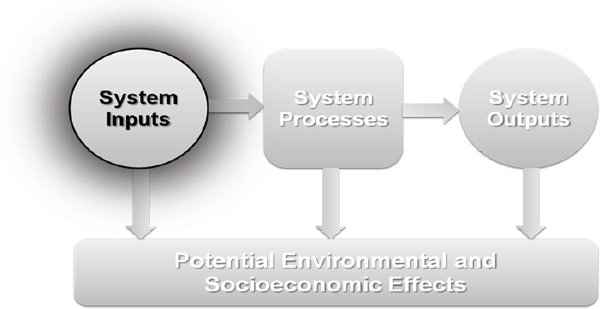
Fuel production from fossil and biological feedstocks is resource intensive, and algal biofuels require resource inputs in the form of water, energy, land, and nutrients. Algal biofuels have been produced at small scale, sufficient to prove that there are a number of possible production pathways. Although production of algal biofuels is technically feasible, they have to be shown to be economically, environmentally, and socially sustainable to become a practical substitution for petroleum-based fuels. The scaling of the pathways for algal biofuel production that are deemed practical for commercial production poses a new demand on natural resources. The levels of nutrients, water, land, and energy necessary to
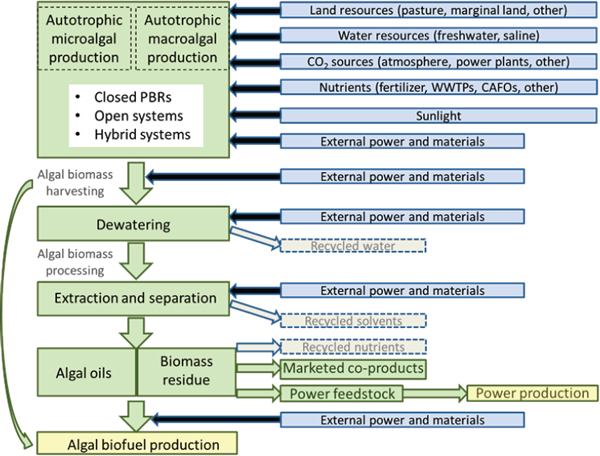
FIGURE 4-1 Resource requirements of algal biodiesel production.
NOTE: WWTPs denote municipal wastewater treatment plants. CAFOs are concentrated animal feeding operations.
produce alternative biofuels in an economically, environmentally, and socially sustainable way would have to be carefully considered (McKone et al., 2011). This chapter focuses on the current sustainability knowledge of the water, nutrient, land, and energy requirements of algal biofuels at all steps in the photoautotrophic algae-based production process (Figure 4-1). Where relevant data are available, quantitative case studies for at least two potential pathways for algal biofuel production are presented in this Chapter. In addition, potential assessment indicators are provided when appropriate, and current knowledge and data gaps are identified for each of the four resource requirement categories.
Major new advancements in the current knowledge base will require multi-hectare scale demonstration facilities to be built and maintained in operation for a period of time sufficient to allow detailed real-time analyses of the key variables required for commercial success (Campbell et al., 2011). Moreover, commercial-scale demonstrations will be necessary to assess and to improve algal biofuel technologies and their integration with the existing energy infrastructure (Sagar and van der Zwaan, 2006; Katzer, 2010). Innovations that result in reduced resource use along the entire algal biofuel supply chain will remove some of the existing barriers to the development of large-scale, sustainable, and economically viable algal biofuel enterprises. In addition, improvements in algal productivity and biofuel yield will help to reduce resource requirements per unit of algal biofuel produced.
Water provides the essential physical environment in which cultivated algae grow and reproduce (Murphy and Allen, 2011). It also acts as a thermal regulator and provides a
medium for essential nutrient resources—carbon dioxide (CO2), nitrogen (N), phosphorus (P), and other nutrients—for algal biomass production. Water has to be pumped to and contained and circulated in mass cultivation systems whether they involve either open ponds or closed photobioreactors. Closed photobioreactors also may use water spraying or submersion to maintain temperature of the culture. Given that the agricultural demand for water in the United States accounts for 85 percent of consumptive water use, large-scale production of biomass, including algae, has the potential for large regional strain on water systems unless nonfreshwater sources are used when possible.
Irrespective of the type of fuel produced, water is an integral element of fuel production, and thus an important nexus exists between fuel production and water supplies (Pate, 2007; Murphy and Allen, 2011). In the case of algal biofuel production, water is necessary for biomass feedstock production, and it can be lost during the processing of the algal biomass to fuels. This section discusses water requirement and consumptive use of fresh water along different steps of the algal biofuel supply chain and throughout the life cycle of algal biofuel production. In this report, water requirement refers to the quantity of water needed throughout the life cycle of algal biofuel production. Consumptive use of fresh water is the quantity of fresh water withdrawn from surface or groundwater sources that is lost to the immediate environment through evaporation or incorporation into products. In a sustainability assessment, the consumptive use of freshwater needs to be assessed in the context of regional water availability. For example, water withdrawn from a fossil aquifer that is declining quickly is less sustainable than the same amount of water withdrawn from an aquifer that replenishes more quickly. Where data are available, estimates for water use are compared to those for other biofuels and petroleum-based fuels.
4.1.1 Water Requirements in the Supply Chain
The water requirements of any algal cultivation system depend on the physical structure and configuration of the system, the local climate, and the ability to reclaim and reuse system water (Table 4-2; Murphy and Allen, 2011). Open ponds are subject to evaporative water losses (Yang et al., 2011) that are influenced by multiple factors including pond area, volume, and water level; water and air temperature; and wind velocity, humidity, and atmospheric pressure (Boyd and Gross, 2000). The average U.S. evaporation rate from a pond system is estimated to be 0.9 cubic meters of water per square meter per year (Murphy and Allen, 2011), but evaporative losses from open ponds vary by geographical region. Moreover, some operation regimes (for example, stirring and sparging) can increase the water loss to levels that are greater than would be predicted by evaporation and purging alone.
In outdoor open-pond algae cultivation, freshwater addition is necessary to compensate for evaporative water loss and to avoid salt buildup. Therefore, fresh water is necessary in any algal cultivation system, irrespective of the type of culture water used (Yang et al., 2011). The linkage between evaporative losses and purging, or blow-down, for an open-pond system is illustrated in Figure 4-2. A significant amount of water (Fout) is lost to evaporation in open ponds thereby concentrating total dissolved solids in the pond water. Whether they are fed with fresh water or with saline water, all algal cultivation systems have a control point for the maximum allowable concentration of dissolved solids that is maintained in the culture. If this set point is based on salinity, evaporation would raise the pond salinity so that steps would have to be taken to compensate for this increase. Addition of water with a lower salt concentration and flushing of water from the pond are two steps that can be taken to maintain salinity below the defined control point (xcontrol). Both steps can increase the water requirement and consumptive water use.
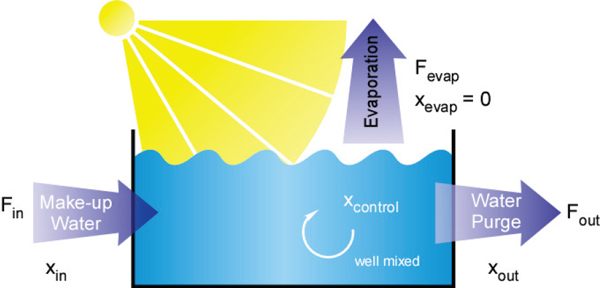
FIGURE 4-2 Dissolved solids control in a simplified open-pond cultivation system.
NOTE: Make-up water addition (Fin) and water purge (Fout) are used to control the critical concentration of total dissolved solids in the pond water (xcontrol). If the pond is well-mixed, the concentration of total dissolved solids in the purge (xout) is equal to the concentration in the pond (xcontrol).
In addition to evaporative losses, water can seep into and out of open ponds, particularly if they are clay-lined or if liner failure occurs. Water percolation is strongly influenced by the composition and texture of the underlying soils (for example, clay versus sand). Seepage rates are typically on the order of 5 to 6 millimeters per day (Weissman et al., 1989; Boyd and Gross, 2000), which is low compared to rates of evaporative water loss in many regions of the United States. In contrast to open ponds, closed photobioreactors are not affected by surface evaporation and seepage, and the lowest reported values for estimated water use are associated with closed systems. However, the water requirements of a photobioreactor system depend on its actual configuration and operating conditions.
The reclamation and recycling of water are key determinants of the total water requirements of both open-pond and closed photobioreactor systems. Whether and how much of the harvest water can be reclaimed and reused depend on the efficiency of separation processes, the quality of the return water, and the sensitivity of the algal culture itself to changes and impurities in the return water, including any waste products produced by the resident algae (Murphy and Allen, 2011).
The water requirement for processing of algal biomass to biofuel is small relative to evaporative losses during cultivation in open-pond systems. Water use for processing algal biomass to fatty-acid methyl ester (FAME) was estimated to be 1 liter per liter of biodiesel produced. Water loss during the drying of algae to prepare the biomass for processing to fuel is unavoidable, and some water also is unavoidably lost during the extraction of oil from algae and esterification of algal oil. However, Pate et al. (2011) stressed that evaporative water loss under operating conditions involving the inland use of water with a high salt content will result in salinity increases unless fresh water is used to make up for the loss or steps are taken either to mitigate or adapt to salt build-up. The use of inland saline water in algal biofuel production also could have other potential environmental effects (see Chapter 5).
4.1.2 Life-Cycle Water Requirements
Quantification of life-cycle water requirements of algal biofuel production would support managing future impacts on water demand and enable comparison of water use for algal biofuel with other fuels. The estimation of life-cycle use of any requirement for algal biofuel production (for example, water, nutrients, and energy), however, is complicated by the developing nature of the technologies. In addition to uncertainty as to how algal biofuel will evolve on the path of commercialization, there is also a lack of data on material and energy requirements of the current technologies.
An additional complication in open-pond algae cultivation is that water use varies significantly with climatic differences in temperature, humidity, and rainfall. Other biofuels and agricultural crops show such variability in water use. For example, regional variability in irrigation results in estimates of life-cycle water requirements to make ethanol from corn varying from 5 to 2,140 liters of water per liter of fuel, depending on in which U.S. state the corn is grown (Chiu et al., 2009). If the national average of water demand for corn-grain production is used, Chiu et al. (2009) estimated the water use for corn-grain ethanol to be 142 liters of water per liter of fuel. However, the geographical distribution of additional corn grown to meet ethanol demand is uncertain so that whether their water demand matches the national average for all corn also is unclear. The water intensity of open-pond algae cultivation depends critically on the future geographic distribution of cultivation. This future distribution is difficult to forecast, however, being based on the conflux of uncertain future technological performance, policy, and industry response. In the absence of a reliable forecast, studies of water intensity can clarify relationships between location, climate, and water use.
4.1.2.1 Life-Cycle Water Use of Freshwater Open-Pond Systems
A number of studies have analyzed water requirements of biofuel produced from algae cultivated in open-pond systems and include different phases of the life cycle (Harto et al., 2010; Wigmosta et al., 2011; Yang et al., 2011). There are large differences in assumptions and results among studies, which is not surprising given the challenges mentioned above. Table 4-1 summarizes the assumptions and results of three studies on open-pond algae cultivation to highlight differences in results and the origins of these differences. The results span over two orders of magnitude, from 32 to 3,650 liters of water per liter of algal biofuel. As a comparison, 1.9-6.6 liters of water are consumed to produce 1 liter of petroleum-based gasoline from crude oil or oil sands (King and Webber, 2008; Wu et al., 2009; Harto et al., 2010). Resolving the variability and uncertainty in these results is beyond the scope of this report. Instead, the goal of this report is to identify and prioritize issues that could affect the long-term sustainability of algal biofuels. Prioritization of research and development (R&D) for issues of concern could contribute to developing algal biofuels as a sustainable part of the energy future.
Harto et al. (2010) analyze life-cycle water requirements of a number of alternative transportation fuels, including corn-grain and switchgrass ethanol, soybean biodiesel, solar and wind generated electricity, and algal biofuels with algae cultivation in open-pond systems and closed photobioreactors. The scope of processes analyzed includes embodied water in facilities and vehicles. In most scenarios, water use in evaporation and fuel production dominate the life cycles. Scenario analyses that combine pessimistic versus optimistic assumptions for productivities with evaporation yield variability from 32 to 656 liters of fresh water per liter of biodiesel. The low-end scenario of 32 liters per liter applies to algae
TABLE 4-1 Summary of Assumptions and Results of Studies for Life-Cycle Water Requirements for Open-Pond Algal Biodiesel
|
|
|||||||||
| Assumptions | Estimated Water Loss or Use | ||||||||
|
|
|
||||||||
| Study | Case | Fuel Productivity (L/m2/yr) |
Net Evaporation (cm/d) |
Cultivation (L/L) |
Harvest (L/L) | Fuel Processing (L/L) |
Coproducts (%) | Other | Life-Cycle Water Use (L/L) |
|
|
|||||||||
|
Harto et al. (2010)a |
Low case |
5.5 |
0 |
0 |
01 |
31 |
46 |
1 |
32 |
|
Base case |
5.5 |
0.25 |
165 |
01 |
50 |
0 |
1 |
216 |
|
|
High case |
2.6 |
0.42 |
575 |
01 |
80 |
0 |
0.58 |
656 |
|
|
Wigmosta |
79.5 gal/yr production |
0.46 |
0.06 |
438 |
01 |
N/Ab |
0 |
0 |
438 |
|
200 gal/yr production |
0.46 |
0.20 |
1613 |
01 |
N/Ab |
0 |
0 |
1613 |
|
|
Yang et al. (2011) |
100% harvest recycle |
3.2 |
0.27 |
450 |
0 |
140 |
0 |
0 |
590 |
|
50% harvest recycle |
3.2 |
0.27 |
450 |
1550 |
140 |
0 |
0 |
2140 |
|
|
No harvest recycle |
3.2 |
0.27 |
450 |
3060 |
140 |
0 |
0 |
3650 |
|
|
|
|||||||||
aHarto et al.(2010) and Wigmosta et al.(2011) assumed 100 percent recycling of harvest water.
bWigmosta et al.(2011) did not estimate water use for processing algal biomass to fuels. They did not include water use upstream of algae cultivation (for example, water use for fertilizer production), but that amount is a small fraction of the total water requirement (Harto et al., 2010).
