6
Methyl Chloride1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory
![]()
1 This document was prepared by the AEGL Development Team composed of Sylvia Talmage (Summitec Corporation), Julie M. Klotzbach (Syracuse Research Corporation), Chemical Manager George Rodgers (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances), and Ernest V. Falke (U.S. Environmental Protection Agency). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold concentrations for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Methyl chloride is a substantially odorless, colorless gas with moderate flammability and explosiveness. Most methyl chloride produced today is used as a chemical intermediate in the production of silicones, agricultural chemicals, methyl cellulose, quaternary amines, butyl rubber, and tetraethyl lead. Previous use in refrigeration systems led to accidental exposures and, in some cases, deaths. In the late 1880s, methyl chloride had limited use as a general and local anesthetic. Data on toxicity to humans were available from accidental exposures, occupational exposures, and clinical studies. Animal studies, primarily with the rat and mouse, generally used a repeat-exposure scenario. Data were available on lethal and sublethal concentrations, neurotoxicity, developmental and reproductive effects, genotoxicity, and carcinogenicity. Metabolism is rapid. The human and animal studies document the central nervous system as the target of acute and chronic exposures. In animal studies, other organs such as the kidneys and testes have been affected by repeat exposures.
Clinical studies show that single exposures of healthy adults to methyl chloride at 200 ppm for 3 or 3.5 h (Putz-Anderson et al. 1981a,b) and a two-day repeat exposure of exercising adults exposed at 150 ppm for 7.5 h/day (Stewart et al. 1980) are without adverse neurotoxic effects. The subjects included both “fast” and “slow” metabolizers of methyl chloride. These exposures failed to elicit physiologic, neurologic, behavioral, or clinical symptoms. Furthermore, in
the absence of a clearly defined odor at these concentrations, the subjects were unable to differentiate between control and exposure days. None of the exposures produced mild, transient effects that define the AEGL-1 values. Because methyl chloride has no clearly defined odor or warning properties at concentrations that might be neurotoxic, an AEGL-1 is not recommended.
The AEGL-2 values were based on several studies with rats; a monitoring study was used as support. The basis for the AEGL-2 values was the absence of clinical signs in rats exposed at 1,500 ppm for 6 h/day for 1 day (Dodd et al. 1982) or 90 days (Mitchell et al. 1979). Because of the greater blood uptake of chemicals by rodents than humans (Landry et al. 1981, 1983; Nolan et al. 1985), an interspecies uncertainty factor of 1 was applied. Although humans differ in the rate at which they metabolize methyl chloride, the difference does not appear to be toxicologically significant (Nolan et al. 1985). Because of differences in uptake and metabolism among the human population, an intraspecies uncertainty factor of 3 was considered sufficient. Time scaling was performed using the equation Cn × t = k, using the default values of n = 3 for shorter durations and n = 1 for longer durations. Because of the long exposure duration of the key study, the 10-min value was set equal to the 30-min value. In a monitoring study, accidental exposures at 1,000-2,000 ppm and repeated exposure at 2,000-4,000 ppm resulted in transient symptoms of blurred vision, dizziness, headache, and nausea in workers (MacDonald 1964). Exposure durations were not reported, but appeared to be throughout the workday. Application of an intraspecies uncertainty factor of 3 to 1,500 ppm, the mean concentration of methyl chloride in the occupational monitoring studies, results in 500 ppm, a value similar to the 4-and 8-h AEGL-2 values.
The only lethality data were 50% lethality (LC50) values for the mouse, a particularly sensitive species. Two studies reported no deaths in rats during the first 4 days of exposures to methyl chloride at 5,000 ppm for 6 h/day (Morgan et al. 1982; Chellman et al. 1986a). A single 6-h exposure at 5,000 ppm was selected as the point-of-departure for the threshold for lethality. Interspecies and intraspecies uncertainty factors of 1 and 3, respectively, were applied as was done in the calculation for AEGL-2 values. Time scaling was performed using the equation Cn × t = k, using n = 3 for shorter durations and n = 1 for longer durations. Because of the long exposure duration of the key study, the 10-min AEGL-3 was set equal to the 30-min value.
The AEGL values for methyl chloride are presented in the Table 6-1.
1. INTRODUCTION
Methyl chloride is a substantially odorless, colorless gas with moderate flammability and explosiveness. Additional chemical and physical properties are listed in Table 6-2. At “high concentrations” it has a mild ethereal odor and sweet taste. Methyl chloride is ubiquitous in the environment because it is produced by wood burning and is released by natural organic processes, such as
microbial fermentation. Most industrially-produced methyl chloride is used as a chemical intermediate. The primary use is in the manufacture of silicones (72%); other products in which it is used as an intermediate include agricultural chemicals, methyl cellulose, quaternary amines, butyl rubber, and tetraethyl lead. Previously, it was used as a refrigerant and as an agricultural pesticide or fumigant (ATSDR 1998; O’Neil et al. 2001; Reid 2001). It had limited use as a general and local anesthetic in the late 1800s. It comprised 16% of the anesthetic “Somnoform” (Henderson 1930). Skin contact with the liquid may cause frostbite (DOT 1985).
Major production methods of methyl chloride involve the reaction of methanol and hydrogen chloride or the chlorination of methane (Holbrook 1992). Production in the United States was 920 million pounds in 1994 (CMR 1995). Methyl chloride (99.5-99.9% purity) is marketed as a liquefied gas under pressure (WHO 2000).
2. HUMAN TOXICITY DATA
The most important route of exposure to methyl chloride in humans is via the respiratory tract. Reported human exposures have primarily been the result of its use as a refrigerant gas and as a blowing agent for plastic foams. Early published reports of acute intoxications involved leaks in domestic refrigerators and overexposures of industrial workers. Uses as a refrigeration gas and as a blowing agent for plastic foams have been discontinued.
TABLE 6-1 Summary of AEGL Values for Methyl Chloride
|
|
||||||
| Classification | 10 min | 30 min | 1h | 4h | 8h | End Point (Reference) |
|
|
||||||
| AEGL-1 (nondisabling) | NRa | NRa | NRa | NRa | NRa | |
| AEGL-2 (disabling) | 1,100 ppm (2,277 mg/m3) |
1,100 ppm (2,277 mg/m3) |
910 ppm (1,884 mg/m3) |
570 ppm (1,180 mg/m3) |
380 ppm (787 mg/m3) |
NOAEL for clinical signs, tissue lesions in rats (Mitchell et al. 1979; Dodd et al 1982) |
| AEGL-3 (lethal) | 3,800 ppm (7,866mg/m3) |
3,800 ppm (7,866 mg/m3) |
3,000 ppm (6,210 mg/m3) |
1,900 ppm (3,933 mg/m3) |
1,300 ppm (2,691 mg/m3) |
Threshold for lethality in rats (Morgan et al. 1982; Chellman et al. 1986a)s |
|
|
||||||
a AEGL-1 values are not recommended because methyl chloride has no odor or warning properties at concentrations that may be neurotoxic.
Abbreviations: NR, not recommended; NOAEL, no-observed-adverse-effect level.
The central nervous system (CNS) is the primary target of methyl chloride, with behavioral symptoms and neurologic effects resulting from both acute and chronic exposures. Overexposures can result in loss of equilibrium, dizziness, semiconsciousness, and delayed death. Case histories show that acute exposures at high concentrations and chronic exposures to moderately high concentrations result in degeneration of portions of the CNS. Symptoms include headache, confusion, ataxia, muscle weakness, and tremor. Gastrointestinal disturbances may also occur, but there is no effect on pulmonary function. Recovery may be protracted. Renal, hepatic, cardiovascular, gastrointestinal, and other complications also have been documented (Repko and Lasley 1979). Data on the toxicity of methyl chloride have been reviewed by the Agency for Toxic Substances and Disease Registry (ATSDR 1998), the International Agency for the Research on Cancer (IARC 1999), the World Health Organization (WHO 2000), and the Hazardous Substances Databank (HSDB 2005).
Although mortalities have been reported as a result of accidental overexposure to methyl chloride, no information was available on measured concentrations.
2.2. Nonlethal Toxicity
Methyl chloride is considered nonirritating to the eyes, nose, and throat; however, the liquid can cause frostbite (DOT 1985).
TABLE 6-2 Chemical and Physical Properties of Methyl Chloride
|
|
||
| Parameter | Value | Reference |
|
|
||
| Synonyms | Chloromethane, monochloromethane | O’Neil et al. 2001 |
| CAS registry no. | 74-87-3 | O’Neil et al. 2001 |
| Chemical formula | CH3Cl | O’Neil et al. 2001 |
| Molecular weight | 50.49 | O’Neil et al. 2001 |
| Physical state | Colorless gas | O’Neil et al. 2001 |
| Melting point | -97.7°C | O’Neil et al. 2001 |
| Boiling point | -23.7°C | O’Neil et al. 2001 |
| Density | Holbrook 1992 | |
| Vapor | 2.3 g/L at 0°C, 1 atm (air = 1) | |
| Liquid | 0.9 g/mL at 20/4°C (water = 1) | |
| Solubility in water | 4.8 g/L at 25°C | O’Neil et al. 2001 |
| Vapor pressure | 3670 mm Hg at 20°C | Holbrook 1992 |
| Flammability limits | Flammable; 8.1-17.2% | DOT 1985 |
| Conversion factors | 1 ppm = 2.07 mg/m3 1 mg/m3 = 0.483 ppm |
ACGIH 2003 |
|
|
||
2.1. Acute Lethality
2.2.1. Odor Threshold and Awareness
Data on the odor and irritation thresholds of methyl chloride are conflicting. The odor threshold has been reported at 10 ppm (Billings and Jonas 1981; Ruth 1986), and an irritation threshold was reported in a literature review as approximately 500 ppm (Ruth 1986). However, the specific source of the odor and irritation thresholds was not reported. In well-conducted clinical studies with male and female subjects, odor was not clearly perceived at concentrations of 150 ppm (Stewart et al. 1980) or 200 ppm (Putz-Anderson et al. 1981a). Several reviews, including one by Repko and Lasley (1979), state that methyl chloride is undetectable at concentrations that are dangerous to breathe. The odor is described as ethereal or sweet (Reid 2001).
2.2.2. Case Reports
Numerous case reports of exposure to methyl chloride as a result of refrigeration losses or industrial leaks have been reported. A few examples are cited here. Symptoms of fatigue, drowsiness, staggered walk, headache, blurred vision, mental confusion, vertigo, muscular cramping and rigidity, and tremor may be preceded by a latent period of 1-4 h. Depending on the severity of the exposure, symptoms may persist for several months, and personality changes, such as depression, may develop (ATSDR 1998).
MacDonald (1964) described nine case reports of employees at a synthetic-rubber plant where he was the medical supervisor. Where concentrations were noted in the work area, measurements were taken by gas chromatography or, in one case, by a Riken indicator, which gives immediate indication of the presence of methyl chloride at concentrations up to 10,000 ppm. In some cases exposures continued for several days before employees reported to the medical department. The cases were reported in short paragraphs, and no further details on exposure durations were reported. In the first case, an employee experienced vision disturbance, headache, dizziness, nausea, and staggering for several days prior to reporting to the medical department. Concentrations of methyl chloride in his work area was <25-1,600 ppm. Symptoms disappeared slowly, and he returned to work after 36 days. Two other employees working in the same area had similar symptoms, but medical examinations were normal. Apprehension and depression occurred for some time following the exposures.
A fourth employee neglected to wear a mask in an area where concentrations of methyl chloride were known to be 1,000-2,000 ppm. He experienced dizziness, blurred vision, headache, nausea, and vomiting. The exposure duration could not be quantified. The symptoms cleared quickly and he returned to work the next day. A second exposure one year later, although considered more moderate, resulted in more persistent symptoms. After a methyl chloride spill, a
fifth employee reported symptoms similar to those described above, and he appeared to be euphoric. He completed his work shift, but reported to the medical department the next day with persistent symptoms. He returned to work 2 weeks later. Over the next 10 years he experienced occasional periods of dizziness and headaches, which he attributed to mild overexposures. Concentrations in his work area rarely exceeded 100 ppm.
Following another accidental spill, an employee repeatedly entered and left an area that had methyl chloride in excess of 10,000 ppm (Riken indicator). Although he experienced symptoms of blurred vision, dizziness, and sight headache, he did not report to the medical department. At another time, he worked in an area with a leak that was not controlled for 13 days. Monitoring data showed concentrations of methyl chloride at 2,000-4,000 ppm. During the first week, the employee slept for long periods; the following week he experienced the typical symptoms described above. Although the symptoms lessened with time, the employee became irritable and depressed. This continued until his reassignment into another area of the plant. Another employee exposed at the same time, but not to the same degree, experienced milder symptoms.
An eighth employee was found unconscious lying in a cloud of escaping methyl chloride gas by other workmen. He was admitted to the hospital where he remained unconscious for several hours. Weakness and headaches were still present when he was discharged 10 days later. Follow-up examinations over the next 5 years revealed persistent symptoms, personality changes, and neurologic damage.
In 1963, 17 male crew members on an Icelandic fishing trawler were exposed for 2 days to methyl chloride from a leaking refrigerator located under their sleeping quarters (Gudmundsson 1977). No estimates of exposure concentrations were made. Fifteen of the crew members had signs of intoxication and abnormal neurologic symptoms. One survivor died within 24 h of exposure, two committed suicide 11 and 18 months later, and one died 10 years later. Six of 10 survivors (one survivor could not be located) still had neurologic deficits 20 months later. All survivors suffered from mild to permanent neurologic or psychiatric sequelae 13 years after the exposure occurred.
Lanham (1982) reported a case of a husband and wife who stored StyrofoamTM insulating boards in the basement of their new home prior to installation. The home was of tight, energy-efficient construction. Several days later they developed symptoms of blurred vision, fatigue, vertigo, tremor, and abnormal gait. Concentrations of methyl chloride measured by three different devices were above 200 ppm.
Battigelli and Perini (1955) described two workers exposed to methyl chloride while repairing a refrigeration system. On the basis of the room size and the amount of gas in the system, the exposure was estimated at >29,000 ppm (duration was not provided). The workers developed vertigo, tremors, dulled senses, nausea, vomiting, and abdominal pain 3-4 h after exposure. Symptoms disappeared 1 day after the exposure.
Four refrigeration-repair workers were exposed to methyl chloride at approximately 39,000, 50,000, 440,000, and 600,000 ppm (Jones 1942). Common symptoms were ataxia, staggering, headache, drowsiness, anorexia, blurred and double vision, convulsions, nausea, and vomiting. The exposure duration was not reported.
2.2.3. Occupational Exposures
Scharnweber et al. (1974) described six cases of prolonged worker exposure to “relatively low levels” of methyl chloride. Exposures were for 2-3 weeks, sometimes with 12-to 16-h workdays, before onset of symptoms. The analysis method was not reported. Two workers exposed at up to 300 ppm (8-h time-weighted average [TWA]) for several weeks were hospitalized with symptoms of confusion, blurry vision, difficulty in eating and swallowing, headache, and combativeness. Some symptoms, such as poor memory and headache, persisted for several months. Four workers exposed at 265 ppm (8-h TWA) for 2-3 weeks, with 12-to 16-h workdays, developed similar symptoms, including impaired memory, gait, and speech and slight elevation in blood pressure. Scharnweber et al. (1974) concluded that 8-h of exposure to methyl chloride at concentrations greater than 200 ppm is necessary for development of chronic methylchloride intoxication.
Continuous monitoring studies (for up to 4 months) during manufacturing operations at nine plants were conducted by the Dow Chemical Co. (personal communication, 1970, as cited in ACGIH 2003). Time-weighted average exposure concentrations were determined for 54 job classifications. The average TWA was 30 ppm with a range of 5-78 ppm; peaks as high as 400 ppm were recorded. Routine, periodic medical examinations did not identify any evidence of overexposure. Methyl chloride concentrations in relation to reported illnesses in StyrofoamTM-manufacturing plants were summarized. On the basis of 100 sample points at 9 plants, illness was reported in plants where average concentrations of methyl chloride were 2-1,500 ppm; the range of average exposures was 195-475 ppm. Symptoms of illness included weakness, drowsiness, staggered gait, thickness of the tongue, and lapses of memory. At 141 plants (1,784 sample points) without reported illnesses, average concentrations at sample points were 2-500 ppm, and the range of average exposures was 15-195 ppm.
Repko et al. (1976) compared neurologic functions in a group of 122 healthy male and female workers exposed to methyl chloride in the manufacture of foam products with 49 workers also engaged in the manufacture of foam products but not exposed to methyl chloride. Average daily air concentrations were determined for each worker individually. Air concentrations were monitored by different methods in different plants and involved continuous and single-sampling techniques. For continuous monitoring with gas or infrared analyzers (five plants), the amount of time each employee spent in an area was used to
calculate TWA exposures. On testing days, carbon tubes were used to collect area samples. Results correlated “reasonably” with concentrations determined from conductivity and infrared analyzers. In the sixth plant, continuous monitoring was conducted with an automated gas chromatograph. Carbon tubes also were used during the battery of tests. The study was not blind; volunteers were paid and were told the objectives of the tests. Functional capacity was evaluated with a series of comprehensive neurologic, electroencephalogram (EEG), and behavioral test batteries.
