5
Methyl Bromide1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory
![]()
1 This document was prepared by the AEGL Development Team composed of Sylvia Talmage (Summitec Corporation), Julie M. Klotzbach (Syracuse Research Corporation), Chemical Manager George Rodgers (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances), and Ernest V. Falke (U.S. Environmental Protection Agency). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold concentrations for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Methyl bromide is a colorless, nonflammable gas, with no taste or odor properties at low concentrations. Methyl bromide is currently used as a fumigant for buildings and soil and as a methylation agent in industry. Methyl bromide is an effective herbicide, rodenticide, nematicide, insecticide, bactericide, and fungicide. In the past, it was used as a refrigerant and fire extinguisher. Worldwide consumption of methyl bromide in 1996 was approximately 68 thousand metric tons. It is available as a liquefied gas in steel cylinders or cans.
Although numerous reports of accidental exposure of humans to methyl bromide that resulted in neurotoxicity or deaths are available in the literature, reliable information on exposure concentrations was not available. Acute, repeat-exposure, subchronic, and chronic studies, primarily with rats and mice, were available. Human case reports and controlled animal studies document that the central nervous system (CNS) is the primary target of methyl bromide. Neurotoxic symptoms can be delayed for several hours. Extremely high concentrations also produce lung edema. The mechanism-of-action of monohalomethanes is not completely understood, but could involve metabolism via the glutathione-S-transferase (GST) pathway to products that alkylate or inactivate cellular proteins. Species with higher cellular concentrations of GST appear to be more sensitive to the effects of methyl bromide than those with lower concentrations. The same is true for humans because of genetic polymorphisms of GST in the human population.
Data from animal studies were available on lethal and sublethal concentrations, neurotoxicity, developmental and reproductive effects, genotoxicity, and potential carcinogenicity. Although genotoxicity studies show that alkylation of DNA and proteins occurs, carcinogenicity has not been established in chronic studies with rats and mice. The dose-response curve for lethality is steep and the margin of safety between no-effect and lethal values is small. Data from rat and mouse studies show that the concentration-response curve for 50% lethality (LC50 values) over exposure durations of 3.5-480 min can be determined with the equation C1.2 × t = k.
Methyl bromide has no odor or irritation properties at concentrations below those that define the AEGL-2 values. Therefore, AEGL-1 values were not established.
The AEGL-2 values are based on the no-observed-adverse-effect level (NOAEL) for neurotoxicity, as evidenced by a lack of clinical signs in studies with rats and dogs. The weight-of-evidence from those studies indicates that 200 ppm of methyl bromide for 4 h is the threshold concentration for neurotoxicity (Hurtt et al. 1988; Hastings 1990; Japanese Ministry of Labour 1992; Newton 1994a). Reversible impairment of olfactory function (lesions of the olfactory epithelium) was observed in the rat (Hurtt et al. 1988; Hastings 1990; Japanese Ministry of Labour 1992). These lesions are specific to the nasal olfactory epithelium of the rat, based on nasal air flow patterns (Bush et al. 1998; Frederick et al. 1998), so it is unlikely that such lesions would occur in primates at the same exposure concentration and duration. Because uptake of methyl bromide is greater in rodents than in humans (based on comparative respiratory rates and comparisons with methyl chloride) and because GST concentrations in rodents are greater than in humans (resulting in more rapid production of toxic metabolites), an interspecies uncertainty factor of 1 was applied. Humans differ in their capacity to metabolize the related chemical methyl chloride; but, toxicologically, the difference is thought to be less than 3-fold (Nolan et al. 1985). Therefore, an intraspecies uncertainty factor of 3 was applied. The resulting 4-h value of 67 ppm was time scaled to the other exposure durations using the equation Cn × t = k, with n = 1.2. The value of n was based on lethality studies in rats. The mechanism for delayed neurotoxic effects (AEGL-2) and death (AEGL-3) are assumed to be the same. Because the time-scaled 8-h AEGL-2 value of 37 ppm is close to the chronic NOAEL of 33 ppm for mice (NTP 1992), is less than the 4-day NOAEL of 55 ppm for clinical signs and tissue lesions in dogs (Newton 1994a), and less than the 36-week NOAEL of 55 ppm for neurobehavioral parameters and nerve conduction velocity in rats (Anger et al. 1981), the 8-h value was set equal to the 4-h AEGL-2 value of 67 ppm.
Because of differences in methyl-halide metabolism between mice and other rodents and the greater sensitivity of mice to the structurally-similar chemical methyl chloride (metabolism is also by the glutathione [GHS] pathway), the mouse was not considered an appropriate model from which to derive AEGL values for methyl bromide. The AEGL-3 values were based on the BMCL05 (benchmark concentration, 95% lower confidence limit with 5% response)
of 701 ppm in a 4-h exposure study of rats (Kato et al. 1986). The BMCL05 was also the highest nonlethal value in the study. An interspecies uncertainty factor of 1 and an intraspecies uncertainty factor of 3 were applied, as was done in the calculation of AEGL-2 values. For time scaling (Cn× t = k), n was set equal to 1.2, based on lethality data in the rat. Because uptake of methyl bromide is greater in rodents than in humans (based on comparative respiratory rates and comparisons with methyl chloride) and because GST concentrations in rodents are higher than in humans (resulting in more rapid production of toxic metabolites), an interspecies uncertainty factor of 1 was considered sufficient. Humans differ in their capacity to metabolize methyl bromide, but toxicologically the difference is not thought to be greater than 3-fold (Nolan et al. 1985). An intraspecies uncertainty factor of 3 is supported by the steep dose-response curve for lethality by methyl bromide, which indicates that there might be little intraspecies variation. Furthermore, a larger uncertainty factor would result in values that would be near the AEGL-2 values. Therefore, an intraspecies uncertainty factor of 3 was considered sufficient. The 8-h AEGL-3 value of 130 ppm is supported by a repeat-dose study in which dogs exposed to methyl bromide at 156 ppm for 7 h/day did not exhibit severe clinical signs until the third day of exposure (Newton 1994a). There were no remarkable histopathologic lesions at autopsy.
The AEGL values for methyl bromide are presented in Table 5-1.
TABLE 5-1 Summary of AEGL Values for Methyl Bromide
|
|
||||||
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
|
|
||||||
| AEGL-1 (nondisabling) | NRa | NRa | NRa | NRa | NRa | |
| AEGL-2 (disabling) | 940 ppm (3,657 mg/m3) |
380 ppm (1,478 mg/m3) |
210 ppm (817 mg/m3) |
67 ppm (261 mg/m3) |
67 ppm (261 mg/m3) |
NOAEL for clinical signs in rats and dogs (Hurtt et al. 1988; Hastings 1990; Japanese Ministry of Labour 1992; Newton 1994a) |
| AEGL-3 (lethal) | 3,300 ppm (12,837 mg/m3) |
1,300 ppm (5,057 mg/m3) |
740 ppm (2,879 mg/m3) |
230 ppm (895 mg/m3) |
130 ppm (506 mg/m3) |
BMCL05 in rats (Kato et al. 1986) |
|
|
||||||
aNumerical values are not recommended because the data indicate that toxic effects might occur below the odor threshold for methyl bromide. Absence of an AEGL-1 value does not imply that exposure below the AEGL-2 value is without adverse effects.
Abbreviations: BMCL05, benchmark concentration, 95% lower confidence limit with 5% response; NOAEL, no-observed-adverse-effect level; NR, not recommended.
1. INTRODUCTION
Methyl bromide is a colorless, highly volatile gas that exists as a liquid below 3.6°C. It is heavier than air. Methyl bromide is nonflammable over a wide range of concentrations in air, and poses practically no fire hazard. These physical properties result in excellent penetration properties and make it a good fumigant. Additional chemical and physical properties are listed in Table 5-2.
Methyl bromide is ubiquitous in the environment, because it is generated naturally by oceans, biomass burning, and plants. For industrial purposes, methyl bromide is produced by direct bromination of methane and by the hydrobromination of methanol (Davis et al. 1977; O’Neil et al. 2001; Ioffe and Kampf 2002). Sulfur or hydrogen sulfide may be added as reducing agents to the methanol and sodium bromide. Anthropogenic methyl bromide is used mainly as a fumigant. It is an effective herbicide, rodenticide, nematicide, insecticide, bactericide, and fungicide, and has been used commercially in the United States for most of the twentieth century for the fumigation of soil, structures (such as warehouses), and food commodities, as well as for quarantine purposes (Duafala and Gillis 1999). Approximately 77% is used in preplanting fumigation of soil (IPCS 1995). In 1995, between 25,000 and 27,000 tons of methyl bromide were applied as a fumigant in the United States. Methyl bromide is also used as an intermediate for the manufacture of pharmaceuticals, in ionization chambers, for degreasing wool, and for extracting oils from nuts, seeds, and flowers (O’Neil et al. 2001; Ioffe and Kampf 2002). In the past, methyl bromide was used in fire extinguishers, as a refrigerant, and even as an anesthetic agent in dentistry (Alexeeff and Kilgore 1983).
In 1996, world consumption of methyl bromide was 68.4 thousand metric tons (Ioffe and Kampf 2002). The U.S. Environmental Protection Agency (EPA 2011) lists the production range of methyl bromide as 16.4 million pounds in 2003 and 1.8 in 2010. Production has decreased because of environmental concerns about depletion of the ozone layer by such chemicals.
Methyl bromide is easily liquefied, and is commercially available as a liquefied gas contained in steel cylinders or cans, usually under its own pressure of about two atmospheres (Braker and Mossman 1980; IPCS 1995; Duafala and Gillis 1999). Nitrogen or carbon dioxide may be added before shipment to permit rapid ejection at low temperatures. Formulations for soil fumigation contain chloropicrin (2%) or amyl acetate (0.3%) as warning agents.
2. HUMAN TOXICITY DATA
2.1. Odor Threshold
Methyl bromide has almost no odor or irritating effect, even at physiologically hazardous concentrations (Reid 2001). Reported odor thresholds vary from 20 to 1,000 ppm (Van den Oever et al. 1982; Sittig 1985; Ruth 1986). The odor of methyl bromide has been described as sweetish and similar to chloroform
(O’Neil et al. 2001), musty or fruity at concentrations above 1,000 ppm (Marraccini et al. 1983; ATSDR 1992), or faintly acrid at around 500 ppm (Hustinx et al. 1993). Methyl bromide has a burning taste, and contact with the skin may cause frostbite (O’Neil et al. 2001). When methyl bromide is used as a structural fumigant, it may react with sulfur-containing materials in buildings to produce a persistent odor (Anger et al. 1986).
The addition of 2% chloropicrin as a warning agent (a potent lacrimator sensed at 1.3 ppm) to some preparations of methyl bromide intended for fumigation (IPCS 1995; Reid 2001) is of limited safety efficacy, because chloropicrin vapor typically disappears before the methyl bromide vapor dissipates.
2.2. Toxicity and Neurotoxicity
The toxicity of methyl bromide has been reviewed by EPA (1980, 1992), Alexeeff and Kilgore (1983), ATSDR (1992), IPCS (1995), Yang et al. (1995), Reid (2001), OECD SIDS (2002), and HSDB (2010). A review of the literature published in 1983 documented 115 fatalities, 523 systemic illnesses, and 242 skin and eye injuries on a worldwide basis (Alexeeff and Kilgore 1983).
TABLE 5-2 Chemical and Physical Properties of Methyl Bromide
|
|
||
| Parameter | Value | Reference |
|
|
||
| Synonyms | Bromomethane, monobromomethane, methyl fume, isobrome | O’Neil et al. 2001 |
| CAS registry no. | 74-33-9 | O’Neil et al. 2001 |
| Chemical formula | CH3Br | O’Neil et al. 2001 |
| Molecular weightl | 94.95 | O’Neil et al. 2001 |
| Physical state | Colorless gas (above 4°C) | O’Neil et al. 2001 |
| Melons pomt | -93.7°C | Reid2001 |
| Boiling pomt | 3.56°C | Reid 2001 |
| Density Vapor Liquid |
3.97g/L at 20°C(air=l) 1.73g/mL at 4°C(water=l) |
O’Neil et al. 2001 |
| Solubility | 1.75 g/100 g in water at 20°C, 748 mm Hg | O’Neil etal. 2001 |
| Vapor pressure | l,420 mm Hg at 20°C | O’Neil et al. 2001 |
| Flammability limits | Practically nonflammable; flame propagation range is 13.5-14.5% by volume in air, ignition temperature is 537°C: bums m 02 | Reid 2001 |
| Conversion factors | 1 ppm = 3.39 mg/m3 at 25°C 1 mg/m 3= 0.257 ppm | Reid 2001 |
Most cases of accidental exposures have involved manufacturing or packaging operations, use of fire extinguishers containing methyl bromide, or fumigation activities. Exposures at high concentrations may occur during fumigation activities, especially when methyl bromide is first released to the environment after fumigation ends, or when fumigated areas are not properly ventilated. When methyl bromide is used as a storage fumigant, its concentrations usually range from 4,112 to 25,700 ppm for 2-3 days; higher concentrations are required to kill eggs and pupae. Accidental inhalation exposure incidents have occurred during atmospheric inversions, which prevent methyl bromide gas from rising and dispersing into the troposphere, or when children, adults, or animals enter sealed, fumigated structures. Most human exposure data on methyl bromide are from its use as an agricultural fumigant. It is applied to soil under plastic sheets or used in space fumigation under tarpaulins. It is also applied to a variety of agricultural commodities in specially designed fumigation chambers. Worker exposure may result from leaks in the plastic sheets or tarpaulin or from failure to allow adequate time for the methyl bromide to dissipate following fumigation (NIOSH 1984). The data from these accidental exposures are generally old, and concentration measurements were either not made or conducted using outdated analytic techniques. Regardless, estimates of concentrations leading to human deaths range from 1,600 to 60,000 ppm, depending on duration of exposure (ATSDR 1992).
The primary target of toxicity in humans accidentally or occupationally exposed to methyl bromide is the CNS (Alexeeff and Kilgore 1983; O’Neil et al. 2001). Symptoms of overexposure by inhalation to methyl bromide are headache, visual disturbance, vertigo, nausea, vomiting, anorexia, irritation of the respiratory system, abdominal pain, malaise, muscle weakness, incoordination, slurring of speech, staggering gait, hand tremor, convulsions, mental confusion, dyspnea, pulmonary edema, coma, and death from respiratory or circulatory collapse (O’Neil et al. 2001). Severe exposures may result in bronchial or pulmonary inflammation and pulmonary edema, which may not appear for 24 h or more after exposure. Death may occur from respiratory or cardiovascular failure. Exposure to methyl bromide has been known to adversely affect the kidneys, eyes, liver, and skin. Methyl bromide is an insidiously-acting chemical because of its lack of odor or immediate irritating properties at low concentrations (Reid 2001), and because signs of exposure are often delayed. In severe cases of poisoning, recovery can be protracted, with persisting neurologic problems.
Inhalation is the most significant route of exposure to methyl bromide, although skin absorption does occur. Standard protective clothing did not protect fumigators wearing respirators from developing skin lesions during two 20-min exposures at concentrations estimated to be about 9,000 ppm (Zwaveling et al. 1987; Hezemans-Boer et al. 1988). Absorption of methyl bromide was indicated by bromide concentrations in the blood.
Numerous case reports of methyl bromide exposure are described in the literature. In these reports, concentrations are either unknown or were measured or calculated after the incident. Analytic methods used in older studies, such as
colorimetry, have limited sensitivity. For example, Watrous (1942) describes both mild symptoms and more severe symptoms of nausea, vomiting, headache, and skin lesions in workers exposed for up to 2 weeks. Measurements of methyl bromide were generally less than 35 ppm, but exposures were based on a color detection method (methanol torch) with a lower detection limit of 35 ppm. Analytic methods for detecting higher concentrations involved flame colorimetry, an imprecise method. The exposures were complicated by accidents and routine dermal contact with the cooled liquid. In a factory where fire extinguishers were filled with methyl bromide, one death occurred (accompanied by convulsions) and another employee suffered less severe effects (Tourangeau and Plamondon 1945). Measurements taken at 30 min and 1 h had methyl bromide at concentrations of 297-390 ppm in front of the hood, where filling took place. Three additional nonfatal cases are described as examples below. Two of these cases also measured or estimated concentrations after the event. These cases are followed by a description of study of neurologic changes in methyl bromide applicators.
Because over 50 cases of methyl-bromide poisoning were reported in date processing and packaging plants in Southern California, Ingram (1951) and Johnstone (1945) conducted a series of surveys in 40 plants. Fumigation took place in a chamber that opened directly into the employee’s workroom. Appropriate amounts of methyl bromide were released from 50-pound drums or by using 2-pound or 1-pound cans. Exhaust systems were generally inadequate to dissipate the fumes following fumigation. Tests in these plants showed methyl bromide at concentrations up to 100 ppm in the general workroom air, up to 500 ppm near the walls of ineffectively sealed chambers, and over 1,000 ppm at the breathing zone of workers entering the fumigation chamber. Semiquantitative measurements were made with a halide torch, and average concentrations over time were measured colorimetrically with a halogenated-hydrocarbon apparatus.
Hustinx et al. (1993) described an accidental exposure during greenhouse fumigation. Nine individuals were inadvertently exposed while working in an enclosed area adjacent to the area being fumigated. The areas were separated by a poorly sealed partition. Three weeks earlier, the portion of the greenhouse in which the accident occurred had been fumigated with methyl bromide at 200 g/m2 (five times greater than the legally allowable concentration of 40 g/m2). At that time, two of the five workers in the nonfumigated section experienced nausea, vomiting, and dizziness. During fumigation, the highest methyl bromide concentration (25 ppm) was measured near the partition in the nonfumigated portion of the greenhouse. Measurements were made with Drager gas detectors (lower detection limit of 3-5 ppm). On the day before the accident (3 weeks after fumigation of the first greenhouse section), all nine workers were in the nonfumigated portion of the greenhouse for an average of 6 h (range of 4-8 h). Most workers experienced nausea and headache that day, and two of them stayed home the following day. The next day, fumigation was carried out in the previously nonfumigated section of the greenhouse, while the laborers worked in the section that had been fumigated 3 weeks earlier. After spending 2 h at work, all but one of the remaining seven workers experienced sudden and almost simultaneous
nausea, dizziness, and vomiting (the one exception experienced only a slight burning sensation in the throat). All seven workers left the greenhouse and went home. Within 2 h, two workers developed twitching of all limbs followed by generalized and continuous seizure activity, necessitating the induction of a sodium thiopental coma to stop the seizures. Methyl bromide concentrations ranged from 200 ppm near the partition to 150 ppm at the far end of the nonfumigated section 5 h after the accident, suggesting that the actual exposure was ≥200 ppm. Three days after admission to the hospital, chest x-rays revealed unilateral infiltration and pleural effusion, which subsided over the next 10-14 days. The thiopental coma was withdrawn after 3 weeks from the two severely affected patients, who then manifested persistent signs of axonal neuropathy. These signs improved only slightly over 6 months. Both workers had exhibited similar rises in serum alanine aminotransferase, aspartate aminotransferase, and lactic acid dehydrogenase activities, which peaked on the sixth day after admission and returned to normal before the thiopental treatment was discontinued, suggesting the increased activities reflected a methyl-bromide-related hepatic effect. The other seven patients experienced remarkably uniform signs, which included headache, nausea, and a “floating” sensation. Within 19 days after the accident, all residual complaints had disappeared in these seven patients. An unused, dry set of drainage pipes that crossed the entire length of both greenhouse sections was identified as the most likely major cause of the spread of methyl bromide to the nonfumigated section.
