5
Microbial Metabolism and Physiology
The vast repertoire of metabolism and physiology allows different kinds of terrestrial microbes to colonize diverse environments. Because of niche competition, individual taxa have evolved to grow optimally under a limited range of conditions. For example, microbes that grow optimally at –15°C do not survive at 122°C, the known upper temperature limit for terrestrial life. Decisions about planetary protection must consider the interplay between availability of water (Decision Point 1), bioavailability of trace elements and sources of energy (Decision Points 2 and 4), microbial metabolism and physiology (Decision Points 2, 4, and 6), the techniques used to reduce bioloads (Decision Point 7), and the environment of the target bodies (Decision Points 1, 2, 3, 4, and 6). Geophysical considerations (Decision Point 5) are less relevant. All require knowledge about the following:
1. The physical and chemical environment of the target body (Chapter 4);
2. The environmental source of organisms on the spacecraft and their ability to survive and grow at temperatures found on icy moons (e.g., at 0°C or below);
3. The relationship between growth at low temperatures and tolerance to heat-mediated bioload reduction; and
4. The survival tactics of microbial life in response to high levels of radiation or extremely low vacuum, which causes desiccation.
In addition to Decision Points 1-7, the location of assembly and launch facilities will influence planetary protection concerns. The cold environments of icy bodies might provide habitats for terrestrial microbes that can grow only at very low temperatures. On Earth, environments capable of supporting microbial growth at 0°C or below typically include temperate and high-latitude marine environments, high-latitude ice, soils, cryopegs, the upper atmosphere, seasonally cold soils, and dairy products, meats, and seafoods that are maintained at low temperature. Because of the location of spacecraft assembly facilities in the United States, soils from temperate environments are the most likely source of spacecraft contamination with an organism capable of growing on an icy planetary body. These organisms would have to grow at 0°C or below on the target bodies and also survive temperatures and other conditions involved with the assembly and launch of the spacecraft. A study of the cultured microorganisms from the spacecraft assembly facility at NASA Kennedy Space Center did not detect psychrophilic or psychrotolerant microorganisms or high-salt-tolerant organisms.1 The predominant groups of organisms isolated included thermophiles, acidophiles, and ultraviolet-C- and H2O2-resistant bacteria. Molecular studies based on
rRNA sequences and shotgun metagenomic analyses (enabled by anticipated improvements in DNA sequencing efficiencies) of low-biomass samples would provide a cultivation-independent assessment of microbial community composition within spacecraft assembly facilities and within and on a spacecraft.
Decision Point 1—Liquid Water
The absolute and unambiguous requirement of liquid water for the propagation of terrestrial organisms on Earth or on icy bodies in the outer solar system constrains the possibility of forward contamination to a relatively small number of objects in the outer solar system. See Chapter 4 for a detailed discussion of this point.
Decision Point 2—Key Elements
The origin and development of life are intimately linked to the periodic table of elements. The most important elements required by living systems for catalysis, organization of macromolecular structure, or energy transduction include, but are not limited to, carbon, hydrogen, nitrogen, oxygen, phosphorus, sulfur, potassium, magnesium, calcium, and iron. Sometimes other elements such as boron participate in chemical signaling between bacteria,2 or elements like selenium can incorporate into specific proteins as selanocysteine, now referred to as the twenty-first essential amino acid.3
Because of its pervasive role in many biological processes, phosphorus plays an indispensible role in living systems. In some marine organisms, arsenic can incorporate into lipids in place of phosphorus,4 but this generally toxic compound does not replace phosphorus in nucleic acids, in protein structures, or in catalytic functions. There is no consensus as to the minimum concentration of phosphorus required for growth by microorganisms. The cultivation of different bacterial taxa in phosphate at varying concentrations reveals a complex set of genes that mediate stress-response to phosphate limitation. In general, phosphorus limitation triggers adaption, including the up-regulation of specific genes involved in phosphate response stress that shift cells from utilization of inorganic phosphate to scavenging of organo-phosphate and polyphosphate from the environment,5,6 substituting sulfur for phosphate in membrane lipids in marine photosynthetic bacteria,7 changes in cell morphology to increase surface-to-volume ratio of the cell,8 and shutting down cell metabolism to survive in a dormant stage.9,10,11 In most cases, these studies were preformed with bacteria that grow in high concentrations of organic nutrients, and the concentrations of phosphorus at which the stress response is stimulated are considerably higher than those measured in oligiotrophic oceans (0.2 to 1 nM inorganic phosphate). Even in studies with the freshwater oligiotrophic bacterium Caulobacter crescentus, 30 μM of phosphate induced an adaptive response.12 In contrast, various strains of Rhizobium species, a nitrogen-fixing soil microbe, grew as rapidly at 0.05 μM phosphate as at 2 mM.13 The data on the effects of phosphate limitation on spore-forming microorganisms includes studies only with the mesophiles Bacillus subtilis and Clostridium perfringens. In B. subtilis, concentrations of phosphorus below 0.1 mM led to reduced growth rates and entry into stationary growth phase,14,15 whereas in C. perfringens, sporulation did not occur when phosphorus concentrations were less than 3 mM.16 There were no studies found that looked at the effects of phosphorus limitations on psychrophilic or psychrotolerant spore-forming bacteria.
The studies on phosphorus limitation using pure cultures of microorganisms have revealed the exquisite complexity of physiological responses and survival mechanisms. However, complex ecosystems exist in oligiotrophic environments where the concentration of phosphate is lower than the lowest concentration that either prevents growth or induces stress responses in most isolated microbes tested. Oligiotrophic oceans such as the Sargasso Sea in the northwestern Atlantic, the North Pacific subtropical gyre, and eastern part of the Mediterranean Sea have extremely low levels of dissolved phosphate. For example, the concentrations of phosphate in surface waters of the subtropical Sargasso Sea are from 0.2 to 1.0 nM.17,18,19 The canonical “Redfield ratio” used in biogeochemical models of the ocean is 106C:16N:1P and does not apply to oligiotrophic oceans where the N:P ratio can be higher than 30.20 Although these environments show limited phosphate stress, they have active ecosystems anchored by Prochlorococcus spp. as the primary producers. These photosynthetic bacteria are highly adapted to low levels of
nutrients, including phosphate, and can compensate by synthesizing sulfur lipids instead of phospholipids.21 It was further observed that the synthesis of membrane lipids normally accounted for 18 to 28 percent of the phosphate utilized by phytoplankton. The dominant heterotrophic bacteria in these oligiotrophic oceans, Pelagobacter ubique, can like the Prochlorococcus species grow in situ in phosphate at low concentrations while utilizing carbon compounds in the low levels found in the dissolved organic compound fraction.22 Both of these organisms are small (<1 μm) and have the smallest genomes of “free-living” organisms and are genetically surprisingly well adapted to grow in the presence of phosphate and other inorganic nutrients at ultralow concentrations. It is apparent that microorganisms have adapted to phosphorus limitations and, in particular, have developed the ability to grow in phosphate at concentrations that are at the edge of researcher’s ability to measure. Consequently, it might not be possible to measure phosphorus in the oceans or ice of icy moons if it is present only at the low levels measured in present day oligiotrophic oceans.
Decision Point 3—Physical Conditions
Radiation flux and temperature extremes that exceed the documented limits tolerated by life on Earth will constrain the potential growth of terrestrial microbial life forms in target body environments. These same parameters have important implications for Decision Points 6 and 7, which consider the survivability of irradiated microbes in oligotrophic environments and at temperature in ranges in which cold-loving organisms can survive.
