Methods and Effects of Fertility Management
This chapter reviews and assesses current options for controlling fertility of free-ranging horses and burros. Investigation of potential fertility-control options was one of the mandates of the previous National Research Council studies. In the late 1970s and early 1980s, the Committee on Wild and Free-Roaming Horses and Burros reviewed the status of contraception, including sterilization, for population control in free-ranging herds. That committee reported on the feasibility of several techniques, including hormone injections for stallions and hormone treatments, intrauterine devices (IUDs), and surgery for mares. It concluded that endocrine contraception in stallions or mares was the most promising approach because IUDs often dislodged and surgery was impractical in field conditions (NRC, 1980). The 1980 report noted that studies of endocrine contraception in stallions were going on at the time and recommended a study of contraception in mares. In 1991, the Committee on Wild Horse and Burro Research reviewed the proposal for and later the results of a study that examined steroid implants in mares captured from the range and held in pens, steroid implants in free-ranging mares, and vasectomies of free-ranging dominant stallions. That committee found some steroid treatments to be effective in mares. Vasectomies were effective in sterilizing individual animals, but the committee questioned the technique’s effectiveness at a population level, given that only dominant stallions were treated (NRC, 1991).
Research on effective methods of fertility control remains important to the Bureau of Land Management (BLM) because fertility control is the major alternative to gathering and removing horses that is generally accepted by the public. In the 20 years since the last National Research Council report was completed, considerable progress has been made in developing and testing fertility control for wild animal populations, both free-ranging and captive. Research with captive animals has been especially valuable in allowing more extensive and careful monitoring and analysis of efficacy and safety of a wide array of products. In particular, pathological conditions associated with some types of contraceptive treatment have been detected and are under systematic investigation, which is difficult to accomplish in free-ranging populations.
Although the committee’s report includes information on burros as well as horses, the need for fertility control in horses is considered more pressing because their populations are much larger (BLM, 2003, revised 2005). In addition, many more studies have focused on horses, so considerably more data are available on them than on burros. Nevertheless, given similarities in reproductive physiology, the efficacy and safety of methods could be expected to be generally similar in the two species. Their social structures differ, however, as described in the following sections, and this could influence the effects of fertility-control methods on behavior and social organization.
Reversible contraception and permanent sterilization are achieved by interrupting reproductive processes, and the committee’s evaluation of these methods is based in part on understanding their effects on an animal’s reproductive physiology and behavior. Accordingly, this chapter starts with two reviews: one on equine social and mating behavior, social relationships, and social structure and a second on reproductive physiology in domestic horses and donkeys, with information on free-ranging horses and burros when available. The brief reviews are intended to serve as background for understanding the potential effects of fertility-control methods on behavior and reproductive processes. The chapter then evaluates available fertility-control treatments for both females and males and summarizes the advantages and disadvantages of the most promising methods.
EQUINE SOCIAL BEHAVIOR AND SOCIAL STRUCTURE
Horses, zebras, and asses (the primogenitors of donkeys and burros) are highly social animals, but their social structures vary. Klingel (1975) was the first to document that equids exhibit two types of social organization. In one, typified by horses and plains and mountain zebras, females and their young live in closed membership groups with one, and occasionally a second, male. In those so-called harem groups, females benefit by receiving material rewards from their males (Rubenstein, 1986). Enhanced male vigilance against potential intruder males not only reduces a male’s chances of being cuckolded but reduces harassment experienced by females. Consequently, females can devote more time to feeding and increase the likelihood that their offspring will survive to independence (Rubenstein, 1986). That type of society emerges under more mesic environmental conditions in which food is relatively abundant and distributed near predictable watering points.
In more arid areas, where abundant food is far from water, the second type of society appears, as typified by Grevy’s zebras and the wild asses, including the African wild ass that is the ancestor of the donkey. Arid and semiarid conditions make it difficult for females, whether with or without young foals, to remain together in closed-membership groups, meet their different physiological needs, and benefit from the extra foraging time that heightened male vigilance provides. Nonlactating females and mares that have older foals need drink only every 3-5 days (Ginsberg, 1989; Becker and Ginsberg, 1990), whereas ones that have foals 3 months old and younger must drink daily. The latter females stay near water whereas the others wander more widely in search of better pasture. Because both types of females are fertile and males cannot be with both simultaneously, males establish territories. The most dominant hold areas near water, where they have exclusive access to females that have young foals and intercept those coming to water every few days. Aridity thus alters the nature of relationships among both females and males and leads to a more fluid, fission-fusion type of social system (Rubenstein, 1994).
Although the two social systems emerge from differences in individual social relationships and environmental conditions, they share some important characteristics. First, the mother-infant bond is strong in all equids. Second, sons and daughters leave their mothers
when they reach sexual maturity; males join bachelor groups, and females are immediately integrated into adult society. Third, the female reproductive state influences female nutritional needs; meeting these needs sometimes permits long-term stable bonds to form but sometimes does not. Much depends on long-term evolutionary responses to ecological circumstances that lead to the emergence of different social systems. In free-ranging horses, the norm is a stable society in which females can meet their needs while benefiting from limited interruptions. In free-ranging burros, fluidity of social relationships is the norm in that close bonds among females and between males and females are precluded by the disjunctive nature of high-quality feeding and drinking locations.
REPRODUCTION IN DOMESTIC HORSES AND DONKEYS
This section provides an overview of the various points in the reproductive processes of male and female horses and burros that can be targeted for fertility control (see Asa, 2010, and Asa and Porton, 2010, for further details).
Sexual maturity in free-ranging male and female horses occurs at the age of about 18 months, but onset of reproduction is dependent on social parameters within the population. First reproduction for males is typically delayed for up to several years while they reach social maturity. Sexual maturity in domestic donkeys and free-ranging burros is reported to occur at the age of 1-2 years in females (Fielding, 1988; Pugh, 2002) and 1.5 years in males (Nipken and Wrobel, 1997). The earliest possible age of puberty in males and females of both species is 1 year, so preventing reproduction in those animals would require that treatment begin before that age.
Both species have seasonal breeding patterns, but seasonality is less pronounced in domestic donkeys and free-ranging burros (Ginther et al., 1987). Seasonal reproduction is controlled primarily by photoperiod, but temperature and body condition can also influence reproductive timing (Sharp and Ginther, 1975; Guillaume et al., 2002). Thus, local conditions can affect the length of the breeding season, especially for female horses. Male domestic horses can produce sperm year round, but the quality declines during winter, the mares’ nonbreeding season (Pickett et al., 1975).
Most female free-ranging horses give birth in the spring, and this is followed within 5-12 days by postpartum estrus (foal heat), when conception is again possible. Female domestic donkeys also show postpartum estrus (Pugh, 2002). Nonpregnant female domestic donkeys also begin to have reproductive cycles in the spring, and domestic horses and donkeys both continue cycling until conception or the end of the breeding season.
For horses and donkeys, as for many other mammals, the ovarian or estrous cycle is divided into phases. During the follicular or estrous phase (when females will stand for mating), follicle growth is stimulated by gonadotropin-releasing hormone (GnRH) from the hypothalamus and follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary. The follicles produce estradiol, which stimulates estrous behavior. The estrous phase in donkeys and horses reportedly lasts about 6-9 days (Ginther, 1979; Vandeplassche et al., 1981).
During estrus, the female is attractive to males and receptive to mating. Courtship behaviors are generally similar in horses and donkeys with some important exceptions. Estrous horses often raise their tails, exposing the genital area, as they approach and follow males (Asa, 1986). Tail raise is not as obvious in female donkeys, but they spend more time in proximity to males and respond to male vocalization by approaching (Henry et al., 1991). Courtship interactions tend to be more vigorous in donkeys and include more elements of aggression, such as kicking and chasing. Female horses urinate more frequently
during estrus, and males assess urine via the flehmen response, which introduces pheromones into the vomeronasal organ for neural processing of the female’s reproductive status (Stahlbaum and Houpt, 1989). Vocalization appears to be more important in donkeys, males of which commonly initiate sexual interactions by vocalizing (Henry et al., 1991).
Ovulation occurs toward the end of the estrous phase, but courtship and mating may continue for an additional couple of days in both horses and donkeys. An LH surge triggers ovulation, which is followed by conversion of the follicles to corpora lutea (CL), which produce progesterone. Progesterone domination during the luteal phase, also called diestrus, inhibits further estrous behavior. The total cycle in horses lasts about 3 weeks but in donkeys may last as long as 28 days (Ginther, 1979; Vandeplassche et al., 1981; Fielding, 1988). Estradiol and progesterone prepare the uterus for implantation and nourishing the embryo.
Fertility rates in domestic horses are reported to range from about 80 to 100 percent per breeding season, depending on factors such as breed, age, and reproductive history (reviewed in Ginther, 1979). Fertility rates are lower in older and very young mares (Carnevale and Ginther, 1992; Vanderwall et al., 1993). Rates are also lower in domestic mares that have not previously foaled than in currently lactating mares (reviewed in Ginther, 1979). In one study of pasture breeding of domestic donkeys, all 14 females that were examined were pregnant (Henry et al., 1991).
Gestation length is 11 months in horses and 12-12.5 months in domestic donkeys (Ginther, 1979; Fielding, 1988). However, possible ovulation or spontaneous luteinization, resulting in the formation of secondary CL, around day 40 can confound calculation of gestation length in field studies. Estradiol secreted by the follicles that precede CL formation can stimulate estrous behavior in a small percentage of pregnant females (Tomasgard and Benjaminsen, 1975) and give the appearance of a natural estrous cycle.
With a gestation length of about a year, horses and donkeys can give birth every year. However, that may not occur, especially in nutritionally stressed females. In particular, nursing females, experiencing the energetic drain of lactation in addition to maintenance, may not succeed in sustaining a pregnancy. But lactation itself does not prevent estrous cycles, so conception may occur, although the embryo may be lost if the female is nutritionally stressed. Early embryo loss (defined as up to day 40 of pregnancy) is reported to be 5-15 percent even in well-fed domestic mares but can be 30 percent or higher in mares that are 18 years old or older (Vanderwall, 2008). Pregnancy loss may also be high in yearling mares (Mitchell and Allen, 1975). In a small study of domestic donkeys, three of 14 pregnant females experienced early embryo loss (Henry et al., 1991).
POTENTIAL METHODS OF FERTILITY CONTROL IN FREE-RANGING HORSES AND BURROS
First, it is important to note that, when the committee prepared its report, no fertility-control methods that were highly effective, easily delivered, and affordable were available for use across all BLM Herd Management Areas (HMAs). In addition, there were no fertility-control methods that did not alter the behavior or physiology of free-ranging horses and burros in some way. Any method that prevents reproduction can do so only by affecting some aspect of the reproductive system. Even if the only effect were to prevent births, that would change the age structure of a herd by reducing the number of young and could enhance the health of females by reducing the caloric demands of reproduction. Thus, in evaluating fertility-control methods, it is important to compare them not only for obvious factors—such as efficacy, mode of delivery, and cost—but for the constellation of
their effects on physiology, behavior, and social structure. It is also critical to extend the comparisons to the social-structure changes and behavioral and health effects that are caused by gathers.
The porcine zona pellucida (PZP) vaccine, an immunocontraceptive, is the most extensively tested method in free-ranging horses and may be the most promising option at present. Several other methods that are potentially useful in horse and burro populations will be considered in this chapter, but more research may be required before their application can be recommended. Fertility-control methods range from other types of vaccines to hormone agonists;1 some methods are more appropriate for treatment of females, and others could be used to control male fertility. Some of the methods are reversible—and allow the possibility of future restoration of fertility—but others are permanent sterilants that have the economic and logistical advantage of making repeated treatment unnecessary. In particular, nonsurgical approaches to sterilization will be evaluated.
Methods that are not considered permanent may not be 100-percent reversible in all animals. Even if a contraceptive, such as an implant, is removed or its effect wears off (in the case of an injectable contraceptive), other factors may slow or even prevent complete restoration of fertility. Many factors affect fertility and time to conception or birth even in females that have never been treated with contraceptives (reviewed in Asa, 2005). Female age is the most obvious factor, but parity (the number of times that a female has given birth), age at production of first offspring, time elapsed since last pregnancy, nutritional status, health, genetics, and other more subtle factors can also influence a female’s ability to conceive and maintain a pregnancy to term. Fertility of previously contracepted females can be affected by those factors and by lingering effects of the contraceptive itself. Individual differences are common.
The process of selecting the best method for the species and situation includes an evaluation of many equally important factors, such as delivery route, efficacy, duration of effect or reversibility, physiological side effects, and possible effects on behavior and social structure. It is also important to know whether a method is safe for prepubertal animals and whether females can be treated during pregnancy or lactation. Although methods can be male- or female-directed, more research in control of fertility in free-ranging equids has targeted females, specifically different formulations of the PZP vaccine, than males. The following review includes methods for both males and females and methods that have been tested with other species that could be considered for use in free-ranging equids.
ADJUSTMENT OF SEX RATIO TO LIMIT REPRODUCTIVE RATES
Adjustment of the sex ratio to favor males has been proposed for managing population growth rates of horse and burro populations. Sex ratio typically is somewhat adjusted after a gather in such a way that 60 percent of the horses returned to the range are male. At that ratio, however, population growth would be only slightly reduced: modeling by Bartholow (2004) suggests that birth rates could decline from about 20 percent to 15 percent a year if the proportion of males increased from 0.50 to 0.57. If more aggressive sex-ratio adjustments are initiated by drastically altering the number of females relative to males beyond a 40:60 ratio, care should be taken to assess possible additional consequences. In the Pryor Mountain Wild Horse Range, Singer and Schoeneker (2000) found that increases in the number of males on this HMA lowered the breeding male age but did not alter the birth rate. Because the existing females were distributed among many more small harems,
____________
1 A hormone agonist binds to a receptor of a cell and has the same action as the native hormone.
estimates of genetic effective population size increased.2 In addition, bachelor males will likely continue to seek matings, thus increasing the overall level of male-male aggression (Rubenstein, 1986). Male condition may decline because of the increase in time spent in competing, and the disruption caused by male-male competition may affect female foraging success. Both those outcomes might reduce overall population growth more than would a reduction in the number of breeding females. Because horses and burros have polygynous mating systems (multiple females mate with one male), additional males would not be expected to affect the likelihood of reproduction in individual females. Reduction in reproductive rate would depend on the number of females remaining. Having a larger number of males competing could favor females by enhancing the opportunities for mate choice, could mean that males of higher genetic quality would achieve harem stallion status, or both. Given that the addition of males or the subtraction of females can lead to a similar sex ratio but have different effects on population growth rates, forecasting models tuned with population-specific survival and fecundity levels can be used to determine how to adjust sex ratios to limit population growth in individual populations effectively.
FEMALE-DIRECTED METHODS OF FERTILITY CONTROL
Potential methods of fertility control directed at female equids include surgical ovariectomy (removal of the ovaries); immunocontraceptives, which trigger the animal’s immune system to prevent pregnancy; GnRH agonists; steroid hormones; and intrauterine devices. The mode of action and effects of each method are reviewed below.
Surgical Ovariectomy
Surgical ovariectomy and ovariohysterectomy are commonly used in domestic species, such as cats and dogs (including feral cats and dogs), but seldom applied to other free-ranging species. Accessing the female reproductive tract, which lies within the body cavity, in contrast with the reproductive tract of males of most species, which have external testes, carries the risk of dehiscence of sutures or inflection. However, an alternative vaginal approach, colpotomy, avoids an external incision and reduces the chances of surgical complications or inflection (Rodgerson and Loesch, 2011). The mare is sedated and tranquilized while standing but restrained; a local anesthetic is sometimes used as well to reduce movement during surgery. An incision is made through the wall of the vagina and then through the peritoneum to access the ovaries. Although the risks are lower than with trans abdominal surgery, episioplasty (suturing to close the vulva) and stall restriction for 2-7 days are recommended to reduce the chance of evisceration. Monitoring for 24-48 hours for signs of hypovolemic shock due to internal bleeding is also recommended. The procedure is not without risk.
Duration and Efficacy
Removal of the ovaries is of course permanent and 100-percent effective. Ovariectomy during the first 2-3 months of pregnancy results in abortion because of the loss of progesterone
____________
2 Effective population size is the size of an idealized population that would experience the same magnitude of random genetic drift as the population of interest. Populations that have experienced fluctuating sizes between generations, unequal sex ratios, or high variance in reproductive success are likely to have effective population sizes that are lower than the number of animals present. The concept of effective population size is discussed in Chapter 5.
from the corpus luteum (Holtan et al., 1979). Ovariectomy during the period of lactation would not be expected to affect milk production, inasmuch as gonadal hormones (estrogen and progesterone) are important during late pregnancy when mammary glands are developing but not after milk production is established.
Side Effects
Typical side effects associated with ovariectomy in many species include decreased activity and weight gain. The absence of gonadal hormones could affect sociosexual behavior but perhaps not as profoundly as in most other species. Although the cyclic production of estrogen by the ovaries is required for stimulation of estrus and mating behavior in virtually all species, the horse is an exception. The full repertoire of courtship and mating behavior has been displayed by ovariectomized mares and by anestrous mares during the nonbreeding season (Asa et al., 1980b; Hooper et al., 1993). The behavior was found to be hormonally supported by adrenal sex steroids (Asa et al., 1980a), for example, estrone and dehydroepiandrosterone, a weak estrogen and an androgen, respectively. In contrast with ovarian hormones, adrenal sex steroids are not secreted cyclically, so estrous behavior is displayed sporadically. No comparable study of the sexual behavior of free-ranging, nonpregnant mares has been conducted during the nonbreeding season. However, if free-ranging ovariectomized mares also show estrous behavior and occasionally allow copulation, interest of the stallion would be maintained, and this would foster band cohesion.
Immunocontraceptives
No other class of contraceptives has been as extensively researched in domestic and free-ranging equids as immunocontraceptives. Immunocontraception relies on the target species’ immune system to produce an immune reaction (usually in the form of antibodies) to some target tissue or biochemical that is required for successful reproduction. The immune response is most often triggered by inoculation of the target species with biochemicals or tissues from other species that are similar in structure to the biochemicals or tissues of the host. The target animal’s immune system responds to the foreign compounds injected into the body by producing antibodies that bind to both the injected, foreign compounds and the structurally similar tissues or biochemicals in the target species. The biological effects of the immunocontraceptive, aside from prevention of conception, depend on which biochemicals or tissues are the intended targets, the ability of the immunocontraceptive to induce an immune response (its immunogenicity), the specificity of the immune response to the target biochemicals or tissues, and the duration of the immune response.
In equids, the two most studied immunocontraceptives are vaccines directed against GnRH, a peptide hormone produced by the hypothalamus, and the zona pellucida, the outer membrane layer surrounding the mammalian oocyte (egg). Both are discussed below in further detail with regard to delivery routes, efficacy, duration of effect or reversibility, and side effects. This review focuses on published studies of captive and free-ranging horses, where available; otherwise, results from studies of other ungulates are used to provide an approximation of what might occur after application of the treatment to horses.
Porcine Zona Pellucida Vaccine
Sperm must bind to the zona pellucida of the oocyte to initiate the sperm acrosome reaction that is required for fertilization. Anti-zona pellucida vaccines prevent conception
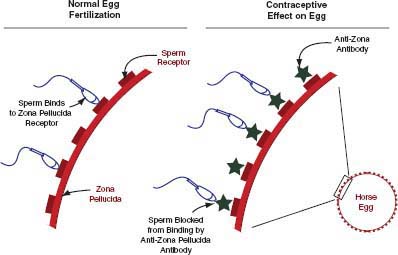
FIGURE 4-1 Mode of action of porcine zona pellucida vaccine.
SOURCE: Illustration provided by I.K.M. Liu.
late in the chain of events required for successful fertilization by preventing sperm from fertilizing eggs (Figure 4-1). There are three formulations of the PZP vaccine: a liquid formulation accompanied by a primer that is effective for 1 year (liquid PZP), a time-release pellet formulation that can be effective for up to 22 months (PZP-22), and a formulation in which PZP is encapsulated in liposomes3 to extend contraception efficacy (SpayVac®; Immunovaccine Technologies, Inc. [IMV], Halifax, Canada).
