2
Overview of Algal Biofuel Supply Chain
Assessing the sustainability of algal biofuels requires an understanding of the individual components that make up potential supply chains. This chapter focuses on the basic processes of algal biofuel production from the biology and traits of the organisms, to methods for cultivation, and to processing into liquid fuels. It discusses algal strains and the attributes of those strains critical for biofuel production, the photoautotrophic methods for algae cultivation through open-pond and closed photobioreactor systems, the processes for collection and dewatering if necessary, and the processing of algal lipid, biomass, or secreted products into fuels. It provides the basic descriptions of the supply chain components used in later chapters and summarizes some critical process improvements that could enhance the overall sustainability of algal biofuels.
The organisms considered as potential feedstock for algal biofuel production belong to a vast and diverse assemblage of aquatic organisms that carry out oxygen-evolving photosynthesis and lack the stems, roots, leaves, and embryos of plants (Leliaert et al., 2011). The category includes eukaryotic species that are related to the plant lineage and can be further categorized as macroalgae that are large structured species (for example, kelps) or microalgae that are microscopic species (for example, Nannochloropsis spp.). In the context of biofuel, the term “microalgae” also includes cyanobacteria, a diverse prokaryotic lineage whose ancestor gave rise to the plant chloroplast (Keeling, 2010). More than 40,000 species of microalgae have been described, and they collectively cover a comprehensive spectrum of habitats and tolerances of ranges of pH, salinity, and temperature (Van den Hoek et al., 1995; Falkowski and Raven, 1997; Paerl, 2000). McKenzie (2011) estimated that prokaryotic and eukaryotic microalgae are responsible for more than 40 percent of net primary productivity on Earth. Algae can be a more appealing biofuel feedstock than land plants because of their faster biomass doubling cycle, their more accessible forms of stored carbon than
the lignocelluloses used for cellulosic biofuels, and their ability to thrive on water sources and on land sites that are unsuitable for terrestrial farming.
Microalgae contain diverse pigments and metabolites that are desirable as nutritional supplements and colorants. Examples of such products include astaxanthin, an antioxidant derived from the alga Haematococcus, and a high-protein powder derived from cyanobacterial species of Spirulina (Arthrospira) (Gershwin, 2008; Guedes et al., 2011). Commercialscale algal ponds that grow these and other microalgae have operated for more than a decade (Del Campo et al., 2007). However, the scale of deployment for algae cultivation for fuel is expected to be much larger than the scale of algae cultivation for nutraceuticals or other specialty products currently available in the market.
Generating biofuels from algae requires exploiting and expanding the demonstrated commercial-scale growth of algal biomass, and harvesting the relatively accessible carbon stored therein. Carbon is stored within algal cells in various forms, and these molecules can be accessed by different technologies. Both eukaryotic and prokaryotic algal cells are rich sources of polar lipids that are associated with membranes; in some cases, the photosynthetic thylakoid membranes are extensive. Carbon is such a crucial element for algae that it is typical for them to store surplus carbon when cellular division is restricted by some factor other than carbon availability—this situation is termed unbalanced growth. In many eukaryotic microalgae, photosynthetic carbon fixation continues under unbalanced conditions. Under extended periods of environmental stress, the excess fixed carbon is stored in the form of neutral lipids called triacylglycerols (TAGs). TAGs are hydrocarbon chains terminated in a carboxylic acid group. The three carboxyl groups are bound to glycerol through an ester linkage. Biofuels containing hydrocarbon chains longer than six carbons are particularly valued because of their high heats of combustion, volatility, and compatibility with existing engines. As discussed later in this chapter, extracted TAGs can be converted to biodiesel using a number of technologies, including transesterification and hydrotreating. Even algal species that do not store large amounts of TAGs can be converted to biofuels through various chemical conversion technologies. For example, species that store polysaccharides can be fermented to yield ethanol, and other biomass processing technologies, such as gasification, pyrolysis, and hydrothermal liquefaction, have shown great utility for the conversion of whole biomass into biofuels.
The incipient algal biofuel industry is emerging and evolving from its early foundations in algae cultivation for fish feedstuff and for human nutraceuticals. Early technology development of processing algae to fuels emphasized the conversion of neutral lipids (TAGs) to biodiesel. Choices of algal feedstocks have been expanding to address the goals of fuel production rather than nutritional content and to exploit new technologies for processing biomass that extend beyond those that focus on TAGs. Ideal attributes for algal feedstock for fuels include rapid and dense growth; efficient use of nutrients, light, and carbon dioxide (CO2) under a range of temperatures; resistance to pests and predators; accumulation of desirable macromolecules that can be processed into fuels; ease of harvest; and the absence of undesirable by-products.
Commercial and research interest in the United States has focused on microalgae, and these species are emphasized in this report. Microalgae have been reported to reach short-term maximum productivities of 50-60 g dry weight per square meter (m2) per day in CO2-enriched open ponds in Hawaii and California (Sheehan et al., 1998). These and other data on productivity from laboratory-scale experiments have promoted the reputation of microalgae as prime candidates for providing cheap biomass feedstocks for food, feedstuff, or energy. Some authors have extrapolated values of maximal biomass productivity and combined them with maximal oil content to predict oil yields of 100 tonnes per hectare
(ha) per year. Such reports have spurred investment in intensive research on algal biofuel production. However, such high productivity projections have yet to be obtained in largescale, long-term experiments. Serious barriers remain for reproducing optimal growth and productivity conditions at a commercial scale. They include maintaining the stability of the culture and delivering the required nutrients and other resources in an efficient manner at such scales. Current yields from large-scale operations range from 40-60 tonnes dry weight of algal biomass production per ha per year, and conservative projections anticipate up to 100 tonnes dry weight of biomass, or 30 tonnes of biodiesel per ha per year in subtropical or tropical, sunny climates (Scott et al., 2010). Estimated yields from a variety of cultivation systems are discussed later in the chapter.
2.1.1 Strain Diversity
The choice of strains for biomass production depends on the desired product and technology to be used for fuel production, the source, and the type of cultivation facility (open versus closed). Initial efforts using outdoor ponds focused on production of biodiesel by the transesterification of TAGs to produce fatty-acid methyl esters (FAME).1 Therefore, strains that accumulate TAGs were selected. Five groups of microalgae were classified as high priority for biofuel production by the U.S. Aquatic Species Program (Sheehan et al., 1998): diatoms (Bacillariophyceae), green algae (Chlorophyceae), golden-brown algae (Chrysophyceae), prymnesiophytes or haptophytes (including Prymnesiophyceae), and eustigmatophytes (Eustigmatophyceae). Many strains and genera of eukaryotic microalgae are potential high-oil producers for large-scale culture (Sheehan et al., 1998; Rodolfi et al., 2009). These include species of Tetraselmis, Dunaliella, Chlorococcum, Scenedesmus, and Chlorella, and particularly Neochloris oleoabundans and Botryococcus braunii from Chlorophyta; the genera of Amphora, Amphiprora, Cylindrotheca, and Navicula, and the species of Nitzschia dissipata, Phaeodactylum tricornutum, and Chaetoceros muelleri from Bacillariophyta; the species of Nannochloropsis ocalata and N. salina from Eustigmatophyceae; and the genera of Isochrysis and Pavlova from Haptophyta.
Improvements of technologies that convert total biomass to yield drop-in fuels—such as those being pursued by companies such as Inventure (Inventure, 2012), Xtrudx (Xtrudx Technologies, 2012), and Solvent Rescue Limited (Solvent Rescue Limited, 2012) and academic institutions such as Old Dominion University (Hatcher, 2011)—are changing the scope of organisms that are being considered for biofuel production. All categories of algae are rich in polar lipids that can be recovered by such processes, and they have cellulose or other polysaccharide cell walls composed of sugars. Cyanobacteria store excess carbon as glycogen rather than TAGs, and cyanobacteria and macroalgae accumulate quantities of other complex polysaccharides. These and other macromolecules are all potential carbon sources for producing drop-in fuels if appropriate processing technologies are available. In addition, algal carbohydrate potentially can be a feedstock for fermentative fuel production processes that are based on heterotrophic organisms, such as those used by LS9, Inc. (LS9 Inc., 2011) and Solazyme (Solazyme, 2012). Cyanobacteria are used directly for ethanol production by Algenol (Chance et al., 2011a; Algenol Biofuels, 2012). As of 2012, a number of marine macroalgal species are being considered for biofuel production in India. An example
_______________
1 As Chapter 3 discusses, algal triacylglycerols are reacted with methanol to form fatty-acid methyl esters (FAME). Due to its higher viscosity compared to conventional liquid transportation fuels, FAME cannot be used as a drop-in fuel, but can be blended with conventional diesel.
is the red algal species Kappaphycus alvarezii, a species cultivated for its high carrageenan2 content (Russell, 1983; Rodgers and Cox, 1999; Woo et al., 2000). Species of Spirulina have properties suitable for aquaculture, and they are grown at relatively large scales for sale as a nutritional supplement (Earthrise Nutritional, 2009a). Still, the spectrum of cyanobacteria that could be suitable for fuel production is largely unexplored. Prokaryotic algal species provide additional diversity in light harvesting, tolerance of growth habitat and pH, and facility of genetic modification.3 Moreover, some cyanobacterial species are diazotrophs; that is, they are able to fix atmospheric nitrogen (N). Although no current commercial operations rely on a nitrogen-fixing strain, several filamentous strains that have good lightharvesting properties and for which genetic methods are well developed are diazotrophic (Heidorn et al., 2011; Ruffing, 2011). The use of these strains as a biofuel feedstock or as a nitrogen provider for non-fixing strains (to reduce nutrient input) has received little attention.
Clear differences exist in carbon storage forms (important as fuel feedstock), dominant pigments (important for solar energy capture), and accessory pigments such as carotenoids (which can be valuable commercial products) among different algal divisions (Table 2-1). Furthermore, their pigmentation and composition are affected by growth conditions and environmental stress.
Emphasizing individual strains that are intended for monoculture discounts potential advantages that could be associated with mixed cultures. A recent study showed increased lipid production in algal cultures as a function of species diversity in mixed cultures under nutrient-limiting growth conditions (Stockenreiter et al., 2012). However, this effect has been demonstrated only at the laboratory scale or in low-density natural algal populations, and requires confirmation for extended periods of time and at relevant volumes. Moreover, lipid production of mixed algal culture could be different under the nutrient-replete conditions of ponds designed for maximal growth. Mixed cultures might facilitate crossprotection, diversity of products through product conversion, flocculation and harvesting improvements, and efficient use of light in the water column (Stomp et al., 2007). However, mixed cultures increase the heterogeneity of the potential product, which could affect the quality of yield and the ability to optimize the diverse characteristics of the mixture for a single product. The potential to enhance the supply chain of algal biofuel through growth of mixed cultures merits additional research to determine the effects on desirable product yield and biomass accumulation (see section Cultivation in this chapter). Because data are not available for large-scale, mixed-species systems, this report introduces the concept of mixed culture systems but focuses primarily on monoculture systems.
Among the biggest challenges for strain selection is the difficulty of translating desirable strain properties from the laboratory to the field. A desirable strain would have robust growth in open ponds under natural weather and cultivation conditions, and would retain attributes that are selected and measured in the controlled conditions of the laboratory. However, the ability to grow well and compete when exposed to environmental conditions is difficult to predict. Few strains are already proven to be robust in outdoor mass cultivation, and years of investment in time and process went into their commercial development.
_______________
2 A gelatinous substance extracted from red algae and widely used as a stabilizing or thickening agent in industrial, pharmaceutical, and food products.
3 Within the text of this report, the committee will distinguish whether it is discussing “genetic modification” or “genetic engineering” specifically. The committee considers genetic modification to be a general term and includes in its definition any organism whose genetic material has been altered through an array of approaches, including traditional cross breeding, mutagenesis, and genetic engineering. Genetic engineering is a modern technique that enables the introduction of a foreign gene or genes into the genome of an organism through recombinant DNA methods in an attempt to introduce a new trait into that organism.
TABLE 2-1 Characteristics of Photoautotrophic Algaea
|
|
|||||
| Division | Dominant Photosynthetic Pigment(s) |
Accessory Pigments (Carotenoids) | Principal Energy Storage Compound | % Proteinb | % Lipidb |
|
|
|||||
|
Cyanoprokaryota (blue-green algae) |
Phycobilins, Chlorophyll a |
Zeaxanthin, beta-carotene, myxoxanthin, echinenone, canthaxanthin |
Glycogen, other polysaccharides, polyhydroxyalkanoates, |
10-70 |
1-20 |
|
Bacillariophyceae |
Chlorophyll a, |
Fucoxanthin, beta-carotene, diadinoxanthin, diatoxanthin |
Lipid |
5-35 |
5-55 |
|
Haptophyceae |
Chlorophyll a, |
Beta-carotene |
Chrysolaminaran |
5-30 |
5-55 |
|
Chlorophyceae |
Chlorophyll a, |
Lutein, |
Starch |
5-30 |
5->50 |
|
Haptophyceae |
Chlorophyll a, |
Fucoxanthin |
Starch |
5-35 |
5-50 |
|
Raphidophyceae |
Chlorophyll a |
Diatoxanthin |
Lipid |
5-35 |
5-55 |
|
Rhodophyceae |
Phycobilins, |
Starch |
5-15 |
5-15 |
|
|
Phaeophyceae |
Chlorophyll a |
Fucoxanthin |
Starch |
5-15 |
5-15 |
|
Chrysophyta |
Chlorophyll a |
Beta-carotene, fucoxanthin |
Lipids (oil) Leucosin |
20-30 |
30-40 |
|
Eustigmatophyta |
Chlorophyll a |
vialaxanthin, beta-carotene |
Lipids (oil) |
10-30 |
40-65 |
|
|
|||||
a Table shows wide ranges in the percentage of lipids and proteins, reflecting that these and other parameters are dramatically affected by growth conditions.
b Percentages are given as a percent of dry weight.
Successful mass cultivation of new strains likewise will require intensive work to commercialize, whether those strains are native, genetically modified, or bred for improved attributes.
2.1.2 Desirable Strain Properties
Regardless of the technology or strain, the goal is to maximize the quantity of a final product per unit time, area, or water volume. Further, the desire is to maximize the product output per unit input of energy, nutrients, and other resources. Biomass and lipid accumulation per unit time are two measures of productivity (see Rodolfi et al., 2009 for example). Many other criteria are important for selecting algal strains for commercial biofuel production, including variables that alter cost in the supply chain that are important for economic viability (for example, AQUAFUEL, 2009). Ideally, the criteria for strain selection are measurable. Among important selection criteria are:
• Photosynthetic efficiency. The most objective measure to compare productivity of algae with land crops is photosynthetic efficiency. Photosynthetic efficiency is defined as the percent of available light (energy) that is converted into biomass energy. However, this definition might not be the most relevant for a given supply chain, depending on how the biomass will be processed and what the final products and coproducts will be (Box 2-1).
BOX 2-1
Relevance of Photosynthetic Efficiency to Biofuel Production
The amount of biofuel produced per unit of land area is a key parameter in the evaluation of any biofuel production process. Photosynthetic efficiency, a measure of how efficiently light energy is converted to chemical energy, is one of the key determinants of overall biomass yield. The measure relevant to biofuel production is the amount of energy contained in biomass expressed as a ratio of the solar energy supplied (Blankenship et al., 2011). The calculation is performed for a typical area integrated over a year or a growing season. When done this way, values of up to 3 percent have been reported for microalgae (Wijffels and Barbosa, 2010). Some authors choose to calculate photosynthetic efficiency based on only the percentage of photosynthetically active radiation (PAR) present (Ort et al., 2011), or even only the PAR absorbed (Janssen et al., 2001). These calculations lead to considerably higher values and lead to some confusion around the potential for biofuel production from algae.
Further complicating this particular discussion is determination of the heat of combustion, or the heating value,a to be used. For measures of total photosynthetic efficiency, the heat of combustion is generally taken to be the higher heating value of the dried biomass (Jenkins et al., 1998).
The critical feature for this discussion is not the exact efficiency, but rather that the value is far below what should be theoretically possible (Robertson et al., 2011). Indeed, many have lamented that photosynthesis uses one of the “slowest metabolic enzymes in the contemporary biosphere” (Parikh et al., 2006; p.113). Considerable improvement in photosynthesis might be realized by any number of techniques of modern biology. Improvements in photosynthesis would lead directly to more prolific production of biofuels, which would consequently reduce the land, water, nutrient, and energy inputs required. Improvements to photosynthesis would directly improve the sustainability of algal biofuels.
_______________
a “The higher heating value (also known as gross calorific value or gross energy) of a fuel is defined as the amount of heat released by a specified quantity (initially at 25°C) once it is combusted and the products have returned to a temperature of 25°C, which takes into account the latent heat of vaporization of water in the combustion products. The lower heating value (also known as net calorific value) of a fuel is defined as the amount of heat released by combusting a specified quantity (initially at 25°C) and returning the temperature of the combustion products to 150°C, which assumes the latent heat of vaporization of water in the reaction products is not recovered” (DOE-EERE, 2012a).
• Quantity of final products. This category includes the total amount of biomass, its composition, and the products to be refined, extracted, or excreted from the biomass:
• Total caloric value of the biomass (for combustion or a total biomass processing technology).
• Percent lipids and lipid composition (for biodiesel).
• Percent starch and carbohydrate composition (for subsequent fermentation and to identify higher value by-products such as agar).
• Percent protein and protein composition (soluble and insoluble protein for food and feedstuff).
• Total secretion of desirable products.4
• Presence of high-value coproducts.
_______________
4 Some companies, such as Joule and Algenol, have taken a dramatically different approach, relying not on accumulation of biomass, but on the secretion of desirable products from stable algal cultures (Robertson, D.E., S.A. Jacobson, F. Morgan, D. Berry, G.M. Church, and N.B. Afeyan. 2011. A new dawn for industrial photosynthesis. Photosynthesis Research 107(3):269-277). In this paradigm that uses photobioreactors, the criteria for strain selection are different from those used for open ponds. Planktonic unicellular species that would be difficult to protect from grazers and to harvest from ponds, are desirable within bioreactors. Well-developed genetic model organisms that are amenable to genetic engineering (such as Synechocystis sp. strain PCC 6803, Synechococcus sp. strain PCC 7002, and Synechococcus elongatus PCC 7942; and the unicellular green alga Chlamydomonas reinhartii) can be used in the controlled environment of photobioreactors.
• Nutrient and other resource requirements. These include the quantity of nutrients, such as CO2, nitrogen, and phosphorus; the type and quality of the water supply; and siting requirements. Strains could be selected because of their nutrientuse efficiency. Strains also might be selected because of their ability to flourish in brackish or wastewater, which would reduce the demand on freshwater supplies, and in the climatic conditions of a particular site.
• Robustness. This term describes the overall stability of the crop, which depends on resistance to extremes of climate and environmental variables (for example, competitors, pathogens and predators, salinity and dissolved solutes, temperature, and pH). Tolerances to these variables vary widely within the diverse spectrum of microalgae. The ability to thrive in water with various salts, metals, and other solutes could become increasingly important as competition for freshwater use among different sectors increases. Resistance to high pH allows growth in alkaline conditions that favor a monoculture crop over sensitive predators and pathogens. Filamentous species or species with large cell size tend to be more resistant to grazers than unicellular species with small cell size (Tillmann, 2004). Tolerance to a broad range of temperatures could be important if the algae are cultivated in regions with high daily or seasonal fluctuation in temperature. To maintain year-round production, it might be desirable to rotate strains that have different temperature tolerance profiles. The wide spectrum of sites that are under consideration for production ponds will require organisms with different light, water quality, and climatic tolerances. Robustness might be assessed by scoring the strain success under a wide range of potentially relevant conditions such as in Evens and Niedz (2011).
• Harvestability. Harvesting cost and energy consumption can vary dramatically among different algal strains (Uduman et al., 2010). Contributing factors include the sedimentation rate and the capability for induced bioflocculation5 or auto-flocculation. Filamentous strains that can be seined, species with positive buoyancy, or species that settle out of the water column quickly once agitation ceases might not require centrifugation, and they can be harvested easily. Growing mat-forming algae or algal films could facilitate harvesting (Tang et al., 1997), but to the committee’s knowledge, such approaches have not been scaled up. Strategies that rely on harvesting secreted products rather than biomass simplify the harvesting step, but such strategies require photobioreactors for algae cultivation to prevent contamination by microorganisms that would consume the product.