Ambient concentrations of methyl chloride were 7.4-70 ppm, with an overall average of 33.6 ppm. There were no significant differences in results of neurologic tests or EEGs. Although the exposed group outperformed the control group on a few tasks, significant performance deficits were observed for most tasks. The concentration of methyl chloride was related to the decrease in performance deficits, primarily cognitive time sharing, and increased finger tremor. Methyl chloride concentration also was correlated with breath concentration, as well as urine pH and hematocrit. The authors concluded that daily exposure to methyl chloride below 100 ppm can cause significant, transitory changes in functional capacity. Because exposures before the study were higher and because questionable statistical methods were used, the study is of limited value (Torkelson and Rowe 1981).
The National Institute of Occupational Safety and Health (Cohen et al. 1980) conducted a survey of four U.S. chemical plants. Three of the plants produced methyl chloride and the fourth used methyl chloride as a blowing agent in the production of polystyrene foam. The personal 8-h TWA concentrations at the first three plants were 8.9-12.4 ppm, <0.2-7.5 ppm, and <0.1-12.7 ppm; personal exposures in the fourth plant were 3.0-21.4 ppm. In a Dutch methyl chloride plant, individual 8-h TWA area samples (which correlated closely with personal samples) were 30-90 ppm during one working week (van Doorn et al. 1980). Symptoms, if present, were not reported in these studies.
2.2.4. Clinical Studies
Clinical studies of methyl chloride are summarized in Table 6-3. As part of a pharmacokinetic study of methyl chloride, six male volunteers were exposed at 10-50 ppm on separate days for 6 h (Nolan et al. 1985). Exposures took place in a 70-m3 chamber. Atmospheres were measured continuously with an infrared spectrometer and at 15-min intervals with a gas chromatograph equipped with a flame ionization detector. There was no recognizable odor or irritation. No adverse effects were reported by the subjects or by the physicians conducting the post-exposure examinations.
Additional clinical studies are discussed below in the section on neurotoxicity (Stewart et al. 1980; Putz-Anderson et al. 1981a,b) or on metabolism (Lof et al. 2000).
TABLE 6-3 Summary of Clinical Studies of Methyl Chloride
|
|
|||
| Concentration (ppm) | Exposure Duration | Effect | Reference |
|
|
|||
| 10 | 2h | No irritation or CNS effects. | Lof et al. 2000 |
| 10,50 | 6h | No recognizable odor or irritation. | Nolan et al. 1985 |
| 0,20,100, 150 | 1,3, or 7.5 h, 2-5 d | No eye, nose, or throat irritation: no effect on physiologic, neurologic, behavioral, or clinical parameters; exercise incorporated into the protocol for male subjects. | Stewart et al. 1980 |
| 0, 100, 200 | 3h | No odor perception; little to no effect on tests of alertness. | Putz-Anderson et al. 1981a |
| 0,200 | 3.5 h | No odor perception; no effect on tests of alertness. | Putz-Anderson et al. 1981b |
|
|
|||
2.3. Neurotoxicity
In a study using a controlled atmospheric chamber, nine male subjects (ages 19-34) were exposed to methyl chloride at 0, 20, 100, or 150 ppm for 1, 3, or 7.5 h, and nine female subjects were exposed at 0 or 100 ppm for identical periods of time (Hake et al. 1977; Stewart et al. 1980). Male subjects were exposed at 150 ppm on 2 consecutive days and male and female subjects were exposed at 100 ppm on 5 consecutive days. An additional exposure of male subjects involved fluctuating concentrations of 50, 100, and 150 ppm (TWA of 100 ppm) for 1, 3, or 7.5 h/day for 5 days. Groups were composed of 2-4 subjects. Groups were defined by exposure duration; for example, the four male subjects exposed for 7.5 h were exposed to methyl chloride at 0, 20, 100, fluctuating 50-150, and 150 ppm on different weeks. The entire testing period was 5 weeks. The male subjects were sedentary except for 11 min of exercise on a bicycle ergometer (6 min at 350 kpm and 5 min at 750 kpm) between the fifth and seventh hour h of exposure on the fourth day at all concentrations (day 2 for the male group exposed at 150 ppm). Concentrations were verified by gas chromatography and infrared analysis. Clinical symptoms and physiologic (EEG and visual evoked response patterns), clinical chemistry and hematology, neurologic, and behavioral effects were monitored; blood and alveolar breath samples were monitored for methyl chloride. Subjective responses were recorded immediately after entering the chamber, at the half hour, and hourly thereafter. The report form contained the descriptors headache; nausea; dizziness; abdominal pain; eye, nose, throat irritation; odor; and other, with modifiers of mild, moderate, and strong (only abnormalities reported). Neurologic studies consisted of a modified Romberg test, equilibrium test, spontaneous EEG, and visual evoked response. Cognitive testing, consisting of time estimation, eye-hand coordination, arithmetic, and number recognition, was performed after 2 and 3 h during
the 3- and 7.5-h exposures, respectively. The physiologic, neurologic, behavioral, clinical, and medical responses revealed no deleterious effects from methyl chloride (blood and breath analysis for methyl chloride are summarized in Section 4.1). The notation of a mild odor was reported as frequently for control exposure (0 ppm) as for test exposures.
Putz-Anderson et al. (1981a) assessed the behavioral effects of inhaled methyl chloride in groups of 8 or 12 healthy male and female subjects. Ages ranged from 18 to 32 years. Methyl chloride was administered at concentrations of 0, 100, or 200 ppm for 3 h. Three performance tests (visual vigilance, dual task, and time discrimination), designed to test attention or alertness, were administered before and during the treatment period. The net impairment resulting from exposure at 200 ppm was marginally significant (4.5%). The authors concluded that exposure at 200 ppm produced little or no behavioral impairment. In a second study (Putz-Anderson et al. 1981b), conducted in the same manner and using the same tests, groups of 12 healthy male and female subjects were exposed at 200 ppm for 3.5 h. The subjects did not experience any significant impairment on the tests. The authors note that the subjects were no more successful than chance in assessing whether they had been exposed to the control or chemical atmosphere.
2.4. Developmental/Reproductive Toxicity
No studies were found regarding reproductive or developmental effects in humans after inhalation of methyl chloride.
2.5. Genotoxicity
No studies were found regarding genotoxic effects in humans after inhalation exposure to methyl chloride. In an in vitro test, methyl chloride at 0.3-5% induced an increase in the frequency of sister chromatid exchanges in human lymphoblasts, but did not induce DNA damage (Fostel et al. 1985). Unscheduled DNA synthesis was induced in primary cultures of human hepatocytes of three individuals exposed at 1%, but not at 0.1-0.3% (Butterworth et al. 1989).
2.6. Carcinogenicity
Holmes et al. (1986) conducted a retrospective study of 852 workers exposed to methyl chloride in a butyl rubber manufacturing plant. Mortality from all causes was lower than expected compared with the U.S. male population. There was no statistical evidence that the death rate from cancer at any site was increased. No concentrations of methyl chloride were specified in this study.
Rafnsson and Gudmundsson (1997) conducted a long-term follow-up study of the survivors of the acute exposure described by Gudmundsson (1977)
in Section 2.2.2. The 24 crew members, which included individuals that had not been heavily exposed, were compared with a matched referent group. The authors found increased mortality from cardiovascular diseases and “vague signs” of cancer risk.
In reviewing the two studies described above for its Integrated Risk Information System (IRIS), EPA (2003) concluded that the studies failed to convincingly demonstrate an increased cancer mortality risk. The agency has not assigned a carcinogenicity classification to methyl chloride. The National Toxicology Program (NTP) has not classified methyl chloride with regard to carcinogenicity. IARC (1999) also concluded that evidence for carcinogenicity in humans is inadequate. IARC reviewed the study by Holmes et al. (1986), as well as a study by Ott et al. (1985). In the Ott et al. (1985) study, exposures were to mixtures of chemicals, including methyl chloride. The standard mortality ratio (SMR) for all cancers was 0.7. IARC (1999) also concluded that there was inadequate evidence for carcinogenicity in animals (see Section 3.6). The overall IARC evaluation is that methyl chloride is “not classifiable as to its carcinogenicity to humans” (Group 3). The American Conference of Governmental Industrial Hygienists (ACGIH 2003) considers methyl chloride “not classifiable as a human carcinogen” (A4). However, NIOSH (1984) has classified methyl chloride as a “potential occupational carcinogen” with no further categorization.
2.7. Summary
Acute and chronic exposures to methyl chloride primarily affect the CNS. Deaths have occurred from accidental exposure to methyl chloride, but there are no reliable data on concentrations and exposure durations. The odor is very faint and may not be noticed by individuals at concentrations that are life-threatening. Like many solvents, methyl chloride is nonirritating to the eyes and mucous membranes. Accidental workplace exposures have documented concentrations of 1,000-2,000, 2,000-4,000 (for up to a week), and 10,000 ppm, the latter concentration for short periods of time (MacDonald 1964). These exposures resulted in typical symptoms of blurred vision, dizziness, headache, nausea, and vomiting. In some cases symptoms were delayed for several hours. Daily, repeated exposures to methyl chloride at ≥265 ppm for 12-to 16-h workdays have resulted in similar symptoms (Scharnweber et al. 1974).
In a well-conducted clinical study, no toxicologically-significant effects were apparent when female and exercising male subjects were exposed to methyl chloride at 100 ppm for 7.5 h on 5 consecutive days, male subjects were exposed at 150 ppm for 7.5 h on two consecutive days, or male subjects exposed at 50-150 ppm (TWA of 100 ppm) for 7.5 h on 5 consecutive days (Stewart et al. 1980). In all cases male subjects exercised on a bicycle ergometer for 11 min per day. A concentration of 200 ppm was also a no-effect level for toxicologically
significant neurobehavioral symptoms during separate 3-and 3.5-h exposures (Putz-Anderson et al. 1981a,b).
No studies on developmental or reproductive effects were found. In in vitro systems, methyl chloride is genotoxic only at very high concentrations. There is inadequate evidence for carcinogenicity from methyl chloride in humans.
3. ANIMAL TOXICITY DATA
Few studies of acute exposure to methyl chloride are available. Most studies address neurotoxicity and use repeat-exposure methods. These studies are summarized in Table 6-4.
3.1. Acute Lethality
In an early, comprehensive study (Smith and von Oettingen 1947a,b; Dunn and Smith 1947), several species were exposed to methyl chloride at 300, 500, 1,000, 2,000, 3,000, or 4,000 ppm for 6 h/day for up to several months. Concentrations were monitored by a titration method, from direct rotameter calibrations, and by weight loss from the methyl chloride tanks. Agreement among the measurement methods was fairly good. Animals were observed for frank toxicity, including ataxia and convulsions. Concentrations of 3,000 and 4,000 ppm were lethal to most species within a few days. For 5 adult monkeys, exposure at 2,000 ppm resulted in convulsive seizures and long periods of unconsciousness; no deaths were recorded during the first week. At 500 ppm, 3 of 4 dogs died after 4 weeks of exposure and both monkeys died after 16 weeks of exposure (with progressive debility and persisting unconsciousness). None of the monkeys, dogs, rats, mice, rabbits, or guinea pigs exposed at 300 ppm for up to 64 weeks died, and no tissue lesions were found. Symptoms developed later in young animals. Deaths were dependent on time and concentration. At high concentrations, mortality correlated with the product of exposure duration and concentration, but at about 500 ppm, mortality was more gradual. Details of this study were insufficient to estimate acute lethal concentrations for each species. Furthermore, although the values appear similar to those of more recent studies, the methods do not meet current standards. Therefore, this study and the following study are not presented in Table 6-4 with other mortality studies.
Additional studies were reported by this group. The average survival time of beagles exposed at 14,661 ppm was 5.9 h (von Oettingen et al. 1949). Death was preceded by an initial increase in heart rate and blood pressure, followed by reduced respiration, decreased heart rate, and progressive fall in blood pressure. This concentration had little narcotic action as measured by disappearance of corneal reflex, pupillary reflex, and muscular action before death. Groups of mice (strain and number not specified) were exposed to methyl chloride at various concentrations for 7 h (von Oettingen et al. 1949). Concentrations were estimated
by the amount of chemical volatilized over the 7-h period and by a titration method. Mice exhibited increased activity soon after exposure began; this was followed by quiescence at 2 h. Death was preceded by clonic convulsions. Most deaths occurred during the first 8 h after exposure; none occurred after 16 h. The 7-h 50% lethality (LC50) was approximately 3,100 ppm. No further details were provided.
3.1.1. Rats
In an unpublished study, groups of 40 male and 40 female Sprague-Dawley rats were exposed continuously to methyl chloride (99.5% purity) at 0, 200, 500, 1,000, or 2,000 ppm for 48 or 72 h (Burek et al. 1981). Twenty rats per sex were killed immediately after exposure and the remaining rats were observed for 12 days before they were also killed for necropsy. At 2,000 ppm, rats were lethargic, moribund, or dead at 48 h, and all were dead at 72 h. No male rats and 1 of 10 female rats exposed at 1,000 ppm for 48 h died during the 12-day post-exposure observation period; mortality in the rats exposed for 72 h was 6 of 10 males and 8 of 10 females. The authors attributed the deaths to kidney failure. No deaths occurred at 0, 200, or 500 ppm for up to 72 h of exposure, and no overt signs of toxicity were observed during the exposures. There were no gross or microscopic changes in organs of rats exposed at 200 ppm for 24 h; livers of female rats exposed at 200 ppm for 72 h showed reversible changes.
Groups of 10 male and 10 female F344 rats were exposed to methyl chloride at 0, 2,000, 3,500, or 5,000 ppm for 6 h/day for up to 12 days (Morgan et al. 1982). The chemical was 99.5% pure; analysis was by an infrared gas analyzer. At 5,000 ppm, clinical signs included diarrhea during the first days of exposure, incoordination of the forelimbs by day 3, hindlimb paralysis on day 5, and a moribund state (6 males and 5 females) on day 5. In the 3,500-ppm group, two females in a moribund state were killed after the fifth day of exposure. No other deaths were reported. Histopathologic changes included cerebellar degeneration in males and females exposed at 5,000 ppm, dose-related degeneration and necrosis of the renal proximal convoluted tubules in males exposed at 2,000 ppm and in both sexes at 3,500 and 5,000 ppm, hepatocellular degeneration in females exposed at 2,000 ppm and in both sexes at 3,500 and 5,000 ppm, testicular degeneration in males exposed at ≥2,000 ppm, and adrenal fatty degeneration in males and females exposed at 3,500 and 5,000 ppm. In a later study by the same group (Chellman et al. 1986a), exposure to methyl chloride at 5,000 ppm for 5 days resulted in weight loss and testicular, brain, liver, and adrenal lesions in male F344 rats (see Section 3.4 for reproductive effects). Tremors, ataxia, and forelimb and hindlimb paralysis were observed, but the day of onset was not specified. One rat of five rats died after the last day of exposure. Exposure to methyl chloride at 7,500 ppm for 6 h/day for 2 days resulted in 67% lethality (8/12 rats) during a 4-day post-exposure observation period.
TABLE 6-4 Summary of Acute, Repeat-Exposure, and Subchronic Inhalation Studies of Methyl Chloride in Laboratory Animals
|
|
||||
| Species | Concentration (ppm) | Exposure Duration | Effecta | Reference |
|
|
||||
| Dog | 0,200, 500 | 23.5 h/d, 3 d | No clinical signs or microscopic lesions at 200 ppm: neurotoxic signs and brain-stem lesions at 500 ppm | McKenna et al. 1981a |
| Dog | 0,50,150,400 | 6 h/d, 5 d/wk,13wk | No clinical signs: no brain or spinal cord lesions. | McKenna et al 1981b |
| Rat | 0,100,500,1,500 | 6h | No clinical signs: no effect on organ weights (up to 18 h post-exposure); no microscopic examinations. | Dodd et al. 1982 |
| Rat | 0,1,000, 5,000 | 6 h/d, 5 d | No deaths; initial weight loss of 3% and 20% in 1,000- and 3.000-ppm groups, respectively, followed by recovery . | Working et al. 1985a |
| Rat | 0, 5,000 | 6h/d,5d | Degenerauon of cerebellar granular cells, lesions m liver and adrenal glands; death of 1 rat after fifth day of exposure | Chellman et al. 1986a |
| 0, 7,500 | 6h/d,2d | 67% lethality. | ||
| Rat | 0,500,1,000,2,000, 3,500, 5,000 | 6 h/d, up to 12 d | No deaths during first 4 d; pathologic changes in cerebellum (5,000 ppm), kidneys (≥2,000 ppm). liver (≥2.000 ppm). adrenal glands (≥3,500 ppm), testes (≥2,000 ppm). | Morgan etal. 1982 |
| Rat | 200 | 48,72 h | No deaths; no cluneal signs. | Burek et al 1981 |
| 500 | 48,72 h | No deaths; no cluneal signs. | ||
| 1.000 | 48 h | 5% mortality. | ||
| 2,000 | 48 h | Moribund state or death- | ||
| Rat | 400 | 6 h/d, 5 d/wk,90 d | No cluneal signs, no organ lesions. | McKennaetal. 1981b |
|
|
||||
| Species | Concentration (ppm) | Exposure Duration | Effecta | Reference |
|
|
||||
| Rat | 0,375, 750,1,500 | 6 h/d, 5 d/wk, 90 d | No cluneal signs oi tissue lesions; mcreased liver weight at 1,500 ppm. | Mitchell et al. 1979 |
| Mouse | 2,250 (males) | 6h | LC50 | White et al. 1982 |
| 8,500 (females) | 6h | LC50 | ||
| Mouse | 2,200 | 6h | LC50 | Chellman et al. 1986b |
| Mouse (3 strains) | 0,500,1,000,2,000 | 6h/d up to l2 d | 2.000 ppm: death of one strain of male mice by day 2; ataxia, hematuria in females, death of males and females of other strains by day 5; pathologic changes in cerebellum (≥1.000 ppm). kidneys (≥1.000 ppm). liver (≥500 ppm). | Morgan etal. 1982 |
| Mouse | 1,500 ppm | 6 h/d, 5 d/wk, 2 wk | 2 of 10 mice died dimng first week, motor incoordination during second week; degenerative changes m cerebellum. | Jiang etal. 1985 |
| Mouse | 0,15,50,100,150,200 | 22 h/d, 11 d | Cerebellar lesions at >100 ppm; 200 ppm lethal in 5 days. | Landry etal. 1985 |
| 0,150,400,800, 1,600,2,400 | 5.5 h/d, 11 d | Cerebellar lesions at j400 ppm; moribund at 2,400 ppm by day 9. | ||
| Mouse | 0,375,750,1,500 | 6 h/d, 5 d/wk, 90 d | Mild liver changes; no brain lesions. | Mitchell et al. 1979 |
| Mouse | 0,50,150,400 | 6 h/d, 5 d>Wk, 90 d | No clinical signs or organ lesions. | McKenna et al. 1981b |
| Guinea pig | 0, 20,000 | 10 min/d, 6 d/wk, 61-70 d | No deaths, neurotoxic signs; lesions of cerebellar cortex | Kolkmann and Volk 1975 |
|
|
||||
3.1.2. Mice
The 6-h LC50 values for methyl chloride in male and female B6C3F1 mice were 2,250 and 8,500 ppm, respectively (White et al. 1982). After exposure to methyl chloride at 2,500 ppm for 30 min, hepatic glutathione (GSH) was reduced to 9% of control values in both sexes. No further data were provided in this abstract. As reported in a later paper by the same authors (Chellman et al. 1986b), groups of five male B6C3F1 mice were exposed for 6 h to methyl chloride at 500-ppm increments from nonlethal concentrations to a concentration that caused 100% mortality (exact concentrations not specified). Animals were killed 18 h after exposure. The LC50 was 2,200 ppm. Tremors, ataxia, and forelimb and hindlimb paralysis occurred prior to death.
