4
Current Status of Surface Measurement Technologies and Potential ACWA Site Applications
There are several overarching system requirements for the detection and monitoring of distilled mustard agent (HD) and distilled mustard mixed with bis(2- chloroethylthioethyl) ether (HT) at the Pueblo Chemical Agent Pilot Plant (PCAPP) and mustard agent (H) and nerve agents GB and VX at the Blue Grass Chemical Agent Pilot Plant (BGCAPP). These include an analytical system that (1) provides demonstrated reliability, (2) meets monitoring figures of merit1 for the agents of interest at the desired concentrations in target matrices, (3) uses sampling intervals sufficient for protection of individuals and the environment, (4) conforms with accepted industrial hygiene principles and regulatory standards, and (5) allows sound statistical sampling selection to capture the exposure conditions or relevant waste contamination levels of the material matrices being characterized (U.S. Army, 2011a).
Distinguishing the degree to which various analytical systems can provide these capabilities for chemical agents on or within specific matrices depends on the ability of the analytical system to characterize different phases of the target species (i.e., gas, liquid, and solid) and the information content derived from such measurements. Levels of information content from measurements are described for a particular system through the monitoring method classification detailed in U.S. Army (2011a), whereby Class I methods provide quantitative and accurate values over the desired concentration range (using, for example, the depot area agent monitoring system [DAAMS]), Class II methods provide semi-quantitative and positive detection above a designated concentration limit (using, for example, the miniature continuous air monitoring system [MINICAMS]), and Class III methods, which are qualitative, provide only a binary positive or negative response for a specific agent at the designated response level (e.g., MINICAMS measurements of headspace VSL).
The current detection technologies selected for process and waste characterization are primarily based on vapor-phase detection, either in the context of (1) direct air sampling in the process stream or ambient environments, or (2) decontamination and decommissioning endeavors through vapor headspace analysis of materials contained within an enclosed space (U.S. Army, 2011a). Two primary technologies are integrated
![]()
1These include meeting required sensitivity, specificity, and response time.
into the design and construction of PCAPP and BGCAPP: DAAMS and MINICAMS. For example, the PCAPP facility has incorporated 137 DAAMS into the air monitoring system, typically with adjacent MINICAMS (Waybright, 2011).
Briefly, DAAMS preconcentrate air samples for preset sampling intervals using a sorbent filled-tube or sample loop, followed by agent desorption and gas chromatography-mass spectrometry (GC-MS) characterization. Typically, samples are preconcentrated for an 8-hr collection time and a full analysis is performed within 24 hr to provide archival analytical results. In contrast, MINICAMS utilize cycled GC measurements to provide near-real-time (NRT) analyses that take approximately 5 to 15 min. Depending on the specific contaminated matrices expected during agent processing, changeover, or closure activities, the specific protocols used primarily conform with those prescribed by the U.S. Environmental Protection Agency.
The samples derived from the anticipated waste streams, including from plant decommissioning activities, vary widely in their physical characteristics (for instance, their state and volume and type of matrix) and the agent contamination spatial resolution desired. Although DAAMS and MINICAMS technologies for vapor analyses have been demonstrated to be reliable, they do not provide good spatial or temporal resolution and are not particularly well suited for the direct analysis of agents in condensed-phase wastes and in occluded or potentially occluded volumes (see Box 3-3).
Recent advances in analytical technologies, as described below, are anticipated to complement existing agent monitoring strategies and may improve workplace and environmental safety. They provide the ability to detect agent contamination directly for a variety of matrices as well as agent in the gas phase, potentially providing high spatial resolution and rapid temporal assessments of agent contamination. These features are particularly relevant for more efficient and timely characterization of wastes during the agent processing and agent changeover phases of demilitarization operations. The ability to rapidly scan protection gear (demilitarization protective ensemble (DPE) suits) as workers exit Class A areas, where the suits may have been contaminated by exposure to chemical agents, could enhance worker safety while greatly reducing the time spent in decontamination and transition. In addition, the committee envisions that the application of these advanced technologies during plant decommissioning (closure) activities could reduce the time required for this phase of the project, yielding significant cost savings. Finally, the spatial resolution and rapid temporal response of the new analytical methods described below might prove to be an invaluable asset in dealing with unanticipated events such as an agent release, facilitating the tracking of any vapor releases to their source.
Two recently developed surface measurement technologies based on mass spectrometry ambient ionization techniques have been commercialized and found widespread application: direct analysis in real time (DART) (Cody et al., 2005) and desorption electrospray ionization (DESI) (Takáts et al., 2004). These are illustrated in Figures 4-1 and 4-2 and discussed in greater detail below. DART, DESI, and related techniques (Harris et al., 2011) allow direct sampling and analysis of a wide variety of liquid or solid matrices and produce high spatial resolution information to localize the target analytes. DAAMS and MINICAMS provide information about time-averaged agent concentration levels in the air or from the vapor headspace of samples in enclosed spaces. Such information represents indirect measurements of where the contamination is
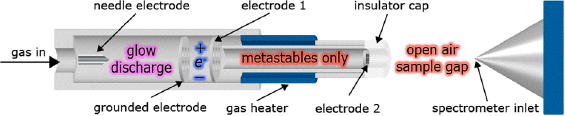
FIGURE 4-1 Schematic diagram of DART ion source. An electrical discharge creates a glow discharge plasma as inert gas flows through the DART chamber. The plasma contains ions, electrons, and excited neutral atoms and molecules. Biased electrodes remove ions and electrons, leaving only long-lived electronically or vibronically excited atoms and molecules (metastables). The gas can be heated to assist desorption of neutrals from surfaces. The heated gas exits the source heading toward the atmospheric pressure inlet of the mass spectrometer. The ions produced in the vapor phase between the DART insulator cap and the mass spectrometer inlet are formed by interaction of the electronic excited-state species of helium or neon or vibronically excited nitrogen with the sample. Samples are placed along the path between the cap and inlet. For larger samples the source is mounted at an angle with respect to the MS inlet.
SOURCE: JEOL USA, Inc., R.B. Cody. Reprinted with permission.
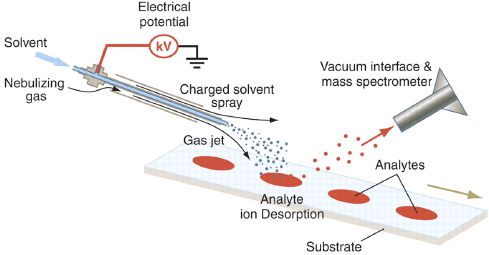
FIGURE 4-2 Schematic diagram of DESI ion source. A nebulizing gas flows around an electrospray source, which is directed at the surface to be analyzed. Charged droplets pick up analyte molecules from the surface and are captured by the vacuum interface of the mass spectrometer through a capillary of variable length. SOURCE: Cooks et al., 2006. Reprinted with permission from AAAS.
localized. In contrast, real-time, spatially resolved techniques such as DART and DESI provide the ability to allow directly identifying the location of agent contamination on solid matrices and, with appropriate modifications, DART may also be used to measure trace components in vapor plumes and locate their sources by tracking airborne agent concentration gradients in real time. Detailed descriptions of these recently developed ambient mass spectrometry technologies are provided later in this chapter.
The ion source is an integral part of most laboratory mass spectrometric instrumentation and is located in close proximity to the mass analyzer in order to maximize ion transport from the source to the analyzer. As a result, the operator must take the sample to the instrument. However, with the new sampling technologies it is possible to perform nonproximate analysis, in which the ion source is some distance (0.5 to 3 m) from the atmospheric pressure inlet, with ions transported through flexible tubing and sampled by remote wands. This approach enables the instrument to be brought to the sample and facilitates the use of a cart-mounted or even personnel-carried instruments capable of identifying localized regions of agent contamination on surfaces. In addition, even though DART and DESI are designed to detect surface species, it might be possible to further develop methods of operating DART and DESI instruments, or combine them with a second ambient ion source specifically for detection of trace species in the vapor phase, producing a single portable instrument able to perform real-time vapor and surface analyses.
In summary, the combination of real-time measurements with multi-state (gases, liquids, and solids) analytical capability suggests that the newer instruments for ambient ionization mass spectrometry incorporating remote sampling capability deserve serious consideration for their potential to supplement the current DAAMS and MINICAMS agent monitoring instruments. Based on the capabilities reviewed in this chapter and potential measurement strategies reviewed in Chapter 5, their potential to enhance workplace safety, improve operational efficiency, and accelerate decontamination activities during both operational and closure activities at the BGCAPP and PCAPP facilities will be assessed.
PROPERTIES OF THE TARGET MOLECULES RELEVANT TO THEIR DETECTION BY AMBIENT MASS SPECTROMETRY
Before examining details of recent advances in ambient mass spectrometry, it is useful to review the ionization schemes and mass spectrometer configurations that are generally used to detect chemical agents. In this section, the properties of the target chemical species GB, VX, and mustard agents are reviewed in relation to their detection by ambient ionization mass spectrometry. This analysis also provides insights into possible interferences from other trace species in the sampled environment that could compromise the detection of the target chemical agents.
The best way to detect a molecule by mass spectrometry is to leave the molecule intact through a soft ionization process—that is, a process that is not energetic enough to fragment the resulting ion. Such soft processes are often chemical ionizations that are only mildly exothermic, leaving products with insufficient internal energy to fragment. These processes are in contrast to electron impact ionization, in which high-energy
electrons excite and ionize the molecule, which then fragments readily. This can lead to ambiguity in the identification of the precursor, especially if several neutral species are simultaneously ionized in a mixture. In addition, chemical ionization, unlike electron impact ionization, seldom produces highly reactive and easily fragmented odd electron species.