DECISION POINT 4—CHEMICAL ENERGY
Determining the minimal energy requirements for life is far more difficult than constraining the possibilities of contamination according to the presence or absence of water or essential elements. Discoveries each year of novel extremophiles with new types of metabolism continuously change understanding of life’s minimal energy criterion and the chemical dimensions of Earth’s “habitable zone.” Two considerations suggest taking an optimistic approach to the presence of energy: (1) work in the fields of electron transport and metabolism in the past 20 years has made it clear that some group of terrestrial organisms has made use of nearly any redox couple that can yield significant energy23-28 and (2) observational, laboratory, and theoretical studies show that icy bodies contain a vast number of oxidized and reduced compounds, many of which might serve as potential electron donors or acceptors (Table 5.1).
Researchers currently lack conclusive information about the presence of energy and elemental sources necessary to support the growth of potential contaminating organisms on icy bodies. Electron donors (e.g., Fe2+, SH−, organic carbon) and electron acceptors (e.g., CO2, SO42−, O2, H2O2)29-33 might be present on some icy bodies. Given the poorly constrained knowledge of the chemistry of these environments, a supply of energy cannot be ruled out. Here the committee takes the conservative assumption that if an environment contains essential elements and water, then a source of energy might exist.
DECISION POINT 6—COMPLEX NUTRIENTS
Radiation-resistant microorganisms might present a special problem when the possibility of forward contamination is considered. “Stowaway” bacteria and archaea on spacecraft targeting the outer planets and their moons will experience exposure to high-level radiation.34 Yet the extreme resistance of some bacteria on Earth to acute and chronic forms of ionizing radiation that feasibly mimic conditions during transit argues that some microbes within sealed spacecraft components might survive such a journey, because of their resistance to gamma rays afforded by biochemical properties and/or by microenvironments of biofilms, and ultimately could reach life-supporting environments.35,36,37 For example, as the level of cell grouping increases, survival characteristics of irradiated organisms typically increase as a result of the effects imposed by the limitation of atmospheric dioxygen, whereby cells within biofilms or within clumps are shielded from oxygen effects. Hence, prokaryotes under oxidative stress tend to adhere to surfaces and to each other during growth.38 Given the possibility that such organisms might survive irradiation exposure and come into contact with a habitable environment, efforts to prevent forward contamination must consider the ultimate fate of these potentially viable organisms on icy moons.
TABLE 5.1 Examples of Electron Donors or Acceptors for Life in Icy Bodies Both Inferred and Measured in Past Work
| Europa | Comments | Enceladus | Comments | Titan | Comments | |
| Electron Donors (observed) | ||||||
| Organics | Detected on surface of Callisto, Ganymede Reference a | Expected on the surface. Interior unknown | Organic compounds Reference j Size of some known; structures not known | Methane and other organics detected in the plume. | Organics and methane Reference l and m suggested as a source of energy for organisms by references n and o | Surface organics from hydrocarbon cycle |
| Hydrogen | n/a | n/a | n/a | n/a | Reference o | |
| Electron Donors (suggested in literature as plausible candidates) | ||||||
| Hydrogen | Reference b | Reference k | ||||
| Electron Acceptors (observed) | ||||||
| Oxygen | Observed on surface Reference c | Interior unknown | ||||
| CO/CO2 | CO2 on surface Reference d | Reference j | Observed in plume; CO and N2 peaks cannot be differentiated | Trace quantities of CO2 observed Reference o | ||
| SO2 | Reference e | |||||
| Electron Acceptors (suggested) | ||||||
| Sulfur | Sulfate inferred on surface Reference f and g Elemental sulfur also suggested Reference h | Interior unknown | ||||
| Fe3+, other metals | Fe3+, other metals Reference b, i | Fe3+, other metals depends on connection with silicate core | ||||
NOTE: Comments are provided as caveats and context.
a K.P. Hand, C. Chyba, J.C. Priscu, R.W. Carlson, and K.H. Nealson, Astrobiology and the potential for life on Europa, pp. 589-630 in Europa (R. Pappalardo, W. McKinnon, and K. Khurana, eds.), University of Arizona Press, Tucson, Ariz., 2009.
b D. Schulze-Makuch and L.N. Irwin, Energy cycling and hypothetical organisms in Europa’s ocean, Astrobiology 2:105-121, 2002.
c J.R. Spencer and W.M. Calvin, Condensed O2 on Europa and Callisto. Astronomy Journal 124:3400-3403, 2002.
d K.P. Hand, R. Carlson, and C.F. Chyba, Energy, chemical disequilibrium, and geological constraints on Europa, Astrobiology 7:1006-1022, 2007.
e A.L. Lane, R.M. Nelson, and D.L. Matson, Evidence for sulfur implantation in Europa’s UV absorption band, Nature 292:38-39, 1981.
f T.B. McCord, G.B. Hansen, F.P. Fanale, R.W. Carlson, D.L. Matson, T.V. Johnson, W.D. Smythe, J.K. Crowley, P.D. Martin, A. Ocampo, C.A. Hibbitts, J.C. Granahan, and the NIMS Team, Salts on Europa’s surface detected by Galileo’s Near Infrared Mapping Spectrometer, Science 280:1242-1245, 1998.
g R.W. Carlson, R.E. Johnson, and M.S. Anderson, Sulfuric acid on Europa and the radiolytic sulfur cycle, Science 286:97-99, 1999.
h R.W. Carlson, W.M. Calvin, J.B. Dalton, G.B. Hansen, R.L. Hudson, R.E. Johnson, T.B. McCord, and M.H. Moore, Europa’s surface composition, pp. 283-327 in Europa (R. Pappalardo, W. McKinnon, and K. Khurana, eds.), University of Arizona Press, Tucson, Ariz., 2009.
i E.J. Gaidos, K.H. Nealson, and J.L. Kirschvink, Life in ice-covered oceans, Science 284:1631, 1999.
j J.H. Waite, Jr., M.R. Combi, W.-H. Ip, T.E. Cravens, R.L. McNutt, Jr., W. Kasprzak, R. Yelle, J. Luhmann, H. Niemann, D. Gell, B. Magee, G. Fletcher, J. Lunine, and W.-L. Tseng, Cassini ion and neutral mass spectrometer: Enceladus plume composition and structure, Science 311:1419-1422, 2006.
k C.P. McKay, C.C. Porco, T. Altheide, W.L. Davis, and T.A. Kral, The possible origin and persistence of life on Enceladus and detection of biomarkers in the plume, Astrobiology 8:909-919, 2008.
l C.C. Porco, E. Baker, J. Barbara, K. Beurle, A. Brahic, J.A. Burns, S. Charnoz, N. Cooper, D.D. Dawson, A.D. Del Genio, T. Denk, et al., Imaging of Titan from the Cassini spacecraft, Nature 434:159-168, 2005.
m H.B. Niemann, S.K. Atreya, S.J. Bauer, G.R. Carignan, J.E. Demick, R.L. Frost, D. Gautier, J.A. Haberman, D.N. Harpold, D.M. Hunten, G. Israel, et al., The abundances of constituents of Titan’s atmosphere from the GCMS instrument on the Huygens probe, Nature 438:779-784, 2005.
n A.D. Fortes, Exobiological implications of a possible ammonia-water ocean inside Titan, Icarus 146:444-452, 2000.
o F. Raulin, Astrobiology and habitability of Titan, Space Science Reviews 135:37-48, 2007.
