To effectively treat patients diagnosed with drug-resistant (DR) tuberculosis (TB) and protect the population from further transmission of this infectious disease, an uninterrupted supply of quality-assured (QA), second-line anti-TB drugs (SLDs) is necessary. Patients diagnosed with multidrug-resistant tuberculosis (MDR TB)—a disease caused by strains of Mycobacterium tuberculosis (M.tb.) resistant to two primary TB drugs (isoniazid and rifampicin)—face lengthy treatment regimens of 2 years or more with daily, directly observed treatment (DOT) with SLDs that are less potent, more toxic, and more expensive than those used to treat drug-susceptible TB. From 2000 to 2009, only 0.2–0.5 percent of the estimated 5 million MDR TB cases globally were treated with drugs of known quality and in programs capable of delivering appropriate care (Keshavjee, 2012). The vast majority of MDR TB patients either died from lack of treatment or contributed to the spread of MDR TB in their communities. A strengthened global supply chain for SLDs could save lives by consistently delivering high-quality medicines to more of the people who need them.
When SLDs are unavailable to a national TB control programme (NTP) and medical providers in a particular country, patients miss critical doses of medicine or never start treatment—risking the escalation of disease and
___________________
1 The planning committee’s role was limited to planning the workshop, and the workshop summary has been prepared by the workshop rapporteurs as a factual summary of what occurred at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants, and are not necessarily endorsed or verified by the Forum or the Institute of Medicine (IOM), and they should not be construed as reflecting any group consensus.
amplification of drug resistance, enhanced infectivity and transmission of disease to others, and death. Ensuring a reliable and affordable supply of high-quality SLDs is a complex public health intervention that, thus far, has not been organized or implemented in a way that allows all providers and patients access to SLDs when they are needed. Some MDR TB patients without access to SLDs through a Green Light Committee (GLC)-approved program may receive appropriate treatment through a government-run or other QA program. However, it is estimated that approximately 90 percent of patients with DR TB are not receiving treatment through a government-run or QA program. In other words, these patients are likely receiving treatment from sources of unknown quality or no treatment at all. In recent years, many countries have been working to scale up MDR TB treatment programs but, as mentioned by several workshop participants, efforts by international organizations and institutions to ensure SLDs are delivered to patients have not kept pace with global MDR TB needs. Challenges facing the global supply chain for SLDs, and the efficient delivery of drugs to patients, include
• The overall market for SLDs is relatively small due to limited diagnostic capacity at the country level.
• Demand-forecasting mechanisms do not fully capture patient needs for SLDs.
• Markets are opaque, with high barriers to entry that may deter manufacturers.
• Drugs to treat MDR TB carry high prices and have a short shelf life (24 months) compared with treatments for drug-susceptible TB.
• Once drugs are ordered, there are lengthy time lines to reach the country.
The July 31–August 1, 2012, workshop was convened by the Forum on Drug Discovery, Development, and Translation (“the Forum”) of the Institute of Medicine (IOM) in Washington, DC, to explore options and opportunities to improve the effectiveness of the global SLD supply chain in delivering drugs to patients. Titled “Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant TB,” the workshop was part of a series sponsored by the Forum to gather information from experts around the world on DR TB prevention, diagnosis, treatment, and management.
The Forum held a foundational workshop in Washington, DC, in 2008. The summary of that workshop, Addressing the Threat of Drug-Resistant Tuberculosis: A Realistic Assessment of the Challenge: Workshop Summary (IOM, 2009), and the accompanying white paper (Keshavjee and Seung, 2008) provided background for and informed the development of four
subsequent workshops in countries with a high burden of DR TB. The first workshop in the international series was held in Pretoria, South Africa, on March 3–4, 2010 (IOM, 2011a). The second workshop was held in Moscow, Russia, on May 26–27, 2010 (IOM, 2011b). The third workshop was held in New Delhi, India, on April 18–19 and 21, 2011 (IOM, 2012), and the final workshop in the series is being planned for January 2013 in Beijing, China. Box 1-1 includes some key themes related to the drug supply chain that emerged from the workshops in Washington, DC, South Africa, Russia, and India.
The workshop summarized in this volume was convened by the Forum to provide a setting for fostering a dialogue on the needs and opportunities for a global supply chain for TB SLDs. The workshop brought together members of the international TB community—including individuals from U.S. federal agencies, international health authorities, nongovernmental organizations (NGOs), the private sector, academia, and advocacy groups, for 2 days of informative presentations and robust discussion. Box 2-1 lists the objectives of the workshop.
In her opening remarks, Gail Cassell, Visiting Professor, Department of Global Health and Social Medicine, Harvard Medical School, warned that failing to address current SLD supply issues would perpetuate the present situation in which the majority of MDR TB patients are undiagnosed and untreated while simultaneously fostering the development of rapid resistance to new TB drugs in the pipeline. Since the 2008 workshop (IOM, 2009), data have emerged to suggest that the burden of MDR and extensively drug-resistant tuberculosis (XDR TB) is underestimated (Wallengren et al., 2011) and has not only reached global pandemic proportions, but is being fueled by patients who are undiagnosed or who are receiving inadequate treatment (Keshavjee and Farmer, 2012). Data from KwaZulu-Natal, South Africa, show that 88 percent of XDR TB cases are untreatable with drugs currently available in South Africa.2 China has the highest annual number of MDR TB cases in the world; a survey published by the Chinese Center for Disease Control and Prevention indicated that 10 percent of Chinese TB patients have MDR TB, and 8 percent of those with MDR have XDR TB (Zhao et al., 2012). The same survey in China also revealed that primary transmission, or person-to-person spread, of DR TB accounted for 78 percent of new MDR TB cases and 86 percent of new XDR TB cases. In sum, Cassell noted that there has been an increasing recognition in recent years that DR TB strains are just as easily transmissible from person-to-person
___________________
2 Data provided via personal communication, October 15, 2012, with Kristina Wallengren, KwaZulu-Natal Research Institute for Tuberculosis and HIV (K-RITH), Nelson R. Mandela School of Medicine, University of KwaZulu-Natal.
BOX 1-1
Key Drug Supply Chain Themes from
Previous IOM Forum Publicationsa
• Diagnosing, treating, and managing DR TB is a complex public health intervention presenting multiple opportunities to improve the scientific, clinical, and organizational aspects of the intervention. Improvements to the efficiency and effectiveness of the global supply chain for SLDs represent a significant opportunity to speed medicines to patients and avoid preventable morbidity and mortality from DR TB.
• The arrival of drugs into beneficiary countries ordered through the GLC mechanism can be delayed for months, during which time patients are transmitting DR TB and dying.
• Limited, inconsistent, and unpredictable demand for SLDs is a key challenge for manufacturers of QA SLDs that results in backlogs, delays, and high prices.
• Increased market volumes could attract more manufacturers of QA drugs to the global SLD market and increase competition, reduce prices, and increase availability.
• Greater transparency and visibility for manufacturers with regard to demand, QA processes, and financing could improve the SLD supply chain.
as drug-susceptible strains, which was previously not believed to be the case, heightening the need to prioritize MDR TB infection control.
BACKGROUND AND HISTORY OF THE CURRENT GLC MECHANISM
History Prior to the Formation of GLC3
The GLC mechanism was developed in response to the widespread emergence of resistance to first-line anti-TB drugs (FLDs) that began in the 1980s and that has gained momentum rapidly since the 1990s. Peter Cegielski, Team Leader for Drug-Resistant TB, International Research and
___________________
3 This subsection is based on the presentation by Peter Cegielski, Team Leader for Drug-Resistant TB, International Research and Programs Branch, Division of Tuberculosis Elimination, U.S. Centers for Disease Control and Prevention (CDC).
• The forecast and demand management aspects of procurement have a high degree of uncertainty.
• Procurement processes for drug provision mechanisms could be improved and streamlined.
• Regulatory processes, quality standards, and treatment regimens could benefit from harmonization among countries in order to reduce barriers to suppliers entering the SLD market.
• Information management systems could improve tracking of operational activities of DR TB supply chains.
• Ensuring the timely delivery of high-quality SLDs to patients is part of a complex health care challenge that includes several steps, from initial testing, diagnosis, and treatment protocols to drug manufacturing and delivery to initiation and completion of treatment.
___________________
a Based on remarks from Gail Cassell, Visiting Professor, Department of Global Health and Social Medicine, Harvard Medical School; and Stemming the Tide of Multidrug-Resistant Tuberculosis: Major Barriers to Addressing the Growing Epidemic (Keshavjee and Seung, 2008); Addressing the Threat of Drug-Resistant Tuberculosis: A Realistic Assessment of the Challenge: Workshop Summary (IOM, 2009); The Emerging Threat of Drug-Resistant Tuberculosis in Southern Africa: Global and Local Challenges and Solutions: Workshop Summary (IOM, 2011a); The New Profile of Drug-Resistant Tuberculosis in Russia: A Global and Local Perspective: Workshop Summary (IOM, 2011b); and Facing the Reality of Drug-Resistant Tuberculosis in India: Challenges and Potential Solutions: Workshop Summary (IOM, 2012).
Programs Branch, Division of Tuberculosis Elimination, U.S. Centers for Disease Control and Prevention (CDC), provided historical background about the formation of the GLC initiative in the late 1990s. The history and design of the GLC mechanism provides context and a basis for understanding the current challenges facing efforts to supply SLDs to the global MDR TB population.
According to Cegielski, the period spanning the 1940s to the 1970s was a “golden era” for TB drug development, in which dozens of new compounds were developed into commercial products. Concurrently, microbiological methods to test for susceptibility to those new drugs were developed. By the early 1970s, the superior efficacy of the three-drug regimen of isoniazid, rifampicin, and pyrazinamide had been established by extensive clinical trials. The establishment of this efficient regimen consequently engendered a sense of optimism that TB had been conquered. Cegielski noted that this optimism led to a general complacency that left the world unprepared for the emergence of strains resistant to the regimen. Efforts to discover and
BOX 1-2
Statement of Task for the Workshop
This public workshop explored innovative solutions to the problem of how to get the right SLDs for MDR TB to people who critically need them. More specifically, the workshop examined current problems and potential opportunities for coordinated international efforts to ensure that a reliable and affordable supply of high-quality SLDs is available. The workshop objectives were to consider
• To what extent and in what ways current mechanisms are or are not effectively accomplishing what is needed, including consideration of bottlenecks
o The advantages and disadvantages of centralization in the management of the global drug supply chain, and potential decentralized approaches to improve operations of the supply chain
o What can be learned from case studies and examples from other diseases (e.g., the Affordable Medicines Facility-malaria [AMFm] and the U.S. President’s Emergency Plan for AIDS Relief [PEPFAR])
• The current allocation of responsibilities and roles of the private (including industry and nonprofit public health organizations) and public sectors, and examination of opportunities for enhancing and optimizing collaboration
• Identification of potential innovative solutions to the problem
develop new drugs ended, and drug production was massively curtailed as the spread of disease slowed, particularly in wealthier countries. This period coincided with many nations’ development into middle-income countries with their own domestic pharmaceutical industries. Because those industries often lacked stringent QA/QC (quality control) standards, the volume of substandard and counterfeit drugs infiltrating the market rose, and an incipient resistance to the standard TB drug regimen resulted. By the 1980s, rifampicin resistance had emerged as a serious problem in many areas of the world. In the 1990s, the Global Drug Resistance surveys carried out by the World Health Organization (WHO) and the International Union Against Tuberculosis and Lung Disease (“The Union”) demonstrated the magnitude of the worldwide MDR TB burden. The consequent surge in demand for SLDs revealed widespread supply, cost, and availability problems.4
___________________
4 An exception is fluoroquinolones. They are effective against TB, but because they were developed for other indications, production is robust and pricing and supply are therefore not an issue.