In the ambient ionization instrumentation discussed later in this chapter, a variety of classes of chemical ionization reactions are likely depending on how the source is configured and operated. Electron transfer involves a precursor ion accepting or donating an electron to the molecule to be ionized. The reaction of a chemical agent, C, with a reagent ion, A+ or A, may be illustrated as follows:
For a positive chemical agent ion, A+:
![]()
For a negative reagent ion, A -
![]()
Charge transfer produces an ion with the same mass as the chemical agent, which is obviously desirable. The important parameters in determining whether reaction 4.1a or 4.1b can occur are ionization potentials (IP) for positive ions and electron affinities for negative ions. The former ionization potential is defined (Lias et al., 1988) as the energy at 0 K for the process in reaction 4.2:
![]()
Reaction 4.1a may then occur if the ionization potential of the chemical agent is less than the ionization potential of the parent molecule of the ion precursor, A. In dry air, a typical reagent ion may be NO+. The important property for negative ion charge transfer is the electron affinity, defined by Lias et al. (1988) as the negative of the 0 K energy for attaching an electron, as illustrated in reaction 4.3:
![]()
In this case, reaction 4.1b occurs if the electron affinity of the chemical agent is greater than that of the reagent ion A.
Proton donors are the most commonly used ionization reagents in ambient ionization mass spectrometry. In air with even a modest amount of humidity, a well- known series of reactions take place that rapidly form protonated water, H3O+, and its hydrates (Ferguson and Fehsenfeld, 1969). Just as its name implies, proton transfer involves moving a proton from the ionizing agent to the chemical agent, as illustrated by reaction 4.4:
![]()
Here the important property in determining the likelihood of reaction is the proton affinity, defined as the negative of the energy at 298 K of adding a proton to a molecule (Lias et al., 1988), as expressed by reaction 4.5:
![]()
Reaction 4.4 will most likely occur if the proton affinity of the chemical agent is greater than that of the H3O+ reagent ion (Bohme, 1975). In the vast majority of exothermic proton transfer reactions, the rate constant is very close to the collisional limit (Anicich, 2003; Su and Chesnavich, 1982). Ambient ion mass spectrometry (like all chemical ionization methodologies) derives its sensitivity from the fact that the ion-molecule collision rate is large. After proton transfer, the mass of the chemical agent in daltons is one number greater than its molecular weight. In sources involving clean air with at least a little water, proton hydrates are the precursor ions unless trace impurities with high proton affinities can sequester the labile proton before it reacts with the target chemical agent (Ketkar et al., 1991a). For example, NH3, present in exhaled breath, can act in this way. In this case, NH4+ and its hydrates are the precursors. These will be more selective ionization agents since NH3 has a considerably higher proton affinity than does H2O and therefore transfers a proton to many fewer molecules than does H3O+ (Linstrom and Mallard, 2007). To avoid situations where atmospheric variation in NH3 affects detection conditions, a trace amount of NH3 may be added to the source to provide consistent performance. Other dopants may be added to fine tune the energetics of proton transfer and further optimize the detection selectivity. There is an equivalent negative ion reaction that is useful for the detection of strong acids. This involves reaction of a proton acceptor such as OH- or O2+ with the target molecule to generate the conjugate base by proton transfer. Since the chemical agents of interest are not strong acids this is not discussed further.
The above reactions detect the agents at either their neutral mass or at 1 dalton higher. There are alternative chemical ionization schemes that involve larger changes in mass. A common one in the atmospheric community involves F- transfer from an appropriate fluoride donor reagent ion. In a prior NRC report on chemical agent monitoring, it was suggested that this class of reaction might be extended to detect chemical agents (NRC, 2005a). Since that time, it has been shown that the fluoride ion affinities of HD, GB, and VX are low, so this process will not be useful (Midey et al., 2008, 2009, 2010).
Studies involving simulants and recent work on agents themselves have shown that clustering is likely to be important (Nilles et al., 2009). Here the chemical agent and reagent ion will simply stick together, as indicated in reaction 4.6,
![]()
where M is an atmospheric molecule that acts as a third body to remove excess energy from the adduct, leading to formation of a stable complex. The work of Nilles et al. (2009) using DART showed that NH4+ clustered to the G series of agents.
All of the above assumes that no trace species is in large enough concentration to deplete the primary ion significantly. In that case, the kinetics becomes more
complicated. The product ions are no longer proportional to concentration. Additionally, one may need to account for reactions of the product ions. Since the interest is to detect contaminants and not large spills, neglecting these effects should not affect the ability to detect an agent.
In detecting chemical agents it is clear from the above discussion that it is extremely important to understand their properties. Table 4-1 extends Table 2-1 from a prior NRC report (NRC, 2005a). It lists a variety of chemical properties for GB (sarin), VX, HD, and HT, including vapor pressure and several ion energetic properties. Except for GB, all the agents of interest have exceedingly low vapor pressures.
The ion energetics properties shown in Table 4-1 indicate that a number of positive chemical ionization reagents should readily ionize the target chemical agents. Electron transfer from an agent molecule to the reagent ion will occur for the common reagent NO+ for VX and H agents since their IPs are lower than that of NO (IP = 9.26 eV) (Linstrom and Mallard, 2007). Since the IPs of the agents are close to that of NO, little fragmentation is expected. Experiments on surrogates confirm this (Midey et al., 2008, 2010). On the other hand, GB has an IP higher than NO, and experiments on surrogates indicate that clustering is likely (Midey et al., 2009). Higher-energy charge transfer ions, such as O2+, will react readily with agents but will lead to fragmentation of the agents, making identification more difficult (Midey et al., 2008, 2009, 2010).
As discussed above, most chemical ionization schemes center on proton transfer. H3O+ is readily formed in ambient mass spectrometry and is the reagent ion most likely to transfer a proton since H2O has a low proton affinity (691 kJ/mol) (Linstrom and Mallard, 2007). All agents have higher proton affinities than H2O and therefore proton transfer is highly probable. This is confirmed in laboratory studies on agent surrogates that show that proton transfer occurs. However, due to the amount of energy available, dissociative proton transfer may also occur, and work on agent surrogates indicates that this may happen (Midey et al., 2008, 2009, 2010). Therefore, it is better to use reagent ions that provide less exothermic proton transfers. NH3 has a proton affinity of 854 kJ/mol (Linstrom and Mallard, 2007). This is considerably lower than the proton affinity of VX, equal to that of GB within error margins, and larger than that of mustard agent. Indeed, the surrogate experiments show that proton transfers from NH4+ to VX and GB are likely and that mustard agent clusters to NH4+ (Midey et al., 2008, 2009, 2010). The proton affinity of VX is near the top of the scale, and almost any protonated reagent ion will ionize it. A reagent ion with a high proton affinity, but slightly less than that of VX, is likely to be very selective by reducing background from interferences and would therefore permit detection of VX with high sensitivity.
Negative ions show much less promise for detection of the chemical agents relevant to ACWA operations. All of the agents have exceedingly low electron affinities, and therefore few parent ions will be formed. In addition, all of the agents have fairly low fluoride affinities. A commonly used reagent ion is SF6". The fluoride affinity of SF5 is 230 kJ/mol, which is much higher than that of any of the chemical agents. It is possible that some other fluoride transfer reagent might be more appropriate, but there is no obvious choice at present.
The above discussion emphasizes that soft ionization techniques are likely to ionize a variety of agents without fragmentation. However, that is not sufficient for conclusive identification of chemical agents. The chemical environment in
TABLE 4-1 Physical Properties of Chemical Warfare Agents
| Agenl Characteristic | Nerve Agents | Blister (Mustard)Agentsa | |||
| GB (Sarin) | VX | HD | HT | ||
| Chemical formula | (CH3)2CHO(CH3) FPO | C11H26NO2PS | (CICH2CH2)2S | 60% HD. 40% ((CICH2CH2SCH2CH2)2O | |
| CAS Registry No. | 107-44-3 | 50782-69-9 | 505-60-2 | N/A | |
| Molecular weight | 140.10 | 267.38 | 159.08 | (Mixture -188.96 based on 60 40 weight percent) | |
| Boiling point (°C) | 150 (extrapolated) | 292 (extrapolated) | 218 | No constant boiling point | |
| Freezing point (°C) | -56 | <-51 | 14.5 | 0 to 1.3 | |
| Vapor pressure (mm Hgat 25 °C) | 2.48 | 0.0009 | 0.106 | 7.7 × 10-2 (calculated based on Raoult's law equation and 60 weight percent HD and 40 weight percent T) | |
| Vapor density (relative to air) | N/A | N/A | 5.5 (calculated) | 6.5 (calculated based on 60 40 weight percent) | |
| Volatility (mg/m3) | 3.370 at 0°C 187,000 at 25 °C | 12.6 at 25 °C | 75 at 0°C (solid) 906 at 20 °C (liquid) | 783 at 25 °C | |
| Surface tension (dyne/cm) | 26.5 at 20°C | 32.0 at 20 °C | 43.2 at 20 °C 42.4 at 25 °C | 42.0 at 25 °C | |
| Kinematic viscosity (cS) | 1.28 at 25 °C | 12.26 at 20°C | 3.52 at 20 °C | 6.50 at 20 °C | |
| Liquid density (g cm3 at20°C) | 1.0SS7 | 1.0083 | 1.27 | 1.26 | |
| Latent heat of vaporization (cal g) | 82.9 | 71.8 | 94.3 | N/A | |
| Solubility (g/L H2O at 25 °C | Completely miscible | 50 at 21.5 °C | 0.92 | Similar to HD | |
| Heat of combustion, (cal/g) | 5,600 | 8,300 | 4,500 | N/A | |
| Ionization potential (eV) | 9.82 | 7.3 | 8.74 | N/A | |
| Proton affinity (kJ/niol) | 857 | 1,039 | 796 | N/A | |
| Fluoride affinity (kJ/mol) | 152 | 111 | 104 | N/A | |
aThe blister agents are labeled H, HD, and HT. The active ingredient in all these agents is bis(2-chloroethyl) sulfide, (ClCH2CH2)2S. HD, called distilled mustard, is nominally pure is(2-chloroethyl)sulfide. H, often called Levinstein mustard, is approximately 70 percent bis(2-chloroethyl) sulfide and 30 percent impurities, which tend to be polysulfides such as (ClCH2CH2)2Sn. HT is a mixture of ca. 60 percent (ClCH2CH2)2S and 40 percent ((CICH2CH2)2SCH2CH2)2O.
bThe surface tensions of HD at both 20°C and 25°C are included to allow the reader to compare the surface tensions of HD and HT under the same physical conditions, while also giving the reader a general comparison of the surface tensions of nerve agents and blister agents across constant physical conditions.
