2
Chloroacetaldehyde1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory
![]()
1 This document was prepared by the AEGL Development Team composed of Peter Bos (RIVM, The Dutch National Institute of Public Health and the Environment), Julie M. Klotzbach (Syracuse Research Corporation), Chemical Manager Marinelle Payton (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances), and Ernest V. Falke (U.S. Environmental Protection Agency). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold concentrations for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Chloroacetaldehyde is a colorless, volatile liquid with an acrid, penetrating odor. It evaporates easily and dissolves in water. It is not flammable, but vapor/ air mixtures may be explosive at temperatures above 88°C. Chloroacetaldehyde can exist in combinations of four forms: monomer, monomer hydrate, dimer hydrate, and cyclic trimer. Commercial aqueous solution of chloroacetaldehyde (45%) contains a 50:50 mixture of the monomer and dimer hydrates. Chloroacetaldehyde is predominantly used as a chemical intermediate in the manufacture of 2-aminothiazole and other compounds, in the control of algae, bacteria, and fungi in water, and in a spinning solution of poly β-alanine.
The toxicity database on chloroacetaldehyde is poor. Apart from a brief statement indicating that a concentration of 10 ppm produced lacrimation and nasal irritation in humans, no information was available on human toxicity. Chloroacetaldehyde is known to be a strong corrosive agent. The predominant effect of chloroacetaldehyde in animals is direct, strong irritation of the eyes, nose, and lungs (resulting in pulmonary edema and death), and has a very steep concentration-response relationship. The best studies of these effects are by Dow Chemical Company (1952) and Arts (1987). The first study exposed mice, rats, and guinea pigs to chloroacetaldehyde at several concentrations and exposure duration, ranging from 400 ppm for 6 min to 10 ppm for 7 h. Rats, mice, and guinea pigs were also exposed repeatedly (eight exposures in 10 days) to
chloroacetaldehyde at 5 ppm for 7 h (Dow Chemical Company 1952). In the second study, rats were exposed at concentrations of 44-2,643 ppm for 1 h (Arts 1987). Both studies focused on mortality and reported nonlethal effects in a general manner. In rats, the lowest concentration-time combinations that induced lethality ranged from 25 ppm for 7 h (19 of 20 rats died) to 400 ppm for 0.1 h (1 of 20 rats died). No deaths were reported at concentration-time combinations ranging from 10 ppm for 7 h to 100 ppm for 12 min. Lethality increased both with concentration and with duration. A 1-h LC50 (lethal concentration, 50% lethality) for rats was estimated to fall between 203 and 243 ppm. Guinea pigs were less sensitive to chloroacetaldehyde than rats.
Nonlethal effects observed in these studies included ocular and nasal irritation. Irritation was slight after repeated exposure to chloroacetaldehyde at 5 ppm, and was more pronounced after single exposures at concentrations greater than 10 ppm. Pulmonary edema was found in some rats 2 weeks after being exposed to chloroacetaldehyde at 44 ppm for 1 h. Animals in this study also exhibited closed eyes and salivation. Pulmonary effects became more severe with increasing concentrations in some animals that died.
Studies of the neurotoxicity of chloroacetaldehyde were not found; however, indirect evidence from experiments with the anticancer drugs ifosfamide and cyclophosphamide (chloroacetaldehyde is a main metabolite of these drugs) suggests that it may have neurotoxic effects. In addition, chloroacetaldehyde was found to be mutagenic in several stains of Salmonella typhimurium, Aspergillus nidulans, Streptomyces coelicolor, and Chinese hamster V79 cells. Little information was available on the carcinogenicity of chloroacetaldehyde.
AEGL-1 values are based on nasal and ocular irritation observed in rats after a single exposure to chloroacetaldehyde at concentrations of 10 ppm and higher. Slight irritation was also observed in rabbits, rats, and mice, but not guinea pigs, after repeated exposure to chloroacetaldehyde at 5 ppm (7 h/day) (Dow Chemical Company 1952). Irritation was reported to be related to both concentration and exposure duration. A concentration of 5 ppm was chosen as the point of departure for the AEGL-1 values. A modifying factor of 2 was applied to reduce that concentration to a no-effect level. With the exception of the 10-min AEGL value, time scaling was performed using the equation Cn × t = k. The value of n was determined to be 1.2 based on mortality data. A total uncertainty factor of 10 (two factors of 3) was considered sufficient for toxicokinetic and toxicodynamic differences between species and for individual variability, and no relevant differences in kinetics were assumed (the effects are attributed to direct interaction of chloroacetaldehyde with the mucous membranes of the nose and eyes). The 10-min AEGL-1 was set equal to the 30-min value, because extrapolation from a 7-h exposure to a 10-min value had too much uncertainty. The report of lacrimation and nasal irritation in humans within a few minutes of exposure to chloroacetaldehyde at 10 ppm (Dow Chemical Company 1952) provided supporting data to derive AEGL-1 values on the basis of the rat data.
AEGL-2 values are based on impaired pulmonary function in rats. Data from a well-performed and adequately documented study in rats (Arts 1987)
were chosen for the point of departure for the 1-h AEGL value. A 1-h exposure at 44 ppm (the lowest concentration tested) resulted in pulmonary edema in some animals that were killed at the end of a 2-week observation period. A modifying factor of 2 was applied to derive a no-effect level. A larger modifying factor was considered unnecessary because of the steep concentration-response curve of chloroacetaldehyde. Analogous to AEGL-1 values, a total uncertainty factor of 10 was used to derive the AEGL-2 values; a larger uncertainty factor would lead to unrealistically low values. AEGL-2 values for other time periods were derived by time scaling.
For AEGL-3 values, two studies of the acute lethality of chloroacetaldehyde (Dow Chemical Company 1952; Arts 1987) were considered. The mortality data showed a steep concentration-response curve; mortality shifted from 0% to close to 100% when concentration or exposure duration was doubled. Mortality data from the Arts (1987) study, in which rats were exposed to chloroacetaldehyde at 44-2,643 ppm for 1 h, was modeled using EPA benchmark dose software (version 1.3.2) (EPA 2005). A benchmark concentration associated with a 5% response (BMC05) of 136 ppm was calculated, with a lower 95% confidence limit (BMCL05) of 99 ppm. Application of a total uncertainty factor of 10, on the same basis as it used in deriving AEGL-1 and AEGL-2 values results, in a 1-h AEGL value of 9.9 ppm. AEGL-3 values for the other time periods were derived by time scaling.
The AEGL values for chloroacetaldehyde are presented in Table 2-1.
TABLE 2-1 Summary of AEGL Values for Chloroacetaldehyde
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| AEGL-1 (nondisabling) | 2.3 ppm (7.4 mg/m3) | 2.3 ppm (7.4 mg/m3) | 1.3 ppm (4.2 mg/m3) | 0.40 ppm (1.3 mg/m3) | 0.22 ppm (0.71 mg/m3) | Ocular and nasal irritation (Dow Chemical Company 1952) |
| AEGL-2 (disabling) | 9.8 ppm (31 mg/m3) | 3.9 ppm (13 mg/m3) | 2.2 ppm (7-1 mg/m3) | 0.69 ppm (2.2 mg/m3) | 0.39 ppm (1.5 mg/m3) | Pulmonary edema (Arts 1937) |
| AEGL-3 (lethal) | 44 ppm (140 mg/m3) | IS ppm (57 mg/m3) | 9.9 ppm (32 mg/m3) | 3.1 ppm (10 mg/m3) | 1.8 ppm (5.6 mg/m3) | Mortality BMCL05 (Dow Chemical Company 1952; Arts 1987) |
1. INTRODUCTION
Chloroacetaldehyde is a colorless, volatile liquid with an acrid, penetrating odor. It evaporates easily and dissolves in water. Chloroacetaldehyde is not flammable, but vapor/air mixtures may be explosive at temperatures above 88°C (see Table 2-2).
Chloroacetaldehyde can exist in combinations of four forms depending on how it was prepared: monomer, monomer hydrate, dimer hydrate, and cyclic trimer (Elmore et al. 1976). The monomer and dimer hydrates are formed instantly when the anhydrous monomer is added to water. The cyclic trimer is formed from the anhydrous monomer upon standing under dry conditions. The cyclic trimer is only slightly soluble in water, but the monomer and dimer hydrates are formed when the cyclic trimer is heated in water. The anhydrous monomer is obtained by cracking the cyclic trimer.
Commercial aqueous solution of chloroacetaldehyde (45%) contains a 50:50 mixture of the monomer and dimer hydrates (Elmore et al. 1976). The two hydrates are dehydrated and converted to the monomer under gas-liquid phase chromatography conditions. Chloroacetaldehyde is sufficiently stable to permit its direct collection on silica gel and subsequent storage in a freezer.
TABLE 2-2 Chemical and Physical Properties for Chloroacetaldehyde
| Parameter | Value | Reference |
| CAS registry no. | 107-20-0 | HSDB 2009 |
| Synonyms | Monochloroacetaldehyde; 2-chloro-l-ethanal | Budavari et al. 1989 |
| Chemical formula | C2H5ClO | Budavari et al. 1989 |
| Molecular weight | 78.50 | Budavari et al. 1989 |
| Physical state | Liquid | Budavari et al. 1989 |
| Color | Colorless | HSDB 2009 |
| Odor | Acrid, penetrating | Budavari et al. 1989 |
| Melting point | -16.3°C (40% aqueous solution) | IPCS 2005 |
| Boiling point | 85-86:C (pure) 85-100°C 90-100°C (40% aqueous solution) |
Budavari et al. IPCS 2005 OSHA 1989 1989 |
| Vapor density (air =1) | 2.7 | IPCS 2005 |
| Liquid density (water = 1) | 1.19 (40% aqueous solution) | IPCS 2005 |
| Solubility in water | Yes | Budavari et al. 1989 |
| Vapor pressure | 100 mm Hg at 45°C (40% aqueous solution) 110 mm Hg at 20°C | MOSH 1991 HSDB 2009 |
| Explosive | Vapor/air mixtures may be explosive (40% aqueous solution) above 88°C | IPCS 2005 |
| Conversion factors | 1 mg/m3 = 0.312 ppm 1 ppm = 3.21 mg/m3 |
MOSH 2011 |
No current information was found on the chemical production of chloroacetaldehyde. The amount of chloroacetaldehyde manufactured or imported in the United States in 1977 was reported to be 1-10 million pounds (EPA 1987).
