The same also holds true for the genetic system that acts to make neuromeres and body segments different from one another. In this case, the conserved function of the Hox genes appears to control regionalization of both the external body segments (including appendages) and the nervous system in all arthropods studied so far.
SUMMARY
As we have described, the mechanisms of segmentation and body regionalization in arthropods function in a manner that allows developmental coordination between ectodermal structures, such as appendages, and the underlying nervous system. We suggest that subdivision of both the body into segments and the nervous system into neuromeres also provides evolutionary flexibility through modular design—any change in one will be mirrored by changes in the other. If segment number is varied by increasing the number of stripes of segment polarity gene expression, the number of neuromeres will also change so that there is still a one-to-one relationship between neural and ectodermal segments. Likewise, a home-otic shift that alters appendage morphology can simultaneously result in a shift in the pattern of neural regionalization. The next step will be to test these ideas in the context of arthropod evolution. Are homeotic-type shifts in appendage specialization during arthropod evolution accompanied by matching shifts in the nervous system that allow coordinated evolution of both appendage morphology and the neural mechanisms that control the locomotion of these appendages? For example, when a crustacean locomotory appendage is transformed to a feeding appendage, is the underlying neural pattern changed as well to ensure that the transformation is functional, not just morphological? Answering these questions will advance our understanding of how macroevolutionary changes in body plans might occur and ultimately help explain how complex nervous systems and behaviors evolve within animals.
ACKNOWLEDGMENTS
We thank Greg Davis for providing the images shown in Fig. 4.5 and the other members of the N.H.P. laboratory for helpful comments and discussion.
![]()
LUKE D. MCGOWAN,* ROULA A. ALAAMA, AMANDA C. FREISE, JOHNNY C. HUANG, CHRISTINE J. CHARVET, AND GEORG F. STRIEDTER
Comparative research has shown that evolutionary increases in brain region volumes often involve delays in neurogenesis. However, little is known about the influence of such changes on subsequent development. To get at this question, we injected FGF2—which delays cell cycle exit in mammalian neocortex—into the cerebral ventricles of chicks at embryonic day (ED) 4. This manipulation alters the development of the optic tectum dramatically. By ED7, the tectum of FGF2-treated birds is abnormally thin and has a reduced postmitotic layer, consistent with a delay in neurogenesis. FGF2 treatment also increases tectal volume and ventricular surface area, disturbs tectal lamination, and creates small discontinuities in the pia mater overlying the tectum. On ED12, the tectum is still larger in FGF2-treated embryos than in controls. However, lateral portions of the FGF2-treated tectum now exhibit volcano-like laminar disturbances that coincide with holes in the pia, and the caudomedial tectum exhibits prominent folds. To explain these observations, we propose that the tangential expansion of the ventricular surface in FGF2-treated tecta outpaces the expansion of the pial surface, creating abnormal mechanical stresses. Two alternative means of alleviating these stresses are tectal foliation and the formation of pial holes. The latter probably alter signaling gradients required for normal cell migration
_____________
Department of Neurobiology and Behavior, University of California, Irvine, CA 92697. *To whom correspondence should be addressed. E-mail: lukemcgowan1@yahoo.com.
and may generate abnormal patterns of cerebrospinal fluid flow; both abnormalities would generate disturbances in tectal lamination. Overall, our findings suggest that evolutionary expansion of sheet-like, laminated brain regions requires a concomitant expansion of the pia mater.
Evolutionary increases in brain region volumes are common (Striedter, 2005). For example, the neocortex is disproportionately enlarged in primates relative to other mammals, and the telencephalon is disproportionately enlarged in parrots and songbirds relative to other birds (Stephan et al., 1981; Boire and Baron, 1994; Iwaniuk and Hurd, 2005; Striedter, 2005). Recent work in evolutionary developmental neurobiology has shown that these evolutionary increases in brain region volumes are often caused by delays in cell cycle exit of neuronal precursors (Finlay et al., 2001; Charvet et al., 2011). Among birds, for example, parrots and songbirds exhibit delayed telencephalic neurogenesis relative to chicken-like birds (Charvet and Striedter, 2008, 2009; Striedter and Charvet, 2008). Among mammals, cell cycle exit in the neocortex is similarly delayed in primates, which have a disproportionately enlarged neocortex (Clancy et al., 2000, 2001, 2007; Finlay et al., 2001).