NOTES: L/L = liters water consumption per liter of biodiesel produced. As a comparison, life-cycle water use to produce gasoline from crude oil and oil sands has been estimated to be about 1.9-6.6 L/L.
TABLE 4-2 Life-Cycle Water Requirements to Produce Biofuel from Corn and Soybean
|
|
|||
| Crop | Study | Water Consumption (L/L) | Notes |
|
|
|||
|
Corn |
Chiu et al., 2009 |
513-1,402 |
Average water use when irrigated. Range comes from different U.S. states. |
|
Dominguez-Faus et al., 2009 |
5-2,138 |
Average irrigated and nonirrigated. Range comes from state variability. |
|
|
Chiu et al., 2009 |
142 |
National average of irrigated and nonirrigated. |
|
|
Soybean |
Dominguez-Faus et al., 2009 |
1,400-2,900 |
Average water use when irrigated. Range comes from different U.S. states. |
|
Harto et al., 2010 |
133 |
National average irrigated and nonirrigated. |
|
|
|
|||
NOTE: L/L = water consumption per liter of ethanol or biodiesel produced. Ethanol has about two-thirds of the energy content of gasoline. Biodiesel has about the same energy content as gasoline.
cultivated in regions in which rainfall makes up for evaporative losses. Allocation of water use to coproducts in addition to fuel can significantly reduce water use associated with the biofuel product.
Wigmosta et al. (2011) developed a geographically resolved model of variability in water and land requirements in different areas in the United States. They estimated water requirements that range from 22 to 3,600 liters of water per liter of oil depending on location of cultivation. Their assumptions for productivity of algae are much lower than those of Harto et al. (2010) and Yang et al. (2011), highlighting the uncertainty associated with critical factors driving material requirements. Wigmosta et al. (2011) also constructed scenarios that build out the geographical distribution of algal biofuel production, starting with areas with lower evaporation and more rainfall. They found steep increases in water requirements as production moves to more water-intensive areas. Yang et al. (2011) explored water recycling, use of saline water instead of fresh water, performance of different algal strains, and geographic variability. They find that recycling harvest water is critical in managing water requirements.
The committee reviewed what is known about water requirements for other algal biofuel pathways. Sapphire Energy estimated that its proposed biorefinery in Columbus, New Mexico, would require 3,500 acre feet (4.32 billion liters) of fresh water to produce 30,000 barrels (4.77 million liters) of green crude each year, or 906 liters of water per liter of green crude. The green crude can be upgraded to drop-in fuels (USDA-RD, 2009). Therefore, Sapphire Energy’s production pathway is comparable to either the reference pathway in Chapter 3 or the alternative pathway #1 depending on whether coproducts are included.
The estimates of life-cycle water use of algal biofuels (Table 4-1) were compared to those of other biofuels to explore whether algal biofuels are more or less water intensive than other biofuels. Table 4-2 shows results of studies of life-cycle water requirements of corn-grain ethanol and soybean biodiesel. For biofuels produced from corn grain, soybean, and algae cultivated in open ponds, water use depends more on the climate (rainfall in particular) where the biomass is grown rather than the type of biomass.
4.1.2.2 Life-Cycle Water Use in Closed Systems—Photobioreactors
Cultivating algae in photobioreactors has the potential to eliminate water consumption from evaporation, which could significantly reduce overall water demand. However, data for closed systems are even scarcer than for open systems. Harto et al. (2010) estimated the
life-cycle water requirements of a photobioreactor system at 30-63 liters fresh water per liter of biodiesel, though this result is based on expert opinion, not empirical measurement of a functioning system. The Algenol process is a closed photobiorector using sea water and fresh water. In its environmental impact assessment for a proposed biorefinery in Fort Meyers, Florida, Algenol estimated that the facility would require 3.6 million gallons of seawater and 210,000 gallons of fresh water to produce 100,000 gallons of algal ethanol each year (or 36 liters of salt water per liter of ethanol and 2.1 liters of fresh water per liter of ethanol) (DOE, 2010a). The freshwater use equals 3.15 liters of fresh water to produce each liter of gasoline-equivalent fuel. The Algenol estimate does not include upstream water use for inputs to their facilities.
4.1.2.3 Algae Cultivation Using Salt or Brackish Water or Wastewater
Using salt-tolerant algal species would allow the use of alternative water sources such as seawater, saline, and brackish groundwater, or coproduced water derived from oil, natural gas, and coal-bed methane wells (DOE, 2010b). This physiological flexibility of algae implies that locating algae production to areas where alternative water sources are available could reduce consumption of fresh water in cultivation. Cultivating saline algae in inland ponds also could reduce the potential for invasion of the ponds by undesirable freshwater organisms.
Vasudevan et al. (2012) estimated the consumption of fresh water in a saline water, open-pond, algae cultivation facility for three cases that they formulated—a base case (nominal, in their language) with reasonable assumptions in technology and system performance, a case with pessimistic assumptions, and a case with optimistic assumptions. The estimated requirement for freshwater make-up was 1,000 liters of freshwater per liter of oil, with a range of 200-2,000 liters from optimistic to pessimistic cases (Vasudevan et al., 2012). This result suggests that the need for freshwater make-up is significant when saline water is used for algae cultivation. However, the make-up water use depends on productivity and salinity limits of algae used, climate, and other uncertainties and variabilities that have yet to be resolved.
Wastewater also can be used in cultivating algae, thereby reducing groundwater and surface water consumption and treating wastewater by reducing nitrogen and phosphorus content. Pittman et al. (2011) discussed the potential benefits and limitations of using wastewater to produce algae for biofuels cost effectively, and concluded that dual-use microalgae cultivation for wastewater treatment and biofuel production has the potential to use up nutrients in wastewater and reduce the amount of fresh water required for biofuel generation from algal biomass. The potential environmental benefits and concerns of algal biofuel production using wastewater as a water and nutrient feed will be discussed further in Chapter 5 of this report, but this concept has not yet been tested at scale.
4.1.3 Scale-up Considerations
The freshwater demands of algal biofuel production will be high if algal biofuels are used to substitute for a significant fraction of annual U.S. liquid transportation fuel consumption, particularly if open ponds are to be used for algae cultivation. If open ponds are used for algae production, then a significant amount of water will be required to replace evaporative losses from the pond surface and to prevent dissolved salt buildup in the biomass cultivation system (Yang et al., 2011). Recent estimates reported by the U.S. Department of Energy (DOE, 2010b) suggest that water losses on the order of several hundred liters of water per liter of algal oil or algal biodiesel produced would result from the operation of open ponds in arid, sunny regions of the continental United States. The most
optimistic production scenario presented in DOE (2010b) was for the southwestern United States. Those estimates were based on high rates of areal production (31 grams per square meter [g/m2] per day) and high average cellular oil contents (50 percent by dry weight). Taking meteorological conditions into account, Wigmosta et al. (2011) estimated the consumptive water use to compensate for evaporative loss from ponds to be 312 trillion liters per year if algae are grown to produce 220 billion liters of algal biofuels. That amount is about twice the quantity of water used for irrigated agriculture in the United States (177 trillion liters in 2005; USGS, 2012). If they limit the algae cultivation to areas with high rainfall, such as areas near the Gulf Coast, the Great Lakes, and most of the eastern seaboard, then consumptive use of fresh water per unit fuel produced can be reduced by 75 percent.
Pienkos (2007) estimated that between 16 and 120 trillion gallons (60 and 454 trillion liters) of water per year would be required to produce the algal oil needed to produce 60 billion gallons (227 billion liters) per year of biodiesel. The amount of petroleum-based fuels consumed by the U.S. transportation sector in 2010 was about 207 billion gallons (783 billion liters) per year (EIA, 2011).
Pate et al. (2011) estimated the consumptive use of fresh water necessary to achieve target biodiesel production levels of 10 billion, 20 billion, and 50 billion gallons (37.8 billion, 75.7 billion, and 189 billion liters, respectively) per year from freshwater algae for four regions of the United States. Their estimates of water use for algal biofuel (Figure 4-3)
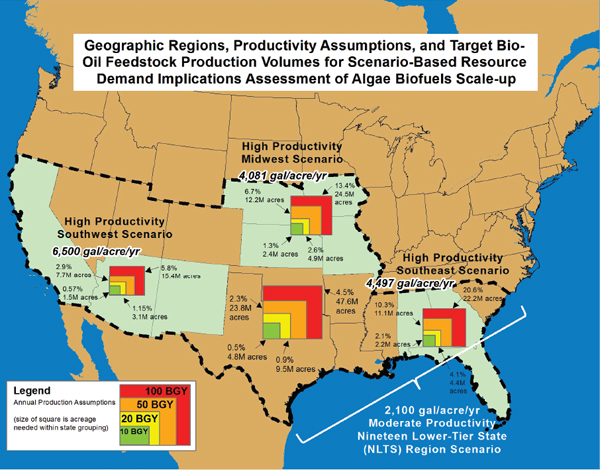
FIGURE 4-3 Algal biofuel scale-up scenarios for four different geographical regions of the United States: the Southwest, Midwest, Southeast, and nineteen lower-tier states.
SOURCE: Pate et al. (2011).
NOTE: 1 gallon = 3.78 liters; 1 acre = 0.40 hectare; 1 gallon per acre = 9.5 liters per hectare.
varied with the geographic region, the volume of feedstock production targeted, and the algal productivity assumed to be achieved. The projections of Pate et al. (2011) suggest that using fresh water only in open-pond algal production systems likely will be a significant sustainability issue if 10 billion gallons (37.8 billion liters) of biodiesel are to be produced each year, depending on the region. Cultivating freshwater algae to 10 billion gallons (37.8 billion liters) per year production appeared to be unattainable in the Midwest and Southeast regions due to water requirements, which represent more than 70 percent and more than 170 percent of the total water used for irrigation in the Midwest and in the Southeast, respectively.
Although water requirements for algal biofuels are estimated to be higher than those of petroleum-based fuels, sustainable use of freshwater needs to be considered in the context of regional availability and other competing uses (NRC, 2011). For example, a petroleum refinery located in a dry region, where groundwater recharge is low with water shortage could be more detrimental to its local supply than would open-pond algal cultivation systems located in a region with rising groundwater level, even though the petroleum refinery uses considerably less water than algae cultivation. The total demand on local water resources by algal biofuel production will depend on management practices for individual facilities (for example, the type and quantity of water used), the number of facilities located within a watershed, and both the existing volume and time trends in the volume of local aquifers, as influenced by competing water uses. Water use and freshwater and salinewater withdrawals in the United States have been estimated by the U.S. Geological Survey (Kenny et al., 2009).
Pate et al. (2011) suggested that the irrigation water from other agricultural applications will need to be diverted to algal biofuel production if 10 billion gallons of fuels are to be produced from algae cultivated in fresh water. Diverting irrigation water from agriculture to algae cultivation for fuels will raise the concern of water use for fuel versus food and feed. Large water withdrawals from surface waters or from groundwater that is connected to surface water systems can affect ecosystems. Many ecosystems require minimum seasonal flows to support life cycles of fish (Jager and Rose, 2003, Nagrodski et al., 2012) and riparian vegetation (Stromberg et al., 1996, Greet et al., 2011). Stream macroinvertebrate communities and diversity also are affected by stream flow (Dewson et al., 2007). In some regions, groundwater depth can affect terrestrial vegetation composition and nutrient cycling (Goedhart and Pataki, 2011). Effects of water withdrawals for algae cultivation on ecological populations and ecosystem processes would be important to consider in concert with effects of irrigation, hydropower, industrial water use, and municipal water use.
Pate et al. (2011) stressed that approaches are needed for algal biofuel production that use nonfreshwater such as coastal marine water; wastewater from agricultural, municipal, and industrial sources; brackish or saline groundwater; and produced water from oil, gas, and coal-bed-methane wells. Cost-effective approaches for reducing evaporative water loss and for dealing with salinity build-up need to be developed. Such approaches will be more important for inland sites where evaporation and salinity build-up are expected to be higher than in coastal marine operational settings that have high relative humidity.
4.1.4 Sustainability Indicators
The sustainability implications of water use are difficult to quantify. Many studies use consumptive water use as a measure. Consumption is withdrawal and subsequent “loss” of ground or surface water through evaporation or runoff. The link between water consumption and sustainability effects, such as ecosystem change or scarcity for human
needs, depends on local conditions. As water supplies are increasingly stressed, there is an increasing need for methods to connect different uses of water to sustainability impacts.
Indicators of sustainability of freshwater requirements for algal biofuel production include the following (Mulder et al., 2010; GBEP, 2011):
• Consumptive freshwater use expressed as kilograms of water per kilogram of fuel produced (biodiesel or ethanol) or liters of water per liter of fuel produced.
• Energy return on water invested (EROWI), megajoule per liter (MJ/L).
These indicators permit general comparisons among sites, feedstocks, and production technologies, but do not provide information about sustainability relative to local supplies. Additional project-specific and site-specific information—total consumptive water use by a facility relative to current supply at the site and relative to projected future demands for all purposes, including biofuel production—will be required for this purpose. For example, a facility estimated to require 1 percent of available supply in an area that is not expected to experience significant population growth or increased agricultural demand for water is likely to be more sustainable than a facility requiring 50 percent of available supply in an area with a rapidly growing population or agricultural demand.
Indicators in addition to water consumption also are used. Water withdrawal refers to the quantity of fresh or ground water withdrawn. The use of green, blue, and gray water footprints are gaining interest in some research communities (Gerbens-Leenes et al., 2009; Hoekstra, 2009). Green water is rainwater evaporated during production such as crop growth. Blue water is irrigation water evaporated during crop growth. Grey water is the quantity of water needed to dilute pollutants from a process to meet water-quality standards. The choice of which of these indicators to use is a matter of debate; it is important that researchers report raw data on water use in addition to processed results for indicators.
4.1.5 Information and Data Gaps
Evaporation during cultivation is a major contributor to life-cycle water requirements. Some studies use pan evaporation to approximate water use in algae cultivation (Harto et al., 2010; Yang et al., 2011). Evaporation from algal ponds could, however, behave differently from pan evaporation. Wigmosta et al. (2011) used mathematical models intended to improve upon the use of pan evaporation data. Empirical data from actual ponds in various operating conditions would enable validation and construction of improved models. The extent to which water can be recycled in harvesting and other process steps also is a critical factor. Empirical data on and actual experience with water recycling in cultivation systems are needed.
