Origin of Radioactivity in Nuclear Plants
Nuclear power reactors1 are fueled with uranium that is slightly enriched in the isotope uranium-235.2 This isotope is capable of sustaining a controlled nuclear chain reaction that is necessary for production of electrical energy. The chain reaction results in the production of neutrons that induce radioactivity in the fuel, cooling water, and structural components of the reactor.
Radioactivity is induced primarily through processes involving the capture of neutrons by uranium atoms in the fuel. Fission occurs when the nucleus of a uranium-235 atom (and less commonly a uranium-238 atom) captures a neutron, becomes unstable, and splits into two and (infrequently) three3 lighter nuclei; these nuclei are referred to as fission products. Uranium fission produces a bimodal mass distribution of fission products shown in Figure D.1. The most common fission products have mass numbers around 90 and 137 (for example, strontium-90 and cesium-137).
The fission products produced in a nuclear power reactor span the periodic table. They include:
- Noble gases, for example, krypton-85 and xenon-133.
- Halogens, for example, iodide-131.
1 The terms nuclear power reactors and nuclear power plants refer to reactors that are used on a commercial basis to produce electricity. Such reactors typically generate on the order of 1000 megawatts of electrical power and 3000 megawatts of thermal power.
2 Natural uranium contains about 99.3 percent uranium-238 and 0.7 percent uranium-235. The fuel used in power reactors is typically enriched in uranium-235 to levels of 3-5 percent.
3 Referred to as ternary fission.
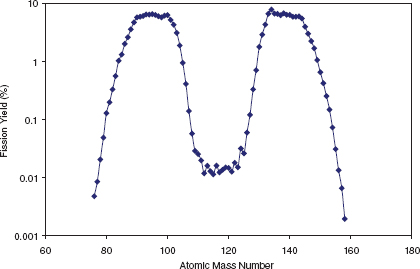
FIGURE D.1 Mass distributions resulting from fission of uranium-235 by thermal neutrons. SOURCE: Data from Joint Evaluated Fission and Fusion File, Incident-neutron data, http://www-nds.iaea.org/exfor/endf00.htm, October 2, 2006; see http://www-nds.iaea.org/sgnucdat/c1.htm.
- Alkali metals, for example, cesium-137.
- Alkaline earth metals, for example, strontium-90.
- Less commonly, hydrogen-3, more commonly referred to as tritium (T), from ternary fission of uranium atoms.
Neutron capture can also induce radioactivity through the transmutation of one chemical element into another. The transmutation process results in the emission of nuclear particles (e.g., protons) and radiation from the nucleus. Some transmutation reactions and products of significance in power reactors include the following:
- Production of nitrogen-16 through the capture of a neutron by the nucleus of an oxygen atom: oxygen-16 + neutron —> nitrogen-16 + proton (abbreviated as 16O(n, p)16N). Nitrogen-16 has a short (7-second) half-life and is primarily a hazard to workers at nuclear plants.
- Production of carbon-14 through the capture of neutrons by the nuclei of nitrogen, oxygen, or carbon atoms: 14N(n, p)14C; 13C(n, y)14C; 17O(n, a)14C.
- Production of tritium (T) by the capture of a neutron by the nu-cleus of a boron atom: 10B(n,2a)T. This is an important reaction in pressurized-water reactors, which use boron in cooling water to control reactivity.
- Production of tritium through capture of a neutron by a deuterium atom that is naturally present in the cooling water of a reactor.
Neutron capture can also induce radioactivity through activation. The capture of a neutron excites the nucleus, which quickly decays to a less energetic state through the emission of radiation. Some activation reactions and products of significance in power reactors include the following:
- Production of cobalt-60 from cobalt-59 through the reaction 59Co(n, y)60Co.
- Production of iron-55 from iron-54 through the reaction 54Fe(n, y)55Fe.
Cobalt-60 and iron-55 are common activation products in the structural components of reactors.
The isotopes produced by these neutron capture processes are almost always radioactive. Their decay involves the emission of alpha, beta, and gamma radiation, to produce both radioactive and nonradioactive decay products. A decay reaction of particular importance in nuclear power reactors is the following:
![]()
This reaction produces plutonium-239 by uranium-238 neutron capture followed by two beta decays.
The particles and other radiation emitted during neutron capture can interact with atoms in the fuel, coolant, and reactor structures to produce additional radioactivity. For example, the interaction of energetic electrons with materials in the reactor results in the emission of photons known as bremsstrahlung. This radiation appears as a faint blue glow when electrons interact with cooling water in the reactor and spent fuel pools.