MDR TB has persisted for 20 years, suggested Cegielski, because during the 1990s and into the 2000s, TB experts, public health leaders, and leadership organizations were polarized about how to respond to MDR TB outbreaks. One faction held the view that services for MDR TB should not be integrated into TB control programs; the other faction held the opposite point of view, that the MDR TB problem should be addressed head on through an MDR-specific strategy. Cegielski stated that the effect of this polarization was an overall ambivalence, with a lack of strategic guidance and policy setting due largely to “a failure of leadership to respond vigorously to the MDR TB problem.” Consequently, low- and middle-income countries failed to develop sufficient diagnostic capacity, clinical expertise, markets, or regulatory capacity to treat MDR TB successfully. Lack of technical expertise therefore became a barrier to MDR TB treatment. Also during this time, many NTP managers did not develop adequate systems to procure SLDs or develop sufficient laboratory capacity.
Cegielski suggested that price also emerged as a primary barrier to expanding MDR TB treatment. He attributed this issue in part to national policies guided by public health leadership organizations opposed to treating MDR TB, which had the result of allocating insufficient resources from ministries of health to NTPs. This landscape began to change slowly in the late 1990s and early 2000s, when GLC; the Global Fund to Fight AIDS, Tuberculosis and Malaria (“the Global Fund”); and other initiatives were developed to address MDR TB by mitigating the barriers of expertise and price.
Formation of GLC5
Salmaan Keshavjee, Director, Program in Infectious Disease and Social Change, Department of Global Health and Social Medicine, Harvard Medical School, described the formation of GLC in response to evidence of the emerging MDR TB epidemic in the 1990s. Initially, GLC was designed as a pilot project mechanism to provide affordable SLDs and to gather data about those projects to inform global policy on the treatment of MDR TB. He also explained the structural and functional evolution of the GLC mechanism, from its initial development as a multi-institutional partnership composed of global stakeholders to its current configuration as an advisory committee to WHO.
___________________
5 This subection is based on the presentation by Salmaan Keshavjee, Director, Program in Infectious Disease and Social Change, Department of Global Health and Social Medicine, Harvard Medical School.
Rationale for Formation of GLC
In the 1990s, WHO introduced promotion of the Directly Observed Treatment-Short course (DOTS) treatment strategy. The DOTS approach in the 1990s included five components: political commitment with increased and sustained financing; case detection through sputum smear microscopy; standardized treatment with supervision; an effective drug supply and management system; and a monitoring and evaluation system and impact measurement.6 In many ways, the introduction of DOTS followed the framework of the selective primary health care movement of the 1980s, which focused on providing low-cost, highly effective interventions for diseases that were identified as particularly threatening to public health (although the primary health care movement itself excluded TB, categorizing it as category II [Walsh and Warren, 1979]). However, the rollout of the DOTS intervention, which was designed specifically for treating drugsusceptible TB, did not address the rising specter of MDR TB. At that time, WHO had advised against treating DR TB, particularly in low-income countries, citing a concern that such a treatment strategy would detract attention and resources from the treatment of drug-susceptible disease. Thus, resistant disease was progressively transmitted and the epidemic continued to grow. In 1993, WHO began conducting annual global surveys to assess drug resistance, which indicated the presence of resistance to TB drugs in all 35 countries reviewed.
In response to an MDR TB epidemic in Lima, Peru, Partners In Health carried out a pilot project for community-based treatment of MDR TB. The project was based on the approach used by New York City to address their MDR TB epidemic in the late 1980s, which had resulted in positive outcomes for MDR TB patients. The success of that pilot project started a movement leading to the creation of the “DOTS-Plus” framework for the treatment of MDR TB, which extended the existing DOTS program to include treatment of MDR TB with second-line anti-TB agents. DOTS-Plus pilot projects were designed to collect data from low-income countries with the objective of enabling WHO to change its policy on the treatment of MDR TB in resource-limited settings.
A mechanism was needed to make SLDs available at a low cost to those projects. GLC was therefore created between 1998 and 2000 as a multi-institutional partnership to address the high cost of MDR TB drugs. Modeled on an approach to make meningitis vaccines available in resource-limited settings, GLC provided access to low-cost SLDs exclusively to DOTS-Plus pilot projects that were meeting certain programmatic benchmarks.
___________________
6 See http://www.who.int/tb/dots/en/index.html (accessed October 18, 2012).
The rationale for this selectivity was to ensure that the drugs were used properly in controlled settings in the pilot programs.
System of Pilot Projects
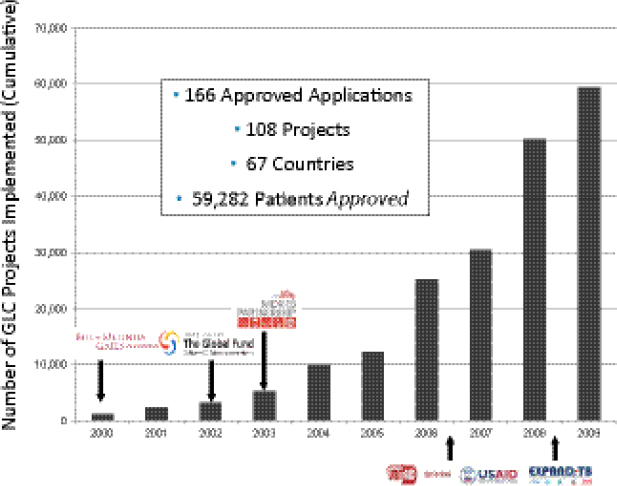
Keshavjee stressed that the GLC mechanism was not intended for scale-up, but was designed to facilitate a series of pilot projects intended to provide data to change global policy about MDR TB. Between 2000 and 2009, the number of pilot projects grew rapidly (Figure 1-1).
Organizational Structure of GLC
GLC’s multi-institutional partnership was hosted by WHO’s TB department as part of one of its working groups and was transferred to the Stop TB Partnership when that partnership was formed in 2001. As the number of pilot projects began to increase in 2005, the Global Drug Facility (GDF) was brought into the system beginning in 2007 to purchase the increasing volume of SLDs through procurement agents, based on a formal agreement with the GLC Secretariat that year.7
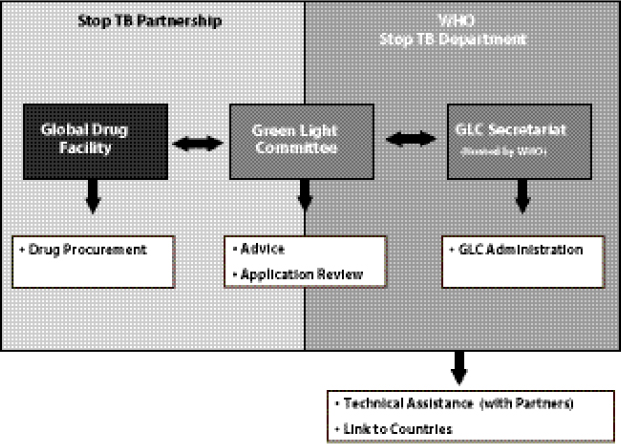
Keshavjee noted that the GLC mechanism had many positive aspects—most notably the success in encouraging programs to provide a high standard of care to patients, and encouraging countries to view the pilot projects as models for national scale-up. However, Keshavjee added, the fact that GLC functioned as a “centralized node of control” (Figure 1-2) had some negative implications. For example, instead of seeing MDR TB as an urgent need that must be rapidly addressed as a public health concern, countries applied to GLC seeking permission to treat their MDR TB patients in a pilot project format. Applications were evaluated on a program’s capacity to diagnose patients and deliver appropriate care. Approved applications were then passed on to the GLC Secretariat, hosted by WHO, which functions in an administrative capacity. WHO’s Stop TB Department provides technical assistance in starting, evaluating, and monitoring beneficiary countries’ MDR TB programs and connects with countries and partners.
Keshavjee noted that all the components of the current system are actually situated within the WHO mechanism (including the Stop TB Partnership, which has no legal identity of its own). He added that eventually WHO’s legal department ruled that GLC could no longer exist as a multi-institutional partnership because GLC and the entire Stop TB Partnership were legally part of WHO, and the current system of involvement of multiple non-WHO institutions was not consistent with WHO rules. As a result, the original system was disbanded. An advisory body to WHO
___________________
7 Médecins Sans Frontières (MSF) purchased drugs for GLC in 2001 and 2002.
FIGURE 1-1 Number of GLC pilot projects implemented around the world between 2000 and 2009.
SOURCE: Keshavjee, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis, adapted from Dr. Ernesto Jaramillo, WHO, Geneva.
with a similar name—designed to keep the GLC “brand” but composed of individual experts, not stakeholders or partners—now exists instead.
Financing and Procurement Requirements
Currently, GLC MDR TB projects are financed by grants from the Global Fund, UNITAID, and other public and private sources of funding. Since 2002, the Global Fund has required that all SLD procurement for MDR TB programs that it funds go through GLC. This policy seeks to control the emergence of SLD resistance by ensuring the use of only QA drugs. Keshavjee emphasized that beneficiary countries are not allowed to use Global Fund or UNITAID monies to purchase SLDs directly from manufacturers, regardless of the QA status of the supplier. He also noted
FIGURE 1-2 Creation of a centralized node of control for the GLC-approved SLD supply chain.
SOURCE: Keshavjee, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
that the WHO system, including use of GDF to oversee procurement, has not successfully optimized pooled procurement—it places orders only when funds are made available by countries/programs. Likewise, it also does not optimize direct price negotiation with manufacturers, and little active negotiation occurred after the initial lowering of prices in 2000.
Change in WHO Policy
Keshavjee stated that GLC’s initial objective of gathering data from pilot programs to effect change in WHO’s MDR TB policy was achieved in 2006. He described how WHO’s global treatment and program recommendations were changed to apply the standard of MDR TB care used in places like New York City in the early 1990s to all countries worldwide. The standard of care included the use of drug sensitivity testing to diagnose drug resistance, the provision of SLDs, and the delivery of care with appropriate monitoring and treatment of adverse events (WHO, 2006).
PRINCIPLES OF DRUG SUPPLY CHAINS8
Understanding the fundamental principles and processes that underlie the efficient operation of drug supply chains in general is a critical first step in developing targeted, concrete strategies for addressing the specific problems that impede the effective operation of the current SLD supply chain. Prashant Yadav, Director, Healthcare Research Initiative, William Davidson Institute, University of Michigan, and Sana Mostaghim, MDR TB Drug Access Project Manager, Clinton Health Access Initiative (CHAI), delivered presentations that provided a technical overview of the structure and principles of drug supply chains. They described the mechanics of scaling up to deliver increased volumes and the effects of limited or “lumpy” demand on manufacturers and on prices. They also explained how market-shaping strategies can improve the efficiency of drug supply chains. In addition, the relationships between key market drivers and elements of price were examined in the context of potential strategies for improving access to treatment by mitigating the barriers of high prices and limited availability of SLDs.