In the third case report, two fumigation workers entered a fumigated building in which the measured concentration (gas chromatography) was 4,370 ppm (Deschamps and Turpin 1996; Garnier et al. 1996). The workers wore cartridge respirators, which are saturable within a few minutes at that concentration (autonomous air flow masks are obligatory under these circumstances). The workers failed to wait until the concentration had decreased to the recommended level of 5 ppm. Both workers opened windows and doors in the nine-floor building over a period of 45 min to 1 h. During the 100-mile journey home, both workers experienced dizziness, fatigue, nausea, vomiting, chest pain, and shortness of breath. They were admitted to a hospital where the condition of the one improved rapidly. The other patient experienced convulsions, ataxia, and kidney failure. His tremors and ataxia were still present 5 months later (he experienced permanent neurologic damage). Bromide concentrations in the blood measured 40-48 h after admittance to the hospital were 47 and 156 mg/L in the first and second patient, respectively. Inspection of the charcoal cartridges of the respirators showed a concentration bromide greater than 10 mg/g; the highest concentration was found in the cartridge of the most injured worker.
Verberk et al. (1979) described bromine in the blood, electroencephalographic (EEG) disturbances, liver function (serum transaminases), serum proteins, and neurologic changes in 33 men engaged in soil disinfection inside greenhouses. Duration of employment ranged from a few months to 11 years. The amount of methyl bromide applied within the past year ranged from 1,500 to 6,000 kg. The relationship between different factors was based on a productmoment
correlation coefficient or Student’s t-test. No relationship was found between bromine concentration in blood and subjective symptoms, general neurologic deficits, or serum proteins. Slight EEG changes and a small increase in serum transaminases were related to blood concentrations of bromine. The authors considered the effects marginal.
2.3. Developmental and Reproductive Toxicity
No studies were found on reproductive or developmental effects in humans after inhalation of methyl bromide.
2.4. Genotoxicity
Liquid methyl bromide tested positive for sister chromatid exchanges (SCE) in in vitro tests with human lymphocytes (Tucker et al. 1986; Garry et al. 1990). When people who are GSH conjugators and nonconjugators were tested, methyl bromide tested positive for SCE in lymphocytes from GSH nonconjugators but not in lymphocytes from GSH conjugators (Hallier et al. 1990). See Section 4.4.2 for an explanation of human variability in GSH conjugation.
2.5. Carcinogenicity
EPA has classified methyl bromide as a Group D carcinogen, “not classifiable as to human carcinogenicity” (EPA 1992). On the basis of animal studies, the National Institute for Occupational Safety and Health characterizes methyl bromide as a “potential occupational carcinogen” (NIOSH 2010). The International Agency for Research on Cancer (IARC 1999) has determined that there is limited evidence for carcinogenicity in animals and inadequate evidence in humans. The overall evaluation states that methyl bromide “is not classifiable as to its carcinogenicity to humans” (Group 3). The American Conference of Governmental Industrial Hygienists (ACGIH 2004) classifies methyl bromide as A4, “not classifiable as a human carcinogen.”
Alavanja et al. (2003) investigated the link between exposure to 45 common agricultural pesticides and the eventual development of prostate cancer in a cohort of 55,332 initially healthy male pesticide applicators in Iowa and North Carolina. The data were collected by self-administered questionnaires that were completed at enrollment (1993-1997). The incidence of cancer in the general population was determined through cancer registries between the time of enrollment through the end of 1999, and a prostate cancer standardized incidence ratio was computed for the cohort. Odds ratios were determined for individual pesticides and for pesticide use patterns identified by the use of factor analysis. Over a period of 4 years, 566 of the men developed prostate cancer, a number greater than the total number of expected prostate cancer cases (494.5; odds ratio
of 1.14), based on state age-adjusted incidence rates. Among the 45 pesticides studied, only methyl bromide use showed a statistically significant exposure-response trend. The data suggested that if methyl bromide is responsible for the increased incidence of prostate cancer, this effect occurs only in those individuals with relatively frequent exposure. Limitations of this study acknowledged by the authors include the fact that the method of data collection was subject to significant recall bias, particularly in participants who had been exposed to the pesticides many years prior to the study. In addition, no direct measurements of pesticide exposure were obtained for the study. The follow-up period for the study was relatively short (an average of 4.3 years), precluding the evaluation of time-dependent exposures and risk. Finally, the authors acknowledged that the finding of increased risk of prostate cancer from the combined effect of exposure to several pesticides and a family history of prostate cancer was somewhat unexpected, and that the study must be replicated before any recommendations can be made.
2.6. Summary
Methyl bromide has been responsible for many occupational poisoning incidents, reflecting its wide use as a fumigant. Although many occupational and accidental exposures to methyl bromide have occurred, few cases have accurately documented exposure concentrations or durations. Methyl bromide is practically odorless, even at lethal concentrations. Descriptive symptoms indicate methyl bromide acts on the CNS (e.g., headache, visual disturbance, mental disturbance, nausea, vomiting) and directly on the lungs (lung edema). Case reports indicate that daily exposure to methyl bromide at 35 ppm (with possible dermal contact) and acute exposures to several hundred ppm can cause mild to severe symptoms.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
Early studies with several species of mammals were carried out by Irish et al. (1940) and Sayers et al. (1929). These reports lack details, used obsolete analytic methods, and used visual inspection rather than standard neurotoxicity tests to assess behavioral deficits. The studies are described here for completeness, but were not considered in the determination of AEGL values. Rats and rabbits were given single exposures to methyl bromide at a series of concentrations which resulted in either 100% mortality or 100% survival (Irish et al. 1940). The postexposure observation period was 4 weeks. Exposure of rats to methyl bromide at 13,000, 5,200, 2,600, 520, 260, 220, or 100 ppm resulted in 100% mortality in 6, 24, and 42 min and 6, 22, 26, and >26 h, respectively. Survival was 100% when exposures at the respective concentrations were 3, 6, and 25 min
and 2, 8, 12, and 22 h. For 8-h exposures, survival was 100% at 240 ppm and 0% at 470 ppm. Survival times for rabbits exposed at the same concentrations were longer by a factor of 2-3. Neurotoxicity was evident in rats exposed at concentrations below 260 ppm, and lung congestion and edema was found at 260-5,200 ppm. Rats withstood repeated exposures to methyl bromide for up to 6 months at 66 ppm. Guinea pigs also survived without demonstrable effects. Rabbits, however, became paralyzed. Results of repeated exposures in monkeys were complicated by deaths from pneumonia and tuberculosis. Similar observations in guinea pigs were reported by Sayers et al. (1929). In addition, Balander and Polyak, (1962) report a 2-h LC50 in mice of 397 ppm; the same data appear to be reported by Izmerov et al. (1982). This value is considerably lower than those reported in more recent studies.
Recent, well-conducted studies with acute exposure durations are discussed below and are summarized in Table 5-3.
TABLE 5-3 Acute Lethality in Laboratory Animals Exposed to Methyl Bromide by Inhalation
|
|
||||
| Species | Concentration (ppm) | Exposure Duration | Effect | Reference |
|
|
||||
| Rat | 19,460 1,880 334 |
3.5 min 1 h 8 h |
LC50 LC50 LC50 |
Zwartetal. 1992; Zwart 1988 |
| Rat | 2,830 | 30 min | LC50 | Bakhishev 1973 |
| Rat | 832 780 701 |
4 h 4 h 4 h |
100% mortality LC50 No deaths |
Katoetal. 1986 |
| Rat | 1,140, 760 506 |
4 h 4 h |
100% mortality No deaths |
Japanese Ministry of Labour 1992 |
| Rat | 402 302 268 |
8 h 8 h 8 h |
100% mortality LC50 No deaths |
Honmaetal. 1985 |
| Mouse | 1,700 | 30 min | LC50 | Bakhishev 1973 |
| Mouse | 1,200 900 |
l h 1 h |
LC50 No deaths |
Alexeeffetal. 1985 |
| Mouse | 760 506 338 |
4 h 4 h 4 h |
100% mortality 90% mortality No deaths |
Japanese Ministry of Labour 1992 |
| Mouse | 500 405 312 |
2 h 4 h 4 h |
No deaths LC50 No deaths |
Yamano 1991 |
|
|
||||
3.1.1. Rats
A concentration-time mortality method was used to estimate LC50 values in male SPF-Wistar rats (Zwart 1988; Zwart et al. 1992). The scheme use approximately 50 rats tested in groups of two at seven exposure durations (3.5-480 min) and various concentrations. Probit analysis allowed calculation of a timescaling value (see Section 4.4.3). LC50 values ranged from 19,460 ppm at 3.5 min to 334 ppm at 480 min. The 1-h LC50 was 1,880 ppm. Most animals showed some clinical signs, such as incoordination immediately after exposure. All mortalities occurred during the first week; these animals exhibited red, discolored lungs. Examination of the remaining rats at 2 weeks after exposure showed clear or light-red fluid in the lungs of some (exposure groups not explained).
Bakhishev (1973) exposed several species of mammals (number and strains not reported) to methyl bromide for 30 min. The 30-min LC50 for rats was 2,830 ppm. Although the details of this study are lacking, the value is similar to that predicted by Zwart et al. (1992) above.
In two separate experiments, groups of 5 male Sprague-Dawley rats were exposed to measured concentrations of methyl bromide at 502, 622, 667, 799, or 896 ppm by inhalation for 4 h, and groups of 10 male Sprague-Dawley rats were exposed at 701, 767, 808, 817, or 832 ppm for 4 h (Kato et al. 1986). The postexposure observation period was 1 week. The 4-h LC50 from the combined studies was 780 ppm (95% confidence limits of 760-810 ppm). Mortalities (estimated from a graph) were 0% at 502-701 ppm, 30% at 767 ppm, 60% at 799 ppm, 70% at 808 ppm, 80% at 817 ppm, and 100% at 832 and 896 ppm. Clinical signs were not described.
Groups of 10 male and 10 female F344 rats inhaled methyl bromide at 150, 225, 338, 506, 760, or 1,140 ppm for 4 h (Japanese Ministry of Labour 1992). At concentrations of 338 ppm or greater, there was decreased locomotor activity, ataxia, nasal discharge, lacrimation, diarrhea, irregular breathing, and bradycardia. All rats exposed at 760 and 1,140 ppm died. Necropsy revealed pulmonary congestion, hepatic degeneration, renal necrosis, myocardial hemorrhages, hemorrhage and necrosis of the adrenal glands, and congestion of the thymus. Rats in the 225-, 338-, and 506-ppm groups exhibited metaplasia of the olfactory epithelium, and rats exposed at 760 and 1,140 ppm (no deaths) exhibited necrosis of the olfactory epithelium. Sublethal effects are summarized in Table 5-4.
Groups of 5 male Sprague-Dawley rats inhaled methyl bromide at 268, 335, 402, 469, or 536 ppm for 8 h (Honma et al. 1985). Atmospheres were monitored with a gas chromatograph fitted with a flame ionization detector. The postexposure observation period was not specified, but it was stated that no deaths occurred later than 6 h after exposure. The 8-h LC50 was 302 ppm (95% confidence limits of 267-340 ppm). All rats survived in the 268-ppm exposure group, and all rats died in the 402-ppm exposure group. Severe hemorrhage was found in the lungs of dead rats. Death was preceded by convulsions in some rats.
Sedation was observed in a concentration-dependent manner. No further details of sedation were provided.
3.1.2. Mice
Bakhishev (1973) reported a 30-min LC50 in mice (number and strain not reported) of 1,700 ppm. Groups of 6 male Swiss-Webster mice were exposed by nose only to methyl bromide at 0, 224, 443, 566, 700, 900, 984, 1,200, 1,486, or 1,527 ppm for 1 h (Alexeeff et al. 1985). Atmospheres were measured with gas chromatography. The mice were observed for 1 week after exposure. The 1-h LC50 was approximately 1,200 ppm (95% confidence limits of 1,058-1,370 ppm). No deaths occurred at ≤900 ppm. Clinical observations were made after exposure. Mice exposed at 700 and 984 ppm exhibited transient hyperactivity during the first 20 h after exposure. At ≥900 ppm, signs of abnormal gait, passivity, lack of grooming, increased respiratory depth, decreased respiratory rate, and tremors appeared that were dose-dependent in number and time of onset. Signs that preceded death included fasciculations, loss of righting reflex, splayed limbs, tonic seizures, muscular rigidity, and rectal bleeding, with the latter effect appearing at the two highest concentrations. The behavior of mice exposed at 224, 443, or 566 ppm was not different from that of the controls (see Table 5-4). Transient weight loss was observed in all treatment groups. One week after exposure, weight loss was observed only at ≥900 ppm. Kidney lesions were observed grossly at concentrations above 900 ppm. Liver congestion and hemorrhage were observed at 1,200 ppm. Cerebral hemorrhage and congestion was observed at ≥984 ppm. Compared with the control group, brain weight to bodyweight ratios were decreased at 443 ppm and increased at 700, 900, and 982 ppm. Liver GSH was reduced at 1,200 and 1,527 ppm. Bromide ion was not detected in tissues at 1 week after exposure to methyl bromide at concentrations up to 700 ppm.
Groups of 10 male and 10 female BDF1 mice inhaled methyl bromide at 100, 150, 225, 338, 506, or 760 ppm for 4 h (Japanese Ministry of Labour 1992). All mice in the 760 ppm group died, and all but two male mice died in the 506 ppm group. Mice in these groups exhibited decreased locomotor activity, tremor, convulsions, diarrhea, irregular breathing, and bradypnoea. Mice in the 100-, 150-, 225-, and 338-ppm groups did not exhibit any clinical signs. Necropsy of the two highest dose groups revealed pulmonary congestion, hepatic degeneration and necrosis, renal tubular necrosis, karyorrhexis of the thymus and lymph nodes, and necrosis of the olfactory epithelium. A single female mouse exposed at 338 ppm exhibited metaplasia of the olfactory epithelium. Sublethal effects are summarized in Table 5-4.
Groups of 6 or 10 ICR-SPF male mice inhaled methyl bromide at 312, 357, 377, 449, or 464 ppm for 4 h (Yamano 1991). No deaths occurred at 312 ppm. Mortality was 10% at 357 and 377 ppm, 90% at 449 ppm, and 100% at 464 ppm. The 4-h LC50 was 405 ppm (95% confidence limits of 386-425 ppm).
The mortality rates of mice exposed at 500 ppm for 105, 120, 130, 140, 150, and 180 min were 0, 0, 11, 15, 85, and 90%, respectively. The post-exposure observation period was not specified. Mortality in mice exposed at 500 ppm for 150 min that had been injected with GSH (500 mg/kg) previously was only 5.3% compared with 85% in mice that were not injected with GSH.
3.2. Acute Nonlethal Toxicity
Many of the acute studies addressed neurotoxicity. These studies are summarized in Section 3.3 (Neurotoxicity) and are listed in Table 5-4. Four studies with rats addressed acute effects on the olfactory epithelium.
Groups of male F344 rats were exposed by inhalation to methyl bromide at 0, 90 (6 rats), or 200 ppm (15 rats) for 6 h (Hurtt et al. 1988). Damage to the olfactory epithelium was assessed in 3 rats/day on day 0 and post-exposure day 1 (90 ppm) or day 0 and post-exposure days 1, 3, 5, and 7 (200 ppm). An additional group of 40 rats were exposed at 200 ppm for 6 h/day for up to 5 days. There were no treatment-related clinical signs during the exposures. Exposure at 90 ppm caused no observable effect on olfactory function or nasal morphology (examined microscopically). Rats exposed at 200 ppm gained less weight than the control group. Exposure at 200 ppm for 6 h resulted in extensive destruction of the olfactory epithelium; however, the basal cells were generally unaffected. A single 6-h treatment with 200 ppm rendered rats unable to find a hidden food pellet; the ability to locate a food pellet returned within 4-6 days. Cellular repair began by day 3 and was essentially complete 10 weeks after the last exposure. At 10 weeks, only small areas of residual damage remained. The absence of lesions in the more anterior respiratory epithelium (where most irritant gases induce damage) indicates specific sensitivity of the olfactory epithelium to methyl bromide. Exposure at 90 ppm for 5 days was also a no-effect level for damage to the olfactory epithelium in an earlier study (Hurtt et al. 1987; see Section 3.3).
Hastings (1990) studied the effect of methyl bromide on olfactory function in rats. A group of 30 rats (sex and strain not identified) were exposed at 200 ppm for 4 h/day, 4 days/week for 2 weeks. After the initial 4-h exposure, rats were unable to locate a hidden food pellet. However, with additional exposures, the rats showed improvement until their performance was equal to that of the control group by day 4. There were no clinical signs or body weight changes. Damage to the olfactory epithelium was extensive and required more than 30 days for repair to near-normal appearance.
Groups of three male Wistar-derived rats were exposed nose-only to methyl bromide at 200 ppm for 6 h, and then killed 25 h later (Reed et al. 1995). There was marked degeneration of over 95% of the olfactory epithelium, with only one or two layers of cells remaining. The lesion did not reach the transitional or respiratory epithelium. No further details of the study were available.
Schwob et al. (1999) studied the reinnervation of the olfactory bulb after near-complete destruction with methyl bromide (330 ppm for 6 h) in male Long-Evans hooded rats. Repair was evaluated for up to 8 weeks after exposure. Repair and reinnervation was nearly complete at 8 weeks in rats that had no diet restrictions.
3.3. Acute Neurotoxicity
Studies of acute exposures to methyl bromide are discussed here and summarized in Table 5-4. Repeat-exposure studies are discussed under Section 3.4.
3.3.1. Dogs
In a one-day range-finding study, beagles were exposed to methyl bromide at 233, 314, 345, 350, or 394 ppm (measured concentrations) for 7 h (Newton 1994a). Two dogs were test at 345 ppm, and one dog was tested at each of the other concentrations. Neurotoxicity (tremors, hunched appearance, and labored breathing) was observed by the seventh hour at all concentrations, with the first signs appearing at 3 h at the three highest concentrations, and during the last 2 and 3 h in the dogs exposed at 233 and 314 ppm, respectively. Postexposure observation of the dog exposed at 233 ppm revealed no remarkable effects.
In the same study, groups of 2-3 dogs inhaled methyl bromide at 55, 156, 268, or 283 ppm, 7 h/day for 4 days (see Table 5-5). At 55 ppm, there were no clinical signs. By the third day at 156 ppm, lacrimation, labored breathing, prostration, and decreased activity were observed. Because toxic effects appeared after repeat exposure at 156 ppm, the authors considered the effects cumulative. For both exposures, postmortem findings, including microscopic examination of brain tissue, were unremarkable. Dogs exposed at the two higher concentrations exhibited decreased activity, labored breathing, and excessive salivation during the exposures and irregular gait, ataxia, emesis, rales, white nasal discharge, and general traumatized behavior postexposure. These effects were not observed during the first day of exposure at 268 ppm. Postexposure examination of the medulla/pons, cerebrum, and cerebellum of these animals showed no methylbromide-related lesions (Newton 1994a).
