Influence of the Microbiome on the Metabolism of Diet and Dietary Components
Although research on the microbiome is considered an emerging science, scientists already have made tremendous progress in understanding the microbial makeup of the microbiome and in associating microbiome diversity with human disease. Moreover, they are beginning to make headway in understanding how the microbiome impacts human health and disease. It is likely that much of this impact is mediated through diet. Growing evidence suggests that gut microbes influence what the human host is able to extract from its diet, both nutritionally and energetically. This chapter summarizes the workshop presentations and discussion that were focused on the influence of the microbiome on diet and dietary components.
DIET, OBESITY, AND THE GUT MICROBIOME1
When the tremendous amount of undigested polysaccharides, lipids, and peptides that pass through the small intestine unabsorbed enter the large intestine, they serve as the perfect medium for growing a rich gut microbiota. “It stands to reason,” Peter Turnbaugh said, “that diet is going to play an important role in shaping the ecology and function of this community.” But how? And how does the gut microbiome, in turn, contribute to dietary energy harvest? Despite considerable interindividual variation in gut microbiome species, all individuals share a core set of microbial genes, according to Turnbaugh. What additional functions, or metabolic capa-
__________________________
1 This section summarizes the presentation of Peter Turnbaugh.
bilities, do these genes afford their human host? How do they allow for metabolism of all of the undigested polysaccharides and other substances inaccessible by human enzymes? And how does that impact human health and disease? Turnbaugh summarized results from a series of experiments designed to address these questions, with a focus on obesity.
Impact of Gut Microbiota on Energetics and Obesity
Turnbaugh’s interest in obesity was sparked by work conducted in Jeffrey Gordon’s laboratory at Washington University, where Fredrik Backhed and colleagues compared body fat in germ-free mice (i.e., mice raised in an isolator and without any exposure to microbes) to body fat in conventionally raised mice (i.e., mice that had been raised their entire life exposed to microbes) (Backhed et al., 2004). Researchers reported lower total body fat in the germ-free mice but were able to recover the body fat by colonizing germ-free mice with microbial communities harvested from conventionally raised mice. In Turnbaugh’s opinion, the most interesting finding of the study was that conventionally raised mice had more body fat even though they were consuming fewer calories. This was true for both female and male mice and across multiple genetic backgrounds.
Turnbaugh was curious about this “perplexing” phenomenon. How does the microbiome affect the ability of its host to harvest energy from the diet? He and colleagues conducted some studies using 16S ribosomal RNA (rRNA) sequencing to identify phylum-level bacteria in the microbiomes in two different mouse models, ob/ob mice (i.e., mice that chronically overeat because they are genetically deficient in leptin) and diet-induced obese mice (i.e., genetically identical mice that are fed a diet high in fat and simple sugars) (Ley et al., 2005; Turnbaugh et al., 2006, 2008). With both models, the researchers found that lean mice had a moderately greater proportion of Firmicutes (60 percent) than Bacteroidetes (40 percent) but that obese mice had an even greater proportion of Firmicutes than Bacteroidetes. Thus, obesity correlates with increased Firmicutes and decreased Bacteroidetes.