It is important to note that PZP vacceines are not a homogeneous set of compounds. The term liquid PZP used below refers to a PZP vaccine prepared according to the methods originally outlined for the horse by Liu et al. (1989) in which pig ovaries are finely sliced to release oocytes from surrounding tissues. The PZP in SpayVac is different in two ways. First, it is prepared differently: whole ovaries are ground and homogenized to separate oocytes from tissues (Yurewicz et al., 1983). Second, the PZP is encapsulated in liposomes to extend the period of release (Brown et al., 1997). In both procedures, the product passes through a series of filters of decreasing pore size to remove other ovarian debris, but it is possible that the SpayVac preparation contains more non-zona pellucida ovarian proteins than liquid PZP produced with the Liu et al. method. Ovarian proteins cannot reliably be separated from zona pellucida proteins by filtration, and the initial grinding and homogenization of whole ovaries in the Yurewicz et al. method results in more non-zona pellucida debris in the initial suspension. Less pure products (containing more ovarian debris) may be more immunogenic than zona pellucida proteins alone and enhance the immune response. Miller et al. (2009) suggested that the difference in antigen preparation might explain the longer duration of efficacy in their SpayVac-treated deer than in deer treated with liquid PZP, but more work is needed to determine whether antigen preparation methods
____________
3 A liposome is an artificially prepared vesicle composed of a lipid bilayer that can incorporate drugs for controlled delivery.
result in differences in PZP efficacy. Ovaries were not examined for pathological effects in horses, deer, or other species treated with SpayVac, nor were any long-term studies done on its reversibility. It is possible that SpayVac prevents fertilization by means in addition to or other than sperm blockage. Reversibility also requires further investigation. All published studies that have used SpayVac liposome preparations in free-ranging horses included the adjuvant AdjuVac™ prepared by Miller at the U.S. Department of Agriculture’s National Wildlife Research Center (NWRC). However, Miller has shown that liposomes are dissolved by the lipid-based adjuvant AdjuVac, which would be expected to shorten its period of efficacy in that the liposomes were designed to prolong contraceptive effect (L. Miller, NWRC, personal communication).
It is also important to note that over the years liquid PZP has been administered to horses with several treatment protocols for the first inoculation, and the effects of the different protocols and of protocols for administering boosters are still not fully understood. For example, in the first study of liquid PZP in domestic mares, Liu et al. (1989) administered the vaccine in four initial injections at 2-week intervals, whereas much of the later work with PZP by Kirkpatrick, Turner, and colleagues (e.g., Kirkpatrick et al., 1991; Turner et al., 1997) involved two initial injections 4 weeks apart. Much of the more recent work (e.g., Liu et al., 2005; Turner et al., 2007) used single-injection protocols that appear to be more feasible in field settings. It is also unclear whether annual booster vaccinations with liquid PZP (e.g., Kirkpatrick et al., 1991) and timed-release PZP pellets (e.g., Turner et al., 2007) generate the same immunologic dynamics needed to prolong the effect of PZP. For example, the total amount of PZP released from a timed-release pellet during the boost period may differ from the amount of PZP in a liquid booster vaccination, and the duration of exposure may not be equivalent. Furthermore, the immune system may respond to these alternative antigen presentations in different ways. The immunologic dynamics induced in the target species with different treatment and boosting protocols are not yet definitively understood.
Delivery Route. Both the liquid and pellet formulations of PZP can be administered by hand to free-ranging equids that have been captured. Liquid PZP can be delivered by dart to animals in the field (Kirkpatrick et al., 1990). Pelleted PZP must be given by hand because darts cannot provide adequate pressure to release pellets into the animal effectively; this was verified in a study of pelleted PZP that was effective for 1 year: the efficacy of the hand-injected PZP was twice that of the dart-injected PZP (Turner et al., 2008). SpayVac (Brown et al., 1997) can be given by hand or dart.
Although the ability to deliver liquid PZP via dart is a useful option, it is not clear how successful attempts would be to dart populations of horses at the desired level of treatment intensity, given the large number of animals needing treatment, variability in the temperament of the horses, and the terrain of HMAs. Two studies of free-ranging horses and one of white-tailed deer have found that over time, with repeated boosters, the difficulty of approaching animals on foot for darting increased (Kirkpatrick and Turner, 2008; Rutberg and Naugle, 2008; Ransom et al., 2011). At the time the report was prepared, the most effective and most reliable method of delivery was hand injection after a gather. However, alternative methods, such as trapping near water holes or blinds, have been used in other areas and could be useful in some HMAs.
Efficacy. Liquid PZP, the first formulation produced, has been assessed for efficacy more often than other PZP formulations. The overall mean of published efficacy values in horses is 88.4 percent (median, 89 percent). Kirkpatrick and Turner’s (2008) value of 95 percent is
based on cumulative experience on Assateague Island4 and represents the most up-to-date information available to the committee on that site. Turner et al. (1997) evaluated several adjuvant formulations.5 If the less effective adjuvants in their study and another study that acknowledged poorly timed boosters in one population (Ransom et al., 2011) are eliminated, the mean efficacy increases to 91.5 percent (median, 90 percent), representing hundreds of animals across several sites. In most of the studies, efficacy was assessed by determining how many treated females had foals in the following foaling season or had pregnancy diagnosed with hormone assays.
Only one study of any PZP formulation has been conducted in burros. Turner et al. (1996) found that liquid PZP significantly reduced fertility for a year after vaccination. A two-shot protocol was more effective (none of 13 females became pregnant) than a one-shot protocol (one of three became pregnant).
Turner et al. (2007) assessed a pelleted form designed to release PZP into the animal’s circulatory system at 1, 3, and 12 months in 96 free-ranging mares in Nevada. Fertility rates over 4 years after vaccination were 5.2 percent, 14.9 percent, 31.6 percent, and 46.2 percent, respectively, in treated mares. The mean fertility rate of untreated females during the study was 53.8 percent. The formulation has come to be called PZP-22 because it remains about 85-percent effective after 22 months. Turner et al. (2008) concluded that the optimal time to administer PZP-22 for maximum duration of effect is fall or winter. BLM began using PZP-22 in free-ranging horses in the late 2000s. However, the efficacy has varied as treatment has been extended to additional field sites. Foaling has been reduced by 30-79 percent in the 2 years after a single injection of PZP-22 at various field sites (J.W. Turner, University of Toledo, personal communication, November 2012). The variability is believed to be due to the time of year of injection, whether delivery was by dart or by hand, the location of the injection (the hip is considered ideal, but that is not always possible when delivery is by dart), and possible differences in preparation in the field. In addition, there has been a change in vaccine production during the last few years: heat extrusion versus cold evaporation (J.W. Turner, University of Toledo, personal communication, November 2012).
Only one published study (Killian et al., 2008a) has evaluated SpayVac efficacy in horses. In a study of captive horses in Nevada, 12 mares received a single hand injection in the neck of 400 μg of SpayVac emulsified with AdjuVac adjuvant for a total volume of 1 mL in March 2003. In fall of each year, treated mares were examined for pregnancy via ultrasonography or rectal palpation, and the observations were later verified by whether a foal was born. In a few cases in which a mare’s behavior prevented that kind of examination, the birth of a foal (or the absence of a birth) in spring of the following year was used to assess fertility and treatment efficacy. In the 4 years of the study, contraception efficacy in the SpayVac-treated mares was 100 percent in year 1 and 83 percent in years 2-4. Bartell (2011) determined that SpayVac in combination with nonaqueous Freund’s modified adjuvant (FMA) induced the strongest immune response in domestic horses as measured by antibody titers and exhibited the strongest suppression of progesterone compared with an aqueous preparation of FMA and non–mycobacterium-based adjuvant, but she did not assess pregnancy or foaling.
____________
4 Assateague Island National Seashore is on a barrier island off the coast of Maryland and operated by the U.S. Department of the Interior’s National Park Service (NPS). A free-ranging herd lives on the island. NPS is not subject to the Wild Free-Roaming Horses and Burros Act of 1971. Nevertheless, because it is a free-ranging population, results of studies of the use of liquid PZP on this herd can inform management of horses under BLM’s jurisdiction.
5 An adjuvant enhances the immune response by encouraging the production of antibodies.
SpayVac has also been evaluated in deer. Miller et al. (2009) evaluated SpayVac and liquid PZP in combination with different adjuvants in 30 captive white-tailed deer grouped into six treatment groups of five does each. SpayVac was administered in three preparations: with liposomes in AdjuVac emulsion, lyophilized with liposomes in AdjuVac suspension, and with liposomes in an alum adjuvant suspension. PZP was produced with two protocols (labeled IVT and NWRC for the providers of the antigen). The SpayVac/AdjuVac emulsion and the IVT-PZP/AdjuVac emulsion had the longest duration of effect: 80 percent of treated deer were contracepted for at least 5 years. Monitoring of the SpayVac/AdjuVac group ceased at 5 years; the IVT-PZP/AdjuVac continued to be effective for 7 years. The estimated decline in fecundity (fawns produced per female) was greater than 90 percent. All other formulations were inferior in performance. The authors concluded that AdjuVac is critical and should be used in emulsion form rather than suspension. They also suggested that, because of production differences, the IVT-PZP probably contained more porcine ovarian tissue and was thus more effective. Fraker et al. (2002) evaluated the efficacy of SpayVac emulsified with Freund’s complete adjuvant (FCA) administered to 41 free-ranging fallow deer. Contraception of treated does was 100 percent over 3 years; however, the samples obtained in the 3 years were from different animals because some animals were culled for analysis. The authors suggested that, on the basis of the antibody titers present after 3 years, the SpayVac vaccination would probably continue to be effective for a longer period. Locke et al. (2007) evaluated SpayVac emulsified with AdjuVac over a 2-year period in wild white-tailed deer (34 treated, 11 controls) and found 100-percent efficacy in both fawning seasons. Killian et al. (2005) cited data from their studies of captive white-tailed deer in Pennsylvania that showed 80-percent efficacy in does for 4 years.
Gray et al. (2010) evaluated a PZP vaccine that was mistakenly referred to as SpayVac (Fraker and Brown, 2011; Gray et al., 2011) in 20 treated and 18 untreated free-ranging mares in Nevada over a 3-year period. The liquid-PZP vaccine was prepared as SpayVac but without liposomes. Efficacy was lower (50-63 percent) than reported by Killian et al. (2008a) for SpayVac. Gray et al. (2010) suggested that the lower efficacy might have been due to their more conservative methods of assessing efficacy in the field; however, in a follow-up published erratum, they acknowledged that the vaccine formulation that they used lacked the liposome compounds included in the SpayVac vaccine (Gray et al., 2011) and suggested that this could explain the differing results. Thus, the studies by Gray et al. (2010) should not be compared to other results for SpayVac specifically, and it is not clear whether these results should be compared to those for liquid PZP. In both the Killian et al. (2008a) and Gray et al. (2010) studies, the AdjuVac adjuvant was combined with the vaccine.
Reversibility. Immunocontraception depends on the immune response to the vaccine reaching and staying above threshold concentration (Adams and Adams, 1990; Zeng et al., 2002). Reversibility of the contraceptive effect depends on the reduction of circulating antibody titers. Substantial variability in reversal time is likely and can be due to the vaccine formulation, the adjuvant used, the treatment protocol, genetic factors, and the nutritional status of the individual animal because these factors may affect the initial and continuing immune response to the vaccine (Homsy et al., 1986; Chandra and Amorin, 1992; Turner et al., 1997, 2001, 2007; Liu et al., 2005; Lyda et al., 2005; Bartell, 2011).
In the first study of liquid PZP in equids, Liu et al. (1989) found that, of 10 feral and six domestic mares, most mares had reversed within 8 months of treatment. Kirkpatrick et al. (1990) first demonstrated that three of seven free-ranging mares became fertile in the first year after 1 year of liquid-PZP treatment, although foaling rates of treated mares overall were lower after treatment than in control mares. Turner et al. (1997) found similar results
in horses in Nevada, where 103 mares were treated with various combinations of PZP and adjuvants and 92 mares served as controls. Data from Assateague Island on reversibility continued to accumulate over the years, and Kirkpatrick and Turner (2002) stated that liquid PZP was 100-percent reversible in three mares treated for 4 consecutive years and two mares treated for 5 consecutive years. The time between final treatment and pregnancy ranged from 1 to 8 years. At the time the committee’s report was prepared, none of the five mares treated for 7 consecutive years had reversed after 7 years of monitoring. In a study of 16 burros, 46.1 percent of treated females were determined to be pregnant via fecal hormone monitoring during the second year after liquid-PZP treatment (Turner et al., 1996).
Studies of longer-acting PZP formulations, such as PZP-22 (pellets) and SpayVac, have assessed reversibility more in the context of measuring the duration of effect of the vaccine; declining infertility in years after vaccination reflects reversibility. In a study by Turner et al. (2007) of 96 treated mares, 15 percent of mares had reversed after 22 months, 31.6 percent after 3 years, and 46.2 percent after 4 years. In that study, however, not every mare was assessed for reversibility every year. Turner et al. (2008) suggested that more rigorous study of reversibility in PZP-22 treated mares is warranted.
Ransom (2012) studied liquid PZP and PZP-22 in three horse populations in the western United States. Twenty-two mares on the Little Book Cliffs HMA and 38 mares on the Pryor Mountain Wild Horse Range were treated with liquid PZP up to 5 consecutive years. At the McCullough Peaks HMA, 28 mares were treated with PZP-22. Among all the sites, in mares that had foaled previously, the probability of not foaling was 74.4 percent after PZP treatment and 35.9 percent in control mares; this indicates that fertility may be suppressed after the planned period of infertility. At Little Book Cliffs and Pryor Mountains, the time from the last liquid-PZP injection to first parturition ranged from 1.5 to 8.1 years and was strongly affected by the total number of years in which the mares were treated. On average, time to parturition increased by 411 days per consecutive year of treatment. At McCullough Peaks, 64 percent of PZP-22 treated mares did not produce a foal during the post-treatment period (5 years). Return to parturition took 1.4-5.5 years. The results reinforce the notion that return to fertility after immunocontraception can be longer than expected.
SpayVac has not been thoroughly assessed for reversibility in captive or free-ranging horses, although the study by Killian et al. (2008a) demonstrated that two of 12 treated mares became pregnant 2-4 years after vaccination. The studies of SpayVac in deer described above did not systematically address reversibility, nor have they been of sufficient duration to detect decreases in vaccine efficacy (animals were contracepted at the same level of efficacy in all years of the study).
Side Effects: Physical and Physiological. Because the antigen target of PZP contraception (liquid, pellet, or SpayVac formula) is highly specific—the egg’s zona pellucida—there appear to be relatively few physical side effects. Barber and Fayrer-Hosken (2000) found that PZP antibodies did not bind to other somatic tissues in horses. Liu et al. (1989) found no evidence of pathological conditions in ovaries of mares treated for 1 year; however, this remains the only study of ovarian pathology in relation to liquid-PZP treatment in horses. Bartell (2011) found that the ovaries of SpayVac-treated domestic mares were lighter, had smaller oocytes, and had thinner zona pellucidae than control mares. Killian et al. (2008a) found that SpayVac-treated mares had unexplained higher rates of uterine edema, but they cited literature (Samper, 1997) suggesting that in healthy mares this is a sign of estrus when mares are under the influence of estrogen produced by ovarian follicles. It is not known whether the extent of edema observed in the SpayVac-treated mares was equivalent to that in normal estrous mares or more severe; the latter might be a possible indication of pathology. Because
of the pathological potential, further research on uterine changes during and after treatment with SpayVac is warranted. There are no documented reports of persistent uterine edema after the use of liquid PZP or PZP-22, but comparable data on the effects identified with the use of SpayVac do not exist.
Mares that have been treated with liquid PZP for 3-7 consecutive years have been reported to have decreased ovulation rates in successive years of treatment (Kirkpatrick et al., 1992, 1995); this suggests that PZP may act at sites other than just the zona pellucida. Powell and Monfort (2001) did not find a statistically significant relationship between the likelihood of ovulatory failure and current contraception status (currently versus previously treated with PZP). It is possible that the likelihood of physiological side effects depends on the delivery of PZP as repeated vaccinations (for example, annually in the case of liquid PZP) as opposed to one long-term vaccination (in the case of PZP-22 and SpayVac).
There are many other possible causes of subfertility in horses (McCue and Ferris, 2011), but in none of the analyses described above were the same mares assessed for cyclicity before and after PZP treatment, so other possible factors contributing to subfertility were not assessed. It is estimated that about 20 percent of domestic horse mares are subfertile (I.K.M. Liu, University of California, Davis, personal communication, August 2012). Ovarian senescence has also been documented in some domestic mares over 20 years old, as evidenced by a longer follicular phase, a prolonged interovulatory interval, and later first ovulation of a breeding season (McCue and McKinnon, 2011)—all of which are reported in mares currently or previously treated with PZP (Powell and Monfort, 2001). Thus, assessing reproductive competence after many years of PZP treatment is confounded by the concomitant effects of aging.
There has been much discussion over the years of the effects of different adjuvants used in combination with PZP in relation to reactions at the injection site, which have included stiffiness, swelling, nodules, and abscesses. The traditional application of liquid PZP involved an initial primer dose administered with FCA and a follow-up booster 2-4 weeks later with Freund’s incomplete adjuvant (FIA). Kirkpatrick et al. (1990) were the first to mention potential concerns with using FCA in wildlife, but in their study only three of 26 treated mares had injection-site abscesses, and all healed within 14 days. One concern with FCA is its ability to produce false positive results in tuberculosis tests; this in part led to the development of FMA, which did not produce such results (Lyda et al., 2005). Chapel and August (1976) also suggested that FCA could be hazardous to people exposed to it when administering injections.
In their study of FCA and FMA use in the primer liquid-PZP dose, Lyda et al. (2005) found only one case of injection-site abscess. The mare was treated with FMA in the primer dose and FIA in the booster. The abscess appeared after the FIA booster dose, and it drained and healed without incident. Antibody titers produced with FMA and FCA did not differ significantly. Neither adjuvant had an effect on the delivery of healthy foals. The authors cited unpublished data suggesting that the incidence of injection-site abscesses was less than 1 percent when injections were given in the hip, but it was higher when injections were given in the neck.
In a large study of free-ranging horses, Roelle and Ransom (2009) found no statistically significant differences in occurrence of dart-site reactions due to adjuvant (FCA or FMA) and suggested that reactions are probably more likely to be due to dart trauma or in some cases a combination of dart trauma and adjuvant. Hand injection led to fewer injection-site reactions than darting. Overall, abscesses in response to darting were rare, in accordance with other studies (Kirkpatrick et al., 1990; Turner and Kirkpatrick, 2002; Lyda et al., 2005). Nodules at the injection site were the most common reaction (25 percent of cases), and these
persisted for up to a year or more but did not appear to affect the animals. Swelling was the second-most common reaction (11 percent and 33 percent at two study sites), and this disappeared within 30 days. Stiffiness was the third-most common (1.4 percent and 11 percent at two study sites) and disappeared within 24 hours.
In their studies of both PZP and GonaCon™ (a GnRH vaccine), Gray et al. (2010, 2011) found no cases of abscesses after hand injection of either compound with AdjuVac as an adjuvant. Similar results have been found in deer when AdjuVac has been used (Locke et al., 2007; Miller et al., 2009).
Contracepted females should generally be in better body condition than uncontracepted females because they do not face the energetic demands of pregnancy and lactation. Turner and Kirkpatrick (2002) found that body-condition scores of mares on Assateague Island were significantly higher in 1999 than in 1988 before PZP contraception was widely applied. Body-condition scores of lactating females at those two times were not significantly different, and this suggests that prevention of pregnancy can enhance body condition. Ransom et al. (2010) found no difference in body-condition scores between treated and untreated mares in three western populations of horses on the basis of a similar body-condition scoring index, but mares that had foals had lower body condition than mares that did not. The most likely reason for the absence of significant body-condition differences between treated and untreated mares is that most treated mares were already pregnant when the study began and therefore did have foals at their sides during the study. In addition, some treated mares that did not respond to contraception and produced foals were exposed to the same energetic demands of gestation and lactation as untreated mares (J. Ransom, National Park Service, personal communication, May 3, 2012). In contrast, Fraker et al. (2002) found that fallow deer does treated with SpayVac had lower stores of kidney fat than untreated does; treated does might have expended more energy during the rut because they were engaged in reproductive behavior more often than untreated does.