3.3.2. Rats
Groups of 15 male and 15 female CD (Sprague-Dawley) rats inhaled methyl bromide at 0, 30, 100, or 350 ppm for 6 h (Driscoll and Hurley 1993). Concentrations were verified by a gas chromatograph equipped with a flame ionization detector. Animals were assessed for clinical signs and changes in body weight. EPA’s functional observational battery of neurotoxicity tests and an automated assessment of motor activity test were conducted 3 h and 7 and 14
TABLE 5-4 Nonlethal Effects of Methyl Bromide in Laboratory Animals Exposed by Inhalation
|
|
||||
| Species | Concentration (ppm) | Exposure Duration | Effect | Reference |
|
|
||||
| Dog | 233.314.345, 350, 394 | 7h | Concentration- and time-dependent increase in Seniors, hunched appearance, and labored breathing- | Newton 1994a |
| Rat | 150 225 |
4h 4h |
No clinical signs. Metaplasia of the olfactory epithelium. |
Japanese Ministry of Labour 1992 |
| 333, 506 | 4h | Decreased motor activity, ataxia, nasal discharge, lacnmation, diarrhea, irregular breathing. bradycardia, metaplasia of the olfactory epithelium. | ||
| Rat | 200 | 4h | No clinical signs or effect on body weight; transient impairment of olfactory function. | Hastings 1990 |
| Rat | 0. 30.100 350 |
6h 6h |
No neurotoxicity or tissue lesions. Changes in neurobehavioral battery 3 h after exposure; no tissue lesions. |
Dnscoll and Hurley 1993 |
| Rat | 90 | 6h | No clinical signs or effect on olfactory epithelium. | Hunt etal. 1988 |
| 200 | 6h | No clinical signs; extensive olfactory -epithelium degeneration and reduced olfactory function. followed by repair. | ||
| Rat | 200 | 6h | Marked degeneration of the olfactory epithelium. | Reed etal. 1995 |
| Rat | 330 | 6h | Near complete destruction of the olfactory epithelium, with repair and reinnervahon at 8 wk postexposure. | Schwob et al. 1999 |
| Rat | 63 | 8h | No effect on body temperature or locomotor activity. | Honmaetal. 1985 |
| 125 | 8h | Transient decrease in body temperature. | ||
| 188 | 8h | Transient decrease in body temperature and body weight gain. | ||
| Mouse | 224,443,566 | lh | No clinical signs; transient weight loss; no gross pathologic lesions; no effect on brain weight; no reduction m liver glutathione at 224 ppm; no detectable tissue bromide at 1 wk postexposure. | Alexeeffet al. 1985 |
| 700 | lh | Transient hyperactivity. | ||
| Mouse | 100.150. 225.338 | 4h | No clinical signs; metaplasia of the olfactorr epithelium at 33Sppm. | Japanese Ministry of Labour 1992 |
|
|
||||
days postexposure. Nasal tissue, brain, spinal cord, and peripheral nerves were examined microscopically at necropsy performed 16-19 days after exposure. There were no effects on mortality, body weight, or organ weights, including brain weights. At 350 ppm exposure, clinical signs consisted of drooping eyelids, decreased arousal, piloerection, decreased rearing, depressed body temperatures, and markedly decreased motor activity. These signs were transient; they occurred only at the 3-h postexposure observation period. No treatment-related histologic findings were seen in nervous-system or nasal tissues. The lowestobserved-adverse-effect level (LOAEL) and NOAEL for neurotoxicity were 350 and 100 ppm, respectively.
Locomotor activity and body temperature of male Sprague-Dawley rats exposed to methyl bromide at 63, 125, 188, or 250 ppm for 8 h were measured (Honma et al. 1985). These end points were unaffected by methyl bromide at 63 ppm, but activity was decreased and strongly inhibited at 188 and 250 ppm, respectively, and body temperature was lowered by 2°C. These effects were reversed by the next day.
3.3.3. Mice
Passive-avoidance and motor-coordination tests were administered to mice following 1-h exposures to methyl bromide (Alexeeff et al. 1985). Concentrations of 224-984 ppm did not affect the ability of mice to recall a single-task passive avoidance test. Results were variable in the rotorod test, but performances were significantly different from the control group, particularly at 1,486 and 1,527 ppm.
3.4. Repeat-Dose Studies
Studies with repeated exposures are summarized in Table 5-5 and discussed below.
3.4.1. Dogs
In a repeat-exposure study with methyl bromide at 5 ppm for 7 h/day for 30 exposures, equivocal evidence of neurotoxicity was reported at the thirtieth exposure (Newton 1994b). A small number of dogs were tested in this study (one per exposure), there was no dose-response relationship, the observations were not part of a standardized protocol, and some of the dogs had been used in a previous study with methyl bromide.
A 6-week study was undertaken to resolve the issues in the Newton (1994a) study (see Table 5-5). Groups of 4 male and 4 female beagles were exposed (whole body) to methyl bromide at concentrations of 0, 5, 10, and 20 ppm (measured by gas chromatography) for 7 h/day, 5 days/week (Schaefer 2002). Potential neurotoxic effects were evaluated with EPA’s functional observational
battery of neurotoxicity tests and an automated motor-activity evaluation during the second, fourth, and sixth week of exposure. Tissues of the nervous system were examined at the end of the study. There were no mortalities. Clinical observations, body weights, food consumption, body temperatures, and the functional-observational-battery and motor-activity parameters were unaffected by exposures. No tissue lesions were observed at necropsy.
Groups of 4 male and 4 female beagles inhaled methyl bromide at 0, 5, 10, 25, 50, or 100 ppm for 7 h/day, 5 days/week for 4-5 weeks (Newton 1994b). During the fifth day of week 5, dogs in the 10-ppm group were exposed at 150 ppm for 6 exposures (analytical concentration of 158 ppm). Physical observations, ophthalmoscopic examinations, neurologic examinations, body weight and food consumption measurements, hematology and clinical chemistry parameters, and urinalysis were performed pretest and during the exposures. At the end of the study, organs were weighed and examined microscopically. No deaths occurred. No treatment-related clinical signs were observed in the 5-, 10-, or 15-ppm groups. Decreased activity was noted in two dogs in the 50-ppm group beginning on exposure day 14, in 3 of 8 dogs in the 100 ppm group beginning on exposure day 9, and in all dogs in the 158-ppm group beginning on exposure day 2. Clinical signs increased in the 158-ppm group as exposure to methyl bromide continued, and included irregular gait, opisthotonos, and convulsions in 3 of 8 dogs. Depression, tremor, and ataxia were observed in the remaining dogs after their sixth exposure. Hematology and clinical chemistry parameters were generally unaffected at ≤100 ppm, and body weight was unaffected at ≤50 ppm. There was no effect on absolute or relative organ weights. Although the signs in the 158-ppm group (examined 2 days after the sixth exposure) suggested diffuse CNS dysfunction, the dominant signs indicated cerebellar or vestibular dysfunction. At autopsy, microscopic examination of tissues revealed lesions in only the group exposed at both 10 and 158 ppm. The lesions included minimal vacuolation of the cerebellum, vacuolation of the adrenal gland, and moderate to moderately severe degeneration of the olfactory epithelium of the nasoturbinates.
3.4.2. Rats
Groups of 10 male F-344 rats were exposed by inhalation to methyl bromide at 0, 90, 175, 250, or 325 ppm for 6 h/day for 5 days (Hurtt et al. 1987). At 250 and 325 ppm, animals developed diarrhea (day 2), hemoglobinuria, and, in a few cases, gait disturbances and convulsions (day 3). Rats in the 325-ppm group died or were sacrificed in extremis before exposure on the fifth day. At ≥175 ppm, vaculolar degeneration of the zona fasciculata of the adrenal glands, cerebellar granule cell degeneration, and nasal olfactory sensory-cell degeneration occurred in a dose-dependent manner. Cerebral degeneration was seen only in the 325-ppm group. Hepatocellular degeneration was seen in the 250-and 325-ppm groups. The 5-day NOAEL for all tissue lesions, including the olfactory epithelium, was 90 ppm.
TABLE 5-5 Repeat-Dose Studies of Methyl Bromide
|
|
|||
| Concentration (ppm) | Exposure Duration | Effects | Reference |
|
|
|||
| Dog | |||
|
|
|||
| 0,55,156,268,283 | 7h/d,4d | 55 ppm: no cluneal signs or lesions. 156 ppm: lacrimation. labored breatluns. irregular sait bv day 3; no brain lesions. 268 and 283: Severe signs: exposure stopped after dav 2. |
Newton 1994a |
| 0,5,10,25,50, 100,158a | 7h/d,5d/wk,4-5wk | 5-25 ppm: no cluneal signs or tissue lesions. 50 ppm: decreased activity on exposure dav 14. 100 ppm: decreased activity on exposure dav 9. 158 ppma : decreased activity bv exposure dav 2. followed bv neurotoxic signs, tremors, convulsions on succeeding days; histopathologic examination indicated minimal cerebellar vacuolauon. adrenal gland vacuoladon. and moderate to moderately severe degeneration of the olfactory epithelium of the nasal passages. |
Newton 1994b |
| 0,5,10, 20 | 7h/d,5d/wk,6wk | No neurotoxicity or tissue lesions. | Schaeffer2002 |
|
|
|||
| Rat | |||
|
|
|||
| 0,90,175,250,325 | 6h/d,5d | 90 ppm: no tissue lesions, including olfactory. 175 ppm: degeneration of adrenal glands. >250 ppm: diarrhea, hepatocellular degeneration, cerebral degeneration; death at 325 ppm. |
Hurtt et al. 1987 |
| 0,150 | 6h/d,5d | No cluneal signs, weight differences, or brain lesions. | Davenport et al. 1992 |
| 0,190, 300 | 4h/d,5d/k,3wk | Compared with controls: minimal body weight gains; difference m spontaneous activity; no brain or nerve tissue lesions; death in 2/12 rats at 300 ppm. | Ikeda et al. 1980 |
| 0,20, 300,400 | 4h/d,5d/wk,6wk | 200 ppm: no cluneal signs or deaths, heart lesions. 300 ppm: paralysis in 3/12 rats, earlv necropsy, heart lesions 400 ppm: ataxia and paralvsis: earlv deaths, heart and brain lesions. |
Katoetal. 1986 |
| 150 | 4h/d,5d/Wk, 11wk | No clinical signs or deaths; heart lesions. | |
| 0,160 | 6h/d,5 d/wk,6wk | Early deaths, numerous tissue and organ lesions. | Eushs et al. 1933; NTP 1992 |
| 0,30, 70,140 | 6h/d,5d/Wk, 13wk | 30 ppm: no sisnificant effects or lesions 70 ppm: decreased motor activity at week 13 and decreased body weight by week 13 140 ppm: earlv deaths, nerve lesions. |
Noms et al. 1993 |
| 0,55 | 7.5h/d,4d/wk,36\wk | No effect on neurobehavioral parameters or nerve conduction velocity. | Anger etal. 1931 |
|
|
|||
| Mouse | |||
|
|
|||
| 0,160 | 6 h/d, 5 d/wk, 6 wk | Early deaths; numerous tissue and organ lesions. | Eushs etal. 1933: NTP 1992 |
| 0,10,33,100 | 6 h/d, 5 d/wk, 2 y | Neurotoxicity, brain lesions, and early deaths at 100 ppm no clinical signs or brain lesions at 10 and H ppm. | NTP 1992 |
|
|
|||
| Rabbit | |||
|
|
|||
| 0,65 | 7.5 h/d, 4 d/wk, 4 wk | Weight loss by week 3. eyeblink response and nerve conduction velocity significantly reduced; partial recovery. | Anger etal. 1931 |
| 0,27 | 7.5h/d,4 d/Wk, 8mon | No changes m neurobehavioral tests; no weight loss. | Russoetal. 1934 |
|
|
|||
aStarting with the last day of exposure of the fifth week, the 10-ppm group was exposed to methyl bromide at 150 ppm (analytical concentration = 158 ppm) for six exposures.
Groups of 8 male and 8 female F-344 rats were exposed to methyl bromide at 0 or 150 ppm for 6 h/day for 5 days (Davenport et al. 1992). Treated animals exhibited no clinical signs, no differences in body weights, and no histologic evidence of brain lesions.
Groups of 12 male Wistar rats were exposed to methyl bromide at 0, 190, or 300 ppm for 4 h/day, 5 days/week for 3 weeks (Ikeda et al. 1980). Body weights were monitored and physiologic responses, equilibrium on the rotorod, and spontaneous activity in an automated activity cage were measured before treatment and at various times up to 29 days after treatment. Brain and nerve tissue of two rats in the 300-ppm group were examined 29 days after treatment. During the exposures, body-weight gains were minimal in the exposure groups compared with the control group (data presented graphically). During the postexposure period, body-weight gains in the treated groups increased, but did not reach that of the control group. Two of the rats in the 300-ppm group died and one exhibited convulsions (time not stated). The remaining rats in the 300-ppm group showed decreased spontaneous motor activity. Physiologic responses (rearing in the open field, defecation) did not differ among groups. Time on the rotorod and the circadian rhythm of spontaneous activity (activity during dark and light periods) were affected in the two treatment groups. Spontaneous activity returned to control values by postexposure day 21. Histologic examinations of the CNS and peripheral nerves revealed no abnormalities.
Kato et al. (1986) conducted repeat inhalation studies with male Sprague-Dawley rats. Groups of 10-12 rats were exposed to methyl bromide by inhalation at 0, 200, 300, or 400 ppm for 4 h/day, 5 days/week for 6 weeks. Another group was exposed at 150 ppm for 11 weeks under the same conditions. Animals were killed 5 days after exposure. No deaths occurred in rats exposed at 150 or 200 ppm, and no clinical signs were observed, although body-weight gains were slightly depressed. Three of 12 rats exposed at 300 ppm developed paralysis and were killed after 4 weeks. Rats in the 400-ppm group exhibited clinical signs of ataxia and paralysis after 2 weeks. Six of 10 rats died or were killed after 5 weeks. At concentrations of 300 ppm and greater, serum-enzyme activities and lipids were affected. Bromide ion accumulated in the kidney and spleen at all concentrations, but there was no clear dose-response relationship. There was no clear dose-response effect on organ weights. Microscopic necrotic lesions were observed in the brain only at 400 ppm, but heart lesions were found at all concentrations.
Target organ toxicity studies were carried out to determine test concentrations for chronic studies by the National Toxicology Program (Eustis et al. 1988; NTP 1992). Groups of 20 F-344 rats/sex/concentration were exposed to methyl bromide at 160 ppm for 6 h/day, 5 days/week for up to 6 weeks. Animals were killed after 3, 10, or 30 exposures or when 50% mortality was reached. Mortality rates exceeded 50% in male rats after 14 exposures. Female rats survived the 30 exposures with less than 50% mortality. The brain, kidneys, nasal cavity, heart, adrenal glands, liver, and testes were the primary target organs. In rats, neuronal necrosis occurred in the cerebral cortex, hippocampus, and thalamus of the
brain. Necrosis of the olfactory epithelium was more severe and extensive in rats than in mice exposed at the same concentrations. Myocardial degeneration was more frequent and severe in male and female rats than in male mice. Cytoplasmic vacuolation of the adrenal cortex was observed in rats. Testicular and thymic degeneration occurred in rats and mice. The authors noted that the effects of methyl bromide were similar to those of methyl chloride.
In a subchronic study, groups of 15 male and 15 female CD rats inhaled methyl bromide at 0, 30, 70, or 140 ppm for 6 h/day, 5 days/week for 13 weeks (Norris et al. 1993). Sacrifice took place 15 days later. At 140 ppm, two male rats died during the first month. Two other males exposed at 140 ppm exhibited salivation, rapid breathing, hyperactivity, and convulsions, and one subsequently died. Males and females in the 140-ppm group had significant depressed body weights and body-weight gain by the end of the study. Body weights of females in the 70-ppm group were also significantly reduced. The body weights of females in the 30-ppm group were lower than those of the controls, but the difference was not statistically significant. Absolute brain weights were reduced in association with the reduced body weights, but brain weights relative to body weights were not affected. The two males that died in the 140-ppm group exhibited moderate to severe brain hemorrhage. Microscopically, neuronal necrosis was observed in several brain areas except the cerebellum. Another male in this group had edema of the hippocampus. No brain lesions were found in females in this group or in males or females exposed at lower concentrations. Slight lesions of the peripheral nerves were observed, primarily in the 140-ppm group, but incidences in the other exposure groups were not concentration related. In the functional observational battery of tests, forelimb grip strength was slightly reduced in males in the 140-ppm group at week 13, and motor activity was decreased in females in the 70-ppm group during week 13, and in the 140 ppm group at most intervals.
3.4.3. Mice
In a 14-day study, groups of 10 male and 10 female B6C3F1 mice were exposed to methyl bromide at 0, 12, 25, 50, 100, or 200 ppm for 6 h/day, 5 days/week (NTP 1992). Neurobehavioral signs of trembling, jumpiness, and paralysis were observed at all test concentrations, but primarily at the three highest concentrations. The time of onset of clinical signs was not described. Nine male and 6 female mice in the 200-ppm group died, with the first death occurring on day 11. Bloody urine was observed on day 6 in mice exposed at 200 ppm. Minimal hyperemia of the lung, liver, and kidneys was seen in females in the 200-ppm group. There were no other lesions, including lesions of the brain and nervous system, attributable to treatment.
Target organ toxicity studies were carried out to determine text concentrations for chronic studies by the National Toxicology Program (Eustis et al. 1988;
NTP 1992). Groups of 20 B6C3F1 mice/sex/concentration were exposed to methyl bromide at 160 ppm for 6 h/day, 5 days/week for up to 6 weeks. Animals were killed after 3, 10, or 30 exposures or when 50% mortality was reached. Mortality rates exceeded 50% in male mice after eight exposures and in female mice after six exposures. The brain, kidneys, nasal cavity, heart, adrenal glands, liver, and testes were the primary target organs. In the brain, neuronal necrosis occurred primarily in the cerebellum. Nephrosis was the likely cause of deaths. Necrosis of the olfactory epithelium was not severe compared with the lesion in rats. Myocardial degeneration, observed in rats at the same concentrations, was not severe in male mice. Atrophy of the adrenal cortex was observed in female mice. Testicular and thymic degeneration occurred in rats and mice. The authors noted that the effects of methyl bromide were similar to those of methyl chloride.
In a chronic study of B6C3F1 mice administered methyl bromide at 0, 10, 33, or 100 ppm (described in Section 3.6), NTP (1992) identified a LOAEL for neurotoxicity of 100 ppm and a NOAEL of 33 ppm. Mice were tested at 3-month intervals throughout the study. Cerebellar and cerebral degeneration were observed in 11/60 and 2/60 mice, respectively, in the 100 ppm group. These lesions were not observed at lower concentrations or in the control groups. Clinical signs of neurotoxicity, tremors, abnormal posture, tachypnea, and hind leg paralysis were observed in mice exposed at 100 ppm.
3.4.4. Rabbits
Sixteen Sprague-Dawley rats and 6 New Zealand white rabbits were exposed to methyl bromide at 65 ppm for 7.5 h/day, 4 days/week for 4 weeks (Anger et al. 1981). Control groups were composed of four rats and two rabbits. Neurobehavioral testing of nerve conduction, eyeblink reflex, activity, and grip/coordination were administered weekly. Another group of 32 rats were exposed to methyl bromide at 55 ppm for 6 h/day, 5 days/week for 36 weeks. Neurobehavioral testing of this group was conducted monthly. Rabbits exposed at 65 ppm began to lose weight by the third week of exposure. By week 4, eyeblink responses and nerve conduction velocity in rabbits were significantly reduced, but rats were unaffected. The symptoms in rabbits partially subsided within 6-8 weeks after exposure ended (Russo et al. 1984). No effects on nerve conduction velocity, open-field activity, or coordination were found in rats exposed at 55 ppm for 36 weeks.
To identify the threshold for chronic neurotoxicity in rabbits, Russo et al. (1984) exposed adult male New Zealand white rabbits to methyl bromide at 27 ppm for 7.5 h/day, 4 days/week, for 8 months. Eyeblink and nerve conduction tests were administered biweekly. The neurobehavioral tests were negative, and rabbits gained weight and appeared healthy.
3.5. Reproductive and Developmental Toxicity
Hurtt and Working (1988) evaluated reproductive parameters of male F-344 rats exposed by inhalation to methyl bromide at 200 ppm for 6 h/day for 5 consecutive days (see Table 5-6). Compared with a control group, plasma testosterone concentration was significantly decreased during the 5-day exposure, but returned to control concentrations by day 8 (3 days postexposure). Concentrations of GSH in the testes and liver were also depressed but returned to control concentrations by day 8. There was no effect on testicular weight or sperm production and motility.