Chloroacetaldehyde is used primarily as a chemical intermediate (EPA 1987) in the manufacture of 2-aminothiazole and other compounds (ACGIH 1991). It is also used in the control of algae, bacteria, and fungi in water, and in a spinning solution of poly β-alanine (ACGIH 1991). Furthermore, it has its application in tree-trunk debarking operations and in analytical chemistry as a fluorescent label (McCann 1975).
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
No case reports on human deaths from acute exposure to chloroacetaldehyde were found.
2.2. Nonlethal Toxicity
Case reports of nonlethal toxicity in humans were not found, nor were occupational or epidemiologic studies available. A report of the Dow Chemical Company (1952) on acute mortality in experimental animals stated the following: “Every concentration employed including the lowest (10 ppm) produced lacrimation and nasal irritation in humans within a few minutes.” No additional details were provided.
Several studies have investigated the toxicity of the antineoplastic agent ifosfamide which indicate a causative role for chloroacetaldehyde (the main metabolite of ifosfamide) in the development of nephrotoxicity (Loebstein et al. 1999; Skinner et al. 2000; Aleksa et al. 2001; Yaseen et al. 2008; Hanly et al. 2009). Additional information on ifosfamide-induced nephrotoxicity is reviewed in Section 4.2.
2.3. Neurotoxicity
No reports on neurotoxicity induced by chloroacetaldehyde were found. Goren et al. (1986), however, suggested a causative role for chloroacetaldehyde (a main metabolite of the anticancer drug ifosfamide) in the development of neurotoxic side-effects of ifosfamide chemotherapy. Cerebellar dysfunction, seizures, and changes in mental status were reported in as many as 30% of patients on high-dose treatment with ifosfamide. Blood concentrations of chloroacetaldehyde were 88 micromoles per liter (μmol/L) at 6 h and 109 μmol/L at 24 h in two patients with neurotoxic effects (somnolescence, urinary incontinence, and inappropriate behavior), compared with 45 μmol/L at 6 h and 22 μmol/L at 24 h
in four patients without such effects. Rieger et al. (2004) conducted a retrospective trial of 60 cancer patients receiving ifosfamide as part of multiple drug chemotherapy regimens to evaluate potential risk factors for ifosfamide-induced encephalopathy. Sixteen patients (26.6%) developed neurologic symptoms; the effects were not correlated with age, sex, hepatic function, or renal function.
2.4. Developmental and Reproductive Toxicity
No studies on developmental or reproductive toxicity of chloroacetaldehyde in humans were found.
2.5. Genotoxicity
No studies on the genotoxicity of chloroacetaldehyde in humans or on human cells were found.
2.6. Carcinogenicity
No carcinogenicity studies of chloroacetaldehyde in humans were found.
2.7. Summary of Human Data
No information on chloroacetaldehyde toxicity in humans was available, other than a brief statement that chloroacetaldehyde at 10 ppm produced lacrimation and nasal irritation in humans (Dow Chemical Company 1952). Studies with ifosfamide suggest a causative role for chloroacetaldehyde (a main metabolite of the drug) in neurologic and renal effects.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
A summary of the data on acute lethality in laboratory animals exposed to chloroacetaldehyde is presented in Table 2-3.
3.1.1. Guinea Pigs
Guinea pigs (10 animals/group; sex and strain not specified) were exposed to chloroacetaldehyde at target concentrations of 25 (for 7 h), 50 (for 4 h), 100 (for 2 h), or 400 ppm (for 0.5 h). No details were provided on the purity the chloroacetaldehyde or the exposure conditions. Target concentrations were monitored during the experiment, but the method and measurements were not specified. It was not clear whether an unexposed control group was used. Mortality was observed only at 400 ppm; seven of 10 guinea pigs died (Dow Chemical Company 1952).
3.1.2. Rats
Rats (19 or 20 animals/group; sex and strain not specified) were exposed to chloroacetaldehyde at target concentrations of 10 (for 7 h), 25 (for 7 h), 50 (for 1, 3.5, or 4 h), 100 (for 0.2 or 2 h), or 400 ppm (for 0.1, 0.25, or 0.5 h). No details were provided on the purity of the chloroacetaldehyde or the exposure conditions. Target concentrations were monitored during the experiment, but the method and measurements were not specified. It was not clear whether an unexposed control group was used. Mortality was related to the concentration and exposure duration (see Table 2-3). No mortality was occurred at concentrations (and durations) of 10 ppm (for 7 h), 50 ppm (for 1 h), and 100 ppm (for 0. 2 h). One animal died at 400 ppm (0.1 h). Almost all of the rats died at 25 ppm (for 7 h), 50 ppm (for 3.5 h and 4 h), 100 ppm (for 2 h), and 400 ppm (for 0.25 and 0.5 h) (Dow Chemical Company 1952).
TABLE 2-3 Acute Lethality in Animals Exposed to Chloroacetaldehyde
| Species | Concentration (ppm) | Exposure Duration | Mortality | Reference |
| Guinea pig (n=10) | 25 50 |
7 h 4 h |
0/10 0/10 |
Dow Chemical Company 1952 |
| 100 | 2 h | 0/10 | ||
| 400 | 0.5 h | 7/10 | ||
| Rat (n = 19-20) | 10 25 |
7 h 7 h |
0/20 19/20 |
Dow Chemical Company 1952 |
| 50 | 1 h 3.5 h 4 h |
0/20 20/20 18/20 |
||
| 100 | 0-2 h 2 h |
0/19 20/20 |
||
| 400 | 0-1 h 0.25 h 0-5 h |
1/20 20/20 19/20 |
||
| Rat (n = 10) | 44 159 203 243 309 596 2.643 |
1 h | 0/10 3/10 4/10 10/10 10/10 10/10 10/10 |
Arts 1987 |
| 203-243 | 1 h | LC50 | ||
3.2. Nonlethal Toxicity
SPF-reared Borr:WISW rats (five animals/sex/group) were exposed (whole body; individually housed) for 1 h to mean concentrations of chloroacetaldehyde (45.4% (w/w) at 44, 159, 203, 243, 309, 596, or 2,643 ppm (0.14, 0.51, 0.65, 0.78, 0.99, 1.91, or 8.47 g/m3, respectively). Concentrations of chloroacetaldehyde were continuously monitored during exposure. No unexposed control animals were used. Relative humidity was high (51-91%) during exposure, due, in part, to the large amount of water in the test material. The animals were observed for up to 2 weeks. Mortality rates of 0% (44 ppm), 30% (159 ppm), 40% (203 ppm), and 100% (≥243 ppm) were found (see Table 2-3). Deaths were observed during exposure (at the two highest concentrations) or within several hours or 1-2 days after exposure. A 1-h LC50 value of 203-243 ppm was estimated. Because of the steep concentration-effect curve and natural variability between groups, it was not possible to determine an LC50 value for chloroacetaldehyde with 95% confidence intervals. The LC50 value was estimated to be closer to 203 ppm, because a considerable decrease in body weight that would probably have lead to death was observed in some animals exposed at 159 and 203 (Arts 1987).
A summary of the nonlethal effects of chloroacetaldehyde in laboratory animals is presented in Table 2-4.
3.2.1. Guinea Pigs and Rabbits
In the acute inhalation experiment with guinea pigs describe earlier (see Section 3.1.1.), ocular and nasal irritation was found very early during exposure at all concentrations tested (25-400 ppm) (Dow Chemical Company 1952). The degree of irritation was related to concentration. Labored breathing was also observed at the higher concentrations, and slight drowsiness was apparent at some concentrations (not specified).
In a repeated-exposure study by Dow Chemical Company (1952), groups of five male guinea pigs and one female rabbit (strains not specified) were exposed to chloroacetaldehyde at 0 or 5 ppm for 7 h/day, 5 days/week, for a total of eight exposures in 10 days. No details were provided on the purity of chloroacetaldehyde, actual or nominal concentrations, or exposure conditions. Slight ocular irritation was observed in the rabbit, but it was unclear from the report whether nasal irritation was also present. No irritating effects were reported in the guinea pigs. No effect on growth, organ weights, and gross pathology were found in either species.
3.2.2. Rats
Rats (19 or 20 animals/group; sex and strain not specified) were exposed to chloroacetaldehyde at target concentrations of 10 (for 7 h), 25 (for 7 h), 50 (for 1, 3.5, or 4 h), 100 (for 0.2 or 2 h), or 400 ppm (0.1, 0.25, or 0.5 h). No
details were provided on the purity of the chloroacetaldehyde or the exposure conditions. Target concentrations were monitored during the experiment, but the method and measurements were not specified. It was not clear whether an unexposed control group was used. Ocular and nasal irritation was observed very early during exposure at all concentrations. Degree of irritation was related to concentration and duration of exposure. Labored breathing was also observed at the higher concentrations, and slight drowsiness was apparent at some concentrations (not specified) (Dow Chemical Company 1952).
