other than cerium and holmium, which is actually one of the rarest of rare earth metals, supplies and demands for the other rare earths are well balanced. It is also important, he added, to remember that the proven reserves of rare earths are growing rapidly.
The solution to the expectation that supplies will become tight as a result of China’s new policies is to increase mining, Shinar stated. That, in fact, is happening because MolyCorp is investing in reopening and expanding its operations at Mountain Pass. Workforce training also will be important.
One lesser-known fact about the critical elements is that the supply of ITO, the quintessential transparent conducting material, is totally dependent on zinc production, and the demand for zinc is not expected to grow much in the future because of macroeconomic factors in China, Canada, Korea, and Japan. Annual production of indium is approximately 900 tons, with primary production of 600 tons and recycling and stockpiles providing the rest. Recycling occurs primarily through removing the thin ITO layer coated on glass used in LCD monitors, flat-screen televisions, and other display devices.
Demand for ITO, which accounts for 85 percent of all indium demand, is expected to grow by 15 percent per year over the next 3 years. Emerging uses are in copper-indium-gallium-selenium solar cells, electrode-less lamps, mercury alloy replacements, and nuclear reactor control rods. Though indium prices are much the same as they were 5 years ago, the price has spiked thanks to China’s recent crackdown on small lead and zinc refiners amid environmental concerns.
Critical Metals
Shifting his focus from the general to the specific, Shinar spoke next about europium red phosphors, particularly europium-doped yttrium oxide, or yttria. Europium accounts for about 0.3 percent of mined rare earth metals, and the demand of about 400 tons of oxide per year is balanced by supply. Yttrium represents 6 percent of mined rare earths, and its supply and demand are balanced as well, at about 8.5 kilotons of oxide per year.
“The main use of red phosphors is as the R in RGB,” said Shinar. He explained that ytrium doped with 4 to 6.5 percent europium produces an intense red phosphorescence at 611 nanometers (nm) (Sylvania, 2010). “Even though RGB monitors and televisions are soon going to be found only in museums, red phosphors are still used extensively in compact fluorescent lamps (CFLs). And hot on the heels of CFLs are white LEDs for solid-state lighting.” But even though the demand for europium and yttrium will continue to grow, proven reserves of these metals are outpacing expected increases in demand.
Erbium accounts for about 0.5 percent of mined rare earths, and the demand of 700 tons of oxide per year is balanced with supply. Erbium is used as a colorant and as a stabilizer for zirconium in jewelry, but its most critical use is in the repeaters used in optical fiber networks that operate with a carrier wavelength of 1.5 microns. These repeaters, which are optically pumped lasers, are incorporated into fiber networks every few kilometers to boost the optical signal. Shinar noted the demand for these lasers may slowly wane as wireless communications steadily grow.
A niche application for rare earths is in neodymium, erbium, and holmium-doped YAG lasers. YAG lasers, which are very robust, efficient solid-state lasers, are staples in optical electronic labs and are used extensively in spectroscopy and ultrafast spectroscopy. Holmium-doped YAG lasers also are used extensively by urologists in laser lithotripsy. Though these are niche applications, they are critical ones nonetheless. Of the three rare earths used in these lasers, only neodymium is in tight supply. Though it represents 16 percent of mined rare earth oxides, it is used extensively in the neodymium-iron-boron magnets incorporated in wind turbines. Of the 23 kilotons of neodymium oxides mined, 2.8 kilotons go into wind turbines alone. Holmium represents about 0.1 percent of mined rare earth oxides, and its supply of 100 tons of oxide per year exceeds demand.
Indium demand is expected to grow rapidly with the development of what are called indium group II-V devices for use in the street lighting market. These devices, which are based on new technology for growing high-quality indium-doped gallium nitride, are superior replacements for fluorescent and high-pressure sodium lamps in overhanging street lights. The City of Anchorage, Alaska, estimates that replacing 25 percent of its streetlights with these new lamps will reduce energy costs by 50 percent and save the city some $360,000 per year. In addition, maintenance costs should drop given that these bulbs have an expected lifetime of 100,000 hours—over 11 years—compared with 5,000 hours for fluorescent and high-pressure sodium lamps. Perhaps more importantly, the new lamps improve visibility dramatically because of superior color rendering.
