Chapter 2
Sustaining Leadership in Innovation
The United States faces new competitive challenges in the 21st century. Globalization is diminishing what once were overwhelming American advantages as the prime location for creating, commercializing, and industrializing technology. Basic research and world-class engineering talent now are highly dispersed around the world, especially in important fields such as nanotechnology, computer science, and renewable energies. How, then, must the U.S. adapt to maintain its leadership in innovation?
IMPROVING FRAMEWORK CONDITIONS
One of America’s most fundamental strengths as a place to commercialize innovation has been its overall investment climate. For much of the post-war era, America’s boasted some of the world’s best transportation, energy, and communication infrastructure.1 In the 1980s, America’s corporate tax rates were among the lowest in the industrialized world.2 The U.S. also has had one of the world’s strongest legal systems for protecting intellectual property rights.3
______________________
1 Michael Porter observed that American communication, power transportation, and transportation infrastructure was “arguably the best in the world” after World War II, and the fact that infrastructure companies were privately owned “was a stimulus to investment and innovation.” See Michael E. Porter, The Competitive Advantage of Nations, New York: Simon and Schuster, 1990, p. 297.
2 The U.S. statutory corporate tax rate dropped from 52 percent to 35 percent in the 1980s, well below the average for OECD nations. See Congressional Budget Office, “Corporate Income Tax Rates: International Comparison,” November 2005 (http://www.cbo.gov/ftpdocs/69xx/doc6902/1128-CorporateTax.pdf). Data from M. P. Devereaux, R. Griffith, and A. Klemm, “Corporate Income Tax Reforms and International Tax Competition,” Economic Policy, vol. 35 (October 2002).
3 The United States still has the lowest rate of computer software piracy in the world, followed by Japan and Luxembourg, according to the International Data Corporation (IDC). See Business
Corporate Taxes: There are concerns that America now is at a competitive disadvantage in some of these areas.4 After the U.S. cut corporate taxes in the 1980s, other industrialized nations cut taxes even further. When state corporate taxes are taken into account, the U.S. corporate statutory rate of 39.3 percent is third highest among OECD nations, which have a median rate of 33 percent.5 What’s more, the tax codes of countries such as Germany, Singapore, Malaysia, and China favor investment in certain industries through such incentives as 10-year tax holidays. While U.S. states offer such tax breaks, the federal government does not. The U.S. is one of the few major trading nations with a tax code that does not treat investment in globally traded industrial activity any differently than non-mobile activity.6 This means “inefficiency and biases in the corporate tax code fail to promote the productivity and innovative capability of businesses in America, hampering the economy and indirectly affecting all Americans.” 7 Business advocacy groups argue that executives find the current tax burden to be an impediment to the competitiveness of their companies operating in the United States.”8
Infrastructure: Some analysts regard America’s aging infrastructure as a competitive disadvantage.9 The U.S. ranks only No. 27 in terms of infrastructure, according to the World Economic Forum, a major factor in America’s falling place in the WEF’s overall global competitiveness rankings.10 That compares to seventh place in 2000, observes the McKinsey Global Institute.11 The American Society of Civil Engineers asserts that most of America’s infrastructure is in poor shape due to delayed maintenance and lack
______________________
Software Alliance and IDC, 08 Piracy Study, May 2009, (http://portal.bsa.org/globalpiracy2008/studies/globalpiracy2008.pdf).
4 It is important to note that the Committee did not conduct a study comparing the U.S. tax system to that of other countries. The Committee did want to draw attention to the growing body of evidence that, in some cases, U.S. tax policy creates a less competitive environment.
5 Congressional Budget Office, op. cit., citing data from Devereaux, Griffith, and Klemm.
6 Robert D. Atkinson, “Effective Corporate Tax Reform in the Global Innovation Economy,” The Information Technology & Innovation Foundation, July 2009, (http://www.itif.org/files/090723_CorpTax.pdf)
7 Ibid.
8 Roth, et al, “2010 Global Manufacturing Competitiveness Survey,” Deloitte Touche Tohmatsu and U.S. Council on Competitiveness, June 2010.
9 For an analysis of the positive link between good infrastructure and innovation and development, see Tony Ridley, Lee Yee-Cheong, Calestous Juma. “Infrastructure, Innovation, and Development,” International Journal of Technology and Globalisation, Volume 2, Number 3-4/2006, Pages 268278. For an industry view, see the interview with Eric Spiegel, the president and CEO of Siemens Corporation in Harvard Business Review, “Investing in Infrastructure Means Investing in Innovation.” March 15, 2012.
10 World Economic Forum, Global Competitiveness Report, op. cit.
11 James Manyika, et al., Growth and Renewal in the United States: Retooling America’s Economic Engine, McKinsey Global Institute, February 2011, (http://www.mckinsey.com/mgi/publications/growth_and_renewal_in_the_us/pdfs/MGI_growth_and_renewal_in_the_us_full_report.pdf).
of modernization.12 The Society reports that an estimated 25 percent of America’s bridges need significant repairs, one-third of major roadways are in substandard condition, and that “America’s sewer systems spill an estimated 1.26 trillion gallons of untreated sewage every year.”13 More recently the Society called for investments in the nation’s transmission, generation, and distribution systems in order to prevent significant costs to businesses and households.14
Likewise, a bipartisan study of America’s aging transportation infrastructure concluded that it is in “bad shape.” The poor condition “compromises our productivity and ability to compete internationally,” it added. The study estimated the U.S. needs to spend $134 billion to $262 billion per year more than current plans call for until 2035 to get this infrastructure into proper condition.15
Other nations are investing aggressively to build and upgrade their transportation infrastructure. China spent $713 billion—twice as much as the U.S.—just on transportation and water infrastructure over the past five years16 and is investing an estimated $500 to 700 billion to build the world’s biggest high-speed rail network.17 In 2008, the European Investment Bank lent 58 billion Euros ($81 billion) to finance infrastructure projects, and had a target of $112 billion in 2009.
______________________
12 ASCE has assigned a C grade to bridges, C- to rail, D+ for energy, D for aviation, dams, transit, dams, and D- to drinking water. See American Society of Civil Engineers, 2009 Report Card for America’s Infrastructure, March 25, 2009, (http://www.infrastructurereportcard.org/sites/default/files/RC2009_full_report.pdf).
13 Data from U.S. federal agencies cited in Eric Kelderman, “Look Out Below! American’s Infrastructure is Crumbling,” Stateline.org, Pew Research Center, January 22, 2008, (http://pewresearch.org/pubs/699/look-out-below).
14 ASCE, Failure to Act: The Economic Impact of Current Investment Trends in Electricity Infrastructure. April, 2012.
15 See Miller Center of Public Affairs, Well Within Reach: America’s New Transportation Agenda, David R. Goode National Transportation Policy Conference. Posted on October 4, 2010 at http://www.infrastructureusa.org/well-within-reach/.
16 Cathy Yan, “Road-Building Rage to Leave U.S. in Dust,” Wall Street Journal, January 18 2011.
17 See Sean Tierney, “High-speed rail, the knowledge economy, and the next growth wave,” Journal of Transport Geography, Volume 22, May 2012, pages 285-287. Tierney notes that failure to invest in economic development “concedes considerable ground to those countries with whom we are trying to compete. Compare the $8 billion that President Obama set aside in the stimulus bill as a down payment for HSR [High Speed Rail], with the estimated $500 - $700 billion that China plans to invest for its 19,000 km HSR network.” For a review of the economic benefits of large scale transportation projects, see T.R. Lakshmanan, “The broader economic consequences of transport infrastructure investments.” Journal of Transport Geography. Volume 19(1), 2011. For a review of recent China’s investments in rail, Will Freeman, “The Big Engine That Can: China’s High-Speed Rail Project,” China Insight Economics, May 28, 2010. Problems have emerged with regard to the rapid construction of China’s rail network, its cost, the revenues it is generating, and its relevance to the needs of the general population. Recent train disasters in China have further spotlighted challenges related to the rapid growth of that nation’s high-speed rail system. See Financial Times, “China’s Rail Disaster.” July 27, 2011 and Keith B. Richburg, “Are China’s High-Speed Trains Heading Off the Rails?” Washington Post, April 23, 2011.
To address this competitive disadvantage in infrastructure, some analysts have called for a U.S. infrastructure bank that, like the EIB, could leverage private capital.18 The purpose of such a National Infrastructure Bank (NIB) would be to invest in merit-based projects of national significance that span both traditional and technological infrastructure by leveraging private capital. Phillips, Tyson and Wolf argue that “the NIB could attract private funds to co-invest in projects that pass rigorous cost-benefit tests, and that generate revenues through user fees or revenue guarantees from state and local governments. Investors could choose which projects meet their investment criteria, and, in return, share in project risks that today fall solely on taxpayers.”19
Energy Efficiency: Reliable, clean, and relatively inexpensive energy has long been an important competitive advantage for the United States. As a recent UNIDO report notes, “Energy efficiency contributes toward reducing overall company expenses, increases productivity, has effects on competitiveness and the trade balance on an economy-wide level, and, by creating a home market for energy efficient technologies, supports the development of successful technology supply industry in that field.”20 Energy efficiency also represents a major opportunity to increase energy security while also limiting carbon dioxide emissions.
An accelerated deployment of existing and emerging energy-supply and end-use technologies has the potential to yield substantial improvements to energy conservation and efficiency.21 America’s buildings, which alone use more energy than any other entire economy of the world except China, are a key area for conservation efforts.22 U.S. buildings are generally grossly inefficient; it has been widely documented that energy use in new and existing buildings can be cut by 50% or more cost-effectively. 23 Lowering the cost base for location of
______________________
18 Felix Rohatyn, The Case for an Infrastructure Bank, Wall Street Journal, September 15, 2010. In the U.S. Senate, legislation, known as the “BUILD Act, was introduced on May 15, 2011 to fund an infrastructure bank.
19 See Charles Phillips, Laura Tyson, and Robert Wolf, “The U.S. Needs an Infrastructure Bank,” Wall Street Journal, January 15, 2010.
20 Wolfgang Eichhammer and Rainer Walz, “Industrial Energy Efficiency and Competitiveness,” Vienna: United Nations Industrial Development Organization, 2011.
21 See National Academy of Sciences, et al., America’s Energy Future, Technology and Transformation, Washington, DC: The National Academies Press, 2009. The report notes that “The deployment of existing energy efficiency technologies is the nearest-term and lowest-cost option for moderating our nation’s demand for energy, especially over the next decade. The committee judges that the potential energy savings available from the accelerated deployment of existing energyefficiency technologies in the buildings, transportation, and industrial sectors could more than offset the Energy Information Administration’s projected increases in U.S. energy consumption through 2030.”
22 U.S. Green Building Council, “Buildings and Climate Change,” Accessed on November 3, 2011 at http://www.documents.dgs.ca.gov/dgs/pio/facts/LA%20workshop/climate.pdf.
23 Greg Kats, Greening Our Built World, Costs, Benefits, and Strategies, Washington, DC: Island Press, 2010.
production in the United States can be fostered by improving conservation, and the techniques learned are themselves marketable globally as innovative services.
Broadband: The U.S. is regarded as lagging in broadband infrastructure. In the U.S., 27 of every 100 households subscribe to high-speed Internet service. In Germany, broadband penetration is at 30 percent. The rate is 31 percent in France, 34 percent in South Korea, 38 percent in Denmark, and 41 percent in Sweden.24 While recognizing that a number of these countries do not have the same geographical spread as the United States, the McKinsey Global Institute nonetheless estimates that the U.S. loses $450 billion in purchasing power annually due to subpar Internet connections.25
Intellectual Property: The U.S. still has one of the best legal systems in the world to protect intellectual property rights. This has made America a leader in IP-intensive industries such as pharmaceuticals, software, and entertainment.26 NDP Consulting estimates that workers in IP-intensive industries generate more than twice the output and sales per employee than do workers in non-IP-based industries. IP-intensive industries also account for around 60 percent of U.S. exports.27
Counterfeiting and patent infringement abroad undermine the economic contribution of these industries, however. An estimated 80 percent of software used in China is pirated, IDC estimates. The piracy rate stands at 61 percent in the entire Asia-Pacific region, 65 percent in Latin America, and 66 percent in Central and Eastern Europe, compared to 21 percent in North America.28 This level of piracy has a substantial effect on U.S. companies’ revenues, and therefore their long-term capacity to innovate and compete.
SUBSTANTIALLY INCREASING R&D FUNDING
As mentioned above, the United States still enjoys a clear lead over other nations in total R&D spending. [See Figure 2.1] But as also noted earlier,
______________________
24 International Telecommunication Union and Federal Communications Commission data cited in Manyika, op. cit.
25 Ibid.
26 In many fields intellectual property protection plays only a small role in enabling firms to reap returns from their innovations. And in some fields it would appear that for the industry as a whole aggressive patenting is a negative sum game. For a survey of the economic literature, both theoretical and empirical, on the choice of intellectual property protection by firms, see Bronwyn H. Hall, Christian Helmers, Mark Rogers, and Vania Sena, “The Choice between Formal and Informal Intellectual Property: A Literature Review,” NBER Working Paper No. 17983, April 2012.
27 See Nam d. Pham, “The Impact of Innovation and the Role of Intellectual Property Rights on U.S. Productivity, Competitiveness, Jobs, Wages, and Exports,” NDP Consulting, April 2010 (http://www.theglobalipcenter.com/sites/default/files/reports/documents/IP_Jobs_Study_Exec_Summary.pdf).
28 Business Software Alliance and IDC, 08 Piracy Study, May 2009, (http://portal.bsa.org/globalpiracy2008/studies/globalpiracy2008.pdf).
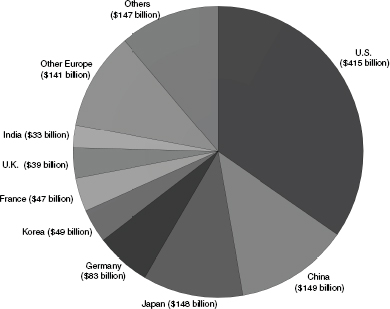
FIGURE 2.1 Total global R&D spending reached $1,252 billion in 2010. SOURCE: Battelle and R&D Magazine, 2012 Global R&D Funding Forecast, December 2011.
this lead is eroding as other nations dramatically increase their investments in research—both in real terms and as a percentage of GDP.
The most dramatic gains are being made by China. R&D spending as a percentage of GDP rose from only 0.6 percent in 1996 to 1.7 percent in 2009—a period during which China’s economy grew by an astounding 12 percent a year.29 Between 2002 and 2007, the percentage of the world’s researchers living in China rose from 13.9 percent to 19.7 percent.30 Since then, China has continued to increase R&D investment by around 10 percent a year, even during the global recession. China’s long-term plans call for boosting R&D to 2.5 percent of GDP by 2020.31 The government also has set an ambitious target of
______________________
29 National Science Foundation Science and Engineering Indicators: 2010 and Ministry of Science and Technology of the People’s Republic of China, China S&T Statistics Data Book 2010, Figure 11.
30 UNESCO Science Report 2010, Paris: United Nations Educational, Scientific and Cultural Organization. Access at http://unesdoc.unesco.org/images/0018/001899/189958e.pdf.
31 China State Council, “National Medium- and Long-Term Program for Science and Technology,” op. cit.
Box 2.1
The European Union’s Growing Investments in Research and Innovation
Complementing the rising R&D expenditures of its member states, the European Union is dramatically increasing its investments in research and innovation. The new Horizon 2020 program, which succeeds the Seventh Framework Program, will invest 80 billion Euros over seven years, beginning in 2013, an increase of some 45 percent. This includes a dedicated budget of € 25 billion to strengthen the EU’s position in science; € 18 billion to strengthen Europe’s industrial leadership in innovation including greater access to capital and support for SMEs; and € 32 billion to help address global challenges such as climate change, renewable energy, and health care.32
According to the European Commissioner for Research, Innovation, and Science Máire Geoghegan-Quinn, the goal of the Horizon 2020 program is designed to transform Europe’s “world-class science base into a world-beating one.”33
producing 2 million patents of inventions, utility models, and designs annually by 2015.34
Investment in R&D has risen sharply in other nations as well. Japanese spending on research and development surged from 2.9 percent of GDP in 1995 to 3.6 percent in 2009.35 India doubled national R&D spending between 2002 and 2008, to Rupees 378 billion ($8.7 billion) annually36, and plans another 220 percent increase by 2012.37 South Korea has boosted R&D spending by an average of 10 percent annually from 1996 to 2007,38 and reportedly plans to increase the R&D-to-GDP ratio from an already-high 3.2 percent to 5 percent by 2012.39 Brazil nearly tripled R&D expenditure between 2000 and 2008, to $24.4 billion.40 Finland has boosted R&D spending from 2 percent of GDP in 1991 to
______________________
32 Access at http://ec.europa.eu/research/horizon2020/index_en.cfm?pg=h2020.
33 Neil McDonald, “Euro Commissioner visits US,” Federal Technology Watch, 10(4) January 23, 2012.
34 China State Intellectual Property Office, “National Patent Development Strategy (2011-2020).”
35 Japanese Ministry of Internal Affairs and Communications, Statistics Bureau, accessed at http://www.stat.go.jp/english/data/kagaku/index.htm. Data refer to fiscal years.
36 UNESCO, UNESCO Science Report 2010, p. 371.
37 Government of India Planning Commission, “Report of the Steering Committee on Science and Technology for Eleventh Five-Year Plan (2007-2012),” December 2006.
38 Battelle, op. cit.
39 Kim Tong-hyung, “5% of GDP Set Aside for Science Research,” Korea Times, December 12, 2009.
40 Brazil Innovation Secretary Francelino Grando, “Brazil’s New Innovation System,” National Academies symposium, Clustering for 21st Century Prosperity, Washington, DC, February 25, 2010.
3.9 percent in 2010, one of the highest levels in the world.41 In 2006, the Singapore government tripled its five-year R&D budget and set a target of pushing national spending to 3.5 percent of GDP by 2015.42
In the United States the growth in pubic R&D funding has been more uneven. Public research spending received an $18.7 billion temporary boost under the 2009 American Recovery and Re-investment Act of 2009. Congress approved significant long-term increases to non-defense R&D investment when it passed the America COMPETES Act, which pledges to double the research budget of the NSF, the DOE’s Office of Science, and NIST over seven years. However, the COMPETES Act has not yet been funded by Congress and its prospects are uncertain in the current budgetary environment.
Federal commitments to higher research spending have been flat or falling. Overall federal funding for R&D in the United States has not increased significantly since 2004, 43 and the full-year continuing resolution passed by Congress for fiscal year 2011 cut R&D spending by 3.5 percent to $144.4 billion. Under the resolution, the NIH budget was reduced by 1.1 percent, the DOE’s energy programs by 14.6 percent, the Office of Science by 1.6 percent, the NSF by 1.3 percent, and NIST by 2.5 percent.44 The Obama Administration proposed a substantial 7.3 percent increase in non-defense R&D spending for fiscal year 2011-2012. Federal support for basic and applied research, in fact, would reach its highest level in history under the proposed budget. Under the President’s plan, the NSF, NIST, and DOE would see especially large percentage increases. 45 However, fiscal challenges, precipitated by concerns about the rapid growth in the federal debt, leave the prospect of rising budgets for research and development uncertain.
These developments come at a time when federal spending on R&D as a share of GDP has been in long-term decline.46 This decline has been masked by rising private-sector R&D spending, which has maintained total U.S. R&D spending as a percentage of GDP at a roughly constant level over the past few decades. [See Figure 2.2] The increased business R&D intensity has enabled
______________________
41 Statistics Finland, Science and Technology Statistics accessed at http://www.research.fi/en/resources/R_D_expenditure/R_D_expenditure_table and Statistics Finland, “R&D Expenditure in the Higher Education Sector Up by 11 Per Cent,” October 27, 2011.
42See Ministry of Trade and Industry, Sustaining Innovation-Driven Growth, Science and Technology, Government of Singapore, February 2006.