Unlike many terrestrial extremophiles that grow within a singular extreme environment, species of the genus Deinococcus demonstrate a suite of extreme survival advantages similar to that needed to survive multiple challenges encountered on missions to the outer planets. One of these species, Deinococcus radiodurans, can survive exposures to ionizing radiation (x rays and gamma rays), ultraviolet C (254 nm) radiation, and charged particles.39,40 For example, D. radiodurans can survive exposure to 12,000 Gy of gamma rays in aqueous preparations; and notably, when desiccated in vacuo or when deeply frozen, D. radiodurans shows significantly increased resistance to gamma rays and ultraviolet C (Figure 5.1).41,42,43,44 Thus, the psychrotolerant, desiccation-resistant D. radiodurans could survive the simultaneous assaults of impinging cosmic radiation (e.g., far-ultraviolet light and charged subatomic particles), drying, deep freezing, and, possibly, the exposure to high-level ionizing radiation anticipated during orbits that transect the radiation belts of Jupiter or Saturn.45,46 There is, however, a prerequisite for recovery of D. radiodurans’s enhanced DNA repair, a capacity that is absolutely dependent on the availability of a rich source of aqueous heterotropic organic growth substrates. Thus, the survival traits of D. radiodurans render it the best currently known model for estimating the outer limits of microbial survival in missions to icy solar system bodies.47-51 Remarkably, the amount of DNA damage caused by most of the physico-chemical insults to which D. radiodurans is most notably resistant is about the same as that in radiation-sensitive cell-types.52 For example, the yield of DNA double-strand breaks (DSB) in D. radiodurans caused by gamma rays is about the same as that seen in sensitive bacteria, simple eukaryotes, and animals (0.004 DSB/Gy/Mbp).53,54,55Deinococcus and other radiation/desiccation-resistant microbes rely on conventional DNA repair enzymes that function extraordinarily efficiently in those species.56-59 Resistant bacteria and archaea have evolved potent chemical defenses that specifically protect their proteins from oxidative damage, thereby preventing inactivation of enzymes, including those needed to repair and replicate DNA.60,61 Under this model, a single system rather than a series of separate repair mechanisms evolved to provide resistance to multiple stressors.62
The survival of D. radiodurans following genotoxic assault depends on the availability of fresh complex nutrients during recovery.63,64,65 Under nutrient-poor conditions, metabolic capabilities limit DNA repair in acutely irradiated D. radiodurans,66,67 and similarly in chronically irradiated D. radiodurans.68 This nutrient-dependent phenotypic reversal from radiation resistance to radiation sensitivity alters scientists’ view of radiation survival in the context of planetary protection. Figure 5.1 illustrates the effects of nutrient conditions and temperature on the recovery of D. radiodurans exposed to gamma radiation. Under standard laboratory conditions, D. radiodurans exposed to 12,000 Gy (from a 60Co source) on wet ice (at 0°C) displays 10 percent survival (D10)69 for radiation inactivation when re-covered in liquid rich medium (TGY, tryptone/glucose/yeast extract) at 32°C;70 the D10 for D. radiodurans irradiated at −79°C and recovered in TGY is 50,000 kGy.71,72 However, if irradiated D. radiodurans cells are transferred to an aqueous solution of 10 mM MgCl2 (without addition of complex carbohydrates, peptides, sugars, or vitamins), the D10 for radiation inactivation falls to 5,000 Gy within a few hours of incubation (Figure 5.1), followed by a progressive loss in viability.73 It follows that the accumulating radiation doses in frozen, hence non-repairing, cells on spacecraft would eventually destroy them unless they were transferred to a liquid environment that contains a rich source of complex organic compounds. Similar arguments and observations apply to radioresistant Bacillus spores74,75 and radioresistant archaea,76,77 which are significantly less resistant than D. radiodurans (Figure 5.1). DNA repair in irradiated Bacillus spores occurs at the onset of germination, which requires heterotrophic organic substrates that would not be present in oceans or liquid water reservoirs of moons of
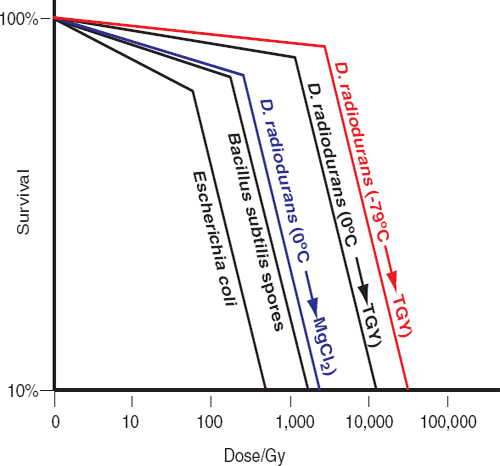
FIGURE 5.1 Gamma-radiation survival profiles of wild-type bacteria. B. subtilis spores and Escherichia coli were irradiated on wet ice (0°C) and recovered on rich medium (Granger et al., 2011; Daly et al., 2004). D. radiodurans was grown in rich medium (TGY) and treated as follows: −79°C→TGY: irradiated frozen on dry ice, then recovered on TGY (Richmond et al., 1999); 0°C→TGY: irradiated on wet ice, then recovered on TGY (Daly et al., 2004); 0°C→MgCl2: irradiated on wet ice, then transferred to 10 mM MgCl2 for 5 hours, then recovered on TGY (Ghosal et al., 2005). Note, the D10 radiation-inactivation values for the radioresistant archaea P. furiosus and H. salinarum NRC-1 are 3 and 5 kGy, respectively (DiRuggiero et al., 1997; Kottemann et al, 2005). SOURCES: Data from A.C. Granger, E.K. Gaidamakova, V.Y. Matrosova, M.J. Daly, and P. Setlow, Effects of Mn and Fe levels on Bacillus subtilis spore resistance and effects of Mn2+, other divalent cations, orthophosphate, and dipicolinic acid on protein resistance to ionizing radiation, Applied and Environmental Microbiology 77:32-40, 2011; M.J. Daly, E.K. Gaidamakova, V.Y. Matrosova, A. Vasilenko, M. Zhai, A. Venkateswaran, M. Hess, M. V. Omelchenko, H.M. Kostandarithes, K.S. Makarova, L.P. Wackett, J.K. Fredrickson, and D. Ghosal. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance, Science 306, 1025-1028, 2004; R.C. Richmond, R. Sridhar, and M.J. Daly, Physicochemical survival pattern for the radiophile Deinococcus radiodurans: A polyextremophile model for life on Mars, SPIE 3755:210-222, 1999; D. Ghosal, M.V. Omelchenko, E.K. Gaidamakova, V.Y. Matrosova, A. Vasilenko, A. Venkateswaran, H.M. Kostandarithes, H. Brim, K.S. Makarova, L.P. Wackett, J.K. Fredrickson, and M.J. Daly, How radiation kills cells: Survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress, FEMS Microbiology Reviews 29:361-375, 2005; J. DiRuggiero, N. Santangelo, Z. Nackerdien, J. Ravel, and F.T. Robb, Repair of extensive ionizing-radiation DNA damage at 95°C in the hyperthermophilic archaeon Pyrococcus furiosus, Journal of Bacteriology 179:4643-4645, 1997; M. Kottemann, A. Kish, C. Iloanusi, S. Bjork, and J. DiRuggiero, Physiological responses of the halophilic archaeon Halobacterium sp. strain NRC1 to desiccation and gamma irradiation, Extremophiles 9:219-227, 2005.