SLD Supply Chain Structure9
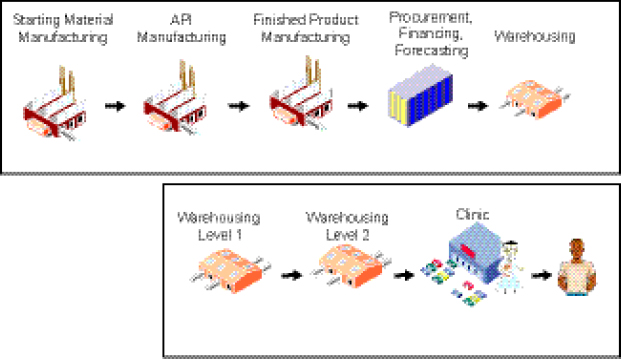
A key characteristic of the supply chain for a predominantly donor-funded drug market, such as the current SLD market MDR TB, is that its two components, the “upstream” segment and the “downstream” segment, are decoupled from one another. This has important implications with regard to issues such as demand, pricing, scale-up, and financing (Figure 1-3).
The structure of the global, or “upstream,” segment of the supply chain comprises
• manufacture of starting material, active pharmaceutical ingredient (API), and finished pharmaceutical product (FPP);
• procurement, financing, and forecasting; and
• warehousing of drugs (prior to shipment to countries).
The domestic, or “downstream,” segment includes the in-country warehousing components, delivery of drugs to clinics, and treatment of patients.
___________________
8 This section is based on the presentations by Prashant Yadav, Director, Healthcare Research Initiative, William Davidson Institute, University of Michigan, and Sana Mostaghim, MDR TB Drug Access Project Manager, Clinton Health Access Initiative (CHAI).
9 This subsection is based on the presentation by Prashant Yadav, Director, Healthcare Research Initiative, William Davidson Institute, University of Michigan.
Challenges of Scaling Up: The “Sequence Question”10
The structure of a well-functioning upstream SLD supply chain should be able to fulfill existing demand and be able to scale up as needed to serve a larger number of patients if there is an increase in demand for SLDs. Two issues, if present, would raise concern about a global supply chain: (1) if the existing supply chain has structural problems (e.g., high prices, long lead times, and poor service levels) and (2) if only a small fraction of the potential demand is currently being served by the supply chain (i.e., when there is a potential latent demand for products that far exceeds the current actual demand).
The presence of those factors could indicate that the supply chain in question is not properly equipped to scale up and cope with that potential demand efficiently. Decisions about how to deal most effectively with this kind of supply chain inefficiency depend on what Yadav called the “sequence question.” This question concerns the best way to bolster the supply chain’s ability to serve a much larger number of people, and hinges on an understanding of the sequential relationship between increasing the demand for products and decreasing the price of those products.
The first option for answering the sequence question is to devote resources to diagnosing and treating the disease (i.e., demand creation) under the assumption that the resulting volume efficiencies will trigger decreases in product prices. The alternative option is a decrease in the price as a prerequisite for demand increase; that is, does the supply chain need to be improved (in terms of pricing, lead times, and service levels) before demand creation will have a “pay-off.”
Price, Cost, and Volume Relationships11
In considering the best way to sequence and optimally allocate resources, Yadav stressed that it is important to understand that the relationship between price and volume is not the same as the relationship between cost and volume. This is due to the market structure that comes in between the cost- and price-volume relationship. In product segments with few suppliers, only a fraction of a producer’s decrease in cost is actually passed on to the buyer in the form of a price decrease. In other words, when producers’ costs decrease due to an increase in volume, the extent to which that decrease in cost will be reflected in a price decrease is dependent on the market structure. The market structure is itself contingent on both
___________________
10 This subsection is based on the presentation by Prashant Yadav, Director, Healthcare Research Initiative, William Davidson Institute, University of Michigan.
11 This subsection is based on the presentation by Prashant Yadav, Director, Healthcare Research Initiative, William Davidson Institute, University of Michigan.
FIGURE 1-3 Existing supply chain for SLDs.
NOTE: API, active pharmaceutical ingredient.
SOURCE: Yadav, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
the degree of competitiveness in the market and the effectiveness of the procurement organization(s). Thus those two factors also determine the extent to which cost decrease due to volume increase will manifest as an actual price decrease.
Market Segmentation and Price Elasticity of Demand12
Yadav emphasized that the current SLD supply chain actually has two types of separately financed supply chains: the internationally financed supply chain operated by GDF and the domestically financed supply chain operated in a given country. The overall SLD drug market therefore faces a unique situation with regard to price and demand elasticity.
Yadav cited a modeling exercise for the market of an unspecified SLD treatment, which revealed that doubling the demand of that treatment led to a decrease in cost of approximately 17 percent. However, market structure dictated that only 50 percent of that decreased cost was reflected in the price, that is, there was a decrease of only about 8 percent as a result of doubling the volume. In the internationally financed portion of the supply
___________________
12 This subsection is based on the presentation by Prashant Yadav, Director, Healthcare Research Initiative, William Davidson Institute, University of Michigan.
chain, demand is relatively inelastic to small changes in price (in other words, small changes in price will not affect overall demand), whereas the domestically financed portion is a very price-elastic market. Thus when examining the overall market, with both supply chains, the demand curve is uniquely shaped. At the beginning it is extremely flat and unresponsive either to large volume changes or to small price changes, but at a certain point in the domestically financed portion of the market, volume will jump when the price point descends below a certain threshold. Yadav noted, however, that extensive modeling work would have to be performed to better understand what that threshold might be in a given market.
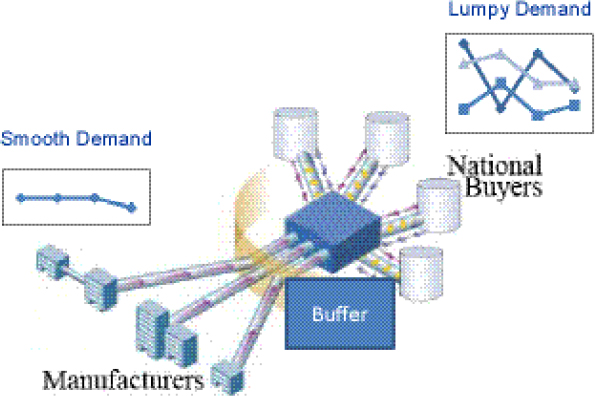
Use of Buffers or Stockpiles to Smooth Demand13
“Lumpy” demand patterns are a key structural challenge for small-volume SLD markets. Demand from NTPs is often very lumpy (i.e., erratic and inconsistent). This lumpy demand presents problems for pharmaceutical manufacturers with respect to costs, batch sizing, and changeovers. Yadav suggested that those problems could be addressed through the development of a buffer inventory or stockpile to smooth demand and thus to help increase batch sizes and decrease changeovers and costs (Figure 1-4). Whether those decreased costs would lead to significantly decreased prices ultimately depends on having the appropriate market structure.
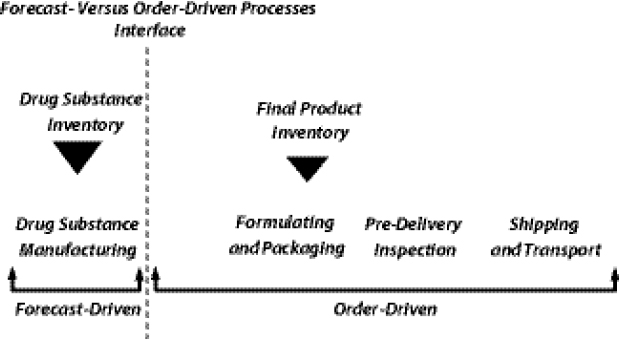
Shifting the Push-Pull Boundary14
Yadav introduced the concept of the “push-pull” boundary in supply chain management (SCM), which delineates between those procedural steps in the chain that are based on forecasts and those that are based on actual orders. Processes in the supply chain include drug substance manufacturing, formulating and packaging, predelivery inspection, and shipping and transport. When most of those processes are order-driven as opposed to forecast-driven, manufacturers must contend with a suboptimal inventory-holding structure and batch-sizing calculus in which demand is low and lumpy, which in turn results in longer lead times and higher costs. In other words, the current market context is not conducive to planning ahead; manufacturers often wait for orders to arrive before initiating API production or FPP manufacturing.
Yadav suggested that to address the imbalance between order- and
13 This subsection is based on the presentation by Prashant Yadav, Director, Healthcare Research Initiative, William Davidson Institute, University of Michigan.
14 This subsection is based on the presentation by Prashant Yadav, Director, Healthcare Research Initiative, William Davidson Institute, University of Michigan.
FIGURE 1-4 A “buffer” supply of SLDs smoothens the lumpiness of demand. Removing lumpiness in demand decreases changeovers, increases batch sizes, and decreases costs. If market structure facilitates, then it would also lead to decreased prices.
SOURCE: Yadav, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
forecast-driven processes in the supply chain, the push-pull boundary could be manipulated such that the interface point is shifted toward expanding the number of forecast-driven steps in the supply chain (Figure 1-5) and away from the number of order-driven steps.
Such a shift would enable manufacturers to plan ahead and facilitate improved inventory-holding patterns and batch sizing. Yadav added that shifting this boundary would require the development of both innovative supply-contracting structures and more accurate demand-forecasting techniques.
SLD Market Drivers and Elements of Price15
Mostaghim addressed two crucial barriers in the upstream portion of the MDR TB drug supply chain—high prices and limited availability of
___________________
15 This subsection is based on the presentation by Sana Mostaghim, MDR TB Drug Access Project Manager, CHAI.
FIGURE 1-5 Shifting the push-pull boundary in the SLD supply chain. The current push-pull boundary leads to a suboptimal inventory-holding structure and a suboptimal batch-sizing calculus.
SOURCE: Yadav, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
QA SLDs. Mitigating those barriers and thus maximizing the operational efficiency of the supply chain could serve to increase the number of patients being treated and, ideally, ultimately contribute to controlling rates of DR TB. He also provided an illustrative case study on cycloserine (Box 1-3).
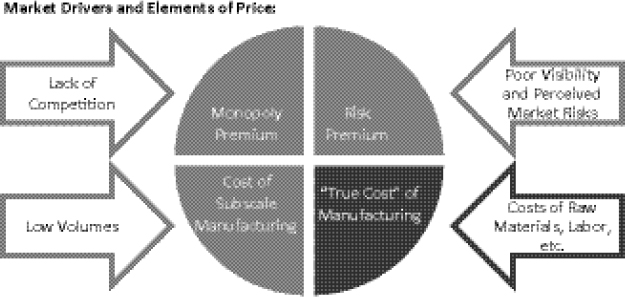
Four key elements factor into pricing of SLDs charged to procurement agents by manufacturers. They each have different market drivers (Figure 1-7). The first element that factors into pricing is the legitimate or “true cost” of manufacturing a given product, such as the costs of raw materials, labor, a reasonable margin, etc. Second, the “monopoly premium” element of price is driven by the lack of competition among manufacturers in the SLD market. Third, “risk premium” is the extent to which manufacturers (or partners) feel that they are in an ambiguous position with regard to investment decisions and confidence about, or visibility into, the market. The fourth element of price relates to economies of scale: the cost of subscale manufacturing, such as suboptimal batch sizes and production campaigns, which are driven by demand and order placement. The challenges facing the supply-and-demand structure for individual drugs in the SLD market are further elucidated with a case study of cycloserine (Box 1-3).