43 Patrick J. Clemens, “Historical Trends in Federal R&D,” in AAAS Report XXXVI: Research and Development FY 2012, Intersociety Working Group, American Association for the Advancement of Science, May 2011.
44 See analysis by American Association for the Advancement of Sciences, “R&D in the FY 2011 year-Long Continuing Resolution,” May 2, 2011.
45 AAAS Report XXXVI, op. cit.
46 Ben Bernanke, “Promoting Research and Development: The Government’s Role.” Issues in S&T, Volume XXVII (4) Summer 2011.
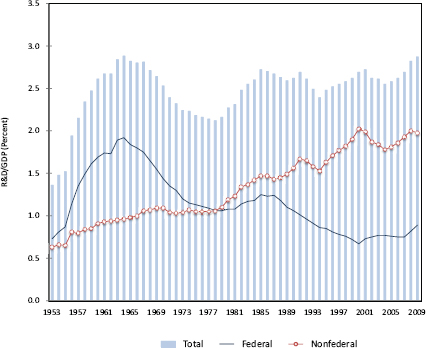
FIGURE 2.2 Federal funding for R&D as a share of GDP has been in long-term decline.
SOURCE: National Center for Science and Engineering Statistics, U.S. R&D Spending Suffered a Rare Decline in 2009 but Outpaced the Overall Economy, NSF 12-310 (March 2012), Figure 4.
total U.S. R&D spending to grow by 3.1 percent in constant dollars over the past 20 years.47
The private sector, however, spends nearly three-fourths of its R&D budget on applied R&D activities. [See Figure 2.3] The federal share, with its greater focus on basic R&D, has fallen steadily since the mid 1980s and now is about 0.7 percent of GDP —its lowest level since World War II.48
______________________
48 National Science Foundation Science and Engineering Indicators, 2010.
47 National Science Foundation, Science and Engineering Indicators: 2010, Chapter 4.
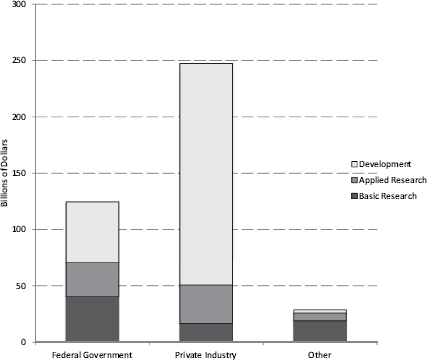
FIGURE 2.3 U.S. R&D spending by source of funding and character of expenditure, 2009.
SOURCE: National Science Foundation, National Center for Science and Engineering Statistics, Science and Engineering Indicators 2012, NSB 12-01 (January 2012), Appendix Tables 4-8, 4-9 and 4-10.
While the overall growth in total absolute R&D spending is good news, the downward trend in federal spending as a percent of GDP is less propitious for it is investments in basic research that generate the discoveries that lie behind future innovation. The burden of funding basic research is increasingly falling upon the federal government as U.S. corporations focus more of their R&D dollars on later-stage development.
The share of federal R&D that is targeted to basic research has also declined. The Department of Defense—which accounted for more than 52 percent of the federal research budget in 2011—invests around 90 percent of its R&D funds on weapons systems development, rather than on basic or applied research. [See Figure 1.4]
This does not mean the federal government can cut back on applied research. It does mean that the United States is spending a great deal less on
early stage research than the official figures might suggest. It also means that much of the U.S. R&D effort is for later-stage military purposes with limited civil applications. The R&D spending of U.S. competitors tends to be the reverse, with heavier emphasis on later-stage R&D for commercial applications. As explained below, a greater emphasis on civilian applied research will be needed in order to compete with other nations that invest more to turn new technology into products and industry, keeping in mind that many of these products eventually have military applications.
These trends in R&D spending are not, of course, entirely uniform. Not all nations are meeting their research investment targets. In 2000, for example, the European Union set a target of 3 percent of GDP by 2010 for its members. But collectively the EU remains at 1.9 percent.49 (There are notable exceptions: Germany and France are both significantly increasing their R&D budgets.50) In addition to the recent recession and financial crises, Battelle attributes the shortfall in part to high labor costs, which equal 70 percent of total R&D spending in Europe compared to 45 percent in the U.S. and 30 percent in non-Japan Asia.51 Despite strong growth since 2002, R&D spending in Brazil remains below 1 percent of GDP, although this is counterbalanced by a substantial investment in FINEP, the Brazilian Technology Agency. FINEP has a $2.5 billion budget and focuses on applied research.52
While governments have increased research funding, some are having a difficult time getting the private sector to do the same. Chinese industry accounts for just 21 percent of the nation’s R&D spending, and the vast majority of enterprises do not conduct continuous R&D.53 In Canada, business spending on R&D has remained at only around 1 percent of GDP—compared to 1.6 percent for average OECD countries54—and fell in 2010 for the third year.55 Singapore also has struggled to increase spending on innovation by private
______________________
49 Börje Johansson, Charlie Karlsson, Mikaela Backman and Pia Juusola, “The Lisbon Agenda from 2000 to 2010,” CESIS Working Paper No., 106, December 2007.
50 Chancellor Merkel’s government in Germany has proposed increasing R&D expenditures to 3 percent of GDP, up from 2.5 percent. See also remarks regarding European R&D targets by the European Commissioner for Research, Innovation, and Science Máire Geoghegan-Quinn, “Innovation for stronger regions: opportunities in FP7 Committee of the Regions” Brussels, July 14, 2011.
51 Battelle and R&D Magazine, 2011 Global R&D Funding Forecast, December 2010.
52 Xinhua, “Financing agency boosts Brazil’s innovation, productivity,” March 6, 2011.
53 See Chunlin Zhang, Douglas Zhihua Zeng, William Peter Mako, and James Seward, Promoting Enterprise-Led Innovation in China, Washington, DC: The International Bank for Reconstruction and Development/The World Bank, 2009.
54 Science, Technology, and Innovation Council, State of the Nation 2008. Ottawa: CSTI Secretariat, 2008.
55 The Daily, “Spending on Research and Development,” Statistics Canada, December 24, 2010. Access at: http://www.statcan.gc.ca/daily-quotidien/101224/dq101224a-eng.htm.
domestic companies.56 In the United States, by contrast, industry’s share of R&D funding has risen steadily and is expected to reach 64 percent in 2012.57 Industrial spending on R&D is forecast to account for all of the increase in U.S. R&D spending from 2011 to 2012.58
INSTITUTIONAL SUPPORT FOR APPLIED RESEARCH
One feature of several successful exporting nations and regions is strong public support for programs that help industries convert new technologies into manufacturing processes and products. In the United States, such collaboration on applied research typically occurs at universities that receive part of their funding from industry. Several other countries and regions have large national institutions employing thousands of scientists and engineers devoted to applied research. In such nations and regions, big public-private research institutes play a vital role in developing globally competitive industries: These institutions can effectively disseminate new technologies to a variety of domestic manufacturers. Small companies can often benefit from the lower cost through shared use of R&D personnel and equipment required to develop proofs-of-concept and to hone the manufacturing processes required for scale production.
As we see below, leading examples of institutions that support applied research include Germany’s Fraunhofer, Taiwan’s ITRI, and South Korea’s ETRI.
Germany’s Fraunhofer Gesellschaft is a network of institutes that offer some of the world’s most successful applied-research programs.59 Fraunhofer employs 4,000 Ph.D. and master’s students and has a $2.2 billion annual budget. It essentially is a contract research organization, but Germany’s federal government supplies a third of its budget. Another third is funded by the Länder, or state, governments. Private companies account for the final third. Fraunhofer operates 59 well-staffed Institutes of Applied Research across the country working closely with German manufacturers in 16 different innovation clusters. Fraunhofer Executive Director Roland Schindler described the organization as a “technology bridge,” helping industry partners develop production processes,
______________________
56 For example, see Richard W. Carney and Loh Yi Zheng, “Institutional (Dis)Incentives to Innovate: An Explanation for Singapore’s Innovation Gap,” Journal of East Asia Studies 9 (2): 291-319.
57 Battelle, op. cit.
58 Ibid.
59 For a case study of the Fraunhofer Gesellschaft, see the annex to Chapter 5 of this volume.
materials, and product designs. Fraunhofer also contributes global market research and helps promote German products abroad.60
Taiwan’s government-owned Industrial Technology Research Institute (ITRI) is one of the foremost institutes of applied industrial research in the world. Half of its $600 million annual operating budget is provided by the government and half is derived from the private sector in the form of licensing fees and payments for contract R&D. It has a staff of 5,728 personnel, of which 1,163 hold PhD’s and 3,152 Master’s degrees. ITRI functions as a technology intermediary between the domestic and international research community, on the one hand, and Taiwanese Industry, on the other hand. It is “arguably the most capable institution of its kind in the world in scanning the global technological horizon for developments of interest in Taiwanese industry, and executing the steps required to import the technology—either under license or joint development…and then absorbing and adopting the technology for Taiwanese firms to use”61. Technology is transferred to Taiwanese industry through licensing arrangements, demonstration of process technologies on internal pilot manufacturing lines, incubation of start-ups spun off from ITRI labs, and the migration of ITRI personnel to Taiwanese companies. ITRI spinoffs were the genesis of Taiwan’s semiconductor industry, a process which has been repeated in personal computers, lighting, displays, and photovoltaics62. ITRI fosters not only the start-up of companies to manufacture new products, but of complete industry chains, including design, materials, process technology development, equipment, packaging, testing, and applications.63
In South Korea, the government-funded Electronics and Telecommunications Research Institute (ETRI) plays a similar role. With
______________________
60 Presentation by Roland Schindler at the National Academies Symposium on ”Meeting Global Challenges: US-German Innovation Policy” November 11, 2010.
61 John A. Matthews and Dong-Sung Cho, Tiger Technology: The Creation of a Semiconductor Industry in East Asia, Cambridge: Cambridge University Press, 2000.
62 Sridhar Kota, “Technology Development and Manufacturing Competitiveness,” Presentation to NIST, Extreme Manufacturing workshop, January 11, 2011. Chun-yen Chang, who founded Taiwan’s first semiconductor research center at National Chiao Tung University, observed in a 2011 oral history interview that “[Y]ou can see that all the Taiwan high tech industry was originally from…the success of the semiconductor industries in Taiwan. We spun off [from the semiconductors] to LCD displays and then to the computer business. “Interview with Chun-yen Chang, Taiwanese IT Pioneers: Chun-yen Chang,” recorded February 16, 2011 (Computer History Museum, 2011), p. 11.
63 Presentation by ITRI Display Technology Center Director John Chen, Hsinchu, Taiwan (February 14, 2012).
roughly 1,700 researchers with doctoral and master’s degrees, ETRI is South Korea’s largest research institute. ETRI was central to the development of the Korean semiconductor industry, participating in the industry-government research consortia that developed Korea’s 256 megabit and 1 gigabit dynamic random access memories64. ETRI currently is number one in the world among public research organizations in terms of patents generated, with second place going to the University of California and third to MIT.65 ETRI laboratories now specialize in fields such as information technology convergence, new materials, next generation semiconductors, and new broadcast and telecom technologies.66 In the emerging field of flexible electronics, in which Korea is becoming a major player, ETRI is developing flexible memristor memory technology, utilizing graphenes, which are highly-conductive carbon nanoparticles seen as having a vast range of potential applications in electronics.67
U.S. Applied Engineering Programs
Federal applied R&D is fragmented among many agencies. A 2010 survey by MIT found that direct manufacturing R&D spending by the federal government, totaling over $700 million, is spread across four agencies. This number has risen significantly with new DARPA and DOE programs in 2011.68
The Manufacturing Extension Program of the U.S. Commerce Department, which helps small businesses apply new techniques and technologies, has a modest $125 million annual budget spread among 66 centers across the country, supported on a matching basis by the states as well as through fees.69
The National Science Foundation supports a network of more than 60 Industry/University Cooperative Research Centers specializing in fields such as advanced electronics, materials, and manufacturing, including a photovoltaic consortium involving four universities, several national laboratories, and 15 industry partners.70 NIST supports programs such as the National
______________________
64 “Taedok to Become Mecca for Venture Firms,” Chonja Sinmun (April 10, 1998).
65 “Korea’s ETRI: World Top Agency in Patents,” Korea Times (April 4, 2012).
66 Electronics and Telecommunications Research Institute, accessed at http://www.etri.se.kr/eng/.
67 “Flexible Graphene Memristors,” Printed Electronics World (December 9, 2010).
68 MIT Washington Office, Survey of Federal Manufacturing Efforts, September 2010. Access at http://web.mit.edu/dc/policy/MIT%20Survey%20of%20Federal%20Manufacturing%20Efforts.pdf.
69 For a comparative assessment of the MEP partnership, see Philip Shapira, Jan Youtie, and Luciano Kay. “Building Capabilities for Innovation in SMEs: A Cross-Country Comparison of Technology Extension Policies and Programs" International Journal of Innovation and Regional Development, 3-4 (2011): 254-272. See also Philip Shapira, “US manufacturing extension partnerships: technology policy reinvented?” Research Policy, Volume 30, Issue 6, June 2001, Pages 977–992.
70 Thomas Peterson, “The NSF Model: The Silicon Solar Consortium.” In National Research Council, The Future of Photovoltaic Manufacturing in the United States, C. Wessner, ed., Washington, DC: The National Academies Press, 2011.
Nanoelectronics Initiative71with a set of four research centers around the country72 in which 35 universities, companies such as IBM and Texas Instruments, and government agencies are striving to develop semiconductor technologies that eventually will replace CMOS as the core technology in most integrated circuits.73
National laboratories also are playing a growing role in helping industry turn technology into products. The National Renewable Energy Laboratory in Boulder, Colo., is one of the few national laboratories where commercializing technology is a top mission. Since it was founded in the 1970s, NREL has helped a number of U.S. businesses pioneer new technologies in solar power, wind energy, and bio-fuels, although its budget has fluctuated widely. Some of America’s largest applied technology programs are run by the military. The U.S. Army Tank Automotive Research, Development and Engineering Center (TARDEC), for example, collaborates extensively with private industry to apply advanced technologies in vehicles it develops.74 TARDEC’s mission, however, is to apply technologies for military needs, not commercial industries.
The U.S. government has recently launched several initiatives to boost federal support for programs aimed at translating new technology into commercial products. The DOE’s Advanced Technology Vehicle Manufacturing program, for example, provides $25 billion in direct loans to automobile and component manufacturers to fund projects aimed at improving fuel-efficiency and reducing dependence on petroleum,75 $2.4 billion of which is being used to develop advanced batteries and electrified vehicles. The Obama Administration’s 2013 Fiscal Year budget request called for $500
______________________
71 For the latest assessment of this initiative, see the President’s Council of Advisors on Science and Technology, “Report to the President And Congress on the Fourth Assessment of the National Nanotechnology Initiative,” Washington, DC: The White House, April 2012. See also Semiconductor Industry Association, “Nanoelectronics Research Initiative: A Model Government-Industry Partnership Promoting Basic Research.” Access at http://www.siaonline.org/clientuploads/One%20Pagers/Nanoelectronics_SRC_FINAL.pdf.
72 The four institutes are the South West Academy of Nanoelectronics (SWAN), headquartered at the Microelectronics Research Center at The University of Texas at Austin; The Western Institute of Nanoelectronics (WIN) in California, headquartered at the UCLA Henry Samueli School of Engineering and Applied Science; The Institute for Nanolectronics Discovery and Exploration (INDEX) in Albany, NY, headquartered at the College of Nanoscale Science and Engineering of the University at Albany; and The Midwest Institute for Nanoelectronics Discovery (MIND), led by the University of Notre Dame and includes Pennsylvania State University, Purdue University, and University of Texas-Dallas.
73 CMOS, patented by Frank Wanlass in 1967, stands for complementary metal-oxide semiconductor. CMOS is a technology for constructing integrated circuits that is used in devices such as microprocessors, static random-access memories, and image sensors.
74 See presentations by Grace Bochenek and Sonya Zanardelli of the U.S. Army Tank and Automotive Research, Development, and Engineering Center at the National Research Council conference on Building the U.S. Battery Industry for Electric-Drive Vehicles: Progress, Challenges, and Opportunities, Livonia, Michigan, July 26, 2010.
75 The Advanced Technology Vehicles Technology Loan Program was authorized under section 136 of the energy Independence and Security Act of 2007 (P. L. 110-140).
million for the DOE to aid advanced manufacturing in flexible electronics and lightweight vehicles, $200 million to DARPA for advanced manufacturing research, and increases for NSF programs relating to cyber physical systems, robotics, and advanced manufacturing.76
Another new U.S. government initiative is aimed at boosting federal assistance to development of commercial drugs. The National Institutes of Health announced Dec. 7, 2010, it would create the National Center for Advancing Translational Sciences (NCATS) by reallocating $700 million from other programs. The aim is to accelerate the pace of new drug development being brought to market by the pharmaceutical industry. 77 However, this reallocation has not taken place; instead other programs, such as Therapeutics for Rare and Neglected Diseases (TRND), have been merged and now continue under the NCATS title. Should this trend continue, it would mean that a lower program level will be available for new translational drug R&D than initially announced.
Several state governments have begun to invest in public-private applied research institutes aimed at stimulating local manufacturing industries. One of the biggest is the Albany NanoTech Complex at SUNY Albany. The complex was launched by the state government in cooperation with corporations such as IBM, Applied Materials, and Tokyo Electron. It includes one of the world’s most advanced 300 mm research fabrication plants devoted to developing prototypes of semiconductors. The complex has generated $5 billion in private investment, has 250 corporate partners, and houses 2,500 researchers, students, faculty, and staff.78 SUNY Albany’s College of Nanoscale Science and Engineering also runs a $50 million prototyping facility for micro-electromechanical systems (MEMs) and optoelectronics devices in Canandaigua, N. Y. The goal is to accelerate development of commercial devices that will be manufactured in the region.79 Other public-private programs for assisting manufacturing at the state level include the Florida Center for Advanced Aero-Propulsion and the Laboratory for Surface Science and Technology at the University of Maine and the Ohio’s Edison Technology
______________________
76 Sridhar Kota, “Opening Remarks” at the National Research Council conference on Building the U.S. Battery Industry for Electric-Drive Vehicles: Progress, Challenges, and Opportunities, Livonia, Michigan, July 26, 2010.
77 See Gardiner Harris, “Federal Research Center Will Help Develop Medicines,” New York Times, January 22, 2011.
78 Source: College of Nanoscale Science and Engineering at the University of the University of New York at Albany (SUNY-Albany). Also Pradeep Haldar “New York’s Nano Initiative,” in National Research Council, Growing Innovation Clusters for American Prosperity, C. Wessner, ed., Washington, DC: The National Academies Press, 2011.
79 College of Nanoscale Science & Engineering press release, October 23, 2010.
Centers, which includes the Northeast Ohio Manufacturing Advocacy and Growth Network (MAGNET).80
Reflecting what they see as an institutional gap in the U.S. innovation system, Germany’s Fraunhofer institutes are helping fill what they see as a gap in the U.S. innovation system by opening a number of U.S. applied technology institutes, often in collaboration with U.S. industries. Fraunhofer USA opened a non-profit state-of-the-art center to develop prototypes for laser components and systems in Plymouth, Mich., for example, and a center in Brookline, Mass., for manufacturing innovation. Other Fraunhofer centers in the U.S. focus on products such as advanced coatings, clean-energy devices, software, and molecular biotechnology applications.81
Lessons and Calls for New U.S. Institutions
The decades-old experience of organizations such as Fraunhofer, ITRI, and ETRI suggest that applied research programs run most effectively with significant, reliable, and steady financial commitment from both the government and the private sector to develop new technological options and sustain new or existing industries. Such programs also require the flexibility to adjust to new technology trends and to capture new commercial opportunities. At the same time, much of the focus of these institutions is on incremental improvements to existing industries and firms to enable them to remain globally competitive.
