Societal Applications and Benefits
Nuclear physics is ubiquitous in our lives: Detecting smoke in our homes, testing for and treating cancer, and monitoring cargo for contraband are just some of the ways that nuclear physics and the techniques it has spawned make a difference in our safety, health, and security. Many of today’s most important advancements in medicine, materials, energy, security, climatology, and dozens of other sciences emanate from the wellspring of basic research and development in nuclear physics. Answers to some of the most important questions facing our planet will come from nuclear science, interdisciplinary efforts in energy and climate, and marketplace innovations. The economic impact of the applications of nuclear physics is significant. As an example, particle beams from accelerators are used to process, treat or inspect a wide range of products with a collective value of more than $500 billion.1 At the same time, approximately 23 million nuclear medicine procedures are carried out each year in the United States to diagnose and treat cancers, cardiovascular disease, and certain neurological disorders. In the future, basic nuclear science will be a key discipline that provides ideas and insights leading to the intellectual properties and patents with which venture capitalists and entrepreneurs will shape the economies of the future.
Between the chapters of this document the committee has highlighted some of the ways that nuclear physics impacts our lives along with some of the individuals poised for leadership in nuclear physics. In this chapter we provide a more detailed
________________________________________________
1 Department of Energy, 2010, Accelerators for America’s Future, Washington, D.C. Available at http://www.acceleratorsamerica.org/report/index.html; last accessed August 31, 2011.
overview of some of the ways in which nuclear physics is being applied to address the nation’s challenges in health, homeland and national security, nuclear energy, and some of the innovations taking place in developing and exploiting new technologies arising from nuclear science.
DIAGNOSING AND CURING MEDICAL CONDITIONS
Nuclear physics techniques have been revolutionary in medical diagnostics and cancer therapy. Of the 23 million nuclear medicine imaging and therapeutic procedures performed each year in the United States, typically 40-50 percent are for cardiac applications, while 25-40 percent are for cancer identification and therapy. In addition, nuclear medicine procedures are used to diagnose Alzheimer’s disease, treat hyperthyroidism, assess coronary artery disease, localize tumors, and diagnose pulmonary emboli.
The science of nuclear medicine, however, goes far beyond the radiopharmaceuticals used for imaging and treatment. Advances in the field are inevitably tied to basic research in nuclear physics at all levels. These advances include accelerators, detectors, understanding the interaction of radiation with matter, and creating complex statistical algorithms for identifying relevant data.
Nuclear Imaging of Disease and Functions
Over the past few decades, new nuclear imaging technologies have enhanced the effectiveness of health care and enabled physicians to diagnose different types of cancers, cardiovascular diseases, and neurological disorders in their early stages. Today there are over 100 nuclear imaging procedures available. These procedures have the additional advantage of being noninvasive alternatives to biopsy or surgery. Unlike other imaging procedures that are designed mainly to identify structure, nuclear medicine can also provide information about the function of virtually every major organ system within the body.
The most important modern advances in nuclear imaging are positron emission tomography (PET) and single-photon emission computed tomography (SPECT). PET, especially when coupled to X-ray computed tomography (CT) scans, has become a highly sensitive probe of abnormal functions, as described in detail in the PET highlight between Chapters 2 and 3.
18F-fluorodeoxyglucose (18F-FDG) is a radiopharmaceutical used in medical-imaging PET scans. This is a glucose analog that is absorbed by cells such as those in the brain and kidneys as well as cancer cells, which use high amounts of glucose. This procedure yields scans such as those displayed in Figure 3.1 and can be used for the study of organ functions and, in the case of cancer cells, for therapeutic applications. The 1.8-hour half-life t1/2 of fluorine-18 results in very high specific
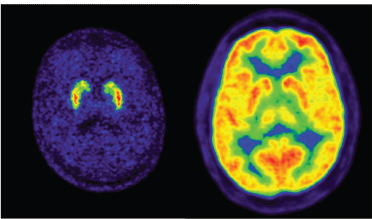
FIGURE 3.1 PET is a powerful tool to probe the functions of the brain. In these images of the brain, the radionuclide is fluorine-18 while the molecules for each image obviously have different biodistributions. The left-hand figure shows fluorodopa (to probe dopamine integrity) while the right-hand figure shows fluorodeoxyglucose (to probe sugar metabolism). SOURCE: Courtesy of Don Wilson, British Columbia Cancer Agency.
activity with no long-term residual activity in the body. However, the short lifetime means that fluorine-18 and 18F-FDG have to be produced very near to where the procedures are to be performed. This often requires in situ small-scale particle accelerators, another capability developed by nuclear physicists, to produce the isotope.
Radionuclides that emit gamma-rays have a long history as imaging tools in the diagnosis of cancer. SPECT has been built around the gamma-ray associated with the decay of molybdenum-99. Molybdenum-99 decays (t1/2 = 66 hours) into an isomer of technetium-99m (m indicating metastable), which in turn decays (t1/2 = 6 hours) by emitting a 140-keV gamma-ray. The cameras for this imaging technique are typically made with a cluster of photomultipliers coupled to a large NaI crystal. In recent years, the semiconductor material CdZnTe (CZT) has gained favor because of its higher energy resolution. Having this type of capability means that multiple tracers can be imaged simultaneously through the use of different energy windows.
In North America, the main radioisotopes needed for imaging and treatment are produced by the Isotope Development & Production for Research and Applications (IDPRA) program, in the Nuclear Physics Program of the Department of
Energy’s (DOE’s) Office of Science, and by two Canadian facilities, TRIUMF and Chalk River.
Worldwide, the molybdenum-99/technetium-99m radionuclide pair is used in four out of five, or in about 12 million diagnostic-imaging procedures in nuclear medicine every year. However, the reactors that have been producing molybde-num-99 are approaching the end of their useful lives, which is expected to trigger an “isotope crisis.” One of the reactors, the Canadian National Research Universal (NRU) reactor at Chalk River, is scheduled to stop isotope production in 2016, while potential replacement reactors around the world may not be available until 2020. Research is now focused on exploring accelerator-based production of molybdenum-99 as an alternative technology using, among other reactions, the 100Mo(g,n)99Mo and the 100Mo(p,2n)99mTc reactions.
Another option centers on rhenium-186, which has a favorable half-life (t1/2 = 90 hours) and emits beta decay electrons of 0.9 MeV with a 10 percent branch emitting a gamma-ray with energy similar to that of technetium-99m. Since rhenium is in the same chemical family as technetium, much of the technology developed for technetium-99m can be applied to rhenium-186. Current efforts are concentrated on reactor production of rhenium-186 via the 185Re(n,g) reaction, followed by mass separation to yield a sample with the high specific activity needed for therapy (see Box 3.1).
New Radioisotopes for Targeted Radioimmunotherapy
Radiopharmaceuticals have been developed that can be targeted directly at the organ being treated. These therapy radiopharmaceuticals rely on the destructive power of ionizing radiation at short ranges, which minimizes damage to neighboring organs.