Groups of five male and five female rats (strain not specified) were exposed to chloroacetaldehyde at 0 or 5 ppm for 7 h/day, 5 days/week, for a total of eight exposures in 10 days. No details were provided on the purity of chloroacetaldehyde, actual or nominal concentrations, or exposure conditions. Exposed rats exhibited slight nasal irritation and very slight ocular irritation. Growth of the male rats was slightly depressed, while the growth of the female rats was comparable to that of the control animals. No effects on organ weight or gross pathology were found (Dow Chemical Company 1952).
SPF-reared Borr:WISW rats (five animals/sex/group) were exposed (whole body; individually housed) for 1 h to mean concentrations of chloroacetaldehyde (45.4% (w/w) at 44, 159, 203, 243, 309, 596, or 2,643 ppm (0.14, 0.51, 0.65, 0.78, 0.99, 1.91, or 8.47 g/m3, respectively). Concentrations of chloroacetaldehyde were continuously monitored during exposure. No unexposed control animals were used. Relative humidity was high (51-91%) during exposure, due, in part, to the large amount of water in the test material. The animals were observed for up to 2 weeks. Descriptions of the observations were generally reported and did not always specify the number of animals affected or the exposure concentrations. Rats were restless and showed signs of discomfort (closed eyes, salivation, and, at the higher concentrations, wet nares, nasal discharge, and wet and soiled heads and breasts). At the highest concentration of 2,643 ppm, all rats exhibited labored respiration, accompanied by dyspnea and mouth breathing. Mortality was observed at all concentration, except the lowest of 44 ppm (see Table 2-3). Many of the rats that died had bloodstains around the nose and mouth. Rats exposed at the highest concentrations that did not die immediately were reported to have breathed “wheezingly”. Two rats exposed at 596 ppm became blind. No chloroacetaldehyde-induced effects on body weight we found, although two animals in the 159-and 203-ppm groups lost a considerable amount of weight. Animals that died during exposure or within the first 2 days of observation had pulmonary edema, which was accompanied in some cases by atelectasis and in most cases by hydrothorax. The investigators suggested that the latter finding could be explained by induced hypertension, although no information was provided to support that conclusion. Pulmonary edema was also observed in some animals exposed at the three lowest concentrations. The investigators concluded that the pulmonary effects suggested an impairment of pulmonary function. The stomachs and intestines were often filled with air because of mouth breathing, and an occasional thrombus was detected in the heart area (Arts 1987).
3.2.3. Mice
Groups of five female mice (strain not specified) were exposed to chloroacetaldehyde at 0 or 5 ppm for 7 h/day, 5 days/week for a total of eight exposures in 10 days. No details were provided on the purity of the chloroacetaldehyde, actual or nominal concentrations, or the exposure conditions. Mice exhibited slight nasal irritation. No effects on growth, organ weights, or gross pathology were found.
TABLE 2-4 Nonlethal Toxicity in Animals Exposed to Chloroacetaldehyde
| Species | Concentration (ppm) | Exposure Duration | Effects | Reference |
| Guinea pig | 25 | 7 h | Concentration-related ocular and | Dow |
| (n= 10) | 53 | 4b | nasal irritation: labored breathing | Chemical |
| 100 | 2 h | at the higher concentrations: slight | Company | |
| 400 | 0.5 h | drowsiness (concentrations not specified) | 1952 | |
| Guinea pig | 5 | 7 h/d, 5 d/wk, | No effects reported | Dow |
| (n=5) | 8 exposures in 10 d | Chemical Company 1952 | ||
| Rabbit | 5 | 7 h/d, 5 d/wk, | Slight ocular irritation | Dow |
| (n=l) | 8 exposures in 10 d | Chemical Company 1952 | ||
| Rat | 10 | 7 h | Concentration- and duration-related | Dow |
| (n=19 or 20) | 25 | 7 h | ocular and nasal irritation; labored | Chemical |
| 53 | 1,3.5,4 h | breathing at higher concentrations: | Company | |
| 100 | 0.2, 2 h | slight drowsiness (concentrations | 1952 | |
| 400 | 0.1, 0.25, 0.5 h | not specified) | ||
| Rat(n = 10) | 5 | 7 h/d, 5 d/wk, | Slight nasal irritation, very slight | Dow |
| 8 exposures | ocular irritation | Chemical | ||
| in 10 d | Company 1952 | |||
| Rat(n = 10) | 44 159 203 243 309 596 2,643 |
1 h | At all concentrations, closed eyes, salivation, and decreased pulmonary function (e.g., pulmonary edema [with some atelectasis and hydrothorax], labored breathing). At higher concentrations, wet nares, nasal discharge, wet and soded heads and breasts. | Arts 1987 |
| Mouse (n = 5) | 5 | 7 h/d, 5 d/wk, 8 exposures in 10 d |
Slight nasal irritation | Dow Chemical Company 1952 |
3.3. Neurotoxicity
No neurotoxicity studies on experimental animals exposed to chloroacetaldehyde were found. However, a study on metabolism in rats found that chloroacetaldehyde specifically affected mitochondrial long-chain fatty acid metabolism. The rate of palmitic-acid oxidation, but not that of succinic-acid or octanoic-acid oxidation, was affected. Such changes in fatty-acid metabolism might play a role in encephalopathy and chronic fatigue observed after treatment with the antitumor alkylating agents ifosfamide and cyclophosphamide, which form chloroacetaldehyde as a main reactive metabolite (Visarius et al. 1999).
3.4. Developmental and Reproductive Toxicity
No studies on developmental or reproductive toxicity of chloroacetaldehyde in experimental animals were found.
3.5. Genotoxicity
Early indications of the genotoxicity of chloroacetaldehyde came from studies on the highly genotoxic compound vinyl chloride. The genotoxicity of vinyl chloride has been attributed to its metabolite chloroacetaldehyde, among others. The genotoxicity of chloroacetaldehyde was reviewed by Bartsch et al. (1976). Mutagenic responses were seen with Salmonella typhimurium TA100, TA1530, and TA1535. Relatively high toxicity in S. typhimurium TA1530 was found for chloroacetaldehyde compared with other mutagenic metabolites of vinyl chloride. A post-mitochondrial mouse-liver fraction decreased the mutagenic effect of chloroacetaldehyde on S. typhimurium TA100. Chloroacetaldehyde was further shown to be a strong mutagen in the Chinese hamster V79 cell system, inducing 8-azaguanine-resistant mutants. The chemical was also high cytotoxicity to those cells. Chloroacetaldehyde reacted covalently with adenine and cytidine in vitro. ACGIH (1991) reported that chloroacetaldehyde was mutagenic in the forward mutation system of Aspergillus nidulans, and in the forward and back mutation system of Streptomyces coelicolor.
3.6. Carcinogenicity
No carcinogenicity studies on inhalation exposure to chloroacetaldehyde were found. For other routes of exposure (skin application, skin initiationpromotion [promoter: phorbol myristate acetate], repeated subcutaneous injections, and intragastric feeding), Van Duuren et al. (1979) found that chloroacetaldehyde was not carcinogenic in male and female Ha:ICR Swiss mice, despite the fact that chloroacetaldehyde was mutagenic in microorganisms and Chinese hamster V-79 cells and was strongly implicated as the proximal carcinogenic
metabolite formed from the animal and human carcinogen vinyl chloride (see Bartsch et al. 1976; Goldschmidt 1984; NRC 2012). However, chloroacetaldehyde was not hepatocarcinogenic in male B6C3F1 mice (Daniel et al. 1992). In that study, chloroacetaldehyde was administered in drinking water at a target concentration of 0.1 g/L (mean measured dose: 0.095 ± 0.006 g/L) for 104 weeks. The mean daily-ingested dose was calculated to be 17 mg/kg/day. The only obvious target organ was the liver, as indicated by an increase in the absolute and relative organ weight. A significant increase in the prevalence of hepatic tumors was found. Prevalence rates for carcinomas, adenomas, and combined tumors (adenomas and carcinomas) were 31, 8, and 38% in treated rats, compared with 10, 5, and 15% among controls. Furthermore, hepatocellular hyperplastic nodules (a preneoplastic lesion) occurred in the liver in the chloroacetaldehyde group (8%) but not in the control group. Histotologic effects in the liver were mild and included cytomegaly, necrosis, and chronic active inflammation.
3.7. Summary of Animal Data
The database on chloroacetaldehyde is poor. Most of the available data are on the lethal and irritant effects of chloroacetaldehyde. In rats, the lowest concentration-duration combinations found to induce lethality ranged from 25 ppm for 7 h (19 of 20 rats died) to 400 ppm for 0.1 h (1 of 20 rats died). No deaths were reported in rats at concentration-duration combinations ranging from 10 ppm for 7 h to 100 ppm for 0.2 h. Lethality increased both with concentration and with duration. A 1-h LC50 for rats was estimated to be between 203 and 243 ppm. Guinea pigs were less sensitive than rats to chloroacetaldehyde.
Chloroacetaldehyde is a strong corrosive agent, and has been shown to be very irritating to the eyes and nose of laboratory animals. These effects occur soon after onset of exposure and are related to concentration and duration of exposure. Repeated exposure to chloroacetaldehyde at 5 ppm induced slight nasal and ocular irritation. Irritation became more pronounced with single exposures to chloroacetaldehyde at concentrations greater than 10 ppm. Pulmonary edema was observed in some rats 2 weeks after exposure to chloroacetaldehyde at 44 ppm for 1-h. Closed eyes and salivation were also observed at that concentration. Pulmonary effects became more severe with increasing concentrations. Animals that died had pulmonary edema, often accompanied by atelectasis and hydrothorax. Although no neurotoxicity studies of chloroacetaldehyde were found, some indirect indications of such effects are suggested by experiments with ifosfamide and cyclophosphamide.