Some experts recommend that the federal government support new public-private intermediary institutions to accelerate industrialization of new technologies. Sridhar Kota, formerly assistant director for advanced manufacturing at the White House Office of Science and Technology Policy, has called for the U.S. to establish “Edison Institutes” modeled after those of Fraunhofer to help make maturing technologies ready for manufacturing. “We need strategic and coordinated investments to transition home-grown discoveries into home-grown products,” Dr. Kota contends.82
______________________
80 The NSF Science and Engineering Indicators for 2012 (Chapter 4) reports that $28.6 billion in 2009, or about 7% of all funding in the US. comes from sources that include academia’s own institutional funds (which support academic institution’s own R&D), other nonprofits (the majority of which fund their own R&D, but also contribute to academic research), and state and local governments (primarily for academic research).
81 Presentation by Roland Schindler at the National Academies Symposium on ”Meeting Global Challenges: US-German Innovation Policy” November 11, 2010.
82 Sridhar Kota, “Opening Remarks” at the National Research Council conference on Building the U.S. Battery Industry for Electric-Drive Vehicles: Progress, Challenges, and Opportunities, Livonia, Michigan, July 26, 2010.
In its most recent report to the President on Advanced Manufacturing, the PCAST characterizes U.S. private sector’s under-investment in important emerging technologies and in the infrastructure to support advanced manufacturing as a market failure. The report notes that individual companies cannot justify such investments because they cannot capture all the benefits for themselves. Instead, the benefits would spill over to many competitors. As a result, PCAST argues, the public sector has an important role in ensuring that new technologies are not only developed but also produced in the U.S.83
A number of government policy proposals have been offered to bolster U.S. manufacturing through support for applied research. The most recent PCAST report, for example, called for an Advanced Manufacturing Initiative spearheaded by the departments of Commerce, Defense, and Energy and coordinated by the Office of Science and Technology Policy, the National Economic Council, or the Office of the Assistant to the President for Manufacturing. Among other things, PCAST calls for federal investment of $1 billion annually for four years to support applied-research programs in potential transformational technologies, public-private partnerships to facility development of broadly applicable technologies, dissemination of new design methodologies, and shared technology infrastructure that would help U.S. manufacturers. PCAST also calls for reforms in corporate income taxes and measures to expand the skilled workforce.84 So far, however, no legislation establishing these programs has been introduced into Congress. Spence and Hlatshwayo advocate co-investment with the private sector to better align private incentives with social objectives. “It is probably a good idea to explicitly target some of the public-sector investment at technologies with the potential to expand the scope of the tradable sector and employment.”85
This call has been followed up with the recently announced National Network for Manufacturing Innovation (NNMI)— an association of precompetitive public-private consortia to conduct applied research on new technologies and design methodologies.86 According the Federal Register
______________________
83 PCAST, Report to the President on Ensuring American Leadership in Advanced Manufacturing, op. cit.
84 Ibid.
85 Michael Spence and Sandile Hlatshwayo, “The Evolving Structure of the American Economy and the Employment Challenge,” Council on Foreign Relations Working Paper, March 2011.
86 NNMI appears to be modeled in concept on Germany’s Fraunhofer-Gesellschaft, NNMI. See Chapter 5 of this report for a description of the Fraunhofer Gesellschaft. See also the presentation by Roland Schindler, Executive Director of Fraunhofer CSE, at the National Academies Symposium on Meeting Global Challenges: U.S.-German Innovation Policy, Washington, DC, November 1, 2010. Germany’s Fraunhofer system has established seven research institutes based at U.S. universities, including Michigan State University, Boston University, Massachusetts Institute of Technology, the University of Maryland, the University of Michigan, Johns Hopkins University,
notice, “The proposed Network will be composed of up to fifteen Institutes for Manufacturing Innovation (IMIs or Institutes) around the country, each serving as a hub of manufacturing excellence that will help to make United States (U.S.) manufacturing facilities and enterprises more competitive and encourage investment in the U.S. … The NNMI program will be managed collaboratively by the Department of Defense, Department of Energy, Department of Commerce’s NIST, the National Science Foundation, and other agencies. Industry, state, academic and other organizations will co-invest in the Institutes along with the NNMI program.” 87
The innovation challenge the United States faces in the 21st century was brought about by the transformation of the global economy in the last decades of the 20th century. Dramatic changes in the location of international production and in the direction of international trade flows resulted from the integration of the emerging economies into world commerce. Foreign direct investment into emerging markets transferred capital and know-how. World trade expanded more rapidly than world output, and trade in high-technology products expanded more rapidly than trade in general. This was due in large part to an increase in the growth of knowledge- and technology-intensive industries worldwide, but especially in emerging economies as they liberalized markets, increased spending on R&D and education, and adopted policies to encourage hightechnology manufacturing production and exports.88 The development of global supply chains initially increased specialization as lower value-added production was moved to lower cost locations.
Emerging economies increasingly have moved up the value-added supply chain so that they are now competing in the same product and technology space as the United States. One measure of this increased competition is the deterioration in the U.S. trade balance in advanced-technology products that began in the late 1990s. [See Figure 2.4] The trade deficit in advanced technology products, based on data through August, will set an all-time high in 2011.
The policy objective of other nations, including emerging economies like China, and India is to move up the manufacturing value-added chain by driving innovation in their economies and increasing the technology intensity of their manufactured exports. As they do so, the United States faces increased
______________________
and the University of Delaware. These institutes provide research and development services to help translate the fruits of research at U.S. academic institutions into products for the marketplace.
87 Federal Register Notice, May 4, 2012. The President’s FY 2013 budget requests $1 billion for the NNMI program.
88 National Science Foundation, Science and Engineering Indicators 2010, chapter 6.
competition in the tradable goods manufacturing sector and increased pressure on domestic manufacturing production and employment.
To be sure, other countries are pursuing these innovation-led policies not out of any desire to cause economic disadvantage to the United States, but because it offers them the best prospects for economic growth and a high standards of living for their citizens. A recent IMF study summarized it as follows: “Technology intensive export structures generally offer better prospects for future economic growth. Trade in high-technology products tends to grow faster than average, and has larger spillover effects on skills and knowledgeintensive activities. The process of technological absorption is not passive but rather ‘capability’ driven and depends more on the national ability to harness and adapt technologies rather than on factor endowments.”89
These changes in technology and trade are massive and are occurring with great rapidity from a historical perspective. In little over a decade, for example, China has increased its share of world high-technology manufactured exports from 6 percent to 22 percent and is now the world’s largest exporter of these products. Over the same period, the U.S. share of high-technology manufactured exports fell from 21 percent to 15 percent.90 [See Figure 2.4]
China’s increase in its share of high-technology exports is reflected in statistics published by the U.S. Census Bureau on trade in advanced-technology products.91 As shown in Table 2.1, the U.S. trade deficit in advancedtechnology products in 2011 was concentrated in China. But this is more a reflection of U.S. loss of competitiveness with the Pacific Rim area in general because China primarily is an assembler of high-technology components made in nations and regions such as Japan, Taiwan, Korea, and the United States.92 China and other emerging economies, however, are continuing to move
______________________
89 The traditional factor endowments are labor and capital. See International Monetary Fund, “Changing Patterns of Global Trade,” June 15, 2011, pp. 8-9. Paul Romer much earlier stated the same idea differently. “But our knowledge of economic history, of what production looked like 100 years ago, and of current events convinces us beyond any doubt that discovery, invention, and innovation are of overwhelming importance in economic growth and that the economic goods that come from these activities are different in a fundamental way from ordinary objects.” Paul Romer, “Idea Gaps and Object Gaps in Economic Development,” Journal of Monetary Economics 32 (1993): 562.
90 National Science Foundation, Science and Engineering Indicators 2010, chapter 6. Data published by the World Bank show similar, but somewhat different results, with China’s share at 20.4 percent in 2008 and the U.S. share at 12.4 percent. World Bank, World Development Indicators at http://data.worldbank.org/indicator/TX.VAL.TECH.CD.
91 The data for advanced technology products put together by the Census Bureau is constructed from more highly disaggregated product definitions allowing for a more precise measure of U.S. trade in technology intensive products than the high technology industry-based OECD classification used in Figure 1.11. National Science Foundation, Science and Engineering Indicators 2010, pp. 6-34.
92 Robert Koopman, William Powers, Zhi Wang and Shang-Jin Wei, “Give Credit Where Credit Is Due: Tracing Value Added in Global Production Chains,” NBER Working Paper No. 16426, September 2010. See also Robert Koopman, Zhi Wang and Shang-Jin Wei, “A World Factory in Global Production Chains: Estimating Imported Value Added in Chinese Exports,” Centre for Economic Policy Research Discussion Paper No. 7430, September 2009.
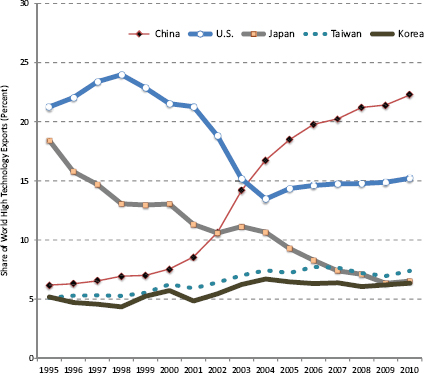
FIGURE 2.4 World export shares of high-technology goods. SOURCE: National Science Foundation, National Center for Science and Engineering Statistics, Science and Engineering Indicators 2012, NSB 12-01 (January 2012), Appendix Table 6-24.
upstream in the global supply chain, increasing competition for U.S. based manufacturing.93
By shifting and reorganizing global supply chains, the globalization of the world economy has also affected the price of products, employment patterns and wages in advanced and emerging economies alike. One of the most significant changes for the United States, as documented in a recent study by Spence and Hlatshwayo, is that from 1990 to 2008, almost all incremental employment growth came from the non-tradable sector of the U.S. economy,
______________________
93 George Tassey, “Rationales and Mechanisms for Revitalizing US Manufacturing R&D Strategies,” Journal of Technology Transfer (2010) 35, pp. 283–333 and International Monetary Fund, “Changing Patterns of Global Trade,” June 15, 2011, pp. 27-29.
primarily government and health care jobs.94 There were job gains in the tradable sector in high-end services (management and consulting, computer systems design, finance and insurance) but these were offset by losses in most areas of manufacturing.95 The authors state that the manufacturing job losses were due to lower value-added positions moving offshore while higher valueadded positions remained in the United States. Looking ahead, with budget constraints at all levels of government and growing pressures to rein in the rate of growth in health care costs, major gains in future employment are unlikely to come from the non-tradable sector. The authors believe the answer lies in expanding the U.S. export sector in both high-end manufacturing and services. “To create jobs, contain inequality, and reduce the U.S. current-account deficit, the scope of the export sector will need to expand. That will mean restoring and creating U.S. competitiveness in an expanded set of activities via heightened investment in human capital, technology, and hard and soft infrastructure. The challenge is how to do it most effectively.”96
Because of the interrelationships between manufacturing and services, expanding the scope of the U.S. export sector will also necessarily expand high value-added services. As manufacturing has become more technology-intensive, the scope and nature of manufacturing has changed, increasing the demand for service occupations and service inputs at the expense of machine operators and assembly-line workers.97 “Data on occupations show that in the last decade there has been a steady increase in the share of employees in the manufacturing sector who are employed in occupations that can be considered as services-related” while at the same time in countries like the United States manufacturing has become more service intensive.98 For example, industrial products increasingly are comprised of a combination of mechanical, electrical and software components that make them more innovative, more capable and more easily updated and enhanced.99 Thus as Gregory Tassey has stated, “the fast-growing high-tech services sector must have close ties to its manufacturing base.”100
______________________
94 Michael Spence and Sandile Hlatshwayo, “The Evolving Structure of the American Economy and the Employment Challenge,” Council on Foreign Relations Working Paper, March 2011.
95 The authors state that the manufacturing job losses were due to the lower value added positions moving offshore while higher value added positions remained. Id. at 31.
96 Id. at 5.
97 OECD, OECD Science, Technology and Industry Scoreboard 2011, Paris: OECD, September 20, 2011, p. 168 and Dirk Pilat and Anita Wölfl, “Measuring the Interaction Between Manufacturing and Services,” OECD STI Working Paper, DSTI/DOC(2005)5, May 31, 2005.
98 OECD, OECD Science, Technology and Industry Scoreboard 2011, id. The OECD estimated that in 2008 services-related occupations in manufacturing in the United States were just over 50 percent of all employees in manufacturing.
99 Jim Brown, “Issue in Focus: Systems and Software Driven Innovation,” Tech-Clarity, 2011. As Janos Sztipanovits, director of Vanderbilt University’s Institute for Software Integrated Systems, stated ”More and more industrial products internal complexity is concentrating in software.” Kate Linebaugh, “GE Makes Big Bet on Software Development,” The Wall Street Journal, November 17, 2011.
TABLE 2.1 U.S. Trade in Advanced Technology Products by Country and Region in 2011
| By Country and Region (Billions of Dollars) | |||
| Country | Exports | Imports | Balance |
| China | 20.1 | 129.5 | -109.4 |
| Ireland | 2.5 | 21.6 | -19.1 |
| Mexico | 31.9 | 47.8 | -15.9 |
| Taiwan | 8.8 | 18.7 | -9.9 |
| Japan | 15.7 | 25.5 | -9.8 |
| Korea | 11.3 | 17.5 | -6.2 |
| Malaysia | 8.0 | 14.1 | -6.1 |
| Thailand | 2.9 | 7.9 | -5.0 |
| France | 10.5 | 11.2 | -0.7 |
| Germany | 13.4 | 12.7 | 0.7 |
| Singapore | 10.3 | 8.1 | 2.1 |
| U.K. | 14.0 | 10.1 | 3.9 |
| Brazil | 11.7 | 1.0 | 10.6 |
| Canada | 30.3 | 13.5 | 16.8 |
| By Region (Billions of Dollars) | |||
| Region | Exports | Imports | Balance |
| Pacific Rim | 95.7 | 219.9 | -124.2 |
| EU | 67.0 | 75.3 | -8.3 |
| Other | 124.0 | 90.8 | 33.2 |
| World | 286.7 | 386.0 | -99.3 |
SOURCE: U.S. Census Bureau, Foreign Trade, Trade in Goods with Advanced Technology Products.
Seen in this context, the innovation challenge that the United States faces is at the same time a trade competitiveness challenge and a high-tech manufacturing and services challenge. Therefore, a fundamental objective of capturing the economic value of innovation has to be increasing the output of manufacturing in the United States for high-technology, high valued-added products to grow U.S. exports and employment.101
______________________
100 Gregory Tassey, “The Manufacturing Imperative,” presentation at NAS Conference on the Manufacturing Extension Partnership, November 14, 2011. Tassey also points out that the manufacturing sector accounts for 67 percent of R&D performed by industry and 57 percent of scientists and engineers in industry are employed by manufacturing.
101 Tassey argues that “Once the premise is accepted that the only way to achieve long-term growth in jobs for a high-income economy such as the United States is through investment in technology,
The Link between Manufacturing and Innovation
Manufacturing is integral to new product development. Production lines are links in an iterative innovation chain that includes pre-competitive R&D, prototyping, product refinement, early production, and full-scale production.102 U.S. corporations still dominate a number of industries, such as personal computers and certain semiconductors, even though end products are produced offshore.103 America’s logic chip-design industry, which includes companies like Qualcomm, Nvidia, and Broadcom, relies almost entirely on silicon wafers fabricated in Asian foundries, while Apple iPods, iPhones, and iPads are assembled in China by the Taiwanese firm Hon Hai Precision Industry. In such products, the greatest economic value is in software, microprocessors, and proprietary designs, while the hardware is generally comprised of standardized parts and assembled with standard production processes.
In many high technology industries, however, design is not so easily separated from manufacturing. Production processes for advanced solar cells, lithium-ion vehicle batteries, and next-generation solid-state lighting devices are highly proprietary to the producing company and often constitute a competitive advantage. If new U.S. companies lack the domestic capability to scale up, Intel founder Andy Grove warns, “we don’t just lose jobs — we lose our hold on new technologies. Losing the ability to scale will ultimately damage our capacity to innovate.” 104
______________________
innovation, and subsequent productivity increases, the key policy issue becomes how to promote desired long-term investment in a domestic economy that must save more and consume less, while reducing budget deficits through decreased spending and increased taxes.” George Tassey, “Rationales and Mechanisms for Revitalizing US Manufacturing R&D Strategies,” Journal of Technology Transfer (2010) 35, pp. 303-304.
102 See President’s Council of Advisors on Science and Technology, “Sustaining the Nation’s Innovation Ecosystems: Information Technology Manufacturing and Competitiveness,” January 2004. (http://www.choosetocompete.org/downloads/PCAST_2004.pdf). See also President’s Council of Advisors on Science and Technology, “Report to the President on Ensuring American Leadership in Advanced Manufacturing,” June 2011.
103 A recent National Research Council study of a range of technology-intensive industries found that in many cases U.S. companies dominated market share, profits, and innovation despite a considerable shift of manufacturing and R&D work offshore. See National Research Council, Innovation in Global Industries: U.S. Firms Competing in the World, Jeffrey T. Macher and David C. Mowery, editors, Washing The National Academies Press, 2008.
104 Andy Grove, “How to Make an American Job Before it is Too Late,” Bloomberg BusinessWeek, July 1, 2010.
Box 2.2
The Case of the Display Industry
A clear example of how loss of one manufacturing industry prevents development of others is computer and TV displays. Asian producers assumed dominance of liquid-crystal displays in the 1990s as U.S. producers abandoned the industry.105
The development by U.S. companies of key technologies and materials for displays on flexible, rather than glass, substrates would seem to present a fresh opportunity for America to re-enter the potentially huge display industry. According to Ross Bringans of the Palo Alto Research Center, “flexible electronics is a very exciting direction, and there will be a lot of new technologies. We are certain that interesting business opportunities will flow out of that.” According to Dr. Bringans, these opportunities are beginning to open, particularly in Europe and East Asia.106
Two major barriers stand in the way of developing a robust U.S. based flexible electronics industry. The first is the commercial challenge of launching the industry. Bob Street of the Palo Alto Research Center has observed that Asian manufacturers such as Samsung will likely dominate this industry because the entry barriers are too high for U.S. production of displays: The ecosystem of production capacity, expertise in volume production, local equipment manufacturers, materials suppliers and technology developers reside in Asia.107 The second challenge concerns the role of the government support. In this regard, a recent study commissioned by the National Science Foundation and the Office of Naval Research of European programs to support the development and commercialization of flexible electronics technologies found that “…the relatively low prevalence of actual manufacturing and advanced systems research and development in the United States has led to an incomplete hybrid flexible electronics R&D scenario for this country….”108
______________________
105 Jeffrey Hart, “Flat Panel Displays,” in National Research Council, Innovation in Global Industries: U.S. Firms Competing in a New World, Jeffrey T. Macher and David C. Mowery, Editors, Washington, DC: The National Academies Press, 2008. For a history of the flat panel display industry, see Thomas P. Murtha, Stefanie Ann Lenway, and Jeffrey A. Hart, Managing New Industry Creation: Global Knowledge Formation and Entrepreneurship in High Technology, Palo Alto: Stanford Business Books, 2002.
106 Ross Bringans, “Challenges and Opportunities for the Flexible Electronics Industry,” Presentation at the National Academies conference on “Flexible Electronics for Security, Manufacturing, and Growth In the United States.” September 24, 2010.
107 See Bob Street, “Next Generation: The Flex Display Opportunity” in The Future of Photovoltaic Manufacturing in the United States, C. Wessner, ed., Washington, DC: The National Academies Press, 2011.
108 Ananth Dodabalpur et al., “European Research and Development in Hybrid Flexible Electronics.” Baltimore MD: WTEC, 2010.
