CAPABILITIES-BASED STRATEGIC PLANNING FOR CHEMICAL AND BIOLOGICAL DEFENSE
As mentioned in Chapter 1, the Chemical and Biological Defense Program (CBDP) mission statement is overly broad and as a result the program appears not to be driven by strategy and planning. It is overly focused on resourcing, and is short on discipline in evaluating the execution. In this chapter, a strategic framework for the CBDP in support of operational capabilities-based planning is described. This approach, combined with some of the programmatic and laboratory-level considerations described in Chapter 5, is intended to provide guidance for coordination and development of consensus within the CBDP community.
The 2004 Joint Defense Capabilities Study on “Improving DoD Strategic Planning, Resourcing, and Execution to Satisfy Joint Capabilities” describes a management approach with increased emphasis on strategy, planning, and accountability (see Figure 4.1). Key elements of this approach are enhanced planning, and execution accountability.
The challenges to the Department of Defense (DoD) in the realm of chemical and biological defense are complex. The Department has responsibilities that span the missions of protecting the warfighter, providing support to the warfighter, defending the United States from attack (i.e., Homeland Defense), and supporting local authorities in executing disaster response following a chemical- or biological-related incident (i.e.,
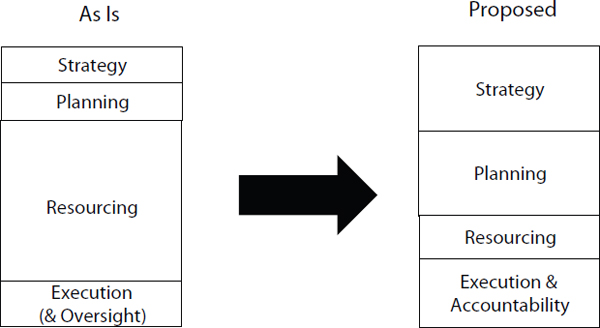
FIGURE 4.1 Notional diagram of the current and proposed management approaches. Box size indicates the relative importance of the element within the approach.
defense support to civil authorities). Events requiring DoD to perform each of these missions could unfold in innumerable, unexpected ways:
- Threats of intentional attack may be unforeseeable.
- Incidents of naturally occurring disease or unintentional chemical exposures cannot be anticipated.
- Where and when the events will occur is largely unknowable.
- Intelligence activities could provide warning of events, but cannot be taken as infallible.
- Adversaries may adopt tactics to counter attempts to defend against attacks.
- Unanticipated events could diminish defense and response capabilities.
The implication of these factors, when considered together, is that it is impossible to describe a concise set of most likely scenarios for which DoD needs to be prepared. In a fiscal environment that demands choices be made among which capabilities DoD can develop and sustain, decision making is even more challenging.
In these contexts, planning often relies on requirements-based processes which describe preferences for individual capabilities based on assumptions of the most likely conditions for which they will be needed.

FIGURE 4.2 Joint Priority List (JPL) from 2011.
These preferences are then translated into ranked lists of priorities (for example, see Figure 4.2, 2011 Joint Priority List). The lists are then used to inform budget decisions via the Program Objective Memorandum (POM) process, with the idea of directing resources toward those capabilities that are higher on the priority lists. This type of approach fails to account for the reality that the scenarios upon which the priorities are predicated are most likely not the events that will unfold and that overall performance depends on interdependencies between the capabilities being developed. In addition, the rigidities of the POM cycle often make the timing of various research, development, test, and evaluation stages critical to project “success,” and are not flexible.
An alternative approach is to use capabilities-based planning (see Figure 4.3). Here, the goal is to adopt strategies that are flexible enough to provide capabilities for events other than those anticipated, adaptive to conditions other than those that are planned, and robust to attempts made to diminish these capabilities. Framing decision making in this way de-emphasizes prioritization and optimization of capabilities. Instead, this framing promotes making choices among portfolios of capabilities that balance tradeoffs among mission performance, risks, and costs. The output of this process approach is guidance on which capabilities to pursue.