TABLE 5-6 Reproductive Toxicity of Methyl Bromide in Animal Models
|
|
|||
| Concentration (ppms) | Exposure Duration | Effects | Reference |
|
|
|||
| Rat | |||
|
|
|||
| 0,200 | 6 h/d for 5 d | Transient decrease in plasma testosterone and testicular and liver glutathione; no effect on testicular weight or sperm production and motility. | Hum and Working 1988 |
| 0, 3, 30, 90 | 6 h/d, 5 d/wk, 2 generations | Body and brain weights of parental (F0) males depressed at 30 and 90 ppm; at 90 ppm, brain weights of F1 offspring decreased without histologic correlates; no effect on litter size, sex ratio, or survival; no gross abnormalities in either generation. | Mayhew 1986 |
| 0, 20,70 | 7 h/d, 5 d/wk, before mating and through day 19 of gestation | No maternal toxicity; no adverse developmental effects. | Hardin et al. 1981; Sikov et al. 1981 |
|
|
|||
| Rabbit | |||
|
|
|||
| 0, 20, 70 | 7 h/d, 5 d/wk, before mating and through gestation | Increased maternal mortality at 70 ppm; no maternal mortalities or clinical signs at 20 ppm; no adverse developmental effects.; | Hardin et al. 1981; Sikov et al. 1981 |
| 0,20,40,80 | 6 h/d, gestation days 7-19 | First study: maternal toxicity at 80 ppm; increase in fetal variations, partially attributed to sire; no effects at lower concentrations. Second study: less maternal toxicity and incidence of fetal variations not statistically significant at 80 ppm. |
Breslin et al. 1990 |
|
|
|||
In a two-generation study, Sprague-Dawley rats were exposed by wholebody inhalation to methyl bromide at 0, 3, 30, or 90 ppm for 6 h/day, 5 days/week (Mayhew 1986). Exposures were for at least 8 weeks before mating and continued over the production of two litters (temporarily suspended in F0 dams from day 21 of gestation until day 5 of lactation). Two litters were produced by both the F0 and F1 generations. There were no clinical signs and no effect on survival in treated animals. In parental animals (F0 generation), body and brain weights of males were decreased in the 30-and 90-ppm groups. Brain weights of F1 parental males and females exposed at 90 ppm were also reduced. No histologic correlates were observed, and no pathologic changes were found in other organs or tissues. At 30 and 90 ppm, pups from the F1 parental generation had reduced body weights compared with controls. Absolute, but not relative, organ weights were also reduced, probably reflecting smaller body sizes. There were no treatment-related effects on litter size, sex ratio, survival through lactation, or grossly observable abnormalities.
No adverse developmental effects in fetuses and no significant maternal toxicity, other than transient lower body weight, compared with controls were noted when female Wistar rats were exposed to methyl bromide at nominal concentrations of 0, 20, or 70 ppm (Hardin et al. 1981; Sikov et al. 1981). Exposures were for 7 h/day, 5 days/week, before mating and through 19 days of gestation. There were no differences in pregnancy rates, embryotoxicity, or fetal viability, and no effect on soft-tissue or skeletal anomalies.
In the same study (Hardin et al. 1981; Sikov et al. 1981), methyl bromide at 70 ppm was highly toxic to pregnant New Zealand rabbits. Exposure was terminated on day 15, but 24 of 25 rabbits died by gestation day 30. There were no deaths or clinical signs in the control or 20-ppm groups. No adverse developmental effects were observed in offspring of dams exposed at 20 ppm or in the offspring of the surviving rabbit in the 70-ppm group.
New Zealand white rabbits were exposed to methyl bromide at 0, 20, 40, or 80 ppm for 6 h/day during gestation days 7-19 (Breslin et al. 1990). No clinical signs of toxicity were observed at the lower concentrations. Clinical signs of maternal toxicity in the 80-ppm group included decreased feces (decreased food intake), lethargy, head tilt, ataxia, and lateral recumbency. Terminal body weight and body weight gain were decreased by 5 and 50%, respectively. Neurotoxicity was observed in 3/26 does after 12 exposures. Severe weight loss in two of dams in the 80-ppm group (464 and 604 g), indicates that decreased feeding began earlier in the study. Fetuses from the 80-ppm group showed decreased weights (4%) and an increased incidence of fused sternebrae, which the authors attributed to maternal stress. Fetuses in the 80-ppm group also had a higher incidence of missing gall bladder and missing caudal lobe of the lung (considered variations). Because these findings were associated with a sire that had a missing gall bladder, the study was repeated to test methyl bromide at 80 ppm. Maternal toxicity appeared to be less severe in this study and the incidences of
missing gall bladder and lung lobe, although increased, were not statistically significant compared with the control group. No maternal or fetal effects were found at the lower concentrations tested in the first study.
3.6. Genotoxicity
Methyl bromide, tested as a gas in sealed desiccators, was mutagenic in Salmonella typhimurium TA100 with and without metabolic activation, but no mutagenic response was observed in TA98 (NTP 1992). Methyl bromide induced sister-chromatid exchanges in bone-marrow cells and micronuclei in peripheral erythrocytes of female B6C3F1 mice exposed at concentrations up to 200 ppm for 6 h/day for 14 days. Results were equivocal in male mice. When exposure duration was lengthened to 4, 8, or 12 weeks, no significant increase in sister-chromatid exchanges or micronuclei was observed in male or female mice.
The mutagenicity and genotoxicity of methyl bromide was reviewed by IPCS (1995) and IARC (1999). Methyl bromide was positive for reverse gene mutation in S. typhimurium TA100 and TA1535, but not in TA98 or TA1538. Metabolic activation was not required for positive results. Methyl bromide was also positive in tests of forward and reverse mutations in Escherichia coli. It bound covalently to DNA in vitro and in vivo in various organs of rats and mice, and induced micronuclei in bone marrow and peripheral blood cells of rats and mice. The frequency of bone marrow cells with chromosomal aberrations was not increased in rats exposed at 70 ppm for 5 days. Methyl bromide did not induce unscheduled DNA synthesis in cultured rat hepatocytes. Assays for dominant and recessive lethal mutations were negative in mice and rats.
3.7. Chronic Toxicity and Carcinogenicity
Several chronic inhalation studies were available to assess the chronic toxicity and oncogenicity of methyl bromide. In one study (Gotoh et al. 1994), groups of 50 male and 50 female F-344.DuCrj rats were exposed to methyl bromide at 0, 4, 20, or 100 ppm (99.9% purity) whole body for 6 h/day, 5 days/week for 104 weeks. Survival in males of the control, 4-, 20-, and 100-ppm groups was 68%, 68%, 62%, and 66%, respectively. Survival in females was 86%, 76%, 78%, and 82%, respectively. The incidence of pituitary adenomas was significantly increased in males exposed at 100 ppm (60%) compared with controls (32%). No increase in treatment-related tumors was observed in female rats. In the same study, 50 male and 50 female BDF1 mice exposed to methyl bromide at 4, 16, or 64 ppm under the same exposure conditions. At 104 weeks, survival was unaffected by treatment and there was no increased incidence of tumors related to treatment.
Reuzel et al. (1987, 1991) exposed male and female Wistar rats to methyl bromide at concentrations of 0, 3, 30, or 90 ppm for 6 h/day, 5 days/week for 29
months. Each group had 90 males and 90 females; 10 rats/sex/group were killed after 13, 52, and 104 weeks. Body weights, clinical signs, hematology, biochemistry, and gross and microscopic effects were examined at those time points. Exposure to methyl bromide at 90 ppm was clearly toxic; early deaths were reported (but were not statistically significant at the end of the study) and body weights were significantly lower than those of respective control groups throughout most of the study. At the end of the study, effects on the heart were apparent in the 90-ppm group. Statistically significant increases in cartilaginous metaplasia (males), moderate to severe myocardial degeneration (females), and thrombi (males and females) were found. Myocardial degeneration also occurred in aged control rats. Therefore, when total incidences of myocardial degeneration were considered, incidences in the control and 90-ppm groups were similar for both sexes. At the end of the study, 3 ppm was the NOAEL for decreases in body weight and absolute and relative brain weight.
Basal-cell hyperplasia of the olfactory epithelium was present in both males and females in a dose-related manner after 29 months, but not at 13, 52, or 104 weeks. The incidence was statistically significant in the 3-ppm group when total incidence was considered (13/48 in males and 19/58 in females compared with 4/46 and 9/58 in the respective control groups). The majority of lesions were characterized as “very slight” in the 3-ppm group, and as “slight” to “moderate” in the higher exposure groups. These lesions were not present in either males or females in the 3-ppm group at 52 weeks and were not significantly increased over those of the respective control groups at 104 weeks. However, these lesions were found in the female control group after 104 weeks (40%), at the incidence observed in females exposed to methyl bromide at 3 ppm for 29 months (40%). At 29 months, the incidence of total olfactory lesions in males exposed at 3 ppm was 27% compared with 9% in males of the control group.
Nasal lesions increased in controls in an age-dependent manner. All but one of the lesions in the 3-ppm group were classified as slight or very slight, and one moderate lesion of the nasal mucosa was observed in controls at 24 months (accompanied by a 40% incidence of total lesions in control females). The incidence in the control males at 24 months was 30%. Thus, the effect in the 3-ppm groups at the end of the study, although dose-related and statistically significant, must be considered slight or equivocal. This study was well-conducted, used a relevant route of administration, used an adequate number of rats of both sexes, and examined all relevant end points of methyl bromide toxicity. The study shows that the nasal lesions occur in aged rats. There was no indication of carcinogenic activity.
A two-year inhalation study of methyl bromide in B6C3F1 mice was conducted by NTP (1992). Groups of 70 male and 70 female mice were exposed to methyl bromide at 0, 10, 33, or 100 ppm. Neurotoxicity tests were performed on 16 mice (8 males and 8 females) per group. Ten animals per group were killed at 6 and 15 months. The exposure at 100 ppm was discontinued after 20 weeks because of neurotoxicity and early deaths. The same organs and tissue that were affected in the Reuzel et al. (1987, 1991) study were targets in this study,
namely the nose, heart, and brain. The bone was additionally affected. Aside from increased mortality in the 100-ppm dose group, statistically significant differences in LOAELs and NOAELs were found for the following end points: cerebellar and cerebral degeneration, 100 and 33 ppm; myocardial degeneration and chronic cardiopathy, 100 ppm and 33 ppm; sternal dysplasia, 100 ppm and 33 ppm, increased but not statistically significant for either males or females in the 33-ppm group compared with controls); and olfactory metaplasia/necrosis, 100 ppm and 33 ppm. Similar to results in the Reuzel et al. (1987, 1991) study, no olfactory lesions were found in the 3-ppm group at the end of 24 months. No increase in tumor incidence occurred. NTP concluded there was no evidence of carcinogenic activity of methyl bromide in male or female B6C3F1 mice exposed at 10, 33, or 100 ppm.
Danse et al. (1984) showed that orally-administered methyl bromide is carcinogenic in the forestomach of Wistar rats. Doses were 0, 0.4, 2, 10, or 50 mg/kg for 13 weeks. At 50 mg/kg, severe hyperplasia of the stratified squamous epithelium in the forestomach was found. A dose of 10 mg/kg resulted in slight epithelial hyperplasia in the forestomach. Adverse effects were not observed at 0.4 or 2 mg/kg. Extrapolation of forestomach cancers to humans is problematic because methyl bromide, a volatile, reactive chemical, was introduced directly into the stomach by gavage and because humans lack a forestomach (Lu and Coulston 1996). Furthermore, Boorman et al. (1986) showed regression of the tumors after discontinuation of exposure.
3.8. Summary
The concentration-response relationship for mortality is steep in all animal species tested, as shown by the margin between LC50s and nonlethal concentrations (Alexeeff et al. 1985; Kato et al. 1986). LC50 values for the rat ranged from 19,460 ppm for 3.5 min to 302-334 ppm for 8 h (Honma et al. 1985; Zwart 1988; Zwart et al. 1992). In rats, no deaths occurred after exposure to methyl bromide at 506 or 700 ppm for 4 h (Kato et al. 1986; Japanese Ministry of Labour 1992) or at 268 ppm for 8 h (Honma et al. 1985). The mouse was a more susceptible species, having LC50 values ranging from 1,200 ppm for 1 h (Alexeeff et al. 1985) to 405 ppm for 4 h (Yamano 1991). Nonlethal concentrations of methyl bromide in mice were 900 ppm for 1 h (Alexeeff et al. 1985) and 312 and 338 ppm for 4 h (Yamano 1991; Japanese Ministry of Labour 1992).
Toxicity to the CNS was evident as clinical and neurotoxic signs and tissue lesions. The nasal olfactory epithelium was also a target of methyl bromide. Clinical or neurotoxic signs were absent in rats exposed at 150 ppm for 4 h (Japanese Ministry of Labour 1992), in rats exposed at 90 or 100 ppm for 6 h (Hurtt et al. 1988; Driscoll and Hurley 1993), in rats exposed at 63 ppm for 8 h (Honma et al. 1985), in mice exposed at 225 ppm for 4 h (Japanese Ministry of Labour 1992), and in mice exposed at 566 ppm for 1 h, although there was a transient weight loss in the mice (Alexeeff et al. 1985). A 6-h exposure of rats to
methyl bromide at 90 ppm was a NOAEL for damage to the olfactory epithelium (Hurtt et al. 1988), whereas the 4-h NOAEL in mice was 150 ppm (Japanese Ministry of Labour 1992). Dogs exhibited clinical signs of tremors, hunched appearance, and labored breathing during the last 2 h of a 7-h exposure at 233 ppm (Newton 1994a). Rats exhibited transient changes in standard neurobehavioral tests after a 6-h exposure at 350 ppm (Driscoll and Hurley 1993). In contrast to the olfactory lesions observed in rats exposed at 200 ppm for 6 h (Hurtt et al. 1988), no lesions were found after a 6-h exposure at 350 ppm (Driscoll and Hurley 1993).
In 5-day repeat-exposure studies with rats, NOAELs were 90 ppm for tissue lesions, including damage to the olfactory epithelium (Hurtt et al. 1987), and 150 ppm for lesions in the brain (Davenport et al. 1992). The 6-week NOAEL for clinical and neurotoxic signs and tissue lesions in dogs was 20 ppm, the highest concentration tested (Schaeffer 2002). The NOAEL for neurobehavioral effects in a 36-week exposure study with of rats was 55 ppm, but there were severe effects on rabbits exposed at 65 ppm for 4 weeks (Anger et al. 1981). In a subchronic study that examined body and organ weights, clinical signs, neurotoxicity, and microscopic tissue lesions, the lowest LOAEL was 30 ppm in females, based on slightly reduced body weight (Norris et al. 1993). The NOAEL for neurotoxicity was 70 ppm for exposures up to week 13 (both sexes), and the NOAEL for motor activity was 30 ppm for females and 140 ppm for males. In a chronic study with mice, 33 ppm was the NOAEL for behavioral neurotoxicity, cerebellar and cerebral degeneration, myocardial lesions, and olfactory metaplasia/ necrosis (NTP 1992).
No reproductive effects were observed in animals after acute exposure to methyl bromide, although plasma testosterone was transiently decreased in rats exposed at 200 ppm for 5 days (Hurtt and Working 1988). Studies with rats and rabbits indicate that inhalation of methyl bromide at up to 70 ppm during gestation does not result in any significant developmental effects, although there was severe maternal toxicity in rabbits (Hardin et al. 1981; Sikov et al. 1981).
Methyl bromide tested positive in numerous mutagenicity and genotoxicity tests. Mutagenicity did not require metabolic activation, which is consistent with direct-acting alkylation of DNA. Alkylation suggests that methyl bromide might be carcinogenic, but carcinogenicity has not been established in chronic studies with rats and mice (Reuzel et al. 1991; NTP 1992; Gotoh et al. 1994).
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
Inhalation studies with rats and dogs have demonstrated that [14C]methyl bromide is rapidly absorbed in the lower respiratory tract and quickly distributed to all tissues, with the lungs, liver, and kidneys being the major organs of distribution immediately after exposure (Bond et al. 1985; Medinsky et al. 1985; Jaskot
et al. 1988). Appreciable amounts of 14C are also found in the nasal turbinates. This deposition, however, might indicate metabolites. Methyl bromide readily crosses cell membranes, whereas the bromide ion does not.
Uptake of methyl bromide has been measured in the rat, dog, and man. Lung uptake in rats is directly proportional to the concentration in air. Andersen et al. (1980) determined the kinetic constants for metabolism of inhaled methyl bromide in male F-344 rats. Concentrations were 100, 1,000, 3,000, and 10,000 ppm. Rats were placed in vacuum chambers and chamber depletion measurements were taken every 10 min for 180 min (chamber atmospheres were recirculated). Methyl bromide exhibited rapid, first-order uptake, with the first-order rate constant decreasing only at concentrations that caused death during the exposure (10,000 ppm). The rate plot was a straight line that was fitted by unweighted linear least squares to estimate the rate constant (0.44/kg/h). This constant was later recalculated as 0.55/kg/h (Gargas and Andersen 1982). The rapid, first-order uptake was considered to be nonenzymatic metabolism. Because methyl bromide had no measurable saturable uptake component, a Km, the ambient concentration at which uptake proceeds at half the maximum rate, and a Vmax, the maximum rate of uptake, could not be calculated.
In contrast to the work of Andersen et al. (1980), Medinsky et al. (1985) observed uptake saturation at higher concentrations. Their 6-h, nose-only inhalation study with male F-344 rats found uptake of 48% at low concentrations (1.6 and 9.0 ppm) which decreased to 38% at 170 ppm and 27% at 310 ppm. Medinsky et al. (1985) proposed that the availability of GSH might be the rate-limiting variable on the pulmonary absorption of methyl bromide at high concentrations. The observation that the rate of pulmonary absorption decreases with increased exposure concentrations suggests there is a saturation point for inhaled methyl bromide. Measurements of respiratory parameters during the study indicated the tidal volume and minute volume were significantly decreased at 310 ppm. Uptake was also measured in the beagle and man. Steadystate uptake in beagles exposed at 174-361 parts per billion (ppb) was 39.5% during a 3-h exposure (Raabe 1986); the clearance half-time was 41 h. Uptake in humans during nasal or oral breathing of methyl bromide at 18 ppb were similar, 52-55% (Raabe 1988).
Metabolism may take place by two pathways. The presence of methanol and bromide ion in tissues implies nucleophilic displacement of the bromide ion (Gargas and Andersen 1982; Honma et al. 1985). However, the primary pathway is probably conjugation with the tripeptide GSH. By analogy with methyl chloride, outlined by Kornbrust and Bus (1983), conjugation of methyl bromide with GSH yields S-methylglutathione cleavage of the glutamic acid and glycine moieties of GSH yields S-methylcysteine, and transamination and decarboxylation yields the mercapturic acid, methylthioacetic acid. The latter sulfurcontaining compounds can be excreted in the urine. Methylthioacetic acid may be further metabolized to methanethiol which may yield, via P-450 metabolism, formaldehyde and formic acid; the latter compounds can enter the one-carbon pool or be excreted as carbon dioxide. The reaction with GSH appears to be
primarily enzyme catalyzed, probably by GST. Formation of formaldehyde appears to be a minor pathway. Additional studies of the metabolism of methyl bromide provide strong evidence that GSH conjugation is the primary metabolic pathway for methyl bromide (Peter et al. 1989; Hallier et al. 1990; Bonnefoi et al. 1991). Several studies have shown that administration of methyl bromide decreases the nonprotein sulfhydryl content (GSH) and, depending on tissue and administered concentration, increases or decreases the GST content of tissues (Roycroft et al. 1981; Alexeeff et al. 1985; Davenport et al. 1992). The concentration of GSH in liver is extremely high (about 10 mM), hence nonenzymatic conjugation of some xenobiotics with GSH can be significant. GSTs are abundant cellular components, accounting for up to 10% of the total cellular protein (Parkinson 2001).
Excretion occurs mainly by expiration of CO2 and urinary excretion. After inhalation of14C-methyl bromide by rats (1.6-310 ppm), 43-50% of elimination of the radiolabel was pulmonary (14CO2) and 20% was renal (Bond et al. 1985; Medinsky et al. 1985; Jaskot et al. 1988). Less than 4% was expired as methyl bromide. Only small amounts are excreted in the feces. A significant amount (25-39%) remains in the tissues after 24-72 h, and is excreted slowly. This fraction presumably represents adducts and incorporation into metabolic pools.