Unfortunately, the downstream effects of delayed cell cycle exit on subsequent developmental processes and adult morphology remain poorly understood. One way to fill this gap in our knowledge is to experimentally recreate the key species differences in the laboratory by means of carefully selected developmental manipulations. A good example of this phenocopy approach was the creation of transgenic mice with a constitutively active form of β-catenin that prolongs proliferation, increases neocortical volume, and generates cortical folds (Chenn and Walsh, 2002). In another example, it has been shown that intraventricular injections of FGF2 in rats delay neocortical cell cycle exit, leading to dramatic increases in neocortex volume and neuron number (Vaccarino et al., 1999). Based in part on these experiments, it is becoming increasingly common to explain human cortical evolution and expansion in terms of delayed and prolonged precursor proliferation (Rakic, 1995a; Kriegstein et al., 2006).
The present study began as an attempt to phenocopy natural variation in telencephalon size among birds. Specifically, we reasoned that FGF2 injections into ventricles of embryonic chicks should, by analogy to the work in mammals (Vaccarino et al., 1999), increase telencephalon volume, effectively creating chickens with a telencephalon as large as that of parrots and songbirds (Striedter and Charvet, 2008; Charvet and Striedter, 2009). However, our FGF2 injections did not significantly alter telencephalon development. Instead, they increased the size of the optic tectum, disrupted tectal lamination, and created tectal gyri and sulci.
It is tempting to dismiss these induced alterations as mere pathologies of no evolutionary significance. However, developmental “monsters” have long been used by evolutionary developmental biologists to identify the kind of variation and generative principles with which natural selection must work (Alberch, 1989). Most of this work has used naturally occurring teratologies, but carefully selected developmental manipulations can likewise be useful. Conrad Waddington, for example, argued that experimental perturbations of development can be used to infer the shape of the epigenetic landscapes that constrain developmental and evolutionary variation (Waddington, 1957; Striedter, 1998), even if the results are deleterious.
In line with Waddington’s approach, here we describe several changes in tectal development that are induced by FGF2 and provide a model to explain them. Our analysis suggests that experimental expansion of the optic tectum is disruptive mainly because it is not accompanied by an equivalent expansion of the pia mater. This mismatch causes tectal foliation and tears holes in pia, which then disrupt laminar development. The presumably maladaptive nature of these alterations probably explains why naturally occurring species differences in optic tectum size are based, as far as we know, on changes in brain patterning, rather than neurogenesis timing (McGowan et al., 2011). Our findings also suggest that the evolutionary expansion of mammalian neocortex must have required a concordant expansion of the neocortical pia.
RESULTS
We first report on changes in tectal volume, ventricular surface area, and proliferative-zone fraction at embryonic day (ED) 7, 3 days after the FGF2 injections (on ED4). We then describe how these alterations manifest on ED12. Finally, we describe qualitative changes in tectal shape and cytoarchitecture that result from FGF2 injection.
FGF2 Expands the Tectal Progenitor Pool
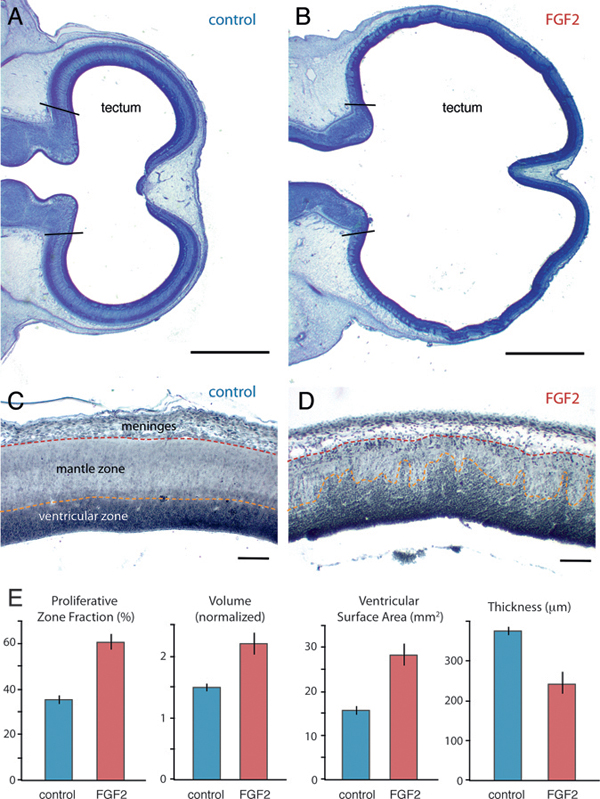
On ED7, FGF2-treated embryos exhibit an expanded tectum compared with controls (Fig. 5.1). Because of substantial variability in absolute volumes at this age, we express tectum volume relative to the rest of the brain (minus telencephalon and medulla), as estimated stereologically (Materials and Methods). By using these methods, we observed a 32% increase in normalized tectum volume [t(11) = 3.9; P < 0.01; n = 13] for the FGF2-treated chicks relative to controls. In contrast, telencephalon volume does not differ significantly between FGF2-treated and control embryos, relative to diencephalon-tegmentum volume [t(11) = 1.3; P = 0.23; n = 13]. FGF2
FIGURE 5.1 On ED7, the optic tectum is expanded in FGF2-treated embryos relative to controls, as illustrated here with Giemsa-stained horizontal sections (A and B; anterior is to the left). Staining with antibodies against PCNA (C and D) reveals that FGF2 treatment increases the proportion of proliferating cells in the tectum (i.e., PZF; E). Normalized tectum volume and tectal ventricular surface area are also larger in FGF2-treated birds than in controls, but tectal radial thickness is reduced. SE bars are shown. (Scale bars: A and B, 1 mm; C and D, 100 μm.) [Note: Figure can be viewed in color in the PDF version of this volume on the National Academies Press website, www.nap.edu.]