the outer planets, and the most radioresistant archaea reported, Halobacterium salinarum NRC-1 and Pyrococcus furiosus, also are chemoorganotrophs.78,79
Thus, for radiation-resistant microbes, the probability of surviving exposure to radiation and their ability to grow are inextricably linked to the availability of growth substrates. For bacteria such as D. radiodurans, it is conceivable that a very small fraction of cells frozen at −79°C, or lower temperatures, could survive 50 to 80 kGy of gamma rays if recovered in complex organic media.80 However, recovery of heavily irradiated D. radiodurans (or other resistant microbes) will not likely occur in simple salt solutions lacking complex organic compounds. For D. radiodurans and the radiation-resistant model archaea H. salinarum NRC-181 and P. furiosus,82 exposure to ionizing radiation at doses in the range and context anticipated for missions to the outer planets would render them incapable of growth or recovery on icy bodies. Similarly, irradiated Bacillus subtilis and Bacillus megaterium spores will not survive, because germination with attendant DNA repair requires complex organic compounds.83,84
Generally speaking, the most radiation-resistant bacteria reported have been gram-positive (e.g., D. radiodurans), and the most sensitive have been gram-negative (e.g., Shewanella oneidensis). However, there are several reported exceptions to this paradigm, including the extremely radiation-resistant gram-negative cyanobacterium Chroococcidiopsis.85 Cyanobacteria are phototrophs, which use the energy from sunlight to convert carbon dioxide and water into organic material to be utilized in cellular functions such as biosynthesis and DNA repair. Any potentially habitable environments of icy bodies would be located beneath the thick external ice I shell and are therefore inaccessible to sunlight. Thus, any terrestrial phototroph potentially able to survive transport to an icy solar system body would not survive there.
DECISION POINT 7—MINIMAL PLANETARY PROTECTION
Current data indicate that the outer solar system objects Europa, Enceladus, and Triton harbor liquid water ice interfaces where persistent temperatures will not exceed 0°C or lower, with the possible exception of deep, interior, localized regions that lie in proximity to heat sources. Terrestrial organisms capable of growth at these low temperatures include psychrophilic (i.e., cold-loving) microbes that have growth temperature optima below 0°C, and psychrotolerant microorganisms also can grow below 0°C, but optimally at 20°C to 30°C. Psychrophilic cultivars can grow at –12°C,86,87 and production of ATP,88 as well as incorporation of thymidine into DNA and leucine into proteins,89 provides proxies for bacterial growth at –15°C. Most of the characterized psychrotolerant microorganisms are gram-negative aerobic heterotrophs, although there are a wide diversity of psychrophilic and psychrotolerant gram-positive spore-forming and non-spore-forming aerobic and anaerobic bacteria and chemolithoautotrophic and anoxygenic photosynthetic bacteria. The following two aspects of microbial physiology have important implications for planetary protection:
1. Psychrophiles can grow only over a limited temperature range that does not exceed 20°C to 30°C, whereas the temperature range of growth for psychrotolerant microorganisms is somewhat broader at 30°C to 40°C. Thus, for both kinds of physiology, the anticipated maximum growth temperature lies between −5°C and 40°C.
2. Non-spore-forming psychrophiles have short survival times at temperatures above their maximum growth temperature and in some cases lyse within minutes after exposure to temperatures a few degrees above their maximum growth temperature.90
Genomic and physiological characterization of cultured species of non-spore-forming psychrophiles shows a diverse range of strategies for growth and survival that can help them to compensate at low temperature but not for temperatures significantly higher than the upper temperature for growth. A single enzyme or group of enzymes for protein synthesis or energy generation can determine the upper temperature limit for psychrophiles or psychrotolerant microorganisms.91 Proteins adapted for optimal activity at low temperature are generally denatured in minutes at 50°C.92 Similarly, the membrane lipids of psychrophiles have adapted to low temperature by lowering their fluidity, but these same modifications cause cell membranes to become leaky and nonfunctional at
elevated temperatures. In some cases, the onset of this leakiness occurs minutes after exposure to temperatures a few degrees centigrade above the maximum growth temperature.93,94
Non-spore-forming psychrophiles will not survive short-time (minutes) exposure to temperatures greater than 20°C. Non-spore-forming psychrotolerant microorganisms will not survive short-time exposure to temperatures above their maximum growth temperature (>20°C to 40°C). Similarly, vegetative cells of fungi and yeasts are generally killed within 10 to 15 minutes of exposure to temperatures of 50°C to 70°C. The overall conclusion is that psychrophiles and psychrotolerant microorganisms are not adapted at the molecular level to grow or survive at temperatures much more than10°C above their maximum growth temperature. Therefore, to meet planetary protection requirements for missions to the icy bodies, heating of the spacecraft or its sealed components to 60°C for 5 hours will provide sufficient bioload reduction for non-spore-forming psychrophiles and psychrotolerant microorganisms.
Spore-forming psychrophiles and psychrotolerant microorganisms include heterotrophs that have complex requirements for organic compounds, and the few described species grow only under anaerobic conditions. These organisms have been isolated from high-latitude permafrost, soil and lake samples, temperate soils, and various animal and dairy products kept at low temperature. In most cases, information is lacking regarding the thermal resistance of spores from psychrophiles. For example, psychrophilic Clostridium species isolated from permafrost have optimum growth temperatures from 4°C to 16°C, with maximum growth temperatures near 20°C, but no discussion of the thermal properties of its spores exist.95 The newly described isolate C. algoriphilum can survive for 24 hours at 20°C but cannot survive at 24°C.96 The spores from six psychrotolerant Bacillus species isolated from dairy products had D1097 values for heat inactivation of only 4.4 to 6.6 minutes at 90°C.98 In contrast, spores from mesophilic Bacillus species had D10 values for heat inactivation of 70 to 200 minutes at 90°C. There appears to be a correlation between the maximum growth temperature of spore formers and the maximum temperature for inactivation of the spores. For 28 strains of Bacillus having different maximum growth temperature, Warth observed that the spores had a D10 value for heat inactivation of 10 minutes at approximately 40°C above the maximum growth temperature (Figure 5.2).99 No psychrophilic Bacillus isolates were used in that study. In fact, most of the studies in which D10 values have been calculated are for spores exposed to high temperatures for short times.
Spores from psychrophilic bacteria are likely to be rendered inactive at 40°C above their maximum growth temperature, which corresponds to 60°C or lower. While there are few specific studies that discuss the length of time of exposure to spore-inactivating temperatures, most exposures are for short periods. For all psychrotolerant microorganisms including spore-forming taxa, heating at 60°C for 5 hours provides a sufficient margin of error to achieve complete inactivation. This temperature and length of heating should pose minimal challenges for spacecraft design, including sensitive instrumentation. There is insufficient data to estimate a D value for spores of psychrotolerant microorganisms at 60°C, and additional research will be necessary to address this knowledge gap. Another factor concerns the nutritional requirements for spore-forming microbes to go from vegetative cells to spores and vice versa. Essentially, all of the spore formers are heterotrophic, and most have complex requirements for growth and for transitioning from spores to vegetative cells.
