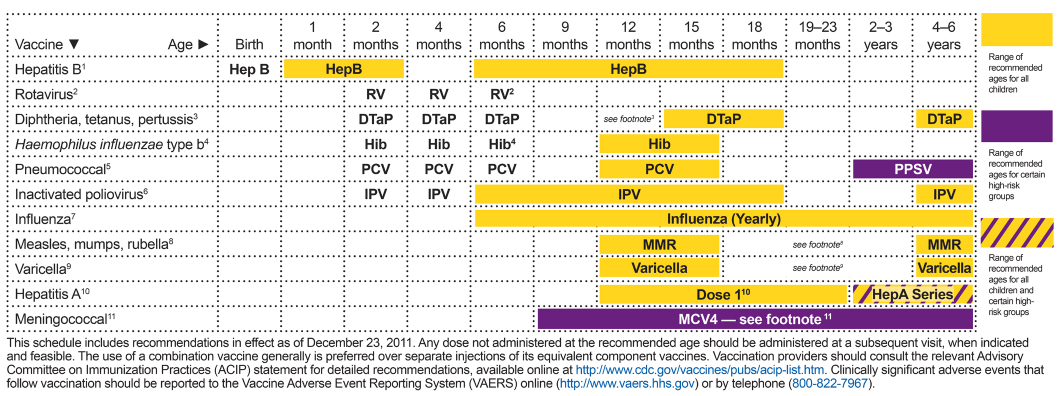
FIGURE A-1 Recommended immunization schedule for individuals aged 0 through 6 years, United States, 2012.
SOURCE: CDC (Centers for Disease Control and Prevention). 2012. Immunization schedules. Atlanta, GA: Centers for Disease Control and Prevention. http://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html.
1. Hepatitis B (HepB) vaccine. (Minimum age: birth)
At birth:
• Administer monovalent HepB vaccine to all newborns before hospital discharge.
• For infants born to hepatitis B surface antigen (HBsAg)–positive mothers, administer HepB vaccine and 0.5 mL of hepatitis B immune globulin (HBIG) within 12 hours of birth. These infants should be tested for HBsAg and antibody to HBsAg (anti-HBs) 1 to 2 months after completion of at least 3 doses of the HepB series, at age 9 through 18 months (generally at the next well-child visit).
• If mother’s HBsAg status is unknown, within 12 hours of birth administer HepB vaccine for infants weighing ≥2,000 grams, and HepB vaccine plus HBIG for infants weighing <2,000 grams. Determine mother’s HBsAg status as soon as possible and, if she is HBsAg-positive, administer HBIG for infants weighing ≥2,000 grams (no later than age 1 week).
Doses after the birth dose:
• The second dose should be administered at age 1 to 2 months. Monovalent HepB vaccine should be used for doses administered before age 6 weeks.
• Administration of a total of 4 doses of HepB vaccine is permissible when a combination vaccine containing HepB is administered after the birth dose.
• Infants who did not receive a birth dose should receive 3 doses of a HepB containing vaccine starting as soon as feasible (see Figure A-2).
• The minimum interval between dose 1 and dose 2 is 4 weeks, and between dose 2 and 3 is 8 weeks. The final (third or fourth) dose in the HepB vaccine series should be administered no earlier than age 24 weeks and at least 16 weeks after the first dose.
2. Rotavirus (RV) vaccines. (Minimum age: 6 weeks for both RV-1 [Rotarix] and RV-5 [Rota Teq])
• The maximum age for the first dose in the series is 14 weeks, 6 days; and 8 months, 0 days for the final dose in the series. Vaccination should not be initiated for infants aged 15 weeks, 0 days or older.
• If RV-1 (Rotarix) is administered at ages 2 and 4 months, a dose at 6 months is not indicated.
3. Diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine. (Minimum age: 6 weeks)
• The fourth dose may be administered as early as age 12 months, provided at least 6 months have elapsed since the third dose.
4. Haemophilus influenzae type b (Hib) conjugate vaccine. (Minimum age: 6 weeks)
• If PRP-OMP (PedvaxHIB or Comvax [HepB-Hib]) is administered at ages 2 and 4 months, a dose at age 6 months is not indicated.
• Hiberix should only be used for the booster (final) dose in children aged 12 months through 4 years.
5. Pneumococcal vaccines. (Minimum age: 6 weeks for pneumococcal conjugate vaccine [PCV]; 2 years for pneumococcal polysaccharide vaccine [PPSV])
• Administer 1 dose of PCV to all healthy children aged 24 through 59 months who are not completely vaccinated for their age.
• For children who have received an age-appropriate series of 7-valent PCV (PCV7), a single supplemental dose of 13-valent PCV (PCV13) is recommended for:
—— All children aged 14 through 59 months.
—— Children aged 60 through 71 months with underlying medical conditions.
• Administer PPSV at least 8 weeks after last dose of PCV to children aged 2 years or older with certain underlying medical conditions, including a cochlear implant. See MMWR 2010; 59(No. RR-11), available at http://www.cdc.gov/mmwr/pdf/rr/rr5911.pdf.