Chloroacetaldehyde was found to be mutagenic in several stains of S. typhimurium, A. nidulans, S. coelicolor, and Chinese hamster V79 cells.
Little information was available on the carcinogenicity of chloroacetaldehyde. However, the target organ for chloroacetaldehyde appears to the liver, as
evidenced by increased absolute and relative liver weights, increased hepatic tumors and preneoplastic lesions, and mild histologic effects.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
Absorption, Distribution, and Excretion
No reports on absorption, distribution, and excretion of chloroacetaldehyde were found.
Metabolism
Joqueviel et al. (1997) summarized information on the metabolic pathways of chloroacetaldehyde. Little information was available on the metabolism of chloroacetaldehyde. What information was available was from in vitro and in vivo studies of rat hepatocytes. The metabolic pathways of chloroacetate and chloroethanol were better documented, and were used to develop a metabolism scheme. In brief, chloroacetaldehyde can be oxidized to chloroacetic acid by aldehyde dehydrogenase, followed by conjugation with glutathione, and finally the formation of the urinary metabolite thiodiglycolic acid. Chloroacetaldehyde may also be directly conjugated with glutathione. This scheme was in close agreement with the metabolic pathways for vinyl chloride (ATSDR 1997; NRC 2012).
4.2. Mechanism of Toxicity
No in vivo studies on the mechanism of toxicity of chloroacetaldehyde following inhalation exposure in humans or animals were found. Studies of other routes of exposure included a study of rats exposed to chloroacetaldehyde by intraperitoneal injection (Visarius et al. 1999) and in vitro studies using isolated rat hepatocytes (Sood and O’Brien 1993, 1994) and liver enzymes (Sharpe and Carter 1993), human kidney tubules (Dubourg et al. 2001, 2002), and perfused rabbit hearts (Joqueviel et al. 1997). In addition, studies of two human lung-cancer cell lines were available (Manzano et al. 1996). In all of these studies, chloroacetaldehyde was investigated as a metabolite of vinyl chloride or the alkylating antitumor agents ifosfamide and cyclophosphamide to understand the mechanism of hepatoxicity, nephrotoxicity, cardiotoxicity, encephalopathy, and chronic fatigue observed after exposure or treatment with those agents. These studies are summarized below. Collectively, they indicate that chloroacetaldehyde has the potential to cause serious effects in different organs once it becomes systemically available. However, because chloroacetaldehyde is very
reactive, effects on the respiratory tract are likely to be predominant following inhalation exposure. Although the potential for chloroacetaldehyde to produce systemic effects cannot be ruled out, it is probable that the respiratory tract will be most sensitive to inhalation exposure; this is supported by results of studies in animals (see Section 3.2).
Hepatoxicity
Chloroacetaldehyde induced a loss in viability of isolated rat hepatocytes in a concentration-and time-dependent manner. Chloroacetaldehyde was metabolized rapidly and the cytotoxic effects were irreversible (Sood and O’Brien 1993). Cytosolic and mitochondrial rat-liver aldehyde dehydrogenases seemed to play a significant role in the metabolism of chloroacetaldehyde (Sharpe and Carter 1993; Sood and O’Brien 1994). Cytotoxicity in isolated rat hepatocytes was enhanced markedly if hepatocyte alcohol or aldehyde dehydrogenase was inhibited before exposure to chloroacetaldehyde. Furthermore, the metabolites chloroacetate and chloroethanol were far less toxic than chloroacetaldehyde. Sood and O’Brien (1993, 1994) found that the concentration of glutathione, reversible thiol protein adduct formation (such as hemithioacetals or thioacetals), mitochondrial toxicity, and lipid peroxidation were involved in chloroacetaldehyde-induced hepatocyte cytotoxicity. Hepatocytes from fasted rats were more susceptible to chloroacetaldehyde. These in vitro results were confirmed in vivo by Visarius et al. (1999).
Nephrotoxicity
Dubourg et al. (2001) investigated the mechanism of nephrotoxicity on isolated tubular (mainly proximal) fragments of human kidney cortex. Chloroacetaldehyde was highly toxic to human kidney tubules. A dramatic decrease in cellular adenosine triphosphate (ATP) concentrations occurred. Concentrations of CoA (substrate of pyruvate dehydrogenase) and acetyl-CoA (activator of pyruvate carboxylase) were virtually depleted. The correlation between the cellular depletion of CoA and acetyl-CoA and nephrotoxic effects demonstrated the importance of thiol compounds in the mechanism of the nephrotoxicity. Chloroacetaldehyde was metabolized at high rates, presumably by oxidation via aldehyde dehydrogenase (a very active enzyme in human kidneys). Chloroacetate, which is less toxic than chloroacetaldehyde, was the only major product of chloroacetaldehyde metabolism by human kidney tubules. Results of this study strongly suggest that chloroacetaldehyde is detoxified by metabolism at high rates to the non-nephrotoxic metabolite chloroacetate and by binding to thiol compounds in isolated human kidney tubules. Dubourg et al. (2002) demonstrated that pediatric tubules are not more sensitive than adult tubules to the toxic effects of chloroacetaldehyde and the rate of chloroacetaldehyde uptake.
Several studies have investigated the mechanism of toxicity of ifosfamide, and indicate a causative role for chloroacetaldehyde in the development of nephrotoxicity (Loebstein et al. 1999; Skinner et al. 2000; Aleksa et al. 2001; Yaseen et al. 2008; Hanly et al. 2009). Ifosfamide is a pro-drug that undergoes intracellular oxidization to its active form by cytotochome P450 monooxygenases in the liver and kidneys; in the process, chloroacetaldehyde and acrolein are formed. Ifosfamide-induced nephrotoxicity might occur in any section of the nephron, but most commonly occurs in the proximal tubule (Hanly et al. 2009). Several mechanisms are probably involved in the development of chloroacetaldehyde-induced nephrotoxicity, including ATP depletion, inhibition of ATPase, collapse of the cellular protein gradient though alterations of mitochondria, the generation of reactive oxygen species, and inhibition of endocytosis (Yaseen et al. 2008; Hanly et al. 2009). Results of a clinical study by Loebstein et al. (1999) suggest that age is a risk factor in the development of ifosfamide-induced nephrotoxicity, with younger patients at greater risk possibly because they have a greater rate of renal metabolism (Loebstein et al. 1999). However, Skinner et al. (2000) did not find an association between age and ifosfamide-induced nephrotoxicity. Differences in study designs and other confounding factors (e.g., concomitant therapy with other antineoplastic agents) might have contributed to conflicting results regarding age dependence of ifosfamide-induced nephrotoxicity (Aleksa et al. 2001).
Cardiotoxicity
Lawrence et al. (1972) reported that intravenous injections of chloroacetaldehyde produced lethal cardiotoxicity in less than 90 min in rabbits; the greater the dose, the earlier the cardiac arrest. The mechanism of cardiotoxicity was studied in more detail in an isolated perfused-rabbit-heart model by Joqueviel et al. (1997). Numerous arrhythmias followed by a decrease in the number and strength of ventricular contractions and swelling of the heart were observed after chloroacetaldehyde treatment. The major metabolite formed was chloroacetate, which was far less toxic than chloroacetaldehyde and did not result in cardiotoxic symptoms. Their findings suggest that the mechanism of cardiotoxicity might be similar to that found in rat hepatocytes and in a rat renal model (lipid peroxidation, glutathione depletion, reversible thiol-protein-adduct formation, and mitochondrial toxicity with ATP depletion).
Lung
Manzano et al. (1996) demonstrated in two human lung-cancer cell lines (large-cell carcinoma cell line COR-L23/R and adenocarcinoma cell line MOR/R04) that chloroacetaldehyde depleted intracellular glutathione concentrations in the lung.
4.3. Other Relevant Information
4.3.1. Irritation and Sensitization
Chloroacetaldehyde vapor was highly irritating to the nose and eyes of guinea pigs, rabbits, rats, and mice (5-400 ppm for 6 min to 7 h; single or repeated exposure) (Dow Chemical Company 1952). Irritation was observed very early in exposure and at low concentrations (single exposure at ≥10 ppm; repeated exposure at ≥5 ppm) (see Section 3.2.). The degree of irritation increased with concentration and exposure duration. Lacrimation and nasal irritation were also reported in humans at 10 ppm within a few minutes (no further details provided). In addition, the following signs of discomfort that might be related to nasal or ocular irritation were reported in rats exposed at 44-2,643 ppm for 1 h: closed eyes, wet nares, nasal discharge (at the higher concentrations), blindness (at 596 ppm), and bloodstains around the nose and mouth of the rats that died (≥159 ppm) (Arts 1987)]. Salivation and labored respiration (at 2,643 ppm), accompanied by dyspnea and mouth breathing, suggest that chloroacetaldehyde was irritating to the throat and respiratory tract.
In the European Union, chloroacetaldehyde has been labeled as C; R34 (corrosive; causes burns) (EC/JRC 2012).
5. DATA ANALYSIS FOR AEGL-1
5.1. Summary of Human Data Relevant to AEGL-1
No information is available on chloroacetaldehyde toxicity in humans other than a brief statement that a concentration of 10 ppm produced lacrimation and nasal irritation in humans within a few minutes of exposure (Dow Chemical Company 1952).
5.2. Summary of Animal Data Relevant to AEGL-1
The database on chloroacetaldehyde is poor. The few studies available indicate that its predominant effect is direct, strong irritation of the eyes, nose, and lungs (resulting in pulmonary edema and death) (Dow Chemical Company 1952; Arts 1987). Effects appeared to be related to concentration and exposure duration, and the studies indicate a very steep concentration-response relationship. The effects occur at concentrations of 10 ppm and higher in rats and guinea pigs and appear soon after onset of exposure. Repeated exposure at 5 ppm (7 h/day, eight exposures in 10 days) induced slight nasal or ocular irritation in rabbits, rats, and mice. Guinea pigs were less sensitive (Dow Chemical Company 1952).