Indium is also used in ITO photovoltaic devices, displays, and SSL industries. Shinar focused his remarks on organic LEDs (OLEDs), which first appeared in a commercial product in 1998 and is now making major inroads in display devices. In 2007, Sony introduced an 11-inch OLED television, but at a cost of $2,500 it was not a big selling item. Since then, at least one company announced it was introducing a much bigger OLED television, but it has yet to appear
on the market. OLED displays can have contrast ratios of 1,000,000:1, but LED technology has not been standing still, and experts continue to argue about which technology produces better displays.
One application of OLED with great potential in the market is the use of white OLED (WOLED) in lighting panels. Potentially, these devices can be more efficient than fluorescent lights and produce more accurate color rendering. In addition, WOLED devices can be flexible and can even become mirrors when turned off. Today’s state-of-the-art devices produce 87 lumens/watt (lumens/W) at 1,000 candelas/m2. The goal is to produce devices that generate 300 lumens/W.
ITO is essential to all of this work because it is the preferred transparent conducting electrode in thin-film photovoltaics, and it is the only transparent conducting electrode in all LCDs and OLEDs. Researchers are making advances in developing transparent zinc oxide and aluminum oxide electrodes, but those efforts are not yet close to producing a commercially viable product.
One of the most promising alternatives is poly(3,4-ethylenedioxythiophene): poly(styrenesulfanate) (PEDOT:PSS). Though this material has been studied for over 10 years, the recent development of a fabrication process that creates multilayered PEDOT:PSS devices has produced a breakthrough in organic photovoltaic and OLED performance (Kim et al., 2011). The new process involves blending these polymers with polyethylene glycol (PEG), immersing the blend in PEG, and then annealing. Even though transmission goes down with each additional layer, sheet resistance also goes down, and that, said Shinar, is important. These PEDOT:PSS sheets are smoother than ITO, which is good for OLEDs, and their refractive index is lower, which reduces internal reflection and increases light output.
In fact, said Shinar, multilayer PEDOT:PSS OLEDs are up to twice as efficient as a standard ITO OLED. And in more recent work, which his group has not yet published, a two-layer PEDOT:PSS OLED produced a maximal luminous power efficiency of 100 lumens/W without coupling enhancement tricks. “With microlens arrays, which typically can double the out-coupling enhancement, these devices would be beyond 200 lumens/W,” said Shinar.
It is important to remember, though, that ITO devices are a moving target with continually improving performance. Recently, for example, chlorinated ITO-based OLEDs showed impressive efficiencies (Helander et al., 2011). The power efficiency reported for these devices exceeded 200 lumens/W, which is approaching the Department of Energy’s goal of 300 lumens/W. Not too long ago, this was considered a pipe dream, said Shinar. The external quantum efficiency for chlorinated ITO is “an amazing 53 percent,” he added. “For every two electrons you inject into the OLED you get one photon out, and not just out, but in the direction you want.”
Heavy Metals
Shinar noted that heavy and rare earth metal atom chelates, using palladium, platinum, iridium, and europium, are going to continue to be important in the display and SSL industries because they produce the best phosphorescent emitters. As he explained, “In all OLEDs, injection into the emitting layer results in 25 percent singlet excitons and 75 percent triplet excitons, and in fluorescence only the singlet excitons are radiative.” As a result, the maximum internal quantum efficiency is only 25 percent. Heavy metal chelate-based phosphors, however, are radiative with triplet excitons, so their use is critical to achieve high internal quantum efficiency for any OLED device.
In fact, when researchers from the Universal Display Corporation successfully fabricated phosphorescent OLEDs using heavy metal chelates, they boosted internal quantum efficiencies into the 90 to 100 percent range. This success, said Shinar, explains why this company is now worth between $500 million and $1 billion.
Shinar noted that iridium, because of its use in these phosphors, should be considered a critical metal. It is the least abundant of the platinum-group elements, yet today it is priced lower than gold. Major commercial sources of iridium are found in South Africa, Russia, and Canada. Iridium is difficult to refine and is produced in small quantities, but supplies have increased in response to a four-fold increase in demand to 334,000 ounces in 2010, largely a result of the inclusion of iridium crucibles in backlit LED televisions and the increased demand for iridium-tipped automobile sparkplugs. “The sharp increase in demand and the small, relatively illiquid market for iridium had a significant impact on price,” said Shinar. In August 2011, iridium was priced at $1,050 per ounce (eBullionGuide.com, 2011).