Blood bromide concentrations in humans normally range up to 15-30 mg/L (EPA 1980; ATSDR 1992; Fuortes 1992). Concentrations of 25-250 mg/L were reported in severe poisoning cases and 83-2,116 mg/L in fatal poisoning cases. When these measurements were taken is unknown in some of these cases. Bromine concentrations in blood are generally lower with poisoning by methyl bromide than by inorganic bromide, indicating that free bromide might not be an indicator of the severity of effects.
Honma et al. (1985) measured methyl bromide and bromine concentrations in blood and tissues of rats after a 2-h exposure at 0, 250, 500, 750, or 1,000 ppm. Methyl bromide in tissues reached maximum values within 1 h, and was rapidly eliminated (half-life of about 30 min). There was a linear relationship between methyl-bromide concentrations in air and blood; the blood concentrations ranged from 0 μg/g at 0 ppm to 0.374 μg/g at 1,000 ppm (measured immediately after exposure). A linear relationship was also found between bromine in tissues and exposure concentrations. Blood bromine ranged from 7 μg/g at 0 ppm to 90 μg/g at 1,000 ppm. In contrast with tissues concentrations of methyl bromide, tissue concentrations of bromine peaked 4-8 h after exposure, and the half-life of elimination was about 5 days. Methanol concentrations remained below those considered responsible for CNS effects.
4.2. Mechanism of Toxicity
The mechanism of methyl-bromide-induced CNS toxicity has not been establish, although it is known that methyl-bromide toxicity results from the methylation of crucial sulfhydryl containing enzymes and proteins in mammalian
tissues and that CNS toxicity might be mediated by CNS glutathione depletion and inhibition of GST activity (Davenport et al. 1992). Methylation of proteins in addition to reduced GSH concentrations might cause cellular disruption. Cellular disruption, especially in the CNS, results in progressive dysfunction. Immunochemical studies of the rat brain show that GST isozymes are present in the cytoplasm, nuclei, and nucleolei of neurons, and the glia of the brainstem, forebrain, and cerebellum. The pattern of GST isozyme distribution throughout regions of the brain is not uniform, which might result in regional susceptibility or resistance to chemical-induced damage (Johnson et al. 1993). In contrast to this proposed mechanism of action, GSH depletion has been found to inhibit toxicity by methyl bromide in some species (Peter et al. 1989). In addition to inhibition of sulfhydryl enzymes, methyl bromide reversibly breaks down adenosine triphosphate (Reigart and Roberts 1999). Another suggested mechanism of toxicity is the formation of methanethiol and formaldehyde as neurotoxic metabolites of methyl bromide (Garnier et al. 1996). In such a case, individuals with greater glutathione-transferase activity might be predisposed to the neurotoxic effects if methyl bromide. However, with the related chemical, methyl chloride, no increase in formaldehyde concentration was observed in the liver or kidneys of mice exposed at 1,000 ppm for 8 h (Jager et al. 1988). Hydrolysis of methyl bromide, resulting in bromide ion, does not appear to be the toxic mechanism. Blood concentrations of bromide after methyl-bromide poisoning are not as high as those associated with intoxication by bromide salts (Gosselin et al. 1984).
In rats, methyl bromide specifically damages the olfactory mucosa lining the posterior regions of the nose, but does not adversely affect the respiratory epithelium (Hurtt et al. 1987, 1988). The reason for the specific sensitivity of the olfactory epithelium is unknown, although methyl bromide is known to inhibit GST activity of olfactory nasal epithelial cells (Thomas et al. 1989). This is a reversible lesion; near complete destruction of the rat olfactory epithelium by methyl bromide at 330 ppm for 6 h was followed by reinnervation and repair after exposure ended (Schwob et al. 1999).
On the basis of chemical similarity, the mechanism of action of methyl bromide is predicted to be the same as that of methyl chloride. Although the exact mechanism of action of methyl chloride is unclear, it appears to involve GSH depletion in target tissues or the production of toxic metabolites, such as formaldehyde or methanethiol, and lipid peroxidation. A lack of GSH could impair the ability of tissues to suppress lipid-peroxidation reactions (Kornbrust and Bus 1983). The buildup of leukotrienes also has been suggested as a toxic mechanism of action. Under conditions of GSH depletion, 5-hydroperoxicosotetraenoic acid, the immediate precursor of leukotrienes, accumulates in the tissues. Leukotrienes are potent vasoconstrictors, and cause increased capillary permeability and tissue edema (AIHA 1997). In contrast with tests of methyl chloride, depletion of GSH before administration of methyl bromide enhanced toxicity (Thomas and Morgan 1988).
Honma (1987; Honma et al. 1991) investigated the effect of methyl bromide on brain neurotransmitters, particularly changes in catecholamine production in the hypothalmus of rats in relation to CNS effects. Rats were exposed to methyl bromide at 0, 16, 31, 63, 125, 188, or 250 ppm for 8 h, and were killed immediately after exposure. There were no effects on catecholamine activity at 16 ppm. At 31-250 ppm, there was a dose-dependent decrease in the neurotransmitter norepinephrine (13-30%), an increase in 3-methoxy-4-hydroxyphenylglycol, a metabolite of norepinephrine, and a decrease in tyrosine hydroxylase activity. As tyrosine hydroxylase catalyzes hydroxylation of tyrosine to L-dopa, the precursor of the catecholamines, the decrease in norepinephrine appears to result from the inhibition of tyrosine hydroxylase. These changes were linked changes in body temperature and appetite noted in Honma et al. (1987).
Two studies show that exogenously-administered GSH protects against the acute lethal effects of methyl bromide. Mortality in mice injected with GSH (500 mg/kg) and exposed to methyl bromide at 500 ppm for 150 min was 5.3%, compared with 85% in mice that were not pretreated with GSH (Yamano 1991). Glutathione injected intraperitoneally (750 mg/kg) resulted in 100% survival of mice exposed at a lethal concentration of 1,850 ppm for 1 h (Kawai and Ueda 1972).
4.3. Structure-Activity Relationships
Toxicity of the monohalomethanes—methyl chloride, methyl bromide, and methyl iodide—appears to be related to atomic weight. All produce similar toxic effects in humans (Gosselin et al. 1984), with the greatest toxicity from methyl iodide, followed by methyl bromide and methyl chloride. Bakhishev (1975) reported 30-min inhalation LC50 values in the rat for methyl chloride, methyl bromide, and methyl iodide of 39,000 ppm, 2,800 ppm, and 2,300 ppm, respectively. The target organs of methyl bromide and methyl chloride in the rat are the same—the brain, liver, adrenal glands, olfactory epithelium, and the testes (Morgan et al. 1982; Hurtt et al. 1987).
4.4. Other Relevant Information
4.4.1. Species Variability
The available data allowed comparisons between rats and mice. Where data were available for the same time periods, mice were more susceptible to the lethal effects of methyl bromide than rats. On the basis of 30-min (Bakhishev 1973), 1-h (Alexeeff et al. 1985; Zwart 1988), and 4-h LC50 values (Kato et al. 1986; Yamano 1991), the mouse is two-fold more sensitive to methyl bromide than the rat. For example, the rat 1-h LC50 of 1,880 ppm (Zwart 1988) is 1.6 times greater than the 1-h mouse LC50 of 1,200 ppm (Alexeeff et al. 1985). This greater sensitivity might be related to the higher concentrations of GST found in
mouse tissues (Griem et al. 2002). Greater sensitivity might also be a reflection of the greater respiratory rate of mice compared with rats. Hori et al. (2002) reported that at methyl bromide concentrations of 500-10,000 ppm for up to 8 hours, survival times of rats were greater than those of mice. The difference in survival times between the species was large at low concentrations, but decreased substantially with increasing concentrations. The animals were killed immediately after the exposures, limiting the usefulness of the data.
The available data also indicate that rabbits are more sensitive than rats or guinea pigs. Guinea pigs and rats withstood 6-month exposures to methyl bromide at 66 ppm without demonstrable effects, but rabbits became paralyzed (Irish et al. 1940). A similar result was reported in a more recent study (Anger et al. 1981). Rabbits exposed at 65 ppm began to lose weight by the third week of exposure and eyeblink responses and nerve-conduction velocity in rabbits were significantly reduced. Rats were unaffected under the same exposure regime. Maternal toxicity was greater in pregnant rabbits exposed to methyl bromide at 70 ppm before to mating and through gestation, whereas no maternal toxicity was evident in rats under the same exposure scenario (Hardin et al. 1981; Sikov et al. 1981).
Both in vitro and in vivo comparisons of different species indicate that concentrations of GST enzymes are much lower in human tissues (liver and lung) than in mice or rats (Andersen et al. 1987; Reitz et al. 1989). The data are consistent with the hypothesis that the rate of activation of mono-and dihalomethanes to toxic metabolites by the GST pathway occurs much more slowly in humans than in rodents. Jager et al. (1988) investigated the concentrations of GSH in rodent tissues. Activities of GSH in the liver were 2-3 times greater in male B6C3F1 mice than in female mice and F-344 rats of both sexes. Griem et al. (2002) compiled ratios of GST activity in rodents to humans in various tissues. The ratios of rat:human and mouse:human GST activity in the liver are 3.95 and 7.64, respectively. On the other hand, nonprotein sulfhydral content (primarily GSH) is similar among human, monkey, and rat tissues on a μmol/mL of tissue basis (Frederick et al. 2002). This was true for major organs, but not nasal tissue. Rat nasal tissue had more nonprotein sulfhydral content than human tissue.
For the related chemical, methyl chloride, blood concentrations of humans, dogs, and rats exposed to 50 ppm for 6 h reached a plateau during the first hours of exposure; elimination was rapid once the exposures were terminated (Landry et al. 1983; Nolan et al. 1985). Blood concentrations of methyl chloride in slow human metabolizers plateaued at 60% of those found in the rat and 70% of those found in the dog. Postexposure elimination was most rapid in the rat (t1/2 = 15 min). The dog and rapid human metabolizers had the same elimination rate (t1/2 = 50 min), and the slow human metabolizers eliminated at the slowest rate (t1/2 = 90 min). At 50 ppm, the rat absorbed 10 μg/min/kg whereas the rapid and slow human metabolizers absorbed 3.7 and 1.4 μg/min/kg, respectively. According to Nolan et al. (1985), differences in the pharmacokinetics between these three species were adequately explained by the differences in respiratory
minute volume and basal metabolic rates (rat > dog > man). Similar comparative studies were not available for methyl bromide, so it should be noted that uptake kinetics of methyl bromide and methyl chloride could be different. Andersen et al. (1980) found uptake of methyl chloride to be saturable, being associated with enzymatic metabolism, whereas the rapid, first-order uptake of methyl bromide was considered to be nonenzymatic metabolism.
Monohalomethanes are not metabolized by the erythrocyte cytoplasm of rats, mice, Rhesus monkeys, cows, pigs, and sheep, but are metabolized by approximately 60% of humans (Redford-Ellis and Gowenlock 1971; Peter et al. 1989). Lack of erythrocytic metabolism might explain the rapid equilibrium between the gas phase of methyl chloride and methyl bromide and whole blood of rats observed in pharmacokinetic studies.
Inhalation studies conducted on various mammalian species have demonstrated clear sex-related differences in susceptibility to methyl bromide. In the 6-week repeat-exposure study of Eustis et al. (1988), survival was much greater in females than in males.
Methyl bromide was toxic only to olfactory epithelium and not the other nasal epithelia of rats (Hurtt et al. 1988). Histochemical techniques revealed degeneration of the sensory and sustentacular cells but not the basal cells from which the former cells are regenerated. This was a reversible effect as the basal cells were not affected. The olfactory region (dorsal meatus) of rats is highly exposed to chemicals because of air-flow characteristics in the nasal turbinates. In rodents, an inhaled vapor traverses a few millimeters of resistant respiratory epithelium before reaching sensitive olfactory tissue; whereas, in humans, an inhaled vapor has to traverse several centimeters and a much larger surface area of respiratory epithelium to reach the olfactory tissue. A mathematical model based on a combination of computational fluid dynamics and physiologicallybased pharmacokinetics showed that the dorsal meatus region of the rat nose receives 12-20% of the inhaled air (Bush et al. 1998; Frederick et al. 1998). A comparison with airflow patterns in the human nose shows that the olfactory epithelium in the dorsal meatus region of the nasal cavity of the rat is exposed to two-to three-fold greater concentrations of chemicals. Therefore, higher concentrations of methyl bromide would likely be required to induce this lesion in humans.
4.4.2. Susceptible Populations
Interindividual variation in the rate of metabolism of methyl halides has been observed in humans. At least two distinct populations of humans with different metabolism rates of the structurally-similar methyl chloride have been identified (Nolan et al. 1985; ATSDR 1992; WHO 2000). The differences in metabolism rate are attributed to a genetic polymorphism of GST. Depending on the presence or absence of GST, humans may be “fast metabolizers” or “slow metabolizers” of methyl chloride. There may be a third phenotype, non-conjugators
(Warholm et al. 1994; Lof et al. 2000). Fast metabolism may lead to the formation of toxic metabolites that can exert their action before they can be eliminated. Slow metabolizers would be expected to be less susceptible to the toxic effects of methyl halides as formation of S-methylglutathione is limited to the nonenzymatic reaction of methyl bromide with GSH. Garnier et al. (1996) reported that of two fumigation workers similarly exposed to methyl bromide during fumigation activities, neurologic and systemic symptoms were greater in the “conjugator.” GST activity was undetectable in the erythrocytes of the nonconjugator. The nonconjugator also had greater concentrations of S-methylcysteine adducts in blood albumin and globin.
For the related chemical, methyl chloride, uptake differed by less than 3-fold between slow and fast metabolizers exposed at 50 ppm (Nolan et al. 1985). Elimination was rapid in both groups after the exposure ended. Elimination was more rapid in volunteers with lower blood and expired-air concentrations. The authors explained the difference in the two groups by a two-fold difference in the rate at which they metabolized methyl chloride. They considered the difference of questionable toxicologic significance.
Among Caucasians, the majority of individuals possess at least one copy of the GST gene; 10-25% are nonconjugators (Nelson et al. 1995; Warholm et al. 1994; Kempkes et al. 1996). Approximately 60% of Asians lack the gene (Nelson et al. 1995).
As noted by ATSDR (1992), people that have kidney or liver disease, anemia, or neurologic deficits might be more susceptible to the toxic effects of methyl chloride. Persons with deficient glucose-6-phosphate dehydrogenase might have reduced concentrations of GSH (Bloom and Brandt 2001). Additionally, accidental exposures suggest that infants are more susceptible than adults (Langard et al. 1996). However, death of an infant was from acute pneumonia resulting from vomiting and aspiration after inhaling methyl bromide.
4.4.3. Concentration-Exposure Duration Relationship
Using approximately 50 male SPF-Wistar rats tested in groups of two at 23 different combinations of time (seven exposure durations, ranging from 3.5 to 480 min) and concentrations, Zwart et al. (1992; Zwart 1988) established a concentration-time–mortality relationship. The probit equation was-30.5 + 6.6 ln C + 5.5 ln T, where C = concentration and T = time. Using the relationship Cn × t = k, the value of n was 1.2. Using all of the LC50 data from rat studies with exposure durations of 3.5 min to 8 h, the best fit of the concentration × time curve results in an n value of 1.23 (see Appendix A). Approximately the same value (1.3) is generated when the mouse data are graphed or when the mouse and rat data are graphed together (graph not shown). Because the mouse is slightly more susceptible than the rat, the graph line for mouse lethality data is parallel to, but just slightly below, the graph line for the rat lethality data.
From the studies summarized in Tables 5-3 to 5-5, it is apparent that there is a threshold concentration at which effects occur or fail to occur. For example, 90 ppm was a NOAEL for olfactory lesions in the rat, both during a 1-day and a 5-day repeat-exposure study, whereas a single exposure at 200 ppm produced such lesions (Hurtt et al. 1987). No neurotoxicity was observed in dogs exposed at 20 ppm for 7 h/day, 5 days/week for 6 weeks (Schaeffer 2002) or in rats exposed at 55 ppm for 7.5 h/day for 36 weeks (Anger et al. 1981). Concentrations of 90 and 175 ppm were the 5-day NOAEL and LOAEL, respectively, for nasal lesions in rats (Hurtt et al. 1988). Concentrations of 33 and 100 ppm were the chronic NOAEL and LOAEL for tissue lesions and neurotoxicity in mice (NTP 1992). There is also a time element. Both rats and mice withstood single exposures to methyl bromide at 140-300 ppm, but deaths occurred when these exposures were repeated (Ikeda et al. 1980; Kato et al. 1986; Hurtt et al. 1987; NTP 1992; Norris et al. 1993).
4.4.4. Concurrent Exposure Issues
No concurrent exposure issues were identified.
5. DATA ANALYSIS FOR AEGL-1
5.1. Summary of Human Data Relevant to AEGL-1
No reliable data relevant to derivation of AEGL-1 values were found. Methyl bromide is not detectable by odor or irritation at concentrations that are thresholds for tissue lesions or neurotoxicity. Lacrimation, an AEGL-1 level effect, was observed in rats exposed for 4 h to methyl bromide concentrations ≥338 ppm, with a no-effect level of 225 ppm (Japanese Ministry of Labour 1992). However, the no-effect level for lacrimation is not an appropriate basis for the derivation of AEGL-1 values, because signs of neurotoxicity (decreased locomotor activity and ataxia) were also observed at concentrations producing lacrimation. Furthermore, lacrimation was observed at methyl bromide concentrations above the point-of-departure used to derive AEGL-2 values (200 ppm). Therefore, derivation of AEGL-1 values would not be appropriate for methyl bromide, because it has no warning properties (e.g., odor or irritation) at concentrations below those that produce neurotoxicity. At one time, the American Conference of Governmental Industrial Hygienists had a threshold limit value-time weighted average (TLV-TWA) for methyl bromide of 20 ppm. The current ceiling standard of the Occupational Safety and Health Administration (OSHA) is 20 ppm (see Section 8.2).
5.2. Summary of Animal Data Relevant to AEGL-1
Only repeat-exposure studies were available on methyl bromide at low concentrations. Several examples of repeat-exposure studies are cited here. No
neurotoxic signs or tissue lesions were observed in dogs exposed at 20 ppm (the highest concentration tested), 7 h/day, 5 days/week for 6 weeks (Schaeffer 2002). In an 8-month study with rabbits, a particularly sensitive species, 27 ppm was the NOAEL for neurobehavioral effects (Russo et al. 1984). In a wellconducted carcinogenicity study with mice, 33 ppm was a NOAEL for any type of effect, including neurotoxicity, tissue lesions, carcinogenicity, and early mortality (NTP 1992). Exposures in that study were for 6 h/day, 5 days/week for 2 years. Finally, in developmental toxicity studies, 20 ppm was a NOAEL for both maternal toxicity and developmental effects in rats and rabbits (Breslin et al. 1990; Hardin et al. 1981).
5.3. Derivation of AEGL-1
Because methyl bromide is not detectable by odor or sensory irritation at concentrations below the AEGL-2 values (see below), AEGL-1 values were not derived. Absence of AEGL-1 values does not imply that exposures below the AEGL-2 values are without adverse effects.
6. DATA ANALYSIS FOR AEGL-2
6.1. Summary of Human Data Relevant to AEGL-2
No reliable human data that address the threshold for neurotoxicity or tissues lesions were available.