5.3. Derivation of AEGL-1
The predominant effect of acute exposures to chloroacetaldehyde is irritation of the eyes and respiratory tract. Because no direct information is available on chloroacetaldehyde toxicity in humans, the AEGL-1 values are based on animal data. A single 7-h exposure to chloroacetaldehyde at concentrations of 10 ppm and higher in rats and at 25 ppm and higher in guinea pigs induced ocular and nasal irritation. However, effects were very slight to slight in rats, mice, and one rabbit exposed daily at 5 ppm (the lowest concentration tested) for 7 h per day for up to eight exposures in 10 days. No effects were reported in guinea pigs (Dow Chemical Company 1952). No effects were found during gross pathologic exams or on organ weights. A concentration of 5 ppm was chosen as the point of departure for calculating the AEGL-1 values, and a modifying factor of 2 was applied to obtain a no-observed-adverse-effect level of 2.5 ppm. Although the critical effect is local irritation, the irritation was related to both concentration and exposure duration. Therefore, it was considered inappropriate to set the same values for all time periods. Instead, time scaling was performed using the equation Cn × t = k. The value of n was determined to be 1.2 based on mortality data (see Section 7.3).
A total uncertainty factor of 10 (two factors of 3) was considered sufficient for toxicokinetic and toxicodynamic differences between species and in individual variability. Irritant effects were attributed to direct interaction of chloroacetaldehyde; therefore, no relevant differences in kinetics were assumed. The resulting AEGL-1 values are presented in Table 2-5. The 10-min AEGL-1 value was set equal to the 30-min value because extrapolation from a 7-h exposure to a 10-min value had too much uncertainty. The report of lacrimation and nasal irritation in humans within a few minutes of exposure to chloroacetaldehyde at 10 ppm (Dow Chemical Company 1952) provides support for deriving AEGL-1 values on the basis of the rat data.
6. DATA ANALYSIS FOR AEGL-2
6.1. Summary of Human Data Relevant to AEGL-2
No human data relevant to effects defined by AEGL-2 were found.
6.2. Summary of Animal Data Relevant to AEGL-2
Decreased pulmonary function, pulmonary edema, closed eyes, and blindness were the relevant effects for deriving AEGL-2 values for chloroacetaldehyde. Effects on the lungs were found in guinea pigs and rats (Dow Chemical Company 1952). Chloroacetaldehyde at nominal concentrations of 10-400 ppm for durations ranging from 7 h to 6 min caused labored breathing in rats at the
TABLE 2-5 AEGL-1 Values for Chloroacetaldehyde
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 2.3 ppm (7.4 mg/m3) | 2.3 ppm (7.4 mg/m3) | 1.3 ppm (4.2 mg/m3) | 0.40 ppm (1.3 mg/m3) | 0.22 ppm (0.71 mg/m3) |
higher concentrations tested. Furthermore, at some concentrations (not specified), slight drowsiness was apparent. No effects on organ weights or gross pathology findings were observed in rats, mice, guinea pigs, or one rabbit exposed to chloroacetaldehyde at 5 ppm for 7 h/day, for eight exposures in 10 days. Pulmonary toxicity was confirmed in a well-performed and adequately reported study of rats exposed to chloroacetaldehyde at concentrations of 44-2,643 ppm (analytically determined) for 1 h (Arts 1987). Shortly after the start of the experiment, labored respiration accompanied by dyspnea and mouth breathing was detected in all animals at the highest concentration (2,643 ppm). Closed eyes were also observed (concentrations not specified). Rats exposed at the highest concentrations that did not die immediately were described as breathing “wheezingly”. The animals that died during exposure or within the first 2 days of observation had pulmonary edema accompanied in some cases by atelectasis and in most cases by hydrothorax. The latter finding could be explained by induced hypertension. Pulmonary edema was also observed in some animals in the three lowest-concentration groups (number of animals affected was not reported) that were killed at the end of the 2-week observation period. Effects indicated impairment of pulmonary function.
6.3. Derivation of AEGL-2
Impaired pulmonary function was the most relevant adverse effect for deriving AEGL-2 values for chloroacetaldehyde. A concentration of 44 ppm was the lowest-observed-adverse-effect level for this effect. A modifying factor or 2 was applied to obtain a point of departure of 22 ppm, because of an incomplete database; specifically, a no-effect level for AEGL-2 effects could not be identified, and effects were more severe than those that define AEGL-2 values. According to NRC (2001), application of an additional modifying factor may be necessary when an incomplete database exists. The modifying factor represents an adjustment for uncertainties in the overall database. It “reflects professional judgment on the entire database available for the specific agent” and is applied on a case-by-case basis. Data from Tables 2-3 and 2-4 indicate that the concentration-response curve for chloroacetaldehyde is very steep (e.g., about a twofold difference in exposure duration or concentration between 0% or 100% mortality). Therefore a relatively small modifying factor of 2 was considered sufficient to derive a no-effect level.
A total uncertainty factor of 10 (two factors of 3) was considered sufficient for toxicokinetic and toxicodynamic differences between species and for individual variability for the following reasons. The effects were attributed to direct interaction of chloroacetaldehyde; therefore, no relevant differences in kinetics were assumed. The concentration-response curve appears to be very steep, indicating that a larger factor is unnecessary. Considering the effects at the lower exposure concentrations, a higher uncertainty factor would lead to unrealistically low values for AEGL-2. For time periods other than 1 h, time scaling was performed using the equation Cn × t = k, with n = 1.2 based on mortality data (see Section 7.3). The resulting AEGL-2 values are presented in Table 2-6.
7. DATA ANALYSIS FOR AEGL-3
7.1. Summary of Human Data Relevant to AEGL-3
No adequate human data that address the level of effects defined by the AEGL-3 were found.
7.2. Summary of Animal Data Relevant to AEGL-3
Two studies of the acute lethality of chloroacetaldehyde were available (Dow Chemical Company 1952; Arts 1987). One study provided mortality data in rats and guinea pigs for exposures varying both in concentration and exposure duration (Dow Chemical Company 1952). At the concentrations tested, mortality was either 0% or close to 100%. The concentration-response curve was very steep, and rats were more susceptible than guinea pigs to chloroacetaldehyde. Slight nasal irritation was observed in rats exposed at 5 ppm for 7 h/day for eight exposures in 10 days, but nearly all of the rats exposed once at 25 ppm for 7 h died (Dow Chemical Company 1952). Arts (1987) studied mortality in rats exposed to chloroacetaldehyde for 1 h at several concentrations. Because of the steep concentration-response curve and the natural variability between the groups, it was not possible to determine an exact LC50 with a 95% confidence interval. The LC50 was between 203 and 243 ppm (mortality rates of 4/10 and 10/10, respectively).
7.3. Derivation of AEGL-3
Mortality data from Dow Chemical Company (1952) show a steep concentration-response curve for chloroacetaldehyde. Doubling of the concentration or the exposure duration shifted mortality from 0% to close to 100%. Concentration-response modeling of the data resulted in confidence intervals that were too
large and was, therefore, inappropriate. An additional problem was a lack of study details (e.g., only nominal concentrations of chloroacetaldehyde were reported). Inclusion of the Arts (1987) data in the modeling only slightly improved the analyses. Using the “Doseresp”-software developed by ten Berge, a value for n of 1.2 (with a 95% confidence interval of 0.88-1.53) for time scaling was derived from the Dow Chemical Company (1952) study in rats (see Table 2-3). Inclusion of the Arts (1987) data did not change the outcome significantly.
Mortality data from the Arts (1987) study (see Table 2-3) were analyzed using EPA benchmark dose software (version 1.3.2) (EPA 2005). Benchmark concentrations for a 1% response (BMC01) and for a 5% response (BMC05) were 118 ppm and 136 ppm, respectively. The lower 95% confidence limit for the BMC05 (BMCL05) for a 1-h exposure was 99 ppm.
A total uncertainty factor of 10 (two factors of 3) was considered sufficient for toxicokinetic and toxicodynamic differences between species and for individual variability for the following reasons. The effects were attributed to direct interaction of chloroacetaldehyde and, therefore, no relevant differences in kinetics were assumed. The concentration-response curve appeared to be very steep, indicating that a larger factor is unnecessary. Doubling the exposure duration or concentration increased the mortality from 0% to 100%. Considering the response at the lower exposure concentrations, a larger uncertainty factor would lead to unrealistically low AEGL-3 values (see Tables 2-3 and 2-4).
The point of departure for calculating the AEGL-3 1-h value was the 1-h BMCL05 of 99 ppm. Application of a total uncertainty factor of 10 results in 9.9 ppm for a 1-h exposure. AEGL-3 values for the other time periods were derived by time scaling according to the dose-response regression equation Cn × t = k, with n = 1.2 based on mortality data (Dow Chemical Company 1952). The resulting AEGL-2 values are presented in Table 2-7.
8. SUMMARY OF AEGLS
8.1. AEGL Values and Toxicity End Points
The AEGLs values for chloroacetaldehyde are summarized in Table 2-8.