A frontier direction is targeted radiopharmaceuticals. This involves attaching a relatively short-lived radioactive isotope that decays via high-energy transfer radiation (alpha-particle emission, for example) to a biologically active molecule, like a monoclonal antibody that has a high affinity for binding to receptors on cancer tumors. When the radioactive nuclei decay, the radiation they produce loses energy quickly and because it does not travel far, a lethal dose of radiation is delivered only to adjoining tumor cells. By careful construction of the targeting molecule, the radioactive nuclei will pass through the body quickly if they do not bind to tumor cells, thus minimizing the exposure of healthy tissue to the high-energy transfer radiation. Presently, the most common radionuclides are iodine-131 and yttrium-90, though neither is ideal. Two radiopharmaceuticals, Bexxar (using iodine-131) and Zevalin (using indium-111 or yttrium-90), are now in use to treat non-Hodgkins lymphoma.
Many research efforts are focused on the production of alternative isotopes
Box 3.1
Suzanne Lapi and Radionuclide Production
Suzanne Lapi is a leader in the effort to develop rhenium-186 for radiation therapy. After receiving her master of science and Ph.D. degrees from Simon Fraser University, British Columbia, she pursued research into the production of rhenium-186 of high specific activity to enhance the therapeutic efficacy of this promising radionuclide. After concluding that accelerator production was not optimal, she focused on increasing specific activity of rhenium-186, produced in a reactor by the 185Re(n,γ) reaction, by mass separation of the postirradiated material. This work is the subject of a patent and is also being applied to increasing the specific activity of molybde-num-99, also produced via the (n,γ) reaction. Presently Dr. Lapi is an assistant professor at the Mallinckrodt Institute of Radiology at Washington University in St. Louis, Missouri. She is a project leader on radionuclide research for cancer applications, oversees production of nonstandard PET radionuclides, and collaborates with internal and external faculty on grants supported by both DOE and the National Institutes of Health (NIH).

FIGURE 3.1.1 Suzanne Lapi. Source Photo courtesy of MIR Photography
with superior cytotoxicity for use in therapy. A promising class of isotopes is those that decay by alpha emission, since alpha particles have a very short range in tissue, resulting in an enhanced cytotoxicity. The radionuclide actinium-225 combines several favorable properties, including a half-life of 10 days, high alpha-particle energy, versatile coordination chemistry, and several alpha-emitting daughter isotopes. Actinium-225 has been used in Phase I and II clinical trials; it is presently being produced at Oak Ridge National Laboratory (ORNL) and at the Institute for Transuranium Elements in Karlsruhe, Germany. Its availability, however, is currently limited, and alternative production mechanisms are being investigated at the Los Alamos National Laboratory (LANL) isotope production facility. More recently, researchers at the Karlsruhe Institute in Germany have reported the efficacy of treating neuroendocrine tumors with the alpha-emitting bismuth-213 nucleus attached to a biological molecule (called DOTATOC) that targets these
particular tumors. They found that the tumors of seven out of nine patients had become smaller with no discernible negative side effects. If this approach can be validated and brought into routine use, the treatment of cancer will have had a major paradigm shift.
In the coming decade, nuclear physics facilities will continue to broaden the range of isotopes for medical applications. For example, the Facility for Rare Isotope Beams (FRIB) at Michigan State University (MSU) will be capable of producing shorter-lived isotopes of key elements for more rapid dose kinetics and new medical applications.
Future Technologies in Nuclear Medicine
The future impact of nuclear science on medical science is difficult to predict. If history is an indicator, one can expect more significant and exciting contributions. At the least, advances in nuclear medicine will likely remain closely connected with advances in nuclear techniques.
One future direction is personalized medicine, the attempt to identify and treat disorders based on an individual’s response to the disease process. This will require more sophisticated nuclear tools. As an example, chemistry systems will be reduced to the size of a postage stamp, thus making patient-specific diagnostic tools and treatment truly individualized. An example of an integrated device, designed for multistep radiosynthesis of PET tracers, is displayed in Figure 3.2.
Other important new directions involve the coupling of advances in genetically engineered antibodies with radionuclides and the use of nuclear imaging to help us understand the underlying causes of disease by extracting functional and anatomical information in the same image.
MAKING OUR BORDERS AND OUR NATION MORE SECURE
Nuclear science has a long tradition in national security, from the Manhattan Project to today’s focus on homeland security. Nuclear devices have determined the outcome of wars and changed the political boundaries of the world. Today, nuclear science plays a critical role in global politics: It protects the borders of the United States, safeguards nuclear material and forestalls the proliferation of nuclear weapons, prevents nuclear terrorism (while at the same time preparing for the “unthinkable”), and ensures that the nation’s nuclear weapons stockpile is reliable.
The past decade has seen an expansion in the types of nuclear security problems facing society. For example, considerable effort has been devoted to exploiting new concepts for nuclear forensics and for border protection. At the same time, traditional fields, such as stockpile stewardship and reactor safeguards, have more needs than ever. The contributions from the nuclear physics community to
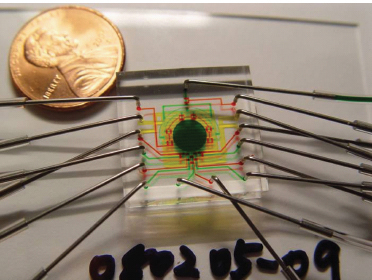
FIGURE 3.2 Future technologies in personalized medicine will require smaller patient-specific diagnostic tools. An example is the chemistry system being designed to produce multiple human doses of FDG, an analog for glucose, on a chip the size of a U.S. penny. In this figure the chip has channels for introducing reagents, channels for opening and closing “pressure valves” by introducing fluids, and channels for venting to allow fluid flow. Flow channels are filled with green dye, control channels with red, and vent channels with yellow. The circle in the center is the reaction chamber. Such devices will reduce time and quantity of reagents and increase efficiency. SOURCE: Courtesy of Arkadij M. Elizarov, Siemens Healthcare. © Copyright Siemens Healthcare 2012. Used with permission.
all of these issues have been both numerous and broad, and a significant number of nuclear physics graduate students express an interest in pursuing careers that address these issues.
Protecting Our Borders from Proliferation of Nuclear Materials
Border Detection of Nuclear Contraband
The priority mission of our nation’s Border Patrol is preventing terrorists and terrorists’ weapons, including weapons of mass destruction, from entering the United States. Currently there are radiation portal monitors installed at approximately 300 ports of entry. These monitors detect gamma-rays and neutrons emitted from nuclear material. However, one can shield such radiation from detection by
placing absorbing material around the nuclear material being smuggled. To deal with such shielding, numerous research groups at universities and national labs are exploring novel detection schemes.
