Highlight: Diagnosing Cancer with Positron Emission Tomography
Atomic nuclei with fewer neutrons than stable isotopes decay predominantly by emitting a positively charged electron, a positron, which is annihilated with electrons, emitting gamma-radiation. For more than 35 years positron emission tomography (PET) has been used as a research tool in neuroscience and in diagnosing cancer.1
PET imaging makes use of the self-collimating nature of positron decay (see Figure PET 1) as two nearly collinear photons are used to locate an annihilation event. PET cameras are typically made of a ring(s) of detectors that are in timed coincidence (resolving time of a few nanoseconds), allowing a line of response to define the chord along which the positron was annihilated (the location of the emission is not known because of the short distance the positron travels before annihilation, typically a few millimeters). By mathematically back-projecting the lines of response, a density map can be generated that reflects the distribution of the positron emitter.
Functional imaging using PET started as a research tool in neuroscience in the late 1970s and remains a major research tool for the neurosciences. However, its main impact recently has been in the diagnosis of cancer. Originally, simple tracer molecules such as water, carbon monoxide, and carbon dioxide were used. The first complex molecule to be used extensively was the glucose analog 18F-fluorodeoxyglucose (FDG), developed at BNL in collaboration
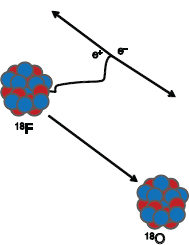
FIGURE PET 1 Illustration of positron decay. One of the protons (red) in the unstable nucleus is converted into a neutron (blue) with the emission of a positive electron (positron). The positron travels a short distance until it is annihilated with a neighboring atomic electron, resulting in two photons (Ð-rays), each with an energy of 511 keV. The photons will travel at nearly 180° from each other to conserve momentum. SOURCE: T. Ruth, 2011, The uses of radiotracers in the life sciences, Reports on Progress in Physics 72: 016701. Printed with permission from IOP Publishing Ltd.
with researchers at the NIH and the University of Pennsylvania around 1975. Since the human brain uses glucose as its primary energy source, the availability of the tracer led to groundbreaking studies of the human brain in health and disease. This effort was driven by the successful use of 14C-labeled deoxyglucose at the NIH by Louis Sokolov in the 1960s. Since 14C is not detectable from outside of the body, the effort went into developing a labeled analog that could be shipped from a cyclotron facility (BNL in this case) and the PET camera (the University of Pennsylvania). Thus 18F with its nearly 2-hour half-life became the radionuclide of choice.
Many more tracers are used to investigate the various neuronal systems, probing both the presynaptic and postsynaptic pathways. Several hundred tracers have been prepared and tested for their utility in investigating various enzymatic and receptor systems although only a handful are routinely used. There are tracers specifically designed to monitor cell proliferation, the hypoxic nature of cells, and cell apoptosis.
The heart of the PET camera is the detection system, which builds on scintillator detector systems developed by nuclear scientists. The vast majority of modern PET scanners make use of segmented inorganic scintillation crystals coupled to multiple photomultiplier tubes (PMT). The ideal crystal will have a high stopping power for the 511-keV annihilation photons (high photoelectric absorption), a high light output with wavelength matched to the PMT, and a fast decay time for the light, and it will be physically robust. For nearly two decades the detector material of choice was bismuth orthogermanate, a scintillator often used in basic nuclear science research. More recently, lutetium orthosilicate was introduced. Owing to its higher light output, the segmentation of the crystals could be finer, thus reducing the crystal element size from approximately (4 mm × 4 mm) to (2 mm × 2 mm). There are proposals to reduce the crystal elements to below 1 mm2. In order to accomplish such a task, the packing fraction of the crystals must be improved—in other words, the empty space between crystal elements must remain a small fraction of the total area.
The typical crystal is segmented into an 8 × 8 grid (or more) coupled to four PMTs. There is an algorithm to identify the location of the event by comparing the light sharing among the PMTs. While this scheme reduces the cost of the scanner, there is a loss in resolution owing to the approximate nature of the light-sharing approach. There are prototype scanners using avalanche photodiodes coupled to individual crystal elements, making the finer pixel identification better. Thus far such systems have been built only for small animal scanners.
As the physical limitations of detection are approached, the remaining avenue is to increase the signal to noise ratio by utilizing tracers that are uniquely suited to imaging the function in question and that otherwise clear rapidly from surrounding tissue. To this end, the development of more specific tracers is believed to be the most critical issue for PET.
One of the main strengths of PET compared to single-photon emission computed tomography (SPECT) is the ability to measure, directly, the attenuation effect of the object being viewed. This is the result of requiring that both photons be detected. Thus, if one photon of the pair is not observed, then there is no line of response. Along the path to the detectors, one or both photons (511 keV each, the rest mass of the electron) can undergo absorption. Thus, in order to be detected as an event, both photons must be detected in temporal coincidence. By using an external source of positron emitter, the attenuating (absorbing) extent of the object to be measured can be determined. All commercial PET cameras are now built with a CT scanner (X-ray tomography) so that a merged image of structure and function can be obtained. Since the CT image is a measure of electron density, it is used to calculate the necessary coefficients for attenuation correction. The primary function of the CT image is to provide a detailed view of the section of the body under investigation. Figure PET 2 illustrates the power of this approach.
There are several physical limitations inherent in PET technology. First, as the emitted positron has kinetic energy, varying from a few hundred keV to several MeV depending on
which radionuclide is used, it will travel a few millimeters to centimeters before annihilating with an atomic electron. As such, the site of annihilation is not the site of emission, resulting in a limitation when defining the origin of the decay. Another limitation is the fact that the positron-electron pair is not at rest when the annihilation occurs; thus by virtue of the conservation of momentum, the two photons are not exactly collinear. Although the lack of collinearity becomes increasingly important with greater detector separation, this effect is ignored, for the most part, in existing tomographs because the detector ring diameter is less than a meter, at which distance the deviation from 180° is a fraction of a millimeter.
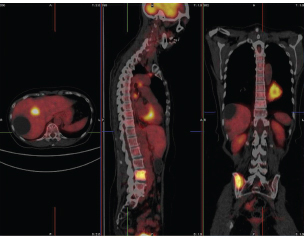
FIGURE PET 2 The three panels from left to right show a combined FDG PET/computed tomography (CT) image in transaxial, saggital, and coronal views. The colored hot metal image is the PET image and the gray image is from the CT camera. The combined image enables physicians to determine the precise location of abnormal function (high uptake in the mass visible on the chest wall in the CT image in this case). In addition, a metastatic tumor is visible in the pelvic region. SOURCE: T. Ruth, 2011, The uses of radiotracers in the life sciences, Reports on Progress in Physics 72: 016701. Photo courtesy of British Columbia Cancer Agency and reprinted with permission from IOP Publishing Ltd.
Because diagnostic imaging is driven by a digital approach (present/absent, yes/no), the desire to have uncluttered images resulting from PET is very important. Nevertheless, the true power of PET lies in its ability to track the distribution of a tracer over time and to extract detailed kinetic data, as in a physical chemistry experiment where rate constants are determined. So, the conflict between using PET technology for clinical diagnosis and using it as an in vivo biochemistry tool will not be easily resolved, nor should it be.
1The information in this vignette is adapted from T.J. Ruth, 2009, The uses of radiotracers in the life sciences, Report on Progress in Physics 72: 016701. Permission granted by IOP Publishing, Ltd.