• Processability and extractability. This parameter includes factors that influence the ease of extracting algal oil or processing algal biomass to fuels, for example, cell volume, thickness and toughness of the cell wall, the presence of tough fibers (for example, cellulose and silica) or cell walls, and the moisture content (Brennan and Owende, 2010). A measure for processability and extractability could be the energy input per gram of dry weight necessary for fractionation and full recovery of all biomass components.
• Added value of coproducts. The algal biomass could be used to produce coproducts that have an intrinsic added value, such as carotenoids, phycobilins, docosahexaenoic acid, or eicosapentaenoic acid (Pal et al., 2011). Coproducts can offset some of the costs of the biofuel product. A specification of the compounds and their expected added value per gram of dry biomass needs to be indicated. However, the market value of coproducts could decrease under an excessive-supply and lowdemand condition.
_______________
5 Bioflocculation is the clumping together of microorganisms through biological interactions.
• Local origin of strains. Using locally selected strains could ease management and improve sustainability (RSB, 2011). Some governments have sought to restrict the importation of nonnative species, for example, the 81st Texas Legislature House Bill 3391 (2009). However, the cosmopolitan nature and wind-borne movements of algae make it unlikely that legislation can reasonably define species as native or nonnative. Regardless of legislation, local strains might have unique adaptations to the local climate, water, and possible parasites that imported or laboratory-grown strains might not have.
• Non-toxic. The selection of non-toxic algal strains will increase social acceptability and reduce the potential impacts related to occupational exposures and accidental releases.
2.1.3 Strain Development and Engineering
Modern agriculture has advanced primarily on the development of improved germplasm, and algae cultivation will likely advance using similar approaches. As with traditional agriculture, advances in breeding, mutagenesis, and genetic engineering are likely to play roles in algal germplasm enhancement. Domestication of algae potentially could change their phenotype dramatically because the desired characteristics for production are different from those that have evolved in the selective pressures of the wild and because hypereutrophic aquaculture conditions will support genotypes that would not be fit in natural environments. Breeding and engineering will enable the stacking of desirable traits within a single species or mixture of species. The definition of desirable traits, product type desired, choice of production organism, and specification of growth and harvesting methods will influence the needs for further development on a case-by-case basis.
The understanding of genetics, physiology, and metabolism at present is uneven across the spectrum of genera and species of algae that might have desirable features for algal biofuel production. Major hurdles include the need to develop genetic technologies for new species that have not been domesticated previously and that have desirable characteristics for large-scale cultivation. The application of genomic approaches could accelerate the analysis of new strains by addressing changes in gene expression for a given organism under various conditions and identifying conserved and nonconserved genes among organisms. Those approaches facilitate the identification of candidate genes that might be relevant for particular pathways of interest (Flaherty et al., 2011; Karpowicz et al., 2011; Lopez et al., 2011; Weckwerth, 2011). Cryogenic storage methods, such as those used at the Culture Collection of Algae at the University of Texas (UTEX, 2012), also may prove important to maintaining germplasm stocks and to replenishing pond inocula with a desired genotype after genetic drift of the crop population. Cultured algae, particularly cultures held for more than 10 years in selective media, have been shown to have reduced growth and production of unexpected secondary metabolites (Martins et al., 2004). A factor that might be overlooked in efforts to genetically engineer metabolic pathways in algae is that both eukaryotic and prokaryotic strains possess circadian clocks that time the peaks of daily rhythmic changes in physiological and metabolic functions (Suzuki, 2001; Ditty et al., 2003; Matsuo and Ishiura, 2010; O-Neill et al., 2011). The mechanisms and the physiological and metabolic consequences of circadian rhythms are insufficiently understood in these organisms.
At present, few eukaryotic algal species are readily amenable to breeding or genetic engineering. Published transformation methods are well developed for Chlamydomonas reinhardtii and Phaeodactylum tricornutum. Solazyme appears to rely on genetically engineered
Chlorella species for heterotrophic fermentation of algal oils. About 30 strains of eukaryotic microalgae have been transformed using biolistic bombardment, vigorous mixing with glass beads, electroporation, or deoxyribonucleic acid (DNA) transfer from Agrobacterium tumefaciens. Strains that have been transformed include representatives of green, red, and brown algae; diatoms; euglenoids; and dinoflagellates (Radakovits et al., 2010). However, in many cases the reported transformation is only transient (Radakovits et al., 2010), and these reports have not led to routine adoption and application for most of those strains. Nevertheless, the transformations demonstrate that developing genetic systems for diverse species is possible with focused effort.
Targeted gene inactivation by homologous recombination has been a long-standing challenge for manipulation of Chlamydomonas and other algal nuclear genes. However, Kilian et al. (2011) made progress in this area when they reported successful knockouts of Nannochloropsis sp. nuclear genes encoding nitrate reductase and nitrite reductase. Various genes have been suppressed successfully in Chlamydomonas by interfering ribonucleic acid (RNAs) (Cerutti et al., 2011). High-throughput methods to introduce interfering RNAs could provide an effective way for gene inactivation in diverse strains that do not exhibit homologous recombination of transgenic DNA. Another challenge for nuclear modification is that gene expression is often silenced when heterologous genes are inserted randomly into the Chlamydomonas reinhardtii nuclear genome (Fuhrmann et al., 1999). Manipulation of the chloroplast genome is facile in C. reinhardtii, but not in other algae (Radakovits et al., 2010). A report of stable chloroplast transformation in Porphyridium suggests that chloroplast transformation via homologous recombination might be a universally applicable approach (Lapidot et al., 2002). Waaland et al. (2004) reviewed macroalgal species as candidates for genomic research and concluded that the red alga Porphyra yezoensis exhibits numerous attributes conducive to further analyses. Extensive biochemical and physiological research has been conducted on the macroalgae because of their use in the food industry. Because there is extensive variation in the extent and type of genetic malleability among different algal species, technologies would have to be developed on a case-by-case basis for individual new algal types whose physiological and metabolic properties suggest their potential as production strains. Moreover, it will be highly desirable to develop methods that can be used to more rapidly develop a genetic system de novo in new strains or species as they are discovered.
Genetic manipulation is more straightforward among cyanobacteria than eukaryotic algae because prokaryotes are amenable to techniques of bacterial genetics (Figure 2-1); some species are naturally transformable and take up exogenous DNA without specific intervention (Heidorn, 2011; Ruffing, 2011). Figure 2-2 shows some of the biochemical pathways in cyanobacteria that can be engineered to produce different desired products. Methods for gene inactivation via homologous recombination and the stable expression of transgenes, from plasmids or integrated into the chromosome, are well established in at least a dozen diverse species (Ducat et al., 2011; Ruffing, 2011). However, the developed model organisms have been maintained in the laboratory for several decades and are not likely to be suitable for growth under outdoor cultivation conditions. The Spirulina species that grow robustly outdoors have proven recalcitrant to manipulation. Despite some reports of transgenic Spirulina (Toyomizu et al., 2001; Kawata et al., 2004), many laboratories have failed to achieve stable transformation of the organism. This failure is likely, at least in part, due to a host of restriction endonucleases that specifically cleave foreign DNA (Zhao et al., 2006). Steps that protect plasmids by methylation while they are in an Escherichia coli host and before they are introduced to the cyanobacterium by conjugation have facilitated genetic technologies for the nitrogen-fixing filamentous strains Anabaena (Nostoc) sp. PCC
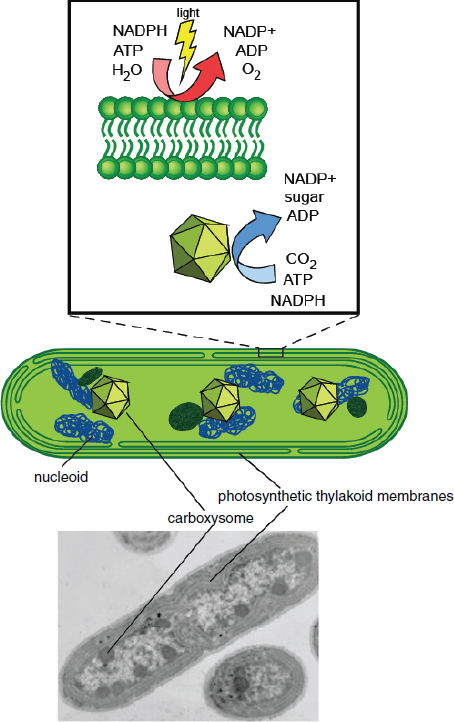
FIGURE 2-1 Overview of cyanobacterial organization.
NOTE: The cartoon diagram in the middle shows the longitudinal section of a representative cyanobacterium (modeled after Synechococcus elongatus). The major features are indicated on the cartoon diagram above and the electron micrograph below.
SOURCE: Adapted from Ducat et al. (2011). Micrograph image courtesy of and reprinted with permission from Lou Sherman, Purdue University.
7120, Anabaena sp. ATCC 29413, and Nostoc punctiforme ATTC 29133 (Elhai et al., 1997). Similar approaches are likely to work for other strains that initially resist transformation. A filamentous cyanobacterium isolated from an outdoor pond that has robust growth properties similar to Spirulina species has been found to be easily manipulated by conjugal introduction of transgenes and transposons (Taton et al., 2012). This finding suggests that diverse cyanobacterial model strains that are more relevant for biofuel development than current laboratory strains could be readily developed.
Genetic engineering holds the promise of transplanting completely novel pathways from heterologous sources and making products of tailored composition (Figures 2-1 and 2-2; Ruffing, 2011). Some demonstrations from genetically engineered cyanobacteria include the production of 1-butanol, isobutyraldehyde, N-alkanes, free fatty acids, and sugars from transformable species of Synechococcus (PCC 7002 and 7942), Thermosynechococcus (BP-1), and Synechocystis (PCC 6803) (Atsumi et al., 2009; Niederholtmeyer et al., 2010; Lan and Liao, 2011). Transgenic strains could play an important role in biofuel production, and some companies are making major investments in these technologies (for example, the Exxon Mobil alliance with Synthetic Genomics, Inc.; Marler, 2011; Roessler, 2011) even though
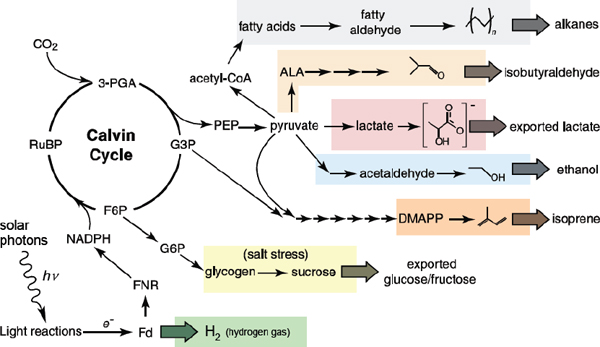
FIGURE 2-2 Schematic representation of engineered biochemical pathways in cyanobacteria.
NOTE: Core metabolism of photosynthetic processes is shown in black text. Branch points used to produce various desired compounds are highlighted in colored boxes. Abbreviations: 3-PGA, 3-phosphoglycerate; FNR, ferredoxin NADP+ reductase.
SOURCE: Adapted from Ducat et al. (2011).
strains have not been used in outdoor systems. The use of engineered strains in outdoor cultivation will be regulated according to the type of genetic modifications applied. The U.S. Environmental Protection Agency (EPA) under the Toxic Substances Control Act (TSCA) recognizes microorganisms that carry sequences from another genus as new organisms that require regulatory permitting (EPA, 2011). Under TSCA, organisms that are modified by technologies based solely on rearranging and reinserting endogenous genetic material into strains of interest are not categorized as genetically modified. Thus, self-cloned species can be used in open ponds without special oversight. Growing genetically modified algae in photobioreactors will follow the same regulatory standards that are common in the fermentation and biotechnology industries.
Irrespective of the algal strain cultivated and its end use, some areas of improvement in strain and cultivation are generally desirable. These include:
• Modulation of carbon allocation.
• Increases in culture density.
• Net increase in photosynthetic efficiency.
• Algal crop protection.
• Other enhancements.
2.1.3.1 Modulation of Carbon Allocation
The basic strategies to adapt microalgae to increased oil production for processing to diesel were summarized by Radakovits et al. (2010). A major target of genetic engineering
is production of algal strains that accumulate and maintain high amounts of oil under high growth rates in continuous cultivation systems. Most eukaryotic algae accumulate increased amounts of oil only in response to nutrient stress or in late exponential growth phase and do so at the expense of a reduced growth rate. Methods to enhance lipid accumulation in algae include enhancing certain enzymatic activities through genetic and transcription engineering approaches (Courchesne et al., 2009; Turchetto-Zolet et al., 2011). Research has focused on identifying the “nutrient stress trigger” that induces TAG accumulation in an effort to make TAG production constitutive. Strains that maintain elevated basal oil content might be produced by mutagenesis or genetic engineering. However, the pathways that regulate stress responses—and key enzymes—that initiate oil production are insufficiently understood at present. Understanding the metabolic regulatory networks that control carbon allocation to carbohydrates and lipid and identifying means to modulate these networks are necessary to achieve constitutively elevated oil yields under continuous growth. Assessments of whether metabolic modifications can be made without genetic tradeoffs that result in suboptimal performance in other aspects of the cells’ metabolism are important.
An array of techniques for improving lipid yields is described in the literature. A few examples are discussed in this section. Genetic manipulation of carbon allocation can enhance lipid production (Li et al., 2011). Starch production is blocked from the sta6 mutant of C. reinhardtii, and its lipid body content increases 30-fold compared to 10-fold in the wild type (Wang et al., 2009). Modifications to improve oil yields have been achieved in oil-seed plants by altering the activities of dozens of genes, each of which results in an increase of a few percent in oil content (Thelen and Ohlrogge, 2002; Lardizabal et al., 2008; Clemente and Cahoon, 2009). Similarly, a broad approach of modifying several genes, which operate in both starch and lipid metabolism, could result in a substantial increase in oil content in algae.
Strategies that target steps in diverse metabolic pathways like starch metabolism, acetyl-coenzyme A (acetyl-CoA) and fatty-acid biosynthesis, and reactions of TAG assembly have shown significant effects on TAG accumulation in some organisms. For example, acetyl-CoA carboxylase overexpression led to “a 40 percent increase in the total fatty acid content of the non-oleaginous yeast Hansenula polymorpha” (Ruenwai et al., 2009). Mutants of Arabidopsis that are deficient in plastid pyruvate kinase had 60 percent less seed oil than the wild type, revealing a major role of this enzyme in pyruvate supply for acetyl-CoA biosynthesis (Baud et al., 2007). Reactions in the latter steps of TAG biosynthetic assembly might provide increased sink strength that could stimulate fatty-acid production (Thelen and Ohlrogge, 2002). Indeed, stimulation in seed oil content of Arabidopsis and rapeseed had been observed when a yeast long chain sn 2 acyltransferase was overexpressed (Zou et al., 1997). The overexpression of a diacylglycerol acyltransferase (DGAT), a committed and final step in TAG biosynthesis, increased seed oil content and seed weight in Arabidopsis (Jako et al., 2001) and tobacco leaves (Andrianov et al., 2009). A specific phenylalanine residue in DGAT was found to be a key determinant of oil content and composition in maize (Zheng et al., 2008), and the corresponding Phaeodactylum gene has been identified. A novel acyl-CoA:diacylglycerol acyltransferase 1-like gene (PtDGAT1) has been cloned and characterized from the diatom P. tricornutum (Guihéneuf et al., 2011) and will be tested in transgenic algae. Structural components of oil globules such as oleosin and caleosin might accelerate oil body formation in oil seeds of higher plants, but an oleosin gene has not been identified in algae. However, a major oil body protein of Haematococcus has been described (Peled et al., 2011).
Manipulating regulatory enzymes (such as transcription factors and signal transduction proteins) has been shown to enhance TAG accumulation in higher plants (Cernac and Benning, 2004) and might be effective in eukaryotic algae. Similar engineering can affect
glycogen accumulation in cyanobacteria (Osanai et al., 2005; Ehira and Ohmori, 2011). Several microRNAs that are differentially expressed in C. reinhardtii, under conditions in which lipid content is changed, were used to develop strains that produce 25 percent more oil than the wild type strain (Maor Sasson, TransAlgae Ltd., Israel, personal unpublished data). The redistribution of carbon from carbohydrates to lipids, higher alcohols, and hydrocarbons requires a better understanding of carbon regulation networks in these species.
2.1.3.2 Increases in Culture Density
The product yield (expressed, for example, as grams per liter per day, or as grams per square meter per day) of an outdoor algal culture is a function of its specific growth rate and its biomass concentration. Thus, maintaining an outdoor culture at its optimal biomass concentration is important to maximizing product yield. However, high cell density reduces light penetration and limits the growth rate of cells below the surface. Although counterintuitive, reducing the light-harvesting ability of individual cells could improve the light availability to the culture and increase overall photosynthetic activity. By reducing wasteful absorption and dissipation of light energy by cells at the surface, excess light is allowed to pass through to cells below. Thus, researchers have proposed selecting and developing strains with low pigmentation level (small light-harvesting antenna6 size) to increase the standing biomass of the culture (Benemann, 1989; Huesemann et al., 2009; Ort et al., 2011). This approach was evaluated in greenhouse conditions and shown to have a positive effect on productivity (Polle et al., 2003). The challenge is to isolate such mutants from the desired strain and to ensure they are stable under long-term outdoor cultivation. More complex culture strategies might facilitate achieving this goal, for example, layering strains that have different antenna sizes and spectra.
Whether factors other than light limit maximum culture densities is unknown. Little work has been done regarding cell-to-cell communication within a given species of microalgae, but evidence of quorum sensing7 (Teplitski et al., 2004; Sharif et al., 2008) and widespread interspecies allelopathic interactions have been reported (Gross, 2003). Endogenous mechanisms that limit population density might exist, in which case genetic modification may improve this aspect for aquaculture purposes.
2.1.3.3 Net Increase in Photosynthetic Efficiency
A long-time goal, as old as the techniques of genetic engineering itself, is to improve photosynthetic efficiency by such alterations as reducing losses from photorespiration, increasing the substrate selectivity of ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco), and enhancing photosystem stability and efficiency. However, 30 years of efforts in this area have not yielded any progress in higher plants or algae. Recent advances in synthetic biology, by fundamentally redesigning prokaryotic photosynthetic organisms to maximize the production of fuel molecules directly driven by photosynthesis (Chance et al., 2011b; Algenol Biofuels, 2012; Joule Unlimited, 2012), might provide some progress in this field. However, only laboratory-scale or small pilot-scale results have been presented. Atsumi et al. (2009) found that overexpression of Rubisco in transgenic Synechococcus elongatus PCC 7942 led to increased production of isobutyraldehyde without negatively
_______________
6 Light-harvesting antennae are protein-pigment complexes that capture sunlight and direct the radiant energy to the reaction centers.
7 Quorum sensing is the process of cell-to-cell communication in microorganisms that involves the production, release, and subsequent detection of chemical-signal molecules.
affecting photosynthetic oxygen evolution, suggesting that net improvements in carbon fixation are reasonable. Chen and Blankenship (2011) made the challenging proposal that photosynthetic capacity might be expanded by engineering cells to use different chlorophylls to capture a broader range of the light spectrum than non-engineered cells.
CO2 abatement is a driver for developing algal biofuels. However, with current practices and species, CO2 often is limited in production ponds and photobioreactors, and addition of a CO2 source is a significant production expense. The effects of CO2 concentrations on algal growth are discussed in the cultivation section later in this chapter, and CO2 requirements and sourcing issues are discussed in Chapter 4. Improved carbon concentrating strategies would address this aspect of photosynthetic efficiency. The enzyme carbonic anhydrase is produced by several divisions of algae (Giordano et al., 2005). The enzyme converts bicarbonate to CO2 that is released intracellularly for fixation by Rubisco. Most algae possess C3 metabolism. That is, the enzyme Rubisco is solely responsible for CO2 fixation. The ability of some plants and microalgae (specifically diatoms and dinoflagellates) to use CO2 directly during C4-intermediate metabolism offers promise for reducing bicarbonate limitation (Zimba et al., 1990; Raven, 2010). A November 2011 press release from Iowa State University reports that Spalding et al. increased algal biomass by 50 to 80 percent in C. reinhartii by artificially increasing the expression of genes that encode components of the carbon-concentrating mechanism, which normally is induced only under low CO2 conditions. The cells presumably continue to actively scavenge CO2 even when it is at relatively abundant levels (Iowa State University, 2011).