Side Effects: Pregnancy, Birth Seasonality, and Survival. Liquid PZP has been demonstrated to be safe to administer to pregnant mares in a number of studies (e.g., Kirkpatrick et al., 1990, 1991). Turner and Kirkpatrick (2002) found that foal survival to 1 year is equivalent between untreated mares and mares treated with liquid PZP during pregnancy; female foals born to PZP-treated females also successfully bred and reared offspring. Kirkpatrick and Turner (2003) analyzed birth records on Assateague Island and found that most foals born to treated and untreated mares are born in season (April-June): 75.8 percent of births to control mares, 64.9 percent of births to treated mares, and 68.9 percent of births attributed to contraceptive failure. None of those differences was significantly different. The authors did note that out-of-season births had been increasing on Assateague Island since 1984 (the contraception management program began there in 1994) for unknown reasons. Turner and Kirkpatrick (2002) found no difference in survival between in-season and out-of-season foals but stated that it probably depends on the environment (Kirkpatrick and Turner, 2003). On Shackleford Banks,6 PZP-treated mares foaled over a broader range of months than untreated mares (Nuñez et al., 2010). Mares given PZP in the year before they conceived gave birth 3-4 months later than untreated mares. Mares that had been on PZP at some point before the year in which they conceived gave birth almost a month later than
____________
6 Shackleford Banks, part of the Cape Lookout National Seashore, is home to a herd of free-ranging horses managed by the U.S. Department of the Interior’s National Park Service. Although they were not treated with PZP for as many years as the Assateague Island horses, the results of behavioral studies of the Shackleford Banks horses can inform management of horses under BLM’s jurisdiction.
untreated mares. However, in an investigation of PZP contraception in free-ranging mares in Nevada, Gray et al. (2010) found no differences in foal survival, birth seasonality, or foal sex ratio between treated and untreated mares. Ransom (2012) also studied the effect of liquid and pelleted PZP (PZP-22) on birth seasonality at three sites in the western United States. Overall, mares that gave birth to foals after treatment (liquid and PZP-22 considered together) did so an average of 31.5 days later (range, 17-46) than untreated mares. Ransom stated that that effect varied among sites and PZP formulations, but these factors were confounded because PZP-22 was used exclusively at one site and not at all at the others. In addition, a monsoon rain at one site allowed a second peak in spring vegetation quality. There was no effect of treatment on foal survival; however, foal survival did decrease the later a foal was born after the peak in spring vegetation quality. Ransom indicated that the average delay in birth of a posttreatment foal results in about a 4.2-percent reduction in survival probability and that this is probably why the treatment effect was not statistically significant (J. Ransom, National Park Service, email communication, July 6, 2012). Ransom also noted that posttreatment mares that gave birth “late” in a given year would often not foal in the following year but then would foal in the third year during the normal birthing season for that site; such factors as photoperiod and temperature might be able to “reset” a mare’s reproductive system so that conception and birth occur during the normal birth season in later years.
Studies of liquid-PZP contraception in the Assateague Island horse population have also revealed effects on survival of mares. In the 4 years before 1994, when management-level contraception began, annual adult mortality was greater than 10 percent; in the first 4 years after contraception, adult mortality decreased to less than 4 percent (Turner and Kirkpatrick, 2002). It should be noted, however, that in 1990 and 1992 many deaths were attributable to an equine encephalitis outbreak and severe storms, respectively. Even so, mare mortality in 1991 and 1993 was about 3-4 percent; from 1994 to 1998, mare mortality was less than 2 percent (Turner and Kirkpatrick, 2002). There was also a shift upward in age classes in the entire herd, which indicated increased survival and the attainment of new, older age classes (Turner and Kirkpatrick, 2002). In a later study (Kirkpatrick and Turner, 2007), untreated mares were compared with mares on PZP for less than 3 years and mares on PZP for more than 3 years. Mean age at death was significantly lower in untreated mares (6.47 years) than in treated mares, and mares on PZP for more than 3 years had a higher mean age at death (19.94 years) than mares on PZP for less than 3 years (10.27 years). At the time the committee’s report was prepared, pelleted PZP and SpayVac had not been examined for effects on adult survival or demographic changes.
Side Effects: Genetic. Concerns have been raised about possible unintended genetic effects of immunocontraception. In a review of ecological and immunogenetic issues surrounding immunocontraception, Cooper and Larsen (2006) suggested that because immunocontraceptives are rarely 100-percent effective and resistance to vaccines (contraceptive failures) might have a genetic basis, managers may be unintentionally selecting for animals that do not respond to immunocontraceptive techniques. Using Falconer’s (1965) equations, they suggested that if the proportion of nonresponding females is 10 percent, which could be considered a valid estimate for liquid PZP in horses, after one generation of selection via immunocontraception, the percentage of female offspring produced that would themselves be resistant would range from 15 to 23 percent, depending on the degree of heritability of resistance to immunocontraception. The authors also suggested that such selection for non-responders could occur in the major histocompatibility complex or in genes that regulate the immune system, either of which could alter resistance to other pathogens.
However, when the committee’s report was prepared, there were no data on resistance to immunocontraception, the heritability of such resistance, or the identity of specific genes that might affect responses to immunocontraceptives. National Park Service staff reported on Assateague Island that there were no indications that resistance was developing or that responses to immunocontraception were changing over time, after 19 years of herd management with PZP. Contraceptive effectiveness continues to be high (A. Turner, Assateague Island National Seashore, email communication, February 24, 2013). The immune response to immunocontraceptives depends on many nongenetic factors, such as nutritional status (Homsy et al., 1986; Chandra and Amorin, 1992; Chandra, 1996; Demas et al., 2003; Houston et al., 2007), and it was not possible for the committee to determine whether resistance to immunocontraception could develop. Similarly, it was not clear whether immunocontraception could inadvertently select for less immune-robust animals because they would not mount a strong response to PZP and would thus remain fertile. Presumably, any genetic background that would predispose animals to being immunocompromised would be under strong selection to be eliminated; even in a small population in which a deleterious mutation that compromised the immune system could become fixed, selection could act against individual animals that have the mutation, although the pressure of selection is smaller in small populations. In addition, Falconer’s (1965) equations apply to threshold or “all-or-none” characters whereas lifetime reproductive success—which contraception affects—is a continuous variable that is not subject to some threshold, so it is not clear whether the Falconer model applies, although other models might. Cooper and Larsen (2006) suggested that immunocontraception could be appropriate for management of species that have long generation times, like horses, because genetic changes (if any) due to immunocontraception would take decades to develop. That would also assume that large numbers of individual animals are contracepted indefinitely and never allowed to breed; this does not seem likely if populations are managed for genetic diversity. However, those concerns highlight the importance of monitoring genetic diversity in immunocontracepted populations (see Chapter 5).
At the population level, removing females even temporarily from the breeding pool is likely to reduce the effective population size (Ne) and genetic diversity of the population. As will be discussed in Chapter 5, reducing the number of breeders or increasing the variance in family size, which will occur as more females bear no young, will reduce Ne and increase the loss of genetic variability. (Tables 5-2 and 5-3 show that some populations display low levels of heterozygosity.)
Side Effects: Behavioral. There are two important considerations in evaluating the literature on contraceptive effects on particular aspects of behavior, particularly bonds between animals and stability of social groups. First, in no published study of immunocontraception have treatment and control groups been matched or balanced with respect to other variables that might affect behavior (such as age, dominance rank, tenure in the group, group size, social or reproductive history, and characteristics of other group members). Rather, investigators have had no control over those variables and thus only compared treated with untreated (or not currently treated) females. Studies in which those factors could be controlled or specifically have their effects measured would require large samples of animals of known history and would be virtually impossible to conduct in the field or even in captivity. Second, no study has been able to differentiate the behavioral effects of a contraceptive compound administered to an animal and the resulting absence of offspring. Thus, in no case can the committee conclude from the published research that the behavioral differences observed are due to a particular compound rather than to the fact that treated animals
had no offspring during the study. That must be borne in mind particularly in interpreting long-term impacts of contraception (e.g., repeated years of reproductive “failure” due to contraception).
Gray (2009) and Gray et al. (2010, 2011) studied the effects of a liquid-PZP vaccine on behavior of free-ranging horses in Nevada during breeding and nonbreeding seasons. There were no treatment effects on activity budget, rates of sexual behavior, proximity between stallions and mares, attempts to initiate proximity, aggression given or received, or band changing by mares. Powell (1999) found no differences in spatial relationships, dominance rank, or aggression between mares currently on PZP and those not currently on PZP on Assateague Island; however, at the time of Powell’s studies, all mares had been treated with PZP at some point in the past, so true controls were not available. On Shackleford Banks, an island where some mares were never treated with PZP, changes in time budgets were observed. Many factors—such as the presence of a foal, the size of a harem, and features of the male associated with the harem—affected time spent in various activities, but a female’s contraceptive status also affected time budgets. In “best fit” general linear models attempting to identify individual and group characteristics that account for variation in the proportion of time spent in grazing and standing, a female’s contraceptive status and an interaction involving contraceptive status and a harem male’s identity had significant effects, as did total harem size and the interaction of male identity and total harem size. In general, PZP-treated females and females in large harems graze less and stand more than non–PZP-treated females and females in smaller groups, but these effects are related to the particular males with which they interact (Madosky et al., in review).
In a study of liquid and pelleted PZP in three populations of horses in the western United States, Ransom et al. (2010) found no effect of treatment on activity budgets, but they did find that treated females engaged in significantly more reproductive behavior (0.05 behavior per hour in control mares versus 0.11 behavior per hour in treated mares), which could be expected with a contraceptive that causes females to cycle repeatedly during the breeding season. Powell (1999) also found no difference in activity budgets between mares currently on PZP and those not currently on PZP. Nuñez et al. (2009) saw significantly more sexual or courtship behavior in treated mares than in controls outside the breeding season but also cited data on other temperate equids that showed that out-of-season cycling is known to occur. Powell (1999) found a nonsignificant trend for currently treated mares to engage in more social behavior overall; however, when only sexual behavior was considered, there was no effect of current contraception status on behavior (Powell, 2000). Turner et al. (1996) did not discern any differences in reproductive behavior between liquid-PZP– treated burros and untreated burros, but they did not provide quantified behavioral data. No other studies of PZP contraception in burros have been published.
The effects of liquid PZP on harem stability in horses have been studied in Nevada during breeding and nonbreeding seasons by Gray (2009) and on Shackleford Banks during the nonbreeding season by Nuñez et al. (2009) and during the breeding season by Madosky et al. (2010). Stability was also assessed on Assateague Island by National Park Service staff (A. Turner, Assateague Island National Seashore, email communication, December 13, 2011). The studies on Shackleford Banks suggest that PZP is associated with increased harem-changing by mares, whereas the Nevada and Assateague studies found no differences between treated and untreated mares in harem-changing. The studies all differ in methodological approaches, definitions of treated and untreated animals, and ecological and social contexts. No studies have been able to control all the factors that could affect harem stability in the field, which could include age, pregnancy status, characteristics of other mares and stallions in the harem, distribution of resources, stallion turnover rates,
population size and demographics, and more. Finally, harem-changing by mares occurs to varied degrees in horse populations in varied ecological contexts in uncontracepted populations (see, e.g., Feist and McCullough, 1975; Berger, 1977, 1986; Nelson, 1978; Rubenstein, 1981; Stevens, 1990; Goodloe, 1991; Jensen, 2000).
Figure 4-2 shows a frequency distribution of the percentage of mares observed changing bands in population studies before or without contraception (Feist and McCullough, 1975; Nelson, 1978; Rubenstein, 1981; Berger, 1986; Rutberg, 1990; Stevens, 1990). Values range from 8 to 61 percent (mean, 27 percent; median, 25 percent). The study by Madosky et al. (2010) found that 70 percent of PZP-treated mares changed bands; that is significantly higher than the percentage of mares that change bands in uncontracepted populations (Wilcoxon signed-rank test, T = –18, p = 0.008, df = 7). The percentage of control mares changing bands (33.3 percent) did not differ from that of mares in uncontracepted herds (Wilcoxon signed-rank test, T = –6, p = 0.44, df = 7) (analysis provided by D. Rubenstein).
Whether Shackleford Banks is a unique case or not, additional study is needed to understand whether the absence of foaling as a result of contraception has an effect on band stability. Gray (2009) argued that sexual behavior and the ability to form consortships were adequate to maintain band stability in her study in Nevada. The studies on Shackleford Banks (Nuñez et al., 2009; Madosky et al., 2010) suggest that there is an interaction between pregnancy and social cohesion. The importance of harem stability to mare well-being is not clear, but considering the relatively large number of free-ranging mares that have been treated with liquid PZP in a variety of ecological settings, the likelihood of serious adverse effects seems low.
Side Effects: Demography and Population Processes. The easiest way to envision the effect of contraception on population processes is to examine its effect on demographic vital rates
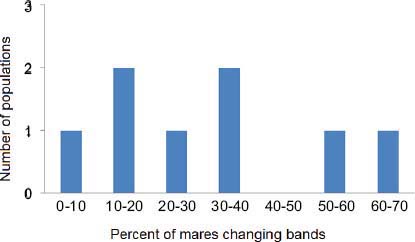
FIGURE 4-2 Percentage of band changes by mares as shown in a review of published literature. DATA SOURCE: Feist and McCullough (1975), Nelson (1978), Rubenstein (1981), Berger (1986), Rut-berg (1990), Stevens (1990).
(e.g., birth and death rates) contained in the equation that approximates the intrinsic rate of population increase (r). The demographic vital rates are related to r via the Lotka-Euler equation; a reasonable approximation is
![]()
where R0= ![]() lxmx is the net reproductive rate, and G =
lxmx is the net reproductive rate, and G = ![]() xlxmx is the generation time, which is proportional to age at first reproduction (α) (May and Rubenstein, 1985); lx and mx are age-specific survival and fecundity rates, respectively (Stearns, 1992; Gotelli, 2001). Intuitively, female fertility control effectively reduces r by reducing mx. The degree to which r is reduced depends on the effectiveness of the fertility-control method used, the proportion of females of a given age class that are treated, and the age classes that are targeted for treatment.
xlxmx is the generation time, which is proportional to age at first reproduction (α) (May and Rubenstein, 1985); lx and mx are age-specific survival and fecundity rates, respectively (Stearns, 1992; Gotelli, 2001). Intuitively, female fertility control effectively reduces r by reducing mx. The degree to which r is reduced depends on the effectiveness of the fertility-control method used, the proportion of females of a given age class that are treated, and the age classes that are targeted for treatment.
Female fertility control would also have indirect and unintended consequences, which may include changes in ages at first (α) and last reproduction (Ω), longevity, and the population’s age structure. If young females are targeted, fertility control can potentially increase the average α. Because treated females no longer have to sustain pregnancies or lactate, their energy needs will be reduced, their body condition will improve (e.g., Kirkpatrick and Turner, 2007), and they can potentially survive better, live longer, and possibly have a longer reproductive life span. Because r correlates negatively with α and positively with α (Oli and Dobson, 2003; Stahl and Oli, 2006), these can have contrasting effects. However, elasticity (or proportional sensitivity) patterns in age-structured populations suggest that the elasticity of population growth rate to changes in age-specific vital rates declines with age and that growth rate generally is more strongly affected by changes in α than in Ω (Caswell, 2001; Oli and Dobson, 2003; Stahl and Oli, 2006). Thus, targeting younger females for contraception would be the most effective strategy if the goal is to reduce r.
Evidence suggests that repeated application of PZP can lead to prolonged infertility (beyond the treatment period), so the effects on population growth may be more dramatic in later years and longer lasting than might have been planned at the start of fertility control. Fertility control via PZP may also increase longevity in females (Kirkpatrick and Turner, 2007), and this would have both direct and indirect ecological effects. Females that survive longer will increase the number of animals using the range, and this is likely to affect the setting of appropriate management levels (see Chapter 7). However, females that live longer may or may not contribute to r via reproduction. In addition, targeting younger age classes for repeated and prolonged fertility control would affect a population’s age structure and the likelihood of a given animal’s contribution to the gene pool (see Chapters 3 and 5). The impact of those consequences will depend on a population’s initial size and structure and should be accounted for when strategies for fertility control are developed.
Many of the behavioral changes associated with fertility control that are discussed in the preceding section are also likely to affect population dynamics. A longer breeding season could affect band stability and would probably extend male sexual activity into months when they normally recover strength and rebuild body condition. Such sexual activity in horses and other equids can involve males herding, pushing, and nudging females (and sometimes even forcing copulations [Berger, 1986]), which lower foraging success and freedom of movement (Rubenstein, 1986, 1994; Linklater et al., 1999; Cameron et al., 2009). Sexual harassment has been seen in many but not all equid populations. Where it
occurs, if levels of harassment remain high year round, both males and females could enter the breeding season in lower condition, and fertility could be compromised. Fecundity (mx) and survival (lx) of nontreated females could be further reduced, again limiting the population growth rate (r). Whether that cascade of events will occur in particular horse or burro populations will depend on the magnitude and interaction of three factors: environmental harshness in the nonbreeding season, social instability, and improvement of body condition in treated females due to absence of energetic demands of pregnancy and lactation. It is known from studies on Assateague Island that PZP-treated mares tend to have higher body-condition scores than females that reproduce regularly (Kirkpatrick and Turner, 2007). More recent results from Shackleford Banks show increased longevity in PZP-treated mares, probably because of their increased body condition and general health (Stuska, 2012). However, it is known that social disruption and harsh conditions during stressful periods can lower body condition (Pollock, 1980). What is not known is how those factors may interact when PZP use is extended to populations in harsher habitats or during periods of harsher climatic conditions, such as drought. It is something that will need to be monitored.
Gonadotropin-Releasing Hormone Vaccine
GnRH stimulates the pituitary gland to produce follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which then stimulate growth of follicles (which produce estrogen) and ovulation. GnRH vaccines prevent the action of GnRH so that in the absence of FSH and LH the failure of follicle growth and ovulation prevents reproduction (Figure 4-3). Two formulations of the most common GnRH vaccine, GonaCon™, have been reported in the literature. Specifically, the GnRH peptide has been conjugated to a keyhole limpet hemocyanin protein (KLH) or to blue mollusk protein (B). Both formulations appear to work well, but the B formulation may be more effective (Killian et al., 2008a; Miller et al., 2008) and is less expensive to produce than the KLH formulation (K. Fagerstone, NWRC, personal communication, April 18, 2012). GnRH vaccines not identified as GonaCon in the literature will be labeled as experimental vaccines because they are formulated in a variety of ways.
Studies of GonaCon as a contraceptive in horses are rare in the published literature; studies of GonaCon in deer are more numerous. Two additional GnRH vaccines are available in other parts of the world: Equity™ and Improvac® are produced by Pfizer Animal Health, Australia. Results of studies of efficacy, reversibility, and side effects of these vaccines are discussed in this section.
Delivery Route. GonaCon™ Equine, developed by NWRC and licensed by the U.S. Environmental Protection Agency (EPA) for use in horses, can be delivered by hand injection or by dart. An experimental version of GonaCon-KLH™ was delivered by dart to white-tailed deer in New York (Curtis et al., 2002).
Efficacy. Killian et al. (2008a) studied the efficacy of GonaCon-KLH in 16 penned horses (eight controls) in Nevada and found that efficacy over the 4 years of the study was 94 percent, 60 percent, 60 percent, and 40 percent, respectively. Gray et al. (2010) evaluated the efficacy of GonaCon-B™ in 24 free-ranging horses in Nevada and found efficacy of 61 percent, 58 percent, and 69 percent during each year of the 3-year study, respectively. As mentioned above, Gray et al. (2010) used a conservative method to estimate efficacy compared with most authors who have assessed contraceptive efficacy and suggested this as one
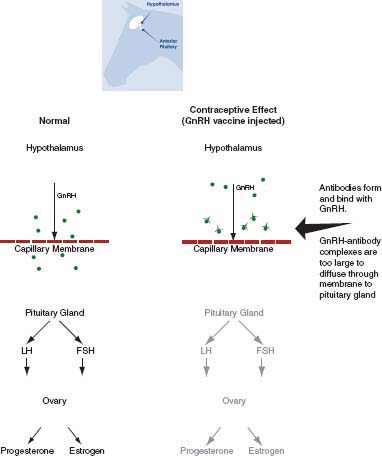
FIGURE 4-3 Mode of action of gonadotropin-releasing hormone (GnRH) vaccines.
NOTE: Without GnRH to stimulate follicle-stimulating hormone (FSH) and luteinizing hormone (LH), there is no production of ovarian estrogen or progesterone and no ovulation.
SOURCE: Adapted from Asa et al. (1996).
possible explanation for the discrepancy between their results and others’ results. A second explanation put forward by the authors was potential differences in body condition between the captive and free-ranging mares used in the two studies. Research suggests that animals that have more energy reserves or are in better body condition have stronger immune systems and thus are able to mount stronger responses to foreign antigens (Chandra, 1996; Demas et al., 2003; Houston et al., 2007). In both studies GonaCon was emulsified with the AdjuVac adjuvant.
Botha et al. (2008) studied Improvac in a large sample (n=55 treated) of mares kept in very large pastures in South Africa. Mares were vaccinated twice (day 0 and day 35) in the middle of the breeding season. By day 35, only 14.5 percent of treated mares showed
evidence of ovarian activity as assessed with ultrasonography; at day 70, no treated mare demonstrated ovarian activity. The authors indicated that the 14.5 percent of treated mares that had evidence of ovarian activity at day 35 received their first vaccination during the luteal phase and suggested that the timing of vaccination in the ovulatory cycle is important. Imboden et al. (2006) also evaluated Improvac in nine mares by vaccinating them twice, 4 weeks apart. Ovarian suppression occurred at 4 weeks and lasted a minimum of 23 weeks, but the authors found significant variability in duration and strength of suppression that did not correlate with antibody titers.
In a study of Equity in Australia, Elhay et al. (2007) vaccinated 24 domestic mares at day 0 and boosted them on day 28. All treated mares showed reduced ovarian activity; by 4 weeks after the booster, ovaries of treated mares resembled those of seasonally anovulatory mares.