One such scheme scans for high-atomic-number (high-Z) materials hidden in vehicles using cosmic ray muons. As energetic cosmic rays impinge on Earth’s atmosphere, they collide with nuclei in the atmosphere to produce copious quantities of muons. Because muons do not interact strongly with the atmosphere, many reach Earth’s surface and even penetrate for some distance into shallow mines. Muon radiography takes advantage of this penetrability and is designed to measure the scattering of these muons as they pass through motor vehicles at border inspection stations as a means of detecting hidden nuclear contraband. As sketched in Figure 3.3, the muons are detected both above and below the vehicle. The muons interact with matter in two ways: (1) with atomic electrons, which results in continuous energy loss, and (2) with the atomic nuclei, which results in large angle changes in the muon’s path. Each of these interactions provides a radiographic signal that can be used to characterize the material inside a truck. For example, very large angle scattering is a signal that the truck contains high-Z material, such as
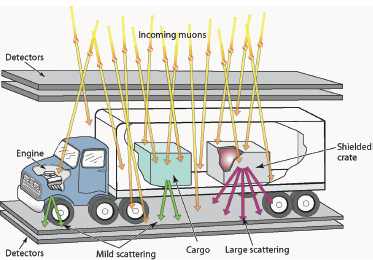
FIGURE 3.3 Muons passing through high-Z materials (like uranium and plutonium) are scattered more than those passing through other materials (such as steel or water). Cosmic ray muons can therefore be used as an active interrogation probe of nuclear materials by detecting muons above and below a truck. SOURCE: Courtesy of C.L. Morris, Los Alamos National Laboratory (LANL).
uranium or plutonium. Muon radiography is proving to be a very efficient border protection tool, and experiments have shown that even high-Z material hidden inside the engine of a vehicle is readily detectable.
Nuclear Safeguards
The International Atomic Energy Agency’s (IAEA’s) safeguards system under the Treaty on the Non-Proliferation of Nuclear Weapons, also known as the Nuclear Nonproliferation Treaty (NPT), is aimed at preventing the diversion of civilian nuclear material into military uses. The IAEA safeguards also include schemes for detecting undeclared nuclear activities, such as illicit operations of nuclear reactors. By signing the NPT Treaty, all of the (currently 184) nonnuclear states agree to IAEA safeguard inspections of their nuclear facilities.
One of the very challenging problems for the IAEA is protecting against repeated thefts of small quantities of material over extended time periods. Accountability safeguards largely rely on the detection of gamma-rays and neutrons from nuclear materials, which can be used to deduce inventory anomalies or materials in unauthorized locations. An important component of these schemes is the coupling of advanced radiation detection physics with large nuclear decay databases (and their uncertainties). Scientists at Lawrence Livermore National Laboratory (LLNL) have demonstrated the practicality of gamma-ray nondestructive isotopic measurements using high-purity germanuim (HPGe) gamma-ray detectors. For homogenous materials, one HPGe detector is sufficient to extract isotopic ratio information; for inhomogeneous materials, external transmission sources and multidetector tomography scanning are needed.
Certifying the Nation’s Nuclear Stockpile
What Happens When Neutrons Interact with Actinides?
To enable certification of the nation’s stockpile in the absence of nuclear testing, a number of nuclear physics measurements, coupled with supporting nuclear theory, are being carried out at university and national laboratories. Many of these studies involve neutron-induced cross sections on fissionable actinides (the 14 chemical elements with atomic numbers from 90 to 103, including uranium and plutonium) and other materials that might be found in a nuclear device. Also of importance is the detailed characterization of the energy resulting from fission and fusion.
Current uncertainties on the important fission cross sections for stockpile stewardship are on the order of 2 to 3 percent. In the case of plutonium-239, it would
be ideal if this uncertainty were reduced to 1 percent, an improved accuracy also important in developing next-generation reactors. Achieving this level of accuracy requires overcoming uncertainties associated with past fission ionization chamber measurements. Accordingly, a team of LLNL, LANL, and university scientists is developing a fission time projection chamber (TPC), sketched in Figure 3.4, that will be capable of three-dimensional event reconstruction with a high background rejection. Once completed, this will represent a major advance in fission physics. Understanding fission cross sections, especially on actinides other than uranium-235, uranium-238, and plutonium-239 is also important for developing the next generation of nuclear reactors.
In addition to fission, several other neutron-reaction cross sections are needed for stockpile stewardship. Perhaps the most important of these is neutron capture in the tens to hundreds of keV neutron energy region. There is an ongoing neutron capture program involving university and national laboratory scientists and
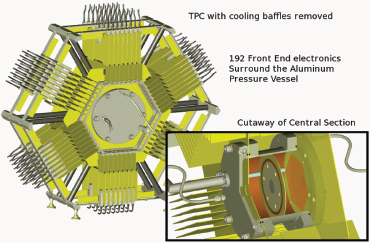
FIGURE 3.4 TPCs are sensitive instruments in basic research in high-energy and nuclear physics used, for example, in the solenoidal tracker at RHIC (STAR). A new application of a TPC is being developed to enable measurements of neutron-induced fission probabilities of actinides with unprecedented accuracies. The TPC will measure the energy, mass, and direction of fission fragments. Upgrades to the baseline TPC, including additional detectors, would also measure the energy, direction, and multiplicities of fission neutrons and will be able to correlate gamma-radiation with fission events. Such measurements of fission probabilities and properties are important in a wide range of disciplines including nuclear energy, nuclear forensics, national security, and basic nuclear science. SOURCE: Courtesy of M. Heffner, LLNL.
the 4-π BaF2 Detector for Advanced Neutron Capture Experiments (DANCE) at the Los Alamos Neutron Science Center (LANSCE). As an example, some amount of americium-241 is present in all weapons-grade plutonium, and reactions on americium-241 are an important diagnostic for weapon performance. Because the americium-241(n,γ) reaction is important for nuclear forensics, there is a close synergy between the stockpile stewardship and nuclear forensics efforts. DANCE is also used to measure neutron cross sections on unstable targets important for s-process nucleosynthesis.
The extreme conditions in a nuclear explosion result in many of the reactions taking place on unstable nuclei. In the coming decade, access to a much broader range of important unstable isotopes will become possible as FRIB comes online. For many short-lived isotopes, direct measurements will provide information on key reactions of interest. However, it will not be possible to measure all of the relevant reactions, and for the very shortest-lived isotopes theory and simulations will be necessary. FRIB data from related reactions will provide important benchmarking and tests of theory, thus lending confidence to the predictions for the very short-lived unstable isotopes.
Using Protons to See Where Light Can Never Shine
Over the last decade, proton radiography has become an increasingly important scientific diagnostic tool for weapons science. First demonstrated in 1995, the technique involves using high-energy protons in flash radiography of dynamic experiments, such as implosion tests of mock-ups of nuclear weapons. Protons have advantages over X-rays for certain radiography experiments because protons can penetrate dense materials more efficiently. A key to the success of proton radiography was the realization that magnetic “lenses” can focus the scattered protons to produce exceptionally high-resolution images. The unique feature of proton radiography is its ability to produce high-resolution “movies” of an explosively driven experiment of up to 32 frames, as displayed in Figure 3.5. This allows scientists to probe and quantify dynamic phenomena important in accessing the nation’s aging stockpile in the absence of nuclear testing. Today, more than 40 proton radiography experiments are conducted at the LANSCE each year. Other experiments have been carried out at the Alternating Gradient Synchrotron (AGS) accelerator at Brookhaven National Laboratory (BNL).
