Determination of the Immunization Schedule
The immunization schedule recommended by the U.S. Advisory Committee on Immunization Practices (ACIP) is determined through consideration of numerous factors and the cooperation of numerous federal agencies in an extensive federal research infrastructure that includes the National Institutes of Health, the Food and Drug Administration (FDA), and the Centers for Disease Control and Prevention (CDC). This chapter provides an overview of some of these factors and the processes in place to help ensure that the immunization schedule benefits the recipients.
The biology of the immune system response to pathogens and foreign substances is complex and was reviewed in a 2012 Institute of Medicine report. A broad overview of how vaccines work to protect the human body against disease is first presented as a prelude to consideration of the safety of the aggregate of vaccines that are part of the immunization schedule from the perspective of immune system responses.
The fundamental goal of vaccination is to prepare the immune system to defend the host against disease by intentionally exposing the body to all or part of an infectious agent in an effort to confer long-term protective immunity against future infection and to protect the most vulnerable individuals against disease.
Immunity protects the body against infectious diseases mainly through the production of specialized protein molecules, known as “antibodies” or “immunoglobulins,” once the immune system has been stimulated by the
presence of foreign substances, called “antigens,” from, for example, pathogens or vaccines (CDC, 2012d; Siegrist, 2008). In addition to immunoglobulins, other parts of the immune system also contribute to protection, including lymphocytes (specialized white blood cells), antigen-presenting cells (which recognize the foreign elements of the vaccine or the virus or bacterium that is the cause of an infectious disease and which help initiate the steps involved in protection), the spleen, and the skin itself, which serves as a protective barrier against bacteria and viruses.
For a vaccine to be efficacious and reduce the incidence of vaccine-preventable diseases, it must elicit the production of high-quality antibodies against the pathogen responsible for disease. Certain vaccines are able to generate an immunologic memory similar to that generated by natural infection, which often confers lifelong protection, whereas other vaccines may require boosters over time to maintain immunity.
The immune response is largely dependent upon the properties of the antigen used to develop the vaccine and on the route of administration. Live attenuated vaccines contain viruses or bacteria that are weakened versions of the naturally occurring infectious agent, whereas inactivated vaccines contain either antigens that are grown in laboratory culture media and inactivated by the use of heat or chemicals, altered bacterial toxins (toxoids) that when administered do not result in natural disease, or antigens that are produced artificially to mimic the surface properties of the pathogen.
Vaccines containing live, attenuated antigens confer a stronger immune response because the antigen is more similar to that encountered during natural infection; however, in rare cases, the virus may replicate uncontrollably in immunocompromised individuals and lead to a severe or fatal reaction. In an inactivated vaccine, the virus or bacterium is not alive and is not able to cause an infectious disease through unintended replication.
The type of vaccine is one factor that determines where the vaccine appears in the recommended immunization schedule. For example, the measles, mumps, rubella (MMR) vaccine is a live attenuated vaccine that for most recipients confers immunity after just one dose. Children following the recommended immunization schedule receive one dose of MMR at between 12 and 15 months of age and a second dose after age 4 years to ensure immunity. An inactivated vaccine such as diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine adsorbed, which contains diphtheria and tetanus toxoids combined with a subunit of the bacterium that causes pertussis, does not confer full immunity until after the second or third dose and requires later booster doses to remain immunologically effective, as the antibody titers that maintain immunity diminish with time.
