1
Butane1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory
![]()
1 This document was prepared by the AEGL Development Team composed of Peter Bos (RIVM, The Dutch National Institute of Public Health and the Environment), Julie M. Klotzbach (Syracuse Research Corporation), Chemical Managers Jonathan Borak and Larry Gephart (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances), and Ernest V. Falke (U.S. Environmental Protection Agency). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold concentrations for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Butane is a colorless gas with a faint disagreeable odor, although it is considered to be odorless by some. It is poorly soluble in water. The lower explosive limit is 1.9%. Butane is produced from natural gas. Its main uses are in the production of chemicals like ethylene and 1,3-butadiene, as a refrigerant, as an aerosol propellant, as a constituent in liquefied petroleum gas, and as the main component of gas lighter refills. Because it is easily accessible, butane is often used in inhalant abuse.
The toxicity of butane is low. Huge exposure concentrations can be assumed in butane abuse. The predominant effects observed in abuse cases are central nervous system (CNS) and cardiac effects. Case studies also reveal that serious brain damage and underdeveloped organs can occur in fetuses in case of high single exposures during the week 27 or 30 of pregnancy. Quantitative data for setting AEGL values are sparse. Quantitative human data include an old study with human volunteers focused on the warning properties of butane.
Mortality from butane in mice and rats is preceded by CNS effects. Some data are available on cardiac effects in dogs, but they are insufficient for setting AEGL values. Data on CNS effects are available for mice and guinea pigs. Butane was negative in the bacterial reverse-mutation assay (Ames test). Carcinogenicity studies and studies on reproductive toxicity are lacking.
The AEGL-1 values for butane are based on observations in a study with volunteers on the warning properties of short exposures to butane (Patty and Yant 1929). It was concluded that 10,000 ppm (10-min exposure) was a boundary for drowsiness. An intraspecies uncertainty factor of 1 is considered adequate because the concentration-response curve for CNS-effects appears to be very steep; thus, interindividual variability will be relatively small. Also, no noticeable irritation was reported at concentrations up to 100,000 ppm (probably for a few min), and a larger uncertainty factor of 3 would lead to unrealistically low AEGL-1 values. Available data suggest a relatively high value for n (Stoughton and Lamson 1936), so time extrapolation was performed using n = 3. Data on butane (Gill et al. 1991) and propane (Stewart et al. 1977) indicate that steady-state plasma concentrations for butane will be reached within 30 min. By analogy to other CNS-depressing substances, the effects of butane are assumed to be solely concentration dependent. Therefore, time extrapolation was performed from 10 min to 30-and 60-min exposures, where the steady-state concentration was calculated. The calculated values for AEGL-1 are presented in Table 1-1. The values are considered protective of the irregular breathing observed in guinea pigs exposed to butane at 21,000-28,000 ppm for up to 2 h (Nuckolls 1933). The calculated 10-min AEGL-1 value is greater than 50% of the lower explosive limit for butane, and the other AEGL-1 values are greater than than 10% of the lower explosive limit.
The AEGL-2 values for butane are based on a study with guinea pigs exposed to butane for 2 h at concentrations between 50,000 and 56,000 ppm (Nuckolls 1933). Animals had a “dazed appearance,” but were able to walk. Therefore, the effects were considered not to be serious enough to impair escape and the lower value in this range (50,000 ppm) was used as starting point for the derivation of AEGL-2 values. Small interindividual differences are expected because the effects are attributed to butane itself and no relevant differences in kinetics are assumed. However, a large uncertainty factor is not necessary considering the steep concentration-response curve; a large factor also would lead to unrealistically low AEGL-2 values that would be similar to the AEGL-1 values. Thus, a total uncertainty factor of 3 is considered sufficient. Time extrapolation was performed using n = 3 for similar reasons as for AEGL-1. No increase in effect from longer durations of exposure is expected for concentrationdependent effects after reaching a steady state. For the same reasons as for AEGL-1, steady-state plasma concentrations will be reached within 30 min of exposure. Thus, the AEGL-2 values for 30-min and 1, 4, and 8 h will be set equal to the 2-h concentration. The AEGL-2 values for the 10-min exposure is derived by time scaling according to the dose-response regression equation Cn × t = k, using n = 3. The calculated 10-min AEGL-2 value is greater than the lower explosive limit and that the other AEGL-2 values are greater than 50% of the lower explosive limit.
The AEGL-3 values for butane are based on an acute exposure study with rats and mice (Shugaev 1969). Mice and rats were exposed to butane for 2 and 4 h, respectively. The reported data allowed the calculation of LC01s (lethal concentrations,
1% lethality). The 2-h LC01 for mice was 160,000 ppm and the 4-h LC01 for rats was 172,000 ppm. The 2-h LC01 for mice was chosen as starting point for AEGL-3 derivation, because mice appear to be the more susceptible species and 160,000 ppm was the lowest concentration tested. A total uncertainty factor of 3 is considered sufficient to account for toxicokinetic and toxicodynamic differences between individuals and interspecies differences for the following reasons. The effects are attributed to butane itself and no relevant differences in kinetics are assumed. The data are from a species with a relatively high susceptibility to butane. The concentration-response curve appears to be very steep indicating that a large uncertainty factor is unnecessary. Further, a larger factor would lead to unrealistically low values that would be similar to the AEGL-2 values. Time scaling was conducted similar to that performed for AEGL-2 values. The AEGL-3 values for 30 min and for 1, 4 and 8 h of exposure were set equal to that for the 2-h AEGL value. The AEGL-3 values for the 10-min exposure were derived by time scaling according to the dose-response regression equation Cn × t = k, using n = 3. The calculated 10-min value of 77,000 ppm is supported by the data from Patty and Yant (1929). They reported that exposure to slowly increasing concentrations of butane up to 50,000 ppm (total exposure duration at least 10 min) and a short exposure (duration not specified) to 100,000 ppm on the same day did not result in serious complaints (Patty and Yant 1929). All of the AEGL-3 values are greater than the lower explosive limit for butane.
The AEGL values for butane are presented in Table 1-1.
TABLE 1-1 Summary of AEGL Values for Butane
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| AEGL-1 (non disabling) | See belowa | 6,900 ppmb (16,000 mg/m3) | 5,500 ppmb (13,000 mg/m3) | 5,500 ppmb (13,000 mg/m3) | 5,500 ppmb (13,000 mg/m3) | Drowsiness in humans (Patty and Yant 1929) |
| AEGL-2 (disabling) | See belowc | See belowd | See belowd | See belowd | See belowd | Dazed appearance in guinea pigs (Nuckolls 1933) |
| AEGL-3 (lethal) | See belowe | See belowe | See belowe | See belowe | See belowe | LCoi in mice (Shugaev 1969) |
a The 10-min AEGL-1 value is 10,000 ppm (24,000 mg/m3) which is greater than 50% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
b The AEGL-1 value is greater than 10% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, safety considerations against the hazard of explosion must be taken into account.
c The 10-min AEGL-2 value is 24,000 ppm (57,000 mg/m3), which is greater than the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
d The AEGL-2 values for 30 min and 1, 4, and 8 h are 17,000 ppm (40,000 mg/m3), which is greater than 50% of the lower explosive limit for butane in air of 19,000 ppm. Therefore,
extreme safety considerations against the hazard of explosion must be taken into account.
e The 10-min AEGL-3 value is 77,000 ppm (180,000 mg/m3). The AEGL-3 values for 30 min and 1, 4, and 8 h are 53,000 ppm (130,000 mg/m3). These values are greater than the lower explosive limit for butane in air of 19,000 ppm). Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
1. INTRODUCTION
Butane is produced from raw natural gas and from petroleum streams obtained by catalytic cracking, catalytic reforming, and other refining processes. Butane is used in the production of ethylene and 1,3-butadiene, in the synthesis of a number of chemicals, as a refrigerant and an aerosol propellant, in the blending of gasoline or motor fuel, as a constituent in liquefied petroleum gas, and as an extraction solvent in deasphalting processes (Low et al. 1987). Butane used in gas lighter refills consists of butane with small amounts of isobutane and propane.
Chemical and physical data for butane are presented in Table 1-2.
2. HUMAN TOXICITY DATA
Most reports of butane intoxication are from cases of butane abuse or suicide attempts. These data are only briefly described because they provide no clear dose-response data and, for abuse cases, subjects generally have a history of repeated exposure, so tolerance to butane could have developed (Evans and Raistrick 1987). In addition, abuse of other volatile organic solvents cannot be excluded. Data on intoxication by liquefied petroleum gas (a mixture of predominantly propane and butane in varying proportions) were not considered.
2.1. Acute Lethality
2.1.1. Case Reports
Substance abuse is one of the predominant causes of death from butane intoxication. Fuel gases containing butane appeared to be responsible for about 30% of deaths from solvent abuse in the United Kingdom and aerosol propellants for about 20% (Adgey et al. 1995). In 2000, 64 deaths were associated with abuse of volatile substances; over 50% of the deaths were attributed to gas fuel inhalation, mainly butane lighter refills (Chaudhry 2002). In Virginia, 39 cases of people who likely died as a direct consequence abusing an inhalant were found between 1987 and 1996. Thirteen of these cases were associated with butane (Bowen et al. 1999). Clear central nervous system (CNS) effects were reported by butane abusers, including disturbed behavior, slow speech, elated mood, hallucinations, and illusionary experiences.
