3
Chlorobenzene1
Acute Exposure Guideline Levels
PREFACE
Under the authority of the Federal Advisory Committee Act (FACA) P.L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review, and interpret relevant toxicologic and other scientific data and develop AEGLs for high-priority, acutely toxic chemicals.
AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes (min) to 8 hours (h). Three levels—AEGL-1, AEGL-2, and AEGL-3—are developed for each of five exposure periods (10 and 30 min and 1, 4, and 8 h) and are distinguished by varying degrees of severity of toxic effects. The three AEGLs are defined as follows:
AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, nonsensory
![]()
1 This document was prepared by the AEGL Development Team composed of J.J.A. Muller and Peter Bos (both from RIVM, The Dutch National Institute of Public Health and the Environment), Julie M. Klotzbach (Syracuse Research Corporation), Chemical Manager Marinelle Payton (National Advisory Committee [NAC] on Acute Exposure Guideline Levels for Hazardous Substances), and Ernest V. Falke (U.S. Environmental Protection Agency). The NAC reviewed and revised the document and AEGLs as deemed necessary. Both the document and the AEGL values were then reviewed by the National Research Council (NRC) Committee on Acute Exposure Guideline Levels. The NRC committee has concluded that the AEGLs developed in this document are scientifically valid conclusions based on the data reviewed by the NRC and are consistent with the NRC guidelines reports (NRC 1993, 2001).
effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure.
AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects or an impaired ability to escape.
AEGL-3 is the airborne concentration (expressed as ppm or mg/m3) of a substance above which it is predicted that the general population, including susceptible individuals, could experience life-threatening health effects or death.
Airborne concentrations below the AEGL-1 represent exposure concentrations that could produce mild and progressively increasing but transient and nondisabling odor, taste, and sensory irritation or certain asymptomatic, nonsensory effects. With increasing airborne concentrations above each AEGL, there is a progressive increase in the likelihood of occurrence and the severity of effects described for each corresponding AEGL. Although the AEGL values represent threshold concentrations for the general public, including susceptible subpopulations, such as infants, children, the elderly, persons with asthma, and those with other illnesses, it is recognized that individuals, subject to idiosyncratic responses, could experience the effects described at concentrations below the corresponding AEGL.
SUMMARY
Chlorobenzene is a flammable liquid with a high vapor pressure and a water solubility of 50 milligrams per liter (mg/L) at 20°C. It is used as a solvent and in the production of nitrochlorobenzene and intermediates for the synthesis of dyestuffs, pharmaceuticals, and products for the rubber and plastic industries. Chlorobenzene has an aromatic, almond-like odor. The odor threshold is 0.050 mg/L in water and is 0.2-1.8 ppm in air, although a value of 62 ppm has also been reported for air.
The toxicity database on chlorobenzene is poor. Information often had to be obtained from descriptions in reviews and summaries, and some older literature could not be obtained (e.g., Rozenbaum et al. [1947]). Human data include to two kinetic studies with volunteers. Animal data included studies on teratogenicity, reproductive toxicity, and mortality. A few studies with experimental animals addressing central nervous system (CNS) depression were reviewed, but were difficult to interpret.
AEGL-1 values are based on kinetic studies with volunteers. Effects in subjects exposed to chlorobenzene at 60 ppm for 7 h (with a 1-h break after 3 h) are indicative of slight CNS depression (drowsiness, heavy head, and headache) and local irritation (Ogata et al. 1991), and are considered evidence of discomfort. These effects were not observed is subjects exposed at 10 ppm for 8 h
(Knecht and Woitowitz 2000). Thus, 10 ppm was chosen as a conservative point of departure for the derivation of AEGL-1 values. Because human data are used, an interspecies uncertainty factor of 1 was used. Despite the fact that only a few subjects were tested, an uncertainty factor of 1 for intraspecies variability was considered appropriate because of the conservatism of the point of departure already provides a margin of safety. (The point of departure of 10 ppm was obtained from a repeated-exposure study, and effects observed at 60 ppm were rather slight.) No information about the time dependency of the effects at 10 or 60 ppm is available. Because the effects at 60 ppm include irritation and CNS effects, the 8-h AEGL-1 value of 10 ppm is considered appropriate for all time points. Furthermore, Knecht and Woitowitz (2000) reported that chlorobenzene concentrations in blood reached a steady-state level within 1 h.
There are no adequate human data for deriving AEGL-2 values. Some studies with experimental animals report subtle CNS effects, but the relevance of these effects to humans is difficult to interpret. The effects reported by Frantik et al. (1994) and De Ceaurriz et al. (1983) are considered effects below those defined by AEGL-2. A more appropriate study is the one by UBTL (1978), in which rats and guinea pigs experienced narcosis and effects that would impair ability to escape. A no-effect concentration of 2,990 ppm for 30 min was selected as the point of departure for calculating AEGL-2 values. An interspecies uncertainty factor of 3 was applied, because data were comparable for rats and guinea pigs, suggesting no large interspecies differences, and the critical effect is CNS depression. The concentration of chlorobenzene in the brain is probably related directly to inhalation rate. Therefore, humans probably require higher external exposures than rodents to obtain a similar concentration of chlorobenzene in the blood or brain. Experience with anesthetic gases shows that interindividual variability in CNS depression caused by these gases is generally not greater than a factor of 2 or 3. Therefore, an intraspecies uncertainty factor of 3 was used. A combined uncertainty factor of 10 was considered appropriate because a larger factor would result in AEGL-2 values below 60 ppm, which a concentration shown to cause only minor effects in humans. The 30-min AEGL-2 was 300 ppm. The 30-min value was extrapolated to 10-min and 1-h values using the equation Cn × t = k, with default values of n = 1 for extrapolation to 1 h and n = 3 for extrapolation to 10 min. The 4-and 8-h AEGL-2 values were set equal to the 1-h value because chlorobenzene concentrations in blood reach a steady-state within 1 h and elimination is rapid. Furthermore, time scaling would result in 4-and 8-h AEGL-2 values that conflict with human data (Ogata et al. 1991).
For the derivation of AEGL-3 values, several mortality studies were found, but most were only available as summaries in other publications and could not be judged on their merits. Bonnet et al. (1979, 1982) reported a 6-h LC50 (lethal concentration, 50% lethality) of 2,965 ppm for male rats and a 6-h LC50 of 1,886 ppm for mice. No deaths were reported in rats or guinea pigs exposed to chlorobenzene at concentrations of up to 7,970 ppm for 30 min (UBTL 1978). Data in rats and guinea pigs reported by UBTL (1978) provide the most
appropriate point of departure for AEGL-3 derivation. A total uncertainty factor of 10 was applied on the same basis it was applied in the derivation of the AEGL-2 values, and time scaling was performed the same as was done for the AEGL-2 values. AEGL-3 values are consistent with the AEGL-2 values and are supported by the 6-h LC01 of 1,873 ppm calculated from the probit equation reported by Bonnet et al. (1982). AEGL values for chlorobenzene are presented in Table 3-1.
1. INTRODUCTION
Chlorobenzene is a flammable liquid with a high vapor pressure and a water solubility of 50 mg/L at 20°C. It is commercially produced by the chlorination of benzene in the presence of a catalyst (ATSDR 1990). Chlorobenzene is used as a solvent and in the production of nitrochlorobenzene and intermediates for the synthesis of dyestuffs, pharmaceuticals, and products for the rubber and plastic industries (BUA 1990). The production volume of chlorobenzene in 1992 was 231 million pounds in the United States (EPA 1995). More current information on production volumes was not available.
Chlorobenzene has an aromatic, almond-like odor. The odor threshold for chlorobenzene in water is 0.050 mg/L and in air is 0.2-1.8 ppm (Verschueren 1983). Odor thresholds for chlorobenzene have been reported as low as 0.2 ppm and as high as 62 ppm (Ruth 1986). Chemical and physical properties for chlorobenzene are presented in Table 3-2.
2. HUMAN TOXICITY DATA
2.1. Acute Lethality
No data were available.
TABLE 3-1 Summary of AEGL Values for Chlorobenzene
| Classification | 10 min | 30 min | 1 h | 4 h | 8 h | End Point (Reference) |
| AEGL-1 (nondisabling) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | No irritant or CNS effects (Ogata et al. 1991; Knecht and Woitowitz 2000) |
| AEGL-2 (disabling) | 430 ppm (2,021 mg/m3) | 300 ppm (1,410 mg/m3) | 150 ppm (705 mg/m3) | 150 ppm (705 mg/m3) | 150 ppm (705 mg/m3) | Narcosis (UBTL 1978) |
| AEGL-3 (lethal) | 1,100 ppm (5,170 mg/m3) | 800 ppm (3,760 mg/m3) | 400 ppm (1,880 mg/m3) | 400 ppm (1,880 mg/m3) | 400 ppm (1,880 mg/m3) | No mortality in rats or guinea pigs (UBTL 1978) |
TABLE 3-2 Chemical and Physical Properties for Chlorobenzene
| Parameter | Value | Reference |
| CAS registry no. | 10S-90-7 | |
| Synonyms | Monochlorobenzene: benzene chloride: phenylchloride: MCB: chlorobenzol | |
| Chemical formula | C6H5Cl | |
| Molecular weight | 112.56 | |
| Physical state | Liquid | ATSDR 1990 |
| Color | Colorless | ATSDR 1990 |
| Odor | Aromatic, almond-like | ATSDR 1990 |
| Melting point | -45.6 °C | ATSDR 1990 |
| Boiling point | 132 °C | ATSDR 1990 |
| Liquid density (water =1) | 1.1058 g/cm3 | ATSDR 1990 |
| Solubility in water | 500 mg/L at 20 °C | ATSDR 1990 |
| Vapor pressure | 8.8 mm Hg at 20 °C | ATSDR 1990 |
| Flammability | 1.8-9.6% | ATSDR 1990 |
| Lower explosive limit | 1.3% | XIOSH2011 |
| Conversion factors | 1 mg.m = 0.22 ppm 1 ppm = 4.7 mg/m |
ATSDR 1990 |
2.2. Nonlethal Toxicity
2.2.1. Case Reports
Several reviews including those of ACGIH (1991) and Hellman (1993) cited reports in which inhalation and oral exposure to chlorobenzene are described as having caused drowsiness, incoordination, and unconsciousness, as well as irritation of the eyes and respiratory tract. However, exposure concentrations were not specified.
Ruth (1986) reported that 205 ppm was an irritating concentration of chlorobenzene, but the source of that information was not provided.
2.2.2. Experimental Studies
In a study investigating urinary metabolites of chlorobenzene, subjects were asked to report subjective effects of the exposure (Ogata et al. 1991). Volunteers were exposed to chlorobenzene at 60.2 ± 3.9 ppm for 3 h in the morning
and 4 h in the afternoon, with a 1-h break between exposures. The concentrations were determined by gas chromatography and were reported to be constant within a 5% range. All of the volunteers complained of a disagreeable odor and drowsiness. Three had a heavy feeling in the head or headache, two had a throbbing pain in the eyes, and one had a sore throat. No information was given about the onset of these complaints. Chlorobenzene did not affect pulse rates or systolic and diastolic pressure. Flicker fusion frequency values (frequency at which successive flashes are seen as continuous) were reduced significantly from 39.1 to 35.9 cycles/second at the end of the 3-h exposure. No further effect was seen in the afternoon. The significance of this finding is difficult to interpret.