6.2. Summary of Animal Data Relevant to AEGL-2
In dogs, neurotoxicity, apparent as tremors, hunched appearance, and labored breathing, was not observed during the first 5 h of exposure to methyl bromide at 233 ppm, during the first 2 days of exposure at 156 ppm for 7 h, during the first day of exposure at 158 ppm, or after 4 daily 7-h exposures at 55 ppm (Newton 1994a). Because signs of neurotoxicity might be delayed, the 233-ppm exposure for 4 h could induce signs at a later time and, therefore, could be considered the threshold for neurotoxicity in dogs. A 4-h exposure at 200 or 225 ppm was a NOAEL for clinical signs in rats during one day, 4-h exposures (Hastings 1990; Japanese Ministry of Labour 1992). In 6-h studies, the NOAELs for neurotoxicity in rats were 200 ppm (Hurtt et al. 1988) and between 100 and 350 ppm (Driscoll and Hurley 1993). No clinical signs were observed in mice after exposure for 1 h at 224 ppm (Alexeeff et al. 1985) or after 4 h at 225 ppm (Japanese Ministry of Labour 1992). In most cases, the exposures were also NOAELs for tissue lesions; the exception being the reversible olfactory lesions in the rat. Because of the differences in the amount and placement of olfactory
epithelium in humans and rodents and the differences in air flow patterns, olfactory lesions in rats might not be relevant to humans.
The developmental study of methyl bromide in rabbits by Breslin et al. (1990) was not considered appropriate for developing AEGL-2 values. The decreased weight of fetuses born to does exposed to methyl bromide at 80 ppm is not considered the result of a single exposure. Furthermore, signs of maternal stress were observed at concentrations below those of other species, such as the rat (Hardin et al. 1981). The reason for the greater sensitivity of the rabbit is unknown.
6.3. Derivation of AEGL-2
For methyl bromide, neurotoxicity leading to an inability to escape is the most relevant end point for AEGL-2 values. Because of the steep dose-response curve for such effects, a NOAEL for neurotoxicity is the appropriate starting point for calculating AEGL-2 values. On the basis of data from three studies in rats (Hurtt et al. 1988; Hastings 1990; Japanese Ministry of Labour 1992) and one study in dogs (Newton 1994a), 200 ppm for 4 h was selected as the point-ofdeparture for derivation of AEGL-2 values. Studies in rats identified the following values as no-effect levels for neurotoxic effects for single exposures: 200 ppm for 6 h in 15 rats/group (Hurtt et al. 1988), 200 ppm for 4 h in 30 rats/group (Hastings 1990), and 225 ppm for 4 h in 20 rats/group (Japanese Ministry of Labour 1992). Thus, the database for rats is robust, with consistent results in all three studies. The study in dogs evaluated effects of a single 7-h exposure to methyl bromide in 1-2 dogs/group and of repeated exposures (7 h/day for 4 days) in 2-3 dogs/group (Newton 1994a). Results of the single exposure study in dogs did not identify a no-effect level for neurotoxicity. Signs of neurotoxicity were observed at all concentrations tested ( ≥233 ppm); however, no signs of neurotoxicity were observed during the first 5 h of exposure at 233 ppm. Results of the repeated-exposure study in dogs (2-3/group) showed the following: no effects at 55 ppm, clinical signs of neurotoxicity on day 3 of exposure at 156 ppm, and severe signs of neurotoxicity on the second day of exposure at 268 ppm. A small number of dogs were tested, so the rat data were considered a stronger basis for selecting a NOAEL for neurotoxicity. The dog data support the selection of 200 ppm for a single 4-h exposure in rats as the point-ofdeparture.
Because uptake of methyl bromide is greater in rodents than in humans (based on comparative respiratory rates and comparisons with methyl chloride) and because GST concentrations in rodents are greater than in humans (allowing more rapid production of toxic metabolites), an interspecies uncertainty factor of 1 was applied. Although humans differ in their capacity to metabolize methyl bromide, the difference is considered to be less than 3-fold from a toxicologic stand point (Nolan et al. 1985). An intraspecies uncertainty factor of 3 was applied. The resulting 4-h value of 67 ppm was time scaled to the other exposure
durations using the equation Cn × t = k, with n = 1.2. Because the time-scaled 8-h value of 37 ppm is close to the chronic NOAEL of 33 ppm for mice (NTP 1992) and is less than the 5-day NOAEL of 55 ppm for clinical signs and tissue lesions in dogs (Newton 1994a) and the 36-week NOAEL of 55 ppm for neurobehavioral parameters and nerve conduction velocity in rats (Anger et al. 1981), the 8-h value was set equal to the 4-h value. AEGL-2 values are presented in Table 5-7, and the calculations are shown in Appendix B. A category graph of AEGL values in relation to animal toxicity data is provided in Appendix C.
7. DATA ANALYSIS FOR AEGL-3
7.1. Summary of Human Data Relevant to AEGL-3
ATSDR (1992) estimates that the concentrations of methyl bromide that lead to death in humans range from 1,600 to 60,000 ppm, depending on the duration of exposure. These estimates are based on older studies with concentrations that were either estimated or measured with techniques of limited sensitivity. Although the human data are not robust, the values can be considered as support for values derived from recent, well-conducted animal studies.
7.2. Summary of Animal Data Relevant to AEGL-3
Data relevant to deriving AEGL-3 values for methyl bromide were available from studies in the rat and mouse. The highest nonlethal concentrations and exposure durations for methyl bromide in the rat were 700 ppm for 4 h (Kato et al. 1986) and 268 ppm for 8 h (Honma et al. 1985). For the mouse, the highest nonlethal concentrations were 900 ppm for 1 h (Alexeeff et al. 1985) and 312 ppm for 4 h (Yamano 1991). For each species, data from different laboratories provide a good fit to a dose-response curve with a time-scaling exponent of 1.2. On the basis of 30-min and 4-h LC50 values (Bakhishev 1975; Kato et al. 1986; Yamano 1991), it appears that the mouse is approximately two-fold more sensitive to methyl bromide than the rat. This greater sensitivity might be related to the higher concentrations of GST in mouse tissues (Griem et al. 2002) and a higher respiratory rate. The mouse was also more sensitive than rats to the related chemical, methyl chloride (see Chapter 6).
7.3. Derivation of AEGL-3
Dividing the highest nonlethal values by any of the commonly used combination of interspecies and intraspecies uncertainty factors of 100, 30, or 10 results in values that are close to the ACGIH TLV-TWA values (currently 1 ppm, but up to 20 ppm in previous years) in the first two cases and below the AEGL-2 values in the latter case.
TABLE 5-7 AEGL-2 Values for Methyl Bromide
|
|
||||
| 10 min | 30 min | 1h | 4h | 8h |
|
|
||||
| 940ppm (3,657 mg/m3) | 380ppm (1,478 mg/m3) | 210ppm (817 mg/m3) | 67ppm (261 mg/m3) | 67 ppm (261 mg/m3) |
|
|
||||
Because of differences in methyl halide metabolism between mice and other rodents and because of the greater sensitivity of mice to the structurallysimilar chemical methyl chloride (metabolism is by the same GSH conjugation pathway), the mouse was not considered an appropriate model for deriving AEGL values for methyl bromide. The AEGL-3 values were based on the BMCL05 (benchmark concentration, 95% lower confidence limit with 5% response) of 701 ppm, calculated using data from a 4-h exposure study in rats (Kato et al. 1986). That concentration was also the highest nonlethal value in the study. As in the derivation of AEGL-2 values, uncertainty factors of 1 and 3 were applied to adjust for interspecies and intraspecies differences, respectively. Time scaling was conducted using the equation Cn × t = k, with n= 1.2, based on lethality data in the rat. Because uptake of methyl bromide is greater in rodents than in humans (based on comparative respiratory rates and comparisons with methyl chloride) and because GST concentrations are higher in rodents than in humans (resulting in more rapid production of toxic metabolites), an interspecies uncertainty factor of 1 was considered sufficient. Humans differ in their capacity to metabolize methyl bromide, but the difference is not considered to be greater than 3-fold from a toxicologic stand point (Nolan et al. 1985). In addition, use of an intraspecies uncertainty factor of 3 is supported by the steep dose-response curve for lethality, which indicates little intraspecies variation (see Chapter 6). Furthermore, larger reduction of the AEGL-3 values would result in values close to the AEGL-2 values. Therefore, an intraspecies uncertainty factor of 3 was considered sufficient. The AEGL-3 values are presented in Table 5-8. The 8-h AEGL-3 value of 130 ppm is supported by repeat-exposure studies, in which dogs exposed to methyl bromide at 156 or 158 ppm for 7 h/day did not exhibit severe clinical signs until the second or third day of exposure (Newton 1994a,b). There were no remarkable histopathologic lesions in the dogs at necropsy after a 4-day exposure. Cerebellar lesions were observed in dogs that were first exposed to methyl bromide at 10 ppm for 7 h/day for 4 weeks, and then exposed at 158 ppm for 6 days.
8. SUMMARY OF AEGLS
8.1. AEGL Values and Toxicity End Points
AEGL values for methyl bromide are presented in Table 5-9, and derivation summaries are provided in Appendix D.
TABLE 5-8 AEGL-3 Values for Methyl Bromide
|
|
||||
| 10 min | 30 min | 1h | 4h | 8h |
|
|
||||
| 3,300 ppm (12,837 mg/m3) |
1,300 ppm (5,057 mg/m3) |
740 ppm (2,879 mg/m3) |
230 ppm (895 mg/m3 ) |
130 ppm (506 mg/m3) |
|
|
||||
TABLE 5-9 Summary of AEGL Values for Methyl Bromide
|
|
|||||
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h |
|
|
|||||
| AEGL-1 (nondisabling] | NRa | NRa | NRa | NRa | NRa |
| AEGL-2 (disabling) | 940 ppm (3,657 mg/m3) |
380 ppm (1,478 mg/m3) |
210 ppm (817 mg/m3) |
67 ppm (261 mg/m3) |
67 ppm (261 mg/m3) |
| AEGL-3 (lethal) | 3,300 ppm (12,837 mg/m3) |
1,300 ppm (5,057 mg/m3) |
740 ppm (2,879 mg/m3) |
230 ppm (895 mg/m3) |
130 ppm (506 mg/m3) |
|
|
|||||
aNumerical values are not recommended because the data indicate that toxic effects might occur below the odor threshold for methyl bromide. Absence of an AEGL-1 value does not imply that exposure below the AEGL-2 value is without adverse effects.
Abbreviations: NR, not recommended.
8.2. Comparison with Other Standards and Guidelines
Guidelines and standards for methyl bromide are presented in Table 5-10. The American Industrial Hygienists Association (AIHA 1997) did not establish a level 1 Emergency Response Planning Guideline (ERPG-1) for methyl bromide because the chemical is not detectable by odor or irritation at concentration below its level 2 guideline (ERPG-2). AEGL-1 values were not derived for the same reason. The 1-h ERPG-2 of 50 ppm is based on animal and human data that suggest no significant respiratory irritation or CNS dysfunction at that concentration. The 1-h ERPG-3 of 200 ppm is based on the observation of Clarke et al. (1945) that exposures of relatively short duration ( ≤2 h), which might have been as low as 250 ppm, have been lethal to animals and humans. However, the observations of Clarke et al. are based on older, poorly cited sources. The AEGL-2 and AEGL-3 values are larger than the corresponding ERPG values and are based on more recent animal data.
The immediately dangerous to life or health (IDLH) standard established by the National Institute for Occupational Safety and Health (NIOSH 1994) is comparable to the corresponding AEGL-2 value. The IDLH of 250 ppm is based on acute inhalation toxicity data from Clarke et al. (1945), which indicated that methyl bromide at 220 ppm can be endured for several hours without serious effects. NIOSH acknowledges that 250 ppm might be a conservative value because there are no data for workers exposed above 250 ppm.
From 1948-1962, the TLV-TWA for methyl bromide was 20 ppm (ACGIH 2004). A skin notation was added in 1961. The TLV-TWA was reduced to 15 ppm in 1973. That standard was subsequently reduced in 1979 to 5 ppm, and a short-term exposure limit (STEL) of 15 ppm was established. In 1996, based on uncertain results in the Reuzel et al. (1991) study, the TLVTWA was lowered to 1 ppm. The 1 ppm concentration protects against mild irritation of the nasal mucosa. ACGIH notes that extensive experience in occupational exposures did not indicate adverse health effects at the previous TLVTWA of 5 ppm.
TABLE 5-10 Extant Standards and Guidelines for Methyl Bromide
|
|
|||||
| Exposure Duration | |||||
| Guideline | 10 min | 30 min | 1h | 4h | 8h |
|
|
|||||
| AEGL-1 | NRa | NRa | NRa | NRa | NRa |
| AEGL-2 | 940 ppm | 380 ppm | 210 ppm | 67 ppm | 67 ppm |
| AEGL-3 | 3,300 ppm | 1,300 ppm | 740 ppm | 230 ppm | 130 ppm |
| ERPG-1 (AIHA)b | NA | ||||
| ERPG-2 (AIHA) | 50 ppm | ||||
| ERPG-3 (AIHA) | 200 ppm | ||||
| IDLH (NIOSH)c | 250 ppm | ||||
| TLV-TWA (ACGIH)d | 1 ppm (skin)e | ||||
| REL-TWA (NIOSH)f | Lowest feasible concentration | ||||
| PEL-TWA (OSHA)g | None | ||||
| PEL-C (OSHA)h | 20 ppm (skin)e | ||||
| MAK (Geimany)i | Skine | ||||
| MAC (The Netherlands)j | 0.3 ppm | ||||
|
|
|||||
a Numerical values are not recommended because the data indicate that toxic effects might occur below the odor threshold for methyl bromide. Absence of an AEGL-1 value does not imply that exposure below the AEGL-2 value is without adverse effects.
b ERPG (emergency response planning guidelines, American Industrial Hygiene Association (AIHA 1997).
The ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1h without experiencing other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor.
The ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible
or other serious health effects or symptoms that could impair an individual’s ability to take protection action.
The ERPG-3 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing lifethreatening health effects.
c IDLH (immediately dangerous to life or health, National Institute for Occupational Safety and Health) (NIOSH 1994) originally represented the maximum concentration from which one could escape within 30 min without any escape-impairing symptoms or any irreversible health effects in the event that protective respiratory equipment failed. NIOSH is currently assessing the diverse uses of IDLHs and determining whether the original criteria used to derive the IDLH values are valid, or if other criteria should be used. Currently, NIOSH considers an IDLH exposure condition as one “that poses a threat of exposure to airborne contaminants when that exposure is likely to cause death or immediate or delayed permanent adverse health effects or prevent escape from such an environment.” The original purpose of the IDLH remains (to ensure worker escape in the event of protective respiratory-equipment failure). The IDLH of 250-ppm is based on acute inhalation toxicity data in humans (Clarke et al. 1945). However, the value is considered possibly “conservative” because there was a lack of relevant acute toxicity data for workers exposed at concentrations above 220 ppm.
d TLV-TWA (threshold limit value-time weighted average, American Conference of Governmental Industrial Hygienists) (ACGIH 2004) is the time-weighted average concentration for a normal 8-h workday and a 40-h workweek, to which nearly all workers may be repeatedly exposed, day after day, without adverse effect. Certain sensitive populations might not be adequately protected from adverse health effects at or below this concentration. This TLV differs from the ceiling of 20 ppm established by OSHA because of ACGIH considered the potential carcinogenicity methyl bromide, its capacity to be absorbed through the skin, its marked neurotoxicity, and its significant nasal and dermal irritation, which warrant a greater degree of caution and a reduction in the previously recommended TLV for occupational exposure. The classification of A4 indicates that methyl bromide is considered “not classifiable as a human carcinogen.” Data were insufficient to recommend a TLV-STEL for methyl bromide.
e Skin notation indicates the danger of cutaneous absorption.
f REL-TWA (recommended exposure limit-time weighted average, National Institute for Occupational Safety and Health). There is no REL-TWA for methyl bromide; NIOSH considers methyl bromide a potential occupational carcinogen, as defined by the OSHA carcinogen policy [29 CFR 1990 [1980].g PEL-TWA (Permissible Exposure Limits-Time Weighted Average, Occupational Safety and Health Administration) (NIOSH 2010 is defined analogous to the ACGIH TLV-TWA, but is for exposures of no more than 10 h/day, 40 h/week.
h PEL-C (permissible exposure limit-ceiling) (NIOSH 2010) is a value that must not be exceeded during any part of the workday. The PEL-TWA of 5 ppm established by OSHA in 1989 was vacated in 1993, and the ceiling limit of 20 ppm, with a skin notation, has been retained.
i MAK (maximale arbeitsplatzkonzentration [maximum workplace concentration]) (Deutsche Forschungsgemeinschaft [German Research Association] (DFG 1999) is analogous to the ACGIH TLV-TWA, but there is no current MAK value for methyl bromide; there is a skin notation and it is considered a Group IIIB substance (“justifiably suspected of having carcinogenic potential”) because of its potential carcinogenicity.
j MAC (maximaal aanvaarde concentratie [maximal accepted concentration]) (Dutch Expert Committee for Occupational Standards, The Netherlands (MSZW 2004) is defined analogous to the ACGIH TLV-TWA.
Abbreviations: NA, not appropriate; NR, not recommended.
8.3. Data Adequacy and Research Needs
Data on human exposures to known concentrations of methyl bromide are lacking. The database of animal studies is robust, containing information on multiple species (dog, rat, mouse, rabbit, and guinea pig) and involving acute and longer-term exposure studies that address lethal and sublethal effects, reproductive and developmental toxicity, genotoxicity, and chronic toxicity and carcinogenicity. Information on metabolism and mechanism of action are supported by studies, including clinical studies, with the related chemical, methyl chloride.
9. REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 2004. Methyl Bromide. Documentation of the Threshold Limit Values and Biological Exposure Indices, Supplement. American Conference of Governmental Industrial Hygienists, Cincinnati, OH.
AIHA (American Industrial Health Association). 1997. Emergency Response Planning Guidelines: Methyl Bromide. Fairfax, VA: AIHA Press.
Alavanja, M.C., C. Samanic, M. Dosemeci, J. Lubin, R. Tarone, C.F. Lynch, C. Knott, K. Thomas, J.A. Hoppin, J. Barker, J. Coble, D.P. Sandler, and A. Blair. 2003. Use of agricultural pesticides and prostate cancer risk in the Agricultural Health Study cohort. Am. J. Epidemiol. 157(9):800-814.
Alexeeff, G.V., and W.W. Kilgore. 1983. Methyl bromide. Residue Rev. 88:101-153.
Alexeeff, G.V., W.W. Kilgore, P. Munoz, and D. Watt. 1985. Determination of acute toxic effects in mice following exposure to methyl bromide. J. Toxicol. Environ.
Health 15(1):109-123.
Andersen, M.E., M.L. Gargas, R.A. Jones, and L.J. Jenkins, Jr. 1980. Determination of the kinetic constants for metabolism of inhaled toxicants in vivo using gas uptake measurements. Toxicol. Appl. Pharmacol. 54(1):100-116.
Andersen, M.E., H.J. Clewell, M.L. Gargas, F.A. Smith, and R.H. Reitz. 1987. Physiologically based pharmacokinetics and the risk assessment process for methylene chloride. Toxicol. Appl. Pharmacol. 87(2):185-205.
Anger, W.K., J.V. Setzer, J.M. Russo, W.S. Brightwell, R.G. Wait, and B.L. Johnson. 1981. Neurobehavioral effects of methyl bromide inhalation exposures. Scand. J. Work Environ. Health 7 (suppl. 4):40-47.
Anger, W.K., L. Moody, J. Burg, W.S. Brightwell, B.J. Taylor, J.M. Russo, N. Dickerson, J.V. Setzer, B.L. Johnson, and K. Hicks. 1986. Neurobehavioral evaluation of soil and structural fumigators using methyl bromide and sulfuryl fluoride. Neurotoxicology 7(3):137-156.
ATSDR (Agency for Toxic Substances and Disease Registry). 1992. Toxicological Profile for Bromomethane. Agency for Toxic Substances and Disease Registry, U.S. Public Health Service [online]. Available: http://www.atsdr.cdc.gov/toxprofiles/tp27.pdf [accessed Jan. 26, 2012].
