The decision hierarchy outlined in this report provides a robust framework, but there are remaining uncertainties in the answers to several of the decision questions. The five research areas described below are important for addressing these uncertainties. The first three areas address the nature of the bioload on the spacecraft before launch, and the last two areas address environmental conditions on icy bodies.
HEAT RESISTANCE OF COLD-LOVING SPORES
Limited data exists on the heat inactivation of spores from psychrophilic and psychrotolerant bacteria. The general strategy for determining the inactivation of spores by heat or other treatment relies on the calculation of D values (inactivation of 90 to 100 percent of the spores) over some period of time for some specific treatment. With heat treatment using high temperatures (90°C or higher) a D value is generally attained in 10 minutes or less. Inactivation of spores will occur over different time intervals depending on the temperature. This kind of analysis, however, has not been done for psychrophilic and psychrotolerant bacterial spores.
Recommendation: The D-value times for heat inactivation of spores from psychrophilic and psychrotolerant spore-forming bacteria should be determined at different temperatures, specifically between 40°C and 80°C. These analyses should include psychrophilic and facultative psychrotolerant bacteria isolated from high-latitude soil, water, and cryopeg samples, as well as psychrotolerant microorganisms isolated from temperate soils, spacecraft assembly sites, and the spacecraft itself.
Recommendation: Studies should be undertaken to better understand the environmental conditions that initiate spore formation and spore germination in psychrophilic and psychrotolerant microorganisms so that these requirements can be compared with the characteristics of target icy bodies.
Recommendation: Searches should be undertaken to discover unknown types of psychrophilic spore-formers and to assess if any of them have tolerances different from those of known types.
ENHANCED RESISTANCE OF BIOFILMS
Biofilm growth confers greater than usual resistance to a diversity of environmental extremes,1 and microbial functional redundancy in biofilms might also confer resilience to environmental extremes.2 Future research can address the extent to which organisms within communities or biofilms may exhibit increased resistance to the high temperatures used for terminal bioload reduction. Although the exterior of a spacecraft that has been assembled in a clean room is unlikely to harbor communities within biofilms, the protected interior of spacecraft might contain microenvironments in which organisms are in contact and behave as biofilms.
Protected microenvironments within spacecraft have to be characterized, and their microbial ecology has to be assessed. Moreover, research is needed to determine whether biofilm growth of organisms associated with spacecraft microhabitats can influence their resistance to heat treatment and other environmental extremes encountered on journeys to icy bodies.
Recommendation: Research should be undertaken to characterize the protected microenvironments within spacecraft and to assess their microbial ecology.
Recommendation: Research should be undertaken to determine the extent to which biofilms might increase microbial resistance to heat treatment and other environmental extremes encountered on journeys to icy bodies.
IMAGING METHODOLOGY TO DETERMINE BIOLOAD
The long-standing NASA standard protocol used to assess microbial contamination on spacecraft during assembly, test, and launch operations uses a Petri-plate-based culturable assay method to determine the number of cultivable aerobic bacterial endospores present on surfaces of interest. This assay takes 72 hours to complete, which can be extremely challenging and costly in a time-constrained hardware assembly environment. Because it relies on swab or wipe sampling, the assay method cannot be used directly for parts that cannot be touched or that are sensitive to the water matrix used for sampling. New techniques for obtaining real-time accurate assessments of microbial burden on flight hardware could provide a significant improvement over the current culture method.
The ideal solution would be a non-invasive, non-destructive technique that can be used to scan a spacecraft’s surfaces and identify living microbes through detection of morphologies of cells that are alive, as indicated by the presence of ribosomal RNA transcripts. There are several techniques that might be stepping stones toward the goal of detecting individual microbes on a spacecraft: e.g., Raman and fourier transform infrared spectroscopy. However, some of these techniques, for example, lack a scanning capability or cannot distinguish between living and dead or metabolically inactive cells.
One particularly promising new technique that might be applicable to the assessment of the bioload on a spacecraft is deep-ultraviolet (224 to 250 nm range) imaging.3,4 The advantage of using short-wavelength ultraviolet radiation is that most minerals and solid surfaces are non-fluorescent at this wavelength, whereas strong fluorescence is seen from the amino acids tryptophan, phenylalanine, and tyrosine, so that any organism containing proteins with these amino acids will be detectable by autofluorescence (i.e., without the addition of fluorescent stains or dyes). The identification of single cells through fluorescence scanning at low magnification can also provide a quantitative measurement of bioload. Real-time analyses of positive targets at higher magnification can enable identification of cells’ morphological properties, including the ability to differentiate bacterial spores from vegetative cells.