TABLE 2-6 AEGL-2 Values for Chloroacetaldehyde
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 9.8 ppm (31 mg/m3) | 3.9 ppm (13 mg/m3) | 2.2 ppm (7.1 mg/m3) | 0.69 ppm (2.2 mg/m3) | 0.39 ppm (1.5 mg/m3) |
TABLE 2-7 AEGL-3 Values for Chloroacetaldehyde
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 44 ppm (140 mg/m3) | 18 ppm (57 mg/m3) | 9.9 ppm (32 mg/m3) | 3.1 ppm (10 mg/m3) | 1.8 ppm (5.6 mg/m3) |
TABLE 2-8 Summary of AEGL Values for Chloroacetaldehyde
| Guideline | Exposure Duration | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h | |
| AEGL-1 (nondisabling) | 2.3 ppm (7.4 mg/m3) | 2.3 ppm (7.4 mg/m3) | 1.3 ppm (4.2 mg/m3) | 0.40 ppm (1.3 mg/m3) | 0.22 ppm (0.71 mg/m3) |
| AEGL-2 (disabling) | 9.8 ppm (31 mg/m3) | 3.9 ppm (13 mg/m3) | 2.2 ppm (7.1 mg/m3) | 0.69 ppm (2.2 mg/m3) | 0.39 ppm (1.5 mg/m3) |
| AEGL-3 (lethal) | 44 ppm (140 mg/m3) | 18 ppm (57 mg/m3) | 9.9 ppm (32 mg/m3) | 3.1 ppm (10 mg/m3) | 1.8 ppm (5.6 mg/m3) |
TABLE 2-9 Extant Standards and Guidelines for Chloroacetaldehyde
| Guideline | Exposure Duration | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h | |
| AEGL-1 (nondisabling) | 2.3 ppm (7.4 mg/m3) | 2.3 ppm (7.4 mg/m3) | 1.3 ppm (4.2 mg/m3) | 0.40 ppm (1.3 mg/m3) | 0.22 ppm (0.71 mg/m3) |
| AEGL-2 (disabling) | 9.8 ppm (31 mg/m3) | 3.9 ppm (13 mg/m3) | 2.2 ppm (7.1 mg/m3) | 0.69 ppm (2.2 mg/m3) | 0.39 ppm (1.5 mg/m3) |
| AEGL-3 (lethal) | 44 ppm (140 mg/m3) | 18 ppm (57 mg/m3) | 9.9 ppm (32 mg/m3) | 3.1 ppm (10 mg/m3) | 1.8 ppm (5.6 mg/m3) |
| IDLH (NIOSH)a | 45 ppm | ||||
| TLV-C (ACGIH)b | 1 ppm | ||||
| PEL-C (OSHA)c | 1 ppm | ||||
| REL-C (NIOSH)d | 1 ppm | ||||
| MAC (The Netherlands)e | 1 ppm | ||||
a IDLH (immediately dangerous to life or health, National Institute for Occupational Safety and Health) (NIOSH 1994) represents the maximum concentration from which one could escape within 30 min without any escape-impairing symptoms, or any irreversible health effects.
b TLV-C (threshold limit value-ceiling, American Conference of Governmental Industrial Hygienists) (ACGIH 2010) is a value that must not be exceeded during any part of the workday.
c PEL-C (permissible exposure limit-ceiling, Occupational Safety and Health Administration) (29 CFR 1910.1000 [2006]) is defined analogous to the ACGIH TLV-C.
d REL-C (recommended exposure limit-ceiling, National Institute for Occupational Safety and Health) (NIOSH 2011) is defined analogous to the ACGIH TLV-C.
e MAC (maximaal aanvaaarde concentratie [maximal accepted concentration]), (Dutch Expert Committee for Occupational Standards, The Netherlands (MSZV 2004 ) is defined analogous to the ACGIH TLV-TWA (the time weighted average concentration for a normal 8-h workday and a 40-ho workweek, to which nearly all workers may be repeatedly exposed, day after day, without adverse effect).
8.2. Comparison with Other Standards and Guidelines
The Immediately Dangerous to Life or Health (IDLH) value for chloroacetaldehyde of 45 ppm is based on an analogy with crotonaldehyde. Crotonaldehyde at 45 ppm was disagreeable to human subjects and caused conjunctival irritation. The IDLH might be a conservative value because acute toxicity data at concentrations greater than 45 ppm are lacking.
The general standards for chloroacetaldehyde are based on the prevention of ocular and nasal irritation. Because of the corrosive properties of chloroacetaldehyde, the standards are set as ceiling values. The 10-and 30-min AEGL-1 values are about two-fold greater than the ceiling value of 1 ppm. However, considering the severity of irritation in relation to the exposure concentrations, as observed in the animal experiments (single and repeated exposure), exposure to chloroacetaldehyde at 2.3 ppm for up to 30 min will probably not lead to significant ocular or nasal irritation in humans.
8.3. Data Quality and Research Needs
No relevant, adequately-documented human data on chloroacetaldehyde were available. AEGL-1 values were based on data from repeated-exposure studies in animals, because no adequate acute-exposure studies of relevant AEGL-1 end points in animals were found. However, one of the repeatedexposure studies had inadequate details and both studies mainly focused on lethality and described nonlethal effects in a general manner without relating them to specific concentrations of chloroacetaldehyde. AEGL-2 values were based on a clear effect level; a no-observed-adverse-effect level could not be determined. However, because the critical end points for chloroacetaldehyde are fairly well understood, the uncertainties in the AEGL values are not expected to be large. An adequate animal experiment focusing on AEGL-1 and AEGL-2 end points after single exposure might be helpful to reduce uncertainties.
9. REFERENCES
ACGIH (American Conference of Government and Industrial Hygienists). 1991. Chloroacetaldehyde (CAS Reg. No. 107-20-0). Pp. 260-261 in Documentation of the Threshold Limit Values and Biological Exposure Indices, 6th Ed. American Conference of Government and Industrial Hygienists, Cincinnati, OH.
ACGIH (American Conference of Governmental Industrial Hygienists). 2010. Chloroacetaldehyde (CAS Reg. No. 107-20-0). TLVs and BEIs: Based on the Documentation of the Threshold Limit Values for Chemical Substances & Biological Exposure Indices. American Conference of Governmental Industrial Hygienists, Cincinnati OH.
Aleksa, K., C. Woodland, and G. Koren. 2001. Young age and the risk for ifosfamideinduced nephrotoxicity: A critical review of two opposing studies. Pediatr. Nephrol. 16(12):1153-1158.
Arts, J.H.E. 1987. Acute (One-Hour) Inhalation Toxicity Study of Chloroacetaldehyde in Rats. Report No. V 87.094/261236. Organization for Applied Scientific Research (TNO), Zeist, The Netherlands [online]. Available: http://yosemite.epa.gov/oppts/epatscat8.nsf/by+Service/731D9542E140E7DC85256F2600655CA2/$File/88870000029.pdf [accessed Feb. 10, 2012].
ATSDR (Agency for Toxic Substances and Disease Registry). 1997. Toxicological Profile for Vinyl Chloride. U.S. Department of Public Health, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA. September 1997.
Bartsch, H., C. Malaveille, A. Barbin, H. Bresil, L. Tomatis, and R. Montesano. 1976. Mutagenicity and metabolism of vinyl chloride and related compounds. Environ. Health Perspect. 17:193-198.
Budavari, S., M.J. O’Neil, A. Smith, and P.H. Heckelman, eds. 1989. Chloroacetaldehyde. P. 326 in The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 11 Ed. Rahway, NJ: Merck.
Daniel, F.B., A.B. DeAngelo, J.A. Stober, G.R. Olson, and N.P. Page. 1992. Hepatocarcinogenicity of chloral hydrate, 2-chloroacetaldehyde, and dichloroacetic acid in the male B6C3F1 mouse. Fundam. Appl. Toxicol. 19(2):159-168.
Dow Chemical Company. 1952. Toxicity of Chloroacetaldehyde, Document No. 8EHQ-0392-2833A. U.S. Environmental Protection Agency, Washington, DC. EPA Document No. 88920001475. Microfiche No. OTS0536151.
Dubourg, L., C. Michoudet, P. Cochat, and G. Baverel. 2001. Human kidney tubules detoxify chloroacetaldehyde, a presumed nephrotoxic metabolite of ifosfamide. J. Am. Soc. Nephrol. 12(8):1615-1623.
Dubourg, L., P. Tanière, P. Cochat, G. Baverel, and C. Michoudet. 2002. Toxicity of chloroacetaldehyde is similar in adult and pediatric kidney tubules. Pediatr. Nephrol. 17(2):97-103.
EC/JRC (European Commission Joint Research Centre). 2012. Chloroacetaldehyde. EINECS No. 203-472-8. European Inventory of Existing Commercial Chemical Substances. European Commission, Joint Research Centre, Institute for Health and Consumer Protection [online]. Available: http://esis.jrc.ec.europa.eu/ [accessed Feb. 9, 2012].
Elmore, J.D., J.L. Wong, A.D. Laumbach, and U.N. Streips. 1976. Vinyl chloride mutagenicity via the metabolites chlorooxirane and chloroacetaldehyde monomer hydrate. Biochim. Biophys. Acta 442(3):405-419.
EPA (U.S. Environmental Protection Agency). 1987. Section 8(e) Submission and Status Report on Chloroacetone and Chloroacetaldehyde. Document No. 8EHQ-0387-0660. Office of Toxic Substances, U.S. Environmental Protection Agency: Washington, DC. April 22, 1987.
EPA (U.S. Environmental Protection Agency). 2005. Benchmark Dose Software, Version 1.3.2. National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC.
Goldschmidt, B.M. 1984. Role of aldehydes in carcinogenesis. J. Environ. Sci. Health C 2(2):231-249.
Goren, M.P., R.K. Wright, C.B. Pratt, and F.E. Pell. 1986. Dechloroethylation of ifosfamide and neurotoxicity. Lancet 2(8517):1219-1220.
Hanly, L., N. Chen, M. Rieder, and G. Koren. 2009. Ifosfamide nephrotoxicity in children: A mechanistic base for pharmacological prevention. Expert Opin. Drug Saf. 8(2):155-168.