Support for Manufacturing Overseas
Some nations aggressively support manufacturing in favored industries with a range of policy tools. They include—
• Financial Incentives: China, Singapore, Malaysia, and other nations offer 10-year tax holidays to foreign companies building factories in desired industries. The use of tax credits that eventually refund a portion of a company’s investment in plants or laboratories also is quite common. In Canada, for example, federal, provincial, and local governments offer some of the world’s most generous tax incentives for aerospace manufacturing, including investment rebates and high depreciation allowances for machinery and equipment. Nondiscretionary tax incentives for aerospace manufacturing equal $1,569 per job in Montreal and $2,617 in Winnipeg, compared to $624 in Seattle and $1,240 in Wichita.109 Canada has become a major global manufacturer of civil helicopters, flight simulators, landing gear, and gas-turbine engines.110
• Workforce Training: Some nations design the curricula of universities and polytechnics to meet the projected needs for skilled workers in desired industries. They also cover the costs of worker training for foreign investors. For example, the mission of Singapore’s Workforce Development Agency (WDA) is to “enhance the employability and competitiveness of everyone in the workforce, from the young to old workers, from the rank-and-file to professionals, managers and executives.” It realizes this mission through training and education programs as well as workshops to upgrade worker skills.111
• Leveraging Domestic Markets: A number of countries use the buying power of the government and consumer subsidies to build local demand for domestic industries. Germany’s feed-in tariffs, which are high enough to guarantee a financial return for both utilities and manufactures, largely explain why that nation has emerged as a global manufacturing leader of photovoltaic systems, for example.112 Indeed,
______________________
109 Invest in Canada Bureau, “Canada—A Strategic Choice: Canada as an Investment Destination for Aerospace” (undated).
110 Ibid.
111 Website of Singapore’s Workforce Development Agency. Access at http://app2.wda.gov.sg/web/Common/homepage.aspx.
112 A feed-in tariff is an incentive structure that sets by law a fixed guaranteed price at which power producers can sell renewable power into the electric power network. The tariff obligates regional or national electricity utilities to buy renewable electricity, such as electricity generated from solar photovoltaic panels, at above-market rates. See presentation by Bernhard Milow of the German Aerospace Center at the National Academies symposium on Meeting Global Challenges: U.S. German Innovation Policy, November 1, 2010. Also see Michael J. Ahearn. “Opportunities and Challenges Facing PV Manufacturing in the United States.” The Future of Photovoltaics
Germany’s renewable-energy sector now employs 340,000, more than the auto industry.113 To help meet its goal of having 2 million electric vehicles on the roads by 2020, the French government awards up to €5,000 to buyers of electric vehicles and plans to have state-owned companies and government agencies order 50,000 such vehicles for their fleets.114 China offers a $9,036 subsidy to buyers of electric cars and subsidizes fleet operations in 25 cities as part of its target of selling 1 million electric vehicles per year by 2020. 115 To promote domestic manufacturers of solid-state lighting, which the government hopes will be a $30 billion export industry by 2015, China is rolling out a program to help 21 major cities install 1 million street lamps using light-emitting diodes.116
• Trade Policy: Although trade barriers have fallen dramatically around the world in recent decades, some nations continue to use a variety of official and unofficial policy tools to support domestic manufacturing. It is common for countries to require foreign defense and aerospace contractors, as well as vendors of big-ticket items such as power plants and rail stock, to source some parts or to perform final assembly domestically, for example. Of major trading nations, China has the most aggressive such “import substitution” policies. The government, which has not signed World Trade Organization protocols on government procurement, essentially compels foreign makers of everything from wind turbines to high-speed trains to manufacture in China and transfer technology to domestic companies.117 Already a big exporter of solar panels, China requires at least 80 percent of equipment for its own solar power plants to be domestically produced.118 A particularly controversial policy directs state agencies to
______________________
Manufacturing in the United States; Summary of Two Symposia, C. Wessner, ed., Washington, DC: The National Academies Press, 2011.
113 Solar Progress, December 2010 Issue. Access at http://www.auses.org.au/wpcontent/uploads/2010/12/SP_DEC10.pdf.
114 David Pearson, “France Backs Battery-Charging Network for Cars,” Wall Street Journal, Oct. 1, 2009.
115 People’s Daily, “China to Sell 1 Million New-Energy Cars Annually by 2015,” Nov. 223, 2010. English translation viewable at http://english.peopledaily.com.cn/90001/90778/90860/7207607.html.
116 China Research and Intelligence, “Brief of the LED Lighting Program of 10,000 Lights in 10 Cities in China,” July 23, 2009. This article can be accessed at http://www.articlesbase.com/pressreleases-articles/brief-of-the-led-lighting-program-of-10000-lights-in-10-cities-in-china1061573.html.
117 See Jason M. Forcier, “The Battery Industry Perspective,” presented at the National Research Council conference on Building the U.S. Battery Industry for Electric-Drive Vehicles: Progress, Challenges, and Opportunities, Livonia, Michigan, July 26, 2010.
118 Keith Bradsher, “China Builds High Wall to Guard Energy Industry.” International Herald Tribune, July 13, 2009.
buy high-technology products that incorporate “indigenous innovation.”119
U.S. Support for Manufacturing
The explicit national support for domestic manufacturing in Asia and European nations such as Germany has been in sharp contrast to the United States, where support for industry has tended to be limited to defense-related manufacturing and enforcing free-trade rules. A recent report by the President’s Council of Advisors for Science and Technology (PCAST) warned that the U.S. is losing leadership in manufacturing, not only in low-tech industries that depend on low-cost foreign labor but also in high-tech products that result from U.S. innovation, inventions, and manufacturing-associated research and development.120
America’s advanced manufacturing base faces formidable competitive challenges. In some cases, according to an analysis by Erica Fuchs and Randolph Kirchain, the cost gaps between manufacturing in the U.S. and Asia are so large that they discourage innovation. It makes more economic sense for companies to import products made with mature technologies than to domestically produce advanced, better-performing products made with new technologies.121
Offshore cost advantages in high-technology products often have little to do with labor rates because manufacturing is highly automated. According to an analysis by the Manufacturing Institute, non-production expenses such as high U.S. corporate taxes, employee benefits, torts, and pollution control put American-based manufacturing at an 18 percent structural cost disadvantage compared to major trading partners and more than a 50 percent disadvantage compared to China, although rising costs elsewhere and a weaker dollar have help narrow these gaps substantially since 2006.122 Manufacturing executives
______________________
119 The State Council, People’s Republic of China, “National Medium- and Long-Term Program for Science and Technology Development, 2006-2020,” (undated).
120 President’s Council of Advisors on Science and Technology, Report to the President on Ensuring American Leadership in Advanced Manufacturing, Executive Office of the President, June 2011.
121Fuchs and Kirchain demonstrated the “dilemma” of manufacturing products with prevailing designs offshore in order to reduce as opposed to manufacturing new-technology products in the U.S. by analyzing the optoelectronic device industry. See Erica R. H. Fuchs and Rondolph Kirchain, “Design for Location? The Impact of Manufacturing Off-Shore on Technology Competitiveness in the Optoelectronics Industry,” Management Science, 56(12), pp. 2323-2349, 2010. In an analysis of optoelectronics devices, Fuchs found that U.S. manufacturing yields would have to increase.
122 Jeremy A. Leonard, “The Tide Is Turning: An Update on Structural Cost Pressures Facing U.S. Manufacturer,” The Manufacturing Institute and Manufacturers Alliance/MAPI, November 2008 (http://www.deloitte.com/assets/DcomUnitedStates/Local%20Assets/Documents/us_pip_TideIsTurning_093009.pdf). A recent Boston Consulting Group report predicts that, with respect to China, some manufacturing operations will return to the United States as wages increase in China and the U.S. dollar weakens. Harold L.
addressing NRC symposia also cited availability of workers, the lack of a domestic supply base, and inadequate access to capital for new plants or expansion as serious obstacles to keeping production in the United States.
State and federal policies and programs can help industry ameliorate these competitive gaps. Strategies for addressing these challenges include—
• Financial Incentives: The U.S. federal and state governments have increased incentives for domestic manufacturing. Michigan’s $1.02 billion Advanced Battery Tax Credits program was instrumental in the state’s success in drawing private investment in lithium-ion battery plants, for example. 123 New York and New Mexico are among the other states that have used aggressive tax credits to lure advanced manufacturing in desired industries. The federal government also has introduced a number of such incentives, especially over the past three years. The 2009 Recovery Act (ARRA) provided a one-time boost of $2.4 billion in grants earmarked for 48 advanced-battery manufacturing projects, for example. The Advanced Energy Manufacturing Tax Credit program provides $2.3 million to companies to cover 30 percent of investments in new, expanded, or refurbished manufacturing plants producing renewable-energy equipment.124
The Department of Energy says the credits, which were matched by $5 billion in private investment, funded 183 projects in 43 states and created tens of thousands of jobs.125 The federal government also has encouraged domestic manufacturing with tax deductions for consumer purchases of electrified vehicles, loan guarantees for green-technology projects, and greater access to export financing.
______________________
Sirkin, Michael Zinser and Douglas Hohner, Made in America, Again: Why Manufacturing Will Return to the U.S., The Boston Consulting Group, August 2011.
123 Michigan’s Advanced Battery Tax Credits initiative was created through an amendment to the Michigan Business Tax Act, Public Act 36 of 2007, to allow the Michigan Economic Development Authority to tax credits for battery pack engineering and assembly, vehicle engineering, advanced battery technology development, and battery cell manufacturing. Under the scheme, Michigan refunds up to $100 million of a company’s capital investment. Battery pack manufacturers receive a credit for each pack they assemble in Michigan. See presentation by Eric Shreffler, “Michigan Investments in Batteries and Electric Vehicles,” at the National Academies Symposium on Building the U.S. Battery Industry for Electric Drive Vehicles, Livonia, Michigan, July 26, 2010.
124 The Advanced Energy Manufacturing Tax Credit was authorized in Section 1302 of the American Recovery and Reinvestment Act and also is known as Section 48C of the Internal Revenue Code. It authorizes the Department of Treasury to award $2.3 billion in tax credits to cover 30 percent of investments in advanced energy projects, to support new, expanded, or re-equipped domestic manufacturing facilities.
125 Carol Browner, “White House Blog: 183 projects, 43 states, Tens of Thousands of High Quality Clean Energy Jobs.” January 8, 2010. Access at http://www.whitehouse.gov/blog/2010/01/08/183projects-43-states-tens-tthousands-high-quality-clean-energy-jobs.
Incentive packages in the U.S. are still unable to match many of those offered by foreign governments, however. These broad-based packages of incentives, which range from tax holidays to free infrastructure, to cheap capital, to lax environmental and labor regulations, offer a coordinated program to create a non-market advantage using state resources. Such practices by China, Korea, and Taiwan (among others) have introduced a fundamental shift in cost and revenue that essentially changes the economic game.
While U.S. states have wide latitude to waive corporate taxes, for example, manufacturing plants still are required to pay federal corporate taxes, which are among the highest in the industrialized world. The Milken Institute argues that reducing the U.S. corporate tax rate to match the OECD average would create 350,000 new manufacturing jobs by 2019, while increasing the R&D tax credit by 25 percent and making it permanent would create 270,000 manufacturing jobs. 126 Financial analyst Steve O’Rourke of Deutsche Bank explained in a National Academies conference on photovoltaic manufacturing that “manufacturing migrates to where companies are most profitable, and the single biggest issue in this analysis is taxes.”127 Federal incentives have closed some of those cost gaps. In the case of photovoltaic manufacturing, Department of Energy official John Lushetsky estimated that the combination of U.S. and state incentives have closed about two-thirds of the cost advantage of operating a factory in China that is attributable to that country’s incentives.128
A major concern voiced in STEP symposia about current federal incentives is that they are too short-term and unpredictable for longterm investments, with funding requiring frequent renewal by Congress. The controversy over the bankruptcy of Solyndra (a manufacturer of novel cylindrical solar panels) after receiving $535 million in federal loan guarantees, moreover, has raised concerns over
______________________
126 Ross DeVol and Perry Wong, “Jobs for America: Investments and Policies for Economic Growth and Competitiveness,” Milken Institute, January 26, 2010. Also see John Neuffer, “China: Intellectual Property Infringement, Indigenous Innovation Policies, and Frameworks for Measuring the Effects on the U.S. Economy,” written testimony to the United States International Trade Commission Investigation No. 332-514 Hearing on behalf of the Information Technology Industry Council, June 15, 2010. (http://www.itic.org/clientuploads/ITI%20Testimony%20to%20USITC%20Hearing%20on%20China%20%28June%2015,%202010%29.pdf).
127 Steve O’Rourke, “Financing Photovoltaics in the United States,” in The Future of Photovoltaics Manufacturing in the United States, C. Wessner, ed., Washington, DC: The National Academies Press, 2011.
128 From presentation by John Lushetsky of the Department of Energy at National Academies symposium “Meeting Global Challenges” in Washington, DC, November 1, 2010.
how such programs are administered—and highlight the political risks of supporting emerging technologies in the face of fierce import competition.129
• Workforce Availability and Location: Availability of engineers and workers with the right skills is another oft-cited reason for America’s declining competitiveness in advanced manufacturing.130 In addition, there is a growing concern that U.S. business school programs are not turning out enough graduates who can run manufacturing operations.131 Availability of talent is the most important factor in a company’s decision where to locate production, according to a recent survey of 400 global manufacturing executives. That report suggested the hollowing out of U.S. manufacturing is taking a toll on America’s skill base. Once this “high degree of accumulated tacit knowledge” is lost, it warned, it “is difficult, if not impossible, to recover.” 132 Some 60 percent of the science and engineering workforce will be eligible for retirement in the next five years, a prospect that former Under Secretary of Energy Kristina Johnson described as “a real national crisis.”133 In the field of electrical power engineering, an essential skill for the advanced-storage industry, an analysis by the Institute of Electrical and Electronics Engineers’ Power & Energy Society concludes that U.S. graduation rates do not meet the nation’s current and future needs.134
______________________
129 See Eric Lipton and John M. Broder, “In Rush to Assist Solar Company, U.S. Missed Signs,” New York Times, Sept. 22, 2011, and Melissa C. Lott, “Solyndra—Illuminating Energy Funding Flaws?”, Scientific American, September 27, 2011.
130 While the overall number of scientists and engineering graduates has grown over the past 3 decades to about 4.3 percent of all U.S. jobs (NSF S&E Indicators 2012, Chapter 3), industry surveys show a shortage of workers with the necessary level and mix of skills needed on the factory floor. See Deloitte and U.S. Council on Competitiveness, “2010 Global Manufacturing Competitiveness Index.” Demand for industrial engineers has remained high even in the recent recession. For a review of the rapidly changing nature of factory employment, see also The Economist, “Factories and Jobs: Back to Making Stuff,” April 12, 2012.
131 See Jack R. Meridith, “Hopes for the future of operations management,” Journal of Operations Management 19 (2001) 397–402. The author notes that “Operations Management has a much longer history than our sister functions in business: finance, marketing, accounting, etc. Yet, we still struggle with fewer majoring students, fewer and newer journals, less academic respect, greater student fear, and fewer professors.”
132 Aleda V. Roth, et. al, “2010 Global Manufacturing Competitiveness Survey,” Deloitte Touche Tohmatsu and U.S. Council on Competitiveness, June 2010.
133 Kristina Johnson, “Advancing Solar Technologies: The U.S. Department of Energy’s Perspective,” in The Future of Photovoltaics Manufacturing in the United States, C. Wessner, ed., Washington, DC: The National Academies Press, 2011.
134 Amy Fischbach, “Engineering Shortage Puts Green Economy and Smart Grid at Risk,” Transmission and Distribution World, April 21, 2009, (http://blog.tdworld.com/briefingroom/2009/04/21/engineer-shortage-puts-green-economy-andsmart-grid-at-risk).
A number of innovative partnerships between industry, schools, and state government agencies are underway to address this skills gap.135 Indiana has launched a new kind of community college called Ivy Tech with 23 campuses and 130,000 students. One of its strengths is working with industry to train “middle-skill workers,” those with two years of college but who did not earn a bachelor’s degree in engineering. Fiftysix percent of demand for all workers in Indiana is classified as middle skill, while only 45 percent of Indiana’s workforce has such training.136 The state of Michigan has a number of programs to train workers and engineers for emerging industries such as advanced batteries, electric vehicles, and solar power with financial support from the DOE and the U.S. Army’s TARDEC.137
The federal government also provides training for trade-affected workers who have lost their jobs as a result of increased imports or shifts in production out of the United States through the Trade Adjustment Assistance Program (TAA).138 Administered by the Department of Labor, this $1 billion a year program includes assistance for displaced workers to find and relocate to new jobs, and training for workers to develop skills demanded in existing labor markets. This includes classroom and on-the-job training, as well as customized training to meet the needs of a specific employer. In some instances, the program also provides income support to workers who are participating in full-time training.
• Promoting Markets: U.S. competitors in Asia and Europe recognize that emerging technologies, such as solar photovoltaics or lithium-ion batteries for vehicles, generally do not have existing market structures and in fact have almost always been established by some sort of nonmarket support.139 This is especially true of the first instantiations of
______________________
135 For a revealing comparative case studies of the importance of social networks in the divergent trajectories of post-industrial regions, see Sean Stafford, Why the Garden Club Couldn’t Save Youngstown: The Transformation of the Rust Belt, Cambridge MA: Harvard UP, 2009.
136 Data from Indiana Department of Workforce Development and U.S. Census Bureau.
137 Presentation by Simon Ng, “Technical Training and Workforce Development,” at the National Academies Symposium on Building the U.S. Battery Industry for Electric Drive Vehicles. July 26, 2010.
138 See Harold F. Rosen, “Strengthening Trade Adjustment Assistance,” Policy Brief 08-02, Peterson Institute for International Economics, 2008. Access at http://www.iie.com/publications/pb/pb082.pdf For a review of issues relating to training programs and global competitiveness focusing on the TAA program, see the transcript of the Hearing before the House Ways and Means Committee, “Promoting U.S. Worker Competitiveness in a Globalized Economy.” June 14, 2007. Serial No. 110-47, Washington, DC: USGPO, 2008. Access at http://www.gpo.gov/fdsys/pkg/CHRG110hhrg43113/pdf/CHRG-110hhrg43113.pdf.
139 The instrumental role of procurement in the development of leading U.S. industries is exemplified by support by the Department of Defense for integrated circuits and advanced
new technologies as contrasted with derivative technologies and products within a technology area (such as the tablet computer as a melding of the laptop computer and cell phone). While other nations pursue active commercial market development strategies through subsidies and other preferential treatment, the debate on this issue continues in the U.S., with some contending that, for example, alternative energy technologies ought not to be subsidized, even though they cannot compete on a cost per kilowatt basis with entrenched incumbent technologies.
In the absence of initial markets of sufficient scale puts the U.S. at a competitive disadvantage in several promising emerging technology industries. Because the largest markets for solar panels have been in Europe and Asia, the U.S. accounts for just 9 percent of global manufacturing capacity of photovoltaic cells and modules, even though American companies are at the forefront of new technologies and production of key materials. European companies control 30 percent of the market.140 Pike Research predicts Asia will account for 53 percent of global demand for lithium-ion batteries for vehicles in 2015, thanks in large part to the supportive policies by governments such as those of China, Japan, South Korea, and Taiwan.141 Roland Berger Strategy Consultants, on the other hand, forecasts that Asia will only account for 26 percent of the global automotive lithium-ion battery market of $8.9 billion in 2015, increasing to 38 percent by 2020 when the market is forecast to reach $15.7 billion.142
If the U.S. does not have a sufficient domestic market in emerging technologies, domestic manufacturers may well lack the scale needed to compete and survive. America’s fledgling advanced battery industry illustrates this paradox. Some 48 factories funded by private investors and government incentives are being established, but industry analysts project serious overcapacity for at least five years before the hybrid and
______________________
computing, including the internet. An extended list also includes jet engines, satellite communications, and the cell phone.
140 Michael J. Ahearn “Opportunities and Challenges Facing PV Manufacturing in the United States.” The Future of Photovoltaics Manufacturing in the United States, C. Wessner, ed., Washington, DC: The National Academies Press, 2011. See also the summary of remarks by Ken Zweibel of the George Washington University Solar Institute, Subhendu Guha of UniSolar, and Dick Swanson of SunPower in the same volume.