SOURCE: NRC, 2005b, 2005c; Midey et al., 2008, 2009, 2010.
demilitarization plants is likely to be complex, and a wide variety of trace species are likely to be present. If one or more of these species has the same nominal mass as the agent when ionized, a false positive could occur with low-resolution mass spectrometric detection. In order to separate the ions derived from agents from those due to other trace species, two techniques are useful. While it may be common to have two species (agent and other) at the same integer (nominal) mass (e.g., CO and N2), it is unlikely that they will have the same exact mass. Suitably compact mass spectrometers with high enough mass resolution to determine exact masses are now common, although more expensive than lower resolution counterparts. A mass spectrometer with a 5 part per million mass resolution is able to identify elemental composition for species up to 200 daltons.
An alternative strategy involving less expensive instrumentation is to isolate ions with the same nominal mass and then fragment them using tandem mass spectrometry (MS/MS). After the ions with the same nominal mass are isolated, they are accelerated and collide with a nonreactive species (e.g., He or Xe), causing fragmentation. Different species with the same nominal mass will have different fragmentation patterns. Fragmentation often occurs to form not one but many ions, as indicated in reaction 4.7:
![]()
where M is the unreactive collision partner used to transfer the energy needed for fragmentation. The mass spectrum of the fragments (B+, C+, D+), which is called the product ion spectrum, determines which species is present. Tandem mass spectrometry can be arranged to occur either in time or in position. Quadrupole traps are common for the former and sequential instrumentation, such as triple quadrupoles, are used for the latter. As an example, a laboratory at the U.S. Army’s Edgewood Chemical Biological Center has used such a scheme to determine VX levels in animal organs. The extract is ionized in a GC-MS equipped with an NH4+ chemical ionization source. Protonated VX is isolated at 268 daltons. However, there are other compounds in the extract with this mass. The 268-dalton ion is passed through a collision cell and the fragment ion at 128 daltons is identified as coming only from VX (McGuire et al., 2008).
Published chemical ionization measurements of chemical agents confirm the ion reaction mechanisms discussed above. Atmospheric pressure ionization MS/MS measurements using protonated water vapor reagent ions by Fite and coworkers (Ketkar et al., 1991b) yielded detection limits for GB of 14.1 parts per trillion by volume (pptv), and detection of VX fragments cleaved to produce the G analog by reaction with a silver fluoride impregnated pad yielded a detection limit of 100 pptv. More recently, Monks and coworkers (Cordell et al., 2007) demonstrated real-time detection of GB and H at detection levels of 3-5 parts per billion by volume (ppbv), also using protonated water vapor as the reagent ion.
EXPERIMENTAL METHODS FOR AMBIENT MASS SPECTROMETRY
Ambient, or open air, surface sampling techniques, sometimes described collectively as “ambient MS techniques,” are a group of ion generation approaches that can be readily coupled to mass spectrometric detectors for both MS and tandem MS (MS/MS) analysis of target molecules. These technologies have been coupled to a variety of mass spectrometers equipped with atmospheric pressure interfaces with only minor modifications, enabling the identification of unknowns by fragmentation pattern matching to databases, elemental formula determination via accurate mass measurements, multianalyte quantitation, spatially resolved measurements, and selective ionization enhancement for target compounds of interest. Ambient MS sampling/ionization techniques such as DART and DESI have grown in popularity because they:
• Enable the sampling of analyte under atmospheric pressure conditions from both liquid and solid states remotely from the mass analyzer.
• Have been used to investigate objects or surface features of a wide range of shapes, sizes and textures.
• Can perform qualitative or quantitative analysis with no or minimal sample preparation, such as dissolution, grinding, extraction, or preconcentration.
• Can conduct all these operations in real time with high sensitivity and minimal unwanted ion fragmentation.
Although this chapter focuses mainly on the two most mature ambient sampling approaches (DESI and DART), a multitude of other approaches have been described in the last 5 yr. Table 4-2 (Harris et al., 2011) lists acronyms for different ambient MS techniques and associated references. This list shows the explosive growth of this field. Schematic illustrations of the experimental methodologies associated with these ambient MS techniques are shown in Figure 4-3 through Figure 4-6. The great variety of techniques reported can be classified into the following broad categories:
• Sampling techniques based on spray and solid-liquid extraction, where ionization proceeds by electrospray ionization (ESI)-like mechanisms,
• Direct and alternating current plasma techniques, where ionization proceeds via chemical ionization mechanisms and sampling proceeds via chemical sputtering or thermal desorption,
• Techniques involving laser desorption or ablation followed by ESI or plasma postionization, and
• Acoustic volatilization methods that involve electrospray or chemical ionization after volatilization.
In terms of technology maturity, only DESI and DART have been commercially available for several years. More recently, laser ablation electrospray ionization (LAESI) and liquid extraction surface analysis (LESA) have also become commercially available. DART and DESI have been by far the most popular techniques in terms of the range of demonstrated applications, the number of users, and the number of scientific publications.
| Acronym | Name | First Report |
| Spray and solid-liquid extraction based | ||
| DAPPI | Desorption Atmospheric Pressure Photo Ionization | Haapala et al., 2007 |
| DESI | Desorption Electrospray Ionization | Takáts et al., 2004 |
| DICE | Desorption Ionization by Charge Exchange | Chan et al., 2010 |
| EASI | Easy Ambient Sonicspray Ionization | Haddad et al., 2006 |
| LESA | Liquid Extraction Surface Analysis | Kertesz and Van Berkel, 2010 |
| LMJ-SSP | Liquid Micro Junction-Surface Sampling Probe | Wachs and Henion, 2001 |
| ND-EESI | Neutral Desorption Extractive Electrospray Ionization | Chen et al., 2007 |
| PESI | Probe Electrospray Ionization | Hiraoka et al., 2007 |
| Plasma-based | ||
| DAPCI | Desorption Atmospheric Pressure Chemical Ionization | Takáts et al., 2005a |
| DART | Direct Analysis in Real Time | Cody et al., 2005 |
| DBDI | Dielectric Barrier Discharge Ionization | Na et al., 2007 |
| DCBI | Desorption Corona Beam Ionization | Wang et al., 2010 |
| FAPA | Flowing Atmospheric Pressure Afterglow | Andrade et al., 2008 |
| LTP | Low-Temperature Plasma probe | Harper et al., 2008 |
| Laser desorption/ablation based | ||
| ELDI | Electrospray-assisted Laser Desorption Ionization | Shiea et al., 2005 |
| IR-LAMICI | Infrared Laser Ablation Metastable-induced Chemical Ionization | Galhena et al., 2010 |
| LADESI | Laser-Assisted Desorption Electrospray Ionization | Rezenom et al., 2008 |
| LAESI | Laser Ablation Electrospray Ionization Mass Spectrometry | Nemes and Vertes, 2007 |
| LDESI | Laser Desorption Electrospray Ionization | Sampson and Muddiman, 2009 |
| MALDESI | Matrix-Assisted Laser Desorption Electrospray Ionization | Sampson et al., 2006 |
| Acoustic-based | ||
| LIAD-ESI | Laser-Induced Acoustic Desorption-Electrospray Ionization | Cheng et al., 2009 |
| RADIO | Radio-frequency Acoustic Desorption and Ionization | Dixon et al., 2009 |
| Other | ||
| AP-TD/SI | Atmospheric Pressure Thermal Desorption/Secondary Ionization | Basile et al., 2010 |
| BADCI | Beta electron-assisted Direct Chemical Ionization | Steeb et al., 2009 |
| DEMI | Desorption Electrospray/Metastable-Induced Ionization | Nyadong et al., 2009 |
| REIMS | Rapid Evaporative Ionization Mass Spectrometry | Schäfer et al., 2009 |
| SwiFerr | Switched Ferroelectric Plasma Ionizer | Neidholdt and Beauchamp, 2011 |
SOURCE: Adapted from Harris et al., 2011.
Coupling of these front-end techniques to detectors providing tandem MS and/or accurate mass capabilities has been shown to significantly improve sensitivity and specificity, as defined in Chapter 5. Detailed descriptions of DART, DESI, and a few closely related approaches are presented in the following sections. While the focus of this review is on surface sampling, it is noteworthy that many of the ambient sampling techniques can directly ionize trace species and provide means for their detection in the vapor phase with high sensitivity.
Direct Analysis in Real Time (DART)
Plasma-based ambient sampling techniques such as DART, FAPA, LTP, DBDI, DCBI and DAPCI (see Table 4-2 for definitions) involve the generation of a direct- current or radio-frequency electrical discharge between a pair of electrodes in contact with a flowing support gas such as N2 or He, generating a constrained flux of ions, radicals, excited-state neutrals, and electrons, which ultimately lead to ionization of the sample. Some or all of these plasma species can be directed toward the surface being sampled, inducing desorption and ionization in a single step. Optional resistive heating of the support gas can further enhance desorption of neutrals, which are then ionized by interaction with plasma species. Plasma-based ambient MS instrumentation tends to be fairly simple and rugged and can be coupled to a variety of mass spectrometers, including, most commonly, quadrupole ion traps, linear quadrupole ion traps, and quadrupole time-of-flight analyzers, providing MS/MS and/or accurate mass-determining capabilities for DART-produced ions. Plasma source mass spectra tend to be relatively simple, because most of the time the analytes are ionized, as described in the previous section, as one or two adduct types, simplifying peak assignment in the case of unknowns. Their applicability is generally limited to molecular weights below 1 kDa. Chemical agent masses are well below this limit.
