3
Core Science and Technology Capabilities for the Chemical and Biological Defense Program
The committee explored the various science and technology capabilities that are relevant to the Chemical and Biological Defense Program (CBDP). In order to address its task, the committee utilized the six science and technology (S&T) capability categories defined in the previous chapter: Enabling CBRN Intelligence, Surveillance, and Reconnaissance; Chemical and Biological Agent Detection; Individual and Collective Protection; Medical Countermeasures; Hazard Assessment, Management, and Decontamination; and Cross-Cutting Science and Technology. In addressing the first five areas, excluding Cross-Cutting Science and Technology, the committee identified four to eight S&T capabilities for each category that “must be available to support Chemical and Biological Defense (CBD) research, development, test and evaluation, and operational activities.”1Table 3.1 shows the S&T capabilities identified by the committee under the six S&T capability categories.
Except where otherwise noted, each of the identified S&T capabilities is of sufficient importance to the CBDP that it should provide support to further the activity. To that end, this chapter describes the committee’s views on which capabilities can and should be obtained in Department of Defense (DoD) laboratories and which are better suited to be obtained outside of the DoD or outside of the government altogether. To reach these conclusions, a decision tree was developed with several elements
_______________________
1 Statement of Task.
TABLE 3.1 Committee Identified S&T Capabilities
| 1. ENABLING CBRN INTELLIGENCE, SURVEILLANCE, AND RECONNAISSANCE | 2. CHEMICAL AND BIOLOGICAL AGENT DETECTION | 3. INDIVIDUAL AND COLLECTIVE PROTECTION |
| Information Acquisition and Analysis | Analytical Methods Discovery | Controlled Molecular Transport Materials Discovery |
| Health Monitoring | Instrumentation Development | Barrier Materials Engineering |
| Environmental Monitoring | Sensor Systems Development | Personal Protective Systems Development |
| Unknown Agent Identification and Characterization | Agent Transport Analysis | Collective Protection Systems Development |
| Physiology | ||
| 4. MEDICAL COUNTERMEASURES | 5. HAZARD ASSESSMENT, MANAGEMENT, AND DECONTAMINATION | 6. CROSS-CUTTING SCIENCE AND TECHNOLOGY |
| Target Discovery | Decontamination Methods Discovery | Acquisition, Maintenance and Transport of Critical Chemical and Biological Reagents |
| Regulatory Science | Decontaminant Development | Agent Simulation |
| Mechanisms of Delivery and Delivery Systems | Decontamination Resilient Materials Development | Informatics |
| Animal Models | Decontamination Systems Engineering | Statistical Measurement Design |
| Host Response | Agent Transport and Viability Analysis | Forensics |
| Pre-Clinical Studies | Education and Training | |
| Clinical Trials (GLP and GMP) | Behavioral Analysis | |
| Medical Product Development | Systems Analysis and Engineering | |
| Repurposing Commercial Technologies | ||
| Systems Biology | ||
| Synthetic Chemistry and Biology | ||
| Materials Science | ||
| Test and Evaluation |
to consider at each node in order to help address the necessity of DoD to maintaining certain capabilities in-house (see Figure 3.1).
For the S&T capabilities listed, which the committee has already determined to be core to the CBDP, the committee asked the following questions:
(1) Should the CBDP be providing funding to support this core S&T capability?
The obvious answer may well be yes, but the committee explored some possible reasons that the CBDP should avoid using its limited resources in a specific area. For example, if another part of DoD or the government, which is viewed as reliable and competent, is already funding this area, maybe the CBDP should not invest heavily in this area, provided the two government units involved communicate with one another and make shared use of the findings.
(2) Assuming the CBDP does decide to support and fund a specific S&T capability, the committee looked at whether this capability can be found outside of the government.
As defined previously, capability is having both competence and capacity to accomplish a goal. Do the extra-governmental sources have the competence and capacity, the ability to ensure safety, security and scale to a given problem? If not, should DoD, as opposed to another governmental agency, be focusing on the S&T capability (see step 4)?
(3) If it can be found outside, should the government do it anyway?
In addressing this question, the committee considered a variety of factors that are of high importance to the program. What is the cost differential? Is there both long- and short-term domestic availability2 in the private sector? Does industry provide the necessary staying power for CBDP? Are there safety and security considerations that would require the program to maintain control? If these issues can be satisfactorily addressed, then perhaps CBDP should obtain this capability primarily from the private sector.
(4) If the above questions direct the government to maintain an internal capability, the CBDP should then consider whether it should maintain the capability itself, within DoD, or obtain it from other
_______________________
2 The meaning of availability will be different for each S&T capability. Considerations for intellectual property rights are important in making this determination. Procedural barriers, such as contracting, should be considered in the short term, but care must be taken not to ascribe all problems to “procedural barriers” to avoid finding a solution.
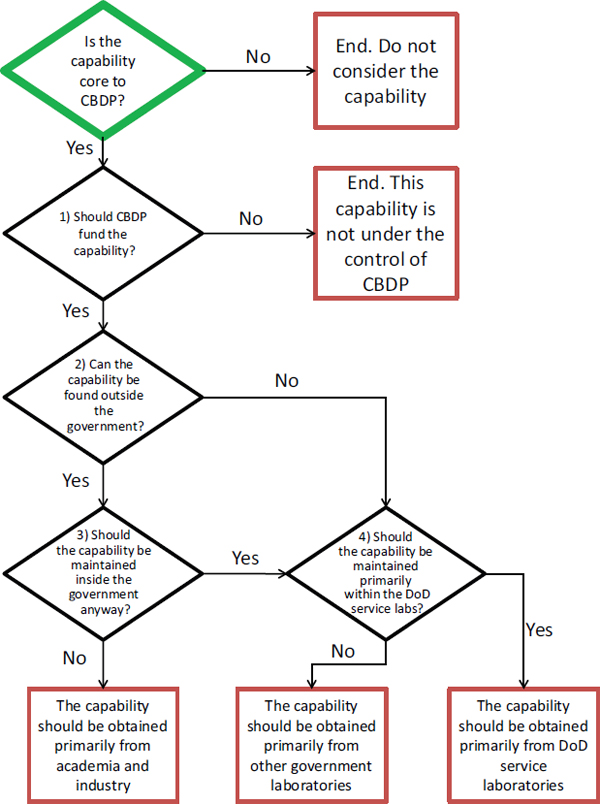
FIGURE 3.1 The decision tree for determining if capabilities should be housed within DoD or leveraged from external sources.
government laboratories3 (e.g., Centers for Disease Control and Prevention, National Institutes of Health, Department of Energy National Laboratories). Items to consider when making this assessment include agency mission, agency resources, and possible public health overlap.
While the committee does not necessarily believe that it should be a high bar for the CBDP to maintain in-house, DoD-controlled capabilities, there are some important questions to consider when discussing the location of the various S&T capabilities.
The committee used the decision tree (Figure 3.1) to determine whether individual capabilities should be maintained by the program and where. Many of the metrics used to address each decision node on the tree are subjective and the committee’s consensus view is described in this chapter. If the CBDP does not agree with an individual assessment of an S&T capability, they are encouraged to undertake a de novo analysis of that capability, using the decision tree above,4 to reach their own conclusion.
ENABLING CBRN INTELLIGENCE, SURVEILLANCE, AND RECONNAISSANCE
The CBDP must have a natural and fundamental role in the prevention of and strategic warning against threats to global and national security through the use of or exposure to priority biological and chemical agents. No other program is better positioned to project capability forward or contribute to global strategic, operational, and tactical warning and prevention. From the force health protection perspective, CBDP plans and provides resources for the research, development, test and evaluation (RDT&E) and delivery of vaccine, prophylaxis, and therapeutic technologies that protect US military personnel and civilians, as required when exposure is determined or suspected. Further, the CBDP supports and directs funding for detection, diagnosis, and biosurveillance, which simultaneously aids in advancing forensic capabilities that inform deci-
_______________________
3 We make a distinction between non-DoD government labs and non-government performers. This is based on an assumption that government labs meet security and surety standards (e.g., ability to work with classified samples, materials, and information) and are designed to be an enduring capability that will be available when needed. Non-government labs have no such assumption of guaranteed continuity, even if security and surety standards are currently met.
4 The real intent of the committee’s approach is to illustrate how to apply a structured, systematic, and consistent process to decision making that allows for a common understanding of priorities and how they were developed. The results also provide a basis for continuity of support.
sions related to medical protection and treatment as well as those that inform intelligence, operations planning, medical intelligence, and attribution. Just as S&T contributes considerably to medical decisions, it also contributes to military and policy decisions related to possibly nefarious activities involving biological and chemical agents. The committee has identified four S&T capabilities that are required to provide technologies needed for chemical, biological, radiological, and nuclear (CBRN) intelligence, surveillance, and reconnaissance (CBRN ISR): information acquisition and analysis, health monitoring, environmental monitoring, and unknown agent identification and characterization.