141 Pike Research, “Asian Manufacturers Will Lead the $8 Billion Market for Electric Vehicle Batteries,” June 1, 2010 (http://www.pikeresearch.com/newsroom/asian-manufacturers-will-lead-the8-billion-market-for-electric-vehicle-batteries).
142 RolandBerger Strategy Consultants, Global Vehicle LiB Market Study, Detroit/Munich, August 2011.
electric-vehicle markets are big enough to absorb the output.143 “We can create the best battery in the world, but without vehicles to put them in this industry will go back overseas and we will have stimulated another country’s industries,” said A123 Systems executive Les Alexander.144
To spur demand, the U.S. General Services Administration has announced a goal to buy more than 40,000 alternative-fuel and fuelefficient vehicles to replace aging, less-efficient sedans, trucks, tankers, and wreckers in the fleets of federal agencies.145 The federal government also is creating a market for advanced batteries through its programs to promote solar and wind projects. Currently, however, there is no requirement that such batteries be purchased from domestic suppliers. This means that national subsidies to foreign manufacturers will have the desired effect by lowering their immediate costs and allowing them to capture overseas markets from less well-subsidized competitors.
• Supporting Exports: Global exports of U.S. manufactured goods and services are important to our balance of payments and economic growth. A key task of the U.S. Commercial Service is to support firms in identifying and exploiting new market opportunities abroad. In 2010, the U.S. Commercial Service directly helped generate $34.8 billion in US exports, assisting over 18,000 business clients. However, while the rest of the world, especially China, India, and Germany, has been augmenting their export assistance, the U.S. has reduced the size of its Commercial Service from over 1,275 employees in 2000 in the international field to barely 900 in 2011.146 By comparison, Germany fields a staff of 100 in Shanghai alone.147 To address the need and opportunity to increase U.S. exports, US Commerce Secretary John Bryson has called for growing and restructuring the Foreign Commercial Service in order to intensify its focus on identifying
______________________
143 See Boston Consulting Group, “Batteries for Electric Cars: Challenges, Opportunities, and the Outlook to 2020,” accessible at http://www.bcg.com/documents/file36615.pdf. Also see presentation by Mohamed Alamgir of Compact Power in Building the U.S. Battery Industry for Electric Drive Vehicles.
144 From presentation by Les Alexander at the National Academies Symposium on Building the U.S. Battery Industry for Electric Drive Vehicles. July 26, 2010.
145 Department of Energy press release, January 26, 2011, (http://www.energy.gov/10034.htm).
146 See testimony of Keith Curtis of the American Foreign Service Association before House Committee on Appropriations. March 22, 2012.
147 American Chamber of Commerce, Shanghai, “US Export Competitiveness in China, Winning in the World’s Fastest-Growing Market,” September 2010. Access at http://www.amchamshanghai.org/ftpuploadfiles/publications/viewpoint/us_export.pdf.
markets where U.S. exports have the best potential for continued growth, including China, Brazil, India, Saudi Arabia and Turkey.148
Export finance, often on concessional terms, is also a major source of support to foreign manufacturers. Many U.S. trade competitors invest significantly more in export credit assistance as both a share of GDP and exports than the United States does.149 The U.S. Export-Import Bank, which provides financing and insurance for export transactions, plays an important role in supporting these manufacturers by expanding the financing of sales of U.S. exports to international buyers.
Why Manufacturing Matters for the U.S.
Concern over America’s declining manufacturing base was a recurring theme of STEP board symposia. Leading executives, industry analysts, and military officials warned that the U.S. is losing competitiveness as a location for new investment in advanced manufacturing capacity, even in industries where the U.S. is at the technological forefront. PCAST also warns that continued erosion of America’s high-tech manufacturing base threatens to undermine U.S. leadership in next-generation technologies.150 Manufacturing matters to the health of the U.S. economy and its innovation ecosystem. The reasons include—
• Jobs: U.S. manufacturing shed 5.5 million jobs between 2000 and 2010. At a time when unemployment remains around 9 percent, the loss of manufacturing jobs takes on greater significance. The Milken Institute estimates that every computer-manufacturing job, for example, creates an additional 15 jobs elsewhere in the economy.151 It also notes that the average manufacturing job in California paid $66,200 a year, roughly 50 percent more than jobs in health care, the state’s fastest
______________________
148 See Department of Commerce Press Release, “Commerce Secretary John Bryson Lays Out Vision for Department of Commerce.” December 15, 2011.
149 Export-Import Bank of the United States, Report to the US Congress on Export Credit Competition and the Export-Import Bank of the United States, June 2010. The U.S. Chamber of Commerce is partnering with the Export-Import Bank of the United States on its Global Access for Small Business initiative to help more than 5,000 small companies export goods and services produced by U.S. workers. For a concise review of the role and performance of the Export-Import Bank in promoting U.S. exports, see Stephen Ezell, “Understanding the Importance of Export Credit Financing to U.S. Competitiveness.” Washington, DC: ITIF, June 2011.
150 President’s Council of Advisors on Science and Technology, Report to the President on Ensuring American Leadership in Advanced Manufacturing, op. cit.
151 Ross C. DeVol, et. al., “Manufacturing 2.0: A More Prosperous California,” Milken Institute, June 2009.
growing industry. Overall, manufacturing contributes $1.6 trillion to GDP and employs 11 million workers.152
• Innovation: A strong manufacturing base is an integral, though often under-appreciated, part of America’s innovation ecosystem. Manufacturing companies account for nearly 70 percent of U.S. industrial research and development153 and employed 63.4% of all domestic scientists and engineers in 2007.154 Domestic manufacturing is a critical element in the creation of new technologies. NIST economist Gregory Tassey notes that most modern technologies are actually “systems” that evolve from an interdependent network of “industries that contribute advanced materials, various components, subsystems, manufacturing systems, and eventually service systems based on sets of manufactured hardware and software.” 155
A 2003 report by to President George W. Bush by the President’s Council of Advisors on S&T, which included past and present CEOs of Dell, Intel, Lockheed Martin, and Autodesk, underscored the link between innovation and manufacturing. The study concluded that nations that manufacture commoditized products increasingly are able to develop the capacities to compete directly with the U.S. on “innovating new products and new industries.” The PCAST report stated “with manufacturing leaving the country, the United States runs the risk of losing the strength of its innovation infrastructure of design, research and development and the creation of new products and industries.”156
• National Security: Large-scale domestic industries also are vital to national defense. Not only does the military need secure supplies of critical components such as semiconductors and sensors. Scale production also is necessary for controlling costs of materiel. Consider the military’s growing requirements for fuel-efficient vehicles. The U.S. Army has committed to cutting fuel consumption by 20 percent in the next 10 to 15 years. At the same time, new weapons and
______________________
152 Gregory Tassey, “The Manufacturing Imperative,” presentation at NAS Conference on the Manufacturing Extension Partnership, November 14, 2011.
153 The Manufacturing Institute, “The Facts About Modern Manufacturing-8th Edition,” Gaithersburg MD: NIST, 2009. Access at http://www.nist.gov/mep/upload/FINAL_NAM_REPORT_PAGES.pdf.
154 Wolfe, 2009, cited by George Tassey, “Rationales and mechanisms for revitalizing US manufacturing R&D strategies,” Journal of Technology Transfer, DOI 10.1007/s10961-009-9150-2, 2010.
155 Tassey, ibid.
156 President’s Council of Advisors on Science and Technology, “Sustaining the Nation’s Innovation Ecosystems: Information Technology Manufacturing and Competitiveness,” January 2004, (http://www.choosetocompete.org/downloads/PCAST_2004.pdf).
communications systems are boosting the need for power in combat and non-combat vehicles.157 Converting much of the Army’s 400,000 vehicle fleet to hybrids would reduce fuel costs, ease dependence on imported petroleum, and provide important logistical advantages in the battlefield. Greater fuel efficiency enabled by light-weight, highdensity lithium-ion batteries would mean fewer dangerous truck convoys through deserts, tanks that can travel and fight longer without refueling while operating next-generation weapons.158 High U.S. production volumes of such batteries will make wide deployment of such equipment more feasible, explained John Pellegrino of the U.S. Army Research Laboratory. “We don’t want each of those vehicles to cost $1 billion,” Dr. Pellegrino said.
The ecosystem for providing risk capital to promising new technology companies not only has been one of the greatest advantages of America’s innovation system—but also one of the most difficult to replicate by other nations. The U.S. still has the world’s biggest pool of private angel, venture capital, and private-equity funds. It also has the strongest equity markets for taking successful start-ups public.
However, the availability of angel and venture funding has shrunk dramatically in the U.S. over the past decade.159 What’s more, investors have become far more averse to risk, and therefore devote more of their capital to later-stage companies that already have established a position in the market. As a result, many promising start-ups—especially in capital-intensive sectors, such as bio-medical, struggle to raise the funds needed to survive the perilous period of transition when a developing technology is deemed promising, but too new to validate its commercial potential and thereby attract the capital necessary for its continued development.
______________________
157 See presentations by Grace Bochenek and Sonya Zanardelli at the National Academies Symposium on Building the U.S. Battery Industry for Electric Drive Vehicles, July 26, 2010.
158 See presentations of John Pellegrino of the Army Research Laboratory and Grace Bochenek in of TARDEC at the National Academies Symposium on Building the U.S. Battery Industry for Electric Drive Vehicles, July 26, 2010.
159 For an analysis of the effect of the recent financial crisis on the venture capital market, see Joern Blockab and Philipp Sandnerc,. “What is the effect of the financial crisis on venture capital financing? Empirical evidence from US Internet start-ups.” Venture Capital: An International Journal of Entrepreneurial Finance, Volume 11, Issue 4, 2009. For a review of the impact of the financial crisis across industries and countries, see Block, Joern Hendrich, De Vries, Geertjan and Sandner, Philipp G., “Venture Capital and the Financial Crisis: An Empirical Study Across Industries and Countries” (January 24, 2010). HANDBOOK OF VENTURE CAPITAL, Oxford University Press, Forthcoming. The authors’ research suggests that the financial crisis has led to a severe ‘funding gap’ in the financing of technological development and innovation around the world.
Box 2.3 The Economic Debate on Manufacturing
Economists hotly debate the degree and significance of America’s decline in manufacturing. Although manufacturing employment dropped sharply over the past decade—after remaining stable at around 17 million for 35 years-some economists contend that decline is explained by higher productivity by U.S. manufacturers.
The National Association of Manufacturers notes that U.S. manufacturing generates $1.6 trillion in value each year, accounts for the lion’s share of exports, directly employs 10 percent of the American workforce, and overall supports one is six private-sector jobs. U.S.-based manufacturers also conduct half of private R&D. 160 Economists also have estimated that manufacturers pay 30 to 40 percent of all corporate taxes collected by the federal, state, and local governments and that each $1 of final manufacturing output creates another $1.43 in economic output when services such as finance, construction, and transportation are included.161
The Heritage Foundation notes that while U.S. manufacturing employment dropped by one-third since 1987, output rose by 46 percent, thanks to a 114 percent increase in productivity. 162 Such data show there is no empirical evidence of a U.S. manufacturing crisis, the RAND Corp. concluded in 2004.163
Recent economic analysis shows, however, that U.S. statistics may be overstating the gains in manufacturing productivity because they fail to adequately reflect the value of imported inputs in manufactured products and because they do not adequately account for the growing use of temporary factory workers. They also note that gains in manufacturing productivity are unevenly distributed, with the significantly higher productivity in computer and electronics manufacturing masking the trends in other sectors.164 Foreign
______________________
160 National Association of Manufacturers data.
161 National Review, “Why Manufacturing Matters,” December 3, 2008.
162 James Sherk, “Technology Explains Drop in Manufacturing Jobs,” Backgrounder #2476, Heritage Foundation, October 12, 2010.
163 Charles Kelley, et al. “High-Technology Manufacturing and U.S. Competitiveness,” TR-136OSTP, prepared for the Office of Science and Technology Policy, RAND Corp., March 2004.
164 See Susan Houseman and others. “Offshoring Bias in U.S. Manufacturing,” Journal of Economic Perspectives 25: 111-132. 2011. Susan Helper, Timothy Krueger, and Howard Wial, “Why Does Manufacturing Matter? Which Manufacturing Matters? A Policy Framework.” Washington, DC: Brookings, February 2012. See also Robert D. Atkinson and others, “Worse than the Great Depression: What Experts Are Missing about American Manufacturing Decline.” Washington, DC: ITIF, March 2012; and Robert D. Atkinson, “Commentary on Gregory Tassey’s ‘Rationales and Mechanisms for Revitalizing U.S. Manufacturing R&D Strategies,’” Journal of Technology Transfer DOI 10.1007/s10961-010-9164-9, 2010 (http://www.itif.org/files/2010-Atkinson-JTT.pdf). See also IDA, “Global Trends in Advanced Manufacturing,” March 2012. Access at https://www.ida.org/upload/stpi/pdfs/p-4603_final2a.pdf.
manufacturing and trade practices, particularly those of China in the first decade of this century, have also negatively impacted U.S. manufacturing employment. Measured in terms of value-added, U.S. production of computer and electronic products—a high-performing sector from 1985 to 2000—dropped by 21 percent in the past decade.165
Public-private partnership programs such as the Small Business Innovation Research program (SBIR) and NIST’s Advanced Technology Program (ATP) have proved successful—and, in the case of SBIR, increasingly important—sources of early-stage capital for new, innovative U.S. companies.
The Technology Innovation Program (TIP) is the successor to NIST’s highly regarded Advanced Technology Program (ATP). Independent evaluations by the National Research Council found ATP to be “an effective federal partnership program.” 166 One of its strengths was to bring together small and large companies (and universities) in partnership to develop new high technology products, such as amorphous silicon detectors that digitally enhance MRI images for improved breast cancer detection. As ATP’s successor, TIP sought to accelerate “innovation in the United States through high-risk, highreward research in areas of critical national need” through “targeted investments in transformational R&D that will ensure our nation’s future through sustained technological leadership.”167 Despite its broad mandate, funding for TIP was modest and no funds were appropriated for this program in the FY 2012.
The Small Business Innovation Research Program (SBIR) provides more than $2.5 billion annually in competitively awarded R&D grants and contracts to qualified small businesses. In comparison, the private venture capital industry invested $919 million at the seed stage in 2011.168 Eleven federal agencies are required by law to provide these funds by setting aside 2.5% of their annual extra-mural R&D budgets for small businesses innovation. In a recent assessment, the National Academies found the program to be “sound in concept and effective in practice.” It highlighted the program’s important role as a source of start-up and seed capital for small businesses to develop new innovative product concepts for the market as well as develop products and
______________________
165 Data cited by Tassey, op. cit.
166 Independent evaluations by the National Research Council found ATP to be “an effective federal partnership program.” See National Research Council, The Advanced Technology Program, Assessing Outcomes, Washington, DC: National Academy Press, 2001. The successor to the Advanced Technology Program, the Technology Innovation Program (TIP) at the National Institute for Standards and Technology “supports, promotes, and accelerates innovation in the United States through high-risk, high-reward research in areas of critical national need” through “targeted investments in transformational R&D that will ensure our nation’s future through sustained technological leadership.” See http://www.nist.gov/tip/.
167 See http://www.nist.gov/tip/.
168 Data from PWC-MoneyTree, January 2012.
carry out contract research for specific agency mission needs. It is valued both as start-up funding and as a “low cost technological probe.”169
A 2008 study by Block and Keller, based on a sample of top inventions recognized by R&D Magazine over 40 years, found that over the past two decades, about two-thirds of the top innovations have roots in governmentindustry partnerships.170 This contrasts with the awards in the previous period that were predominantly funded by either private or federal sources. Their study also found that 20 to 25 percent of the R&D 100 inventions awards over the past decade benefitted from SBIR awards. [See Figure 2.5]
SBIR is increasingly seen as “Best Practice” around the world. As we see below, a growing list of countries have adapted the SBIR program within their own innovation systems.171
• Brazil: The Brazilian Innovation Agency, better known by its acronym FINEP, operates the PIPE and Pappe programs that provide grants to hundreds of small companies that are commercializing technologies.172
• Japan: Japan is expanding the scope and scale of the Small Research Innovation Research program, which was established in 2003 and is directly modeled after the U.S. SBIR. The Small and Medium Enterprise Agency manages the program, and funds are contributed by various ministries involved in areas such as energy, information and communications technologies, and bio and medical sciences. Plans call for increased lending to small- and medium-sized enterprises, more hands-on support for start-ups, and making the application process more flexible.173
______________________
169 National Research Council, An Assessment of the SBIR Program, C. Wessner, ed., Washington, DC: The National Academies Press, 2008. Also from the National Research Council, see An Assessment of the SBIR Program at the Department of Defense (2009) and An Assessment of the SBIR Program at the National Institutes of Health (2009).
170 Block and Keller, op. cit. The authors analyze a sample of innovations recognized by R&D Magazine as being among the top 100 innovations of the year over the last four decades. They find that while in the 1970s almost all winners came from corporations acting on their own, more recently over two-thirds of the winners have come from partnerships involving business and government, including federal labs and federally-funded university research. The authors note that “the R&D 100 Awards carry considerable prestige within the community of R&D professionals, comparable to the Oscars for the motion picture industry. Organizations nominate their own innovations. All entries are initially evaluated by outside juries that include representatives of business, government, and universities.” (Block and Keller, 2008, page 6).
171 See OECD Innovation Policy Platform, “Public Procurement Programmes for Small Firms— SBIR-type programs,” which can be accessed at http://www.oecd.org/dataoecd/33/37/48136807.pdf. This publication describes the evaluation programs for SBIR in the United Kingdom and the Netherlands.
172 An explanation of these activities can be found in Odilon Antonio Marcuzzo do Canto, “Incentives to Support Innovation in the Private Sector: The Brazilian Experience,” Brazil Innovation Agency. Access at http://idbdocs.iadb.org/wsdocs/getdocument.aspx?docnum=976023.
173 See Ministry of Economy, Trade and Industry, “!00 Actions to Launch Japan’s New Growth Strategy,” Action 73, pg. 23, August 2010. Access at http://www.meti.go.jp/english/aboutmeti/policy/2011policies.pdf.
FIGURE 2.5 Percentage of R&D 100 Awards to firms with SBIR Awards.
SOURCE: Fred Block and Matthew R. Keller, “Where Do Innovations Come From? Transformations in the U.S. National Innovation System, 1970-2006,” Washington, DC: The Information Technology and Innovation Foundation, July 2008.
• India: The Small Business Innovation Research Initiative (SBIRI), launched in 2005, supports high-risk R&D projects by Indian biotech start-ups in sectors such as health care, agriculture, industrial technology, and environment. The program has phases for early-stage research and for later development and commercialization.174
• Netherlands: The Dutch government launched the Small Business Innovation Research program in 2004. The program fully funds the first phases of pre-commercial R&D up to €50,000 and up to €450,000 for a second two-year phase.175
• Finland: Of the €343 million that the government invested directly in enterprises in 2009, 61 percent went to small and midsized companies.
______________________
174 See Indian government Web site http://india.gov.in/sectors/science/sbiri.php.
175 See SCI-Network, “Case Study: Small business Innovation Research (SBIR) in the Netherlands,” March 2011. Access at http://www.sci-network.eu/fileadmin/templates/scinetwork/files/Resource_Centre/Case_Studies/Case_Study_-_Dutch_SBIR_-_Final.pdf.
Eighty-seven percent of those companies had fewer than 500 employees. Companies use the funds to develop technologies in partnership with universities.176
• United Kingdom. The Small Business Research Initiative, run by the Technology Strategy Board, was established in 2000 to earmark a share of the government’s procurement budget to contracts with small- and midsized enterprises. The program was revised and expanded in 2009.