2.1.3.4 Algal Crop Protection
Events in which the crop dies (pond “crash” or culture collapse) take a toll on resources and could threaten the economic sustainability and the future potential of the algal biofuel industry (see section Cultivation in this chapter). One cause of such culture collapse is the activity of predators on high-density biomass cultures (see section Contamination and Stability of Culture in this chapter). Simple genetic modifications that affect cell size can improve resistance to grazers and could improve harvesting properties at the same time (Jurgens et al., 1999). Focused screens to find mutations that confer resistance to specific pathogens and grazers are likely to improve crop protection. Because their carbon- and nutrient-allocating traits are the results of domestication, crop algae might carry a heavier metabolic burden than invading weed species. For these reasons, trait modification to instill resistance to herbicides, production of antifungals, and anti-grazer properties could be important. Indeed, at least one company has developed a genetically engineered algal strain for use in open ponds that is resistant to herbicides (IP Monitor, 2009; Aravanis, 2011). Some algae are known to increase lipid content when they are exposed to low levels of herbicide (Ma et al., 2002, 2006). However, if residual biomass is to be used for food or feedstuff, possible negative consequences of these traits would have to be considered. Algal species production of allelopathic chemicals could be exploited to enhance or inhibit growth of other organisms in crop cultures (Gross, 2003). The activities, pathways, and genes related to the secondary metabolites of strains of interest need to be characterized to harness the potential of intrinsic growth modulators.
2.1.3.5 Other Enhancements
The list of potential enhancements is open-ended and will expand as the specific algal species are chosen for cultivation and their attributes become apparent and as the
technologies to modify them increase. Some clearly desirable modifications would provide increases in tolerance to temperature, salinity, pH ranges, and metal concentrations. Tolerances to a range of conditions contribute to crop robustness. Prior demonstrations of such modifications include the conversion of freshwater cyanobacteria to use saline water sources (Waditee et al., 2002; Laloknam et al., 2006). Other aspects of the supply chain can be targeted through genetic modifications, including genetic engineering. For example, groups at Los Alamos National Laboratory have transplanted genes from magnetotactic bacteria. These genes direct the production of magnetic nanoparticles in green algae, which allows simple harvesting by magnetic collection of cells and reduces energy input for centrifugation and dewatering steps (Los Alamos National Laboratory, 2011).
Evaluating the sustainability of algal cultivation systems for biofuel production requires examining the various material and energy inputs needed for the cultivation systems to maintain scalable productivity, maximize system robustness, and minimize costs (Figure 2-3). Scalable productivity refers to a cultivation system’s ability to maintain productivities with respect to algal biomass and algal product (mass/area-time or mass/volume-time) from the laboratory scale to the commercial scale. System robustness refers to a cultivation system’s ability to reliably and dependably deliver consistent productivity and avoid system crashes or failures as a result of either biological or physicochemical causes. Costs pertain to capital and operating costs for a cultivation system.
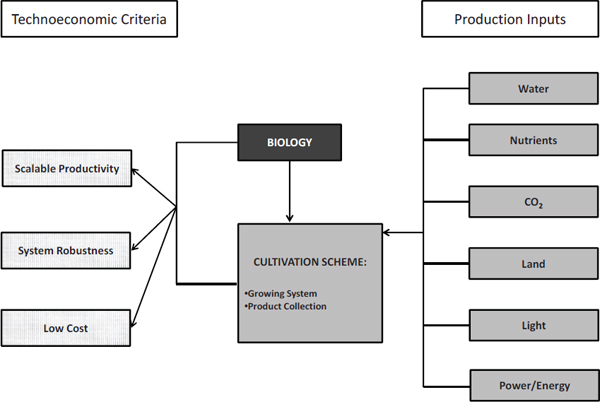
FIGURE 2-3 Material and energy inputs required by a cultivation scheme. Together with the biological scheme, these inputs determine the cultivation system’s productivity, robustness, and cost.
2.2.1 Overview of Algae-Growing Systems
The commercial large-scale cultivation of microalgae began in earnest in the 1960s with the cultivation of Chlorella in Japan (Tsukuda et al., 1977) and the use of phytoplankton as a feedstuff for animals reared in aquaculture (Duerr et al., 1998). In the 1970s, Spirulina was harvested from Lake Texcoco in Mexico (Durand-Chastel, 1980) and produced in Thailand (Kawaguchi, 1980). By 1980, 46 large-scale facilities operated in Asia producing more than 1,000 kg of microalgae each month (Kawaguchi, 1980). The global production of microalgal biomass was estimated to be more than 5,000 dry tonnes in the year 2005 with a value of more than U.S. $1.25 billion, which excludes the value of processed products (Spolaore et al., 2006). About 3,000 dry tonnes of Spirulina are produced in China, India, Myanmar, the United States, and Japan; 2,000 dry tonnes of Chlorella are produced in Taiwan, Germany, and Japan; and 1,200 dry tonnes of Dunaliella salina are produced in Australia, Israel, the United States, and China (Spolaore et al., 2006). In 2008, the global production of microalgal biomass was estimated to be about 9,000 dry tonnes per year (Benemann, 2008).
In addition to algal biology and the intended algal products, numerous factors are considered in selecting the particular algal cultivation system to be used. These include the availability and cost of land, water, energy, nutrients, and labor, and the climate of the location (Borowitzka, 1992). The characteristics of each cultivation system, including its mixing or hydrodynamic characteristics, light utilization efficiency, ability to control temperature, ability to maintain a unialgal culture, and ease of scaling from laboratory to pilot and commercial scales also are considered (Borowitzka, 1999). The two general types of algal cultivation systems discussed in this report are open-pond systems and closed photobioreactor systems.
2.2.2 Open-Pond Systems
The majority of the large-scale microalgal production systems in commercial operation today are open-pond systems, mainly due to economic factors and ease of scale up. Most commercial-scale microalgal cultivation operations are for producing nutraceuticals, and none of them are for producing fuel. The number of microalgal species that can be grown effectively in open-pond systems is limited by the species’ ability to thrive in particularly selective environments while the ponds remain relatively free of protozoan and other algal species contamination (Borowitzka, 1999; Milledge, 2011). For example, Chlorella is grown in a nutrient-rich medium, Spirulina at high pH and bicarbonate concentration, and Dunaliella salina at high salinity (Borowitzka, 1999; Milledge, 2011).
The two most common types of open-pond systems are circular ponds and raceway ponds. Circular ponds are round ponds, with depths of 30-70 centimeters (Moheimani and Borowitzka, 2006). They are typically agitated through a centrally pivoted rotating arm. Ponds up to 45 meters in diameter have been operated in Japan and Taiwan (Becker, 1994). Oscillatoria grown in a circular pond achieved a productivity of about 15 grams dry weight per m2 per day (Sheehan et al., 1998). Mixing efficiency is poor in ponds with diameters greater than 50 meters (Shen et al., 2009). Raceway ponds (Figure 2-4 a-e) are constructed either as single units (Figure 2-4 b-e) or a group of continuous units that are joined together (Figure 2-4a). The raceway channels enable culturing algae in ponds with depths of 15-40 centimeters. The channels are constructed from concrete or compacted earth that might be lined with plastics. A paddle wheel, a propeller, or an air-lift pump operates at all times to agitate and circulate the mixture to prevent algae sedimentation (Becker, 1994; Chen et al., 2009). A key factor in open-pond design and operation is mixing, which evenly distributes
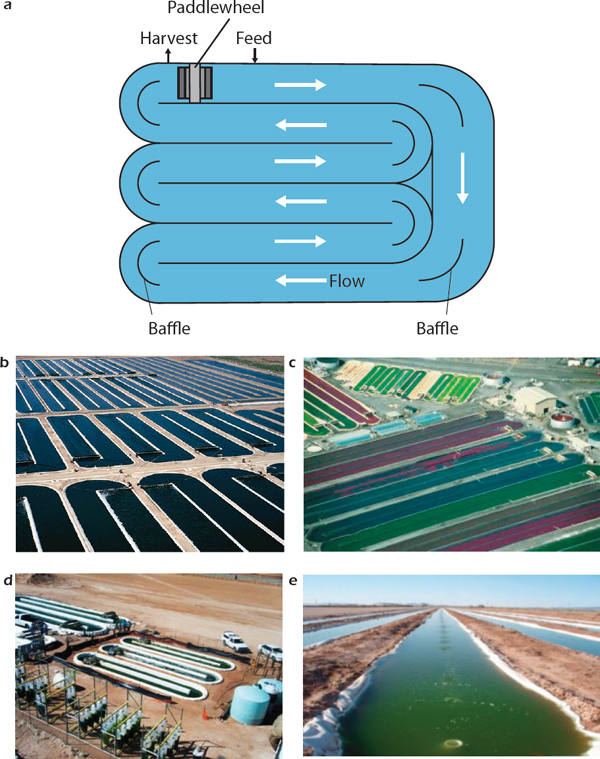
FIGURE 2-4 Open-pond designs for algae cultivation: schematic of raceway design (a), Earthrise raceways (b), Cyanotech raceways (c), Sapphire Energy raceways (d), and Phyco raceways (e).
SOURCES: (a) Adapted from Spirulina Source (Spirulinasource, 1999).
(b) Earthrise (Algae Energy, 2012a). Reprinted with permission from Algae Energy.
(c) Cyanotech (2012). Reprinted with permission from Cyanotech.
(d) Sapphire (Mveda, 2011). Reprinted with permission from Sapphire and Mveda.
(e) Phyco Biosciences (Edwards, 2010). Reprinted with permission from Algae Industry Magazine.
nutrients and exposes algal cells to sunlight and CO2. A velocity of 10-20 centimeters per second (cm/s) prevents algal cells from depositing and settling (Shen et al., 2009). Higher velocities are preferred, but a velocity greater than 30 cm/s could consume too much energy to be economically viable (Sheehan et al., 1998). Earthrise Nutritional, LLC, in California, and Cyanotech Corporation, in Hawaii, have some of the largest algal open ponds lined with plastic liners. Earthrise maintains 30 production ponds each about 5,000 m2 and a series of research ponds (1,000 m2, 200 m2, and 50 m2) (Earthrise Nutritional, LLC, 2009b). Cyanotech has more than 60 ponds, each of which is about 2,900 m2 (Lorenz, 2002; Enay, 2011). The depth of these ponds varies from 30 to 40 centimeters. For raceway ponds, a cell concentration of up to 1 gram dry weight per liter can be achieved, and productivities of 10 to 25 grams dry weight per m2 per day have been reported (Shen et al., 2009). Table 2-2 shows algal productivities for open systems, which vary widely depending on numerous factors, including the type of open system and the algal species grown. Although a productivity of 50 to 60 g dry weight per m2 per day is possible with open systems, achieving even 10 to 20 g dry weight per m2 per day in large-scale systems is difficult on an annual basis because of operational conditions and seasonal variations in temperature and sunlight intensity (Shen et al., 2009).
In a raceway pond of 100 m2, a paddle wheel driven by an electric motor has a power demand of 600 watts (W) (Becker, 1994). The overall energy requirement for paddle wheels in a pond with a roughness coefficient of 0.025 has been estimated at 20 kilowatt hour (kWh) per ha per day for a mixing velocity of 15 centimeters per second and 160 kilowatt
TABLE 2-2 Microalgae Productivities in Open Ponds
|
|
|||||
| Pond Type | Volume (L) | Microalgal Species | Areal Productivity (g DW/m2/d) | Volumetric Productivity (g DW/L/d) | Reference |
|
|
|||||
|
Circular |
1,960 |
Chlorella spp. |
1.61–16.47 |
0.02–0.16 |
Kanazawa et al. (1958) |
|
Circular |
|
Oscillatoria |
15 |
|
Sheehan et al. (1998) |
|
Sloped (cascade) |
1,970 |
Chlorella spp. |
25 |
10 |
Lee (2001) |
|
Slope |
1,990 |
Scenesdesmus obliquus |
24.8 |
|
Becker (1994) |
|
Raceway |
|
Spirulina (Arthrospira) |
9–13 |
|
Olguín et al. (2003) |
|
Raceway |
282 |
Spirulina platensis |
14.47 |
0.183 |
Pushparaj et al. (1997) |
|
Raceway |
300 |
Anabaena spp. |
9.4–23.5 |
0.031–0.078 |
Moreno et al. (2003) |
|
Raceway |
135,000 |
Spirulina (Arthrospira) spp. |
2–17 |
0.006–0.07 |
Jiménez et al. (2003) |
|
Raceway |
|
Dunaliella salina |
1.6–3.5 |
|
García-González et al. (2003) |
|
Raceway |
750 |
Spirulina platensis |
15–27 |
0.06–0.18 |
Richmond et al. (1990) |
|
Raceway |
4,150 |
Phaeodactylum tricornutum |
2.4–11.3 |
0.0028–0.13 |
Laws et al. (1988) |
|
Hybrid system (open ponds and closed photobioreactors) |
|
unknown |
30 (anticipated) |
|
Phycal (2011) |
|
Raceway (proprietary lined “Super Trough System”) |
|
Cyanobacteria spp. |
15.36 (anticipated) |
|
Phyco BioSciences, Inc. (Cloud, 2011a,b) |
|
|
|||||
SOURCE: Adapted from Chen et al. (2009).
hours per ha per day for a mixing velocity of 30 centimeters per second (Benemann, 1986). Other estimates of power requirements for large ponds (for example, Cyanotech’s 2,900 m2 ponds mentioned earlier) range from about 1,200-3,700 W/ha for mixing velocities of 20 to 30 centimeters per second (Pedroni et al., 2001; Frank et al., 2011). A raceway pond of 85 m2 that uses an air-lift pump for circulation has a power consumption of 195 W based on a compressor efficiency of 70 percent and an air demand of 120 liters per second. Ponds in Chile and Brazil have used motor-driven drag boards as an alternative to paddle wheels; the energy requirement was reported to be only 20 percent of the energy needed for a comparable agitation with paddle wheels (Becker, 1994). Laws et al. (1983) introduced the concept of foils that create circular vortices to effectively mix the pond suspension from top to bottom. This is the type of agitation device that Algenol uses in its plastic and covered photobioreactor design (Chance et al., 2011b; see also Chapter 3).
2.2.3 Photobioreactors
Mass cultivation of microalgal species that lack pronounced environmentally selective advantages might require the use of photobioreactors (Milledge, 2011). Photobioreactors are transparent containers or vessels designed to have reduced light path to enhance the amount of available light to the algal cells, and the cultures within are continuously mixed to enhance nutrient distribution and gas exchange. Photobioreactors for microalgae production have an optimal thickness of about 2-4 centimeters (Borowitzka, 1999). The tubular and the flat-plate are the two most common types of microalgal photobioreactors.
All photobioreactors have large surface to volume ratio (SVR). Because of their widespread availability, tubes long have been used as a basic photobioreactor material. The geometric configurations of tubular photobioreactors span a wide range from straight horizontal, straight vertical, helical, to triangular configurations (Figures 2-5, 2-6). One of the world’s largest photobioreactor facilities is in a greenhouse in Klotze, Germany. This facility consists of straight horizontal tubes stacked in vertical fence-like arrays (Figure 2-7). The facility has a total volume of 700 cubic meters (m3), occupies a total land area of 10,000 m2, and produces 35-41 grams dry weight per m2 per day or 120-140 dry tonnes per year. Algae wall adhesion, biofouling, large pressure drop, and gradients in pH, dissolved oxygen, or CO2 can occur along the tube length. These factors are potential disadvantages of tubular photobioreactors (Chen et al., 2009), which might be resolved by innovative engineering designs.
Flat-plate (or flat-panel) photobioreactors are transparent rectangular containers (usually vertical or inclined) with a light path of 1-30 centimeters (Figure 2-8). Flat-plate photobioreactors mix substrate by vigorous air sparging from the bottom.
Productivities of algal biomass in photobioreactors vary with the type of geometric configuration used and the algal species grown (Table 2-3). Many novel production systems have been designed and currently are being developed and tested. The new production systems aim to lower construction and maintenance costs close to those of open-pond systems and maintain the high, stable productivity and reduced contamination risk of closed photobioreactors. These systems include the Solix, ACCORDION, Algenol, and the National Aeronautics and Space Administration’s (NASA) Offshore Membrane Enclosure for Growing Algae (OMEGA), and Photon8’s traveling wave system.
The Solix photobioreactor is an elongated (low height-to-length ratio) flat-panel photobioreactor made of plastics. It is designed to bridge the gap from flask to open raceway pond by serving as a controlled-environment test bed or as an algae inoculum scale-up device (Figure 2-9; Solix Biofuels, 2011). The Solix photobioreactor allows for open-pond deployment by using the water as a thermal regulator for open-air field applications. Air sparging for aeration and mixing occurs along the full length of each panel.
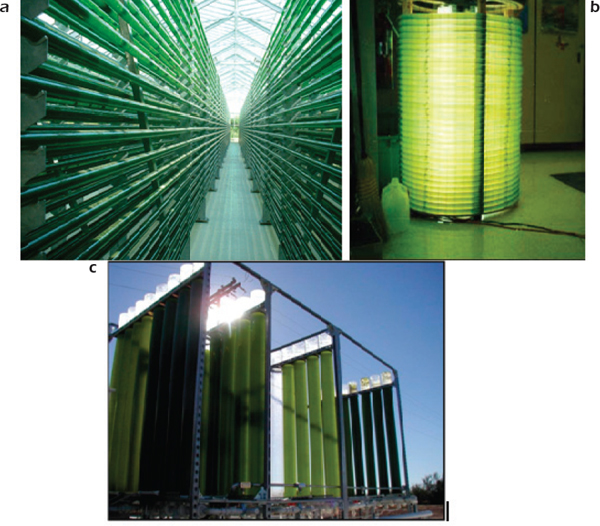
FIGURE 2-5 Tubular photobioreactors.
SOURCES: Clockwise from top left:
(a) California Polytechnic State University;
(b) Kennedy et al. (1995); and
(c) NanoVoltaix (2012). Reprinted with permission from Qiang Hu and Arizona State University/NanoVoltaix.
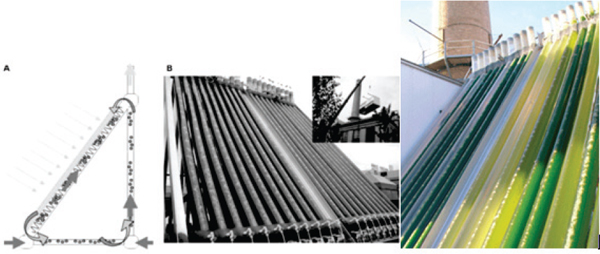
FIGURE 2-6 Triangular external air-lift tubular photobioreactors.
SOURCES: Vunjak-Novakovic et al. (2005). Reprinted with permission from American Chemical Society.

FIGURE 2-7 Tubular photobioreactors of straight horizontal tubes stacked in vertical fence-like arrays housed in a greenhouse in Klotze, Germany.
SOURCE: Algomed. Reprinted with permission from AGU.
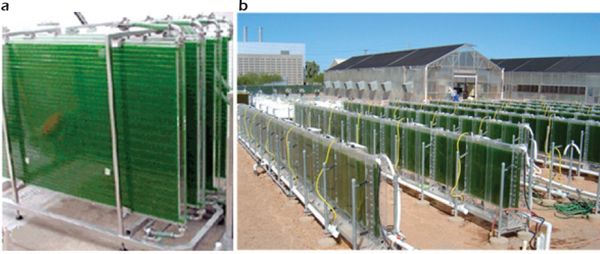
FIGURE 2-8 Flat-plate photobioreactors.
SOURCES: (a) Algae Energy (2012b);
(b) NanoVoltaix (2012). Reprinted with permission from Qiang Hu and Arizona State University/NanoVoltaix.