DART (Figure 4-1) uses a point-to-plane atmospheric pressure glow discharge to generate metastable species in a chamber that is physically separated from the ionization region. The discharge support gas, containing metastables, is heated and directed through a grid electrode that filters ions and electrons to mitigate ion-ion and ion-electron recombination of species generated within the DART ionization source. DART can be used to sample gases, liquids, and solids. In laboratory settings, gases are directly injected into the ionization region following the grid electrode, whereas liquids are generally sampled by dipping a glass capillary in the sample and placing it in the ionization region. Solids can be directly analyzed by being exposed to the stream of ionizing gas, or conveyed in a transmission mode geometry, where, for example, the sample may be coated on an open mesh (Perez et al., 2010). Powders can be mixed with metal particles, adhered to a permanent magnet, and exposed to the DART gas. Foam swabs (Edison et al., 2011), solid-phase extraction materials (e.g., adsorbent fibers) (D’Agostino and Chenier, 2010), and polydimethylsiloxane-coated stir bars (Haunschmidt et al., 2010) can also be directly placed within the DART ionization region. Desorption can also be achieved via infrared (IR) laser ablation of the surface being examined, or IR-LAMICI, resulting in higher spatial resolution (Figure 4-5) (Galhena et al., 2010).
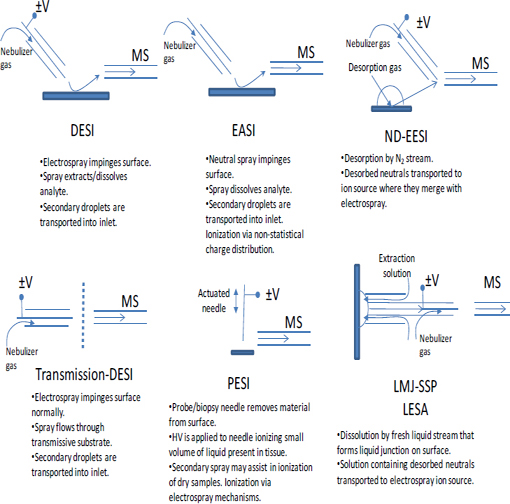
FIGURE 4-3 Schematic illustrations showing the operation of several different ion sources and sampling schemes for ambient mass spectrometry. NOTE: HV = high voltage. SOURCE: Harris et al., 2011. Reprinted with permission. Copyright 2011 American Chemical Society.
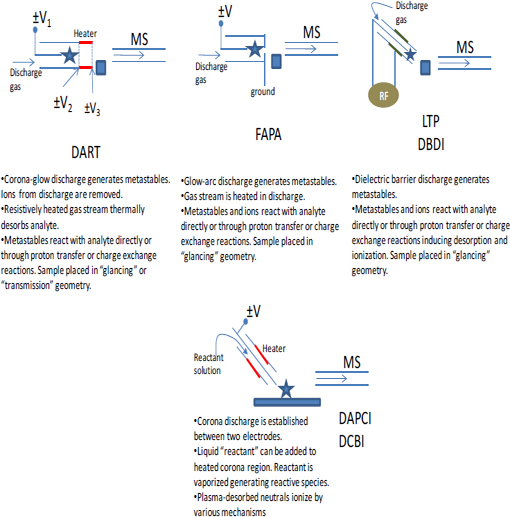
FIGURE 4-4 Additional illustrations showing the operation of several differention sources and sampling schemes for ambient mass spectrometry. SOURCE: Harris et al., 2011. Reprinted with permission. Copyright 2011 American Chemical Society.
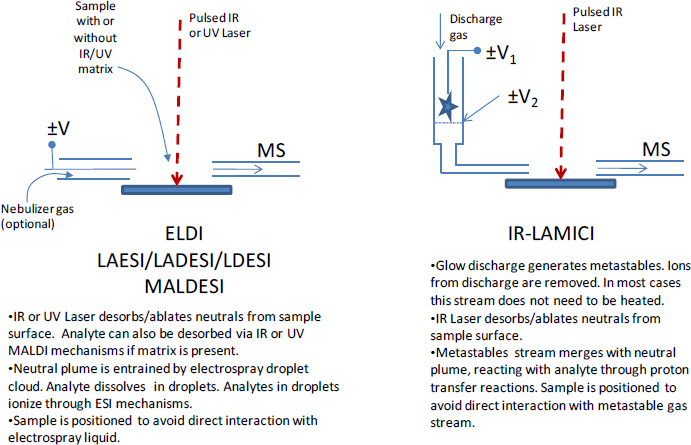
FIGURE 4-5 Laser-based ambient ionization techniques: (left) Laser Ablation-Electrospray Ionization (LAESI) and (right) Infrared Laser Ablation Metastable- induced Chemical Ionization (IR-LAMICI). SOURCE: Harris et al., 2011. Reprinted with permission. Copyright 2011 American Chemical Society.
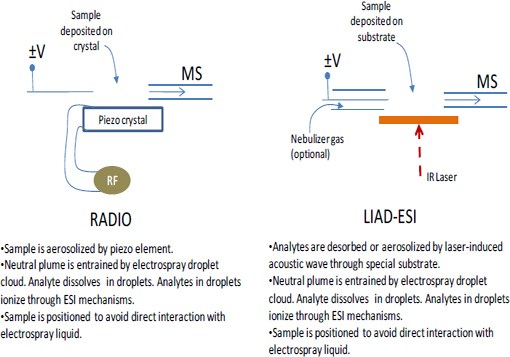
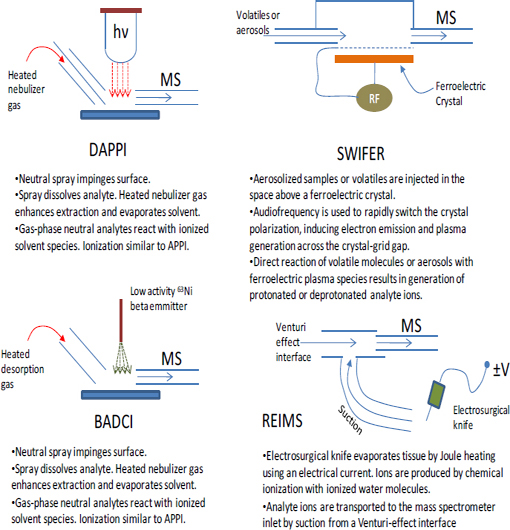
FIGURE 4-6 Schematic illustrations (this page and facing page) showing the operation of several differention sources and sampling schemes for ambient mass spectrometry. SOURCE: Harris et al., 2011. Reprinted with permission. Copyright 2011 American Chemical Society.
The most prevalent mechanism proposed for the formation of positive ions by DART involves Penning ionization of atmospheric water molecules in collisions with electronically excited metastable helium atoms (He* 3S1, 19.8 eV; eq. 4.8) (Cody et al., 2005):
![]()
Protonated water clusters of different sizes react with thermally desorbed molecules, AB (g) (eq. 4.9a), by proton transfer (eq. 4.9b).
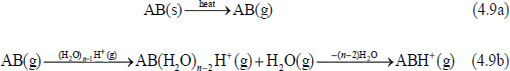
Dissociative proton transfer can also occur; i.e., AB does not stay intact.
Another ionization mechanism that has been observed under high grid voltages, small DART to mass spectrometer inlet spacing, and low-humidity conditions is direct Penning ionization of desorbed analytes (eq. 4.10) (Cody, 2009). This mechanism produces electron ionization-like spectra for low-polarity compounds with low proton affinities. Under these conditions, charge exchange reactions with diatomic oxygen molecular ions may also occur (eq. 4.11a and eq. 4.11b).
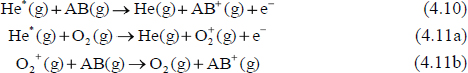
Bartmess and Song et al. have thoroughly discussed the ion generation mechanisms in DART beyond those initially discussed by Cody (Song et al., 2009a, 2009b), suggesting a “transient microenvironment” mechanism. The proposed transient microenvironment mechanism hypothesizes that analyte ionization in DART occurs by proton transfer from protonated microenvironment solvent clusters rather than proceeding by proton transfer from protonated ambient water clusters. Therefore, any solvent used to dissolve the sample can have a significant effect on the ability to detect a given species depending on its proton affinity. Solvent molecular ions produced by reactions of neutral solvent molecules with metastables can also act as reagent ions, leading to both protonated analytes and analyte molecular ions. In the case of negative ion mode experiments, electron capture, dissociative electron capture, proton transfer, and anion attachment were hypothesized to be prevalent reaction pathways (Song et al., 2009a). However, as discussed above, negative ions are not expected to be important for the sensitive detection of chemical warfare agents.
The complexity of the various ionization pathways, combined with intricacies of the coupled fluid and thermal dynamics, electrical fields, and sample positioning effects
present in DART ionization have been shown to lead to marked differences in the efficiency of ion transmission and hence sensitivity (Harris and Fernandez, 2009). Through simulations and experiments, it has been shown that optimum sample placement is a fine balance between (1) adequate and rapid sample heating to induce efficient thermal desorption and (2) limiting losses produced by disturbances in the ion/neutral trajectories induced by the presence of a sizeable solid sample placed within the DART ionization region. These effects, in the case of liquid or transmissive samples, can be largely mitigated by sampling in transmission mode when possible (Perez et al., 2010). In terms of ion activation, ion thermometry and computational fluid dynamics simulations have shown that DART generates ions with higher internal excitation than DESI, with a certain overlap between the two techniques depending on the operational conditions chosen. In DART, the internal energy of the analyte ion increases with increasing gas temperatures and flow rates, leading to increased in-source fragmentation within the first region of the mass spectrometer (Harris et al., 2010).
The viability of using DART for detection of chemical warfare agents was demonstrated as early as 2005 (Cody et al., 2005). This seminal DART publication describes the successful ionization and detection of members of G- and V-series nerve agents and HN-series blister agents from a variety of surfaces, including concrete. These agents were detected as their protonated molecules after a few seconds of exposure of the sample surface to the DART stream. Several recent research articles describe more extensively the application of DART to the analysis of chemical warfare agents (Nilles et al., 2009, 2010). In Nilles et al. (2009), the researchers demonstrated the detection and quantitation of GA, GB, VX, and HD in liquid samples, with recoveries better than 97 percent, linear correlation coefficients of 0.99 or better, and dynamic ranges of three orders of magnitude. Ionization chemistries were chosen such that the analyte was distributed into as few ion masses as possible. In the case of VX (Figure 4-7d), the protonated neutral species is readily, and uniquely, formed under typical DART conditions. This can be attributed to VX having a relatively high proton affinity. However, GA and GB have proton affinities close to that of ammonia (854 kJ/mol) and have a greater tendency to form the ammonium adduct. So, when analyzing these compounds, conditions were tuned to produce only [M + NH4]+ (Figure 4-7b, c). Under positive ion DART conditions, HD produces the [M + OH]+ ion (Figure 4-7a). It is likely that the DART environment produces reactive species that easily oxidize HD to the sulfoxide, which has a higher proton affinity and is detected as the protonated species. Structures of the neutral molecules along with the exact masses of the detected quasimolecular ions are displayed in Figure 4-8. The production of oxidizing species is a mitigating issue in many of the plasma-based ambient ionization methods. Since factors such as relative humidity may influence the importance of oxidation chemistry (Neidholdt and Beauchamp, 2009), the viability of a detection method should be confirmed over the full range of environmental parameters in areas where it is deployed. However, should there be any doubt about the HD identification, HD can easily be confirmed with DART in negative ion mode as the chloride (Cl-) adduct.