TABLE 1-2 Chemical and Physical Properties for Butane
|
|
||
| Parameter | Value | Reference |
|
|
||
| Synonyms | Diethyl; methylethylmethane | Lewis 1999 |
| CAS registry no. | 106-97-8 | |
| Chemical formula | C4H10 | |
| Molecular weight | 58.14 | Lewis 1999 |
| Physical state | Gas | Lewis 1999 |
| Color | Colorless | Lewis 1999 |
| Odor | Odorless Faint disagreeable odora | Lewis 1999 |
| Melting point | -138.35°C | Cavender 1994 |
| Boiling point | -0.5°C | Cavender 1994 |
| Density | Low et al. 1987; Lide 1999 | |
| Vapor Liquid |
2.07 (air = 1) 0.601 g/cm3 at-0.5°C (water = 1) 0.573 g/cm3 at-25°C (water = 1) |
|
| Solubility | 61 mg/L in water at 25°C | Low et al. 1987 |
| Vapor pressure | 243 kPa (25°C) | ECB 2000 |
| Flammability | Extremely flammable | ECB 2000 |
| Explosive | Lower explosive limit = 1.9% | Lewis 1999 |
| Conversion factors | 1 mg/m3 = 0.422 ppm 1 ppm = 2.37 mg/m3 |
Low et al. 1987 |
|
|
||
a Although butane is considered odorless by some, it has been reported that the odor of butane can be detected at concentrations of 1.2-6.2 ppm (2.85-14.63 mg/m3) (Ruth 1986).
Graefe et al. (1999) described a fatal case of a 19-year-old male. He had a history of butane abuse. Froth was present in the trachea and bronchi; pulmonary edema was also reported. The highest concentrations of butane were found in the liver (310 μg/g), brain (282 μg/g), blood (129 μg/mL), and kidneys (54 μg/g).
A 14-year-old boy was found unconsciousness as a result of butane abuse; he died 34 h after the exposure despite resuscitation efforts (Rieder-Scharinger et al. 2000). Death was attributed to multiple organ failure involving the CNS (brain edema), cardiovascular system, pulmonary system, and the liver.
A 13-year-old boy died from butane abuse (Nishi et al. 1985). The cause of death was cardiac arrhythmia and lung edema. The boy had undergone an operation for a cardiac ventricular septal defect at the age of 10. Butane concentrations in his tissues were highest in fat (4.5 μL/g) and brain (3.9 μL/g), followed by kidney (2.1 μL/g), liver (2.0 μL/g), spleen (1.5 μL/g), heart (1.2 μL/g), and blood (0.9 μL/g). Propane and isobutane were also detected.
2.2. Nonlethal Toxicity
2.2.1. Case Reports
In nonfatal cases, butane appears to have frequently affected the heart and brain. Most of these cases involved inhalant abusers with repetitive exposure to butane.
Severe encephalopathy was observed in a 15-year-old girl as the result of abusive butane inhalation. She had been inhaling butane repeatedly for 4 weeks until an acute incident occurred. Cardiopulmonary resuscitation was needed. Repeated magnetic-resonance-imaging scans revealed disintegration of gray matter, increasing cerebral atrophy, and destruction of basal ganglia. Electroencephalography showed strongly diminished basal activity with flat amplitude (Döring et al. 2002). A 15-year-old boy, who was known to inhale butane from a plastic bag, had bilateral hemispheric infarcts (Bauman et al. 1991). Another 15-year-old boy suffered from right-sided hemiparesis after butane abuse; he did not lose consciousness. A computed tomography (CT) head scan on the day following admission to the hospital was normal. At the time of discharge (after 5 days), he still had pronounced upper limb, proximal muscle weakness and a hemiplegic gait (Gray and Lazarus 1993). In another case of butane abuse, a swollen brain was observed in a 15-year-old girl without a history of butane abuse (Williams and Cole 1998), while a CT head scan showed no abnormalities in a 17-year-old male with a 3-year history of butane abuse (Edwards and Wenstone 2000).
Ventricular tachycardia and ventricular fibrillation were noted in a 15-year-old boy, who was found unresponsive and cyanotic. He was known to inhale butane from a plastic bag. During his hospitalization his cardiac status improved but brain functions remained disturbed (Bauman et al. 1991). A 17-yearold male with a 3-year history of butane abuse was found collapsed and showing ventricular fibrillation. He was resuscitated during which he received epinephrine. An electrocardiogram showed an acute anterolateral infarction. Recovery was slow and complicated by acute renal failure and recurrent pulmonary edema (Edwards and Wenstone 2000). Roberts et al. (1990) described a 16-year-old boy who was found unconscious. He had been abusing lighter fuel for two months. The boy suffered from asystolic cardiac arrest and cardiopulmonary resuscitation was commenced. The patient was discharged 10 days after admittance to the hospital. Gunn et al. (1989) described ventricular fibrillation in a 15-year-old boy with a habit of lighter-fuel abuse. A few moments after one such episode of abuse he experienced severe anterior chest pain. He ran downstairs where he collapsed. An ambulance arrived within 5 min. The boy suffered from sinus tachycardia and a widespread ST-segment elevation was noted. A 15-yearold girl, without history of butane abuse, had been inhaling butane intermittently over a period of 2 h. She collapsed when running away from the police (Williams and Cole 1998). On admission to the hospital, there was no spontaneous respiration; an electrocardiogram showed sinus tachycardia with marked T-wave
inversion in the anterolateral leads. A CT scan showed a very tight swollen brain. For 5 days she remained cardiovascularly stable with persistent T-wave inversion on the electrocardiogram. It was concluded that the most plausible cause was a direct effect of butane on the myocardium. Butane could have caused cardiac sensitization, and a surge of adrenaline would have caused the arrhythmia rather than hypoxic arrest. Adgey et al. (1995) describe a case of a 16-year-old boy who collapsed following inhalation of butane from a cigarette lighter refill. The initial cardiac rhythm was ventricular asystole. Cardiopulmonary resuscitation was commenced. The electrocardiogram showed T-wave inversion across the anterior chest leads.
Cartwright et al. (1983) reported pleural effusions and pulmonary infiltrates in a 19-year-old man who had been “fire-breathing.” He had filled his oral cavity with butane from a cigarette lighter and forcefully expelled it over a flame. Because butane is heavier than air, the pulmonary effects were considered to be the result of descending butane gas into the tracheobronchial tree by gravity.
2.2.2. Experimental Studies
Patty and Yant (1929) studied the warning properties of several alkanes, including butane. In a continuous exposure test, subjects were exposed to butane at slowly increasing concentrations up to 50,000 ppm for an unknown duration (but at least 10 min). In an intermittent exposure test, subjects were exposed at fixed concentration for a short, unspecified duration. The concentrations of butane in the intermittent exposure test were approximately 1,000, 2,000, 5,000, 7,000, 10,000, 20,000, and 100,000 ppm. Exposure groups consisted of 3-6 laboratory or clerical personnel (males and females, 20-30 years of age). Subjects first underwent the continuous exposure test, followed on the same day by the intermittent exposure test after a recovery period. The chamber concentration was periodically analyzed. Odor detection was rated by means of an odor scale ranging from 0 (no detectable odor) to 5 (intense effect, may bite or irritate). Individual scores did not differ much from the average scores. Butane could not be detected in the continuous exposure test at concentrations up to 50,000 ppm. In the intermittent exposure test, butane at 18,000 ppm was described as having a “weak odor, readily perceptible” (mean score of 2). The score for odor detection was below 4 (cogent, forcible odor) at 100,000 ppm. The physiologic responses were very briefly reported. Although a table in the report indicated that exposure to butane at 10,000 ppm for 10 min caused drowsiness, this was contradicted by a statement in the text that 10-min exposure to butane 10,000 ppm caused no symptoms.
2.2.3. Occupational and Epidemiological Studies
No data were available.
2.3. Neurotoxicity
Several case reports of intentional butane exposure indicate that butane induces neurotoxicity. Severe encephalopathy was observed in a 15-year-old girl as the result of butane abuse. She had been inhaling butane repeatedly for 4 weeks, until an acute incident occurred that required cardiopulmonary resuscitation. Repeated magnetic resonance imaging over the following weeks revealed disintegration of gray matter, increasing cerebral atrophy, and destruction of basal ganglia. An electroencephalogram showed strongly diminished basal activity with flat amplitude (Döring et al. 2002). A 15-year-old boy who was known to inhale butane from a plastic bag had bilateral hemispheric infarcts (Bauman et al. 1991). Another 15-year-old boy suffered from right-sided hemiparesis after butane abuse; he did not lose consciousness. A CT head scan on the day following admission to the hospital was normal. At the time of discharge (5 days later), he still had pronounced upper limb, proximal muscle weakness and a hemiplegic gait (Gray and Lazarus 1993). In another case, a swollen brain was observed in a 15-year-old girl without a history of butane abuse (Williams and Cole 1998), whereas a CT head scan showed no abnormalities in a 17-year-old male with a 3-year history of butane abuse (Edwards and Wenstone 2000).
A 15-year-old boy was found unresponsive and cyanotic after reportedly inhaling butane from a plastic bag. Ventricular tachycardia and ventricular fibrillation were noted. Cardiac status improved during hospitalization, but brain functions remained disturbed (Bauman et al. 1991). A 15-year-old girl without a history of butane abuse inhaled butane intermittently over a 2-h period. She collapsed when running away from the police (Williams and Cole 1998). On admission to the hospital, there was no spontaneous respiration; a CT scan showed a very tight, swollen brain and an electrocardiogram showed sinus tachycardia with marked T-wave inversion in the anterolateral leads.
2.4. Developmental and Reproductive Toxicity
A pregnant 34-year-old woman accidentally inhaled butane in during week 27 of her pregnancy. She was found unconscious and required mechanical ventilation for 5 h. The exposure duration and concentration of butane were not reported, nor was the amount of time that elapsed before resuscitation commenced. She gradually regained consciousness and was discharged 48 h after admission. An ultrasound at week 39 of the pregnancy showed an almost complete absence of brain tissue in the fetus. The female child was delivered normally and appeared in good condition. A CT scan at 7 days post-partum revealed an almost complete absence of both cerebral hemispheres in the newborn. The thalamus, brainstem, and cerebellum were preserved (Fernàndez et al. 1986).