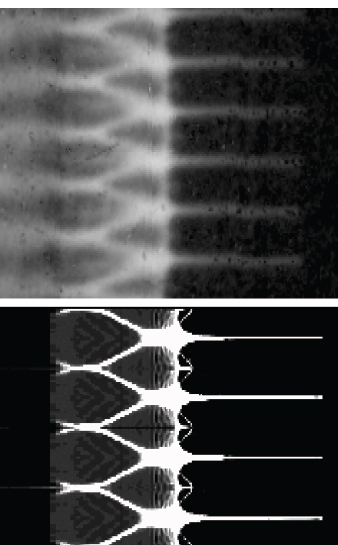
FIGURE 3.5 Understanding the growth of instabilities in shocked material is a major area of research that is being probed with proton radiography. Shown is a comparison of a proton radiograph of spikes and bubbles that are formed by the growth of Rayleigh-Taylor instabilities of a shocked tin surface (top) and a hydrodynamic simulation of the experiment (bottom). SOURCE: Courtesy of W. Buttler, LANL, and B. Grieves, Atomic Weapons Establishment.
The Greatest Challenge: Nuclear Devices in the Hands of Terrorists or a Rogue Nation
What if the unthinkable happens? A nuclear device is exploded by terrorists or a rogue nation. Or a radiological bomb is detonated in a large U.S. city. Nuclear scientists are using the concepts and tools of nuclear science to assess the risks, monitor for contraband nuclear material, and analyze the devices and materials that could be detonated. The goal is to develop a deterrent for candidate devices for such horrendous actions, as well as to discover what, how, and who should a detonation occur. Nuclear forensics comprises the technical means and set of scientific capabilities that, in the event of an attack, would be used to answer these questions. Nuclear scientists and the tools of nuclear science are keys to addressing the challenges of nuclear forensics, as described in detail in the Nuclear Forensics highlight between Chapters 5 and 6.
CARBON-EMISSION-FREE ENERGY FOR THE FUTURE
Fossil fuel emissions from power plants foul the air and are central to the discussion of global warming. The emissions contribute to dense brown clouds that hang over cities like Los Angeles and Phoenix, triggering asthma and other respiratory problems. Nuclear energy is an important component of the nation’s mission to produce safe, secure, economic, and sustainable energy. Broadly speaking, nuclear energy involves both fission reactors and nuclear fusion. Research in reactor physics spans a broad set of specialties including fuel damage, fuel recycling, safeguards, and waste management. Nuclear physics plays a direct role in addressing each of these. For nuclear fusion, the plasma physics community is exploring a number of methods to achieve the necessary conditions for controlled energy release. In nuclear fusion, plasma conditions approaching those in burning stars are required, and nuclear physics plays a significant role in diagnosing the conditions achieved in the plasma.
The majority of the world’s nuclear power is generated using reactors based on designs originally developed for naval use. These and other so-called second-generation nuclear reactors are safe and reliable, but they are being superseded by improved designs. Over the past decade, nuclear engineers have been researching advanced reactor designs, and there is a worldwide movement toward to a new generation of reactors. Some of the advanced designs include fast reactors, high-temperature graphite-moderated reactors, thorium-uranium-fueled reactors, pebble bed designs, and mixed oxide fuel (MOX) plutonium reactors. Developing
these designs requires detailed information about the reactions and other physics involved in the processes that are expected to take place. Such measurements are quite challenging. The fact that many of them are also important to stockpile stewardship and nuclear forensics greatly enhances our ability to bring together the teams of scientists needed for these experiments.
Decay Heat
The energy released during radioactive decay in postfission processes, commonly called “decay heat,” accounts for about 8 percent of the energy produced in the fission process itself. The accurate characterization of decay heat is crucial for the reactor shutdown process, since it is the main source of heating after neutron-induced fission is terminated. The decay heat, and in particular the high-energy part of the radiation, is a key aspect in the proper design of shielding and storage casks for transporting and storing spent nuclear fuel.2
A team of scientists and engineers, led by ORNL, constructed a high-efficiency modular total absorption spectrometer (MTAS), displayed in Figure 3.6, to measure the decay heat of fission products. MTAS complements other instruments designed to directly measure neutron emissions following beta decay of fission fragments, neutrons that contribute to the neutron budget in a reactor and help to ensure stable reactor operation. The decay heat and beta-delayed neutron measurements are also important for understanding r-process nucleosynthesis.
Reactor Material Damage
Irradiation of both nuclear fuel and structural materials in reactors produces material defects that limit the safe lifetime of these materials. Numerous irradiation effects can cause material damage, and a number of ongoing collaborations between nuclear physicists, material scientists, and reactor engineers are examining and characterizing these effects in detail.
One example is the buildup of helium at grain boundaries and its effect on the embrittlement of reactor structural materials. The embrittlement of metals such as nickel, iron, and copper has been demonstrated to be a function of both temperature and helium concentration. Most of the helium is produced by neutron-induced reactions (n, α), but many of the cross sections for these reactions were not well known. New cross section measurements have resulted in significant changes in estimates of the probable safe lifetime of structural reactor materials.
In nuclear fuels, a major cause of damage is the buildup of bubbles of noble
________________________________________________
2 The text of this paragraph is adapted from K.P. Rykaczewski, 2010, Viewpoint: Conquering nuclear pandomenium, Physics 3:94.
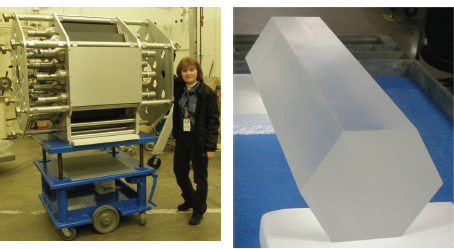
FIGURE 3.6 In a nuclear reactor, a sizeable fraction of the energy when the reactor is on and after it is shut down comes from the radioactive decay of fission products, also known as decay heat. A state-of-the-art device is being commissioned at ORNL to measure the decay heat of fission fragments with high accuracy. The MTAS consists of 19 NaI(Tl) modules with 48 photomultipliers (left panel). Its total volume will be about seven times that of the largest existing total absorption spectrometer. The right panel shows the first MTAS crystal manufactured at Saint Gobain Crystals, in Hiram, Ohio. In the coming decade, FRIB will produce a greatly expanded set of fission fragments and enable precision measurements of their detailed decay modes. SOURCE: Courtesy of K. Rykaczewski and M. Wolinska-Cichocka, ORNL.
gases and their migration through the fuel. Gas bubbles can cause changes in internal gas pressure, thermal conductivity, temperature gradients, and material stress and strain, thus inducing damage or even failure in fuel and cladding materials over time. Understanding the formation and properties of these bubbles and how to detect the gases if released is the focus of a joint collaboration between materials and nuclear scientists.