Information Acquisition and Analysis
The core concept for this capability is that multiple dense sources of information pertinent to CBRN ISR exist and need to be properly mined, managed, integrated, distilled, and efficiently queried. A few examples of information sources in the biological domain include ProMed Mail (outbreak reports), ARGUS (a system that searches World Wide Web news media for signs of social unrest that could be due to pathogen outbreaks), Global Public Health Information Network, social media such as Facebook or Twitter,5 and global weather forecasts and reports. To be fully effective, an information acquisition and analysis system needs to be capable of extracting pertinent facts from a large number of languages and information source types. This involves numerous difficult and largely unsolved problems in natural language processing and information fusion.
DoD needs to be well informed of potential and actual biological and chemical outbreaks and incidents as they relate to both existing and potentially needed detection capabilities. There are multiple government and non-government sources of relevant information for enabling CBRN ISR. Additionally, there are multiple government-funded groups that already attempt various levels of information integration in the chemical and biological domain. It is likely that no existing systems individually meet all current DoD needs. However, DoD should strive to leverage as much as possible from collaborations with existing systems, rather than launching any de novo efforts at developing such capabilities. The committee noted that the current program plans to search social media for outbreak-relevant information did not appear to have a plan for integrating with other available information sources. CBDP should be better aligned with other relevant government information acquisition and analysis efforts in the biological and chemical domains.
_______________________
5 We note that legal issues involving the Privacy Act are faced by any information systems that mine information about US citizens, even if such information is in the public domain.
Health Monitoring
US forces and civilian personnel are projected and mobilized in and out of a diverse array of operational settings and environments. The DoD must be aware of, and be able to protect and respond to, threats to health and readiness. Not only should the DoD support protection and prevention against routine diseases common within the continental United States,6 it must also be able to provide agile medical and S&T responses to “surprise” in any location worldwide. Thus, programs to address those diseases that are intentionally caused, endemic or presenting elsewhere in the world where DoD operates, need to be incorporated into CBDP planning, resourcing, and delivery. While every new disease cannot be anticipated, a system and capabilities must be in place for CBDP to respond effectively and in a timely manner. This applies to medical treatment approaches as well as diagnostics, biomarkers to exposure, and biosurveillance.
One aspect of this is to consider health monitoring in the context of “One Health,”7 which considers human health in the global context of animals (domestic and wild) and includes all reservoirs and vectors (e.g., mammals, birds, ticks, mosquitoes, etc.). This implies increased global biosurveillance of both human and animal baselines to determine what is currently endemic so that new pathogens with pandemic potential can be more rapidly detected and characterized, ideally before they are spread widely via global air transportation. Effective health monitoring implies the development and use of a broad hierarchy of diagnostics that includes not only inexpensive point-of-care presumptive diagnostic kits (akin to home pregnancy tests), but also sensitive and precise multiplex confirmatory diagnostics (symptomatic panels such as respiratory, fever, enteric, encephalitis, etc.; also panels for food-borne pathogens, zoonotic pathogens, etc.), broad-spectrum microarray diagnostics (as a safety net to cover unusual/unexpected known single pathogens or combinations), and finally, sequencing to detect and begin to characterize unknown pathogens. It is important to note that global biosurveillance cannot consist of merely the high-consequence pathogens that the United States is most concerned with. It should offer real benefit to both the local communities and the US warfighters who may be serving there, in terms of diagnosing endemic pathogens of local concern. To be effective, the
_______________________
6 Traditionally, force protection against natural health threats was primarily addressed by WRAIR, and force protection against biological weapons was the main focus of USAMRIID; recently, however, biothreat agent funding has been approved for use in addressing pandemic threats, such as H1N1. The committee recognizes that this decision has broad implications, but believes that an examination of this issue is beyond the scope of this report.
7 Also called “species-neutral.”
United States cannot expect to merely “cherry-pick” samples of potential pandemic or bioterror cases.
Another aspect of health monitoring is that it requires the ability to be adaptive in order to respond to unknown pathogens as they appear. There may be a truly novel heretofore undiscovered/unrecognized pathogen, or bio-engineered pathogen. New “signals” must be identified and the proper responses provided for. “Signals in noise” must be discerned and put in context with and measured against routine disease outbreaks. A robust system of integrated biosurveillance, through the proper “kits” of low-, medium-, and high-resolution diagnostics, best position the CBDP to provide agile and comprehensive support to the warfighter. This is true whether the focus required is force health protection, humanitarian assistance, global biosurveillance, or intelligence to support operational or policy decisions.
Despite the fact that other government agencies have been involved with pathogen detection for many years, the CBDP does not currently have the required completed hierarchy of Food and Drug Administration (FDA)-approved diagnostics outlined above. Nor does it appear that there is any defined path through other government agencies to achieve this goal, for reasons that are beyond the scope of this committee.8 Thus, it appears to be imperative for CBDP to take up the charge of ensuring that the warfighters are provided optimal protection by ensuring that appropriate FDA-approved diagnostics are available to global biosurveillance efforts, as well as available for use in DoD medical facilities in theaters of operation worldwide. To the extent that US regulatory policies and procedures impede the timely delivery of modern diagnostics and effective countermeasures to the warfighter, CBDP should continue to actively engage with the FDA to provide needed improvements.
The responsibility to ensure that the DoD health monitoring needs9 are achieved clearly lies within the DoD. Ideally, more diagnostics could be leveraged from the Department of Health and Human Services (HHS) via the National Institutes of Health (NIH) and the Centers for Disease Control and Prevention (CDC), and/or the United States Department of Agriculture (USDA) for animal or other host/vector diagnostics, but it does not appear likely that timely completion of the required hierarchy of
_______________________
8 As discussed in Chapter 5, research-development-acquisition of medical products face unique challenges, including obtaining FDA approval. FDA-approved diagnostics may be an unrealistic goal, especially for diseases endemic to developing countries, as funding to support the development of FDA-approved diagnostics is not supported by the eventual potential market for those tests.
9 DoD health monitoring needs for the warfighter and civilian personnel are to be aware of, and be able to protect and respond to, threats to health and readiness, including those diseases that are endemic or presenting wherever DoD operates.
diagnostics will be achieved via these routes.10 Much of the work to create and test pathogen diagnostics could be performed outside of DoD and other government agencies (under appropriate government contracting methods). However, live-agent testing for many high-consequence pathogens may need to continue to be performed within government facilities to ensure the ability to do so.
Environmental Monitoring
The DoD desires to operate in a “detect to warn” mode, which translates most broadly into “detect an approaching cloud of chemical or biological agent swiftly enough to issue a warning for the warfighters to don their protective gear.” Thus, the committee’s operational definition of environmental monitoring is what is known as “standoff detection,” and typically would be utilized in the event of either a deliberate attack (chemical or biological) or an industrial accident (most likely chemical, but potentially biological).
Standoff detection is a perennial high-priority request for CBDP for base protection. Standoff detection of potentially dangerous chemical or biological materials is a difficult goal to achieve considering the relationship of distance and agent concentration upon signal detection. Achieving acceptable false positive and negative rates, considering the enormous confounding background of potential aerosolized materials, also adds to the challenge. The high concentrations of agent that are likely required for standoff detection to provide a clear signal requires the dispersion of large quantities of material—something that is less likely to be the case in an asymmetric warfare situation.11 In light of these challenges, it is clear that expertise must be obtained via collaborations between experts outside of the government with expertise and testing facilities within DoD and potentially other government agencies. Unexplored, but of potential promise, is adapting the multimodal architecture which evolved in countering improvised explosive devices to the chemical or biological detection problem (i.e., using more conventional ISR techniques that identify and track suspicious activities to queue forward deployment of chemical or biological detection systems).
_______________________
10 While it may be possible for DoD to acquire existing diagnostics from other government agencies, assay inadequacies due to age and issues related to the particular platforms used by other agencies make adoption difficult. No other agency is currently funding the range and hierarchy of modern diagnostics that the DoD requires for its worldwide missions.
11 The committee is in general agreement that active point detection (e.g., sensors in a drone) may be more likely to achieve desired results within reasonable time/cost/error constraints.
