This chapter provides an overview of issues related to the financing of the MDR TB supply chain discussed at the workshop. The first section provides an overview of the experiences of organizations currently funding the MDR TB chain. The second section describes the financing models for supply and details specific innovative financing approaches and applications used by other programs to consider how those innovative models and approaches might be applied to financing of SLD supply.
FUNDING OF THE MDR TB SUPPLY CHAIN
Each step in the supply chain process, from selecting a pharmaceutical for manufacture to patient use, requires support and funding from multiple organizations in the public and private sector. During the workshop, speakers and participants from organizations that provide funding for SLDs described challenges and barriers that their respective organizations have faced in funding MDR TB and explained some of the funding strategies that they have used to address those issues.
USAID’s Funding Perspective1
Cheri Vincent, TB Team Leader, USAID, described SLD supply chain challenges from the perspective of USAID. She characterized the lack of demand for SLDs as urgently essential because “it really means that patients
___________________
1 This subsection is based on the presentation by Cheri Vincent, TB Team Leader, USAID.
are not getting lifesaving treatment,” and she observed that resolving the problem would require concerted effort to scale up MDR TB treatment programs: WHO/Stop TB Department data indicate that only 19 percent2 of the estimated MDR TB patients worldwide were initiated on treatment in 2011.
Funding Inflexibility and Loss to Use
Inflexibility and mismatch of funding for SLDs is another key concern. An analysis of a randomly selected set of countries that compared the number of available Global Fund or UNITAID SLD treatment regimens to the number of MDR TB patients enrolled in 20113 revealed that only 39 percent of the available funds were actually used by countries for MDR TB treatment regimens. In other words, of the selected countries, only 30 percent met their planned MDR TB treatment objectives. This situation makes for an unpredictable market for manufacturers, donors, and technical assistance providers. Unused Global Fund funding can be reprogrammed for other important activities, but most of the time the country loses these valuable resources to treat MDR TB. However, it is also clear that a few countries are scaling up MDR TB treatment faster than SLDs are available in the Global Fund grants. These countries are not able to benefit from the drugs not used in other countries so they can continue to provide diagnosed patients with the lifesaving drugs. Vincent argued that more flexibility for available funding would allow resources to be matched more appropriately with enrollment and allow some countries to scale up faster, saving more lives and reducing the amount of MDR TB funding lost.
Country Capacity Barriers
Another challenge centers on insufficient country capacities for scaleup, forecasting, procurement, and in-country logistics. Global scale-up trends are erratic; some countries are scaling up more quickly than expected and many others more slowly. Due to the slower and unpredictable rates of national MDR TB program scale-up in countries like India, estimates about the number of patients to be treated are often unrealistically optimistic. The resulting discrepancies between forecasted demand and actual orders placed are problematic for manufacturers. Vincent noted the need for more regular reporting on the pace of MDR TB enrollments by country. WHO and GLC are now starting to monitor MDR TB enrollments by country every 6 months instead of yearly.
___________________
2 Representing approximately 55,000 MDR TB patients.
3 Per WHO enrollment data.
Many countries also struggle with the complicated Global Fund procedural and financial requirements, increasing lead times in procurement and causing drug stock-outs, which suggests that the processes need to be harmonized and simplified. On the manufacturing side, limited production of QA API and FPP SLDs is caused by a lack of incentives for suppliers and a lack of competitiveness in the SLD supplier market, which yields higher prices.
USAID’s Approach
Vincent described USAID’s three-pronged approach for making affordable, quality SLDs available around the world. The first approach is to improve and expand the global SLD supply chain, which mainly involves
• coordinating with GDF4 and other partners on SLD SCM at the global level;
• improving procurement procedures;
• harmonizing treatment regimens; and
• improving the data on SLD needs.
The second approach is to encourage the quality of QA SLDs by taking the following steps:
• supporting GDF in the market development of quality SLDs;
• providing technical assistance to FPP and API manufacturers to become PQ;
• developing public pharmacopeia standards; and
• improving diagnostic technology.
The third approach, technical assistance at the country level, involves
• reducing bottlenecks through better quantification and stock-out early warning systems;
• in-country and regional technical assistance in pharmaceutical management for MDR TB (SLDs and ancillary medicines);
• development of new training platforms and information systems for SLD management; and
• promoting active indicator-based surveillance of use of SLDs, the formularies, and co-medication safety.
___________________
4 USAID is a major donor to GDF, providing 31 percent ($14.97 million) of GDF’s budget in 2011.
Vincent offered several suggestions geared toward accelerating progress in the MDR TB arena. NTPs would benefit from a sustainable, flexible, and pooled global SLD procurement mechanism, an approach that would need major support from the Global Fund as one of the largest funders of SLDs globally. She maintained that because the Global Fund is the major donor for SLD procurement outside of countries’ direct procurement, it needs a restructured focus with new procedures and processes put into place.
Vincent suggested that there is a need for four interventions that the Global Fund and others should support through GDF, including
1. a revolving stockpile to expedite delivery to countries and provide flexibility to countries for scale-up;
2. an APC to motivate suppliers to place full-batch orders to bring prices down;
3. incentives to manufacturers of QA API by guaranteeing orders; and
4. incentives to BRICS countries (Brazil, Russia, India, China, and South Africa) to procure QA medicines by supporting domestic QA manufacturing.