What is the nature of the association? Are the altered microbial communities affecting their hosts in different ways? To answer this question, Turnbaugh and colleagues conducted a microbiota transplantation experiment, where they started with a panel of germ-free recipient mice, all of the same weight and age and with similar other features, and colonized the mice with microbiota samples taken from either an obese or a lean mouse donor (i.e., both ob/ob and diet-induced obese donors). Then they observed the impact of the transplantation over time (Turnbaugh et al., 2006, 2008). Researchers observed about twice as much gain in body fat in mice receiving microbiota transplanted from either ob/ob or diet-induced obese donors, compared to mice receiving microbiota from lean donors (see
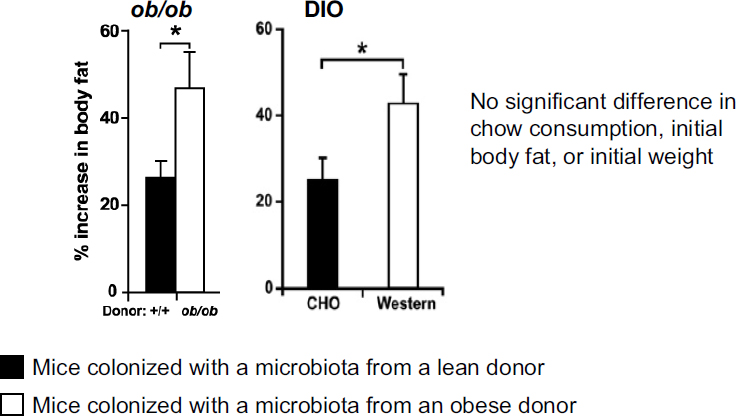
FIGURE 4-1 Difference in body fat gain between initially germ-free mice that receive a microbiota transplanted from either an obese donor (either a genetically obese donor [ob/ob] or a diet-induced obese donor [“Western”]) or a lean donor (again, either a genetically lean donor [+/+] or a diet-induced lean donor [“CHO”]).
NOTE: DIO = diet-induced obesity.
SOURCE: Turnbaugh et al., 2006, 2008.
Figure 4-1). Again, the mice that gained more fat tissue did so even though they were consuming the same amount of calories as the mice with microbiota from lean donors. These results suggest that microbial communities derived from obese versus lean mice impact the energy balance of their new hosts in different ways.
Human twin data provide additional evidence to support the hypothesis that the microbiome impacts host energetics. Using 16S rRNA data from both monozygotic and dyzygotic twin pairs, Turnbaugh and colleagues (2009a, 2010) found greater than 300 microbial genes associated with obesity. Many of these genes “make sense,” Turnbaugh said, given the shift in the relative abundance of Firmicutes and Bacteroidetes associated with obesity in mice. Some of them also indicate a microbial contribution to host carbohydrate and other metabolic pathways.
In another human study, Greenblum et al. (2012) analyzed metagenomic data from twin pairs and other individuals and identified network-level differences in microbial metabolism genes between obese and lean individuals. That is, they identified specific networks of genes that were associated with obese individuals and other networks associated with lean individuals. Most of the differences between the obese- and lean-associated networks were on
the periphery of the networks. Turnbaugh explained that the periphery represents interaction with the host or environment (e.g., intake of a substance from the host or environment), as opposed to glycolysis or some other core component of metabolism. As was the case with the obesity-associated genes detected by Turnbaugh et al. (2009a, 2010), many of the obesity network genes are involved in carbohydrate metabolism as well as carbohydrate transport, nitrate reduction, and xenobiotic metabolism.
Impact of Gastric Bypass on Gut Microbiota: More Evidence of Co-Metabolism by Microbes and Their Host?
Evidence from gastric bypass surgery experiments suggests that gut microbiota impact more than host energetics—they impact host metabolism at large. Turnbaugh reiterated what Jeremy Nicholson had mentioned during his presentation about the metabolic consequences of gastric bypass surgery happening too quickly to be explained by the change in caloric intake. Turnbaugh was curious about the potential role of the gut microbiota as a mediator of these rapid metabolic changes. Using Roux-en-Y-operated mice and comparing them to two different types of controls (sham and weight-matched sham mice), Turnbaugh and colleagues (Alice Liou and Lee Kaplan, Massachusetts General Hospital) observed a significant difference in how quickly the microbial community structure changed after surgery. The microbiota of all the mice changed following their respective surgeries, but the microbiota of the Roux-en-Y-operated mice changed much more dramatically within the first week following surgery. Turnbaugh remarked that the next step is to see if some of the metabolic outcomes triggered by gastric bypass surgery can be transferred to germ-free mice via the gut microbiota.