Bakhishev, G.N. 1973. Relative toxicity of aliphatic halohydrocarbons to rats [in Russian]. Farmakol. Toksikol. 8:140-142.
Bakhishev, G.N. 1975. Relation between the chemical structure and toxicity of some halogenated aliphatic hydrocarbons [in Russian]. Fiziol. Akt. Vesh. 7:35-36.
Balander, P.A., and M.C. Polyak. 1962. Toxicological characteristics of methyl bromide. J. Gig. I. Toksikol. 60:412-419.
Bloom, J.C., and J.T. Brandt. 2001. Toxic responses of the blood. Pp. 389-418 in Casarett & Doull’s Toxicology: The Basic Science of Poisons, 6th Ed., C.D. Klaassen, ed. New York: McGraw-Hill.
Bond, J.A., J.S. Dutcher, M.A. Medinsky, R.F. Henderson, and L.S. Birnbaum. 1985. Disposition of [14C]methyl bromide in rats after inhalation. Toxicol. Appl. Pharmacol. 78(2):259-267.
Bonnefoi, M.S., C.J. Davenport, and K.T. Morgan. 1991. Metabolism and toxicity of methyl iodide in primary dissociated neural cell cultures. Neurotoxicology 12(1):33-46.
Boorman, G.A., H.L. Hong, C.W. Jameson, K. Yoshitomi, and R.R. Maronpot. 1986. Regression of methyl bromide induced forestomach lesions in the rat. Toxicol. Appl. Pharmacol. 86(1):131-139.
Braker, W., and A.L. Mossman. 1980. Matheson Gas Data Book, 6th Ed. Lyndhurst, NJ: Matheson.
Breslin, W.J., C.L. Zablotny, G.J. Bradley, and L.G. Lomax. 1990. Methyl Bromide Inhalation Teratology Study in New Zealand White Rabbits. K-000681-033. Study prepared by The Toxicology Research Laboratory, Dow Chemical Company, Midland, MI, for The Methyl Bromide Industry Panel, Chemical manufacturers Association, Washington, DC.
Bush, M.L., C.B. Frederick, J.S. Kimball, and J.S. Ultman. 1998. A CFD-PBPK hybrid model for simulating gas and vapor uptake in the rat nose. Toxicol. Appl. Pharmacol. 150(1):133-145.
Clarke, C.A., C.G. Roworth, and H.E. Holling. 1945. Methyl bromide poisoning: An account of four recent cases met with in one of H.M. ships. Br. J. Ind. Med. 2(1):17-23.
Danse, L.H., F.L. van Velsen, and C.A. van der Heijden. 1984. Methylbromide: Carcinogenic effects in the rat forestomach. Toxicol. Appl. Pharmacol. 72(2):262-271.
Davenport, C.J., S.F. Ali, F.J. Miller, G.W. Lipe, K.T. Morgan, and M.S. Bonnefoi. 1992. Effect of methyl bromide on regional brain glutathione, glutathione-S-transferase, monoamines, and amino acid in F344 rats. Toxicol. Appl. Pharmacol. 112(1):120-127.
Davis, L.N., J.F. Strange, J.E. Hoecker, P.H. Howard, and J. Santodonato. 1977. Investigation of Selected Potential Environmental Contaminants: Monohalomethanes. EPA-560/2-77-007. TR 77-535. NTIS PB 276 483. Prepared for Office of Toxic Substances, U.S. Environmental Protection Agency, Washington, DC.
Deschamps, F.J., and J.C Turpin. 1996. Methyl bromide intoxication during grain store fumigation. Occup. Med. 46(1):89-90.
DFG (Deutsche Forschungsgemeinschaft). 1999. List of MAK and BAT Values 1999. Maximum Concentrations and Biological Tolerance Values at the Workplace Report No. 35. Weinheim, Federal Republic of Germany: Wiley-VCH.
Driscoll, C.D., and J.M. Hurley. 1993. Methyl Bromide: Single Exposure Vapor Inhalation Neurotoxicity Study in Rats. Lab Project 92N1197. Prepared by Bushy Run Research Center, Export, PA, for The Methyl Bromide Industry Panel, Chemicals Manufacturers Association, Washington, DC.
Duafala, T., and M. Gillis. 1999. Properties, applications, and emissions of man-made methyl bromide. Pp. 191-202 in Reactive Halogen Compounds in the Atmosphere, P. Fabian, and O.N. Singh, eds. The Handbook of Environmental Chemistry Vol. 4, Part E. Berlin: Springer.
EPA (U.S. Environmental Protection Agency). 1980. Ambient Water Quality Criteria Document: Halomethanes. EPA 440/5-80-051. U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://water.epa.gov/scitech/swguidance/standards/criteria/upload/2001_10_12_criteria_ambientwqc_halomethanes80.pdf [accessed Jan. 31, 2012].
EPA (U.S. Environmental Protection Agency). 1992. Bromomethane (CAS Reg. No. 74-83-9). Integrated Risk Information System, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/iris/subst/0015.htm [accessed Jan. 31, 2012].
EPA (U.S. Environmental Protection Agency). 2011. Methyl Bromide Inventory. U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/ozone/mbr/otherreginfo.html [accessed Jan. 31, 2012].
Eustis, S.L., S.B. Haber, R.T. Drew, and R.S. Yang. 1988. Toxicology and pathology of methyl bromide in F344 rats and B6C3F1 mice following repeated inhalation exposure. Fundam. Appl. Toxicol. 11(5):594-610.
Frederick, C.B., M.L. Bush, L.G. Lomax, K.A. Black, L. Finch, J.S. Kimbell, K.T. Morgan, R.P. Subramaniam, J.B. Morris, and J.S. Ultman. 1998. Application of a hybrid computational fluid dynamics and physiologically based inhalation model for interspecies dosimetry extrapolation of acidic vapors in the upper airways. Toxicol. Appl. Pharmacol. 152(1):211-231.
Frederick, C.B., L.G. Lomax, K.A. Black, L. Finch, H.E. Scribner, J.S. Kimbell, K.T. Morgan, R.P. Subramaniam, and J.B. Morris. 2002. Use of a hybrid computational fluid dynamics and physiologically based inhalation model for interspecies dosimetry comparisons of ester vapors. Toxicol. Appl. Pharmacol. 183(1):23-40.
Fuortes, L.J. 1992. A case of fatal methyl bromide poisoning. Vet. Hum. Toxicol. 34(3):240-241.
Gargas, M.L., and M.E. Andersen. 1982. Metabolism of inhaled brominated hydrocarbons: Validation of gas uptake results by determination of a stable metabolite. Toxicol. Appl. Pharmacol. 66(1):55-68.
Garnier, R., M.O. Rambourg-Schepens, A. Muller, and E. Hallier. 1996. Glutathione transferase activity and formation of macromolecular adducts in two cases of acute methyl bromide poisoning. Occup. Environ. Med. 53(3):211-215.
Garry, V.F., R.L. Nelson, J. Griffith, and M. Harkins. 1990. Preparation for human study of pesticide applicators: Sister-chromatid exchanges and chromosome aberrations in cultured human lymphocytes exposed to selected fumigants. Teratog. Carcinog. Mutagen. 10(1):21-29.
Gosselin, R.E., R.P. Smith, and H.C. Hodge. 1984. Methyl bromide. Pp. II-280 to II-284 in Clinical Toxicology of Commercial Products, 5th Ed. Baltimore, MD: Williams & Wilkins.
Gotoh, K., T. Nishizawa, T. Yamaguchi, H. Kanou, T. Kasai, M. Ohasawa, H. Ohbayashi, S. Aiso, N. Ikawa, S. Yamamoto, T. Noguchi, K. Nagano, M. Enomoto, K. Nozaki, and H. Sakabe. 1994. Two-year toxicological and carcinogenesis studies
of methyl bromide in F344 rats and BDF1 mice-inhalation studies. Pp. 185-191 in Environmental and Occupational Chemical Hazards (2): Proceedings of the Second Asia-Pacific Symposium on Environmental and Occupational Health, K. Sumino, ed. Singapore, Kobe University School of Medicine/National University of Singapore (as cited in IARC 1999).
Griem, P., M. Hassauer, F. Kaberlah, J. Oltmanns, J. Scheibner, K. Schneider, and U. Schuhmacher-Wolz. 2002. Quantitative Differences in Xenobiotic Metabolism between Experimental Animals and Humans. Federal Institute for Occupational Safety and Health, Dortmund, Germany [online]. Available: http://www.baua.de/SharedDocs/Downloads/en/Publications/Research-reports/2002/Fb963.pdf?_bloob=publicationFile [accessed Jan. 27, 2012].
Hallier, E., S. Deutschmann, C. Reichel, H.M. Bolt, and H.A. Peter. 1990. A comparative investigation of the metabolism of methyl bromide and methyl iodide in human erythrocytes. Int. Arch. Occup. Environ. Health 62(3):221-225.
Hardin, B.D., G.P. Bond, M.R. Sikov, F.D. Andrew, R.P. Beliles, and R.W. Niemeier. 1981. Testing of selected workplace chemicals for teratogenic potential. Scand. J. Environ. Health 7(suppl. 4):66-75.
Hastings, L. 1990. Sensory neurotoxicology: Use of the olfactory system in the assessment of toxicity. Neurotoxicol. Teratol. 12(5):455-459.
Hezemans-Boer, M., J. Toonstra, J. Meulenbelt, J.H. Zwaveling, B. Sangster, and W.A. van Vloten. 1988. Skin lesions due to exposure to methyl bromide. Arch. Dermatol. 124(6):917-921.
Honma, T. 1987. Alteration of catecholamine metabolism in rat brain produced by inhalation exposure to methyl bromide. Sangyo Igaku 29(3):218-219.
Honma, T., M. Miyagawa, M. Sato, and H. Hasegawa. 1985. Neurotoxicity and metabolism of methyl bromide in rats. Toxicol. Appl. Pharmacol. 81(2):183-191.
Honma, T., M. Miyagawa, and M. Sato. 1987. Methyl bromide alters catecholamine and metabolite concentrations in rat brain. Neurotoxicol. Teratol. 9(5):369-375.
Honma, T., M. Miyagawa, and M. Sato. 1991. Inhibition of tyrosine hydroxylase activity by methyl bromide exposure. Neurotoxicol. Teratol. 13(1):1-4.
Hori, H., T. Hyakudo, T. Oyabu, S. Ishimatsu, H. Yamato, and I. Tanaka. 2002. Acute effects of methyl bromide gas in rats and mice-studies on survival time [in Japanese]. J. UOEH 24:71-75.
HSDB (Hazardous Substances Data Bank). 2010. Methyl Bromine (CASRN 74-83-9). TOXNET, Specialized Information Services, U.S. National Library of Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen? HSDB [accessed Jan. 27, 2012].
Hurtt, M.E., and P.K. Working. 1988. Evaluation of spermatogenesis and sperm quality in the rat following acute inhalation exposure to methyl bromide. Fundam. Appl. Toxicol. 10(3):490-498.
Hurtt, M.E., K.T. Morgan, and P.K. Working. 1987. Histopathology of acute toxic responses in selected tissues from rats exposed by inhalation to methyl bromide. Fundam. Appl. Toxicol. 9(2):352-365.
Hurtt, M.E., D.A. Thomas, P.K. Working, T.M. Monticello, and K.T. Morgan. 1988. Degeneration and regeneration of the olfactory epithelium following inhalation exposure to methyl bromide: Pathology, cell kinetics, and olfactory function. Toxicol. Appl. Pharmacol. 94(2):311-328.
Hustinx, W.N., R.T. van de Laar, A.C. van Huffelen, J.C. Verwey, J. Meulenbelt, and T.J. Savelkoul. 1993. Systemic effects of inhalational methyl bromide poisoning:
A study of nine cases occupationally exposed due to inadvertent spread during fumigation. Br. J. Ind. Med. 50(2):155-159.
IARC (International Agency for Research on Cancer). 1999. Methyl bromide. Pp. 721-736 in Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 71, Part 2. Lyon: IARC [online]. Available: http://monographs.iarc.fr/ENG/Monographs/vol71/mono71.pdf [accessed Jan. 27, 2012].
Ikeda, T., R. Kishi, K. Yamamura, H. Miyake, M. Sato, and S. Ishizu. 1980. Behavioural effects in rats following repeated exposure to methyl bromide. Toxicol. Lett. 6(4-5):293-299.
Ingram, F.R. 1951. Methyl bromide fumigation and control in the date-packing industry. Arch. Ind. Hyg. Occup. Med. 4(3):193-198.
Ioffe, D., and A. Kampf. 2002. Bromine, Organic Compounds. Kirk-Othmer Encyclopedia of Chemical Technology. Indianapolis, IN: John Wiley & Sons.
IPCS (International Programme on Chemical Safety). 1995. Methyl Bromide, Environmental Health Criteria 166. Geneva, Switzerland: World Health Organization [online]. Available: http://www.inchem.org/documents/ehc/ehc/ehc166.htm [accessed Jan. 30, 2012].
Irish, D.D., E.M. Adams, H.C. Spencer, and V.K. Rowe. 1940. The response attending exposure of laboratory animals to vapors of methyl bromide. J. Ind. Hyg. Toxicol. 22(6):218-230.
Izmerov, N.F., I.V. Sanotsky, and K.K. Siderov. 1982. Toxicometric Parameters of Industrial Toxic Chemicals under Single Exposure. Moscow: United Nations Environment Programme, Centre of International Projects, GKNT.
Jager, R., H. Peter, W. Sterzel, and H.M. Bolt. 1988. Biochemical effects of methyl chloride in relation to its tumorigenicity. J. Cancer Res. Clin. Oncol. 114(1):64-70.
Japanese Ministry of Labour. 1992. Toxicology and Carcinogenesis Studies of Methyl Bromide in F344 Rats and BDF Mice (Inhalation Studies). Industrial Safety and Health Association, Japanese Bioassay Laboratory, Tokyo. 197pp (as cited in IPCS 1995).
Jaskot, R.H., E.C. Grose, B.M. Most, M.G. Menache, T.B. Williams, and J.J. Roycroft. 1988. The distribution and toxicological effects of inhaled methyl bromide in the rat. J. Am. Coll. Toxicol. 7(5):631-642.
Johnson, J.A., A. el Barbary, S.E. Kornguth, J.F. Brugge, and F.L. Siegel. 1993. Glutathione S-transferase isoenzymes in rat brain neurons and glia. J. Neurosci. 13(5):2013-2023.
Johnstone, TR. 1945. Methyl bromide intoxication of a large group of workers. Ind. Med. 14(6):495-497.
Kato, N., S. Morinobu, and S. Ishizu. 1986. Subacute inhalation experiment for methyl bromide in rats. Ind. Health 24(2):87-103.
Kawai, M., and K. Ueda. 1972. Effect of glutathione on acute intoxication due to methyl bromide [in Japanese]. Nippon Noson Igakkai Zasshi 21(2):314-315.
Kempkes, M., F.A. Wiebel, K. Golka, P. Heitmann, and H.M. Bolt. 1996. Comparative genotyping and phenotyping of glutathione S-transferase GSTT 1. Arch. Toxicol. 70(5):306-309.
Kornbrust, D.J., and J.S. Bus. 1983. The role of glutathione and cytochrome P-450 in the metabolism of methyl chloride. Toxicol. Appl. Pharmacol. 67(2):246-256.
Landry, T.D., T.S. Gushow, P.W. Langvardt, J.M. Wall, and M.J. McKenna. 1983. Pharmacokinetics and metabolism of inhaled methyl chloride in the rat and dog. Toxicol. Appl. Pharmacol. 68(3):473-486.
Langard, S., T. Rognum, O. Flotterod, and V. Skaug. 1996. Fatal accident resulting from methyl bromide poisoning after fumigation of a neighbouring house; leakage through sewage pipes. J. Appl. Toxicol. 16(5):445-448.
Lof, A., G. Johanson, A. Rannug, and M. Warholm. 2000. Glutathione transferase T1 phenotype affects the toxicokinetics of inhaled methyl chloride in human volunteers. Pharmacogenetics 10(7):645-653.
Lu, F.C., and F. Coulston. 1996. Safety assessment based on irrelevant toxicologic data: An extraordinary case of bromomethane use as a fumigant. Ecotoxicol. Environ. Saf. 33(1):100-101.
Marraccini, J.V., G.E. Thomas, J.P. Ongley, C.D. Pfaffenberger, J.H. Davis, and L.R. Bednarczyk. 1983. Death and injury caused by methyl bromide, an insecticide fumigant. J. Forensic Sci. 28(3):601-607.
Mayhew, D.A. 1986. Two-Generation Reproduction Study via Inhalation in Albino Rats using Methyl Bromide (Final Report). Report 450-1525. Decatur, IL: American Biogenics Corporation.
Medinsky, M.A., J.S. Dutcher, J.A. Bond, R.F. Henderson, J.L. Mauderly, M.B. Snipes, J.A. Mewhinney, Y.S. Cheng, and L.S. Birnbaum. 1985. Uptake and excretion of [14C]methyl bromide as influenced by exposure concentration. Toxicol. Appl. Pharmacol. 78(2):215-225.
Morgan, K.T., J.A. Swenberg, T.E. Hamm, Jr., R. Wolkowski-Tyl, and M. Phelps. 1982. Histopathology of acute toxic response in rats and mice exposed to methyl chloride by inhalation. Fundam. Appl. Toxicol. 2(6):293-299.
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: Broommethaan. Den Haag: SDU Uitgevers [online]. Available: http://www.lasrook.net/lasrookNL/maclijst2004.htm [accessed Jan. 30, 2012].
Nelson, H.H., J.K. Wiencke, D.C. Christiani, T.J. Chang, Z.F. Zuo, B.S. Schwartz, B.K. Lee, M.R. Spitz, M. Wang, X. Xu, and K.T. Kelsey. 1995. Ethnic differences in the prevalence of the homozygous deleted genotype of glutathione S-transferase theta. Carcinogenesis 16(5):1243-1245.
Newton, P.E. 1994a. An Up-and-Down Acute Inhalation Toxicity Study of Methyl Bromide in the Dog. Study No. 93-6067. Pharmaco LSR, East Millstone, NJ.
Newton, P.E. 1994b. A Four Week Inhalation Toxicity Study of Methyl Bromide in the Dog. Study No. 93-6068. Pharmaco LSR, East Millstone, NJ.
NIOSH (National Institute for Occupational Safety and Health). 1984. Monohalomethanes: Methyl Chloride CH3Cl, Methyl Bromide CH3Br, Methyl Iodide CH3I. Current Intelligence Bulletin 43, September 27, 1984 [online]. Available: http://www.cdc.gov/noish/84117_43.html [accessed Jan. 30, 2012].
NIOSH (National Institute for Occupational Safety and Health). 1994. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs): Methyl bromide. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH [online]. Available: http://www.cdc.gov/niosh/idlh/74839.html [accessed Jan. 30, 2012].
NIOSH (National Institute for Occupational Safety and Health). 2010. NIOSH Pocket Guide to Chemical Hazards: Methyl Bromide. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH [online]. Available: http://ww.cdc.gov/niosh/npg/npgd0400.html [accessed Jan. 30, 2012].
Nolan, R.J., D.L. Rick, T.D. Landry, L.P. McCarty, G.L. Agin, and J.H. Saunders. 1985. Pharmacokinetics of inhaled methyl chloride (CH3Cl) in male volunteers. Fundam. Appl. Toxicol. 5(2):361-369.
Norris, J.C., C.D. Driscoll, and J.M. Hurley. 1993. Methyl Bromide: Ninety-day Vapor Inhalation Neurotoxicity Study in CD rats. Project No. 92N1172. Bushy Run Research Center, Export, PA.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NTP (National Toxicology Program). 1992. Toxicology and Carcinogenesis Studies of Methyl Bromide (CAS Reg. No. 74-83-9) in B6C3F1 Mice (Inhalation Studies). Technical Report No. 385. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, Research Triangle Park, NC [online]. Available: http://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr385.pdf [accessed Jan. 30, 2012].