Unknown Agent Identification and Characterization
Continuing human encroachment upon remaining wild lands worldwide, particularly in tropical or subtropical climatic regions, has contributed to a continuing stream of “novel” pathogen outbreaks in recent decades. Severe acute respiratory syndrome (SARS), Ebola, and Nipah are three biological examples of novel pathogens. Non-traditional agents are similarly unpredictable in the chemical domain. DoD requires that there be a robust (available, timely, and skilled) capability to identify and characterize unknown agents, both biological and chemical. This capability can be generalized as a hierarchy of detection approaches: [low resolution] presumptive field assays (rapid and inexpensive) that can rule dangerous agents in or out; [medium resolution] sensitive laboratory confirmatory tests for all known dangerous agents (biological or chemical); and [high resolution] robust suite of broad-spectrum analysis technologies to detect and characterize new, mutated, or engineered dangerous agents.
In this area today, DoD collaborates with many of the best researchers outside of government. However, it may well be necessary that DoD have an in-house capability so that classified samples can be processed. It is important that a robust identification and characterization capability for unknown agents be maintained. DoD has facilities and competent staff who should certainly be part of this capability. There are other non-DoD government agencies such as the Department of Homeland Security’s (DHS) National Biodefense Analysis and Countermeasures Center (NBACC) facilities that have competence with unknown agent identification and characterization that can be considered part of the overall capability.
CBDP is currently sponsoring strong collaborations outside DoD for unknown biological agent identification and characterization. This collaboration involves scientists at DoD laboratories and is providing valuable training and technology transfer to their facilities. This successful model should be sustained and enlarged to include other key US participants as part of a robust US response to novel biothreats.
CHEMICAL AND BIOLOGICAL AGENT DETECTION
Detection of relevant chemical and biological agents is an important capability that underpins the CBDP. Sensors are needed that not only detect and identify agents over a wide range of concentrations, but also quantify airborne, waterborne, and surface contamination levels with adequate specificity to minimize false positives while responding as near to real time (seconds) as possible. The program has developed a wide range of point detectors with varying levels of sensitivity, specificity, and response times for a variety of relevant agents. In many cases improvements in sensitivity, specificity, response time, automation, robustness, size, weight, power consumption, and consumable reagent use would be beneficial. The
development of standoff detection instruments with adequate sensitivity, specificity, response times, and multiagent capability has been difficult.
Systems that substitute real-time point agent detection deployed on unmanned air or ground vehicles may supplement or replace traditional remote sensing systems. Agent detection instrumentation systems that include automated sample collection, instrument control, data acquisition, and analysis and handling must be engineered for both laboratory and field use; and suites of instruments may need to be integrated with either static or mobile platforms for field deployment. Accurate agent transport analysis models to predict future agent distributions based on current agent distributions are also required.
The committee has identified four primary S&T capabilities that are required for the CBDP to achieve their Chemical and Biological Agent Detection objectives. The S&T capabilities are Analytical Methods Discovery, Instrumentation Development, Sensor Systems Development, and Agent Transport Analysis.
Analytical Methods Discovery
Current and anticipated chemical and biological agents must be characterized to identify and assess unique molecular and/or biological features that can be exploited to allow detection at acceptable levels of sensitivity and specificity in as near real time as possible. Chemical agent detection might be based on spectroscopic, mass spectrometric and/or chromatographic or mobility properties of the molecular agent or a derivative agent reaction product. For biological agents, detection may be based on DNA or other biomarker identification (e.g., protein, RNA). The capability to determine which structural and/or reactive agent properties can be exploited for reliable agent detection is the critical first step in developing reliable analytical agent detection methods. Subsequent experimental work can determine which identified analytical methods are reproducible, robust, and inexpensive enough that they could be developed into reliable laboratory and field agent detection technologies.
Analytical methods discovery is primarily a basic science endeavor in molecular recognition. This capability can be obtained in DoD laboratories, other government laboratories and in academic and private-sector laboratories. However, the ability to provide and safely handle the chemical and biological agents12 necessary to test and assess analytical methods is reliably found in DoD facilities (including test ranges).
_______________________
12 The committee recognizes that there are many laboratories throughout the United States with the capability to work with up to Biosafety Level 4 (BSL-4) pathogens. These private laboratories, however, can be closed at any time for any reason (e.g., an accident at a privately owned BSL-4 laboratory may lead to immediate, and possibly permanent, closure).
DoD laboratories will often benefit by collaborating on innovative analytical methods development with leading academic or private-sector analytical methods development laboratories. A formal requirement for the laboratories to engage the extended analytical research and development community will likely increase the success of this S&T capability for DoD. Conversely, non-DoD laboratories will likely need to collaborate with DoD facilities for live-agent testing efforts.
Instrumentation Development
Promising analytical research methods capable of detecting and quantifying chemical agents need to be developed into reliable instruments capable of routine, and ideally automated, laboratory and field measurements. For detection of many biological agents, effective laboratory assays must be periodically re-evaluated to ensure that recently accessible strains are appropriately classified within the assay. The development of robust and reliable instruments requires a wide range of scientific and engineering skills that are often not all available in individual laboratories. Soon after proof-of-principle experiments have been successful, developers of analytical methods that may serve as the basis for innovative instrumentation should involve potential collaborators (especially potential end-users) to define mission-realistic measurement requirements and potential instrument specifications.
Since successful instrumentation development requires such a diverse range of scientific and engineering skills, collaboration is often required. While the basic analytical methodology may have been developed in the DoD, other government, academic, or private-sector laboratories, the full range of skills required to develop and demonstrate robust and reliable instruments are found predominantly either at selected government or private-sector laboratories. Further, cost-effective routine production of instruments is usually only available in the private sector.
Sensor Systems Development
Individual agent detection instruments have limited utility, especially outside a laboratory. To be fully useful, field instruments need to be integrated into a suite of instruments and deployed as a measurement system with automated sample acquisition, instrument control and data acquisition, quality assurance, analysis, transmission, and archiving capabilities. Laboratory instruments may not need to be as fully integrated. Agent-detection instrument suites may be deployed at fixed sites or on mobile platforms, including ground vehicles, ships, and piloted or unmanned aircraft.
Some sensor system development capabilities are available at DoD laboratories, other government and government-sponsored laboratories, and in the private sector and often require collaboration between these entities to acquire the required range of scientific and engineering skills. Systems integration with mobile platforms is often done by private-sector engineers. Instrument system testing and evaluation with actual agents usually require DoD facilities or collaboration.
Agent Transport Analysis
Understanding the present or future distribution of dispersed chemical or biological agent is an important capability for DoD personnel dealing with an attack. Airborne agents can be dispersed by the wind, as can evaporating or re-aerosolized agents from contaminated surfaces. In these cases atmospheric dispersion and meteorological models can be used to predict how fast and how far agent contamination might spread. Agent-contaminated surface waters can also be dispersed by stream flow and/or wave action and contaminated soil can be aerosolized as dust or be transported by vehicle tracks or tires. Precipitation can help transport agent into exposed soil. The development and testing of models to assess agent dispersal mechanisms and rates in various environmental media is a multidisciplinary challenge, and understanding these effects will have implications both for detection system and method development and the testing and evaluation of those systems. Biological agents can be dispersed by both the physical mechanisms listed above and by infected animal, bird, insect, and/or human vectors, creating additional challenges (see section on agent transport and viability analysis in Hazard Assessment, Management and Decontamination).
DoD laboratories have limited capabilities to develop or refine environmental agent dispersion or infection outbreak models. The Defense Threat Reduction Agency (DTRA) has recently funded significant research and development (R&D) activity to develop and test improved atmospheric dispersion models, including inverse models that predict agent release point and size from downwind concentration data. Most ground-breaking environmental dispersion research is currently performed in other US government laboratories (e.g., National Center for Atmospheric Research, Lawrence Livermore National Laboratory (LLNL), Sandia National Laboratory, Los Alamos National Laboratory (LANL)) or in academic or private-sector laboratories.
Many biological agents (excluding toxins) have a reproductive capability that differentiates them from chemical agents. This requires analysis of both agent fate, transport (effect on individual spores/cells/virions, etc. due to physical processes), and transmission via reproduction in hosts
and/or vectors. Agent fate studies examine the impact of factors such as ultraviolet light, desiccation, and re-aerosolization upon biological agents at the individual level. Agent fate studies are performed in academia, in DoD and other government laboratories, and in industry. Agent transmission studies focus on population behavior of biological agents across large or small outbreaks that may span time scales far longer than those of individual agent particles. Such epidemiological modeling of infectious disease outbreaks is performed in academic centers, Department of Energy (DOE) laboratories, and in other government agencies (e.g., CDC and USDA). The CBDP should consider continuing to outsource most of their R&D in this area, specifically for biological agents, and focus on collaborative relevant live agent testing.