Two strains of mice (C3H and C57Bl/6) and the cross between these two strains (B6C3F1) were exposed to methyl chloride at 0, 500, 1,000, or 2,000 ppm for 6 h/day for up to 12 days (Morgan et al. 1982). Groups were composed of five mice of each sex. All male B6C3F1 mice exposed at 2,000 ppm were moribund or dead by day 2, and all male and female mice in the remaining groups exposed at 2,000 ppm were moribund by day 5. The animals exhibited ataxia and had hematuria, the latter primarily in females. Histopathologic changes included: cerebellar degeneration in male and female C57Bl/6 mice exposed at 1,000 ppm and female B6C3F1 mice exposed at 2,000 ppm (the cerebellums of C3H mice were unaffected at all concentrations); degeneration and necrosis of the renal proximal convoluted tubules in male C3H mice exposed at 1,000 ppm and in both sexes of all three strains exposed at 2,000 ppm; basophilic renal tubules in both sexes of all three stains (except female C57Bl/6 mice) exposed at 1,000 ppm, but not at 2,000 ppm; and hepatocellular degeneration at ≥500 ppm, with greatest severity in male mice exposed at 2,000 ppm. Jiang et al. (1985) also found degenerative changes in the cerebellum of female C57Bl/6 mice exposed to methyl chloride at 1,500 ppm for 6 h/day, 5 days/week for 2 weeks. Renal lesions were minimal or absent.
In a study of neurotoxicity, female C57Bl/6 mice were exposed either “continuously” for 11 days (22 h/day without interruption) to methyl chloride at 0, 15, 50, 100, 150, or 200 ppm or “intermittently” (5.5 h/day) at 0, 150, 400, 800, 1,600, or 2,400 ppm for 11 consecutive days (Landry et al. 1985). At the end of exposure, cerebellar lesions were observed in mice continuously exposed at ≥100 ppm and in mice intermittently exposed at ≥400 ppm. Thymus weights were reduced at the lower concentrations, but there were no histologic correlates. Continuous exposure at 200 ppm was lethal in 5 days, and intermittent exposure at 2,400 ppm resulted in moribund mice by day 9.
3.2. Nonlethal Toxicity
3.2.1. Dogs
Groups of three male beagle dogs were exposed to methyl chloride at 0, 200, or 500 ppm for approximately 23.5 h/day for 3 days (McKenna et al.
1981a). After 23.5 h of treatment, dogs exposed at 500 ppm appeared more tranquil, with one exhibiting intermittent tremor and slight excess salivation. After 72 h, the behavior of the control and 200-ppm dogs was comparable. Dogs exposed at 500 ppm for 72 h appeared weak and displayed signs of forelimb stiffness and incoordination, occasional slipping and falling, inability to sit up or walk, limb tremor, and excess salivation. Partial recovery was observed during the 27-day post-exposure period. Microscopic examination of tissues revealed no treatment-related abnormalities in the control dogs or dogs exposed at 200 ppm. All three dogs exposed at 500 ppm displayed lesions in the brain stem and spinal cord, consisting of vacuolization, swollen eosinophilic axons, axon loss, demyelinization, and microglial cells with phatocytosed debris. There were no lesions in the cerebrum, cerebellum, or peripheral nerves.
In a second study by the same investigators (McKenna et al. 1981b), there was no evidence of brain or spinal-cord lesions in dogs exposed to methyl chloride at 0, 50, 150, or 400 ppm for 6 h/day, 5 days/week for approximately 13 weeks. Groups of four young male beagles were exposed to each concentration for a total of 66 days. Chamber concentrations were monitored by infrared spectrometry. Dogs were monitored daily for clinical signs, and body weight and hematologic-and clinical-chemistry parameters were evaluated before and after exposure. The animals were killed at the end of the study, and tissues and organs were examined microscopically. No dogs died during the study. There were no clinical signs, weight gains were comparable to that of controls, and no organ or tissue lesions attributable to methyl chloride were found.
3.2.2. Rats
As part of a mechanism of toxicity study (see Section 4.3), groups of 20 male F344 rats were exposed to methyl chloride at 0, 100, 500, or 1,500 ppm for 6 h or at 500 ppm for 1, 2, or 4 h (Dodd et al. 1982). All rats appeared normal after the exposures. No differences were observed in lung, liver, or kidney weights between control and treated rats when measured 0, 2, 4, 8, or 18 h after exposure (groups of four animals). Organs were not examined microscopically.
No deaths occurred in male F344 rats exposed to methyl chloride at 1,000 or 3,000 ppm for 6 h/day for 5 days (Working et al. 1985a). Weight loss was 3% in the 1,000-ppm group, but it was recovered 3 weeks after exposure ended. In the 3,000-ppm group, animals experienced a 20% weight loss, with recovery 4 weeks later (see Section 3.4 for reproductive effects).
In a 90-day pilot study, groups of 10 male and 10 female F344 rats were exposed to methyl chloride at 0, 375, 750, or 1,500 ppm for 6 h/day, 5 days/week (Mitchell et al. 1979). Animals were observed for food consumption, body weight changes, and mortality. Hematology and clinical-chemistry tests were performed. Organs were weighed and all tissues and organs from animals in the control and high-concentration groups were examined microscopically. Effects on the liver were mild; no necrosis was observed. There were no exposure
-related histopathologic lesions of the brain and spinal cord and no effect on brain weight. In a similar 90-day study, the no-effect concentration for methyl chloride in Sprague-Dawley rats was 400 ppm (McKenna et al. 1981b). The exposure regimen was the same as that described for dogs (see earlier discussion). In addition, rats were evaluated weekly for motor and sensory response. The results of the evaluations were normal except for a slight decline in the performance of female rats (primarily those in the 400-ppm group) over time in the wire maneuver test. The significance of this effect is questionable, given the weight increase in female rats over 90 days and that the same tendency was present in the control group.
3.2.3. Mice
In a 90-day pilot study, male and female B6C3F1 mice were exposed to methyl chloride at 0, 375, 750, or 1,500 ppm for 6 h/day, 5 days/week (Mitchell et al. 1979). Moderate changes in the liver included increased liver weight and cytoplasmic vacuolation in male and female mice exposed at 1,500 ppm, and to a lesser degree in mice exposed at 750 ppm. Female mice were more affected than male mice. There were no exposure-related histopathologic lesions of the brain or spinal cord and no effect on brain weight at any concentration. In a similar study, the no-effect level for clinical signs and organ lesions in CD-1 mice was 400 ppm (McKenna et al. 1981b). The protocol of this study is the same as that for dogs and rats (described earlier).
3.2.4. Guinea Pigs
Kolkmann and Volk (1975) exposed guinea pigs to methyl chloride at 20,000 ppm for 10 min/day for 21 days. Microscopic examination of the cerebellum revealed edema with necrosis of the granular cells.
3.3. Neurotoxicity
Neurotoxic effects were observed before death in many of the studies described earlier. In a study that addressed neurotoxicity at less than lethal concentrations, Landry et al. (1985) exposed female C57Bl/6 mice to various concentrations of methyl chloride either continuously (22 h/day) for 11 days or for 5.5 h/day for 11 days (see Table 6-4). The authors noted that female C57B1/6 mice are particularly sensitive to the neurotoxic effects of methyl chloride and do not have the complications of hepatic and renal toxicity. The mice were tested for their ability to maintain balance on an accelerating, rotating rod after 4, 8, and 11 days of exposure. Performance decrements on the rotating rod were observed after continuous exposure at 150 ppm for 4 and 8 days; mice were moribund or dead by the eleventh day. For intermittent exposures, slight decrements in performance
were observed at 800 and 1,600 ppm after 4 days, but not after 8 or 11 days. Cerebellar damage was observed at 400 ppm.
3.4. Developmental and Reproductive Toxicity
Methyl chloride has been shown to be a reproductive toxicant in a variety of animal studies. These studies are described below and in Table 6-5.
TABLE 6-5 Reproductive Toxicity of Methyl Chloride in Animal Models
|
|
|||
| Concentration (ppm) | Exposure Duration | Effects | Reference |
|
|
|||
| Dog | |||
|
|
|||
| 0, 200, 500 | 23.5 h/d for 3 d | No change in testes weight; no histopathologic lesions of testes. | McKenna et al. 1981a |
|
|
|||
| Rat | |||
|
|
|||
| 0, 150,475, 1.500 | 6 h/d, 5 d/wk for 10 wk | No statistically significant effect on fertility of males exposed at 150 or 475 ppm for two generations (but dose-related trend of reduced fertility); 100% sterility in males at 1.500 ppm; no clear effect on fertility in females. | Hammet al. 1985 |
| 200,500, 1,000 | 43 or 72 h | No testicular lesions at 200 ppm; inflammation of the epididymides with testicular atrophy at 500 and 1.000 ppm. | Burek et al. 1981 |
| 3,500 | 6 h/d, 5 and 4 d, with 3-d break between exposures | Epididymal and testicular lesions; interference with neuroendocrine control of spermatogenesis; first observed at day 9. | Chapm et al. 1984 |
| 5,000, 7,500 | 6 h/d for 2 d | Teshcular or epididymal granulomas: testicular lesions. | Chelhnan et al. 1986a; Working and Chellman 1989 |
| 3,000 | 6 h/d for 5 d | Epididymal inflammation and granulomas, sperm cytotoxicity, preiniplantauon loss m mated females. | Chelhnan et al. 1986c; 1987 |
| 0, 2,000, 3,500, 5,000 | 6 h/d for 5 and 4 d with 2-d break between exposures | Concentration-related testicular degeneration with reduced numbers of sperm. | Morgan et al. 1982 |
| 0, 1,000, 3,000 | 6 h/d for 5 d | At 1.000 ppm: no sperm granulomas, no effect on fertilization of females At 3.000 ppm: epididymal granulomas, preimplantauon loss m mated females from fertilization failure (decrease m sperm quality), recover)- by week 16. | Working et al. 1985a,b; Working and Bus 1986 |
|
|
|||
The reproductive toxicity of methyl chloride in the male rat has been well characterized in studies performed at the Chemical Industry Institute of Toxicology (Burek et al. 1981; Morgan et al. 1982; Chapin et al. 1984; Working et al. 1985a,b; Chellman et al. 1986b,c, 1987; Working and Chellman 1989). Male F344 rats exposed to methyl chloride at concentrations of 1,000 ppm or greater developed testicular degeneration, epididymal inflammation, and sperm granulomas. Fertility might be decreased at 500 ppm. Recovery occurred 16 weeks after exposure. Unexposed females bred to treated males in a dominant lethal assay exhibited increased rates of postimplantation embryonic death during the first 2 weeks post-exposure and increased preimplantation embryonic loss during weeks 2 to 8 post-exposure. Studies on the mechanism of action suggest that preimplantation loss is from cytotoxic effects of methyl chloride on sperm in the testes (a significant decrease in the number of motile sperm of normal morphology 2 to 8 weeks post-exposure) and not to genotoxic effects on the sperm. Chellman et al. (1986a) showed that the effects of methyl chloride were virtually absent when male rats were pretreated with the anti-inflammatory agent 3-amino-1-[m(trifluoromethyl)phenyl]-2-pyrazoline (BW755C).
No changes in testes weights and no histopathologic lesions of the testes were observed in beagles after exposure to methyl chloride at 500 ppm almost continuously for 3 days (McKenna et al. 1981a; see Section 3.1.1 for study description).
A decrease in male fertility was also observed in a two-generation study with rats (Hamm et al. 1985). Groups of 40 male and 80 female F344 rats were exposed to methyl chloride at 0, 150, 475, or 1,500 ppm for 6 h/day, 5 days/week during a 10-week premating period. The only clinical sign during that time was a 10-20% reduced body weight gain in both sexes exposed at 1,500 ppm for 2 weeks and a 5-7% depression in body weight gain after day 57 in rats exposed at 475 ppm. During a 2-week mating period, both sexes were exposed for 7 days/week. Males were mated to two exposed females. Necropsies of 10 males in each group (at 12 weeks) showed severe bilateral testicular degeneration (10/10) and epididymal granulomas (3/10) in the 1,500-ppm group. Males exposed at 1,500 ppm were sterile when mated to either unexposed or exposed females. Fewer F0 males exposed at 475 ppm and mated to unexposed females were fertile (12/28) than in the control (23/28) or 150-ppm (21/28) group. Also, fewer males exposed at 475 ppm and mated to exposed females were fertile (12/40) than in the control (18/40) or 150-ppm (20/39) group. However, there was no difference in fertility in the F1 generation (the number of fertile males in the control, 150-ppm, and 475-ppm groups was 31/40, 26/40, and 14/23, respectively) although there was a trend toward decreased fertility. After a 9-or 18-week recovery period, fertility remained low in F0 males previously exposed at 1,500 ppm (5 and 10%, respectively).
Several studies addressed developmental effects of methyl chloride (see Table 6-6). In the Hamm et al. (1985) study described earlier, no significant differences in litter size, sex ratio, pup viability, or pup growth were observed
TABLE 6-6 Developmental Effects of Methyl Chloride in Animal Models
|
|
|||
| Concentration (ppm) | Exposure Duration | Effects | Reference |
|
|
|||
| Rat | |||
|
|
|||
| 0,150,475,1,500 | 6 h/d, 5 d/wk premating; 6 h/d, 7 d/wk from mating to postnatal day 28 | No significant differences m litter size, sex ratio, pup viability, or pup growth. | Hammet al. 1985 |
| 0, 100,500,1,500 | 6 h/d, gestation days 7-19 | Maternal and fetal toxicity at 1,500 ppm; no external, skeletal, or visceral abnormalities in fetuses. | Wolkowski-Tyl et al. 1983a |
|
|
|||
| Mouse | |||
|
|
|||
| 0, 100,500,1,500 | 6 h/d, gestation days 6-17 | Mortality in dams at 1.500 ppm; survival m other groups with no evidence of maternal or fetal toxicity; no external malformations; small, statistically significant increase m heart defects m litters of 500-ppm group. | Wolkowski-Tyl et al. 1983a |
| 0, 250, 500,750 | 6 h/d, gestation days 6-18 | Decreased body weight gam in dams at 750 ppm; heart malformations in fetuses litters at 500 and 750ppm. | Wolkowski-Tyl et al. 1983b |
| 0, 250. 300 | 24 h. gestation days 11.5-12.5 | No heart malformations | John-Green et al. 1985 |
| 1.000 | 12 h. gestation days 11.5-12 | No heart malformations | |
|
|
|||
between control or treated (150, and 475 ppm) rats. Females were exposed for 6 h/day, 7 days/week from the start of mating to postnatal day 28, but were not exposed from gestation days 18 to postnatal day 4 and the pups were not exposed directly.