Water balance and management, along with issues associated with potential salt buildup and salt management, are essential areas for future research, modeling, and field assessment (NRC, 2008; Gerbens-Leenes et al., 2009). If nonfreshwater is to be used in algae cultivation to alleviate consumptive use of fresh water, then current knowledge of the extent, the water quality and chemistry, and the sustainable withdrawal capacity of those nonfreshwater resources needs to be expanded (DOE, 2010b). For example, Subhadra and Edwards (2011) described the principal fresh and saline aquifers located in the southwestern United States, but comprehensive information on the geography and availability of fresh and saline aquifers in other regions suitable for algal biofuel production is needed. Although saline aquifers in the United States were mapped in the 1960s (Feth, 1965), the depth of the aquifer and other factors, such as aquifer hydraulic conductivity and well
yield, largely are unknown. The distribution and physical and chemical characteristics of saline groundwater resources need to be defined to predict the effects of extracting saline groundwater on freshwater resources and on the environment (Alley, 2003). Without such information, coastal regions may be more suitable for large-scale saltwater algal production systems than inland regions (Darzins et al., 2010).
Data on the regional availability of fresh water, salt water, and other nonfreshwater (for example, wastewater) and on the regional demand of water for agriculture and other uses are needed to assess the potential availability of different water resources for algae cultivation.
4.1.6 Potential Effect on Social Acceptability
Water security is a pressing concern globally and an emerging concern in the United States. Algal biofuel production will, to a still unspecified extent, affect consumptive use of fresh water. Freshwater availability and quality are intricately related to agricultural productivity, human health, and safety. The security impacts to this system from largescale algal biofuel production could be significant. As global weather patterns continue to become more and more extreme, resulting in harsh, prolonged drought in arid climates, uncertainty over water availability has begun to threaten geopolitical stability and represents a serious risk to human health. While the relative abundance of freshwater resources and advanced water transportation and irrigation infrastructure has insulated the United States from the immediate and severe public health and water security issues that many nations currently face, access to fresh water in multiple regions of the country is increasingly limited and likely will become a major national security concern in the future. The Ogallala Aquifer in the Midwestern United States, yielding approximately 30 percent of the U.S. groundwater used for irrigation and supplying 82 percent of the potable water for those living within the aquifer boundary, could be completely depleted in as little as two to three decades (Guru and Horne, 2001). The Southwest has been facing and will continue to face serious water shortages in the coming decades, as aquifers are drained and surface water resources become increasingly scarce.
In recent years, public concern over energy security generally has overshadowed those for water security as oil prices have fluctuated. However, in the coming years, public concerns over water availability and the associated food security and health risks could increase and override those for energy security. If the algal biofuel industry relies heavily on freshwater resources, it could face a considerable setback as the increased use of freshwater resources becomes less acceptable to the public. This will be particularly damaging if infrastructure is already in place and capital already has been deployed in facilities that are subsequently shut down over concerns for their consumptive use of fresh water. Therefore, water recycling and use of nonfreshwater resources are important to ensuring the social acceptability of the large water requirements for algal biofuel production.
Algae require key elemental nutrients for metabolic maintenance and growth, as is true of terrestrial plants. The exact elemental stoichiometry of algal cells varies from one environment to another and among different algal species. However, photoautotrophic algae use photosynthesis to convert light energy into new algal biomass with an elemental stoichiometry that on average obeys the following equation (Stumm and Morgan, 1988):
![]()
Rearranging Eq. 4-1, the elemental content of algae can be expressed more simply as:
(CH2O)106(NH3)16(H3PO4) (Eq. 4-2)
The carbon-to-nitrogen-to-phosphorus stoichiometry of algae can be considered to average C106:N16:P1 by moles, a value that is commonly known as the Redfield ratio (Redfield, 1958). Although the Redfield ratio is not a universal biochemical optimum (see Sardans et al., 2011b), Eq. 4-2 allows quantitative predictions to be made about the carbon, nitrogen, and phosphorus demands of algal biomass production. As implied in Eq. 4-1, CO2 is essential for the photosynthetic production of algal biomass, providing elemental carbon that is required for the cellular synthesis of organic biomolecules, including the carbohydrates and lipids that can be converted into liquid biofuels (Falkowski and Raven, 2007). Nitrogen-containing molecules are involved in energy capture and release, cell structure, and metabolism. Phosphorus-rich molecules are essential to energy transfer. Both nitrogen and phosphorus are essential components of the genetic polymers ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) (Sterner and Elser, 2002). Providing sufficient and temporally stable supplies of CO2, nitrogen, and phosphorus is essential if algal biofuel production is to be deployed at large scale. If the species being cultivated are dominated by silicon (Si)-requiring taxa, such as diatoms, then adequate Si would have to be provided. Unlike water requirements, the amount of nutrients needed to promote algal growth can be expected to be the same whether open ponds or closed photobioreactors are used. However, nutrients can be recycled more readily in closed photobioreactors than in open ponds. The extent and efficiency of nutrient recycling that is used in the post-cultivation processing of algal biomass into biofuels and coproducts will affect the net nitrogen and phosphorus requirements of biofuel production systems. Nutrients cannot be recycled 100 percent because of losses as a result of precipitation1 and nutrients tied up in dead algal biomass. The dead algal biomass cannot be left in the pond to mineralize because of undesirable consequences to the culture medium. The formation of such suspended sludge and the accompanying dissolved organic matter is a sink for nutrients and reduces light availability for the growth of live algae. These practical problems of nutrient recycling have not been discussed in the literature.
Understanding the limitations and needs for CO2 is critical in addressing productivity of feedstock formation (that is, biomass or lipid yield depending on the processing pathway used to produce fuel). Feedstock productivity, in turn, affects the economic viability of algal biofuels. If relatively pure streams of supplemental CO2 are required for algae cultivation, siting algae cultivation facilities close to this resource could reduce transportation costs. However, the proximity to this resource could exacerbate the siting limitations imposed by flat land and sufficient water resources. (See section on Land in this chapter.) Most published studies on algal biofuels acknowledge that efficient algae production requires external sources of concentrated CO2 (supplied in gaseous form or as bicarbonate), in part because the rate of resupply of CO2 to algal cultivation systems from the atmosphere can be limited by diffusion across the air-water interface (DOE, 2010b; Williams and Laurens, 2010; Pate et al., 2011). As noted in Chapter 2, the provision of supplementary CO2 can stimulate algal biomass yield. For example, Yue and Chen (2005) observed more than a doubling
_______________
1 Amount of nutrient loss due to precipitation depends on the chemical composition of the rainwater and the nutrient composition of culture water.
of Chlorella biomass when CO2 concentrations were increased over ambient atmospheric levels (see Figure 2-11 in Chapter 2). From an engineering perspective, maximum biomass yields can be achieved only with aeration or supplementation. Companies responding to the committee’s solicitation of input on resource requirements indicated that they rely on supplemental CO2 to maximize algae production.
Because atmospheric CO2 concentration has been increasing, aquatic scientists have begun to assess the potential effects of CO2 concentration on algal productivity and stoichiometry. In most algae, the activity of the primary photosynthetic enzyme ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco) is less than half-saturated under CO2 levels in equilibrium with the atmosphere, suggesting that CO2 is a factor that limits the rate of photosynthetic carbon fixation (Urabe et al., 2003). For example, by using experimental manipulations of the CO2 concentration in supersaturated boreal lakes, Jansson et al. (2012) demonstrated that rates of phytoplankton primary production were up to tenfold higher in CO2-supersaturated lake water relative to water containing CO2 at equilibrium concentrations. Moreover, the supplementation of algal production systems with CO2 potentially can enhance algal biomass yields per unit nitrogen and phosphorus in algal cells, especially under low-light conditions, thereby increasing nitrogen and phosphorus use efficiency. Even a moderate increase in CO2 could potentially yield increased C:N and C:P ratios in algae (Leonardos and Geider, 2005; Hessen and Anderson, 2008; Van De Waal et al., 2010). In a recent large-scale outdoor experiment, a threefold increase in CO2 relative to current atmospheric concentrations resulted in algal C:N ratios (8.0 by moles) that exceeded Redfield stoichiometry (Riebesell et al., 2007).
4.2.1 Estimated Nutrient Requirements
4.2.1.1 Carbon Dioxide
The estimated CO2 requirements for algal biofuel production are substantial. For example, Pienkos (2007) estimated that 14-21 kilograms of CO2 is required to produce the algal biomass needed to create 1 gallon of biodiesel (3.69-5.54 kilograms of CO2 per liter). Pate et al. (2011) estimated that 14-35 kilograms of CO2 is required to produce 1 gallon of algal oil (3.69-9.23 kilograms of CO2 per liter of algal oil). Both of these values can be compared with estimates made by algal biofuel companies for their demonstration facilities. For example, Algenol estimated that 734 tonnes of CO2 would be required to produce 100,000 gallons (378,000 liters) of ethanol each year in all its photobioreactors (DOE, 2010a); this is equivalent to 1.94 kilograms of CO2 per liter of ethanol or 2.91 kilograms of CO2 per liter of gasoline equivalent. Sapphire Energy estimated that 15,000 to 30,000 tonnes of CO2 would be used annually as an additive to promote algal growth for the production of 30,000 barrels (4.78 million liters) of green crude (USDA-RD, 2009); this is equivalent to 3.14-6.28 kilograms of CO2 per liter of green crude or gasoline equivalent, assuming that 1 liter of green crude can be upgraded to 1 liter of gasoline-equivalent fuel product.
4.2.1.2 Nitrogen and Phosphorus
Pate et al. (2011) analyzed the nitrogen and phosphorus requirements of a production system that uses open ponds for algae cultivation to produce algal oil. (This is analogous to the reference pathway or alternative pathway #1 in Chapter 3). They assumed Redfield C:N:P stoichiometry (Eq. 4-2) in algal biomass production. For simplicity, they assumed 100-percent nutrient uptake efficiencies, and did not account for the potentially higher
nitrogen and phosphorus inputs necessary to compensate for inefficiencies and losses in the biofuel production system. Nutrient recycling was not assumed in their model projections. Calculating by these assumptions, they projected that each metric tonne of dry weight algal biomass produced by their system required 88 kg N and 12 kg P. Assuming an algal biomass with 50-percent oil content, these nitrogen and phosphorus requirements are equivalent to 0.61 kg N and 0.083 kg P per gallon of algal oil produced (or 0.16 kg N and 0.022 kg P per liter of algal oil produced). However, if a 20 percent algal oil content is assumed, then these nutrient requirements increase to 1.5 kilogram of nitrogen and 0.21 kilogram of phosphorus per gallon of oil produced (or 0.40 kg N and 0.055 kg P per liter of algal oil produced). Similar estimates of 1.1 kilogram of nitrogen and 0.24 kilograms of phosphorus per gallon of biodiesel produced (0.29 kg N and 0.063 kg P per liter of biodiesel) have been reported by Yang et al. (2011).
Luo et al. (2010) suggested that direct ethanol synthesis could have lower nitrogen and phosphorus requirements than the reference pathway because cyanobacterial biomass is not removed from the production system during biofuel harvesting. Therefore, nitrogen and phosphorus are needed only for the growth and maintenance of the standing biomass; nutrients incorporated into the algal biomass are not lost during ethanol capture. Luo et al. (2010) estimated that an ethanol production of 56,000 liters per hectare (ha) per year would correspond to a nitrogen and phosphorus requirement of 0.065 g N/MJ and 0.0024 g P/MJ (0.002 kg N and 0.0001 kg P per liter of gasoline equivalent).
4.2.2 Scale-up Considerations
4.2.2.1 Carbon Dioxide
Anthropogenically produced CO2 can be used in algal biofuel production (DOE, 2010b). For example, flue gas from coal-fired power plants is a potential source of CO2 (Benemann et al., 2003a,b). The potential advantages of colocating algal production facilities with stationary industrial CO2 sources and potential barriers to their use are discussed in the report, National Algal Biofuels Technology Roadmap (DOE, 2010b). A map of power plant sources of CO2 located within 20 miles (32 kilometers) of municipal wastewater facilities in the preferred climate regions identified in the Sandia National Laboratories’ scoping assessment is also included in that report. If colocating with stationary sources of CO2 is not feasible, then the cost of capturing and transporting CO2 would have to be considered.
Under the scenario assumptions that Pate et al. (2011) used, about half of the stationary emission sources in the 19 lower-tier states would be needed to supply sufficient CO2 during daylight hours to support the production of 10 billion gallons (37.8 billion liters) per year of algal oil. For all other scenario regions (Figure 4-3), 90 percent to 150 percent of all CO2 emission sources would be needed to produce 10 billion gallons of algal oil. They concluded that only a small number of stationary emission sources would be within a reasonably affordable access range in regions of the United States that are best suited for large-scale algal biomass cultivation, unless a costly infrastructure for CO2 capture and pipelining is in place. Pienkos (2007) estimated that 0.9 billion to 1.5 billion tonnes of CO2 would be needed to produce algal oil for the much larger target of 60 billion gallons (227 billion liters) of biodiesel per year. This demand for CO2 is equivalent to 36 to 56 percent of all current CO2 emissions from all U.S. power plants.
If CO2 supplementation is required for high-yield production of algae, the extent to which algal biomass can be produced affordably at a commercial scale in the United States would be constrained. Moreover, as noted by Campbell et al. (2011), future commitments to
reducing atmospheric CO2 emissions under the Kyoto Protocol suggest that there could be a substantial move toward electricity generation with low CO2 emissions, for example, using renewable electricity (NAS-NAE-NRC, 2010) or coal-generated electricity with carbon capture and storage (NAS-NAE-NRC, 2009). Decarbonizing electricity generation would reduce the number of locations in which flue gas could be provided economically for algae cultivation for biofuels. Other sources and forms of inorganic carbon, such as the provision of solid bicarbonate, might be developed affordably for large-scale autotrophic microalgal biomass production (Pate et al., 2011). However, if the solid bicarbonate is mined from fossil sources, its use to produce algae for fuels could increase fossil energy input and the life-cycle of greenhouse-gas (GHG) emissions of algal biofuels (see Chapter 6 for discussion on GHG emissions).