Fuel Performance and Next-Generation Reactors
One advanced concept is the fast reactor, wherein the neutron flux is considerably higher in energy than in standard thermal reactors. The dominant neutron energies for a fast reactor are 0.1-0.6 MeV. Fission cross sections are considerably less well known at fast reactor energies than at thermal energies. And the situation is most serious for the transuranic fuels. New programs are under way to measure
the fission cross sections, where experimentally feasible, on less abundant isotopes of plutonium and uranium, as well as the minor actinides such as isotopes of americium, curium, and neptunium. In addition to the fission cross sections, accurate knowledge of the neutron capture cross sections on the minor actinides is important. Many of these actinides are radioactive, restricting measurements to small targets. International collaborations are addressing these problems using the DANCE detector at LANSCE, which is designed to study neutron capture reactions on small quantities, about 1 mg, of radioactive and rare stable nuclei. Others are using the TPC displayed in Figure 3.4 to determine fission cross sections with unprecedented accuracy.
One major attractive characteristic of fast reactors is their enhanced ability to burn up highly toxic transuranic fuel produced as waste from light water reactors. At these higher neutron energies, there are a number of nuclear properties of reactor fuels that need to be determined to considerably higher accuracy than is presently possible, including reactions of neutrons with unstable fission products. In the future, FRIB will extend capabilities by allowing studies of a considerably larger class of unstable isotopes. For several key nuclides that have longer half-lives, FRIB will provide separated samples that can be used to measure neutron capture probabilities at neutron beam facilities. For isotopes with shorter lifetimes, indirect reaction measurements at FRIB will provide information to help constrain theoretical models for neutron-capture probabilities, using techniques that will also advance basic nuclear science and nuclear astrophysics.
When two light nuclei interact, they can fuse to form a heavier nucleus, accompanied by the release of a large amount of energy. The conditions found in stellar environments are ideal for sustained fusion chains, and our sun is a natural fusion reactor. However, achieving these hot, dense conditions in the laboratory is very challenging, and to date the only successful terrestrial events have been thermonuclear explosions. Currently there are two main research approaches to fusion: magnetically driven fusion and laser-driven fusion. The National Ignition Facility (NIF) at LLNL is a laser-driven inertial confinement fusion (ICF) facility.
High-Energy-Density Physics
Probing physics at high energy densities is central to several subfields of nuclear physics, including the study of nucleosynthesis, the quark-gluon plasma, and neutron stars. The NIF provides a unique regime in the temperature-density (T-ρ) plane of high-energy-density physics (see Figure 3-7). NIF is designed to compress capsules containing a mixture of deuterium (d) and tritium (t) to temperatures and
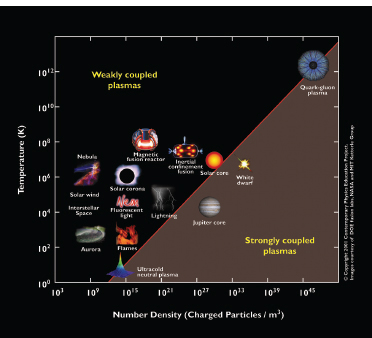
FIGURE 3.7 Plasmas are an important phase of matter, from the flames of candles to the quark-gluon plasma generated for a fraction of a second in a relativistic heavy ion collision. The temperature in kelvins as a function of the number of charged particles per cubic meter for a wide range of physical systems is displayed. The National Ignition Facility produces plasmas via inertial confinement fusion that are comparable to the interior of the sun. SOURCE: Courtesy of the Contemporary Physics Education Project.
densities high enough to ignite thermonuclear reactions. Laser pulses, directed into a hohlraum cylinder containing the target capsule, create an X-ray bath sufficient to compress the capsule through ablation of an outer layer of material. Achieving the conditions needed for ignition is challenging but made more tractable with the use of advanced diagnostics, many of which are based on nuclear physics.
Using Neutrons to See Fusion
The main fusion reaction at NIF is the d + t ![]() n + α reaction, which releases 17.6 MeV of energy per reaction in the form of a 14-MeV neutron (n) and a 3.6-MeV alpha particle. If successfully ignited, an NIF capsule will burn about 1018 d + t
n + α reaction, which releases 17.6 MeV of energy per reaction in the form of a 14-MeV neutron (n) and a 3.6-MeV alpha particle. If successfully ignited, an NIF capsule will burn about 1018 d + t
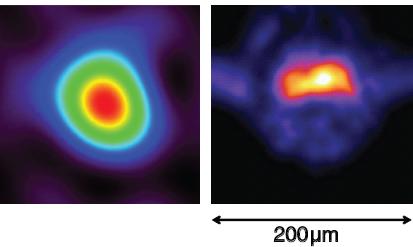
FIGURE 3.8 The NIF at LLNL is striving to use high-powered lasers to fuse deuterium and tritium, recreating in the laboratory the source of energy in our sun. Neutrons are one of the main products of the fusion reactions. Nuclear physicists are developing tools to diagnose the conditions in the NIF d-t capsules. On the left is a simulation of an expected neutron image; on the right is a reconstruction of an actual neutron image of a capsule taken at NIF. The image determines the size of the hotspot and the asymmetry of the implosion. SOURCE: NIF, LLNL.
reactions, with a corresponding release of over 2.5 MJ of fusion energy. One of the important diagnostics for understanding capsule behavior is neutron imaging. The system being built for NIF will image both the primary 14-MeV neutrons from the d + t reaction and the lower energy 6- to 13-MeV neutrons resulting from these primary neutrons losing energy in the material, or downscattering. The image of the primary 14-MeV neutrons determines the size of the burning fuel region (the hotspot). The lower-energy, downscattered neutrons provide information on the average density as a function of radial distance from the center of the fuel and on how symmetric or asymmetric an implosion was achieved. The imaging system, a set of pinhole apertures placed close (within 20 cm) to the capsule and an imaging camera placed far (28 m) from the capsule, will be capable of imaging neutrons from capsules that burned as few as 1014 d + t reactions, as shown in Figure 3.8.
INNOVATIONS IN TECHNOLOGIES AND APPLICATIONS OF NUCLEAR SCIENCE
Nuclear physics is fundamentally cross-disciplinary in nature, providing experimental and theoretical tools and concepts for countless other sciences and
contributing to the quality of life across a wide spectrum of social and economic needs. The applications and manifestations are so entrenched in our daily lives as to be ubiquitous, from simple everyday household items to technologies that provide significant portions of the foundation of medical procedures. Nuclear science has and will continue to play a substantial role in developing solutions for energy, climate, and environmental challenges. Further, the primary tools of modern nuclear science—accelerators and computers—have spawned many applications and economic benefits, some of which are discussed here.