Eight volunteers were exposed to chlorobenzene at 10 ppm for 8 h per day for five consecutive days to determine the relationship between chlorobenzene and urinary concentrations of its metabolites 4-chlorocatechol and chlorophenols (Knecht and Woitowitz 2000). None of the subjects complained of irritant or CNS effects (U. Knecht, Justus Liebig University Giessen, Germany, personal commun., 2005).
2.2.3. Occupational and Epidemiologic Studies
The potential consequences of occupational exposure to chlorobenzene are described in a report by Izmerov et al. (1988). These cases are not included in the chapter because concentrations of chlorobenzene in those studies were unclear and coexposure to other chemicals was possible.
2.3. Neurotoxicity
Izmerov et al. (1988) described changes in electroencephalogram (EEG) readings as “evident on an individual basis” during exposure to chlorobenzene and as near-term and long-term effects. The specific changes were not described. On the basis of changes in electrical brain activity, 0.2 mg/m3 (0.044 ppm) appeared to be a threshold concentration (exposure duration unknown), and 0.1 mg/m3 (0.022 ppm) was a no-effect concentration. No further details were provided in the Izmerov report, and the original publications were not available. Therefore, these results are considered supplementary information.
2.4. Summary
No information is available on the acute lethality of chlorobenzene in humans. Chlorobenzene can be irritating to the eyes and respiratory tract, and signs of CNS effects (drowsiness, heavy feeling in the head, and headache) have been report in people exposed at 60 ppm for 7 h. Odor might have interfered with subjective complaints of irritation. No complaints of irritation were described in another study in which volunteer were exposed to chlorobenzene at 10 ppm for 8 h/day for 5 days.
3. ANIMAL TOXICITY DATA
3.1. Acute Lethality
The acute lethality data on chlorobenzene in laboratory animals is presented in Table 3-3.
TABLE 3-3 Acute Lethality Data on Chlorobenzene in Laboratory Animals
| Species (sex) | Concentration (ppm) | Exposure Duration | Effect | Reference |
| Single exposure | ||||
| Rats (male) | 2,965 | 6 h | LC50 | Bonnet etal. 1982 |
| Rats (or mice) | 4.400 | 2 h | LC100 | Rozenbaum et al. 1947 |
| Guinea pigs | 7.970 | 30 mm | No mortality | UBTL 1978 |
| Rats | 22,000 | 3.5 h | 2 of 3 died | Eastman Kodak Co. 1994 |
| Rats | 9.000 | 6 h | 2 of 3 died | Eastman Kodak Co. 1994 |
| Rats | 7.970 | 30 mm | No mortality | UBTL 1978 |
| Mice (female) | 1.886 | 6h | LC50 | Bonnet et al. 1979 |
| Mice | 7.832 | 2h | LC84 | Sanotsky and Ulanova 1975 |
| 4.070 | 2 h | LC50 | ||
| 2.244 | 2 h | LC16 | ||
| Related exposures | ||||
| Rat (two-generation study) | 450 | 6 h/d, 7 d/wk for up to 17 wk | No mortality | Nair et al. 1987 |
| Rabbits (pregnant) | 3.000 1.000 |
6 h/d for 13 d | Mortality No mortality |
John et al. 1984 |
| Rats (pregnant) | 3.000 1.000 |
6 h/d for 10 d | Mortality No mortality |
John et al. 1984 |
| Rats | 248 | 7 h/d, 5 d/wk for 24 wk | No mortality | Dilley 1977 |
3.1.1. Rabbits
A description of a study by Rozenbaum et al. (1947) was obtained from a report by ATSDR (1990), because the original publication could not be obtained. Rabbits (sex and number not specified) exposed to chlorobenzene (head only or whole body) at 550-660 ppm for 4 h died after 2 weeks, but no effects were observed at 110-220 ppm. Rabbits were also reported to have died 2 weeks after exposure to chlorobenzene at 537 ppm for 2 h. These results contrast with findings in other studies. For example, repeated exposure of 32 male rabbits to chlorobenzene at 248 ppm for up to 24 weeks did not increase mortality (Dilley 1977). In addition, no mortality was observed in a teratogenicity study of rabbits exposed at 1,000 ppm (6 h/day for 10 days), but deaths were observed at 3,000 ppm (John et al. 1984) (see Section 3.3 for further details of this study).
3.1.2. Guinea Pigs
Groups of five guinea pigs per sex were exposed (whole body) to chlorobenzene at mean (± standard deviation [SD]) analytic concentrations of 2,990 ± 53, 5,850 ± 1,350, or 7,970 ± 355 ppm for 30 min, and were observed for 14 days. No deaths were observed at any concentration (UBTL 1978).
3.1.3. Rats
Bonnet et al. (1982) determined the 6-h LC50 for chlorobenzene in male Sprague-Dawley rats. Twelve rats per concentration were exposed (whole body) and observed for 14 days. Nominal test concentrations were not provided. Actual concentrations were determined using gas chromatography, but information on the exposure concentrations was limited to a graph on log scale. It was estimated that the lowest concentration tested in rats was approximately 2,000 ppm and was associated with 8% mortality. The LC50 was 2,965 ppm (95% confidence interval [CI]: 2,787-3,169 ppm), with a regression line of probit =-33 + 10.9 logC (the paper presented a positive intercept [+33] but the data indicate that it should be-33). Hypotony, stereotypy, somnolence, tremor, and muscle contractions were observed during exposure.
A 2-h LC100 value of 4,400 ppm for rats was determined by Rozenbaum et al. (1947, as reported by BUA 1990). However, according to ATSDR (1990), this study was performed in mice. The original publication could not be retrieved to clarify the discrepancy.
The following statement was found in a submission to the U.S. Environmental Protection Agency (Eastman Kodak Co 1994): “Acute exposure to 22,000 ppm for 3½ h killed 2/3 rats in 2½ h while 9,000 ppm for 6 h killed 2/3 rats in 3 h.” A reference to unpublished data from the Eastman Kodak Company was cited, but the original study was not available.
Groups of five rats per sex were exposed (whole body) to chlorobenzene at mean (± SD) analytic concentrations of 2,990 ± 53, 5,850 ± 1,350, or 7,970 ± 355 ppm for 30 min, and animals were observed for 14 days. No deaths were observed at any concentration (UBTL 1978).
Repeated exposure of 32 male rats to chlorobenzene at 248 ppm for up to 24 weeks did not result in mortality (Dilley 1977). In addition, no mortality was observed in a two-generation study (450 ppm, 6 h/day, 7 days/week for 17 weeks) (Nair et al. 1987) or in a rat developmental toxicity study (1,000 ppm, 6 h/day for 10 days) (John et al. 1984). However, in the latter study, increased mortality was observed at 3,000 ppm (John et al. 1984).
3.1.4. Mice
Bonnet et al. (1979) determined the 6-h LC50 of chlorobenzene in female mice (OF1). Groups of 25 mice were exposed to chlorobenzene (whole body) and observed for 14 days. Nominal test concentrations were not provided. Actual concentrations were determined using gas chromatography. The analytic concentrations were 90-100% of the nominal concentrations. No details on the exposure concentrations were provided other than a graph on log scale. It was estimated that the lowest concentration tested was approximately 1,500 ppm and caused approximately 20% mortality. The LC50 was 1,886 ppm (95% CI: 1,781 -1,980 ppm), with a regression line of probit =-17.06 + 6.734 logC (the paper presented a positive intercept [+17.06] but the data indicate that it should be -17.06).
Izmerov et al. (1988) described a study by Sanotsky and Ulanova (1975) that found a 2-h LC50 of 4,070 ppm, an LC16 of 2,244 ppm, and an LC84 of 7,832 ppm for chlorobenzene in mice. Izmerov also reported that another study reported that exposure to chlorobenzene at 2,200 ppm (duration unknown) failed to kill mice, but that at 4,400 ppm three of four mice died. Neither of the primary studies could be obtained.
A 2-h LC100 value of 4,400 ppm for mice was reported by Rozenbaum et al. (1947, as cited by ATSDR 1990). However, according to BUA (1990), this study was performed in rats. The original publication could not be retrieved to clarify the discrepancy.
3.2. Nonlethal Toxicity
The acute nonlethal effects of chlorobenzene in laboratory animals are summarized in Table 3-4.
3.2.1. Guinea Pigs
Groups of five guinea pigs per sex were exposed (whole body) to chlorobenzene at mean (± SD) analytic concentrations of 2,990 ± 53, 5,850 ± 1,350, or
7,970 ± 355 ppm for 30 min, and were observed for 14 days. No deaths were observed at any concentration. At 2,990 ppm, slight ocular and nasal irritation was observed, but none of the animals were judged to have an impaired ability to escape. At the next higher concentration of 5,850 ppm, all guinea pigs suffered from narcosis and were judged to have impaired ability to escape. No deaths occurred at the highest concentration but ataxia occurred within 10 min and narcosis was evident after 15 min (UBTL 1978).
TABLE 3-4 Acute Nonlethal Effects of Chlorobenzene in Laboratory Animals
| Species (sex) | Concentration (ppm) | Exposure Duration | Effect | Reference |
| Guinea pigs | 2,990 | 30 min | Slight ocular and nasal irritation; no impaired ability to escape. | UBTL 1978 |
| 5,850 | 30 min | Narcosis in all guinea pigs. | ||
| 7,970 | 30 min | Ataxiawimin 10 min and narcosis within 15 min. | ||
| Rats | 2,990 | 30 min | Slight ocular and nasal irritation; no impaired ability to escape. | UBTL 1978 |
| 5,850 | 30 min | Narcosis in most rats. | ||
| 7,970 | 30 min | Ataxia at 10 min and narcosis within 25 min. | ||
| Rats (male) | 1,500 | 8 h/d for 5d | Reduction in auditory-evoked response. | Rebertetal. 1995 |
| 1,000 | No effect. | |||
| Rats (male) | 611 | 4h | Shortening of the tonic extension of the hind limbs by 37.5% after electrical stimulation. | Frantiketal. 1994 |
| Mice (male) | 1,054 | 5 min | RD50+ for sensory irritation. | De Ceaurriz et al. 1981 |
| Mice | 75 | 3 h, once or for 5 d | No effect on host defense. | Aranyi etal. 1986 |
| Mice (female) | 610 | 2h | Increased velocity of the tonic extension of the hind limbs by 30% after electrical stimulatior | Frantiketal. 1994 1. |
| Mice (male) | 650 | 4h | Decrease in immobility in the "behavioral despair" swimming test by 2. | De Ceaurriz et al. 1983 |
3.2.2. Rats
Groups of five rats per sex were exposed (whole body) to chlorobenzene at mean (± SD) analytic concentrations of 2,990 ± 53, 5,850 ± 1,350, or 7,970 ± 355 ppm for 30 min, and were observed for 14 days. No deaths were observed at any concentration. At 2,990 ppm, slight ocular and nasal irritation was observed but none of the animals were judged to suffer from impaired ability to escape. At the next higher concentration of 5,850 ppm, most rats suffered from “narcosis” and were judged to have impaired ability to escape; the animals recovered quickly after exposure ended. No deaths occurred at the highest concentration, but ataxia was present at 10 min and narcosis was evident in all animals after 25 min of exposure (UBTL 1978).