Sampling in Nilles et al. (2009) was performed by dipping the closed end of a glass capillary into a solution containing the agent of interest and a corresponding
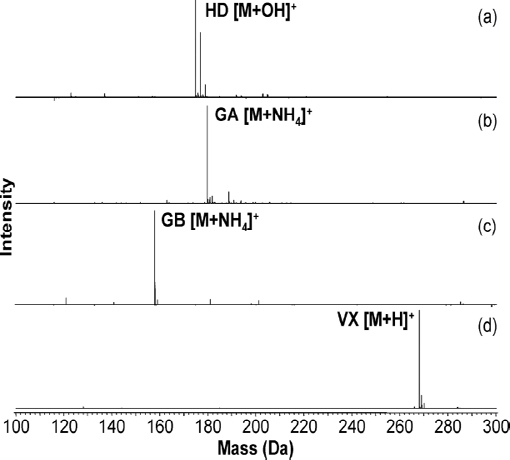
FIGURE 4-7 DART mass spectra of agent standards. (a) sulfur mustard (HD), detected as the protonated sulfoxide—the three peaks are due to the chlorine isotopes; (b) tabun (GA), detected as the ammonium ion adduct; (c) sarin (GB), detected as the ammonium ion adduct; and (d) VX, detected as the protonated species. SOURCE: Nilles et al., 2009. Reprinted with permission. Copyright 2009 American Chemical Society.
internal standard. Isotopically labeled agents D5-GA, D7-GB, 13 C2-HD, and D5-VX were used as internal standards, and the ratio of analyte to internal standard areas used as the response variable. An expanded statistical analysis of the summary data in Nilles et al. (2009), along with additional data supplied by the authors, is provided in Chapter 5. A follow-up article by the same team demonstrated the possibility of performing rapid separations within the DART ionization region by ramping the DART gas temperature, which made it possible to distinguish the [M + NH4]+ ion of GB from isobaric analytes that could otherwise only be resolved via high-resolution mass measurements or tandem mass spectrometric experiments (Nilles et al., 2010).
The applicability of DART for detection of chemical warfare agents (CWA) on a range of real-world samples has been thoroughly evaluated (Nilles et al., 2010). Figure 4-9 illustrates high-resolution mass spectra obtained using DART for the detection of 800 ng of VX spotted on aluminum, concrete, and a bird feather. Similar to the data shown in Figure 4-7, VX is detected as the protonated molecular ion at m/z = 268.150. Aluminum and concrete yield cleaner spectra than does the bird feather, where the more complex matrix yields a wide range of detected molecules. Such species may give rise to false
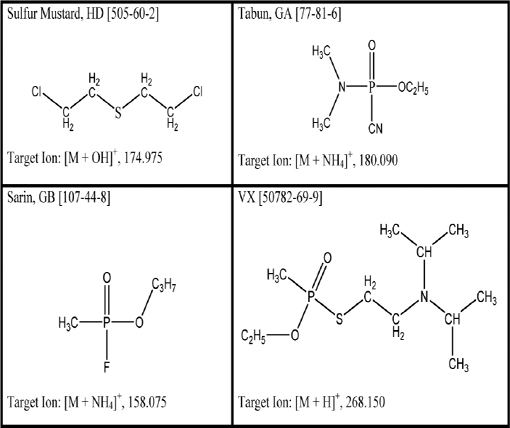
FIGURE 4-8 Structures of HD, GA, GB, and VX, with CAS designations in brackets. In each case, the target ion exact mass is given for the ions observed by DART and displayed in Figure 4-6. SOURCE: Nilles et al., 2009. Reprinted with permission. Copyright 2009 American Chemical Society.
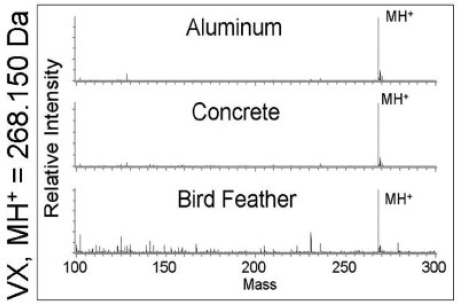
FIGURE 4-9 High-resolution mass spectra obtained by DART for 800 ng VX on aluminum, concrete, and a bird feather. VX appears as the MH+ molecular cation at m/z = 268.150 Da. SOURCE: Larame’e et al., 2008. Reprinted with permission.
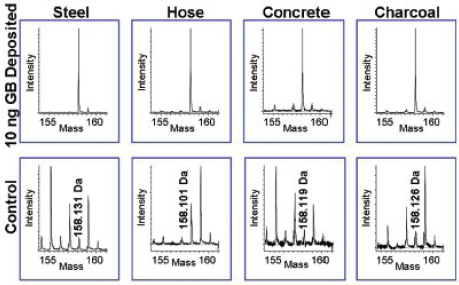
FIGURE 4-10 Surfaces of steel, rubber hose, concrete, and charcoal spiked with 10 ng GB (top row) and unspiked surfaces (bottom row). GB appears as the [MH+NH3]+ cation at mass 158.075 Da. Other peaks not related to GB are seen at nominal mass = 158 but do not interfere or cause a false positive measurement because of the high-resolution mass measurement. SOURCE: Larame’e et al., 2008. Reprinted with permission.
positives at the same nominal m/z of the detected CWA quasi-molecular ion. Figure 4-10 illustrates this problem when 10 ng of GB is spiked onto steel, rubber hose, concrete, and charcoal surfaces and compared to blanks run for the same substrates. In every case species are identified at the same nominal m/z = 268. Accurate mass measurement shows in each illustrates this problem when 10 ng of GB is spiked onto steel, rubber hose, concrete, and charcoal case that the major detected species in the blanks has a different elemental composition from GB. Comparison of intensities for the spiked and blank spectra suggests that detection limits at low picogram levels are readily achieved. The time-of-flight instrument used in these studies had a measured mass resolution of 6,000 (m/Am, where Am is the full width of the peak at half maximum) in the m/z region appropriate for the agents investigated.
Desorption Electrospray Ionization (DESI)
DESI (Figure 4-2) was first introduced by Cooks and coworkers in 2004 (Takáts et al., 2004). DESI combines the features of ESI with those of the family of desorption ionization methods (Karas and Hillenkamp, 1988). An electrosonic spray ionization (ESSI) emitter (Takáts et al., 2005a) is used to create gas-phase solvent ions, ionic clusters, and charged microdroplets, which are directed to the surface to be sampled. An electrical potential of several kilovolts is applied to the spray solution, and pneumatic nebulization is used to assist in desolvation.
Droplet pickup has been suggested as the primary ionization mechanism in DESI, although there is evidence for chemical sputtering (reactive ion/surface collisions) and gas-phase ionization processes (e.g., charge transfer, ion-molecule reactions, and
volatilization/desorption of neutrals followed by ionization) (Takáts et al., 2004; Cotte- Rodriguez et al., 2005; Takáts et al., 2005a; Costa and Cooks, 2007, 2008). According to the droplet pickup mechanism, the surface is prewetted by initial droplets (velocities in excess of 100 m/sec and diameters of less than 10 m), forming a solvent layer that dissolves analytes on the surface. These dissolved analytes are picked up by later arriving droplets that are impacting the surface, creating secondary droplets containing the dissolved analytes. Gas-phase ions are then formed from these charged secondary droplets by ESI-like mechanisms (Takáts et al., 2005a; Costa and Cooks, 2007, 2008). The resulting gas-phase ions have low internal energies, similar to those in ESI and ESSI (Nefliu et al., 2008). The formation of cold ions gives DESI its soft ionization character that affords ESI-like spectra. DESI is able to detect species where thermal desorption is not possible, such as nucleic acids and proteins. In this respect it is very different from DART, where molecules are thermally desorbed by a hot gas stream and subsequently chemically ionized in the vapor phase.
The desorption and ionization steps occurring simultaneously in DESI can be spatially and temporally separated for further control and optimization of the experimental parameters. This is the case in a technique known as laser ablation/desorption electrospray ionization (LADESI), also referred to as electrospray- assisted laser desorption ionization (ELDI), or laser-assisted electrospray ionization (LAESI). In LADESI (Figure 4-5), a neutral plume of molecules and particles from a matrix-free sample surface are entrained with a charged solvent plume. The steps involved in LADESI ion formation are believed to encompass a desorption/ablation process induced by rapid absorption of laser energy generating a neutral plume that undergoes (1) ion/molecule reactions with ions, usually proton donors, generated by an electrospray emitter and/or (2) fusion with charged electrosprayed droplets undergoing solvent evaporation and Rayleigh discharge (Smith et al., 2002) to form ions (Huang et al., 2010).
DESI is most applicable to a solid sample or surface-bound analytes which are ionized by the charged species generated from the ESSI emitter. Recently, DESI has been extended to allow the direct analysis of liquid samples (Chipuk and Brodbelt, 2008; Ma et al., 2008; Miao and Chen, 2009; Miao et al., 2010; Zhang and Chen, 2010; Miao et al., 2011). DESI can also be coupled with solid-phase microextraction (SPME) probes to analyze gaseous species adsorbed onto the SPME fiber from the headspace above a liquid or solid surface (D’Agostino et al., 2006; D’Agostino et al., 2007; D’Agostino and Chenier, 2010).