TABLE 2-3 Microalgae Productivities in Photobioreactors
|
|
||||
| Photobioreactor | Volume (L) | Microalgal Species | Productivity (g DW/L/d) |
Reference |
|
|
||||
|
Airlift tubular |
200 |
Porphyridium cruentum |
1.50 |
Camacho Rubio et al. (1999) |
|
Airlift tubular |
200 |
Phaeodactylon tricornutum |
1.20 |
Acién Fernández et al. (2001) |
|
Airlift tubular |
200 |
Phaeodactylon tricornutum |
1.90 |
Molina et al. (2001) |
|
Inclined tubular |
6.0 |
Chlorella sorokiniana |
1.47 |
Ugwu et al. (2002) |
|
Undular row tubular |
11 |
Arthrospira platensis |
2.70 |
Carlozzi (2003) |
|
Helical tubular |
75 |
Phaeodactylon tricornutum |
1.40 |
Hall et al. (2003) |
|
Parallel tubular |
25,000 |
Haematococcus pluvialis |
0.05 |
Olaizola (2000) |
|
Bubble column |
55 |
Haematococcus pluvialis |
0.06 |
López et al. (2006) |
|
Flat plate |
440 |
Nannochloropsis spp. |
0.27 |
Cheng-Wu et al. (2001) |
|
Flat plate |
100 |
Nannochloropsis spp. |
0.30 |
Rodolfi et al. (2009) |
|
ACCORDION |
60 |
Monodus subterraneous |
0.40 |
Cuello and Ley (2011) |
|
|
||||
SOURCE: Adapted from Ugwu et al. (2007). Reprinted with permission from Elsevier.

FIGURE 2-9 Solix photobioreactor.
SOURCE: Reprinted with permission from Solix Biosystems.
NASA’s OMEGA is a flat-panel photobioreactor made of plastic. Inserts of forwardosmosis membranes allow the exit flow of oxygen and water while the photobioreactor is laid down horizontally on a water surface (Figure 2-10; Trent, 2011). OMEGA is designed for deployment on the surface of bodies of saline water (for example, sea and ocean) where it exploits wave movements for mixing culture and regulating temperature. This photobioreactor currently is undergoing redesign to overcome technical challenges; the final design likely will be more complex than its original design and could include some significant deviations (Trent, 2011).
The ACCORDION photobioreactor is a vertical series of flat plastic panels through which the algal suspension is grown in batch or grown continuously recirculated in batch, semicontinuous, or continuous modes (Figure 2-11; Cuello and Ley, 2011). The adjustable alternating vertical and angled flat plates, or alternating angled flat plates, are designed to improve the light incidence on surfaces and enhance the mixing and flow patterns inside the plates. For example, a treatment for a 60-liter ACCORDION photobioreactor with 45° plate angle and liquid flow rate of 14 liters per minute resulted in algal productivity of 0.30 grams of dry weight per day that was statistically indistinguishable from that of a 1-liter shake-flask control. The ACCORDION photobioreactor is a modular design that can be scaled up by adding modules. This is equivalent to open raceway ponds achieving scale up by adding raceway units. The ACCORDION photobioreactor currently is undergoing further design and structural optimization (Cuello and Ley, 2011).
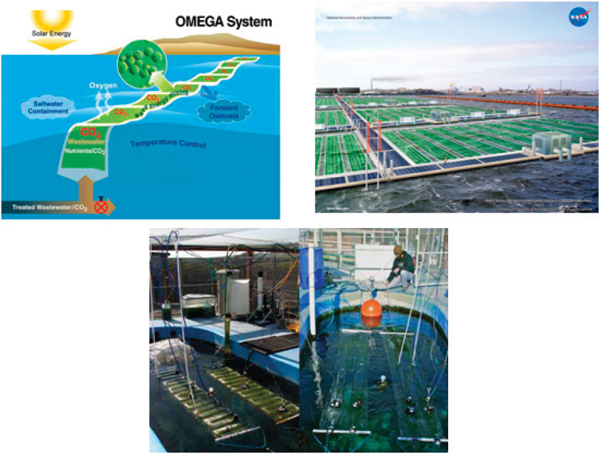
FIGURE 2-10 NASA’s OMEGA photobioreactor.
SOURCE: Trent (2011).
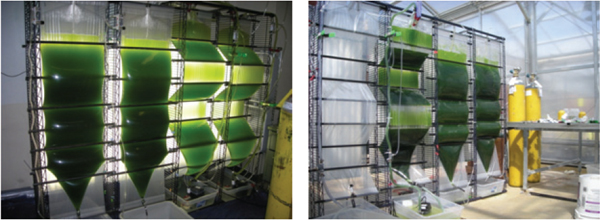
FIGURE 2-11 ACCORDION photobioreactor.
SOURCE: Cuello et al. (2011).
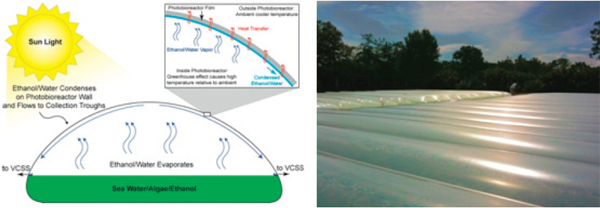
FIGURE 2-12 Algenol photobioreactor.
SOURCES: Chance et al. (2011b); Algenol Biofuels (2012). Reprinted with permission from Ron Chance, Algenol Biofuels.
The Algenol photobioreactor is a plastic, horizontal, half-cylinder vessel that uses a hydrofoil that moves back and forth along the longitudinal axis of the photobioreactor to mix substrate (Figure 2-12). The photobioreactor, which is designed for direct ethanol production, is used to culture enhanced cyanobacteria that excrete ethanol into an aqueous medium. The ethanol-water mixture evaporates to form liquid condensate on the photobioreactor’s concave ceiling and flows down both the sides of its internal walls where plastic sleeves catch the ethanol-water condensate and convey it to a collection port at one end of the photobioreactor (Chance et al., 2011b).
2.2.4 Comparison of Open Systems and Closed Systems
Table 2-4 compares open-pond systems and closed photobioreactor systems for photoautotrophic microalgae production. Although outside the scope of this report, the low costs of construction and maintenance constitute one of the biggest advantages of open-pond
TABLE 2-4 Comparison of Open and Closed Algal Cultivation Systems
|
|
|||
| Parameter | Open System | Closed System | Reference |
|
|
|||
|
Cost |
Lower |
Higher |
Shen et al. (2009) |
|
Pumping energy |
Lower |
Higher |
Becker (1994); Shen et al. (2009) |
|
Ease of scale up |
Greater |
Lower |
Shen et al. (2009) |
|
Evaporative water loss |
up to 10 L/m2/day |
Negligible where wind cooling is sufficient; 1-2 L/m2/day when water-spray cooling is used; or similar to open systems if photobioreactors are submerged in ponds for cooling |
Becker (1994) |
|
Land area required |
Higher |
Lower |
|
|
Contamination risks |
Higher |
Lower |
Borowitzka (1999); |
|
Productivity |
Lower |
Higher |
See Tables 2-1 and 2-2 |
|
Productivity stability |
More variable |
Less variable |
Shen et al. (2009) |
|
Sparged CO2 loss |
Higher |
Lower |
Becker (1994); Shen et al. (2009) |
|
|
|||
systems compared to photobioreactors. The conventional view was that the use of openpond cultivation is more likely to achieve the goal of technoeconomic feasibility for producing microalgae for biofuels than the use of photobioreactors. Because of their lower capital costs and simpler designs, open-pond systems are easier to scale up to increase production than photobioreactors. Most photobioreactor configurations are scaled up by multiplying units and by increasing the unit volume. Increasing unit volume of photobioreactors requires adjustments of physical variables to achieve appropriate flow dynamics within the new unit volume. Disadvantages of open-pond systems include losses of water to evaporation, risk of contamination by competing microorganisms, loss of algal biomass due to weather, and loss of introduced CO2. (See Chapter 4 for details on evaporative water loss.)
Advantages of photobioreactors include significantly higher microalgal biomass productivity and greater production stability over time than open-pond systems. For example, the volumetric productivity of Nannochloropsis spp. in photobioreactors could exceed that in open raceways by as much as 16 times (Table 2-4). The risk of biological contamination is much greater in open-pond systems than in closed photobioreactor systems. With the exception of Spirulina and Dunaliella salinas, which are cultivated in open systems under highly selective growing conditions, the lack of competitive advantages of many of the microalgal species being tested for biofuel production in open ponds and their susceptibility to culture crashes are concerns. Thus, the low volumetric productivity and susceptibility to contamination could constitute a substantial risk to the economic sustainability of openpond cultivation systems compared to closed photobioreactor systems.
The utilization efficiencies of some vital input resources in terms of production per unit input—particularly for water and land—are in general lower in open cultivation systems than in closed photobioreactors (Table 2-5; Davis et al., 2011). As noted above, evaporative water loss is of particular concern in open-pond systems. These losses could be as much as 10 liters per m2 per day. Thus, a one hectare open pond could lose 100,000 L of water per day or 36,500,000 liters of water per year. When the cooling of photobioreactors is achieved through water-spray cooling or through submergence in open ponds, the evaporative water loss associated with photobioreactors also can be substantial and as much as in open systems. Table 2-5 further compares the land area requirement, energy consumption, net energy ratio, and other criteria for cultivating Nannochloropsis spp. in an open raceway, flat-plate photobioreactor, and tubular photobioreactor to produce 100,000 kg dry weight (DW) of algal biomass per year (Jorquera et al., 2010). The land area required for the open raceway exceeded that of the tubular photobioreactor by 241 percent and that of the flatplate photobioreactor by 256 percent. The total energy consumption for the open raceway, flat-plate photobioreactor, and tubular photobioreactor were 3.72 watt per cubic meter (W/m3), 53 W/m3, and 2,500 W/m3, respectively. The resulting net energy ratios for oil production, defined as the total energy produced divided by the total energy requirement, were 3.05, 1.65, and 0.07 for the open raceway pond, flat-plate photobioreactor, and tubular photobioreactor, respectively. While the tubular photobioreactor had a net energy ratio of less than 1, and thus consumed more energy than it produced, the net energy ratios for flatplate photobioreactors and open raceway ponds were both greater than 1. Therefore, the favorable energy balance might persist through mass cultivation of Nannochloropsis using either of these methods. However, the 2010 study by Jorquera et al. did not consider the harvest costs and the cost of oil extraction that add significantly to energy consumption. A thorough discussion of life-cycle assessment (LCA) of energy balance for algal biofuels is in Chapter 4. The Jorquera study was part of a meta-analysis that reanalyzed published data to provide an estimate of the energy requirement for fuel production (Liu et al., 2012). The true energy return may not fully be known until full-scale commercial production has been
TABLE 2-5 Comparison of Raceway Ponds, Flat-plate, and Tubular Photobioreactors in Cultivating Nannochloropsis spp. to Produce 100,000 kg DW Per Year
|
|
|||
| Variable | Raceway Ponds | Flat-Plate Photobioreactors | Tubular Photobioreactors |
|
|
|||
|
Annual biomass production (kg/year) |
100,000 |
100,000 |
100,000 |
|
Volumetric productivity (g/L per day) |
0.035 |
0.27 |
0.56 |
|
Illuminated areala productivity (kg/m2 per day) |
0.011 |
0.014 |
0.0081 |
|
Occupied arealb productivity (kg/m2 per day) |
0.011 |
0.027 |
0.025 |
|
Occupied arealb productivity (t/ha per year) |
39 |
99 |
93 |
|
Illuminated areala volume (per m2) |
300 |
50 |
14 |
|
Illuminated areaa/volume ratio (per m) |
3.3 |
19 |
69 |
|
Occupied areab/volume ratio (per m) |
2.3 |
10 |
22 |
|
Biomass concentration (g/L) |
0.35 |
2.7 |
1.02 |
|
Dilution rate (per day) |
0.1 |
0.1 |
0.1 |
|
Area required for biomass production of |
26,000 |
11,000 |
11,000 |
|
100,000 kg/yr (m2) |
|||
|
Reactor volume required for biomass production of |
7,800 |
1,000 |
490 |
|
100,000 kg/yr (m3) |
|||
|
Flow rate required to maintain a 0.1 /day dilution |
780 |
100 |
49 |
|
rate (m3/day) |
|||
|
Hydraulic retention time (volume/flow rate) |
10 |
10 |
10 |
|
Relative oil content (%) |
30 |
30 |
30 |
|
Net oil yield (m3/year) |
33 |
33 |
33 |
|
Oil yield per area (m3/ha per year) |
13 |
32 |
31 |
|
Energy consumption (W/m3) |
3.7 |
53 |
2,500 |
|
Energy consumption required for accumulation of |
29,000 |
54,000 |
1,200,000 |
|
100,000 kg/year biomass (W) |
|||
|
Total energy consumption (kWh/month) |
8,700 |
16,000 |
370,000 |
|
Total energy consumption (GJ/year) |
378 |
700 |
16,000 |
|
Energy produced as oil (GJ/year) |
1,200 |
1,200 |
1,200 |
|
Total energy content in 100,000 kg biomass (GJ/year) |
3,200 |
3,200 |
3,200 |
|
NER for oil production |
3.1 |
1.7 |
0.07 |
|
NER for biomass production |
8.3 |
4.5 |
0.20 |
|
|
|||
aIlluminated area refers to the surface area of a raceway pond or photobioreactor subject to illumination.
bOccupied area refers to the land area occupied by the raceway pond or photobioreactor.
NOTE: Net Energy Ratio (NER) = total energy produced/total energy requirement.
SOURCE: Adapted from Jorquera et al. (2010). Reprinted with permission from Elsevier.
realized. Even then, uncertainty in the estimates of energy return might remain, as in the case of corn-grain ethanol (Hall et al., 2011). Where biofuel feedstocks consist of genetically modified organisms or other organisms of potential societal concern (for example, organisms that have been invasive in one or more environments), photobioreactors may be more acceptable to some communities or individuals.
In summary, open-ponds and closed photobioreactors each offer distinct advantages and disadvantages. Open-pond systems allow for larger scale units at lower capital investments, lower operating costs, and lower energy demands than closed photobioreactors. However, the open nature of such ponds makes them vulnerable to the natural elements, including loss of water through evaporation and invasion of undesirable species. Closed systems offer some protection of the cultivated algae from the natural elements. Because they have pipes and tubes, closed photobioreactors are more expensive to construct and require more energy to operate than open-pond systems. But closed photobioreactors can improve the sun exposure and take advantage of specialized species, thereby improving
productivity. It is premature to draw conclusions as to which system is preferable at this nascent state of the development of algal biofuels. Other aspects of sustainability (for example, economics) would have to be considered in selecting the cultivation systems for algal biofuel production.
2.2.5 Design Considerations for Algal Cultivation Systems
2.2.5.1 Supplemental Carbon Dioxide
Because supplemental gaseous CO2 significantly enhances algal biomass growth rates, supplementation is recognized as a universal practice in mass cultivation (Ono and Cuello, 2004a,b, 2006; Williams and Laurens, 2010). Yue and Chen (2005) reported, for instance, that the maximum growth rate for Chlorella ZY-1 strain was achieved when an air flow enriched with 10 percent (v/v) CO2 was used (Figures 2-13 and 2-14). The linear growth rate under that condition was about 1.17 g dry weight per liter per-day (Figure 2-14), and the cell concentration reached 5.77 g dry weight per liter after 6 days of cultivation. Growth rates and cell concentrations were higher in cultures grown with 5, 20, 30, and 60 percent CO2 with all CO2 treatments delivered at a flow rate of 2.5 L/min than without CO2 supplementation (Figure 2-13).
Supplemental carbon sources for algal cultures include bicarbonate dissolved in water or CO2 gas. If carbon is supplied as CO2 gas in open ponds, then optimizing the size of gas bubbles is critical to ensure that the CO2 remains in the water and is taken up by algae. Open-pond cultivation has used sintered porous stones or PVC pipes with a line of fine holes on the upper part. In shallow ponds, however, these methods result in significant losses of CO2 to the atmosphere because the aqueous algal suspension retains the gas bubbles only for a short time so that CO2 cannot be absorbed completely unless countercurrent carbonation sumps are used. Achieving a utilization rate of more than 10 percent of the supplied CO2 under working conditions has been difficult (Becker, 1994). In addition, mounting pipes or tubes at the bottom of ponds is technically challenging, and porous
FIGURE 2-13 Variations in growth of Chlorella ZY-1 strain with CO2 concentrations (v/v).
SOURCE: Yue and Chen (2005). Reprinted with permission from Elsevier.
FIGURE 2-14 Variations in growth rate of Chlorella ZY-1 strain with CO2 concentrations (v/v).
SOURCE: Yue and Chen (2005). Reprinted with permission from Elsevier.
materials used for gas distribution tend to get clogged by algae and other debris and require regular cleaning (Becker, 1994). Weissman et al. (1988) demonstrated that the rate of CO2 outgassing from an open raceway varied and could be reduced, based on pH, alkalinity, and ionic strength. An alternative is the use of a floating CO2 injector, such as one developed in Peru (Vasquez and Heussler, 1985). The device consists of a floating compartment with a hollow enclosure (preferably made from sealed PVC pipes and covered with transparent sheeting). Flotation is achieved because of the gas cushion under the cover. Through a ballvalve system, the compartment is filled with pure CO2. When the device emerges through its buoyancy, the valve is shut, and vice versa. The resulting CO2 loss is as low as 4 percent of total CO2 supply in some cases (Becker, 1994).
The amount of CO2 offgassing from photobioreactors depends on the specific type of photobioreactor and how it is operated. Flat-plate photobioreactors, for instance, generally have much shorter gas paths than tubular photobioreactors (Sierra et al., 2008). Unlike in open raceways, CO2 offgassing from photobioreactors could be minimized by recycling the effluent gas stream. If the recycled gas stream happens to have a high oxygen concentration, the challenge of stripping the oxygen to prevent inhibition of culture growth needs to be addressed.
2.2.5.2 Contamination and Stability of Culture
If the product yield (for example, algal oil or ethanol) depends on the growth of a particular cultivated algal species, then growing and maintaining that monoculture is critical. Citing Moheimani and Borowitzka (2006), Stephens et al. (2010) noted that some open-pond systems could be run over 6 months without significant levels of contamination. Nonetheless, maintaining dominance and high biomass productivity of the preferred cultivated species in open and non-aseptic algal monocultures remains one of the most formidable challenges in open-pond mass cultivation. Like monocultures of terrestrial crops, large algal monocultures tend to be invaded by undesirable pests and pathogens, and crop protection is a major challenge to algal pond sustainability (Hannon et al., 2010). The principal types
of contaminants in algal cultures include other algal species, bacteria, zooplankton, fungi, insects, and viruses. The types and populations of contaminants in algal cultures depend on the local environmental conditions, the algal species cultivated, and the specific cultivation system in use (Baptist et al., 1993; Becker, 1994; Mahan et al., 2005).
Contamination of open-pond algal cultures by other algal species is unavoidable because the growth conditions inevitably are suitable for the cultivated algal species and other local species. Even in the case of the cyanobacterium Spirulina, which is cultivated at highly selective growing conditions of high alkalinity and pH, contamination by another cyanobacterium Oscillatoria or by algae has been reported at suboptimal growth conditions such as at bicarbonate concentrations below 15 grams per liter (Becker, 1994). In extreme cases, an invading algal species could become dominant and overgrow the intended species. In cultures of Scenedesmus spp., the common contaminants include Chlorella spp., Selenastrum spp., and some species of diatoms (Becker, 1994). In Japan, it has been reported that maintaining Chlorella cultures required frequent start-up of the culture with uncontaminated inoculum (Becker, 1994). While it is impossible to keep other algal species away from an open-pond system, strategies can be adopted to keep the contamination at acceptable levels, including using high concentrations of the inoculum, periodic cleaning of the cultivation system, and implementing a specific nutrient or a combination of environmental conditions that favor the desired species (Becker, 1994).