• Germany: Among several government programs to help start-ups commercialize technology are the Central Innovation Programme SME (ZIM), which has an annual budget of €300 million and received another €900 million in 2009.177 Another program, EXIST, awards grants to technology start-ups and stipends to cover costs of equipment, materials, coaching, and childcare for scientists.178
Despite its validation though the National Academies assessment as well through its emulation abroad, the U.S., the U.S. Congress delayed reauthorization of SBIR for several years, creating uncertainty about the future of the program. The SBIR/STTR Reauthorization Act of 2011 has extended the programs through September 2017.. NIST’s Technology Innovation Program, which replaced the Advanced Technology Program, is now unfunded, despite a proven track record of success, at least for the ATP antecedent. A major advantage of ATP was its focus on linking small and large companies, along with universities to develop new high technology products.
DEVELOPING TWENTY-FIRST CENTURY UNIVERSITIES
A major asset for America’s innovation system is the strength and independence of its research universities. U.S. research universities serve as the funnel point for the entry into the U.S. of foreign talent in Science, Technology, Engineering, and Mathematics (STEM). Twenty-first century universities are also playing a growing role in supporting regional innovation ecosystems by transferring new technologies to the private sector. Universities such as Stanford, the Massachusetts Institute of Technology, Georgia Tech, and the University of Texas-Austin have acted as powerful engines of innovation, often
______________________
176 Tekes Annual Review 2009, (http://www.tekes.fi/en/community/Annual%20review/341/Annual%20review/1289).
177 Federal Ministry of Economics and Technology, Central Innovation Programme (ZIM), January 2011 (http://www.zim-bmwi.de/download/infomaterial/informationsbroschuere-zim-englisch.pdf). For an analysis of ZIM, see European Commission, ZIM, the Central Programme for SMEs (Zentrales Innovationsprogramm Mittelstand), PRO INNO Europe, INNO-Partnering Forum, Document ID: IPF 11-005, 2010.
178 Details of the EXIST program can be found on the German federal government Web site http://www.hightech-strategie.de/en/879.php.
generating promising new companies and industries.179 Passage of the Bayh Dole Act of 1980, which incentivized universities to sell and license technology generated from federally funded research, is widely associated with contributing to a boom in new technology companies.180
America’s 198 public research universities have long been the backbone of this system, conducting 62 percent of federally funded research.181 These institutions educate 85 percent of undergraduates and 70 percent of graduate students enrolled in all research universities. They account for 60 percent to 80 percent of doctorates degrees in computer and information sciences, engineering, math and statistics, physical sciences, and security—and from 78 percent to 95 percent of bachelor’s degrees in all of these areas of national need.182
U.S. Universities Face Financial Challenges
Yet this invaluable national asset is in financial trouble. 183 Charles M. Vest recently summed up the problem: “In the last decade, the real state appropriation to public colleges and universities per student has dropped by 20 percent overall. But the total cost to students and their families of attending a state university has increased by 52 percent during this same decade. Such declining state support and the resultant tuition increases are not a sustainable
______________________
179 See Edward P. Roberts and Charles Eesley, Entrepreneurial Impact; the Role of MIT, Kauffman Foundation Report (2009). Access at http://web.mit.edu/dc/policy/MIT-impact-full-report.pdf.
180 According to Arundeep S. Pradhan of the Association of University Technology Managers, “since 1980, American universities have spun off more than 5,000 companies, which have been responsible for the Introduction of 1.25 products per day into the marketplace and have contributed to the creation of more than 260,000 jobs. The result has been a contribution of over $40 billion dollars annually to the American economy.” Testimony before the House Committee on Science and Technology, July 17, 2007. For an academic assessment of the impact of the Bayh-Dole Act of 1980, see Rosa Grimaldi, Martin Kinney, Donald S. Siegel, and Mike Wright, “30 years after Bayh– Dole: Reassessing academic entrepreneurship” Research Policy, Volume 40, Issue 8, October 2011, Pages 1045–1057. See also the analysis of Mowery and Sampath who note that the catalytic effects of the Bayh-Dole Act may be overrated. Mowery, David C. and Sampat, Bhaven N., The Bayh-Dole Act of 1980 and University Industry Technology Transfer: A Model for Other OECD Governments? (2005). The Journal of Technology Transfer, Vol. 30, Issue 1-2, p. 115-127 2005. Finally see Roberts and Eesley, Entrepreneurial Impact, Kauffman Foundation Report (2009), http://web.mit.edu/dc/policy/MIT-impact-full-report.pdf
181 Association of Public Land-Grant Universities, “Ensuring Public Research Universities Remain Vital,” November 2010. Access at https://www.aplu.org/NetCommunity/Document.Doc?id=2819.
182 Association of Public and Land-grant Universities data. See Peter McPherson, David Shulenburger, Howard Gobstein, and Christine Keller, “Competitiveness of Public Research Universities & the Consequences for the Country: Recommendations for Change,” Association of Public and Land-Grant Universities, March 2009, (http://www.aplu.org/NetCommunity/Document.Doc?id=1561).
183 The National Academies of Sciences is undertaking a competitiveness study focusing on the health of U.S. research universities at the request of Senators Barbara Mikulski (D-MD) and Lamar Alexander (R-TN) and Representatives Bart Gordon (D-Tenn.) and Ralph Hall (R-Texas).
situation.”184 An article in The Chronicle of Higher Education noted that state funding for public universities has been declining for two decades on a perstudent basis and is reaching levels that are “threatening to cripple many leading public universities and erode their world-class quality.” 185
• State Budget Cutbacks: Cutbacks have been especially harsh in the past few years, as state-government budget deficits widened dramatically as a result of the recession. In 2009, the University of California’s budget was cut by 20 percent, or $813 million. The university expects a further 16.4 percent cut in Fiscal Year 2011, reducing state funding to 1999 levels even though there now are 73,000 more students.186 At the University of Georgia, state funds per student have dropped from $8,191 in FY 2009 to $6,242 in FY 2011 and also now are at 1999 levels not adjusted for inflation, despite 4,000 more students, additional buildings, and higher teacher salaries.187 Arizona State University’s budget was slashed by $88 million in 2009, and a further cut of 20 percent for four-year colleges in universities in the state has been proposed for 2011.188
In all, 32 U.S. states cut their support for higher education in 2010 by between 0.3 percent and 13.5 percent, with double-digit declines in Missouri, Delaware, Iowa, Minnesota, Arizona, and Oregon.189 “Given the national reliance on public universities for majority contributions to the nation’s need to advance knowledge and prepare new scientists and engineers,” warns the Association of Public and Land-Grant Universities, “a serious decline in the capacity of public research universities critically risks the attainment of these national goals.”190
• Limited use of Dedicated Taxes: Funding from states for universities is especially vulnerable to state budget cuts because it often comes
______________________
184 Charles M. Vest, “Chancellor’s Colloquium,” University of California, Davis, November 30, 2011, p. 8.
185 See Paul Courant, James Duderstadt, and Edie Goldenberg, “Needed: A National Strategy to Preserve Public Universities,” The Chronicle of Higher Education, January 3, 2010, (http://milproj.dc.umich.edu/pdfs/2010/2010-Chronicle-Commentary.pdf).
186 Carolyn McMillan, “Regents Scrutinize Fiscal Crisis,” UC Newsroom, March 16, 2011 (http://www.universityofcalifornia.edu/news/article/25150).
187 University of Georgia President Michael F. Adams Budget Update, May 19, 2010 (http://www.uga.edu/Budgetslides_05-19-2010.pdf).
188 The Arizona Republic, “ Arizona Board Approves Steep Tuition Hikes,” April 8, 2011.
189 Center for the Study of Education Policy data cited in Inside Higher Ed, “The Sinking States,” January 24, 2011, (http://www.insidehighered.com/news/2011/01/24/states_make_more_cuts_in_spending_on_higher_education).
190 Peter McPherson, Howard J. Gobstein, and David E. Schulenburger, “Forging a Foundation for the Future: Keeping Public Research Universities Strong,” Association of Public and Land-Grant Universities, 2010 (http://www.aplu.org/NetCommunity/Document.Doc?id=2263).
from general tax revenues. While programs such as highway maintenance and construction of sports stadiums and convention centers often are funded from recurring revenue streams such as lotteries, casino proceeds, and gasoline and alcohol taxes, only a handful of states dedicate such funds to universities.191
The advantage of these dedicated taxes is that these sources provide recurring revenue streams for important programs, even during times of economic downturn, and are not subject to government budgetary restraints. Notably, the State of Texas uses a Permanent University Fund (PUF), established in its 1876 Constitution, to fund higher education. Currently, PUF land assets deliver proceeds through oil, gas, sulfur, and water royalties, rentals on mineral leases, and gains on fiduciary investments.
• Declines in private funding: Reversing a three-decades-long trend of increasingly strong ties between industry and universities, the absolute value of industrial R&D dollars to academic institutions—funds provided directly to academic institutions for the conduct of research— began to decline beginning in 2002 after reaching a high of $2.2 billion in 2001. Also, industrial R&D support to academia has historically been concentrated in relatively few institutions.192 Leading university and industry leaders have pointed out that U.S. companies increasingly choose to work with foreign rather than U.S. universities, encouraged by the more favorable IP rights that foreign universities offer and the strong incentives for joint industry-university research that foreign governments provide. 193
Growing Investments in Universities Abroad
As U.S. universities struggle, other nations are increasing investment and overhauling their higher-education systems to turn universities into engines of innovation-led growth. Strengthening university commercialization programs, breaking down barriers between academia and industry, and freeing university researchers to start or join companies are standard features of many national innovation strategies. Governments in emerging economies also are aiming to
______________________
191 Alene Russell, “Dedicated Funding for Higher Education: Alternatives for Tough Economic Times,” American Association of State Colleges and Universities, Higher Education Policy Brief, December 2008. Access at http://www.aascu.org/uploadedFiles/AASCU/Content/Root/PolicyAndAdvocacy/PolicyPublications/08.decpm(2).pdf.
192 See National Science Foundation, “Where has the Money Gone? Declining Industrial Support of Academic R&D,” InfoBrief, NSF 06-328, September 2006.
193 GUIRR, “Re-Engineering the Partnership: Summit of the University-Industry Congress,” Meeting of 25 April 2006, Washington, DC.
upgrade universities to world standards and establish innovative new ones more attuned to needs of the global economy. For example—
• Flanders: The Flemish government launched a large €232 million program in 2006 for strategic basic research at universities of benefit to industry, the non-profit sector, and government policy objectives. The biggest investments have gone to large university-based R&D centers for microelectronics, biotechnology, and broadband technology. Each Flemish university has been instructed to keep a portfolio of industryoriented projects and operate a technology-transfer office. 194 At the Katholieke University Leuven, which has launched 100 companies, each of the 50 research divisions can reinvest proceeds from industrial involvement into equipment and infrastructure.195
• India: India’s 358 universities and famed Indian Institutes of Technology (IIT)traditionally have played little role in commercializing technology.196 The government is starting to overhaul the entire system of science and engineering education to promote collaboration with industry and allow faculty to work with industry. 197 A committee studying reforms of IITs is expected to call for granting them greater management and financial autonomy from the government and to encourage research partnerships with private companies.198 India’s Five-Year plan, for example, calls for establishing a network of globally competitive “centers of excellence” in certain technologies based at universities.199
• Canada: As part of Canada’s efforts to promote commercialization by universities, the Foundation for Innovation since 1997 has allocated $5.2 billion to research projects, new laboratories, industry collaborations, and recruitment of foreign faculty. The government also has increased the number of Centers of Excellence based at universities
______________________
194 Fientje Moerman ”Keynote Address,” National Research Council, Innovative Flanders, Innovation Policies for the 21st Century, C. Wessner, ed., Washington, DC: The National Academies Press, 2008.
195 Koenraad Debackere, ”Leuven as a Hotspot for Regional Innovation,” in Innovative Flanders, op. cit.
196 Martin Gruber, and Tim Studt. “2011 Global R&D Funding Forecast: The Globalization of R&D,” R&D Magazine, December 15, 2010.
197 Ramesh Mashelkar I, “Renewing the National Laboratories,” in India’s Changing Innovation System, op. cit. Also see P. V. Indiresan, “National and State Investments in Science and Engineering Education,” in India’s Changing Innovation System, op. cit.
198 Hindustan Times, “More Autonomy, New Programmes for IITs,” January 16, 2011.
199 Government of India Planning Commission, “Report of the Steering Committee on Science and Technology for Eleventh Five Year Plan (2007-2012),” December 2006, (http://planningcommission.gov.in/plans/planrel/fiveyr/11th/11_v1/11th_vol1.pdf). Also see Hindustan Times, “More Autonomy, New Programmes for IITs,” January 16, 2011. India’s Eleventh Five-Year plan also sets high targets for expanding university enrollment. http://planningcommission.gov.in/plans/planrel/fiveyr/11th/11_v1/11th_vol1.pdf.
devoted to research collaborations with industry. The centers, in fields ranging from optics to brain research, are credited with spinning off more than 100 companies.200
• Singapore: Singapore is seeking to upgrade schools such as the National University of Singapore and Nanyang Technological University, which are strong in science and technology, to become world-class research institutions and fonts of entrepreneurialism. The government established a high-level Enterprise Board at each university and an innovation fund to supplement each school’s own resources to finance entrepreneurship education, technology incubators, commercialization programs, and entrepreneurs-in-residence programs. Polytechnics are receiving grants to help universities bring research to the market.201
• Japan: In 1999, Japan enacted a law similar to the Bayh-Dole Act of 1980 allowing universities and research institutes to patent investments derived from publicly funded research.202 The government also boosted funding for joint university-industry research programs and helped universities set up 45 centers for commercializing research. In 2004, universities were given more autonomy to allocate resources, collaborate with industry, and set their own research priorities.203 The reforms led to a sharp rise in university spin-offs and industry research collaborations.204
• Brazil: Between 2000 and 2008, the number of master’s degrees and doctorates awarded by Brazilian universities annually has both doubled, to more than 36,000 and 10,000, respectively. From 2002 to 2010, the government invested $550 million to build 226 new technology schools.205
______________________
200 Networks of Centers of Excellence Web site. Also see Peter J. Nicholson, “Converting Research into Innovation,” in Innovation Policies for the 21st Century, op. cit.
201 National Research Foundation, “National Framework for Innovation and Enterprise,” Prime Minister’s Office, Republic of Singapore, 2008, (http://www.nrf.gov.sg/nrf/otherProgrammes.aspx?id=1206).
202 See Sadao Nagaoka and Kenneth Flamm, “The Chrysanthemum Meets the Eagle— The Coevolution of Innovation Policies in Japan and the United States,” in National Research Council, 21st Century Innovation Systems for Japan and the United States: Lessons from a Decade of Change, Masayuki Kondo, Kenneth Flamm, and Charles Wessner, Editors, Washington, DC: The National Academies Press, 2009.
203 A concise analysis of Japan’s shift in innovation policy is found in National Research Council, S&T Strategies of Six Countries: Implications for the United States, Committee on Global Science and Technology Strategies and Their Effect on U.S. National Security, Washington, DC: The National Academies Press, 2010.
204 Masayuki Kondo, “Kyutenkaishihajimeta Nippon no Daigakuhatsubencha no Genjou to Kadai” (“The Current State and Issues of Rapidly Increasing University Spin-offs in Japan”), Venture Review, No. 3, 101-107. 2002.
205 Francelino Grando, “Brazil’s New Innovation System,” National Academies symposium, Clustering for 21st Century Prosperity, Washington, DC, February 25, 2010.
• China: China has an ambitious $2.8 billion plan called Project 211 to create 100 higher education facilities that are on par with the best in the world.206
• The United States is one of the few industrial nations without a national strategy for sustaining the quality of its research universities.207 Some higher-education experts contend that public research universities’ reliance on state funding is a flaw in the U.S. innovation system because state lawmakers do not recognize a direct payoff from such investments. The authors of the Chronicle of Higher Education article note that “many of the benefits from graduate training—like the benefits of research—are public goods that provide only limited returns to the states in which they are located. The bulk of the benefits are realized beyond state boundaries.” Several higher-education advocates contend the U.S. federal government needs to assume more responsibility for funding public research universities. 208 To provide more stable funding for higher education, the American Association of State Colleges and Universities has called upon states to earmark more revenue from recurring sources such as excise taxes, gaming, and landuse rights.209
New Models of 21st Century Universities
While it’s often the case that other nations are adapting best practices from the United States, new schools are being established around the world based on innovative models designed to meet the needs of the 21st century global economy. Several of these new institutions deserve study by American educators. Finland’s Aalto University, for example, merges three existing universities that specialized in technology, economics, and art and design to integrate students and faculty in all of these disciplines into a single community.210 Since the 1990s, Sweden’s Chalmers University of Technology has transformed itself into one of Europe’s most entrepreneurial universities.211 The new Singapore University of Technology and Design, a collaboration with MIT and China’s Zhejiang University, will have a multi-disciplinary curriculum
______________________
206 See China Education and Research Network, “Project 211: A Brief Introduction,” (http://www.edu.cn/20010101/21852.shtml).
207 Courant, Duderstadt, and Goldenberg, op. cit.
208The chancellor and vice-chancellor of the University of California at Berkeley, for example, have called for the federal government to provide basic funding for a limited number of top public research universities. See Robert J. Birgeneau and Frank D. Yeary, “Rescuing Our Public Universities,” Washington Post, September 27, 2009.
209 American Association of State Colleges and Universities, op. cit.
210 Aalto University Web site: http://www.aalto.fi/en/.
211 See Merle Jacob, Mats Lundqvist, and Hans Hellsmark, “Entrepreneurial Transformations in the Swedish University System: The Case of Chalmers University of Technology,” Research Policy, Volume 32, Issue 9, October 2003, pp. 1555-1568.
and research focus that will strive to teach students to be creative and solve problems.212 The university will house an International Design Center modeled after a smaller facility at MIT and intends to “become the world’s premier hub for technologically intensive designs.” MIT will help design programs to encourage innovation and entrepreneurship.
New Opportunities for Global Collaboration
The globalization of universities is helping to foster a 21st century learning environment for American students by proving them greater opportunities to work with partners and in teams that are cross cultural as well as cross functional. Technological advances are also allowing for students and faculty to work across borders while avoiding the time and costs of travel. These potentials can be further developed through encouraging U.S. faculty and students to collaborate more extensively with their peers abroad, as demonstrated by leading U.S. universities like MIT and Carnegie Mellon University.
Indeed, a number of U.S. academic institutions are now operating internationally, addressing not only potential students individually (per the traditional paradigm), but increasingly addressing foreign universities, foreign local authorities and governments, in order to develop new types of institutional arrangements. These include helping creating, monitoring or evaluating emerging institutions in other countries, transferring organizational skills, operating training programs for teachers and researchers, contributing to higher education and research capacity building abroad and to the marketing of its benefits for economic and social progress in other societies. Such new arrangements may also include the coaching and steering of research programs in emerging countries, their early inclusion in international networks, and the affiliation of private companies to academic and research programs.
On the other hand, many emerging nations are now facing the need and the opportunity of large investments in science, technology and higher education (public and private), aiming at responding to the explosive social demand for higher education and to the vast social and political transformations already induced by new waves of educated youth. These investments not only seek new skills and but also the certification of quality that may be expected from working along together with well established academic and scientific institutions from the United States. For these institutions, including the American universities, such institutional arrangements provide new forms of expansion, as they tend to help securing new financial or human
______________________
212 Brochure of Singapore University of Technology and Design, 2010. Access at http://www.sutd.edu.sg/cmsresource/brochure/undergraduate_brochure.pdf.
resources, and to challenge their own traditional competences and agendas. Above all, they provide unique access to new pulls of talent worldwide, benefiting above all leading American universities. 213
The culture of academic collaboration with industry is well established in the United States. Notable among these is the Semiconductor Research Corporation Focus Center Research Program, a multi-million dollar, 30 university research collaboration to address long-term technology issues of relevance to the semiconductor industry.214
University-industry collaboration, particularly with regard to technology-transfer programs, offers a mixed picture.215 Over all, the number of start-ups spun out of elite research universities in the United States has risen from 200 in 1994 to 651 in 2010. Successful patent applications and new technology licenses, had remained flat for a decade, but were up in the latest survey by the Association of University Technology Managers (AUTM). According to AUTM’s 2010 survey, the number of startups formed increased 10.6 percent and the number of licenses/options executed to startups increased 14 percent. At the same time, “the total number of licenses and options executed remained essentially flat, increasing only 0.6 percent. The number of licenses executed decreased two percent, whereas the number of options increased 13 percent. However, there was a strong 15 percent increase in the total number of active licenses and options through the close of 2010.”216 Of 20,309 invention disclosures by universities in 2010, about 22 percent resulted in issued U.S. patents.