The efficacy of GnRH vaccines has also been studied in other species. In an early study with an experimental version of GonaCon-KLH, Miller et al. (2000) reported an 88-percent reduction in fawning in eight white-tailed does. In a series of studies of white-tailed deer in Maryland (n=28, Gionfriddo et al., 2009) and New Jersey (n=32, Gionfriddo et al., 2011a), GonaCon-KLH emulsified with AdjuVac resulted in 67- to 88-percent contraceptive efficacy in year 1 and 43- to 47-percent efficacy in year 2. Those values were lower than the ones reported for captive deer. Miller et al. (2008) found 100-percent efficacy in years 1 and 2 and 80-percent efficacy in years 3-5 for five does treated with GonaCon-B compared with 100 percent in year 1, 60 percent in year 2, 50 percent in years 3 and 4, and 25 percent in year 5 for GonaCon-KLH given as a single injection to five does. A two-injection protocol of GonaCon-KLH was identical in efficacy to GonaCon-B in years 1-2. Gionfriddo et al. (2011a) suggested that their efficacies were lower because their wild deer were in poorer nutritional condition and living in overgrazed habitats. However, Perry et al. (2006) found only 60-percent efficacy over 3 years in 28 captive black-tailed deer, so species differences also seem possible. Curtis et al. (2002) reported an 87-percent efficacy in 32 white-tailed deer over 2 years using an experimental version of GonaCon-KLH administered as a two-shot series in year 1 and a booster at year 2. In years 3 and 4 of their study, efficacy declined to 71 percent and 43 percent, respectively, in the absence of a booster. Fawning rates were significantly lower than those of controls in years 1 and 2.
Killian et al. (2009) evaluated two doses (1,000 or 2,000 μg) of GonaCon-KLH in 22 captive female elk over a 3-year period. Low-dose efficacy was 92 percent, 90 percent, and 100 percent over the 3 years compared with high-dose efficacy of 90 percent, 100 percent, and 100 percent; these differences were not significantly different. Ten captive female Rocky Mountain elk treated with GonaCon-B had significantly reduced pregnancy rates for 3 years (90-percent reduction in year 1, 75-percent in year 2, and 50-percent in year 3) compared with controls (Powers et al., 2011).
Efficacy of GonaCon-KLH was 100 percent in six female bison for 1 year (Miller et al., 2004). In a short-term study (12-14 weeks) of six female wild boar, 100 percent of GonaCon-treated sows became infertile (Massei et al., 2008). In another short-term study (36 weeks) of feral swine treated with two different doses of GonaCon-KLH, Killian et al. (2006) found that none of the nine sows receiving the higher dose was pregnant at the end of the study and only 10 percent gave birth during the study. Of the 11 sows receiving the lower dose, 56 percent gave birth during the study and 11 percent were pregnant by the end of the study. The authors reported 80- to 90-percent efficacy in domestic pigs in previously published studies from their laboratory.
Reversibility. Elhay et al. (2007) found that in mares treated with Equity the duration of ovarian quiescence ranged from 4 to 23 weeks in 10 of 16 treated mares. The remaining six
mares did not return to cyclicity during the study (the duration was about 34 weeks for a sample of mares monitored over a longer term). Three mares with short-duration effects (4-8 weeks) were characterized by low antibody titers. The most frequent duration of contraceptive effects was 23 weeks.
Massei et al. (2008) cited their own unpublished data on GonaCon treatment in wild boar sows that suggest that the vaccine works for several years. Miller et al. (2000) stated that their experimental version of GonaCon-KLH appeared to be reversible in white-tailed does and that infertility appeared to last for 2 years without boosting.
Side Effects: Physical and Physiological. GonaCon-B–treated free-ranging mares showed no evidence of injection-site reactions to vaccination (Gray et al., 2010). Mares treated with Improvac demonstrated significantly reduced progesterone concentrations that were still at baseline at day 175; in addition, treated mares had reduced ovarian volume (Botha et al., 2008). Injection-site reactions were transient and disappeared by day 6. In the Imboden et al. (2006) study of Improvac, vaccination significantly affected the number, size, and types of ovarian follicles, corpora lutea, and progesterone concentrations but not estradiol. Most mares showed reactions to the injections, including swelling, pain, stiffiness, pyrexia, and apathy, but these signs disappeared within 5 days. The difference between these Improvac studies in occurrence and severity of injection-site reactions could be related to injections being given in the neck (Imboden et al., 2006) instead of the hip (Botha et al., 2008). Mares treated with Equity have demonstrated reduced progesterone concentrations, reduction in ovary and follicle size, and absence of corpora lutea (Elhay et al., 2007).
Kirkpatrick et al. (2011) expressed concerns about GnRH vaccines, pointing out that GnRH receptors are found in various body tissues and that GnRH can act as a neuro-transmitter. GnRH can affect olfaction in rodents, can depress activity of the cerebral cortex, and is associated with two genetic disorders of the cerebellum. However, many of the results mentioned are from studies that used GnRH agonists that result in supranormal concentrations of GnRH. GnRH vaccines block rather than enhance any effects of GnRH, so the effects of the two methods would be expected to be opposite in some or all tissues that have GnRH receptors (see section below “Gonadotropin-Releasing Hormone Agonists”).
Side Effects: Pregnancy, Birth Seasonality, and Survival. In probably the earliest study of a GnRH vaccine, Goodloe (1991) found no differences in birth seasonality between treated and untreated mares on Cumberland Island, a barrier island off the coast of Georgia. She did observe significantly higher mortality in foals born to treated mares in 1 year and a non-significant trend in the same direction in the second year, but other possible effects (such as age, body condition, dominance rank, and habitat quality) were not considered. Gray et al. (2010) found no effects of GonaCon-B on birth seasonality, foal survival, or foal sex ratio in free-ranging horses. In a review of contraceptive vaccines in wildlife, Kirkpatrick et al. (2011) stated that GnRH vaccines should be safe for pregnant horses because pregnancy is maintained by the placenta in this species, but they presented no data. However, pituitary LH, which depends on GnRH, is needed for pregnancy maintenance during about the first 6 weeks of pregnancy, after which equine chorionic gonadotropin (eCG) takes over this role.
In other species, Powers et al. (2011) found that GonaCon-B administered mid-gestation to captive female Rocky Mountain elk did not affect calving or calf survival. Miller et al. (2000) found that fawns born to white-tailed does treated with an experimental version of GonaCon-KLH were normal and healthy. They did find indications that some treated does were able to produce enough LH to conceive, but the progesterone produced by the corpus luteum was not adequate to carry pregnancy to term. In a study of an experimental
GonaCon-KLH, Curtis et al. (2002) found that fawning dates of treated white-tailed does were later than those of control does in the first 2 years of the study when efficacy was high but not significantly different when efficacy was lower (less than 71 percent). Female bison treated with GonaCon-KLH in the final months of pregnancy delivered healthy calves at calving dates comparable with those of controls (Miller et al., 2004); this suggests that it can be used safely in the last trimester of pregnancy in this species.
Side Effects: Genetic. Because a GnRH vaccine is an immunocontraceptive, its potential genetic side effects (that is, its selection against a stronger immune response) would be similar to those of PZP mentioned above.
Side Effects: Behavioral. Reviews of the effects of GnRH vaccines and independent studies have suggested that GnRH vaccines have a stronger suppressive effect on LH than on FSH, so sexual behavior may not be suppressed completely in females (Thompson, 2000; Stout and Colenbrander, 2004; Imboden et al., 2006; Powers et al., 2011). That is, continued production of FSH, and later of estradiol, may support estrous behavior but without ovulation, which requires LH. An additional or alternative explanation might be continued production of adrenal sex steroids in the absence of ovarian steroids; this has been shown to support estrous behavior in domestic horses during the nonbreeding season or after ovariectomy (Asa et al., 1980b). In Gray’s (2009) study of the effects of GonaCon on behavior of free-ranging horses in Nevada during both breeding and nonbreeding seasons, there were no treatment effects on activity budget, rates of sexual behavior, proximity between stallions and mares, attempts to initiate proximity, aggression given or received, or band-changing by mares. In white-tailed does previously treated with GonaCon, the recovery of estrous behavior in years 3, 4, and 5 after vaccination was suppressed when does received an additional vaccination with an anti–follicle-stimulating, hormone-releasing hormone (Killian et al., 2008b), a peptide similar in structure to GnRH.
Effects of GonaCon in Other Ungulate Species. Because GonaCon has not been tested extensively in equids, its effects in other ungulate species are reviewed in this section. Killian et al. (2008b) found that 10 white-tailed does treated with either formulation of GonaCon exhibited estrous behavior less frequently in the first 2 years after treatment, but in later years estrous behavior was displayed more often, even though does were still infertile; this suggests that estrous behavior may return before fertility is fully restored. Miller et al. (2000) found that eight does treated with an experimental version of GonaCon-KLH demonstrated the same number of estrous events, defined by bucks sniffing and chasing does, as control does during 30-44 days of observation during the rut. In their study of an experimental GonaCon-KLH, Curtis et al. (2002) found that treated does cycled later in the year during the second year of treatment than in the first year. Perry et al. (2006) found significantly reduced progesterone in female black-tailed deer treated with GonaCon-KLH. Gionfriddo et al. (2006) found no histopathological effects in a variety of tissues in 28 female white-tailed deer treated with GonaCon-KLH; 29 percent of treated does had injection-site reactions, but they were not discernible externally and were not considered serious. Gionfriddo et al. (2011a,b) found that ovaries and uteri of 32 GonaCon-KLH–treated white-tailed does were smaller than those of controls. Major organs, organ systems, and blood-chemistry parameters were normal in most treated deer (Gionfriddo et al., 2011b). When abnormalities were seen, they could not be clearly related to treatment, and treated does had higher body-condition scores than controls.
Captive female Rocky Mountain elk treated with GonaCon-B did not differ from controls in biochemistry or hematology parameters, and there was no effect on female precopulatory behavior (Powers et al., 2011). There was a nonsignificant trend for males to direct more precopulatory behavior toward treated does than at controls. Treated females did have more follicles than controls, but the follicles were smaller and fewer corpora lutea were present. The authors also commented that GonaCon-B used in conjunction with AdjuVac can cause a positive result on Johne’s disease antibody testing. Injection-site abscesses occurred in 35 percent of treated does, and some lasted for years, but most treated or sham-treated animals showed some level of reaction.
Adams and Adams (1990) vaccinated 30 heifers with GonaCon-KLH mixed with Freund’s complete adjuvant. All treated animals had significantly reduced progesterone, reduced uterine and ovarian tissue mass, and reduced GnRH receptor numbers. GonaCon-KLH–vaccinated female bison demonstrated suppressed progesterone (Miller et al., 2004).
Massei et al. (2008) found no effects of GonaCon on activity budgets, social rank, injection-site reactions, or hematology and biochemistry parameters in a 14-week study of wild boar sows. Treated sows gained more weight, but the gain was considered modest. In a short-term study (36 weeks) of feral swine treated with two different doses of GonaCon-KLH, Killian et al. (2006) found that treated sows had significantly reduced progesterone and numbers of corpora lutea, although females in both treatment groups showed some evidence of follicular activity. There was also evidence of regression of the uterine epithelium.
In studies of GonaCon, injection-site reactions were likely in most species, even if they were not externally visible, but these reactions appeared to be minor and relatively short-lived in most cases. Miller et al. (2008) explained that the water-in-oil emulsion that is often mixed with GonaCon is necessary to induce a long-term immune response, and it is generally accepted that some local reactions (cysts, granulomas, or sterile abscesses) at the injection site are common.
Gonadotropin-Releasing Hormone Agonists
As described above, GnRH, which is produced in the hypothalamus, initiates the cascade of reproductive hormones by causing pituitary release of FSH, which enhances follicle growth, and LH, which triggers ovulation. GnRH agonists (synthetic versions of GnRH that have activity similar to the natural hormone) are commonly used in many domestic species to stimulate follicle growth, estrus, and ovulation. Ovuplant® (deslorelin in a short-acting implant; Peptech Animal Health, Australia, now part of Virbac, France) was developed specifically to induce ovulation in domestic mares. Another GnRH agonist product, Suprelorin® (deslorelin in a slow-release implant matrix; Peptech Animal Health), was developed for use in domestic dogs and is now widely used for contraception in a broad array of captive wildlife species, including female ungulates. GnRH agonists can act as reversible contraceptives when treatment is extended for more than a few days. After the initial stimulation phase, continued administration results in down-regulation of the pituitary cells that synthesize FSH and LH. Without FSH and LH support, the ovaries become quiescent; this condition is sometimes referred to as reversible chemical ovariectomy.
Delivery Route and Efficacy
Suprelorin implants, similar in size to animal ID microchips, are inserted with a trocar, which requires brief restraint but not anesthesia. Two formulations that are active for a minimum of 6 or 12 months are available, but experience has shown that the duration of
contraception is longer in most animals—an average of 12 and 18 months, respectively.7 At an adequate dose, GnRH agonists are effective in females of virtually all mammal species, but they have not been tested specifically as contraceptives in horses, burros, or wild equids. Short-term treatment to control ovulation and to investigate their action on pituitary function indicates that GnRH agonists could be effective in suppressing reproduction in mares (Montovan et al., 1990; Fitzgerald et al., 1993). For example, even the short-acting product Ovuplant, designed merely to stimulate but not down-regulate reproduction in mares, has delayed return to cycling in some animals (Johnson et al., 2002). That observation suggests that continued treatment with a long-acting, slow-release implant, such as Suprelorin, would be effective for fertility control, even though the mare appears to be more resistant to pituitary desensitization than other species (Porter and Sharp, 2002).
Reversibility
GnRH agonists are considered generally reversible, primarily on the basis of studies of domestic dogs (Junaidi et al., 2003; Ludwig et al., 2009), cats (Toydemir et al., 2012), and humans (Plosker and Brogden, 1994). However, the duration of effect is greater in some individual animals, and this confounded documentation of reversal before data collection stopped in a study of domestic cats (Munson et al., 2001). In addition, long-term treatment is associated with a longer time to recovery (Nejat et al., 2000). Other studies have reported what may be permanent effects, for example, during treatment of prostate cancer in men (Murthy et al., 2007).
Side Effects
GnRH agonists have not been used often during pregnancy, so potential effects have not been systematically investigated. Possible effects can be predicted by examining another role of LH: maintenance of corpora lutea (CL) that produce the progesterone required for pregnancy to become established. However, around day 40, increasing concentrations of eCG produced by specialized cells in the uterine endometrium assume the role of stimulating CL progesterone production. Later, the feto-placental unit takes over progesterone synthesis from the CL for the remainder of gestation. Because LH is needed for support of progesterone secretion only during very early pregnancy, treatment with a GnRH agonist after that time would be unlikely to cause abortion.
Data from captive wild canids (African wild dogs and Mexican wolves) treated with Suprelorin during pregnancy revealed an unexpected consequence of GnRH agonist treatment. Females given Suprelorin implants in early pregnancy gave birth but did not produce sufficient milk to feed their pups; this indicates that some aspect of mammary development and milk production was affected.8 However, initiation of treatment during lactation after milk production has been established appears to have no effect.
Effects of GnRH agonists on behavior, after the initial stimulation phase when estrous behavior might result, should be similar to those associated with ovariectomy. That is, estrous cycles would be absent, but sporadic expression of estrus supported by adrenal sex steroids might occur.
____________
7 Database managed by the Association of Zoos and Aquariums Wildlife Contraception Center (St. Louis, MO). Accessed July 20, 2012.
8 Association of Zoos and Aquariums Wildlife Contraception Center database. Accessed July 20, 2012.
Repeated administration of various formulations of GnRH agonists (e.g., deslorelin acetate) for the induction and enhancement of ovulation and for the initiation of cyclicity in the transitional and anestrous phases of the estrous cycle in domestic mares is a standard and routine procedure used on broodmare farms worldwide (Squires, 2011). No adverse effects of repeated administration of these GnRH agonists have been reported in the literature over the last 2 decades since its acceptance, and they continue to be used in the manipulation of the estrous cycle in domestic mares (I.K.M. Liu, University of California, Davis, personal communication, August 2012). Because of the possibility of species differences in response, the relevance to free-ranging wildlife is unclear and deserves further study.
Steroid Hormone Treatments
Progesterone and estrogen are the hormones that change with estrous cycles and support pregnancy in mammals. However, administration of natural or synthetic forms can prevent pregnancy, usually by negative feedback on the reproductive hormone axis.
Natural and Synthetic Progestagens
In the luteal or diestrous phase of the ovarian cycle and during pregnancy, high levels of progesterone suppress the final stages of follicle growth and ovulation. Thus, synthetic progestagens are attractive candidates for contraception and in fact are widely used for that purpose in women (e.g., Implanon® implants, etonorgestrel; Depo-Provera®, medroxy-progesterone acetate in a depot vehicle for injection) and in captive wild animals (MGA implants, melengestrol acetate, Wildlife Pharmaceuticals).
Delivery Route, Efficacy, and Reversibility. Progesterone or its synthetic equivalents can be administered as implants or as injections that might be delivered remotely by dart. With a sufficient dose, the efficacy rate approaches 100 percent. Silastic implants containing a progestagen can be effective for 2 years or more9 and generally have a high reversal rate. The likelihood that a female will reproduce after such treatment is subject to other factors that affect fertility, such as age, health, and parity before treatment. Reversal can be hastened by removing the implant.
The vast number of studies on the treatment of mares with progesterone or synthetic progestagens have been for short-term control and timing of ovulation, not for contraception (e.g., Pinto, 2011). However, results of this body of work have shown that only one synthetic progestagen, altrenogest, is consistently effective in suppressing reproductive function in mares. Two others have been effective at very high concentrations in only some studies (Storer et al., 2009; Pinto, 2011). Those results are attributed to the specificity of the progesterone receptor in mares (Nobelius, 1992). At the time this report was prepared, the only progestagen product approved for use in domestic mares was altrenogest (Regu-Mate®). The only studies of progestagen contraception in mares used native progesterone in silastic implants to treat feral mares in holding pens in Nevada. Those placed subcutaneously in the neck area were lost, became infected, or both and so were not effective for limiting reproduction (Plotka et al., 1988). In a later study of the same population of captive feral mares, insertion into the peritoneal cavity prevented loss, and no evidence of inflection was reported (Plotka et al., 1992). However, the doses of progesterone used (implants contained either 8 or
____________
9 Wildlife Pharmaceuticals, Association of Zoos and Aquariums Wildlife Contraception Center database. Accessed July 20, 2012.
24 g of progesterone) suppressed signs of estrous behavior but did not prevent ovulation and conception. That work was suspended also because of the invasive nature of the surgery and the unacceptable stress placed on mares (BLM, 2003, revised 2005). It is possible that treatment with altrenogest would be more successful than progesterone because synthetic steroid hormones typically have substantially higher bioactivity and affinity for the receptor and a lower metabolic clearance rate. The consequence is that smaller doses are needed for increased binding and efficacy. However, at the time of the committee’s study, there was no altrenogest product that was active for more than 30 days.
Side Effects. Progesterone and synthetic progestagens support pregnancy but interfere with parturition by suppressing contractility of uterine smooth muscle. At doses high enough to be contraceptive, progestagens can block parturition, as documented, for example, in white-tailed deer (Plotka and Seal, 1989). Altrenogest is often used to maintain pregnancy and delay parturition in horses, but a study by Neuhauser et al. (2008) found that it did not prevent parturition, raising the question of its efficacy for maintaining pregnancy. However, there were some differences in health and survival of foals born to altrenogest-treated mares in that study. Although progesterone (as the “progestational” hormone) supports gestation, synthetic progestagens often have affinity for other steroid hormone receptors as well. For example, binding to androgen receptors might masculinize female fetuses, depending on the dose and stage of fetal development. However, fillies born to mares treated with the synthetic progestagen altrenogest during pregnancy (but not around the time of expected parturition) showed normal reproductive development, hormone production, and fertility (Naden et al., 1990). Progestagen treatment during lactation would not be expected to have a deleterious effect on milk production and in fact might enhance it. There are no data specifically on horses, but progestagens are a preferred method of contraception in women (Tankeyoon et al., 1984) and are not contraindicated in other species.
Side effects of progestagens vary taxonomically. Progestagen treatment of carnivores is associated with life-threatening mammary and uterine pathological conditions, whereas several uterine pathological conditions in primates (including women) are reversed by treatment with progestagens. Information on long-term administration of progestagens in equids is lacking, but extrapolation of results in other ungulates suggests that hydrometra (fluid accumulation in the uterus) might be expected.
Natural and Synthetic Estrogens
Estrogen is instrumental in the sexual characteristics of mammals and in the regulation of the menstrual cycle. Estrogen treatment can reduce concentrations of FSH and LH in the bloodstream and thus decrease the development of viable eggs.