4.2.2.2 Nitrogen and Phosphorus
The nitrogen and phosphorus demands that Pate et al. (2011) concluded would be necessary to support four levels of algal biodiesel produced from algae grown in four different geographical regions of the United States are shown in Table 4-3. The projected nitrogen requirements for the producing 10 billion gallons per year (37.8 billion liters per year; assuming a biomass oil content of 50-percent dry weight) of algal oil represents about half of
TABLE 4-3 Estimates of the Nitrogen and Phosphorus Resource Demands (in millions of metric tonnes/year) Required to Produce Different Levels of Algal Biodiesel Production in Different Geographical Regions of the United States
|
|
||||||||
| Scenario Region |
Total biomass (BM) produced and projected nitrogen (N) and phosphorus (P) neededa in millions of metric tonnes per year (M mt/year) | Projected nitrogen and phosphorus demand for algae as percent of total U.S. use in 2006b | ||||||
|
|
|
|||||||
| Nutrient use | 10 BGY (37.8 BLY) |
20 BGY (75.7 BLY) |
50 BGY (189.3 BLY) |
100 BGY (378.5 BLY) |
10 BGY (37.8 BLY) |
20 BGY (75.7 BLY) |
50 BGY (189.3 BLY) |
100 BGY (378.5 BLY) |
|
|
||||||||
|
SW, MW, and |
BM:70 |
BM:140 |
BM:350 |
BM:700 |
||||
|
SEc with 50% |
N:6.1 |
N:12.3 |
N:31 |
N:61 |
N:44 |
N:88 |
N:221 |
N:436 |
|
lipid |
P:0.8 |
P:1.7 |
P:4.2 |
P:8.3 |
P:20 |
P:41 |
P:102 |
P:202 |
|
NLTSd region |
BM:170 |
BM:350 |
BM:870 |
BM:1740 |
||||
|
with 20% lipid |
N:15 |
N:31 |
N:77 |
N:153 |
N:107 |
N:221 |
N:550 |
N:1093 |
|
P:2.1 |
P:4.2 |
P:10 |
P:21 |
P:51 |
P:102 |
P:244 |
P:512 |
|
|
|
||||||||
aAssuming elemental algal biomass composition C:N:P ratio of 106:16:1 (Redfield, 1934) and 100-percent nutrientuptake efficiency independent of algal productivity and cultivation system area at 50-percent dry-weight biomass lipid content for SW (Southwest), MW (Midwest), and SE (Southeast) scenarios, and 20-percent lipid content for scenario region.
bTotal U.S. consumption in 2006 estimated as 14.0 M mt elemental N consumed as ammonia and 4.1 M mt elemental P consumed as phosphate rock from Mineral Commodity Summaries in 2010 (USGS, 2010).
cWith scenario lipid productivities of ~6,500 (SW), ~4,100 (MW), and ~4,500 (SE) gal/acre (~24,600 [SW], ~15,500 [MW], and ~17,000 [SE] L/0.40 hectares) per year at 50-percent lipid.
dNLTS (nineteen lower-tier state region) scenario assumes moderate annual average algal lipid productivity of ~2,100 gal/acre (~7,950 L/0.40 hectares) per year at 20-percent lipid content over nineteen lower-tier states of AZ, AK, AL, CA, CO, FL, GA, IA, KS, LA, MO, MS, NE, NM, NV, OK, SC, TX, and UT.
Note: The table shows the four different levels of algal biofuel production (10, 20, 50, and 100 billion gallons per year; 37.8, 75.7, 189, and 379.5 billion liters per year (BLY), respectively) in four different geographical regions of the United States (Southwest, Midwest, Southeast, and nineteen lower-tier states). Bold values show problem levels for nutrient availability from commercial fertilizer.
SOURCE: Table 2 from Pate et al. (2011).
the total U.S. consumption of nitrogen from ammonia and about one-fifth of total U.S. consumption of phosphorus from phosphate rock in 2006. If the assumed average oil content decreases from 50 to 20 percent, these requirements are about 107 percent for nitrogen from ammonia and 51 percent for phosphorus of the total U.S. consumption in 2006. Assuming that nutrients in harvest water are not recycled, Pate et al. (2011) concluded that these algal biofuel-elevated demands for phosphorus likely would be unsustainable due to limited natural resource supplies (for example, Cordell et al., 2009; Vaccari, 2009).
4.2.3 Opportunities for Mitigation
Recycling of spent growth medium and of the residual nitrogen and phosphorus that remain in post-process algal biomass residuals will be essential for the sustainable production of algal biofuels. If anaerobic digestion is used to process waste algal biomass after lipid extraction, then cellular nitrogen and phosphorus can be recovered and recycled. The methane produced can be used to generate electricity and contribute to improving the energetic balance in the overall algae-to-biofuel process (Sialve et al., 2009). In addition, municipal and agricultural wastewater potentially can be used as nutrient feedstocks, thereby reducing external inputs of nitrogen and phosphorus fertilizers (see Chapter 5).
Sturm et al. (2012) performed a pilot-scale algal biomass production experiment using four outdoor bioreactors fed by effluent from a Lawrence, Kansas, wastewater treatment plant. Using wastewater for algae cultivation is expected to induce nitrogen-limited conditions for algal growth. These actively aerated bioreactors were run as continuous-flow systems at a hydraulic residence time of 10 days, without additional CO2 supplementation. In contrast to natural freshwater and marine ecosystems, the algal biomass produced in these wastewater cultivation systems had a lower average biomass C:P ratio of 67:1 by moles (and thus a higher phosphorus demand per unit carbon sequestered) than would be predicted either from Redfield stoichiometry or from the analysis of Sterner et al. (2008). That ratio is also at the lower end of the range of C:P ratios observed in nutrient-replete phytoplankton cultures (C:P = 64-86; see Table A2 in Geider and La Roche, 2002). In contrast, the algae produced by these bioreactors contained a higher average C:N ratio of 17:1 by moles (and thus a lower nitrogen demand) than predicted by Redfield or by Sterner et al. (2008). In addition, this value is at the upper limit of the range of C:N ratios observed in nutrient-replete phytoplankton cultures (C:N = 4-17; see Table A2 in Geider and La Roche, 2002). The algal biomass produced in these wastewater cultivation systems (Sturm et al., 2012) was rich in phosphorus (low C:P ratios), and algal growth appeared to be limited by nitrogen (high C:N ratios and low N:P ratios). It is thus unclear what C:N:P stoichiometry should be assumed when calculating the potential nitrogen and phosphorus demands of large-scale algal biomass production efforts, which potentially may be supplied with nutrient feedstocks having widely varying N:P supply ratios and levels of inorganic carbon supplementation.
Colocation of biofuel production facilities with power plants could provide the facilities with a ready source of supplemental CO2, but the number of sites suitable for colocation would constrain the use of these CO2 sources. The use of fossil bicarbonate could mitigate the CO2 constraint, but with consequent potential impacts on fossil energy input and lifecycle GHG emissions.
4.2.4 Sustainability Indicators
The key nutrients required for algae cultivation are CO2, nitrogen, and phosphorus. Corresponding general indicators of sustainability of nutrient requirements include (GBEP, 2011):
• kg CO2 required/L of fuel produced.
• kg CO2 required/tonne dry biomass of algae.
• kg N required/L of fuel produced.
• kg N required/tonne dry biomass of algae.
• kg P required/L of fuel produced.
• kg P required/tonne dry biomass of algae.
Additional indicators for assessment of nutrient requirements for diatoms:
• kg Si required/L of fuel produced.
• kg Si required/tonne dry biomass of algae.
Additional indicators that could be developed, analogous to EROWI, are the energy return per amount of nutrient input (MJ/kg, or its inverse—nitrogen or phosphorus use, kg/MJ), and nutrients required to meet various production targets relative to existing national usage or supply.
4.2.5 Information and Data Gaps
Earlier sections discussed the use of wastewater and recycling nutrients from lipidextracted algae as opportunities to reduce synthetic nitrogen and phosphorus inputs. Those opportunities are critical to meeting the sustainability objective of enhancing or maintaining (rather than depleting) natural resources. However, integrating wastewater treatment by algae with algae cultivation for fuels needs to undergo R&D and demonstration to optimize the systems and establish the feasibility of the concept. Similarly, R&D is needed to incorporate nutrient recycling into algal biofuel production systems. The potential for combining the use of wastewater in algae cultivation and the production of a fertilizer coproduct is worth further investigation.
With respect to estimating nutrient requirements, additional calculations of the potential nitrogen and phosphorus demands of algal biofuel production need be performed to confirm or modify the values suggested by Pate et al. (2011). (See Box 4-1 for detailed discussion.) The C:N:P stoichiometry of algae is critical to estimating the quantities of nutrients required for large-scale deployment of algal biofuels.
4.2.6 Potential Effects on Social Acceptability
Large-scale deployment of algal biofuels requires large inputs of nitrogen and phosphorus fertilizers. If the nutrients are not recycled or supplied from waste sources, nutrient requirements of algae for fuels could incur indirect and unintentional impacts on food prices (Pate et al., 2011). The long-term supply of phosphorus is also cause for concern, as many believe that the world’s supply of phosphate may have peaked or that a peak in supply is impending (Craswell et al., 2010). It will be detrimental to the algal biofuel industry if it is viewed as massive sink for nutrients that are in short supply, particularly if it is perceived that they are in direct competition with food producers. Technology development to use wastewater in algae cultivation and to recycle nutrients tied up in lipid-extracted algae could minimize potential competition for fertilizers between agricultural crops and algae cultivation.
BOX 4-1
Estimating Quantities of Nitrogen and Phosphorus Needed to Support an Algal Biofuel Industry
Pate et al. (2011) estimated the quantities of nitrogen and phosphorus needed to produce at least 38 billion liters of algal biofuel. Their analysis was based on the assumption of Redfield stoichiometry determined in marine systems (C106:N16:P1). However, the canonical Redfield ratio recently has been called into question by Sterner et al. (2008), who reviewed more than 2,000 measurements of the chemical content of suspended particulate matter (seston) from freshwater and marine ecosystems worldwide and documented an enormous level of variability in nutrient use efficiency. They found that small freshwater lakes exhibited higher average ratios of C:P = 224 (standard deviation = 156) and C:N = 10.0 (standard deviation = 3.0) than the Redfield stoichiometry (C:P = 106 and C:N = 6.6 by moles). Across their entire dataset, a non-Redfield proportionality of C166:N20:P1 best described the elemental composition of algae.
These trends potentially imply a higher nitrogen- and phosphorus-use efficiency (a higher yield of algal biomass per unit nitrogen and phosphorus consumed by algal cells) in artificial algal biomass production systems than was assumed in the study by Pate et al. (2011). Stoichiometric data provided in a recent algal biofuel study by Sturm et al. (2012) do not support this conclusion for phosphorus, however. Sterner et al. (2008) suggest that algal stoichiometry varies significantly with habitat type: freshwater seston tends to have a greater nutrient use efficiency (higher C:P and C:N ratios) than marine seston, implying that the future nitrogen and phosphorus demands of freshwater- versus marine-based algal biomass cultivation systems potentially could differ.
Another key question revolves around the potential effects of CO2 enrichment on algal nutrient-use efficiency. The responses of both vascular plants and phytoplankton to enhanced CO2 are variable and often species-dependent (Sardans et al., 2011a), and the consistency of CO2 effects remains uncertain. Given the observed variation in algal C:N:P stoichiometry that has been reported in the literature, three key questions therefore arise:
• What expected values (or what ranges of expected values) of C:P and C:N would best be used to update the analyses of Pate et al. (2011)?
• At what CO2 levels will consistent and predictable effects of CO2 enrichment on algal nutrient-use efficiency occur?
• Will the net effects of CO2 enrichment differ in single-strain algal cultivation systems versus systems that contain mixed-species assemblages?
These three unanswered questions represent research needs that can be filled only by field-based measurements of algal biomass yield and C:N:P stoichiometry, using pilot-scale or commercial-scale large outdoor photobioreactor systems operating under a wide range of environmental conditions.
A major constraint on the future expansion of biofuel production is likely to be the limited amount of land suitable for producing bioenergy crops and for expanding related refinery and transportation infrastructure (Cai et al., 2011). Much greater efforts will be needed to develop a comprehensive picture of the ideal siting locations for algae cultivation facilities (Darzins et al., 2010). Careful land-use planning to create specific locations where all-important resource demands can be met can help to build capacity and allow algae to make a vital, even if only modest, contribution to the U.S. biofuels industry (Lundquist et al., 2010).
The future development and scale-up of algal biofuels needs to be assessed from the multiple perspectives of site location, resource availability, and resource demands (DOE, 2010b). Key land considerations (Cai et al., 2011) can be expected to include:
• What total land area will be required for the proposed algal biofuel facility or facilities?
• What land and sites are potentially available?
• Where is this land located?
• What is this land currently being used for?
• What is the price of this land?
• What is the topography associated with this land?
• What are the climatic conditions associated with this land?
• Are the water resources required to support algal biofuel production available, either on or sufficiently near this land?
• Are the nutrient resources required to support algal biofuel production available, either on or sufficiently near this land?
• Which of the lands meeting all suitability criteria are available for actual facility siting and development?
4.3.1 Siting Requirements
The mass cultivation of algae is likely to be technically feasible in many regions of the United States (DOE, 2010b). However, the actual siting of future algal biofuel production facilities will be influenced by numerous economic, legal, political, physical, and social factors (Darzins et al., 2010). Complete detailed life-cycle assessments (LCA) and an environmental-impact assessment before any large-scale deployment are useful to and important for ensuring a smooth path to commercialization, particularly if the land being considered for siting has not ever been developed (Pienkos and Darzins, 2009). In addition, the sites where algal cultivation systems can be installed will be constrained by high land cost, agricultural activity, environmental value, and intrinsic cultural value of the land being considered (Darzins et al., 2010). Biofuel-driven land-use change (LUC) also potentially could create significant environmental impact and sustainability concerns (see Chapter 6). A diverse set of site-specific factors (Figures 4-4, 4-5, and 4-6; Table 4-4) would have to be matched carefully to the cultivation systems used for algal biofuel production if the essential requirements for successful large-scale algal biomass production (suitable land and climate, sustainable water supplies, and sustainable nutrient supplies) are to be aligned in terms of their geographical location (DOE, 2010b). For example, local variation in solar irradiance, day length, and air temperature determine the effective length of the growing season for high-yield algae cultivation, as is the case for natural lakes (Marshall and Peters, 1989). In addition, surface water evaporation rates (Figure 4-6), which affect water losses if open-pond systems are used for algae cultivation, vary geographically.