Adjuvants can provide improved immunity by delaying the absorption of the antigens or by arousing or boosting the immune system response (IOM, 2012; Melvold, 2009). The immunoglobulin M (IgM) isotype is the
primary immunoglobulin generated after immunization, quickly followed by the IgG isotype. To demonstrate the immunogenicity of a vaccine, serum antivaccine (or antigenic marker) IgG antibodies are measured. For example, in studies of immunization with pandemic swine influenza A virus (H1N1) vaccines, detection of antibodies demonstrating inhibition of hemagglutination at a serum titer of 1:40 or greater provides evidence of seroprotection to the individual (Liang et al., 2010). These antibodies reduce infection by blocking the interaction between the influenza virus antigen, hemagglutinin, and cell surface receptors that it will use to enter the cell (Reddy et al., 2011). For the group of subjects studied, after a single immunization of 7.5 μg of a nonadjuvant split-virion formulation of the H1N1 vaccine in children ages 3 to <12 years, the increase in the hemagglutination titer over the baseline titer by 3 weeks postimmunization was robust (from a baseline mean titer of 6 to a postimmunization titer of 178) (Liang et al., 2010). When the titers are presented as geometric means to account for the distribution of responses, the baseline geometric mean titer was 5.3 and the postimmunization geometric mean titer was 178, a 32-fold response achieved by 3 weeks postimmunization. In adolescents (individuals ages 12 to <18 years), the geometric mean titers increased even more over the first 3 weeks postimmunization, from a baseline of 7 to 578, an 82-fold change (Liang et al., 2010).
The response to vaccination can be blunted in individuals who lack critical components of the immune system. For example, the responses to influenza immunization can be nonexistent or poor in patients who have received rituximab, which is an antibody to CD20, a membrane surface marker on B cells, present from early to full maturation of B cells and plasma cells, which secrete immunoglobulins (Bedognetti et al., 2011). Rituximab is useful therapeutically for the treatment of multiple conditions, including forms of lymphoma and collagen vascular diseases. However, the number of B (CD19+) cells can be reduced for 6 months or longer after discontinuation of rituximab in patients who are in remission from lymphoma. Treatment with rituximab was found to greatly reduce the number of memory B cells characterized as CD27+ and was associated with a poor or absent response to influenza immunization (Bedognetti et al., 2011). Although the patients had detectable CD4+ and CD8+ lymphocytes, they did not have CD19+ B cells and did have reduced numbers of CD27+ memory B cells, a condition which was associated with failure to mount a protective response after immunization (Bedognetti et al., 2011).
Another factor used to determine where a vaccine appears in the immunization schedule is vulnerability to the vaccine-preventable disease by age. This determination requires some knowledge of the pattern of disease in the community, which may differ by region of the world. As the immunity afforded by maternal antibodies at birth wanes, infants become
more susceptible to pathogens, many of which may lead to serious or fatal infections. Therefore, to be effective, a vaccine should be administered early enough to protect the infant or child against preventable diseases.
The age range for which a childhood vaccine is developed and recommended as part of the immunization schedule takes into account the age at which the immune system can tolerate vaccine components, potential interference with the immune response from maternal antibodies, and the age at which a child is most at risk for disease transmission and mortality. ACIP recommends vaccines “for members of the youngest age group at risk for experiencing the disease for which efficacy and safety have been demonstrated,” and its recommendations are based on the best evidence available (CDC, 2011a, p. 4).
IMMUNIZATION AT THE POPULATION LEVEL
For immunizations to adequately protect individuals and the individuals in the communities in which they live against outbreaks of vaccine-preventable diseases, a high proportion of vaccinated individuals needs to be maintained in the general population. The success of vaccination to preserve low levels of disease incidence depends on the population level of “community immunity,” also commonly known as herd immunity, which refers to the immunity of a group that is afforded when a high proportion of individuals are not susceptible to infection. Community immunity is maintained by vaccination against communicable diseases, and this concept is expertly discussed in other sources (Fine, 1993; Fine et al., 2011).
It is possible to quantify the fraction of the population that needs to be protected to prevent disease spread on the basis of the epidemiological traits of the pathogen in question (such as its transmissibility and duration of infectivity). The calculation requires an understanding of the so-called basic reproduction ratio, or R0, which quantifies the maximum transmission potential of an infectious disease. It is strictly defined as the number of secondary cases generated by a typical primary case in a fully susceptible population. If R0 is >1, then the pathogen is predicted to transmit to more than one other person and successfully invade the population. For the major childhood infectious diseases, such as measles, mumps, rubella, chickenpox, and polio, a variety of methods have been devised to estimate R0 from longitudinal incidence reports, outbreak data, and age-stratified serology (Anderson and May, 1982, 1992; Becker, 1989; Keeling and Rohani, 2008).