BOX 1-3
Cycloserine Case Studya
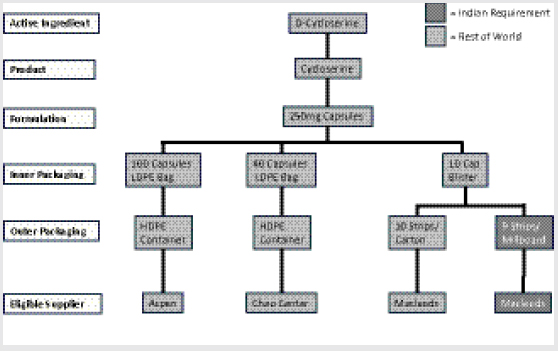
Examining the market for cycloserine is instructive for understanding the principles and challenges that underlie other major SLD markets (e.g., kanamycin, capreomycin, PAS [4-aminosalicylic acid] products), although each has unique characteristics. Upon examination, the market for QA cycloserine initially appears to be promisingly healthy and potentially competitive, with three formulators that are approved either by WHO prequalification (WHO PQ) or by a stringent regulatory authority (SRA) and more than one qualified API manufacturer. However, in reality its fundamental supply-and-demand structure is operating counter to the ultimate objective of affordable and sustainable access. For example, market fragmentation begins to occur at the drug packaging stage, where the same capsule formulation requires inner packaging in three different ways within four separate types of outer packaging. By the bottom level of the market, the eligible suppliers for each variety of packaged product have fragmented into four different silos that procurement agents must work with, effectively deteriorating the potential for healthy competition. A single manufacturer, Macleods, actually accounts for two of the silos, having developed a different variety of packaged product to comply with specific Indian requirements (Figure 1-6).
The upshot of this market fragmentation is effectively a monopoly situation. Despite having three eligible manufacturers of cycloserine, due to the incongruent technical specifications required by different treatment programs and countries, there is in practice only a single eligible manufacturer for each specific mode of packaging and tendering.
___________________
a This box is based on the presentation by Sana Mostaghim, MDR TB Drug Access Project Manager, CHAI.
BARRIERS, CHALLENGES, AND NEEDS
Cegielski moderated a multi-stakeholder discussion examining the specific barriers, challenges, and needs that prevent the SLD supply chain from operating at optimal efficiency to deliver drugs to MDR TB patients. The first part of this section sets forth an overview of the barriers and challenges that were raised during the individual presentations delivered by Keshavjee; Lucica Ditiu, Executive Secretary, Stop TB Partnership, WHO;
NOTE: HDPE, high-density polyethylene; LDPE, low-density polyethylene.
SOURCE: Mostaghim, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
Yadav; Mostaghim; Rifat Atun, Professor of International Health Management, Imperial College London; and succeeding workshop discussions. The section is organized thematically to capture general topics covered by individual workshop participants, including the identification of bottlenecks and other supply chain structural issues; upstream supply chain barriers; financing needs; clinical challenges; and regulatory challenges. After covering these thematic issues, the section focuses on the perspectives of different stakeholders who elaborated on the specific supply chain barriers during
FIGURE 1-7 Key barriers to improving SLD access: high prices and limited availability of QA MDR TB drugs. Sufficiently deep volumes must be identified and leveraged to improve the situation and deflate the non-essential elements of price.
SOURCE: Mostaghim/CHAI, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
panel discussions. Finally, the section compiles key messages and suggested ways forward as offered by the individual speakers and participants.16
Plenary Overview of Barriers and Challenges
Structural Challenges and Needs
Delay points. Keshavjee described an analysis carried out by GLC that identified multiple delay points for SLDs occurring within the GLC mechanism. The GLC analysis explored the average length of delays that occur among different cogs in the system, from the country’s or TB program’s initial application (which could be made by either the NTP directly, or an NGO working with NTP approval) to GLC, to the arrival of the drugs at treatment sites. For example, depending on when an application requesting permission to treat patients is received by the GLC Expert Committee,
___________________
16 Although a few WHO employees were able to participate in the meeting, the leadership of the WHO Stop TB Department and the Global Fund, who had been invited to participate, were regrettably unable to attend. The viewpoints of stakeholders expressed in this summary are, per IOM guidelines, those views specifically expressed at the workshop. Several workshop participants noted that it will be important to follow up with and include WHO and the Global Fund in ongoing and future discussions.
it could take 1–3 months for a decision to be made and then referred to the GLC Secretariat to address any required technical issues. At that point there is a potential 2- to 6-month wait for the provision of WHO technical support (including receipt of a final report to the country). Once technical issues are resolved—and this sometimes takes months—there is often another delay averaging 1–4 months for the country or program to organize a procurement order with GDF, and a similar additional delay occurring between GDF and the GDF procurement agent. Keshavjee cited delays of days or months for the funding agency to release money, for the drugs to pass through customs and regulatory processes upon arrival in country, and for the drugs actually to be delivered to treatment sites (Figure 1-8).
Bottlenecks. Ditiu outlined a list of potential points in the system at which patients can face bottlenecks or barriers to receiving MDR TB treatment through the GLC mechanism. First, she noted, the patient must be empowered to seek help for his or her disease and have access to health care, diagnosis (testing, results, and follow-up), and good treatment advice; then the patient must receive appropriate therapeutic recommendations (e.g., drug
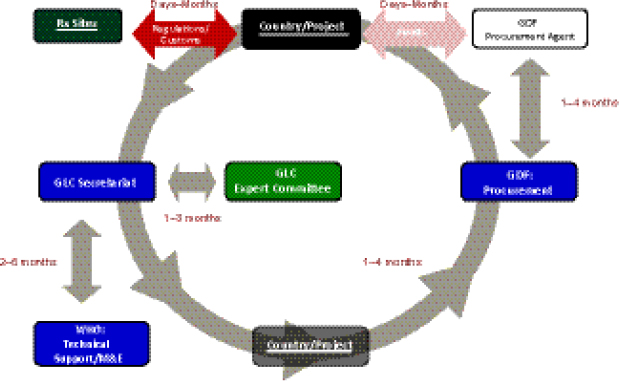
FIGURE 1-8 The GLC initiative flow through cycle.
NOTE: M&E, monitoring and evaluation.
SOURCE: Keshavjee, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
regimen). Patients in a given country must then have access to the SLD supply chain, and SLDs must actually be available to that country through the supply chain. The latter requires that national governments acknowledge the problem of DR TB and translate this into a political commitment to obtain SLDs.
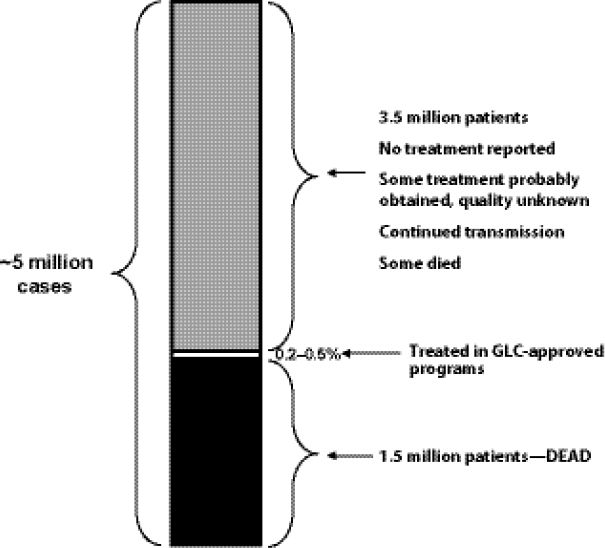
Challenges of GDF/GLC system design. Keshavjee emphasized that GLC and GDF were initially designed to support pilot projects of DR TB programs (i.e., GLC was not designed for scale-up to meet global demand, and GDF was mandated to provide SLDs only to GLC-approved projects/cohorts). From that perspective, GLC accomplished its primary goal, which was to facilitate access to low-cost SLDs in order to collect data that could support WHO endorsement of a policy of treating MDR TB in resource-poor settings as part of an integrated TB treatment and management strategy. However, he noted, because much-needed operational changes to support conversion from a pilot project system were never made, the GLC mechanism became structurally incapable of facilitating treatment for the vast majority of MDR TB patients. He noted that GLC and GDF continue to function on a scale more consistent with pilot projects than with the scale of actual patient need. Keshavjee estimated that the GLC mechanism treated only 0.2–0.5 percent of the estimated number of patients in need of SLDs worldwide in the past 10 years (Figure 1-9). He noted that many of those patients who did not receive treatment with drugs of known quality and in programs capable of delivering appropriate care either died from a lack of treatment or have contributed to the spread of MDR TB in their communities.
Keshavjee noted that the spread of MDR and XDR TB is partly a consequence of the lack of scale-up of high-quality treatment programs. This has led to both ongoing transmission of drug-resistant strains in the community, and the acquisition of drug resistance through patients purchasing SLDs of unknown quality in the private sector and taking short and irregular courses of medicines without appropriate medical supervision and support.
Ditiu suggested that, in her opinion, as a consequence of the pilot project system, few countries—with the exceptions of Brazil, India, and South Africa—are currently aiming to diagnose all of their cases of MDR TB and enroll patients in treatment programs. She noted that the pilot project system has the result that countries often consider MDR TB to be a separate issue from the drug-susceptible TB issue and continue to focus on treating cohorts of MDR TB patients rather than addressing treatment of patients across the entire disease spectrum. She also noted that GDF was designed as an agency for preplanned orders, not as an emergency response
SOURCE: Keshavjee, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
organization, which is a role that it is being called on to play with increasing frequency.
Prices. Keshavjee noted that prices for SLDs available through GLC-approved programs have increased significantly since initial negotiations with manufacturers in 2000 and 2001.17 He argued that two factors contributed to this. First, as an institution WHO and the mechanisms sitting within it were not capable of undertaking the type of hard bargaining with
___________________
17 Amikacin: +991 percent; kanamycin: +617 percent; cycloserine: 321 percent; capreomycin: +292 percent; ethionamide, prothionamide, PAS: stable (July 2001–March 2011) (MSF and IUATLD, 2011).
the pharmaceutical industry that has been the hallmark of the HIV drug supply. Second, the maintenance of high prices can be linked to the Global Fund’s mandate that all MDR TB drug procurement for all its financed programs must go through the GLC mechanism (i.e., approval of the technical capabilities of a TB program followed by ordering of drugs to implement the treatment program). He noted that a motivation for this decision was to help contain the spread of broad-spectrum resistance that could arise if the Global Fund supported the provision of SLDs that were then used improperly.18 Beneficiary countries were not permitted to purchase drugs directly from suppliers, regardless of their QA status, and could only purchase through WHO/GDF. Keshavjee characterized this as GLC being granted a “monopoly,” which gave rise to what he deemed a “moral hazard” in the sense that “it created a monopoly, and a situation where the people buying the medicines and the people paying for them were different.” He suggested that this moral hazard discouraged the development of a strategy to reduce the price of SLDs: “[s]omebody else was paying an unlimited amount, a check was coming to pay for it, and basically you could buy whatever you wanted at whatever price.” He described a scenario whereby, on handing over purchasing of MDR TB medications to WHO/GDF, the Global Fund lost the ability to bundle the purchase of these drugs with its purchase of large volumes of HIV and malaria medications and to use this type of market mechanism to drive down prices while maintaining quality.