A preliminary high-level assessment of microalgal biomass production potential was performed by Sandia National Laboratories and reported in DOE (2010b). The climate criteria used in this spatially explicit analysis were: annual average cumulative sun hours of 2,800 hours or above, annual average daily temperature of 55°F (12.8°C) or higher, and annual average freeze-free days of 200 days or more. In terms of adequate sunlight and suitable climate, parts of Hawaii, California, Arizona, New Mexico, Texas, Louisiana, Georgia, and Florida appeared to be the most promising in the United States. Northern states such as Minnesota, Wisconsin, Michigan, and the New England states experienced strong seasonal variability in insolation and temperature (see Exhibit 9.4 in DOE, 2010b). The apparent lack
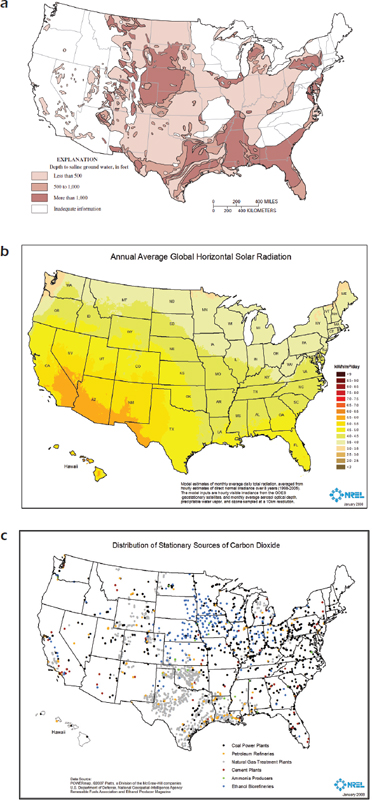
FIGURE 4-4 Resource availability for large-scale algae cultivation.
NOTE: (a) Depth to saline groundwater (units); (b) Annual average solar radiation (units); (c) Large stationary sources of CO2.
SOURCE: Pienkos and Darzins (2009).
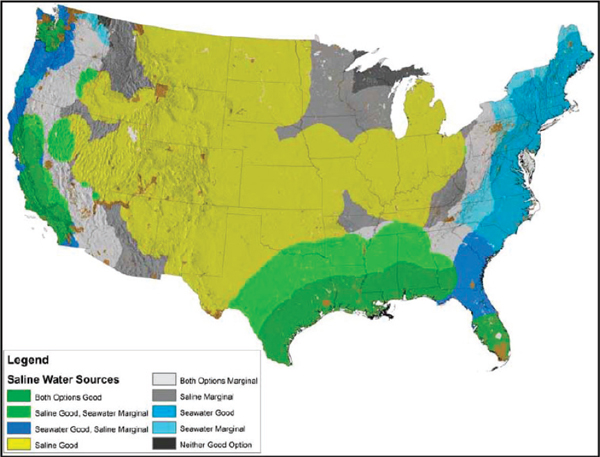
FIGURE 4-5 Map of seawater and saline groundwater resources in the continental United States, based on estimated potential energy production versus energy needed to transport water.
SOURCE: Pate (2011); map created by the Pacific Northwest National Laboratory.
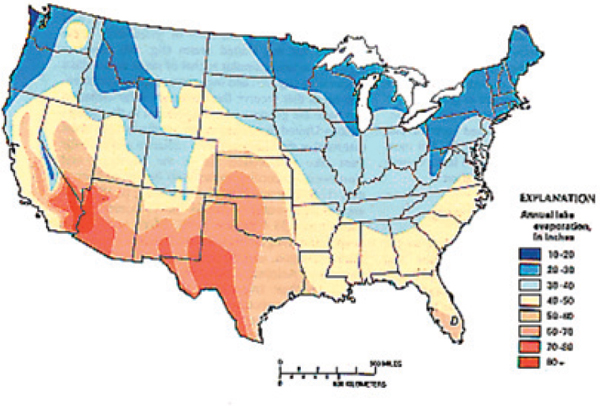
FIGURE 4-6 Mean annual lake evaporation in the continental United States.
SOURCE: Hanson (1991).
NOTE: 1 inch = 2.54 cm; 1 mile = 1.61 km.
TABLE 4-4 Key Land, Climate, and Water Resource Elements for Large-Scale Algal Biofuel Production
|
|
||
|
Land (Siting) |
Local Climate |
Water |
|
|
||
|
Topography |
Solar irradiance |
Location |
|
Land use and land cover |
Temperature |
Supply and demand |
|
Land ownership |
Evaporation rate |
Salinity |
|
Soil type and geology |
Severe weather |
Allocation |
|
|
||
SOURCE: Adapted from Exhibit 9.2 in DOE (2006).
of attractiveness of the Gulf Coast region in the Sandia study was attributed to lower annual average solar insolation. In contrast, another study by the Pacific Northwest National Laboratory (Wigmosta et al., 2011) suggested that annual productivities in this region were higher than those estimated by the Sandia study. However, the predictions by Sandia National Laboratories are consistent with the map of potential algal oil production developed by Wigmosta et al. (2011) for open-pond production systems (discussed in the next section Estimated Land Requirement).
At the site level, local topography and soils also can potentially limit the availability and suitability of land for open-pond cultivation. The installation of large shallow ponds requires flat terrain that has a slope of no more than 5 percent because of the intrinsic needs of the technology and because of increased costs of site development and fluid pumping (Darzins et al., 2010). Local wind conditions affect mixing and temperature in ponds and the integrity of pond liners. High wind in a dusty or sandy location could contribute to sediment loading to open-pond cultivation systems, which may require frequent clean up.
Darzins et al. (2010) stressed that siting biofuel production systems close to water and nutrient resources would place additional limits on algal biofuels’ contributions to future liquid transportation markets. They noted that even where land suitable for large-scale algal biofuel production exists, the local availability of essential resources could affect the economics of production, net energy return, and GHG emissions. The optimal sites for commercial-scale algal biofuel production would have either the required resources in close proximity or mechanisms in place to ensure adequate and uninterrupted supplies of these resources. In particular, access to large volumes of fresh water, saline water, or both will be essential for algae cultivation (see Figures 4-4a and 4-5). Darzins et al. (2010) expressed concern that the availability of sufficient supplies of supplemental CO2 is uncertain (see Figure 4-4c) in the geographical regions that are best suited to year-round algae cultivation.
In addition to the photosynthetic surface area necessary for algae cultivation, estimates of the total land required for an algae cultivation facility would include the space required for inoculum cultivation; systems for delivering inoculum to cultivation vessels; harvesting systems; reservoirs for holding water; waste management, storage, and recycling facilities; and other support systems (Murphy and Allen, 2011). For example, basins may be needed to hold water releases from blowdown in an open-pond system. Similarly, additional land for berm formation is required if the raceway ponds are constructed with earthworks. Murphy and Allen (2011) accounted for the land required for infrastructure to support the primary cultivation facilities by using a scaling factor of 1.6:1 when estimating the total area burden for open raceway ponds. This scaling factor is the ratio of photosynthetic pond surface area to the area of associated land needed to support those ponds. Clarens et al. (2011) used a smaller scaling factor of 1.25:1.
Algae cultivation facilities could be sited on unprofitable cropland (typically land that is not suitable for commodity crops), in which case the potential for large-scale algal biofuel production to affect food production would have to be considered. Pre-existing surface
water resources (for example, aquaculture ponds and coastal zone waters) also could be considered. In addition, algal biofuel facilities could be sited on lands that are highly suitable for solar energy production, such as arid lands in the southwest or sunny coastal regions. However, insufficient information is currently available to assess the viability or production capacity of these potential pathways. Moreover, potential tradeoffs (for example, water availability) would have to be considered.
Nutrient removal from wastewater by algae can be coupled with biofuel production (Pittman et al., 2011). For example, more than 15,000 existing U.S. domestic wastewater treatment plants (WWTPs) collectively produce about 34 billion gallons (128.7 billion liters) of wastewater effluent per day (EPA, 2008). Several thousand small (less than 10 ha) and a few large-scale (more than 100 ha) algal pond systems currently are being operated in the United States for municipal wastewater treatment (Lundquist et al., 2010). Food processing facilities and agricultural dairy and feedlot operations are other sources of nutrient-rich liquid wastes. Operations such as these potentially provide desirable colocation sites in which wastewater could be used as a water and nutrient source for integrated algal biomass production systems. One problem is that sufficient land is rarely available in WWTP locations in urban areas. Two types of systems can be envisioned: dedicated algae cultivation facilities, whose primary purpose would be the production of algal biomass (such systems also would require wastewater treatment and nutrient recycling); and wastewater treatment facilities, whose primary goal would be to perform wastewater treatment, but would produce harvestable algal biomass as a consequence of the treatment process (see Exhibit 9.7 and associated text in DOE, 2010b). Such integrated systems would be expected to have multiple benefits, including providing an inexpensive nutrient source, reducing demands for other sources of fresh water, potentially reducing operating costs for wastewater facilities, improving the quality of treated effluents, and potentially reducing GHG emissions (Craggs et al., 2011; Wiley et al., 2011). However, the number of potential colocation sites with sufficient adjacent land area that would be suitable for large-scale algal biomass cultivation is unclear.
Other innovative siting efforts might be feasible if suitable supplies of light and other resources are available. For example, aquaculture is an extensive industry in the United States; many catfish production ponds are out of production and thus potentially are available for alternative use in algal biofuel production. More than 100,000 hectares of catfish production ponds potentially could be available in the state of Mississippi alone (Hanson, 2006) if these existing facilities were found to be suitable for algal biomass production and harvesting.
4.3.2 Estimated Land Requirements
Algal biomass production potentially requires much less land area than terrestrial biofuel feedstock cultivation. The land requirements for algal biofuel production are potentially 1-2 orders of magnitude lower than any crop-based biofuel, whether based on volumetric yield or energy yield per unit area (Table 4-5; see also Table 1 in Singh et al., 2011). Future improvements in algal oil content and areal productivity (kg/ha per year) of algal cultivation systems can be expected to further lessen the amount of land needed to produce a given quantity of liquid biofuel (Pienkos and Darzins, 2009).
Wigmosta et al. (2011) performed a national-scale assessment of potential algal biofuel production and its resource uses in open-pond systems. The assessment was based on a theoretical facility consisting of 100 30-centimeter deep, 4-hectare ponds that required about 400 hectares of land for the ponds themselves, and an additional 90 hectares to
TABLE 4-5 Ranked Comparison of the Oil Yield and Land-Use Requirements of Microalgae with Nine Agricultural Crop-Based Biodiesel Feedstocks
|
|
||||
| Plant Source | Seed Oil Content (% oil by weight in biomass) | Oil Yield (L oil/ha year) |
Land Use (m2 year/kg biodiesel) | Biodiesel Productivity (kg biodiesel/ ha year) |
|
|
||||
|
Soybean (Glycine max L.) |
18 |
636 |
18 |
562 |
|
Camelina (Camelina sativa L.) |
42 |
915 |
12 |
809 |
|
Canola/rapeseed (Brassica napus L.) |
41 |
974 |
12 |
862 |
|
Sunflower (Helianthus annuus L.) |
40 |
1,070 |
11 |
946 |
|
Microalgae (low oil content) |
30 |
58,700 |
0.2 |
51,927 |
|
Microalgae (medium oil content) |
50 |
97,800 |
0.1 |
86,515 |
|
Microalgae (high oil content) |
70 |
136,900 |
0.1 |
121,104 |
|
|
||||
SOURCE: Adapted from Mata et al. (2010). Reprinted with permission from Elsevier.
accommodate operational infrastructure. Potential algal oil production and its associated land and water resource requirements were simulated on the basis of the dominant physical processes that affect algal growth. The supplies of water, nutrients, and CO2 were not limited in the simulations to provide theoretical estimates for annual mean and annual maximum open-pond microalgae production if all the sites with suitable land and water requirements are developed.
The analysis by Wigmosta et al. (2011) identified 11,588 non-competitive areas totaling 430,830 km2 that potentially could be used for large-scale, open-pond algae production. Strong geographical variation in their theoretical annual mean levels of biofuel production (L/ha per year) was projected (Figure 4-7)—the land and freshwater availability favors locations in the Gulf Coast region. The highest predicted rates of annual mean production occurred in South Central Texas and much of Florida, but land prices in those regions could affect the feasibility of their use for algae cultivation. Their study suggested that under the assumptions of their analysis and current technology, algae can potentially be cultivated at large scale in 5.5 percent of the land in the conterminous United States to generate 220 billion liters of oil per year (Wigmosta et al., 2011). That amount of algal oil is equivalent to 28 percent of total U.S. petroleum consumption for transportation.
Pate et al. (2011) also performed quantitative assessments of the land demands resulting from algal biofuel generation and concluded that land requirements were likely to be the most manageable among the resource demands (water, nutrients, and land) considered in their study. As is the case with water requirements, land demands vary with the geographic region, the quantity of feedstock to be produced, and the level of algal productivity assumed to be achieved. They concluded that the Southwestern and the Nineteen Lower-Tier State regions in the United States potentially were more likely to meet the siting requirements for algal biofuel production scale-up than the Midwestern and Southeastern regions. However, the total land area required to meet targeted biofuel production levels is expected to be inversely correlated with the annual biomass productivity and algal lipid contents that actually can be achieved in practice (see Figure 2 in Pate et al., 2011) (see also Figure 3a in Xu et al., 2011). Batan et al. (2010; 2011) used a detailed, industrial-scale engineering model for photobioreactor-grown Nannochloropsis to calculate a land requirement of 4.41 million hectares (10.9 million acres) to produce 40 billion gallons (151.4 billion liters) of microalgae-derived biodiesel per year. That land area is equivalent to 16 percent of the total land area in Colorado and 0.45 percent of U.S. land area.
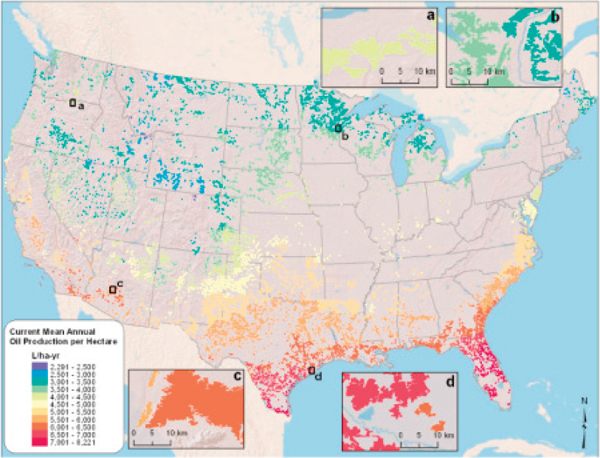
FIGURE 4-7 Estimated mean annual algal oil production (L/ha per year) under current technology in the continental United States, predicted by a GIS-based analysis. The insets illustrate finer-level detail at two northern and two southern locations.