These interventions, she estimates, would reduce procurement time delays from 6 to 8 months to 3 to 4 months.5
Vincent noted that continued communication and coordination among donors through existing working groups and entities should move from talk to action, citing the successful example of the Xpert® Buy-Down. In August 2012, Cepheid, along with partners BMGF, PEPFAR, USAID, and UNITAID, announced their agreement to support a buy-down of the price of the Xpert MTB/RIF (rifampicin) molecular test,6 with the objective of improving diagnosis and identification of MDR TB patients, a key first step in patient management and treatment that can thus reduce transmission (Cepheid, 2012). The buy-down agreement makes the test available in 145 high-burden countries for less than $10 per test (down from $16.86). However, the important work of partners to scale up MDR TB treatment must happen at the country level with appropriate in-country technical assistance.
___________________
5 Since the workshop, according to Vincent, there has been progress on moving some of these interventions forward with the Global Fund.
6 Endorsed by WHO in 2010 for use in countries with high TB burdens.
Benefit of National-Level FLD Procurement
At the national level, countries (that are capable of doing so) could be helped to transition to taking over responsibility for FLD procurement, allowing more donor funds to be channeled toward SLDs. There were more countries with government budget line items for FLDs before the Global Fund started than there are now. Although the Global Fund has ensured the scale-up of FLDs, the effort may have also crowded out some governments from providing even their minimal levels of funding for anti-TB medicines. Vincent noted that all of her suggestions ultimately hinge upon PMDT scale-up, particularly in countries like China (1,000 of 50,000 estimated cases put on treatment in 2011) and India (3,300 of 64,000–100,000 patients treated in 2011), and that countries need intensified support and capacity building to scale up.
Perspective from BMGF7
Michael Kimerling, Senior Program Officer, Tuberculosis Global Health Program, BMGF, described two key challenges related to the over-fragmentation of the market at multiple levels. The first is a “collective inability” to consolidate demand and ensure the predictable and timely treatment delivery needed to incite the necessary volume growth. The second challenge is to find a way for stakeholders and funders to cooperate more effectively and leverage their strengths to stimulate price competition in the market.
He suggested that three integrated strands of innovation are necessary to spur progress toward resolving these issues:
1. Continued development of the right diagnostics to include expanding laboratory infrastructure and investing in R&D for point of care and rapid DST.
2. Consideration of new drugs and improved therapeutic regimens currently under development (he suggested the need to shift focus from old drugs to inclusion of new drugs).
3. Regimen harmonization and standardization to improve forecasting, simplification of the complex MDR TB patient management system, and encouragement of deeper country ownership.
___________________
7 This subsection is based on the presentation by Michael Kimerling, Senior Program Officer, Tuberculosis Global Health Program, BMGF.
Cost of Drugs Versus Cost of Delivery
Kimerling urged the MDR TB community to look beyond the price of SLDs to consider the overall cost of patient care and treatment (i.e., the price of delivery8), because the SLD pricing problem is only one factor. He noted huge variation in cost of delivery in different countries and warned that “we can’t be naive and think that by solving the SLD-pricing problem we actually are solving the care and treatment problems.”
Impact of New Drugs on Market Fragmentation
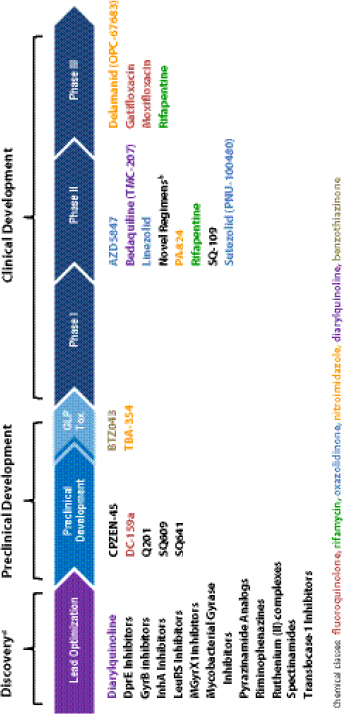
The SLD market is fragmented by country-to-country variations in treatment regimen, and in many cases multiple regimens within countries themselves. Therefore, achieving market stability and reliable forecasting is likely to be further challenged and fragmented by the introduction of new drugs and regimens that are currently under development as part of the existing pipeline (Figure 3-1).
Because, Kimerling said, at least two of the new drugs in Phases II and III of clinical development could be on the market in the next 1 or 2 years, there will be a need for forecasting and modeling about the impact of their market entry. Currently, there are three key funders in the MDR TB space: Global Fund, UNITAID, and individual governments. He suggested interacting directly with those governments that are developing economically and have their own resources and health budgets that could potentially be combined with funding organizations in an innovative way.
BMGF Program-Related Investments (PRIs)
Kimerling remarked that the model of BMGF-supported PRIs, which take the form of loans that must be repaid (not grants), could potentially serve as an innovative financial intervention to help break the price-inflation cycle in the MDR TB market (Figure 3-3). PRIs are financial mechanisms offered by foundations like BMGF in the form of equity or debt guarantee as a way of using endowments to support charitable market interventions. For example, PRIs could help to counteract monopoly premiums by facilitating competition. Volume guarantees could be used to offset the costs of subscale manufacturing and to address risk premiums. PRIs could help manufacturers by supporting a product’s manufacturing line or by taking an equity position through a loan or direct investment.