The quantity R0 has been used to guide vaccination policy with recognition that it is defined when the entire population is susceptible to the pathogen. That is, the number of susceptible individuals (S) is equal to the population size (N). To determine the size to which the pool of susceptible individuals needs to be reduced (via immunization) to control the infection,
researchers consider the following expression: R0 × S/N. This quantity takes into account both the fundamental transmission potential of the pathogen (quantified by the use of R0) and the fraction of the population that is susceptible (S/N). The aim of vaccination is therefore to ensure that R0 × S/N remains less than 1; that is, less than one transmission will result from an infection. To achieve this goal, immunization needs to reduce the proportion of the population unprotected (S/N) to less than 1/R0, which implies that the fraction of the population that needs to be immunized is 1 – 1/R0 (Fine et al., 2011). This principle is illustrated in Figure 2-1.
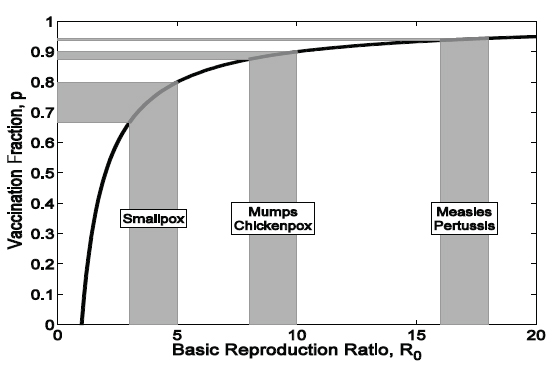
The relationship between the estimated R0 and the targeted vaccination coverage is illustrated in Figure 2-2. This calculation has been of practical use in guiding the setting of immunization targets, albeit with the recognition that for any infectious disease, R0 is likely to change in different settings and is determined by population density, contact patterns, and access to health care (Anderson and May, 1982). The gray regions translate ranges of the estimated R0 into vaccination targets. For instance, on the basis of
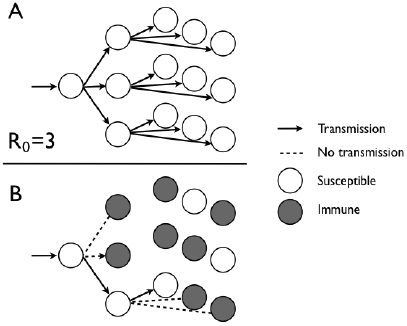
FIGURE 2-1 Different transmission outcomes with immunization when R0 is equal 0 to 3. (A) With the entire population susceptible, successive generations lead to one, three, and, eventually, nine transmissions. (B) When 1 – 1/R0 is equal to 2/3 of the 0 population protected by immunization, each infected individual will infect only one other individual.
SOURCE: Committee on the Assessment of Studies of Health Outcomes Related to the Recommended Childhood Immunization Schedule.
FIGURE 2-2 Relationship between the estimated R0 and the immunization target 0 (solid black line). The gray regions translate ranges of the estimated R0 into the 0 vaccination target.
SOURCE: Adapted and reprinted by the Committee on the Assessment of Studies of Health Outcomes Related to the Recommended Childhood Immunization Schedule.
historical records, the estimated R0 values for mumps and chickenpox in North America prevaccination were 8 to 10, leading to a vaccination target threshold of 87.5 to 90 percent of the population. Similarly, for measles and pertussis in England and Wales before the introduction of immunization, R0 values ranged from 16 to 18 (Anderson and May, 1992), leading to a vaccination target of between 93.75 and 94.4 percent of the population.