Impact of Diet on the Human Gut Microbiota
Turnbaugh and colleagues (2009b) conducted an extensive set of diet shift comparisons using a “humanized” mouse model, that is, an initially germ-free mouse that was colonized with a human microbiome. They compared 16S rRNA sequences in humanized mice that were fed conventional mouse chow versus humanized mice fed a “Western” diet (i.e., high fat) and observed a rapid effect of diet shift on the gut microbiome, with only a single day of a high-fat diet having a significant effect. According to Turnbaugh, the results suggest that at least in the mouse model, the gut microbiome is “incredibly dynamic” and can respond to dietary perturbations very quickly. The researchers also found a significant effect of host diet on microbial gene abundance and expression.
The same data reported in Turnbaugh et al. (2009b) were further analyzed using a novel methodology described in Reshef et al. (2011) that enabled the researchers to identify and compare the strength of all possible relationships among all species-level operational taxonomic units (OTUs) across all samples. For example, one possible relationship is what the researchers called a “mutual exclusion” relationship, whereby a particular OTU is present in a microbiome only in the absence of another particular OTU. Reshef and colleagues (2011) concluded that a large proportion of the microbial species relationships could be explained by diet (i.e., conventional diet versus Western diet) as well as by sex and age. So there appear to be networks of diet-dependent microbial species.
Impact of Diet on Gut Microbiota in Other Mammals
The relationship between diet and the gut microbiota is not limited to humans and mice but extends across a wide range of mammalian species. Ley et al. (2008) reported differences in microbiome structure between omnivorous, herbivorous, and carnivorous species (among a total of 60 species, including humans). Subsequently, Muegge et al. (2011) collected data indicating that the microbial communities among these three different groups of mammals have evolved different suites of genes that allow their hosts to better process their respective diets. For example, gut microbiomes in carnivores tend to be enriched with amino acid catabolism genes.
Implications of the Association Between Diet and the Microbiome
Evidence collected by Turnbaugh and others suggests that the human ability to extract and store calories from food as fat is at least partially impacted by gut microbes. In turn, dietary choices impact the gut microbiome. This diet-microbiome interaction suggests to Turnbaugh that nutrition might be better viewed from a metagenomic perspective—one that takes into account both host and microbial genetics. It also raises questions about (1) the definition of a calorie (e.g., Do scientists need to redefine a calorie in relation to the gut microbiome?), (2) the future of personalized nutrition (e.g., Can nutritionists use knowledge of the human microbiome to design personalized diets?), and (3) next-generation medical treatments (e.g., Can medical researchers use this knowledge to design microbiome-targeted interventions?).
MICROBIAL METABOLITES OF DIETARY COMPONENTS2
When assessing diet-disease risk relationships, Johanna Lampe said, “We really can’t ignore the contribution of the gut microbiome.” Not only do gut microbes influence host energetics, as Peter Turnbaugh elaborated, they also play key roles in multiple other areas of host metabolism. Microbes contribute to host fermentation, reduction of nitrate and sulfate, esterification, aromatic fission, and hydrolysis and deconjugation (i.e., not just of glycosides in our plant food, but also of steroid hormones and other endogenous compounds that are excreted in bile and end up in the colon). Qin et al. (2010) identified a number of host metabolic pathways handled by gut microbes, many of which are involved with carbohydrate or amino acid metabolism or xenobiotic biodegradation. But how do microbes influence metabolites? And how do microbial metabolites of dietary components contribute to disease prevention and disease risk?