To be implemented, these types of mechanisms must function in conjunction with all players involved. Therefore, Kimerling suggested,
___________________
8 Including hospital and clinic care, training and personnel costs, lab tests, etc.
FIGURE 3-1 Global TB drug pipeline, as of June 18, 2012.
NOTE: GLP, Good Laboratory Practices; Tox., toxicity.
a Ongoing projects without a lead compound series can be viewed at http://www.newtbdrugs.org/pipeline-discovery.php (accessed November 19, 2012).
b Combination regimens: first clinical trial (NC001) of a novel TB drug regimen testing the three drug combination of PA-824, moxifloxacin, and pyrazinamide was initiated November 2010 and completed in 2011 with promising results. The second clinical trial (NC002) of this regimen was launched in March 2012 and will test the efficacy of the regimen in drug-sensitive and multidrug-resistant patients. The third clinical trial (NC003) will evaluate PA-824, TMC-207, pyrazinamide, and clofazimine in combinations and is scheduled to begin September 2012.
SOURCE: Kimerling, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
they could usefully foster a needed shift from thinking about the cost-effectiveness of a single component of MDR TB treatment to thinking about aggregate affordability.
UNITAID’s Financing and Spending Approaches9
Brenda Waning, UNITAID, WHO, presented two different functional characteristics of UNITAID: that the organization is itself funded through an innovative mechanism (the air ticket levy) and that the organization then spends those funds in an innovative way.
Innovative Funding Mechanism
The air ticket funding mechanism involves application of progressive levies10 to all flights departing from contributing countries, levies from 9 of which represent 80 percent of UNITAID’s funding11 (the other 20 percent comes from various sources12). Air ticket levies have several advantages, including that they
• are equitable, predictable, and untied;
• are cost-effective to collect;
• do not affect airline profitability or sales; and
• have the unique characteristic of constituting a South-to-South cooperation.
Innovative Spending Approach
UNITAID’s strategy for innovative spending consists of a market-only approach to increase access to the commodities used to prevent, diagnose, and treat TB, HIV, and malaria. Market conditions for those products are analyzed to expose their underlying causes and to seek opportunities to apply leverage and resources effectively. Although, as Waning noted, differences among individual markets can make it difficult to draw comparisons (e.g., between first-line ARVs and MDR SLDs), UNITAID’s experience in
___________________
9 This subsection is based on the presentation by Brenda Waning, Coordinator, Market Dynamics, UNITAID, WHO.
10 Ranging from 1 euro for a domestic ticket to 40 euros for an international business class ticket.
11 Cameroon, Chile, Democratic Republic of the Congo, France, Korea, Madagascar, Mali, Mauritius, and Niger.
12 BMGF, Brazil, Cyprus, Luxembourg, Norway from a carbons emissions tax, Spain, and the United Kingdom. Waning quoted a leading group on innovative financing for development as strongly recommending an increase in the number of countries that pledge to UNITAID.
the second-line ARV and pediatric markets (implemented by CHAI), might be applicable to MDR TB SLDs. To that end, Waning offered the following as successful attributes of CHAI’s pediatric ARV effort13:
• Drugs, treatment regimens, and product selections were harmonized within and among countries.
• Good cooperation with WHO was secured with regard to guidelines, consolidation agreements, and prioritization of WHO PQ registration for priority products.
• CHAI collaborated with multiple purchasing organizations (e.g., PEPFAR/USAID’s Supply Chain Management System [SCMS], UNICEF [United Nations Children’s Fund]) to improve quantification for country-level demand forecasting and global-level demand consolidation.
In that effort, it was important to understand supply chain cost structures in order to address their insufficiencies and also to understand interactions across markets (e.g., diagnostics, medicines, nutrition) and the impact of introducing new market entries. Manufacturers’ confidence in the market was bolstered by UNITAID’s coming to the table with a large pool of funding and resources, which resulted in successful price negotiations, improved price transparency, and development of new formulations. Procurement was innovative despite institutional constraints similar to those in the MDR TB supply chain.
Suggested Ways Forward
Waning offered her view on potential ways forward, emphasizing the need to minimize uncertainty as a crucial strategy for the SLD market. Adopting a proactive rather than reactive approach toward managing uncertainty could be a real opportunity in the SLD MDR TB market (e.g., improving clarity of funding and disbursements). Collecting both upstream and downstream data will help maximize efficiency and market certainty. Drawing on new diagnostic technology (e.g., GeneXpert) will help determine actual need more accurately. Connections with the private sector could be further strengthened (e.g., with IMS Health) to impact global market drivers.
Finally, she suggested creating new ways of working directly with those countries outside the donor-funded market (e.g., China and India) where products like API and FPP are being made and new technology is being developed. To deal with the global market space and “capitalize on the
___________________
13 Treatment expanded from 60,000 to 360,000 patients within the UNITAID programs.
bigger world,” UNITAID is making efforts to engage these middle-income countries that are dominating market anchors and not restricting its focus to the much smaller market of donor-funded programs.
MODELS FOR FINANCING AND SUPPLY
During the workshop, speakers and participants presented innovative models of financing and supply used by other organizations and programs to supply medical products for other diseases. The workshop discussions considered successes of and lessons learned from these other initiatives, the extent to which those lessons could or would not apply to MDR TB SLDs, and possible applications.