Frantik et al. (1994) investigated the relative neurotoxicity of several solvents. Groups of four adult male rats (albino, specific pathogen free) were exposed at least three concentrations of chlorobenzene (analytic purity) or to ambient air. Inhalation exposure was performed in a dynamic system for 4 h, and concentrations were measured by gas chromatography. The actual exposure concentrations were not specified. Most animals were tested three or four times at intervals of 3 weeks. Immediately after exposure, the animals received a short electrical pulse through ear electrodes. The duration of subsequent tonic extension of the hind limbs was determined. This parameter was shown to be the most sensitive and consistent. The study authors calculated the concentration required to induce a 37.5% change in the neurologic response (decrease in duration of the tonic extension from 8 to 5 seconds). A 37.5%-effect concentration of 611 ppm (90% CI: 538-684 ppm) was reported for chlorobenzene. The slope was 0.061%/ppm. A 37.5% response corresponds, according to the study authors, to a concentration that does not influence normal locomotor activity or induce behavioral excitation, so it may be considered a sensitive neurologic end point.
Rebert et al. (1995) studied the effect of chlorobenzene on the auditory system of rats. Groups of eight or nine male Long Evans rats were exposed (whole body) at target concentrations of chlorobenzene of 500-2,400 ppm for 8 h per day for 5 days. Analytic concentrations determined by gas chromatography were within 10% of the target concentrations. Auditory function was assessed 3-13 days after exposure using the brainstem auditory-evoked response (integrated amplitude) elicited by 16-kilohertz (kHz) tone pips over a range of 25-95 decibels (dB), with 10 dB increments. The average response over 55-85 dB was compared with controls. A reduction in the integrated amplitude of the response was found in animals exposed at 2,000 ppm or 2,400 ppm in one experiment and at approximately 1,500 or 2,000 ppm in another (estimated from a figure) but not at 500 or 1,000 ppm (estimated from a figure). For one of the experiments, the effect was still present 4 weeks after exposure. Although it was not a subject in the Rebert et al. (1995) study, it is known that exposure to other organic solvents can result in permanent hearing loss from the destruction of cochlear hair cells. A reduction in body weight gain was observed at 2,000 and 2,400 ppm. No information was available on body weights of animals exposed at 1,500 ppm or less. Other effects
are not described in this study. The highest concentration of 2,400 ppm is close to the 6-h LC50 of 2,965 ppm in the rat study by Bonnet et al. (1982).
3.2.3. Mice
Frantik et al. (1994) investigated the relative neurotoxicity of several solvents. Groups of eight female H-strain mice were exposed to at least three concentrations of chlorobenzene (analytic purity) or to ambient air. Inhalation exposure was performed in a dynamic system for 2 h and concentrations were measured by gas chromatography. The concentrations of chlorobenzene were not defined. Most animals were used three or four times at intervals of 3 weeks. Immediately after inhalation, the animals received a short electrical pulse through ear electrodes. The velocity of tonic extension from toxicity was determined. This parameter was shown to be the most sensitive and consistent. The authors calculated the concentration needed to induce a 30% change in the neurologic response (decrease in velocity of the tonic extension). For chlorobenzene, a 30%-effect concentration of 610 ppm was reported (90% CI: 320-900 ppm). The slope was 0.041%/ppm. This 30% response level corresponds, according to the study authors, to a concentration that does not influence normal locomotor activity or induced behavioral excitation.
De Ceaurriz et al. (1983) tested the effect of chlorobenzene on the duration of immobility during a 3-min “behavioral despair” swimming test. Groups of 10 male Swiss OF1 mice were exposed (whole body) for 4 h to chlorobenzene at 0, 650, 785, 875, or 1,000 ppm. The analytic concentrations were determined using gas-liquid chromatography but the results were not provided, so it was assumed that the stated concentrations were the analytic concentrations. After exposure, mice were placed in water, and duration of immobility was determined over 3 min and compared with that of control animals. A significant and concentration-dependent decrease in immobility of-28,-45,-53, and-82% was found at 650, 785, 875, and 1,000 ppm, respectively. The concentration at which there was a 50% decrease in immobility was estimated to be 804 ppm (95% CI: 718-887 ppm).
De Ceaurriz et al. (1981) determined the concentration of chlorobenzene that reduced the respiratory rate by 50% (RD50) in mice. Groups of six male Swiss OF1 mice were exposed (head only) for 5 min to at least four different concentrations of chlorobenzene. Respiratory rate was determined during exposure with a plethysmograph. The analytic concentration was determined using gas chromatography, but it is unclear whether the RD50 of 1,054 ppm was based on target, nominal, or analytic concentrations.
Aranyi et al. (1986) examined the effect of chlorobenzene on murine host defenses. Groups of approximately 150 female mice were exposed to chlorobenzene at 75 ppm for 3 h once or five times on 5 consecutive days. Analytic concentrations were determined using gas chromatography and were in close agreement with the target concentrations. Host defense status was determined by challenge with Streptococcus zooepidemicus and Klebsiella pneumonia. Deaths
were recorded daily over a 14-day observation period. No effect on mortality from streptococcus challenge or on bactericidal activity was found.
3.3. Developmental and Reproductive Toxicity
Groups of 32-33 pregnant female F344 rats were exposed by inhalation (whole body) to chlorobenzene at 0, 75, 210, or 590 ppm (99.982% pure, nominal concentrations) for 6 h per day on gestation days 6-15 (Hayes et al. 1982; John et al. 1984). Animals were killed on day 21 of gestation and the fetuses examined. Chlorobenzene concentrations in the chamber were determined with infrared spectrophotometry. The time-weighted average analytic concentrations were within 7-8% of the target concentrations. The exposure conditions of this study were chosen on the basis of a preliminary range-finding study in which test atmospheres of 0, 300, 1,000, and 3,000 ppm were generated, and 10 rats per concentration were exposed 6 h/day on gestation days 6-5 and sacrificed on day 16.
In the range-finding study, 3,000 ppm induced severe irritation of the eyes and nasal area, signs of narcosis, and mortality (or a moribund state). The time of onset of these effects was not specified. Effects observed at 1,000 ppm included a reduction in absolute body weight, reduced food consumption, internal and external lesions, an increase in relative kidney and liver weights, reduction in thymus size, and an increase in the number of resorptions. At 300 ppm, only a small decrease in body weight gain (on days 6-8) and an increase in relative liver weight were observed.
In the main study, maternal toxicity observed only at the highest concentration of 590 ppm, and consisted of a significant reduction in weight gain on days 6-8 and a significant increase in absolute and relative liver weights. No effects were found on pregnancy rate, litter size, resorptions, fetal body weights, or the incidence of external or soft-tissue alterations. At the highest concentration some increases in skeletal variations, such as a delay in ossification, were found (Hayes et al. 1982; John et al. 1984).
In another study, groups of 30 pregnant New Zealand white rabbits were exposed (whole body) to chlorobenzene at 0, 75, 210, or 590 ppm (99.982% pure, nominal concentrations) for 6 h/day on gestation days 6-18 (Hayes et al. 1982; John et al. 1984). Animals were killed on day 29 of gestation and the fetuses were examined. Chlorobenzene concentrations in the chamber were determined with infrared spectrophotometry. The time-weighted average concentrations were within 7-8% of the target concentrations. These exposure conditions were chosen on the basis of a preliminary range-finding study in which groups of seven rabbits were exposed at 0, 300, 1,000, and 3,000 ppm for 6 h/day on gestation days 6-18, and sacrificed on day 19.
The effects observed at 3,000 ppm in the range-finding study with rabbits were mortality (or moribund state) during exposure, severe systemic toxicity and hepatotoxicity, reduced weight gain, and macroscopic changes of the liver. Effects observed at 1,000 ppm included reduced bodyweight gain on days 6-8 and
macroscopic changes of the liver. Slight liver effects (not described) were found at 300 ppm.
Maternal toxicity observed only in the 210-and 590-ppm groups, and consisted of a significant increase in absolute and relative liver weights. No effects were found on pregnancy rate, litter size, resorptions, or fetal body weights. A small but not significant increase in head and facial anomalies and heart defects was found in the 210-and 590-ppm groups. The incidence of an extra (thoracic) rib (variation) was significantly increased in the offspring from does exposed at 580 ppm (Hayes et al. 1982; John et al. 1984).
Because of the small increase in malformations, the rabbit study was repeated using concentrations of chlorobenzene at 10, 30, 75, and 590 ppm. Maternal toxicity was found only in the 590-ppm group, and consisted of an increase in liver weight. The percentage of litters with resorptions was significantly increased at 590 ppm, but this observation was within the historical-control range. No effects were found on pregnancy rate, litter size, fetal body weights, or the incidence of external, skeletal, or soft-tissue alterations (Hayes et al. 1982 John et al. 1984). It was concluded from these studies in rats and rabbits that chlorobenzene does not induce teratogenic or embryolethal effects at up to maternally-toxic concentrations.
Nair et al. (1987) performed a two-generation reproduction study in rats exposed to chlorobenzene by inhalation. Groups of 30 male and 30 female CD rats were exposed (whole body) to target concentrations of 0, 50, 150, or 450 ppm for 6 h/day, 7 days per week. The analytic concentrations of chlorobenzene were determined using a MIRAN 1A organic vapor analyzer, and were found to be approximately 10% higher than target concentrations. No effects on mortality, body weight, food consumption, reproductive parameters, pup viability, or survival were found. Hepatic toxicity was mainly seen at 150 and 450 ppm. Increases in the incidence of small flaccid testes and in the incidence and severity of unilateral or bilateral degeneration (minimal to severe) of the germinal epithelium were found at 450 ppm in both generations. Three of six affected rats exposed at the highest concentration in each generation sired litters. Small increases in these effects were also seen at 150 ppm. An increased incidence of dilated renal pelvis was observed in males of the F0 generation exposed at the highest concentration and in all treated males of the F1 generation. Microscopically, an increase in renal degeneration and inflammatory lesions was found at the two highest concentrations.
The transfer of chlorobenzene to the fetus of pregnant mice after inhalation exposure at 500 ppm for 1 h was shown by Shimada (1988b). It can be concluded that chlorobenzene does not affect fertility in rats at concentrations up to those that induce over maternal toxicity.
3.4. Genotoxicity
The genotoxicity of chlorobenzene has been evaluated in several in vitro and in vivo models, as reviewed by NTP (1985), ATSDR (1990), BUA (1990),
IPCS (1991), and Hellman (1993), reported in more recent studies. A summary of the information presented in these reviews are presented in the sections below.
3.4.1. In Vitro Studies
Results of several in vitro genotoxicity studies in nonmammlian test systems, including Salmonella typhimurium (Lawlor et al. 1979; Haworth et al. 1983; Shimizu et al. 1983; Simmon et al.1979, NTP 1985), Escherichia coli (Lawlor et al. 1979), Aspergillus nidulans (Prasad 1970; Prasad and Pramer 1968), and Saccharomyces cerevisiae (Monsanto Company 1976), indicate that chlorobenzene does not induce DNA damage or gene mutations. However, chlorobenzene did induce mutations in Actinomycetes antibioticus (Keskinova 1968) and in another study of S. cerevisiae (Simmon et al. 1979).
Results of in vitro studies in mammalian cells have yielded conflicting results regarding the genotoxic potential of chlorobenzene. Chlorobenzene did not induce gene mutations in mouse lymphoma cells (Monsanto Company 1976), unscheduled DNA repair in rat liver cells (Shimada et al. 1983); Williams et al. 1989), or chromosome aberrations (Loveday et al. 1989). However, chlorobenzene induced gene mutations in mouse lymphoma cells (McGregor et al. 1988) and sister-chromatid exchange (Loveday et al. 1989). Chlorobenzene also produced decreases in cell proliferation and mitotic indices and an increase in sister-chromatid exchange rat bone-marrow cells (Khalil and Odeh 1994).