Efforts to develop room-temperature phosphors free of heavy metals have begun, but the best results so far still fall short of the mark. Shinar wondered if more research in this direction should be initiated. One possibility would be to exploit triplet-triplet annihilation to produce singlet excitons that could increase the internal quantum efficiency well beyond 25 percent.
In closing, Shinar noted that “for optoelectronics, the critical in critical resources is questionable. There is no single silver bullet because the situation has improved, and instead there are many potential silver bullets for different problems.”
One reason that PV technology is such an exciting area today, said Ken Zweibel, is that solar energy is such an abundant resource (Figure 5-1). The sheer size of the solar energy “reserve” dwarfs all other potential sources of renewable energy and is more than an order of magnitude larger than all coal, uranium, petroleum, and natural gas reserves combined.
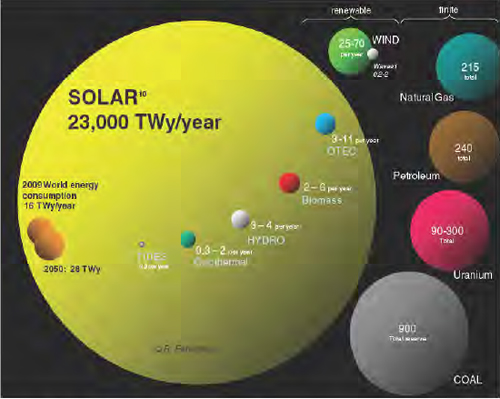
FIGURE 5-1 Solar energy dwarfs all other sources of renewable and finite energy sources.
SOURCE: Perez (2009); Perez et al. (2011).
Furthermore, an important characteristic of solar energy has been its historical reduction in price, Zweibel explained.
A plot of the price of the global average solar energy power module in constant dollars versus cumulative sales shows a consistent relationship from 1976 to 2009 (Figure 5-2). Over 35 years, each doubling in sales volume has produced a 20 percent drop in price. Today, said Zweibel, “prices are dropping like a stone, with the cost now down to $1.30 or $1.40 per watt for the modules, whereas only a few years ago, it was closer to $3 per watt.”
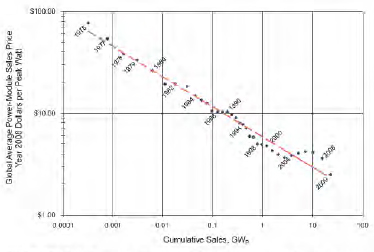
FIGURE 5-2 Between 1976 and 2009, the price of solar energy power modules has declined as cumulative sales increased as a result of aggressive pricing for market share.
SOURCE: Paula Mints, Principal Analyst, Navigant Solar Services Program, March 8, 2010.
At the same time, worldwide photovoltaic module production has risen exponentially over the past decade, with China’s entry into the market providing a huge boost in world output. In large part because of increased production from China, output nearly doubled in 2010. China first entered the market in 2006 and now accounts for over half of the world’s production of PV modules. Chinese production has grown so quickly that prices worldwide have plummeted. As a result, margins are now very thin, and many PV module manufacturers, including those in China, are facing financial difficulties.
The beneficiaries of the plummeting cost of PV modules are those who install PV systems, and as production has increased so has the number of PV installations (Figure 5-3). In 2010 alone, the total peak megawatts installed more than doubled, with Germany leading this dramatic uptake in PV use. In 2011, growth in the amount of PV installed returned to more normal levels, which accounts for the glut in supplies.
In terms of cumulative installations, PV now produces about 40 gigawatts (GW) of power worldwide. Growth in output is similar to that seen decades earlier when natural gas
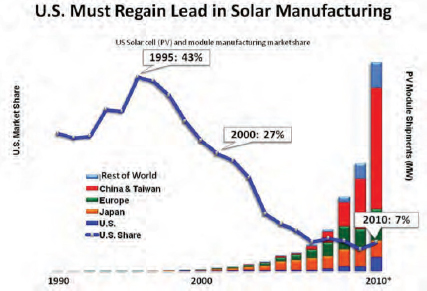
FIGURE 5-3 Production of photovoltaics has exploded over the past decade. (Consider U.S. solar cell (PV) and module manufacturing market share.)
SOURCE: PV News and Navigating Consulting, http://energy.gov/articles/competition-worth-winning [accessed December 22, 2011].
and nuclear power started becoming significant components of the energy infrastructure.