Results of other developmental toxicity tests with methyl chloride are conflicting. Wolkowski-Tyl et al. (1983a,b) found no external, skeletal, or visceral abnormalities in the fetuses of pregnant F344 rats exposed at 0, 100, 500, or 1,500 ppm on gestation days 7-19. Maternal and fetal toxicity were apparent at the highest concentration, as evidenced by reduced food consumption and decreased body weight and weight gain in dams and reduced fetal body weight. However, a small but statistically significant number of heart malformations were found in the B6C3F1 fetuses of pregnant C57Bl/6 mice (bred to C3H males) exposed at 500 ppm on gestation days 6-17. The malformation involved the bicuspid and tricuspid valves, and primarily involved a reduced number of papillary muscles on the right side of the heart. No abnormalities were found in fetuses when dams were exposed at 100 ppm. Dams exposed at 1,500 ppm were
moribund and killed. In a subsequent study, the same malformations were observed in fetuses when mouse dams were exposed at 500 or 750 ppm. No other embryotoxicity, fetotoxicity, or malformations were observed at 250 ppm. In another study, no heart malformations were found in fetuses of C57Bl/6 mouse dams exposed at 250, 300, or 1,000 ppm during gestation days 11.5-12.5, considered the critical period for heart development by John-Green et al. (1985). Wolkowski-Tyl et al. (1983b) considered gestation day 14 the critical developmental period. John-Green et al. (1985) also noted the variability in the appearance of the papillary heart muscles of fetal mice and, owing to their small size, the difficulty in confirming their presence. When examinations of fetal hearts were conducted with technicians that were blind to the test conditions, the technicians failed to find either an absence of or a reduction in size of the papillary muscles of the tricuspid valve. Small numbers of animals were used in the John-Green et al. (1985) study, and the exposure was for 24 h at 250 or 300 ppm or for 12 h at 1,000 ppm.
3.5. Genotoxicity
Genotoxicity assays have been reviewed by ATSDR (1998) and EPA (2003). In in vitro assays, methyl chloride is a weak genotoxin at high concentrations. Methyl chloride has been shown to be mutagenic in several strains of Salmonella typhimurium and to induce unscheduled DNA synthesis in several types of cells at concentrations >3%, but not at 1%. It has not been shown to methylate the DNA of rat tissues. Exposures of male B6C3F1 mice to methyl chloride at 1,000 ppm for 6 h/day for 4 days failed to induce DNA-protein crosslinks in the kidneys, and gave only minor evidence of single-strand DNA breaks (Jager et al. 1988).
In in vivo assays, cytotoxicity appears to dominate potential genotoxicity. At 15,000 ppm, but not at 3,500 ppm, methyl chloride was weakly positive for the induction of unscheduled DNA synthesis in rat liver. Concentrations of 2,000-3,000 ppm (but not 1,000 ppm) produced dominant lethal effects in several strains of rats. The authors of the individual studies, including Chellman et al. (1986c), stated that the effects appeared to be attributable to cytotoxic effects on sperm in the testes rather than to direct genotoxicity, and to the effects of genotoxic oxidative metabolites resulting from an induced inflammatory response in the epididymides.
3.6. Chronic Toxicity and Carcinogenicity
In a 2-year study, methyl chloride was tested for carcinogenicity in F344 rats (120 per sex) and B6C3F1 mice (120 per sex) (Pavkov et al. 1981). Vapor concentrations were 0, 50, 225, or 1,000 ppm, and exposures were for 6 h/day. Mouse survival was affected at 1,000 ppm, whereas rat survival was unaffected. This outcome for mice might have been influenced by fighting for dominance
among males. Neurofunctional impairment was observed in mice exposed at 1,000 ppm beginning with the 18-month interim sacrifice. Histopathologic examinations of male and female mice at the 18-month sacrifice revealed cerebellar lesions and atrophy. These lesions were not observed at lower concentrations or in male or female rats. Beginning at 6 months, male rats exposed at 1,000 ppm developed bilateral atrophy of the testicular seminiferous tubules. However, this lesion, as a result of aging, was present in all male rats at the 24-month sacrifice. At 12 months, renal degenerative changes consisting of cortical tubular epithelial hypertrophy and hyperplasia and hepatocellular degeneration were observed in male mice exposed at 1,000 ppm. These lesions progressed in severity and prevalence throughout the study. At 24 months, the only evidence of carcinogenicity was a statistically significant increased incidence of benign and malignant renal tumors in male mice exposed at 1,000 ppm; two male mice in the 225-ppm group also had renal adenomas. EPA (2003) considered 250 ppm a no-observed-adverse-effect level (NOAEL) for any effect in rats. EPA did not designate a NOAEL for mice, but noted that the tumors observed at 225 ppm might be related to treatment.
3.7. Summary
Acute toxicity data on methyl chloride are sparse. The 6-h LC50 values for male and female B6C3F1 mice were 2,200 and 8,500 ppm, respectively (White et al. 1982).
Studies with laboratory animals have shown adverse effects on the brain, kidneys, liver, testes, and spleen. These effects are generally seen at neurotoxic concentrations or after repeated exposures at lower concentrations. In laboratory animals, no-effect levels were 1,500 ppm for clinical signs in rats exposed for 6 h (Dodd et al. 1982), 400 ppm for brain and spinal cord lesions in dogs exposed for 6 h/day, 5 days/week for 13 weeks (McKenna et al. 1981b), 400 ppm for cerebellar lesions in female mice exposed for 5.5 h/day for 11 days (Landry et al. 1985), 1,500 ppm in rats and mice exposed for 6 h/day for 13 weeks (Mitchell et al. 1979), and 200 ppm for clinical signs and brain lesions in dogs and rats exposed continuously for 3 days (Burek et al. 1981; McKenna et al. 1981a).
In reproduction studies with rats, exposures to methyl chloride that did not cause inflammation of the epididymides did not affect reproduction. A nearly 72-h continuous exposure of dogs to methyl chloride at 500 ppm had no effect on testicular weight or function (McKenna et al. 1981a). In developmental and teratology studies, results were conflicting. In one study, exposures of mice to methyl chloride at ≥500 ppm on gestation days 6-18 caused a reduced number of papillary muscles of the tricuspid valve of the fetal heart (Wolkowski-Tyl et al. 1983b); whereas in a study performed in a slightly different manner, these malformations were not observed (John-Green et al. 1985). However, it is possible the critical day of heart development was not chosen in the latter study.
The cancer potential is low and species specific (Pavkov et al. 1981). Consistent with the low cancer potential demonstrated in laboratory animals, alkylation
of DNA appears to be minimal. Mutagenicity is considered weak to moderate. Although positive in dominant lethal assays, the effect appears to be secondary to injury to a specific area of the epididymides.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
Human and animal studies show that methyl chloride is rapidly absorbed from the lungs (Andersen et al. 1980; Stewart et al. 1980; Landry et al. 1981, 1983; Nolan et al. 1985; ATSDR 1998). In humans exposed at up to 200 ppm, steady state was reached during the first hour of exposure (Putz-Anderson et al. 1981a; Nolan et al. 1985; Lof et al. 2000). Methyl chloride is extensively distributed throughout the body, with greatest deposition in the liver, kidneys, and testes (Kornbrust et al. 1982; Landry et al. 1983). This deposition, however, might refer to metabolites. In the rat, methyl chloride is metabolized by conjugation with GSH to yield S-methylglutathione. Cleavage of the glutamic acid and glycine moieties of GSH yields S-methylcysteine, and transamination and decarboxylation yields the mercapturic acid, methylthioacetic acid (Kornbrust and Bus 1983). The latter sulfur-containing compounds can be excreted in the urine. Methylthioacetic acid might be further metabolized to methanethiol which might yield formaldehyde and formic acid via P-450 metabolism; the latter compounds can enter the one-carbon pool or be excreted as carbon dioxide. The reaction with GSH appears to be primarily enzyme catalyzed, probably by glutathione transferase. Formation of formaldehyde appears to be a minor pathway. Human kidneys lack detectable CYP2E1 protein which suggests that formaldehyde formation might not occur in this organ. In summary, methyl chloride is rapidly absorbed and metabolized (does not bioaccumulate) and is excreted as numerous metabolites indistinguishable from normal metabolites.
The metabolite S-methylcysteine has been identified in the urine of occupationally exposed humans (van Doorn et al. 1980), in humans during clinical exposures (Nolan et al. 1985), and exposed rats (Landry et al. 1983). Urinary Smethylcysteine excretion is extremely variable in human subjects and is not a good indicator of the concentrations to which subjects are exposed (Nolan et al. 1985). Methylmercapturic acid was not detectable in the urine of exposed workers (van Doorn et al. 1980). In the rat, the metabolite14CO2 may account for nearly 50% of inhaled14C-labeled methyl chloride (Kornbrust and Bus 1983). Unmetabolized methyl chloride is excreted via the lungs in human subjects (Stewart et al. 1980; Nolan et al. 1985; Lof et al. 2000).
4.2. Blood Concentrations and Pharmacokinetic Models
Blood and alveolar-air concentrations of methyl chloride are difficult to correlate with exposure (ATSDR 1998). Differences in uptake between individuals (Stewart et al. 1980; Nolan et al. 1985; Lof et al. 2000), as well as differences
in measurement methods (Nolan et al. 1985), might be responsible for the lack of correlation.
The pharmacokinetics of methyl chloride in six male subjects exposed at 10 or 50 ppm were studied by Nolan et al. (1985). Air concentrations of methyl chloride in the test chamber were compared with venous-blood and expired-air concentrations. Blood concentrations increased rapidly and reached a plateau during the first hour of exposure and were proportional to the exposure concentration. On the basis of blood and expired-air concentrations, the subjects could be divided into two distinct groups. Two of the subjects had blood concentrations three-fold greater than the other subjects. At 10 ppm, blood concentrations were approximately 30 and 8 ng/mL in the two groups and, at 50 ppm, blood concentrations were approximately 100 and 35 ng/mL (values read from graphs). Blood:air partition coefficients of 2.1 and 2.5 were calculated for the groups with the higher and lower blood values exposed at 10 ppm, respectively. At 50 ppm, blood:air partition coefficients of 1.7 and 1.8 were calculated for the respective groups. Following a single breath of methyl chloride at 500 ppm, held for 20 seconds, blood:air and serum:air partition coefficients were both 0.8 (Morgan et al. 1970). In the Nolan et al. (1985) study, expired-air concentrations exhibited the same temporal uptake; differences in expired-air concentrations were two-fold between the two groups, with higher concentrations in the group with the greater uptake. In slow and rapid metabolizers, expired air contained 30-40% or 70% of the concentration in inhaled air, respectively. Absorption rates of 1.4 μg/min/kg (slow metabolizers) and 3.7 μg/min/kg (rapid metabolizers) were calculated using a two-compartment model. Elimination was rapid in both groups after exposure ended. Elimination was more rapid in volunteers with the lower blood and expired-air concentrations. The authors explained the difference in the two groups by a two-fold difference in the rate at which they metabolized methyl chloride. They considered the difference of questionable toxicologic significance.
In a second controlled chamber study, breath and venous-blood samples were taken before exposure, immediately after exiting from the exposure chamber, and 15-and 30-min after exposure (Stewart et al. 1980). Subjects were exposed at 0, 20, 100, or 150 ppm for 1, 3, or 7.5 h (see study description details in Section 2.3). Blood and breath concentrations varied among individuals and, to a lesser degree, among days. For male subjects with low uptake (see Section 4.2), the mean breath concentrations measured 1 min after exposure to methyl chloride at 100 ppm for 1, 3, or 7.5 h were 33 (1 subject), 40, and 44 ppm, respectively. Two male subjects had considerably higher alveolar concentrations (up to 77 ppm). Weekly measurements of breath concentrations of methyl chloride in females with low uptake ranged from 33 to 47 ppm, with no correlation to exposure duration. Only one of the nine females was a “high-level responder” (up to 76 ppm). At 100 ppm for 7.5 h, blood concentrations pre-exit ranged from 0.1 to 1.3 ppm in low-responder males. Two males with higher alveolar concentrations (one each in the 1-and 3-h exposure groups) also had higher blood concentrations, 9.8 ppm after 1 h and 15.1 ppm after 3 h. Blood concentrations dropped
rapidly during the 15-min post-exposure period, and methyl chloride concentration in expired air dropped so rapidly as to be of little or no value in quantifying exposure. In four low-responder females exposed at 100 ppm for 7.5 h, the average pre-exit blood concentration was 6 ppm. Concentrations for the high-level female responder were 5 and 18 ppm on two different occasions.
The average venous-blood concentrations of methyl chloride in 24 healthy male and female subjects exposed at 100 or 200 ppm for 3 h were 36 and 63 ppm, respectively (Putz-Anderson et al. 1981a). Three of the individuals exposed at 200 ppm had breath concentrations greater than 100 ppm (50% of the exposure concentration). Blood concentrations for the respective exposures were 11.5 and 7.7 ppm. In a second study (Putz-Anderson et al. 1981b), the breath and blood concentrations of male and female subjects exposed at 200 ppm were 74 and 14.5 ppm. The number of high and low responders was not cited, but breath and blood standard deviations of the mean were 43 and 100%, respectively.
Blood concentrations also were studied in 24 subjects who differed in glutathione- S-transferase (GST) genotype (Lof et al. 2000). At a concentration of 10 ppm, blood concentrations were similar in individuals with high, intermediate, and low GSH activity (0.5-0.6 μmoL/L); however, uptake was greater and clearance was more rapid in the group with high GSH activity.
The average concentration of methyl chloride in the blood of five beagles exposed at 15,000 ppm over a 6-h period was 6.1 mg% (von Oettingen et al. 1949). Concentrations ranged from 5.1 mg% at 10 min to 7.3 mg% at 240 min. While exposed at 40,000 ppm for 4 h, blood concentrations rose from 12.0 mg% at 10 min to 18.9 mg% at 150 min.
Andersen et al. (1980) determined the kinetic constants for metabolism of inhaled methyl chloride in male F344 rats. Exposure concentrations were not specified (however, for the related chemical, methyl bromide, concentrations were 100, 100, 3,000, and 10,000 ppm); chamber depletion measurements were taken every 10 min for 180 min. Methyl chloride exhibited a mixed-form rate curve, possessing both a saturable and a first-order component. However, the overall uptake rate from first-order processes was negligible and all of the observed uptake was accounted for by the saturable term. Saturation dependence appeared to be associated with enzymatic metabolism. Data were transformed by modified Eadie-Hofstee plots to calculate the inhalational Km (the ambient concentration at which uptake proceeds at half the maximum rate) and the inhalational Vmax (the maximum rate of uptake). The authors developed a fourcompartment, steady state, pharmacokinetic model to describe gas uptake in general. Km was 640 ppm and Vmax was 120 ppm.
In male F344 rats and beagles exposed at 50 or 1,000 ppm for 3 h, blood concentrations rapidly reached steady-state concentrations which were proportional to the exposure concentration (Landry et al. 1983). Blood concentrations were similar in the two species at each concentration (values were slightly higher for the rat than the dog). A linear two-compartment model with zero order uptake and first-order output described methyl chloride pharmacokinetics in
both species. Apparent steady-state blood values after exposure to methyl chloride at 50 or 1,000 ppm were 160 and 3,690 ng/g (dogs) and 194 and 3,930 ng/g (rats). The total areas under the blood-concentration curve (AUCs) were 70 and 3,930 μg/g/min for rats exposed at 50 and 1,000 ppm for 6 h, and 28 and 659 μg/g/min for dogs exposed at 50 and 1,000 ppm, respectively, for 3 h. The AUCs estimated for rats at 3 h are 35 and 710 μg/g/min, respectively. There was no indication of saturable metabolism based on blood concentrations. Endexposure blood concentrations for dogs were approximately 20-fold greater at 1,000 ppm compared with 50 ppm, reflecting the ratio of exposure concentrations. Metabolism ratios, normalized to body weight, were similar for the rat and dog.
Landry et al. (1983) and Nolan et al. (1985) reported that the relative steady-state blood concentrations of methyl chloride after a 3-h exposure was 194 ng/g in rats, 160 ng/g in dogs, 100 ng/g in rapidly metabolizing humans, and 35 ng/g in slowly metabolizing humans. The rat absorbed 10 μg/min/kg, and humans absorbed 3.7 μg/min/kg (rapid metabolizers) and 1.4 μg/min/kg (slow metabolizers).
4.3. Mechanism of Toxicity
Acute CNS effects might be from methyl chloride (this compound was once used as an anesthetic in combination with other anesthetics), but rapid metabolism probably limited its efficacy as an anesthetic.
Recent reviews state that the mechanism of neurotoxicity from methyl chloride is unclear, but most probably involves the metabolism of methyl chloride (i.e., the conjugation of methyl chloride with GSH). Acute exposures of rats and mice cause significant reductions in GSH concentrations in numerous organs, including the liver, kidneys, lungs, and brain (Dodd et al. 1982; Landry et al. 1983; Kornbrust and Bus 1984). A 6-h exposure of male F344 rats to methyl chloride at 1,500 ppm decreased the nonprotein sulfhydryl (primarily GSH) content of liver, kidney, and lungs to 17, 27, and 30% of control values, respectively (Dodd et al. 1982). At 500 ppm, values were 41, 59, and 55% of control values, respectively. At 1,500 ppm, recoveries to control concentrations occurred within 8 h. There were no changes in tissue GSH following a 6-h exposure to methyl chloride at 100 ppm. Blood concentrations of nonprotein sulfhydryl were not affected by the exposures. In a related study with male F344 rats, brain concentrations of GSH were reduced to a much lesser extent compared with the liver and kidney (Kornbrust and Bus 1984). The same tissues of male B6C3F1 mice were affected to a much greater degree. In another study, tissue nonprotein sulfhydryl in the liver, kidney, testis, and epididymis of rats decreased in a concentration-dependent manner in rats exposed at 225, 600, or 1,000 ppm for 6 h (Landry et al. 1983). No significant decreases of GSH were observed in the brain or blood.