GAVI’s Innovative Financing Mechanisms14
David Ferreira, Managing Director for Innovative Finance, and Head of the Washington, DC, Office, GAVI, provided an overview of GAVI, a public–private partnership founded in 2000 with the mission of increasing access to immunization in poor countries. In addition to increasing access, GAVI aims to serve as a developmental pathway for beneficiary countries toward “graduating” from the system and providing full access independently.
In contrast to the GLC mechanism, GAVI was initially designed and structured with the specific objective of not following a pilot model, but to roll out at the same scale as routine immunization. GAVI was originally part of UNICEF but broke off to become an independent foundation in 2008 and has sought to establish a diverse range of governing partners.15 The inclusion of independent voices (e.g., investment bankers from Goldman Sachs) from outside the vaccine world better informs GAVI’s planning and decision-making processes.
Ferreira introduced three innovative financing initiatives employed by GAVI to try to improve predictability and flexibility of funding, ensure sufficient and uninterrupted drug supply, aggregate and forecast demand accurately, and engage the private sector. The prevalent theme in those initiatives is market shaping, that is, creating a sustainable market with affordable prices and volumes sufficient for global coverage. Next, Nina
___________________
14 This subsection is based on the presentations by David Ferreira, Managing Director for Innovative Finance, and Head of the Washington, DC, Office, GAVI; and Nina Schwalbe, Managing Director, Policy and Performance, GAVI.
15 Among the partners are multilateral agencies, BMGF, developing country governments, donor country governments, civil society organizations, research institutes, and the vaccine industry.
Schwalbe, GAVI, described the GAVI vaccine market-shaping approach and its possible applications for MDR TB.
International Finance Facility for Immunization (IFFIm)
IFFIm is a vehicle for long-term legally binding government commitments. GAVI’s donors (including Australia, France, Italy, the Netherlands, Norway, South Africa, Spain, Sweden, the United Kingdom, and soon-to-include Brazil) aggregate $6.3 billion in nominal terms, some of whom have made commitments for terms over 20 years. IFFIm uses these commitments to borrow money backed by those assets from bond markets in Australia, Japan, the United Kingdom, the United States, and elsewhere. This instrument’s high degree of predictability over the long term enables GAVI to have the extreme flexibility to use money when needed. Ferreira noted that this also enables countries to plan more effectively and allows GAVI to make more sensible deals in the market, ultimately leading to improved public health outcomes.
Advance Market Commitment (AMC)
The AMC instrument is designed to incentivize manufacturers to move quickly from a low-volume, high-margin market (e.g., a three-dose pneumococcal vaccine being produced for rich countries at a very high price) to a business model with high volumes and low margins—in other words, to produce more, differently formulated, vaccines at lower prices. A “war chest” of donor funding ($1.5 billion) was created to present a feasible, sustainable, and attractive market for producers.
The AMC is essentially a long-term secure contract; manufacturers are offered a donor-funded16 price guarantee and purchase commitment as a supply incentive to develop new formulations according to stringent quality standards. In exchange, manufacturers commit to supplying the product at a significantly lower price, long term, to developing countries (paid by both GAVI and beneficiary countries). The incentives to invest in R&D for new formulations stimulate innovation from manufacturers, and the contracting structure secures long-term supply. Ferreira noted that GAVI was able to achieve dramatic price reduction through the use of AMC and that rollout is proceeding rapidly (18 countries so far).
___________________
16 Or in GAVI’s case, a collective pool of donor funds.
The GAVI Matching Fund was launched in 2011 with BMGF and the UK government. GAVI solicits funds from corporations or organizations with the promise that all funds raised within a given time frame will be doubled by the donors. This type of instrument is useful in diversifying the funding base and raising public awareness by encouraging participants to raise funds from their employers, suppliers, etc., which would also be doubled.
Ferreira noted that the GAVI Matching Fund has also been a useful tool for leveraging non-cash resources from partners, which are also matched with cash under certain conditions. By tapping into the core business skills of those partners, the supply chain is strengthened in various ways, such as logistics, delivery, and technology deployment to manage stock. This is a means of having the in-country logistics and in-country supply chain feed back to the global procurement part of the supply chain.
Market-Shaping Objectives
Schwalbe explained that because GAVI’s initial strategy of pooling procurement of vaccines had not resulted in sufficient price reductions after 10 years, the organization adopted a market-shaping approach (in addition to pooling procurement). This approach has three key objectives: (1) to balance supply and demand to ensure sufficient uninterrupted market supply; (2) to control vaccine prices to minimize costs to GAVI and beneficiary countries; and (3) to ensure that the products are appropriate and QA and that innovation is fostered. Once the vaccines are procured, the countries have the full responsibility for actually delivering them, which also guides the market-shaping efforts.
Market-Shaping Tools
Maintaining the appropriate balance between those market-shaping objectives is dependent on the ability to communicate market information in a timely, transparent, and accurate way, and GAVI relies on certain tools to achieve that balance.
• Predictability of funding is enhanced through strategic long-term demand forecasting, which includes corroborating manufacturers’ forecasts against GAVI’s own forecasts.
• Demand pooling17 among 73 countries allows leveraging of high demand volumes.
• Flexible contracting is achieved by adapting procurement tactics to the market environment (e.g., using AMCs to enter into multi-year contracts, demand guarantees, stockpiling, incentivizing new manufacturers).18
• QA is ensured through collaboration with WHO to strengthen national regulatory pathways and support the development of global quality standards.