injections also expand the tectum’s ventricular surface area by 79% [t(11) = 4.9; P < 0.01; n = 13] and reduce tectal thickness by 35% [t(11) = -4.5; P < 0.01; n = 13].
Postproliferative cells in the developing brain’s mantle zone are less densely packed than their proliferating precursors in the ventricular zone (Fig. 5.1C). Therefore, the FGF2-induced tectal thinning is consistent with FGF2 delaying tectal cell cycle exit. To test this hypothesis, we computed the fraction of all tectal cells that is proliferative, rather than postproliferative. We have previously used this proliferative zone fraction (PZF) measure to demonstrate species differences in neurogenesis timing (Striedter and Charvet, 2008; Charvet and Striedter, 2009). Here we extend the approach by staining ED7 brains with proliferating cell nuclear antigen (PCNA), a relatively specific marker for proliferating cells (Valero et al., 2005) (Fig. 5.1C and D). As very few PCNA-negative cells were observed within the PCNA-positive zone (and vice versa), we calculated the tectum’s PZF as the volume of the PCNA-positive zone divided by total tectum volume. Our analysis revealed that the tectum’s PZF is 67% larger in FGF2-treated embryos than in controls [t(9) = 7.2; P < 0.01; n = 11; Fig. 5.1E).
FGF2-Induced Alterations Persist to ED12
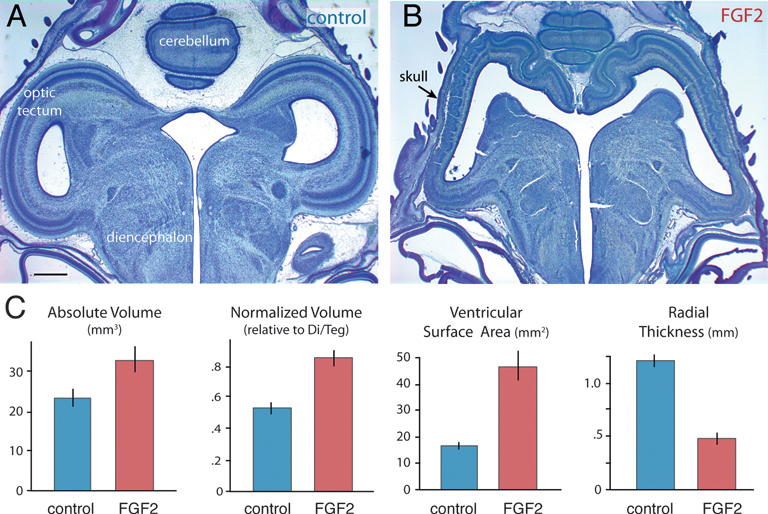
On ED12, FGF2-treated embryos still exhibit abnormally large and thin optic tecta (Fig. 5.2). At this age, absolute tectum volume is 40% larger in FGF2-treated chicks than in controls [t(14) = 2.4; P < 0.05; n = 16]. Tectum volume relative to rest-of-brain volume (minus telencephalon) is increased by 57% [t(14) = 5.9; P < 0.01; n = 16], and tectum volume relative to the entire brain is boosted by 33% [t(14) = 6.6; P < 0.01; n = 16]. Again, telencephalon volume is not significantly different between FGF-treated embryos and controls, regardless of how this volume is measured (absolute, P = 0.94, n = 16; normalized, P = 0.15, n = 16; volume fraction, P = 0.53, n = 16). The tectum’s ventricular surface area is increased by 181% in FGF2-treated chicks [t(14) = 5.3; P < 0.01; n = 16], but tectal thickness is reduced by 60% [t(14) = -8.61; P < 0.01; n = 16].