CONCLUSIONS AND RECOMMENDATIONS
Beyond the absolute requirement for liquid water, life cannot propagate on icy bodies in the outer solar system in the absence of phosphorus or without redox couples for energy. Physiological capabilities of potential contaminants determined through metagenomic surveys of microbial taxa in component and spacecraft assembly facilities could impact many different aspects of planetary protection. Molecular surveys could determine the presence of cold-tolerant organisms and the identification of radiation-resistant taxa. However, the ability of organisms to resist the effects of exposure to radiation during flight or on icy bodies in the outer solar system would require the unlikely availability of complex heterotrophic organic substrates. Energy requirements also pose a challenge to the propagation of potential contaminants, but at this time, currently available data about redox-couples or the presence or absence of key elements is not sufficiently informative to guide planetary protection policies. In contrast, a growing body of evidence argues that psychrophiles grow over a limited temperature range of ~20°C, and most lyse within minutes after exposure to temperatures a few degrees above their maximum growth temperature. The
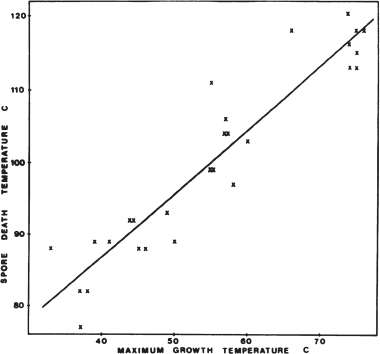
FIGURE 5.2 The spore death temperature (the temperature at which D = 10 minutes) in relation to maximum growth temperature for 28 strains of Bacillus. SOURCE: A.D.Warth, Relationship between the heat resistance of spores and the optimum and maximum growth temperatures of Bacillus species, Journal of Bacteriology 134:699-705, 1978.
few examples of cold-tolerant, spore-forming organisms that are known require complex organic compounds to grow. The preponderance of currently available data supports the view that heating at 60°C will be sufficient to inactivate spores from psychrophilic microorganisms.
Finding: If the preponderance of data eliminates the presence of liquid water, the likelihood of bioavailable phosphorus, sources of redox-couples for energy, or complex organics required for radiation resistance on icy bodies in the outer solar system, planetary protection will require only routine spacecraft cleaning and minimal monitoring.
Recommendation: Molecular-based inventories of bioloads, including both living and dead taxa, must be collected in order to document the range of physiological capabilities of potential contaminants in component and spacecraft assembly facilities.
Recommendation: If the probability of contamination by psychrophilic and psychrotolerant spore-forming organisms exceeds 10– 4 after treatment at 60°C for 5 hours, full Viking-level, terminal bioload reduction procedures must be undertaken for planetary protection.
1. M.T. La Duc, A.E. Dekas, S. Osman, C. Moissl, D. Newcombe, and K. Venkateswaran, Isolation and characterization of bacteria capable of tolerating the extreme conditions of clean-room environments, Applied Environmental Microbiology 73:2600-2611, 2007.
2. X. Chen, S. Schauder, N. Potier, A. Van Dorsseiaer, I. Pelczer, B.L. Bassier, and F.M. Hughson, Structural identification of a bacterial quorum-sensing signal containing boron, Nature 415:545-549, 2002.
3. J.F. Stolz, P. Basu, J.M. Santini, and R.S. Oremland, Arsenic and selenium in microbial metabolism, Annual Reviews of Microbiology 60:107-130, 2006.
4. B.P. Rosen, A.A. Ajees, and T.R. McDermott, Life and death with arsenic, Bioessays Journal 33:350-357, 2011.
5. M. Sebastian and J.W. Ammerman, The alkaline phosphatase PhoX is more widely distributed in marine bacteria than the classical PhoA, The ISME Journal 3:563-572, 2009.
6. B. Temperton, J.A. Gilbert, J.P. Quinn, and J.W. McGrath, Novel analysis of oceanic surface water metagenomes suggests importance of polyphosphate metabolism in oligiotrophic environments, Public Library of Science One 6(1):1-14, 2011.
7. B.A.S. Van Mooy, G. Rocap, H.F. Fredricks, C.T. Evans, and A.H. Devol, Sulfolipids dramatically decrease phosphorus demand by picocyanobacteria in oligiotrophic marine environments, Proceedings of the National Academy of Sciences USA 103:8607-8612, 2006.
8. S. Le Blastier, A. Hamels, M. Cabeen, L. Schille, F. Tilquin, M. Dieu, M. Raes, and J.-Y. Matroule, Phosphate starvation triggers production and secretion of an extracellular lipoprotein in Caulobacter crescentus, Public Library of Science One 5:1-11, 2010.
9. T. Nyström, K. Flärdh, and S. Kjellenberg, Responses to multiple-nutrient starvation in marine Vibrion sp. strain CCUG 15956, Journal of Bacteriology 172:7085-7097, 1990.
10. R.Y. Morita, Starvation and miniaturization of heterotrophs, with special emphasis on maintenance of the starved viable state, pp. 111-130 in Bacteria in Their Natural Environment (M.M. Fletcher and G.D. Floodgate, eds.), Academic Press, Inc., London, 1985.
11. R. Repaske and A.C. Repaske, Quantitative requirements for exponential growth of Alcaligenes eutrophus, Applied and Environmental Microbiology 32:585-591, 1976.
12. S. Le Blastier, A. Hamels, M. Cabeen, L. Schille, F. Tilquin, M. Dieu, M. Raes, and J.-Y. Matroule, Phosphate starvation triggers production and secretion of an extracellular lipoprotein in Caulobacter crescentus, Public Library of Science One 5:1-11, 2010.
13. K.G. Cassman, D.N. Munns, and D.P. Beck, Growth of Rhizobium strains at low concentrations of phosphate, Soil Science Society of America Journal 45:520-523, 1981.
14. W.R. Abdel-Fattah, Y. Chen, A. Eldakak, and F.M. Hulett, Bacillus subtilis phosphorylated PhoP: Direct activation of the EσA- and repression of the EσE- responsive phoB-PS+V promoters during Pho response, Journal of Bacteriology 187:5166-5178, 2005.
15. B. Kaushal, S. Paul, and F.M. Hulett, Direct regulation of Bacillus subtilis phoPR transcription by transition state regulator ScoC, Journal of Bacteriology 192:3103-3131, 2010.
16. V.A. Philippe, M.B. Mendez, I.H. Huang, L.M. Orsaria, M.R. Sarker, and R.R. Grau, Inorganic phosphate induces spore morphogenesis and enterotoxin production in the intestinal pathogen Clostridium perfringens, Infection and Immunity 74:3651-3656, 2006.
17. S. Dyhrman, J.W. Ammeerman, and B.A.S. Van Mooy, Microbes and the marine phosphorus cycle, Oceanography 20:110-116, 2007.
18. D.M. Karl, Nutrient dynamics in the deep blue sea, Trends in Microbiology 10:410-418, 2002.
19. J. Wu, W. Sunda, E.A. Boyle, and D.M. Karl, Phosphate depletion in the Western North Atlantic Ocean, Science 289:759-762, 2000.
20. J. Wu, W. Sunda, E.A. Boyle, and D.M. Karl, Phosphate depletion in the Western North Atlantic Ocean, Science 289:759-762, 2000.