INDIVIDUAL AND COLLECTIVE PROTECTION
Deployed troops confronted or threatened with dispersed chemical or biological agents need both personal protective gear and collective protective shelters that will mitigate agent effects to the extent feasible. Personal protective gear may include respiratory masks and controlled-permeability suits, boots, and gloves. Collective protection may be afforded by air-locked and sealed temporary battlefield shelters with filtered air supplies, as well as by more permanent sealable, air-filtered spaces shipboard or at operational bases and other military facilities. Both personal and collective protective gear must be designed to minimize physiological stresses induced by their use, including heat stress, respiratory difficulties, excess weight burdens, cardiac stress, and sweat and waste accumulation.
The committee has identified five S&T Capabilities that support Individual and Collective Protection. These include Controlled Molecular Transport Materials Discovery, Barrier Materials Engineering, Personal Protective Systems Development, Collective Protection Systems Development, and Physiology.13
Controlled Molecular Transport Materials Discovery
Materials scientists can now design and characterize materials, fabrics, polymers, and construction materials tailored to restrict agent penetration while allowing passage of oxygen, water, carbon dioxide, and other substances that humans need to consume or emit. Robust, lightweight materials with controlled chemical and heat transfer properties
_______________________
13 Prophylactic medications and topical creams are covered in Medical Countermeasures below.
can be used to develop improved absorbents, fabrics, and other materials required for improved personal and collective protection gear. Materials can also be designed to trap or deactivate chemical or biological agents. The design of new tailored materials may greatly improve the effectiveness and greatly reduce the stressors associated with the use of current protective gear.
While DoD laboratories have some capability to achieve the fundamental materials science advances required to design and demonstrate new materials with tailored mass, heat transport, and agent deactivation properties, much of the nation’s ground-breaking capabilities are found in academic and private-sector laboratories. The committee recognizes that significant collaborative efforts in this area are ongoing and continued collaboration with selected non-government laboratories is advised.
Barrier Materials Engineering
New prototype materials with controlled molecular transfer properties have to be engineered into fabrics, plastics, filters, and other robust materials that can be used to develop and test better protective gear. Properly engineered new materials with tailored mass and heat transport properties are the key to more effective and usable personal protective gear. This directly affects the mission capability of the warfighter.
DoD laboratories do have significant capabilities in barrier materials engineering, but collaboration with selected academic and private-sector scientists and engineers is advised to ensure in-house capabilities are regularly renewed and expanded. DoD personnel and facilities will usually be required to test new materials against relevant threat agents.
Personal Protective Systems Development
New material formulations have to be incorporated into prototypes of improved protective gear by teams of experienced materials scientists and engineers. DoD laboratories have suitable expertise and should continue to lead in the production of initial prototypes. Still, they should seek to collaborate with private-sector technical experts as they may be required to produce sufficient items for extensive test and evaluation activities. Skillful prototype development and extensive and realistic testing is critical to achieving improved personal and collective chemical and biological (CB) protection products.
Given the current lack of commercial markets and the relatively small size of the DoD market for CB protective gear, limited prototype engineering capability is available outside DoD. In addition, DoD personnel and facilities will usually be required for extensive prototype testing.
Collective Protection Systems Development
Collective protection systems development involves some additional considerations compared to personnel individual protection. New material formulations have to be incorporated into prototypes of improved collective protection systems (e.g., shelters) by teams of experienced materials scientists and engineers. Shelter materials must have structural strength as well as agent protection properties. Skillful prototype development and extensive and realistic testing is critical to achieving improved collective CB protection systems. These systems directly impact warfighter survivability in both base and forward environments.
For the reasons discussed in the personnel protection systems development section above, limited prototype engineering capability is available outside DoD and close collaboration with the few commercial suppliers is needed.
Physiology
The ability of individual protective gear (e.g., suits, masks, gloves) to permit operations in the presence of chemical or biological contamination must be weighed against the effects of the protective gear (e.g., increased heat load, increased moisture retention, decreased manual dexterity and overall maneuverability, potential psychological effects, etc.). Expertise in human physiology is required in all stages of design, development, testing, and operational transitioning. To a somewhat lesser degree this also can apply to collective protection (e.g., shelters).
It is imperative that the warfighter be provided with effective individual and collective protective solutions, and access to skilled human physiology expertise is extremely important in the design, testing, and deployment phases. Basic human physiology expertise can be found outside of the DoD and government. To be maximally relevant to CBDP, extensive on-site collaboration with the designers, testers, and ultimate end users of the protective gear is mandatory for any physiology collaborators.
Development of countermeasures, both prophylactic and therapeutic, is vital to provide the warfighter protection against either biological or chemical agents. The S&T capabilities required for development of these countermeasures have increased in complexity14 due to the requirement
_______________________
14 The complexity arises from the need to perform efficacy testing of a candidate compound. Because of the inability to perform this testing in human subjects, the FDA Animal Rule for efficacy has been introduced (vida infra).
that the DoD acquire FDA licensure for products given to military service members. Many of these capabilities should be coordinated with ongoing activities with NIH and the Biomedical Advanced Research and Development Authority (BARDA), agencies that are developing CB countermeasures for the civilian populations and have many overlapping capabilities required for countermeasure development that can be leveraged. The committee found eight S&T Capabilities that are needed to achieve CBDP objectives in Medical Countermeasures: Target Discovery, Regulatory Science, Mechanisms of Delivery and Delivery Systems, Animal Models, Host Response, Pre-Clinical Studies, Clinical Trials (GLP and GMP), and Medical Product Development.
Target Discovery
Identification of critical targets for the development of new vaccines, antibiotics, diagnostics, and other pharmaceuticals are required for the development of countermeasures to protect the warfighter. Continued target identification for new and existing DoD-relevant biothreats will be critical for development of new innovative countermeasure drugs, vaccines, and potentially new therapeutic platforms.
In general, and particularly for biological agents, there are many outside sources that can provide this service either within the pharmaceutical or biotechnology industries or academia. The caveat is that this capability may be limited by the biosafety level required for the development of the targets. For example, if target development or antibiotic screening requires live, virulent agents and it is a BSL-4 agent this significantly limits where the studies can be performed. In many cases initial screens have been performed and reported that can be leveraged by the DoD laboratories for licensing and further development. The most productive route is likely through collaborations with industrial, and in some cases academic, partners willing to apply their target identification strategies to pathogens with high DoD relevance.
Regulatory Science
Over the last decade there has been recognition by many of the FDA regulatory challenges involved in countermeasure product development due to the multiple deficits in our basic understanding of many of the biothreat diseases and the application of the “Animal Rule”15 to the process. The acknowledgment of the impact of the FDA requires a product development process that embeds an element of regulatory science with
_______________________
15 12 CFR Parts 314 and 601.
appropriate expertise related to the application of Good Laboratory Practice (GLP)-based assays and protocols. In many cases the implementation of this capability requires a significant addition to current infrastructure and a shift in the research culture at appropriate institutions. Full understanding of key elements of regulatory science will be critical for the successful development of countermeasures. The committee was informed that DTRA is now supporting an FDA effort to establish a new genome reference database to accelerate the validation process for highly multiplexed assays utilizing modern technologies. This is a laudable example of interagency collaboration.
There are multiple contract research organizations that are well prepared and have the capabilities of applying GLP work for product development up to BSL-3. While this covers the vast majority of potential biothreats, BSL-4 biothreats and some chemical agents, a number of other biothreats, and chemical agents may require DoD expertise and facilities. Maintenance of these latter capabilities focusing on specific high-priority pathogens and chemical agents should be maintained for test and evaluation (T&E).
Mechanisms of Delivery and Delivery Systems
This set of capabilities is concerned with being able to deliver pathogens, drugs, and vaccines by multiple routes that will mimic likely exposure and therapeutic delivery modalities. For example, one clear modality of concern to DoD is aerosol delivery which can be used in exposure protocols or as a strategy for therapy delivery to a target organ.
It is vital for model development to maintain expertise in delivery strategies that are likely to be the route of exposure for human populations. Aerosol delivery has been the focus for decades, but all routes need to be considered (e.g., food may be seen as a bioagent delivery mechanism by terrorists). Further, progress in therapeutics is occurring not only by breakthroughs in target identification and chemical modifications, but also in delivery mechanisms. For example, the use of nanoparticle-based delivery mechanisms is being investigated by both pharmaceutical companies and basic research scientists worldwide. The capability to perform the delivery may be as important to CBDP as studying the basic science behind the delivery. Strong connections to appropriate institutions that perform this work should be developed so that DoD personnel can be trained and current infrastructure updated as needed.