Vaccines are held to the highest possible standard of safety, in part because they are provided to healthy individuals with the purpose of preventing illness (Chen et al., 2005). In the United States, before a vaccine is introduced into the population as part of the recommended childhood immunization schedule, it must undergo careful analysis and evaluation, principally by FDA and ACIP. The review examines the immunologic properties of the vaccine and its probable effect on population health.
Each new vaccine is tested within the context of the existing immunization schedule, for example, by identification of the biologically optimal
time during childhood when the immunization should be received and then testing of the new vaccine by incorporation of the vaccine into the existing schedule within that time frame. Selection of a particular moment within that biologically appropriate time frame is done mainly on the basis of considerations of safety and effectiveness (i.e., it should not be administered too early, when a child cannot generate an effective immune response, yet it should be administered soon enough to protect the child against the disease) (Siegrist, 2008).
Logistics and feasibility also need to be considered; for example, a vaccine could be scheduled for administration at times when a child is already likely to be visiting a provider for a normal periodic health care visit based on conventional guidelines (CDC, 2011a). Thus, approval of each new vaccine is premised on evaluation of the vaccine itself and of the entire schedule within which it is situated.
Although this process results in an evaluation of whether the observed benefits outweigh the observed risks for the new vaccine and, by extension, for the schedule, it does not include studies specifically designed to test variations in the schedule in an effort to identify the optimal schedule. Chapter 5 reviews researchers’ efforts in testing variations in immunization schedules. An overview of the licensure and recommended practice review is discussed below.
VACCINE DEVELOPMENT AND APPROVAL SPECIFICS
Role of FDA
Since 1902, the U.S. government has exercised increasingly strict control over the development, manufacture, and sale of vaccines (Baylor and Midthun, 2004). At present, all vaccines marketed in the United States must be licensed by FDA. The licensing requirement provides the means by which FDA exercises authority over the testing and approval of new vaccines, as well as the manufacture, labeling, and continued safety and effectiveness of approved vaccines (Baylor and Midthun, 2004; FDA, 2010).
The clinical development of a vaccine in the United States begins when a sponsor submits the required Investigational New Drug (IND) application to FDA. The IND application includes information on the vaccine’s safety and immunogenicity in animal trials, its manufacturing details, and the proposed protocol of clinical trials for testing in humans.
When the FDA accepts an IND application, manufacturers proceed with premarketing Phase I, II, and III clinical trials (Baylor and Midthun, 2004). Phase I and II clinical trials enroll fewer than 1,000 participants and are designed to draw conclusions about the vaccine’s components, dosing effectiveness and the need for booster doses, and route of administration
and to evaluate common reactions. The results of these trials may influence the choice of the candidate vaccine to be used in subsequent studies, such as additional Phase I or II trials or after Phase III trials. Phase III trials are large-scale trials conducted to provide a more thorough assessment of safety. Sample sizes for Phase III trials are determined to evaluate a vaccine’s efficacy, and therefore such trials have larger sample sizes (up to 100,000 participants in some rare cases) than do Phase I or II trials for vaccines or other premarketing trials for therapeutic drugs. Because Phase III trials are primarily powered for determination of efficacy (Hudgens et al., 2004), conclusions about vaccine safety derived from these trials are limited and may best extrapolate to common adverse events (Chen and Orenstein, 1996; Chen et al., 2005).
Throughout this process, FDA has the authority to request additional information about the clinical trials or to interrupt the clinical trials if concerns about safety or effectiveness emerge. If the clinical trials demonstrate that a vaccine is safe and effective, the licensing procedures begin with the submission of a Biologics License Application and a review of the immunization benefits and risk demonstrated by the clinical evidence. If FDA’s Center for Biologics Evaluation and Research is convinced that the vaccine’s benefits significantly outweigh potential risks for use in the general population, the vaccine is licensed and will undergo further evaluation of product safety through activities such as periodic facility inspections, as well as postmarketing clinical safety evaluation (FDA, 2010). Manufacturers may be asked to undergo Phase IV studies, which include a larger population and are used to assess less common adverse events or the length of time for which the vaccine induces immunity (Baylor and Midthun, 2004). Following vaccine licensure, manufacturers are required by 21 CFR 600.80 to report to the FDA serious or unexpected adverse events within 15 days of the event occurring, and to report other adverse events quarterly for the first 3 years after the vaccine is licensed, and then once per year thereafter (Baylor and Midthun, 2004; Farizo, 2012; FDA, 2010). Postmarketing surveillance efforts that are coordinated as part of the federal research infrastructure are discussed in detail in Chapter 3.