OECD SIDS (Organization for Economic Co-operation and Development, Screening Information Data Set). 2002. Methyl Bromide (CAS No. 74-83-9). SIDS Initial Assessment Report. United Nations Environment Programme [online]. Available: http://www.chem.unep.ch/irptc/sids/OECDSIDS/Methyl_bromide.pdf [accessed Jan. 31, 2012].
O’Neil, M.J., A. Smith, and P.E. Heckelman, eds. 2001. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th Ed. Whitehouse Station, NJ: Merck.
Parkinson, A. 2001. Biotransformation of xenobiotics. Pp. 133-224 in Casarett & Doull’s Toxicology, The Basic Science of Poisons, 6th Ed., C. Klaassen, ed. New York: McGraw Hill.
Peter, H., S. Deutschmann, C. Reichel, and E. Hallier. 1989. Metabolism of methyl chloride by human erythrocytes. Arch. Toxicol. 63(5):351-355.
Raabe, O.G. 1986. Inhalation Uptake of Selected Chemical Vapors at Trace Levels. UCD-472-507. A3-132-33. Laboratory for Energy-Related Health Research, University of California, Davis, CA. Submitted to the Biological Effects Research Section, California Air Resources Board, Sacramento, CA [online]. Available: http://www.arb.ca.gov/research/apr/past/a3-132-33.pdf [accessed Jan. 30, 2012].
Raabe, O.G. 1988. Inhalation Uptake of Xenobiotic Vapors by People: Final Report. UCD-472-509, A5-155-33. Laboratory for Energy-Related Health Research, University of California, Davis, CA. Submitted to the Biological Effects Research Section, California Air Resources Board, Sacramento, CA[online]. Available: http://www.arb.ca.gov/research/apr/past/a5-155-33.pdf [accessed Jan. 30, 2012].
Redford-Ellis, M., and A.H. Gowenlock. 1971. Studies on the reaction of chloromethane with human blood. Acta Pharmacol. Toxicol. 30(1):36-48.
Reed, C.J., B.A. Gaskell, K.K. Banger, and E.A. Lock. 1995. Olfactory toxicity of methyl iodide in the rat. Arch. Toxicol. 70(1):51-56.
Reid, J.B. 2001. Saturated methyl halogenated aliphatic hydrocarbons. Pp. 12-26 in Patty’s Toxicology, 5th Ed., Vol. 5. New York: John Wiley & Sons.
Reigart, J.R., and J.R. Roberts. 1999. Recognition and Management of Pesticide Poisonings, 5th Ed. EPA 735-R-98-003. U.S. Environmental Protection Agency, Washington,
DC [online]. Available: http://www.epa.gov/oppfead1/safety/healthcare/handbook/handbook.pdf [accessed Jan. 30, 2012].
Reitz, R.H., A.L. Mendrala, and F.P. Guengerich. 1989. In vitro metabolism of methylene chloride in human and animal tissues: Use in physiologically based pharmacokinetic models. Toxicol. Appl. Pharmacol. 97(2):230-246.
Reuzel, P.G.J., C.F. Kuper, H.C. Dreef-van der Meulen, and V.M.H. Hollanders. 1987. Chronic (29-month) Inhalation Toxicity and Carcinogenicity Study of Methyl Bromide in Rats. Report No. V86.469/221044. Zeist, The Netherlands: TNOCIVO Toxicology and Nutrition Institute.
Reuzel, P.G.J., H.C. Dreef-van der Meulen, V.M.H. Hollanders, C.F. Kuper, V.J. Feron, and C.A. van der Heijden. 1991. Chronic inhalation toxicity and carcinogenicity study of methyl bromide in Wistar rats. Food Chem. Toxicol. 29(1):31-39.
Roycroft, J.H., R.H. Jascot, E.C. Grose, and D.E. Gardner. 1981. The effects of inhalation exposure of methyl bromide in the rat. Toxicologist 1(1):79[Abstract 285].
Russo, J.M., W.K. Anger, J.V. Setzer, and W.S. Brightwell. 1984. Neurobehavioral assessment of chronic low-level methyl bromide exposure in the rabbit. J. Toxicol. Environ. Health 14(2-3):247-255.
Ruth, J.H. 1986. Odor thresholds and irritation levels of several chemical substances: A review. Am. Ind. Hyg. Assoc. J. 47(3):A142-A151.
Sayers, R.R., W.P. Yant, B.G.H. Thomas, and L.B. Berger. 1929. Physiological Response Attending Exposure to Vapors of Methyl Bromide, Methyl Chloride, Ethyl Bromide, and Ethyl Chloride. U.S. Public Health Service Public Health Bulletin 185. Washington, DC: U.S. Government Printing Office.
Schaefer, G.J. 2002. A 6-Week Inhalation Toxicity Study of Methyl Bromide in Dogs. Report WIL-440001, WIL Research Laboratories, Inc., Ashland, OH.
Schwob, J.E., S.L. Youngentob, G. Ring, C.L. Iwema, and R.C. Mezza. 1999. Reinnervation of the rat olfactory bulb after methyl bromide-induced lesion: Timing and extent of reinnervation. J. Comp. Neurol. 412(3):439-457.
Sikov, M.R., W.C. Cannon, D.B. Carr, R.A. Miller, L.F. Montgomery, and D.W. Phelps. 1981. Teratologic Assessment of Butylene Oxide, Styrene Oxide and Methyl Bromide. DHHS Publication (NIOSH) 81-124. PB 81-168-510. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, Cincinnati, OH.
Sittig, M. 1985. Pp. 587-588 in Handbook of Toxic and Hazardous Chemicals and Carcinogens, 2nd Ed. Park Ridge, NJ: Noyes Publications.Thomas, D.A., and K.T. Morgan. 1988. Olfactory toxicity: Studies of methyl bromide. CIIT Activities 8(1):3-7.
Thomas, D.A., S.A. Lacy, and K.T. Morgan. 1989. Studies on the mechanism s of methyl bromide induced olfactory toxicity. Toxicologist 9:37.
Tourangeau, F.J., and S.R. Plamondon. 1945. Cases of exposure to methyl bromide vapours. Can. J. Pub. Health 36:362-367.
Tucker, J.D., J. Xu, J. Stewart, P.C. Baciu, and T.M. Ong. 1986. Detection of sister chromatid exchanges induced by volatile genotoxicants. Teratog. Carcinog. Mutagen. 6(1):15-21.
Van den Oever, R., D. Roosels, and D. Lahaye. 1982. Actual hazard of methyl bromide fumigation in soil disinfection. Br. J. Ind. Med. 39(2):140-144.
Verberk, M.M., T. Rooyakkers-Beemster, M. de Vlieger, and A.G. van Liet. 1979. Bromine in blood, EEG and transaminases in methyl bromide workers. Br. J. Ind. Med. 36(1):59-62.
Warholm, M., A.K. Alexandrie, J. Hogberg, K. Sigvardsson, and K. Rannug. 1994. Polymorphic distribution of glutathione transferase activity with methyl chloride in human blood. Pharmacogenetics 4(6):307-311.
Watrous, R.M. 1942. Methyl bromide-local and mild systemic toxic effects. Ind. Med. 11:575-578.
WHO (World Health Organization). 2000. Methyl Chloride. Concise International Chemical Assessment Document No. 28. Geneva, Switzerland: World Health Organization [online]. Available: http://whqlibdoc.who.int/publications/2000/9241530286.pdf [accessed Jan. 31, 2012].
Yamano, Y. 1991. Experimental study on methyl bromide poisoning in mice. Acute inhalation study and the effect of glutathione as an antidote [in Japanese]. Sangyo Igaku 33(1):23-30.
Yang, R.S., K.L. Witt, C.J. Alden, and L.G. Cockerham. 1995. Toxicology of methyl bromide. Rev. Environ. Contam. Toxicol. 142:65-85.
Zwart, A. 1988. Acute Inhalation Study of Methyl Bromide in Rats. CIVO Report No. V88. 127/27, CIVO Institutes, TNO, Zeist, The Netherlands. 17 pp (as cited in IPCS 1995).
Zwart, A., J.H. Arts, W.F. ten Berge, and L.M. Appelman. 1992. Alternative acute inhalation toxicity testing by determination of the concentration-time-mortality relationship: Experimental comparison with standard LC50 testing. Regul. Toxicol. Pharmacol. 15(3):278-290.
Zwaveling, J.H., W.L. de Kort, J. Meulenbelt, M. Hezemans-Boer, W.A. van Vloten, and B. Sangster. 1987. Exposure of the skin to methyl bromide: A study of six cases occupationally exposed to high concentrations during fumigation. Hum. Toxicol. 6(6):491-495.
APPENDIX A
TIME-SCALING CALCULATION FOR METHYL BROMIDE
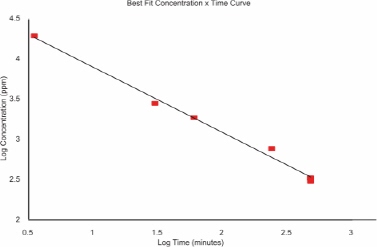
FIGURE A-1 Regression line of LC50 data in rats. Source: Data from Bakhishev 1973; Honma et al. 1985; Kato et al. 1986; Zwart 1988).
Data:
|
|
|||
| Time (min) | Concentration (ppm) | Log time | Log concentration |
|
|
|||
| 3.5 | 19.460 | 0.5441 | 4.2891 |
| 30 | 2,830 | 1.4771 | 3.4518 |
| 60 | 1,8S0 | 1.7782 | 3.2742 |
| 240 | 780 | 2.3802 | 2.8921 |
| 480 | 302 | 2.6812 | 2.48O0 |
| 480 | 334 | 2.6812 | 2.5237 |
|
|
|||
Regression Output:
|
|
|
| Intercept | 4.7129 |
| Slope | -0.8115 |
| R Squared | 0.9914 |
| Correlation | -0.9957 |
| Degrees of Freedom | 4 |
| Observations | 6 |
|
|
|
n = 1.23
k = 642,119
APPENDIX B
DERIVATION OF AEGL VALUES FOR METHYL BROMIDE
Derivation of AEGL-1 Values
AEGL-1 values are not recommended because toxic effects might occur below the odor threshold for methyl bromide. Absence of AEGL-1 values does not imply that exposures below the AEGL-2 values are without adverse effects.
Derivation of AEGL-2 Values
| Key studies: | Hurtt, M.E., D.A. Thomas, P.K. Working, T.M. Monticello, and K.T. Morgan. 1988. Degeneration and regeneration of the olfactory epithelium following inhalation exposure to methyl bromide: pathology, cell kinetics, and olfactory function. Toxicol. Appl. Pharmacol. 94(2):311-328. |
| Hastings, L. 1990. Sensory neurotoxicology: Use of the olfactory system in the assessment of toxicity. Neurotoxicol. Teratol. 12(5):455-459. | |
| Japanese Ministry of Labour. 1992. Toxicology and Carcinogenesis Studies of Methyl Bromide in F344 Rat and BDF Mice (Inhalation Studies). Industrial Safety and Health Association, Japanese Bioassay Laboratory, Tokyo (as cited in IPCS 1995). | |
| Newton, P.E. 1994a. An Up-and-Down Acute Inhalation Toxicity Study of Methyl Bromide in the Dog. Study No. 93-6067. Pharmaco LSR, East Millstone, NJ. | |
| Toxicity end points: | Clinical signs of neurotoxicity, NOAEL is 200 ppm for 4 h |
| Time scaling: | C1.2 × t = k, based on rat lethality data k = (200 ppm ÷ 3)1.2 × 240 min k = 37,059.7 ppm-min |
| Uncertainty factors: | 1 for interspecies differences 3 for intraspecies differences |
| Modifying factor: | Not applied |
| Calculations: | |
| 10-min AEGL-2: | C1.2 = 37,059.7 ppm-min ÷ 10 min C = 940 ppm |
| 30-min AEGL-2: | C1.2 = 37,059.7 ppm-min ÷ 30 min C = 380 ppm |
| 1-h AEGL-2: | C1.2 = 37,059.7 ppm-min ÷ 60 min C = 210 ppm |
| 4-h AEGL-2: | C1.2 = 37,059.7 ppm-min ÷ 240 min C = 67 ppm |
| 8-h AEGL-2: | Set equal to the 4-h AEGL-2 of 67 ppm (If based on a chronic NOAEL of 33 ppm for mice (NTP 1992), a 4-day NOAEL of 55 ppm for clinical signs and tissue lesions in dogs (Newton 1994a), and the 36-week NOAEL of 55 ppm for neurobehavioral parameters and nerve conduction velocity in rats (Anger et al. 1981), the time-scaled value would be 37 ppm.) |
Derivation of AEGL-3 Values
| Key study: | Kato, N., S. Morinobu, and S. Ishizu. 1986. Subacute inhalation experiment for methyl bromide in rats. Ind. Health 24(2):87-103. |
| Toxicity end point: | BMCL05 of 701 ppm in the rat |
| Time scaling: | C1.2 × t = k, based on rat lethality data. k = (701 ppm ÷ 3)1.2 × 240 min k = 166,927.3 ppm-min |
| Uncertainty factors: | 1 for interspecies differences 3 for intraspecies variability |
| Modifying factor: | Not applied |
| Calculations: | |
| 10-min AEGL-3: | C1.2 = 166,927.3 ppm-min ÷ 10 min C = 3,300 ppm |
| 30-min AEGL-3: | C = 166,927.3 ppm-min ÷ 30 min C = 1,300 ppm |
| 1-h AEGL-3 | C = 166,927.3 ppm-min ÷ 60 min C = 740 ppm |
| 4-h AEGL-3: | C = 166,927.3 ppm-min ÷ 240 min C = 230 ppm |
| 8-h AEGL-3: | C = 166,927.3 ppm-min ÷ 480 min C = 130 ppm |
APPENDIX C
CATEGORY GRAPH OF TOXICITY DATA AND
AEGL VALUES FOR METHYL BROMIDE
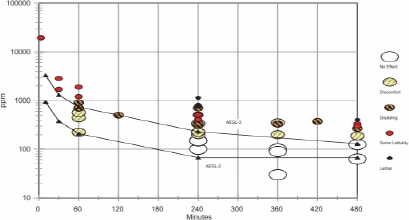
FIGURE C-1 Category graph of toxicity data on methyl bromide compared with AEGL values. All of the toxicity data pertain to laboratory animals; no clinical data were available.
APPENDIX D
ACUTE EXPOSURE GUIDELINE LEVELS FOR METHYL BROMIDE
Derivation Summary for Methyl Bromide
AEGL-1 VALUES
AEGL-1 values are not recommended because toxic effects might occur below the odor threshold for methyl bromide. Absence of AEGL-1 values does not imply that exposures below the AEGL-2 values are without adverse effects.
| AEGL-2 VALUES | ||||
|
|
||||
| 10 min | 30 min | 1h | 4h | 8h |
|
|
||||
| 940 ppm | 380 ppm | 210 ppm | 67 ppm | 67 ppm |
|
|
||||
Key references:
(1) Hurtt, M.E., D.A. Thomas, P.K. Working, T.M. Monticello, and K.T. Morgan. 1988. Degeneration and regeneration of the olfactory epithelium following inhalation exposure to methyl bromide: Pathology, cell kinetics, and olfactory function. Toxicol. Appl. Pharmacol. 94(2):311-328.
(2) Hastings, L. 1990. Sensory neurotoxicology: Use of the olfactory system in the assessment of toxicity. Neurotoxicol. Teratol. 12(5):455-459.
(3) Japanese Ministry of Labour. 1992. Toxicology and Carcinogenesis Studies of Methyl Bromide in F344 Rat and BDF Mice (Inhalation Studies). Industrial Safety and Health Association, Japanese Bioassay Laboratory, Tokyo (as cited in IPCS 1995).
(4) Newton, P.E. 1994a. An Up-and-Down Acute Inhalation Toxicity Study of Methyl Bromide in the Dog. Study No. 93-6067. Pharmaco LSR, East Millstone, NJ.
Test species/Strain/Number: (1) dog/beagle/1 (with support from higher and lower exposures; (2) rat/not specified/30; (3) rat/F-344/10 male and 10 female; and (4) rat/male F-344/15
Exposure route/Concentrations/Durations: Inhalation, (1) 233 ppm for 5 h; (2) 200 ppm for 4 h; (3) 225 ppm for 4 h; and (4) 200 ppm for 6 h
Effects:
(1) No toxic signs or brain lesions; (2) no clinical signs; (3) reversible metaplasia of the olfactory epithelium; and (4) no clinical signs, reversible olfactory epithelium degeneration.
End point/Concentration/Rationale: Threshold for neurotoxic signs is 200 ppm for 4 h. Neurotoxicity (e.g., ataxia) would inhibit the ability to escape. Olfactory lesions were considered specific to the rat.
|
|
||||
| 10 min | 30 min | 1h | 4h | 8h |
|
|
||||
| 940 ppm | 380 ppm | 210 ppm | 61 ppm | 61 ppm |
|
|
||||
Uncertainty factors/Rationale:
Total uncertainty factor: 3
Interspecies: 1, based on studies with methyl chloride, uptake is greater in rodents than in humans; GST concentrations are greater in rodents than humans, resulting in faster production of toxic metabolites.
Intraspecies: 3, differences in metabolism among humans are no greater than 3-fold (Nolan et al. 1985).
Modifying factor: Not applied
Animal-to-human dosimetric adjustment: Not applied
Time scaling: C1.2 × t = k, based on lethality studies with the rat.
Data adequacy: Although there are no controlled clinical studies, the database of experimental animal studies is robust. Studies of dogs exposed to methyl bromide at 156 ppm (no clinical signs for first 2 days) or 268 ppm (severe signs during first day) indicate that 200 ppm for 4 h would be near the threshold for neurotoxicity in dogs (Newton 1994a).
AEGL-3 VALUES |
||||
|
|
||||
| 10 min | 30 min | 1h | 4h | 8h |
|
|
||||
| 3,300 ppm | 1,300 ppm | 740 ppm | 230 ppm | 130 ppm |
|
|
||||
Key Reference: Kato, N., S. Morinobu, and S. Ishizu. 1986. Subacute inhalation experiment for methyl bromide in rats. Ind. Health 24(2):87-103.
Test species/Strain/Number: Male rat/Sprague-Dawley/5 or 10
Exposure route/Concentrations/Durations: Inhalation at 502, 622, 667, 701, 767, 808, 832, or 896 ppm for 4 h
|
|
||||
| 10 min | 30 min | 1h | 4h | 8h |
|
|
||||
| 3,300 ppm | 1,300 ppm | 740 ppm | 230 ppm | 130 ppm |
|
|
||||
End point/Concentration/Rationale: BMCL05, 701 ppm, threshold for lethality
Uncertainty factors/Rationale:
Total uncertainty factor: 3
Interspecies: 1, based on studies with methyl chloride, uptake is greater in rodents than in humans; GST concentrations are greater in rodents than humans, resulting in faster production of toxic metabolites.
Intraspecies: 3, differences in metabolism among humans are no greater than 3-fold (Nolan et al. 1985). Use of an intraspecies uncertainty factor of 3 is supported by the steep dose-response curve for lethality which indicates that there might be little intraspecies variation (see Chapter 6). Furthermore, larger reduction of the AEGL-3 values would result in values near the AEGL-2 values.
Modifying factor: Not applied
Animal-to-human dosimetric adjustment: Not applied
Time scaling: C1.2 × t = k, based on several lethality studies with the rat
Data adequacy: Although reliable human data are lacking, the database of animal studies is robust, consisting of acute, repeat-exposure, subchronic, chronic, neurotoxicity, genotoxicity, and carcinogenicity studies.






























