Germany, said Zweibel, is a special case and is far ahead of every other country in the world in terms of PV uptake. PV now supplies about 10 percent of peak energy use in the middle of the day, or about 3 percent of the country’s total energy usage. Using comparable measures, wind provides about twice the total amount of power as solar does in Germany.
Photovoltaics are semiconductors operating at about 10 percent efficiency. It takes 10 km2 of PV to produce 1 GW of power. For thinner PV modules, that translates into 10 cubic meters of crystalline silicon per gigawatt of power.
The Department of Energy’s Solar Vision calls for PV to account for 10 percent of U.S. electricity output by 2030, which would be approximately 600 GW, compared to today’s output of around 20 GW. The International Energy Agency predicts that solar will generate 50 percent of the world’s electricity by 2050, though Zweibel found this figure hard to accept.
Meeting Future Demand
Zweibel listed a number of key technologies that will play a major role in the future growth of PV as an electricity source. Crystalline silicon currently holds about 90 percent of the market, with cadmium telluride accounting for the remaining 10 percent. Emerging and promising materials include copper indium selenide alloys with gallium and sulfur, and type III-V multi-junction semiconductors for concentrator-containing solar cells. Commercially viable but less competitive are amorphous and thin-film silicon on glass. Emerging technologies that are less assured of success include organic dye-based “Graetzel” cells and plastic cells.
The most important materials that are needed to produce solar power today include silicon, silver, tellurium, and cadmium, with steel, aluminum, and copper being used in the contacts and housings and other bulk components. Emerging technologies could require significant quantities of indium, selenium, molybdenum, gallium, germanium, arsenic, and ruthenium. Many other metals, such as nickel, zinc, and tin, are used in minor amounts.
There are a number of bottlenecks. For example, the risk and timing of investment, including the unpredictability and rapid alteration in demand, have led to silicon shortages. “Because of the capital cost for making purified silicon, there was a time when the silicon purification industry was out of sync with the demand from solar,” said Zweibel. The demand for silicon by the solar industry is now larger than the demand from all the other semiconductors in the world. “That transition was hard for traditional silicon purification companies to grasp.”
Other bottlenecks involve extraction of the elements needed to meet growing demands from the solar industry. If there is not enough of a particular element available to meet the immediate or short-term future demand, it may not be economically feasible to increase extraction in a timely manner. Similarly, there may not be refining capability to meet increased demand, or it may not be economical to refine ores with low concentrations of the needed element. Finally, supplies may exist, but they may not be accessible for purchase because of market or political forces.
Specific materials have unique issues. The availability of coated glass substrates has been a problem for short periods of time. Materials such as tellurium used in cadmium telluride, indium used in copper indium selenide alloys, and gallium could have price and supply issues at mid- and long-term time frames. Of these, indium is also used in significant amounts in liquid crystal displays.
Today, materials such as tellurium, indium, gallium, molybdenum, and selenium add between $0.002/W to $0.03/W to PV costs, with silver adding $0.09/W, numbers that Zweibel characterized as being small. However, the costs of each of these materials can rise dramatically in response to even small changes in supply and demand. Given that $0.10/W to $0.20/W separates the best PV modules from the least competitive modules, changes as small as $0.03/W can be significant. “Something that goes from 3 cents to 15 cents per watt could be prohibitive,” said Zweibel.
Using Less Material
There are various strategies for reducing the amount of material needed to produce electricity. One approach is to use thinner layers of material, though that reduces the amount of light absorbed. That problem can be solved by adding backside mirrors, which can enable the thickness of the semiconductor layer to be reduced in some cases by a factor of 10.
Boosting device efficiency reduces the amount of material needed. Doubling the efficiency of a device means using half of the material to generate the same amount of electricity. Recycling, both internally in terms of manufacturing waste and externally as far as recovering materials from old modules, can have a significant impact on supplies.
Already, such strategies have played a role in reducing material demands from PV manufacturing. For example, the cadmium telluride layer has been decreased from 3 microns to 0.67 microns with little difficulty in light absorption. Thinner copper indium selenide alloys—0.75 microns compared to 2.0 microns—have reduced demand for indium, selenide, and gallium. Silver replacements are now being tested, and Zweibel predicted that silver will be replaced eventually in PV modules.
By 2030, such efforts could reduce the per-watt demand for rare metals. Zweibel predicted that tellurium use could drop from 100 to 16 metric tons/GW. Indium usage could drop from 30 to 9.4 metric tons/GW. Gallium usage could fall from 8 to 2.3 metric tons/GW.