Delivery Route, Efficacy, and Reversibility. Both natural estradiol (a specific estrogen) and synthetic ethinyl estradiol, incorporated into silastic implants, have been tested as contraceptives in captive and free-ranging feral horses (Plotka et al., 1988, 1992; Eagle et al., 1992). In the trial with 8-g estradiol implants placed in the neck of 30 feral mares in holding pens (Plotka et al., 1988), loss of many implants compromised results, but most of the mares that retained the implants mated and conceived, probably because the dose was insufficient. In a subsequent trial at the same facility, 1.5-g, 3-g, and 8-g ethinyl estradiol implants were placed intraperitoneally to prevent loss in three groups of 8-10 mares each. Contraceptive efficacy of those implants was 75 percent, 75 percent, and 100 percent, respectively (Plotka et al., 1992). Extrapolation from assays of ethinyl estradiol from blood samples up
through 21 or 30 months suggested contraceptive efficacy from 16 months (1.5-g implants) to 60 months (8-g implants). Efficacy was judged by the number of mares ovulating or pregnant according to cyclic or sustained increases in progesterone, respectively. On the basis of data on duration of efficacy, it appears that all treated mares returned to cycling, and this suggests reversibility. However, follow-up did not extend to production of young. Behavioral data were not collected, and no deleterious effects were reported.
Side Effects. Estrogens are more effective in suppressing follicle growth than progestagens, but at contraceptive doses they have been associated with serious side effects. A general action of estrogen is to stimulate cell proliferation, but it also can be mutagenic (Liehr, 2001). At the high doses required to achieve contraception, the result can be abnormal growth (hyperplasia) and even cancer (neoplasia) of organs that have estrogen receptors, such as the uterine endometrium, mammary glands, pituitary, and liver (Gass et al., 1964; Santen, 1998). In mares, estrogen is associated with uterine edema (Pelahach et al., 2002). Therefore, unopposed estrogen treatment is not prescribed; instead, estrogen is typically combined with a progestagen, which tempers its effect on most target tissues. Almost all formulations of human birth-control pills contain synthetic estrogen plus progestagen; one contains only progestagen.
Treatment of mares with estrogen stimulates estrous behavior (Asa et al., 1984), but male-like behavior has been observed with continued treatment (Nishikawa, 1959), suggesting a shift in steroid metabolism to favor conversion to an androgen. Such male-type behavior was observed (C. Asa, unpublished) in free-ranging mares in Nevada treated with ethinyl estradiol (study by Eagle et al., 1992). However, no systematic observations were conducted on expression of social or sexual behavior in the studies by Plotka, Eagle, and colleagues (Plotka et al., 1988, 1992; Eagle et al., 1992).
Combination Estrogen Plus Progestagen
As mentioned above, all formulations of human birth-control pills except one contain synthetic estrogen plus progestagen. The major contraceptive action of estrogen is to inhibit follicle growth, whereas progestagen prevents ovulation, so the combination is more effective than progestagen-only contraceptive formulations (because of the associated pathological changes, there are no commercially available estrogen-only contraceptives). The addition of a progestagen allows the use of a lower estrogen dose and reduces the probability of side effects. In addition, progestagen counters some estrogen effects, such as inhibition of estrous behavior. In general, the hormonal effect of the combination is most analogous to pregnancy.
A combination of natural progesterone and ethinyl estradiol in silastic implants was tested in captive and free-ranging mares (Plotka et al., 1992; Eagle et al., 1992) and found to be effective in preventing pregnancy or foaling, respectively. Efficacy was 100 percent in captive mares and 84-90 percent in free-ranging mares; the discrepancy was attributed to the less exact methods of assigning foals to mares in the helicopter surveys of the free-ranging herds. The combination implants, inserted intraperitoneally, were effective for 2 or 3 years. As mentioned above in connection with estrogen alone, it appears that all treated mares returned to cycling, but follow-up did not extend to production of young. Although there are no other published reports on estrogen plus progesterone treatment of equids or other ungulates, results of studies of nonhuman primates indicate a high rate of reversal (Porton and DeMatteo, 2005). No behavioral data were collected, so effects on behavior or social organization are not available.
Intrauterine Devices
Intrauterine devices were first used in domestic animals (such as camels) perhaps thousands of years ago. IUDs were a nonhormonal alternative for women in the 1960s and early 1970s that fell out of favor in the late 1970s, mostly because of problems with the Dalkon Shield (Sivin, 1993). Later analyses of IUD use in women have shown the method to be both highly effective and safe (Chi, 1993; Sivin, 1993; Rivera and Best, 2002). The precise mechanism of action of IUDs is not well described but is thought to be low-grade inflammation of the uterine endometrium provoked by the presence of the foreign object. Thus, IUDs may more appropriately be considered antigestational devices in that endometrial inflammation is not conducive to embryo implantation. Although there have been few studies of IUD use in nonhuman animals, some species may be well suited to this method.
Delivery Route, Efficacy, and Reversibility
Two studies have evaluated IUDs in domestic and captive feral horses. The first (Daels and Hughes, 1995) used a flexible, silastic O-ring, fabricated specially for the study, in six domestic mares; when compressed, it could be easily inserted into the cervix and later removed in the same way. During the breeding season after the IUDs were in place, none of the mares conceived, but all conceived after IUD removal during the next 2 years. Uterine health was monitored with palpation, ultrasonography, and vaginoscopy when samples were taken for uterine cytology and culture. Cytology and culture results were consistent with inflammation, which reversed within a week of IUD removal. It was concluded that the inflammatory response was sufficient to interfere with fertility. Mares that had IUDs in place continued to exhibit estrous cycles with the same frequency as control mares.
The second study (Killian et al., 2004), of 15 feral mares in a holding facility, used a commercially available copper-containing IUD, which is considered more effective because of the spermicidal action of copper ions (O’Brien et al., 2008). In a pilot study, the authors tested three types of copper-containing products on four mares and selected the copper T for the larger study of 15 mares. After 60 days with a stallion, 20 percent of the IUD-treated mares were pregnant compared with 75 percent of the control mares. After the second and third years, 71 and 86 percent were pregnant, respectively (Killian et al., 2006). The authors believed the pregnancies of the IUD-treated mares were due to loss of the relatively small IUDs, not to failure of efficacy, because no IUDs were found on ultrasound examination of the pregnant treated mares.
MALE-DIRECTED METHODS OF FERTILITY CONTROL
Potential methods of fertility control directed at male equids include castration, vasectomy (chemical or surgical), and immunocontraceptives. The mode of action and effects of each method are reviewed below.
Surgical or Chemical Sterilization
Sterilization of male equids can be accomplished through removal of the testes, permanent disruption of spermatogenesis, or blockage of the vas deferens to prevent the passage of sperm.
Castration
Castration, also referred to as gelding in equids, eliminates the organs that produce sperm, thereby making the male infertile. Surgical castration has been common husbandry practice for domestic equids for over 2,000 years.
Delivery, Efficacy, and Reversibility. Castration (gelding) is a routine operation for domestic male horses and is much less invasive or risky than the comparable surgery in mares. However, complications can occur at a rate of about 10 percent, including hemorrhage from the spermatic artery if not properly crushed; inadequate postoperative drainage that results in swelling, inflection, or hydrocele (fluid accumulation); or even evisceration in rare cases (Blodgett, 2011). Surgical castration is, of course, permanent and is 100-percent effective in eliminating the source of sperm.
An agent for chemical castration (formerly Neutersol®, now Esterilsol™, Ark Sciences, New York City) developed for and extensively tested in domestic dogs might also be effective in stallions. A solution of zinc gluconate with l-arginine is injected into each testicle, where it causes permanent disruption of the seminiferous tubules, where spermatogenesis occurs. However, given the much larger volume of stallion testes, the technique might need modification and would require testing under controlled conditions before application in the field could be considered.
Efficacy of Esterilsol is not well established, even in dogs, in that the product is relatively new. Available data indicate that efficacy depends primarily on proper injection of the solution so that it is distributed adequately throughout the testis. It is claimed to be virtually painless (ACC&D, 2012).
Side Effects. Because castration removes the primary source of androgen production, male-type aggressive and sexual behaviors are usually reduced. Adrenal androgens (such as dehydroepiandrosterone) are still produced, but they are weaker and have much less effect on behavior than testosterone. Some geldings show less alteration in behavior after castration, potentially because of the adrenal androgen action but more probably because of individual differences in temperament, prior experience, or both and because of development of behavior patterns that are slow to disappear. Males that do not retain sufficient sex drive and aggressive competitiveness to acquire and maintain a harem could be outcompeted or supplanted by intact, fertile males.
The effects of chemical castration on testosterone production are not clear. The mechanism of action (spermicidal action of zinc gluconate) is supposed to spare the Leydig cells, which produce testosterone. However, the generalized scarring that occurs, and that is necessary for the permanent changes in testicular architecture to prevent further sperm production or release, could also affect Leydig cell structure and compromise hormone synthesis and release. The extent of the effect on testosterone production would determine the possible effects on male-type behavior.
Individual males vary in their behavioral response to castration—for example, in the loss of male-type behavior, such as aggression and sexual interest, depending on the age and sexual experience of the male. However, some or total loss of sex drive would be likely in castrated stallions, and this is counter to the often-stated public interest in maintaining natural behaviors in free-ranging horses. The effect that gelding a portion of the males in a herd would have on reproduction and behavior could not be predicted at the time this report was prepared. Aside from variability in how much male-type behavior is lost in gelded animals, the effects of gelding on reproduction and behavior in the population will also
depend on the roles that the males selected for gelding (whether harem males or bachelors) hold in the population, their reproductive and social history, and possibly their age. Keeping a portion of the male population nonreproducing by gelding could increase aggression and competition in herds or decrease it. Similarly, reproductive success may be reduced or increased. With respect to effects at the population level, it is not clear how castration of males would be better than vasectomy, which does not affect testosterone or male-type behaviors. Ultimately, the growth rate of any population that includes reproductive horses of both sexes will be commensurate with the number of fertile females in the population.
Vasectomy
Vasectomy, whether surgical or chemical, does not affect the production of sperm but does prevent ejaculation of sperm by blocking the epididymis (where sperm leave the testis) or the vas deferens (the duct that carries sperm to the urethra for ejaculation).
Delivery and Efficacy. A potential disadvantage of both surgical and chemical castration is loss of testosterone and consequent reduction in or complete loss of male-type behaviors necessary for maintenance of social organization, band integrity, and expression of a natural behavior repertoire. Vasectomy blocks passage of sperm without affecting testosterone synthesis or secretion, sparing androgen-supported natural behaviors. The most widely used vasectomy method is surgical, although there are several variations that are meant to increase efficacy, reduce production of sperm granulomas, or facilitate microsurgical vasectomy reversal (Esho and Cass, 1978; Frenette et al., 1986; Silber, 1989; Moss, 1992). After either chemical or surgical vasectomy, the average delay to passage of all remaining sperm from the vas deferens is about 6 weeks, so treatment should occur well in advance of the mares’ breeding season to ensure infertility.
Surgical vasectomy in dominant stallions has been used successfully to control fertility in bands of free-ranging horses (Eagle et al., 1993; Asa, 1999). The vasectomy procedure was 100-percent effective in preventing foal production in stable bands that had no subordinate stallions, but some of the bands that had intact subordinate stallions contained foals. The stability of bands did not differ between treated and untreated groups. However, limiting treatment to dominant stallions leaves subordinate band stallions and bachelors fertile and thus reduces overall efficacy. In particular, bands that had subordinate stallions were vulnerable (Asa, 1999). The probability that subordinate stallions will mate is higher in bands that have a vasectomized dominant stallion because the females continue to have estrous cycles throughout the entire breeding season, whereas females with intact, fertile stallions are likely to conceive in the first month or so of the breeding season. Thus, females with vasectomized dominant stallions present many more opportunities for mating with a subordinate. For population control, a more effective approach would be to vasectomize a larger proportion of males, regardless of age or social status. The target number or proportion of males treated could be adjusted to achieve the level of population control recommended for each HMA.
Chemical vasectomy is a simpler, less invasive alternative to a surgical approach, but both require anesthesia. Several chemical agents have been assessed in domestic dogs and cats (Pineda et al., 1977; Pineda and Dooley, 1984). There are no published reports on chemical vasectomy in horses, but the procedure should not be difficult to adapt.
Reversibility. Both surgical vasectomy and chemical vasectomy should be considered permanent if properly done. Vasectomy reversal has been successful in humans in some cases
(Silber, 1989), but it requires microsurgery by a highly skilled surgeon, so it would not be practical for field application. Spontaneous reversal has been reported after some surgical approaches—resulting from recanalization of the vas deferens (Esho and Cass, 1978)—so the choice of technique is critically important.
Side Effects. There are no reported side effects of vasectomy, a procedure that is considered safe and effective even in humans, in whom it has become commonplace. However, in free-ranging horse herds that have vasectomized males, females that do not conceive continue to undergo estrous cycles until the end of the breeding season and continue to attract and mate with males (Asa, 1999). Thus, the number of months that males compete for and defend females is increased, and this increases the risk of injury to males and diverts time from foraging that, in some environments, could compromise a male’s body condition going into winter. Those problems did not occur in the single study of vasectomy for fertility control (Asa, 1999) but might be more likely under some conditions for some males.
Winter survival of males that do lose condition may be reduced. That is likely to have a number of consequences for a population’s dynamics. A lost stallion would probably be replaced quickly by a bachelor male or the mares would be taken in by dominant stallions of other bands. However, the stability of the harems taken over by younger, less experienced males would be more likely to decline (Rubenstein, 1994), and this could reduce female fecundity via increased levels of male harassment. Turnover might enhance the genetic diversity of populations, in that more males would be contributing to the gene pool and thus enhancing effective population size.10
Steroid Hormone Treatments
High doses of androgen can suppress endogenous production of testosterone via negative feedback and have a suppressive effect on spermatogenesis. Turner and Kirkpatrick (1982) treated 10 free-ranging stallions with microencapsulated testosterone propionate. Only 28.4 percent of bands that had treated stallions had foals compared with 87.5 percent of the untreated bands. Although increased concentrations of androgen could be expected to cause increased aggression, it was not reported. However, only territorial marking and sexual behaviors were analyzed. All stallions showed evidence of reversal in about 8 months. No side effects were noted.
GnRH Vaccines
As described in the section on the use of GnRH vaccines in females, treatment with GnRH vaccines interferes with the production of LH and FSH from the pituitary; in males, that results in failure of stimulation of testosterone, which is necessary for stimulation of spermatogenesis and expression of sexual behavior. However, the use of GonaCon or other experimental GnRH vaccines has not completely eliminated sperm production (Malmgren et al., 2001; Turkstra et al., 2005). Stout and Colenbrander (2004) reported that mature stallions treated with GnRH vaccines continued to produce sufficient semen to impregnate a mare.
____________
10 Genetic diversity and effective population size are discussed further in Chapter 5.
Delivery Route, Efficacy, and Physical Side Effects
In possibly the first study of GnRH immunization in domestic stallions, Malmgren et al. (2001) evaluated an experimental GnRH vaccine used with the adjuvant Equimune® in four domestic stallions (one control, three treated) during the nonbreeding season. The vaccination protocol involved five shots at intervals of 2-4 weeks. All stallions showed a response, but one male had a significantly lower antibody response than the other two. Two of the treated stallions demonstrated decreases in testosterone and more pronounced decreases in testis size and semen quality as well as changes in testicular histology, but these effects did not appear until 7-9 weeks after initial vaccination. There was no clear change in ejaculate volume.
Turkstra et al. (2005) evaluated two different adjuvants (Carbopol® and CoVaccine™ HT) with an experimental GnRH vaccine in previously hemicastrated stallions. Four animals were treated with Carbopol, and four animals were treated with CoVaccine HT. Stallions were treated during the breeding season with an initial vaccination, boosted at 6 weeks, and monitored for a total of 14 weeks after the initial vaccination. There were no injection-site reactions and no changes in body weight. The CoVaccine HT treatment was superior; treated stallions had undetectable testosterone from 2 weeks after the booster until the end of the study. Those stallions also had reduced sperm motility, but there were no adjuvant-related differences in semen volume, sperm concentration, or sperm count. Both adjuvants appeared to reduce testis size and alter testis histology in ways that would reduce fertility. The authors suggested that, aside from superior performance, CoVaccine HT is also desirable because time to effect was better defined.
Janett et al. (2009) evaluated the effects of Equity, given to five domestic stallions as three injections at intervals of 4-8 weeks, on testosterone concentrations, sexual behavior, and semen characteristics. Two stallions exhibited minor injection-site reactions that resolved in 2-3 days. Adverse effects on sperm quality were observed in four stallions, although there was individual variation in the strength and type of effect (lower sperm numbers, lower motility, and increased sperm defects), and one stallion had a weak immune response. Overall, those inhibitory effects lasted from 24 weeks to under 46 weeks.
Although not tested in stallions, GonaCon-KLH has been evaluated in a number of studies of male deer. Typical results include reduced testosterone concentrations and testis size (Killian et al., 2005; Miller et al., 2000, but see Gionfriddo et al., 2011a). Killian et al. (2005) found inactive Leydig cells and regressed seminiferous tubules that did not contain mature sperm in eight treated bucks. Gionfriddo et al. (2011b) found that 10 GonaCon-KLH–treated bucks had higher body-condition scores than untreated bucks.
One interesting finding in the Killian et al. (2005) study was that there was a high prevalence of pulmonary disease, the leading cause of mortality, in bucks in their Pennsylvania study site. The incidence of the disease was higher in treated bucks, but the authors reported that the microorganisms that cause the disease are endemic in captive deer herds in Pennsylvania. They speculated that vaccination with GonaCon could have lowered resistance to the disease.
Reversibility
In four stallions treated with Equity, testosterone remained suppressed for 24, 36, 45, and 46 weeks (excluding one low-responding stallion) (Janett et al., 2009). In a study of eight deer bucks that received different treatment protocols, Killian et al. (2005) reported that suppressive effects of GonaCon-KLH on male reproductive physiology appear to last
for 3 years, with testicular function beginning to recover in year 4; however, the authors suggested that a low level of sperm production might have persisted.
Behavioral Side Effects
Malmgren et al. (2001) found that four stallions vaccinated with an experimental GnRH vaccine first began to demonstrate reduced sexual interest and behavior 4 weeks after the initial vaccination, and the reduction appeared to persist for about 13 weeks. Libido was reduced in four stallions treated with Equity, including one that did not respond with high vaccine titers. The fifth stallion had a strong immune response and significantly reduced testosterone concentrations but maintained very strong, sustained sexual behavior (Janett et al., 2009). Kirkpatrick et al. (2011) expressed concern about the application of GnRH vaccines in stallions because testosterone-supported behaviors, which are necessary for keeping bands together, are suppressed; however, no data or citations are provided for this claim. It appears from the available data that sexual behaviors may be suppressed to various degrees by individual animal, but the effect of the suppression on other behaviors has not been assessed.
In other species, Killian et al. (2005) reported that eight GonaCon-KLH–treated white-tailed bucks had reduced libido and interest in estrous does; bucks might mount does but not completely. Miller et al. (2000) found similar effects with an experimental version of GonaCon-KLH in four white-tailed bucks and remarked that the rutting season was not extended in treated bucks. The inability of GnRH vaccines to suppress FSH completely, although central to maintenance of sexual behavior in treated females, is not likely to affect males. The possible effects on male behavior are probably limited to suppression of LH, inasmuch as LH alone is needed to support testosterone production. Thus, an adequate vaccine dose that suppressed LH should be accompanied by elimination of testosterone, a situation similar to castration. Whether male-type behavior would continue without testosterone support depends on the temperament and prior experience of the male.
Gonadotropin-Releasing Hormone Agonists
As discussed in the section on their use in females, GnRH agonists first stimulate then suppress production of pituitary and gonadal hormones involved in reproductive function. The pituitary hormones, LH and FSH, are the same as in females, but in males the gonadal hormone affected is testosterone; without testosterone, spermatogenesis is not supported. The outcome can be likened to a potentially reversible chemical castration (Junaidi et al., 2009). Although effective in males of some species, GnRH agonist treatment has had mixed results in male ungulates. In domestic stallions given various GnRH agonist formulations, some studies reported transient stimulation followed by return to baseline or lower concentrations of LH and testosterone (Montovan et al., 1990; Boyle et al., 1991), whereas others showed enhanced LH secretion or sexual behavior (Roser and Hughes, 1991; Sieme et al., 2004). No suppressive effects of what were considered high doses were detected by Brinsko et al. (1998); this led them to conclude that stallions are remarkably resistant to reproductive suppression by GnRH agonist treatment. Nevertheless, the ability of some agonists at some doses to achieve even slight suppression suggests that more potent analogues or higher doses might be effective. Newer, more potent agonists have not yet been tested adequately in stallions.
Delivery Route and Duration of Efficacy
Recent formulations, such as Suprelorin, in slow-release implants are more practical for contraceptive treatment than osmotic pumps or injections. As described in the section “Female-Directed Methods of Fertility Control,” Suprelorin is produced in 6-month and 12-month formulations. Those durations of efficacy represent minimums, and suppression continues for about twice as long in most species.