Animal Models
Direct human testing will not be possible with most, if not all, biothreat agents to allow for the evaluation of countermeasure efficacy in humans. Therefore, the capability to develop high-quality animal models of infectious diseases in multiple species is critical for countermeasure development. Animal models against chemical agents and toxins are required for similar reasons. The maintenance of this capability requires multiple disciplines including pathology, immunology, systems biology, and aerobiology. The latter skill is required in order to accurately mimic the most likely exposure routes that military personnel will face. These models should be readily available to rapidly assess new antibiotics or vaccines that are developed, whether developed by DoD or elsewhere.
The “Animal Rule” requires that sponsors of products in development demonstrate efficacy in at least two animal species that accurately represent the infectious disease in humans. In some cases, this requirement is extremely difficult because of the limited knowledge base related to some of the infections (e.g., viral hemorrhagic diseases) most relevant to military personnel. Thus, it is important to remember that there remains a significant amount of basic pathophysiology that needs to be performed in animal model research as well as efficacy testing.
This capability is one that can be achieved through multiple routes as there are multiple outside parties that can perform both proof-of-principle and GLP models in multiple species, including primates. Furthermore, HHS, including NIH and BARDA, has invested in academic institutions to develop capabilities in model development for a variety of biothreats. Many universities have now developed the infrastructure for producing reports compatible with product development. While there is now broad development and use of animal models in academia and industry, the DoD laboratories needs to maintain, at least in part, this capability for pathogens and chemical threats that require highly specialized (bio)containment facilities to ensure availability.
Host Response
Ordinarily this would be a component built in to the animal model development phase; however, there are specific elements of the “Animal Rule” that make this capability of critical importance particularly as it relates to vaccine development. Thus, the “Animal Rule” requires that the protection elicited by a countermeasure in an animal model must mimic a similar type of response in the human. Typically this is referred to as “correlates of protection.” While this is more easily proven for antibiotics and chemical agents, vaccines generate host-driven immune responses that can be quite variable and unpredictable among species. Therefore,
understanding the mechanisms of protection in the animal models, as well as developing innovative strategies for collecting as much information regarding the mechanisms of vaccine protection by humans, is critically important for countermeasure development.
Since the correlates of protection are a major milestone for countermeasure development through the “Animal Rule,” this capability is critical for countermeasure development. It is important to realize that the anticipated design of procedures needed to meet the Animal Rule requirements shall begin early in the R&D process as it may change how pivotal experiments are performed.
Since at this point there is no roadmap for developing correlates of protection, this process is likely to be a multidisciplinary approach requiring multiple collaborations with agencies that can provide strong computing power and relevant data from in vivo and in vitro assays. Such strategies are probably best performed via strong project management coordination with funded academic centers and other agencies.
Pre-Clinical Studies
This capability encompasses a broad range of activities including GLP studies. Such activities include proof-of-principle studies for efficacy, dose-ranging studies, pharmacodynamics and pharmacokinetics, and toxicology. These activities are critical to down selection of new therapeutics. Optimally, these would be performed in a head-to-head fashion to compare potential products for further development.
Some of these studies are small in size and scope, therefore, lending themselves to outsourcing. Except in cases where the BSL category would limit its location, these studies could be performed inside or outside of DoD. The construction of the new BSL-4 at the University of Texas Medical Branch provides an example of a conduit for performing even high biocontainment agent studies outside of DoD. The new DoD laboratories will have expanded capacity to do this work as well.
Clinical Trials (GLP and GMP)
While efficacy trials for threat agents will not be performed in humans, the FDA still requires appropriate human safety trials. This capability will be required for advancement of drugs beyond animals, and requires access to appropriate infrastructure to handle human clinical trials (i.e., institutional review boards, informed consent, and safety monitoring). Included in this activity is post-marketing surveillance (phase 4) where if the product needs to be utilized, there is an infrastructure in place to monitor outcomes regarding safety and efficacy and potential delayed effects during large-scale use.
Advanced development and acquisition of FDA licensure will require multiple stages of clinical trials. Demonstration of safety is a critical component, not only to the FDA, but also to the military personnel who will be receiving the new pharmaceutical.
This capability has been performed for decades outside of DoD by medical schools and agencies that perform this activity under contract. The DoD should leverage the external capabilities (within or outside of government) available for clinical trials rather than replicate it.
Medical Product Development
Methodologies of product production have evolved over the last decade. Because of the evolving nature of the threat, the ability to rapidly scale up manufacturing to provide safe and effective countermeasures for military personnel is needed. This capability would allow the military to have flexibility with regard to its manufacturing strategies and allow for rapid implementation of new product production without major delay.
This capability is likely best found, or developed, outside of DoD with collaborations with academia, industry, or combinations thereof where the experience of product development and appropriate personnel are maintained.
HAZARD ASSESSMENT, MANAGEMENT, AND DECONTAMINATION
In the DoD context, hazard management is basically limited to avoidance, quarantine, or decontamination. This section will focus primarily on the S&T aspects of decontamination. Hazard assessment has two aspects: detection of the hazard (which was covered above) and determination of how well the hazard has been decontaminated, which is discussed here. In order for the CBDP to address the area of Hazard Assessment, Management, and Decontamination the committee identified five S&T Capabilities that are needed by the CBD Program. These include Decontamination Methods Discovery, Decontaminant Development, Decontamination Resilient Materials Development, Decontamination Systems Engineering, and Agent Transport and Viability Analysis.
Decontamination Methods Discovery
Methods discovery for decontamination concerns the research and development to identify what will neutralize or kill chemical or biological agents. Ideally, decontamination methods will be effective against a broad array of potential agents and pathogens. Also, it is desirable that methods
will not be corrosive or otherwise destroy the functionality of materials and equipment being decontaminated.
Without effective decontamination methods, the only choice will be to sequester any material, equipment, or area that has been contaminated. For some agents (e.g., anthrax spores which can survive for decades) this could effectively mean near-permanent denial of use.
Methods discovery can and does occur outside of the government. CBDP should partner with the best expertise available to obtain the needed methods discovery. However, testing of the effectiveness of any method can and should be done with real agents in a DoD facility. Testing at scale for any decontamination methods requires the use of extremely focused DoD facilities.
Decontaminant Development
In order to be an effective decontaminant, a substance must be capable of being applied in an appropriate manner, be effective in real-world environments where surfaces will not be in a pristine condition, and result in an ability to be functionally restored (e.g., electronics are not destroyed).
Without decontamination substances there will be a lack of ability to continue missions in both the near and long term. While fighting contaminated might be effective for an individual campaign, it is not effective for a war. Having effective decontamination substance(s) available when necessary is essential for the warfighting equipment and for all supporting equipment. It is also necessary to have wide-area decontamination substances and delivery methods available for battle spaces, and especially necessary for civilian areas. Appropriate consideration of concept of operations (CONOPs) will enable DoD to develop different decontaminants to address different operational needs (i.e., decontamination of a military aircraft in a campaign is different than decontamination of a building).
To obtain this capability, CBDP should partner with the best expertise available to enable the needed decontaminant development. However, testing of the effectiveness of any substance can and should be done with real agents in a DoD laboratory or facility.
Decontamination Resilient Materials Development
The development of materials that can withstand decontamination processes, or even self-decontaminate (e.g., paint or fabric embedded with neutralizing additives), is needed for warfighting and support equipment. Such materials would survive, with full functionality, a standard low-cost decontamination process or would not need to be decontaminated
at all. Resilience is especially important for high-value, sophisticated equipment such as electronics in aircrafts, or for porous materials, such as painted surfaces or seat cushions, that are quite difficult to effectively decontaminate.
In the absence of developing materials that are resilient or self-healing, decontamination substances and systems will require much more sophistication and time to develop. Resilient material development could greatly reduce the time, cost, viability, and effectiveness of decontamination.
Resilient materials development is an important capability that can be advanced in parallel with other decontamination strategies. The CBDP should leverage outside sources that are already working on this problem. Rigorous testing of the effectiveness of any material can and should be done with real agents in a DoD governmental laboratory or facility.
Decontamination Systems Engineering
The decontamination system includes the means of delivery of a decontamination substance, the logistics for the decontamination, i.e., moving the material to the proper location, cleanup after the decontamination process, and an ability to determine the decontamination has been effective.
There are two types of systems: (1) material and equipment and (2) wide-area battlefield, containment area, or urban area. For material and equipment, the logistics are quite different and easier to develop and implement than those for a wide area. In a wide area there likely needs to be decision aids to help determine the priorities and methods (a completely different type of CONOPs from material or equipment) for effective decontamination. In all cases, the tracking of contaminated substances to other areas and the potential for re-suspension of materials must be considered.
Decontamination substances alone will not be enough to fully utilize and maintain warfighting capabilities during wartime. Without the systems decontamination ability, decontamination will not work and material, equipment, or wide areas will simply need to be declared unusable.