A 25-year-old pregnant woman tried to commit suicide by inhaling butane at 30-weeks gestation. She was found comatose and needed resuscitation. The
duration and concentration of butane exposure were not reported, nor was the amount of time that elapsed before resuscitation commenced. Spontaneous labor occurred at 36 weeks. The infant did not breathe spontaneously; he was resuscitated, intubated, and ventilated artificially, but died 11 h after birth (Gosseye et al. 1982). The infant’s brain weighed 99 g (mean normal weight is 308 g), and the general appearance of the convolutions corresponded to about 30 weeks of maturation. A severe encephalomalacia was noted. The kidneys were underdeveloped, and the heart showed some foci of fibrosis in the subendocardial myocardium. The lungs were poorly aerated and the alveoli contained a number of squamous cells. Other viscera were reported to be unremarkable.
2.5. Genotoxicity
No data were available.
2.6. Carcinogenicity
No data were available.
2.7. Summary
A number of fatal and nonfatal cases related to butane abuse or suicide attempts have been described. Quantitative exposure estimates are lacking for all cases. In the case of butane abuse, most of the health effects described in case reports are thought to be induced by repeated exposures and abuse of other chemicals cannot be ruled out. Organs that were most often seriously affected in these cases were the brain and heart.
A 10-min exposure to butane at 10,000 ppm caused drowsiness in human volunteers. These were probably rather minor effects. Butane was reported to be “readily perceptible” at a concentration of 18,000 ppm. No irritation was noted at 100,000 ppm (exposure duration not specified but was probably for a few minutes).
Inhalation of butane during pregnancy (weeks 27 and 30 of gestation) at concentrations that produced unconsciousness in the mother caused clearly underdeveloped brains in two fetuses. In both cases, the effects were attributed intrauterine anoxia.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
3.1.1. Monkeys
No data were available.
3.1.2. Dogs
Butane at concentrations of 200,000-250,000 ppm produced “relaxation” in dogs (number and sex not specified), but caused death after a short time (Stoughton and Lamson 1936). No further details were given.
3.1.3. Rats
Shugaev (1969) exposed rats (sex and strain not specified) to varying concentrations of butane for 4 h. The number of animals was not specified but the results suggest that the exposure groups consisted of 6 animals. Exposure concentrations were reported to be controlled by gas chromatography, but no information about the concentrations of butane tested or the duration of the postexposure observation period was provided. The experimental data were analyzed by probit analysis. A 4-h LC50 (lethal concentration 50% lethality) of 278,000 ppm (658 g/m3) was reported, with 95% confidence limits of 252,000-302,000 ppm. Most rats died during the third or fourth hour of exposure. The LC16 was calculated to be 227,000 ppm (537 g/m3) and the LC84 to be 333,000 ppm (790 g/m3). Mean butane concentrations in organs at the LC50 were 7.5 μg/g in the brain, 4.9 μg/g in the liver, 4.4 μg/g in the kidneys, 5.2 μg/g in the spleen, and 20.9 μg/g in perinephric fat.
3.1.4. Mice
Shugaev (1969) exposed mice (sex and strain not specified) to various butane concentrations for 2 h. The number of animals was not specified but the results suggest that exposure groups consisted of 6 animals. Exposure concentrations were reported to be controlled by gas chromatography, but no information about the concentrations of butane tested or the duration of the post-exposure observation period was provided. The experimental data were analyzed by probit analysis. A 2-h LC50 of 287,000 ppm (680 g/m3) was reported, with 95% confidence limits of 252,000-327,000 ppm. Most of the mice died during the second hour of exposure. The LC16 was calculated to be 224,000 ppm (530 g/m3) and the LC84 to be 363,000 ppm (860 g/m3). The mean butane concentration in the brain of dead mice at the LC50 was 7.8 μg/g.
Groups of mice (sex and strain not specified) were exposed to butane at concentrations of 130,000, 220,000, 270,000, or 310,000 ppm; 6 mice were tested at the lowest concentration, and 10 mice at each of the higher concentrations (Stoughton and Lamson 1936). The animals were observed for 24-48 h after exposure. Although it was not clearly described, the study description suggests that the animals were exposed under static conditions in a closed-chamber setting. The animals were observed for 48 h after exposure. Effects observed were “light anesthesia,” “loss of posture” (complete anesthesia), and death. Exposure to butane at 270,000 ppm for 2 h was lethal to 4 of 10 mice; the average
time of death was 84 min. Exposure at 310,000 ppm was lethal to 60% of the mice, and the average time of death was 65 min. No deaths occurred in mice exposed for 2 h at 130,000 or 220,000 ppm. All deaths occurred during exposure. Surviving mice recovered rapidly, within 5 min after exposure ended (Stoughton and Lamson 1936).
A summary of data on lethality from acute inhalation of butane is provided in Table 1-3.
3.2. Nonlethal Toxicity
3.2.1. Dogs
Six dogs were exposed to butane for various durations to study its potency as a cardiac sensitizer (Chenoweth 1946). Anesthetized dogs were exposed to butane by a tracheal cannula, and epinephrine (0.01 or 0.02 mg/kg) was injected intravenously at different intervals during exposure. Of the 15 trials with individual dogs, three resulted in ventricular fibrillation. The lowest concentration at which ventricular fibrillation occurred was approximately 35,000 ppm (estimated from a graph); epinephrine was injected after 2 min. Other dogs showed no ventricular fibrillation after injection with epinephrine when exposed to butane at about 50,000 ppm. Because details of the study were lacking and because the tests were conducted in anesthetized dogs, no clear conclusions can be drawn from this study. Krantz et al. (1948) also reported cardiac sensitization by butane in dogs, but the exposure conditions were not specified.
TABLE 1-3 Lethality in Laboratory Animals after Acute Inhalation of Butane
| Species | Concentration (ppm) | Exposure Duration | Lethality | Reference |
| Rat | 227,000 278,000 333,000 |
4h | LC16 LC50 LC84 |
Shugaev 1969 |
| Mouse | 224,000 287,000 363,000 |
2h | LC16 LC50 LC84 |
Shugaev 1969 |
| Mouse | 130,000 | 2h | 0/6 deaths | Stoughton and Lamson 1936 |
| Mouse | 220,000 | 2h | 0/10 deaths | Stoughton and Lamson 1936 |
| Mouse | 270,000 | 2h | 4/10 deaths | Stoughton and Lamson 1936 |
| Mouse | 310,000 | 2h | 6/10 deaths | Stoughton and Lamson 1936 |
Abbreviation: LC%, lethal concentration, % lethality.
The hemodynamic effects of butane were studied in groups of 7 anesthetized (pentobarbital at 30-35 mg/kg) adult mongrel dogs. Dogs were artificially ventilated via an endotracheal cannula and several parameters of cardiac function (e.g., pulmonary arterial pressure, atrial pressure, ventricular pressure, heart rate, stroke volume) were studied (Zakhari 1977). Each dog was exposed butane at nominal concentrations of 0.5, 1.0, 2.5, 5.0, and 10.0% (5,000, 10,000, 25,000, 50,000, and 100,000 ppm, respectively) via the respirator for 5 min; each exposure immediately following the preceding one. No further details were presented on actual exposure concentrations. Concentration-related decreases in cardiac output and left ventricular pressure were observed starting at 5,000 ppm. Myocardial contractility (the rate of rise in left ventricular pressure) and mean aortic pressure showed a concentration-related decrease starting at 25,000 ppm. The individual contribution of butane exposure (as opposed to coexposure with anesthesia) to produce these effects is unclear.
3.2.2. Guinea Pigs
Nuckolls (1933) exposed groups of three guinea pigs to butane at 21,000-28,000 ppm or 50,000-56,000 ppm for 5, 30, 60, or 120 min. The animals were observed during exposure and for 10 days after exposure. The concentrations were analyzed periodically and adjustments made to maintain the predetermined concentrations. Guinea pigs exposed at 21,000-28,000 ppm showed occasional chewing movements and irregular or rapid breathing, but the effects did not worsen as the exposure duration increased. Animals recovered quickly and appeared normal after exposure ended. Guinea pigs exposed for 5 min at 50,000-56,000 ppm showed no significant effects. Continuation of exposure resulted in irregular breathing, occasional retching, and chewing movements, and the animals showed a “dazed appearance” in the second hour of exposure but were able to walk. The description of the results suggests that the effects did not increase in severity with continuation of exposure. One guinea pig exposed for 2 h at 50,000-56,000 ppm was examined histopathologically 7 days after exposure; no effects were found.
3.2.3. Mice
Groups of mice (sex and strain not specified) were exposed to butane at concentrations of 130,000, 220,000, 270,000, or 310,000 ppm; 6 mice were tested at the lowest concentration and 10 mice at each of the higher concentrations (Stoughton and Lamson 1936). The study description suggests that the concentrations reported were initial concentrations and that the animals were exposed in a closed-chamber setting. The animals were observed for 48 h after exposure. Effects observed were “light anesthesia” and “loss of posture” (complete anesthesia). Light anesthesia was defined as being unable to maintain an upright position in a rotating bottle (2 mice in a 2-L bottle). Complete anesthesia
(loss of posture) was defined as the inability to regain an upright position after shaking the bottle in which the mice were placed (5 mice in a 20-L bottle). Exposure to butane at 130,000 ppm induced light anesthesia within 25 min (on average). Light anesthesia was observed within 1 min of exposure to butane at 220,000 ppm, and loss of posture was observed within 15 min. Loss of posture occurred within 4 min at 270,000 ppm and within 3 min at 310,000 ppm. Butane at concentrations of 270,000 and 310,000 ppm caused mortality (see Section 3.1.5). Surviving mice recovered within a few min after exposure ended.
A summary of nonlethal effects from acute inhalation of butane is provided in Table 1-4.
3.3. Neurotoxicity
No data other than that described in Sections 3.2.2 and 3.2.3 were available.
3.4. Developmental and Reproductive Toxicity
No were data available.