Role of ACIP
In the United States, immunization policy is developed and implemented through collaborations among federal partners, state and local governments, professional medical associations, and other relevant organizations. These organizations are represented on ACIP, which is the federal advisory committee that provides expert external advice and guidance on the use of FDA-licensed vaccines and related agents in the U.S. population
to the director of CDC and the secretary of the U.S. Department of Health and Human Services.
Each year CDC issues recommendations on the use of vaccines and immunization schedules for children, adolescents, and adults (Kroger et al., 2011; NVAC, 2011). A number of liaison organizations, such as the American Academy of Pediatrics (AAP), the American College of Physicians, and the American Academy of Family Physicians (AAFP), issue recommendations on the immunization schedule that are harmonized to the greatest extent possible with the annual recommendations from CDC (NVAC, 2011; Smith, 2010; Smith et al., 2009). A representative from ACIP serves as a liaison on the National Vaccine Advisory Committee, which is the federal advisory committee responsible for advising the National Vaccine Program Office (NVPO) on priorities of vaccine supply and enhancing vaccine safety and efficacy (HHS, 2012).
In the process of making recommendations for new vaccines, ACIP first reviews a wide range of data associated with the vaccine, including the rates of morbidity and mortality from the disease that the vaccine protects against in the general U.S. population and specific high-risk groups; cost-effectiveness; the results of clinical trials, including indicators of safety, efficacy, and effectiveness; information on vaccine use provided by the manufacturer in the product’s labeling or package insert; and the feasibility of incorporating the vaccine into the existing immunization program. Expert opinions from voting members and other experts may also be incorporated into the deliberations (NVAC, 2011; Smith, 2010; Smith et al., 2009).
As of October 2010, ACIP has adopted an evidence-based framework, Grading of Recommendations Assessment, Development, and Evaluation (GRADE), which it uses when making new recommendations or substantial revisions of vaccination recommendations. The GRADE framework is a method for ACIP to systematically assess the type or quality of evidence about the health outcomes after immunization with a vaccine. The evidence that ACIP reviews is grouped into four categories that reflect the reviewers’ level of confidence in the estimated effect of vaccination on health outcomes on the basis of the strength of the design of the study used to provide the evidence considered. The GRADE categories are as follows (CDC, 2012b):
- randomized controlled trials or overwhelming evidence from observational studies;
- randomized controlled trials with important limitations or exceptionally strong evidence from observational studies;
- observational studies or randomized controlled trials with notable limitations; and
- clinical experience and observations, observational studies with important limitations, or randomized controlled trials with several major limitations.
Recommendations from ACIP are also categorized into Category A or B recommendations, although the distinction does not reflect the quality of the evidence reviewed. Category A recommends vaccination for all people in a particular age group or for a group at increased risk for vaccine-preventable diseases. Category B recommendations do not apply to all members of a group; rather, they are intended to provide guidance to a clinician when determining if vaccination is appropriate for an individual. After review, if CDC accepts the recommendations of ACIP, they are published in Morbidity and Mortality Weekly Reports (MMWR) (Smith, 2010; Smith et al., 2009).
PAST AND PRESENT IMMUNIZATION SCHEDULES
The current schedule of recommended immunizations for infants and children from birth through age 6 years comprises vaccines that prevent 14 infectious diseases, a remarkable achievement compared with the schedule in 1948, when immunizations against only diphtheria, tetanus, pertussis, and smallpox were available and recommended for administration for protection. In 1955, the polio vaccine was licensed and added to the recommended immunizations to eliminate yearly outbreaks. Over the next 40 years, vaccines were added to the recommended schedule as they were licensed, including MMR, the hepatitis B vaccine, and the Haemophilus influenzae type b vaccine (Hib). Smallpox vaccine was removed from the U.S. recommended schedule in 1972, as the disease had been eliminated as a result of great public health efforts.