Decontamination systems engineering can and does occur outside of the government. CBDP should partner with the best expertise available to obtain the needed decontamination systems engineering. However, testing of the effectiveness of any engineered system and getting feedback from operators on the process and decision aids will be essential. While the ability to test decontamination systems on real equipment exists within the DoD laboratories and facilities, the reluctance (due to overall cost of test and decontamination) to actually conduct the tests seemed very high, thereby making them unavailable for full-scale, live-agent testing.
Agent Transport and Viability Analysis
Decontamination of biological agents is highly agent specific. Some biological agents are quite fragile and will only survive a brief exposure to the environment (e.g., ultraviolet light kills many bacteria) while others have extremely robust survival mechanisms (e.g., anthrax spores can survive for decades). Many robust operational molecular detection methods for biological agents are based on nucleic acids and will correctly detect both live and intact DNA as well as dead non-intact DNA—without distinguishing between the two. Thus, robust, timely viability assays are important for successful hazard assessment, management, and decontamination. CBDP needs to be able to deliver robust viability assays for a wide range of potential biological contaminants. Methods for characterizing chemical agent contamination on either military equipment or environmental surfaces also require continued development. Recent advances in ambient ionization mass spectrometry methods provide real-time surface contamination measurements that can determine distributions of agent contamination both before and after decontamination treatments.16
To the DoD, a critical question after a biological contamination incident will be “Is it safe now for people to remove their protective gear and resume normal operations?” Thus, the ability to provide robust viability assays for any biological agents that could be reasonably anticipated is of high importance to CBDP. Of equally high importance is the ability to rapidly create robust viability assays for any additional biological agents that were not anticipated or known in advance of their use.
As with detection assay development, viability assay development can and does occur outside of the government. CBDP should partner with the best expertise available to obtain the needed viability assay capabilities for program specific needs (i.e., threat agents). Ambient ionization mass spectrometry systems that can be adapted to characterize chemical agent contamination of surfaces is now available from several commercial sources.
CROSS-CUTTING SCIENCE AND TECHNOLOGY
Numerous critical aspects of CBDP underlie many of the specific capabilities discussed above. These foundational capabilities are discussed in this last section; however, they should be recognized as being of crucial importance to each of the operational capability sections discussed above. It should also be noted that many of these infrastructural capabilities are
_______________________
16 The National Research Council Committee on Assessment of Agent Monitoring Strategies for the Blue Grass and Pueblo Chemical Agent Destruction Pilot Plants recently completed a study in this area. Their report can be found online at www.nap.edu.
assumed to be fully available by other program elements for their use (often at no cost or at less than full-cost recovery), yet funding for infrastructure may have dropped below critical mass in some cases. The thirteen cross-cutting issues identified by the committee include Acquisition, Maintenance and Transport of Critical Chemical and Biological Reagents; Simulation; Informatics; Forensics; Education and Training; Behavioral Analysis; Systems Analysis and Engineering; Repurposing Commercial Technologies; Systems Biology; Synthetic Chemistry and Biology; Materials Science; Statistical Measurement Design; and Test and Evaluation.
Acquisition, Maintenance, and Transport of Critical Chemical and Biological Reagents
CBDP needs to be able to acquire or generate, maintain, and transport all chemical and biological reagents necessary to support the development and testing of detection, characterization, and viability assays. This includes traditional chemical agents, non-traditional agents, and biological agents and toxins, as well as all other relevant agents needed for test panels and other research, development, test, and evaluation purposes. Lack of required reagents in a timely fashion can slow or seriously derail nearly all major CBDP research, development, test, and evaluation programs. This cross-cutting S&T capability supports nearly all other programs within CBDP.
Certain aspects of both chemical and biological reagent creation, use, and transportation are severely limited by US and international law and conventions.17 There are very few facilities permitted to create or work with threat agents. Similarly, there are relatively few facilities where BSL-4 Select Agent use is permitted. In the case of Select Agents, there are facilities outside of the government that are permitted to create and work with these agents. There are numerous facilities that are permitted to work with BSL-3 biological agents (including relevant Select Agents).
The current DoD Critical Reagents Program maintains a robust capability to grow, maintain, and transport biological reagents. This includes live agent, DNA/RNA, and antibodies. Similarly, the ECBC is where controlled chemical agents are manufactured and transported for DoD research and live-agent testing needs. The DoD is a primary source of capability for live-agent production and testing of both chemical and biological agents, particularly in terms of the scale of the testing facilities.
_______________________
17 The Chemical Weapons Convention (CWC) permits member-states to operate a single small-scale facility for the production of chemical agents that are included in the schedules of the CWC. The Edgewood Chemical Biological Center (ECBC) is currently the designated facility in the United States.
Agent Simulation
Many of the chemical agents cannot be tested in full-scale outside exercises. Information about them must be inferred from “simulants”—that is, compounds that closely approximate the properties of the molecule in as many properties as possible other than toxicity—or by “simulation”—that is, computing the behavior and characteristics of the compound from study of the behavior and characteristics of compounds of similar structure. Although computer-based simulation has developed extraordinarily rapidly, accurate simulation still depends on a foundation of empirical knowledge obtained through experiment. The DoD laboratories are uniquely equipped to carry out calibrating studies with actual agents in secure laboratories; but some types of tests require large-scale experiments, or experiments under a variety of conditions that cannot be simulated accurately in the laboratory.
The state of development of chemical simulants could be substantially improved. A number of compounds have been examined, but work in this area has been limited for reasons unrelated to the science: (1) simulants do not replicate the properties of the agents; (2) even if good simulants were developed, their use would require complex environmental protocols to demonstrate acceptable environmental impact; and (3) they might be expensive. These statements may well be correct, but given the importance of the problem, they are not acceptable reasons to neglect the development of accurate simulants. This activity is one that has the potential to be the basis for a good collaboration with university laboratories, with the universities combining physical organic chemistry, synthesis, and computer-based simulation of properties, and the DoD laboratories carrying out comparisons with active agent. Alternatives have to be developed based on considerations of structures. It is important to emphasize that a simulant does not have to behave indistinguishably in all respects from the chemical agent to be useful. Thus, a compound that would simulate permeability through fabric would not have to replicate volatility; what is required is that the differences are known and can be corrected for.
With these points of calibration, it should be possible to build more elaborate and predictive computer models for dispersal, migration, environmental deactivation, skin permeability, and so on; the technology for such models has developed very rapidly in the last years, but has not permeated the DoD laboratories concerned with CBD, other than in some areas of dispersal and related hydrodynamic issues.
It is critical, in the committee’s view, to pursue a program in simulants and simulations aggressively; not to do so risks making claims for effectiveness of technology and doctrine that may not hold up in conditions that have not been tested.
Informatics
Biology has recently matured to become an information-dominated science. Chemistry has been in that state already for many years. Both bioinformatics and cheminformatics are essential underpinnings for all research and most development efforts in CBDP. Bioinformatics is a broad term that can be used to cover both infrastructure (e.g., sample tracking, laboratory information systems, web sites, and databases) and more domain-specific computation (e.g., genomic comparisons, protein structure modeling, and pathway analysis). Similarly, cheminformatics can describe, for example, atomistic modeling, small molecule docking, and drug compound in silico screening.
The existence or non-existence of sufficient informatics capability can literally make or break critical projects and programs in chemical and biological defense. In the biological domain, information is undergoing exponential growth driven by a recent 1,000 times increase in DNA sequence data production rates, and informatics will become an increasingly important tool for CBDP in the coming years.
Currently most of the informatics capabilities are found outside of government proper. Some DoD laboratories have made large strides increasing their in-house capabilities in the past few years, particularly in terms of de novo annotation of bacterial genomes. It appears that increased collaborations with outside experts would be the most efficient way to ensure that CBDP programs are receiving the required informatics expertise in both the chemical and biological domains.
Statistical Measurement Design
Proper experimental design underlies testing and evaluation of all CBDP products and systems. How many times and at what concentrations of agent should a new piece of protective gear be tested? What percentage of production lots should be tested to ensure high confidence that those lots can be delivered to warfighters for effective use? Rigorous statistical expertise should be applied to both design the right number and nature of experiments as well as to accurately assess the results.
The lives of warfighters are at stake for virtually every product that results from a CBDP. The proper design and analysis of test experiments is therefore of very high importance to CBDP.
Statistical testing expertise is available at multiple locations outside of government. The committee observed evidence that there could be greater use of external expertise to review testing plans that are currently created internally and apparently not subject to independent external review (see also Test and Evaluation).