Algal cultures contaminated by Monas spp. or other species of protozoans often are totally decimated within 12 to 18 hours after the corruption is first detected (Baptist et al., 1993). The fungi chytrids have been detected in several algal cultures and often occur as epidemics, which sometimes result in the complete loss of cultures. The fungus Chytridium spp. is the most dangerous fungus for cultures of Chlorophyceae, and often appears together with the zooflagellate Aphelidium spp. Infections of Scenedesmus cultures by these organisms have been detected practically worldwide. Such infection is characterized by heavy flocculation of the algal suspension, a brown color of the culture medium, and decreased oxygen evolution (Becker, 1994). By the time these symptoms appear in the algal culture, it is already too late to control the parasite. The only biological control for Aphelidium infection in an early stage is a many-fold dilution of the culture with fresh medium, enabling the algal population to remain in an exponential growth phase so that its multiplication rate exceeds that of the parasite. Infection of Scenedesmus spp. cultures with Chytridium spp. have been treated successfully in Israel by applying the fungicide Benomyl (methyl 1-butylcarbomoyl benzimidazolecarbamate) at a dosage of 1 milligram per liter (mg/L) (Becker, 1994). Fungal contamination of C. reinhardtii can be controlled using the fungicides carbendazim (1 mg/L), thiophanate-methyl (20 mg/L), and benomyl (20 mg/L) (Mahan et al., 2005). A combination of carbendazim and the antibiotics ampicillin and cefotaxime also has been shown to remove or reduce contamination of C. reinhardtii by three different bacteria and two different fungi tested (Kan and Pan, 2010).
There have been occasional reports of contamination of algal cultures by the zooplankton Lycrymanis spp., Colpidium spp., and Vorticella spp., though these organisms had negligible effects on algal growth (Becker, 1994). Contamination by a group of rotifers called Branchionus, however, may impede the growth of algal cells and in extreme cases could spoil the entire algal culture. The most effective control has been to lower the pH of the culture to about 3.0 by adding acid and keeping the culture at that pH for 1 to 2 hours before the pH is readjusted back to 7.5 with potassium hydroxide (KOH). The treatment effectively eliminates the rotifers without deleterious effects on the algal cultures (Becker, 1994).
2.2.5.3 Open-Pond Operations
Open-pond facilities for the large-scale production of algal biofuels likely will need to be managed as complex, bioengineered systems by applying known principles of population, community, and ecosystem ecology (cf. Graham and Smith, 2004; Smith et al., 2010a). Numerous configurations for open-pond systems can be envisioned, two of which are discussed below.
2.2.5.3.1 Single species (target strain inoculum) hybrid system
One hybrid facility design for algae cultivation involves the front-end use of a photobioreactor that is later linked to open ponds. The upstream photobioreactor would be a breeder or feeder system that provides an influx of high-density, target algae for production and harvest in downstream open-raceway systems (DOE, 2010). However, several key ecological factors potentially may complicate the stable long-term use of single-strain hybrid or single-strain all open-pond systems.
First, nominally single-strain hybrid systems would be subjected constantly to potential invasions by microflora and microfauna present in the local landscape. For example, resting stages or live individuals from taxonomically diverse groups of cyanobacteria and algae can be deposited onto the surface of the open ponds, either via the direct deposition of atmospheric particulates, or in association with rain and snowfall (for example, Brown et al., 1964). In addition, open ponds will be invaded by a diverse community of aquatic consumers, including rotifers, ciliates, insect larvae, and crustacean zooplankton. These animal invaders can be transmitted primarily via insects, migratory waterfowl, and other regionally mobile animals (for example, Frisch et al., 2007) and by wind and rainfall (Jenkins and Underwood, 1998). The taxonomic identities and total number of species that ultimately become resident in these ponds depend on the size and composition of regional species pools (Chase, 2003; Ptacnik et al., 2010), allelopathic interactions with the desired species, competition for available resources, and the productivity and surface area of the pond system (Hoffmann and Dodson, 2005; Smith et al., 2005). Some species of herbivorous invaders are highly undesirable because their unrestricted growth can strongly suppress algal growth (see the predator-prey dynamics discussion later in this chapter).
Second, the primary goal of the upstream photobioreactor is to provide a constant supply of the target algal strain. However, once invasions by cyanobacteria and algae occur in the downstream open pond, the target algal strain may not continue to dominate the algal community. This problem could exist whether the target strain is genetically modified or naturally occurring. Such invasions could reduce overall biomass yield and require costly interruptions of biomass production for system closure, cleaning, and strain re-establishment.
In the most desired case, the target strain would be highly competitive and would continuously remain the dominant primary producer in the pond while the hybrid cultivation system is in operation. However, strong temporal dynamics in species composition are observed in most polycultures. Individual species tend to exhibit major changes in relative abundance as conditions change over ecological time. Occasionally some species increase from nearly undetectable abundance to strong numerical dominance during a period of only weeks (for example, Reynolds, 1997). Such strong species dynamics are undesirable because the oscillation of target strain abundance could create strong instability in algal biofuel production rates via changes in algal biomass, lipid content and molecular composition, lipid extractability, and lipid harvestability. Zmora and Richmond (2003) summarized the production of Nannochloropsis for rotifers and as a direct aquaculture feed. They
reported that the highest lipid content in Nannochloropsis was observed in the summer, but the highest eicosapentaenoic acid level was observed in winter. Daily harvesting of 25 to 30 percent of the culture volume during the summer yielded the most biomass. Contaminants were controlled by pH shifts and use of low levels of chlorine.
2.2.5.3.2 Mixed species (natural plankton community inoculum) systems
Another facility design for algae cultivation uses open-pond systems that are inoculated with planktonic assemblages obtained from natural water bodies in the nearby landscape. Such inocula contain a representative subset of the indigenous species pool of microflora and microfauna. This species pool will include aquatic viruses, bacteria, and fungi; cyanobacteria and eukaryotic microalgae and macroalgae; and aquatic consumers such as rotifers, ciliates, insect larvae, and crustacean zooplankton. Just as in the hybrid ponds discussed earlier, mixed-species algal production systems potentially would be subjected to daily invasions by species of microflora and microfauna that might not have been in the original starting inoculum.
A mixed species assemblage provides potential advantages of improved biomass yield and increased culture stability. The agricultural literature has long demonstrated that the joint cultivation of multiple plant species (polyculture) typically provides a greater total biomass yield than a single crop. This phenomenon is known as “overyielding” (Bessler et al., 2009). Overyielding of biomass has been observed in algal assemblages (Weis et al., 2008), leading Smith et al. (2010b) to hypothesize that mixed-species cultures could produce higher yields of algal biomass and lipids than single-species algal cultures. Stockenreiter et al. (2012) tested this hypothesis in both natural and laboratory microalgal communities and found higher lipid production in diverse algal communities relative to algal monocultures grown under the same resource supply conditions. This study supports the suggestion that naturally occurring, multispecies, microalgal communities grown in open ponds potentially could store more solar energy than single species communities cultivated in closed photobioreactors (Smith et al., 2010b). Incorporating the ecological advantages of diversity-related, resource-use dynamics into algal biomass production might provide a cost-effective way to improve yield and the robustness of algae cultivation for biofuel production (Stockenreiter et al., 2012).
Another key ecological interaction that applies to commercial-scale algae production in open ponds is the predator-prey dynamic. Similar to their natural analogues, artificially constructed open-pond systems will develop diverse biological communities. In particular, because they will contain primary producers (algae) and primary consumers (herbivorous zooplankton), these pond communities will tend to exhibit predator-prey population oscillations similar to insect outbreaks that damage crop yields in terrestrial agriculture. In particular, unrestrained growth of large herbivorous zooplankton, such as Daphnia pulex or Daphnia magna, is analogous to placing an excess of grazing animals in a field. They can over-graze algal cells and result in order-of-magnitude reductions in algal biomass yields. Selective grazing of desirable species coupled with nutrient release from grazers can alter the composition of the resident algae to increased levels of undesirable forms. From a crop protection point of view, this is a highly undesirable outcome. However, aquatic food webs can be altered to lessen losses of algal biomass via top-down control (Carpenter and Kitchell, 1988). For example, the carnivorous mosquitofish (Gambusia affinis) can be added to pond production systems to remove large zooplankton and help maximize the pond-grown algal biomass for biofuel production (Smith et al., 2010b). The mixed community could contain variable nutritional value as taxonomic composition changes. Changes in composition likely would alter the lipid content and the potential quality of algal biomass for making fuels.
2.3 PROCESSING ALGAL BIOMASS INTO FUELS
Fuel production from algal biomass is most commonly assumed to involve cultivation of microalgal species that have high lipid productivity and the processing of the lipid to biodiesel. In this case, production of biofuel requires the algae to be concentrated and subsequently treated to cause the release of the intracellular lipids. The concentration, or harvest, step involves the separation and typically drying of the algal cells to prepare them for lipid collection. Lipid collection usually is accomplished by rupturing the algal cells. Subsequent extraction of the biomass might be required for economical oil recovery. Thus, biodiesel production from algae requires two distinct separation steps—harvest and product collection—regardless of whether growth occurs in open or closed photobioreactors.
The important feature in harvest and extraction is that the algae and the lipids are insoluble in water. The technical problem in the production of biodiesel is simply producing a pure, dry triacylglycerol stream for subsequent processing to biofuels. Because the algal biomass and the algal oils are immiscible in water, harvest can be completely spontaneous, and there is no key thermodynamic separation energy to be overcome. The constraints on the system are purely engineering-related, and better engineering can reduce the energy expenditure required for separating the algal biomass from the culture water and drying it for subsequent oil collection. Relatively low algal biomass concentrations and the small size of microalgae make separation challenging and energy intensive. A meta-analysis of published studies shows that more than 40 percent of the total energy required for biodiesel production can be attributed to harvest and product collection (Clarens et al., 2010). (See Chapter 4 for details on energy use.)
Purity of the algal lipid is an important parameter for processing into liquid transportation fuel. Inorganic materials that stay with the oil are a concern, and the method of harvest and collection can influence the impurity levels. Inorganic salts and phospholipids are two known impurities that could affect processing. Inorganic salts are in the culture medium and occur naturally in algae, but they also can be introduced as flocculants.
2.3.1 Harvesting and Dewatering Methods
Microalgal cultures are about neutrally buoyant suspensions of microscopic particles. As noted earlier in this chapter (see Tables 2-1, 2-2, and 2-4), algal cell biomass is most commonly reported to be up to about 0.4 grams per liter in open ponds and 3 g/L in photobioreactors, though concentrations up to 40 grams per liter have been reported (Brennan and Owende, 2010). These concentrations require that almost a liter of water be removed from the algae to produce a few grams of dry biomass. The pumping and processing of water are energy intensive, and reducing the energy required to collect the algae directly affects the sustainability of microalgae cultivation.
Microalgae are grown as insoluble particles in an aqueous medium. Furthermore, the lipids present in the algae are similarly immiscible in water. In principle, the separation of algal oils from the aqueous growth media can be spontaneous and require little energy. In practice, the separation of algae from the growth media and the separation of lipids from algal biomass in a timely manner is energy intensive. Reducing the energy required can be accomplished through improvements in the algal strains, through engineering improvements, and through favorable interplay of the two. As an example, improvements to algae that increase the density of cells in the culture, in principle, reduce the amount of water that has to be eliminated during recovery. Reducing the water processed would, all other things being equal, reduce the energy expended during algae collection.
Methods for harvest vary greatly depending on whether macroalgae or microalgae are grown. Though the focus of this report is on microalgae, macroalgae are harvested mechanically with relatively low-energy input today (Roesijadi et al., 2010). Harvesting macroalgae is a more than 400-year-old industry (McHugh, 2003) with innovations still being proposed to improve efficiency (Garthwaite, 2012; OneWater, 2012). For microalgae, the methods used for harvest rely on size exclusion or separation based on density (Table 2-6). These include filtration, centrifugation, sedimentation, flotation, flocculation (coagulation), and electrophoresis techniques (Uduman et al., 2010). Flocculation and gravity sedimentation are similar. Natural density separation can be sped up by adding agents that cause the microalgae to aggregate. Triggers for aggregation include changes in pH or other chemical triggers. Algae either may settle or will float to the surface of the liquid. The separation is a relatively low-energy decanting that produces a majority water phase and an algal phase. Inorganic or organic (synthetic) flocculants both are used, with the nature and disposition of these materials being the sustainability concerns.
Use of inorganic flocculants, such as ferric chloride and alum, pose environmental and processing concerns. Flocculants travel through the process with the algal biomass and would have to be accounted for in the process. High concentrations of metals present in residual algal biomass would limit its use as coproducts because of safety concerns. Organic flocculants may be susceptible to anaerobic digestion, removing them from the recycle stream. The presence of flocculants may affect the suitable uses for the algal biomass as coproducts.
Centrifugation and filtration can be used alone or in concert with a preliminary densitydriven separation. Centrifugation rapidly concentrates organisms but requires high capital and operating costs. Filtration may be inefficient because the microscopic algal cells tend to clog the filter. Centrifugation and filtration are receiving considerable focus, and innovations are being reported (for example, Heaven et al., 2011; Milledge, 2011; Bhave et al., 2012). Other methods for harvest, such as acoustic manipulation of algal cells or electrophoresis techniques including electrolytic coagulation and electrolytic flocculation, have been reviewed, but their harvest rate and reliability vary (Sukenik and Shelef, 1984; Chen et al., 2009; Uduman et al., 2010; Vandamme et al., 2011; Leckey and Hinders, 2012). As noted earlier in this chapter, there also have been efforts to develop genetically engineered algae incorporating magnetic nanoparticles to reduce energy costs for harvesting and dewatering.
TABLE 2-6 Characteristics of Microalgae Harvesting Techniques.
|
|
||||
| Harvest Methods | Suspended Solids Concentration (%) |
Operating Costs per Gallon of Water |
Cell Harvesting Efficiency |
Algal Species |
|
|
||||
|
Centrifuging |
High (< 22%) |
Very high ($20 to $50) |
> 90% |
Almost all except the very fragile |
|
Filtration/screening |
Medium to high |
Medium to high |
20% to 90% |
Algae with large |
|
Flocculation |
Low to medium |
Low to medium |
50% to 90% |
Algae with low density |
|
Bioflocculation |
Low to medium |
Low |
About 90% |
|
|
Sedimentation/settling |
Low |
Low to medium |
10% to 90% |
Algae with high density |
|
|
||||
SOURCE: Adapted from Shen et al., 2009. Reprinted with permission from the American Society of Agricultural and Biological Engineers.
2.3.2 Extraction
Once the algal biomass has been harvested, oil needs to be extracted if lipids are the desired primary product. For processing methods that use whole cells, harvest might be all that is required for the next stage of fuel production. Biodiesel production is a technology that in most variants requires collection of the algal lipids for post processing. Extraction of oil from algal biomass has proven to be difficult. Unlike oil recovery from oilseed plants, there is no well-defined, commercial lipid extraction process for algae on the market. As in the case of oil recovery from seeds, extraction with organic solvents has been tested with algal systems. There are two differences between oil extraction from seeds and algae. First, simple milling of oilseeds creates an extractable meal. Second, the oilseed meal is about 90 percent solids. In contrast, algal biomass is high in water content, and cell membranes are not easily ruptured to yield readily extractable oil.
High levels of oil expression have been reported for algae grown heterotrophically. Levels reported up to 80 percent are said to be so high that only cell lysing is required for collection, avoiding extraction (Dillon, 2011). There are no reports indicating that photoautotrophic cultivation has attained such high oil fractions.
Oil extraction can be done with dried algae or with the wet paste from harvest. Drying is energy intensive, but yields a material that can be mechanically treated to open up access for oil extraction (Viswanathan et al., 2011). Once dried, oils are extracted. Characteristics of a desirable solvent include high solvent power, low toxicity, low specific heat, low heat of vaporization, low cost, high availability, and preferably nonflammable. Examples of solvents include hexane (most frequently cited as the solvent used for algal-oil extraction), chloroform, methanol, ethanol, butanol, ethyl acetate, and petroleum ether. The solvent is boiled away from the algal oil, recovered, and reused. Solvent recovery is high but is not 100 percent. A 0.5 to 5 percent loss can be assumed. The large volume of solvents and solvent vapors in the process can represent a fire and explosion risk.
Solvents used for wet extraction usually are immiscible with water to save on energy use in solvent recovery. The biomass generally is processed as whole cells because mechanical membrane rupture is difficult in the wet pastes. The solution of algal lipid in solvent is recovered for purification. The solvent is boiled away from the algal oil, as in oilseed processing.
2.3.3 Combined Harvest and Collection
Several methods that seek to use electric fields, heating, or other means to free oil from algae without having to harvest the algae are being developed. Origin Oil, Open Algae, and Diversified Technologies are a few of the companies that seek to substantially reduce the energy requirement of harvest and oil collection. The premise of those methods is to supply energy to an algal culture to rupture (lyse) the cells. Lipids in the cells then spontaneously separate from the biomass, rising to the surface while the biomass sinks. The anticipated result is a solid sediment, an aqueous layer, and a free oil layer so that simple, cost-effective, and energy-efficient gravity separation recovers the oil. These systems offer the promise of substantial reduction in energy use and the elimination of solvent use. A new extraction-harvest technique, microwave-assisted drying, uses microwaves to excite liquid within algal cells, causing the cell wall to rupture and release lipids. Solvents then are used to extract the lipids. Microwave-assisted drying can save time because algal biomass does not have to be dehydrated before the lipid extraction. Whether microwaving would diminish the quality of the lipids or the practicality of scale-up to quantities needed for liquid transportation fuel is unknown (Mercer and Armenta, 2011). Little information currently is available about the effectiveness and energy balance of these processes.
2.3.4 In-Situ Esterification
Treatment of wet algal biomass with alcohols can result in direct collection of fatty-acid methyl esters. To date, this technology has shown little benefit when compared with the more common oil recovery technologies (Ehimen et al., 2010).
2.3.5 Processing
The majority of the presentations to this committee and the published literature focus on producing fuels from lipid-producing microalgae (Benneman, 2011; Clarens, 2011). These, however, do not represent all available options for processing algae to fuels. Many of the processing options are studied but not described in the literature, making a thorough analysis difficult. Three key parameters influence the algal biofuel supply chain (Figure 2-15):
• Whether microalgae or macroalgae is cultivated for fuel production (micro versus macro).
• Whether the algae are cultivated for short duration and harvested for processing or cultivated in stable conditions while desired products emitted by algae are continuously harvested (short duration versus stable).
• Whether biochemical or thermochemical processes are used to produce biofuels from algae (biological versus chemical).
Although the sustainability challenges identified in this report frequently cannot be addressed without quality data, categorizing the algal biofuel production pathways by these three key parameters allows some general comments to be made about each combination of parameters (Figure 2-15).
The categories used to distinguish the processing depend on the nature of the algae, whether a stable culture is used, and whether the post processing is chemical or biological. Algae can be divided into microalgae or macroalgae, independent of whether the algae are fresh or salt water species. The dynamics of the culture are important and largely can be divided into whether the desire is for a time-stable culture or whether the algae are killed in the collection of the product. This designation, therefore, is largely determined by whether
FIGURE 2-15 Matrix showing combinations of key parameters that define algal biofuel processing pathways.
NOTE: The grey boxes indicate combinations of pathways that are not pursued, to best of the committee’s knowledge.
the desired products are retained within the algal cell walls or emitted extracellularly. Currently all processes described that use stable cultures and emit desirable products use closed photobioreactors. Presentations to the committee raised doubts that extracellular products could be collected in open-pond systems because of potential microbial consumption of the product (Benemann, 2011). Chemical processes, including conventional extraction of oil from microalgae and processing the oil to biodiesel, are typically used. Biological processes, such as fermentation of microalgal biomass, have been demonstrated. Other combinations have been described as a means for producing algal fuels.
2.3.5.1 Microalgae Harvested with Product Collected for Chemical Processing
The harvest of lipid-producing microalgae cultivated for short duration and the chemical processing of algal oil into fuel represent the most commonly discussed method for production of algal biofuels. The expression level of the oil as a fraction of the total biomass determines what processing will be required. High oil-expression levels sufficient to avoid extraction were not found in published data. Extraction with volatile alkane, ester, or alcohol solvents will recover lipids and phospholipid fractions from the algal biomass. The lipid recovery begins by boiling away the solvent, leaving the lipids for subsequent processing (Sheehan et al., 1998). Oil collected then is subjected to degumming. Most degumming methods involve a water wash step, creating an aqueous waste stream, which is reported to be 10-30 kilograms per 1,000 kilograms of degummed oil for typical seed oil processes (Crown Iron Works, 2008). Other component additions may be required, but are similarly small. This step, while necessary, is not likely to have a big impact on the energy or raw material requirements of the process.