Qualitative Changes in Lamination, Folding, and Pial Integrity
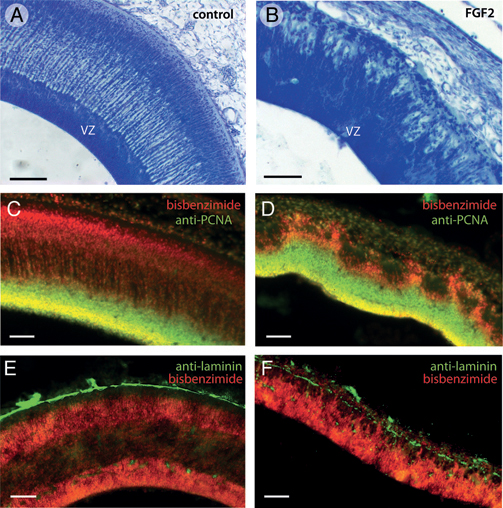
The tectum of FGF2-treated embryos exhibits not only quantitative changes in volume and thickness, but also altered morphology. As mentioned earlier, FGF2-treated embryos on ED7 have an abnormally thin mantle zone in the optic tectum. This effect is most extreme in the lateral tectum, where the tectal surface approaches the developing skull. In this region, the ventricular zone of FGF2-treated embryos is not smooth, as in control embryos, but contains irregular radial protrusions that resemble
FIGURE 5.2 FGF2-treated embryos on ED12 have a significantly enlarged and abnormal tectum (A and B). Absolute and normalized tectum volumes, as well as the tectum’s ventricular surface, are increased significantly in FGF2-treated chickens relative to controls (C). Tectal thickness of FGF2-treated chickens is reduced relative to controls. SE bars are shown. (Scale bar: 1 mm.) [Note: Figure can be viewed in color in the PDF version of this volume on the National Academies Press website, www.nap.edu.]
mountains (Fig. 5.3B). These mountainous protrusions consist mainly of dividing precursor cells of the VZ (Fig. 5.1C). However, double labeling with anti-PCNA and bisbenzimide, a fluorescent counterstain, reveals that the tops of the cellular mountains in the FGF2-treated tecta consist primarily of postproliferative cells (Fig. 5.3C and D).
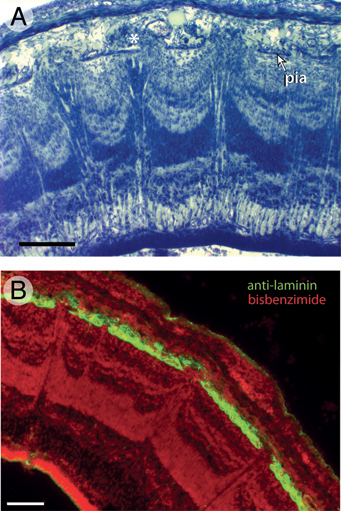
Nissl staining further revealed that the pia mater in the mountainous tectal region of FGF2-treated embryos is abnormally thin. To examine this more closely, we used an antibody against laminin, an ECM protein secreted by pial cells (Halfter et al., 2002; Siegenthaler et al., 2009). Control embryos on ED7 show a continuous layer of laminin at the tectum’s outer surface (Fig. 5.3E). This layer of laminin is disorganized in the mountainous regions of the FGF2-treated tecta and exhibits numerous discontinuities (Fig. 5.3F).
FIGURE 5.3 In lateral portions of FGF2-treated tecta on ED7, the superficial surface of the ventricular zone (VZ) is not smooth, as it is in control embryos (A), but irregular or “mountainous” (B). Double-staining with anti-PCNA to label proliferating cells and bisbenzimide to label all cell nuclei reveals that the tops of the mountains in the FGF2-treated tecta contain mainly postproliferative cells (C and D). Staining with antibodies against laminin, which delineates the pia mater, reveals that FGF2 treatment disrupts the pia overlying the mountainous tectal regions (E and F). (Scale bars: 100 μm.) [Note: Figure can be viewed in color in the PDF version of this volume on the National Academies Press website, www.nap.edu.]