Structural procurement barriers. Keshavjee further suggested that because all procurement using Global Fund dollars is required to go through GLC, and GLC is an agency of WHO, WHO restrictions about conducting pooled procurement and the ability to negotiate prices with suppliers are key barriers to SLD price improvements. For example, because there was no real pooled procurement, nor were advance purchase contracts used, manufacturers had no idea about a given year’s orders—no real forecast of the potential demand. These conditions, in turn, prevented manufacturers from being able to produce the necessary volumes to decrease prices. Keshavjee suggested that a significant barrier imposed by the current organizational structure of the global SLD supply chain is that there is no direct procurement relationship between countries and QA manufacturers.
Ditiu remarked that in July 2011, the GLC mechanism was reorganized. As a result of the reorganization, countries can procure directly from GDF, including purchasing partial regimens if necessary. She expressed concern, however, that countries currently are not availing themselves of this
___________________
18 “To help contain resistance to second-line anti-TB drugs and consistent with the policies of other international funding sources, all procurement of medications to treat MDR-TB must be conducted through the GLC” (Third Board Meeting, October 10–11, 2002).
option, which could significantly scale up the number of MDR TB patients receiving treatment. She suggested improving communication to countries about this new, more open, option for SLD procurement.
Competing organizational incentives. Keshavjee cited the need to more clearly understand the differing incentives across the players in the supply chain and the potential implications of achievement of those disparate goals. He suggested that the search for solutions to the MDR TB problem requires examining and aligning the interests of all players. He presented a chart that elucidates his characterization of those incentives and the barriers to effective dispatch of their respective responsibilities (Table 1-1).
Upstream Supply Chain Barriers
Individual speakers described several key barriers to efficiency in the upstream SLD supply chain, which was defined by Mostaghim as the segment encompassing the point at which a company decides to produce a drug to the point an order is placed and the drug is tendered. The primary barriers identified include limited demand, restricted market structure, and low volumes of API and FPP production, all of which contribute to the key challenges of high prices and limited availability of SLDs.
Limited and unpredictable demand. Atun and Yadav noted that a major reason for the high prices of SLDs is that manufacturers are challenged by problems of limited and unpredictable demand from NTPs and procurement agencies, which stems from a lack of pooled procurement and poor forecasting results. Mostaghim noted that the range of product specifications, in terms of individual countries developing different DR TB treatment regimens, further fragments the already limited demand for specific SLDs.
Yadav explained that accurately estimating the point at which demand for SLDs would trigger the necessary price decrease is a challenge because it varies from manufacturer to manufacturer, depending on the plant configuration they use. Mostaghim elaborated that such a prediction is also highly product-dependent (e.g., fermentation- versus chemically-based products19). Moreover, the internationally and domestically financed segments of the SLD supply chain have very different price and demand elasticity, which combine to create a unique demand curve for the overall market.
Yadav noted that potential demand for SLDs far exceeds the demand fulfilled by the current supply chain. He estimated that, according to published sources (WHO, 2010), potential demand is likely at around 440,000
___________________
19 This distinction is explained in the section “Perspectives of Suppliers/Manufacturers” later in this chapter.
TABLE 1-1 SLD Supply Chain Stakeholders: Responsibilities, Interests, and Barriers
| Responsibilities | Interests | Barriers | |
| WHO |
• Help countries • Set global standards |
• Retain current system structure due to financial interests and “self-perception as central convener” |
• Conflict of interest in reforming the system linked to receipt of substantial overhead from GDF funds • Control of funding provides leverage/power over countries |
| Global Fund |
• Help countries overcome barriers • Use donor funds appropriately |
• Improve current system structure due to financial situation • Avoid blame for XDR TB |
• Lacks in-house technical expertise on MDR TB • Fears conflict with WHO and resulting effect on donors |
| Stop TB Partnership |
• Bring partners together to improve access to TB care • Use donor funds appropriately |
• WHO connection facilitates access to countries • Benefits from size/scale of GDF operations |
• Has operated essentially as a subsidiary of WHO rather than a true partnership • Coordinating board not responsive to MDR TB SLD problems |
| Donors |
• Accountability to home taxpayers • Ensure good outcomes from funded projects |
• Avoid perception of exerting overt/undue influence on WHO, other multilateral bodies |
• Political risk of backing away from existing strategy they have funded |
| Countries |
• Protect citizens' interests • Deal with epidemics |
• Support local industries and interests • Avoid hard currency |
• Fear that complaining would lead to loss of WHO/donor funds • Funding TB not high priority |
| Patients |
• Access highquality treatment • Gain representation in the system • Avoid transmitting disease |
• Access to adequate SLD regimens • Highly stigmatized • Unaware of their rights |
|
SOURCE: Keshavjee, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
patients, numbers diagnosed at about 30,000, and current fulfilled demand at approximately 15,000 patients. Closing the gap between potential and currently fulfilled demand would require a significant scale-up of the existing SLD supply chain and the rapid diagnosis of more patients using drug susceptibility testing (DST). He suggested that the barriers of high prices, long lead times, and poor service levels20 would need to be mitigated for such a scale-up to occur. Yadav suggested that bold, upfront financing would be required to facilitate both demand creation and the necessary scale-up to cope with that increased demand.
Restricted market structure. Restricted market structure—for example, having only one eligible supplier for a product—and lack of competition among suppliers in the SLD market are key barriers to improving price and availability. Mostaghim remarked that restricted market structure hinders price improvement in that even if sufficiently deep product volumes were attained, the extent to which that volume is channeled into a single-supplier market structure would curtail improvements in price.
Mostaghim and Yadav noted that the current market for SLDs is also severely restricted because the drugs are not typically used for any other indications in humans. Therefore, demand for QA versions of those products is limited exclusively to MDR TB treatment (e.g., kanamycin).
Low volumes of API and FPP production. Mostaghim noted that economies of scale with regard to batch sizing and production campaigns have a direct impact on production cost. Limited actual demand (i.e., drug orders) results in low batch sizes that are not cost-effective for manufacturers to produce, necessitating a higher price for the finished product. An overall lack of visibility into the SLD market also diminishes manufacturer confidence to produce greater volumes based on forecasts.
Yadav explained that another reason that prices for SLDs are inflated is because the API batch sizes that are currently produced by manufacturers are so relatively small21 and therefore expensive to produce, and the cost of API manufacturing represents a significant portion of the FPP cost. He noted that the markets for producing APIs that are not used exclusively for manufacturing SLDs (i.e., that are also used for other types of FPPs) are healthier than those API markets that are linked only to TB drugs. Yadav suggested that a healthy API market requires specific one-time donor
___________________
20 Service levels refer to the length of time between an NTP’s order placement and receipt, and the fraction of those orders that can be fulfilled within a specified time frame.
21 For example, an API batch size might run 5 out of 250 operating days per year, whereas a product with a “healthy” market might run for 2 or 3 months.
interventions to correct deficiencies and does not require ongoing donor support.
Financing and Systems Challenges
Atun identified a significant funding shortfall as the major challenge for MDR TB diagnosis and treatment. Projected funding requirements for MDR TB between 2011 and 2015 are estimated at $7.1 billion, around $3.6 billion of which has been approved by the Global Fund in 116 countries. However, the Global Fund’s total TB control funding will decline substantially after 2012, despite the increasing prevalence of MDR TB in a number of countries around the world that depend on the Global Fund for financing their TB control programs.22 Hence, if domestic funding is not mobilized to address the MDR TB emerging needs, the Global Fund may be the only funding source for MDR TB management and SLDs in countries such as Armenia, Bangladesh, Bulgaria, Georgia, Kyrgyzstan, Tajikistan, and Uzbekistan.
Atun suggested there is an urgent need to invest in innovative MDR TB financing and in appropriate push and pull mechanisms to use these funds for adoption of new medicines and health products for MDR TB. Push mechanisms are required to generate supply-side incentives for creating new innovations in TB control, new medicines and health technologies, and as new service delivery models; pull mechanisms are required for demand creation and market signaling to show that financing is available in countries and in health systems for adopting new innovations for TB control and MDR TB; and crosscutting initiatives are required for international- and national-level systems strengthening. For example, strengthening procurement and supply chains, building staff capacity, and developing appropriate regulatory policies can ensure that innovations that have been developed can be adopted when financing is available (Figure 1-10).
Atun noted that the current funding shortfall and state of unpredictable financing are detrimentally affecting the employment of push and pull mechanisms. He explained that there is an imbalance in industrial, health, and financing policies whereby industrial policies encourage technology
___________________
22 At the time of the workshop, a 16 percent cap on Global Fund financing for TB had been proposed. However, in September 2012, the Board of the Global Fund voted not to implement a funding cap but instead to develop a new funding model that would allocate funds to country “bands” according to income level and disease burden, among other factors. The model envisions greater predictability and flexibility in the funding application and allocation process. For more information, see http://www.theglobalfund.org/en/mediacenter/newsreleases/2012-09-14_Global_Fund_Adopts_New_Approach_to_Funding_Grants (accessed November 14, 2012) and http://www.stoptb.org/news/stories/2012/ns12_058.asp (accessed November 14, 2012).
push mechanisms, but regulatory policies and a lack of incentives for health systems constrain adoption of innovation. He suggested the following reasons for this imbalance:
• insufficient emphasis on demand-side factors that influence innovation adoption;
• inadequate incentives and downstream rewards for adopting innovation;
• toleration of inefficiency and ineffectiveness in service delivery, where much waste exists; and
• no incentives for innovation.
Another barrier is that crosscutting initiatives, such as training of human resources and development of appropriate regulatory policies, which are critical for strengthening platforms for adoption and scale-up
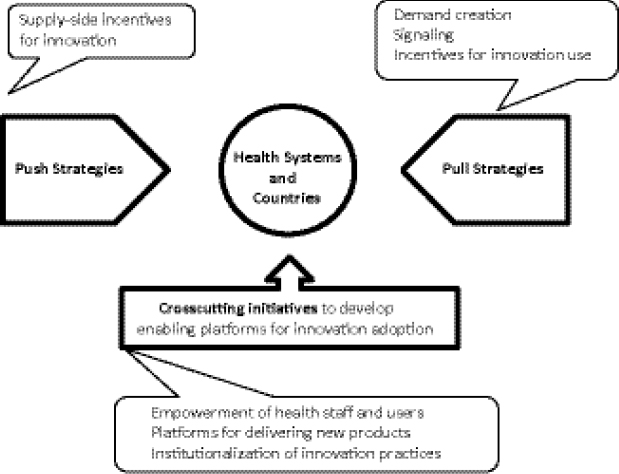
FIGURE 1-10 Push and pull mechanisms for adopting innovations for MDR TB.