Inhibition of GSH conjugation decreases the toxicity of methyl chloride (White et al. 1982; Chellman et al. 1986b). Mice exposed to methyl chloride at 1,500 ppm for 6 h/day, 5 days a week for 2 weeks developed multiple degenerative, necrotic foci in the internal granule cell layer of the cerebellum. Tremors, ataxia, and forelimb and hindlimb paralysis were associated with the cerebellar damage. Pretreatment of mice with buthionine-S,R-sulfoxime, a GSH depleter, protected mice from the cerebellar degeneration. Methanethiol and formaldehyde have been suggested as the toxic metabolites. Treatment of mice with methanethiol produces the same CNS symptoms of tremors, convulsion, and coma, as seen in animals and humans acutely intoxicated with methyl chloride (Heck et al. 1982; Kornbrust and Bus 1983; Chellman et al. 1986b).
Methyl chloride induces dominant lethal effects in several strains of rats. The mechanism of reproductive toxicity appears attributable to cytotoxic effects on sperm in the testes and to the effects of genotoxic oxidative metabolites resulting from an induced inflammatory response in the epididymides (Working et al. 1985b; Chellman et al. 1986c). Using both male and female F344 rats and male and female B6C3F1 mice exposed to methyl chloride at 1,000 ppm for 6 h/day for 4 days, Jager et al. (1988) found no biochemical sex differences in enzymatic transformation with respect to formaldehyde dehydrogenase or formaldehyde-induced genetic damage that would explain tumor formation. Mice, which have higher GSH activity in the kidneys than other species, appear to be more susceptible to methyl chloride.
Although the exact mechanism of action of methyl chloride is unclear, it appears to involve GSH depletion in target tissues and the production of toxic metabolites, such as formaldehyde or methanethiol, as well as lipid peroxidation. A lack of GSH might impair the ability of tissues to suppress lipid peroxidation reactions (Kornbrust and Bus 1984). The buildup of leukotrienes also has been suggested as a toxic mechanism of action. Under conditions of GSH depletion, 5-hydroperoxicoso-tetraenoic acid, the immediate precursor of leukotrienes, accumulates in the tissues. Leukotrienes are potent vasoconstrictors, and cause increased capillary permeability and tissue edema (AIHA 2003). Pretreatment of male F344 rats with the anti-inflammatory agent BW755C prevented inflammatory lesions of the brain, kidney, liver, and testis after exposure to methyl chloride at 5,000 ppm for 5 days (Chellman et al. 1986a).
A suggested mechanism of action for renal tumors in male mice is GSH depletion in the target tissue, increased lipid peroxidation, and formation of formaldehyde-induced DNA lesions (Bolt and Gansewendt 1993). Once GSH is depleted in the tissues, the alternate oxidative pathway via P-450 leads to formation of formaldehyde. Renal microsomes of male mice are more active than those of females in transforming methyl chloride to formaldehyde (Jager et al. 1988). Although Jager et al. (1988) did not observe increased formaldehyde concentrations in mouse liver or kidneys after a single, 8-h exposure at 1,000 ppm or increased DNA protein crosslinks after 4 days of exposure at 1,000 ppm, Ristau et al. (1989) observed an increase in DNA-protein crosslinks immediately
after an 8-h exposure. These crosslinks were attributed to the action of formaldehyde and were rapidly repaired.
4.4. Structure-Activity Relationships
von Oettingen et al. (1950) compared the toxicity of methyl chloride with that of dichloro-, trichloro-, and tetrachloro-methane in an unidentified strain of mice. The 7-h LC50 values were 3,080, 16,186, 5,761, and 9,528 ppm, respectively. The authors noted that methyl chloride had little narcotic action compared with the other chlorinated methanes. In the same study, dogs were found to be more susceptible to trichloromethane than methyl chloride, as measured by survival when exposed at 15,000 ppm.
For halogenated methanes, toxicity increases in the order methyl chloride, methyl bromide, and methyl iodide, but all produce similar toxic syndromes in man (Gosselin et al. 1984). However, few studies using comparable concentrations and exposure durations were available to make comparisons.
4.5. Other Relevant Information
4.5.1. Species Variability
There are differences in the rate at which species metabolize methyl chloride. The kinetics of methyl chloride in man are similar to those described for the rat and dog (Landry et al. 1983; Nolan et al. 1985). Blood concentrations reached a plateau during the first hour of exposure in all species and elimination was rapid once the exposures were terminated. Blood concentrations in slow human metabolizers reach a plateau at 60% of those found in the rat and 70% of those found in the dog. Post-exposure elimination was most rapid in the rat (t1/2 = 15 min). The dog and rapid human metabolizers eliminated methyl chloride at the same rate (t1/2 = 50 min), and the slow human metabolizers eliminated at the slowest rate (t1/2 = 90 min). During exposures to methyl chloride at 50 ppm, the rat absorbed 10 μg/min/kg whereas the rapid and slow human metabolizers absorbed 3.7 and 1.4 μg/min/kg. According to Nolan et al. (1985), differences in the pharmacokinetics between these three species were adequately explained by the differences in respiratory minute-volume and basal-metabolic rates (rat >dog > man). Using measurements of liver and kidney cytosol, Thier et al. (1998) placed the metabolism of methyl chloride in the following order: B6C3F1 female mouse > B6C3F1 male mouse > F344 rat > hamster. Activity was two to seven times greater in liver cytosol than in kidney cytosol.
As noted in several laboratory studies, mice are more susceptible to the toxic effects of methyl chloride than rats. In both control and exposed animals (1,000 ppm, 6 h/day for 4 days), specific activity of GST was greater in the livers of male B6C3F1 mice than in female mice (by a factor of 2) and greater in the livers of male mice than in F344 rats of either sex (by a factor of 3). GST
activity was similar in the kidneys of male and female mice. Formaldehyde dehydrogenase (FDH) activity was higher in the liver and kidneys of mice than in rats. There were no sex differences in activity of FDH. Exposure to methyl chloride at1,000 ppm for 1 or 4 days reduced the activity of GST in the kidneys of male mice by approximately 10%; activity levels in the livers of male mice and kidneys of female mice and male and female rats did not change (Jager et al. 1988). In in vitro assays, sex-, strain-and species-specific differences in bioactivation of methyl chloride by cytochrome P-450 was seen in the liver and kidneys of rats and mice (Dekant et al. 1995). In kidney microsomes, the rate of oxidation of methyl chloride in mice was significantly greater in males than in females, and in rats of both sexes than in mice. The rate of oxidation in kidney microsomes was faster in CD-1 mice and NMRI mice than in C3H/He or C57Bl/6J mice. Oxidative dechlorination of methyl chloride via the P-450 system might lead to the formation of formaldehyde.
The available data for the related chemical, methyl bromide, allowed comparisons between rats and mice. Where data were available for the same exposure durations, mice were more susceptible to the lethal effects of methyl bromide than rats (see Chapter 5). On the basis of 30-min and 1-and 4-h LC50 values, the mouse is two-fold more sensitive to methyl bromide than the rat. This increased sensitivity may be related to the higher concentrations of GST found in mouse tissues (Griem et al. 2002). Increased sensitivity might also be a reflection of the higher respiratory rate of mice compared with rats.
Erythrocyte cytoplasm of rats, mice, Rhesus monkeys, cows, pigs, and sheep does not metabolize monohalomethanes, whereas the erythrocyte cytoplasm of approximately 60% of humans does (Redford-Ellis and Gowenlock 1971; Peter et al. 1989). Lack of erythrocytic metabolism might explain the rapid equilibrium between the gas phase of methyl chloride and methyl bromide and concentrations in whole blood of rats observed in pharmacokinetic studies.
For the related chemical, methyl bromide, the available data indicate that rabbits are more sensitive than rats or guinea pigs. Guinea pigs and rats withstood 6-month exposures to methyl chloride at 66 ppm without demonstrable effects, but rabbits became paralyzed (Irish et al. 1940). A similar result was observed in a more recent study (Anger et al. 1981). Rabbits exposed at 65 ppm began to lose weight by the third week of exposure and eyeblink responses and nerve conduction velocity in rabbits were significantly reduced. Rats were unaffected under the same exposure regime. Maternal toxicity was high in pregnant rabbits exposed to methyl bromide at 70 ppm before mating and through gestation, whereas no maternal toxicity was evident in rats exposed under the same scenario (Sikov et al. 1981; Hardin et al. 1981).
Methyl bromide was specifically toxic to the olfactory epithelium of the rat, whereas the other nasal epithelia were unaffected (Hurtt et al. 1988). Histochemical techniques revealed degeneration of the sensory and sustentacular cells but not the basal cells from which the former cells are regenerated. This was a reversible effect as the basal cells were not affected. The olfactory region (dorsal meatus) of rats is highly exposed to chemicals because of the air-flow characteristics
in the nasal turbinates. In rodents, an inhaled vapor traverses a few millimeters of resistant respiratory epithelium before reaching sensitive olfactory tissue; whereas, in humans, an inhaled vapor has to traverse several centimeters and a much larger surface area of respiratory epithelium to reach the olfactory tissue. A mathematical model based on a combination of computational fluid dynamics and physiologically-based pharmacokinetics showed that the dorsal meatus region of the rat nose receives 12-20% of the inhaled air (Bush et al. 1998; Frederick et al. 1998). A comparison with airflow patterns in the human nose shows that the olfactory epithelium in the dorsal meatus region of the nasal cavity of the rat is exposed at two-to three-fold greater concentrations of chemicals. Therefore, compared with the rat, it is likely that higher concentrations of methyl bromide would be required to induce this lesion in humans.
Comparison of data from in vitro and in vivo studies indicates that GST enzymes are much lower in human tissues (liver and lung) than in mice or rats (Andersen et al. 1987; Reitz et al. 1989). The data are consistent with the hypothesis that the rate of activation of mono-and di-halomethanes to toxic metabolites by the GST pathway occurs much more slowly in humans than in rodents. Jager et al. (1988) investigated the concentration of GSH in rodent tissues. Activities of GSH were 2-3 times greater in the liver of male B6C3F1 mice than in female mice or F344 rats of both sexes. Griem et al. (2002) compiled rodent-to-human ratios of GST activity in various tissues. Ratios of rat-tohuman and mouse-to-human GST activity in liver are 3.95 and 7.64, respectively. On the other hand, nonprotein sulfhydral content (primarily GSH) is similar in human, monkey, and rat tissues on a μmol/mL of tissue basis (Frederick et al. 2002). This was true for major organs, but not nasal tissue. Nonprotein sulfhydral content was greater in the nasal tissues of rats than humans.
4.5.2. Susceptible Populations
Interindividual variation in the rate of metabolism of methyl chloride has been observed in humans. At least two distinct populations of humans with different rates of metabolism of methyl chloride have been identified (Nolan et al. 1985; ATSDR 1998; WHO 2000). Differences in metabolic rate are attributed to the genetic polymorphism of GST. Depending on the presence or absence of GST, humans may be “fast metabolizers” or “slow metabolizers” of methyl chloride. There might be a third phenotype, non-conjugators (Warholm et al. 1994; Lof et al. 2000). Fast metabolism might lead to the formation of toxic metabolites that can exert their action before they can be eliminated. Slow metabolizers would be expected to be less susceptible to the toxic effects of methyl chloride. Because the elimination of methyl chloride is rapid in both populations, the difference is of questionable toxicologic significance. In addition, Nolan et al. (1985) noted that an exposure to methyl chloride at 50 ppm for 6 h did not affect blood GST concentrations of either population.
Among Caucasians, the majority of individuals possess at least one copy of the GST gene; 10-25% are non-conjugators (Warholm et al. 1994; Nelson et al. 1995; Kempkes et al. 1996). Approximately 60% of Asians lack the gene (Nelson et al. 1995).
Some forms of GST are present in the human fetal liver and other forms are present at birth at a low rate and increase after birth (Cresteil 1998).
As noted in ATSDR (1998), populations that have kidney or liver disease, anemia, or neurologic deficits might be more susceptible to the toxic effects of methyl chloride. Persons with a deficiency of glucose-6-phosphate dehydrogenase might have reduced concentrations of GSH (Bloom and Brandt 2001). Additionally, accidental exposures suggest that infants are more susceptible than adults (Langard et al. 1996). However, in the latter case, death of an infant was from acute pneumonia resulting from vomiting and aspiration after methyl bromide inhalation.
4.5.3. Concentration-Exposure Duration Relationship
At constant concentrations of methyl chloride at ≤200 ppm, the highest concentration in clinical studies, blood concentrations reach steady state within 1 h (Putz-Anderson et al. 1981a). A similar relationship between air and blood values was observed in animal studies.
4.5.4. Concurrent Exposure Issues
Putz-Anderson et al. (1981a,b) assessed the behavioral effects of inhaled methyl chloride alone (200 ppm for up to 3.5 h) or in combination with 10 mg oral diazepam (a CNS depressant), alcohol (0.8 mL/kg), or caffeine (3 mg/kg). Subjects were groups of 8 or 12 healthy males and females, ages 18 to 32 years. Three performance tests (visual vigilance, dual task, and time discrimination), designed to test attention or alertness, were administered before and during the treatment period. Exposure to methyl chloride at 200 ppm produced little or no behavioral impairment, whereas diazepam and alcohol alone produced a 10% (statistically significant) impairment. Caffeine produced a small, but significant impairment on only one test. When the drugs were administered in combination with methyl chloride, the effect was equivalent to the sum of the separate effects (there were no behavioral interactions).
5. DATA ANALYSIS FOR AEGL-1
5.1. Summary of Human Data Relevant to AEGL-1
There are few data on acute exposures to methyl chloride. In wellconducted clinical studies, methyl chloride at 10 ppm for 2 h (Lof et al. 2000),
10 or 50 ppm for 6 h (Nolan et al. 1985), 100 ppm for 7.5 h/day for 5 days (Stewart et al. 1980), 150 ppm for 7.5 h/day for 2 days (Stewart et al. 1980), or 200 ppm for 3.5 h (Putz-Anderson et al. 1981b) produced no discernible irritant or neurotoxic effects in human subjects. Both male and female subjects were tested, exercise was incorporated into the protocol of the Stewart et al. (1980) study, and adequate numbers of subjects were tested.
In reports of accidental exposures, the threshold for neurotoxic symptoms such as blurred vision and sleepiness appeared to be 1,000-2,000 ppm (Mac-Donald 1964). In occupational monitoring studies, chronic exposures to ≤200 ppm TWA (15-195 ppm) with peaks up to 500 ppm did not produce neurotoxic symptoms, whereas prolonged exposures to a TWA ≥265 ppm for 12-16 h workdays for 2-3 weeks resulted in neurologic symptoms (Dow Chemical Co. 1970, personal communication, as cited in ACGIH 2003; Scharnweber et al. 1974).
5.2. Summary of Animal Data Relevant to AEGL-1
Most animal studies of methyl chloride involved a repeat-exposure scenario. In the only acute study, no clinical signs were observed in male F344 rats exposed to methyl chloride at 100, 500, or 1,500 ppm for 6 h (Dodd et al. 1982). No histopathologic examinations were performed in this study. No clinical signs or tissue or organ lesions were observed in F344 rats exposed to methyl chloride continuously at 200 ppm for 48 h (Burek et al. 1981). No microscopic lesions were observed in rats exposed at 1,000 ppm for 6 h/day for 12 days (Morgan et al. 1982). Mice were more sensitive than rats; some strains showed hepatocellular degeneration after exposure at 500 ppm for 6 h/day for 12 days (Morgan et al. 1982). Lesions were not observed in other tissues. The no-effect concentration in rats, mice, and dogs exposed to methyl chloride for 6 h/day, 5 days/week for 13 weeks was 400 ppm (McKenna et al. 1981b).
5.3. Derivation of AEGL-1 Values
No adverse neurotoxic effects were observed in a clinical study in which healthy adults were given a single exposure to methyl chloride at 200 ppm for 3 or 3.5 h (Putz-Anderson et al. 1981a,b) or in a 5-day repeated exposure study of exercising adults exposed at 150 ppm for 7.5 h/day (Stewart et al. 1980). The subjects included both “fast” and “slow” metabolizers. The exposures failed to elicit physiologic, neurologic, behavioral, or clinical symptoms. Furthermore, in the absence of a clearly defined odor at the concentrations tested, the subjects were unable to differentiate between control and exposure days. None of the exposures produced mild, transient effects on which to base the AEGL-1 values. Because methyl chloride has no clearly defined odor or warning properties at concentrations that might be neurotoxic, AEGL-1 values are not recommended.