21. B.A.S. Van Mooy, G. Rocap, H.F. Fredricks, C.T. Evans, and A.H. Devol, Sulfolipids dramatically decrease phosphorus demand by picocyanobacteria in oligiotrophic marine environments, Proceedings of the National Academy of Sciences USA 103:8607-8612, 2006.
22. S.J. Giovannoni, H.J. Tripp, S. Givan, M. Podar, K.L. Vergin. D. Baptista, L. Bibbs, J. Eads, T.H. Richardson, M. Noordewier, M.S. Rappe, J.M. Short, J.C. Carrington, and E.J. Mathur, Science 309:1242-1245, 2005.
23. K.H. Nealson and R. Rye, Evolution of metabolism, in Treatise on Geochemistry (W.H. Schlesinger, ed.), Elsevier Press, Amsterdam, 2004.
24. C. Myers and K.H. Nealson, Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor, Science 240:1319-1321, 1988.
25. D. Lovley and E. Phillips, Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese, Applied and Environmental Microbiology 54:1472-1480, 1988.
26. R.S. Oremland, J.F. Stolz, and T. Hollibaugh, The microbial arsenic cycle in Mono Lake, California, FEMS Microbiology Ecology 48:15-27, 2006.
27. J.G. Kuenen, Anammox bacteria: From discovery to application, Nature Reviews of Microbiology 6:320-326, 2008.
28. A. Boetius, K. Ravenschlag, C.J. Schubert, D. Rickert, F. Widdel, A. Gieseke, R. Amann, B.B. Joergensen, U. Witte, and O. Pfannkuche, A marine microbial consortium apparently mediating anaerobic oxidation of methane, Nature 407:623-626, 2000.
29. R.W. Carlson et al., Hydrogen peroxide on the surface of Europa, Science 283:2062-2064, 1999.
30. R.E. Johnson, T.I. Quikenden, P.D., Cooper, A.J., McKinley, and C.G. Freeman, The production of oxidants in Europa’s surface, Astrobiology 3:823-850, 2003.
31. F. Postberg, J. Schmidt, J. Hillier, S. Kempf, and R. Srama, A salt-water reservoir as the source of a compositionally stratified plume on Enceladus, Nature 474:620-622, 2011.
32. J.R. Spencer and W.M. Calvin, Condensed O2 on Europa and Callisto, The Astronomical Journal 124:3400-3403, 2002.
33. K.P. Hand, C.F. Chyba, J.C. Priscu, R.W. Carlson, and K.H. Nealson, Astrobiology and the potential for life on Europa, in Europa (R. Pappalardo, W. McKinnon, and K. Khurana, eds.), University of Arizona Press, Tucson, Ariz., 2009.
34. National Research Council, Preventing the Forward Contamination of Europa, National Academy Press, Washington, D.C., 2000.
35. D. Slade and M. Radman, Oxidative stress resistance in Deinococcus radiodurans, Microbiology and Molecular Biology Reviews 75:133-191, 2011.
36. M.J. Daly, A new perspective on radiation resistance based on Deinococcus radiodurans, Nature Reviews of Microbiology 7:237-245, 2009.
37. K.S. Makarova, L. Aravind, Y.I. Wolf, R.L. Tatusov, K.W. Minton, E.V. Koonin, and M.J. Daly, Genome of the extremely radiation resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics, Microbiology and Molecular Biology Reviews 65:44-79, 2001.
38. D. Ghosal, M.V. Omelchenko, E.K. Gaidamakova, V.Y. Matrosova, A. Vasilenko, A. Venkateswaran, H.M. Kostandarithes, H. Brim, K.S. Makarova, L.P. Wackett, J.K. Fredrickson, and M.J. Daly, How radiation kills cells: Survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress, FEMS Microbiology Reviews 29:361-375, 2005.
39. M.J. Daly, L. Ouyang, P. Fuchs, and K.W. Minton, In vivo damage and recA-dependent repair of plasmid and chromosomal DNA in the radioresistant bacterium Deinococcus radiodurans, Journal of Bacteriology 176:3508-3517, 1994.
40. D. Slade and M. Radman, Oxidative stress resistance in Deinococcus radiodurans, Microbiology and Molecular Biology Reviews 75:133-191, 2011.
41. R.C. Richmond, R. Sridhar, and M.J. Daly, Physicochemical survival pattern for the radiophile Deinococcus radiodurans: A polyextremophile model for life on Mars, SPIE 3755:210-222, 1999.
42. L.R. Dartnell, S.J. Hunter, K.V. Lovell, A.J. Coates, and J.M. Ward, Low-temperature ionizing radiation resistance of Deinococcus radiodurans and Antarctic Dry Valley bacteria, Astrobiology 10:717-732, 2010.
43. I.G. Paulino-Lima, E. Janot-Pacheco, D. Galante, C. Cockell, K. Olsson-Francis, J.R. Brucato, G.A. Baratta, G. Strazzulla, T. Merrigan, R. McCullough, N. Mason, and C. Lage, Survival of Deinococcus radiodurans against laboratory-simulated solar wind charged particles, Astrobiology 11:875-882, 2011.
44. A. Bauermeister, R. Moeller, G. Reitz, S. Sommer, and P. Rettberg, Effects of relative humidity on Deinococcus radiodurans’ resistance to prolonged desiccation, heat, ionizing, germicidal, and environmentally relevant UV radiation, Microbial Ecology 61:715-722, 2011.
45. E. Roussos, N. Krupp, T.P. Armstrong, C. Paranicas, D.G. Mitchell, S.M. Krimigis, G.H. Jones, K. Dialynas, N. Sergis, and D.C. Hamilton, Discovery of a transient radiation belt at Saturn, Geophysical Research Letters 35:L22106, doi:10.1029/2008GL035767, 2008.
46. R.J. Sault, D. Santos-Costa, S.J. Bolton, R.M. Thorne, and S.M. Levin, Observation of Jupiter’s electron radiation belts dynamics on time-scales of days, American Geophysical Union Fall Meeting, Abstract #SM23B-1611, American Geophysical Union, Washington, D.C., 2009.
47. E. Roussos, N. Krupp, T.P. Armstrong, C. Paranicas, D.G. Mitchell, S.M. Krimigis, G.H. Jones, K. Dialynas, N. Sergis, and D.C. Hamilton, Discovery of a transient radiation belt at Saturn, Geophysical Research Letters 35:L22106, doi:10.1029/2008GL035767, 2008.
48. R.J. Sault, D. Santos-Costa, S.J. Bolton, R.M. Thorne, and S.M. Levin, Observation of Jupiter’s electron radiation belts dynamics on time-scales of days, American Geophysical Union Fall Meeting, Abstract #SM23B-1611, American Geophysical Union, Washington, D.C., 2009.
49. V. Mattimore and J.R. Battista, Radioresistance of Deinococcus radiodurans: Functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation, Journal of Bacteriology 178:633-637, 1996.
50. J.K. Fredrickson, S.W. Li, E.K. Gaidamakova, V.Y. Matrosova, M. Zhai, H.M. Sulloway, J.C. Scholten, M.G. Brown, D.L. Balkwill, and M.J. Daly, Protein oxidation: Key to bacterial desiccation resistance? ISME Journal 2:393-403, 2008.
51. D. Slade and M. Radman, Oxidative stress resistance in Deinococcus radiodurans, Microbiology and Molecular Biology Reviews 75:133-191, 2011.
52. M.J. Daly, A new perspective on radiation resistance based on Deinococcus radiodurans, Nature Reviews of Microbiology 7:237-245, 2009.