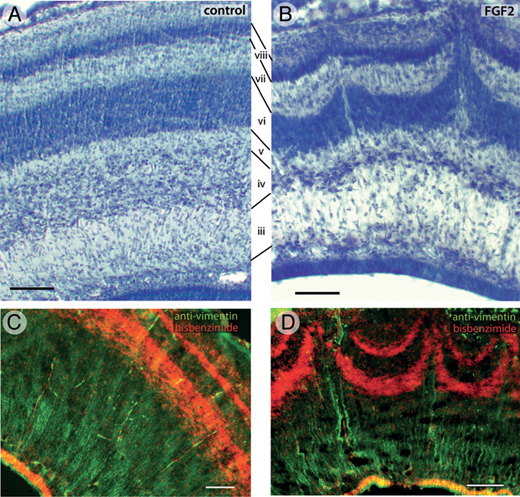
FGF2-induced abnormalities in tectal lamination are even more pronounced by ED12 (Fig. 5.4). At this age, the tectum of controls contains numerous laminae that are clearly delineated, smooth, and of constant thickness (Fig. 5.4A). Corresponding laminae can be identified in FGF2-treated animals, but some of the layers are thinned, especially in the lateral tectum. Furthermore, in these lateral tectal regions, layers vi and viii exhibit protrusions where neurons appear to have migrated too far in the radial dimension (Fig. 5.4B). The center of each such protrusion
FIGURE 5.4 On ED12, the tectum of control embryos contains numerous laminae (A). The same laminae, numbered according to the nomenclature of LaVail and Cowan (1971a), are present in FGF2-treated animals, but some of them are thinner than normal (B). In addition, the lateral tectum of FGF2-treated embryos exhibits radial protrusions that resemble volcanoes (B). Running up through the center of each volcano are cell-sparse zones containing numerous vimentin-positive radial glia fibers (D). The radial glia are more homogenously distributed in control embryos (C). (Scale bars: 100 μm.) [Note: Figure can be viewed in color in the PDF version of this volume on the National Academies Press website, www.nap.edu.]
contains a radially oriented cell-sparse zone. Analysis of serial transverse and sagittal sections reveals these protrusions to be elongate at their base but relatively short and punctate at their tips (McGowan et al., 2012, Fig. S1). Therefore, we refer to these protrusions as “volcanoes.” Staining with antibodies against vimentin, an intermediate filament found mainly in radial glial cells, shows that the central channel of each volcano contains numerous radial glia processes (Fig. 5.4D). In control birds, these radial processes are more homogeneously distributed (Fig. 5.4C).
FIGURE 5.5 Individual volcanoes in FGF2-treated tecta are aligned with holes in the pia mater on ED10. This is evident in Giemsa-stained sections (A), but even more obvious in sections stained with antibodies against laminin (B). The image in A also depicts several cell clusters in the space between the pia and the overlying dura mater (asterisk). These ectopias typically extend through the pial holes from the top of individual volcanoes. (Scale bars: 100 μm.) [Note: Figure can be viewed in color in the PDF version of this volume on the National Academies Press website, www.nap.edu.]
The volcano-like laminar disturbances at ED12 tend to be located in the lateral and dorsal tectum, where the tectal surface approaches the developing skull (McGowan et al., 2012, Movie S1). This location corresponds, at least roughly, to the position of mountainous laminar disturbances observed on ED7 (McGowan et al., 2012, Movie S2). In addition, the peaks of individual volcanoes at ED12 tend to be in register with discontinuities in, or regional thinning of, the overlying pia mater. This alignment between individual volcanoes and holes in the pia is even more obvious at ED10 (Fig. 5.5), suggesting that pial holes are partially repaired between ED10 and ED12. Ectopic cells are often found in the subdural space above each pial hole, especially on ED10 (Fig. 5.5A).
FIGURE 5.6 Folding of the caudomedial tectum in FGF2-treated embryos is seen most often at ED12 (A) but also in a few cases on ED7 (B). (Scale bars: 1 mm.) [Note: Figure can be viewed in color in the PDF version of this volume on the National Academies Press website, www.nap.edu.]