6. Inactivated poliovirus vaccine (IPV). (Minimum age: 6 weeks)
• If 4 or more doses are administered before age 4 years, an additional dose should be administered at age 4 through 6 years.
• The final dose in the series should be administered on or after the fourth birthday and at least 6 months after the previous dose.
7. Influenza vaccines. (Minimum age: 6 months for trivalent inactivated influenza vaccine [TIV]; 2 years for live, attenuated influenza vaccine [LAIV])
• For most healthy children aged 2 years and older, either LAIV or TIV may be used. However, LAIV should not be administered to some children, including (1) children with asthma, (2) children 2 through 4 years who had wheezing in the past 12 months, or (3) children who have any other underlying medical conditions that predispose them to influenza complications. For all other contraindications to use of LAIV, see MMWR 2010; 59(No. RR-8), available at http://www.cdc.gov/mmwr/pdf/rr/rr5908.pdf.
• For children aged 6 months through 8 years:
—— For the 2011–12 season, administer 2 doses (separated by at least 4 weeks) to those who did not receive at least 1 dose of the 2010–11 vaccine. Those who received at least 1 dose of the 2010–11 vaccine require 1 dose for the 2011–12 season.
—— For the 2012–13 season, follow dosing guidelines in the 2012 ACIP influenza vaccine recommendations.
8. Measles, mumps, and rubella (MMR) vaccine. (Minimum age: 12 months)
• The second dose may be administered before age 4 years, provided at least 4 weeks have elapsed since the first dose.
• Administer MMR vaccine to infants aged 6 through 11 months who are traveling internationally. These children should be revaccinated with 2 doses of MMR vaccine, the first at ages 12 through 15 months and at least 4 weeks after the previous dose, and the second at ages 4 through 6 years.
9. Varicella (VAR) vaccine. (Minimum age: 12 months)
• The second dose may be administered before age 4 years, provided at least 3 months have elapsed since the first dose.
• For children aged 12 months through 12 years, the recommended minimum interval between doses is 3 months. However, if the second dose was administered at least 4 weeks after the first dose, it can be accepted as valid.
10. Hepatitis A (HepA) vaccine. (Minimum age: 12 months)
• Administer the second (final) dose 6 to 18 months after the first.
• Unvaccinated children 24 months and older at high risk should be vaccinated.
See MMWR 2006; 55(No. RR-7), available at http://www.cdc.gov/mmwr/pdf/rr/rr5507.pdf.
• A 2-dose HepA vaccine series is recommended for anyone aged 24 months and older, previously unvaccinated, for whom immunity against hepatitis A virus infection is desired.
11. Meningococcal conjugate vaccines, quadrivalent (MCV4). (Minimum age: 9 months for Menactra [MCV4-D], 2 years for Menveo [MCV4-CRM])
• For children aged 9 through 23 months (1) with persistent complement component deficiency; (2) who are residents of or travelers to countries with hyperendemic or epidemic disease; or (3) who are present during outbreaks caused by a vaccine serogroup, administer 2 primary doses of MCV4-D, ideally at ages 9 months and 12 months or at least 8 weeks apart.
• For children aged 24 months and older with (1) persistent complement component deficiency who have not been previously vaccinated; or (2) anatomic/functional asplenia, administer 2 primary doses of either MCV4 at least 8 weeks apart.
• For children with anatomic/functional asplenia, if MCV4-D (Menactra) is used, administer at a minimum age of 2 years and at least 4 weeks after completion of all PCV doses.
• See MMWR 2011; 60:72–76, available at http://www.cdc.gov/mmwr/pdf/wk/mm6003.pdf, and Vaccines for Children Program resolution No.6/11-1, available at http://www.cdc.gov/vaccines/programs/vfc/downloads/resolutions/06-11meningmcv.pdf, and MMWR 2011; 60:1391–1392, available at http://www.cdc.gov/mmwr/pdf/wk/mm6040.pdf, for further guidance, including revaccination guidelines.
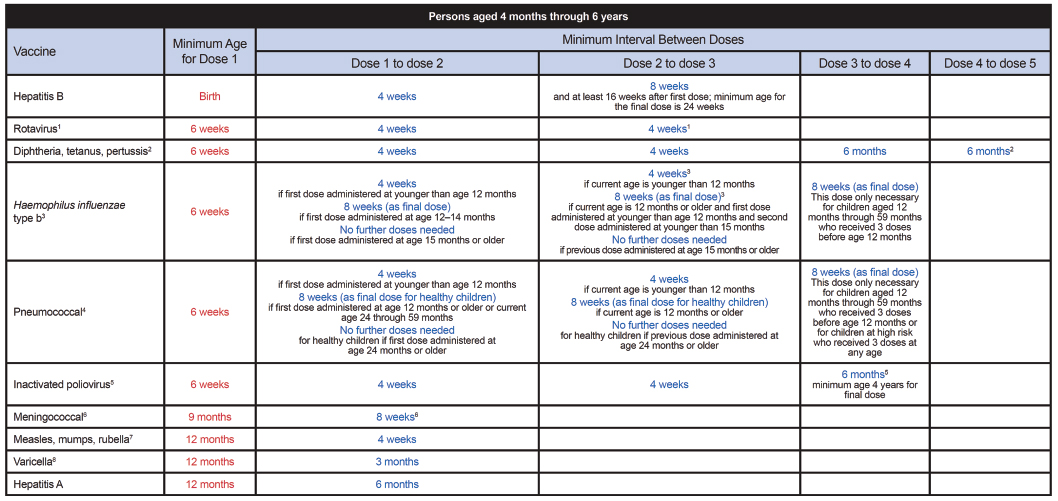
FIGURE A-2 Catch-up immunization schedule for persons aged 4 months through 18 years who start late or who are more than 1 month behind—United States, 2012. The figure provides catch-up schedules and minimum intervals between doses for children whose vaccinations have been delayed. A vaccine series does not need to be restarted, regardless of the time that has elapsed between doses.