The performance of university technology-transfer offices varies. Fiftytwo percent of the 130 technology-transfer programs studied do not have revenues to cover their costs. Some 16.2 percent of U.S. institutions surveyed reported that their programs are financially self-sustaining, meaning they do not
______________________
213 D. Bruce Johnstone et al., eds., Higher Education in a Global Society, New York: Northampton MA: Edward Elgar Publishing, 2010.
214 The Focus Center Research program is aimed at solving the long-range (normally 5 years or more), difficult challenges outlined in the International Technology Roadmap for Semiconductors, which is a forward looking assessment that is sponsored by several industry groups.
215 For a review of the challenges universities face in technology transfer, see DiGregorio, D., and Shane, S. “Why do some universities generate more start-ups than others?” Research Policy, 32(2), 209-227, 2003. (Reprinted in D. Siegel (ed.) Technological Entrepreneurship: Institutions and Agents Involved in University Technology Transfer, Aldershot, UK: Edward Elgar) and Siegel, D. et al, 2003. “Assessing the impact of organizational practices on the relative productivity of university technology transfer offices.” Research Policy, 32(1):27-48. See also Thursby, J. and Thursby M. “Who is Selling the Ivory Tower? Sources of Growth in University Licensing,” Management Science, 48:1, January 2002, 90-104.
216 See AUTM U.S. Licensing Survey Highlights, 2010.
depend on the university’s operating budget.217 Many technology transfer offices are not only underfunded but also labor under federal rules that make it difficult for principal investigators to commercialize federally funded research.218 In addition, the system for allocating federal R&D funds and for rewarding faculty focuses overwhelmingly on scientific discovery, rather than on applied research or development of prototypes. 219
A recent National Research Council study affirmed that the primary mission of university technology transfer activities is the dissemination of technologies for the public good and recommended that the current system of technology transfer be improved.220 To this end, the study on Managing Intellectual Property in the Public Interest noted that university leadership should more clearly articulate the mission of technology transfer activities and adopt organizational changes to make them more effective.221
The United States pioneered the use science and technology parks— typically with research universities at their core—as platforms for launching new companies and creating regional innovation clusters. Now, research parks are proliferating across the world. While key aspects are borrowed from successful U.S. science and technology parks, many new parks overseas have a greater scope and scale, and in many cases benefit from substantial government funding. Here are some examples.222
• Singapore: Singapore is building a network of science parks in a 500 acre urban district called One North, located close to the National University of Singapore, National University Hospital, and Singapore Polytechnic. The $10 billion master plan includes Biopololis, a 4.5 million-square-foot campus that aspires to be Asia’s biomedical hub. The complex houses 5,000 life science researchers from universities, hospitals, and multinationals such as Eli Lilly and Novartis in disciplines ranging from X-ray crystallography to DNA sequencing. One North also includes Fusionopolis, a futuristic 24-story tower intended as a one-stop R&D shop mixing companies in energy
______________________
217 Presentation by Ashley Stevens at the National Academies Symposium on Clustering for 21st Century Prosperity, February 25, 2010.
218 National Research Council, Managing University Intellectual Property in the Public Interest, Stephen A. Merrill and Ann-Marie Mazza, editors, Washington, DC: National Academy Press, 2010.
219 Darmody, “University Based Clusters,” op. cit.
220 National Research Council, Managing University Intellectual Property in the Public Interest, op. cit.
221Ibid.
222 See National Research Council, Understanding Research, Science, and Technology Parks, op. cit. Also, see National Research Council, Innovative Flanders: Innovation Policies for the 21st Century, Charles W. Wessner, ed., Washington, DC: The National Academies Press, 2008.
technologies, aerospace, nanotechnology, sensors, cognitive science, and devices for wired homes.223
• Russia: The government is investing $3 billion over three years in an attempt to develop a 400-hectare Skolkovo district in Moscow into an innovation hub for multinationals and Russian start-ups. Siemens, GE, and Nokia-Siemens have all pledged to build R&D centers, and Dow, Intel, and Cisco have signed memorandums of understanding to do so. Skolkovo will include a new university being developed in a partnership with MIT that is to open in 2014. The central government also has earmarked $172 million to be given to 130 start-ups.224
• China: China has a number of mega-parks larger in size than North Carolina’s Research Triangle and that typically feature a diversity of industries and a high concentration of R&D facilities by universities, corporations, and government research institutes. The Chinese government invested $1.4 billion in Suzhou Industrial Park, for example, home to operations of 113 of the Fortune 500 companies. The more established Zhongguancun Science Park in Beijing hosts more than 20,000 enterprises and 950,000 employees, and has produced $110 billion worth of income as of 2009. 225 The Zhangjiang High-Tech Park in Shanghai’s Pudong district, which was farmland in 1992, has more than 4,000 companies and 100,000 workers and covers 17 square kilometers. Zhangjiang includes more than 30 government research institutes and 91 R&D centers by multinational corporations in such industries as life sciences, information technology, semiconductors, and multimedia gaming. It also has a $2.5 billion venture capital fund for start-ups and nearly 100 multinational corporate R&D centers, including major expansions by Novartis, General Electric, Pfizer, Novartis, and AstraZeneca.226
• Mexico: The new Research and Technology Innovation Park (PIIT) on the outskirts of Monterrey, Mexico, has strong ties to Tecnologico de Monterrey, the nation’s premier engineering school. Spread over 172 acres near the airport, PIIT will the first in Mexico to integrate the laboratories in an array of technologies by leading universities, foreign and domestic corporations, small-business incubators, and national laboratories at a single site. PIIT’s first $145 million phase includes major laboratories by companies as diverse as Motorola, PepsiCo, and India’s Infosys. It also is building public R&D centers for electronics,
______________________
223 See Yena Lim, “The Singapore S&T Park,” in National Research Council, Understanding Research, Science and Technology Parks, op. cit.
224 Courtney Weaver, “Welcome to Russia’s Silicon Valley,” Financial Times, August 21, 2011.
225 See Zhu Shen, “China: Navigating the Frontier of Life Sciences Silk Road,” in National Research Council, Understanding Research, Science, and Technology Parks, op. cit.
226 Data from Zhangjiang High-Tech Park Web site, http://www.zjpark.com/zjpark_en/zjgkjyq.aspx?ID=7.
biotechnology, mechatronics, advanced materials, the food industry, product design, IT, and water research. The University of Texas at Austin will run an IC2 business incubator.227
• France: Minatec, a campus of 3,000 students and researchers in Grenoble, has emerged as one of Europe’s premier hubs for nanotechnology and micro-system research. The French government has invested €3.2 billion and regional and local governments have provided another €150 million for the 20-hectare campus, which in the lynchpin of a €4 billion government initiative to make Grenoble a world center for development of next-generation chips. Minatec has 200 industrial partners, including Mitsubishi, Philips, Bic, and Total, and has launched startups in fields such as optronics, biotechnology, circuit design, and sensing.228
Measuring the performance of science and research parks is difficult, and empirical literature on the topic has been described as “embryonic.”229 Several experts note that better metrics are required to evaluate research parks in order to justify the substantial public investment.230 In their seminal study of research parks, Michael I. Luger and Harvey A. Goldstein observed that one reason measuring performance is difficult is that “there is no consensus about the definition of success.” Goals cited by developers, universities, and public officials include economic development, technology transfer, land development, and enhancement of research capabilities at affiliated universities.231
Companies in similar industries have long tended to locate close to each other for centuries .232 In the United States, innovation clusters in regions such as Silicon Valley and greater Boston have tended to flourish close to major research universities without government coordination. In the past two decades,
______________________
227 Jaime Parada, “Monterrey-International City of Knowledge Program,” National Research Council, Understanding Research, Science and Technology Parks, op. cit.
228 David Holden, “Initiatives in France,” National Research Council, Understanding Research, Science, and Technology Parks. op. cit.
229 For a review of the empirical literature on research parks, see Albert Link, “Research, Science, and Technology Parks: An Overview of the Academic Literature,” in National Research Council, Understanding Research, Science, and Technology Parks. op. cit.
230In his presentation at the March 13, 2008, National Academies symposium “Understanding Research, Science, and Technology Parks,” William Kittredge of the U.S. Department of Commerce described effective performance-measurement metrics for research parks and for economic development in general remains a “work in progress.”
231 Michael I. Luger and Harvey A. Goldstein, Technology in the Garden, Chapel Hill: University of North Carolina Press, 1991, p. 34.
232 Alfred Marshall was one of the first economists to develop a theory about regional agglomerations of industries. See Principles of Economics, London: Macmillan, 1920. The first edition of Marshall’s classic textbook appeared in 1890.
however, regional innovation clusters have become a matter of more focused public policy in the U.S. and around the world.233 Of 260 cluster initiatives studied in 2003, government supported two-thirds. In 52 percent, government was the primary funder.234
International Cluster Initiatives
Regional cluster initiatives linking universities, industry, government economic-development agencies, and investor groups now are found across Asia-Pacific, Europe, and Latin America. The European Union even operates a European Cluster Observatory that maps clusters across the continent.235 In some cases, clusters receive significant government financial assistance and are integral components of comprehensive national or regional innovation strategies.
These examples offer a flavor of the public-private strategies being deployed around the world—
• Germany: The German government is investing €500 million and private industry €2.6 billion in “innovation alliances” that aim to develop nine innovation clusters.236 An initiative for a cluster in molecular imaging for medical engineering, for example, includes Bayer Schering Pharma, Goehringer Ingelheim Pharma, and Siemens. Other innovation alliances include photovoltaic cells, lithium-ion batteries for energy storage, and automotive electronics.237 Germany’s Fraunhofer has established pilot production centers in a program to accelerate development of cluster in organic electronics in Heidelberg involving a coalition of universities and companies. 238
• Brazil: Brazil’s Minas Gerais state is supporting emerging clusters in microelectronics, bio-fuels, and software. The state also has identified
______________________
233 See Örjan Solvell, Göran Lindqvist, and Christian Ketels, “The Cluster Initiative Greenbook” (Stockholm: The Competitiveness Institute, 2003). Of 260 cluster initiatives around the world studied for this report, 72 percent had been established in 1999 or later.
234 Ibid.
235 Presentation by Andrew Reamer, “Stimulating Regional Economies: The Federal Role,” in the National Academies Symposium, Growing Innovation Clusters for American Prosperity, June 3, 2009.
236 German Federal Ministry for Education and Research, Ideas. Innovation. Prosperity: High Tech Strategy 2020 for Germany, Berlin: BMBF, 2010 (http://www.bmbf.de/pub/hts_2020_en.pdf). Details of the “European Cluster Alliance” can be found at http://www.proinnoeurope.eu/index.cfm?fuseaction=page.display&topicID=223&parentID=50.
237 Information on Germany’s Innovation Alliances is found on the Research in Germany Web site, http://www.research-in-germany.de/coremedia/generator/research-landscape/rpo/networks-andclusters/41832/10-3-innovation-alliances.html.
238 Presentation by Christian May, “German Policy Initiatives,” in the National Academies Symposium on Flexible Electronics for Security, Manufacturing, and Growth in the United States. September 24, 2010.
several hundred “poles of excellence” in traditional industries that it is seeking to consolidate into hubs based in one location so that they can achieve bigger scale and support larger concentrations of public and private investment. To advance these clusters, the new agency Sistema Mineiro de Inovação, or SIMI, is supporting science parks, incubators, and training programs and helping establish linkages between government programs, researchers, and investors across the state.239
• Taiwan: The Taiwanese government was instrumental in launching the island’s semiconductor, notebook computer, and liquid-crystal display industrial clusters, among others in the 1980s and 1990s.240 Now, the Industrial Technology Research Institute is coordinating public-private to establish Taiwan as a global leader in industrials such as flexible displays, solid-state lighting devices, and solar modules.241 The government has invested more than $50 million to help Taiwan develop a comprehensive supply chain for flexible electronics, for example, and has helped acquire key U.S. technologies.242
• Hong Kong: The Hong Kong government began a concerted clusterdevelopment program following the 1997 Asian financial crisis. It began by targeting areas like green technology, precision engineering, communications technologies, and biotechnology. The goal is to leverage Hong Kong’s strategic location on the border of mainland China. Hong Kong is promoting such new clusters as thin-film photovoltaic panels, chips wireless telecom devices, and smart cards. The government has invested $1.5 billion in a science park that is the focal point of these clusters.243
• Singapore: Singapore’s Ministry of Trade and Industry announced $10 billion in R&D spending over five years to accelerate development of clusters such as life sciences, environmental and water technologies, interactive and digital media. The government wants Singapore to become a “global talent hub” in these industries and expects they will employ 80,000 by 2015 and that their value-added will triple to
______________________
239 Alberto Duque Portugal, “An Integrated Approach: Brazil’s Minas Gerais Strategy,” in the National Academies Symposium on Clustering for 21st Century Prosperity, February 25, 2010.
240 See Alice H. Amsden, “Taiwan’s Innovation System: A Review of Presentations and Related Articles and Books,” Memorandum on the National Academies Symposium, “21st Century Innovation Systems for the U.S. and Taiwan: Lessons From a Decade of Change,” Taipei, January 46, 2006.
241 Chu Hsin-Sen, “The Taiwanese Model: Cooperation and Growth” in National Research Council, Innovation Policies for the 21st Century. Op. cit.
242 See presentation by John Chen, “Taiwan’s Flexible Electronics Program,” at the National Academies Symposium on Flexible Electronics for Security, Manufacturing, and Growth in the United States.” September 24, 2010.
243 Presentation by Nicholas Brooke “Optimizing Synergies: The Hong Kong Science Park” at the National Academies Symposium on Clustering for 21st Century Prosperity, February 25, 2010.
$27 billion.244 Government support for these clusters includes new incubators and funding for early-state capital programs.245
U.S. Regional Cluster Initiatives
As previously mentioned, many promising regional innovation cluster initiatives are underway across the U.S. Many of cluster-building strategies at the state level reflect a holistic understanding of what it takes to build a 21st century innovation ecosystem and compete globally in specific industries. 246 Promising state and regional initiatives often involve public-private partnerships in which corporations, universities, and governments pool resources to establish R&D centers, train workforces, develop supply and support industries, and provide risk capital to starts-ups where angel and venture funding is lacking. 247
State governments are deploying a wider range of policy tools, from tax credits and R&D grants to low-cost loans to free workforce training, in the attempt to close the gap with financial incentives offered by offshore locations in the intense competition for investment.248 Few of these initiatives, however, can match the financial resources and policy support of those in other nations.249
In remarks at a STEP Board symposium, then Commerce Secretary Gary Locke declared that “regional innovation clusters have a proven track record of getting good ideas more quickly into the marketplace. The burning question becomes, ‘How do we create more of them?’”250
______________________
244 Singapore Ministry of Trade and Industry, Sustaining Innovation-Driven Growth, Science, and Technology, Government of Singapore, February 2006, (http://app.mti.gov.sg/data/pages/885/doc/S&T%20Plan%202010%20Report%20(Final%20as%20of%2010%20Mar%2006).pdf).
245 Singapore National Research Foundation, “National Framework for Innovation and Enterprise,” Prime Minister’s Office, Republic of Singapore, 2008, (http://www.nrf.gov.sg/nrf/otherProgrammes.aspx?id=1206.
246 For review of cluster growth in the U.S. states, see Mary Jo Waits, “The Added Value of the Industry Cluster Approach to Economic Analysis, Strategy Development, and Service Delivery.” Economic Development Quarterly, 14(1):35-50, February 2000.
247A National Research Council Committee led by Gordon Moore concluded that “Public-private partnerships, involving cooperative research and development activities among industry, government laboratories, and universities, can play an instrumental role in accelerating the development of new technologies to the market.” See National Research Council, Government-Industry Partnerships for the Development of New Technologies, C. Wessner, ed., Washington, DC: The National Academies Press, 2003, page 23.
248 See National Research Council, Growing Innovation Clusters for American Prosperity, Charles W. Wessner, Rapporteur, Washington, DC: The National Academies Press, 2011.
249 For a review of scope, as well as advantages and disadvantages of state capitalism, See The Economist, The Rise of State Capitalism, January 21, 2012.
250 Keynote address by then Commerce Secretary Gary Locke at the National Academies Symposium on Clustering for 21st Century Prosperity, Washington, DC, February 25, 2010.
A number of analysts, policy institutes, and non-government organizations have published studies in recent years urging the federal government to make regional initiatives a core element in economic development.251 Rather than calling for massive new funding, several of these same studies call on federal agencies to make more effective and efficient use of scattered resources they already deploy. Michael Porter, for instance, has criticized existing federal programs as “often fragmented, duplicative, and inefficient.”252
One new federal approach is for several agencies to pool efforts with state and local governments and universities to support specific regional clusters aimed at meeting national needs. Under White House leadership, the SBA, NIST, EDA, NSF, and EDC, for example, are joining an effort by the DOE to establish “energy-innovation hubs,” regional innovation clusters in solar power, energy-efficient buildings, nuclear energy, and advanced batteries. The first $129.7 million project seeks to create an innovation hub devoted to developing technologies, designs, and systems for energy-efficient buildings that will be based at the Philadelphia Navy Yard Clean Energy.253 President Barack Obama’s 2009 budget also allocated $50 million in funds administered by the Commerce Department’s Economic Development Agency to assist regional cluster initiatives,254 while the SBA is working with state agencies and the DOD to help launch robotics clusters in Michigan, Virginia, and Hawai’i.255
______________________
251 For example, see Karen G. Mills, Elisabeth B. Reynolds, and Andrew Reamer, “Clusters and Competitiveness: A New Federal Role for Stimulating Regional Economies,” Metropolitan Policy Program at Brookings, April 2008. Also see Michael E. Porter, “Clusters and Economic Policy: Aligning Public Policy with the New Economics of Competition,” Institute for Strategy and Competitiveness White Paper, revised May 18, 2009. Mark Muro and Bruce Katz, “The New Cluster Moment: How Regional Innovation Clusters Can Foster the Next Economy,” Washington, DC: Brookings Institution, September 2010, http://www.brookings.edu/papers/2010/0921_clusters_muro_katz.aspx.
252 Porter, op. cit.
253 Department of Energy press release, “Penn State to Lead Philadelphia-Based Team that will Pioneer New Energy-Efficient Building designs,” August 24, 2010, (http://www.energy.gov/news/9380.htm). Details on the energy innovation research cluster can be found in the funding opportunity announcement for FY 2010 on the DOE Web site. See http://www.energy.gov/hubs/documents/eric_foa.pdf.
254 President Obama’s fiscal 2009 budget provided $50 million in regional planning and matching grants within the Economic Development Administration to “support the creation of regional innovation clusters that leverage regions’ existing competitive strengths to boost job creation and economic growth.” See Executive Office of the President, “A Strategy for American Innovation: Driving Towards Sustainable Growth and Quality Jobs,” National Economic Council Office of Science and Technology Policy, September 2009.
255 Presentation by Karen Mills, “Building Regional Innovation Clusters” at the National Academies Symposium on Clustering for 21st Century Prosperity, February 25, 2010.