DESI has been used for a wide variety of direct analysis, including the analysis of pharmaceutical products (Chen et al., 2005; Weston et al., 2005; Williams and Scrivens, 2005; Kauppila et al., 2006; Rodriguez-Cruz, 2006; Cotte-Rodriguez et al., 2007; Venter and Cooks, 2007), dyes on thin layer chromatography plates (Van Berkel et al., 2005), polymers (Nefliu et al., 2006), alkaloids on plant tissue (Talaty et al., 2005), pesticides spiked onto immobilized powders or various surfaces (Hagan et al., 2008), explosives on a variety of surfaces (Cotte-Rodriguez et al., 2005; Takáts et al., 2005b; Cotte-Rodriguez and Cooks, 2006; D’Agostino et al., 2006; Mulligan et al., 2006; Nefliu et al., 2006), and CWAs as well as their hydrolysis products (Cotte-Rodriguez and Cooks, 2006; D’Agostino et al., 2006; Mulligan et al., 2006; D’Agostino et al., 2007; Song and Cooks, 2007; D’Agostino and Chenier, 2010). The analytical capabilities of DESI-MS are
illustrated by an ability to distinguish diseased and nondiseased tissue based on their chemical signatures (Wiseman et al., 2005). In these studies, qualitative changes in the lipid profiles were obtained that distinguished the tumor from the nontumor region of a biopsied liver adenocarcinoma tissue.
Analysis of CWAs by DESI has been reported using two different approaches, involving the use of SPME fibers (D’Agostino et al., 2006; D’Agostino et al., 2007; D’Agostino and Chenier, 2010) and direct sampling (Cotte-Rodriguez and Cooks, 2006; Mulligan et al., 2006; Cotte-Rodriguez et al., 2007; Song and Cooks, 2007). The first report of an analysis of CWAs by DESI appeared in early 2006. It reported the detection of the nerve agent simulant triethyl phosphate as well as two nerve agents, GB and soman. The acquired DESI-MS/MS data for CWAs was similar to that obtained by traditional liquid chromatography ESI-MS/MS. SPME headspace sampling followed by the direct DESI-MS analysis took less time for sample preparation and analysis than did aqueous extraction and liquid chromatography ESI-MS/MS analysis, indicating that DESI could be a good candidate for high-throughput analysis (D’Agostino et al., 2006). Later, more complex CWA samples were analyzed by SPME DESI-MS/MS, with detection limits in the sub-nanogram range (D’Agostino et al., 2007; Song and Cooks, 2007). Very recently, ion mobility separations were used as a part of rapid SPME DESI- MS confirmation method for some common organophosphorus CWAs (D’Agostino and Chenier, 2010). In terms of direct sampling of CWAs, the detection specificity can be significantly enhanced by “reactive” DESI. In Song and Cooks (2007), this mode of DESI analysis involved the addition of boric acid to the spray solvent to enhance the analysis of the hydrolysis products of phosphonate esters through heterogeneous ion/molecule reactions. On average, sub-nanogram detection limits were obtained for methylphosphonic acid (MPA), ethylphosphonic acid (EMPA), and isopropyl methylphosphonic acid (IMPA) from complex matrices (Song and Cooks, 2007). When a methanolic solution of these compounds was deposited on a clean Teflon surface, 10 pg each of EMPA, IMPA, and MPA could be readily detected (Song and Cooks, 2007).
When charged droplets impinge on a surface from an electrospray source driven by a flow of sheath air, they pick up material and will eventually remove soluble deposits from the surface. Organic material on the surface is removed by impinging charged droplets that rebound and repel each other, developing into a spreading plume that can distribute desorbed material over a wide area. Specially designed electrospray sources have been developed to clean surfaces for high-value end products such as silicon wafers. It would not be desirable to disperse localized CWA deposits over a larger area, leading to more widespread contamination, which might include the detection instrumentation. Due to their exceptionally low flow rates, use of nanospray sources could help to alleviate the spread of sampled species from the surfaces (Roach et al., 2010).
Nonproximate Analysis by Ambient Mass Spectrometry
The need for field-portable nonproximate detection is apparent for compounds harmful to health or the environment, such as toxic industrial species, explosives, CWA and environmental toxins (Badman and Cooks, 2000; Gao et al., 2006; Mulligan et al., 2006; Chaudhary et al., 2006). Remote detection of these classes of compounds increases
safety and is more convenient and time responsive than the laboratory proximate analysis of field samples. Several systems providing this capability have become commercially available. For example, the Bruker Scanning Infrared Gas Imaging System 2 is a sensing system for remote infrared detection of hazardous gases in industrial, environmental, and homeland security applications.2 In traditional MS analysis of field samples with ambient mass spectrometry techniques, the ionization source is located close to the atmospheric pressure inlet of the mass spectrometer, limiting the analysis to small, well-defined sample substrates (Dixon et al., 2007). A highly desirable feature to enhance the utility of ambient mass spectrometry is implementation of a system having capability for nonproximate analysis, whereby the ion source is remotely coupled to the MS detector.
DESI has been used for the direct and nonproximate detection of trace amounts of explosives (RDX, HMX, PETN, TNT, Composition C4, and TATP) and the nerve agent simulant dimethyl methylphosphonate (DMMP) from ambient surfaces up to 3 m from the mass spectrometer using a stainless steel transfer tube (Figure 4-11a) (Cotte- Rodriguez and Cooks, 2006). In that study, the analyte of interest was desorbed from the surface and ionized by charged droplets generated using a DESI spray source. The resulting droplet plume, along with entrained analyte ions, was transported for analysis through the stainless steel transfer tube with the help of the vacuum of the mass spectrometer. Using this apparatus, the limits of detection for explosives RDX, HMX, PETN, TNT, Composition C4, and TATP and of the CWA simulant DMMP were in the low-nanogram range with a 3-m transfer tube. With a shorter 1-m transfer tube, limits of detection for RDX and DMMP were in the subnanogram range (Cotte-Rodriguez and Cooks, 2006). The same research group later expanded this experimental methodology for nonproximate analysis by performing reactive DESI to increase both selectivity and sensitivity for the detection of target analytes with proton affinities above a certain value. For example, in the analysis of 2,4,6-triphenylpyridine, ammonia vapor was introduced into the ion transfer tube to remove abundant ions (chemical noise) arising from neutral species with proton affinities lower than ammonia (Figure 4-11b) (Cotte-Rodriguez et al., 2007). In addition, by optimizing the temperature of the transfer tube, the fragmentation and the formation of stable adduct ions could be controlled (Cotte-Rodriguez et al., 2007).
Ion transfer in nonproximate analysis can also be assisted by controlling airflow to enhance transport of ions to the atmospheric sampling interface of the mass spectrometer. Implementations include the use of a commercial air ejector (Dixon et al., 2007) (Figure 4-12 [top]) and airflow-assisted ionization (AFAI) (He, 2011) (Figure 4-12 [bottom]). As shown in Figure 4-12 (top), the commercial air ejector is used to transport ions formed either by ESI or by DESI from a surface, from the point of formation to the MS inlet and then through a flexible polyethylene tube to the MS. In the AFAI method, high-flow-rate airflow extraction effectively captures the charged droplets containing analytes from the DESI desorption site, which are then transferred 0.5 m to the inlet of the MS.
Analytes in a remote location can also be transferred as neutral species for MS detection. Either the remote analyte sampling, transport, and ionization relay (RASTIR) method (Dixon et al., 2008) (Figure 4-13 [top]) or neutral desorption-extractive
![]()
2Additional information is available at http://www.brukeroptics.com/gas_detection.html.
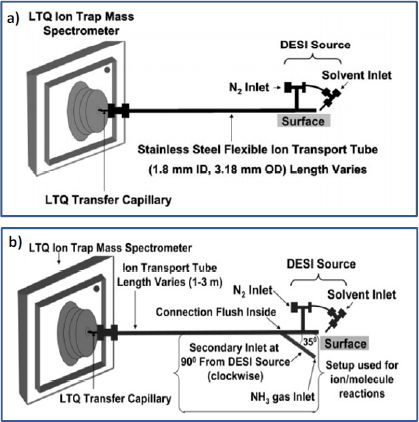
FIGURE 4-11 DESI remote sampling techniques. (a) Experimental arrangement for non-proximate analysis using ambient mass spectrometry. The DESI source is remotely located from the mass spectrometer, with ions transported over distances up to 3 m through a flexible stainless steel tube. SOURCE: Cotte-Rodriguez and Cooks, 2006. Reproduced with permission of the Royal Society of Chemistry. (b) Modified experimental arrangement for implementation of reactive DESI in the transfer tube, in which a secondary inlet allows the introduction of a neutral reagent gas. Ammonia, for example, scavenges protons from trace species with lower proton affinity to remove chemical noise from the spectra. SOURCE: Cotte-Rodriguez et al., 2007. Reprinted with permission. Copyright 2007 American Chemical Society.
electrospray ionization (ND-EESI) (Chen et al., 2009) (Figure 4-13 [bottom]) can be used to implement this approach to nonproximate analysis. Based on the venturi effect, the RASTIR source creates an induced vacuum from high-pressure nitrogen gas flow, which can be used to transfer low-volatility analytes for subsequent ionization by ESI. In the ND-EESI experiment, a trace amount of explosives from biological surfaces such as human skin can be detected rapidly through a long transfer tube (up to 4 m) after interacting with a charged droplet beam generated by ESSI. In the ND-EESI experiment, selective ion/molecule reactions can also be implemented, which further enhances the detection selectivity and the sensitivity.