3.4.2. In Vivo Studies on Animals
Results of in vivo studies indicate that chlorobenzene has some potential to induce genotoxicity, although conflicting results have been reported. In studies of interactions with DNA, chlorobenzene has been reported to bind DNA in mouse liver, kidneys, and lungs (Grilli et al. 1985), and a guanine DNA adduct was found in the urine of rats after exposure to chlorobenzene (Krewet et al. 1989). Chlorobenzene also inducted DNA damage in peripheral lymphocytes, but not bone-marrow cells from mice following in vivo exposure (Vaghef and Hellman 1995). Chlorobenzene induced a dose-related increase in the formation of micronucleated polychromatic erythrocytes in the femoral bone marrow of mice (Mohtashamipur et al. 1987). In contrast, in vivo exposure to chlorobenzene did not induce mirconucleus formation in erythrocytes of the bone marrow of mice (Shelby et al. 1993), dominant lethal mutations or sister-chromatid exchange in mice (Feldt 1985), or recessive lethal mutations in Drosophila melanogaster (Valencia 1982).
3.4.3. Conclusion
The available information indicates that chlorobenzene has some potential to induce DNA damage, which is further underpinned by the formation of
epoxide-containing metabolites. However, because of conflicting results from mutagenic tests in vitro and in vivo, it is unclear whether the genotoxic activity could represent a risk to human health.
3.5. Carcinogenicity
No inhalation carcinogenicity studies are available for chlorobenzene. However, a gavage study in rats and mice was performed by NTP (1985). That study concluded that chlorobenzene increased the occurrence of neoplastic nodules of the liver at high dose (120 milligrams per kilogram of body weight per day) in male F344/N rats, providing some, but not clear evidence, of carcinogenicity in male rats. Carcinogenic effects of chlorobenzene were not observed in female F344/N rats or in male or female B6C3F1 mice.
3.6. Summary
Only a few animal studies provide LC50 values. Bonnet et al. (1979) reported 6-h LC50 values of 2,965 ppm and 1,886 ppm for male rats and female mice, respectively. The concentration-response curve in rats was steep; the extrapolated concentration range between 0-100% was covered by a factor of approximately 3. However, in mice the concentration-response curve was comparatively shallow and was covered by a factor of approximately 10. No deaths were observed in rats or guinea pigs exposed at concentrations up to 7,970 ppm for 30 min (UBTL 1978). Other studies were had limitations in their reporting or were only available as a summary.
An RD50 for sensory irritation of 1,054 ppm in mice was assessed for chlorobenzene (De Ceaurriz et al. 1981). Chlorobenzene was moderately irritating to the skin and not irritating to the eye in standard tests (Mihail 1984). Slight ocular and nasal irritation was observed in rats and guinea pigs exposed at 2,990 ppm for 30 min (UBTL 1978). Ocular irritation in the rat was also observed after repeated exposure to chlorobenzene at 3,000 ppm (John et al. 1984).
Similar to many other volatile organic compounds, exposure to sufficiently high concentrations of chlorobenzene can induce signs of CNS depression. CNS effects are concentration-related in a continuum of slight effects (lightheadedness) to narcosis, and eventually lead to death from paralysis of the respiratory center.
Animal data related to neurotoxicity are available include clinical effects observed in standard studies and as measurements of specific parameters in specific studies. Ataxia and narcosis were reported in most rats and all guinea pigs exposed to chlorobenzene at 5,850 ppm for 30 min; the effects occurred earlier in guinea pigs than in rats (UBTL 1978). Several clinical effects indicative of neurotoxicity were reported in the acute toxicity study on rats by Bonnet et al. (1982). However, no information on the concentrations at which those effects were observed was provided. Frantik et al. (1994) studied chlorobenzene exposure in relation to the duration of the tonic extension of the hind limbs after a
short electrical pulse in rats and mice. Neurologic responses (decrease in velocity of the tonic extension) of 30% and 37.5% were found in rats and mice, respectively, exposed at 610 ppm for 4 h. This effect is considered a relatively mild end point. Rebert et al. (1995) determined that the concentration threshold for auditory changes in rats was 1,500 ppm (exposed for 8 h/ day for 5 days) and that the no-observed effect level was 1,000 ppm. The concentration of chlorobenzene that caused a 50% decrease in the duration of immobility in a “behavioral despair” swimming test in mice was 804 ppm for 4 h (De Ceaurriz et al. 1983). This effect is considered a relatively mild end point.
The developmental studies of chlorobenzene in rats and rabbits (John et al. 1984) indicates that chlorobenzene does not induce irreversible structural effects in animals when tested at up to maternally toxic concentrations of 590 ppm (for 6 h). In rats, an increase in fetal skeletal variations, such as delayed ossification, was found at 590 ppm.
A two-generation test of chlorobenzene in rats (Nair et al. 1987) indicates that chlorobenzene does not influence fertility at up to concentrations inducing systemic toxicity in the parents of 450 ppm (for 6 h).
In vitro studies indicate that chlorobenzene does not induce DNA damage or gene mutations in bacterial tests. Contradicting results were found in gene mutation tests on yeast and DNA damage and gene-mutation tests using mammalian cells. Results of an in vitro test showed that chlorobenzene did not induce chromosome damage. Based on the in vitro tests, DNA damage and gene mutations cannot be excluded. In vivo studies indicate that chlorobenzene has some potential for DNA damage. The results of the chromosome mutation tests were contradictory. A mutagenic potential in vivo for chlorobenzene cannot be excluded. Overall, the available information indicates that chlorobenzene has some potential to induce DNA damage, perhaps associated with the formation of epoxide-containing metabolites. However, because of conflicting results of the in vitro and in vivo mutagenic tests, it is unclear whether the potential measured in some of the studies represents a risk to human health.
The carcinogenicity of chlorobenzene was only tested in an oral study in rats and mice (NTP 1985). NTP concluded that chlorobenzene increased the occurrence of neoplastic nodules of the liver at 120 mg/kg/day in male F344/N rats, but not in female F344/N rats or in male or female B6C3F1 mice.
4. SPECIAL CONSIDERATIONS
4.1. Metabolism and Disposition
4.1.1. Absorption
Ogata and Shimada (1983) determined the metabolites excreted in the urine of two workers exposed to chlorobenzene at 0.84 ppm for 415 min or 0.5 ppm for 228 min and compared it with their estimated intake. The main chlorobenzene metabolites recovered in the urine were p-chlorobenzenemercaptic acid
(MA) and 4-chlorocatechol (4-CC). Comparison of combined urinary metabolites to the estimated dose of chlorobenzene ([MA + 4-CC] ÷ estimated dose of chlorobenzene expressed as mmole/kg body weight) were 0.38 and 0.45 for the 0.84 ppm (415 min) and 0.5 ppm (228 min) exposures, respectively.
Knecht and Woitowitz (2000) exposed eight volunteers to chlorobenzene at10 ppm for 8 h/day, with an interruption of 45 min, for 5 successive days to determine a biologic tolerance level. Chlorobenzene concentrations in the blood were determined at the end of each day and every 10 min after the last exposure for 3.5 h. In one volunteer, the blood concentration was determined every hour up to 4 h during exposure. Urinary concentrations of metabolites were collected daily at before, during, and after exposure, and for 24 h after the last exposure. Chlorobenzene in blood reached a steady-state level within 1 h during a 4-h exposure at 10 ppm. No estimate of pulmonary absorption was provided.
4.1.2. Distribution
Sullivan et al. (1983) determined the distribution of 14C-labeled chlorobenzene in rats after single or repeated 8-h exposures. Radioactivity in all tissues, except fat, increased in proportion to the exposure concentration. The amount of radiolabel in fat increased 30-fold between 100 and 700 ppm. Immediately after exposure, radioactivity was highest in fat tissues. The preferential distribution of chlorobenzene to adipose tissue reflects the lipophilic nature of this compound, and is confirmed in a study with mice by Shimada (1988a).
4.1.3. Metabolism
The metabolism of chlorobenzene has been investigated in several species (summarized in ATSDR 1990; BUA 1990; IPCS 1991). The first step in chlorobenzene metabolism is hepatic oxidation by cytochrome P450 to mainly chlorobenzene-3,4-epoxide, but also to chlorobenzene-2,3-epoxide and 3-chlorophenol. The epoxides are converted enzymatically by glutathione-transferase to watersoluble mercapturic-acid derivates and by epoxide hydratase via dihydrodihydroxychlorobenzene to chlorocathechols. Nonenzymatic rearrangement of the epoxides results in the formation of chlorophenols. The chlorophenols and chlorocathechols can be eliminated in the urine directly or after conjugation with glucuronic acid or sulfate. There was some indication that metabolism was saturated in rats exposed at 400 ppm. Metabolism can be affected by pretreatment with microsomal enzyme-inducing agents and by glutathione depletion. Binding of chlorobenzene to protein, RNA, and DNA was shown in several studies and were probably caused by the reactivity of the epoxide (BUA 1990).
4.1.4. Excretion
Chlorobenzene is eliminated unchanged via the lungs and as metabolites principally in the urine and to a smaller extent in the feces. The main urinary
metabolites are 4-chlorocatechol conjugates and 4-chlorophenylmercapturic acid. The percentage eliminated via the lungs increases with concentration. The elimination of inhaled chlorobenzene consists of a quick first phase (probably exhalation of chlorobenzene) and a second slower phase (probably metabolism and urinary excretion) (ATSDR 1990).
4.2. Mechanism of Toxicity
Acute inhalation exposure to chlorobenzene results in contact irritation and CNS depression. Repeated also results in toxicity to several organs including the liver, kidneys, and white blood cells.
As with many volatile organic compounds, the concentration of chlorobenzene in the brain is probably the pivotal factor for CNS depression. According to De Jongh et al. (1998), the calculated concentration of chlorobenzene in the lipid phase of the brain shows a good correlation with acute mortality data for several volatile organic compounds. So, the concentration of chlorobenzene in the brain lipid phase probably determines the potential for CNS depression and, eventually, death from paralysis of the respiratory center.
No information is available on the mechanism of the toxicity to several organs including liver, kidneys, and white blood cells after inhalation exposure. However, studies (Reid 1973; Reid and Krishna 1973) with intraperitoneal exposure to radioactive chlorobenzene show covalent binding to proteins. Also, pretreatment with cytochrome-P450 inhibitors or inducers reduced or potentiated the binding and the renal and hepatic necrosis. This suggests that toxic effects in the liver and kidneys and probably also white blood cells (or bone marrow) are from reactive metabolites. The reactive epoxides are normally removed by the enzymatic reaction with glutathione or by epoxide hydratase. Repeated exposure can, therefore, result in glutathione depletion. A decrease in glutathione can result in an increase in covalent binding and toxicity.
The mechanism of action for the toxicity of chlorobenzene is unknown and, therefore, the appropriate dose metric cannot be assessed. Physiologicallybased pharmacokinetic (PBPK) modeling could provide some additional insight by evaluating correlations between selected dose metrics and dose-response data from toxicity studies. However, not all metabolites can be modeled in sufficient detail currently and the available data are scarce for appropriate dose-response modeling. Therefore, the uncertainty was judged to be too large for using the PBPK-modeling to derive AEGL values.
4.3. Structure-Activity Relationships
No quantitative structure-activity relationships were found for chlorobenzene and its congeners. However, chlorobenzene shares its CNS-depressing action with other aromatic compounds like benzene, toluene, and xylene. A comparison of LC50 values for several aromatic compounds (Bonnet et al. 1979,
1982), indicated that chlorobenzene was one of most toxic compounds. Only dichlorobenzene was more toxic than chlorobenzene.