3.5. Genotoxicity
Butane appears to be negative in the Ames test, with and without metabolic activation (citation of an unpublished report in Moore 1982). Shimizu et al. (1985) reported that butane at concentrations up to 25,000 ppm was negative in tests with Salmonella typhimurium TA98, TA100, TA1535, TA1537, and TA1538, and in Escherichia coli (WP2uvrA), with and without metabolic activation.
TABLE 1-4 Nonlethal Effects in Laboratory Animals after Inhalation of Butane
| Species | Concentration (ppm) | Exposure Duration | Effect | Reference |
| Guinea pigs | 21,000-28,000 | Up to 2 h | Increased respiration rate; increased sniffing and chewing behavior- | Nuckolls 1933 |
| Guinea pigs | 50,000-56,000 | Up to 2 h | Increased respiration rate; increased retching and chewing behavior; dazed appearance. | Nuckolls 1933 |
| Mice | 130,000 | 2h | Light anesthesia within 25 min. | Stoughton and Lamson 1936 |
| Mice | 220,000 | 2h | Light anesthesia within 1 min; complete anesthesia within 15 min. | Stoughton and Lamson 1936 |
| Mice | 270,000 | 2h | Complete anesthesia within 4 min. | Stoughton and Lamson 1936 |
| Mice | 310,000 | 2h | Complete anesthesia within 3 min. | Stoughton and Lamson 1936 |
3.6. Carcinogenicity
No were data available.
3.7. Summary
Butane was reported to cause cardiac sensitization in dogs, but the studies did not provide detailed information on exposure concentrations and duration or were performed on anesthetized dogs. Because of these limitations, no clear conclusions can be drawn. Butane caused hemodynamic effects in anesthetized dogs but considering the exposure conditions of the study it cannot be used as a basis for setting AEGL values.
Slight effects on the respiratory rate were reported in guinea pigs exposed to butane at 21,000-28,000 ppm for up to 2 h. Guinea pigs exposed at 50,000-56,000 ppm for 2 h showed a “dazed appearance” but were able to walk. Light and complete anesthesia occurred in mice exposed to initial concentrations of butane at 130,000 ppm (within 25 min) and 220,000 ppm (within 15 min). Light anesthesia was defined as “being unable to maintain an upright position in a rotating bottle.”
A steep concentration-response curve for mortality was observed in mice and rats; the LC84:LC16 ratio was 1.6 for mice (2-h exposure) and 1.5 for rats (4-h exposure). The response in mice and rats were remarkably comparable at similar concentrations, despite the difference in exposure duration. In another study, no deaths occurred in mice exposed to initial concentrations of butane at 130,000 or 220,000 ppm for 2 h; mortality was 40 and 60% at concentrations of 270,000 and 310,000 ppm, respectively. No deaths occurred in guinea pigs exposed to butane at 50,000-56,000 ppm for 2 h.
Butane was negative in the bacterial reverse mutation (Ames) test with and without metabolic activation.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
4.1.1. Absorption
Four human subjects (3 male, 1 female; 20-21-years of age) were exposed to butane at 600 ppm for 4 h. The chamber concentration was monitored continuously. Pulmonary uptake of butane appears to increase quickly within the first 5 min of exposure and reaches a plateau within the first 30 min. Pulmonary uptake remained fairly constant during the remainder of the exposure, ranging from 30% to 50% (Gill et al. 1991). Butane concentration in exhaled breath decreased rapidly to less than 5 ppm, 20 min after exposure ended. Blood concentrations of butane also decreased rapidly to less than 0.02 μg/mL after 20 min (data were estimated from graphs).
4.1.2. Metabolism
Tsukamoto et al. (1985) exposed male ICR mice (number of animals not specified) for 1 h to a mixture of butane, air, and oxygen (in the proportion of 2:1:1; thus, the butane concentration was 500,000 ppm). Animals were killed immediately after exposure. In addition to butane, methyl ethyl ketone and secbutanol were detected in blood and tissues as metabolites. Tissue concentrations of methyl ethyl ketone were 2.9-4 μg/g, with highest concentrations in blood, followed by the liver, kidneys, and brain. The concentration of sec-butanol in these tissues was 30-35 μg/g, with highest concentrations in blood and brain. Exposure to the butane mixture killed 40% of the animals despite the high oxygen content (>25%).
In vitro studies with rat liver microsomes showed that hydroxylation results for nearly 100% in 2-butanone over 1-butanone (Frommer et al. 1970).
4.1.3. Species Variability
Shugaev (1969) determined LC50 values for butane for 2-h exposures in mice and 4-h exposures in rats. The different exposure duration for the two species was based on a comparison of the ratio of minute ventilation (volume of gas exchanged from the lungs per minute) to body weight, which is approximately two-fold greater in mice. The 2-h LC16, LC50, and the LC84 for the mouse were similar to the corresponding 4-h values for the rat (see Table 1-3). Most of the mice died during the second hour of exposure, whereas rats mainly died during the third and fourth hour of exposure. Mean brain concentrations of butane in dead rats (7.5 μg/g) and mice (7.8 μg/g) were similar. However, tissue concentrations of butane in rats were not determined after a 2-h exposure and the reported brain concentration could already have been reached with a shorter exposure duration. Results of lethality studies suggest that mice might be more sensitive than rats; mice had a shorter time to death and their 2-h LC50 values were close to the 4-h LC50 values in rats.
5. DATA ANALYSIS FOR AEGL-1
5.1. Summary of Human Data Relevant to AEGL-1
Case reports of butane exposure do not provide any quantitative data that could be used for deriving AEGL-1 values. Patty and Yant (1929) studied the warning properties of short-term exposures to butane. Physiologic responses were only briefly described, and a discrepancy was noted. It was stated in a table that exposure to butane at 10,000 ppm for 10 min produced drowsiness in volunteers (n = 3 or 6), but the text indicated that no symptoms occurred. Several alkanes (C3 to C7) were studied in this experiment and more severe effects were reported for hexane and heptanes, suggesting that if effects were observed with butane they would have been described more explicitly. It is therefore concluded
that any drowsiness associated with butane was of a very minor severity. Odor detection was assessed by means of an odor scale ranging from 0 (no detectable odor) to 5 (intense effect, may bite or irritate). No noticeable irritation was reported at concentrations up to 100,000 ppm for a short but undefined exposure period; the odor detection score was below 4 (cogent, forcible odor).
5.2. Summary of Animal Data Relevant to AEGL-1
Nuckolls (1933) exposed groups of three guinea pigs to butane at 21,000-28,000 ppm or 50,000-56,000 ppm for 5, 30, 60, or 120 min. The animals were observed during exposure and for 10 days after exposure. Guinea pigs exposed at 21,000-28,000 ppm for up to 2 h showed occasional chewing movements and irregular or rapid breathing, while animals exposed at 50,000-56,000 ppm also had a “dazed appearance” in the second hour of exposure but were still able to walk. The description of the results suggests that the effects did not increase in severity with continuation of exposure. Animals recovered quickly and appeared normal after exposure ended. One guinea pig exposed for 2 h at 50,000-56,000 ppm was examined histopathologically 7 days after exposure; no effects were found.
5.3. Derivation of AEGL-1
The human data presented by Patty and Yant (1929) form the basis of the AEGL-1 values. Butane at a concentration of 10,000 ppm (10-min exposure) can be regarded as a boundary for the drowsiness reported; although some drowsiness may be noticed, it will not be experienced as discomfort. Further, no noticeable irritation was reported at concentration up to 100,000 ppm for a short exposure duration (exact duration unknown). Although the study was performed with small groups of volunteers (3 or 6 people) of a relatively young age (20-30 years), an intraspecies uncertainty of 1 is considered adequate for the following reasons. First, the concentration-response curve for CNS-effects appears to be very steep (see Section 6.3) and, thus, interindividual variability will be relatively small. Second, no noticeable irritation was reported at concentrations up to 100,000 ppm for a short duration (exact duration unknown). Third, the use of an intraspecies factor of 3 would lead to AEGL-1 values that are unrealistically low (e.g., in comparison with the occupational standards, see Section 8.2). For similar reasons and because subjects exposed at slowly increasing concentrations of butane up to 50,000 ppm for at least 10 min did not experience any significant adverse effects, a modifying factor is not considered necessary.
The rationale for time scaling in the derivation of AEGL-1 values and the choice of n in the dose-response regression equation is similar to that for AEGL-2 (see Section 6.3). By analogy to other CNS-depressing substances, the effects of butane are assumed to be solely concentration dependent. Thus, after reaching steady state (within 30 min of exposure), no increase in effect size is expected at
4 and 8 h. The other exposure duration-specific values were derived by time scaling according to the dose-response regression equation Cn × t = k, using n = 3. Time extrapolation was performed from 10 min to 30-and 60-min exposures. The resulting values for AEGL-1 are presented in Table 1-5. These values are considered protective of the irregular breathing observed in guinea pigs exposed to butane at 21,000-28,000 ppm for up to 2 h.
6. DATA ANALYSIS FOR AEGL-2
6.1. Summary of Human Data Relevant to AEGL-2
No adequate human data relevant to AEGL-2 effects were found. Two case reports indicate that single exposure to high concentrations of butane might cause severe brain damage in the fetus, but these studies are not suitable as basis for AEGL-2 values because exposure data are lacking.
6.2. Summary of Animal Data Relevant to AEGL-2
Nuckolls (1933) exposed groups of three guinea pigs for 5, 30, 60, or 120 min to 50,000-56,000 ppm for 2 h. Guinea pigs had an increase in respiratory rate and sniffing and chewing behavior and showed a “dazed appearance” in the second hour of exposure, but the animals were still able to walk. The description of the effects appears to indicate that the effects did not increase in severity with continuation of exposure. Animals recovered quickly and appeared normal after exposure ended. One guinea pig exposed for 2 h was examined histopathologically 7 days after exposure; no effects were found.