Addressing Challenges in Medicine, Industry, and Basic Science with Accelerators
Beams of high-energy particles, produced by accelerators, are essential for both fundamental and applied research and for technical and industrial fields. Accelerators have become prevalent in our lives, and there are now over 30,000 accelerators worldwide. Of these, the largest number (about 44 percent) are used for radiotherapy, while 41 perecnt are used for ion implantation, 9 percent for industrial research, and about 4 percent for biomedical research. The remaining 1 to 2 percent of accelerators are very high-energy accelerators used in nuclear and particle physics to probe the fundamental nature of the matter making up our universe.
All accelerators can be described as devices that use electric fields to accelerate charged particles (such as electrons or ions) to high energies, in well-defined beams. Since the discovery of the X-ray in 1895 by Roentgen, many famous nuclear physicists have made seminal contributions to new accelerator technologies, including John D. Cockcroft, Ernest Walton, Earnest O. Lawrence, and Robert Van de Graaff. Today accelerator technologies range from the Large Hadron Collider (LHC) capable of producing TeV particles to the lowest energy accelerators used by industry.
Accelerators and Medicine
Accelerators form the basis for many diagnostic systems, from chest X-ray machines to whole-body X-ray scanners capable of creating a three-dimensional image of the living body. Accelerators such as cyclotrons enable protons and other light nuclei to be used to produce radioactive nuclei that are used in diagnostic medicine. Radioisotopes such as thallium-201 are used to diagnose heart disease. The production of the unstable isotopes of the elements of life, such as oxygen-15, carbon-11, nitrogen-13, and the pseudo-hydrogen fluorine-18, has led to the field of PET. These positron-emitting radionuclides are attached to biologically active molecules. When the tagged molecules are injected, the annihilation radiation can be imaged and the functional capacity of the patient can be determined, as discussed in the PET highlight, located between Chapters 2 and 3. Today PET scanners
are combined with computed tomography (CT) scanners so that in one setting, the structural (CT) and functional (PET) capacity of the patient can be determined. CT and PET scanners have revolutionized nuclear medicine.
Intense X-rays are now one of the primary modes of treating cancer. Accelerators throughout the world generate beams of electrons that are directed to targets that create X-rays, which are then directed at the tumors to destroy them. The modern therapy machine has become extremely sophisticated in that the electron beam can be modulated to increase and decrease the flux to alter the dose of X-rays and thereby spare healthy tissue while maximizing the dose to the tumor. While the standard of care for cancer treatment includes X-ray therapy, there is a growing use of high-energy protons to ablate the tumors. The idea is to deposit as much energy as possible in the tumor cells while sparing the surrounding tissues.
In the United States, partnerships between industry and nuclear science laboratories have led to new accelerator developments for medical applications. For example, the National Superconducting Cyclotron Laboratory (NSCL) at Michigan State University has pioneered the application of superconducting accelerator technology in medicine. This work has resulted in the miniaturization of the cyclotron so that it will fit on a gantry and rotate around the subject, simplifying beam delivery and allowing for tighter control of radiation dose delivery. NSCL has also designed and constructed a gantry-mounted, superconducting K100 medical cyclotron, funded by Harper Hospital in Detroit, for neutron therapy. The NSCL’s conceptual design for a superconducting cyclotron for proton therapy has been adopted and further refined by Varian Medical Systems/ACCEL Corporation, with technical advice from NSCL faculty and staff.
The success of proton therapy has stimulated interest in using heavier hadrons, such as carbon ions, with the potential of depositing more energy to a small area. Several synchrotrons delivering carbon-12 for therapy have been installed in Europe and Japan. At Brookhaven National Laboratory (BNL), home of the Relativistic Heavy Ion Collider (RHIC), next-generation accelerators for precise, safe cancer radiotherapies are being developed.
Accelerators in Industry and for Energy
There is a vast enterprise of techniques that use accelerators in a wide range of industries to polymerize plastics, to sterilize food and medical equipment, to weld materials using an electron beam, to implant ions into materials, to etch circuits on electronic devices, to examine the boreholes of oil wells, and to search for dangerous goods. There are approximately 8,500 such devices worldwide.
Electron beams dominate the industrial uses, with the curing of wire-cable tubing and of ink accounting for more than 60 percent of the market. Other electron beam uses include shrinking films, cross bonding of fibers in tires, and irradiation
of food. Here, electron beams replace traditional thermal heating approaches because of the gain in efficiency that comes from the more uniform distribution of energy.
A number of major accelerator developments related to nuclear energy are being pursued, including plasma heating for fusion reactors, inertial fusion reactors, nuclear waste transmutation, electronuclear breeding, and accelerator-driven subcritical reactors.
Basic and Applied Science
The breadth of scientific disciplines that make use of accelerators to perform their studies is considerable. Cutting-edge materials research makes use of synchrotron radiation having a wide range of wavelengths. Muon beams and neutrons produced from spallation sources probe the properties of materials such as the high-temperature superconductors. Mass spectroscopy is a standard analytical technique for chemists. As discussed at the end of this chapter, high-resolution mass spectrometry is used in archaeology and geology for dating artifacts by determining the ratio of stable to long-lived isotopes.
A free-electron laser (FEL) is a powerful source of coherent electromagnetic radiation that is produced by a relativistic electron beam propagating through a periodic magnetic field (see Figure 3.9). FELs are capable of producing intense radiation over a wide range of the electromagnetic wave spectrum, from microwave to hard X-ray, with average beam powers up to tens of kilowatts and peak powers up to tens of gigawatts. FELs are used for research in many fields, including materials science, surface and solid-state physics, chemical, biological and medical sciences, and nuclear physics. While the principle of operation of all FELs is the same, each device is optimized for its main application. FELs that are used in applications that require high average power are typically operated in the infrared (IR) region and are driven by a high-repetition-rate linear accelerator with an optical resonator. Nuclear physics accelerator facilities are leading new developments in FEL technologies.
New investigations in condensed matter studies at accelerator labs in the United States and Germany have already identified previously unknown interstellar molecular emission lines, developed new processes for production of boron nitride nanotubes, and produced nonthermal pulsed laser deposition of complex organics on arbitrary substrates. Superconducting radiofrequency technology developed at the Continuous Electron Beam Accelerator Facility (CEBAF) nuclear physics accelerator is now being commercialized for future implementation in weapons
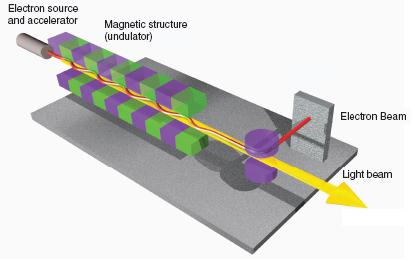
FIGURE 3.9 FELs are a powerful source of coherent electromagnetic radiation that is produced by a relativistic electron beam propagating through a magnetic field. They are used in numerous basic and applied science applications, including probing materials, biological systems, and nuclei. Shown is a schematic diagram of the basic layout of an FEL. The electron beam is transported through the periodically varying magnet field of an undulator magnet. Microbunching inside the electron beam at a spacing equal to that of the light’s wavelength enables electrons to radiate coherently in order to establish lasing. An FEL can be operated with either an optical resonator or in a single-pass configuration with a long undulator section. SOURCE: Image courtesy of Deutsches Elektronen-Synchrotron (DESY) in Hamburg, Germany. Copyright: DESY 2006.
systems for the U.S. Navy. And FEL technologies and applications are strongly coupled to nuclear physics research, including the technologies needed for a future electron-ion collider.