In order to scan large surface areas for trace chemical detection, modified DESI has been introduced, including multiple sprayer DESI (Cotte-Rodriguez et al., 2007) (Figure 4-14 [top]) and large-area sprayer enclosure DESI (Figure 4-14 [bottom]) (Soparawalla et al., 2009). Although DESI can be used for nonproximate analysis of individual samples of interest within a small surface area, the total analysis time required will be substantial if large objects are to be examined. A multiple DESI sprayer setup (using three sprayers covering areas of about 70 mm2) (Figure 4-14 [top]) was designed to address this issue, and it could also be used for high-throughput analysis. An
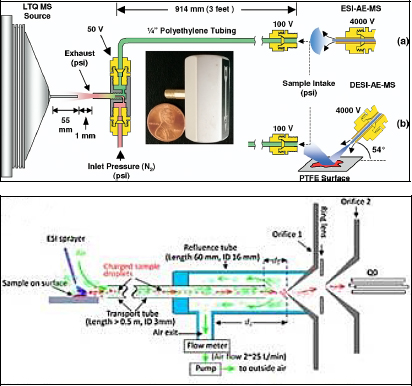
FIGURE 4-12 DESI remote sampling techniques using AE and AFAI. (Top) Schematic of the commercial air ejector (AE) interfaced to a linear quadrople ion trap-MS with an ESI or DESI ion source; the inset photograph shows the actual injector. SOURCE: Dixon et al., 2007. Copyright 2007. Reprinted with permission from Elsevier. (Bottom) Schematic showing operation of the non-proximate airflow-assisted ionizer (AFAI) SOURCE: He et al., 2011. Copyright 2011 John Wiley & Sons. Reprinted with permission.
FIGURE 4-13 RASTIR and ND-EESI schematics. (Top) Labeled schematic of a RASTIR assembly showing the high-pressure region (stage 1), high-velocity jet/entrainment/exhaust region (stage 2), and the vacuum region (stage 3). SOURCE: Dixon et al., 2008. Reprinted with permission. Copyright 2008 American Chemical Society. (Bottom) Schematic of an air-tight neutral desorption enclosure for the ND- EESI method. SOURCE: Chen et al., 2009. Copyright 2009. Reprinted with permission from Elsevier.
FIGURE 4-14 Multiple-sprayer and nonproximate large-area sprayer DESI setups. (Top) Multiple-sprayer DESI setup used for high-surface-area screening as well as emitters that are welded together. SOURCE: Cotte-Rodriguez et al., 2007. Reprinted with permission. Copyright 2007 American Chemical Society. (Bottom) Nonproximate large-area DESI setup consisting of a large-area sprayer coupled to an ion transport tube. SOURCE: Soparawalla et al., 2009. Copyright 2009 John Wiley & Sons, Inc. Reprinted with permission.
alternative large-area analysis DESI source is shown in Figure 4-14 [bottom]. This system uses two coaxial stainless steel tubes, with the internal tube having an inner diameter of 1 cm. High-pressure nitrogen gas (300 psi) and solvent were propelled through the annular space, covering a large-area (up to 3 cm2 with effective ionization) screening surface, which was interlocked with the transfer tube via a plastic enclosure (Soparawalla et al., 2009). These large-area nonproximate analysis modes, which have been particularly well implemented with DESI, could find uses in mapping chemical agent contamination on equipment or building surfaces.
Preliminary reports on the remote tethering of a handheld DART ion source to a mass spectrometer through a 1.5-m-long transfer line have recently been presented (Musselman, 2011), but extensive testing has not yet been reported. Owing to its inherent simplicity, robustness, and suitability for small molecule detection, remote-sampling DART has the potential for becoming a key means for surface sampling and analysis under harsh field conditions.
Most of the ACWA scenarios for ambient ionization MS suggested in this report will require a sampling wand. However, the degree of target species dispersion caused by these wand based-based sampling systems has not been described in the literature, and probably has not been characterized. The suspected proclivity of DESI for dispersing
target species more extensively than DART is based on their respective fundamental operational properties and the basic physics of each technique, and not on data for specific wand designs (which are not available).
Overall, DART, DESI, and other nonproximate sampling and analysis approaches have expanded the capability of mass spectrometry in analyzing dangerous compounds with improved safety for examiners. In addition, portable (Mulligan et al., 2006) or miniaturized (Gao et al., 2006) mass spectrometers could be coupled to the variety of the remote sampling devices and operated at distance for increased flexibility during in situ analysis (Cotte-Rodriguez et al., 2007).
POTENTIAL ROLES FOR AMBIENT MASS SPECTROMETRY IN THE ACWA PROGRAM
Virtually all early applications of ambient mass spectrometry were based on thermal desorption combined with atmospheric-pressure chemical ionization (APCI) (Van Berkel et al., 2008). Pioneering studies by Horning and coworkers in the 1970s employed samples collected on platinum wires, which were desorbed using a heated gas flow and ionized by APCI (Dzidic et al., 1975). A variety of commercial systems were developed based on ambient mass spectrometry, mainly to detect explosives and drugs. Nonproximate analysis was accomplished using a heated surface sampler coupled to a mass spectrometer through a 10-m heated transfer line (Stott et al., 1993). These investigations promoted the concept of near-real-time analysis of target species without need for sample preparation. With complex mixtures, selected reaction monitoring using MS/MS analysis performed using triple quadrupole mass spectrometers provided confident identifications and avoided false positives. Of particular note is an early study by Fite and coworkers at Extrel Corporation, supported by the U.S. Army Program Manager for Chemical Demilitarization, in which an atmospheric pressure ionization tandem quadrupole mass spectrometer system was used to directly detect GB and VX in air with a 15-sec response time and with detection limits of 7.2 ppt for GB and 6 ppt for VX (Ketkar et al., 1991b). A corona discharge provided protonated water reagent ions. That selected reaction monitoring could be used to ensure positive detections was also demonstrated, but the detection limit was reduced for GB to 14.1 ppt and for VX (cleaved to its G-analog) to 100 ppt. The same group provided an insightful analysis of the influence of coexisting analytes with higher proton affinities than the target molecules (Ketkar, 1991a).
This evolutionary growth of ambient mass spectrometry spiked beginning with the development of DESI, whose first publication was in November 2004, and DART, whose first publication was in March 2005. The commercialization of these experimental methodologies and the many variations they inspired have led to widespread applications of ambient mass spectrometry. While DESI in many ways is unique, it is of interest to note that DART is a variation of thermal desorption combined with APCI. The design of the DART source is unique, however, in that it isolates the corona discharge from the target sample, filters out directly formed ions from the discharge, and relies on excited neutral species to generate reactant species that subsequently chemically ionize species present in the vapor phase or thermally desorbed from surfaces by a variable temperature
stream of heated gas. This avoids having target molecules interact with energetic electrons and ions produced in the corona discharge. The use of DART’s gas phase desorption/ionization process is also logistically less complex than DESI’s liquid jet- based splatter desorption and ionization process.
As detailed above, ambient ionization mass spectrometry technology has made great strides since its introduction in 2004/2005. A range of systems and system components are now commercially available from a variety of U.S.-based analytical instrument companies. A number of these companies and their major commercial offerings are listed in Appendix C.
Table 4-3 provides a detailed summary of the capabilities and limitations of ambient mass spectrometry (DART and DESI) compared to the existing vapor monitoring technology and measurement strategies (DAAMS and MINICAMS). Table 4-4 summarizes some of the relevant strengths and weaknesses of DESI and DART for the chemical agent contamination characterization scenarios described in this report. Both DESI and DART have demonstrated an impressive capability to detect trace levels of ACWA-relevant chemical agents in solution and adsorbed on surfaces. DART can also be modified to detect trace levels of airborne agent in real time. Finally, because most of the published applications of both DESI and DART, including the chemical warfare agent and agent simulant studies described above, are laboratory-scale experiments performed under carefully controlled conditions, in which very small amounts of the target species are present on sample substrates of modest extent, the mobilization and dispersion of target species were seldom either a scientific or a safety issue and were not generally considered or commented on in those studies. However, the potential ACWA applications defined in this report may involve much higher levels of chemical agent contamination where target species dispersion is potentially much more serious. That is why target species dispersal is potentially much more important for envisioned ACWA plant applications than in much smaller scale laboratory demonstrations.
These summaries, along with the discussions of ambient mass spectrometry relevant to the detection of chemical warfare agents presented in this chapter, form the basis for the committee’s findings and recommendations offered below. If the Assembled Chemical Weapons Alternatives managers decide that the potential advantages of ambient ionization technology applied in any of the scenarios developed in Chapter 3 and discussed further in Chapter 5 are worth the cost and effort required to acquire and integrate new detection technology, the following findings and recommendations should help guide specification, acquisition, and integration activities.
| Ambient Mass Spectrometry (DART and DESI) | Vapor Monitoring (DAAMS and MINICAMS) | |
| Capabilities | ||
|
• Direct measurements from vapor (DART), liquid (DART and DESI), or solid (DART and DESI) matrices |
• Demonstrated reliability for bulk vapor analyses in chemical demilitarization plants |
|
|
• Possibility of surface spatial discrimination with user-defined resolution (ca. 50 μm-several centimeters, only DESI) |
• Established STEL values based on toxicological data that correspond directly with action protocols |
|
|
• Ability to track agent concentration gradients in the vapor phase (only DART) |
• False positive rates reasonably well characterized through long record of utilization |
|
|
• Ability to remotely sample through wands |
||
|
• Simultaneous measurement of multiple agents during changeover and decommissioning with high selectivity through analytical and confirmatory signals |
• Agent calibration reference standards are readily available and utilized with regular frequency |
|
|
• Potential for rapid changeover of multiple types of ion sources for enhanced selectivity and spatial resolution |
• Established regulatory guidelines are available for vapor and vapor headspace analyses |
|
|
• Potential for validation through monitoring both agent ions and product degradation fragmentation ions |
• Potentially easier instrumental clean-up from contamination by analyzing concentrated samples |
|
|
• Analysis in near real time (msec or sec) |
||
| Limitations | ||
|
• Potential for instrumental contamination owing to high sensitivity |
• Primarily utilized for vapor analysis (limited for liquids and solids) |
|
|
• Lack of standard reference materials for agents on various surfaces |
• Indirect analysis of liquid and solid samples by vapor measurements (e.g., via swabs, etc.) |
|
|
• Limited knowledge regarding correspondence of surface concentrations with those in the gas-phase |
• Significant time and exposure is dedicated to changeover activities (e.g., changing detectors from FID to halogen specific detection, etc.) |
|
|
• Lack of regulatory guidelines for concentrations of agent on surfaces |
• Typically process detection is set to monitor a single agent |
|
|
• Expensive consumables: solvents, highpurity gases |
• Relatively slow analysis rates (ca. 5 min for MINICAMS) |
|
| Capability/Limitation | DART | DESI |
| Real-time mapping of surface adsorbed agent distributions | Yes - with moderate spatial resolution (~1 cm) | Yes - with high spatial resolution (~50 μm to 1 cm) |
| Real-time analysis of liquid solvent agent concentrations | Yes - with appropriate liquid-phase reference standards | Yes - with appropriate liquid-phase reference standards |
| Near-real-time vapor-phase agent concentration measurements | Yes - with direct sampling | Yes - by bubbling vapor through solvent and liquid-phase analysis or direct analysis of SPME adsorbed sample |
| Real-time vapor-phase agent concentration measurements and airborne plume tracking | Yes - with direct sampling | No - requires liquid- or solid- phase sample collection step |
| Non-proximate liquid pool or contaminated surface measurements | Yes - but few demonstrated wand configurations | Yes - a range of demonstrated wand configurations |
| Ionization source logistics | Gas phase - relatively simple to deploy and control | Liquid phase - moderately more difficult to deploy and control |
| Agent dispersion during measurement | Moderate issue - can control agent vaporization rates and atmospheric pressure gas-phase diffusion is slow | Moderate issue - liquid jet can be miniaturized, and dispersed agent can be intercepted by shields |
Finding 4-1. When compared to existing vapor monitoring (DAAMS and MINICAMS) measurement strategies, ambient ionization mass spectrometry provides the following capabilities for detection and quantitation of chemical agents and their degradation products (see also Table 4-3):
• Exceptional sensitivity and selectivity.