Reversibility
Suprelorin reversal rates have not been established for equids, but in male dogs the rate nears 100 percent. However, the rate has been lower in some other species,11 so caution is recommended in treating a species for the first time.
Side Effects
The side effects of GnRH agonists are similar to those of castration, inasmuch as the treatment can be considered chemical gonadectomy. Because inhibition of spermatogenesis requires suppression of testosterone, any testosterone-supported secondary sex characteristics and behavior would be affected. However, as explained in the section on side effects of surgical castration, males with prior sexual experience may continue to show interest in estrous females but would probably not be able to compete successfully with untreated, intact males.
ADDITIONAL FACTORS IN EVALUATING METHODS OF FERTILITY CONTROL
The sections above included the information most relevant to understanding and choosing a method for fertility control: delivery route, efficacy, duration of effect, and possible side effects. There are, however, some additional effects that should be considered in evaluating the methods. For example, data on the effects of some contraceptive approaches on general health and longevity are accumulating. The energetic costs of pregnancy and lactation are high, and this burden is much greater on free-ranging females that must subsist on lower-quality forage than on domestic animals that have calorie- and nutrient-rich diets. Mares on Assateague Island treated with PZP that did not regularly produce foals were in better body condition and lived longer than females that were not contracepted and continued to reproduce (Turner and Kirkpatrick, 2002).
Several methods (such as vasectomy, PZP vaccines, and GnRH vaccines) are likely to be associated with a prolonged breeding season. That is, mares that are not pregnant continue to undergo estrous cycles until late summer or fall, when day length is decreasing and no longer stimulates cycling (Sharp and Ginther, 1975). Although nonpregnant females that continue to cycle expend time and energy in courtship and mating, the expenditure is considerably lower than the energetic demands of pregnancy and lactation. Thus, any effect on health and well-being of females should be negligible. In contrast, the burden on males could be greater in that the length of the breeding season, and thus the time in which males compete for and defend estrous females, is prolonged. Time spent in defending and courting females also diverts males from grazing, and this could affect health and
____________
11 Association of Zoos and Aquariums Wildlife Contraception Center database. Accessed July 20, 2012.
body condition under some conditions. However, no study has focused specifically on that issue, and it warrants further investigation.
Early studies of fertility control focused on steroid hormone treatments, mirroring approaches to contraception in humans (such as birth-control pills that contain synthetic estrogen and progestagen). However, serious concerns arose regarding the tissue accumulation of synthetic steroids (testosterone in males, estrogen and progestagen in females) because they become concentrated in fat and muscle (Lauderdale et al., 1977; Hageleit et al., 2000). The potential for those compounds to enter the food chain argues against their use in free-ranging wildlife.
IDENTIFYING THE MOST PROMISING FERTILITY-CONTROL METHODS
The fertility-control methods discussed in this chapter vary considerably. The criteria most important in selecting promising fertility-control methods for free-ranging horses and burros are delivery method, availability, efficacy, duration of effect, and potential physiological and behavioral side effects. The relative importance of those criteria will probably vary with characteristics of the site (the HMA or HMA complex) and population characteristics of the equids at the site. The importance of a given criterion may also change.
The first criterion is delivery method. As they exist now, fertility-control methods can be distinguished by whether it is necessary to have an animal in hand for administration. In most cases, treatments must be delivered when animals are gathered. There are HMAs in which remote delivery (e.g., darting) is possible, but these seem to be exceptions, and investigators have reported increasing difficulty in darting animals repeatedly, as would be necessary with vaccines that require periodic boosters. In addition, some data suggest that hand injection of some contraceptives is more reliable than delivery by dart even if darting is possible for the method in question. Thus, given the current fertility-control options, remote delivery appears not to be a practical characteristic of an effective population-management tool, but it could be useful in some scenarios. However, alternative methods to gathering, such as trapping near water sources, should be considered. At the time the committee’s report was prepared, no product for oral delivery was available that would be species-specific and gender-specific. Although altrenogest, an oral progestagen product, has been used successfully in domestic mares to control estrus, it requires daily dosing during the breeding season. There is no mechanism to assure delivery to mares only, so consumption by stallions, nontargeted wildlife, and domestic grazing livestock could have deleterious effects.
The second criterion, availability of the fertility-control product, includes not only the ability to obtain the product but skilled personnel to administer or conduct it correctly. The methods discussed above range from experimental products to well-established surgical procedures. Two contraceptive vaccines (liquid PZP and GonaCon) are registered with EPA for use in horses; other immunocontraceptives are available only for research application (see Box 4-1). An ideal population-management tool for horses and burros would be readily available in sufficient quantities to achieve population-level effects with little regulatory and administrative burden.
The third criterion, efficacy, is important for calculating the number or percentage of animals that must be treated to reach the target population for an HMA. Efficacy also depends on the ability to administer the treatment to a sufficient percentage of animals to achieve population-management objectives. Fertility-control methods that are highly effective (such as vasectomy) in preventing fertility may have no effect on population growth if a sufficient number of animals cannot be treated. Thus, efficacy involves both the efficacy of
BOX 4-1
Regulatory Considerations Regarding Immunocontraceptives
Licensing and registration of contraceptive products are necessary to ensure that safe and efficacious agents are used as tools for managing free-ranging horse and burro herds. In the United States, before 2006, the Food and Drug Administration Center for Veterinary Medicine was responsible for registration and licensing of such products, but the U.S. Environmental Protection Agency (EPA) has since assumed that responsibility (Eisemann et al., 2006). Extensive data are necessary for successful registration, including safety and efficacy for target species, effects on nontarget species, effects of environmental residue, and human safety. Registration is a long and expensive enterprise that discourages licensing of products that have low expected sales. Government agencies and industry have largely discontinued pursuing registration for important products that are useful to a small consumer base because of the quantity of data required and the associated expense (Fagerstone et al., 1990). Because such products are not widely used and therefore have low profit margins, they cannot generate enough profit to finance the studies required or the annual registration maintenance fees (Fagerstone et al., 1990). The cost for registration of GonaCon™ has been estimated to be $200,000-$500,000 (K. Fagerstone, NWRC, personal communication, 2012). Unregistered products can be used in field studies, although permits for experimental field trials are required. At the time this report was prepared, liquid PZP and GonaCon were licensed. Application to EPA for licensing pelleted PZP-22 for free-ranging horses was being prepared.
the treatment at the level of the individual animal and the efficacy at the population level, determined by the ability to administer the treatment successfully. For example, studies have found that a substantial percentage of a population (more than 50 percent) must be effectively treated to achieve reductions in population size (e.g., Garrott and Siniff, 1992; Pech et al., 1997; Hobbs et al., 2000; Kirkpatrick and Turner, 2008). It is critical that information on efficacy be integrated with population modeling to determine how many individuals in a population must be treated to achieve population goals.
Duration of fertility inhibition has major practical importance. Shorter-acting methods require substantially more effort and financial resources to implement even if the cost of the contraceptive itself is low. Longer-acting methods are preferable to minimize requirements for personnel and financial resources and to decrease the frequency of animal handling. Longer-acting methods should be used more judiciously because they remove animals from the gene pool for a longer period, perhaps permanently.
Several types of side effects were covered in the sections on the different methods in this review. Potential pain associated with administration is one consideration, although the use of anesthetics, analgesics, or both during administration may address this problem (e.g., during vasectomy). The discomfort of injections and darting is transitory and is not generally considered unacceptable. The potential of a method to cause disease or debilitation is not acceptable. That IUDs may provoke undue uterine inflammation warrants caution and would require further testing before application in the field could be considered. In addition, evidence concerning loss rates of IUDs, especially during copulation, would be needed. The possibility that ovariectomy may be followed by prolonged bleeding or peritoneal inflection makes it inadvisable for field application. Potential effects of GnRH vaccines and agonists on other tissues than the pituitary gonadotrophs have not been well studied or documented and warrant caution until further research has been conducted.
Any of the methods described may also affect behavior. Because all methods affect sexual function in some way, changes in expression of sexual and social behavior should be
considered. The ideal method would not eliminate sexual behavior or change social structure substantially. Castration, ovariectomy, and the GnRH products (vaccines and agonists) eliminate or substantially reduce steroid hormone production and so have a potentially profound effect on the expression of sexual behavior. In contrast, vasectomy and the PZP vaccines result in a prolonged breeding season, with increased sexual interaction, because females continue to undergo estrous cycles but fail to conceive. That is not ideal because a prolonged breeding season can result in more fighting among males over access to females. However, the many studies of PZP vaccines and the single study of vasectomized stallions have not reported problems with increased aggression (e.g., more injuries or deaths among stallions).
Considering the above criteria, the methods judged most promising for application to free-ranging horses or burros are PZP vaccines, GonaCon vaccine, and chemical vasectomy. The advantages and disadvantages of each of these methods and their effects on behavior are shown in Tables 4-1 and 4-2, respectively. PZP vaccines are female-directed, chemical vasectomy is male-directed, and GnRH vaccines can be used to treat either males or females. Of the PZP vaccines, PZP-22 and SpayVac seem most appropriate and practical because of their longer duration of effect (especially PZP-22). They could be applied to herds immediately in a research framework, which is required because the products are not yet licensed. Research should address efficacy, duration, and side effects at the population and individual levels where possible. At the time the committee’s report was prepared, there was no evidence to suggest that PZP-22 or SpayVac would have different effects from liquid PZP apart from reports of uterine edema in SpayVac-treated animals. Although GonaCon can be used and has been tested in males, the effects are similar to those of chemical castration. To achieve the suppression of spermatogenesis needed to ensure infertility, testosterone must be suppressed to at or near zero. As with surgical castration, although sexually experienced males may continue to express learned behavioral patterns, they would probably not be successful in competing with intact males. Because preserving natural behaviors is an important criterion, GonaCon seems more appropriate for use in females. Although vaccines against GnRH interfere with its action on the pituitary (stimulating FSH and LH), FSH secretion is partially independent of GnRH (Padmanabhan and McNeilly, 2001). FSH is not required for stimulation of testosterone; LH is sufficient. In females, however, FSH is important for stimulating growth of follicles, which secrete estradiol, the hormone that supports estrous behavior. The role of LH is in the final stages of follicle growth and in inducing ovulation, so blockage of LH is sufficient to prevent conception. Investigations of GonaCon treatment of mares have reported continued estrous behavior and secretion of estradiol consistent with at least partial FSH independence from GnRH control. Thus, to the extent that GonaCon preserves natural behavior patterns while effectively preventing reproduction, it is a promising candidate as a female-directed fertility-control method. However, further studies of its behavioral effects are needed. Chemical vasectomy is promising as an alternative to or in combination with treating females. However, as stated above, vasectomizing more than dominant males would be practical in application at the population level. The effects of surgical vasectomy, and presumably of chemical vasectomy, on sexual behavior closely parallel those of the PZP vaccines and possibly GonaCon.
Although all three methods extend the breeding season, the implications of this effect after vasectomy are more serious because the likelihood of late-season mating and late births would be greater. Foals born later have less time to grow and accumulate fat stores for winter, and this jeopardizes their survival. The more intact males there are in a population, the more likely late-season birth would be because mares would have a greater chance of encountering and mating with a fertile male as the season progressed. Thus, vasectomy
TABLE 4-1 Advantages and Disadvantages of the Most Promising Fertility-Control Methods
| Method | Advantages | Disadvantages |
| PZP-22 and SpayVac®a | Research and application in both captive and free-ranging horses | Capture needed for hand injection of PZP-22 |
| Allows estrous cycles to continue so natural behaviors are maintained | Extended breeding season requires males to defend females longer | |
| High efficacy | With repeated use, return to fertility becomes less predictable | |
| Can be administered during pregnancy or lactation | Out-of-season births are possible | |
| Chemical Vasectomy | Simpler than surgical vasectomy Permanent | Requires handling and light anesthesia Permanent |
| No side effects expected | Only surgical vasectomy has been studied in horses, so side effects of the chemical agent are unknown | |
| Normal male behaviors maintained | Extended breeding season requires males to defend females longer and may result in late-season foals if remaining fertile males mate | |
| Should have high efficacy | Only surgical vasectomy has been studied in horses, so efficacy rate is unknown | |
| GonaCon™ for Females | Capture may be needed for hand injection of initial vaccine and any boosters | |
| Effective for multiple years | Lower efficacy than PZP-vaccine products, especially after first year | |
| Sexual behavior exhibited | Sexual behavior may not be cyclic, inasmuch as ovulation appears to be blocked | |
| Social behaviors not affected in the single field study | Should not be administered during early pregnancy because abortion could occur Few data on horses | |
| aPZP-22 and SpayVac® are formulated for longer efficacy and require further documentation of continued efficacy and of rate of unexpected effects. SOURCE: Asa et al. (1980b), Kirkpatrick et al. (1990), Thompson (2000), Kirkpatrick and Turner (2002, 2003, 2008), Stout and Colenbrander (2004), Imboden et al. (2006), Turner et al. (2007), Killian et al. (2008a), Gray (2009), Nuñez et al. (2009, 2010), Gray et al. (2010, 2011), Powers et al. (2011), Ransom (2012). |
||
might be more appropriate in populations in which a relatively large percentage of males could be treated. The strategy of treating only dominant stallions should be avoided.
Late-season births could occur in mares treated with one of the vaccine products if reversal occurred during the breeding season, but because most free-ranging mares give birth every other year rather than yearly, conceptions and births should become re- established in spring or early summer. For mares that are able to maintain a pregnancy and give birth annually, reversal late in the season could have long-term consequences for all her future foals in that the 11-month gestation and the one or two ovulatory cycles needed to conceive can result in an about 12-month repeating cycle (see Garrott and Siniff, 1992).
TABLE 4-2 Behavioral Effects of Fertility-Control Methods
| Behavior | PZPa,b | GonaCon™ for Females | Vasectomy | |
| Male sexual | Increase or no change reported | No change reported | Longer breeding season | |
| Female sexual | Increase or no change reported | Decrease or no change | Longer breeding season reported | |
| Social structure | Possible decrease in band stability | No change reported | No change reported | |
| Activity budget | Females may graze less | No change reported | No change reported | |
| Aggression | Males may defend females longer | No change reported | Males defend females longer | |
| Spatial relationships | Females may spend more time near male | No change reported | No change reported | |
| aIncludes results of studies of both liquid and pelleted (PZP-22) formulations; not all studies reported results in all the behavioral categories, and not all studies detected changes. aThere are no published reports on behavioral effects of SpayVac®. SOURCE: Rubenstein (1994), Turner et al. (1996), Asa (1999), Powell (1999, 2000), Thompson (2000), Stout and Colenbrander (2004), Imboden et al. (2006), Killian et al. (2008b), Gray (2009), Nuñez et al. (2009), Gray et al. (2010, 2011), Madosky et al. (2010, in review), Ransom et al. (2010), Powers et al. (2011). |
||||
Given that chemical vasectomy appears to be an effective means of reducing male reproduction with side effects that are likely to be minimal and not socially different from controlling female fertility, strategies that simultaneously control male and female fertility are likely to be most biologically and economically cost-effective. Because of the polygynous nature of horse and burro societies, the effect of chemically vasectomizing any one dominant harem-holding or territorial stallion will have a greater effect than contracepting any one fertile female. Moreover, because eventual male turnover is ensured, any long-term problems associated with chemical reproductive interventions are likely to be more reliably self-correcting in males than in females. When that safety factor is added to the problem of procuring large supplies of PZP vaccine in the short term, strategies of dual control allow large-scale and aggressive interventions that modeling (see Chapter 6) suggests will be necessary for regulating population growth in humane and ecologically sound ways.
Most of the PZP-vaccine research in horses (as reviewed in this chapter) has used the older, shorter-acting formulation that requires two initial injections and annual boosters. That formulation was the one licensed for use in horses at the time of the committee’s study. The longer-acting formulations (PZP-22 and SpayVac) were not licensed in the United States, so they were restricted to use for research purposes and not available for widespread application for management purposes. Similarly, GonaCon was registered with EPA for use in free-ranging horses in January 2013. Many state veterinary licensing agencies require that a vasectomy be performed by a licensed veterinarian, although the surgery is straightforward, but the simpler chemical vasectomy has not been systematically evaluated in horses, so testing in captive horses would be needed before widespread application in the field.
On the basis of the peer-reviewed literature and direct communication with scientists who are studying fertility control in horses and burros, the committee considers the three
most promising methods of fertility control to be PZP vaccines (in the forms of PZP-22 and SpayVac), GonaCon, and chemical vasectomy. Chemical vasectomy requires capture and handling, which could be straightforward in areas where BLM regularly gathers horses. It is more problematic in areas where it could be difficult or impossible to capture a sufficient number of animals for treatment to achieve a population effect. In addition, the efficacy of the two vaccines is higher if they are hand-injected rather than delivered by dart. Even in the case of liquid formulations of the vaccines that can in principle be delivered by dart, adequate delivery cannot be ensured. In addition, darting typically entails following animals by helicopter, which could be as stressful as gathering. Alternative methods for gaining closer access to animals for delivering injections should be sought for areas where gathering is not practical or possible.
The vaccines can be effective for multiple years, but chemical vasectomy should be considered permanent. In cases in which reversibility is important and repeated treatment is practical, one of the vaccines would be preferable, with the caution that treatment for more than a few years may prolong recovery of fertility. A single treatment that induces lifetime infertility could be preferable in other situations.
Even if a large fraction of a population’s males are chemically vasectomized and the sterility is permanent, the effects of such an extensive intervention on the dynamics of the population will be self-correcting. If gathers are an average of 5 years apart, younger males rising through the ranks as bachelors or adopting alternative routes to adulthood (Rubenstein and Nuñez, 2009) will be adding new genes to the pool at an increasing rate. Given that virtually all burro and some horse populations exhibit low levels of genetic heterozygosity, virtual elimination of local male fertility for short periods to allow translocations of males that have desired genetic characteristics into the population may be warranted. Such large-scale local chemical vasectomies would allow managers to enhance genetic diversity and reduce inbreeding of populations at risk. Moreover, it would be a self-correcting process as younger males that have the original genetic constitution mature and compete for reproductive opportunities with translocated males. Managing genetic diversity through translocation is discussed more thoroughly in the next chapter.
All three methods should preserve the basic social unit and expression of sexual behavior, although there have been conflicting reports on various effects of the vaccines on social interactions and on the cyclicity of estrous behavior. The major effect of the methods is that the typical breeding season would be extended for females that do not conceive (the implications are discussed at length above). No method has yet been developed that does not have some effect on physiology or behavior. However, the effects of not intervening to control or manage population numbers are potentially harsher than contraception; in the absence of natural predators, population numbers are likely to be limited by starvation (see Chapter 3 for discussion of the effect of density-dependent factors). Even if there were a method that had no effect other than preventing the production of young, the absence of young would alter the age structure of the population and could thereby affect harem dynamics. The most appropriate comparison that should be made in assessing the effects of any method of fertility control is with the current approach, gathering and removal. That is, to what extent does the prospective method affect health, herd structure, and the expression of natural behaviors relative to the effects of gathering? Three methods (PZP-22 and SpayVac, GonaCon, and chemical vasectomy) are considered the most promising for managing fertility in free-ranging horses and burros because they have the fewest and least serious effects on those parameters. In addition, although their application requires handling the animals— gathering—that process is no more disruptive than the current method for controlling numbers, and it lacks the further disruption of removal
and relocation to long-term holding facilities. Considering all the current options, the three methods, either alone or in combination, offer the most acceptable alternative for managing population numbers. However, further research is needed before they are ready for widespread deployment for horse population management.
The current major gaps in knowledge about PZP-22, SpayVac, and GonaCon include a thorough understanding for each vaccine of percentage and duration of efficacy and the extent of its reversibility. GonaCon should be examined to evaluate the extent to which treated females continue to exhibit sexual behavior, which is important for maintaining natural social interactions. A study is needed to assess the efficacy and safety of potential agents for chemical vasectomy before it is used in free-ranging stallions during gathers.
In light of the extensive research that has been conducted with liquid PZP, the likelihood that PZP-22 or SpayVac will produce new or unexpected effects, other than an extended duration of action, is small, and this should reduce the scope of research that would be needed. Furthermore, given the decades of research on the earlier liquid formulation of PZP and its successful application in numerous free-ranging horse herds, liquid PZP can be used in many herd areas now. It might be applied not only in herds that are amenable to darting but during gathers for horses that are turned back onto the range. Even without a booster in the months just after a gather, any later inoculation will serve as a booster and initiate a period of infertility (J.W. Turner, University of Toledo, personal communication, August 2012). Thus, liquid PZP could serve as an interim fertility-control method until one of the other longer-acting methods is available.