Lampe addressed these questions using xenobiotic degradation of phytochemicals as an example. There are an estimated 25,000 phytochemicals, with both negative and positive effects. As an example of a phytochemical with negative effects, most people associate nitrates with processed meats, but in fact vegetables are a major source of nitrates in the human diet. Water is another major source. Nitrates can be converted into nitrites, which in turn can interact with a number of different compounds to form nitrosamines, nitrosamides, and nitrosoguanidine; these, in turn, can form DNA adducts and cause DNA damage, creating the potential for carcinogenesis. On the flip side, there are a whole host of dietary bioactive phytochemicals with potential beneficial human health effects (Scalbert et al., 2011). These include the phenolics (phenolic acids, stilbenes, curcuminoids, chalcones, lignans, flavonoids, isoflavones), terpenoids (phenolic terpenes, carotenoids, saponins, phytosterols), organosulfurs (thiosulfinates), and nitrogen-containing compounds (glucosinolates, indoles). Lampe focused on three specific phytochemicals: glucosinolates in cruciferous vegetables, soy isoflavones, and plant lignins.
Glucosinolates and the Human Gut Microbiome
Cruciferous vegetables—that is, broccoli and its vegetable relatives—are the poster children of cancer-preventing vegetables. Both epidemiological and animal data show consistent associations between intake of cruciferous vegetables, whether broccoli, cauliflower, cabbage, or something else, and lower risks of various cancers, primarily of epithelial origin (e.g., lung, colorectal, breast, prostate, and pancreatic cancers). The epidemiological
__________________________
2 This section summarizes the presentation of Johanna Lampe.
data have shown associations across many population groups in Asia, Western Europe, and North America. Animal studies have shown that both cruciferous vegetable extracts and isothiocynate and indole isolates are chemopreventive. Various mechanisms have been proposed to explain the association, including decreased inflammation and oxidative stress, induced cell differentiation and apoptosis, and improved carcinogen metabolizing capacity.
From a human dietary perspective, one of the challenges to deriving chemopreventive benefit from cruciferous vegetables is that isothiocynate, the active component that actually imparts the protective benefit, is difficult to access. It exists in the plant as a glucosinolate, which the plant enzyme myrosinase cleaves into isothiocynate. However, myrosinase is active only in raw vegetables. Cooking inactivates it. On the basis of measurements of excreted total isothiocyanates in urine, Shapiro et al. (2001) reported that chewing uncooked broccoli results in much greater recovery of isothiocynates than swallowing unchewed sprouts and that cooking decreases isothiocyanate availability relative to both chewed and unchewed raw broccoli. They also found that pretreating cooked broccoli with myrosinase dramatically increases availability (i.e., to a point where excretion is more than double what it is with chewed uncooked broccoli). Lampe noted that one could thus argue the value of pretreating all cruciferous vegetables with myrosinase, but that would pose yet another challenge—that is, free isothiocyanate in the diet tends to cause gastritis, with nausea and vomiting, in some individuals. Also, it is not really clear where in the gastrointestinal (GI) tract, or how, isothiocyanates exert their chemopreventive effect and whether introducing pure isothiocyanate would be ideal.
Given that very few human populations regularly consume raw cruciferous vegetables, how are humans deriving the chemopreventive benefit of broccoli and other cruciferous vegetables? The answer, Lampe said, is in our gut microbiome. Lampe and her colleagues reported a wide range of recovery of isothiocynates (ITCs) in urine after eating 200 grams of cooked broccoli (Li et al., 2011). Some individuals excreted almost no ITC (i.e., the ITC was not available to them), whereas others excreted nearly 30 percent (i.e., indicating high availability). To determine whether the gut microbiome might be contributing to this variation, researchers analyzed fecal samples from the low- versus high-ITC excretors and observed that fecal bacteria in the low-ITC excretors have a lower capacity to degrade glucoraphanin compared to the high-ITC excretors. So clearly there is something going on at the level of the gut microbiome, Lampe remarked, with high excretors containing the enzymatic machinery necessary for cleaving glucosinolate into ITC. The researchers did not find any major taxonomic differences in bacterial composition. However Li et al. (2011) was a pilot study, Lampe
remarked. She hopes to examine bacterial composition differences between low- and high-ITC excretors in more detail in a future study.