HSDB (Hazardous Substances Data Bank). 2009. Chloroacetaldehyde (CASRN 107-20-0). TOXNET, Specialized Information Services, U.S. National Library of
Medicine, Bethesda, MD [online]. Available: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB. [accessed Feb. 9, 2012].
IPCS (International Programme on Chemical Safety). 2005. Chloroacetaldehyde (40% solution). International Chemical Safety Card IPCS 0706. International Programme on Chemical Safety, Commission of the European Communities [online]. Available: http://www.inchem.org/documents/icsc/icsc/eics0706.htm [accessed Feb. 9, 2012].
Joqueviel, C., M. Malet-Martino, and R. Martino. 1997. A13C NMR study of 2-13C-chloroacetaldehyde, a metabolite of ifosfamide and cyclophosphamide, in the isolated perfused rabbit heart model. Initial observations on its cardiotoxicity and cardiac metabolism. Cell. Mol. Biol. 43(5):773-782.
Lawrence, W.H., E.O. Dillingham, J.E. Turner, and J. Autian. 1972. Toxicity profile of chloroacetaldehyde. J. Pharm. Sci. 61(1):19-25.
Loebstein, R., G. Atanackovic, R. Bishai, J. Wolpin, S. Khattak, G. Hashemi, M. Gobrial, S. Baruchel, S. Ito, and G. Koren. 1999. Risk factors for long-term outcome of ifosfamide-induced nephrotoxicity in children. J. Clin. Pharmacol. 39(5):454-461. Manzano, R.G., K.A. Wright, and P.R. Twentyman. 1996. Modulation by acrolein and chloroacetaldehyde of multidrug resistance mediated by the multidrug resistanceassociated protein (MRP). Clin. Cancer Res. 2(8):1321-1326.
McCann, J., V. Simmon, D. Streitwieser, and B.N. Ames. 1975. Mutagenicity of chloroacetaldehyde, a possible metabolic product of 1,2-dichloroethane (ethylene dichloride), chloroethanol (ethylene chlorohydrin), vinyl chloride, and cyclophosphamide. Proc. Natl. Acad. Sci. USA 72(8):3190-3193.
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: Chlooraceetaldehyde. Den Haag: SDU Uitgevers [online]. Available: http://www .lasrook.net/lasrookNL/maclijst2004.htm [accessed Feb. 9, 2012].
NIOSH (National Institute for Occupational Safety and Health). 1991. Occupational Health Guidelines for Chemical Hazards: Chloroacetaldehyde. DHHS (NIOSH) 81-123. Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Atlanta, GA [online]. Available: http://www.cdc.gov/niosh/docs/81-123/ [accessed Feb. 9, 2012].
NIOSH (National Institute for Occupational Safety and Health). 1994. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs): Chloroacetaldehyde. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH [online]. Available: http://www.cdc.gov/niosh/idlh/107200.html [accessed Feb. 9, 2012].
NIOSH (National Institute for Occupational Safety and Health). 2011. NIOSH Pocket Guide to Chemical Hazards: Chloroacetaldehyde. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH [online]. Available: http://www .cdc.gov/niosh/npg/npgd0118.html [accessed Feb. 09, 2012].
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NRC (National Research Council). 2012. Vinyl Chloride in Acute Exposure Guideline Levels for Selected Airborne Chemicals, Vol. 11. Washington, DC: National Academies Press.
OSHA (Occupational Safety and Health Administration). 1989. Chloroacetaldehyde. Sampling and Analytical Methods: Method No. 76. Occupational Safety & Health Administration, Washington, DC [online]. Available: http://www.osha.gov/dts/sltc/methods/organic/org076/org076.htm [accessed Feb. 9, 2012].
Rieger, C., M. Fiegl, J. Tischer, H. Ostermann, and X. Schiel. 2004. Incidence and severity of ifosfamide-induced encephalopathy. Anticancer Drugs 15(4):347-350.
Sharpe, A.L., and D.E. Carter. 1993. Substrate specificity of rat liver aldehyde dehydrogenase with chloroacetaldehydes. J. Biochem. Toxicol. 8(3):155-160.
Skinner, R., S.J. Cotterill, and M.C. Stevens. 2000. Risk factors for nephrotoxicity after ifosfamide treatment in children: A UKCCSG Late Effects Group study. Br. J. Cancer 82(10):1636-1645.
Sood, C., and P.J. O’Brien. 1993. Molecular mechanisms of chloroacetaldehyde-induced cytotoxicity in isolated rat hepatocytes. Biochem. Pharmacol. 46(9):1621-1626.
Sood, C., and P.J. O’Brien. 1994. Chloroacetaldehyde-induced hepatocyte cytotoxicity. Mechanisms for cytoprotection. Biochem. Pharmacol. 48(5):1025-1032.
Van Duuren, B.L., B.M. Goldschmidt, G. Loewengart, A.C. Smith, S. Melchionne, I. Seldman, and D. Roth. 1979. Carcinogenicity of halogenated olefinic and aliphatic hydrocarbons in mice. J. Natl. Cancer Inst. 63(6):1433-1439.
Visarius, T.M., J.W. Stucki, and B.H. Lauterburg. 1999. Inhibition and stimulation of long-chain fatty acid oxidation by chloroacetaldehyde and methylene blue in rats. J. Pharmacol. Exp. Ther. 289(2):820-824.
Yaseen, Z., C. Michoudet, G. Baverel, and L. Dubourg. 2008. Mechanisms of the ifosfamide-induced inhibition of endocytosis in the rat proximal kidney tubule. Arch. Toxicol. 82(9):607-614.
APPENDIX A
DERIVATION OF AEGL VALUES FOR CHLOROACETALDEHYDE
Derivation of AEGL-1 Values
| Key study: | Dow Chemical Company. 1952. Toxicity of Chloroacetaldehyde. Document No. 8EHQ-0392-28338. EPA Document No. 88920001475. Microfiche No. OTS0536151. |
| Toxicity end point: | 5 ppm for 7 h, lowest-observed-adverse-effect level for nasal and ocular irritation. The point of departure was 2.5 ppm after a modifying factor of 2 was applied (see below). |
| Time scaling: | Cn × t = k; n = 1.2 based on lethality data k = (2.5 ppm)1.2 × 420 min = 1,261 ppm-min |
| Uncertainty factors: | 3 for interspecies differences 3 for intraspecies variability Total uncertainty factor of 10 |
| Modifying factor: | A modifying factor of 2 was applied to reduce the lowest-observed-adverse-effect level to a no-effect level. |
| Calculations: | |
| 10-min AEGL-1: | Set equal to the 30-min AEGL of 2.3 ppm (= 7.4 mg/m3) |
| 30-min AEGL-1: | C1.2 × 30 min = 1,261 ppm-min C = 22.5 ppm 22.5 ÷ 10 = 2.3 ppm (rounded) (= 7.4 mg/m3) |
| 1-h AEGL-1: | C1.2 × 60 min = 1,261 ppm-min C = 12.7 ppm 12.7 ÷ 10 = 1.3 ppm (rounded) (= 4.2 mg/m3) |
| 4-h AEGL-1: | C1.2 × 240 min = 1,261 ppm-min C = 4.0 ppm 4.0 ÷ 10 = 0.40 ppm (rounded) (= 1.3 mg/m3) |
| 8-h AEGL-1: | C1.2 × 480 min = 1,261 ppm-min C = 2.2 ppm 2.2 ÷ 10 = 0.22 ppm (rounded) (= 0.71 mg/m3) |
Derivation of AEGL-2 Values
| Key study: | Arts, J.H.E. 1987. Acute (One-Hour) Inhalation Toxicity Study of Chloroacetaldehyde in Rats. Report No. V 87.094/261236. Organization for Applied Scientific Research (TNO), Zeist, The Netherlands [online]. Available: http://yosemite.epa.gov/oppts/epatscat8.nsf/by+Service/731D9542E140E7DC85256F2600655CA2/$File/88870000029.pdf [accessed Feb. 10, 2012]. |
| Toxicity end point: | 44 ppm for 1 h, lowest-observed-adverse-effect level for impaired pulmonary function. The point of departure was 22 ppm after a modifying factor of 2 was applied (see below). |
| Time scaling: | Cn × t = k; n = 1.2 based on lethality data k = (22 ppm) 1.2 × 60 min = 2,449 ppm-min |
| Uncertainty factors: | 3 for interspecies differences 3 for intraspecies variability Total uncertainty factor of 10 |
| Modifying factor: | A modifying factor of 2 was applied to reduce the lowest-observed-adverse-effect level, because the pulmonary effects were more severe than those that define AEGL-2 values. |
| Calculations: | |
| 10-min AEGL-2: | C1.2 × 10 min = 2,449 ppm-min C = 97.9 ppm 97.9 ÷ 10 = 9.8 ppm (rounded) (= 31 mg/m3) |
| 30-min AEGL-2: | C1.2 × 30 min = 2,449 ppm-min C = 39.2 ppm 39.2 ÷ 10 = 3.9 ppm (rounded) (= 13 mg/m3) |
| 1-h AEGL-2: | 22 ppm ÷ 10 = 2.2 ppm (= 7.1 mg/m3) |
| 4-h AEGL-2: | C1.2 × 240 min = 2,449 ppm-min C = 6.9 ppm 6.9 ÷10 = 0.69 ppm (= 2.2 mg/m3) |
| 8-h AEGL-2: | C1.2 × 480 min = 2,449 ppm-min C = 3.9 ppm 3.9 ÷10 = 0.39 ppm (= 1.5 mg/m3) |
Derivation of AEGL-3 Values