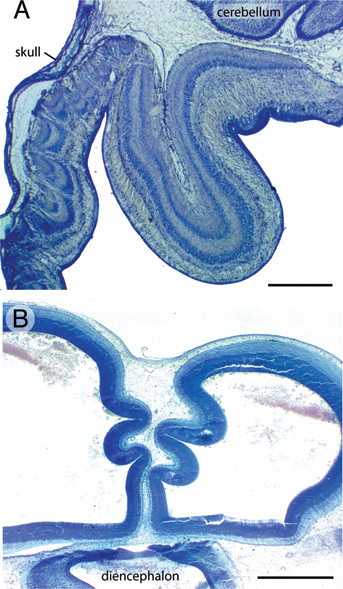
Caudal to the volcanoes, the tectum of FGF2-treated embryos frequently exhibits folds resembling cortical gyri and sulci. The extent of folding is variable across embryos, but the folds are usually located in the caudomedial tectum, where the tectal surface lies far from the skull and, by ED12, close to the cerebellum [Fig. 5.6A and McGowan et al. (2012, Movie S1)]. Folding is more often seen at ED12 than at ED7, but a few FGF2-treated embryos exhibit prominent tectal folds even at ED7 [Fig. 5.6B and McGowan et al. (2012, Movie S2)]. In the folded parts of the tectum, the laminae are consistently smooth and devoid of volcanoes.
FGF2 is a secreted growth factor that in vitro prolongs proliferation and delays differentiation for various neuronal and glial precursors (Deloulme et al., 1991; Vescovi et al., 1993; Ray and Gage, 1994; Bouvier and Mytilineou, 1995). It also delays neurogenesis in vivo. Specifically, intraventricular injections of FGF2 in embryonic rats and mice increase neocortical neuron numbers (Ohmiya et al., 2001; Chenn and Walsh, 2002), as one would expect if FGF2 prolongs precursor proliferation. Several studies suggest that proliferation rate and neuronal migration are relatively unaffected by FGF2 manipulations in vivo (Raballo et al., 2000; Ohmiya et al., 2001; Chenn and Walsh, 2002; Kang et al., 2009), although in vitro studies have reported more complex effects (Martín et al., 2006).
Given the mammalian data, it is surprising that our injections of FGF2 into chicken embryos altered tectal, rather than telencephalic, development. The most likely explanation for this species difference is that some of the receptors binding FGF2 are only weakly expressed in the chick telencephalon at the age when we inject exogenous FGF2 (Walshe and Mason, 2000; Nishita et al., 2011). We also note that the rodent studies focused exclusively on neocortical development, leaving some uncertainty about whether FGF2 affects mammalian midbrain development.
Our injections of FGF2 on ED4 appear to delay tectal neurogenesis. The principal evidence for this conclusion is that the tectum’s PZF, as determined from the PCNA-stained sections, is significantly higher in FGF2-treated embryos than in controls. Neuronal birth-dating studies to confirm the delay in neurogenesis are in progress. FGF2 treatment also affects neuronal migration in the lateral tectum, where the mountains and volcanoes are observed, but these effects are likely to be downstream consequences of the delay in tectal neurogenesis. Our principal evidence in favor of this hypothesis is that FGF2 injections on ED5 do not induce the migratory abnormalities seen after injections on ED4. However, at this point, we cannot exclude the possibility that FGF2 also affects other developmental parameters, such as cell cycle rate or developmental cell death.
An intriguing aspect of our findings is that FGF2 induces large folds in the caudomedial tectum, but laminar disruptions without folding in the lateral tectum. This differential effect is unlikely to be caused by a difference in FGF2 levels, as the embryonic tectum produces very little endogenous FGF2 (Martín et al., 2006) and the injected FGF2 appears to diffuse homogeneously through the cerebral ventricles. However, the differential FGF2 effect could be related to spatial differences in FGF2 receptor distribution (Walshe and Mason, 2000; Nishita et al., 2011) or to the normal rostroventral to caudodorsomedial gradient of neurogenesis observed within the avian tectum (LaVail and Cowan, 1971b). Alternatively, the
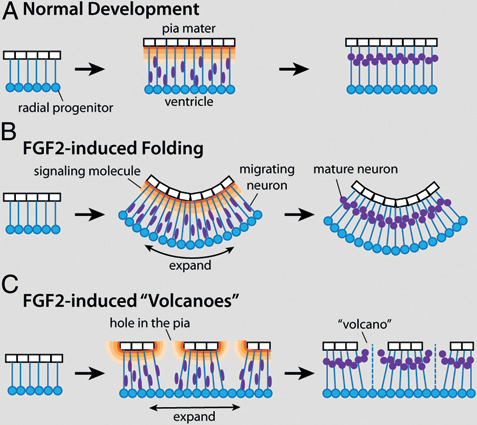
effects of FGF2 on tectal morphology may depend on interactions between the developing tectal surface and overlying nonneural tissues. The latter hypothesis is supported mainly by the observation that mountains and volcanoes are consistently observed in close apposition to the developing skull, whereas the macroscopic folds develop where the skull lies far from the tectum. Taking this observation into account, as well as the finding that FGF2 injections disrupt pial morphology in lateral tectum, we propose the following model to explain most of the observed FGF2 effects (Fig. 5.7).