Although such reductions are important, demand will nonetheless soar if the world truly meets the goal of getting 10 percent of its electricity from PV. Reaching this goal implies 600 GW/year annual production, which would require 10,000 metric tons of telluium per year, compared to 120 metric tons used today. The total availability of tellurium is about 2,000 metric tons per year today. Indium use would reach 6,000 metric tons per year, gallium 1,500 metric tons per year, molybdenum 18,000 metric tons per year, and selenium 9,600 tons per year.
Research may yield solutions to these impending supply problems, Zweibel said. If not, the most important recent commercial PV technology—the development of cadmium telluride—will have an uncertain future because of tellurium availability. In addition, the most important emerging commercial technology—copper indium gallium selenide—will have an uncertain future because of indium, selenium, and gallium availability. It is essential, he said, to find new supplies and develop new refining capabilities for tellurium and indium. Otherwise, all scenarios to meet demand for PV will be highly challenging. “The lack of U.S. commitment to extraction and refining inside our borders is a concern for U.S. PV competitiveness,” Zweibel concluded.
In response to a question about the role of chemistry in developing new materials, Shinar said that chemistry lies at the heart of developing WOLEDs and OLEDs, since each of these devices depends on complex organic chemistry and polymer chemistry. As an example, he said that chemists have been studying polyanilines as transparent conducting organics and are trying to solve stability issues. The problem is not that chemists do not want to work on developing these materials but that there is not much funding available to do so. In terms of inorganic materials, research is proceeding slowly, and these materials are hard to develop.
In response to a question about potential environmental issues associated with the widespread use of cadmium, Zweibel said that, because the manufacturers take the modules back at the end of their lifespan and recycle all of the cadmium telluride, the system is actually a closed loop. Also, cadmium telluride is much less dangerous than cadmium. For those reasons, there has been no backlash against the use of very thin, very stable layers of cadmium telluride in PV.
Zweibel replied to a question about the impact of the dramatically lower price for silicon cells on the technology development by noting that copper indium selenide technology is already suffering a good deal of push-back because it has to make price goals that have moved down so quickly. He added that, although he once thought that thin films would dominate the future of PV, “I no longer think that, and I would say that it is going to be a horserace for the next 20 years.”
In response to a question about mining bismuth telluride as a significant source of tellurium, Zweibel noted that there are places in the world where this mineral is accessible and where the tellurium is highly concentrated, as high as 17 percent in at least one instance. These deposits are likely to change the supply issue dramatically when the current production sources—copper, zinc, lead, and gold mining—start proving inadequate. He noted, too, that tellurium is the most
abundant metal in the universe with an atomic weight over 40 Daltons, even though it is depleted in the Earth’s crust (Cohen, 1984). There are large deposits in undersea ridges, and some companies are starting to show interest in undersea mining at these ridges, which are rich in many other materials as well (Hein et al., 2001).
In response to a question about the role of chemistry in developing new materials, Zweibel said that chemists play a critical role in two areas. In the development of mainstream PVs, the most effective researchers in this area are chemists. It is chemists who develop the understanding of how materials behave during processing and about the fundamental behavior of the materials themselves. The second area where chemistry is important is in manufacturing, which is really a chemical engineering problem and relies heavily on the knowledge of chemical engineers.
When asked about installation costs, Zweibel said that large-scale installations are largely mechanized and that the cost per watt can be as low as $0.20/W out of a total cost of $2.50/W to $3.50/W, with the module’s cost running at $1.00/W to $1.50/W. For residential applications, installation costs are higher. The Germans have worked out the most efficient installation methods, and their costs run about $1.00/W to $1.25/W with total system costs at $3.50/W. Residential installation costs also run higher because of the soft costs involved, such as marketing and sales. He noted that the United States can learn much from the German experience.
Finally, when he was asked about the role of chemists in the development of photovoltaics, Zweibel said that they are at the center of that process, despite the substantial involvement of electrical engineers, physicists, and others. It is “important to understand how that chemistry happens both in terms of the processing and in terms of the nature of the material itself.” Chemists also are needed in the large-scale manufacturing of photovoltaics, which is very dependent on chemical engineering. “Chemists are really the heart and soul, to a great degree, of this technology and of photovoltaics.”
This page intentionally left blank.