| Key studies: | Arts, J.H.E. 1987. Acute (One-Hour) Inhalation Toxicity Study of Chloroacetaldehyde in Rats. Report No. V 87.094/261236. Organization for Applied Scientific Research (TNO), Zeist, The Netherlands [online]. Available: http://yosemite.epa.gov/oppts/epatscat8.nsf/by+Service/731D9542E140E7DC85256F2600655CA2/$File/88870000029.pdf [accessed Feb. 10, 2012]. Dow Chemical Company. 1952. Toxicity of Chloroacetaldehyde. Document No. 8EHQ-0392-28338. EPA Document No. 88920001475. Microfiche No. OTS0536151. |
| Toxicity end point: | Lethality in rats exposed for 1 h. The 1-h BMC05 is 136 ppm, with a lower 95% confidence limit of 99 ppm (the point of departure). |
| Time scaling: | Cn × t = k; n = 1.2 based on lethality data k = (99 ppm)1.2 × 60 min = 14,891 ppm-min |
| Uncertainty factors: | 3 for interspecies differences 3 for intraspecies variability Total uncertainty factor of 10 |
| Calculations: | |
| 10-min AEGL-3: | C1.2 × 10 min = 14,891 ppm-min C = 441 ppm 441 ÷ 10 = 44 ppm (rounded) (= 140 mg/m3) |
| 30-min AEGL-3: | C1.2 × 30 min = 14,891 ppm-min C = 176 ppm 176 ÷ 10 = 18 ppm (rounded) (= 57 mg/m3) |
| 1-h AEGL-3: | 99 ppm ÷ 10 = 9.9 ppm (= 32 mg/m3) |
| 4-h AEGL-3: | C1.2 × 240 min = 14,891 ppm-min C = 31 ppm 31 ÷ 10 = 3.1 ppm (= 10 mg/m3) |
| 8-h AEGL-3: | C1.2 × 480 min = 14,891 ppm-min C = 18 ppm 18 ÷ 10 = 1.8 ppm (= 5.6 mg/m3) |
APPENDIX B
ACUTE EXPOSURE GUIDELINE LEVELS FOR CHLOROACETALDEHYDE
Derivation Summary for Chloroacetaldehyde
AEGL-1 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 2.3 ppm (7.4 mg/m3) | 2.3 ppm (7.4 mg/m3) | 1.3 ppm (4.2 mg/m3) | 0.40 ppm (1.3 mg/m3) | 0.22 ppm (0.71 mg/m3) |
| Key reference: Dow Chemical Company. 1952. Toxicity of Chloroacetaldehyde. Document No. 8EHQ-0392-28338. EPA Document No. 88920001475. Microfiche No. OTS0536151. | ||||
| Test species/Strain/Number: Guinea pigs (strain not specified): 5 Rabbit (strain not specified): 1 Rats (strain not specified): 10 Mice (strain not specified): 5 |
||||
| Exposure route/Concentrations/Durations: Inhalation, chloroacetaldehyde at 0 or 5 ppm for 7 h/d, 5 d/wk for a total of eight exposures in 10 d. | ||||
| Effects at 5 ppm: Guinea pigs: no effects Rabbit: slight ocular irritation Rats: slight nasal irritation, very slight ocular irritation Mice: slight nasal irritation |
||||
| End point/Concentration/Rationale: Rabbit, rats, and mice had slight nasal and ocular irritation after 7 h of exposure at 5 ppm (lowest-observed-adverse-effect level). | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 10 was considered sufficient for toxicokinetic and toxicodynamic differences between species and individual variability. The effects were attributed to direct interaction of chloroacetaldehyde and, therefore, no relevant differences in kinetics between species and between humans were assumed. Interspecies: 3 Intraspecies: 3 |
||||
| Modifying factor: A modifying factor of 2 was applied to reduce 5 ppm to a no-effect level of 2.5 ppm. | ||||
| Animal-to-human dosimetric adjustment: Not applied | ||||
| Time scaling: Cn × t = k; n = 1.2 based on lethality data | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 2.3 ppm (7.4 mg/m3) | 2.3 ppm (7.4 mg/m3) | 1.3 ppm (4.2 mg/m3) | 0.40 ppm (1.3 mg/m3) | 0.22 ppm (0.71 mg/m3) |
| Data adequacy: No human data were available. Lacrimation and nasal irritation reported in humans within a few minutes of exposure to chloroacetaldehyde at 10 ppm (Dow Chemical Company 1952) provides supporting data for deriving AEGL-1 values on the basis of rat data. No adequate animal data identifying a no-effect level for ocular and nasal irritation were available. | ||||
AEGL-2 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 9.8 ppm (31 mg/m3) | 3.9 ppm (13 mg/m3) | 2.2 ppm (7.1 mg/m3) | 0.69 ppm (2.2 mg/m3) | 0.39 ppm (1.5 mg/m3) |
| Key reference: Arts, J.H.E. 1987. Acute (One-Hour) Inhalation Toxicity Study of Chloroacetaldehyde in Rats. Report No. V 87.094/261236. Organization for Applied Scientific Research (TNO), Zeist, The Netherlands. | ||||
| Test species/Strain/Number: SPF-reared Borr:WISW rats (5 animals/sex/group) | ||||
| Exposure route/Concentrations/Durations: Inhalation, mean actual concentrations of chloroacetaldehyde at 44, 159, 203, 243, 309, 596, and 2,643 ppm for 1 h. | ||||
| Effects: 44 ppm: closed eyes, salivation; pulmonary edema (still presented 2 weeks after exposure) 159 ppm: 3/10 deaths 203 ppm: 4/10 deaths 243 ppm: 100% mortality 309 ppm: 100% mortality 596 ppm: 100% mortality 2,643 ppm: 100% mortality |
||||
| End point/Concentration/Rationale: Pulmonary edema at 44 ppm, the lowest concentration tested. | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 10 was considered sufficient for toxicokinetic and toxicodynamic differences between species and individual variability. The effects were attributed to direct interaction of chloroacetaldehyde and, therefore, no relevant differences in kinetics between species and between humans were assumed. Interspecies: 3 Intraspecies: 3 |
||||
| Modifying factor: A modifying factor of 2 was applied because of an incomplete database (a no-effect level was not identified). A factor of 2 was considered sufficient because of the steep concentration-response curve. | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 9.8 ppm (31 mg/m3) | 3.9 ppm (13 mg/m3) | 2.2 ppm (7.1 mg/m3) | 0.69 ppm (2.2 mg/m3) | 0.39 ppm (1.5 mg/m3) |
| Animal-to-human dosimetric adjustment: Not applied | ||||
| Time scaling: Cn × t = k; n = 1.2 based on lethality data | ||||
| Data adequacy: No human data were available. A no-effect level for effects defined by the AEGL-2 could not be determined. However, because of the steep concentration-response curve and the additional animal data, the uncertainties in the AEGL-2 values probably not very large. | ||||
AEGL-3 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 44 ppm (140 mg/m3) | 18 ppm (57 mg/m3) | 9.9 ppm (32 mg/m3) | 3.1 ppm (10 mg/m3) | 1.8 ppm (5.6 mg/m3) |
| Key references: (1) Dow Chemical Company. 1952. Toxicity of Chloroacetaldehyde. Document No. 8EHQ-0392-28338. EPA Document No. 88920001475. Microfiche No. OTS0536151. (2) Arts, J.H.E. 1987. Acute (One-Hour) Inhalation Toxicity Study of Chloroacetaldehyde in Rats. Report No. V 87.094/261236. Organization for Applied Scientific Research (TNO), Zeist, The Netherlands. | ||||
| Test species/Strain/Number: SPF-reared Borr: WISW rats (5 animals/sex/group) | ||||
| Exposure Route/Concentrations/Durations: Inhalation, mean actual concentrations of 44, 159, 203, 243, 309, 596, and 2,643 ppm for 1 h | ||||
| Effects: 44 ppm: 0/10 deaths 159 ppm: 3/10 deaths 203 ppm: 4/10 deaths 243 ppm: 100% mortality 309 ppm: 100% mortality 596 ppm: 100% mortality 2,643 ppm: 100% mortality |
||||
| End point/Concentration/Rationale: A BMCL05 of 99 ppm for 1 h was calculated using EPA benchmark dose software (EPA 2005). | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 10 was considered sufficient for toxicokinetic and toxicodynamic differences between species and individual variability. The effects were attributed to direct interaction of chloroacetaldehyde and, therefore, no relevant differences in kinetics between species and between humans were assumed. Interspecies: 3 Intraspecies: 3 |
||||
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 44 ppm (140 mg/m3) | 18 ppm (57 mg/m3) | 9.9 ppm (32 mg/m3) | 3.1 ppm (10 mg/m3) | 1.8 ppm (5.6 mg/m3) |
| Modifying factor: Not applied | ||||
| Animal-to-human dosimetric adjustment: Not applied | ||||
| Time scaling: Cn × t = k; n = 1.2 based on lethality data | ||||
| Data adequacy: Sufficient for deriving AEGL-3 values. | ||||
APPENDIX C
CATEGORY GRAPH OF TOXICITY DATA AND AEGL VALUES FOR CHLOROACETALDEHYDE
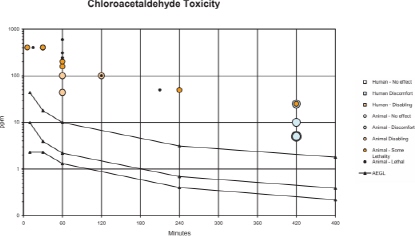
FIGURE C-1 Category graph of toxicity data and AEGLs values for chloroacetaldehyde.


