Bacterial Metabolism of Daidzein, a Soy Isoflavone
Soy protein has generated long-standing interest for its potential effects on bone loss and hot flashes in perimenopausal women because of the weak estrogenic properties of the two major soy isoflavones, daidzein and genistein. Like many flavonoides, isoflavones are metabolized by gut bacteria.
Daidzein can be metabolized in two ways, via either the formation of equol, which is an isoflavone, or the formation of O-desmethylangolensin. Only about 30 to 50 percent of individuals produce equol, depending on gut microbial composition and depending on the population. For example, in Asia, the percentage of individuals who produce equol is closer to 50 percent, compared to the United States, where it is closer to 25 to 30 percent. Interestingly, Lampe noted, the percentage of individuals in Japan that produce equol appears to be decreasing and is now down to about 30 to 35 percent. It is unknown whether the shift is a result of dietary and associated gut microbiome changes in the younger generation. While not everyone produces equol, most individuals produce O-desmethylangolensin. To determine whether any specific microbial communities are associated with the capacity to produce equol, Hullar and Lampe (unpublished) identified individuals as equol producers or nonproducers based on a soy protein challenge, collected fecal samples, and analyzed 16S rRNA as part of what Lampe described as a “quick and dirty” evaluation of the gut microbiome. Their data suggest that fecal bacterial communities in equol producers differ from those of nonproducers. Moreover, within the equol producers, they found that equol production is associated with differences in the fecal microbiome makeup. Lampe speculated that several different bacteria consortia may be capable of equol production.
A number of research groups have looked at whether equol production is associated with any health outcomes. For example, Aktinson et al. (2003) and Frankenfeld et al. (2004b) reported positive associations between equol production and 2-OH/16alphaOHE1 (16alpha-hydroxyestrone) ratios in premenopausal and postmenopausal women. Frankenfed et al. (2004a) reported that mammographic density was 39 percent lower in equol producers. Akaza et al. (2002) reported that plasma equol concentrations were inversely associated with prostate cancer risk in Japanese men. Lastly, Fuhrman et al. (2008) reported a significant interaction between soy intake and equol-producer status in predicting breast density in postmenopausal women. Lampe noted that not all reported associations hold up across all populations. It is not clear why so many associations have been reported between equol production and disease risk in the Japanese population,
perhaps because of early life exposures and “priming” of the microbial systems.
Gut Microbial Metabolism of Plant Lignins
As a final example of the impact of the microbiome on host metabolism of dietary components, Lampe mentioned plant lignins. A whole host of plant lignins can be found in seeds, nuts, berries, grains, and other foods, most of which are metabolized into enterodiol and sometimes further converted into enterolactone. Kuijsten et al. (2005) reported highly variable enterodiol and enterolactone production among individuals. Lampe and colleagues measured microbiome diversity for low-, intermediate-, and high-enterolactone excretors among 115 women and detected significant differences between the low and high excretors and between the intermediate and low excretors. The greatest diversity was among high excretors and the least diversity among low excretors. suggesting that microbial diversity may be associated with enterolactone production. That diversity appears to be distributed across phyla, with high excretors having 20 unique genera.
During the open panel discussion at the end of the first day, there was some discussion around the fact that most human microbiome studies to date are based on fecal sampling. Workshop participants expressed varying opinions about whether microbes and metabolites in the feces reflect what is happening in the gut. One audience member said, “I think you are looking in the wrong place, checking stool.” He suggested sampling the small intestine, where bacterial overgrowth is a problem in patients with small-bowel disturbances. There are sampling technologies available, he said. Another audience member agreed and noted that he and his gastroenterology colleagues are beginning to collect these samples in some of their pediatric patients. Yet another audience member asked if anyone has ever compared feces microbiota to microbiota from various portions of the gut.
Vincent Young described feces as the “summary statement of your gut.” In his opinion, feces has most of what exists elsewhere in the GI tract. It may not provide any indication of the relative abundance of species at various points upstream, but it does provide “some indication” of what is there. However, he emphasized that the usefulness of feces sampling depends on the research question. In some cases, it may not be a good choice. He noted that samples have been collected from various places along the length of the human GI tract and that they reveal both longitudinal and axial differences in both 16S rRNA and metagenomic microbial sequences.