4.4. Other Relevant Information
4.4.1. Irritation and Sensitization
Chlorobenzene was considered moderately irritating to the skin and nonirritating to the eye in the OECD 404 and 405 tests (Suberg 1983). A maximization test gave no indication of a sensitizing potential (Mihail 1984).
5. DATA ANALYSIS FOR AEGL-1
5.1. Summary of Human Data Relevant to AEGL-1
Ogata et al. (1991) exposed four volunteers to a chlorobenzene at 60 ppm for 3 h in the morning and 4 h in the afternoon, with a 1-h break between exposures. All volunteers complained of a disagreeable odor and of drowsiness after exposure ended. Three subjects had a heavy feeling in the head or had headaches, two reported throbbing pain in the eyes, and one complained of sore throat. No information was given about the time of onset of these complaints. No effect on pulse rate or systolic and diastolic pressure was found. Flicker fusion frequency values were reduced significantly at the end of the 3-h exposure period in the morning, indicating lowered perception, but no further effect was seen in the afternoon. The significance of this finding is difficult to interpret.
Knecht and Woitowitz (2000) exposed eight volunteers to chlorobenzene at 10 ppm for 8 h/day for 5 days to determine the relationship between exposure and urinary concentrations of 4-chlorocatechol and chlorophenols. None of the subjects complained of irritant or CNS effects (U. Knecht, Justus Liebig University Giessen, Germany, personal commun. 2005).
5.2. Summary of Animal Data Relevant to AEGL-1
No adequate animal data relevant to the type of effects defined by the AEGL-1 were found.
5.3. Derivation of AEGL-1
Effects in subjects exposed to chlorobenzene at 60 ppm are indicative of slight CNS depression and local irritation (Ogata et al. 1991), and are considered evidence of discomfort. These effects were not observed is subjects exposed at 10 ppm for 8 h (Knecht and Woitowitz 2000). Thus, 10 ppm was chosen as a conservative point of departure for the derivation of AEGL-1 values. Because human data are used, an interspecies uncertainty factor of 1 was used. Despite
the fact that only a few subjects were tested, an uncertainty factor of 1 for intraspecies variability was considered appropriate because of the conservatism of the point of departure already provides a margin of safety. (The point of departure of 10 ppm was obtained from a repeated-exposure study, and effects observed at 60 ppm were rather slight.) No information about the time dependency of the effects at 10 or 60 ppm is available. Because the effects at 60 ppm include irritation and CNS effects (drowsiness, heavy feeling in the head, and headache), the 8-h AEGL-1 value of 10 ppm is considered appropriate for all time points. Furthermore, Knecht and Woitowitz (2000) reported that chlorobenzene concentrations in blood reached a steady-state level within 1 h. AEGL-1 values for chlorobenzene are presented in Table 3-5.
6. DATA ANALYSIS FOR AEGL-2
6.1. Summary of Human Data Relevant to AEGL-2
No human data were available that adequately address toxicity end points as defined by AEGL-2.
6.2. Summary of Animal Data Relevant to AEGL-2
Slight ocular and nasal irritation was found in guinea pigs exposed to chlorobenzene at a mean (± SD) analytic concentration of 2,990 ± 53 ppm, but none of the animals was judged to suffer from impaired ability to escape. At the next higher concentration tested of 5,850 ± 1,350 ppm, all guinea pigs and most rats suffered from narcosis and were judged to have impaired ability to escape (UBTL 1978). Frantik et al. (1994) determined that a 37.5% decrease in the duration of tonic extension of the hind limbs of rats after a short electrical pulse was induced after a 4-h exposure at 611 ppm. A comparable study in mice found a 30% effect level at 610 ppm. The investigators noted that these concentrations were not expected to influence normal locomotor activity or to induce behavioral excitation, based on extrapolation from concentration-response data. Rebert et al. (1995) determined a no-observed-effect level of 1,000 ppm for auditory effects in rats exposed for 8 h/day for 5 days. Because organic solvents can induce permanent hearing loss, this was considered a relevant end point for AEGL-2 derivation. However, whether permanent hearing loss can be induced by single exposure is unclear. De Ceaurriz et al. (1983) conducted a study to evaluate behavioral response to swimming in an enclosed space (escape behavior followed by immobility), and estimated the concentration of chlorobenzene to elicit a 50% decrease in immobility to be 804 ppm. However, no signs of toxicity, such as motor impairment, were reported in response to chlorobenzene exposure. Thus, this effect was considered a subtle change in neurobehavior.
Repeated exposure of rats and rabbits to chlorobenzene resulted in severe effects at concentrations of 1,000 ppm and greater, in limited effects around 500 ppm, and no effects at 200 ppm (Hayes et al. 1982; John et al. 1984).
TABLE 3-5 AEGL-1 Values for Chlorobenzene
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) |
6.3. Derivation of AEGL-2
There are no adequate human data for deriving AEGL-2 values, so animal data were used. The threshold for AEGL-2 effects (impaired ability to escape) in rats and guinea pigs appears to be between 2,900 ppm, which produced slight ocular and nasal irritation, 5,980 ppm, which produced narcosis (UBTL 1978).
Behavioral and neurophysiologic effects were observed in rats and mice at concentrations below 2,990 ppm (De Ceaurriz et al. 1983; Frantik et al. 1994; Rebert et al. 1995); however, these observations were not considered suitable bases for a point of departure for AEGL-2 values for the following reasons. Effects on brainstem auditory-evoked potential in rats were associated with repeated exposures at >1,000 ppm (8 h/day for 5 days) (Rebert et al. 1995), and represent changes in sensitivity of the brain (or cochlear hair cells) to detect sound at specific frequencies. The effects cannot be associated reliably with concentrations would cause hearing loss sufficient to impair escape, nor can they be extrapolated to single exposures as the effect observed might reflect cumulative exposures over 5 days (Rebert et al. 1995).
Inhibition of electrically-evoked seizures in rats and mice (Frantik et al. 1994) was observed with chlorobenzene at 611 and 610 ppm, respectively. Suppression of such seizure activity has been shown to be a predictor of CNS depressing activity (e.g., narcosis) at higher concentrations for a variety of substances (Frantik et al. 1994). However, the CNS effects observed occurred at concentrations below the levels that would be expected to produce CNS depression of sufficient magnitude to impair mobility or escape (based on results of UBTL 1978). In addition, narcosis was not reported in the Frantik et al. (1994) study.
Shortening the duration of the immobility response in mice occurred in association with a 4-h exposure to chlorobenzene at 650 ppm (De Ceaurriz et al. 1983). This effect has been reported in rats and mice after treatment with antidepressant drugs (De Ceaurriz et al. 1983). The mechanism for the response to chlorobenzene is not understood, although a CNS effect is suggested (De Ceaurriz et al. 1983). The effects in the De Ceaurriz et al. (1983) study would not be sufficient to affect mobility (or escape) in the absence of a panic stimulus. In mice, the effect was to shorten the duration of immobility, not to produce immobility or lengthen the period of immobility. In addition, narcosis was not reported in the study.
Given that the neurosensory and behavioral and effects observed at lower concentrations are not directly relevant to AEGL-2 effects, the most appropriate point of departure is the absence of AEGL-2 related effects in rats and guinea
pigs exposed at 2,990 ppm for 30 min (UBTL 1978). An interspecies uncertainty factor of 3 was applied because data were comparable for rats and guinea pigs, suggesting no large interspecies differences, and the critical effect is CNS depression. The concentration of chlorobenzene in the brain is probably related directly to inhalation rate. Therefore, humans probably require higher external exposures that rodents to obtain a similar concentration of chlorobenzene in the blood or brain. Experience with anesthetic gases shows that interindividual variability in CNS depression is generally not greater than a factor of 2 or 3. Therefore, an intraspecies uncertainty factor of 3 was used. A combined uncertainty factor of 10 was considered appropriate because a larger factor would result in AEGL-2 values that would conflict with human data (values would be lower than 60 ppm, a concentration shown to cause only minor effects in humans). With a combined uncertainty actor of 10, the 30-min AEGL-2 is 300 ppm.
The 30-min value was extrapolated across time periods using the equation Cn× t = k, with default values of n = 1 for extrapolation to 10-min and n = 3 for extrapolation to 1-h values. The 4-and 8-h values were set equal to the 1-h value because a steady-state chlorobenzene concentration in blood is reached within 1 h and its elimination is rapid. Furthermore, time scaling would result in 4-and 8-h AEGL-2 values (37 and 19 ppm, respectively) that conflict with human data (Ogata et al. 1991). AEGL-2 values are presented in Table 3-6.
7. DATA ANALYSIS FOR AEGL-3
7.1. Summary of Human Data Relevant to AEGL-3
No human mortality data on chlorobenzene were available.
7.2. Summary of Animal Data Relevant to AEGL-3
Several animal studies relevant to deriving AGEL-3 values were available, but most were available only from descriptions in secondary sources or were from older literature that lacked adequate details. Mortality data on rabbits exposed to chlorobenzene at 550-660 ppm (Rozenbaum et al. 1947 could not be used because the results were from a secondary source and the findings conflicted with a developmental-toxicity study in which no mortality occurred in rabbits exposed at 1,000 ppm (John et al. 1984). No deaths occurred in guinea pigs exposed at 2,990, 5,850, or 7,970 ppm for 30 min (UBTL 1978).
Bonnet et al. (1982) estimated a 6-h LC50 of 2,965 ppm for chlorobenzene in male Sprague-Dawley rats. This estimate is consistent with the results of a range-finding developmental-toxicity study in rats, in which deaths were observed at 3,000 ppm but not at 1,000 ppm (John et al. 1984). However, the results of Bonnet et al. (1982) conflict with those of Rebert et al. (1995), who reported no deaths in male Long Evans rats exposed at 1,000-2,400 ppm for 8 h/day for 5 days. Strain differences might have contributed to these conflicting results.
There are several studies of acute mortality in mice exposed to chlorobenzene, but most descriptions lack adequate detail. A 6-h LC50 of 1,886 ppm in mice was reported by Bonnet et al. (1979). Mice appear to be more sensitive than rats to chlorobenzene.
7.3. Derivation of AEGL-3
Data in rats and guinea pigs reported by UBTL (1978) provide the most appropriate point of departure for AEGL-3 derivation. No mortality occurred in either species after exposure to chlorobenzene at 7,970 ppm for 30 min. Interspecies and intraspecies factors of 3 were applied on the same basis they were applied in the derivation of AEGL-2 values. Time scaling was also performed in the same fashion as for the AEGL-2 calculations, and the 4-and 8-h AEGL-3 values were set equal to the 1-h value. Time scaling with n = 1 would result in an 8-h AEGL-3 value of 50 ppm, which would not be consistent with the observation that only slight CNS depression and irritation occurred in humans exposed at 60 ppm for 7 h. Table 3-7 summarizes the AEGL-3 values. These values are consistent with the AEGL-2 values (see Table 3-6) and are supported by the 6-h LC01 of 1,873 ppm calculated from the probit equation reported by Bonnet et al. (1982).
8. SUMMARY OF AEGLS
8.1. AEGL Values and Toxicity End Points
AEGL values for chlorobenzene are presented in Table 3-8.