• A tiered pricing model is used to scale prices according to a country’s relative income.
Factors that influence GAVI’s decisions about potential market-shaping interventions include the production complexity of vaccines (which has some similarities with MDR TB injectables), the market environment, and GAVI’s relative market power. Schwalbe also cited the importance to suppliers of transparent and timely WHO PQ processes.
GAVI’s Cofinancing Policy
Schwalbe detailed GAVI’s financing policy, which falls under the auspices of the organization’s objective to increase the predictability of global financing and improve the sustainability of national financing for immunizations.
Beneficiary countries cofinance with GAVI according to their ability to pay and in compliance with a very strict system. All countries must pay for at least a portion of every vaccine that GAVI funds. The copay for the lowest tier of countries is 20 cents per vaccine.19 The next group of countries starts out paying 20 cents for the first year and then pays a progressive increase of 15 percent per year, and the final group includes “graduated”20 countries that commit to a 5-year trajectory to full pricing. In other words, as countries develop economically, explained Ferreira, they are required to finance larger and larger proportions of their vaccines until they ultimately graduate out of the GAVI system and fully fund their own vaccines. Schwalbe noted that GAVI has ensured, through communication and connection with the pharmaceutical industry, that after graduation,
___________________
17 In cooperation with procurement partner UNICEF.
18 Countries can self-procure if they choose and are reimbursed for the price that GAVI pays.
19 Established based on the cost of the DPT (diphtheria, pertussis, and tetanus) vaccine alone, which is what those countries were already paying for as a minimum.
20 Countries with a gross national income (GNI) of $1,500 per capita or less are eligible for GAVI cofinancing.
countries will have guaranteed access to the same price they were paying within the program.
GAVI’s disincentives for defaulting have thus far generally been effective. Ferreira explained that benefits of the cofinancing requirement include enabling country ownership and forcing the relative interests into alignment. Ministries of health and finance serve as signatories on grant proposals as a way of guaranteeing legislative accountability. Schwalbe also noted that within the cofinancing system, countries do not pay in cash but rather by procuring a proportion of the vaccines (using their own procurement systems or through UNICEF), which is aimed at establishing sustainable self-procurement systems.
Lessons from GAVI’s Model
Schwalbe listed several suggestions for MDR TB that could be gleaned from GAVI’s model:
• clarifying short- versus long-term objectives for each market;
• increasing funding predictability;
• improving demand transparency and credibility of forecasts;
• gathering forward-looking market intelligence on supply and suppliers; and
• engaging in partnerships and regular interaction with manufacturers.
Waning remarked, however, that an attempt to apply GAVI’s people, systems, and resources to the Global Fund would yield very different results because of the Global Fund’s structural and cultural constraints. Specifically, effecting change such as implementing pooled procurement would require the Global Fund to make procurement and SCM plans available. She praised GAVI’s commitment to “sitting at the table” with industry in what she called a safe way, and suggested that WHO and UNITAID should seek a similar level of engagement with industry in order to move forward.
Supply Chain Management System21
SCMS Objectives
PEPFAR/USAID’s SCMS was mandated to establish and operate a safe, secure, reliable, and sustainable supply chain and to develop country-level self-sustaining supply chain skills and capabilities. Gordon Comstock, Director, Global Supply Chain, Partnership for Supply Chain Management,
___________________
21 This subsection is based on the presentation by Gordon Comstock, Director, Global Supply Chain, Partnership for Supply Chain Management.
noted that the challenges faced in the area of HIV/AIDS that led to the establishment of SCMS are similar to those faced in the MDR TB supply chain:
• poor coordination among governments, funders, and donors;
• insufficient long-range forecasting or planning capabilities;
• limited in-country SCM capacity;
• long procurement cycles;
• inability to place timely orders; and
• potentially inadequate infrastructure for warehousing and distribution.
SCMS Design
The impact of the barriers on treatment programs are also similar, including problems with product quality, cost, delays, delivery, and stock management. SCMS was designed to eliminate supply chain barriers, stock-outs, and overstocks by integrating the supply chain’s four key levers:
1. Financial structure enables continuous product supply by pooling procurement through a USAID working capital fund.
2. The business model aligns the requirements of countries with the values of donors (in the case of SCMS, the U.S. government) to enhance flexibility in strategy, structure, people, and processes.
3. Operational strategies employ private-sector supply chain best practices, the key to which is knowing the “customer.”
4. SCMS considers quality systems to be critical and thus adheres to stringent regulatory standards for public health.22
Working Capital Fund
The USAID working capital fund is critical to the SCMS procurement strategy and has the advantage of allowing countries to develop operating plans annually. Funds are deposited up-front and are not time-bound (thus they can be rolled over to the next fiscal cycle) or performance-restricted. Implementing partners are not charged until the product is actually delivered, allowing orders to be placed in advance according to a country’s supply plan and enabling prompt vendor payment. The inventory methodology facilitates a blended inventory price to all countries rather than each country paying a unique price.
___________________
22 The WHO model quality system for procurement agencies.
SCMS’s business model enables procurement decisions and tender splitting based on the best value for components, such as price, transportation costs, vendor performance, vendor lead time, product registrations, common harmonized labeling, and consideration of market dynamics. The logistics model integrates the global supply chain with local supply chains in each country to ensure that products reach their final destination. Central to this effort is prepositioning aggregated operating inventory in hubs or sustainable regional distribution centers to improve efficiency, to save costs (by moving product from the producer to the regional distribution center by sea transport), and to ensure an uninterrupted supply of ARVs by moving the product closer to its target market. Smaller, regular shipments protect local supply chain infrastructure and systems, and ensure the safe, secure, and timely transport of cold or cool chain commodities. The system also allows a rapid response to emergency requests.