Stoughton and Lamson (1936) exposed mice to butane at various concentrations for 2 h. Light anesthesia, defined as “being unable to maintain an upright position in a rotating bottle,” occurred within 25 min (on average) at 130,000 ppm, the lowest concentration tested, and within 1 min at 220,000 ppm. Complete anesthesia, defined as “the inability to regain an upright position after shaking the bottle in which the mice were placed,” was observed within 15, 4, and 3 min in mice exposed at 220,000, 270,000, and 310,000 ppm, respectively. Light anesthesia can be considered serious enough to impair escape, and could be used as basis for AEGL-2. However, the experimental procedure is poorly described but suggests that reported concentrations are probably initial concentrations in a closed-chamber setting. Butane concentration will have decreased during exposure; thus, the effects observed cannot be related to a specific exposure concentration.
6.3. Derivation of AEGL-2
Case reports indicate that single exposure to high concentrations of butane might cause severe brain damage in the fetus, but no adequate human or animal
data are available for a quantitative evaluation of this end point. Because human data are lacking, the AEGL-2 values are based on animal data. Two animal studies are available, a study with guinea pigs (Nuckolls 1933) and a study with mice (Stoughton and Lamson 1936). In the latter study, “light anesthesia” was observed after a mean exposure duration of 25 min to butane at 130,000 ppm and after 1 min at 220,000 ppm. The use of this study is hampered by the possibility that exposure concentration decreased during the study.2
The only available starting point adequate for AEGL-2 values is provided by the study of Nuckolls (1933), in which guinea pigs were exposed for 2 h to butane at concentration of 50,000-56,000 ppm. Because the animals were able to walk, their “dazed appearance” is considered not to be sufficiently serious to impair escape. A concentration of 50,000 ppm is considered an appropriate starting point for the derivation of AEGL-2 values. Because the anesthetic effects of butane are considered to be predominantly concentration dependent, a total uncertainty factor of 3 is considered sufficient for toxicokinetic and toxicodynamic differences between individuals and interspecies differences. The effects are attributed to butane itself and no relevant differences in kinetics are assumed, so only small interindividual differences are expected. The concentration-response curve appears to be very steep, indicating that a large uncertainty factor is unnecessary. Further, a larger uncertainty factor would lead to unrealistically low
TABLE 1-5 AEGL-1 Values for Butane
| 10 min | 30 min | 1 h | 4 h | 8 h |
| See belowa | 6,900 ppmb (16,000 mg/m3) | 5,500 ppm (13,000 mg/m3) | 5,500 ppmb (13,000 mg/m3) | 5,500 ppmb (13,000 mg/m3) |
a The 10-min AEGL-1 value is 10,000 ppm (24,000 mg/m3), which is greater than 50% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
b The AEGL value is greater than 10% of the Lower explosive limit for butane in air of 19,000 ppm. Therefore, safety considerations against the hazard of explosion must be taken into account.
![]()
2 During the evaluation of “light anesthesia,” two mice were placed in a 2-L bottle. Assuming a minute volume for mice of 25 mL/min per animal (Paulussen et al. 1998), it can be calculated that the two animals breathe 2.5% (50/2,000) of the available air per minute. Assuming that the pulmonary retention in mice is comparable to the approximately 50% reported in humans (Gill et al. 1991), about 1.25% of the butane present in the air is retained by the two animals per minute. Thus, the butane concentration will have decreased to approximately 73% of the initial concentration (a decrease from 130,000 to 95,000 ppm) after 25 min. Although the reduction in concentration can be regarded as negligible for short-exposure durations because of general variation in actual exposure concentrations, a mean exposure duration of 1 min is inadequate as starting point for time scaling to 8 h of exposure. Therefore, the study by Stoughton and Lamson (1936) does not provide an adequate starting point for AEGL-2 values.
values for AEGL-2 and would be similar to the AEGL-1 values. The relationship between concentration and duration of exposure as related to lethality was examined by ten Berge et al. (1986) for approximately 20 irritant or systemically-acting vapors and gases. The authors subjected the individual animal data sets to probit analysis with exposure duration and exposure concentration as independent variables. An exponential function (Cn × t = k), where the value of n ranged from 0.8 to 3.5 for different chemicals was found to be an accurate quantitative descriptor for the chemicals evaluated. Approximately 90% of the values of n range between n = 1 and n = 3. Consequently, these values were selected as the reasonable lower and upper bounds of n to use when data are not available to derive an empirical value for n. An indication for the value of n for CNS-depressing effects can be obtained from the study by Stoughton and Lamson (1936). Complete anesthesia from butane was reported to occur at an initial concentration of 220,000 ppm after 15 min, 270,000 ppm after 4 min, and 310,000 ppm after 3 min. On the basis of these data, n is estimated to be greater than 4 (after accounting for a small decrease in concentration during the 15-min exposure3 ). Although the data cannot be used to provide an estimate for n, it can be concluded that n will be relatively high and that the upper bound of n = 3 is an appropriate estimate for time scaling. This is consistent with other anesthetics for which effects are assumed to be concentration dependent rather than time dependent.
No increase in the severity of response by duration is expected for concentration-dependent effects after reaching a steady state. Although no appropriate kinetic data are available on butane to assess the duration needed to reach a steady state, it can be estimated from the pulmonary-uptake data from Gill et al. (1991) that a steady-state uptake, and hence, steady-state plasma values, will be reached within 30 min. In addition, it has been stated that gases which are relatively insoluble in blood increase rapidly toward equilibrium with the inhaled concentration and the less soluble in blood the faster the narcotic action of the gas (Drummond 1993). The increase to a quick equilibrium has been confirmed for propane, which has properties comparable to butane. Concentrations of propane were approximately similar in blood samples taken 15 min before the end of 1-, 2-, and 8-h exposures to propane at 250 and 500 ppm (Stewart et al. 1977).
Because of the poor solubility of butane in water (61 mg/L), it is expected that exposure to butane will lead to a rapid equilibrium and that there will be no increase in the severity of response for duration of 30 min to 8 h. Therefore, the AEGL-2 values for 30 min and 1, 4 and 8 h are set equal to the 2-h concentration. The AEGL-2 value for the 10-min exposure is derived by time scaling according to the dose-response regression equation Cn × t = k, using n = 3.
![]()
3 “Complete anesthesia” was evaluated with five mice in a 20-L bottle. Using the assumptions made in footnote 1, approximately 0.3% of the butane will be retained by the five animals per min. The concentration of butane will have decreased to about 95% of the initial concentration (from 220,000 to 209,000 ppm) after 15 min.
The AEGL-2 values for butane are presented in Table 1-6.
7. DATA ANALYSIS FOR AEGL-3
7.1. Summary of Human Data Relevant to AEGL-3
Case reports indicate that butane’s effects on the brain (e.g., encephalopathy) or effects on the heart (either direct cardiotoxicity or cardiac sensitization) might cause death. It is difficult to distinguish between direct toxicity of butane and the effects caused by hypoxia. The case reports do not provide adequate quantitative data to derive AEGL-3 values. Exposure to butane at slowly increasing concentrations up to 50,000 ppm (total exposure duration was at least 10 min) or at 100,000 ppm (short exposure, exact duration unknown) on the same day did not result in serious complaints (Patty and Yant 1929).
7.2. Summary of Animal Data Relevant to AEGL-3
Experiments with dogs (Chenoweth 1946; Krantz et al. 1948) support the cardiac sensitization potency of butane, but neither study provides an adequate basis for deriving AEGL-3 values.
Two relevant animal studies are available, a study with rats and mice by Shugaev (1969) and a study in mice by Stoughton and Lamson (1936). Shugaev (1969) reported a 2-h LC50 of 287,000 ppm (680 g/m3) in mice and a 4-h LC50 of 278,000 ppm (658 g/m3) in rats. The concentration-response curve was very steep, with LC84:LC16 ratios of approximately 1.5 for both species. The 2-h lethality data in mice obtained by Stoughton and Lamson (1936) were remarkably similar to those obtained by Shugaev (1969). Stoughton and Lamson (1936) observed no mortality in mice after a 2-h exposure to butane at 130,000 or 220,000 ppm, whereas 4/10 and 6/10 mice died during a 2-h exposure at 270,000 and 310,000 ppm, respectively. The experimental procedure in the study by Stoughton and Lamson (1936) is poorly described, but suggests that the reported concentrations of butane are probably initial concentrations in a closedchamber setting. Thus, the concentration of butane will have decreased during exposure.4
7.3. Derivation of AEGL-3
There are no adequate human data for derivation of AEGL-3 values. Although case reports indicate that humans exposed to butane at high concentrations
![]()
4 Lethality was studied with five mice in a 20-L bottle. The concentration of butane will have decreased to about 83% of the original concentration after 60 min and to 69% after 120 min (see footnote 1). Deaths occurred after 84 min (on average) at an initial butane concentration of 270,000 ppm and after 65 min (on average) at an initial concentration of 310,000 ppm.
might develop cardiac arrhythmias, which are potentially fatal, the data were inadequate to evaluate this end point. Therefore, AEGL-3 values are based on animal data.
7.3. Derivation of AEGL-3
There are no adequate human data for derivation of AEGL-3 values. Although case reports indicate that humans exposed to butane at high concentrations might develop cardiac arrhythmias, which are potentially fatal, the data were inadequate to evaluate this end point. Therefore, AEGL-3 values are based on animal data.
