Appendix B
Possible Factors Underlying
Chronic Multisymptom Illness
Chronic multisymptom illness (CMI)—like several other syndromes, including fibromyalgia, chronic fatigue syndrome (CFS), and irritable bowel syndrome (IBS)—lacks characteristic biomarkers. In the absence of other diseases that would explain the symptoms, its diagnosis is based on symptom criteria. CMI can consist of diverse symptoms that vary among people or even within the same person over time. The symptoms may be related to central nervous system upregulation (amplification) of neural signals that have a somatic or visceral origin rather than originating exclusively in bodily conditions. That mechanism is similar to the current understanding of IBS in deployed Gulf War veterans, which appears to develop by the combination of gastroenteritis, leading to gut mucosal immune dysfunction and inflammation, and impairment of the brain’s ability to reduce or downregulate neural signals from the gastrointestinal tract related to the deployment experience (Drossman, 1999; IOM, 2010; Spiller and Garsed, 2009). The dysregulation of the “brain–gut axis,” malfunctioning of the visceral signaling regulatory system, leads to the characteristic symptoms of IBS.
Although the degree to which the brain enhances neurologic signals from the body in CMI has yet to be determined, it is recognized that the brain’s ability to filter incoming signals is highly modifiable by environmental and psychologic factors. For example, the number of symptoms that people report correlates with psychologic and environmental factors, including levels of anxiety and depression and the degree of stress after exposure to abuse or war trauma (Bair, 2003; IOM, 2008; Vaccarino et al., 2009; Zaubler and Katon, 1996). Such correlations do not imply that the symptoms reported are indicative of a psychiatric disorder. In those
who have pain, these correlations are explained in part by the effects of stressors on limbic areas of the brain (in particular, the cingulate cortex) that are associated with pain regulation. For example, a history of physical and sexual abuse is associated with increased reports of pain in people who have IBS, and increased pain correlates with enhanced activation of the midcingulate cortex, an area at the interface of pain regulation and emotional input (Ringel et al., 2008). In addition, peripheral inflammatory states associated with increased cytokine activation may in turn alter brain functioning, producing “sickness behavior.” These phenomena may help to explain the emotional distress and increased symptom awareness associated with CMI (Dantzer et al., 2008). That putative mechanism is illustrated in Figure B-1, which demonstrates that somatic and visceral sensations (for example, muscle pain, fatigue, and abdominal pain) are experienced as symptoms only when the signal amplitude is above the brain’s perception threshold. Thus, peripheral neural signals arising from an injury might be above the perception threshold and be experienced as a symptom, and other regulatory signals (for example, increased gut signals after eating) might be received in the brain but not experienced as a symptom unless one overeats or has a gastrointestinal disorder, such as functional dyspepsia. In addition, the brain’s ability to downregulate incoming signals (that is, to raise the threshold level) will depend on regulatory processes and the person’s cognitive and emotional state. Thus, injuring oneself might not be experienced as a symptom when one is distracted during a sports event until the event is
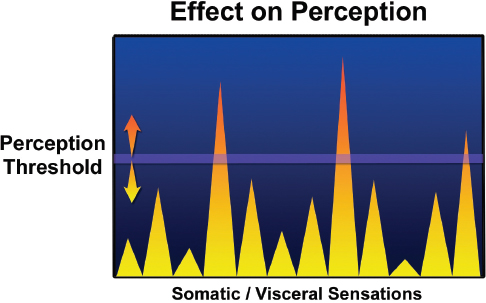
FIGURE B-1 Putative mechanism by which the body perceives symptoms.
over. Conversely, anxiety about an injury and hypervigilance to the affected part can lead to increased pain. In CMI, central factors may lead to a lower sensation threshold and, if this is the case, centrally targeted treatments would probably have therapeutic value by increasing sensation thresholds, as occurs in treatment for other conditions, such as fibromyalgia, CFS, and IBS. There is some evidence that patients who have similar somatic symptoms have higher concentrations of substance P (which transmits pain) in their blood and cerebrospinal fluid than people who do not have such symptoms (Clauw, 2009). In those patients, direct pressure on the skin or inflation of a balloon in the esophagus causes pain at a much lower level of pressure than in people who do not have these somatic symptoms.
In addition, central measures of hormonal stress, such as an altered response of the hypothalamic-pituitary-adrenal axis, reveal an inappropriate flattened response to additional stress that may reflect a more sustained central overactivity in patients who have visceral hypersensitivity (such as IBS) or somatic hypersensitivity (such as fibromyalgia) (Clauw, 2009).
Nonrestorative sleep can be reproduced in normal volunteers who are subjected to disruption of deep stage four sleep and is a typical sleep pattern in patients who have IBS. When deep stage four sleep is repeatedly disrupted, affected people can develop myalgia, heightened pain, presence of tender points on examination, and disrupted sleep patterns afterward. Further research is needed to assess whether use of programs and agents that restore a better sleep pattern may also lead to improvement in other symptoms.
In summary, the multiple symptoms of CMI, like the symptoms associated with other functional somatic syndromes, may arise from at least two factors: an impairment of the brain in its downregulating of incoming nerve signals originating in the body and an increase in or amplification of bodily nerve signals for any of a variety of reasons (such as injury or infection). The degree to which those factors interact in CMI is an important topic for future research.
REFERENCES
Bair, M. J. 2003. Depression and pain mortality: A literature review. Archives of Internal Medicine 163(20):2433-2445.
Clauw, D. J. 2009. Fibromyalgia: An overview. American Journal of Medicine 122(12 Suppl.):S3-S13.
Dantzer, R., J. C. O’Connor, G. G. Freund, R. W. Johnson, and K. W. Kelley. 2008. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience 9(1):46-56.
Drossman, D. A. 1999. Mind over matter in the postinfective irritable bowel. Gut 44(3):306-307.
IOM (Institute of Medicine). 2008. Gulf War and Health, Volume 6: Physiologic, Psychologic, and Psychosocial Effects of Deployment-related Stress. Washington, DC: The National Academies Press.
IOM. 2010. Gulf War and Health, Volume 8: Update of Health Effects of Serving in the Gulf War. Washington, DC: The National Academies Press.
Ringel, Y., D. A. Drossman, J. L. Leserman, B. Y. Suyenobu, K. Wilber, and W. Lin. 2008. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: An FMRI study. Gastroenterology 134(2):396-404.
Spiller, R., and K. Garsed. 2009. Postinfectious irritable bowel syndrome. Gastroenterology 136(6):1979-1988.
Vaccarino, A. L., T. L. Stills, K. R. Evans, and A. H. Kalali. 2009. Multiple pain complaints in patients with major depressive disorder. Psychosomatic Medicine 71(2):159-162.
Zaubler, T. S., and W. J. Katon. 1996. Panic disorder and medical comorbidity: A review of the medical and psychiatric literature. Bulletin of the Menninger Clinic 20(2 Suppl. A):A12-A38.