Pooled Procurement
The in-country SCMS supply chain model aligns with health care services and strengthens national systemic infrastructure. It seeks to balance country ownership and pooled procurement through a national–organizational partnership. Through SCMS pooled procurement, central stock-outs have been virtually eliminated, and $1.3 billion was saved on procurement of nearly 700 million ARVs. The use of pooled procurement in SCMS, when appropriately aligned with national interests, also serves to increase flexibility, mitigate risk, implement QA requirements, strengthen the market, enhance purchasing power, and improve product availability and the ability to focus on harmonization.
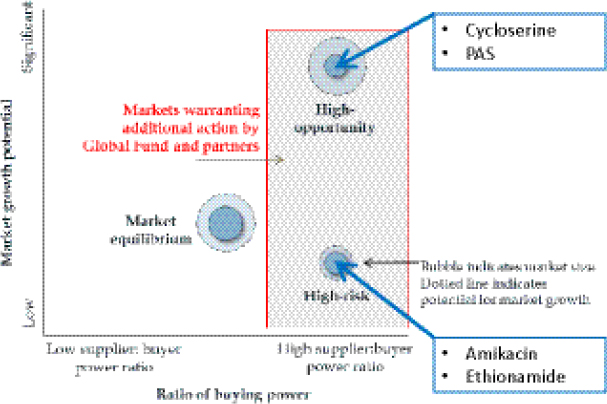
However, SCMS has encountered challenges with pooled procurement that echo those faced by similar MDR TB efforts; examples include limited and fragmented demand, registration requirements leading to country-specific constraints, and a lack of harmonization regarding donor and procurement regulations. Comstock indicated that within SCMS a clear link was created between performance and results, noting that under PEPFAR, ARV stock-outs are not acceptable. He remarked that in the case of the market for MDR TB drugs like cycloserine and PAS, which feature a high supplier-to-buyer power ratio, market action such as pooled procurement could be warranted to gain supplier leverage and to shift the market toward a healthier equilibrium (Figure 3-2).
SOURCE: Comstock, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis, based on presentation to Global Fund Market Dynamics Committee by Results for Development Institute, March 28, 2011.
CHAI’s Model for Improved Access to Pediatric HIV Treatment23
David Ripin, CHAI, recounted several procurement challenges faced by CHAI’s UNITAID-funded project to scale up and catalyze the market for pediatric HIV treatment. When the project was implemented, there was a very low treatment coverage rate and a low-volume, fragmented market with few pediatric-friendly QA formulations or in-country product registrations. Noting that certain barriers to procurement existed that mirror those in the MDR TB supply chain, Ripin described the efforts that CHAI used to mitigate those barriers.
___________________
23 This subsection is based on the presentation by David Ripin, Executive Vice President, Access Programs, and Chief Scientific Officer, CHAI.
Guidance for Prioritizing Regimens
As the market grew, the proliferation of unique treatment products fragmented a demand that was already relatively small, but the development and provision of clear normative guidance about regimen prioritization from WHO, through an IATT (Inter-Agency Task Team), was effective in reducing this fragmentation (e.g., creating a rationalized list to reduce the number of formulations from 45 to 15). CHAI also used that prioritization guidance to help specific countries and communities focus their formulary lists only on optimal products for regimens across all patient weight bands as well as for pediatric formulations.
Ripin remarked that a similar strategy of consolidation and rationalization could be used for MDR TB regimens, which are fragmented both between countries24 and within the same country, noting that some countries have guidelines for more than five standard regimens. Product-level procurement data could be used to identify high-impact opportunities for harmonization of regimens (e.g., he noted that PAS and sodium PAS are the same active ingredient) and to make recommendations about packaging formats to improve market efficiency.
Aggregating Procurement
Another key challenge was the long lead times caused by low-volume individual orders from countries that would often fail to meet minimum production thresholds for manufacturers. In some cases, even aggregate global demand was insufficient for certain products. As the CHAI-UNITAID program transitioned funding and procurement responsibilities to countries, the program adopted the practice of aggregated (as opposed to pooling) procurement among a group of buyers through collaboration with PEPFAR and the Global Fund. CHAI coordinated and aligned orders in a predictable way, consolidated demand around fewer formulations, and reallocated product to manage shortages. Two or more suppliers were selected to supply products where demand was sufficient to support multiple suppliers. In some cases of low demand, where total market volume would not be greater than a few production batches, a single supplier was selected. The result was greater access to low-volume products, lower risk of stock-outs, and more consistent lead times. Feedback from suppliers indicated that they preferred tendering practices that split supply across more than one supplier in a predictable manner to allow higher confidence in allocating production capacity.
___________________
24 He noted that countries have guidelines for regimens based on different drugs: kanamycin versus capreomycin, PAS-Acid versus sodium PAS, cycloserine versus terizidone.
Long and variable lead times were addressed by encouraging robust forecasting and considering non-price factors in evaluation of suppliers’ tender bids. A weighted system was used to account for past performance factors such as supplier delivery metrics and breadth of country registrations (e.g., 70 percent weight on price, 15 percent weight on delivery metrics, and 15 percent on breadth of registration). The weighting strategy encouraged suppliers to improve their delivery performance and reduce delays in trying to improve their weighted scores for the next round of tendering.