SOURCE: Wigmosta et al. (2011). Reprinted with permission from the American Geophysical Union.
4.3.3 Opportunities for Mitigation
Opportunities for mitigating land requirements for algal biofuel systems lie in productivity— the only way to reduce the area required is to increase the productivity per unit area. Thus, developing more highly productive algal strains, engineering different pond designs, or using photobioreactors rather than ponds all would reduce land requirements. The related topics of impacts of land use and how to mitigate those effects are discussed in Chapter 5.
4.3.4 Sustainability Indicators
Indicators of sustainability of land requirements for algal biofuel production include the following:
• Liters of fuel produced per hectare.
• Kilograms of fuel produced per hectare.
• Energy return on land invested (MJ/ha), analogous to EROWI.
• Land required to meet various production targets relative to existing national usage or supply.
The definition of the indicators would need to specify whether energy needed to prepare land and to transport resources to the site is included. Land indicators are highly dependent on where the land is, though all the indicators have at least some site specificity. DOE could help address these indicators through more detailed studies of the potential biofuel production capacities of specific technologies in various regions of the country.
4.3.5 Information and Data Gaps
Land-use availability databases, land-suitability databases, and geo-spatial resource maps compiled specifically for the purpose of assessing the potential for algal biofuel production are incomplete (Darzins et al., 2010). A number of relevant resource maps for the United States and the world can be found in Lundquist et al. (2010), who also present a detailed geographic information systems (GIS)-based analysis of algal biofuel production in California. Continued efforts to perform meta-analyses of existing LCAs are desirable to comprehensively assess the land requirements and the most likely site locations for future algal biofuel production.
4.3.6 Effects on Social Acceptability
Part of the appeal of algae production as a renewable fuel source is the potential small land area required to produce a given quantity of energy relative to terrestrial crops. However, commercial-scale algal biofuel facilities, especially those relying on open-pond cultivation systems, still will require thousands of hectares of land to achieve sufficient economies of scale. Despite the algal industry’s general focus on using marginal and degraded land for development of commercial-scale facilities, questions still may be raised as to the potential social and health-related risks of developing such large areas for the purpose of algae production.
Land in the desert Southwest is suitable for the scale-up of algae cultivation facilities because of favorable climatic conditions for algal growth. While much of the land in this region has been designated as marginal due to its inability to support food production, public support for large-scale land developments, even in the renewable-energy sector, is not guaranteed. The arid conditions and infertile soil in the desert Southwest support highly fragile ecosystems that take far longer to recover following major disturbances than ecosystems in wetter climates. These areas also contain a number of threatened or endangered species that are the focus of major conservation efforts and public awareness campaigns, such as the desert tortoise. Setbacks to the construction of commercial-scale photovoltaic facilities have resulted over concern for the vulnerability of these ecosystems, forcing solar developers to purchase additional land as conservation easements and to create wildlife rehabilitation and protection programs (BLM and DOE, 2010). The algal biofuel industry likely will face similar hurdles as it continues toward commercialization; actively engaging the public and conservation organizations during the site selection and permitting process could help overcome those barriers.
Even if algae developers have gained acceptance from the public, the definition of marginal or degraded land is fluid and depends on both the technology available for farming and the macroeconomic and geopolitical conditions at a given time. For example, land that is considered marginal and unsuitable for traditional freshwater farming today may be considered suitable in the future if a cultivar of a food crop is developed to tolerate dryer or less fertile soils. Pressure to use that land for food rather than energy production
may be intensified if food security concerns escalate. This pressure would be magnified significantly should breakthroughs in desalination technology make farming viable in areas where it was previously uneconomical to move fresh ground or surface water. In such a scenario, the acceptability of an algae development that has been permitted successfully and previously in good standing with local communities and the public at large could be called into question.
Land may have more value for development or recreational purposes than for massive open-pond systems. Local municipalities and communities may not be open to production facilities being located close to areas that might be developed to attract retirees, tourists, or other economic development projects. Wind turbines are perceived by some to be aesthetically unpleasing, and so could these large open or closed algal production systems. The advantages and disadvantages of using prime recreational or production lands for algal biofuel production will have to be discussed, debated, and decided upon by the stakeholder community. If oil prices continue to rise, or if foreign oil supplies suddenly were no longer available, then the argument to use land for algae production for biofuels over any economic development or aesthetic might be more pro than con.
To exploit the high photosynthetic efficiency of algae, energy must be invested in cultivation systems and biorefineries to grow the algae, to manage water utilizations, and to process algae into the desired fuel. Given the considerable energy use in the supply chains of other biofuels, (for example, Farrell et al., 2006), analysis of the full fuel cycle of algal supply chains is critical to understanding energy implications. This section reviews the state of knowledge of the energy properties of algal biofuel production systems.
4.4.1 Life Cycle Energy Studies of Algal Biofuels
The method to assess energy and material flows of supply chains is LCA (see also Chapter 1). The primary challenge in applying LCAs to algal biofuel production is the early stage of development of the technology. It is not yet clear what technologies along the processing chain will emerge as the most commercially feasible. Also, many technologies are in the laboratory or pilot-scale stage. Their technical (and energy) characteristics when scaled up to the industrial level are not yet clear. Nonetheless, there is a great deal of recent research activity to assess life-cycle energy use of algal biofuel production. Table 4-6 shows the energy return on investment (EROI) from a selection of recent publicly available studies of raceway systems. EROI is defined as the ratio of total energy outputs (biofuel + coproducts) to energy inputs, where energy inputs are summed over the life cycle: cultivation, nutrient procurement, harvesting, extraction, processing, and associated supply chains (Eq. 4-3).
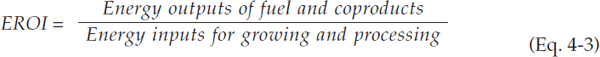
Unless the EROI for an energy production system is greater than 1.0, energy needed to make a fuel is greater than energy contained in the fuel and coproducts. Thus, production pathways for algal biofuels that have EROI less than 1 clearly are unsustainable. Ideally, the EROI of an algal biofuel is at least comparable to the EROI of other alternative fuels.
TABLE 4-6 Energy Return on Investment (EROI) Values for Open-Pond Systems
|
|
|||||
| Source | Nutrients | Harvesting | Extraction/ Processing | Products and Coproducts | EROI |
|
|
|||||
|
Brentner et al. (2011) |
Flue gas |
Centrifuge, drying, |
Hexane/ |
BDb |
0.13 |
|
reference case Brentner et al. (2011) reference case with coproducts |
Flue gas |
press |
esterification |
BDb, electricity, nutrients |
0.28 |
|
Brentner (2011) best case swap raceways |
Flue gas |
Chitosan-flocculation |
Supercritical methanol |
BDb, electricity, nutrients |
0.96 |
|
Clarens et al. (2010) |
Industrial CO2 |
Alum flocculation, centrifuge |
- |
Algal biomass |
1.06 |
|
Clarens et al. (2011) C-2 |
CCa coal plant |
Auto-flocculation, |
Hexane/ esterification |
BDb, electricity, nutrients |
1.36 |
|
Clarens et al. (2011) C-3 |
Flue gas |
Auto-flocculation, |
Hexane/ esterification |
BDb, electricity, nutrients |
1.99 |
|
Clarens et al. (2011) C-4 |
WWc, flue gas |
Auto-flocculation, |
Hexane/ esterification |
BDb, electricity, nutrients |
2.32 |
|
GREET baseline pathway |
Flue gas |
Bio-flocculation, flotation, centrifuge, homogenization |
Hexane/ esterification |
BDb, electricity, nutrients |
0.39 |
|
Jorquera et al. (2010) |
N/A |
N/A |
N/A |
Algal biomass |
7.01 |
|
Sander and Murthy (2010) filter press |
N/A |
Filter press, drying |
Hexane/ esterification |
BDb, fermentation stock |
3.33 |
|
Sander and Murthy (2010) centrifuge |
N/A |
Centrifuge, drying |
Hexane/ esterification |
BDb, fermentation stock |
1.77 |
|
Stephenson et al. (2010) |
Flue gas |
Alum flocculation, |
Hexane/ esterification |
BDb, electricity, nutrients |
1.60 |
|
Vasudevan et al. (2012) dry extraction, nominal |
Flue gas |
Dissolved air flotation, centrifuge |
Belt dryer |
BD, electricity |
0.3 |
|
Vasudevan et al. (2012) wet extraction, nominal |
Flue gas |
Dissolved air flotation centrifuge |
Stream lysing, centrifuge, wash |
BD, electricity |
2.51 |
|
|
|||||
aCC = carbon capture.
bBD = biodiesel.
cWW = wastewater.
Notes: The GREET (Greenhouse Gases, Regulated Emissions, and Energy Use in Transportation) model is a spreadsheet model developed at the Argonne National Laboratory for evaluating energy and emission impacts of advanced vehicle technologies and new transportation fuels.
Results in EROI show tremendous variation. The values at the low end suggest that the technology in its current form is not feasible for net energy return. In contrast, values at the high end suggest algal biofuel could have EROI considerably higher than corn-grain ethanol (EROI = 1.2; see Farrell et al., 2006). One source of variation in EROI is differences in choices of processes and inputs in the supply chain. Table 4-6 summarizes some results from comparison of technology systems. The three scenarios from Clarens et al. (2011) show changes as a function of carbon source and use of wastewater as replacement of nutrient input. Brentner et al. (2011) and Sander and Murthy (2010) highlighted how changing harvesting (and extraction) technologies have major effects on energy use.
TABLE 4-7 Energy Use in Cultivation Stage in Closed Photobioreactors
|
|
||
|
Source |
Bioreactor Type |
Cultivation Energy |
|
|
||
|
Stephenson et al. (2010) |
Air lift tubular |
6 |
|
Jorquera et al. (2010) |
Air lift tubular |
14 |
|
Jorquera et al. (2010) |
Flat plate |
0.61 |
|
Brentner et al. (2011) |
Annular |
19 |
|
Brentner et al. (2011) |
Tubular |
57 |
|
Brentner et al. (2011) |
Flat plate |
1.4 |
|
|
||
Coproducts significantly affect the energy analysis. The typical scenario analyzed is anaerobic digestion of algae residuals to produce electricity and recover nutrients. One can see from Brenter et al. (2011) that changing from landfilling of algae residuals to anaerobic digestion nearly doubles EROI in their calculations. In Sander and Murthy (2010), the energy credit for using algae residuals is 10 times larger than the energy content of the produced biodiesel. This result can be interpreted as an assertion that the algal biorefinery becomes primarily a source of fermentation inputs and biodiesel is a secondary output.
There is considerable variability within similar technology or supply pathways—for example, Sander and Murphy’s EROI of 1.77 versus Brentner et al.’s EROI of 0.28. This variability is related to the use of different data or assumptions for the same processes and different boundary choices for supply chains. Variability in data has two sources. One source of variability is generic in LCA. Different analysts choose different data sources, and there is not a systematic way to realize convergence (Williams et al., 2009). The second source of variability is tied to the emerging nature of the technology. LCA is a method designed to assess existing technologies through chaining process input-output tables. Many processes in algal biofuel production systems are still in the laboratory or pilot phase. There is much uncertainty in how these technologies will evolve and scale up in the future; actual energy use could be much higher or much lower than suggested by the current suite of initial LCA studies.
The results in Table 4-6 address open-pond cultivation. Given the potential for closed photobioreactors to mitigate other resource and environmental issues such as water consumption (Harto et al., 2010), the energy use of closed systems is important to consider. A few studies estimated the direct energy use for the feedstock cultivation step in algal biofuel production systems that use photobioreactors (Table 4-7; Jorquera et al., 2010; Stephenson et al., 2010; Brentner et al., 2011). Tubular and annular reactors are thought to require far more energy to operate than is contained in the biodiesel product. Flat-plate reactors are thought to require far less energy, though Brentner et al. (2011) reported a net energy input higher than contained in biodiesel output (1.4 megajoules per megajoule of biodiesel). While the caveats for other results apply here as well, these studies suggest that the energy use of photobioreactors could fundamentally affect the net energy balance of algal biofuels (Jorquera et al., 2010).
4.4.2 Energy Requirements in the Supply Chain and Credits for Coproducts
Analyses of prior studies provide insight into the current understanding of what production stages are important contributors to energy requirements despite the large uncertainties and variability associated with energy requirements of algal biofuel production. Table 4-8 abstracts results from a meta-analysis of LCA studies to summarize the range in energy requirements of different stages (Liu et al., 2011). This meta-analysis included data
TABLE 4-8 Range in Energy Requirements by Process Stage for Open-Pond Cultivation of Algae to Produce Biodiesel (Plus Coproducts)
|
|
|
|
Production Stage |
Energy Requirement |
|
|
|
|
Nutrients (fertilizer + CO2) |
0.2-1.6 |
|
Cultivation |
0.04-3.14 |
|
Separation |
0.01-0.26 |
|
Extraction |
0.19-0.51 |
|
Conversion |
0.03-0.22 |
|
|
|
SOURCE: Liu et al. (2011). Reprinted with permission from Elsevier.
from several studies (Lardon et al., 2009; Clarens et al., 2010; Jorquera et al., 2010; Sander and Murthy, 2010; Stephenson et al., 2010; Campbell et al., 2011).
The high end of the range of energy requirements for nutrients corresponds to the use of virgin fertilizers and industrial CO2 in production. Cultivation consumes energy primarily for mixing and pumping water. Murphy and Allen (2011) suggested that energy needs for water management could be substantially higher than current LCA studies indicate. Extraction is generally assumed to be via hexane solvent and conversion to biodiesel via transesterification.
The treatment of the energy credits for coproducts is critical in the energy balance of algal biofuels. For production of bio-electricity, the energy credit per megajoule of biodiesel ranges from 0.3-1.3 megajoule/megajoules biodiesel (Lardon et al., 2009; Stephenson et al., 2010; Campbell et al., 2011). Sander and Murthy (2010) assume that residual biomass from algae production substitutes for corn used in ethanol production, yielding an energy credit for the coproduct of 10.7 megajoules/megajoule biodiesel.