The study by Stoughton and Lamson (1936) provides a no-observedadverse-effect level for lethality in mice exposed to butane for 2 h. However, the mice were probably exposed in a closed-chamber setting and the reported butane concentrations might refer to initial concentrations. Hence, this study does not provide an adequate starting point for deriving AEGL-3 values. Shugaev (1969) exposed mice and rats to butane for 2 and 4 h, respectively. The reported data (LC16, LC50, and LC84) indicate that the concentration-response curve for a 2-h exposure in mice and a 4-h exposure in rats are very similar (see Table 1-3). Further, brain concentrations of butane in dead mice and rats exposed at the LC50 appeared to be comparable. This might be explained by the difference in the ratio of minute ventilation to body weight, which is approximately two-fold greater in mice. However, it might be an indication that mice are more susceptible to butane toxicity, because a steady state is expected to be reached rapidly with butane (see Section 6.2). Because no further data are available, it is assumed that mice are more susceptible than rats. Because the study by Shugaev (1969) reports only LC16, LC50, and the LC84 values obtained by probit analysis and not the individual experimental data, benchmark dose-response modeling is not possible. However, the LC01 can be calculated because the mean is known and the standard deviation of the underlying lognormal distribution can be derived from these data. The 2-h LC01 for mice is 160,000 ppm and the 4-h LC01 for rats is 172,000 ppm. The 2-h LC01 for mice is chosen as the starting point for the AEGL-3 values, because it is the lowest concentration tested in what appears to be a more susceptible species.
TABLE 1-6 AEGL-2 Values for Butane
| 10 min | 30 min | 1 h | 4 h | 8 h |
| See belowa | See belowb | See belowb | See belowb | See belowb |
a The 10-min AEGL-2 value is 24,000 ppm (57,000 mg/m3), which is greater than the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
b The AEGL-2 values for 30-min and 1-, 4-, and 8-h are 17,000 ppm (40,000 mg/m3), which is greater than 50% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
A total uncertainty factor of 3 is considered sufficient for toxicokinetic and toxicodynamic differences between individuals and interspecies differences for the following reasons. The effects are attributed to butane itself and no relevant differences in kinetics are assumed. A species with a relatively high susceptibility is used as starting point. The concentration-response curve appears to be very steep indicating that a large uncertainty factor is unnecessary. Further, a larger uncertainty factor would lead to unrealistically low values for AEGL-3, which would be similar to the AEGL-2 values.
As indicated by the study by Stoughton and Lamson (1936), mortality is preceded by CNS-depression. Hence, the rationale described in Section 6.2 for determining the value of n for time scaling to derive AEGL-2 values is considered also appropriate for AEGL-3 values. After a steady state has been reached, no increase in effect severity by exposure duration is expected. Therefore, the AEGL-3 values for 30 min and for 1, 4 and 8 h of exposure will be set equal to that for the 2-h exposure. The AEGL-3 values for the 10-min exposure are derived by time scaling according to the dose-response regression equation Cn × t = k, using n = 3. The 10-min AEGL of 77,000 ppm is supported by the data from Patty and Yant (1929). They reported that exposure to butane at slowly increasing concentrations up to 50,000 ppm (total exposure duration at least 10 min) and to 100,000 ppm (short exposure, exact duration unknown) on the same day did not result in serious complaints (Patty and Yant 1929).
The AEGL-3 values for butane are presented in Table 1-7.
8. SUMMARY OF AEGLS
8.1. AEGL Values and Toxicity End Points
The AEGL values for butane are summarized in Table 1-8.
8.2. Comparison with Other Standards and Guidelines
Standards and guidelines for short-term exposures to butane are presented in Table 1-9.
8.3. Data Quality and Research Needs
The database for butane is poor and important studies date back to the 1920s or 1930s. Although case reports indicate that butane might cause arrhythmias in humans exposed at high concentrations, no adequate human or animal data are available to evaluate this end point in a quantitative way. Similarly, case reports indicate that single exposure to high concentrations of butane might cause severe brain damage in the fetus, but no adequate data are available for a quantitative evaluation.
TABLE 1-7 AEGL-3 Values for Butane
| 10 min | 30 min | 1 h | 4 h | 8 h |
| See belowa | See belowa | See belowa | See belowa | See belowa |
a The 10-min AEGL-3 value is 77,000 ppm (180,000 mg/m3), and the 30-min and 1-, 4-, and 8-h AEGL-3 values are 53,000 ppm (130,000 mg/m3). All of these values are greater than the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
TABLE 1-8 Summary of AEGL Values for Butane
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h |
| AEGL-1 (nondisabling) | See belowa | 6,900 ppmb (16,000 mg/m3) | 5,500 ppmb (13,000 mg/m3) | 5,500 ppmb (13,000 mg/m3) | 5,500 ppmb (13,000 mg/m3) |
| AEGL-2 (disabling) | See belowc | See belowd | See belowd | See belowd | See belowd |
| AEGL-3 (lethal) | See belowe | See belowe | See belowe | See belowe | See belowe |
a The 10-min AEGL-1 value is 10,000 ppm (24,000 mg/m3), which is greater than 50% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
b The AEGL-1 value is greater than 10% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, safety considerations against the hazard of explosion must be taken into account.
c The 10-min AEGL-2 value is 24,000 ppm (57,000 mg/m3), which is greater than the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
d The AEGL-2 values for 30 min and 1, 4, and 8 h are 17,000 ppm (40,000 mg/m3), which is greater than 50% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
e The 10-min AEGL-3 value is 77,000 ppm (180,000 mg/m3). The AEGL-3 values for 30 min and 1, 4, and 8 h are 53,000 ppm (130,000 mg/m3). These values are greater than the lower explosive limit for butane in air of 19,000 ppm). Therefore, extreme safety considerations against the hazard of explosion must be taken into account
TABLE 1-9 Extant Standards and Guidelines for Butane
| Guideline | Exposure Duration | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h | |
| AEGL-1 | See belowa | 6,900 ppmb (16,000 mg/m3) | 5,500 ppmb (13,000 mg/m3) | 5,500 ppmb (13,000 mg/m3) | 5,500 ppmb (13,000 mg/m3) |
| AEGL-2 | See belowc | See belowd | See belowd | See belowd | See belowd |
| AEGL-3 | See belowe | See belowe | See belowe | See belowe | See belowe |
| TLV-TWA (ACGIH)f | 1,000 ppm | ||||
| Guideline | Exposure Duration | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h | |
| REL-TWA (NIOSH)g | 800 ppm | ||||
| MAK (Germany)h | 1,000 ppm | ||||
| MAK Peak Limit (Germany)i | 2,000 ppm | ||||
| MAC (The Netherlands)j | 600 ppm | ||||
a The 10-min AEGL-1 value is 10,000 ppm (24,000 mg/m3), which is greater than 50% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
b The AEGL-1 value is greater than 10% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, safety considerations against the hazard of explosion must be taken into account.
c The 10-min AEGL-2 value is 24,000 ppm (57,000 mg/m3), which is greater than the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
d The AEGL-2 values for 30 min and 1, 4, and 8 h are 17,000 ppm (40,000 mg/m3), which is greater than 50% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
e The 10-min AEGL-3 value is 77,000 ppm (180,000 mg/m3). The AEGL-3 values for 30 min and 1, 4, and 8 h are 53,000 ppm (130,000 mg/m3). These values are greater than the lower explosive limit for butane in air of 19,000 ppm). Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
f TLV-TWA (threshold limit value - time weighted average, American Conference of Governmental Industrial Hygienists) (ACGIH 2007) is the time-weighted average concentration for a normal 8-h workday and a 40-h workweek, to which nearly all workers may be repeatedly exposed, day after day, without adverse effect.
g REL-TWA (recommended exposure limit - time weighted average, National Institute for Occupational Safety and Health) (NIOSH 2010) is defined analogous to the ACGIH TLV-TWA.
h MAK (maximale arbeitsplatzkonzentration [maximum workplace concentration]) (Deutsche Forschungsgemeinschaft [German Research Association] (DFG 2007) is defined analogous to the ACGIH TLV-TWA.
i MAK spitzenbegrenzung (peak limit,German Research Association (DFG 2007) constitutes the maximum concentration to which workers can be exposed for a period up to 60 min with no more than three exposure periods per work shift; total exposure may not exceed the 8-h MAK.
j MAC (maximaal aanvaaarde concentratie [maximal accepted concentration]) (Dutch Expert Committee for Occupational Standards, The Netherlands (MSZV 2004) is defined analogous to the ACGIH TLV-TWA.
The case reports do not provide an adequate basis for AEGL values. The only study with human volunteers (Patty and Yant 1929) is rather old and focused on the warning properties of butane.
9. REFERENCES
ACGIH (American Conference of Government and Industrial Hygienists). 2007. Butane (CAS Reg. No. 106-97-8). TLVs and BEIs. Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. American Conference of Government and Industrial Hygienists: Cincinnati, OH.
Adgey, A.A.J., P.W. Johnston, and S. McMechan. 1995. Sudden cardiac death and substance abuse. Resuscitation 29(3):219-221.
Bauman, J.J., B.S. Dean, and E.P. Krenzelok. 1991. Myocardial infarction and neurodevastation following butane inhalation. Vet. Hum. Toxicol. 33(4):389.
Bowen, S.E., J. Daniel, and R.L. Balster. 1999. Deaths associated with inhalant abuse in Virginia from 1987 to 1996. Drug Alcohol Depend. 53(3):239-245.
Cartwright, T.R., D. Brown, and R.E. Brashear. 1983. Pulmonary infiltrates following butane ’fire-breathing’. Arch. Intern. Med. 143(10):2007-2008.
Cavender, F. 1994. Aliphatic hydrocarbons. Pp. 1221-1239 in Patty’s Industrial Hygiene and Toxicology, 4th Ed., Vol. 2B, G.D. Clayton, and F.E. Clayton, eds. New York: John Wiley & Sons.
Chaudhry, S. 2002. Deaths from volatile substance misuse fall. BMJ 325(7356):122.
Chenoweth, M.B. 1946. Ventricular fibrillation induced by hydrocarbons and epinephrine. J. Ind. Hyg. Toxicol. 28:151-158.
DFG (Deutsche Forschungsgemeinschaft). 2007. List of MAK and BAT Values 2007. Maximum Concentrations and Biological Tolerance Values at the Workplace Report No. 43. Weinheim, Federal Republic of Germany: Wiley VCH.