It became increasingly evident that as the schedule became more complex, providers would benefit from annual updates with detailed information about new vaccines, who should receive each vaccine, the age(s) at the time of receipt, the dose, and the use of combination vaccines in their practices. In 1995, CDC, AAP, and AAFP created a harmonized immunization schedule. Since then, the ACIP-recommended schedule has been adopted by the CDC and both professional associations, along with others (CDC, 2012c). Today, combination vaccines deliver immunizations against up to five separate diseases in a single injection, including DTaP-Hib-inactivated poliovirus vaccine (IPV) and DTaP-hepatitis B (recombinant) virus vaccine-IPV.
Immunization rates for children in the United States are generally high, with some variation occurring depending on geography and the specific vaccine. The majority of children are fully immunized with the recommended
component of vaccines (not including influenza and hepatitis A virus vaccines) by age 3 years. According to the National Immunization Survey (NIS), less than 1 percent of U.S. children aged 19 to 35 months receive no vaccinations at all (CDC, 2011c). However, not every vaccine on the schedule has equal coverage in this population. In the NIS study population for children born between January 2008 and May 2010, vaccines with higher coverage included the poliovirus vaccine (93.9 percent), MMR (91.6 percent), the hepatitis B vaccine (91.1 percent), and the varicella vaccine (90.8 percent). In contrast, the rotavirus vaccine was received by only 67.3 percent of children, and just 80.7 percent of children received the full series of the Hib vaccine, which is an increase from previous years during which a shortage of the vaccine was experienced (CDC, 2012a). A review of data from the 2003 NIS revealed that more than one in three children were undervaccinated (missing age-appropriate doses from the recommended immunization schedule) during the first 24 months of life and that only 18 percent of U.S. children received all vaccinations at the recommended times or acceptably early (Luman et al., 2005). Immunizations are recommended to protect children when they are most vulnerable to vaccine-preventable diseases, and delays in timely immunization leave children susceptible to disease.
For some children, vaccination on the recommended schedule may be contraindicated, either permanently or temporarily, and the CDC offers guidelines on conditions that may require that vaccination with certain vaccines be postponed or avoided altogether (CDC, 2011b).
Most health care providers encourage adherence with the recommended immunization schedule for children; however, a compelling motivator to see that children receive their full immunizations is their requirement to attend school. Since the early 1980s, all 50 states have made policy decisions to require immunizations for school entry. These immunization requirements were originally enacted to prevent and control frequent outbreaks of vaccine-preventable diseases. Furthermore, during outbreaks, officials have removed unvaccinated children from school, which has proved to be a successful control measure (Omer et al., 2006).
Because school-based immunization requirements are determined on a state-by-state basis, differences in age requirements, processes for adding new vaccines, and exemptions to immunizations exist across the country. Exemptions may be medical in nature, such as exemptions for delayed or skipped immunization if the child has a condition that contraindicates immunization with the vaccine, as referenced above.
Currently, every state law covering immunization requirements has a provision that allows medical exemptions. Parents may also request an exemption on religious grounds, and such exemptions are permitted in 48 states. Exemptions because of personal beliefs, which include religious,
philosophical, or other nonmedical beliefs, are granted in 20 states, including Colorado and Washington, two states that saw localized outbreaks of pertussis in 2012 (Omer et al., 2006).
Although rates of medical exemptions are relatively constant nationwide, rates of nonmedical exemptions vary considerably (CDC, 2010, 2011d). For example, from 2006 to 2007, the average nonmedical exemption rate in the state of Washington was 6 percent, although some counties had exemption rates as high as 27 percent (Omer et al., 2006).