Peter Turnbaugh remarked that based on his observations in mice, while
there are some cases in which a proximal gut sample can be distinguished from a distal gut sample, overall the picture derived from fecal samples is similar to that derived from colon or cecum sampling of either the mucosal or the luminal content. He noted data collected by Paul Eckburg and colleagues (2005) showing that microbial communities can be matched to individuals regardless of whether the microbial sequencing data came from biopsy or fecal samples. Turnbaugh speculated that some biogeographic structure probably exists, but at a finer scale.
Akaza, H., N. Miyanaga, N. Takashima, S. Naito, Y. Hirao, T. Tsukamoto, and M. Mori. 2002. Is daidzein non-metabolizer a high risk for prostate cancer? A case-controlled study of serum soybean isoflavone concentration. Japanese Journal of Clinical Oncology 32(8):296-300.
Atkinson, C., H. E. Skor, E. Dawn Fitzgibbons, D. Scholes, C. Chen, K. Wahala, S. M. Schwartz, and J. W. Lampe. 2003. Urinary equol excretion in relation to 2-hydroxyestrone and 16alpha-hydroxyestrone concentrations: An observational study of young to middle-aged women. Journal of Steroid Biochemistry and Molecular Biology 86(1):71-77.
Backhed, F., H. Ding, T. Wang, L. V. Hooper, G. Y. Koh, A. Nagy, C. F. Semenkovich, and J. I. Gordon. 2004. The gut microbiota as an environmental factor that regulates fat storage. PNAS 101(44):15718-15723.
Eckburg, P. B., E. M. Bik, C. N. Bernstein, E. Purdom, L. Dethlefsen, M. Sargent, S. R. Gill, K. E. Nelson, and D. A. Relman. 2005. Diversity of the human intestinal microbial flora. Science 308(5728):1635-1638.
Frankenfeld, C. L., A. McTiernan, E. J. Aiello, W. K. Thomas, K. LaCroix, J. Schramm, S. M. Schwartz, V. L. Holt, and J. W. Lampe. 2004a. Mammographic density in relation to daidzein-metabolizing phenotypes in overweight, postmenopausal women. Cancer Epidemiology, Biomarkers & Prevention 13(7):1156-1162.
Frankenfeld, C. L., A. McTiernan, S. S. Tworoger, C. Atkinson, W. K. Thomas, F. Z. Stanczyk, S. M. Marcovina, D. S. Weigle, N. S. Weiss, V. L. Holt, S. M. Schwartz, and J. W. Lampe. 2004b. Serum steroid hormones, sex hormone-binding globulin concentrations, and urinary hydroxylated estrogen metabolites in post-menopausal women in relation to daidzein-metabolizing phenotypes. Journal of Steroid Biochemistry and Molecular Biology 88(4-5):399-408.
Fuhrman, B. J., B. E. Teter, M. Barba, C. Byrne, A. Cavalleri, B. J. Grant, P. J. Horvath, D. Morelli, E. Venturelli, and P. C. Muti. 2008. Equol status modifies the association of soy intake and mammographic density in a sample of postmenopausal women. Cancer Epidemiology, Biomarkers & Prevention 17(1):33-42.
Greenblum, S., P. J. Turnbaugh, and E. Borenstein. 2012. Metagenomic systems biology of the human gut microbiome reveals topological shifts associated with obesity and inflammatory bowel disease. PNAS 109(2):594-599.
Kuijsten, A., I. C. Arts, T. B. Vree, and P. C. Hollman. 2005. Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. Journal of Nutrition 135(4):795-801.
Ley, R. E., F. Backhed, P. Turnbaugh, C. A. Lozupone, R. D. Knight, and J. I. Gordon. 2005. Obesity alters gut microbial ecology. PNAS 102(31):11070-11075.