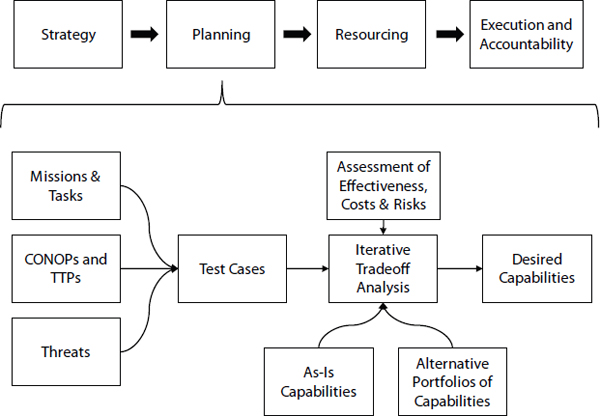
FIGURE 4.3 Diagram of an approach for planning in a capabilities-based process.
This approach has proven useful in cases of deep uncertainty and fiscal constraints in areas such as planning for capabilities related to missile defense and global strike. Capabilities-based planning is hard and can only be undertaken if the resources, expertise, and will are available. When done correctly, it is a powerful approach, but if done poorly, it will lead to confusion and result in new gaps in the program. It should be noted that if the capabilities-based approach is adopted, there may be elements of the current program that should be transferred.
Components of the new approach include identifying a meaningful set of test cases, selecting and assessing sound measures of effectiveness, building creative portfolios of capabilities, and developing tools to conduct iterative tradeoff analysis.1
Identifying Meaningful Test Cases
Proliferation of missions, ambiguity about threats, and multiplicity of CONOPS can lead to innumerable potential scenarios against which program portfolios can be assessed. Practicality demands that assessment be constrained to a concise set of test cases. Capabilities-based planning addresses this challenge in two ways. First, the cases used for analysis are selected not because of belief that they are inherently more likely or more important than other possible scenarios. Instead, they are selected based on a view that an option that performs well in the conditions specified in the case will exhibit a capability deemed important—i.e., the case represents a test. Second, those test cases are selected based on the same type of deliberation among analysis communities that is required to build creative portfolios. Striking a balance between relevant and not overly constrained test cases is obviously difficult and requires iteration during the analysis. In selecting the test cases, it is also important to consider the findings of relevant intelligence and threat assessments. Test case development should include red-teaming. Red-teaming should help ensure that casualties are not the sole measure of risk, and that asymmetric and terrorist threats are sufficiently considered.
Selecting and Assessing Sound Measures of Effectiveness
Sound measures of effectiveness are grounded in a clear logic of how mission success is defined and how capabilities are combined to achieve
_______________________
1 Joint Defense Capabilities Study, Improving DoD Strategic Planning, Resourcing, and Execution to Satisfy Joint Capabilities: Final Report, 2004; Davis, Paul K., Lessons from RAND’s Work on Planning Under Uncertainty for National Security. Santa Monica, CA: RAND Corporation, 2012.
mission success. When tied to such logic models, measures are more likely to be valid and less likely to promote perverse or unintended decisions. Sound measures should also be reliably measureable, particularly when linked to program evaluation. Only then can estimates of the measures for different programs and at different times be trusted. Additionally, to be useful, measurement must be feasible given time and resources consistent with the decisions they are being used to effect. Red-teaming is integral to completing a valid assessment of effectiveness.
Building Creative Portfolios of Capabilities
Policy makers can only expect good outcomes if they have options that include opportunities to balance across performance and costs tradeoffs. Options that are optimized for a specific scenario or capability are unlikely to be flexible, adaptive, and robust. However, developing creative portfolios of alternatives requires iterative deliberation between the warfighter and support operations, science and engineering, systems analysis, and cost analysis communities. Incorporating this deliberation into strategic planning is critical to sound analysis.
Developing Tools to Support Tradeoff Analysis
Capabilities-based analysis requires many types of tools. The multiplicity of test cases requires tools that can allow exploration of performance across a large number of conditions and can be reconfigured quickly to be used for other cases throughout the iterative analytic process. These assessments must be grounded in valid estimates of performance costs and risks. These estimates could come from many sources including red-teaming, modeling and simulation, field demonstrations, and reliably conducted expert elicitation. Finally, tools are needed to illustrate the tradeoffs inherent in choices among alternative program portfolios. The choices supported by this analysis provide guidance for desired capabilities and are the starting point for identifying capability gaps.
Once the desired capabilities are identified, the execution and accountability stage can be started (see Figure 4.4).