The degummed oil then can be processed in several ways. Two main products are commonly mentioned: traditional transesterified biodiesel and hydrotreated or so-called green diesel. In traditional biodiesel production, methanol and a base catalyst react with the triacylglycerol algal oil to produce a fatty-acid methyl ester. Homogeneous base catalysts, commonly in the form of sodium or potassium hydroxide, are being replaced by heterogeneous catalysts, reducing waste (Ondrey, 2004). Glycerol is produced as a coproduct in both methods.
Recent trends have been toward the production of hydrotreated diesels rather than esters. In hydroprocessing, hydrogen reacts with the raw algal oil to produce alkanes, propane, and water. Hydrotreated diesels are more similar to petroleum-based diesel and are said to offer better performance than esters (Kalnes et al., 2007; Pew Center on Global Climate Change, 2011). Hydrogenated diesels are assumed to be compatible with existing petroleum infrastructure (DOE-EERE, 2012b), and whether they are sufficiently similar enough to petroleum-based fuels to be considered drop-in fuels would have to be tested.
2.3.5.2 Extracellular Secretion of Products by Microalgae for Chemical Processing
Algenol and Joule Technologies are two companies exploiting the ability of algae to secrete products extracellularly (Algenol Biofuels, 2012; Joule Unlimited, 2012). The products collect in the growth media for subsequent recovery. Algenol addressed the committee and has described its production method in journal articles and patents (Chance et al., 2011b). Algenol uses cyanobacteria that directly produce ethanol. Joule Technologies has patented cyanobacteria that directly produce alkane (Reppas, 2012). Eukaryotic organisms also have been described (Ramachandra et al., 2009). Algae and cyanobacteria emit a range of materials that could be used for fuel production. The best-described process in the published
literature is Algenol’s method of producing ethanol. Ethanol requires only purification and does not require subsequent processing. Recovery of ethanol from an aqueous solution is energy intensive, even with the solar still arrangement used by Algenol to provide primary concentration (Chance et al., 2011b).
The production methods described all use closed photobioreactors. This is likely a result of the desire to maintain stable producing cultures for long periods of time. Introduction of competing algal species or microbial contamination would be detrimental. Closed systems are a means to ensure culture purity and consistent product quality.
In principle, the energy required for separation in this mode of operation can be very low. Engineering organisms that express immiscible products would result in spontaneous separations that do not require energy input. Stable cultures can be maintained requiring minimal water inputs. Water clearly is required to replenish any water lost to photosynthesis and during processing. While the promise is large, the available published studies are insufficient for an accurate appraisal of the overall energy and LCAs to be performed.
2.3.5.3 Microalgae Harvested with Product Collected for Biological Processing
Although fermentation of microalgal biomass has been studied (Harun, 2010), it is not being developed at commercial scale at present. Microalgae provide carbon sources in the form of proteins and carbohydrates that can be exploited using fermentation. Some advantages include rapid growth rates, short harvesting cycles, and the absence of lignin. Like other biomass fermentations, a wide variety of products could be produced. There is a lack of detailed studies on this processing pathway.
2.3.5.4 Extracellular Emission of Products by Microalgae for Biological Processing
Proterro (2012) describes a method that uses microalgae in a photobioreactor to generate sugars that can be recovered for subsequent use as a feedstock for other fermentations. The sugar produced can be used for any fermentation process to produce fuels. Ethanol is currently the largest volume fuel produced by fermentation. Butanol (Butamax Advanced Biofuels, 2012; Gevo, 2012), farnesene (Amyris, 2012), alkanes (LS9 Inc., 2011; Solazyme, 2012), and other products also can be produced by sugar fermentation. Direct production of sugars requires that the algal culture be protected from opportunistic microorganisms and requires an environmentally sealed photobioreactor.
2.3.5.5 Macroalgae Harvested with Product Collected for Biological Processing
Bio Architecture Labs recently announced the development of a technology for the fermentation of macroalgal biomass (Wargacki et al., 2012). Use of macroalgae as feedstock for biochemical conversion is made possible by the development of organisms capable of metabolizing alginate polysaccharides. Organisms engineered for alginate transport and metabolism were further engineered for ethanol synthesis. This enables direct ethanol synthesis from macroalgae. Brown macroalgae are said to be attractive feedstocks because they do not require fertilizer input, arable land, and freshwater resources, and therefore do not compete with existing food crops for those resources. Sugars can be released in simple mechanical operations, like crushing and milling, because macroalgae do not contain lignin (Wargacki et al., 2012). Furthermore, cultivation methods are established because macroalgae are harvested for food ingredients, animal feedstuff, and fertilizers. Saccharina japonica
was demonstrated as a fermentation substrate. Gracilaria salicornia also has been shown to be a suitable substrate for microbial fermentation to ethanol (Wang et al., 2011).
2.3.5.6 Microalgae or Macroalgae Harvested for Whole-Biomass Processing
Interest is increasing in whole-biomass conversion for processing of terrestrial biomass (Marker et al., 2010; Wright et al., 2010). Pyrolysis of whole biomass yields an upgradeable biocrude. A recent review (Anex et al., 2010) shows that these routes have cost advantages relative to other biomass conversion technologies. The material produced potentially can be used in an existing refinery, saving capital relative to other options.
Several forms of pyrolysis have been explored. Hydropyrolysis is reported to be appropriate for use in processing of algal biomass (Marker et al., 2012). The low aromatic content in algal biomass (because of the absence of lignin) is said to make algal biomass a particularly good feedstock for hydropyrolysis.
The development of two pilot-scale alternatives has been reported, both producing fossil fuel-compatible materials (Hatcher, 2011). These synthetic crudes are stated to be compatible with existing refineries. In one of the processes, fertilizer is a coproduct.
Insufficient documentation is available for a detailed mass and energy balance of the processes. It can be presumed that the detailed studies on terrestrial biomass will yield similar results when algae are the feedstock. That is, whole-cell processing provides a potentially viable means of producing drop-in replacement fuels, taking advantage of existing refinery infrastructure to reduce risk and costs.
2.3.6 Fuel Products and Coproducts
The processes described above make many potential fuel components. Table 2-7 summarizes some of the dominant inputs and outputs for the technologies described.
2.3.7 Status of Algal Biofuel Production
Algal biofuel production is rapidly evolving, and as such, any status report is outdated at the moment of its completion. There are currently no operating algal biofuel production facilities that are comparable in scale to the average capacity of about 13.5 million gallons (51 million liters) per year for U.S. biodiesel refineries, much less rivaling the largest at about 100 million gallons (378 million liters) per year (NBB, 2012). Many projects are still in the research and development phase and exact production numbers are difficult to obtain.
An integrated coordination of biological (for example, algal strain selection and development of algae cultivation) and engineering processes (for example, reactor design, harvesting and dewatering methods, and processing) is needed to realize the potential of algal biofuels. However, the domestication of algae poses a special challenge as investigation into key biological and ecological aspects of algal biofuel production has lagged far behind the progress in feedstock processing design, system engineering, and life-cycle analyses over the past few decades. Nonetheless, increased core understanding of algae and their potential for improvements is fundamental to accelerating the entire algal biofuel enterprise. Relative to the vast and diverse spectrum of potentially available organisms, only a narrow range of currently cultivated microalgal strains are considered for commercial production
TABLE 2-7 Dominant Inputs and Outputs for Algal Processing Technologies.
|
|
|||||
| Duration of Cultivation System | Type of Algae | Type of Processing | Product | Major Inputs for Processing | Coproducts |
|
|
|||||
|
Short |
Microalgae |
Chemical |
Hydrogenated diesel |
Hydrogen, extraction solvent |
Algal biomass, propane |
|
Short |
Microalgae |
Chemical |
Biodiesel |
Methanol, base catalyst |
Algal biomass, glycerol |
|
Stable |
Microalgae |
Chemical |
Ethanol |
||
|
Stable |
Microalgae |
Chemical |
Alkanes |
||
|
Short |
Macroalgae |
Biochemical |
Ethanol, other products |
||
|
Short |
Microalgae |
Biochemical |
Ethanol, other products |
||
|
Short |
Microalgae |
Chemical |
Pyrolysis oil |
Char, off-gases |
|
|
Short |
Macroalgae |
Chemical |
Pyrolysis oil |
Char, off-gases |
|
|
|
|||||
of biofuels. Extensive new genomic analyses and physiological studies will be useful for screening and expanding the range of candidate species of microalgae and macroalgae that can be used for commercial-scale production of biofuels.
The presence of dramatically different photosynthetic efficiencies and chemistries across algal species underscores a critical need for basic and applied research to expand the spectrum of germplasm available for the enterprise. Research on expanding the light spectrum useful for photosynthesis, improving the distribution of incident light to various aquatic photosynthetic scale-up processes, and enhancing the efficiency of Rubisco or other basic physiological processes to better utilize carbon could lead to dramatic improvements in productivity. Additional new breakthroughs in areas such as the capability of algae to convert nutrients into biomass more efficiently or the reduction of processing costs associated with harvesting and dewatering (for example, via genetic enhancements that favor autoflocculation) also have the potential to further improve the energy balance and to enhance the overall sustainability of an expanding algal biofuel industry.
Equally important is crop protection research that focuses on reducing biomass losses to pathogens and grazers. Because contamination by other algal species is largely unavoidable, especially in open-pond algal cultures, improving the existing understanding of how algal biomass production systems can be managed as complex bioengineered systems would be helpful. This can be achieved in part via the application of principles of population, community, and ecosystem ecology. Identifying which ecophysiological parameters and genes best provide protection against grazers and pathogens at commercial-scale production levels also would be helpful.
Improvements in algae cultivation methods and the physical processes used to harvest, dewater, and convert algal biomass into fuels are as important to the sustainable development of algal biofuels as improvements in algal strains. New ways to reduce the energy requirements for converting cultivated algae in an aqueous solution into a dewatered state that can then be processed into fuel could be explored. Research and development in understanding how dewatered algae can be processed into a fuel and whether algae can produce a useful hydrocarbon directly without the need for harvest and dewatering and with minimal processing could be an important contributor to reducing production costs. Fundamentally, the questions involve integrating biology, ecology, and engineering into a systematic understanding and improvement of the entire algal biofuel enterprise (Box 2-2).
BOX 2-2
Research and Development for Enhancing Algal Biofuel Production
Research and development directed at domestication of algae for biofuel production is vitally important.
This effort will require improving the functional understanding of the biology, physiology, and ecology of microalgae. This upstream research and development will help inform and guide downstream engineering methods and designs for cultivation and processing systems that will enhance the entire algal biofuel production chain. Thus, concerted, complementary efforts in algal domestication and biofuel production will include:
• Development of strategies to improve carbon fixation rates and yields of algal crops at commercial production-level scale.
• Development of algal strains or multi-species assemblages that achieve high productivity and high volumetric concentrations over a wide range of environmental conditions (including variations in temperature and light levels) and are as easily harvested and processed as possible.
• Evaluation and development of improved crop protection methods.
• Design and development of robust, low-cost, long-lasting production systems for algal strains or multi-species assemblages that demand minimal regulations and control of environmental parameters.
• Development of strains that excrete oil or other fuel precursors, especially immiscible products.
• Development of improved harvest technologies that reduce energy required during collecting and processing.
• Design and development of integrated biological and engineering production strategies that obviate algae harvesting, drying, and oil-extraction processes.
• Design and development of integrated biological and engineering production strategies that continually reuse the algae, water, and nutrients.
• Design and development of systems that can process whole biomass into fuels.
SUMMARY FINDING FROM THIS CHAPTER
Algal strain development is needed to enhance traits that contribute to increasing fuel production per unit resource use, reducing the environmental effects per unit fuel produced, and enhancing economic viability. Improvements in biomass or product (lipid, alcohol, or hydrocarbons) yield, culture density, nutrient uptake, ease of harvest, and photosynthetic efficiency are some of the improvements that would improve sustainability of algal biofuels.
81st Texas Legislature House Bill 3391, Bill introduced by the Texas House of Representatives relating to the continuation and functions of the Parks and Wildlife Department; changing the elements of an offense. § Chapter 952. (Regular Session, June 19, 2009).
Acién Fernández, F.G., J.M. Fernández Sevilla, J.A. Sánchez Pérez, E. Molina Grima, and Y. Chisti. 2001. Airliftdriven external-loop tubular photobioreactors for outdoor production of microalgae: Assessment of design and performance. Chemical Engineering Science 56(8):2721-2732.
Algae Energy. 2012a. Open cultivation system for growing algae. Available online at http://algae-energy.co.uk/biofuel_production/cultivation/. Accessed May 15, 2012.
____________. 2012b. Photobioreactors. Available online at http://algae-energy.co.uk/biofuel_production/pbrs/. Accessed May 15, 2012.
Algenol Biofuels. 2012. Homepage. Available online at http://algenol.com/. Accessed June 16, 2012.
Algomed. 2012. Microalgae cultivated in a 500 km long system of glass tubes—A German innovation. Available online at http://www.algomed.de/index.php?lang=eng&op=algenfarm_anlage. Accessed May 15, 2012.
Amyris. 2012. Homepage. Available online at http://www.amyris.com/. Accessed June 17, 2012.
Andrianov, V., N. Borisjuk, N. Pogrebnyak, A. Brinker, J. Dixon, S. Spitsin, J. Flynn, P. Matyszczuk, K. Andryszak, M. Laurelli, M. Golovkin, and H. Koprowski. 2009. Tobacco as a production platform for biofuel: Overexpression of Arabidopsis DGAT and LEC2 genes increases accumulation and shifts the composition of lipids in green biomass. Plant Biotechnology Journal 8:1-11.
Anex, R.P., A. Aden, F.K. Kazi, J. Fortman, R.M. Swanson, M.M. Wright, J.A. Satrio, R.C. Brown, D.E. Daugaard, A. Platon, G. Kothandaraman, D.D. Hsu, and A. Dutta. 2010. Techno-economic comparison of biomass-to-transportation fuels via pyrolysis, gasification, and biochemical pathways. Fuel 89(Supplement 1):S29-S35.
AQUAFUEL. 2009. Deliverable 1.4 Report on biology and biotechnology of algae with indication of criteria for strain selection. Available online at http://www.aquafuels.eu/attachments/079_D%201.4%20Biology%20Biotechnology.pdf. Accessed September 14, 2012.
Aravanis, A. 2011. Near-Term Commercialization of Algal Biofuel. Presentation to the NRC Committee on the Sustainable Development of Algal Biofuels on June 13.
Atsumi, S., W. Higashide, and J.C. Liao. 2009. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nature Biotechnology 27(12):1177-1180.
Baptist, G., D. Meritt, and D. Webster. 1993. Growing Microalgae to Feed Bivalve Larvae. North Dartmouth: University of Massachusetts, Dartmouth.
Baud, S., S. Wuilleme, B. Dubreucq, A. de Almeida, C. Vuagnat, L. Lepiniec, M. Miquel, and C. Rochat. 2007. Function of plastidial pyruvate kinases in seeds of Arabidopsis thaliana. Plant Journal 52(3):405-419.
Becker, E.W. 1994. Microalgae: Biotechnology and Microbiology. Cambridge, UK: Cambridge University Press.
Benemann, J.R. 1986. Microalgae Biotechnology: Products, Processes, and Opportunities. Washington, DC: OMEC International Inc.
____________. 1989. The future of microalgal biotechnology. In Algal and Cyanobacterial Biotechnology, edited by Cresswell R.C., N. Shah, and T.A. Rees. Harlow, England: Longman Scientific and Technical.
____________. 2008. Opportunities and challenges in algae biofuel production. Available online at http://www.fao.org/uploads/media/algae_positionpaper.pdf. Accessed October 21, 2011.
____________. 2011. Promise and Challenges in Sustainable Development of Algal Biofuels: Questions and Answers. Presentation to the NRC Committee on Sustainable Development of Algal Biofuels on July 5.
Bessler, H., V.M. Temperton, C. Roscher, N. Buchmann, B. Schmid, E.D. Schulze, W.W. Weisser, and C. Engels. 2009. Aboveground overyielding in grassland mixtures is associated with reduced biomass partitioning to belowground organs. Ecology 90(6):1520-1530.
Bhave, R., T. Kuritz, L. Powell, and D. Adcock. 2012. Membrane-based energy efficient dewatering of microalgae in biofuels production and recovery of value added coproducts. Environmental Science and Technology 46(10):5599-5606.
Blankenship, R.E., D.M. Tiede, J. Barber, G.W. Brudvig, G. Fleming, M. Ghirardi, M.R. Gunner, W. Junge, D.M. Kramer, A. Melis, T.A. Moore, C.C. Moser, D.G. Nocera, A.J. Nozik, D.R. Ort, W.W. Parson, R.C. Prince, and R.T. Sayre. 2011. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332(6031):805-809.
Borowitzka, M.A. 1992. Algal biotechnology products and processes—matching science and economics. Journal of Applied Phycology 4(3):267-279.
____________. 1999. Commercial production of microalgae: ponds, tanks, tubes and fermenters. Journal of Biotechnology 70(1-3):313-321.
Brennan, L., and P. Owende. 2010. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and coproducts. Renewable and Sustainable Energy Reviews 14(2):557-577.
Brown, R.M., D.A. Larson, and H.C. Bold. 1964. Airborne algae—Their abundance and heterogeneity. Science 143(360):583-585.
Butamax Advanced Biofuels, LLC. 2012. Homepage. Available online at http://www.butamax.com/. Accessed June 17, 2012.
California Polytechnic State University. 2012. Tubular Photobioreactor. Available online at http://brae.calpoly.edu/CEAE/biofuels.xhtml. Accessed May 15, 2012.
Camacho Rubio, F., F.G. Acién Fernández, J.A. Sánchez Pérez, F. García Camacho, and E. Molina Grima. 1999. Prediction of dissolved oxygen and carbon dioxide concentration profiles in tubular photobioreactors for microalgal culture. Biotechnology and Bioengineering 62(1):71-86.
Carlozzi, P. 2003. Dilution of solar radiation through “culture” lamination in photobioreactor rows facing south-north: A way to improve the efficiency of light utilization by cyanobacteria (Arthrospira platensis). Biotechnology and Bioengineering 81(3):305-315.
Carpenter, S.R., and J.F. Kitchell. 1988. Consumer control of lake productivity. Bioscience 38(11):764-769.
Cernac, A., and C. Benning. 2004. WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant Journal 40(4):575-585.
Cerutti, H., X.R. Ma, J. Msanne, and T. Repas. 2011. RNA-mediated silencing in algae: Biological roles and tools for analysis of gene function. Eukaryotic Cell 10(9):1164-1172.
Chance, R.R., B. McCool, and J. Coleman. 2011a. Questionnaire reply from Algenol Biofuels Inc. Received by the NRC Committee on Sustainable Development of Algal Biofuels on July 7.
Chance, R.R., B. McCool, and J.D. Coleman. 2011b. A Cyanobacteria-Based Photosynthetic Process for the Production of Ethanol. Presentation to the NRC Committee on Sustainable Development of Algal Biofuels on June 13.
Chase, J.M. 2003. Community assembly: when should history matter? Oecologia 136(4):489-498.
Chen, M., and R.E. Blankenship. 2011. Expanding the solar spectrum used by photosynthesis. Trends in Plant Science 16(8):427-431.
Chen, P., M. Min, Y.-F. Chen, L. Wang, Y. Li, Q. Chen, C. Wang, Y. Wan, X. Wang, Y. Cheng, S. Deng, K. Hennessy, X. Lin, Y. Liu, Y. Wang, B. Martinez, and R. Ruan. 2009. Review of the biological and engineering aspects of algae to fuels approach. International Journal of Agricultural and Biological Engineering 2(4):1-30.
Cheng-Wu, Z., O. Zmora, R. Kopel, and A. Richmond. 2001. An industrial-size flat plate glass reactor for mass production of Nannochloropsis sp. (Eustigmatophyceae). Aquaculture 195(1-2):35-49.
Clarens, A.F. 2011. Life cycle assessment of algae-to-energy technologies. Presentation to the NRC Committee on Sustainable Development of Algal Biofuels on June 13.
Clarens, A.F., E.P. Resurreccion, M.A. White, and L.M. Colosi. 2010. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environmental Science and Technology 44(5):1813-1819.
Clemente, T.E., and E.B. Cahoon. 2009. Soybean oil: Genetic approaches for modification of functionality and total content. Plant Physiology 151(3):1030-1040.
Cloud, B. 2011a. Questionnaire reply from Phyco Biosciences Inc. Received by the NRC Committee on Sustainable Development of Algal Biofuels on September 27.