Tender Splitting to Aid Market Entry
Ripin explained that minimum batch sizing is a particularly crucial barrier in the pediatric HIV market (as it is for MDR TB), both for new products and for suppliers seeking to enter the market. The batch-size requirements for validation purposes are often greater than preexisting volume of demand for a new product. This increases the risk that some of the material produced in the validation batches will expire before being sold, potentially resulting in higher pricing at the time of market introduction to offset this risk. The higher cost for a new supplier that has not yet achieved efficiencies of scale can put new market entrants at a disadvantage when entering the market. Therefore CHAI used a strategy of splitting tenders to encourage new market entrants; Ripin illustrated the benefits of that strategy on price using a case study (Figure 3-3).
For this product (LPV/r), there was a monopoly price in the market that was low enough to prevent new suppliers from entering the market at a competitive price. A second supplier was added to the program by splitting the tender award across a primary and a secondary supplier, with the secondary supplier paid a higher price than the primary supplier for the first 2 years. The investment in bringing additional suppliers into the market led to price declines for both the primary and secondary suppliers, which has resulted in an overall market savings. Ripin emphasized that though splitting tenders may result in a marginal increase in short-term spending, it can result in a long-term decrease in the amount that will be spent.
Affordable Medicines Facility-malaria (AMFm)25
AMFm was founded in response to the developing resistance to chloroquine and to the fact that the newer class of treatments (artemisinin-
___________________
25 This subsection is based on the presentation by Olusoji Adeyi, Sector Manager, Health, Nutrition, and Population, World Bank.
FIGURE 3-3 Price benefits of splitting tenders. Despite an initial investment of approximately $2 million for a premium price, this strategy led to a broader supply base and significant and sustainable price reductions.
SOURCE: Ripin, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
combination therapies, or ACTs) was too expensive to treat the large numbers of patients in need. Olusoji Adeyi, Sector Manager, Health, Nutrition, and Population, World Bank, offered some lessons from AMFm that could be relevant to the treatment of MDR TB. The functions of AMFm include
• reducing prices via negotiations with manufacturers;
• subsidizing buyers from the public, NGOs, and the private sector26; and
• providing in-country supporting interventions such as promoting uptake and ensuring proper use.
Public- and Private-Sector Interaction
The involvement of the private sector is crucial in provision of supporting interventions because 40–90 percent of treatment, depending on the country, is carried out in the private sector. Adeyi stressed that engaging
___________________
26 Essentially, AMFm makes a copayment to registered suppliers on behalf of the buyer.
with the private sector has been very successful in reducing prices (thus increasing affordability) and increasing availability of subsidized ACT, even in remote areas, and it has facilitated an increased market share for ACTs.27
The public sector has also benefited from the private-sector involvement, as public-sector purchases of AMFm-subsidized ACTs from the private sector have served to offset public-sector procurement delays.28 Adeyi warned, however, that a mechanism like AMFm, designed to work upstream to change the architecture of financing, cannot by itself change the downstream portion (the community level), which requires a reinforcement of quality at the case-management level.
Applications for MDR TB
Adeyi suggested that AMFm-style strategies that might improve access to MDR TB treatment include the use of wider public-sector channels for distribution, new approaches to financing and reducing prices, and determining the lowest-quality level of delivery system that should be eligible to participate in the system. He characterized the latter as constituting a necessary trade-off between “reach” and “richness” in the short term in order to progress toward the ideal extent of access to treatment. All of the above strategies would require pre-agreed scope, measures of success, and approaches to evaluation.
Adeyi emphasized the need to question and address the fundamental assumption that beneficiary countries necessarily have well-functioning central public medical systems. This assumption underpins the central medical store procurement approach that often causes major bottlenecks in the supply chain. As a possible alternative, he suggested taking advantage of and improving existing drug distribution systems in-country. Other efforts are under way to gather information and initiate improvements in the operation of the SLD supply chain, and workshop participants discussed these related efforts and their goals (Box 3-1).
___________________
27 With the attendant effect of “crowding out” oral artemisinin monotherapy, which carries an increased probability of widespread onset of resistance.
28 Adeyi remarked that concerns about middlemen taking advantage of the subsidy, rural areas being excluded from subsidized ACTs, and the private sector depriving the public sector of ACTs were ultimately unfounded.
BOX 3-1
Related Efforts in SLD Supply Chain and Accessa
Second-Line Drug Access Improvement Initiative (SLDAII)
SLDAII is a joint effort of many leading organizations from the public, private, NGO, and allied fields that was founded on the vision that all patients would be able to access affordable, QA treatment within a sustainable MDR TB SLD drug market. SLDAII focuses on developing the technical information and knowledge needed to make rational decisions and to document why certain risks need to be taken.
The initiative comprises six workstreams, concentrated on the supply side, which are each tied to a goal or set of goals. The manufacturer scale-up and operational forecast workstreams are connected to the goal of increased affordability of IQA SLDs. Supply chain diagnosis and financing mechanisms aim to increase the supply of IQA SLDs. The IQA recommendation and private-sector engagement workstreams target greater operational efficiency. Each goal is also underpinned with a set of specific outcomes that need to be accomplished in order to achieve each goal itself (Figure 3-4).