8.2. Comparison with Other Standards and Guidelines
The immediate danger to life or health (IDLH) value of 1,000 ppm was based on acute inhalation toxicity studies. With exception of the 10-min AEGL-3 value, all AEGL-values are below the IDLH value. The 8-h AEGL-2 value is about twice as high as the PEL-TWA (permissible exposure limits-time weighted average) established by the Occupational Safety and Health Administration. The TLV-TWA (threshold limit value-time weighted average) of the American Conference of Governmental Industrial Hygienists and analogous German and Dutch standards are equal to the 8-h AEGL-1 value. A summary of extant standards and guidelines for chlorobenzene is provided in Table 3-9.
TABLE 3-6 AEGL-2 Values for Chlorobenzene
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 430 ppm (2,021 mg/m3) |
300 ppm (1,410 mg/m3) |
150 ppm (705 mg/m3) |
150 ppm (705 mg/m3) |
150 ppm (705 mg/m3) |
TABLE 3-7 AEGL-3 Values for Chlorobenzene
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 1,100 ppm (5,170 mg/m3) | 800 ppm (3,760 mg/m3) | 400 ppm (1,880 mg/m3) | 400 ppm (1,880 mg/m3) | 800 ppm (1,880 mg/m3) |
TABLE 3-8 Summary of AEGL Values for Chlorobenzene
| Classification | Exposure Duration | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h | |
| AEGL-1 (nondisabling) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) |
| AEGL-2 (disabling) | 430 ppm (2,021 mg/m3) | 300 ppm (1,410 mg/m3) | 150 ppm (705 mg/m3) | 150 ppm (705 mg/m3) | 150 ppm (705 mg/m3) |
| AEGL-3 (lethal) | 1,100 ppm (5,170 mg/m3) | 800 ppm (3,760 mg/m3) | 400 ppm (1,880 mg/m3) | 400 ppm (1,880 mg/m3) | 400 ppm (1,880 mg/m3) |
TABLE 3-9 Extant Standards and Guidelines for Chlorobenzene
| Guideline | Exposure Duration | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h | |
| AEGL-1 | lOppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) |
| AEGL-2 | 430 ppm (2,021 mg/m3) | 300 ppm (1,410 mg/m3) | 150 ppm (705 mg/m3) | 150 ppm (705 mg/m3) | 150 ppm (705 mg/m3) |
| AEGL-3 | 1,100 ppm (5,170 mg/m3) | 800 ppm (3,760 mg/m3) | 400 ppm (1,880 mg/m3) | 400 ppm (1,880 mg/m3) | 400 ppm (1,880 mg/m3) |
| ERPG-1 (AIHA)a | 30 ppm | ||||
| ERPG-2 (AIHA) | 500 ppm | ||||
| ERPG-3 (AIHA) | 1,000 ppm | ||||
| IDLH (NIOSH)b | 1,000 ppm | ||||
| TLV-TWA (ACGIH)c | 10 ppm | ||||
| PEL-TWA (OSHA)d | 75 ppm | ||||
| MAK (Germany)e | 10 ppm | ||||
| MAC (The Netherlands)f | 10 ppm | ||||
a ERPG (emergency response planning guidelines, American Industrial Hygiene Association)(AIHA 2010)
The ERPG-1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing other than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. The
ERPG-2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms that could impair an individual’s ability to take protection action.
The ERPG-3 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed for up to 1 h without experiencing or developing lifethreatening health effects.
b IDLH (immediately dangerous to life or health, National Institute for Occupational Safety and Health) (NIOSH 1994) represents the maximum concentration from which one could escape within 30 min without any escape-impairing symptoms, or any irreversible health effects.
c TLV-TWA (threshold limit value–time weighted average, American Conference of Governmental Industrial Hygienists) (ACGIH 2010) is the time-weighted average concentration for a normal 8-h workday and a 40-h workweek, to which nearly all workers may be repeatedly exposed, day after day, without adverse effect.
d PEL-TWA (permissible exposure limits-time weighted average, Occupational Safety and Health Administration) (29 CFR 1910.1000 [2006]) is defined analogous to the ACGIH TLV-TWA, but is for exposures of no more than 10 h/day, 40 h/week. .
e MAK (maximale arbeitsplatzkonzentration [maximum workplace concentration]) (Deutsche forschungsgemeinschaft [German Research Association] (DFG 2005) is defined analogous to the ACGIH TLV-TWA.
f MAC (maximaal aanvaaarde concentratie [maximal accepted concentration]) Dutch Expert Committee for Occupational Standards,, The Netherlands (MSZW 2004) is defined analogous to the ACGIH TLV-TWA.
8.3. Data Quality and Research Needs
Human data are scarce. Only two kinetic studies of chlorobenzene in humans were found, and no adequate studies of toxic effects in human were available. Of the studies relevant to AEGL-2 values, only one study was of sufficient quality, in which rats and guinea pigs were exposed for 30 min to nonlethal concentrations.
9. REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 1991. Chlorobenzene. Pp. 271-274 in Documentation of the Threshold Limit Values (TLVs) for Chemical Substances and Physical Agents and Biological Exposure Indices (BEIs), 6th Ed. American Conference of Governmental Industrial Hygienists, Cincinnati OH.
ACGIH (American Conference of Governmental Industrial Hygienists). 2010. Chlorobenzene (CAS Reg. No. 108-90-7). TLVs and BEIs Based on the Documentation of the Threshold Limit Values for Chemical Substances & Biological Exposure Indices. American Conference of Governmental Industrial Hygienists, Cincinnati OH.
AIHA (American Industrial Hygiene Association). 2010. Emergency Response Planning Guidelines (ERPG): Chlorobenzene. Fairfax, VA: AIHA Press.
Aranyi, C., W.J. O'Shea, J.A. Graham, and F.J. Miller. 1986. The effects of inhalation of organic chemical air contaminants on murine lung host defenses. Fundam. Appl. Toxicol. 6(4):713-720.
Arbetslivsinsinstitutet (National Institute for Working Life). 2003. Consensus report for chlorobenzene. Pp. 48-54 in Scientific Basis for Swedish Occupational Standards XXIV, J. Montelius, ed. Arbete och Hälsa (Work and Health) No. 16. Stockholm: National Institute for Working Life [online]. Available: http://www.inchem.org/documents/kemi/kemi/ah2003_16.pdf [accessed Feb. 27, 2012].
ATSDR (Agency for Toxic Substances and Diseases Registry). 1990. Toxicological Profile for Chlorobenzene. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Agency for Toxic Substances and Diseases Registry, Atlanta, GA. December 1990 [online]. Available: http://www.atsdr.cdc.gov/ToxProfiles/TP.asp?id=489&tid=87#top [accessed Feb. 27, 2012].
Bonnet, P., G. Raoult, and D. Gradiski. 1979. LC50s of common aromatic hydrocarbons [in French]. Arch. Mal. Prof. 40(8-9):805-810.
Bonnet, P., G. Morele, G. Raoult, D. Zissu, and D. Gradiski. 1982. Determination of the median lethal concentration of the main aromatic hydrocarbons in the rat [in French]. Arch. Mal. Prof. 43(3):461-465.
BUA (Beratergremium für Umweltrelevante Altstoffe [Advisory Committee for Environmental Existing Chemicals of the German Chemical Society]). 1990. Chlorobenzene (CAS Reg. No. 108-90-7). BUA Substance Report 54. Weinheim, Federal Republic of Germany: VCH.
De Ceaurriz, J.C., J.C. Micillino, P. Bonnet, and J.P. Guenier. 1981. Sensory irritation caused by various industrial airborne chemicals. Toxicol. Lett. 9(2):137-143.
De Ceaurriz, J., J.P. Desiles, P. Bonnet, B. Marignac, J. Muller, and J.P. Guenier. 1983. Concentration-dependent behavioral changes in mice following short-term inhalation exposure to various industrial solvents. Toxicol. Appl. Pharmacol. 67(3):383-389.
DeJongh, J., H.J. Verhaar, and J.L. Hermens. 1998. Role of kinetics in acute lethality of nonreactive volatile organic compounds (VOCs). Toxicol. Sci. 45(1):26-32.
DGF (Deutsche Forschungsgemeinschaft). 2005. List of MAK and BAT Values, 2005. Maximum Concentrations and Biological Tolerance Values at the Workplace Report No. 41. Weinheim, Federal Republic of Germany: Wiley VCH.
Dilley, J.V. 1977. Toxic Evaluation of Inhaled Chlorobenzene (Monochlorobenzene). Stanford Research Institute, Menlo Park, CA. NTIS PB-276623.
Eastman Kodak Co. 1994. Toxicity and Health Hazard Summary of Chlorobenzene with Cover Letter Dated 04/05/94. Submitted by Eastman Kodak Co, Rochester, NY, to U.S. Environmental Protection Agency, Washington, DC. EPA Document No. 86940000289. Microfiche No. OTS0572392.
EPA (U.S. Environmental Protection Agency). 1985. Health Assessment Document for Chlorinated Benzenes. EPA 600/8-84-015F. Office of Health and Environmental Assessment, U.S. Environmental Protection Agency, Washington, DC.
EPA (U.S. Environmental Protection Agency). 1995. Chlorobenzene Fact Sheet: Support Document (CAS No. 108-90-7). EPA 749-F-95-007a. Office of Pollution Prevention and Toxics, U.S. Environmental Protection Agency, Washington, DC [online]. Available: http://www.epa.gov/chemfact/chlor-sd.pdf [accessed Feb. 27, 2012].
Feldt, E.G. 1985. Evaluation of mutagenic hazards of benzene and some of its derivates. Gig. Sanit. 7:21-23 (as cited in BUA 1990).
Frantik, E., M. Hornychova, and M. Horvath. 1994. Relative acute neurotoxicity of solvents: Isoeffective air concentrations of 48 compounds evaluated in rats and mice. Environ. Res. 66(2):173-185.
Grilli, S., G. Arfellini, A. Colacci, M. Mazzullo, and G. Prodi. 1985. In vivo and in vitro covalent binding of chlorobenzene to nucleic acids. Jpn. J. Cancer Res. 76(8):745-751.
Haworth, S., T. Lawlor, K. Mortelmans, W. Speck, and E. Zeiger. 1983. Salmonella mutagenicity test results for 250 chemicals. Environ. Mutagen. (suppl. 1):3-142.
Hayes, W.C., T.S. Gushaw, K.A. Johnson, T.R. Hanley, J.H. Ouellette, and J.A. John. 1982. Monochlorobenzene: Inhalation Teratology Study in Rats and Rabbits. Dow Chemical Company, Midland, MI (as cited in NTP 1985).
Hellman, B. 1993. NIOH and NIOSH Basis for an Occupational Health Standard: Chlorobenzene. DHHS (NIOSH) 93-102. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH. January 1993 [online]. Available: http://www.cdc.gov/niosh/docs/93-102/pdfs/93-102.pdf [accessed Feb. 29, 2012].
IPCS (International Programme on Chemical Safety). 1991. Chlorobenzenes Other than Hexachlorobenzene. Environmental Health Criteria 128. Geneva, Switzerland: Health Organization [online]. Available: http://www.inchem.org/documents/ehc/ehc/ehc128.htm [accessed Feb. 27, 2012].
Izmerov, N.F., N.M. Vasilenko, N.N. Semiletkina, and L.A. Timofiyevskaya. 1988. Chlorobenzenes (Chlorobenzene, Dichlorobenzene, Trichlorobenzene). Scientific Reviews of Soviet Literature of Toxicity and Hazards of Chemicals No. 108. Moscow: GKNT (Centre for International Projects).
John, J.A., W.C. Hayes, T.R. Hanley, Jr., K.A. Johnson, T.S. Gushow, and K.S. Rao. 1984. Inhalation teratology study on monochlorobenzene in rats and rabbits. Toxicol. Appl. Pharmacol. 76(2):365-373.