• Direct measurements from vapor, liquid, or solid samples.
• Real-time measurement with minimum response times of milliseconds to seconds.
• Multiagent detection capability and degradation product monitoring.
• Measurements of variations in spatial and temporal concentration.
Finding 4-2. DART, DESI, and other emergent ambient ion sources can be considered for surface analysis. A range of different technologies can be employed for liquid and ambient vapor ionization. Both DESI and DART are able to sample and identify molecular species on surfaces in real time (less than 5 sec per measurement).
Finding 4-3. DESI requires a solvent, usually an organic or organic/water mixture, with a few percent acid to enhance protonation of the target molecules. A high-velocity gas flow concentric with the electrospray tip directs charged droplets toward the sample. This plume can easily scatter liquid and solid material. Further dispersal of chemical agent that might lead to contamination of adjacent surfaces, especially the mass spectrometer, is not a desirable result.
Finding 4-4. DART, using a controlled flow of heated gas typically comprising helium or a nitrogen/helium mixture, provides the capability to heat, evaporate, and subsequently ionize the target species with modest target agent dispersal. Furthermore, it is possible to vary the temperature of the flowing gas to carry out temperature programmed desorption, observing in turn species with a wide range of volatilities. In addition to surface sampling capabilities, the DART source is an efficient atmospheric pressure chemical ionization source and hence can ionize and detect species at low concentrations in the vapor phase.
Recommendation 4-1. While both DESI and DART have been demonstrated to have excellent sensitivity for detecting chemical agents in liquids and on a wide range of surfaces, if only one technique is adopted, DART is preferable for the potential ACWA utilization scenarios, based on its lower dispersion of target species, utilization of a gas rather than a liquid as the working medium, and ability to efficiently ionize and detect trace levels of species in the gas phase. A DESI system with a cover shield to intercept dispersed contaminants may also be applicable. The use of instrument shields to minimize agent contamination would have to be investigated during instrument test and evaluation activities.
Finding 4-5. The platform configuration most likely to satisfy the analytical needs put forward in the various scenarios in Chapter 3 for different waste streams consists of a cart-mounted or handheld mass spectrometer equipped with a modified interface to accommodate a special remote sampling wand, a surface ambient ionization source combined with a vapor ambient ionization source, and any sampling accessories. Ambient ionization mass spectrometry systems backed by an uninterrupted power supply will allow portability between different rooms or site areas without breaking vacuum. Careful attention to instrument shielding and sampling wand design and implementation can reduce the possibility of agent contamination during instrument use.
Finding 4-6. At the highest levels of sensitivity, detection of chemical agents requires high mass resolution to distinguish the agents from isobaric trace species with the same nominal mass. A minimum resolution of 10,000 (m/μm, where μm is the full width at half maximum) is required for this purpose. This value can be accomplished with an orthogonal sampling time-of-flight (TOF) mass spectrometer incorporating a reflectron. High-end instruments of this type can provide mass resolution 1.5-5 times this value and would be preferable if not for their cost and size. Selective reaction monitoring, in which an ion structure is confirmed by specific fragmentation pathways, can avoid false positives for chemical agent identification without requiring high mass resolution. While this can be accomplished with newer TOF-TOF instruments, the suggested mass analyzers are either a triple quadrupole or a linear ion trap.
Recommendation 4-2. The Army should specify a list of requirements for an ambient ionization mass spectrometer system that would implement analytical capabilities specifically designed to respond to the challenges summarized in the different scenarios described in Chapter 3. Suggested mass analyzers are either triple quadrupole or linear ion trap, because both can be operated in selective reaction monitoring modes for validation of agent identification and reduction of false positives without requiring high mass resolution. The instrument’s atmospheric pressure interface should be fitted with an optionally heated transfer line designed to serve as a multifunction sampling wand. Ionization can occur either at the end of the wand (desorbed surface species or ambient vapor) or at the atmospheric pressure sampling orifice of the mass spectrometer (vapor). In the first case, ions are transported, whereas in the second case, neutrals are generated and then transported to the ionizer. A system employing a wand should be tested for the efficacy of the analytical methodology to trace an expanding vapor plume back to its source. This would be especially beneficial in identifying and locating Type I and possibly Type II occluded spaces (see Box 3-3) as well as in identifying leaker munitions in both storage and processing areas. The overall ambient ionization mass spectrometric system should be portable, either cart-mounted or handheld, for maximum utility.
Recommendation 4-3. The sampling wand should accommodate a variety of sampling modes and interchangeable ion sources:
• A compact DART (and possibly DESI) source that can be mounted either at the end of the wand, with ions sampled through the wand, or directly on the mass spectrometer, with neutrals sampled through the wand.
• A thermal neutral desorption mode where hot gas is blown toward the surface and the desorbed neutrals are collected by a suction interface that directs them toward the ionization source attached to the mass spectrometer.
• A gas sampling mode where ambient air is drawn into the interface but no gas is blown to any surfaces.
• A sampling port that allows the user to manually wipe an area and either place the wipe directly on the sampling wand or insert it through the port into the plasma of the DART source attached to the mass spectrometer.
• A high-sensitivity air monitoring mode where a solid-phase-microextraction fiber is exposed to the vapor to be sampled and directed into the plasma source.
• A high-sensitivity surface sampling mode where a polydimethylsiloxane membrane is attached to the surface to be sampled for an extended period of time and then exposed directly to the plasma for desorption ionization.
• A liquid sampling mode that allows manual sampling of a liquid pool or drip.
Finding 4-7. The chemical agents VX, GB, and HD have markedly different acid-base properties in the gas phase. This is reflected in the quasimolecular ions observed and monitored when DART is employed for their detection. Only VX is observed as the protonated molecular ion, GB is detected as an adduct with ammonium ion, and HD is
detected as the oxidized sulfoxide derivative, which has a proton affinity significantly higher than that of the parent molecule. While it has been demonstrated that all three species can be detected with high sensitivity in laboratory studies using the same instrumental conditions, it is not obvious that this multiagent detection capability will be possible when this experimental methodology is deployed and used in working environments. For example, ammonia in laboratories can originate from human breath and would likely not be as abundant in Class A environments where workers are in DPE suits and might have to be added to the sampling flow. In addition, the extent of sample oxidation in ambient ionization sources is known to be dependent on source operating conditions and environmental factors such as the relative humidity.
Finding 4-8. MINICAMS are designed to monitor relatively large areas and alarm when levels exceed a specified limit. Although regarded as near-real-time detectors, they typically require 5 to 10 min for a single analysis. This is not particularly efficient when attempting to locate and define an area of agent contamination. For example, when exposure to agent is possible, MINICAMS sampling ports are moved over worker DPE suits to see if agent levels exceed 1 vapor screening level, pausing in four quadrants to take a measurement. This is a time-consuming procedure.
Recommendation 4-4. Procedures developed and optimized in laboratory environments for the real-time detection of chemical agents using ambient ionization mass spectrometry should be verified in all working environments where they are likely to be deployed, using actual sample materials (e.g., activated charcoal from filter beds and worker masks, DPE suit material, and polymer-coated concrete).
Recommendation 4-5. Procedures should be developed for using ambient ionization mass spectrometry (e.g., DART, large-area DESI) to check worker DPE suits for contamination when workers are exiting Class A work areas. This approach could greatly reduce the time required for this activity, including verification of the effectiveness of decontamination procedures carried out prior to DPE suit removal when agent is detected.
Finding 4-9. Mass spectrometric detection methodology is able to provide relative quantification of analyte species, including chemical agents, over several orders of magnitude with excellent linearity between concentration and response for both gas- and liquid-phase analytes with appropriate sampling and ionization methods. Absolute quantification can be provided with appropriate reference standards. This is true of the ambient ionization mass spectrometric methods as well. However, quantifying amounts of target species on surfaces and adsorbed in solid substrates is more problematical.
Recommendation 4-6. The Army should develop reference standards to permit calibration of mass spectrometric instruments using DART (or other deployed ambient ionization sources) for analysis of chemical agents in gases and liquids. In the case of gas-phase samples, it would be useful to develop a reference standard that reliably provides a vapor-phase concentration equal to 1 vapor screening level of the target agent,
both to quantify measurements and to verify acceptable performance during critical operations. Even though they may be less reliable for quantitative analysis, calibration standards and procedures should also be developed that ensure acceptable sensitivity for detection of trace amounts of agents on relevant surfaces.




