6. DATA ANALYSIS FOR AEGL-2
6.1. Summary of Human Data Relevant to AEGL-2
No reliable human data on acute exposures to methyl chloride were available. Accidental exposures at chemical plants provide an indication of the threshold for neurotoxic symptoms. In these reports, only workplace TWA concentrations were provided; personal monitors were not utilized. Most of the cases involved repeat exposures. These data indicate that sleepiness, dizziness, and blurred vision might occur at concentrations of 1,000-2,000 ppm, although repeated exposure at 2,000-4,000 ppm induced similar symptoms; very short exposures at 10,000 ppm also were tolerated with similar symptoms (Mac-Donald 1964). Reports of accidental exposures at a chemical plant document neurotoxic symptoms following a short exposure to methyl chloride at 1,000-2,000 ppm, after work for several days in an area where the measured area concentration ranged from <25 to 1,600 ppm, during 13 days of exposure at 2,000-4,000 ppm, and after brief excursions into an area where the measured concentration was >10,000 ppm. Prolonged exposures to methyl chloride for 12-16 h over several weeks at concentrations as low as 300 ppm might cause symptoms in some workers (Scharnweber et al. 1974).
6.2. Summary of Animal Data Relevant to AEGL-2
Except for an acute exposure of F344 rats to methyl chloride at 1,500 ppm for 6 h, which resulted in no clinical signs (Dodd et al. 1982), all other animal studies involved repeat exposures. No clinical signs or tissue or organ lesions were observed in rats exposed at 1,500 ppm for 6 h/day, 5 days/week for 90 days (Mitchell et al. 1979). Slight decrements in performance on a rotating rod were observed in female mice exposed at 800 and 1,600 ppm for 5.5 h/day for 4 days, but not after 8 or 11 days of exposure (Landry et al. 1985). No brain or spinal-cord lesions were observed in dogs exposed at 400 ppm for 5 h/day for 13 weeks (McKenna et al. 1981b).
6.3. Derivation of AEGL-2 Values
The AEGL-2 values for methyl chloride were based on several rat studies, and a monitoring study was used as support. The basis for the AEGL-2 values was the absence of clinical signs in rats exposed to methyl chloride at 1,500 ppm for 6 h/day for 1 day (Dodd et al. 1982) or 90 days (Mitchell et al. 1979). Because of the greater blood uptake of methyl chloride by rodents compared with humans (Landry et al. 1983; Nolan et al. 1985), an interspecies uncertainty factor of 1 was applied. Although humans differ in the rate at which they metabolize methyl chloride, the difference does not appear to be toxicologically significant (Nolan et al. 1985). Furthermore, most humans are rapid metabolizers, the more susceptible group. Therefore, an uncertainty factor of 3 was considered
appropriate for intraspecies differences. In the absence of time-scaling information, the AEGL values were scaled using the equation Cn × t= k, with default values of n = 3 for shorter durations and n = 1 for longer durations (NRC 2001). Because of the long exposure duration of the key study, the 10-min value was set equal to the 30-min value (see Table 6-7). Accidental exposures to methyl chloride at 1,000-2,000 ppm and repeated exposure at 2,000-4,000 ppm resulted in transient symptoms of blurred vision, dizziness, headache, and nausea in workers (MacDonald 1964). Exposure durations were not reported, but appeared to be throughout the workday. The application of an intraspecies uncertainty factor of 3 to the mean concentration of 1,500 ppm reported in this occupational monitoring report results in a value of 500 ppm, which is similar to the 4-and 8-h AEGL-2 values. Calculations are provided in Appendix A, and a category graph of the human and animal toxicity data in relation to the AEGL values are provided in Appendix B.
7. DATA ANALYSIS FOR AEGL-3
7.1. Summary of Human Data Relevant to AEGL-3
Although deaths have occurred from accidental exposures to methyl chloride, no reliable information on concentrations or exposure durations were available. Monitoring data indicate that healthy workers can withstand concentrations of methyl chloride at 2,000-4,000 ppm for several days and brief excursions at 10,000 ppm (MacDonald 1964).
7.2. Summary of Animal Data Relevant to AEGL-3
Only one group of researchers has published recent data on lethality (White et al. 1982; Chellman et al. 1986b). Older studies (before 1950) do not meet current testing standards, although results are similar to those reported by Chellman et al. (1986b). The LC50 values for methyl chloride in male and female B6C3F1 mice are 2,250 and 8,500 ppm, respectively (White et al. 1982; Chellman et al. 1986b). Few acute lethality data were available for the rat. Repeat exposures of B6C3F1 mice to methyl chloride at 1,000 ppm for 6 h/day for up to 12 days resulted in microscopic tissue lesions, but no deaths (Morgan et al. 1982). Similar results were observed when F344 rats were exposed to methyl chloride at 5,000 ppm for 5 days, although one rat died following the last exposure (Chellman et al. 1986a). The next highest concentration of 7,500 ppm for 6 h/day for 2 days resulted in 67% mortality during the 4-day post-exposure period (Chellman et al. 1986a).
TABLE 6-7 AEGL-2 Values for Methyl Chloride
|
|
||||
| 10 min | 30 min | 1h | 4h | 8h |
|
|
||||
| 1,100 ppm (2,277 mg/m3) | 1,100 ppm (2,277 mg/m3) | 910 ppm (1,884 mg/m3) | 570 ppm (1,180 mg/m3) | 380 ppm (787 mg/m3) |
|
|
||||
7.3. Derivation of AEGL-3 Values
Data on lethality are limited to LC50 values for the mouse, a particularly sensitive species. Because of the higher respiratory rate in mice and the higher concentrations of GSH in mouse liver and kidney, particularly the male mouse, compared with human tissues, the mouse is not considered an appropriate surrogate for human response to methyl chloride. On the basis of data from a monitoring study in which humans withstood repeated exposures to methyl chloride at 1,000-4,000 ppm, the 6-h LC50 of 2,200 ppm in male mice is not realistic for application to humans.
Two studies reported no deaths in rats during the first 4 days of 5-and 12-day exposures to methyl chloride at 5,000 ppm for 6 h/day (Morgan et al. 1982; Chellman et al. 1986a). Chellman et al. (1986a) reported that 1/5 rats died following the fifth day of exposure. Morgan et al. (1982) reported that no deaths occurred in a 12-day exposure study, although 6/10 males and 5/10 females exposed at 5,000 ppm (6 h/day) and 2/10 females exposed to 3,500 ppm (6 h/day) were moribund and were killed after the fifth day of exposure. Thus, similar responses (death or near death) were observed in both studies. However, since a single 6-h exposure to methyl chloride at 5,000 ppm did not produce death in either study, this value was considered the point-of-departure for lethality. As was done for the AEGL-2 values, interspecies and intraspecies uncertainty factors of 1 and 3, respectively, were applied. Time-scaling was performed using the equation Cn × t= k, with n = 1 for longer durations and n = 3 for shorter durations (NRC 2001). Because of the long exposure duration in the key studies, the 10-min value was set equal to the 30-min value (see Table 6-8). Calculations are provided in Appendix A, and a category graph of the toxicity data in relation to AEGL values are provided in Appendix B.
8.2. Comparison with Other Standards and Guidelines
Standards and guidelines for methyl chloride are summarized in Table 6-10. The American Industrial Hygiene Association did not derive a level-1 emergency response planning guideline (ERPG-1) for methyl chloride “because the odor threshold is greater than 200 ppm” (AIHA 2003). The 1-h ERPG-2 value of 400 ppm is one-half of the 1-h AEGL-2 value. The basis for the ERPG-2 value was several studies with laboratory animals; in particular, the basis was the noobserved effect level of 400 ppm for clinical signs and tissue lesions in rats, mice, and dogs exposed for 6 h/day, 5 days/week for 13 weeks (McKenna et al. 1981b). The AEGL-2 is based on a combination of human and animal data, but
TABLE 6-8 AEGL-3 Values for Methyl Chloride
|
|
||||
| 10 min | 30 min | 1h | 4h | 8h |
|
|
||||
| 3,800 ppm (7,866 mg/m3) | 3,800 ppm (7,866 mg/m3) | 3,000 ppm (6,210 mg/m3) | 1.900 ppm (3,933 mg/m3) | 1,300 ppm (2,691 mg/m3) |
|
|
||||
particularly on the single and repeat exposures to methyl chloride at 1,500 ppm. The ERPG-3 was based on LC50 values for the mouse and rat which were apparently reduced by a factor of 2. The mouse and rat are more sensitive to methyl chloride than humans; therefore, although the AEGL-3 values were based on animal data, values were chosen that were supported by occupational exposure data.
8. SUMMARY OF AEGLS
8.1. AEGL Values and Toxicity Endpoints
AEGL values are listed in Table 6-9. A derivation summary is provided in Appendix C.
The NIOSH immediately dangerous to life and health (IDLH) value for methyl chloride is 2,000 ppm (NIOSH 1994). The IDLH is based on the report by MacDonald (1964) that a worker exposed to methyl chloride at 2,000 to 4,000 ppm for 13 days became sleepy and dizzy during the first week. A second worker exposed at 1,000-2,000 ppm during the workshift experienced dizziness, blurred vision, headache, nausea, and vomiting. The same study was used as support for the AEGL-3 values.
Chronic workplace standards for methyl chloride are lower than the AEGL values, and range from 25 ppm to 50 ppm. ACGIH (2003) noted that an exposure to methyl chloride at 100 ppm would be without health effects, but reduced the value to 50 ppm on the basis of a potentially limited margin of safety. ACGIH also recognized that repeated exposures might lead to cumulative effects. The German maximum workplace concentration (i.e., MAK) carries carcinogen, pregnancy, and skin absorption notations.
TABLE 6-9 Summary of AEGL Values for Methyl Chloride
|
|
|||||
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h |
|
|
|||||
| AEGL-1 (nondisabling) | NRa | NRa | NRa | NRa | NRa |
| AEGL-2 (disabling) | 1,100 ppm (2,277 mg/m3) | 1,100 ppm (2,277 mg/m3) | 910 ppm (1,884 mg/m3) | 570 ppm (1,180 mg/m3) | 380 ppm (787 mg/m3) |
| AEGL-3 (lethal) | 3,800 ppm (7,866 mg/m3) | 3,800 ppm (7,866 mg/m3) | 3,000 ppm (6,210 mg/m3) | 1,900 ppm (3,933 mg/m3) | 1,300 ppm (2,691 mg/m3) |
|
|
|||||
aAEGL-1 values are not recommended (NR) because methyl chloride has no odor or warning properties at concentrations that may be neurotoxic.
TABLE 6-10 Extant Standards and Guidelines for Methyl Chloride
|
|
|||||
| Exposure Duration | |||||
| Guideline | 10 min | 30 min | 1h | 4h | 8h |
|
|
|||||
| AEGL-1 | NRa | NRa | NRa | NRa | NRa |
| AEGL-2 | 1,100 ppm | 1,100 ppm | 910 ppm | 570 ppm | 380 ppm |
| AEGL-3 | 3,800 ppm | 3,800 ppm | 3,000 ppm | 1,900 ppm | 1,300 ppm |
| ERPG-1 (AIHA)b | NA | ||||
| ERPG-2 (AIHA) | 400 ppm | ||||
| ERPG-3 (AIHA) | 1,000 ppm | ||||
| IDLH (NIOSH) c | 2,000 ppm | ||||
| TLV-TWA (ACGLH) d | 50 ppm (skin notation)e | ||||
| REL-TWA (N10SH)f | Lowest feasible concentration | ||||
| PEL-TWA (OSHA)g | 100 ppm | ||||
| PEL-C (OSHA)h | 200 ppm 300 ppm (5-min maximum peak in anv 3h) | ||||
| TLV-STEL (ACGLH)i | 100 ppm | ||||
| MAK (Germany)j | 50 ppm (skin notation)e | ||||
| MAK Peak Limit (Geimany)k | Excursions of 15 mm duration at twice the MAK may take place 4 times'work shift at 1-h intervals | ||||
| MAC (The Netherlands)l | 25 ppm | ||||
|
|
|||||
a AEGL-1 values are not recommended (NR) because methyl chloride has no odor or warning properties at concentrations that may be neurotoxic.
b ERPG (emergency response planning guidelines, American Industrial Hygiene Association (AIHA 2003).
The ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor.
The ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protective action.
The ERPG-3 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing lifethreatening health effects.
c IDLH (immediately dangerous to life or health, National Institute for Occupational Safety and Health) (NIOSH 1994) represents the maximum concentration from which one could escape within 30 min without any escape-impairing symptoms or any irreversible health effects.
dTLV-TWA (threshold limit value-time weighted average, American Conference of Governmental Industrial Hygienists) (ACGIH 2003) is the time-weighted average concentration for a normal 8-h workday and a 40-h workweek, to which nearly all workers may be repeatedly exposed, day after day, without adverse effect.
e Dermal absorption might contribute substantially to systemic intoxication.
f REL-TWA (recommended exposure limit-time weighted average, National Institute for Occupational Safety and Health) (NIOSH 2010) is defined analogous to the ACGIH TLV-TWA.
g PEL-TWA (permissible exposure limit–time weighted average, Occupational Safety and Health Administration) (NIOSH 2010) is defined analogous to the ACGIH TLVTWA, but is for exposures of no more than 10 h/day, 40 h/week.
h PEL-C (permissible exposure limit-ceiling, Occupational Safety and Health Administration) (NIOSH 2010) is a value that must not be exceeded during any part of the workday.
i TLV-STEL (threshold limit value-short-term exposure limit, American Conference of Governmental Industrial Hygienists) (ACGIH 2003) is defined as a 15-min TWA exposure which should not be exceeded at any time during the workday even if the 8-h TWA is within the TLV-TWA. Exposures greater than the TLV-TWA and up to the STEL should not be longer than 15 min and should not occur more than four times per day. There should be at least 60 min between successive exposures in this range.
j MAK (maximale arbeitsplatzkonzentration [maximum workplace concentration]) [German Research Association (DFG 2003)] is defined analogous to the ACGIH TLV-TWA.
k MAK spitzenbegrenzung (peak limit) [German Research Association (DFG 2003)] constitutes the maximum average concentration to which workers can be exposed for a period up to 30 min with no more than two exposure periods per work shift; total exposure may not exceed 8-h MAK.
l MAC (maximaal aanvaarde concentratie [maximal accepted concentration]) (Dutch Expert Committee for Occupational Standards, The Netherlands) (MSZW 2004) is defined analogous to the ACGIH TLV-TWA.
Abbreviations: NA, not applicable; NR, not recommended.
8.3. Data Adequacy and Research Needs
The data from two well-conducted clinical studies define a no-effect concentration in healthy adults, and additional data were available from monitoring studies. Because there is inherent uncertainty in monitoring data, AEGL-2 and AEGL-3 values were based on both animal toxicity data and human monitoring studies. Most animal toxicity studies used a repeat-exposure scenario. Using
such studies for assess single exposures provides a margin of safety for AEGL-2 and AEGL-3 values. Although the neurotoxic mechanism of action of methyl chloride is not completely understood, metabolism is similar in various species and rate of metabolism appears related to body size and respiratory rate. Data were available on relative uptake and blood concentrations among species.
9. REFERENCES
ACGIH (American Conference of Government and Industrial Hygienists). 2003. Methyl Chloride. Documentation of the Threshold Limit Values and Biological Exposure Indices. American Conference of Government and Industrial Hygienists, Cincinnati, OH.
AIHA (American Industrial Health Association). 2003. Emergency Response Planning Guidelines: Methyl Chloride. Fairfax, VA: AIHA Press.
Andersen, M.E., M.L. Gargas, R.A. Jones, and L.J. Jenkins, Jr. 1980. Determination of the kinetic constants for metabolism of inhaled toxicants in vivo using gas uptake measurements. Toxicol. Appl. Pharmacol. 54(1):100-116.
Andersen, M.E., H.J. Clewell, M.L. Gargas, F.A. Smith, and R.H. Reitz. 1987. Physiologically based pharmacokinetics and the risk assessment process for methylene chloride. Toxicol. Appl. Pharmacol. 87(2):185-205.
Anger, W.K., J.V. Setzer, J.M. Russo, W.S. Brightwell, R.G. Wait, and B.L. Johnson. 1981. Neurobehavioral effects of methyl bromide inhalation exposures. Scand. J. Work Environ. Health 7 (suppl. 4):40-47.
ATSDR (Agency for Toxic Substances and Disease Registry). 1998. Toxicological Profile for Chloromethane. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA [online]. Available: http://www.atsdr.cdc.gov/toxprofiles/tp106.pdf [accessed Feb. 2, 2012].
Battigelli, M.C., and A. Perini. 1955. Two cases of acute methyl chloride intoxication [in Italian]. Med. Lav. 46(11):646-652.
Billings, C.E., and L.C. Jonas. 1981. Odor threshold in air as compared to threshold limit values. Am. Ind. Hyg. J. 42(6):479-480.
Bloom, J.C., and J.T. Brandt. 2001. Toxic responses of the blood. Pp. 389-418 in Casarett & Doull’s Toxicology: The Basic Science of Poisons, 6th Ed., C.D. Klaassen, ed. New York: McGraw-Hill.
Bolt, H.M., and B. Gansewendt. 1993. Mechanisms of carcinogenicity of methyl halides. Crit. Rev. Toxicol. 23(3):237-253.
Burek, J.D., W.J. Potts, T.S. Gushow, D.G. Keyes, and M.J. McKenna. 1981. Methyl Chloride: 48 and 72 Hour Continuous Inhalation Exposure in Rats Followed by up to 12 Days of Recovery. Five Reports Dealing with Studies of Methyl Chloride Pharmacokinetics and Inhalation Toxicity Studies, with Cover Letter Dated July11, 1981. Submitted by Toxicology Research Laboratory, Dow Chemical Company, Midland, MI, to U.S. Environmental Protection Agency, Washington, DC. EPA Document No. 408120723. Microfiche No. OTS0511317.