Keskinova, D. 1968. The action of dimethylcyclodiazomethane in chlorobenzene solutions on the mutation process in Actinomycetes antibioticus-400. Sov. Gen. 4:1082-1085 (as cited in NTP 1985).
Khalil, A.M., and M.M.T. Odeh. 1994. Genetic toxicology of benzene and its derivatives in rat bone marrow cell cultures. Toxicol. Environ. Chem. 45(3-4):157-166.
Knecht, U., and H.J. Woitowitz. 2000. Human toxicokinetics of inhaled monochlorobenzene: Latest experimental findings regarding re-evaluation of the biological tolerance value. Int. Arch. Occup. Environ. Health 73(8):543-554.
Krewet, E., G. Müller, and K. Norpoth. 1989. The excretion of chlorophenylmercapturic acid, chlorophenols and a guanine adduct in the urine of chlorobenzene-treated rats after phenobarbital pretreatment. Toxicology 59(1):67-79 (as cited in Arbetslivsinsinstitutet 2003).
Lawlor, T., S. Hanworth, and P. Voytek. 1979. Evaluation of the genetic activities of nine chlorinated phenols, seven chlorinated benzenes and three chlorinated hexanes [abstract]. Environ. Mutagen. 1:143(A) (as cited in NTP 1985).
Loveday. K.S., M.H. Lugo, M.A. Resnick, B.E. Anderson, and E. Zeiger. 1989. Chromasome aberration and sister chromatid exchange tests in chinese hamster ovary cells in vitro: II. Results with 20 chemicals. Environ. Mol. Mutagen. 13(1):60-94 (as cited in BUA 1990).
McGregor, D.B., A. Brown, P. Cattanach, I. Edwards, D. McBride, C. Riach. and W.J. Caspary. 1988. Responses of the L5178Y tk+/tk-mouse lymphoma cell forward mutation assay: III. 72 coded chemicals. Environ. Mol. Mutagen. 12(1):85-154 (as cited in BUA 1990).
Mihail, F. 1984. Monochlorbenzol, Untersuchung auf Hautsensibilisierende Wirkung bei Meerschweinchen. BAYER AG, Institut für Toxikologie, Bericht Nr. 13057, Wuppertal-Elberfeld 19. 11. 1984 (as cited in BUA 1990)
Mohtashamipur, E., R. Triebel, H. Straeter, and K. Norpoth. 1987. The bone marrow clastogenicity of eight halogenated benzenes in male NMRI mice. Mutagenesis 2(2):111-113 (as cited in ICPS 1991).
Monsanto Company. 1976. Litton Bionetics Mutagenicity Evaluation of Bio-75-86-CP 5535 (WGK): Monochlorobenzene. Office of Pesticides and Toxic Substances, U.S. Environmental Protection Agency, Washington, DC. TSCA Sec 8(d) Submission 8DHQ-1078-0214(1) (as cited in EPA 1985).
MSZW (Ministerie van Sociale Zaken en Werkgelegenheid). 2004. Nationale MAC-lijst 2004: Chloorbenzeen. Den Haag: SDU Uitgevers [online]. Available: http://www.lasrook.net/lasrookNL/maclijst2004.htm [accessed Feb. 27, 2012].
Nair, R.S., J.A. Barter, R.E. Schroeder, A. Knezevich, and C.R. Stack. 1987. Twogeneration reproduction study with monochlorobenzene vapor in rats. Fundam. Appl. Toxicol. 9(4):678-686.
NIOSH (National Institute for Occupational Safety and Health). 1994. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs): Chlorobenzene. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH [online]. Available: http://www.cdc.gov/niosh/idlh/108907.html [accessed Feb. 28, 2012].
NIOSH (National Institute for Occupational Safety and Health). 2011. NIOSH Pocket Guide to Chemical Hazards: Chlorobenzene. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati, OH [online]. Available: http://www.cdc.gov/niosh/npg/npgd0121.html [accessed Feb. 28, 2012].
NRC (National Research Council). 1993. Guidelines for Developing Community Emergency Exposure Levels for Hazardous Substances. Washington, DC: National Academy Press.
NRC (National Research Council). 2001. Standing Operating Procedures for Developing Acute Exposure Guideline Levels for Hazardous Chemicals. Washington, DC: National Academy Press.
NTP (National Toxicology Program). 1985. Toxicology and Carcinogenesis Studies of Chlorobenzene in F344/N Rats and B6C3F1 Mice (Gavage Studies). Technical Report No. 261. NIH 86-2517. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Toxicology Program, Research Triangle Park, NC [online]. Available: http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/tr261.pdf [accessed Feb. 27, 2012].
Ogata, M., and Y. Shimada. 1983. Differences in urinary monochlorobenzene metabolites between rats and humans. Int. Arch. Occup. Environ. Health 53(1):51-57.
Ogata, M., T. Taguchi, N. Hirota, Y. Shimada, and S. Nakae. 1991. Quantitation of urinary chlorobenzene metabolites by HPLC: Concentrations of 4-chlorocatechol and chlorophenols in urine and of chlorobenzene in biological specimens of subjects exposed to chlorobenzene. Int. Arch. Occup. Environ. Health 63(2):121-128.
Prasad, I. 1970. Mutagenic effects of the herbicide 3,4-dichloropropionanilide and its degradation products. Can. J. Microbial. 16(5):369-372.
Prasad, I., and D. Pramer. 1968. Mutagenic activity of some chloroanilines and chlorobenzenes. Genetics 20:212-213.
Rebert, C.S., R.W. Schwartz, D.J. Svendsgaard, G.T. Pryor, and W.K. Boyes. 1995. Combined effects of paired solvents on the rat's auditory system. Toxicology 105(2-3):345-354.
Reid, W.D. 1973. Mechanism of renal necrosis induced by bromobenzene or chlorobenzene. Exp. Mol. Path. 19(2):197-214.
Reid, W.D., and G. Krishna. 1973. Centrolobular hepatic necrosis related to covalent binding of metabolites of halogenated aromatic hydrocarbons. Exp. Mol. Pathol. 18(1):80-99.
Rozenbaum, N.D., R.S. Blekh, S.N. Kremneva, S.L. Ginzburg, and L.V. Pozhatiskii. 1947. Use of chlorobenzene as a solvent from the standpoint of industrial hygiene [in Russian]. Gig. Sanit. 12(1):21-24 (as cited in ATSDR 1990, BUA 1990).
Ruth, J.H. 1986. Odor thresholds and irritation levels of several chemical substances: A review. Am. Ind. Hyg. Assoc. J. 47(3):A142-A151.
Sanotsky, I.V., and L.P. Ulanova. 1975. Criteria of Safety in Assessing the Danger of Chemical Compounds [in Russian]. Moscow: Meditsina (as cited in Izmerov et al. 1988).
Shelby, M.D., G.L. Erexson, G.J. Hook, and R.R. Tice. 1993. Evaluation of a threeexposure mouse bone marrow micronucleus protocol: Results with 49 chemicals. Environ. Mol. Mutagen. 21(2):160-179.
Shimada, Y. 1988a. Studies on monochlorobenzene poisoning: Part III. Distribution of monochlorobenzene in the organs of pregnant mice and transfer to the fetus through the placenta: Comparison with trichloroethylene and 1, 1, 1-trichloroethane. Okayama Igakkai Zasshi 100(1-2):147-153.
Shimada, Y. 1988b. Studies on monochlorobenzene poisoning: Part II. Distribution of monochlorobenzene among the organs of mice. Okayama Igakkai Zasshi 100(1-2):135-146.
Shimada, T., C.A. McQueen, and G.M. Williams. 1983. Study of Effects on Cultured Liver Cells of Three Chlorinated Benzenes. Final Report. Prepared by American Health Foundation, for Chemical Manufacturer Association.
Shimizu, M., Y. Yasui, and N. Matsumoto. 1983. Structural specificity of aromatic compounds with special reference to mutagenic activity in Salmonella typhimurium: A series of chloro-or fluoro-nitrobenzene derivatives. Mutat. Res. 116(3-4):217-238.
Simmon, V.F., E.S. Riccio, and M.V. Peirce. 1979. In Vitro Microbiological Genotoxicity Assays of Chlorobenzene, m-Dichlorobenzene, o-Dichlorobenzene and p-Dichlorobenzene. Report No. EPA 560/1979 SRI/002. Contract No 68-02-2947. Menlo Park, CA: SRI International (as cited in EPA 1985 and BUA 1990).
Suberg, H. 1983. Chlorbenzol rein, Prüfung auf primär reizende/ätzen de Wirkung am Kaninchenauge. Briefbericht der Bayer AG, Institut für Toxikologie (as cited in BUA 1990) Sullivan, T.M., G.S. Born, G.P. Carlson, and W.V. Kessler. 1983. The pharmacokinetics of inhaled chlorobenzene in the rat. Toxicol. Appl. Pharmacol. 71(2):194-203.
UBTL (Utah Biomedical Test Laboratory). 1978. Utah Biomedical Test Laboratory Report on NIOSH Sponsored Inhalation Study for IDLH Values (Final Report) with Cover Letter Dated 10/22/91. Submitted by Shell Oil Company to U.S. Environmental Protection Agency, Washington, DC. EPA Document No. TSCATS 88-920000156. Microfiche No. OTS 0534605.
Vaghef, H., and B. Hellman. 1995. Demonstration of chlorobenzene-induced DNA damage in mouse lymphocytes using the single cell gel electrophoresis assay. Toxicology 96(1):19-28.
Valencia, R. 1982. Drosophila Sex Linked Recessive Lethal Test on Monochlorobenzene. Prepared by University of Wisconsin, Madison, WI. Submitted by Bioassay System Corporation, Woburn, MA, to U.S. Environmental Protection Agency, Washington, DC. EPA Document 40-8320545. Microfiche No. OTS0511274.
Verschueren, K. 1983. Pp. 350-359, 712-717 in Handbook of Environmental Data on Organic Chemicals, 2nd Ed. New York: Van Nostrand Reinhold Company (as cited in ATSDR 1990).
Williams, G.M., H. Mori, and C.A. McQueen. 1989. Structure-activity relationships in the rat hepatocyte DNA-repair test for 300 chemicals. Mutat. Res. 221(3):263-286 (as cited in BUA 1990).