Adverse Effects of Vaccines
Parents may be what is referred to as vaccine-hesitant (refusing, delaying, or feeling unsure about some immunizations) because vaccines, like other drugs and biologicals, can in some cases be associated with adverse events (Opel et al., 2011). Vaccines that are commonly associated with serious adverse events are never licensed. Likewise, if a serious or frequent adverse event is discovered during postmarketing surveillance, the vaccine is taken off the schedule (e.g., the first rotavirus vaccine).
Most adverse events are mild or self-limited, for example, fever after measles vaccine or a sore, swollen injection site after the tetanus booster. Many events may occur in the days and weeks following vaccination, however, typically few are a result of vaccination, and most are coincidental. Ongoing research continues to examine such adverse events (IOM, 2012).
In the 1980s, the United States experienced an increase in civil lawsuits filed against vaccine manufacturers for injury compensation, which led to hesitancy on the part of the manufacturers to produce enough vaccines to keep the supply stable at a reasonable price. To streamline the legal process and maintain the vaccine supply, the U.S. Congress enacted the National Childhood Vaccine Injury Act in 1986 to establish a no-fault system for compensating individuals for vaccine-related injuries, the National Vaccine Injury Compensation Program (VICP). Individuals or parents of children who experience a vaccine-related injury must first file their petition with VICP before pursuing a civil case. As a no-fault system, the possible negligence of the manufacturer or physician is not considered in determination of compensation, which is funded by an excise tax on vaccines.
VICP covers all vaccines routinely administered to children as part of the recommended childhood immunization schedule and all injuries listed in its injury table. A claimant who seeks compensation for an adverse event that has not been established and placed in this table has the option of providing evidence to establish causation (Cook and Evans, 2011). The National Childhood Vaccine Injury Act also established the Vaccine Adverse Event Reporting System to track adverse events and created NVPO
to coordinate immunization-related activities among federal agencies (Cook and Evans, 2011).
Anderson, R.M., and R.M. May. 1982. Directly transmitted infectious diseases: Control by vaccination. Science 215(4536):1053-1060.
Anderson, R.M., and R.M. May. 1992. Infectious Diseases of Humans: Dynamics and Control, New York: Oxford University Press.
Baylor, N.W., and K. Midthun. 2004. Regulation and testing of vaccines. In Vaccines, edited by S.A. Plotkin and W.A. Orenstein. Philadelphia, PA: Saunders Elsevier. Pp. 1611-1627.
Becker, N.G. 1989. Analysis of infectious disease data, vol. 33. Boca Raton, FL: Chapman & Hall/CRC.
Bedognetti, D., G. Zoppoli, C. Massucco, E. Zanardi, S. Zupo, A. Bruzzone, M.R. Sertoli, E. Balleari, O. Racchi, and M. Messina. 2011. Impaired response to influenza vaccine associated with persistent memory B cell depletion in non-Hodgkin’s lymphoma patients treated with rituximab-containing regimens. Journal of Immunology 186(10):6044-6055.
CDC (Centers for Disease Control and Prevention). 2010. The school entry immunization assessment report, 2004-05. Atlanta, GA: National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention.
CDC. 2011a. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and Reports 60(2):1-64.
CDC. 2011b. Guide to vaccine contraindications and precautions. Atlanta, GA: Centers for Disease Control and Prevention. http://www.cdc.gov/vaccines/recs/vacadmin/contraindications.htm (accessed July 9, 2012).
CDC. 2011c. National and state vaccination coverage among children aged 19-35 months— United States, 2010. Morbidity and Mortality Weekly Reports 60(34):1157-1163.
CDC. 2011d. Vaccination coverage among children in kindergarten—United States, 2009-10 school year. Morbidity and Mortality Weekly Reports 60(21):700-704.
CDC. 2012a. National, state, and local area vaccination coverage among children aged 19-35 months—United States, 2011. Morbidity and Mortality Weekly Reports 61(35):689-696.