SOURCE: Atun, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis (edited).
of innovations, are similarly underfunded. He warned that if resources are not devoted to achieving strengthened health systems, functional SCM systems, and adequate country-level capacity for delivering services, SLDs will continue to fail to reach the people who need them—regardless of whether funding is available for the purchase of innovative medicines and health products.
Yadav stated that the SLD supply chain is limited by an antiquated contracting structure between GDF and manufacturers that is based on public procurement rules from decades ago and that is no longer supported by current empirical or analytical research. He maintained that the contracting practices need to be modernized with more innovative structures, but that such efforts are often met with the response that institutional constraints and the nature of available financing do not allow such innovation.
Because financing is currently dominated by domestic investments, Ditiu stressed the need to engage with stakeholders in those countries to clarify, for example, the importance of scale-up and QA drugs. Yadav further asserted that concrete financing must back up demand forecasting. To achieve this, he suggested that the nature of that financing must be reorganized to allow for longer-term commitments.
Clinical Challenges
Regimen harmonization for MDR TB treatment is challenged by the lack of a clinically established standard/best regimen of SLDs, said Cegielski. Thomas Moore, GDF, Stop TB Partnership, WHO, noted that GDF carries out efforts to harmonize WHO-recommended regimens in the programmatic management of drug-resistant tuberculosis (PMDT) catalog, but that it is ultimately at the discretion of NTPs whether they follow WHO recommendations or not. Myriam Henkens, International Medical Coordinator, Médecins Sans Frontières (MSF), suggested that a range of different regimen options is necessary because the drugs are weak and have to be adapted for each individual patient’s treatment.
Mostaghim stated that lack of harmonization of treatment regimens for SLDs both within and among countries results in fragmentation of overall patient demand. For example, there are cases of countries with six standard approved regimens, or that have accepted the use of all existing WHO Prequalification of Medicines Programme (WHO PQP) SLDs in the treatment of DR TB.
Regulatory Challenges23
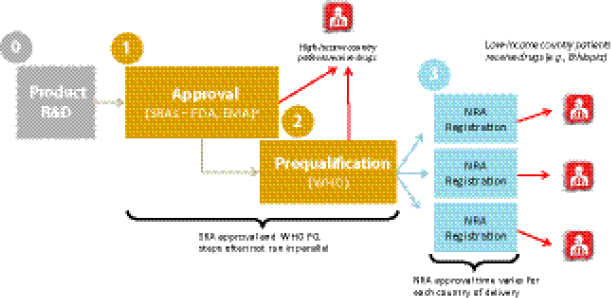
Vincent Ahonkhai, Senior Regulatory Affairs Officer, Bill & Melinda Gates Foundation (BMGF), described the existing drug QA regulatory mechanism for low-resource countries with donor-funded MDR TB programs. After development, a drug first acquires approval from the U.S. Food and Drug Administration (FDA) or European Medicines Agency (EMA). To be eligible for procurement with donor funding, the drug must then pass through the WHO PQ process to assure its quality, safety, and efficacy per WHO’s standards. After WHO PQ, the drug must then be registered in the beneficiary country before it can be procured by a donor agency. There are thus multiple bottlenecks in this regulatory process, particularly with respect to WHO’s central regulatory authority,24 that affect the overall global availability of QA drugs. Limited availability of SLDs is causally linked with high prices, as multiple participants noted.
Regulatory pathways vary widely among countries and have a direct impact on MDR TB patients’ access to SLDs. Ahonkhai remarked that the MDR TB burden primarily affects low-income countries that are highly dependent on donor-funded regulatory and procurement pathways to deliver drugs to patients, as opposed to the “one-step” national regulatory pathways that are typical of middle- and high-income countries. Companies wishing to distribute in low-income countries therefore face multiple regulatory steps after drug development and before they can deliver the product in countries (Figure 1-11):
• approval by stringent regulatory authorities (e.g., FDA, EMA);
• WHO PQ25; and
• national registration in the recipient country.
In the amount of time that often elapses to achieve the SRA and WHO PQ regulatory steps, patients in higher-income countries would have usually received their drugs already, yet in lower-income countries, the additional step of national registration remains. This lengthy regulatory process is not attractive to manufacturers or procurers because the delays at each regulatory stage—also termed “drug lag”—contribute to slow product uptake and inadequate coverage. Ahonkhai suggested that regulatory harmonization
___________________
23 This subsection is based on the presentation by Vincent Ahonkhai, Senior Regulatory Affairs Officer, Bill & Melinda Gates Foundation (BMGF).
24 As Lisa Hedman, Project Manager, WHO, noted, the WHO PQ process is dealing with a “bandwidth” problem that is causing delays in registering new suppliers.
25 Ahonkhai noted that the SRA approval and WHO PQ processes are often not run in parallel, which can result in further delay.
FIGURE 1-11 Regulatory steps from drug development to delivery. Typical time line for present-day medications.
NOTE: EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; NRA, national regulatory authority; R&D, research and development; SRA, stringent regulatory authority; WHO PQ, WHO prequalification.
a SRAs—typically FDA and EMA.
SOURCE: Ahonkhai, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
efforts (e.g., the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use [ICH], the Pan American Network for Drug Regulatory Harmonization) are needed to standardize and harmonize production regulation and registration.
The remainder of this section provides an overview of the specific barriers and challenges faced from the unique perspectives of different stakeholders in the SLD supply chain, as identified by individual presenters during Session I of the workshop and during discussions between the speakers and the audience. Moore delivered a presentation describing the perspective of GDF in its role as a procurement mechanism for GLC-supported programs. Iain Richardson, Senior Director, Global Supply Chain and Logistics, Eli Lilly and Company, and Robert Sebbag, Vice President, Access to Medicines, Sanofi, described challenges and barriers from the perspective of supplying and manufacturing SLDs. Anne Goldfeld, Professor of Medicine, Harvard Medical School, and Co-founder, Global Health Committee (GHC) (an NGO that has worked in Cambodia as the Cambodian Health Committee since 1994 and is based in the United States at Harvard Medical
School), Henkens, and Andrew Gray, Senior Lecturer, Pharmaceutical Sciences, School of Health Sciences, University of KwaZulu-Natal, offered their perspectives on the challenges faced by MDR TB providers and collaborating organizations operating in countries dealing with high MDR TB burdens.
Perspective of GDF26
Moore outlined the functions that GDF, as an initiative of the Stop TB Partnership launched in 2001, has fulfilled in coordinating and managing TB drug procurement. GDF has promoted harmonization of WHO’s TB recommended treatment regimens, facilitated rapid DOTS expansion, and developed the market for FLDs. GDF strives to maintain stringent QA standards and by 2011 had increased the number of QA SLDs available for procurement for MDR TB treatment to 32 products, 75 percent of which have 2 QA suppliers. Through its commercial partners, GDF encourages competitive and sustainable pricing for SLDs through international bidding. More than 19,000 MDR TB patients were supplied with SLD treatment by GDF in 2011—with all these patients having been part of the previously approved GLC cohorts. GDF also monitors and provides technical assistance to TB management programs in-country.27 GDF is an exclusively donor-supported system that does not derive profit from the funding it receives to procure SLDs.
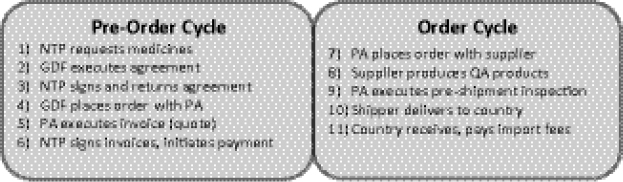
GDF Procurement Cycle Barriers
Moore distinguished between two sections in the GDF procurement cycle—“pre-order” and “order” (Figure 1-12)—in describing specific supply chain barriers. Within the “pre-order” component, he noted that delays occur often within the country, including delays by the NTP in making the initial requests for medicines to GDF and in obtaining the necessary authorization and signatures from local government officials for grant and procurement agreements. Lack of funding from countries or donors to pay for the drugs is also a key barrier to an effective procurement cycle with GDF.
___________________
26 This subsection is based on the presentation by Thomas Moore, GDF, Stop TB Partnership, WHO.
27 Ditiu characterized GDF’s role in this context as a kind of “call center” or reference point for drug management.
FIGURE 1-12 GDF procurement cycle. The cycle is made up of two components and normally requires 6–8 months.
NOTE: NTP, national TB control programme; PA, Procurement Agent; QA, quality-assured.
SOURCE: Ditiu, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
GDF’s SLD Stockpile
To support the order segment of the procurement cycle, GDF established an SLD stockpile with UNITAID funding to expedite emergency orders (delivery within 30 days on average) and accelerated orders (delivery within 31–89 days on average). The stockpile consists of 5,800 patient treatments. Moore noted that the stockpile has been successful in reducing lead times thus far, with 60 programs ordering from the stockpile in 2011 (including 25 emergency orders placed by 20 different programs). Moore emphasized that the stockpile will require new funding if it is to continue after 2012, when its current UNITAID funding expires and, ideally, should consist of an increased number of patient treatments.
Expanding the SLD Market: GDF Role
Moore commented on GDF’s potential ability to promote expansion of the global market for SLDs. Through its DOTS expansion effort, GDF was able to reach 41 percent of the FLD public market. A serious challenge for achieving similar results in the SLD market is the current lack of demand and resultant high prices. In 2011, the average price of drugs delivered through the GDF system ranged from $2,070 to $7,891 per patient. The high cost borne by suppliers to establish or upgrade production facilities and the lengthy (18- to 24-month) WHO PQ process remain significant barriers to growth in the SLD market. Because of the weak procurement systems of many NTPs and partners requesting QA products, Moore maintained
that GDF’s ability to support expansion of the SLD market would be contingent upon
• significant increase in demand;
• the provision of accurate information about numbers of patients, time lines, regimens, and patient enrollment rates from TB programs and partners;
• sufficient funding to allow NTPs to engage in direct procurement from GDF;
• whether funding is received from a donor, alignment of processes relating to the transfer of funds, paperwork, donor requests, and plans;
• new funding for the existing SLD stockpile; and
• attracting new suppliers to the SLD market.
Moore noted that GDF is actively attempting to recruit new potential SLD suppliers through direct meetings at their facilities, conferences, and workshops organized to engage suppliers in the SLD market.
Perspectives of Suppliers/Manufacturers
Perspective of Large Pharmaceutical Companies
Challenges for manufacturers in the SLD market.28 Richardson reflected on key concerns from the manufacturer’s standpoint about entering into and remaining in the SLD market, emphasizing that manufacturing SLDs is not yet attractive from an economic perspective. Absence of accurate demand forecasting makes it difficult to shape investment, production, and time lines. Richardson noted that Eli Lilly considers an adequate demand forecast to be one that is 80 percent accurate 2 months into the future, and projects at least 24 months. Currently, there is no forecast of SLD orders available, nor is the accuracy of the global annual forecasts measured in a meaningful way.