APPENDIX A
DERIVATION OF AEGLS VALUES FOR CHLOROBENZENE
Derivation of AEGL-1 Values
| Key studies: | Ogata, M., and Y. Shimada. 1983. Differences in urinary monochlorobenzene metabolites between rats and humans. Int. Arch. Occup. Environ. Health 53(1):51-57. Knecht, U., and H.J. Woitowitz. 2000. Human toxicokinetics of inhaled monochlorobenzene: Latest experimental findings regarding re-evaluation of the biological tolerance value. Int. Arch. Occup. Environ. Health 73(8):543-554. |
| Toxicity end point: | Slight CNS effects (drowsiness, heavy feeling in the head, and headache) and local irritation at 60 ppm (7 h with a 1-h break after 3 h) and no effects at 10 ppm (8 h/day for 5 days). The latter concentration was used as the point of departure. |
| Time scaling: | None, because chlorobenzene concentrations in blood reach a steady-state level within 1 h. |
| Uncertainty factors: | 1 for interspecies differences 1 for intraspecies variability |
| Calculations: | |
| 10-min AEGL-1: | Set equal to 8-h AEGL-1 value of 10 ppm |
| 30-min AEGL-1: | Set equal to 8-h AEGL-1 value of 10 ppm |
| 1-h AEGL-1: | Set equal to 8-h AEGL-1 value of 10 ppm |
| 4-h AEGL-1: | Set equal to 8-h AEGL-1 value of 10 ppm |
| 8-h AEGL-1: | 10 ppm |
Derivation of AEGL-2 Values
| Key study: | UBTL (Utah Biomedical Test Laboratory). 1978. Utah Biomedical Test Laboratory Report on NIOSH Sponsored Inhalation Study for IDLH Values (Final Report) with Cover Letter Dated 102291. Submitted by Shell Oil Company to U.S. Environmental Protection Agency, Washington, |
| Values (Final Report) with Cover Letter Dated 102291. Submitted by Shell Oil Company to U.S. Environmental Protection Agency, Washington, DC. EPA Document No. TSCATS 88-920000156. Microfiche No. OTS 0534605. | |
| Toxicity end point: | Narcosis in rats and guinea pigs. |
| Time scaling: | 2,990 ppm for 30 min was extrapolated across time periods using the equation Cn × t = k, with default values of n = 1 for extrapolation to 10-min and n = 3 for extrapolation to 1-h values. The 4-and 8-h values were set equal to the 1-h value because a steady-state chlorobenzene concentration in blood is reached within 1 h and for reasons of consistency with human data. C3 × t = k: k = (2,990 ppm) 3 × 30 min = 8.02 × 1011 ppm-min C1 × t = k: k = 2,990 ppm × 30 min = 89,700 ppm-min |
| Uncertainty factors: | 3 for interspecies differences 3 for intraspecies variability |
| Calculations: | |
| 10-min AEGL-2: | [(8.02 × 1011 ppm-min) ÷ 10 min]1/3 ÷ 10 = 430 ppm (rounded) |
| 30-min AEGL-2: | 2,990 ppm ÷ 10 = 300 ppm (rounded) |
| 1-h AEGL-2: | (89,700 ppm-min ÷ 60 min) ÷ 10 = 150 ppm |
| 4-h AEGL-2: | Set equal to 1-h AEGL-2 value of 150 ppm |
| 8-h AEGL-2: | Set equal to 1-h AEGL-2 value of 150 ppm |
Derivation of AEGL-3 Values
| Key study: | UBTL (Utah Biomedical Test Laboratory). 1978. Utah Biomedical Test Laboratory Report on NIOSH Sponsored Inhalation Study for IDLH Values (Final Report) with Cover Letter Dated 102291. Submitted by Shell Oil Company to U.S. Environmental Protection Agency, Washington, |
| DC. EPA Document No. TSCATS 88-920000156. Microfiche No. OTS 0534605 | |
| Toxicity end point: | No mortality in rats or guinea pigs. |
| Time scaling: | 7,970 ppm for 30 min was extrapolated across time periods using Cn × t = k, with default values of n = 1 for extrapolation to 10-min and n = 3 for extrapolation to 1-h values. The 4-and 8-h values were set equal to the 1-h value because a steadystate chlorobenzene concentration in blood is reached in 1 h and for reasons of consistency with AEGL-2 values. C3 × t = k: k = (7,970 ppm)3 × 30 min = 1.52 × 1013 ppm-min C1 × t = k: k = 7,970 ppm × 30 min = 239,100 ppm-min |
| Uncertainty factors: | 3 for interspecies differences 3 for intraspecies variability |
| Calculations: | |
| 10-min AEGL-3: | [(1.52 × 1013 ppm-min) ÷ 10 min]1/3 ÷ 10 = 1,100 ppm (rounded) |
| 30-min AEGL-3: | 7,970 ppm ÷ 10 = 800 ppm (rounded) |
| 1-h AEGL-3: | (239,100 ppm-min ÷ 60 min) ÷ 10 = 400 ppm (rounded) |
| 4-h AEGL-3: | Set equal to 1-h AEGL-3 value of 400 ppm |
| 8-h AEGL-3: | Set equal to 1-h AEGL-3 value of 400 ppm |
APPENDIX B
CATEGORY PLOT FOR CHLOROBENZENE
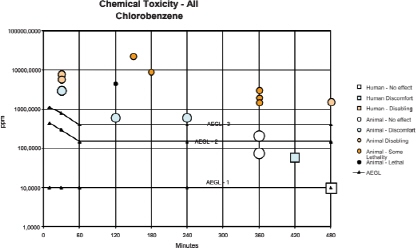
FIGURE B-1 Category plot of animal and human data and AEGL values for chlorobenzene.
APPENDIX C
ACUTE EXPOSURE GUIDELINE LEVELS FOR CHLOROBENZENE
Derivation Summary for Chlorobenzene
AEGL-1 VALUES
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) | 10 ppm (47 mg/m3) |
| Key references: | ||||
| Study 1: Ogata, M., and Y. Shimada. 1983 Differences in urinary | ||||
| monochlorobenzene metabolites between rats and humans. Int. Arch. Occup. | ||||
| Environ. Health 53(1):51-57. | ||||
| Study 2: Knecht, U., and H.J. Woitowitz. 2000. Human toxicokinetics of inhaled | ||||
| monochlorobenzene: Latest experimental findings regarding re-evaluation of the | ||||
| biological tolerance value. Int. Arch. Occup. Environ. Health 73(8):543-554. | ||||
| Test species/Strain/Number: Humans, 4 subjects (study 1), 8 subjects (study 2) | ||||
| Exposure route/Concentrations/Durations: Study 1: inhalation, 60 ppm, 7-h exposure with a 1-h break after 3 h; Study 2: inhalation, 10 ppm, 8 h/day for 5 days | ||||
| Effects: Study 1: slight CNS effects (drowsiness, heavy feeling in the head, and | ||||
| headache) and local irritation at 60 ppm; Study 2: no effects at 10 ppm. | ||||
| End point/Concentration/Rationale: No discomfort effects at 10 ppm | ||||
| Uncertainty factors/Rationale: | ||||
| Total uncertainty factor: 1 | ||||
| Interspecies: 1, data are from a human study | ||||
| Intraspecies: 1, effects at 60 ppm were very slight | ||||
| Modifying factor: None | ||||
| Animal-to-human dosimetric adjustment: Not relevant | ||||
| Time scaling: None. No information on time dependency is available in the studies, | ||||
| but the observed effects do not indicate a strong time dependency; this is also | ||||
| supported by absorption data. | ||||
| Data adequacy: The key studies were evaluations of the kinetics of chlorobenzene, | ||||
| and were not designed to determine toxicity. | ||||
| AEGL-2 VALUES | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 430 ppm (2,021 mg/m3) | 300 ppm (1,410 mg/m3) | 150 ppm (705 mg/m3) | 150 ppm (705 mg/m3) | 150 ppm (705 mg/m3) |
| Key reference: UBTL (Utah Biomedical Test Laboratory). 1978. Utah Biomedical Test Laboratory Report on NIOSH Sponsored Inhalation Study for IDLH Values | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 430 ppm (2,021 mg/m3) | 300 ppm (1,410 mg/m3) | 150 ppm (705 mg/m3) | 150 ppm (705 mg/m3) | 150 ppm (705 mg/m3) |
| (Final Report) with Cover Letter Dated 102291. U.S. EPA/OPTS Public Files OTS0534605. Submitted by Shell Oil Company to U.S. Environmental Protection Agency, Washington, DC. EPA Document No. TSCATS 88-920000156. Microfiche No. OTS 0534605. | ||||
| Test species/Strain/Number: Rats and guinea pigs, strains unknown, five per sex per species. | ||||
| Exposure route/Concentrations/Durations: Inhalation, 2,990, 5,850, or 7,970 ppm for 30 min; 14-day observation. | ||||
| Effects: 2,990 ppm: Slight ocular and nasal irritation 5,850 ppm: Narcosis and impaired ability to escape 7,970 ppm: No deaths; ataxia and narcosis |
||||
| End point/Concentration/Rationale: No narcosis at 2,990 ppm for 30 min; no animals suffered from impaired ability to escape. |
||||
| Uncertainty factors/Rationale: Total uncertainty factor: 10 (a larger uncertainty factor would lead to AEGL-2 values that conflict with human data) Interspecies: 3, data were comparable for rats and guinea pigs suggesting no large interspecies differences, and the critical effect is CNS depression. Intraspecies: 3, interindividual variability for CNS depression by comparable gases generally will not be greater than a factor of 2 or 3. |
||||
| Modifying factor: None | ||||
| Animal-to-human dosimetric adjustment: None | ||||
| Time scaling: Cn × t = k; default values of n = 3 for 10-min value and n = 1 for 60-min value. AEGL-2 values for 4 and 8 h are set equal to the 1-h value because chlorobenzene concentrations in blood reach a steady-state within 1 h and its elimination is rapid. Time scaling would result in 4-and 8-h AEGL-2 values that would conflict with human data. | ||||
| Data adequacy: Only one study (30-min exposures at three concentrations) aimed at identifying an immediately dangerous to life and health value. | ||||
| AEGL-3 VALUES | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 1,100 ppm (5,170 mg/m3) | 800 ppm (3,760 mg/m3) | 400 ppm (1,880 mg/m3) | 400 ppm (1,880 mg/m3) | 400 ppm (1,880 mg/m3) |
| Key reference: UBTL (Utah Biomedical Test Laboratory). 1978. Utah Biomedical Test Laboratory Report on NIOSH Sponsored Inhalation Study for IDLH Values | ||||
| 10 min | 30 min | 1 h | 4 h | 8 h |
| 1,100 ppm (5,170 mg/m3) | 800 ppm (3,760 mg/m3) | 400 ppm (1,880 mg/m3) | 400 ppm (1,880 mg/m3) | 400 ppm (1,880 mg/m3) |
| (Final Report) with Cover Letter Dated 102291. Submitted by Shell Oil Company to U.S. Environmental Protection Agency, Washington, DC. EPA Document No. TSCATS 88-920000156. Microfiche No. OTS 0534605 |
||||
| Test species/Strain/Number: Rats and guinea pigs, strains unknown, five per sex per species. | ||||
| Exposure route/Concentrations/Durations: Inhalation, 2,990, 5,850, or 7,970 ppm for 30 min; 14-day observation. | ||||
| Effects: 2,990 ppm: Slight ocular and nasal irritation 5,850 ppm: Narcosis and impaired ability to escape 7,970 ppm: No deaths; ataxia and narcosis |
||||
| End point/Concentration/Rationale: No deaths after 30-min exposure at 7,970 ppm. | ||||
| Uncertainty factors/Rationale: Total uncertainty factor: 10 (a larger factor would lead to AEGL-3 values that would conflict with AEGL-2 values) Interspecies: 3, data were comparable for rats and guinea pigs suggesting no large interspecies differences, and the critical effect is CNS depression. Intraspecies: 3, interindividual variability for CNS depression by comparable gases generally will not be greater than a factor of 2 or 3. |
||||
| Modifying factor: None | ||||
| Animal-to-human dosimetric adjustment: None | ||||
| Time scaling: Cn × t = k; default values of n = 3 for 10-min value and n = 1 for 1-h value. AEGL-3 values for 4-and 8-h are set equal to the 1-h value because chlorobenzene concentrations in blood reach a steady-state within 1 h and its elimination is rapid. Furthermore, time scaling would result in 4-and 8-h AEGL-3 values that would conflict with AEGL-2 values and human data. |
||||
| Data adequacy: Sufficient | ||||






