SOURCE: CDC. 2012. Immunization schedules. http://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html.
1. Rotavirus (RV) vaccines (RV-1 [Rotarix] and RV-5 [Rota Teq]).
• The maximum age for the first dose in the series is 14 weeks, 6 days; and 8 months, 0 days for the final dose in the series. Vaccination should not be initiated for infants aged 15 weeks, 0 days or older.
• If RV-1 was administered for the first and second doses, a third dose is not indicated.
2. Diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine.
• The fifth dose is not necessary if the fourth dose was administered at age 4 years or older.
3. Haemophilus influenzae type b (Hib) conjugate vaccine.
• Hib vaccine should be considered for unvaccinated persons aged 5 years or older who have sickle cell disease, leukemia, human immunodeficiency virus (HIV) infection, or anatomic/functional asplenia.
• If the first 2 doses were PRP-OMP (PedvaxHIB or Comvax) and were administered at age 11 months or younger, the third (and final) dose should be administered at age 12 through 15 months and at least 8 weeks after the second dose.
• If the first dose was administered at age 7 through 11 months, administer the second dose at least 4 weeks later and a final dose at age 12 through 15 months.
4. Pneumococcal vaccines. (Minimum age: 6 weeks for pneumococcal conjugate vaccine [PCV]; 2 years for pneumococcal polysaccharide vaccine [PPSV])
• For children aged 24 through 71 months with underlying medical conditions, administer 1 dose of PCV if 3 doses of PCV were received previously, or administer 2 doses of PCV at least 8 weeks apart if fewer than 3 doses of PCV were received previously.
• A single dose of PCV may be administered to certain children aged 6 through 18 years with underlying medical conditions. See age-specific schedules for details.
• Administer PPSV to children aged 2 years or older with certain underlying medical conditions. See MMWR 2010; 59(No. RR-11), available at http://www.cdc.gov/mmwr/pdf/rr/rr5911.pdf.
5. Inactivated poliovirus vaccine (IPV).
• A fourth dose is not necessary if the third dose was administered at age 4 years or older and at least 6 months after the previous dose.
• In the first 6 months of life, minimum age and minimum intervals are only recommended if the person is at risk for imminent exposure to circulating poliovirus (i.e., travel to a polio-endemic region or during an outbreak).
• IPV is not routinely recommended for U.S. residents aged 18 years or older.
6. Meningococcal conjugate vaccines, quadrivalent (MCV4). (Minimum age: 9 months for Menactra [MCV4-D]; 2 years for Menveo [MCV4-CRM])
• See Figure 1 (“Recommended immunization schedule for persons aged 0 through 6 years”) and Figure 2 (“Recommended immunization schedule for persons aged 7 through 18 years”) for further guidance (http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6105a5.htm).
7. Measles, mumps, and rubella (MMR) vaccine.
• Administer the second dose routinely at age 4 through 6 years.
• Administer the second dose routinely at age 4 through 6 years. If the second dose was administered at least 4 weeks after the first dose, it can be accepted as valid.
9. Tetanus and diphtheria toxoids (Td) and tetanus and diphtheria toxoids and acellular pertussis (Tdap) vaccines.
• For children aged 7 through 10 years who are not fully immunized with the childhood DTaP vaccine series, Tdap vaccine should be substituted for a single dose of Td vaccine in the catch-up series; if additional doses are needed, use Td vaccine. For these children, an adolescent Tdap vaccine dose should not be given.
• An inadvertent dose of DTaP vaccine administered to children aged 7 through 10 years can count as part of the catch-up series. This dose can count as the adolescent Tdap dose, or the child can later receive a Tdap booster dose at age 11-12 years.
10. Human papillomavirus (HPV) vaccines (HPV4 [Gardasil] and HPV2 [Cervarix]). • Administer the vaccine series to females (either HPV2 or HPV4) and males (HPV4) at age 13 through 18 years if patient is not previously vaccinated.
• Use recommended routine dosing intervals for vaccine series catch-up; see Figure 2 (“Recommended immunization schedule for persons aged 7 through 18 years,” http://www.cdc.gov/vaccines/schedules/downloads/child/7-18yrs-schedule-pr.pdf).