Forensics
An important goal of CBDP is detecting and mitigating chemical and biological threats to the warfighter. A secondary goal, however, is to develop answers to key intelligence questions related to the perpetrators, origin of the source materials, and how the biological or chemical weapon was produced and disseminated. For example: How did a food poisoning event transpire? Was anthrax used on US or allied forces from a natural exposure in contaminated soil or was it engineered in some fashion to be more lethal? Was it a strain one would expect to find in that location of the world? Could it be associated with a strain that has been or could be identified as having originated from a known facility? Could any chemical signatures in the agent used on US or allied forces indicate what production process was used, who might have the capacity and competency to produce or disseminate agent, and who might have aided the perpetrators?
Chemical and biological weapons forensics may leverage many of the techniques and technologies used for detection diagnostics, plus other techniques that examine orthogonal dimensions (e.g., isotopes in the water used in manufacturing might provide a clue to origin). CBDP has invested in some of these activities. For example, DNA sequence analysis may drive both the development of detection diagnostics and supply evidence of potential genetic engineering, which is of forensic value. For several reasons, the DoD chemical and biological laboratories may be involved in attempts to augment other US government capabilities to perform forensic analysis of incidents affecting US forces in order to inform attribution decisions and mission planning and decision making.
The committee is aware that chemical and biological forensics is not currently in the purview of CBDP, but is handled by other DoD and DOE elements. Chemical forensics is handled primarily at two sites (ECBC and LLNL). Bioforensics is presently in a more nascent form. Greater synergy between the CBDP, DHS, and intelligence community programs, including the Defense Intelligence Agency, could prove useful to improve overall DoD diagnostics and forensics capabilities.
Education and Training
Implementation of capability in CBD will usually have components in both technology and training in use of the technology: the latter may be the more difficult, but depends on the former. Ease of use is a key concern in the development of most CBD programs.
The development of aids for education and training has been a long-standing interest in the DoD, with programs such as SIMNET and the Medical Management of Biological Casualties “Blue Book” being pioneer-
ing efforts. Adapting computer-aided programs from a wide variety of apparently unrelated activities (e.g., for sonar operators, helicopter pilots, and sniper detection) could provide useful methodology at relatively low cost. The development of realistic training protocols, with long-lasting impact, is a more complex subject. Since technology for CBD cannot be tested and learned in a “real” environment (as in live-fire exercises), and since a serious CB attack, or even a threat of one, produces great confusion and misuse of technology (as judged from experiences in the early days of the Iraq wars), the development of adaptable, durable, robust training protocols for users is an important issue, but one from which there is little established technology.
Behavioral Analysis
Earlier we discussed how physiology was important to study the impact of chemical and biological protection on the physical ability of the warfighter to function in protective gear. Similarly, behavioral analysis is important to understand the mental state of those required to perform their missions under the added mental and physical stress of an impending or actual chemical or biological attack.
Even if individual and collective protection gear functions perfectly as designed, there may be individuals who increase their risk of exposure due to behavioral factors (e.g., claustrophobia, extreme irritability due to discomfort, etc.). It is important for CBDP to learn as early as possible in the design, development, and test cycle whether new equipment has any characteristics that may increase the likelihood of such behaviors. A thorough understanding of behavioral factors may also provide useful design requirements.
Most human behavioral expertise resides in academia. Close collaboration between academia and DoD researchers, product developers, and operators will be needed to effectively translate this expertise into a useful outcome for the DoD.
Systems Analysis and Engineering
System analysis refers to an overall analysis of the various alternative means of meeting the mission requirements. Cost/benefit, threats, CONOPs, manufacturability, human factors, and behavioral analysis are all capabilities needed to conduct an appropriate systems analysis. Systems engineering refers to the detailed examination of how the totality of an individual system is likely to perform in its operational environment. Computer simulations are a critical part of systems engineering and include many factors of informatics.
A system analysis performed early in the R&D process can often eliminate technically interesting but operationally inadequate solutions. Integrating end-user expertise into a system analysis can lead to discovery of entirely new solutions to mission requirements.
Without systems engineering, the performed solution at best may be overly expensive, and at worst will fail to meet operational requirements.
System analysis and engineering are critical to CBDP, especially when incorporated into the process at early stages and updated throughout the RDT&E process. High-quality system engineering and analysis capability exists both inside and outside the government, although it does not seem to be resident throughout the CBDP.
Repurposing Commercial Technologies
The committee encourages the entire CBD community to take an active approach to following scientifically related fields of R&D and product development in an effort to identify non-CBDP projects, products, and personnel that may aid the CBDP meet its mission without direct, or with reduced, investment and shorten time to solution. Most of the activities involved (literature and public press tracking, active engagements in societal meetings, and engagement of fellow US government colleagues) are likely performed already by nearly every CBDP scientist and manager. Additionally, actively engaging pre-competitive alliances (particularly in the pharmaceutical industry) could provide the CBDP with sufficient direct awareness of the research-of-interest and partnership connectivity to justify the relatively modest membership fees.
In the procurement-type strategy much of the innovation for the required DoD capability or product is developed during the early stages of research and then subsequently transitioned into a scale that is needed. In some cases however, due to the rapidity of technology development, such as the materials sector and medical research, there can be a significant breakthrough that could rapidly be incorporated to provide a needed product.18 The procurement mechanism lends itself toward the development of “blinders” that may mask the incorporation of concurrent innovative solutions to ongoing needs by limiting the ability of incorporating new concepts or emerging technologies into the process. The incorporation of a “tech watch” concept into the existing practice would have two elements, (1) mechanisms for searching and identifying relevant breakthroughs in the literature and private sector and (2) mechanisms
_______________________
18 As an example, the National Cancer Institute (NCI) has recently issued a solicitation for testing their older and unused drug candidates for new purposes. In this case, AZT was in the NCI drug archive and later found new use as the best therapeutic for HIV/AIDS.
and processes in place for incorporating the innovation into a T&E for the capability needed.
The DoD researchers might help focus the off-label requirement and specify the need for detectors with new assay capabilities (and contribute to the new assay development itself). It is not clear that a major role exists for the DoD laboratories to run large-scale repurposing panels or to perform detector repurposing development. The culture of program managers and scientists within the DoD should dictate that they are “smart buyers” first before a major RDT&E investment is made.
Systems Biology
Systems biology studies how the many different networks or systems within one or more living organisms interact. One example might be how proteins from some viral pathogens spoof their way past human immune defenses and hijack human proteins in order to accomplish replication of the attacking virus, with a byproduct being human illness. By definition, systems biology requires a complex collaborative interconnection and integration of diverse sets of knowledge and expertise. One hope for systems biology is the future ability to automatically analyze pathways in multiple pathogens (and their corresponding responses in hosts) to determine potential broad-spectrum medical countermeasures. (Note that this was a goal of the former Transformational Medical Technologies program.)
Systems biology is still very early in development, and most systems biology efforts in the pharmaceutical companies are conventional drug development programs that have been extended to include understanding of pathways rather than knowledge of single protein targets. Systems biology requires a strong base in fundamental science to be useful; it is not a silver bullet. Since chemical agents—especially nerve agents—attack multiple pathways, the topic is an ideal candidate for study in a systems biology program.
Systems biology is being performed worldwide throughout the various biology communities. CBDP should be able to leverage much of this work. However, it appears probable that not all of the desired aspects of systems biology related to pathogenesis will be supplied by the academic research community or other government agencies. Thus, it is likely that CBDP will need to be selective in supporting necessary systems biology efforts related to key mission needs.
Synthetic Chemistry and Biology
Synthetic chemistry is now capable of synthesizing almost any small-to medium-sized molecule. Chemical synthesis is no longer rate limiting for chemical and biological defense.
“Synthetic biology” is a name given to the rational, biology-based synthesis of compounds (small molecules or large) that requires manipulation of synthetic pathways using metabolic and genomic tools. The ability to use synthetic biology to recreate viruses and to create novel bacterial platforms should indicate to DoD that a useful fundamental capability is to be able to detect and characterize the application of synthetic biology applied for both beneficial and nefarious purposes. The timeline of when full mastery of synthetic biology will be achieved is unclear, but recent rapid advances in other aspects of biotechnology make it appear imprudent to suggest that massively engineered organisms will not be a potential threat in the near future, whether due to design or unintended consequences of a beneficial intention.
Materials Science
Materials science has a role to play in many of CBDP’s R&D endeavors. Areas as diverse as temporary building construction materials to nanomaterials for targeted drug delivery and agent-surface transport modeling to studies on the degradation effects of decontamination methods on textiles draw on the field. As a result, it is important that the DoD maintain at least a limited in-house infrastructure to perform research in materials science and to be available to consult and collaborate with scientists as needed across the program. This is also an area, however, where its very ubiquity has resulted in a great deal of expertise within the government and commercial sectors. Many examples can be found in the field of nanotechnology, which has received heavy investment over the past decade. To leverage these resources effectively, it will be necessary for CBDP to encourage collaboration and engagement with those broad communities.