4.4.3 LCA Issues Related to Algal-Lipid Processing
Pathways described in Chapter 3 describe two different ways of converting crude algal lipids to liquid fuels. Both yield fuels suitable for use in diesel applications. The majority of the published LCAs assumed the production of FAME diesels, which are less desirable than “drop-in” fuels because of FAME’s incompatibility with existing infrastructure for petroleum-based fuels. Given the lack of LCA work on green diesel products from algae, differences in energy use between FAME and green diesel are addressed by analogy with conventional diesel processing. A number of studies of conventional diesel processing that have been reviewed allow comparisons to be made (Kalnes et al., 2009).
Comparison between seed oil-derived diesel fuels treated by esterification and by hydrotreating show that there is little difference in either the energy return or carbon emissions. These studies start with the same raw oils, meaning that the differences only reflect the processing to finished fuel. Figure 4-8 shows that these are nearly identical and, therefore, life-cycle impacts will be similar between esterification processes and hydrotreating.
4.4.4 Opportunities for Mitigation
Keeping other factors constant, increasing the productivity of algal growth drives down energy use for cultivation and harvesting. This said, if achieving higher productivity involves major process changes, such as using photobioreactors instead of raceways, the
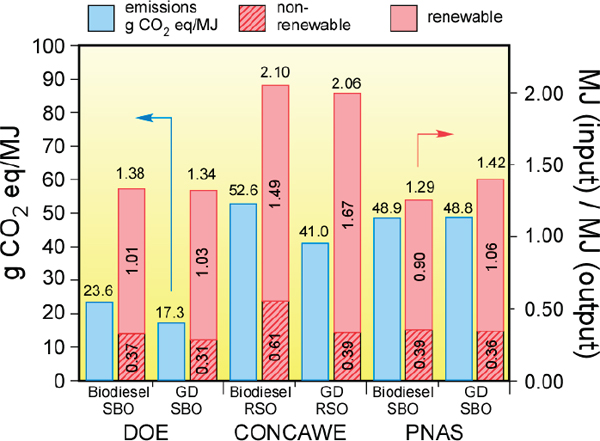
FIGURE 4-8 Comparison of conventional esterified biodiesel production with green diesel (GD) production based on soybean oil (SBO) and rapeseed oil (RSO).
NOTE: Each of the three completed studies shows little difference on energy use or carbon emissions between green diesel and biodiesel. By analogy, green diesel production from algal lipids relative to conventional biodiesel processing is likely to have similar life-cycle impacts.
SOURCE: Adapted from Kalnes et al. (2009).
embodied energy of the process change needs to be assessed and compared to direct energy savings. Water management is clearly an important factor in energy use. Efficient pumps and gravity-driven designs, for example, could mitigate high energy use for water management. The embodied energy in providing nutrients, including CO2, can be substantial. The extent to which waste products can be used as nutrients has the potential to substantially reduce energy use. Separating algal products from water is a major factor driving energy use in separation and extraction. Drying processes in particular are energy intensive. Brentner et al. (2011) called attention to the potential of supercritical processes to reduce energy use for processing algae. Given the importance of coproducts in the net energy balance, developing higher “energy value” coproducts could be an important mitigation strategy.
4.4.5 Sustainability Indicators
A number of different metrics are already in use to assess energy systems (Farrell et al., 2006; GBEP, 2011; Baral et al., 2012), including:
• EROI.
• Net Energy Value (NEV).
• Fossil Inputs.
Before discussing the different measures in more detail, it is important to first recapitulate the definition and quantification of energy use. The key issue is that energy inputs and outputs come in different forms having differing utility, in particular chemical (for example, heating value of fuel), electricity, and heat. While one could simply add different energy types by unit conversion, different forms of energy are not interchangeable. For example, it takes more than one unit of heat to make one unit of electricity. One approach to put different energy forms on a comparable basis is the idea of primary (or source) energy. The precise definition of primary energy varies, but in general it includes the heat or fossil inputs needed to make electricity. In some cases, it also includes indirect energy use associated with delivering fossil fuels. For the U.S. energy system, one common conversion used is 3.4 megajoules of upstream primary energy per kilowatt hour of electricity. Analysts often use different definitions of energy and do not always explicitly state which definition is being used. Care is needed when comparing energy results from different studies.
Energy outputs generally include only those utilized; that is, waste heat is not included. Energy output is estimated by direct unit conversion, conversion to source energy, or in some cases, using the energy needed to make products that coproducts replace. NEV forms the difference.
NEV = Energy outputs of fuels and products – Energy inputs.
Fossil inputs measure quantities of fossil fuels used in processing. EROI and NEV refer generically to energy, which could be supplied by fossil or renewable forms. Fossil inputs are thus specific to how heat and electricity are supplied and thus require definition of the enveloping energy system.
4.4.6 Information and Data Gaps
Much uncertainty remains as to the current and future energy properties of algal cultivation systems, pointing to critical gaps. Scarcity of data on material flows at existing scales of algal biofuel production presents a challenge in assessing EROI. LCA studies of algae use process modeling to estimate energy consumption. Additional empirical data can help validate these models.
Although there are gaps in data, data collection by itself will not resolve the uncertainties of life-cycle energy implications of algal biofuels. The true energy behavior is a result of scale-up and learning processes that bring algae from the laboratory and pilot scale to industrial scale. While future energy behavior is challenging to forecast, given the substantial investment and path dependence associated with bringing an energy technology to scale, due diligence demands that a serious forecasting effort be made. While there are a variety of cost forecasting methods available, such as learning curves and scaling factors, methods to forecast energy and material flows of developing technologies are undeveloped. Efforts need to be made to develop such methods. Increased data availability from laboratory and pilot scales is critical to calibrate and validate the forecasting methods that emerge.
A review of published literature suggests that the scale-up of algal biofuel production to yield 37.8 billion liters of algal oil (10 billion gallons) would place an unsustainable demand on energy, water, and nutrients with current technology and knowledge. Estimated
values for EROI range from 0.13 to 3.33. The estimated consumptive use of fresh water for producing 1 liter of gasoline equivalent of algal biofuel is 3.15-3,650 liters. The estimated requirement for nitrogen and phosphorus needed to produce 37.8 billion liters of algal biofuels ranges from 6 million to 15 million metric tons of nitrogen and from 1 million to 2 million metric tons of phosphorus if the nutrients are not recycled or included and used in coproducts.
Freshwater use for production of algal biofuel is inevitable because fresh water is necessary to compensate for water loss and to avoid salt buildup as a result of evaporation during cultivation. Two key drivers for freshwater requirement in algal biofuel production are evaporative loss in open-pond cultivation and discharge of harvest water in biofuel production systems that harvest the algae. Therefore, water use would be a serious concern in an algal biofuel production system that uses fresh water in open ponds without recycling harvest water. Freshwater use also could be reduced by using saltwater or brackish water in algae cultivation. However, information on the availability of inland saline water resources is sparse.
Recycling of harvest water also is important in reducing nitrogen and phosphorus input to algae cultivation. To promote high yield, algae are cultivated in nutrient-rich media. The nutrients will be lost if harvest water is not recycled. If algal biomass is harvested to process to fuels, there could be another opportunity to recover nitrogen and phosphorus after processing because the fuel products do not contain those two elements.
Recycling nutrients via reuse of harvest water or the use of wastewater from agricultural or municipal sources provides an opportunity to reduce energy use, as synthetic fertilizer input contributes to energy input over the life cycle of algal biofuels. Energy inputs for water management (for example, pumping groundwater, and moving water in cultivation systems), harvesting, and water extraction (for example, drying of algal biomass) are two key drivers in the overall energy balance of algal biofuel production systems.
A key aspect to sustainable development of algal biofuels is siting (for example, suitable climate and colocation of key resources) and recycling of key resources. Siting of algal biofuel production facilities needs to account for climate, topography, and proximity to water and nutrients. Siting algae cultivation far away from water and nutrient resources would incur additional costs and energy consumption for transporting those resources to the cultivation facilities. Although several studies assessed land, water, and nutrient requirements and energy balance of algal biofuel production, few studies considered all four requirements simultaneously.
A national assessment of land requirements for algae cultivation that takes into account climatic conditions; fresh water, inland and coastal saline water, and wastewater resources; sources of CO2; and land prices is needed to inform the potential amount of algal biofuels that could be produced economically in the United States.
SUMMARY FINDINGS FROM THIS AND EARLIER CHAPTERS
Based on a review of literature published until the authoring of this report, the committee concluded that the scale-up of algal biofuel production sufficient to meet at least 5 percent of U.S. demand for transportation fuelsa would place unsustainable demands on energy, water, and nutrients with current technologies and knowledge. However, the potential to shift this dynamic through improvements in biological and engineering variables exists. (See also Chapters 2 and 3 on improvements in biological and engineering variables.)
Sustainable development of algal biofuels would require research, development, and demonstration of the following:
• Algal strain selection and improvement to enhance desired characteristics and biofuel productivity. (See Chapter 2.)
• An EROI that is comparable to other transportation fuels, or at least improving and approaching the EROIs of other transportation fuels.
• The use of wastewater for cultivating algae for fuels or the recycling of harvest water, particularly if freshwater algae are used.
• Recycling of nutrients in algal biofuel pathways that require harvesting unless coproducts that meet an equivalent nutrient need are produced.
A national assessment of land requirements for algae cultivation that takes into account climatic conditions; fresh water, inland and coastal saline water, and wastewater resources; sources of CO2; and land prices is needed to inform the potential amount of algal biofuels that could be produced economically in the United States.
_______________
a U.S. consumption of fuels for transportation was about 784 billion liters in 2010. Five percent of the annual U.S. consumption of transportation fuels, which would be about 39 billion liters, is mentioned to provide a quantitative illustration of the water and nutrients required to produce algal biofuels to meet a small portion of the U.S. fuel demand.
Alley, W.M. 2003. Desalination of Ground Water: Earth Science Perspectives. Reston: U.S. Geological Survey.
Baral, A., B.R. Bakshi, and R.L. Smith. 2012. Assessing resource intensity and renewability of cellulosic ethanol technologies using Eco-LCA. Environmental Science and Technology 46 (4):2436-2444.
Batan, L., J. Quinn, T. Bradley, and B. Willson. 2011. Erratum: Net energy and greenhouse gas emissions evaluation of biodiesel derived from microalgae (Environmental Science and Technology (2010) 44 (7975-7980)). Environmental Science and Technology 45(3):1160.
Batan, L., J. Quinn, B. Willson, and T. Bradley. 2010. Net energy and greenhouse gas emission evaluation of biodiesel derived from microalgae. Environmental Science and Technology 44(20):7975-7980.
Benemann, J., P.M. Pedroni, J. J. Davison, H. Beckert, and P. Bergman. 2003a. Technology roadmap for biofixation of CO2 and greenhouse gas abatement with microalgae. Paper read at the Second Annual Conference on Carbon Sequestration, May 5-8, Alexandria, VA.
Benemann, J.R., J.C. Van Olst, M.J. Massingill, J.C. Weissman, and D.E. Brune. 2003b. The controlled eutrophication process: Using microalgae for CO2 utilization and agricultural fertilizer recycling. Paper read at the 6th Greenhouse Gas Control Technologies Conference (GHGT6), October, Kyoto Japan.
BLM (Bureau of Land Management) and DOE (U.S. Department of Energy). 2010. Solar Energy Development Draft Programmatic Environmental Impact Statement (Draft Solar PEIS). Washington, D.C.: Bureau of Land Management and U.S. Department of Energy.
Boyd, C.E., and A. Gross. 2000. Water use and conservation for inland aquaculture ponds. Fisheries Management and Ecology 7(1-2):55-63.
Brentner, L.B., M.J. Eckelman, and J.B. Zimmerman. 2011. Combinatorial life cycle assessment to inform process design of industrial production of algal biodiesel. Environmental Science and Technology 45:7060-7067.
Cai, X.M., X.A. Zhang, and D.B. Wang. 2011. Land availability for biofuel production. Environmental Science and Technology 45(1):334-339.
Campbell, P.K., T. Beer, and D. Batten. 2011. Life cycle assessment of biodiesel production from microalgae in ponds. Bioresource Technology 102(1):50-56.
Chiu, Y.-W., B. Walseth, and S. Suh. 2009. Water embodied in bioethanol in the United States. Environmental Science and Technology 43(8):2688-2692.
Clarens, A.F., H. Nassau, E.P. Resurreccion, M.A. White, and L.M. Colosi. 2011. Environmental impacts of algae-derived biodiesel and bioelectricity for transportation. Environmental Science and Technology 45:7554-7560.
Clarens, A.F., E.P. Resurreccion, M.A. White, and L.M. Colosi. 2010. Environmental life cycle comparison of algae to other bioenergy feedstocks. Environmental Science and Technology 44(5):1813-1819.
Cordell, D., J.O. Drangert, and S. White. 2009. The story of phosphorus: Global food security and food for thought. Global Environmental Change-Human and Policy Dimensions 19(2):292-305.
Craggs, R.J., S. Heubeck, T.J. Lundquist, and J.R. Benemann. 2011. Algal biofuels from wastewater treatment high rate algal ponds. Water Science and Technology 63(4):660-665.
Craswell, E.T., P.L.G. Vlek, and H. Tiessen. 2010. Peak phosphorus—Implications for soil productivity and global food security. Paper read at the 19th World Congress of Soil Science, Soil Solutions for a Changing World, August 1-6, Brisbane, Australia.
Darzins, A., P.T. Pienkos, and L. Edye. 2010. Current status and potential for algal biofuels production: A report to IEA Bioenegy Task 39. Available online at http://www.fao.org/uploads/media/1008_IEA_Bioenergy_-_Current_status_and_potential_for_algal_biofuels_production.pdf. Accessed September 14, 2012.
Dewson, Z.S., A.B.W. James, and R.G. Death. 2007. A review of the consequences of decreased flow for instream habitat and macroinvertebrates. Journal of the North American Benthological Society 26(3):401-415.
DOE (U.S. Department of Energy). 2006. Energy Demands on Water Resources: Report to Congress on the Interdependency of Energy and Water. Washington, DC: U.S. Department of Energy.
____________. 2010a. Algenol Integrated Biorefinery for Producing Ethanol from Hybrid Algae. Washington, DC: U.S. Department of Energy.
____________. 2010b. National Algal Biofuels Technology Roadmap. Washington, DC: U.S. Department of Energy, Energy Efficiency and Renewable Energy.
Dominguez-Faus, R., S.E. Powers, J.G. Burken, and P.J. Alvarez. 2009. The water footprint of biofuels: A drink or drive issue? Environmental Science and Technology 43(9):3005-3010.