Deriving Proposed Solutions and Specifications
After supporting analysis tools have been applied and desired capabilities have been identified, the acquisition community is in a position to propose solutions and develop specifications for those solutions. To be effective, this process should incorporate realistic red-teaming and deliberation between the user and science and technology (S&T) communities
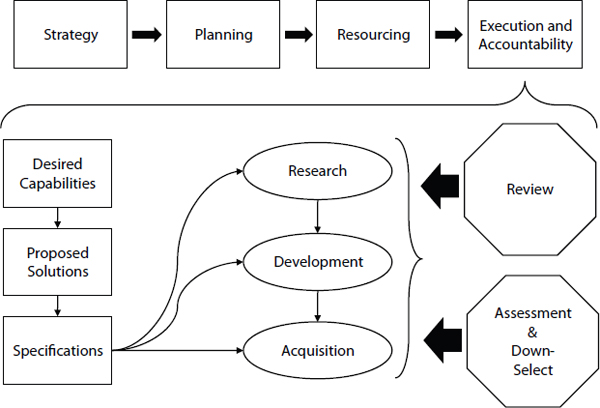
FIGURE 4.4 Diagram of execution and accountability in a capabilities-based process.
so that the process considers both innovation and technical feasibility—the art of the possible and the art of the probable (see “Maintaining a Connection to the End User”). Depending on the urgency, difficulty, and capability base, decisions should be made about the degree of specification needed before a research-development-acquisition (R-D-A) process begins. Specifications should consider whether or not 100% survivability is needed or possible.
From Specification to the Research-Development-Acquisition Process
The R-D-A process is well established within DoD, and many elements of the established process are adequate for the CBDP. For the R-D-A process to be effective for the medical countermeasures program, however, R-D-A should be done as a team approach with end-to-end involvement, including regulatory processes considered in the earliest phases.
Once specifications are derived based on a solid analysis of capability gaps and tradeoffs, then the maturity of existing products can be assessed against the specification to determine whether new, innovation research is necessary (Research) or whether development or furthering of an exist-
ing idea is appropriate (Development), or if a developed product is ready and simply needs to be acquired (Acquisition). It may be the case that activities could be started at more than one R-D-A level to build in a need to address near-term needs with a spiral development process that will fundamentally change the product in the future. It would be expected that more projects would be started in the research phase than the development phase, and even less in the acquisition phase. If the R-D-A process is conducted so there are multiple projects and available options (“shots on goal”) then it is essential that a robust, independent down-selection process is established. In the development and acquisition phases, regular assessments are also essential. These assessments should evaluate technical quality as well as progress toward project and program goals. Such assessments provide the most credible way to make down-selection decisions.
In considering the strategic planning process necessary to support the Chemical and Biological Defense Program, the committee identified the following principle findings and recommendations.
Capabilities-Based Planning, Development, and Acquisition
Finding 4.1: A requirements-driven S&T process is not a good match for the CBDP. The planning and experimentation carried out by the CBDP is usually so removed from plausible use that it is difficult to believe that the Combatant Commands would know how to understand and evaluate the program’s impact, how best to protect their forces, to carry out their operations in the face of current and/or high-probability future threats. Planning tends to focus on narrow conceptions of threats and responses derived from historical events. Outcomes tend to be described in terms of consequences which can be easily measured, such as fatalities and injuries. Options tend to be developed based on incremental modifications to current materiel and operations. Each of these approaches is inadequate for addressing the evolving and innovative nature of chemical and biological threats. Moreover, the perceived goal of “100% protection” appears to impact all aspects of the program such that few products reach the field in a timely manner, especially in the medical countermeasures part of the program.
Recommendation 4.1: The Office of the Secretary of Defense (through the Assistant Secretary of Defense for Nuclear, Chemical, and Biological Defense Programs) should evaluate a shift to capabilities-based
planning, as a more appropriate approach for this program. The goal is to adopt strategies that are flexible to provide capabilities for events other than those anticipated, adaptive to conditions other than those that are planned, and robust to attempts made to diminish these capabilities. Planning should expand the range of options considered; iterative review and realistic red-teaming should challenge assumptions built into plans and promote innovations in defense to correspond to that in the threats. The scope of red-teaming and review should encompass the threats and activities against which performance is assessed and the evaluations of performance are made. The overall S&T focus should shift from “zero casualties” to “mission success.”