Bush, M.L., C.B. Frederick, J.S. Kimball, and J.S. Ultman. 1998. A CFD-PBPK hybrid model for simulating gas and vapor uptake in the rat nose. Toxicol. Appl. Pharmacol. 150(1):133-145.
Butterworth, B.E., T. Smith-Oliver, L. Earle, D.J. Loury, R.D. White, D.J. Doolittle, P.K. Working, R.C. Cattley, R. Jirtle, G. Michaloupoulos, and S. Strom. 1989. Use of primary cultures of human hepatocytes in toxicology studies. Cancer Res. 49(5):1075-1084.
Chapin, R.E., R.D. White, K.T. Morgan, and J.S. Bus. 1984. Studies of lesions induced in the testis and epididymis of F-344 rats by inhaled methyl chloride. Toxicol. Appl. Pharmacol. 76(2):328-343.
Chellman, G.J., K.T. Morgan, J.S. Bus, and P.K. Working. 1986a. Inhibition of methyl chloride toxicity in male F-344 rats by the anti-inflammatory agent BW755C. Toxicol. Appl. Pharmacol. 85(3):367-379.
Chellman, G.J., R.D. White, R.M. Norton, and J.S. Bus. 1986b. Inhibition of the acute toxicity of methyl chloride in male B6C3F1 mice by glutathione depletion. Toxicol. Appl. Pharmacol. 86(1):93-104.
Chellman, G.J., J.S. Bus, and P.K. Working. 1986c. Role of epididymal inflammation in the induction of dominant lethal mutations in Fischer-344 rat sperm by methyl chloride. Proc. Natl. Acad. Sci. USA 83(21):8087-8091.
Chellman, G.J., M.E. Hurtt, J.S. Bus, and P.K. Working. 1987. Role of testicular versus epididymal toxicity in the induction of cytotoxic damage in Fischer-344 rat sperm by methyl chloride. Reprod. Toxicol. 1(1):25-35.
CMR (Chemical Marketing Reporter). 1995. Chemical Profile: Methyl Chloride. CMR 6:44-45 (as cited in ATSDR 1998).
Cohen, J.M., R. Dawson, and M. Koketsu. 1980. Extent-of-Exposure Survey for Methyl Chloride. Technical Report No. 80-134. National Institute for Occupational Safety and Health, Centers for Disease Control, U.S. Department of Health and Human Services, Washington, DC.
Cresteil. T. 1998. Onset of xenobiotic metabolism in children: Toxicological implications. Food Addit. Contam. 15(suppl.):45-51.
Dekant, W., C. Frischmann, and P. Speerschneider. 1995. Sex, organ and species specific bioactivation of chloromethane by cytochrome P450E1. Xenobiotica 25(11):1259-1265.
DFG (Deutsche Forschungsgemeinschaft). 2003. List of MAK and BAT Values 2003. Maximum Concentrations and Biological Tolerance Values at the Workplace Report No. 39. Weinheim, Federal Republic of Germany: Wiley-VCH.
Dodd, D.E., J.S. Bus, and C.S. Barrow. 1982. Nonprotein sulfhydryl alterations in F-344 rats following acute methyl chloride inhalation. Toxicol. Appl. Pharmacol. 62(2):228-236.
DOT (U.S. Department of Transportation). 1985. CHRIS Hazardous Chemical Data: Methyl Chloride. U.S. Department of Transportation, Washington, DC.
Dunn, R.C., and W.W. Smith. 1947. The acute and chronic toxicity of methyl chloride: Histopathological observations. Arch. Pathol. 43(3):296-300.
EPA (U.S. Environmental Protection Agency). 2003. Methyl Chloride. Integrated Risk Information System. U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/iris/index.html [accessed Feb. 6, 2012].
Fostel, J., P.F. Allen, E. Bermudez, A.D. Kligerman, J.L. Wilmer, and T.R. Skopek. 1985. Assessment of the genotoxic effects of methyl chloride in human lymphoblasts. Mutat. Res. 155(1-2):75-81.
Frederick, C.B., M.L. Bush, L.G. Lomax, K.A. Black, L. Finch, J.S. Kimbell, K.T. Morgan, R.P. Subramaniam, J.B. Morris, and J.S. Ultman. 1998. Application of a hybrid computational fluid dynamics and physiologically based inhalation model for
interspecies dosimetry extrapolation of acidic vapors in the upper airways. Toxicol. Appl. Pharmacol. 152(1):211-231.
Frederick, C.B., L.G. Lomax, K.A. Black, L. Finch, H.E. Scribner, J.S. Kimbell, K.T. Morgan, R.P. Subramaniam, and J.B. Morris. 2002. Use of a hybrid computational fluid dynamics and physiologically based inhalation model for interspecies dosimetry comparisons of ester vapors. Toxicol. Appl. Pharmacol. 183(1):23-40.
Gosselin, R.E., R.P. Smith, and H.C. Hodge. 1984. Clinical Toxicology of Commercial Products, 5th Ed. Baltimore, MD: Williams & Wilkins.
Griem, P., M. Hassauer, F. Kaberlah, J. Oltmanns, J. Scheibner, K. Schneider, and U. Schuhmacher-Wolz. 2002. Quantitative Differences in Xenobiotic Metabolism between Experimental Animals and Humans. Federal Institute for Occupational Safety and Health, Dortmund, Germany [online]. Available: http://www.baua.de/SharedDocs/Downloads/en/Publications/Research-reports/2002/Fb963.pdf?__blob=publiccationFile [accessed Jan. 27, 2012].
Gudmundsson, G. 1977. Methyl chloride poisoning 13 years later. Arch. Environ. Health 32(5):236-237.
Hake, C.L., R.D. Stewart, A. Wu, H.V. Forester, and P.E. Newton. 1977. Experimental human exposures to methyl chloride at industrial environmental levels. Toxicol. Appl. Pharmacol. 41(1):198[Abstract 160].
Hamm, T.E., T.H. Raynor, M.C. Phelps, C.D. Auman, W.T. Adams, J.E. Proctor, and R. Wolkowski-Tyl. 1985. Reproduction in Fischer-344 rats exposed to methyl chloride by inhalation for two generations. Fundam. Appl. Toxicol. 5(3):568-577.
Hardin, B.D., G.P. Bond, M.R. Sikov, F.D. Andrew, R.P. Beliles, and R.W. Niemeier. 1981. Testing of selected workplace chemicals for teratogenic potential. Scand. J. Environ. Health 7(suppl. 4):66-75.
Heck, H.D., E.L. White, and M. Casanova-Schmitz. 1982. Determination of formaldehyde in biological tissues by gas chromatography/mass spectrometry. Biomed. Mass Spectrom. 9(8):347-353.
Henderson, Y. 1930. “Somnoform.” J. Am. Med. Assoc. 95(19):1445.
Holbrook, M.T. 1992. Chlorocarbons, hydrocarbons. Pp. 1028-1040 in Kirk-Othmer Encyclopedia of Chemical Technology, 4th Ed., Vol. 5, J.I. Kroschwitz, and M. Howe-Grant, eds. New York: John Wiley & Sons.
Holmes, T.M., P.A. Buffler, A.H. Holguin, and B.P. His. 1986. A mortality study of employees at a synthetic rubber manufacturing plant. Am. J. Ind. Med. 9(4):355-362.
HSDB (Hazardous Substances Databank). 2005. Methyl Chloride (CASRN 74-87-3). TOXNET, Specialized Information Services. U.S. National Library of Medicine: Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen? HSDB [accessed Feb. 2, 2012].
Hurtt, M.E., D.A. Thomas, P.K. Working, T.M. Monticello, and K.T. Morgan. 1988. Degeneration and regeneration of the olfactory epithelium following inhalation exposure to methyl bromide: Pathology, cell kinetics, and olfactory function. Toxicol. Appl. Pharmacol. 94(2):311-328.
IARC (International Agency for Research on Cancer). 1999. Methyl chloride. Pp. 737-748 in Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide. IARC Monographs on Evaluation of Carcinogenic Risk to the Humans Vol. 71. Lyon, France: IARC [online]. Available: http://monographs.iarc.fr/ENG/Monographs/vol71/mono71.pdf [accessed Feb. 3, 2012].
Irish, D.D., E.M. Adams, H.C. Spencer, and V.K. Rowe. 1940. The response attending exposure of laboratory animals to vapors of methyl bromide. J. Ind. Hyg. Toxicol. 22(6):218-230.
Jager, R., H. Peter, W. Sterzel, and H.M. Bolt. 1988. Biochemical effects of methyl chloride in relation to its tumorigenicity. J. Cancer Res. Clin. Oncol. 114(1):64-70.
Jiang, X.Z., R. White, and K.T. Morgan. 1985. An ultrastructural study of lesions induced in the cerebellum of mice by inhalation exposure to methyl chloride. Neurotoxicology 6(1):93-104.
John-Green, J.A., F.F. Welsch, and J.S. Bus. 1985. Comments on heart malformations in B6C3F1 mouse fetuses induced by methyl chloride-continuing efforts to understand the etiology and interpretation of an unusual lesion. Teratology 32(3):483-487.
Jones, M.A. 1942. Methyl chloride poisoning. Quart. J. Med. 41:29-43.
Kempkes, M., F.A. Wiebel, K. Golka, P. Heitmann, and H.M. Bolt. 1996. Comparative genotyping and phenotyping of glutathione S-transferase GSTT 1. Arch. Toxicol. 70(5):306-309.
Kolkmann, F.W., and B. Volk. 1975. Necroses in the granular cell layer of the cerebellum due to methylchloride intoxication in guinea pigs [in German]. Exp. Pathol. 10(5-6):208-308.
Kornbrust, D.J., and J.S. Bus. 1983. The role of glutathione and cytochrome P-450 in the metabolism of methyl chloride. Toxicol. Appl. Pharmacol. 67(2):246-256.
Kornbrust, D.J., and J.S. Bus. 1984. Glutathione depletion by methyl chloride and association with lipid peroxidation in mice and rats. Toxicol. Appl. Pharmacol. 72(3):388-399.
Kornbrust, D.J., J.S. Bus, G. Doerjer, and J.A. Swenberg. 1982. Association of inhaled 14C methyl chloride with macromolecules from various rat tissues. Toxicol. Appl. Pharmacol. 65(1):122-134.
Landry, T.D., T.S. Gushow, P.W. Langvardt, J.M. Wall, and M.J. McKenna. 1981. Pharmacokinetics of Inhaled Methyl Chloride in Dogs. Five Reports Dealing with Studies of Methyl Chloride Pharmacokinetics and Inhalation Toxicity Studies, with Cover Letter Dated July 11, 1981. Submitted by Toxicology Research Laboratory, Dow Chemical Co., Midland, MI, to U.S. Environmental Protection Agency, Washington, DC. EPA Document No. 40-8120723. Microfiche No. OTS0511317.
Landry, T.D., T.S. Gushow, P.W. Langvardt, J.M. Wall, and M.J. McKenna. 1983. Pharmacokinetics and metabolism of inhaled methyl chloride in the rat and dog. Toxicol. Appl. Pharmacol. 68(3):473-486.
Landry, T.D., J.F. Quast, T.S. Gushow, and J.L. Mattsson. 1985. Neurotoxicity of methyl chloride in continuously versus intermittently exposed female C57BL/6 mice. Fundam. Appl. Toxicol. 5(1):87-98.
Langard, S., T. Rognum, O. Flotterod, and V. Skaug. 1996. Fatal accident resulting from methyl bromide poisoning after fumigation of a neighbouring house; leakage through sewage pipes. J. Appl. Toxicol. 16(5):445-448.
Lanham, J.M. 1982. Methyl chloride: An unusual incident of intoxication. Can. Med. Assoc. J. 126(6):593.
Lof, A., G. Johanson, A. Rannug, and M. Warholm. 2000. Glutathione transferase T1 phenotype affects the toxicokinetics of inhaled methyl chloride in human volunteers. Pharmacogenetics 10(7):645-653.
MacDonald, J.D. 1964. Methyl chloride intoxication: Report of 8 cases. J. Occup. Med. 6:81-84.
McKenna, M.J., T.S. Gushow, T.J. Bell, et al. 1981a. Methyl Chloride: A 72-Hour Continuous (23.5 hr/day) Inhalation Toxicity Study in Dogs and Cats. Submitted by Toxicology Research Laboratory, Dow Chemical USA, Midland, MI, to U.S. Environmental
Protection Agency, Washington, DC. EPA Document No. 87820010220, Microfiche No. OTS0206129.
McKenna, M.J., J.D. Burek, J.W. Henck, D.L. Wackerle, and R.C. Childs. 1981b. Methyl Chloride: A 90-Day Inhalation Toxicity Study in Rats, Mice and Beagle Dogs. Five Reports Dealing with Studies of Methyl Chloride Pharmacokinetics and Inhalation Toxicity Studies, with Cover Letter Dated July11, 1981. Submitted by Toxicology Research Laboratory, Dow Chemical USA, Midland, MI, to U.S. Environmental Protection Agency, Washington, DC. EPA Document No. No. 40-8120723. Microfiche No. OTS0511317.
Mitchell, R.I., K. Pavkov, R.M. Everett, and D.A. Holzworth. 1979. A Ninety Day Inhalation Toxicology Study in F-344 Albino Rats and B6C3F1 Mice Exposed to Atmospheric Methyl Chloride Gas. Battelle Columbus Laboratories for the Chemical Industry Institute of Toxicology, Research Triangle Park, NC. Microfishe No. OTS0205952.
Morgan, A., A. Black, and D.R. Belcher. 1970. The excretion in breath of some aliphatic halogenated hydrocarbons following administration by inhalation. Ann. Occup. Hyg. 13(4):219-233.
Morgan, K.T., J.A. Swenberg, T.E. Hamm, Jr., R. Wolkowski-Tyl, and M. Phelps. 1982. Histopathology of acute toxic response in rats and mice exposed to methyl chloride by inhalation. Fundam. Appl. Toxicol. 2(6):293-299.
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: Methylchloride. Den Haag: SDU Uitgevers [online]. Available: http://www.lasrook.net/lasrookNL/maclijst2004.htm [accessed Feb. 3, 2012].
Nelson, H.H., J.K. Wiencke, D.C. Christiani, T.J. Chang, Z.F. Zuo, B.S. Schwartz, B.K. Lee, M.R. Spitz, M. Wang, X. Xu, and K.T. Kelsey. 1995. Ethnic differences in the prevalence of the homozygous deleted genotype of glutathione S-transferase theta. Carcinogenesis 16(5):1243-1245.
NIOSH (National Institute for Occupational Safety and Health). 1984. Monohalomethanes: Methyl Chloride CH3Cl, Methyl Bromide CH3Br, Methyl Iodide CH3I. Current Intelligence Bulletin 43, September 27, 1984 [online]. Available: http://www.cdc.gov/niosh/84117_43.html [accessed Feb. 3, 2012].
NIOSH (National Institute for Occupational Safety and Health). 1994. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs): Methyl chloride. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH [online]. Available: http://www.cdc.gov/niosh/idlh/74873.html [accessed Feb. 3, 2012].
NIOSH (National Institute for Occupational Safety and Health). 2010. NIOSH Pocket Guide to Chemical Hazards: Methyl chloride. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH [online]. Available: cdc.gov/niosh/npg/npgd0403.html [accessed Feb. 03, 2012].
Nolan, R.J., D.L. Rick, T.D. Landry, L.P. McCarty, G.L. Agin, and J.H. Saunders. 1985. Pharmacokinetics of inhaled methyl chloride (CH3Cl) in male volunteers. Fundam. Appl. Toxicol. 5(2):361-369.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
O’Neil, M.J., A. Smith, and P.E. Heckelman, eds. 2001. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th Ed. Whitehouse Station, NJ: Merck.
Ott, M.G., G.L. Carlo, S. Steinberg, and G.G. Bond. 1985. Mortality among employees engaged in chemical manufacturing and related activities. Am. J. Epidemiol. 122(2):311-322.
Pavkov, K.L., W.D. Kerns, R.I. Mitchell, M.M. Connell, R.L. Persing, C.E. Chrisp, and H.H. Harroff. 1981. Final Report on a Chronic Inhalation Toxicology Study in Rats and Mice Exposed to Methyl Chloride. Dow Chemical Company/Battelle Columbus Laboratories for Chemical Industry Institute of Toxicology. Microfiche No. OTS0535223.
Peter, H., S. Deutschmann, C. Reichel, and E. Hallier. 1989. Metabolism of methyl chloride by human erythrocytes. Arch. Toxicol. 63(5):351-355.
Putz-Anderson, V., J.V. Setzer, J.S. Croxton, and F.C. Phipps. 1981a. Methyl chloride and diazepam effects on performance. Scand. J. Work Environ. Health 7(1):8-13.
Putz-Anderson, V., J.V. Setzer, and J.S. Croxton. 1981b. Effects of alcohol, caffeine and methyl chloride on man. Psychol. Rep. 48(3):715-725.
Rafnsson, V., and G. Gudmundsson. 1997. Long-term follow-up after methyl chloride intoxication. Arch. Environ. Health 52(5):355-359.
Redford-Ellis, M., and A.H. Gowenlock. 1971. Studies on the reaction of chloromethane with human blood. Acta Pharmacol. Toxicol. 30(1):36-48.
Reid, J.B. 2001. Saturated methyl halogenated aliphatic hydrocarbons: Methyl chloride. Pp. 2-12 in Patty’s Toxicology, 5th Ed., Vol. 5. New York: John Wiley & Sons.