Information and Computer Technologies
Both nuclear physics experiments and theory have been enabled by and, in turn, have spawned, advances in computer science and technology. For experimentalists, the enormous quantity of data that characterize modern nuclear physics experiments has required that systems be devised to handle and make such data meaningful. RHIC experiments now routinely collect petabyte scale data sets each year, at rates of 1 GB per second. Analysis of such data sets drives technology development for the sustained use of data grids. For example, the computing groups
for the STAR collaboration at RHIC have developed a data movement service to achieve sustained and robust automated data transfers of 5 TB a week, with peak data transfer rates reaching 30 MB per second. This allows next-day access to fresh data from the experiments for analysis.
Analogous progress has come out of the need for massive and reliable computational approaches to address some of the fundamental problems in nuclear theory. Lattice quantum chromodynamics (QCD) calculations of the structure and properties of protons and hot quark-gluon plasmas that begin with fundamental quark and gluon building blocks are among the most demanding numerical computations in nuclear physics. Advancing this basic science drives innovation in computer architectures. In a lattice QCD calculation, space and time are rendered as a grid of points, and the quarks and gluons at one point interact only directly with those at other nearby points. This localization of the particles and their interactions makes these numerical computations particularly well suited for massively parallel supercomputers, with communications between processors having a simple pattern that enables the efficient use of a very large number of processors.
This characteristic of lattice QCD calculations drove some physicists to design special-purpose supercomputers that attracted attention in the broader computer hardware arena by achieving lower price-to-performance ratios than contemporary commercial supercomputers. A particularly successful group designing special-purpose lattice QCD supercomputers was based at Columbia University, working in partnership with IBM, which manufactured the computer chips. Originally, the group built a machine based on a low-power, simple, digital signal-processing chip (similar to those in cell phones) and a special-purpose serial communication network. This partnership laid the foundation for a new machine called the QCDOC (QCD on a chip), displayed in Figure 3.10, in which the whole processing unit, including a newer more powerful microprocessor, the communication network, and memory, was integrated on one chip.
Recently, the LHC, which enables particle and heavy-ion nuclear physics research at the energy frontier, has reached unprecedented volumes of data and requirements for data transfer rates and data processing power. This has led to the development of technology that allows extraordinary data transfer rates at large distances. At Super Computing 2011, the International Conference for High Performance Computing, Networking, Storage and Analysis, held in Seattle, Washington, in November 2011, a new world record of bidirectional data transfer rate was achieved: 23 GB per second between the University of Victoria Computing Centre located in Victoria, British Columbia, and the Washington State Convention Center in Seattle. Such technology eventually will influence the Internet infrastructure used in our everyday life.
Lattice QCD machines, QCDOC in particular, became the paradigm for a new generation of world-leading massively parallel supercomputers that are currently
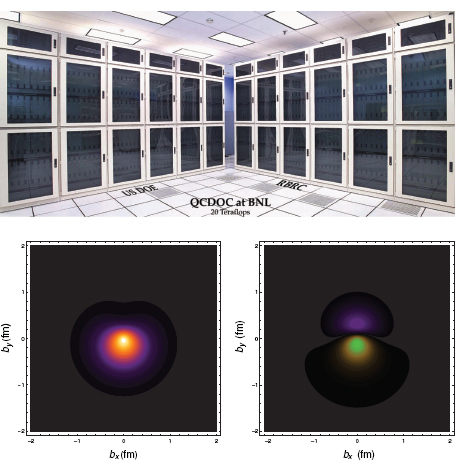
FIGURE 3.10 Nuclear science computing needs have led the community to develop new and innovative communication networks, data transport and manipulation systems, and computer architectures. An example is the QCDOC supercomputer at BNL (shown in the upper figure), a joint venture between RIKEN in Japan and the U.S. Department of Energy, in partnership with IBM. Examples of calculations now possible with the most powerful computers are given in the lower figures. Displayed are lattice QCD calculations of the transverse charge distributions of a proton (lower left) and a neutron (lower right), polarized in the x-direction, as a function of the radial distance from the center of the nucleon computed. These transverse charge densities are shown in a reference frame in which the observer is riding along with the photon (the Breit frame). In both cases, the charge distribution has an electric dipole component in the y-direction. This effect is entirely due to the interplay of special relativity and the internal structure of the nucleon. SOURCE: (top) Courtesy of Brookhaven National Laboratory; (bottom) Courtesy of Huey-Wen Lin and Saul D. Cohen, University of Washington.
being used in a vast array of applications having impacts in science and on the broader economy. In particular, IBM built the successful commercial Blue Gene line of computers, which engaged several former Columbia students and postdoctoral scholars. In addition to lattice QCD calculations, these supercomputers have been just as successful in simulating exploding stars or nuclear reactors, both of which require enormous computing power. Climate science researchers at BNL are using a Blue Gene named New York Blue to make significant progress in understanding today’s climate and to better predict climate evolution. Genomic sequencing, protein folding, materials science, and brain simulations are also prominent on the list of successful Blue Gene applications. Special-purpose supercomputers for lattice QCD have also been designed in Europe (the Array Processor Experiment) and Japan (the CPPACS and PACS-CS projects in Tsukuba).
Cosmic Rays, Electronic Devices, and Nuclear Accelerators
Cosmic rays are continuously bombarding Earth: more during active solar periods, more at the poles, and less at the equator. When cosmic rays, or radiation from their secondary products, interact with an electronic device, the function of that device can be compromised. The resulting errors in the functionality of an electronic device, such as the one displayed in Figure 3.11, can have very serious consequences for technologies used by such disparate industries as aerospace and autos.
A single event upset (SEU) refers to a change in the state of the logic or support circuitry of an electronic device caused by radiation striking a sensitive location or node in the device. SEUs can range from temporary nondestructive soft errors to hard error damage in devices. The detailed physics determining the rate at which SEUs occur is both complicated and device dependent. Circuit manufacturers try to design around the risks posed by cosmic ray interactions by introducing redundancy or other protective measures to compensate for the radiation-induced errors. To do so requires detailed knowledge of the expected rates and types of SEUs that can occur. Thus, experimental testing of semiconductor device response to radiation requires beams of particles that provide realistic analogs of cosmic rays and their secondary products. The main particles responsible for SEUs are neutrons, protons, and alpha particles, as well as heavy ions. Thus, the beams needed for this large experimental program require a range of nuclear accelerator facilities to test for device vulnerabilities and to characterize the radiation-induced failure modes of the electronic chips. For this, nuclear physics accelerator facilities are a unique resource, and agencies and companies from all over the world purchase beam time at accelerator facilities to test for device vulnerabilities and to characterize the radiation-induced failure modes of the electronic chips. In the United States
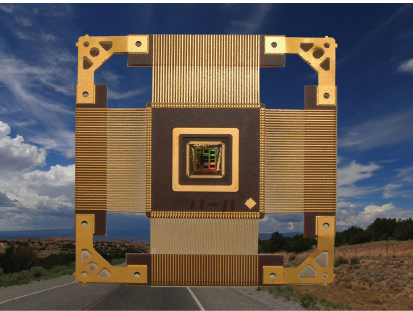
FIGURE 3.11 Nuclear physics laboratories across the world are working in collaboration with the aerospace and semiconductor industries to assess the impact of cosmic rays on electronic devices such as computer chips. Ongoing research programs are involved in testing the effects of heavy ions and neutrons on microelectronic devices, such as this one being studied at Texas A&M University. SOURCE: Zig Mantell and Texas A&M University.
alone, each year national and university nuclear physics laboratories provide almost 10,000 hours of accelerator time for this important service.