53. M.J. Daly, E.K. Gaidamakova, V.Y. Matrosova, A. Vasilenko, M. Zhai, A. Venkateswaran, M. Hess, M.V. Omelchenko, H.M. Kostandarithes, K.S. Makarova, L.P. Wackett, J.K. Fredrickson, and D. Ghosal, Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance, Science 306:1025-1028, 2004.
54. M.J. Daly, Death by protein damage in irradiated cells. DNA Repair 11(1):12-21, 2012.
55. E. Gladyshev and M. Meselson, Extreme resistance of bdelloid rotifers to ionizing radiation, Proceedings of the National Academy of Sciences USA 105:5139-5144, 2008.
56. K.S. Makarova, L. Aravind, Y.I. Wolf, R.L. Tatusov, K.W. Minton, E.V. Koonin, and M.J. Daly, Genome of the extremely radiation resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics, Microbiology and Molecular Biology Reviews 65:44-79, 2001.
57. M.J. Daly, E.K. Gaidamakova, V.Y. Matrosova, A. Vasilenko, M. Zhai, R.D. Leapman, B. Lai, B. Ravel, S.W. Li, K.M. Kemner, and J.K. Fredrickson, Protein oxidation implicated as the primary determinant of bacterial radioresistance, Public Library of Science Biology 5(4):e92, 2007.
58. A. Kish, G. Kirkali, C. Robinson, R. Rosenblatt, P. Jaruga, M. Dizdaroglu, and J. DiRuggiero, Salt shield: intracellular salts provide cellular protection against ionizing radiation in the halophilic archaeon, Halobacterium salinarum NRC-1, Environmental Microbiology 11:1066-1078, 2009.
59. D. Slade and M. Radman, Oxidative stress resistance in Deinococcus radiodurans, Microbiology and Molecular Biology Reviews 75:133-191, 2011.
60. M.J. Daly, E.K. Gaidamakova, V.Y. Matrosova, J.G. Kiang, R. Fukumoto, D.Y. Lee, N.B. Wehr, G.A. Viteri, B.S. Berlett, and R.L. Levine, Small-molecule antioxidant proteome-shields in Deinococcus radiodurans, Public Library of Science One 5(9):e12570, 2010.
61. C.K. Robinson, K. Webb, A. Kaur, P. Jaruga, M. Dizdaroglu, N.S. Baliga, A. Place, and J.J. Diruggiero, A major role for nonenzymatic antioxidant processes in the radioresistance of Halobacterium salinarum, Journal of Bacteriology 193:1653-1662, 2011.
62. M.J. Daly, E.K. Gaidamakova, V.Y. Matrosova, A. Vasilenko, M. Zhai, R.D. Leapman, B. Lai, B. Ravel, S.W. Li, K.M. Kemner, and J.K. Fredrickson, Protein oxidation implicated as the primary determinant of bacterial radioresistance, Public Library of Science Biology 5(4):e92, 2007.
63. M.J. Daly, L. Ouyang, P. Fuchs, and K.W. Minton, In vivo damage and recA-dependent repair of plasmid and chromosomal DNA in the radioresistant bacterium Deinococcus radiodurans, Journal of Bacteriology 176:3508-3517, 1994.
64. A. Venkateswaran, S.C. McFarlan, D. Ghosal, K.W. Minton, A. Vasilenko, K. Makarova, L.P. Wackett, and M.J. Daly, Physiologic determinants of radiation resistance in Deinococcus radiodurans, Applied and Environmental Microbiology 66:2620-2626, 2000.
65. D. Ghosal, M.V. Omelchenko, E.K. Gaidamakova, V.Y. Matrosova, A. Vasilenko, A. Venkateswaran, H.M. Kostandarithes, H. Brim, K.S. Makarova, L.P. Wackett, J.K. Fredrickson, and M.J. Daly, How radiation kills cells: Survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress, FEMS Microbiology Reviews 29:361-375, 2005.
66. M.J. Daly, L. Ouyang, P. Fuchs, and K.W. Minton, In vivo damage and recA-dependent repair of plasmid and chromosomal DNA in the radioresistant bacterium Deinococcus radiodurans, Journal of Bacteriology 176:3508-3517, 1994.
67. D. Ghosal, M.V. Omelchenko, E.K. Gaidamakova, V.Y. Matrosova, A. Vasilenko, A. Venkateswaran, H.M. Kostandarithes, H. Brim, K.S. Makarova, L.P. Wackett, J.K. Fredrickson, and M.J. Daly, How radiation kills cells: Survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress, FEMS Microbiology Reviews 29:361-375, 2005.
68. A. Venkateswaran, S.C. McFarlan, D. Ghosal, K.W. Minton, A. Vasilenko, K. Makarova, L.P. Wackett, and M.J. Daly, Physiologic determinants of radiation resistance in Deinococcus radiodurans, Applied and Environmental Microbiology 66:2620-2626, 2000.
69. The decimal reduction factor, D10, is defined as the exposure—e.g., to heat or radiation—required to reduce the microbial population to one-tenth of its initial number. D10 values are measured experimentally and quoted values apply only to the exact conditions under which they are measured. The exposure may be quoted in terms of the time subjected to heat at a particular temperature or to the total absorbed radiation dose. Thus D10 values are frequently quoted in minutes (when determined by heating) or in grey (when determined by exposure to ionizing radiation).
70. D. Slade and M. Radman, Oxidative stress resistance in Deinococcus radiodurans, Microbiology and Molecular Biology Reviews 75:133-191, 2011.
71. R.C. Richmond, R. Sridhar, and M.J. Daly, Physicochemical survival pattern for the radiophile Deinococcus radiodurans: A polyextremophile model for life on Mars, SPIE 3755:210-222, 1999.
72. L.R. Dartnell, S.J. Hunter, K.V. Lovell, A.J. Coates, and J.M. Ward, Low-temperature ionizing radiation resistance of Deinococcus radiodurans and Antarctic Dry Valley bacteria, Astrobiology 10:717-732, 2010.
73. D. Ghosal, M.V. Omelchenko, E.K. Gaidamakova, V.Y. Matrosova, A. Vasilenko, A. Venkateswaran, H.M. Kostandarithes, H. Brim, K.S. Makarova, L.P. Wackett, J.K. Fredrickson, and M.J. Daly, How radiation kills cells: Survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress, FEMS Microbiology Reviews 29:361-375, 2005.
74. A.C. Granger, E.K. Gaidamakova, V.Y. Matrosova, M.J. Daly, and P. Setlow, Effects of Mn and Fe levels on Bacillus subtilis spore resistance and effects of Mn2+, other divalent cations, orthophosphate, and dipicolinic acid on protein resistance to ionizing radiation, Applied and Environmental Microbiology 77:32-40, 2011.
75. S. Ghosh, A. Ramirez-Peralta, E. Gaidamakova, P. Zhang, Y.Q. Li, M.J. Daly, and P. Setlow, Effects of Mn levels on resistance of Bacillus megaterium spores to heat, radiation and hydrogen peroxide, Journal of Applied Microbiology 111(3):663-670, 2011.
76. J. DiRuggiero, N. Santangelo, Z. Nackerdien, J. Ravel, and F.T. Robb, Repair of extensive ionizing-radiation DNA damage at 95°C in the hyperthermophilic archaeon Pyrococcus furiosus, Journal of Bacteriology 179:4643-4645, 1997.