One of the keys to America’s post-war dominance of high-technology industries has been its ability to attract the world’s best and brightest scientific, technological, and entrepreneurial talent. European immigrants such as Alexander Graham Bell helped fuel America’s industrial takeoff, and the U.S. assumed world leadership in physical sciences with the help of an influx of physicists who fled European fascism, including such Albert Einstein and Enrico Fermi.256 Since the 1970s, immigrant engineers and scientists from India, Taiwan, South Korea, and then China have been instrumental to the success of the U.S. semiconductor, computer, software industries, and biotechnology industries and have founded an inordinate share of U.S. technology companies.257
America is as dependent as ever on imported brainpower as a pipeline for future innovation: Foreign students earned 40 percent of U.S. science and engineering doctorate degrees in 2005, compared to 16 percent in 1980. In engineering, the share was 61 percent.258 One telling sign of this foreign dominance is to look at where recipients of U.S. engineering Ph.D. have earned their bachelor’s degrees. Of the 10 schools with the highest representation of alumni in 2008, six are from China. 259The Massachusetts Institute of
______________________
256 These scientists and engineers were highly esteemed by society though public perceptions may have changed. Recent research suggests that public perceptions of science are highly contextual, with people making judgments about the relative trust to be placed in traditional scientific expertise (which often is generated by government institutions) and in local knowledge based in the local context. See, Lewenstein, Bruce V. 1992. “The Meaning of ’Public Understanding of Science’ in the United States After World War II.” Public Understanding of Science 1 (1):45-68. Recent research also reveals that that social support contributes directly to men’s and women’s ability to envision themselves in a future science career, which, in turn, predicted their interest in and motivation for a science career. See Sarah K. Buday, Jayne E. Stake and Zoë D. Peterson, “Gender and the Choice of a Science Career: The Impact of Social Support and Possible Selves.” Sex Roles-Journal of Research, 66(3-4):197-209, 2012.
257 AnnaLee Saxenian of the University of California at Berkeley estimated that Chinese and Indian engineers were represented on the founding teams of 24 percent of Silicon Valley technology businesses founded between 1980 and 1998. See AnnaLee Saxenian, Silicon Valley’s New Immigrant Entrepreneurs, San Francisco: Public Policy Institute of California, 1999. A follow-up study found that in one-quarter of all U.S. technology companies founded between 1995 and 2005, one-quarter had chief executive officers or chief technology officers who were foreign-born. See Vivek Wadhwa, Ben Rissing, AnnaLee Saxenian, Gary Gereffi, “Education, Entrepreneurship and Immigration: America’s New Immigrant Entrepreneurs, Part II,” Duke University Pratt School of Engineering, U.S. Berkeley School of Information, Ewing Marion Kauffman Foundation, June 11, 2007.
258 Robert V. Hamilton presentation at Brookings Institution conference on “Immigration Policy: Highly Skilled Workers and U.S. Competitiveness and Innovation,“ Washington, February 7, 2011.
259 Semiconductor Industry Association, Maintaining America’s Competitive Edge: Government Policies Affecting Semiconductor R&D and Manufacturing Activity, prepared by Dewey & LeBoeuf, March 2009, (http://www.sia-online.org/galleries/defaultfile/Competitiveness_White_Paper.pdf).
Technology ranks No. 10. Chinese students alone accounted for 30 percent of all U.S. doctorate degrees granted in natural sciences.260
Now the competition for non-native talent is becoming global as more countries take an activist approach to recruiting talent.261 To address skill shortages exacerbated by an aging population, the European Union has promulgated a “blue card” that allows highly skilled migrants from non-EU nations to live and work on a temporary base, and also allows them to move freely among most member countries.262 The EU also is simplifying procedures for obtaining legal resident status for foreign workers to by setting up a “onestop-shop” system for applicants.263 Canada has made recruiting foreign talent a top priority in its national innovation strategy. 264 Forty percent of the 8,053 new faculty members and 44 percent of the 1,806 new researches recruited by Canadian universities and the Foundation for Innovation as of the fall of 2009 came from other nations, for example.265 Thirty percent of the nearly 2,000 department chairs hired the Canada Research Chairs program also were recruited outside of Canada.266 Singapore’s innovation strategy puts a heavy emphasis on “drawing creative and talent people from all corners of the world to live and work in Singapore.”267 Among its prize recruits are eminent scientists from the National Cancer Institute, MIT, and the University of California at San Diego.268
While other nations step up recruiting, it has been getting more difficult for highly skilled foreigners to live and work in the U.S. The backlog for permanent resident visas grew so long amid tightened scrutiny after the Sept. 11,
______________________
260 Robert V. Hamilton, “Foreign Natural Sciences Doctoral Attainment at U.S. Universities, 1980 to 2005, George Mason University, prepared for Brookings Institution conference on “Immigration Policy: Highly Skilled Workers and U.S. Competitiveness and Innovation, “ Washington, February 7, 2011.
261 See Devesh Kapur and John McHale, Give us Your Best and Brightest, Washington, DC: Center for Global Development, 2005.
262 The Blue European Labour Card is an approved EU-wide work permit (Council Directive 2009/50/EC) allowing high-skilled non-EU citizens to work and live in any country within the European Union, with the exception of UK, Denmark, and Ireland.
263 Europa, “Making Europe More Attractive to Highly Skilled Immigrants and Increasing the Protection of Lawfully Residing and Working Migrants,” Brussels, October 23, 2007, (http://europa.eu/rapid/pressReleasesAction.do?reference=IP/07/1575.
264 Industry Canada, Achieving Excellence: Investing in People, Knowledge and Opportunity— Canada’s Innovation Strategy, 2001. (http://dsp-psd.pwgsc.gc.ca/Collection/C2-596-2001E.pdf).
265 Canada Foundation for Innovation, 2009 Report on Result, op. cit.
266 Canada Research Chairs data http://www.chairs-chaires.gc.ca/home-accueil-eng.aspx.
267 Ministry of Trade and Industry, Sustaining Innovation-Driven Growth, Science, and Technology, Government of Singapore, February 2006, (http://app.mti.gov.sg/data/pages/885/doc/S&T%20Plan%202010%20Report%20(Final%20as%20of%2010%20Mar%2006).pdf).
268 Lim Chuan Poh, “Singapore Betting on Biomedical Science,” Issues in Science and Technology, Spring 2010.
2001, terrorist attacks that an estimated 1 million people were waiting for 120,120 visas issued a year as of 2006—a backlog of nine years.269
The tougher immigration climate comes despite forecasts of looming skill shortages due to demographic changes and declining interest by U.S. students in science and engineering. The McKinsey Global Institute, for instance, projects a possible shortfall of nearly 2 million technical and analytical workers in the U.S. over the next 10 years. 270 The National Association of Manufacturers and Deloitte & Touche reported that higher immigration will be necessary to meet a projected need for new skilled workers in manufacturing by 2020. The alternative could be “a significant decrease in manufacturing’s competitiveness.”271 The Brookings Institution concludes that the “the U.S. immigration priorities and outmoded visa system discourage skilled immigrants and hobble the technology-intensive employers who would hire them.” As a result, these policies “work against urgent national priorities.”272
Not all analysts agree that dramatic increases in immigration are required to meet future skill needs. Research by Lindsey Lowell and Harold Salzman, for example, concluded that the U.S. actually graduates more STEM students than are hired each year, and that many graduates find work in other fields for economic reasons.273 Nor is there yet firm evidence that Chinese, Indian, and other foreign nations are returning home in significant numbers after receiving advanced U.S. science and technology degrees. 274 Other studies, however, suggest a significant risk of a “brain drain” as highly skilled Chinese and Indians leave to take advantage of greater career opportunities in their home countries.275 Continued inaction and complacency threatens over time to undermine an essential pillar of U.S. competitiveness.
Several proposals seek to reform U.S. immigration rules that tilt heavily toward granting citizenship to relatives of current citizens, regardless of
______________________
269 See Vivek Wadwha, Guillermina Jasso, et. al, “Intellectual Property, the Immigration Backlog, and a Reverse Brain-Drain,” Ewing Marion Kauffman Foundation, August 2007, (http://www.kauffman.org/uploadedFiles/reverse_brain_drain_101807.pdf).
270 James Manyika, et. al, Growth Renewal in the United States: Retooling America’s Economic Engine, McKinsey Global Institute, February 2001.
271 The National Association of Manufacturers, the Manufacturing Institute, and Deloitte & Touche, “Keeping America Competitive: How a Talent Shortage Threatens U.S. Manufacturing,” April 21, 2003.
272 Darrell M. West, “Creating a ‘Brain Gain’ for U.S. Employers: The Role of Immigration,” Brookings Policy Brief Series #178, Brookings Institution, January 2011.
273 B. Lindsay Lowell, Hal Salzman, Hamutal Bernstein, and Everett Henderson, “Steady as She Goes? Three Generations of Students Through the Science and Engineering Pipeline,” paper presented at annual meets of the Association for Public Policy Analysis and management, Washington, DC, October 2009.
274 See Patrick Gaule, “Return Migration: Evidence From Academic Statistics,” National Bureau of Economic Research fellow, draft paper, November 17, 2010.
275 Vivek Wadhwa, AnnaLee Saxenian, Richard Freeman, and Alex Salkever, “Losing the World’s Best and Brightest: America’s New Immigrant Entrepreneurs,” Ewing Marion Kauffman Foundation, March 2009.
skills. Only 6.5 percent of U.S. immigrant visas are for skilled workers, compared to 36 percent in Canada. And of those holding H-1B visas, only 7 percent are able to change to permanent resident status, notes Darrell West of Brookings.276 Common reform proposals include easing limits on temporary work visas, streamlining visa procedures, and giving priority for green cards to foreigners with advanced science and technology degrees and needed skills.277 The McKinsey Global Institute observes that nations such as Australia, the United Kingdom, and Canada have moved to a point-based system for allocating residency based heavily on skill levels. It suggests the U.S. do the same.278
Proposed changes in U.S. immigration policy, however, have aroused intense political passions that make it difficult for Congress to consider reform of rules that would attract and retain highly skilled immigrants to the Unites States.279 In this context, the recent initiatives by the Department of Homeland Security and the Bureau of Citizenship and Immigration Services are welcome. Announced in August 2011, these initiatives now make it possible for foreign entrepreneurs to obtain an EB-2 immigrant visa if they can demonstrate that their business endeavors will be in the national interest of the United States. Also, H-1B beneficiaries who are sole owners of the petitioning company may petition for H-1B non-immigrant visas to employ foreign workers in specialty occupations that require theoretical or technical knowledge.280
The world of innovation is changing rapidly. Old assumptions about how investments in research result in commercial products and domestic industries are becoming less valuable as frameworks for U.S. science and technology policy.
A New Approach: A new policy approach is required, one based on a richer understanding of the complexity and global dimensions of innovation. While greater investments in research and development are needed to keep the United States at the technology forefront, that alone will not guarantee globally competitive U.S. industries and a prosperous U.S. economy. Intermediating
______________________
276 Darrell M. West, “Creating a ‘Brain Gain’ for U.S. Employers: The Role of Immigration,” Brookings Policy Brief Series #178, Brookings Institution, January 2011.
277 Ibid. Some analysts have emphasized the need to strengthen the U.S. pipeline of scientists and engineers and to create a more competitive immigration policy that admit the “best and brightest” from around the world. See the statement of B. Lindsay Lowell before the House Judiciary Committee “Immigration and the Science & Engineering Workforce: Failing Pipelines, Restrictive Visas, and the ‘Best and Brightest’”October 5, 2011.
278 James Manyika, et. al., Growth and Renewal in the United States: Retooling America’s Economic Engine, McKinsey Global Institute, February 2011
279 For a review of potential reforms concerning the H-1B visa, which enables U.S. employers to hire temporary, foreign workers in specialty occupations, see GAO, “Reforms Are Needed to Minimize the Risks and Costs of Current Program.” GAO-11-26.
280 Wall Street Journal, “U.S. to Assist Immigrant Job Creators.” August 3, 2011.
institutions and new initiatives, both at the state and federal levels, as well as by private foundations, are needed for the United States to capture the benefits of its public investments in research and development.
Indeed, the way forward for the United States is to build on its strengths: open competition, deep private capital markets, leadership in academic research, a flexible labor force, intellectual property protections, and an environment that allows entrepreneurs to quickly respond to new market and investment opportunities. Importantly, these strengths need to be renewed and reinforced, as they have in the past, with federal programs to nurture and grow new technologies and new industries of the future.
The Role of Partnerships: Public-private partnerships have long been a key element of successful U.S. innovation policy.281 Public-private partnerships can provide incentives for closer collaboration among government industry, higher education, the military, private investment groups, and other institutions to foster an environment in which the United States can thrive in an era of open and global innovation.282 Well designed public-private partnerships not only can help insure that the U.S. remains a world leader in creating knowledge, but they also can enable America to capture more of the economic value of innovation by making U.S. regions more competitive places to translate inventions into products, companies, industries, and jobs.
This report documents several examples of successful U.S. collaboration between government, industry, and academia. They include federal programs such as the SBIR and the NIST Advanced Technology Program, research consortia such as Sematech, and newer institutions such as the Flexible Display Center at Arizona State University.283 This report also highlights a number of promising and innovative state and regional publicprivate initiatives to bolster competitiveness.284 Such initiatives include regional innovation clusters, new kinds of science parks, workforce-training programs, and efforts to help entrepreneurs obtain access to the facilities, technical assistance, and early-stage capital they need to convert U.S. innovation into a new wave of U.S. industries. Federal agencies can play a valuable support role in aiding these regional initiatives.
What are others doing? American policymakers also need to learn from the experiences of other nations and discern which best practices can be
______________________
281 National Research Council, Government-Industry Partnerships for the Development of New Technologies, Summary Report, C. Wessner, ed., Washington, DC: National Academy Press, 2001.
282 National Research Council, Government-Industry Partnerships for the Development of New Technologies: Summary Report, C. Wessner, ed., Washington, DC: National Academy Press, 2001.
283 See Chapter 6 for an illustrative review of national policies and programs to support emerging industries abroad.
284 See Chapter 7 for an illustrative review of national and regional policies to develop innovation clusters around the world.
adapted to the American context.285 Well-designed public-private partnerships can address many of the challenges facing the myriad actors of the U.S. innovation ecosystem and can help ensure that more of the fruits of America’s tremendous investments in research flow into the American economy.
The bold and innovative strategies being deployed abroad offer valuable lessons for policymakers in the U.S. This report details a great variety of actions governments are taking around the world to both increase their nations’ innovation capacity and global competitiveness in emerging technology-intensive industries. In some cases, governments are adapting the most successful features of the U.S. innovation ecosystem—such as universityindustry collaboration, public provision and support for early-stage risk capital, strong protection of intellectual property rights, and well-funded, scalable research parks. In other cases, nations in Asia and Europe are pioneering new models of public-private partnerships that far exceed the scale and scope of comparable U.S. programs. This is especially true when it comes to applied technology and support for large-scale manufacturing.
This unprecedented focus around the world on innovation means that American science and technology policies can no longer be based on the outdated assumption that the United States is naturally destined to remain the global center of innovation activity. Nor can it be based on the assumption that bolstering American industrial competitiveness is merely a matter of increasing R&D spending. As innovation becomes more globalized, absorbing and capitalizing on product and process innovations from abroad will become increasingly important for U.S. competitiveness.
Importance of Collaboration: policies also need to take into account the increasingly global and open nature of the innovation process, much of which takes place within collaborative international networks of researchers in universities, companies, and other institutions. As nations around the world increase their innovation capacity and R&D workforces, leveraging technology and brainpower abroad will become increasingly important for the U.S. to achieve its own science and technology goals.
Collaboration in research and development can greatly accelerate discoveries of cures for chronic disease, the development of renewable energies, and technologies to curb the negative impacts of climate change. Open crossborder innovation networks, meanwhile, can help corporations turn new technologies into innovative products faster, at greater variety and at lower cost. It is important, therefore, to insure that the United States can compete, cooperate, and prosper in this new world of innovation. That will require a fresh approach to innovation policy.
______________________
285 See Chapter 5 for case study reviews of programs and policies of leading nations and regions, including China, India, and Germany.
Box 2.4
A History of Public Private Partnerships
Public-public-private collaborations have been woven into the fabric of the U.S. economic system from the beginning of the Republic. What became known as the American System of Manufacturing, in which goods from muskets to clocks were made of interchangeable parts, was pioneered in the early 1800s through War Department contracts.286 Congress funded Samuel Morse’s demonstration of the first telegraph with a substantial grant in 1842. America’s aircraft industry was nurtured by the 1925 U.S. Air Mail Act.287 RCA was founded in 1919 at the initiative of the Navy Department, which also held equity and a board seat, so that the U.S. could have a radio communication industry to compete with Britain’s Marconi Co.288 The U.S. Signal Corps funded most of the initial research for transistors and semiconductors, and the military funded the first production lines of Western Electric, General Electric, Raytheon, and Sylvania. It also bought most of the output for weapons and communications systems.289 Admiral Hyman Rickover and his naval reactor group oversaw the design and construction of America’s first civilian light-water nuclear power plant at Shippingport, Penn., in the 1950s. 290 Military research and weapons contracts also have been instrumental in establishing America’s aerospace and computer industries and the forerunner of the Internet.291 Federal programs have been instrumental as well to the U.S. pharmaceutical industry. A recent study found that public-sector research institutions made important contributions to
______________________
286 See David A. Hounshell, From the American System to Mass Production, 1800-1932: The Development of Manufacturing Technology in the United States, Baltimore, Maryland, USA: Johns Hopkins University Press, 1984.
287 A stated purpose of the U.S. Air Mail Act of 1925 (also known as the Kelly Act), which authorized the U.S. Postal Service to contract with private aviation companies, was “to encourage commercial aviation.” The federal role in their early airline industry is explained in Roger E. Bilstein, Flight in America: From the Wrights to the Astronauts, Baltimore: Johns Hopkins University Press, 1984, and in Tim Brady, editor, The American Aviation Experience: A History, Southern Illinois University Press, 2001.
288 An early account of the U.S. Navy’s role in establishing RCA and the U.S. radio communication system is found in The World’s Work, “The March of Events,” Volume XLIV, May 1922.
289 A concise history of U.S. government involvement in establishment of America’s electronics industry is found in Kenneth Flamm, Mismanaged Trade?: Strategic Policy and the Semiconductor Industry, Washington, DC, Brookings Institution, 1996. pp. 27-38.
290 Richard Hewlett and Francis Duncan, The Nuclear Navy, Chicago: University of Chicago, 1974.
291 See National Research Council, Funding a Revolution, Government Support for Computing Research, Washington, DC: National Academy Press, 1999. The extensive NRC review documents the seminal role o federal funding for the information and communications industries of today. See also the presentation by Kenneth Flamm of the University of Texas at Austin in National Research Council, Innovation Policies for the 21st Century, op. cit.
the discovery of up to 21.2 percent al all new FDA-approved drugs from 1990 through 2007.292
Capturing the value of U.S. investments in R&D: The assumption that the output of the U.S. innovation process will be captured by U.S.-based industry has been rendered obsolete by globalization and the rise of corporate open innovation practices. In today’s world, knowledge created through federally funded research at universities and national laboratories can be commercialized and industrialized virtually anywhere. The key is to take measures to provide the funding, support services, and to anchor new and existing companies in clusters of competency here in the United States.
This report highlights the features of a more comprehensive innovation policy. It calls for a better understanding by government of the real factors behind corporate decisions on where to develop new technologies, commercialize products, and locate production and help close competitive gaps with other nations to the degree possible. Some of these gaps can be closed with more enlightened tax policy, in others through incentives such as research grants, loans, and credits for U.S.-based manufacturing.
The committee’s formal findings and recommendations on how to sustain a strong American innovation system for the 21st century are found in the next two chapters.
______________________
292 Ashley J. Stevens, Jonathan J. Jensen, Katrine Wyller, Patrick C. Kilgore, Sabarni Chatterjee, and Mark L. Rohrbaugh, “The Role of Public-Sector Research in the Discovery of Drugs and Vaccines,” The New England Journal of Medicine, February 9, 2011, (http://healthpolicyandreform.nejm.org/?p=13730&query=home).


































