Ley, R. E., M. Hamady, C. Lozupone, P. J. Turnbaugh, R. R. Ramey, J. S. Bircher, M. L. Schlegel, T. A. Tucker, M. D. Schrenzel, R. Knight, and J. I. Gordon. 2008. Evolution of mammals and their gut microbes. Science 320(5883):1647-1651.
Li, F., M. A. Hullar, S. A. Beresford, and J. W. Lampe. 2011. Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. British Journal of Nutrition 106(3):408-416.
Muegge, B. D., J. Kuczynski, D. Knights, J. C. Clemente, A. Gonzalez, L. Fontana, B. Henrissat, R. Knight, and J. I. Gordon. 2011. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 332(6032):970-974.
Qin, J., R. Li, J. Raes, M. Arumugam, K. S. Burgdorf, C. Manichanh, T. Nielsen, N. Pons, F. Levenez, T. Yamada, D. R. Mende, J. Li, J. Xu, S. Li, D. Li, J. Cao, B. Wang, H. Liang, H. Zheng, Y. Xie, J. Tap, P. Lepage, M. Bertalan, J. M. Batto, T. Hansen, D. Le Paslier, A. Linneberg, H. B. Nielsen, E. Pelletier, P. Renault, T. Sicheritz-Ponten, K. Turner, H. Zhu, C. Yu, S. Li, M. Jian, Y. Zhou, Y. Li, X. Zhang, S. Li, N. Qin, H. Yang, J. Wang, S. Brunak, J. Dore, F. Guarner, K. Kristiansen, O. Pedersen, J. Parkhill, J. Weissenbach, H. I. T. C. Meta, P. Bork, S. D. Ehrlich, and J. Wang. 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464(7285):59-65.
Reshef, D. N., Y. A. Reshef, H. K. Finucane, S. R. Grossman, G. McVean, P. J. Turnbaugh, E. S. Lander, M. Mitzenmacher, and P. C. Sabeti. 2011. Detecting novel associations in large data sets. Science 334(6062):1518-1524.
Scalbert, A., C. Andres-Lacueva, M. Arita, P. Kroon, C. Manach, M. Urpi-Sarda, and D. Wishart. 2011. Databases on food phytochemicals and their health-promoting effects. Journal of Agricultural and Food Chemistry 59(9):4331-4348.
Shapiro, T. A., J. W. Fahey, K. L. Wade, K. K. Stephenson, and P. Talalay. 2001. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: Metabolism and excretion in humans. Cancer Epidemiology, Biomarkers, & Prevention 10(5):501-508.
Turnbaugh, P. J., R. E. Ley, M. A. Mahowald, V. Magrini, E. R. Mardis, and J. I. Gordon. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444(7122):1027-1031.
Turnbaugh, P. J., F. Backhed, L. Fulton, and J. I. Gordon. 2008. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3(4):213-223.
Turnbaugh, P. J., M. Hamady, T. Yatsunenko, B. L. Cantarel, A. Duncan, R. E. Ley, M. L. Sogin, W. J. Jones, B. A. Roe, J. P. Affourtit, M. Egholm, B. Henrissat, A. C. Heath, R. Knight, and J. I. Gordon. 2009a. A core gut microbiome in obese and lean twins. Nature 457(7228):480-484.
Turnbaugh, P. J., V. K. Ridaura, J. J. Faith, F. E. Rey, R. Knight, and J. I. Gordon. 2009b. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Science Translational Medicine 1(6):6ra14.
Turnbaugh, P. J., C. Quince, J. J. Faith, A. C. McHardy, T. Yatsunenko, F. Niazi, J. Affourtit, M. Egholm, B. Henrissat, R. Knight, and J. I. Gordon. 2010. Organismal, genetic, and transcriptional variation in the deeply sequenced gut microbiomes of identical twins. PNAS 107(16):7503-7508.
This page intentionally left blank.