EIA (Energy Information Adminstration). 2011. September 2011 Monthly Energy Review. Washington, DC: U.S. Energy Information Administration.
EPA (U.S. Environmental Protection Agency). 2008. Clean Watersheds Needs Survey 2004 Report to Congress. Washington, DC: U.S. Environmental Protection Agency.
Falkowski, P.G., and J.A. Raven. 2007. Aquatic Photosynthesis. 2nd ed. Princeton, NJ: Princeton University Press.
Farrell, A.E., R.J. Plevin, B.T. Turner, A.D. Jones, M. O’Hare, and D.M. Kammen. 2006. Ethanol can contribute to energy and environmental goals. Science 311:506-508.
Feth, J.H.F. 1965. Preliminary map of the Conterminous United States Showing Depth to and Quality of Shallowest Ground Water Containing More Than 1,000 Parts Per Million Dissolved Solids. Reston, VA: U.S. Geological Survey.
GBEP (Global Bioenergy Partnership). 2011. GBEP Sustainability Indicators for Bioenergy. Rome: FAO (Food and Agriculture Organization of the United Nations).
Geider, R.J., and J. La Roche. 2002. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. European Journal of Phycology 37(1):1-17.
Gerbens-Leenes, W., A.Y. Hoekstra, and T.H. van der Meer. 2009. The water footprint of bioenergy. Proceedings of the National Academy of Sciences of the United States of America 106(25):10219-10223.
Goedhart, C.M., and D.E. Pataki. 2011. Ecosystem effects of groundwater depth in Owens Valley, California. Ecohydrology 4 (3):458-468.
Greet, J., J.A. Webb, and R.D. Cousens. 2011. The importance of seasonal flow timing for riparian vegetation dynamics: a systematic review using causal criteria analysis. Freshwater Biology 56(7):1231-1247.
Guru, M., and J. Horne. 2001. The Ogallala Aquifer. Pp. 321-328 in Water Resource Management, R.A. Falconer, W.R. Blain, C.A. Brebbia, P. Anagnastopoulos, K. Katsifarakis, and A.H.D. Cheng, eds. Haldiki, Greece: WIT Press.
Hanson, R.L. 1991. Evapotranspiration and droughts. U.S. Geological Survey Water-Supply Paper 2375:99-104.
Hanson, T.R. 2006. Catfish farming in Mississippi. Mississippi History Now (April). Available online at http://mshistory.k12.ms.us/articles/217/catfish-farming-in-mississippi. Accessed March 15, 2012.
Harto, C., R. Meyers, and E. Williams. 2010. Life cycle water use of low-carbon transport fuels. Energy Policy 38(9):4933-4944.
Hessen, D.O., and T.R. Anderson. 2008. Excess carbon in aquatic organisms and ecosystems: Physiological, ecological, and evolutionary implications. Limnology and Oceanography 53(4):1685-1696.
Hoekstra, A.Y., A.K. Chapagain, M.M. Aldaya, and M.M. Mekonnen. 2009. Water Footprint Manual: State of the Art 2009. Enschede, The Netherlands: Water Footprint Network.
Jager, H.I., and K.A. Rose. 2003. Designing optimal flow patterns for fall Chinook salmon in a central valley, California, river. North American Journal of Fisheries Management 23(1):1-21.
Jansson, M., J. Karlsson, and A. Jonsson. 2012. Carbon dioxide supersaturation promotes primary production in lakes. Ecology Letters 15:527-532.
Jorquera, O., A. Kiperstok, E.A. Sales, M. Embiruçu, and M.L. Ghirardi. 2010. Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresource Technology 101(4):1406-1413.
Kalnes, T.N., K.P. Koers, T. Marker, and D.R. Shonnard. 2009. A technoeconomic and environmental life cycle comparison of green diesel to biodiesel and syndiesel. Environmental Progress and Sustainable Energy 28(1):111-120.
Katzer, J.R. 2010. Sustainable research, development, and demonstration. Industrial and Engineering Chemistry Research 49(21):10154-10158.
Kenny, J.F., N.L. Barber, S.S. Hutson, and K.S. Linsey. 2009. Estimated Use of Water in the United States in 2005. Reston, VA: U.S. Geological Survey.
King, C.W., and M.E. Webber. 2008. Water intensity of transportation. Environmental Science and Technology 42(21):7866-7872.
Lardon, L., A. Helias, B. Sialve, J.P. Stayer, and O. Bernard. 2009. Life-cycle assessment of biodiesel production from microalgae. Environmental Science and Technology 43(17):6475-6481.
Leonardos, N., and R.J. Geider. 2005. Elevated atmospheric carbon dioxide increases organic carbon fixation by Emiliania huxleyi (Haptophyta), under nutrient-limited high-light conditions. Journal of Phycology 41(6):1196-1203.
Liu, X., A.F. Clarens, and L.M. Colosi. 2011. Algae biodiesel has potential despite inconclusive results to date. Bioresource Technology 104:83-86.
Lundquist, T.J., I.C. Woertz, N.W.T. Quinn, and J.R. Benemann. 2010. A Realistic Technology and Engineering Assessment of Algae Biofuel Production. Berkeley, CA: Energy Bioscience Institute.
Luo, D.X., Z.S. Hu, D.G. Choi, V.M. Thomas, M.J. Realff, and R.R. Chance. 2010. Life cycle energy and greenhouse gas emissions for an ethanol production process based on blue-green algae. Environmental Science and Technology 44(22):8670-8677.
Marshall, C.T., and R.H. Peters. 1989. General patterns in the seasonal development of chlorophyll a for temperate lakes. Limnology & Oceanography 34(5):856-867.
Mata, T.M., A.A. Martins, and N.S. Caetano. 2010. Microalgae for biodiesel production and other applications: A review. Renewable and Sustainable Energy Reviews 14(1):217-232.
McKone, T.E., W.W. Nazaroff, P. Berck, M. Auffhammer, T. Lipman, M.S. Torn, E. Masanet, A. Lobscheid, N. Santero, U. Mishra, A. Barrett, M. Bomberg, K. Fingerman, C. Scown, B. Strogen, and A. Horvath. 2011. Grand challenges for life-cycle assessment of biofuels. Environmental Science and Technology 45:1751-1756.
Mulder, K., N. Hagens, and B. Fisher. 2010. Burning water: A comparative analysis of the energy return on water invested. Ambio 39(1):30-39.
Murphy, C.F., and D.T. Allen. 2011. Energy-water nexus for mass cultivation of algae. Environmental Science and Technology 45(13):5861-5868.
Nagrodski A., G.D. Raby, C.T. Hasler, M.K. Taylor, and S.J. Cooke. 2012. Fish stranding in freshwater ecosystems: Sources, consequences, and mitigation. Journal of Environmental Management 103:133-141.
NAS-NAE-NRC (National Academy of Sciences, National Academy of Engineering, and National Research Council). 2009. America’s Energy Future: Technology and Transformation. Washington, DC: The National Academies Press.
____________. 2010. Electricity from Renewable Resources. Washington, DC: The National Academies Press.
NRC (National Research Council). 2008. Water Implications of Biofuels Production in the United States. Washington, DC: The National Academies Press. ____________. 2011. Renewable Fuel Standard. Potential Economic and Environmental Effects of U.S. Biofuel Policy. Washington, DC: The National Academies Press.
Pate, R. 2007. Overview of energy-water interdependencies and the emerging energy demands on water resources. Albuquerque: Sandia National Laboratories.
____________. 2011. U.S. Department of Energy, Office of Biomass Program (OBP) Sponsor Perspectives for NRC Study on Algae Biofuels Sustainability. Presentation to the NRC Committee on Sustainable Development of Algal Biofuels on March 17.
Pate, R., G. Klise, and B. Wu. 2011. Resource demand implications for U.S. algae biofuels production scale-up. Applied Energy 88(10):3377-3388.
Pienkos, P.T. 2007. The potential for biofuels from algae. Paper read at the Algal Biomass Summit, November 15, San Francisco, CA.
Pienkos, P.T., and A. Darzins. 2009. The promise and challenges of microalgal-derived biofuels. Biofuels Bioproducts and Biorefining-Biofpr 3(4):431-440.
Pittman, J.K., A.P. Dean, and O. Osundeko. 2011. The potential of sustainable algal biofuel production using wastewater resources. Bioresource Technology 102(1):17-25.
Redfield, A.C. 1934. On the proportions of organic derivations in sea water and their relation to the composition of plankton. Pp. 177-192 in James Johnstone Memorial Volume, R.J. Daniel, ed. University Press of Liverpool.
____________. 1958. The biological control of chemical factors in the environment. American Scientist 46:205-221.
Riebesell, U., K.G. Schulz, R.G.J. Bellerby, M. Botros, P. Fritsche, M. Meyerhöfer, C. Neill, G. Nondal, A. Oschlies, J. Wohlers, and E. Zöllner. 2007. Enhanced biological carbon consumption in a high CO2 ocean. Nature 450(7169):545-548.
Sagar, A.D., and B. van der Zwaan. 2006. Technological innovation in the energy sector: Research and Development, deployment, and learning-by-doing. Energy Policy 34(17):2601-2608.
Sander, K., and G.S. Murthy. 2010. Life cycle analysis of algae biodiesel. International Journal of Life Cycle Assessment 15(7):704-714.
Sardans, J., A. Rivas-Ubach, and J. Peñuelas. 2011a. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspectives in Plant Ecology, Evolution and Systematics. DOI: 10.106/j.ppees.
____________. 2011b. The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: A review and perspectives. Biogeochemistry. DOI: 20.11.08.002 10.1007/s10533-011-9640-9
Sialve, B., N. Bernet, and O. Bernard. 2009. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnology Advances 27(4):409-416.
Singh, A., P.S. Nigam, and J.D. Murphy. 2011. Renewable fuels from algae: An answer to debatable land based fuels. Bioresource Technology 102(1):10-16.
Stephenson, A.L., E. Kazamia, J.S. Dennis, C.J. Howe, S.A. Scott, and A.G. Smith. 2010. Life-cycle assessment of potential algal biodiesel production in the United Kingdom: A comparison of raceways and air-lift tubular bioreactors. Energy and Fuels 24:4062-4077.
Sterner, R.W., T. Andersen, J.J. Elser, D.O. Hessen, J.M. Hood, E. McCauley, and J. Urabe. 2008. Scale-dependent carbon: Nitrogen: phosphorus seston stoichiometry in marine and freshwaters. Limnology and Oceanography 53(3):1169-1180.
Sterner, R.W., and J.J. Elser. 2002. Ecological Stoichiometry. Princeton, NJ: Princeton University Press.
Stromberg, J.C., R. Tiller, and B. Richter. 1996. Effects of groundwater decline on riparian vegetation of semiarid regions: The San Pedro, Arizona. Ecological Applications 6(1):113-131.
Stumm, W., and J.J. Morgan. 1988. An introduction emphasizing chemical-equilibria in natural-waters. Agriculture Biology and Environmental Sciences(41):18.
Sturm, B.S., E. Peltier, V. Smith, and F. De Noyelles. 2012. Controls of microalgal biomass and lipid production in municipal wastewater-fed bioreactors. Environmental Progress and Sustainable Energy 31:10-16.
Subhadra, B.G., and M. Edwards. 2011. Coproduct market analysis and water footprint of simulated commercial algal biorefineries. Applied Energy 88(10):3515-3523.
Urabe, J., J. Togari, and J.J. Elser. 2003. Stoichiometric impacts of increased carbon dioxide on a planktonic herbivore. Global Change Biology 9(6):818-825.
USDA-RD (U.S. Department of Agriculture Rural Development). 2009. Environmental Assessment for Sapphire Energy Inc.’s Integrated Algal Biorefinery (IBR) Facility in Columbus, New Mexico. Washington, DC: U.S. Department of Agriculture.
USGS (U.S. Geological Survey). 2010. Mineral Commodity Summaries 2010. Reston, VA: U.S. Geological Survey.
Vaccari, D.A. 2009. Phosphorus: A looming crisis. American Scientist 300:54-59.
Van De Waal, D.B., A.M. Verschoor, J.M.H. Verspagen, E. Van Donk, and J. Huisman. 2010. Climate-driven changes in the ecological stoichiometry of aquatic ecosystems. Frontiers in Ecology and the Environment 8(3):145-152.
Vasudevan, V., R.W. Stratton, M.N. Pearlson, G.R. Jersey, A.G. Beyene, J.C. Weissman, M. Rubino, and J.I. Hileman. 2012. Environmental performance of algal biofuel technology options. Environmental Science and Technology 46(4):2451-2459.
Weissman, J.C., D.T. Tillett, and R.P. Goebel. 1989. Design and Operation of an Outdoor Microalgae Test Facility. Golden, CO: Solar Energy Research Institute.
Wigmosta, M.S., A.M. Coleman, R.J. Skaggs, M.H. Huesemann, and L.J. Lane. 2011. National microalgae biofuel production potential and resource demand. Water Resources Research 47(4):W00H04.
Wiley, P.E., J.E. Campbell, and B. McKuin. 2011. Production of biodiesel and biogas from algae: A review of process train options. Water Environment Research 83(4):326-338.
Williams, E., Weber, C., and Hawkins, T. 2009. Hybrid approach to managing uncertainty in life cycle inventories. Journal of Industrial Ecology 15(6):928-944.
Williams, P.J.L.B., and L.M.L. Laurens. 2010. Microalgae as biodiesel and biomass feedstocks: Review and analysis of the biochemistry, energetics and economics. Energy and Environmental Science 3(5):554-590.
Wu, M., M. Mintz, M. Wang, and S. Arora. 2009. Water consumption in the production of ethanol and petroleum gasoline. Environmental Management 44(5):981-997.
Xu, L., D.W.F.W. Brilman, J.A.M. Withag, G. Brem, and S. Kersten. 2011. Assessment of a dry and a wet route for the production of biofuels from microalgae: Energy balance analysis. Bioresource Technology 201:5113-5122.
Yang, J., M. Xu, X. Zhang, Q. Hu, M. Sommerfeld, and Y. Chen. 2011. Life-cycle analysis on biodiesel production from microalgae: Water footprint and nutrients balance. Bioresource Technology 102(1):159-165.
Yue, L., and W. Chen. 2005. Isolation and determination of cultural characteristics of a new highly CO2 tolerant fresh water microalgae. Energy Conversion and Management 46(11-12):1868-1876.








