Döring, G., F.A. Baumeister, J. Peters, and J. von der Beek. 2002. Butane abuse associated encephalopathy. Klin. Paediatr. 214(5):295-298.
Drummond, I. 1993. Light hydrocarbon gases: A narcotic, asphyxiant, or flammable hazard? Appl. Occup. Environ. Hyg. 8(2):120-125.
ECB (European Chemicals Bureau). 2000. Butane, pure. EINECS No. 203-448-7. IUCLID Dataset. European Commission, European Chemicals Bureau [online]. Available: http://esis.jrc.ec.europa.eu/doc/existing-chemicals/IUCLID/data_sheets/106978.pdf [accessed Jan. 12, 2012]
Edwards, K.E., and R. Wenstone. 2000. Successful resuscitation from recurrent ventricular fibrillation secondary to butane inhalation. Br. J. Anaesth. 84(6):803-805.
Evans, A.C., and D. Raistrick. 1987. Phenomenology of intoxication with toluene-based adhesives and butane gas. Br. J. Psychiatry 150:769-773.
Fernàndez, F., A. Pèrez-Higueras, R. Hernàndez, A. Verdú, C. Sánchez, A. González, and J. Quero. 1986. Hydrancephaly after maternal butane-gas intoxication during pregnancy. Dev. Med. Child Neurol. 28(3):361-363.
Frommer, U., V. Ullrich, and H.J. Staudinger. 1970. Hydroxylation of aliphatic compounds by liver microsomes, I. The distribution pattern of isomeric alcohols. H.-S. Z. Physiol. Chem. 351(8):903-912.
Gill, R., S.E. Hatchett, C.G. Broster, M.D. Osselton, J.D. Ramsey, H.K. Wilson, and A.H. Wilcox. 1991. The response of evidential breath alcohol testing instruments
with subjects exposed to organic solvents and gases. I. Toluene, 1,1,1-trichloroethane and butane. Med. Sci. Law 31(3):187-200.
Gosseye, S., M.C. Golaire, and J.C. Larroche. 1982. Cerebral, renal and splenic lesions due to fetal anoxia and their relationship to malformations. Dev. Med. Child Neurol. 24(4):510-518.
Graefe, A., R.K. Müller, R. Vock, H. Trauer, and H.J. Wehran. 1999. Fatal propaneputane poisoning [in German]. Arch. Kriminol. 203(1-2):27-31.
Gray, M.Y., and J.H. Lazarus. 1993. Butane inhalation and hemiparesis. J. Toxicol. Clin. Toxicol. 31(3):483-485.
Gunn, J., J. Wilson, and A.F. Mackintosh. 1989. Butane sniffing causing ventricular fibrillation. Lancet 1(8638):617.
Krantz, J.C., Jr., C.J. Carr, and J.F. Vitcha. 1948. Anesthesia. XXXI. A study of cyclic and noncyclic hydrocarbons on cardiac automaticity. J. Pharmacol. Exp. Ther. 94(3):315-318.
Lewis, R.J., ed. 1999. Sax’s Dangerous Properties of Industrial Materials, 10th Ed. New York: Wiley.
Lide, D.R., ed. 1999. Handbook of Chemistry and Physics, 80th Ed. Boca Raton, FL: CRC Press.
Low, L.K., J.R. Meeks, and C.R. Mackerer. 1987. n-Butane (CAS Reg. No. 106-97-8). Pp. 267-272 in Ethel Browning’s Toxicity and Metabolism of Industrial Solvents, 2nd Ed., Vol. 1. Hydrocarbons R. Snyder, ed. New York: Elsevier.
Moore, A.F. 1982. Final report of the safety assessment of isobutane, isopentane, nbutane, and propane. J. Am. Coll. Toxicol. 1(4):127-142.
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: n-Butaan. Den Haag: SDU Uitgevers [online]. Available: http://www.lasrook.net/lasrookNL/maclijst2004.htm [accessed Jan. 12, 2012].
NIOSH (National Institute for Occupational Safety and Health). 2010. NIOSH Pocket Guide to Chemical Hazards: n-Butane. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH [online]. Available: http://www.cdc.gov/niosh/npg/npgd0068.html [accessed Jan. 12, 2012].
Nishi, K., N. Ito, J. Mizumoto, K. Wada, T. Yamada, Y. Mitsukuni, and S. Kamimura. 1985. Death associated with butane inhalation: Report of a case. Nihon Hoigaku Zasshi 39(3):214-216.
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
Nuckolls, A.H. 1933. Underwriters’ Laboratoris Report on the Comparative Life, Fire, and Explosion Hazards of Common Refrigerants. Miscellaneous Hazards No. 2375. Chicago, IL: National Board of Fire Underwriters.
Patty, F.A., and W.P. Yant. 1929. Odor Intensity and Symptoms Produced by Commercial Propane, Butane, Pentane, Hexane, and Heptane Vapor. U.S. Bureau of Mines Report of Investigation No 2979. Washington, DC: U.S. Department of Commerce, Bureau of Mines.
Paulussen, J.J.C., C.M. Mahieu, and P.M.J. Bos. 1998. Default Values in Occupational Risk Assessment. TNO Report V98.390. TNO Nutrition and Food Research Institute, Zeist, The Netherlands.
Rieder-Scharinger, J., R. Peer, W. Rabl, W. Hasibeder, and W. Schrobersberger. 2000. Multiple organ failure following inhalation of butane gas: A case report [in German]. Wien Klin. Wochenschr. 112(24):1049-1052.
Roberts, M.J., R.A. McIvor, and A.A. Adgey. 1990. Asystole following butane gas inhalation. Br. J. Hosp. Med. 44(4):294.
Ruth, J.H. 1986. Odor thresholds and irritation levels of several chemical substances: A review. Am. Ind. Hyg. Assoc. J. 47(3):A142-A151.
Shimizu, H., Y. Suzuki, N. Takemura, S. Goto, and H. Matsushita. 1985. The results of microbial mutation test for forty-three industrial chemicals. Sangyo Igaku 27(6):400-419.
Shugaev, B.B. 1969. Concentrations of hydrocarbons in tissues as a measure of toxicity. Arch. Environ. Health 18(6):878-882.
Stewart, R.D., A.A. Hermann, E.D. Baretta, H.V. Foster, J.J. Sikora, P.E. Newton, and R.J. Soto. 1977. Acute and Repetitive Human Exposure to Isobutane and Propane. Report no. CTFA-MCOW-ENVM-BP-77-1, April 1977. National Clearinghouse for Federal Scientific and Technical Information, Springfield, VA.
Stoughton, R.W., and P.D. Lamson. 1936. The relative anesthetic activity of the butanes and pentanes. J. Pharmacol. Exp. Ther. 58:74-77.
ten Berge, W.F., A. Zwart, and L.M. Appelman. 1986. Concentration-time mortality response relationship of irritant and systemically acting vapours and gases. J. Hazard. Mater. 13(3):301-309.
Tsukamoto, S., S. Chiba, T. Muto, T. Ishikawa, and M. Shimamura. 1985. Studies on the metabolism of volatile hydrocarbons in propane gas (LPG) inhalation: Detection of the metabolites. Nihon Hoigaku Zasshi 39(2):124-130.
Williams, D.R., and S.J. Cole. 1998. Ventricular fibrillation following butane gas inhalation. Resuscitation 37(1):43-45.
Zakhari, S. 1977. Butane. Pp. 55-59 in Non Fluorinated Propellants and Solvents for Aerosols, L. Goldberg, ed. Cleveland, OH: CRC Press.
APPENDIX A
DERIVATION OF AEGL VALUES FOR BUTANE
Derivation of AEGL-1 Values
| Key study: | Patty, F.A., and W.P. Yant. 1929. Odor Intensity and Symptoms Produced by Commercial Propane, Butane, Pentane, Hexane, and Heptane Vapor. U.S. Bureau of Mines Report of Investigation. No 2979. Washington, DC: U.S. Department of Commerce, Bureau of Mines. |
| Toxicity end point: | 10-min exposure to 10,000 ppm is the no-observed-adverse-effect level for CNS depression. |
| Time scaling: | C3 × t = k for extrapolation to 30 min and 60 min; flatlining assumed from 60 min to 4-and 8-h exposure (based on 60-min steady-state concentration). k = (10,000 ppm)3 × 10 min = 1013 ppm3-min |
| Uncertainty factors: | 1 for interindividual variability |
| Calculations: | |
| 10-min AEGL-1: | 10,000 ppma (24,000 mg/m3) |
| 30-min AEGL-1: | C3 × 30 min = 1013 ppm3-min C = 6,900 ppmb (rounded) (16,000 mg/m3) |
| 1-h AEGL-1: | C3 × 60 min = 1013 ppm3-min C = 5,500 ppmb (rounded) (13,000 mg/m3) |
| 4-h AEGL-1: | Set equivalent to 1-h AEGL-1 of 5,500 ppmb (13,000 mg/m3) |
| 8-h AEGL-1: | Set equivalent to 1-h AEGL-1 of 5,500 ppmb (13,000 mg/m3) |
a The AEGL-1 value is greater than 10% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, safety considerations against the hazard of explosion must be taken into account.