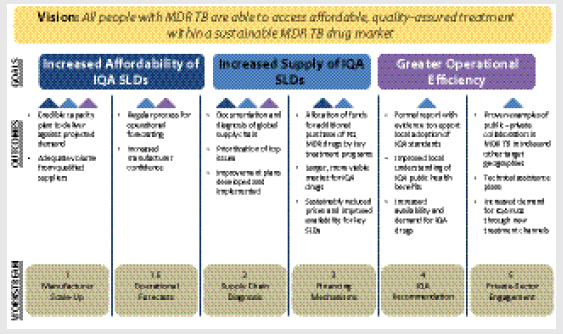
FIGURE 3-4 SLDAII vision and goals.
NOTES: Arrows represent connection between workstreams to different goals; IQA, internationally quality-assured; PQ, prequalified; SLD, second-line anti-TB drug.
SOURCE: Kimerling, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
Michael Kimerling, Senior Program Officer, Tuberculosis Global Health Program, BMGF, suggested that SLDAII fills an important niche in the current environment in helping to ensure coordination and actionoriented progress, but noted that it is open to revision; evaluation is currently underway with the view to ensuring that the right partners are being involved and properly engaged. He said that in moving forward strategically, a shift in focus is needed from identifying barriers to creating innovative solutions and from writing documents to writing business plans. Integrating the private sector will require learning its language and analyzing its needs, which will aid in adapting to a changing market. All efforts should be focused on working urgently and systematically toward a common and clearly established set of principles: ensuring uninterrupted and universal access to the QA drugs at affordable prices.
MDR TB Innovation Summit
Tracy Sims, Vice President, Eli Lilly & Co. Foundation, described ideas that were generated in an MDR TB Innovation Summit that was convened in May 2012.b The Innovation Summit tackled the following clearly defined problem statement:
What innovative approaches/products can be implemented (either directly or indirectly) that would overcome the supply chain barriers that today prevent IQA SLDs from reaching the entire global population of MDR TB patients?
The Summit included participants from both within and outside the sphere of MDR TB expertise who were tasked with dissecting the problem, analyzing it, and producing actionable outcomes. The underlying strategic concepts driving the Summit were the need to take action, develop new approaches, involve new people, engage in partnership, and take well-informed risks.
The initial step in the process employed at the 2-day Summit was to clearly identify the problem and segregate the problem space into five pieces:
1. demand for care;
2. purchasing and procurement;
3. production, QA, and QC;
4. logistics; and
5. delivery of care.
Barriers to the delivery of medication from both the supply and demand sides were identified, and the participants were divided into teams to address each of the identified barriers separately. The teams developed
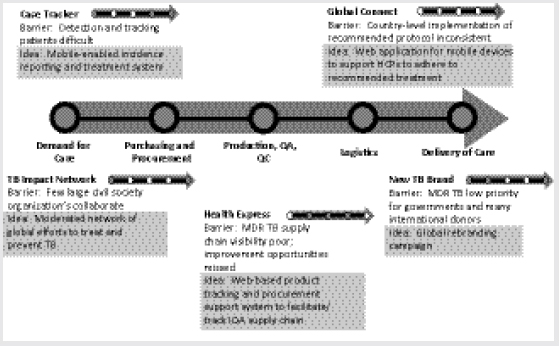
idea fragments, which were then accumulated into unique idea resumes against each barrier and ultimately refined into 12 idea platforms. Five of those platforms were identified as having the strongest potential for resolving major challenges and were developed into emergent, actionable strategies (Figure 3-5).
Sims explained how the five strategies might address their respective barriers in the SLD supply chain:
• Case Tracker: A mobile-enabled incident-reporting and treatment system to pool data, stimulate action, track demand for care, and enable more detailed forecasting.
• Global Connect: A mobile, Web-based system to support HCP adherence to recommended treatment and improve delivery of care.
• TB Impact Network: A moderated network of global efforts to treat and prevent TB and MDR TB and to improve collaboration.
• Health Express: A Web-based product-tracking and procurement support system to improve QA SLD supply chain visibility.
• New TB “Brand”: Development of a consolidated voice or campaign for MDR TB to drive accountability and heighten awareness of the urgency of MDR TB nationally and internationally.
___________________
a This box is based on the presentations by Michael Kimerling, Senior Program Officer, Tuberculosis Global Health Program, BMGF, and Tracy Sims, Vice President, Eli Lilly & Co. Foundation.
b Partners included the Eli Lilly & Co. Foundation, the Stop TB Partnership, and the Innosight Consulting Firm.
Key Messagesa
• Current barriers in access to SLDs include a lack of demand, inflexibility and a mismatch of expectations from stakeholders, and country capacity barriers.
• Collective inability to consolidate demand and a lack of collaboration among stakeholders, including funders, are key barriers to an effective SLD supply chain.
FIGURE 3-5 Owner/participant teams for the top five MDR TB Innovation Summit ideas.
NOTE: HCP, health care professional; IQA, internationally quality-assured; QA, quality-assured; QC, quality control.
SOURCE: Sims, 2012. Presentation at IOM workshop on Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis.
• Unique funding mechanisms and spending strategies offered thought-provoking options for further consideration.
• Successful innovative funding mechanisms used in other disease programs were presented, including immunization, HIV/AIDS, and malaria.
___________________
a Identified by individual speakers.