Reitz, R.H., A.L. Mendrala, and F.P. Guengerich. 1989. In vitro metabolism of methylene chloride in human and animal tissues: Use in physiologically based pharmacokinetic models. Toxicol. Appl. Pharmacol. 97(2):230-246.
Repko, J.D., and S.M. Lasley. 1979. Behavioral neurological and toxic effects of methyl chloride: A review of the literature. CRC Crit. Rev. Toxicol. 6(4):283-302.
Repko, J.D., P.D. Jones, L.S. Garcia, E.J. Schneider, E. Roseman, and C.R. Corum. 1976. Behavioral and Neurological Effects of Methyl Chloride. NIOSH (DHEW) Publication No. 77-125, PB-274-770. U.S. Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health, Cincinnatti, OH.
Ristau, C., H.M. Bolt, and R.R. Vangala. 1989. Detection of DNA-protein crosslinks in the kidney of male B6C3F1 mice after exposure to methyl chloride. Arch. Toxicol. Suppl. 13:243-245.
Ruth, J.H. 1986. Odor thresholds and irritation levels of several chemical substances: A review. Am. Ind. Hyg. Assoc. J. 47(3):A142-A151.
Scharnweber, H.C., G.N. Spears, and S.R. Cowles. 1974. Chronic methyl chloride intoxication in six industrial workers. J. Occup. Med. 16(2):112-113.
Sikov, M.R., W.C. Cannon, D.B. Carr, R.A. Miller, L.F. Montgomery, and D.W. Phelps. 1981. Teratologic Assessment of Butylene Oxide, Styrene Oxide and Methyl Bromide. DHHS Publication (NIOSH) 81-124. PB 81-168-510. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, Cincinnati, OH.
Smith, W.W., and W.F. von Oettingen. 1947a. The acute and chronic toxicity of methyl chloride. I. Mortality resulting from exposure to methyl chloride in concentrations of 4000 to 300 parts per million. J. Ind. Hyg. Toxicol. 29(1):47-52.
Smith, W.W., and W.F. von Oettingen. 1947b. The acute and chronic toxicity of methyl chloride. II. Symptomatology of animals poisoned by methyl chloride. J. Ind. Hyg. Toxicol. 29(2):123-128.
Stewart, R.D., C.L. Hake, A. Wu, S.A. Graff, H.V. Forster, W.H. Keeler, A.J. Lebrun, P.E. Newton, and R.J. Soto. 1980. Methyl Chloride: Development of a Biologic Standard for the Industrial Worker by Breath Analysis. PB 81167 686. Medical College of Wisconsin, Milwaukee, WI.
Thier, R., F.A. Wiebel, A. Hinkel, A. Burger, T. Bruning, K. Morgenroth, T. Senge, M. Wilhelm, T.G. Schulz. 1998. Species differences in the glutathione transferase GSTT1-1 activity towards the model substrates methyl chloride and dichloromethane in liver and kidney. Arch. Toxicol. 72(10):622-629.
Torkelson, T.R., and V.K. Rowe. 1981. Halogenated aliphatic hydrocarbons containing chlorine, bromine, and iodine. Pp. 3433-3601 in Patty’s Industrial Hygiene and Toxicology, Vol. IIB. Toxicology, 3rd Ed., G.D. Clayton, and F.E. Clayton, eds. New York: John Wiley & Sons.
van Doorn, R., P.J. Borm, C.M. Leijdekkers, P.T. Henderson, J. Reuvers, and T.J. van Bergen. 1980. Detection and identification of S-methylcysteine in urine of workers exposed to methyl chloride. Int. Arch. Occup. Environ. Health 46(2):99-109.
von Oettingen, W.F., C.C. Powell, N.E. Sharpless, W.C. Alford, and L.J. Pecora. 1949. Relation Between the Toxic Action of Chlorinated Methanes and their Chemical and Physicochemical Properties. National Institutes of Health Bulletin No. 191. Washington, DC: U.S. Government Printing Office.
von Oettingen, W.F., C.C. Powell, N.E. Sharpless, W.C. Alford, and L.J. Pecora. 1950. Comparative studies of the toxicity and pharmacodynamic action of chlorinated methanes with special reverence to their physical and chemical properties. Arch. Int. Pharmacodyn. Ther. 81(1):17-34.
Warholm, M., A.K. Alexandrie, J. Hogberg, K. Sigvardsson, and K. Rannug. 1994. Polymorphic distribution of glutathione transferase activity with methyl chloride in human blood. Pharmacogenetics 4(6):307-311.
White, R.D., R. Norton, and J.S. Bus. 1982. Evidence for S-methyl glutathione metabolism in mediating the acute toxicity of methyl chloride (MeCl). Pharmacologist 24(3):172 [Abstract 429].
WHO (World Health Organization). 2000. Concise International Chemical Assessment Document 28: Methyl Chloride. World Health Organization: Geneva, Switzerland.
Wolkowski-Tyl, R., M. Phelps, and J.K. Davis. 1983a. Structural teratogenicity evaluation of methyl chloride in rats and mice after inhalation exposure. Teratology 27(2):181-195.
Wolkowski-Tyl, R., A.D. Lawton, M. Phelps, and T.E. Hamm, Jr. 1983b. Evaluation of heart malformations in B6C3F1 mouse fetuses induced by in utero exposure to methyl chloride. Teratology 27(2):197-206.
Working, P.K., and J.S. Bus. 1986. Failure of fertilization as a cause of preimplantation loss induced by methyl chloride in Fischer 344 rats. Toxicol. Appl. Pharmacol. 86(1):124-130.
Working, P.K., and G.J. Chellman. 1989. The use of multiple endpoints to define the mechanism of action of reproductive toxicants and germ cell mutagens. Prog. Clin. Biol. Res. 302:211-227.
Working, P.K., J.S. Bus, and T.E. Hamm, Jr. 1985a. Reproductive effects of inhaled methyl chloride in the male Fischer 344 rat. I. Mating performance and dominant lethal assay. Toxicol. Appl. Pharmacol. 77(1):133-143.
Working, P.K., J.S. Bus, and T.E. Hamm, Jr. 1985b. Reproductive effects of inhaled methyl chloride in the male Fischer 344 rat. II. Spermatogonial toxicity and sperm quality. Toxicol. Appl. Pharmacol. 77(1):144-157.
APPENDIX A
DERIVATION OF AEGL VALUES FOR METHYL CHLORIDE
Derivation of AEGL-1 Values
AEGL-1 values are not recommended because methyl chloride has no odor or warning properties at concentrations that might be neurotoxic.
Derivation of AEGL-2 Values
| Key studies: | Mitchell, R.I., K. Pavkov, R.M. Everett, and D.A. Holzworth. 1979. A Ninety Day Inhalation Toxicology Study in F-344 Albino Rats and B6C3F1 Mice Exposed to Atmospheric Methyl Chloride Gas. Battelle Columbus Laboratories for the Chemical Industry Institute of Toxicology, Research Triangle Park, NC.Microfiche No. OTS0205952. |
| Dodd, D.E., J.S. Bus, and C.S. Barrow. 1982. Nonprotein sulfhydryl alterations in F-344 rats following acute methyl chloride inhalation Toxicol. Appl. Pharmacol. 62(2):228-236. | |
| Toxicity end point: | No clinical signs in rats exposed at 1,500 ppm for 6 h. No clinical signs in rats exposed at 1,500 ppm for 6 h/day, 5 days/week for 90 days. |
| Time scaling: | Cn × t = k, default values of n = 3 for shorter exposure durations and n = 1 for longer exposure durations. |
| (1,500 ppm/3)3× 360 min = 4.5 × 1010 ppm3-min (1,500 ppm/3) × 360 = 1.8 × 105 ppm-min |
|
| Uncertainty factors: | 1 for interspecies differences; uptake is greater in rats than in humans. 3 for intraspecies variability; differences in metabolism were not considered toxicologically significant. |
| Modifying factor: | Not applicable |
| Calculations: | |
| 10-min AEGL-2: | Set equal to the 30-min value because of the long exposure durations of the key studies. |
| 30-min AEGL-2: | C = (4.5 ×x 1010 ppm3-min ÷ 30min)1/3 C = 1,100 ppm |
| 1-h AEGL-2: | C = (4.5 × 1010 ppm3-min ÷ 60 min)1/3 C = 910 ppm |
| 4-h AEGL-2: | C = (4.5 × 1010 ppm3-min ÷ 240 min)1/3 C = 570 ppm |
| 8-h AEGL-2: | C = (1.8 × 105 ppm-min) ÷ 480 min C = 380 ppm |
| Derivation of AEGL-3 Values | |
| Key studies: |
Morgan, K.T., J.A. Swenberg, T.E. Hamm, Jr., R. Wolkowski-Tyl, and M. Phelps. 1982. Histopathology of acute toxic response in rats and mice exposed to methyl chloride by inhalation. Fundam. Appl. Toxicol. 2(6):293-299. |
| Chellman, G.J., K.T. Morgan, J.S. Bus, and P.K. Working. 1986a. Inhibition of methyl chloride toxicity in male F-344 rats by the antiinflammatory agent BW755C. Toxicol. Appl. Pharmacol. 85(3):367-379. | |
| Toxicity end point: |
5,000 ppm for 6 h/day for 12 days was nonlethal to rat for 5 days, one death following the fifth day of exposure. |
|
5,000 ppm for 6 h/day for 5 days was nonlethal to rats. |
|
| Time scaling: |
Cn × t = k; default values of n = 3 for shorter exposure durations and n = 1 for longer exposure durations. (5,000 ppm ÷ 3)3 × 360 min = 1.67 ×1012 ppm3-min (5,000 ppm ÷ 3) × 360 = 6.0 × 105 ppm-min |
| Uncertainty factors: |
1 for interspecies difference; uptake is greater in rats than in humans 3 for intraspecies variability; differences in metabolism were not considered toxicologically significant. |
| Modifying factor: | Not applicable |
| Calculations: | |
| 10-min AEGL-3: | Set equal to the 30-min value because of the long exposure durations of the key studies. |
| 30-min AEGL-3: | C = (1.67 × 1012 ppm3-min ÷ 30 min)1/3C = 3,800 |
| 1-h AEGL-3: | C = (4.5 × 1012 ppm3-min ÷ 60 min)1/3C = 3,000 ppm |
| 4-h AEGL-3: | C = (1.67 × 1012 ppm3-min ÷ 240 min)1/3C = 1,900 ppm |
| 8-h AEGL-3: | C = (6.0 × 105 ppm3-min) ÷ 480 min C = 1,300 ppm |
APPENDIX B
CATEGORY GRAPH OF HUMAN AND ANIMAL TOXICITY DATA
AND AEGL VALUES FOR METHYL CHLORIDE
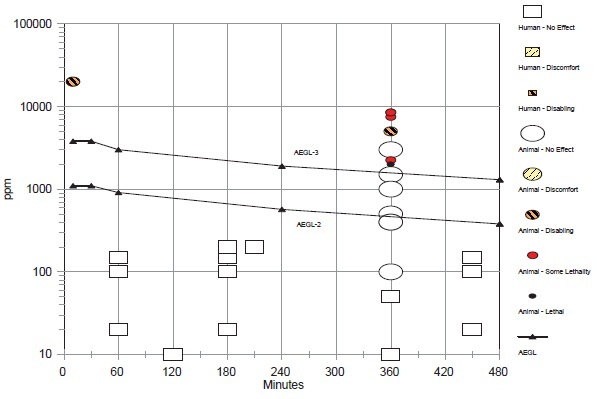
FIGURE B-1 Category graph of human and animal toxicity data in relation to AEGL values for methyl chloride. Some of the data points represent repeat exposures. The two lethal concentrations are for the mouse, a particularly sensitive species.
APPENDIX C
ACUTE EXPOSURE GUIDELINE LEVELS FOR METHYL CHLORIDE
Derivation Summary for Methyl Chloride
AEGL-1 VALUES
AEGL-1 values are not recommended because methyl chloride has no odor or warning properties at concentrations that might be neurotoxic.
AEGL-2 VALUES |
||||
|
|
||||
| 10 min | 30 min | 1h | 4h | 8h |
|
|
||||
| 1,100 ppm | 1,100 ppm | 910 ppm | 570 ppm | 380 ppm |
|
|
||||
Key references:
(1) Dodd, D.E., J.S. Bus, and C.S. Barrow. 1982. Nonprotein sulfhydryl alterations in F-344 rats following acute methyl chloride inhalation. Toxicol. Appl. Pharmacol. 62(2):228-236.
(2) Mitchell, R.I., K. Pavkov, R.M. Everett, and D.A. Holzworth. 1979. A Ninety Day Inhalation Toxicology Study in F-344 Albino Rats and B6C3F1 Mice Exposed to Atmospheric Methyl Chloride Gas. Battelle Columbus Laboratories. Microfiche No. OTS0205952.
Test species/Strain/Number: (1) groups of 20 male F344 rats; (2) 10 male and 10 female F344 rats/group
Exposure route/Concentrations/Durations: Inhalation, (1) 0, 100, 500, or 1,500 ppm for 6 h; (2) 0, 375, 750, or 1,500 ppm, 6 h/day, 5 days/week for 90 days.
Effects: (1) no clinical signs; (2) no clinical signs, no tissue lesions, increased liver weight at 1,500 ppm.
End point/Concentration/Rationale: NOAEL for clinical signs and tissue lesions.
Uncertainty factors/Rationale:
Total uncertainty factor: 3
Interspecies: 1, uptake is greater in rodents than in humans as measured by blood concentrations.
Intraspecies: 3, differences in uptake (by a factor of 2-3) and metabolism among humans are not considered toxicologically significant.
Modifying factor: Not applicable
Animal-to-human dosimetric adjustment: Not applied
Time scaling: Cn × t = k; default values of n = 3 for durations shorter than 6 h and n = 1 for durations longer than 6 h. Because of the long exposure durations of the key studies, the 10-min value was set equal to the 30-min value.
| 10 min | 30 min | 1h | 4h | 8h |
|
|
||||
| 1,100 ppm | 1,100 ppm | 910 ppm | 570 ppm | 380 ppm |
|
|
||||
Data adequacy: The animal studies were well conducted. The 90-day exposure duration of one of the key studies ensures a true NOAEL after a single 6-h exposure. Animal tissues were examined microscopically after the 90-day exposure. The AEGL values are supported by a monitoring study in which accidental, repeat exposures to methyl chloride at 1,000-4,000 ppm (duration not known) resulted in transient neurotoxic symptoms (MacDonald 1964). The values are also supported by clinical studies in which no effects were observed after a 3.5-h exposure at 200 ppm (Putz-Anderson et al. 1981b) or after a 7.5-h exposure (with exercise) at 150 ppm (Stewart et al. 1980).
AEGL-3 VALUES |
||||
|
|
||||
| 10 min | 30 mins | 1h | 4h | 8h |
|
|
||||
| 3,800 ppm | 3,800 ppm | 3,000 ppm | 1,900 ppm | 1,300 ppm |
|
|
||||
Key references:
(1) Morgan, K.T., J.A. Swenberg, T.E. Hamm, Jr., R. Wolkowski-Tyl, and M. Phelps. 1982. Histopathology of acute toxic response in rats and mice exposed to methyl chloride by inhalation. Fundam. Appl. Toxicol. 2(6):293-299.
(2) Chellman, G.J., K.T. Morgan, J.S. Bus, and P.K. Working. 1986a. Inhibition of methyl chloride toxicity in male F-344 rats by the anti-inflammatory agent BW755C. Toxicol. Appl. Pharmacol. 85(3):367-379.
Test species/Strain/Number: (1) Groups of 10 male and 10 female F344 rats; (2) groups of 5 male F344 rats
Exposure route/Concentrations/Durations: Inhalation, (1) 5,000 ppm for 6 h/day, 12 days; (2) 5,000 ppm for 6 h/day, 5 days.
Effects: (1) moribund state in 11/20 rats on day 5, tissue and organ lesions; (2) death of 1/5 on day 5.
End point/Concentration/Rationale: Threshold for lethality on day 1 at exposure concentration of 5,000 ppm.
Uncertainty factors/Rationale:
Total uncertainty factor: 3
Interspecies: 1, uptake is greater in rodents than in humans as measured by blood concentrations.
Intraspecies: 3, differences in uptake (by a factor of 2-3) and metabolism among humans are not considered toxicologically significant.
Modifying factor: Not applicable
Animal-to-human dosimetric adjustment: Not applied
Time scaling: Cn × t = k; default values of n = 3 for durations shorter than 6 h and n = 1 for durations longer than 6 h. Because of the long exposure durations of the key studies, the 10-min value was set equal to the 30-min value.
| 10 min | 30 min | 1h | 4h | 8h |
|
|
||||
| 3,800 ppm | 3,800 ppm | 3,000 ppm | 1,900 ppm | 1,300 ppm |
|
|
||||
Data adequacy: Lethality data are sparse and, with the exception of the mouse, usually occurred after repeat exposures. The AEGL values are supported by a monitoring study in which accidental, repeat exposures to methyl chloride at 1,000- 4,000 (durations not known) resulted in transient neurotoxic symptoms (MacDonald 1964).























