Helping to Understand Climate Effects One Nucleus at a Time
Applications of nuclear techniques are used to advance other scientific disciplines, including climate science, cosmochemistry, geochronology, paleoclimate, paleo-oceanography, and geomorphology. Since 1949, when Willard Libby first demonstrated carbon dating, the field of trace analyses of long-lived cosmogenic isotopes has steadily grown. Because they are chemically inert, noble gases play a particularly important role as tracers in environmental studies. Owing to their inertness, the geochemical and geophysical behavior of these gases and their distribution on Earth is simpler to understand than that of reactive elements. In addition,
their inertness facilitates recovery of minute quantities from very large volumes of other material. Precision tools and techniques developed for basic nuclear physics continue to be applied to answer open questions in climatology, geology, and oceanography.
Probing Ancient Aquifers in Egypt
A challenging problem in earth science is the determination of the residence times and flow velocities of groundwater circulating deeply through Earth’s crust. Krypton-81, which is produced by cosmic-ray-induced spallation in the atmosphere, has been identified as an ideal chronometer for determining fluid residence times on the 105-106 year timescale. However, since krypton-81 is such a rare isotope it has been extremely difficult to measure its abundance.
A new method, atom trap trace analysis (ATTA), was developed at Argonne National Laboratory to analyze krypton-81 in environmental samples. With a half-life of 230,000 years and an atmospheric isotopic abundance of one part per trillion, krypton-81 can provide unique information on terrestrial issues involving million-year timescales. Individual krypton-81 atoms can be selectively captured and detected with a laser-based atom trap. Joining low-level counting and accelerator mass spectrometry (AMS), two methods previously developed by nuclear physicists, ATTA is the newest method to detect tracers with an isotopic abundance at parts per trillion.
Using ATTA, krypton-81 atoms in environmental samples can now be counted and the isotopic abundance of krypton-81 measured. In the first application of ATTA to a groundwater study, a team of geologists and physicists from the United States, Switzerland, and Egypt sampled krypton from the Nubian Aquifer ground-water (displayed in Figure 3.12), which is of unknown age. Following extraction of krypton from thousands of liters of water at six deep wells, the krypton-81/Kr ratios measured by ATTA indicated groundwater ages ranging from 200,000 to 1,000,000 years. These results characterized the age and hydrologic behavior of this huge aquifer, with important implications for climate history and water resource management in the region. The success of this project suggests that widespread application of krypton-81 in earth sciences is now feasible.3
Tracing Ocean Circulation
It is becoming more apparent that the oceans are a major regulator for our world’s climate. One of these “motors” is the Atlantic conveyor belt system, whereby
________________________________________________
3 Portions of the discussion in this section are adapted from the Argonne National Laboratory, 2003, Physics Division Annual Report, Chapter IV.
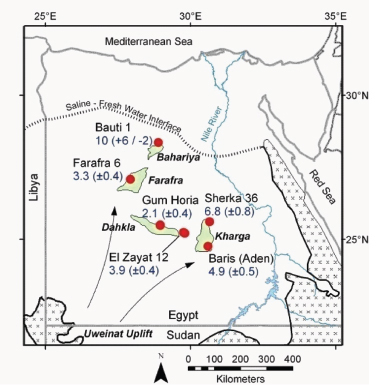
FIGURE 3.12 Understanding the flow of groundwater that circulates through Earth’s crust is an open question in geology. In a collaboration of nuclear scientists and geoscientists, the precision technique of atom-trap analysis was used to measure the radioactive isotope krypton-81 in deep wells of the Nubian Aquifer in Egypt. The map shows sample locations and their krypton-81 ages (in 100,000 years) in relation to oasis areas (shaded green). Groundwater flow in the Nubian Aquifer is toward the northeast. SOURCE: Adapted from N.C. Sturchio et al., 2004, One million year old groundwater in the Sahara revealed by krypton-81 and chlorine-36, Geophysical Research Letters 31. Copyright 2004 American Geophysical Union. Reproduced/modified by permission of American Geophysical Union.
warm water is chilled in the far North Atlantic, sinks to greater depths, and flows down the Atlantic across the Indian Ocean into the Pacific, where it heats up, rises to the surface, and flows back to the North Atlantic, as displayed in Figure 3.13. The whole cycle takes about 1,000 years. It is becoming increasingly clear that the amount of heat transported from the tropics to the polar regions by the oceans
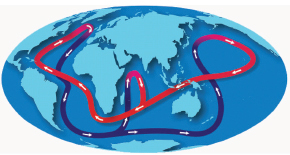
FIGURE 3.13 Thermohaline circulation, commonly referred to as the ocean “conveyor belt,” is made up of ocean currents that transport heat from the tropics to the polar regions. AMS of the radioactive isotope argon-39 will be used to explore this conveyor belt and its impact on climate. SOURCE: National Oceanic and Atmospheric Administration.
is comparable to the amount transported by the atmosphere. Therefore, it is very important to understand this system. With a half-life of 269 years, argon-39 is particularly well suited to study questions related to ocean circulation. However, its extremely low concentration (argon-39/Ar = 8.1 × 10−16), coupled to its long half-life, makes it impossible to measure the argon-39 decay in any sample of reasonable size.4
AMS using the ATLAS heavy ion accelerator at Argonne National Laboratory has been successful in separating argon-39 from its ubiquitous potassium-39 iso-baric background, the latter being 6-7 orders of magnitude more intense. Measurement of isotopic ratios as small as argon-39/Ar = 4 × 10−17 have been achieved. This program is now poised to measure argon-39 concentrations in ocean water samples in order to explore the oceanic “conveyor belt.”
________________________________________________
4 Portions of this paragraph have been adapted from M. Gaelens, M. Loiselet, G. Ryckewaert, et al., 2004, Oceans circulation and electron cyclotron resonance sources: Measurement of the AR-39 isotopic ratio in seawater, Review of Scientific Instruments 75: 1916.





