Test and Evaluation
The materials and space required, access to live agents, and knowledge of the operational realities and environments in which the warfighters function make test and evaluation a core S&T capability for CBDP. However, based on limited exposure to the program, the committee infers that T&E is an area that requires serious strengthening in areas relevant to CBD. Upon examination of the T&E structures in place, a number of questions emerge as difficult to answer: (1) Under what conditions of actual use (a combat soldier, in MOP gear, with weapons, pack, ammo, comms, under fire, in an environment with mud, thorns, rocks, sweat, and water) are the suits protective, and against what agents? (2) How, exactly, are these evaluations carried out (and they must incorporate simulants or
“hazards” that are already environmentally common, e.g., diesel smoke as a particulate, poison oak/ivy as ground cover, fluorescent particles in dirt in an alley, and food dye in the wet sand of a landing zone). Test and evaluation protocols should be designed with consideration of the CONOPs the gear is intended to support. Data collected under controlled test conditions (and even the test conditions themselves) must be evaluated to aid in estimates of real world mission consequences and effectiveness.
The point of urging much more realistic and demanding T&E is to encourage development of a culture in which T&E produces accurate evaluations of how protective equipment, detectors, and operational doctrine function under conditions of use in conflict. This applies not just to the development of new equipment or materials, but also to currently fielded equipment that may have to protect against new threats. For example, established CONOPs may need to be revised if fielded equipment does not provide the same level of protection against an emerging agent as against the traditional agents. In order for a commander to plan and execute operations, it is important that test results can be collected, evaluated, and presented in a way that provides accurate information regarding possible casualties under a variety of conditions (lightly or heavily contaminated, damp or dry environment, etc.) and any restrictions or limitations on mission-critical activities that protective gear may introduce.
SUMMARY OF CBDP CORE CAPABILITIES
The committee identified 39 core chemical and biological defense S&T capabilities and created a framework that groups them in six categories. Using the decision framework discussed above the committee found that almost all of the capabilities can be found outside of the service laboratories. For each capability, R&D and T&E are discussed separately and typically were not best suited to the same organization.
The committee considered four types of institutions with laboratories that may be suited to provide CBDP core capabilities and organized them from typically having the most fundamental-science-focused to the most product-focused research. These institutions are (1) academia, (2) other governmental facilities (e.g., NIH, CDC, DOE National Labs, NIST), (3) DoD laboratories and facilities, and (4) industry (e.g., pharmaceutical companies). For some capabilities, T&E requires use of actual agent;19
_______________________
19 Actual agent testing refers to the actual chemical or biological agent the capability is being tested against (e.g., Vx, Sarin, sulfur mustard, anthrax, tularemia, botulinum toxin), as opposed to testing with simulants.
institutions other than DoD laboratories may be well suited to do the work but would need to do so in close collaboration with DoD.
Table 3.2, which summarizes the committee’s judgments about how well suited the types of institutions are for R&D and for T&E with respect to 26 of the core capabilities, is reproduced below. Dark shades indicate an institutional category that the committee views as well suited to maintain a given capability for the CBDP, while the lighter shade indicates less well-suited locales. The white boxes indicate that the institutional category is, in the committee’s view, not well suited to maintain the capability. The other 13 capabilities are cross-cutting science and technology that the committee views as necessary for effective RDT&E for any of the capabilities defined in the preceding capability categories. Discussion of the potential locales for the cross-cutting science and technology capabilities were discussed previously in this chapter.
The committee does not intend to imply that each of the thirteen cross-cutting capabilities be maintained exclusively, or indeed at all, within DoD.
When considering the various locales for obtaining S&T capabilities, it is important to recognize that
- the analysis of the various laboratory locales is general, and individual performers within a category may be exceptions;
- the color coding of each category represents the aggregate of reasons considered, including but not limited to
- reputation and experience at providing the given capability,
- the extent to which the capability requires work with classified information,
- limitations on the locale of the capability resulting from international treaties or other laws, and
- the need to maintain important capabilities, at least in part, at government facilities to ensure availability (e.g., BSL-4 facilities).
In identifying the science and technology capabilities necessary to support the Chemical and Biological Defense Program, the committee identified the following principle findings and recommendations.
Scientific Collaboration
Finding 3.1: Little of the fundamental science required for CBD lies primarily in the DoD. The vast majority of the scientific research performed in the United States occurs in academic and industrial laboratories. This is
particularly true for the biological and chemical sciences which lie at the nexus of the S&T requirements of the CBDP.
Finding 3.2: The military laboratory community is not as strongly partnered with key external research institutions and programs as it could and should be. As the United States has a robust S&T sector, the CBDP can and does engage with individuals and organizations external to DoD and the US government, but this typically occurs at the individual project or principal investigator level, and not necessarily on a sustained basis. The CBDP has not systematically promoted institutional ties with academic, industrial (especially pharmaceutical companies), and other non-DoD laboratories or related federal programs.
Recommendation 3.1: The Director, JSTO-CBD, should ensure that the development of a Culture of Collaboration is a high priority for all elements of the chemical and biological defense enterprise. Although information control requirements and contracting concerns have been stated as barriers on both sides to collaboration, these are issues that can and should be addressed. To ensure that the program delivers products based on the best S&T available, the CBDP needs to find ways to partner with the broader scientific community and other federal agencies in areas relevant to chemical and biological defense.
Tech Watch and Adopt
Finding 3.3: There is the potential to significantly improve chemical and biological defense capabilities by using existing technology. Despite the nation’s superb biomedical research establishment and the explosive growth of biological and biomedical science that is relevant to DoD as well as the public health community, relatively little of this broad competency has been applied to problems relevant to chemical and biological defense.
Recommendation 3.2: The DASD(CBD) should establish an effective “tech watch and adopt” component within the CBDP to bring innovative solutions to ongoing needs. Program managers and scientists within the CBDP should recognize the importance of technology watch and adoption before a major new RDT&E investment is made. The incorporation of a “tech watch and adopt” concept would have at least the following three elements: (1) mechanisms for searching and identifying relevant breakthroughs in the literature and from the private sector; (2) mechanisms and processes in place for incorporating innovation into the ongoing program
for the capability needed; and (3) processes for rapid adoption of “tweaks” that would significantly improve existing capabilities. An adjunct objective would be to get the external performers interested in CBD problems such that they might be recruited to work on the problem.
Linking R&D Community to Operators
Finding 3.4: Separation of S&T performers from the end user is impeding their ability to meet the user’s needs. Individuals in the military laboratories noted that understanding more fully the context of their work could assist S&T personnel in developing operationally relevant products, identifying variables or factors that would otherwise be overlooked, and possibly shortening development time. In addition, a stronger relationship between operators and R&D performers could support innovation by enabling informed, collaborative “blue sky thinking.”
Recommendation 3.3: The DASD(CBD) should survey the military laboratories and associated facilities to identify strong relationships between S&T performers and the warfighters, and support replication of such interactions across the program.
Simulants for Test and Evaluation
Finding 3.5: Broadly speaking, the capacity for test and evaluation to support the needs of the CBDP exists within DoD. Test and evaluation is a core component of the program and important to maintain within DoD at a high level of competency and responsiveness.
Finding 3.6: Much of the current T&E is based on unrealistic expectations of how the material or equipment being tested would actually be used. The threat, although long-standing, is uncertain. In addition, the lack of connection with the military operators often leads to the omission of realistic simulation of deployment and use environments.
Recommendation 3.4: Because of the economic, logistical, and environmental concerns with actual agent testing, DASD(CBD) should give priority to the active development and production of realistic and relevant threat agent simulants for both outdoor and large-chamber tests. A single simulant, especially for chemical agents, is unlikely to possess all of the same physical, chemical, and/or transport properties of an actual agent; therefore, multiple simulants may be required to fully stress critical design parameters during T&E.
Review of Test and Evaluation Plans
Finding 3.7: Test and evaluation plans apparently are not subject to independent external review. These plans are created internally, and the committee observed little evidence of the use of external expertise to review testing plans.
Recommendation 3.5: For CBD products to be viable for fielding, the Deputy Under Secretary of the Army for Test and Evaluation should require that (1) T&E activities be based on testing protocols that accurately emulate actual operating environments (both threat properties and operator employment) and (2) independent reviews of testing protocols be conducted.






