Information is lacking about product use in country and patient enrollment rates and plans, and data like these from in-country DR TB programs are not being fed back into any forecasting mechanism. Despite the usually ideal circumstance of having a single primary customer (WHO) for SLDs, the ordering process remains unpredictable with little to no forecast visibility. With no reliable forecast, in most cases manufacturers will not begin
___________________
28 This subsection is based on the presentation by Iain Richardson, Senior Director, Global Supply Chain and Logistics, Eli Lilly and Company.
activities, or even order raw materials, until an order is received. This in turn leads to long lead times of several months to satisfy orders, which puts more pressure on countries to accurately forecast their demand up to a year or more in advance. The result is drug management challenges in-country, creating either shortages due to an inaccurately low forecast or the destruction of expired goods when a country forecasts its needs too high.
Richardson offered three suggestions for improving the operation of the SLD market, assuming significantly larger numbers of patients were to be enrolled in treatment and the demand for internationally quality-assured (IQA) drugs were to grow substantially:
1. attract more manufacturers to the market to avoid the monopoly premium effect;
2. develop improved forecasting mechanisms to make demand more predictable; and
3. improve supply chain design, including creating buffer inventories, which would allow manufacturers to produce larger, more economic production batches and reduce other lead times for customers.
More manufacturers would enter the market and engage in fruitful price negotiations, assuming the following conditions were met: substantial growth of overall demand, improvement of predictability of orders, and manufacturers’ optimization of production scale.
Regulatory challenges.29 Challenges to major manufacturers are also posed by an increase in regulatory barriers at the local and national levels in high–MDR TB burden countries. For example, at least 17 of the top 27 high-burden countries currently require at least one level of local registration (some countries also request that production information on the label be in the local language). Even for what are called “accelerated” regulatory approvals, there are still requirements for dossiers and filing fees, and such approval processes present opportunities for delays and market fragmentation. In contrast, countries that do not have additional local mechanisms, relying instead on the recognition of another regulatory authority’s approval (e.g., WHO PQ), enjoy more rapid access to IQA medicines without generating additional costs.
Complexity of SLD manufacture. An important technical issue faced by manufacturers is the complexity of the manufacturing process for SLDs.
___________________
29 This subsection is based on the presentation by Iain Richardson, Senior Director, Global Supply Chain and Logistics, Eli Lilly and Company.
Richardson clarified the distinction between chemically produced drugs (e.g., cycloserine), which are relatively straightforward to scale up in terms of volume of production, and injectable drugs, which are fermentationbased and require freeze-drying of the active ingredient. Fermentation chemistry and freeze-drying are expensive and complex processes. Cassell remarked that such injectable MDR TB drugs are made by microorganisms and have associated problems with batches, reproducibility, and yields (and thus QA). Patrick Lukulay, Vice President, Global Health Impact Programs, U.S. Pharmacopeial Convention (USP), noted that manufacturing the API for SLDs is also very complicated and extremely expensive due to the crystallization steps required.
Need for systems strengthening and capacity building.30 SLDs for MDR TB are a particular challenge for pharmaceutical companies. Small volumes are required, they are expensive, and they are difficult to manage and to ensure proper treatment compliance. Sebbag stressed that reliable diagnosis and ensuring compliance are crucial for protecting SLDs from misuse, which can lead to further spread of MDR and XDR TB. In that vein, drugs are only one part of the MDR TB challenge, in that education and enhanced patient communication are also needed, calling for systems strengthening and capacity building, such as training programs, at all levels of the supply chain.
Using malaria treatment as an example, Sebbag described how a complex organizational structure of institutions, partners, and funding entities (which is similar to the MDR TB structure) makes facilitating communication among the multiple partners a challenge. One strategy for systems strengthening that is used in malaria treatment is capacity building to implement best practices at the local supply chain and case management levels to ensure that patients have access to QA drugs. Sebbag noted that this would require both workforce training and active partnership of all players in the SLD supply chain.
Perspective of a Midsize Pharmaceutical Company
Paul Ryu, Dong-A Pharmaceutical Co., Ltd., provided the perspective of a midsize pharmaceutical company. According to Ryu, Dong-A is currently producing drugs to meet 80 percent of the world’s demand for cycloserine; the company has made investments of about $3 million to upgrade its facilities and has already been inspected by WHO. He commented that the company is willing to make the investment to enter the
___________________
30 This subsection is based on the presentation by Robert Sebbag, Vice President, Access to Medicines, Sanofi.
Sidebar:
Eli Lilly’s SLD Technology Transfera
In 2003, Eli Lilly and Company launched the Eli Lilly MDR TB partnership (the “Partnership”). As part of the Partnership, Eli Lilly committed to transfer the technology necessary to manufacture two SLDs (cycloserine and capreomycin) to partners globally, some of whom would be located in countries with the highest MDR TB burden. In this way, Eli Lilly hoped to improve the availability of these drugs and the sustainability of their supply. In addition, between 2003 and 2011, Eli Lilly manufactured and supplied these two medicines to the WHO mechanism at concessionary prices. Eli Lilly stopped supplying cycloserine in 2006 and stopped manufacturing capreomycin in 2011, by which time several of its partners were able to supply the market.
To illustrate the rationale behind Eli Lilly’s decision to transfer the technology for the manufacture of capreomycin, Iain Richardson, Senior Director, Global Supply Chain and Logistics, Eli Lilly and Company, explained that by 2011, despite having already doubled the company’s internal capacity, manufacturing was “running flat out” and still only supplying sufficient medicine to treat approximately 7,000 patients. However, the installed capacity across Eli Lilly’s partners far exceeds Eli Lilly’s own capacity, thereby better assuring supply. Richardson also illustrated that Eli Lilly’s cost structure for cycloserine manufacture was simply not competitive when compared with other manufacturers that had different scale and overhead drivers.
Such factors drove Eli Lilly’s decision to transfer the technology for the manufacture of those two drugs, with the intention of creating a sustainable long-term supply and increasing market volume, by shifting production to low-cost manufacturing partners in high-burden countries. Richardson described how that technology was transferred via the Partnership to seven manufacturers, including several in high-burden countries (China, India, Russia, and South Africa). All of those manufacturers now have regulatory approvals, six have stringent regulatory approvals or WHO PQ, and one has national regulatory authority (NRA) and pending WHO PQ approval. Eli Lilly has continued its involvement with those partners in their ongoing attempts to expand the SLD market and provide treatments to patients.
___________________
a This box is based on the presentation by Iain Richardson, Senior Director, Global Supply Chain and Logistics, Eli Lilly and Company.
SLD market, despite its small size. However, it is currently facing a regulatory barrier, delay in WHO registration, and is seeking options to expedite the process. Lisa Hedman, Project Manager, WHO, commented that the Expert Review Panel process can grant a provisional prequalified (PQ) status while registration processes are pending. She noted that WHO is dealing with a “bandwidth” problem due to the large number of pending applications and inspections. This, compounded by the percentage of poor applications received from facilities deemed unacceptable (all of which must be processed and inspected), exhausts WHO resources and generates further delays.
Perspective of Providers and Collaborating Organizations
Challenges of Implementing a New MDR TB Program in a Country That Went Through the Proper GLC Mechanism31
Goldfeld related her experiences about how GHC initiated an MDR TB treatment in Ethiopia in 2009 in partnership with the Federal Ministry of Health. Ethiopia is a high TB- and MDR TB–burdened country. The GHC program employed a hospital and community-based MDR TB care model that GHC has pioneered in Cambodia, and in so doing overcame major obstacles, including the delay of WHO/GLC-approved SLDs from GDF and issues of diagnostic lab capacity, human resources, isolation beds, and access to traditional funding mechanisms such as the United States Agency for International Development (USAID).
Until this GHC program initiated treatment in February 2009, no MDR TB treatment was available in Ethiopia despite its large estimated burden and a successful application by the country for SLDs. Forty-five patients were approved for treatment, and the drugs had been anticipated for delivery in October 2008; however, they did not arrive until almost a year later. In this vacuum, by leveraging an initial donation of 18 doses of capreomycin from Eli Lilly, GHC, with the support of the Jolie-Pitt Foundation, purchased additional SLDs, provided funds for the inpatient and outpatient management of patients, and performed the first countrywide MDR training, including training personnel in its program in Cambodia. The South-to-South partnership that GHC established, with ongoing technical and program design input and funding for additional SLDs, ancillary medication, food baskets, social support, laboratory tests, and procedures, was supported by charitable contributions, including a grant from the Eli Lilly MDR-TB Partnership, continued support from the Jolie-Pitt Foundation,
___________________
31 This subsection is based on the presentation by Anne Goldfeld, Professor of Medicine, Harvard Medical School, and Co-founder, GHC.
305 courses of capreomycin from Eli Lilly, a donation of cycloserine from the Chao Foundation, and paser from Jacobus. These resources, in addition to the leveraged expertise, were key in removing bottlenecks to drug procurement from the Ministry of Health. When GLC drugs finally arrived, in-country expertise in treatment and management of MDR had been established, allowing rapid adoption.
The Ethiopian/GHC MDR TB treatment partnership has been scaled up and was expanded to Gondar in northern Ethiopia in August 2010. As of July 2012, 500 patients had been initiated on therapy, with only 6 defaults and a death rate of less than 10 percent in a very sick population (24 percent HIV co-infection and the majority of patients with bilateral cavitary disease). Though some GLC-imposed procedures (e.g., requirements that patients be hospitalized in isolation wards that were not yet completed at program initiation) were extremely problematic due to lack of funding and capacity limitations, Goldfeld noted that it was possible to find “work-arounds,” such as setting up interim isolation wards in unused facilities and receiving drug donations. Goldfeld reported that scale-up of the MDR TB program, countrywide training programs, and health systems capacity building are ongoing in Ethiopia, with outstanding program results. She also noted that of the original 221 patients who were waiting for therapy in fall 2008, her team was able to find and initiate only 66 (30 percent) on MDR therapy; 42 had died waiting for drugs (20 percent), and the team was unable to find 110 (50 percent) of those on the list despite a house-to-house search, many of whom thus presumably died. She emphasized the urgency of providing access to high-quality MDR care to interrupt preventable death from a curable infectious disease and to interrupt further transmission.
Challenges of Supplying QA SLDs (MSF)32
Henkens described MSF’s role in providing MDR TB treatment worldwide. In 1999, MSF became involved with procuring SLDs. The organization committed to pooling procurement of 2,000 treatments from QA sources by negotiating a price drop with manufacturers and buying drugs to guarantee complete treatment.33 MSF currently treats approximately 1,200 patients in 17 countries, using both the GDF procurement mechanism and its own pooled procurement mechanism for certain projects. MSF maintains a stringent QA structure by adhering to the requirements for SLDs determined by
___________________
32 This subsection is based on the presentation by Myriam Henkens, International Medical Coordinator, MSF.
33 After 2,000 treatments were attained, procurement was taken over by IDA in 2002 and transferred to GDF in 2006.
approved regulatory bodies (e.g., WHO PQ, SRA registration, U.S. FDA tentative approval, Global Fund/GDF Expert Review Panel) or evaluated following its own internal qualification scheme. Henkens also suggested that countries should commit to using only QA drugs, noting that given the relatively low efficacy of current treatment protocols and given the high rate of adverse reactions, patients have the right to be sure that at least the quality of drugs is not questionable.