Young embryonic brains consist mostly of radial glia progenitors that surround the ventricle and extend radial processes toward the pia mater (Kriegstein and Alvarez-Buylla, 2009). As these radial glia divide, the brain tissue expands tangentially (Rakic, 1995a; Kriegstein et al., 2006; Kriegstein and Alvarez-Buylla, 2009). At some point, radial glia begin to leave the cell cycle and become young neurons, which migrate away from the ventricular surface along the radial processes. The neurons are thought to stop their migration when they encounter a molecular signal, such as reelin or retinoic acid, secreted from specialized cells near the pial surface (Halfter et al., 2002; Siegenthaler et al., 2009) (Fig. 5.7A).
To explain our observations, we propose that intraventricular FGF2 injections cause the ventricular surface of the developing tectum to expand tangentially more quickly than the pial surface with its attendant laminin-positive basement membrane. Because the radial glia processes are attached to the pial surface (Halfter et al., 2002; Siegenthaler et al., 2009; Georges-Labouesse et al., 1998; Radakovits et al., 2009), the differential tangential expansion creates laterally directed tension between the ventricular and pial surfaces. One way to relieve this tension is to let the tectum buckle into the ventricle, creating macroscopic folds (Fig. 5.7B). Alternatively, the differential expansion of the pial and ventricular surfaces can be accommodated by stretching and thinning the pia, which can cause it to become perforated in some locations. We suggest that this second solution is adopted in the lateral tectum of FGF2-treated embryos, perhaps because adhesive interactions between the tectal surface and the overlying skull prevent the tectal infolding.
The formation of mountains and volcanoes in the lateral tectum is likely linked to the pial holes (or small tears), with which they are spatially aligned. One possibility is that the holes in the pia mater lead to gaps in signaling gradients that originate directly from pial cells or from cells associated with them (e.g., Cajal–Retzius cells). If the signal instructs young neurons when to stop their migration, neurons born beneath the gaps would migrate abnormally far, thereby forming the observed volcanoes as well as ectopias in the subdural space (Fig. 5.7C). In addition, or alternatively, cerebrospinal fluid may flow through the gaps in the pia and carry young neurons with it through bulk flow. This second hypothesis implies
FIGURE 5.7 A working model to explain the two major effects of FGF2 injections on tectal development. (A) During normal development both radial glia progenitors (blue) and pia mater cells (white) proliferate, causing coordinate tangential expansion of the tectum’s pial and ventricular surfaces. After exiting the cell cycle, young neurons (purple) migrate away from the ventricular surface until they encounter a molecular signal (orange) secreted by specialized cells near the pial surface. By delaying neurogenesis, exogenous FGF2 causes the ventricular surface of the developing tectum to expand tangentially more quickly than the pial surface can expand (B and C). One way to relieve the tension caused by this differential tangential expansion is to fold the tectum inward, into the ventricle (B). Alternatively, the pia can stretch and, in some places, break (C). The resultant holes in the pia create gaps in the signaling gradient, which in turn leads to aberrant migration and the formation of cellular volcanoes. [Note: Figure can be viewed in color in the PDF version of this volume on the National Academies Press website, www.nap.edu.]
that intraventricular pressure is relatively high and that the pia mater, rather than the ventricular surface (i.e., the ependymal layer), provides the major resistance to cerebrospinal fluid efflux. Evidence thus far exists only for the former assumption (Desmond et al., 2005). The vimentin-positive radial glia fibers running up the center of individual volcanoes (Fig. 5.4D) most likely represent glial processes that grew toward the pial surface after
the holes had formed, sometimes extending beyond the pial surface into the meningeal space (Halfter et al., 2002).
Our model ties together the two major types of changes seen in FGF2-treated tecta, namely tectal folding and volcano-like disturbances in tectal lamination. However, our model does not explain why the tectum remains abnormally thin on ED12, when one might expect tectal differentiation and migration to be largely complete (Crossland et al., 1975), even if tectal neurogenesis is briefly delayed. Perhaps exogenous FGF2 delays neuronal differentiation and migration more than we expect. To test this hypothesis, one would have to examine tectal thickness in older FGF2-treated embryos. Alternatively, FGF2-treated tecta may exhibit increased rates of developmental cell death. Finally, it is possible that tectal cell density is higher in the FGF2-treated embryos than in controls. These hypotheses have not yet been tested.