b The 10-min AEGL-1 value is greater than 50% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
Derivation of AEGL-2 Values
| Key study: | Nuckolls, A.H. 1933. Underwriters’ Laboratoris Report on the Comparative Life, Fire, and Explosion Hazards of Common Refrigerants. Miscellaneous Hazards No. 2375. Chicago, IL: National Board of Fire Underwriters. |
| Toxicity end point: | CNS depression, no effects consistent with definition of AEGL-2 in guinea pigs exposed to butane at 50,000 ppm for 2 h. |
| Time scaling: | C3 × t = k for extrapolation to 10 min, flatlining assumed from 30 min to 8-h exposure (based on 2-h steady-state concentration). k = (50,000 ppm)3 × 30 min = 3.8 × 1015 ppm3-min |
| Uncertainty factors: | Total uncertainty factor of 3 for differences between species and individuals. |
| Calculations: | |
| 10-min AEGL-2: | C3 × 10 min = 3.8 × 1015 ppm3-min C = 72,112 ppm 72,112 ÷ 3 = 24,000 ppma (rounded) (= 57,000 mg/m3) |
| 30-min AEGL-2: | C = 50,000 ppm (2-h steady state concentration) 50,000 ppm ÷ 3 = 17,000 ppmb (rounded) (40,000 mg/m3) |
| 1-h AEGL-2: | Set equivalent to the 30-min AEGL-2 of 17,000 ppmb (40,000 mg/m3) |
| 4-h AEGL-2: | Set equivalent to the 30-min AEGL-2 of 17,000 ppmb (40,000 mg/m3) |
| 8-h AEGL-2: | Set equivalent to the 30-min AEGL-2 of 17,000 ppmb (40,000 mg/m3) |
a The 10-min AEGL-2 value is greater than the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
b The AEGL-2 value is greater than 50% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
Derivation of AEGL-3 Values
| Key study: | Shugaev, B.B. 1969. Concentrations of hydrocarbons in tissues as a measure of toxicity. Arch. Environ. Health 18(6):878-882. |
| Toxicity end point: | Lethality study in mice exposed for 2 h. The calculated 2-h LC01 is 160,000 ppm. |
| Time scaling: | C3 × t = k for extrapolation to 10 min, flatlining assumed from 30 min to 8-h exposure (based on 2-h steady-state concentration). k = (160,000 ppm)3 × 30 min = 1.2 × 1017 ppm3-min |
| Uncertainty factors: | Total uncertainty factor of 3 for differences between species and individuals. |
| Calculations: | |
| 10-min AEGL-3: | C3 × 10 min = 1.2 × 1017 ppm3-min C = 230,760 ppm 230,760 ÷ 3 = 77,000 ppm (rounded) (18,000 mg/m3) |
| 30-min AEGL-3: | C = 160,000 ppm (2-h steady state concentration) 160,000 ppm ÷ 3 = 53,000 ppma (rounded) (130,000 mg/m3) |
| 1-h AEGL-3: | Set equivalent to the 30-min AGEL-3 of 53,000 ppma (130,000mg/m3) |
| 4-h AEGL-3: | Set equivalent to the 30-min AGEL-3 of 53,000 ppma (130,000mg/m3) |
| 8-h AEGL-3: | Set equivalent to the 30-min AGEL-3 of 53,000 ppma (130,000mg/m3) |
aThe AEGL-3 values are greater than the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
APPENDIX B
CATEGORY GRAPH FOR BUTANE
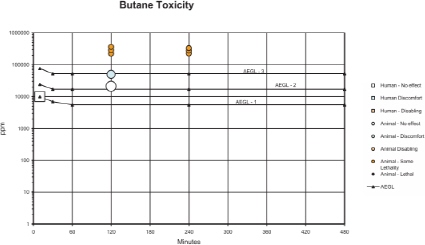
FIGURE B-1 Category graph of toxicity data and AEGLs values for butane.
APPENDIX C
ACUTE EXPOSURE GUIDELINE LEVELS FOR BUTANE
Derivation Summary for Butane
AEGL-1 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| See belowa | 6,900 ppmb (16,000 mg/m3) | 5,500 ppmb (13,000 mg/m3) | 5,500 ppmb (13,000 mg/m3) | 5,500 ppmb (13,000 mg/m3) |
| Key reference: Patty, F.A., and W.P. Yant 1929. Odor Intensity and Symptoms Produced by Commercial Propane, Butane, Pentane, Hexane, and Heptane Vapor. U.S. Bureau of Mines Report of Investigation. No 2979. Washington, DC: U.S. Department of Commerce, Bureau of Mines. | ||||
| Test species/Strain/Number: Groups of 3- 6 human subjects (male and female, ages 20-30 years). The study was on the warning properties of several alkanes. | ||||
| Exposure route/Concentrations/Durations: Subjects were exposed at slowly increasing concentrations up to 50,000 ppm (continuous exposure test, total exposure was at least 10 min), followed by exposure to fixed exposure concentrations for a short duration (exact duration unknown) on the same day (intermittent exposure test). The fixed exposure concentrations were approximately 1,000, 2,000, 5,000, 7,000, 10,000, 20,000, and 100,000 ppm. | ||||
| Effects: No odor detection and no irritation were reported during the continuous exposure test. Drowsiness reported after a 10-min exposure to butane 10,000 ppm was considered to be of minor severity. No details on CNS effects were presented for the higher exposure concentrations. No irritation was reported at 100,000 ppm for 10 min. | ||||
| End point/Concentration/Rationale: No AEGL-1 effects at 10,000 ppm for 10 min. | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 1 Interspecies: 1, test subjects were humans. Intraspecies: 1, concentration-response curve appears to be very steep indicating small interindividual variability; no irritation at 100,000 ppm for 10 min; larger factor will result in unrealistically low values compared with occupational standards. |
||||
| Modifying factor: Not applicable | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time scaling: n = 3 for time scaling from 10 to 60 min (animal data suggest high value of n); because steady state is reached within 30 min, the values for the 4- and 8-h exposures were set equivalent to the 60-min value. | ||||
| Data adequacy: Database is relatively poor but the values are supported by the available animal data. | ||||
a The AEGL-1 value is 10,000 ppm (23,700 mg/m3), which is greater than 50% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
b The AEGL value is greater than 10% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, safety considerations against the hazard of explosion must be taken into account.
AEGL-2 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| See belowa | See belowb | See belowb | See belowb | See belowb |
| Key reference: Nuckolls, A.H. 1933. Underwriters’ Laboratoris Report on the Comparative Life, Fire, and Explosion Hazards of Common Refrigerants. Miscellaneous Hazards No. 2375. Chicago, IL: National Board of Fire Underwriters. | ||||
| Test species/Strain/Number: Guinea pigs, 3 animals per every concentration-time combination. | ||||
| Exposure route/Concentrations/Durations: Inhalation for 5, 30, 60, or 120 min at 21,000-28,000 ppm or 50,000-56,000 ppm. | ||||
| Effects: At 21,000-28,000 ppm, occasional irregular and rapid breathing, did not worsen during exposure, and rapid recovery after exposure ended. At 50,000-56,000 ppm, irregular breathing and dazed appearance during second hour of exposure, but animals were able to walk | ||||
| End point/Concentration/Rationale: Animals had dazed appearance but were able to walk at 50,000 ppm (lower concentration in the exposure range). | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: A total uncertainty factor of 3 is considered sufficient for toxicokinetic and toxicodynamic differences between individuals and interspecies differences. The effects are attributed to butane itself and no relevant differences in kinetics are assumed, so only small interindividual differences are expected. The concentration-response curve appears to be very steep indicating that a large uncertainty factor is unnecessary. Further, a larger uncertainty factor would lead to unrealistically low values for AEGL-2 that would be similar to the AEGL-1 values. |
||||
| Modifying factor: Not applicable | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time scaling: A steady state is reached within 30 min, and the effects are considered to be concentration dependent. Therefore, the starting point for the 30-min and the 1-, 4-, and 8-h values were the 2-h steady-state value of 50,000 ppm. For extrapolation from 30 to 10 min, n = 3. | ||||
| Data adequacy: Although case reports of butane exposure indicate potential for cardiac sensitization (analogous to propane), this end point has not been studied. | ||||
a The 10-min AEGL-2 value is 24,000 ppm (57,000 mg/m3), which is greater than the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
b The AEGL-2 value for 30-min and 1-, 4-, and 8-h exposures is 17,000 ppm (40,000 mg/m3), which is greater than 50% of the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.
AEGL-3 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| See belowa | See belowa | See belowa | See belowa | See belowa |
|
|
||||
| Key reference: Shugaev, B.B. 1969. Concentrations of hydrocarbons in tissues as a measure of toxicity. Arch. Environ. Health 18(6):878-882. | ||||
| Test species/Strain/Number: Mice, strain and number not specified. | ||||
| Exposure route/Concentrations/Durations: Inhalation for 2 h, butane concentrations not specified. | ||||
| Effects: LC16 = 224,000 ppm; LC50 = 287,000 ppm; LC84 = 363,000 ppm A 2-h LC01 was calculated to be 160,000 ppm. | ||||
| End point/Concentration/Rationale: Lethal concentration, 1% lethality | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: A total uncertainty factor of 3 is considered sufficient because the effects are attributed to butane itself, and no relevant differences in kinetics are assumed. A species with a relatively high susceptibility is used. The concentration-response curve appears to be very steep indicating that a large factor s unnecessary. Further, a larger uncertainty factor would lead to unrealistically low values for AEGL-3, which would be similar to the AEGL-2 values. |
||||
| Modifying factor: Not applicable | ||||
| Animal-to-human dosimetric adjustment: Not applicable | ||||
| Time scaling: A steady state is reached within 30 min, and the effects are considered to be concentration dependent. Therefore, the starting point for the 30-min and the 1-, 4-, and 8-h values were the 2-h steady-state value of 160,000 ppm. For extrapolation from 30 to 10 min, n = 3. | ||||
| Data adequacy: The results of the key study in mice are comparable with the results from a second study in mice. The 10-min value is supported by human data. Exposure to slowly increasing concentrations of butane up to 50,000 ppm (total exposure duration at least 10 min) and a short exposure (exact duration unknown) at 100,000 ppm on the same day did not result in serious complaints. | ||||
a The 10-min AEGL-3 values is 77,000 ppm (180,000 mg/m3), and the AEGL-3 value for 30 min and 1, 4, and 8 h is 53,000 ppm (130,000 mg/m3). All of the AEGL-3 values are greater than the lower explosive limit for butane in air of 19,000 ppm. Therefore, extreme safety considerations against the hazard of explosion must be taken into account.



