A major challenge facing the supply chain for QA SLDs is that despite an increase in the number of QA manufacturers, the prices of QA products have not improved since 2001. Henkens suggested that the fact that Eli Lilly no longer subsidizes capreomycin and cycloserine could account for the increases in price of those drugs, noting that the prices for products that had the same manufacturers in 2001 and 2011 (ethionamide, prothionamide, and PAS) are the ones that have remained stable. Improved coordination among countries and producers could help ensure that increased competition does not lead to increased prices, given limited availability. Henkens suggested that “breaking the vicious cycle” would require strategies such as improved forecasting, transparent market allocation, and implementing a revolving fund and rotating stockpile.
Challenges for South Africa’s MDR TB Policy34
Decentralizing MDR TB care. The South African National Department of Health has announced that every facility treating MDR TB will have a GeneXpert® machine for diagnosing M.tb. and rifampicin resistance by the end of the 2012 financial year. Gray speculated that the expected significant increase in the number of diagnoses and the number of patients requiring MDR TB treatment will present a serious challenge to the current treatment capacity in the country. Only 2,500 beds are available in specialist TB hospitals in the country (for a population of 50 million). To address this problem, South Africa has developed a new policy aimed at decentralizing the management of MDR TB patient care. Gray expressed his concern that the policy also includes, in certain cases, treating patients on an ambulatory basis even in the intensive phase of treatment.
Challenges of public tendering. Most TB drugs in South Africa are procured on a competitive basis through the public sector, which generates a specific set of problems. First, there is a dearth of domestic producers, despite the tender system being geared toward nationally registered products. Second, many tenders for first- and second-line products go unawarded; for
___________________
34 This subsection is based on the presentation by Andrew Gray, Senior Lecturer, Pharmaceutical Sciences, School of Health Sciences, University of KwaZulu-Natal.
example, in view of the limited number of products available, a company might opt not to put forward a tender in the hope that the government would then approach the company to negotiate a price higher than the one the company would have offered in a tender. Third, there is a concentration of suppliers—particularly suppliers of API. Though there are multiple formulators, the current market is overreliant on a single, limited supplier. It was also noted by several workshop participants that individual countries have different approaches for managing MDR TB and the SLD drug supply; country-specific experiences can inform efforts to improve the global supply chain (Box 1-4).
Need for New SLD Formulations and Regimens
The individual panelists offered the following additional concerns and problems relating to SLD formulations and regimens:
• prevalence, severity, and lack of diagnosis of SLD-related adverse reactions (e.g., ototoxicity due to injectable agents);
• problems with the effectiveness of existing SLDs;
• absence of pediatric formulations for SLDs; and
• lengthy and complex nature of treating DR TB with SLDs.
In light of such disadvantages, several panelists suggested there is an urgent need to develop new and improved regimens. Over the course of the session identifying barriers and challenges to the supply chain, individual speakers and participants also identified potential ways forward to address some of the key barriers (Box 1-5).
BOX 1-4
Country Approaches to the MDR TB SLD Supply Chaina
Several speakers delivered presentations describing the approaches of specific countries with a high burden of MDR TB. Norbert Ndjeka, MDR TB Director, Department of Health, South Africa, described the challenges faced in South Africa’s approach to MDR TB management. Joël Keravec, Brazil Country Program Director, Management Sciences for Health (MSH), explained Brazil’s model for TB treatment and drug management. Andreas Seiter, Senior Health Specialist, Pharmaceuticals, Health, Nutrition, and Population, World Bank, described India’s approach to cofinancing its NTP with the World Bank.
South Africa
Ndjeka addressed South Africa’s approach to the challenges it has faced in treating MDR TB patients and its attempts to shift from pilot projects to a nationally owned program. With the highest TB burden in the world, South Africa still struggles with a gap between diagnosed and treated MDR TB patients and a treatment success rate of less than 50 percent.b A first step is the standardization of treatment guidelines throughout the country, along with a means of ensuring compliance. To address the current bed shortage for MDR TB patients (due to an inadequate number of specialized facilities), South Africa has adopted a national strategic plan to transition to a decentralized model of MDR TB management. The essence of the policy is to de-institutionalize care of MDR TB patients, to “bring treatment to the communities where they live.” A parallel objective is to implement nurse-initiated MDR TB treatment to further facilitate early diagnosis, initiation of treatment, community DOT, and patients’ education about their disease. Under this policy, drug procurementc and quality testing are performed by the Department of Health and universities, not through global mechanisms of QA (e.g., WHO PQ, GLC, GDF).
A huge discrepancy remains between the cost of treating an MDR/XDR TB patient and the cost of treating a drug-susceptible TB patient in South Africa; approximately 100 drug-susceptible TB patients can be treated for the cost of treating a single MDR/XDR TB patient. This gives rise to challenges of prioritization, in that arguments exist for focusing on drug-susceptible TB and for questioning the high cost of treatment for MDR TB. A further issue is that TB funding in South Africa is not securely dedicated to TB only, and funds are often used for other health-related programs. Patients’ high pill burden (especially when co-infected) and antipathy toward receiving injections is also a challenge, as is problematic
packaging of medicines. Ndjeka outlined another stream of systemic challenges related to the supply chain for MDR TB drugs, specifically stock-outs. Poor forecasting leads to drug stock-outs, and there are no effective mechanisms to track those stock-outs or drug usage in general; “unreal” stock-outs often occur when there are drugs available at pharmaceutical depots but not at treatment facilities.
Brazil
In Brazil, the state is the key player in all aspects of drug management. In his presentation, Keravec explicated Brazil’s national policy for TB management and control, which could provide a model for protecting the effectiveness of new TB drugs as they enter the market. Brazil currently carries a relatively low level of DR TB burden. Despite ranking 17th of the 22 highest-burdened countries for drug-susceptible TB, only 700 DR TB patients are notified and treated each year. All TB drugs are available only in the public sector, free to patients, and QA; both TB and DR TB treatment is similarly restricted only to the public sector. A system of reference centers is responsible for creating and monitoring DR TB treatment centers.
Keravec noted that Brazil’s NTP quantifies need and the Ministry of Health’s pharmacy department is responsible for procuring QA drugs. Local procurement is prioritized via direct agreements with public government manufacturers and via an open-tendering process at market prices for SLDs that are produced by local private-sector manufacturing firms.d However, he described the following major challenges facing Brazil’s public SLD manufacturers: manufacturers have limited access to APIs that are not produced in the country, tax burdens and production costs are high compared to India and China, and the small internal DR TB market makes investment in SLD production by local manufacturers less attractive. Such barriers have led to the need to procure some SLDs that are not produced in-country (i.e., capreomycin, cycloserine, PAS, and clofazimine). In such cases the GDF/GLC mechanism is employed using the Pan American Health Organization as a mediator. To enable improved demand forecasting, procurement, and distribution, all DR TB
patients and SLDs are managed within a single integrated, Web-based platform (e-TB Manager/Site TB). This platform integrates all levels of TB control within the public sector and reference centers treating DR TB patients, including information on diagnosis, clinical management, and drug supply components. The system contributes to multiple programmatic aspects of DR TB control including, pharmacovigilance and precise drug consumption monitoring.
India
India is employing a different type of approach to financing its TB program, as described by Seiter. The World Bank finances loans (not grants) to the Indian government that are used to procure drugs, but in a way that is implemented within India’s own national policy and in accordance with its own set of regulatory standards. The World Bank is not involved in any phase of procurement except for financing. This strategy is aimed at system strengthening, essentially functioning as a bridge between scale-down of the prior Global Fund program and scale-up of Indian investment in its own national program. The World Bank itself does not have the in-house technical expertise to help achieve such goals, but it can partner relatively flexibly to bring in the appropriate technical and organizational support as required. This financing framework could serve as an example of how countries might transition from external grants to autonomous financing and in the process build a stronger NRA. In India, pharmaceutical manufacturers are regulated at the state level, but the central authority plays a role in ensuring quality for centrally procured medicines. Neither the state regulators nor the central Food and Drugs Authority are yet accepted as “stringent regulatory authorities” by the international community.
___________________
a This box is based on presentations by Joël Keravec, Brazil Country Program Director, Management Sciences for Health (MSH); Norbert Ndjeka, MDR TB Director, Department of Health, South Africa; and Andreas Seiter, Senior Health Specialist, Pharmaceuticals, Health, Nutrition, and Population, World Bank.
b A new national strategic plan has a target of 60 percent within the next 5 years.
cNdjeka noted that the companies that supply the drugs to the Department of Health are the same ones supplying those drugs for WHO PQ.
dAll manufacturers are validated and registered by Anvisa, Brazil’s NRA.
BOX 1-5
Suggested Ways Forwarda
Over the course of the session identifying barriers and challenges to the supply chain, individual speakers and participants also identified potential ways forward to address some of the key barriers. A number of their suggestions are also addressed later in this report. Those suggestions are compiled here in an integrated summary of those individually identified themes. These suggestions should not be construed as reflecting consensus or endorsement by the planning committee, the Forum, the workshop participants, or the IOM.
• Implementation of a rotating stockpile or buffer stock could smooth demand and improve predictability in manufacturing and delivery processes.
• Improved demand forecasting and predictability could help to attract new manufacturers to the market and improve competition.
• Donors should consider increasing the allocation of funding toward program implementation and improvement and/or shortening of the current treatment regimen.
• Pooled procurement and direct procurement from manufacturers employed within the GLC mechanism could alleviate the problems of limited availability and high prices.
• Taking steps to accelerate registration, adoption, and uptake could reduce drug lag in the regulatory process.
Key Messagesa
• Failure to address the current SLD supply chain issues will lead to increased drug resistance, morbidity, and mortality.
• GLC was designed to foster pilot projects and was not meant as a mechanism for scale-up.
• Market-shaping strategies, including shifting the push-pull boundary, may improve the drug supply chain.
• Crucial barriers to addressing SLDs include high cost, limited supply, and manufacturing complexities.
• Difficulties in meeting multiple, complex regulatory requirements contribute to delays in delivery of SLDs.
___________________
a Identified by individual speakers.
• Training programs implemented at all levels of the supply chain could strengthen systems and build in-country capacity.
• Development of innovative financing and contracting structures to shift the push-pull boundary SLD supply chain toward forecast-driven manufacturing processes could improve efficiency and cost.
• Market fragmentation could be addressed by consolidating and harmonizing product specifications.
Mostaghim offered four strands of market structure adjustments that might help to facilitate patients’ access to QA SLDs. He noted that successful implementation of these types of structural adjustments would require tight coordination among all partners and stakeholders, including donors, procurers, manufacturers, NTPs, regulators, and so on.
• Demand-side activities: Consolidating and harmonizing NTPs’ technical specifications for products and facilitating in-country registrations.
• Manufacturer engagement: Providing clear guidance regarding entry and eligibility for key product specifications.
• Regulatory engagement: Expediting priority dossiers for key products by maximizing the number of eligible participants in a tender.
• Procurement and supplier selection: Proactively splitting tenders, ensuring transparency, and providing clearer demand forecasting.
___________________
a Identified by individual speakers.