Another open question is whether evolution ever increased tectum size by delaying tectal neurogenesis. To our knowledge, there have been no published reports of foliated tecta in nature. Furthermore, we have previously shown that chicken-like birds expanded their midbrain tectum, relative to other birds, by shifting an early gene expression boundary, rather than by selectively delaying tectal neurogenesis (McGowan et al., 2011). This does not, of course, prove that evolution never increased tectum size by delaying neurogenesis. However, our present findings suggest that the avian tectum is vulnerable to morphological disruptions if tectal neurogenesis is delayed dramatically. To create viable increases in tectum size by delaying neurogenesis, those delays would have to be small or coupled with increased pial proliferation.
Finally, our study sheds light on the evolution of neocortical folding in mammals. The foliation in our FGF2-treated tecta resembles that observed in the neocortex of mice modified to exhibit increased neocortical progenitor proliferation (Chenn and Walsh, 2002; Kingsbury et al., 2003). However, in all these cases, the pial and ventricular surfaces are equally folded. In contrast, in naturally occurring cortical gyri the ventricular surface is much smoother (and smaller) than the pial surface (Welker, 1990; Kriegstein et al., 2006). Why does evolution prefer the latter mode of cortical foliation? One possible explanation is that involution of the ventricular surface would make it more difficult for axons to cross from one side of a gyrus to the other (Van Essen, 1997). It would also obstruct the path of long-range axons that normally pass down the center of individual cortical gyri (Prothero and Sundsten, 1984). Alternatively, the downside of the natural mode of cortical foliation is that it requires an enormous expansion of the pial surface because, without it, gyral growth would likely rupture the pia and disrupt lamination. Thus, the present study highlights that cortical foliation can be accomplished by various developmental means and that pial development is a critical variable.
Fertile chicken eggs (Gallus gallus domesticus) were obtained from a commercial supplier and incubated in a rotating egg incubator (PROFI-I; Lyon Technologies) at 38° and 50% to 60% humidity. On ED4, 0.5 to 1 μL of human recombinant bFGF (100 ng/μL, dissolved in 0.1 M PBS solution and dyed with methylene blue; R&D Systems) was injected into the lateral or tectal ventricles. The injected FGF2 rapidly diffused throughout the ventricles, regardless of injection site. Control chicks were injected with 0.5 to 1 μL of dyed 0.1 M PBS solution. After injection, the eggs were resealed and transferred to the incubator until ED7, ED10, or ED12. The embryos were then immersion-fixed overnight in methacarn (by volume 60% methanol, 30% chloroform, 10% glacial acetic acid), dehydrated, embedded in paraffin, and sectioned at 18 μm. Approximately 40 to 70 evenly spaced sections from each brain were mounted onto Superfrost Plus slides (Fisher Scientific).
For morphometric measurements, sections were stained with Giemsa stain (Sigma-Aldrich) and coverslipped. Brain regions were delineated and volumes estimated using the Cavalieri method, as described previously (Striedter and Charvet, 2008). As defined here, the telencephalon includes the evaginated hemispheres and midline telencephalic structures; the tectum corresponds to what others have called the optic or dorsal tectum (Delgado et al., 2005). Telencephalon and tectum volumes for the ED12 embryos were normalized by comparing them to the rest of the brain, including diencephalon, pretectum, tegmentum, torus semicircularis, and hindbrain (but excluding tectum or telencephalon, respectively). The hindbrain was excluded from the normalization factor for the ED7 embryos, because it had not been sectioned completely in all the embryos. Ventricular surface area was estimated by summing ventricular surface lengths from a series of regularly spaced sections and multiplying the sum by the section spacing. The tectum’s radial thickness was quantified by dividing tectum volume by the tectum’s ventricular surface area.
To examine whether FGF2 injections delay tectal neurogenesis, we measured the proliferative and postproliferative zones in FGF2-treated and control chickens at ED7. As development proceeds, cells exit the proliferative ventricular zone and form a postproliferative mantle zone. As the mantle zone expands, the ventricular zone wanes. Therefore, a region’s PZF is a good measure of how far neurogenesis has progressed; the higher the PZF, the more neurogenesis has been delayed (Striedter and Charvet, 2008). To estimate the tectum’s PZF, we stained sections with antibodies against PCNA. Measurements were made on four equally spaced sections through each tectum and then averaged. Mounted sections were incubated with anti-PCNA (clone PC10; mouse; 1:500; Zymed), followed by a secondary antibody (anti-mouse IgG; 1:200; Vector Labs). They were then