77. M. Kottemann, A. Kish, C. Iloanusi, S. Bjork, and J. DiRuggiero, Physiological responses of the halophilic archaeon Halobacterium sp. strain NRC1 to desiccation and gamma irradiation, Extremophiles 9:219-227, 2005.
78. J. DiRuggiero, N. Santangelo, Z. Nackerdien, J. Ravel, and F.T. Robb, Repair of extensive ionizing-radiation DNA damage at 95°C in the hyperthermophilic archaeon Pyrococcus furiosus, Journal of Bacteriology 179:4643-4645, 1997.
79. M. Kottemann, A. Kish, C. Iloanusi, S. Bjork, and J. DiRuggiero, Physiological responses of the halophilic archaeon Halobacterium sp. strain NRC1 to desiccation and gamma irradiation, Extremophiles 9:219-227, 2005.
80. R.C. Richmond, R. Sridhar, and M.J. Daly, Physicochemical survival pattern for the radiophile Deinococcus radiodurans: A polyextremophile model for life on Mars, SPIE 3755:210-222, 1999.
81. J. DiRuggiero, N. Santangelo, Z. Nackerdien, J. Ravel, and F.T. Robb, Repair of extensive ionizing-radiation DNA damage at 95°C in the hyperthermophilic archaeon Pyrococcus furiosus, Journal of Bacteriology 179:4643-4645, 1997.
82. M. Kottemann, A. Kish, C. Iloanusi, S. Bjork, and J. DiRuggiero, Physiological responses of the halophilic archaeon Halobacterium sp. strain NRC1 to desiccation and gamma irradiation, Extremophiles 9:219-227, 2005.
83. A.C. Granger, E.K. Gaidamakova, V.Y. Matrosova, M.J. Daly, and P. Setlow, Effects of Mn and Fe levels on Bacillus subtilis spore resistance and effects of Mn2+, other divalent cations, orthophosphate, and dipicolinic acid on protein resistance to ionizing radiation, Applied and Environmental Microbiology 77:32-40, 2011.
84. S. Ghosh, A. Ramirez-Peralta, E. Gaidamakova, P. Zhang, Y.Q. Li, M.J. Daly, and P. Setlow, Effects of Mn levels on resistance of Bacillus megaterium spores to heat, radiation and hydrogen peroxide, Journal of Applied Microbiology 111(3):663-670, 2011.
85. D. Ghosal, M.V. Omelchenko, E.K. Gaidamakova, V.Y. Matrosova, A. Vasilenko, A. Venkateswaran, H.M. Kostandarithes, H. Brim, K.S. Makarova, L.P. Wackett, J.K. Fredrickson, and M.J. Daly, How radiation kills cells: Survival of Deinococcus radiodurans and Shewanella oneidensis under oxidative stress, FEMS Microbiology Reviews 29:361-375, 2005.
86. A.J. Auman, J.L. Breezee, J.J. Gosink, P. Kämpfer, and J.T. Staley, Psychromonas ingrahamii sp. nov., a novel gas vacuolate, psychrophilic bacterium isolated from Arctic polar sea ice, International Journal of Systemics and Evolutionary Microbiology 56:1001-1007, 2006.
87. J. Breeze, N. Cady, and J.T. Staley, Subfreezing growth of the sea ice bacterium Psychromonas ingrahamii, Microbial Ecology 47:300-304, 2004.
88. P. Amato and B.C. Christner, Energy metabolism response to low-temperature and frozen conditions in Psychrobacter cryohalolentis, Applied Environmental Microbiology 75:711-718, 2009.
89. B.C. Christner, Incorporation of DNA and protein precursors into macromolecules by bacteria at –15 degrees C, Applied and Environmental Microbiology 68:6435-6438, 2002.
90. See, for example, R.D. Haight and R.Y. Morita, Thermally induced leakage from Vibrio marinus an obligately psychrophilic marine bacterium, Journal of Bacteriology 92:1388-1393, 1966.
91. N.J. Russell, Cold adaptation of microorganisms, Philosophical Transactions of the Royal Society of London B 326:595-611, 1990.
92. For additional details see, for example Figures 1 and 2 in C. Gerday, M. Aittaleb, J.-L. Arpigny, E. Baise, J.-P. Chessa, G. Garssoux, I. Petrescu, and G. Feller, Psychtrophilic enzymes: A thermodynamic challenge, Biochemica et Biophysica Acta 1342:119-131, 1997; Figure 2 in C. Gerday, M. Aittaleb, M. Bentahir, J.-P. Chessa, P. Claverie, T. Collins, S. D’Amico, J. Dumont, G. Garsoux, D. Georlette, A. Hoyoux, T. Lonhienne, M.-A. Meuwis, and G. Feller, Cold-adapted enzymes: From fundamentals to biotechnology, Trends in Biotechnology 18:103-107, 2000; and Figure 2 in D. Georlette, V. Blaise, T. Collins, S. D’Amico, E. Gratia, A. Hoyoux, J.-C. Marx, G. Sonan, G. Feller, C. Gerday, Some like it cold: Biocatalysis at low temperatures, FEMS Microbiology Reviews 28:25-42, 2004.
93. R.D. Haight and R.Y. Morita, Thermally induced leakage from Vibrio marinus an obligately psychrophilic marine bacterium, Journal of Bacteriology 92:1388-1393, 1966.
94. For discussions of the molecular adaptations of membrane lipids and the effects of temperature on their stability see, for example, N.J. Russell, Psychrophilic bacteria—Molecular adaptations of membrane lipids, Comparative Biochemistry and Physiology 118A:489-493, 1997.
95. V.A. Shcherbakova, N.A. Chuvilskaya, E.M. Rivkina, S.A. Pecheritsyna, K.S. Laurinavichius, N.E. Suzina, G.A. Osipov, A.M. Lysenko, D.A. Gilichinsky, and V.K. Akimenko, Novel psychrophilic anaerobic spore-forming bacterium from the overcooled water brine in permafrost: Description of Clostridium algoriphilum sp. nov, Extremophiles 9:239-246, 2005.
96. V.A. Shcherbakova, N.A. Chuvilskaya, E.M. Rivkina, S.A. Pecheritsyna, K.S. Laurinavichius, N.E. Suzina, G.A. Osipov, A.M. Lysenko, D.A. Gilichinsky, and V.K. Akimenko, Novel psychrophilic anaerobic spore-forming bacterium from the overcooled water brine in permafrost: Description of Clostridium algoriphilum sp. nov, Extremophiles 9:239-246, 2005.
97. The decimal reduction factor, D10, is defined as the exposure—e.g., to heat or radiation—required to reduce the microbial population to one-tenth of its initial number. D10 values are measured experimentally and quoted values apply only to the exact conditions under which they are measured. The exposure may be quoted in terms of the time subjected to heat at a particular temperature or to the total absorbed radiation dose. Thus D10 values are frequently quoted in minutes (when determined by heating) or in grey (when determined by exposure to ionizing radiation).
98. T.E. Shehata and E.B. Collins, Sporulation and heat resistance of psychrophilic strains of Bacillus, Journal of Dairy Science 54:1405-1409, 1972.
99. A.D. Warth, Relationship between the heat resistance of spores and the optimum and maximum growth temperatures of Bacillus species, Journal of Bacteriology 134:699-705, 1978.