The deep-ultraviolet imaging method should be applicable to the assessment of bioload on the surfaces of spacecraft. As shown in the adjacent images (of Figure 6.1), bacteria “hiding” in the matrices of well-scrubbed surfaces of stainless steel are easily seen using deep-ultraviolet fluorescence imaging. In addition, because of their high tyrosine content (and probably other chemical differences), the spores have a fluorescent signal that differs from the signal of vegetative cells, making the approach valuable for direct determination of bacterial spores on
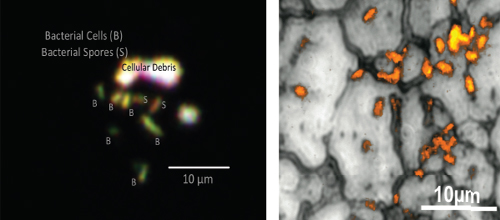
FIGURE 6.1 Bacterial cells and spores on the same metal substrate (left). Vegetative cells “hiding” on a plasma-cleaned surface (right). SOURCE: Courtesy of R. Bhartia, Jet Propulsion Laboratory/California Institute of Technology.
surfaces. Low-resolution scanning could identify areas of fluorescence followed by high-resolution imaging to count cells.
Maturing and validating deep-ultraviolet fluorescence imaging to the level of automatic quantitative sampling of spacecraft surfaces would be a major positive addition in the area of planetary protection quality assurance.
Recommendation: Technologies should be developed to directly detect and enumerate viable microorganisms on spacecraft surfaces.
AVAILABILITY OF BIOLOGICALLY IMPORTANT ELEMENTS
The availability of key elements necessary for life (carbon, hydrogen, oxygen, nitrogen, and phosphorus, as well as trace nutrients) within liquid environments on icy bodies represents a key uncertainty in the decision hierarchy for planetary protection. In future missions, observation techniques applied to different icy bodies can determine the concentration of these elements, as well as of compounds containing these elements. Further progress in understanding the chemistry of the early solar nebula from which the icy bodies accreted will also be important for constraining the abundances of key elements. An especially important research area for constraining the availability of key elements for terrestrial biological contaminants is the solubility of these elements and compounds under the conditions found within icy bodies. Theoretical modeling and laboratory analog studies will further constrain aqueous solubility and water-rock interactions under the pressures, temperatures, pH, and solute conditions expected within icy bodies. Such studies are especially needed at the high pressures encountered within large icy bodies, because little is known about the possible interactions of rocks and brines with high-pressure ice phases.
Recommendation: Research should be undertaken to determine the concentrations of key elements or compounds containing biologically important elements on icy bodies in the outer solar system through observational technologies and constraints placed on the range of trace elements available through theoretical modeling and laboratory analog studies.
Understanding global chemical cycles and global material transport on icy bodies is important for several planetary protection decision points, notably the availability of elements, the availability of chemical energy
sources, and the possibility of transport of spacecraft components on an icy body’s surface into a subsurface liquid environment. The key to understanding this transport is to examine the geologic processes that can promote surface-subsurface exchange, to determine the rate at which they occur, the depth to which they penetrate, the influence of materials other than water ice mixed into the icy shells, and the role of liquid water in their operation. The concept of a “no-mans land” that bars transport of material into the subsurface also deserves closer scrutiny. When examined through this lens, and in combination with observed surface geology, several icy bodies may fall into the category of no concern. Further spacecraft exploration and reconnaissance of icy body geology and surface characteristics will continue to improve understanding of the global material transport cycles of icy bodies.
Recommendation: Research should be undertaken to understand global chemical cycles within icy bodies and the geologic processes occurring on these bodies that promote or inhibit surface-subsurface exchange of material.
1. P. Watnick and R. Kolter, Biofilm, City of microbes, Journal of Bacteriology 182:2675-2679, 2000.
2. M.-E.Y. Boivin, B. Massieux, A.M. Breure, G.D. Greve, M. Rutgers, and W. Admiraal, Functional recovery of bacterial biofilm communities after copper exposure, Environmental Pollution 140:239-246, 2006.
3. R. Bhartia, W.F. Hug, E.C. Salas, R.D. Reid, K.K. Sijapati, A. Tsapin, W. Abbey, P.G. Conrad, K.H. Nealson, and A.L. Lane, Classification of organic and biological materials with deep UV excitation, Applied Spectroscopy 62:1070-1077, 2008.
4. R. Bhartia, E.C. Salas, W.F. Hug, R.D. Reid, A.L. Lane, K.J. Edwards, and K.H. Nealson, Label-free imaging with deep UV laser induced native fluorescence, Applied Environmental Microbiology 76:7231-7237, 2010.