____________. 2011b. Questionnaire reply from Phyco BioSciences Inc. Received by the NRC Committee on Sustainable Development of Algal Biofuels on July 25.
Courchesne, N.M.D., A. Parisien, B. Wang, and C.Q. Lan. 2009. Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. Journal of Biotechnology 141(1-2):31-41.
Crown Iron Works. 2008. MultiPure degumming/neutralizing system. Available online at http://www.crown-iron.com/userimages/DegNeuN1.pdf. Accessed June 16, 2012.
Cuello, J., and J. Ley. 2011. ACCORDION Photobioreactor for Algae Production of Biofuels and Biochemicals. Presentation to the Conference EPA’s GHG Emissions Standards for Power Plants and Oil Refineries on February 2.
Cyanotech. 2012. Hawaiian Spirulina Pacifica® Available online at http://www.cyanotech.com/spirulina.xhtml. Accessed April 5, 2012.
Davis, R., A. Aden, and P.T. Pienkos. 2011. Techno-economic analysis of autotrophic microalgae for fuel production. Applied Energy 88(10):3524-3531.
Del Campo, J.A., M. Garcia-Gonzalez, and M.G. Guerrero. 2007. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Applied Microbiology and Biotechnology 74(6):1163-1174.
Dillon, H. 2011. Presentation in the session “Broadening the Bionergy Horizon.” In DOE Biomass Summit: Replace the Whole Barrel, Supply the Whole Market, July 26, 2012, National Harbor, MD.
Ditty, J.L., S.B. Williams, and S.S. Golden. 2003. A cyanobacterial circadian timing mechanism. Annual Review of Genetics 37:513-543.
DOE-EERE (U.S. Department of Energy, Energy Efficiency and Renewable Energy). 2012a. Lower and higher heating values of fuels. Available online at http://hydrogen.pnl.gov/cocoon/morf/hydrogen/site_specific/fuel_heating_calculator?canprint=false. Accessed June 18, 2012.
____________. 2012b. Drop-in biofuels. Available online at http://www.afdc.energy.gov/fuels/emerging_dropin_biofuels.xhtml. Accessed August 21, 2012.
DOE (U.S. Department of Energy). 2010. National Algal Biofuels Technology Roadmap. Washington, D.C.: U.S. Department of Energy, Energy Efficiency and Renewable Energy.
Ducat, D.C., J.C. Way, and P.A. Silver. 2011. Engineering cyanobacteria to generate high-value products. Trends in Biotechnology 29(2):95-103.
Duerr, E.O., A. Molnar, and V. Sato. 1998. Cultured microalgae as aquaculture feeds. Journal of Marine Biotechnology 6(2):65-70.
Durand-Chastel, H. 1980. Production and use of Spirulina in Mexico. Pp. 51-64 in Algae Biomass. Amsterdam, The Netherlands: Elsvier/North-Holland Biomedical Press.
Earthrise Nutritional, LLC. 2009a. Earthrise. Available online at http://www.earthrise.com/. Accessed February 9, 2012.
____________. 2009b. About Earthrise: Our farm. Available online at http://earthrise.com/farm.xhtml. Accessed August 26, 2012.
Edwards, M. 2010. Phyco Biosciences super trough growing algae. Available online at http://www.algaeindus-trymagazine.com/abundance-food/. Accessed May 15, 2012.
Ehimen, E.A., Z.F. Sun, and C.G. Carrington. 2010. Variables affecting the in situ transesterification of microalgae lipids. Fuel 89(3):677-684.
Ehira, S., and M. Ohmori. 2011. NrrA, a nitrogen-regulated response regulator protein, controls glycogen catabolism in the nitrogen-fixing cyanobacterium Anabaena sp strain PCC 7120. Journal of Biological Chemistry 286(44):38109-38114.
Elhai, J., A. Vepritskiy, A.M. MuroPastor, E. Flores, and C.P. Wolk. 1997. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. Journal of Bacteriology 179(6):1998-2005.
Enay, S. 2011. Hawaii’s Natural Energy Laboratory fuels innovation. Available online at http://www.hawaiibusiness.com/Hawaii-Business/November-2011/Hawaiis-Natural-Energy-Laboratory-fuels-innovation/. Accessed August 26, 2012.
EPA (U.S. Environmental Protection Agency). 2011. Microbial products of biotechnology: Final regulations under the Toxic Substances Control Act summary (fact sheet). Available online at http://www.epa.gov/biotech_rule/pubs/fs-001.htm. Accessed March 2, 2012.
Evens, T.J., and R.P. Niedz. 2011. Mapping the fundamental niches of two freshwater microalgae, Chlorella vulgaris (Trebouxiophyceae) and Peridinium cinctum (Dinophyceae), in 5-dimensional ion space. International Journal of Ecology DOI:10.1155/2011/738035.
Falkowski, P.G., and J.A. Raven. 1997. Aquatic Photosynthesis. Malden, MA: Blackwell Science.
Flaherty, B.L., F. Van Nieuwerburgh, S.R. Head, and J.W. Golden. 2011. Directional RNA deep sequencing sheds new light on the transcriptional response of Anabaena sp strain PCC 7120 to combined-nitrogen deprivation. BMC Genomics 12:332.
Frank, E.D., J. Han, I. Palou-Rivera, A. Elgowainy, and M.Q. Wang. 2011. Life-Cycle Analysis of Algal Lipid Fuels with the GREET Model. Oak Ridge: TN: U.S. Department of Energy.
Frisch, D., A.J. Green, and J. Figuerola. 2007. High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds. Aquatic Sciences 69(4):568-574.
Fuhrmann, M., W. Oertel, and P. Hegemann. 1999. A synthetic gene coding for the green fluorescent protein (GFP) is a versatile reporter in Chlamydomonas reinhardtii. Plant Journal 19(3):353-361.
García-González, M., J. Moreno, J.P. Cañavate, V. Anguis, A. Prieto, C. Manzano, F.J. Florencio, and M.G. Guerrero. 2003. Conditions for open-air outdoor culture of Dunaliella salina in southern Spain. Journal of Applied Phycology 15(2-3):177-184.
Garthwaite, J. 2012. Unlocking Seaweed’s Next-Gen Crude: Sugar. Available online at http://green.blogs.nytimes.com/2012/01/23/unlocking-seaweeds-next-gen-crude-sugar/. Accessed June 13, 2012.
Gershwin, M.E., and A.B. Belay. 2008. Spirulina in human nutrition and health. Boca Raton: CRC Press.
Gevo. 2012. Homepage. Available online at http://www.gevo.com/. Accessed June 17, 2012.
Giordano, M., J. Beardall, and J.A. Raven. 2005. CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annual Review of Plant Biology 56:99-131.
Graham, D.W., and V.H. Smith. 2004. Designed ecosystem services: Application of ecological principles in waste-water treatment engineering. Frontiers in Ecology and the Environment 2(4):199-206.
Gross, E.M. 2003. Allelopathy of aquatic autotrophs. Critical Reviews in Plant Sciences 22(3-4):313-339.
Guedes, A.C., H.M. Amaro, and F.X. Malcata. 2011. Microalgae as sources of high added-value compounds—A brief review of recent work. Biotechnology Progress 27(3):597-613.
Guihéneuf, F., S. Leu, A. Zarka, I. Khozin-Goldberg, I. Khalilov, and S. Boussiba. 2011. Cloning and molecular characterization of a novel acyl-CoA:diacylglycerol acyltransferase 1-like gene (PtDGAT1) from the diatom Phaeodactylum tricornutum. FEBS Journal 278(19):3651-3666.
Hall, C.A., B.E. Dale, and D. Pimentel. 2011. Seeking to understand the reasons for different energy return on investment (EROI) estimates for biofuels. Sustainability 3(12):2413-2432.
Hall, D.O., F.G. Acién Fernández, E.C. Guerrero, K.K. Rao, and E.M. Grima. 2003. Outdoor helical tubular photobioreactors for microalgal production: Modeling of fluid-dynamics and mass transfer and assessment of biomass productivity. Biotechnology and Bioengineering 82(1):62-73.
Hannon, M., J. Gimpel, M. Tran, B. Rasala, and S. Mayfield. 2010. Biofuels from algae: Challenges and potential. Biofuels 1(5):763-784.
Harun, R., M.K. Danquah, and G.M. Forde. 2010. Microalgal biomass as a fermentation feedstock for bioethanol production. Journal of Chemical Technology and Biotechnology 85(2):199-203.
Hatcher, P.G. 2011. The ODU algae to biodiesel project. Presentation to the NRC Committee on the Sustainable Development of Algal Biofuels on August 24.
Heaven, S., J. Milledge, and Y. Zhang. 2011. Comments on “Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable.” Biotechnology Advances 29(1):164-167.
Heidorn, T., C. Daniel, H. Hsin-Ho, L. Pia, O. Paulo, S. Karin, and L. Peter. 2011. Synthetic biology in cyanobacteria: Engineering and analyzing novel functions. Pp. 539-579 in Methods in Enzymology, C. Voigt, ed. Academic Press.
Hoffmann, M.D., and S.I. Dodson. 2005. Land use, primary productivity, and lake area as descriptors of zooplankton diversity. Ecology 86(1):255-261.
Huesemann, M.H., T.S. Hausmann, R. Bartha, M. Aksoy, J.C. Weissman, and J.R. Benemann. 2009. Biomass productivities in wild type and pigment mutant of Cyclotella sp (Diatom). Applied Biochemistry and Biotechnology 157(3):507-526.
Inventure, Inc. 2012. Homepage. Available online at http://inventurechem.com/home.xhtml. Accessed June 16, 2012.
Iowa State University. 2011. Iowa State University scientists genetically increase algae biomass by more than 50 percent. Available online at http://www.news.iastate.edu/news/2011/nov/spaldingdario. Accessed June 17, 2012.
IP Monitor. 2009. Patent application no. 209333021—genetically engineered herbicide resistant algae. Available online at http://www.ipmonitor.com.au/patents/case/2009333021. Accessed June 15, 2012.
Jako, C., A. Kumar, Y.D. Wei, J.T. Zou, D.L. Barton, E.M. Giblin, P.S. Covello, and D.C. Taylor. 2001. Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiology 126(2):861-874.
Janssen, M., P. Slenders, J. Tramper, L.R. Mur, and R.H. Wijffels. 2001. Photosynthetic efficiency of Dunaliella tertiolecta under short light/dark cycles. Enzyme and Microbial Technology 29(4-5):298-305.
Jenkins, B.M., L.L. Baxter, T.R. Miles Jr., and T.R. Miles. 1998. Combustion properties of biomass. Fuel Processing Technology 54(1-3):17-46.
Jenkins, D.G., and M.O. Underwood. 1998. Zooplankton may not disperse readily in wind, rain, or waterfowl. Hydrobiologia 387:15-21.
Jiménez, C., B.R. Cossío, and F.X. Niell. 2003. Relationship between physicochemical variables and productivity in open ponds for the production of Spirulina: A predictive model of algal yield. Aquaculture 221(1-4):331-345.
Jorquera, O., A. Kiperstok, E.A. Sales, M. Embiruçu, and M.L. Ghirardi. 2010. Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresource Technology 101(4):1406-1413.
Joule Unlimited. 2012. Frequently asked questions. Available online at http://www.jouleunlimited.com/faq. Accessed March 9, 2012.
Jurgens, K., J. Pernthaler, S. Schalla, and R. Amann. 1999. Morphological and compositional changes in a plank-tonic bacterial community in response to enhanced protozoan grazing. Applied and Environmental Microbiology 65(3):1241-1250.
Kalnes, T., T. Marker, and D.R. Shonnard. 2007. Green diesel: A second generation biofuel. International Journal of Chemical Reactor Engineering 5(1): ISSN (Online) 1542-6580.
Kan, Y., and J. Pan. 2010. A one-shot solution to bacterial and fungal contamination in the green alga Chlamydomonas reinhardtii culture by using an antibiotic cocktail. Journal of Phycology 46(6):1356-1358.
Kanazawa, Z., C. Fujita, T. Yuhara, and T. Sasa. 1958. Mass culture of unicellular algae using the open pond circulation method. Journal of General and Applied Microbiology 4:135-139.
Karpowicz, S.J., S.E. Prochnik, A.R. Grossman, and S.S. Merchant. 2011. The GreenCut2 resource, a phylogenomically derived inventory of proteins specific to the plant lineage. Journal of Biological Chemistry 286(24):21427-21439.
Kawaguchi, K. 1980. Microalgae production systems in Asia. Pp. 25-33 in Algae Biomass Production and Use, G. Shelef and C.J. Soeder, eds. Amsterdam, The Netherlands: Elsevier/North Holland Biomedical Press.
Kawata, Y., S. Yano, H. Kojima, and M. Toyomizu. 2004. Transformation of Spirulina platensis strain C1 (Arthrospira sp PCC9438) with Tn5 transposase-transposon DNA-cation liposome complex. Marine Biotechnology 6(4):355-363.
Keeling, P.J. 2010. The endosymbiotic origin, diversification and fate of plastids. Philosophical Transactions of the Royal Society B: Biological Sciences 365(1541):729-748.
Kennedy, C.A., K. Thurston, D. Gahl, T. Gordon, and S. Julian. 1995. The Biocoil Project—1994-95. Available online at http://advbio.cascadeschools.org/94-95/biocoil.xhtml. Accessed May 15, 2012.
Kilian, O., C.S.E. Benemann, K.K. Niyogi, and B. Vick. 2011. High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proceedings of the National Academy of Sciences of the United States of America 108(52):21265-21269.
Laloknam, S., K. Tanaka, T. Buaboocha, R. Waditee, A. Incharoensakdi, T. Hibino, Y. Tanaka, and T. Takabe. 2006. Halotolerant cyanobacterium Aphanothece halophytica contains a betaine transporter active at alkaline pH and high salinity. Applied and Environmental Microbiology 72(9):6018-6026.
Lan, E.I., and J.C. Liao. 2011. Metabolic engineering of cyanobacteria for 1-butanol production from carbon dioxide. Metabolic Engineering 13(4):353-363.
Lapidot, M., D. Raveh, A. Sivan, S. Arad, and M. Shapira. 2002. Stable chloroplast transformation of the unicellular red alga Porphyridium species. Plant Physiology 129(1):7-12.
Lardizabal, K., R. Effertz, C. Levering, J. Mai, M.C. Pedroso, T. Jury, E. Aasen, K. Gruys, and K. Bennett. 2008. Expression of Umbelopsis ramanniana DGAT2A in seed increases oil in soybean. Plant Physiology 148(1):89-96.
Laws, E.A., D.G. Redalje, D.M. Karl, and M.S. Chalup. 1983. A theoretical and experimental examination of the predictions of two recent models of phytoplankton growth. Journal of Theoretical Biology 105(3):469-491.
Laws, E.A., S. Taguchi, J. Hirata, and L. Pang. 1988. Optimization of microalgal production in a shallow outdoor flume. Biotechnology and Bioengineering 32(2):140-147.
Leckey, C.A.C., and M.K. Hinders. 2012. Viscous effects in the acoustic manipulation of algae for biofuel production. Journal of Applied Phycology 24(1):145-156.
Lee, Y.K. 2001. Microalgal mass culture systems and methods: Their limitation and potential. Journal of Applied Phycology 13(4):307-315.
Leliaert, F., H. Verbruggen, and F.W. Zechman. 2011. Into the deep: New discoveries at the base of the green plant phylogeny. BioEssays 33(9):683-692.
Li, Y., D. Han, M. Sommerfeld, and Q. Hu. 2011. Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresource Technology 102(1):123-129.
Liu, X., A.F. Clarens, and L.M. Colosi. 2012. Algae biodiesel has potential despite inconclusive results to date. Bioresource Technology 104:803-806.
Lopez, D., D. Casero, S.J. Cokus, S.S. Merchant, and M. Pellegrini. 2011. Algal functional annotation tool: A web-based analysis suite to functionally interpret large gene lists using integrated annotation and expression data. BMC Bioinformatics 12:282.
López, M.C.G.M., E.D.R. Sánchez, J.L. Casas López, F.G.A. Fernández, J.M.F. Sevilla, J. Rivas, M.G. Guerrero, and E.M. Grima. 2006. Comparative analysis of the outdoor culture of Haematococcus pluvialis in tubular and bubble column photobioreactors. Journal of Biotechnology 123(3):329-342.
Lorenz, R. 2002. GRAS notification: “Spirulina” microalgae. Available online at http://www.accessdata.fda.gov/scripts/fcn/gras_notices/grn_101.pdf. Accessed August 26, 2012
Los Alamos National Laboratory. 2011. LANL develops first genetically engineered “magnetic” algae. Los Alamos News Center: News, Releases, Video, Publications. Available online at http://www.lanl.gov/news/stories/magnetic_algae.xhtml. Accessed February 8, 2012.
LS9 Inc. (Life Sciences Sustaining 9 Billion). 2011. Homepage. Available online at http://www.ls9.com/. Accessed February 9, 2012.
Ma, J.Y., S.F. Wang, P.W. Wang, L.J. Ma, X.L. Chen, and R.F. Xu. 2006. Toxicity assessment of 40 herbicides to the green alga Raphidocelis subcapitata. Ecotoxicology and Environmental Safety 63(3):456-462.
Ma, J.Y., L.G. Xu, S.F. Wang, R.Q. Zheng, S.H. Jin, S.Q. Huang, and Y.J. Huang. 2002. Toxicity of 40 herbicides to the green alga Chlorella vulgaris. Ecotoxicology and Environmental Safety 51(2):128-132.
Mahan, K.M., O.W. Odom, and D.L. Herrin. 2005. Controlling fungal contamination in Chlamydomonas reinhardtii cultures. Biotechniques 39(4):457-458.
Marker, T., M. Linck, and L. Felix. 2010. Integrated hydropyrolysis and hydroconversion (IH2) process for direct production of gasoline and diesel fuel from biomass. Paper read at the BIOMASS 2010, March 30-31, Arlington, VA.
Marker, T.L., L.G. Felix, M.B. Linck, and M.J. Roberts. 2012. Integrated hydropyrolysis and hydroconversion (IH2) for the direct production of gasoline and diesel fuels or blending components from biomass, Part 1: Proof of principle testing. Environmental Progress and Sustainable Energy 31(2):191-199.
Marler, D. 2011. An Assessment of the Environmental Performance of Algal Biofuels. Presentation to the NRC Committee on Sustainable Development of Algal Biofuels on September 8.
Martins, C.A., D. Kulis, S. Franca, and D.M. Anderson. 2004. The loss of PSP toxin production in a formerly toxic Alexandrium lusitanicum clone. Toxicon 43(2):195-205.
Matsuo, T., and M. Ishiura. 2010. New insights into the circadian clock in Chlamydomonas. International Review of Cell and Molecular Biology 280(C):281-341.
McHugh, D.J. 2003. A guide to the seaweed industry. Rome, Italy: FAO.
McKenzie, F.T. 2011. Our changing planet: An introduction to earth system science and global environmental change, 4th edition. Upper Saddle, NJ: Prentice-Hall.
Mercer, P., and R.E. Armenta. 2011. Developments in oil extraction from microalgae. European Journal of Lipid Science and Technology 113(5):539-547.
Milledge, J.J. 2011. Commercial application of microalgae other than as biofuels: A brief review. Reviews in Environmental Science and Biotechnology 10(1):31-41.
Moheimani, N.R., and M.A. Borowitzka. 2006. Limits to productivity of the alga Pleurochrysis carterae (haptophyta) grown in outdoor raceway ponds. Biotechnology and Bioengineering 96(1):27-36.
Molina, E., J. Fernández, F.G. Acién, and Y. Chisti. 2001. Tubular photobioreactor design for algal cultures. Journal of Biotechnology 92(2):113-131.
Moreno, J., M.A. Vargas, H. Rodríguez, J. Rivas, and M.G. Guerrero. 2003. Outdoor cultivation of a nitrogen-fixing marine cyanobacterium, Anabaena sp. ATCC 33047. Biomolecular Engineering 20(4-6):191-197.
Mveda. 2011. Sapphire Energy photo. Available online at http://www.mveda.com/blog/2011/03/sapphire-develops-research-center/. Accessed May 15, 2012.
NanoVoltaix. 2012. Photo-Bioreactors. Available online at http://www.nanovoltaix.com/markets/algae.php. Accessed May 15, 2012.