ACC&D (Alliance for Contraception in Cats & Dogs). 2012. Esterisol™ (“Zinc Neutering”). Product Profile and Position Paper. Available online at http://www.acc-d.org/ACCD%20docs/PPPP-Esterilsol.pdf/. Accessed November 14, 2012.
Adams, T.E. and B.M. Adams. 1990. Reproductive function and feedlot performance of beef heifers actively immunized against GnRH. Journal of Animal Science 68:2793-2802.
Asa, C.S. 1986. Sexual behavior of mares. Veterinary Clinics of North America: Equine Practice 2:519-534.
Asa, C.S. 1999. Male reproductive success in free-ranging feral horses. Behavioral Ecology and Sociobiology 47:89-93.
Asa, C.S. 2005. Assessing efficacy and reversibility. Pp. 53-65 in Wildlife Contraception: Issues, Methods and Application, C.S. Asa and I.J. Porton, eds. Baltimore, MD: Johns Hopkins University Press.
Asa, C.S. 2010. Reproductive physiology. Pp. 411-428 in Wild Mammals in Captivity: Principles and Techniques for Zoo Management, 2nd edition. D.G. Kleiman, K.V. Thompson, and C.K. Baer, eds. Chicago: University of Chicago Press.
Asa, C.S., D.A. Goldfoot, M.C. Garcia, and O.J. Ginther. 1980a. Dexamethasone suppression of sexual behavior in ovariectomized mares. Hormones and Behavior 14:55-64.
Asa, C.S., D.A. Goldfoot, M.C. Garcia, and O.J. Ginther. 1980b. Sexual behavior in ovariectomized and seasonally anovulatory mares. Hormones and Behavior 14:46-54.
Asa, C.S., D.A. Goldfoot, M.C. Garcia, and O.J. Ginther. 1984. The effect of estradiol and progesterone on the sexual behavior of ovariectomized mares. Physiology & Behavior 33:681-686.
Asa, C.S., I. Porton, A.M. Baker, and E.D. Plotka. 1996. Contraception as a management tool for controlling surplus animals. Pp. 451-467 in Wild Mammals in Captivity: Principles and Techniques, D.G. Kleiman, M.E. Allen, K.V. Thompson, and S. Lumpkin, eds. Chicago: University of Chicago Press.
Asa, C.S. and I.J. Porton. 2010. Contraception as a management tool for controlling surplus animals. Pp. 469-482 in Wild Mammals in Captivity: Principles and Techniques for Zoo Management, 2nd edition. D.G. Kleiman, K.V. Thompson, and C.K. Baer, eds. Chicago: University of Chicago Press.
Barber, M.R. and R.A. Fayrer-Hosken. 2000. Evaluation of somatic and reproductive immunotoxic effects of the porcine zona pellucida vaccination. Journal of Experimental Zoology Part A: Comparative and Experimental Biology 286:641-646.
Bartell, J.A. 2011. Porcine Zona Pellucida Immuncontraception Vaccine for Horses. M.S. thesis. Oregon State University.
Bartholow, J.M. 2004. Economic Analysis of Alternative Fertility Control and Associated Management Techniques for Three BLM Wild Horse Herds. Reston, VA: U.S. Geological Survey.
Becker, C.D. and J.R. Ginsberg. 1990. Mother-infant behaviour of wild Grevy’s zebra: Adaptations for survival in semi-desert East Africa. Animal Behaviour 40:1111-1118.
Berger, J. 1977. Organizational systems and dominance in feral horses in the Grand Canyon. Behavioral Ecology and Sociobiology 2:131-146.
Berger, J. 1986. Wild Horses of the Great Basin: Social Competition and Population Size. Chicago: University of Chicago Press.
BLM (Bureau of Land Management). 2003, revised 2005. Strategic Research Plan: Wild Horse and Burro Management. Fort Collins, CO: U.S. Department of the Interior.
Blodgett, G.P. 2011. Normal field castration. Pp. 1557-1561 in Equine Reproduction, A.O. McKinnon, E.L. Squires, W.E. Vaala, and D.D. Varner, eds. Oxford: Wiley-Blackwell.
Botha, A.E., M.L. Schulman, H.J. Bertschinger, A.J. Guthrie, C.H. Annandale, and S.B. Hughes. 2008. The use of a GnRH vaccine to suppress mare ovarian activity in a large group of mares under field conditions. Wildlife Research 35:548-554.
Boyle, M.S., J. Skidmore, J. Zhang, and J.E. Cox. 1991. The effects of continuous treatment of stallions with high levels of a potent GnRH analogue. Journal of Reproduction and Fertility Supplement 44:169-182.
Brinsko, S.P., E.L. Squires, B.W. Pickett, and T.M. Nett. 1998. Gonadal and pituitary responsiveness of stallions is not down-regulated by prolonged pulsatile administration of GnRH. Journal of Androlology 19:100-109.
Brown, R.G., W.D. Bowen, J.D. Eddington, W.C. Kimmins, M. Mezei, J.L. Parson, and B. Pohajdak. 1997. Temporal trends in antibody production in captive grey, harp and hooded seals to a single administration immunocontraceptive vaccine. Journal of Reproductive Immunology 35:53-64.
Cameron, E.Z., T.H. Setsaas, and W.L. Linklater. 2009. Social bonds between unrelated females increase reproductive success in feral horses. Proceedings of the National Academy of Sciences of the United States of America 106:13850-13853.
Carnevale, E.M. and O.J. Ginther. 1992. Relationships of age to uterine function and reproductive efficiency in mares. Theriogenology 37:1101-1115.
Caswell, H. 2001. Matrix Population Models, 2nd edition. Sunderland, MA: Sinauer Associates.
Chandra, R.K. 1996. Nutrition, immunity and inflection: From basic knowledge of dietary manipulation of immune responses to practical application of ameliorating suffering and improving survival. Proceedings of the National Academy of Sciences of the United States of America 93:14304-14307.
Chandra, R.K. and S.A.D. Amorin. 1992. Lipids and immunoregulation. Nutritional Research 12:S137-S145.
Chapel, H.M. and P.J. August. 1976. Report of nine cases of accidental injury to man with Freund’s complete adjuvant. Clinical and Experimental Immunology 24:538-541.
Chi, I. 1993. What we have learned from recent IUD studies: A researcher’s perspective. Contraception 48:81-108.
Cooper, D.W. and E. Larsen. 2006. Immunocontraception of mammalian wildlife: Ecological and immunogenetic issues. Reproduction 132:821-828.
Curtis, P.D., R.L. Pooler, M.E. Richmond, L.A. Miller, G.F. Mattfeld, and F.W. Quimby. 2002. Comparative effects of GnRH and porcine zona pellucida (PZP) immunocontraceptive vaccines for controlling reproduction in white-tailed deer (Odocoileus virginianus). Reproduction Supplement 60:131-141.
Daels, P.F. and J.P. Hughes. 1995. Fertility control using intrauterine devices: An alternative for population control in wild horses. Theriogenology 44:629-639.
Demas, G.E., D.L. Drazen, and R.J. Nelson. 2003. Reductions in total body fat decrease humoral immunity. Proceedings of the Royal Society of London Series B 270:905-911.
Eagle, T.C., E.D. Plotka, D.B. Siniff, and J.R. Tester. 1992. Efficacy of chemical contraception in feral mares. Wildlife Society Bulletin 20:211-216.
Eagle, T.C., C.S. Asa, R.A. Garrott, and D.B. Siniff. 1993. Efficacy of dominant male contraception to reduce reproduction in feral horses. Wildlife Society Bulletin 21:116-121.
Eisemann, J.D., K.A. Fagerstone, and J.R. O’Hare. 2006. Wildlife contraceptives: A regulatory hot potato. Pp. 63-66 in Proceedings of the 22nd Vertebrate Pest Conference, R.M. Tim and J.M. O’Brien, eds. Davis: University of California.
Elhay, M., A. Newbold, A. Britton, P. Turley, K. Dowsett, and J. Walker. 2007. Suppression of behavioural and physiological oestrus in the mare by vaccination against GnRH. Australian Veterinary Journal 85:39-45.
Esho, J.O. and A.S. Cass. 1978. Recanalization rate following methods of vasectomy using interposition of fascial sheath of vas deferens. Journal of Urology 120:178-179.
Fagerstone, K.A., R.M. Bullard, and C.A. Ramcy. 1990. Politics and economics of maintaining pesticide registrations. Vertebrate Pest Conference 14:8-11.
Falconer, D.S. 1965. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Annals of Human Genetics 29:51-76.
Feist, J.D. and D.R. McCullough. 1975. Reproduction in feral horses. Journal of Reproduction and Fertility Supplement 23:13-18.
Fielding, D. 1988. Reproductive characteristics of the jenny donkey—Equus asinus: A review. Tropical Animal Health and Production 20:161-166.
Fitzgerald, B.P., K.D. Peterson, and P.J. Silvia. 1993. Effect of constant administration of a gonadotropin-releasing hormone agonist on reproductive activity in mares: Preliminary evidence on suppression of ovulation during the breeding season. American Journal of Veterinary Research 54:1746-1751.
Fraker, M.A. 2012. SpayVac® for Wild Horses: A Lost-Lasting, Single-Dose pZP Contraceptive Vaccine. Webinar Presentation to the Bureau of Land Management and Members of the National Academy of Sciences’ Committee to Review the Bureau of Land Management Wild Horse and Burro Management Program, May 3.
Fraker, M.A., R.G. Brown, G.E. Gaunt, J.A. Kerr, and B. Pohajdak. 2002. Long-lasting single-dose immunocontraception of feral fallow deer in British Columbia. Journal of Wildlife Management 66:1141-1147.
Fraker, M.A. and R.B. Brown. 2011. Efficacy of SpayVac® is excellent: A comment on Gray et al. (2010). Wildlife Research 38:537-538.
Frenette, M.D., M.P. Dooley, and M.H. Pineda. 1986. Effect of flushing the vasa deferentia at the time of vasectomy on the rate of clearance of spermatozoa from the ejaculates of dogs and cats. American Journal of Veterinary Research 47:463-470.
Garrott, R.A. and D.B. Siniff. 1992. Limitations of male-oriented contraception for controlling feral horse populations. Journal of Wildlife Management 56:456-464.
Gass, G.H., D. Coats, and N. Graham. 1964. Carcinogenic dose-response curve to oral diethylstilbestrol. Journal of the National Cancer Institute 33:971-977.
Ginsberg, J.R. 1989. The ecology of female behaviour and male mating success in the Grevy’s zebra. Symposium of the Zoological Society of London 61:89-110.
Ginther, O.J. 1979. Reproductive Biology of the Mare: Basic and Applied Aspects. Cross Plains, WI: EquiServices.
Ginther, O.J., S.T. Scraba, and D.R. Bergfelt. 1987. Reproductive seasonality of the jenny. Theriogenology 27:587-592.
Gionfriddo, J.P., J.D. Eisemann, K.J. Sullivan, R.S. Healey, and L.A. Miller. 2006. Field test of GonaCon™ immunocontraceptive vaccine in free-ranging female white-tailed deer. Pp. 78-81 in Proceedings of the 22nd Vertebrate Pest Conference, R.M. Timm and J.M. O’Brien, eds. Davis: University of California.
Gionfriddo, J.P., J.D. Eisemann, K.J. Sullivan, R.S. Healey, L.A. Miller, K.A. Fagerstone, R.M. Engeman, and C.A. Yoder. 2009. Field test of a single-injection gonadotrophin-releasing hormone immunocontraceptive vaccine in female white-tailed deer. Wildlife Research 36:177-184.
Gionfriddo, J.P., A.J. DeNicola, L.A. Miller, and K.A. Fagerstone. 2011a. Efficacy of GnRH immunocontraception of wild white-tailed deer in New Jersey. Wildlife Society Bulletin 35:142-148.
Gionfriddo, J.P., A.J. DeNicola, L.A. Miller, and K.A. Fagerstone. 2011b. Health effects of GnRH immunocontraception of wild white-tailed deer in New Jersey. Wildlife Society Bulletin 35:149-160.
Goodloe, R.B. 1991. Immunocontraception, Genetic Management, and Demography of Feral Horses on Four Eastern U.S. Barrier Islands. Ph.D. dissertation. University of Georgia.
Gotelli, N. 2001. A Primer of Ecology, 3rd edition. Sunderland, MA: Sinauer Associates.
Gray, M.E. 2009. The Influence of Reproduction and Fertility Manipulation on the Social Behavior of Feral Horses (Equus caballus). Ph.D. dissertation. University of Nevada, Reno.
Gray, M.E., D.S. Thain, E.Z. Cameron, and L.A. Miller. 2010. Multi-year fertility reduction in free-roaming feral horses with single-injection immunocontraceptive formulations. Wildlife Research 37:475-481.
Gray, M.E., D.S. Thain, E.Z. Cameron, and L.A. Miller. 2011. Corrigendum to: Multi-year fertility reduction in free-roaming feral horses with single-injection immunocontraceptive formulations. Wildlife Research 38:260.
Guillaume, D., G. Duchamp, J. Salazar-Ortiz, and P. Nagy. 2002. Nutrition influences the winter ovarian inactivity in mares. Theriogenology 58:593-597.
Hageleit, M., A. Daxenberger, W.D. Kraetzl, A. Kettler, and H.H.D. Meyer. 2000. Dose-dependent effects of melengestrol acetate (MGA) on plasma levels of estradiol, progesterone and luteinizing hormone in cycling heifers and influence on oestrogen residues in edible tissues. Acta Pathologica Microbiologica and Immunologica Scandinavica 108:847-854.
Henry, M., S.M. McDonnell, L.D. Lodi, and E.L. Gastal. 1991. Pasture mating behaviour of donkeys (Equus asinus) at natural and induced oestrus. Journal of Reproduction and Fertility Supplement 44:77-86.
Hobbs, N.T., D.C. Bowden, and D.L. Baker. 2000. Effects of fertility control on populations of ungulates: General, stage-structured models. Journal of Wildlife Management 64:473-491.
Holtan, D.W., E.L. Squires, D.R. Lapin, and O.J. Ginther. 1979. Effect of ovariectomy on pregnancy in mares. Journal of Reproduction and Fertility Supplement 27:457-463.
Homsy, J., W.J.W. Morrow, and J.A. Levy. 1986. Nutrition and autoimmunity: A review. Clinical Experimental Immunology 65:473-488.
Hooper, R.N., T.S. Taylor, D.D. Varner, and T.L. Blanchard. 1993. Effects of bilateral ovariectomy via colpotomy in mares: 23 cases (1984-1990). Journal of the American Veterinary Medical Association 203:1043-1046.
Houston, A.I., J.M. McNamara, Z. Barta, and K.C. Klasing. 2007. The effect of energy reserves and food availability on optimal immune defence. Proceedings of the Royal Society of London Series B 274:2835-2842.
Imboden, I., F. Janett, D. Burger, M.A. Crowe, M. Hassig, and R. Thun. 2006. Influence of immunization against GnRH on reproductive cyclicity and estrous behavior in the mare. Theriogenology 66:1866-1875.
Janett, F., R. Stump, D. Burger, and R. Thun. 2009. Suppression of testicular function and sexual behavior by vaccination against GnRH (Equity™) in the adult stallion. Animal Reproduction Science 115:88-102.
Jensen, H. 2000. Social Structure and Activity Patterns among Selected Pryor Mountain Wild Horses. Report to the Billings Field Office, Bureau of Land Management.
Johnson, C., S. McMeen, and D. Thompson. 2002. Effects of multiple GnRH analogue (deslorelin acetate) implants on cyclic mares. Theriogenology 58:469-471.
Junaidi, A., P.E. Williamson, J.M. Cummins, G.B. Martin, M.A. Blackberry, and T.E. Trigg. 2003. Use of a new drug delivery formulation of the gonadotrophin-releasing hormone analogue Deslorelin for reversible long-term contraception in male dogs. Reproduction, Fertility and Development 15:317-322.
Junaidi, A., P.E. Williamson, G.B. Martin, M.A. Blackberry, J.M. Cummins, and T.E. Trigg. 2009. Dose-response studies for pituitary and testicular function in male dogs treated with the GnRH superagonist, deslorelin. Reproduction in Domestic Animals 44:725-734.
Killian, G., L.A. Miller, N.L. Diehl, J. Rhyan, and D. Thain. 2004. Evaluation of three contraceptive approaches for population control of wild horses. Pp. 263-268 in Proceedings of the 21st Vertebrate Pest Conference, R.M. Timm and W.P. Gorenzel, eds. Davis: University of California.
Killian, G., D. Wagner, and L. Miller. 2005. Observations on the use of the GnRH vaccine Gonacon™ in male white-tailed deer (Odocoileus virginianus). Pp. 256-263 in Proceedings of the 11th Wildlife Damage Management Conference, D.L. Nolte and K.A. Fagerstone, eds. Fort Collins, CO: National Wildlife Research Center.
Killian, G., L. Miller, J. Rhyan, and H. Doten. 2006. Immunocontraception of Florida feral swine with a single-dose GnRH vaccine. American Journal of Reproductive Immunology 55:378-384.
Killian, G., D. Thain, N.K. Diehl, J. Rhyan, and L. Miller. 2008a. Four-year contraception rates of mares treated with single-injection porcine zona pellucida and GnRH vaccines and intrauterine devices. Wildlife Research 35:531-539.
Killian, G., D. Wagner, K. Fagerstone, and L. Miller. 2008b. Long-term efficacy and reproductive behavior associated with Gonacon™ use in white-tailed deer (Odocoileus virginianus). Pp. 240-243 in Proceedings of the 23rd Vertebrate Pest Conference, R.M. Timm and M.B. Madon, eds. Davis: University of California.
Killian, G., T.J. Kreeger, J. Rhyan, K. Fagerstone, and L. Miller. 2009. Observations on the use of Gonacon™ in captive female elk (Cervus elaphus). Journal of Wildlife Diseases 45:184-188.
Kirkpatrick, J.F. and A. Turner. 2002. Reversibility of action and safety during pregnancy of immunization against porcine zona pellucida in wild mares (Equus caballus). Reproduction Supplement 60:197-202.
Kirkpatrick, J.F. and A. Turner. 2003. Absence of effects from immunocontraception on seasonal birth patterns and foal survival among barrier island wild horses. Journal of Applied Animal Welfare Science 6:301-308.
Kirkpatrick, J.F. and A. Turner. 2007. Immunocontraception and increased longevity in equids. Zoo Biology 26:237-244.
Kirkpatrick, J.F. and A. Turner. 2008. Achieving population goals in a long-lived wildlife species (Equus caballus) with contraception. Wildlife Research 35:513-519.
Kirkpatrick, J.F., I.K.M. Liu, and J.W. Turner, Jr. 1990. Remotely-delivered immunocontraception in feral horses. Wildlife Society Bulletin 18:326-330.
Kirkpatrick, J.F., I.M. Liu, J.W. Turner, Jr., and M. Bernoco. 1991. Antigen recognition in feral mares previously immunized with porcine zona pellucida. Journal of Reproduction and Fertility Supplement 44:321-325.
Kirkpatrick, J.F., I.M.K. Liu, J.W. Turner, Jr., R. Naugle, and R. Keiper. 1992. Long-term effects of porcine zonae pellucidae immunocontraception on ovarian function in feral horses (Equus caballus). Journal of Reproduction and Fertility 94:437-444.
Kirkpatrick, J.F., R. Naugle, I.K.M. Liu, M. Bernoco, and J.W. Turner, Jr. 1995. Effects of seven consecutive years of porcine zona pellucida contraception on ovarian function in feral mares. Biology of Reproduction Monographs 1:411-418.
Kirkpatrick, J.F., R.O. Lyda, and K.M. Frank. 2011. Contraceptive vaccines for wildlife: A review. American Journal of Reproductive Immunology 66:40-50.
Klingel, H. 1975. Social organization and reproduction in equids. Journal of Reproduction and Fertility Supplement 23:7-11.
Lauderdale, J.W., L.S. Goyings, L.F. Krzeminski, and R.G. Zimbelman. 1977. Studies of a progestogen (MGA) as related to residues and human consumption. Journal of Toxicology and Environmental Health 3:5-33.
Liehr, J.G. 2001. Genotoxicity of the steroidal oestrogens oestrone and oestradiol: Possible mechanism of uterine and mammary cancer development. Human Reproduction Update 7:273-281.
Linklater, W.L., E.Z. Cameron, E.O. Minot, and K.J. Stafford. 1999. Stallion harassment and the mating system of horses. Animal Behavior 58:295-306.
Liu, I.K.M., M. Bernoco, and M. Feldman. 1989. Contraception in mares heteroimmunized with pig zona pellucidae. Journal of Reproduction and Fertility 85:19-28.
Liu, I.K.M., J.W. Turner, Jr., E.M.G. Van Leeuwen, D.R. Flanagan, J.L. Hedrick, K. Murata, V.M. Lane, and M.P. Morales-Levy. 2005. Persistence of anti-zonae pellucidae antibodies following a single inoculation of porcine zonae pellucidae in the domestic equine. Reproduction 129:181-190.