NBB (National Biodiesel Board). 2012. Plants listings. Available online at http://www.biodiesel.org/production/plants/plants-listing. Accessed June 15, 2012.
Niederholtmeyer, H., B.T. Wolfstadter, D.F. Savage, P.A. Silver, and J.C. Way. 2010. Engineering cyanobacteria to synthesize and export hydrophilic products. Applied and Environmental Microbiology 76(11):3462-3466.
O-Neill, J.S., G. Van Ooijen, L.E. Dixon, C. Troein, F. Corellou, F.Y. Bouget, A.B. Reddy, and A.J. Millar. 2011. Circadian rhythms persist without transcription in a eukaryote. Nature 469(7331):554-558.
Olaizola, M. 2000. Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. Journal of Applied Phycology 12(3-5):499-506.
Olguín, E.J., S. Galicia, G. Mercado, and T. Pérez. 2003. Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. Journal of Applied Phycology 15(2-3):249-257.
Ondrey, G. 2004. Biodiesel production using a heterogeneous catalyst. Chemical Engineering Journal, Chemantator (October).
OneWater, Inc. 2012. OneWater. Available online at http://onewaterworks.com/. Accessed June 14, 2012.
Ono, E., and J.L. Cuello. 2004a. Design parameters of solar concentrating systems for CO2-mitigating algal photobioreactors. Energy 29(9-10):1651-1657.
____________. 2004b. Feasibility assessment of open-pond microalgal CO2 mitigation technology with implemention of the Kyoto Protocol: A case study for Japan. Environment Control in Biology 42(3):161-168.
____________. 2006. Feasibility assessment of microalgal carbon dioxide sequestration technology with photobioreactor and solar collector. Biosystems Engineering 95(4):597-606.
Ort, D.R., X. Zhu, and A. Melis. 2011. Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiology 155(1):79-85.
Osanai, T., Y. Kanesaki, T. Nakano, H. Takahashi, M. Asayama, M. Shirai, M. Kanehisa, I. Suzuki, N. Murata, and K. Tanaka. 2005. Positive regulation of sugar catabolic pathways in the cyanobacterium Synechocystis sp PCC 6803 by the group 2 sigma factor sigE. Journal of Biological Chemistry 280(35):30653-30659.
Pal, D., I. Khozin-Goldberg, Z. Cohen, and S. Boussiba. 2011. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Applied Microbiology and Biotechnology 90(4):1429-1441.
Parikh, M.R., D.N. Greene, K.K. Woods, and I. Matsumura. 2006. Directed evolution of Rubisco hypermorphs through genetic selection in engineered E. coli. Protein Engineering Design and Selection 19(3):113-119.
Paerl, H.W. 2000. Marine plankton. Pp. 121-148 in The Ecology of Cyanobacteria: Their Diversity in Time and Space, Whitton, B.A. and M. Potts, eds. Boston, M.A.: Kluwer Academic.
Pedroni, P.M., J. Davison, H. Beckert, P. Bergman, and J. Benemann. 2001. A proposal to establish an international network on biofixation of CO2 and greenhouse gas abatement with microalgae. Available online at http://www.netl.doe.gov/publications/proceedings/01/carbon_seq/p17.pdf. Accessed August 26, 2012.
Peled, E., S. Leu, A. Zarka, M. Weiss, U. Pick, I. Khozin-Goldberg, and S. Boussiba. 2011. Isolation of a novel oil globule protein from the green alga Haematococcus pluvialis (chlorophyceae). Lipids 46(9):851-861.
Pew Center on Global Climate Change. 2011. Advanced biohydrocarbon fuels. Available online at http://www.c2es.org/docUploads/AdvancedBiohydrocarbonFuels.pdf. Accessed August 21, 2012.
Phycal. 2011. Questionnaire reply from Phycal. Received by the NRC Committee on the Sustainable Development of Algal Biofuels on July 6.
Polle, J.E.W., S.D. Kanakagiri, and A. Melis. 2003. tla1, a DNA insertional transformant of the green alga Chlamydomonas reinhardtii with a truncated light-harvesting chlorophyll antenna size. Planta 217(1):49-59.
Proterro. 2012. Homepage. Available online at http://proterro.com/. Accessed June 17, 2012.
Ptacnik, R., T. Andersen, P. Brettum, L. Lepisto, and E. Willen. 2010. Regional species pools control community saturation in lake phytoplankton. Proceedings of the Royal Society B-Biological Sciences 277(1701):3755-3764.
Pushparaj, B., E. Pelosi, M.R. Tredici, E. Pinzani, and R. Materassi. 1997. An integrated culture system for outdoor production of microalgae and cyanobacteria. Journal of Applied Phycology 9(2):113-119.
Radakovits, R., R.E. Jinkerson, A. Darzins, and M.C. Posewitz. 2010. Genetic engineering of algae for enhanced biofuel production. Eukaryotic Cell 9(4):486-501.
Ramachandra, T.V., D.M. Mahapatra, B. Karthick, and R. Gordon. 2009. Milking diatoms for sustainable energy: Biochemical engineering versus gasoline-secreting diatom solar panels. Industrial and Engineering Chemistry Research 48(19):8769-8788.
Raven, J.A. 2010. Inorganic carbon acquisition by eukaryotic algae: Four current questions. Photosynthesis Research 106(1-2):123-134.
Reppas, N.B. 2012. Methods and compositions for the recombinant biosynthesis of N-Alkanes. Patent US8183027, filed September 23, 2011, and issued May 22, 2012.
Reynolds, C.S. 1997. Vegetation processes in the pelagic: A model for ecosystem theory. Vol. 9, Excellence in Ecology. Oldendorf, Germany: Ecology Institute.
Richmond, A., E. Lichtenberg, B. Stahl, and A. Vonshak. 1990. Quantitative assessment of the major limitations on productivity of Spirulina platensis in open raceways. Journal of Applied Phycology 2(3):195-206.
Robertson, D.E., S.A. Jacobson, F. Morgan, D. Berry, G.M. Church, and N.B. Afeyan. 2011. A new dawn for industrial photosynthesis. Photosynthesis Research 107(3):269-277.
Rodgers, S.K., and E.F. Cox. 1999. Rate of spread of introduced rhodophytes Kappaphycus alvarezii, Kappaphycus striatum, and Gracilaria salicornia and their current distribution in Kane’ohe Bay, O’ahu, Hawai’i. Pacific Science 53(3):232-241.
Rodolfi, L., G.C. Zittelli, N. Bassi, G. Padovani, N. Biondi, G. Bonini, and M.R. Tredici. 2009. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnology and Bioengineering 102(1):100-112.
Roesijadi, G., S.B. Jones, L.J. Snowden-Swan, and Y. Zhu. 2010. Macroalgae as a Biomass Feedstock: A Preliminary Analysis. Richland, WA: Pacific Northwest National Laboratory.
Roessler, P. 2011. Algal Strain Selection and Development for Biofuel Production. Presentation to the NRC Committee on Sustainable Development of Algal Biofuels on September 8.
RSB (Roundtable on Sustainable Biofuels). 2011. Principles and criteria. Available online at http://rsb.epfl.ch/page-24929.xhtml. Accessed July 18, 2011.
Ruenwai, R., S. Cheevadhanarak, and K. Laoteng. 2009. Overexpression of acetyl-CoA carboxylase gene of Mucor rouxii enhanced fatty acid content in Hansenula polymorpha. Molecular Biotechnology 42(3):327-332.
Ruffing, A.M. 2011. Engineered cyanobacteria: Teaching an old bug new tricks. Bioengineered Bugs 2(3):136-149.
Russell, D.J. 1983. Ecology of the imported red seaweed Eucheuma striatum Schmitz on Coconut Island, Oahu, Hawaii. Pacific Science 37(2):87-107.
Scott, S.A., M.P. Davey, J.S. Dennis, I. Horst, C.J. Howe, D.J. Lea-Smith, and A.G. Smith. 2010. Biodiesel from algae: Challenges and prospects. Current Opinion in Biotechnology 21(3):277-286.
Sharif, D.I., J. Gallon, C.J. Smith, and E. Dudley. 2008. Quorum sensing in cyanobacteria: N-octanoyl-homoserine lactone release and response, by the epilithic colonial cyanobacterium Gloeothece PCC6909. ISME Journal 2(12):1171-1182.
Sheehan, J., T. Dunahay, J. Benemann, and P. Roessler. 1998. A Look Back at the U.S. Department of Energy’s Aquatic Species Program: Biodiesel from Algae. Golden, CO: National Renewable Energy Laboratory.
Shen, Y., W. Yuan, Z.J. Pei, Q. Wu, and E. Mao. 2009. Microalgae mass production methods. Transactions of the ASABE 52(4):1275-1287.
Sierra, E., F.G. Acien, J.M. Fernandez, J.L. Garcia, C. Gonzalez, and E. Molina. 2008. Characterization of a flat plate photobioreactor for the production of microalgae. Chemical Engineering Journal 138(1-3):136-147.
Smith, A.G., S.A. Scott, M.P. Davey, J.S. Dennis, I. Horst, C.J. Howe, and D.J. Lea-Smith. 2010a. Biodiesel from algae: Challenges and prospects. Current Opinion in Biotechnology 21(3):277-286.
Smith, V.H., B.L. Foster, J.P. Grover, R.D. Holt, M.A. Leibold, and F. deNoyelles, Jr. 2005. Phytoplankton species richness scales consistently from laboratory microcosms to the world’s oceans. Proceedings of the National Academy of Sciences of the United States of America (102):4393-4396.
Smith, V.H., B.S.M. Sturm, F.J. deNoyelles, and S.A. Billings. 2010b. The ecology of algal biodiesel production. Trends in Ecology and Evolution 25:301-309.
Solazyme. 2012. Homepage. Available online at www.solazyme.com. Accessed February 9, 2012.
Solix Biofuels. 2011. The Lumian™ AGS4000: A high productivity algae growth system. Available online at http://www.solixbiofuels.com/content/products/lumian-ags4000. Accessed February 9, 2012.
Solvent Rescue Limited. 2012. Homepage. Available online at http://solventrescue.co.nz/. Accessed June 16, 2012.
Spirulinasource. 1999. Yaeyama farm in Southern Japan grows chlorella in circular ponds. New Ambadi farm in India grows spirulina in raceway style ponds. Available online at http://www.spirulinasource.com/earth-foodch6c.xhtml. Accessed May 15, 2012.
Spolaore, P., C. Joannis-Cassan, E. Duran, and A. Isambert. 2006. Commercial applications of microalgae. Journal of Bioscience and Bioengineering 101(2):87-96.
Stephens, E., I.L. Ross, J.H. Mussgnug, L.D. Wagner, M.A. Borowitzka, C. Posten, O. Kruse, and B. Hankamer. 2010. Future prospects of microalgal biofuel production systems. Trends in Plant Science 15(10):554-564.
Stockenreiter, M., A.K. Grabe, H. Haupt, and H. Stibor. 2012. The effect of species diversity on lipid production by micro-algal communities. Journal of Applied Phycology 24(1):45-54.
Stomp, M., J. Huisman, L.J. Stal, and H.C.P. Matthijs. 2007. Colorful niches of phototrophic microorganisms shaped by vibrations of the water molecule. ISME Journal 1(4):271-282.
Sukenik, A., and G. Shelef. 1984. Algal autoflocculation—Verification and proposed mechanism. Biotechnology and Bioengineering 26(2):142-147.
Suzuki, L., and C.H. Johnson. 2001. Algae know the time of day: Circadian and photoperiodic programs. Journal of Phycology 37(6):933-942.
Tang, E.P.Y., W.F. Vincent, D. Proulx, P. Lessard, and J. de la Noue. 1997. Polar cyanobacteria versus green algae for tertiary waste-water treatment in cool climates. Journal of Applied Phycology 9(4):371-381.
Taton, A., E. Lis, D.M. Adin, G. Dong, S. Cookson, S.A. Kay, S.S. Golden, and J.W. Golden. 2012. Gene transfer in Leptolyngbya sp. Strain BL0902, a cyanobacterium suitable for production of biomass and bioproducts. Plos One 7(1):e30901.
Teplitski, M., H. Chen, S. Rajamani, M. Gao, M. Merighi, R.T. Sayre, J.B. Robinson, B.G. Rolfe, and W.D. Bauer. 2004. Chlamydomonas reinhardtii secretes compounds that mimic bacterial signals and interfere with quorum sensing regulation in bacteria. Plant Physiology 134(1):137-146.
Thelen, J.J., and J.B. Ohlrogge. 2002. Metabolic engineering of fatty acid biosynthesis in plants. Metabolic Engineering 4:12-21.
Tillmann, U. 2004. Interactions between planktonic microalgae and protozoan grazers. Journal of Eukaryotic Microbiology 51(2):156-168.
Toyomizu, M., K. Suzuki, Y. Kawata, H. Kojima, and Y. Akiba. 2001. Effective transformation of the cyanobacterium Spirulina platensis using electroporation. Journal of Applied Phycology 13(3):209-214.
Trent, J. 2011. Offshore Membrane Enclosure for Growing Algae. Presentation to the NRC Committee on Sustainable Development of Algal Biofuels on September 8.
Tsukuda, O., T. Kawahara, and S. Miyachi. 1977. Mass culture of Chlorella in Asian countries. Pp. 363-365 in Biological Solar Energy Conversion, A. Mitsui, S. Miyachi, A. San Pietro and S. Tamura, eds. New York: Academic Press.
Turchetto-Zolet, A.C., F.S. Maraschin, G.L. de Morais, A. Cagliari, C.M.B. Andrade, M. Margis-Pinheiro, and R. Margis. 2011. Evolutionary view of acyl-CoA diacylglycerol acyltransferase (DGAT), a key enzyme in neutral lipid biosynthesis. BMC Evolutionary Biology 11:263.
Uduman, N., Y. Qi, M.K. Danquah, G.M. Forde, and A. Hoadley. 2010. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. Journal of Renewable and Sustainable Energy 2(1): ISSN (Online) 1941-7012.
Ugwu, C.U., H. Aoyagi, and H. Uchiyama. 2007. Photobioreactors for mass cultivation of algae. Bioresource Technology 99:4021-4028.
Ugwu, C.U., J.C. Ogbonna, and H. Tanaka. 2002. Improvement of mass transfer characteristics and productivities of inclined tubular photobioreactors by installation of internal static mixers. Applied Microbiology and Biotechnology 58(5):600-607.
UTEX (The Culture Collection of Algae at the University of Texas at Austin). 2012. Cryopreservation of algae. Available online at http://web.biosci.utexas.edu/utex/protocols.aspx. Accessed June 16, 2012.
Van den Hoek, C., D.G. Mann, and H.M. Jahns. 1995. Algae: An Introduction to Phycology. Cambridge, UK: Cambridge University Press.
Vandamme, D., S.C.V. Pontes, K. Goiris, I. Foubert, L.J.J. Pinoy, and K. Muylaert. 2011. Evaluation of electro-coagulation-flocculation for harvesting marine and freshwater microalgae. Biotechnology and Bioengineering 108(10):2320-2329.
Vasquez, V., and P. Heussler. 1985. Carbon dioxide balance in open air mass culture of algae. Archiv für Hydrobiologie, Ergebnisse der Limnologie, Beiheft 20:95-113.
Viswanathan, T., S. Mani, K.C. Das, S. Chinnasamy, and A. Bhatnagar. 2011. Drying characteristics of a microalgae consortium developed for biofuels production. Transactions of the ASABE 54(6):2245-2252.
Vunjak-Novakovic, G., Y. Kim, X. Wu, I. Berzin, and J.C. Merchuk. 2005. Air-lift bioreactors for algal growth on flue gas: Mathematical modeling and pilot-plant studies. Industrial and Engineering Chemistry Research 44(16):6154-6163.
Waaland, J.R., J.W. Stiller, and D.P. Cheney. 2004. Macroalgal candidates for genomics. Journal of Phycology 40(1):26-33.
Waditee, R., T. Hibino, T. Nakamura, A. Incharoensakdi, and T. Takabe. 2002. Overexpression of a Na+/H+ antiporter confers salt tolerance on a freshwater cyanobacterium, making it capable of growth in sea water. Proceedings of the National Academy of Sciences of the United States of America 99(6):4109-4114.
Wang, X., X. Liu, and G. Wang. 2011. Two-stage hydrolysis of invasive algal feedstock for ethanol fermentation. Journal of Integrative Plant Biology 53(3):246-252.
Wang, Z.T., N. Ullrich, S. Joo, S. Waffenschmidt, and U. Goodenough. 2009. Algal lipid bodies: stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii. Eukaryotic Cell 8(12):1856-1868.
Wargacki, A.J., E. Leonard, M.N. Win, D.D. Regitsky, C.N.S. Santos, P.B. Kim, S.R. Cooper, R.M. Raisner, A. Herman, A.B. Sivitz, A. Lakshmanaswamy, Y. Kashiyama, D. Baker, and Y. Yoshikuni. 2012. An engineered microbial platform for direct biofuel production from brown macroalgae. Science 335:308-313.
Weckwerth, W. 2011. Green systems biology—From single genomes, proteomes and metabolomes to ecosystems research and biotechnology. Journal of Proteomics 75(1):284-305.
Weis, J.J., D.S. Madrigal, and B.J. Cardinale. 2008. Effects of algal diversity on the production of biomass in homogeneous and heterogeneous nutrient environments: A microcosm experiment. Plos One 3(7):e2825.
Weissman, J.C., D.T. Tillett, and R.P. Goebel. 1989. Design and Operation of an Outdoor Microalgae Test Facility. Golden, CO: Solar Energy Research Institute.
Wijffels, R.H., and M.J. Barbosa. 2010. An outlook on microalgal biofuels. Science 329(5993):796-799.
Williams, P.J.L.B., and L.M.L. Laurens. 2010. Microalgae as biodiesel and biomass feedstocks: Review and analysis of the biochemistry, energetics and economics. Energy and Environmental Science 3(5):554-590.
Woo, M., C. Smith, and W. Smith. 2000. Ecological interactions and impacts of invasive Kappaphycus striatum in Kane’ohe Bay, a tropical reef. Pp. 186-192 in Marine Bioinvasions, J. Pedersen, ed. Cambridge, MA: Massachusetts Institute of Technology, Sea Grant College Program.
Wright, M.M., D.E. Daugaard, J.A. Satrio, and R.C. Brown. 2010. Techno-economic analysis of biomass fast pyrolysis to transportation fuels. Fuel 89:S11-S19.
Xtrudx Technologies, Inc. 2012. Homeage. Available online at http://www.xtrudx.com/about.xhtml. Accessed June 16, 2012.
Yue, L., and W. Chen. 2005. Isolation and determination of cultural characteristics of a new highly CO2 tolerant fresh water microalgae. Energy Conversion and Management 46(11-12):1868-1876.
Zhao, F.Q., X.W. Zhang, C.W. Liang, J.Y. Wu, Q.Y. Bao, and S. Qin. 2006. Genome-wide analysis of restriction-modification system in unicellular and filamentous cyanobacteria. Physiological Genomics 24(3):181-190.
Zheng, P., W.B. Allen, K. Roesler, M.E. Williams, S. Zhang, J. Li, K. Glassman, J. Ranch, D. Nubel, W. Solawetz, D. Bhattramakki, V. Llaca, S. Deschamps, G.Y. Zhong, M.C. Tarczynski, and B. Shen. 2008. A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nature Genetics 40(3):367-372.
Zimba, P.V., M.J. Sullivan, and H.E. Glover. 1990. Carbon fixation in cultured marine benthic diatoms. Journal of Phycology 26(2):306-311.
Zmora, O., Richmond, A. 2003. Microalgae production for aquaculture. Pp. 365-379 in Handbook of Microalgal Culture, Biotechnology and Applied Phycology. A. Richmond, ed. Boston, MA: Blackwell Publishing.
Zou, J.T., V. Katavic, E.M. Giblin, D.L. Barton, S.L. MacKenzie, W.A. Keller, X. Hu, and D.C. Taylor. 1997. Modification of seed oil content and acyl composition in the brassicaceae by expression of a yeast sn-2 acyltransferase gene. Plant Cell 9(6):909-923.


















