Locke, S.L., M.W. Cook, L.A. Harveson, D.S. Davis, R.R. Lopez, N.J. Silvy, and M.A. Fraker. 2007. Effectiveness of Spayvac® for reducing white-tailed deer fertility. Journal of Wildlife Diseases 43:726-730.
Ludwig, C., P.O. Desmoulins, M.A. Driancourt, S. Goericke-Pesch, and B. Hoffmann. 2009. Reversible down-regulation of endocrine and germinative testicular function (hormonal castration) in the dog with the GnRH-agonist azagly-nafarelin as a removable implant “Gonazon”; a preclinical trial. Theriogenology 71:1037-1045.
Lyda, R.O., J.R. Hall, and J.F. Kirkpatrick. 2005. A comparison of Freund’s Complete and Freund’s Modified Adjuvants used with a contraceptive vaccine in wild horses (Equus caballus). Journal of Zoo and Wildlife Medicine 36:610-616.
Madosky, J., D. Rubenstein, J. Howard, and S. Stuska. 2010. The effect of immunocontraception on harem fidelity in a feral horse (Equus caballus) population. Applied Animal Behaviour Science 128:50-56.
Madosky, J., J. Howard, D. Rubenstein, and S. Stuska. In review. Synchrony of time budgets, social interactions, and harem stability in a feral horse population.
Malmgren, L., O. Andresen, and A.M. Dalin. 2001. Effect of GnRH immunisation on hormonal levels, sexual behaviour, semen quality and testicular morphology in mature stallions. Equine Veterinary Journal 33:75-83.
Massei, G., D.P. Cowan, J. Coats, F. Gladwell, J.E. Lane, and L.A. Miller. 2008. Effect of the GnRH vaccine GonaCon on the fertility, physiology and behaviour of wild boar. Wildlife Research 35:540-547.
May, R.M. and D.I. Rubenstein. 1985. Reproductive strategies. Pp. 1-23 in Reproduction in Mammals, C.R. Austin and R.V. Short, eds. Cambridge, UK: Cambridge University Press.
McCue, P.M. and R.A. Ferris. 2011. The abnormal estrous cycle. Pp. 1754-1768 in Equine Reproduction, 2nd edition. A.O. McKinnon, E.L. Squires, W.E. Vaala, and D.D. Varner, eds. Hoboken, NJ: Wiley-Blackwell.
McCue, P.M. and A.O. McKinnon. 2011. Ovarian abnormalities. Pp. 2123-2136 in Equine Reproduction, 2nd edition. A.O. McKinnon, E.L. Squires, W.E. Vaala, and D.D. Varner, eds. Hoboken, NJ: Wiley-Blackwell.
Miller, L.A., B.E. Johns, and G.J. Killian. 2000. Immunocontraception of white-tailed deer with GnRH vaccine. American Journal of Reproductive Immunology 44:266-274.
Miller, L.A., J.C. Rhyan, and M. Drew. 2004. Contraception of bison by GnRH vaccine: A possible means of decreasing transmission of brucellosis in bison. Journal of Wildlife Diseases 40:725-730.
Miller, L.A., J.P. Gionfriddo, J.C. Rhyan, K.A. Fagerstone, D.C. Wagner, and G.J. Killian. 2008. GnRH immunocontraception of male and female white-tailed deer flawns. Human-Wildlife Conflicts 2:93-101.
Miller, L.A., K.A. Fagerstone, D.C. Wagner, and G.J. Killian. 2009. Factors contributing to the success of a single-shot, multiyear PZP immunocontraceptive vaccine for white-tailed deer. Human-Wildlife Conflicts 3:103-115.
Mitchell, D. and W.R. Allen. 1975. Observations on reproductive performance in the yearling mare. Journal of Reproduction and Fertility Supplement 23:531-536.
Montovan, S.M., P.F. Daels, J. Rivier, J.P. Hughes, G.H. Stabenfeldt, and B.L. Lasley. 1990. The effect of a potent GnRH agonist on gonadal and sexual activity in the horse. Theriogenology 33:1305-1321.
Moss, W.M. 1992. A comparison of open-ended versus closed-end vasectomies: A report on 6220 cases. Contraception 46:521-525.
Munson, L., J.E. Bauman, C.S. Asa, W. Jochle, and T.E. Trigg. 2001. Efficacy of the GnRH analogue deslorelin for suppression of oestrus cycle in cats. Journal of Reproduction and Fertility Supplement 57:269-273.
Murthy, V., A.R. Norman, Y. Barbachano, C.C. Parker, and D.P. Dearnaley. 2007. Long-term effects of a short course of neoadjuvant luteinizing hormone-releasing hormone analogue and radical radiotherapy on the hormonal profile in patients with localized prostate cancer. British Journal of Urology International 99:1380-1382.
Naden, J., E.L. Squires, and T.M. Nett. 1990. Effect of maternal treatment with altrenogest on age at puberty, hormone concentrations, pituitary response to exogenous GnRH, oestrous cycle characteristics and fertility of fillies. Journal of Reproduction and Fertility 88:185-195.
Nejat, R.J., H.H. Rashid, E. Bagiella, A.E. Katz, and M.C. Benson. 2000. A prospective analysis of time to normalization of serum testosterone after withdrawal of androgen deprivation therapy. Journal of Urology 164:1891-1894.
Nelson, K.J. 1978. On the Question of Male-Limited Population Growth in Feral Horses (Equus caballus). M.S. thesis. New Mexico State University, Las Cruces.
Neuhauser, S., F. Palm, F. Ambuehl, and C. Aurich. 2008. Effects of altrenogest treatment of mares in late pregnancy on parturition and on neonatal viability of their foals. Experimental and Clinical Endocrinology and Diabetes 116:423-428.
Nipken, C. and K.H. Wrobel. 1997. A quantitative morphological study of age-related changes in the donkey testis in the period between puberty and senium. Andrologia 29:149-161.
Nishikawa, Y. 1959. Studies on reproduction in horses. Tokyo: Japan Racing Association.
Nobelius, A.M. 1992. Gestagens in the Mare: An Appraisal of the Effects of Gestagenic Steroids on Suppression of Oestrus in Mares. M.S. thesis. Monash University, Australia.
NRC (National Research Council). 1980. Wild and Free-Roaming Horses and Burros: Current Knowledge and Recommended Research. Phase I Final Report. Washington, DC: National Academy Press.
NRC (National Research Council). 1991. Wild Horse Populations: Field Studies in Genetics and Fertility. Washington, DC: National Academy Press.
Nuñez, C.M.V., J.S. Adelman, C. Mason, and D.I. Rubenstein. 2009. Immunocontraception decreased group fidelity in a feral horse population during the non-breeding season. Applied Animal Behavior Science 117:74-83.
Nuñez, C.M.V., J.S. Adelman, and D.I. Rubenstein. 2010. Immunocontraception in wild horses (Equus caballus) extends reproductive cycling beyond the normal breeding season. PLoS One 5:e13635.
O’Brien, P.A., R. Kulier, F.M. Helmerhorst, M. Usher-Patel, and C. d’Arcangues. 2008. Copper-containing, framed intrauterine devices for contraception: A systematic review of randomized controlled trials. Contraception 77:318-327.
Oli, M.K. and F.S. Dobson. 2003. The relative importance of life-history variables to population growth rate in mammals: Cole’s prediction revisited. American Naturalist 161:422-440.
Padmanabhan, W. and A. McNeilly. 2001. Is there an FSH-releasing factor? Reproduction 121:21-30.
Pech, R., G.M. Hood, J. McIlroy, and G. Saunders. 1997. Can foxes be controlled by reducing their fertility? Reproduction, Fertility and Development 9:41-50.
Pelahach, L.M., H.E., Greaves, M.B. Porter, A. Desvousges, and D.C. Sharp. 2002. The role of estrogen and progesterone in the induction and dissipation of uterine edema in mares. Theriogenology 58:441-444.
Perry, K.R., W.M. Arjo, K.S. Bynum, and L.A. Miller. 2006. GnRH single-injection immunocontraception in black-tailed deer. Pp. 72-77 in Proceedings of the 22nd Vertebrate Pest Conference, R.M. Timm and J.M. O’Brien, eds. Davis: University of California.
Pickett, B.W., L.C. Faulkner, and J.L. Voss. 1975. Effect of season on some characteristics of stallion semen. Journal of Reproduction and Fertility Supplement 23:25-28.
Pineda, M.H. and M.P. Dooley. 1984. Surgical and chemical vasectomy in the cat. American Journal of Veterinary Research 45:291-300.
Pineda, M.H., T.J. Reimers, L.C. Faulkner, M.C. Hopwood, and G.E. Seidel, Jr. 1977. Azoospermia in dogs induced by injection of sclerosing agents into the caudae of the epididymides. American Journal of Veterinary Research 38:831-838.
Pinto, C.R.F. 2011. Progestagens and progesterone. Pp. 1811-1819 in Equine Reproduction, 2nd edition. A.O. McKinnon, E.L. Squires, W.E. Vaala, and D.D. Varner, eds. Oxford: Wiley-Blackwell.
Plosker, G.L. and R.N. Brogden. 1994. Leuprorelin. A review of its pharmacology and therapeutic use in prostatic cancer, endometriosis and other sex hormone-related disorders. Drugs 48:930-967.
Plotka, E.D. and U.S. Seal. 1989. Fertility control in female white-tailed deer. Journal of Wildlife Diseases 25:643-646.
Plotka, E.D., T.C. Eagle, D.N. Vevea, A.L. Koller, D.B. Siniff, J.R. Tester, and U.S. Seal. 1988. Effects of hormone implants on estrus and ovulation in feral mares. Journal of Wildlife Diseases 24:507-514.
Plotka, E.D., D.N. Vevea, T.C. Eagle, J.R. Tester, and D.B. Siniff. 1992. Hormonal contraception of feral mares with Silastic rods. Journal of Wildlife Diseases 28:255-262.
Pollock, J.I. 1980. Behavioral ecology and body condition changes in New Forest ponies. Royal Society for the Prevention of Cruelty to Animals Scientific Publication 6. 113 pp.
Porter, M. and D. Sharp. 2002. Gonadotropin-releasing hormone receptor trafficking may explain the relative resistance to pituitary desensitization in mares. Theriogenology 58:523-526.
Porton, I.J. and K. DeMatteo. 2005. Contraception in non-human primates. Pp. 119-148 in Wildlife Contraception: Issues, Methods, and Applications, C.S. Asa and I.J. Porton, eds. Baltimore, MD: Johns Hopkins University Press.
Powell, D.M. 1999. Preliminary evaluation of porcine zona pellucida (PZP) immunocontraception for behavioral effects. Journal of Applied Animal Welfare Science 2:321.
Powell, D.M. 2000. Evaluation of Effects of Contraceptive Population Control on Behavior and the Role of Social Dominance in Female Feral Horses, Equus caballus. Ph.D. dissertation. University of Maryland, College Park.
Powell, D.M. and S.L. Montfort. 2001. Assessment: Effects of porcine zona pellucida immunocontraception on estrous cyclicity in feral horses. Journal of Applied Animal Welfare Science 4:271-284.
Powers, J.G., D.L. Baker, T.L. Davis, M.M. Conner, A.H. Lothridge, and T.M. Nett. 2011. Effects of gonadotropin-releasing hormone immunization on reproductive function and behavior in captive female Rocky Mountain elk (Cervus elaphus nelsoni). Biology of Reproduction 85:1152-1160.
Pugh, D. 2002. Donkey reproduction. Pp. 113-114 in the 48th Annual Convention Proceedings of the American Association of Equine Practitioners. Lexington, KY: AAEP.
Ransom, J.I. 2012. Population Ecology of Feral Horses in an Era of Fertility Control Management. Ph.D. dissertation. Colorado State University, Fort Collins.
Ransom, J.I., B.S. Cade, and N.T. Hobbs. 2010. Influences of immunocontraception on time budgets, social behavior, and body condition in feral horses. Applied Animal Behavior Science 124:51-60.
Ransom, J.I., J.E. Roelle, B.S. Cade, L. Coates-Markle, and A.J. Kane. 2011. Foaling rates in feral horses treated with the immunocontraceptive porcine zona pellucida. Wildlife Society Bulletin 35:343-352.
Rivera, R. and K. Best. 2002. Current opinion: Consensus statement on intrauterine contraception. Contraception 65:385-388.
Rodgerson, D.H. and D.A. Loesch. 2011. Ovariectomy. Pp. 2564-2573 in Equine Reproduction, 2nd edition. A.O. McKinnon, E.L. Squires, W.E. Vaala, and D.D. Varner, eds. Oxford: Wiley-Blackwell.
Roelle, J.E. and J.I. Ransom. 2009. Injection-site reactions in wild horses (Equus caballus) receiving an immunocontraceptive vaccine. U.S. Geological Survey Scientific Investigations Report 2009-5038. Fort Collins, CO: U.S. Geological Survey.
Roser, J.F. and J.P. Hughes. 1991. Prolonged pulsatile administration of gonadotropin-releasing hormone (GnRH) to fertile stallions. Journal of Reproduction and Fertility Supplement 44:155-168.
Rubenstein, D.I. 1981. Behavioural ecology of island feral horses. Equine Veterinary Journal 13:27-34.
Rubenstein, D.I. 1986. Ecology and sociality in horses and zebras. Pp. 282-302 in Ecological Aspects of Social Evolution, D.I. Rubenstein and L.W. Wrangham, eds. Princeton, NJ: Princeton University Press.
Rubenstein, D.I. 1994. The ecology of female social behaviour in horses, zebras and asses. Pp. 13-28 in Animal Societies, Individuals, Interactions, and Organization, P.J. Jarman and A. Rossiter, eds. Kyoto, Japan: Kyoto University Press.
Rubenstein, D.I. and C.M. Nuñez. 2009. Sociality and reproductive skew in horses and zebras. Pp. 196-226 in Reproductive Skew in Vertebrates: Proximate and Ultimate Causes, R. Hager and C.B. Jones, eds. Cambridge, UK: Cambridge University Press.
Rutberg, A.T. 1990. Intergroup transfer in Assateague pony mares. Animal Behaviour 40:945-952.
Rutberg, A.T. and R.E. Naugle. 2008. Population-level effects of immunocontraception in white-tailed deer (Odocoileus virginianus). Wildlife Research 35:494-501.
Samper, J.C. 1997. Ultrasonographic appearance and the pattern of uterine edema to time ovulation in mares. Pp. 189-191 in the 43rd Annual Convention Proceedings of the American Association of Equine Practitioners. Lexington, KY: AAEP.
Santen, R. 1998. Biological basis of the carcinogenic effects of estrogen. Obstetrical and Gynecological Survey 53:10S-18S.
Sharp, D.C. and O.J. Ginther. 1975. Stimulation of follicular activity and estrous behavior in anestrous mares with light and temperature. Journal of Animal Science 41:1368-1372.
Sieme, H., M.H.T. Troedsson, S. Weinrich, and E. Klug. 2004. Influence of exogenous GnRH on sexual behavior and frozen/thawed semen viability in stallions during the non-breeding season. Theriogenology 61:159-171.
Silber, S.J. 1989. Pregnancy after vasovasostomy for vasectomy reversal: A study of factors affecting long-term return of fertility in 282 patients followed for 10 years. Human Reproduction 4:318-322.
Singer, F.J. and K.A. Schoenecker, compilers. 2000. Managers’ Summary—Ecological Studies of the Pryor Mountain Wild Horse Range, 1992-1997. Fort Collins, CO: U.S. Geological Survey.
Sivin, I. 1993. Another look at the Dalkon Shield: Meta analysis underscores its problems. Contraception 48:1-12.
Squires, E.L. 2011. Gonadotropin releasing hormones. Pp. 1820-1824 in Equine Reproduction, 2nd edition. A.O. McKinnon, E.L. Squires, W.E. Vaala, and D.D. Varner, eds. Oxford: Wiley-Blackwell.
Stahl, J.T. and M.K. Oli. 2006. Relative importance of avian life-history variables to population growth rate. Ecological Modelling 198:183-194.
Stahlbaum, C.C. and K.A. Houpt. 1989. The role of the flehmen response in the behavioral repertoire of the stallion. Physiology and Behavior 45:1207-1214.
Stearns, S.C. 1992. The Evolution of Life Histories. Oxford: Oxford University Press.
Stevens, E.F. 1990. Instability of harems of feral horses in relation to season and presence of subordinate stallions. Behaviour 112:149-161.
Storer, W.A., D.L. Thompson, R.M. Gilley, and P.J. Burns. 2009. Evaluation of injectable sustained release progestin formulations for suppression of estrus and ovulation in mares. Journal of Equine Veterinary Science 29:33-36.
Stout, T.A.E. and B. Colenbrander. 2004. Suppressing reproductive activity in horses using GnRH vaccines, antagonists or agonists. Animal Reproduction Science 82-83:633-643.
Stuska, S. 2012. Shackleford Banks Horse Herd Update. September 25. Beaufort, NC: Foundation for Shackleford Horses.
Tankeyoon, M., N. Dusitsin, S. Chalapati, S. Koetsawang, S. Saibiang, M. Sas, J.J. Gellen, O. Ayeni, R. Gray, A. Pinol, and L. Zegers. 1984. Effects of hormonal contraceptives on milk volume and infant growth: WHO Special Programme of Research and Development and Research Training in Human Reproduction. Contraception 30:505-522.
Thompson, D.L. 2000. Immunization against GnRH in male species (comparative aspects). Animal Reproduction Science 60:459-469.
Tomasgard, G. and E. Benjaminsen. 1975. Plasma progesterone in mares showing estrus during pregnancy. Nordisk Veterinærmedicin 27:570-574.
Toydemir, T.S.F., M.R. Kiliçarslan, and V. Olgaç. 2012. Effects of the GnRH analogue deslorelin implants on reproduction in female domestic cats. Theriogenology 77:662-674.
Turkstra, J., F. Van Der Meer, J. Knaap, P. Rottier, K. Teerds, B. Colenbrander, and R. Meloen. 2005. Effects of GnRH immunization in sexually mature pony stallions. Animal Reproduction Science 86:247-259.
Turner, A. and J.F. Kirkpatrick. 2002. Effects of immunocontraception on population, longevity and body condition in wild mares (Equus caballus). Reproduction Supplement 60:187-195.
Turner, J.W., Jr. and J.F. Kirkpatrick. 1982. Androgens, behavior and fertility control in feral stallions. Journal of Reproduction and Fertility Supplement 32:79-87.
Turner, J.W., Jr., I.K.M. Liu, and J.F. Kirkpatrick. 1996. Remotely delivered immunocontraception in free-roaming feral burros (Equus asinus). Journal of Reproduction and Fertility 107:31-35.
Turner, J.W., Jr., I.K.M. Liu, A.T. Rutberg, and J.F. Kirkpatrick. 1997. Immunocontraception limits foal production in free-roaming feral horses in Nevada. Journal of Wildlife Management 61:873-880.
Turner, J.W., Jr., I.K.M. Liu, D.R. Flanagan, A.T. Rutberg, and J.F. Kirkpatrick. 2001. Immunocontraception in feral horses: One inoculation provides one year of infertility. Journal of Wildlife Management 65:235-241.
Turner, J.W., Jr., I.K.M. Liu, D.R. Flanagan, A.T. Rutberg, and J.F. Kirkpatrick. 2007. Immunocontraception in wild horses: One inoculation provides two years of infertility. Journal of Wildlife Management 71:662-667.
Turner, J.W., A.T. Rutberg, R.E. Naugle, M.A. Kaur, D.R. Flanagan, H.J. Bertschinger, and I.K.M. Liu. 2008. Controlled-release components of PZP contraceptive vaccine extend duration of infertility. Wildlife Research 35:555-562.
Vandeplassche, G.M., J.A. Wesson, and O.J. Ginther. 1981. Behavioral, follicular and gonadotropin changes during the estrous cycle in donkeys. Theriogenology 16:239-249.
Vanderwall, D.K. 2008. Early embryonic loss in the mare. Journal of Equine Veterinary Science 28:691-702.
Vanderwall, D.K., G.L. Woods, D.A. Freeman, J.A. Weber, R.W. Rock, and D.F. Tester. 1993. Ovarian follicles, ovulations, and progesterone concentrations in aged versus young mares. Theriogenology 40:21-32.
Yurewicz, E.C., A.G. Sacco, and M.G. Subramanian. 1983. Isolation and preliminary characterization of a purified pig zona antigen (PPZA) from porcine oocytes. Biology of Reproduction 29:511-523.
Zeng, W., S. Ghosh, Y.F. Lau, L.E. Brown, and D.C. Jackson. 2002. Highly immunogenic and totally synthetic lipopeptides as self-adjuvanting immunocontraceptive vaccines. Journal of Immunology 169:4905-4912.


















