CDC. 2012b. New framework (GRADE) for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Reports 61(18):327.
CDC. 2012c. Past immunization schedules. Atlanta, GA: Centers for Disease Control and Prevention. http://www.cdc.gov/vaccines/schedules/past.html (accessed June 12, 2012).
CDC. 2012d. Principles of vaccination. In Epidemiology and prevention of vaccine-preventable diseases. Atlanta, GA: Centers for Disease Control and Prevention.
Chen, R.T., and W.A. Orenstein. 1996. Epidemiologic methods in immunization programs. Epidemiologic Reviews 18(2):99-117.
Chen, R.T., R.L. Davis, and P.H. Rhodes. 2005. Special methodological issues in pharmacoepidemiology studies of vaccine safety. In Pharmacoepidemiology, Fourth Edition, edited by B.L. Strom. West Sussex, England: John Wiley & Sons Ltd. Pp. 455-485.
Cook, K.M., and G. Evans. 2011. The National Vaccine Injury Compensation Program. Pediatrics 127(Suppl 1):S74-S77.
Farizo, K. 2012. Assessing the safety of vaccines at the FDA: Pre- and post-licensure evaluation. Presentation to Meeting 2 of the Institute of Medicine Committee on the Assessment of Studies of Health Outcomes Related to the Recommended Childhood Immunization Schedule, Seattle, WA, March 8.
FDA (Food and Drug Administration). 2010. Biologics License Applications (BLA) process (CBER). Rockville, MD: Food and Drug Administration. http://www.fda.gov/BiologicsBloodVaccines/DevelopmentApprovalProcess/BiologicsLicenseApplicationsBLAProcess/default.htm (accessed November 2, 2012).
Fine, P.E. 1993. Herd immunity: History, theory, practice. Epidemiologic Reviews 15(2): 265-302.
Fine, P., K. Eames, and D.L. Heymann. 2011. “Herd immunity”: A rough guide. Clinical Infectious Diseases 52(7):911-916.
HHS (U.S. Department of Health and Human Services). 2012. National Vaccine Advisory Committee. Washington, DC: U.S. Department of Health and Human Services. http://www.hhs.gov/nvpo/nvac/index.html (accessed July 30, 2012).
Hudgens, M.G., P.B. Gilbert, and S.G. Self. 2004. Endpoints in vaccine trials. Statistical Methods in Medical Research 13(2):89-114.
IOM (Institute of Medicine). 2012. Adverse effects of vaccines: Evidence and causality. Washington, DC: The National Academies Press.
Keeling, M.J., and P. Rohani. 2008. Modeling Infectious Diseases: Of Humans and Animals. Princeton, NJ: Princeton University Press.
Kroger, A.T., C.V. Sumaya, L.K. Pickering, and W.L. Atkinson. 2011. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and Reports 60(2):1-64.
Liang, X.F., H.Q. Wang, J.Z. Wang, H.H. Fang, J. Wu, F.C. Zhu, R.C. Li, S.L. Xia, Y.L. Zhao, F.J. Li, S.H. Yan, W.D. Yin, K. An, D.J. Feng, X.L. Cui, F.C. Qi, C.J. Ju, Y.H. Zhang, Z.J. Guo, P.Y. Chen, Z. Chen, K.M. Yan, and Y. Wang. 2010. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 375(9708):56-66.
Luman, E.T., L.E. Barker, K.M. Shaw, M.M. McCauley, J.W. Buehler, and L.K. Pickering. 2005. Timeliness of childhood vaccinations in the United States: Days under-vaccinated and number of vaccines delayed. Journal of the American Medical Association 293(10):1204-1211.
Melvold, R.W. 2009. The immune system: Biologic and clinical aspects. In Patterson’s allergic diseases, vol. 7, edited by L. C. Grammer and P. A. Greenberger. Philadelphia, PA: Walters Kluwer/Lippincott Williams & Wilkins. Pp. 1-21.
NVAC (National Vaccine Advisory Committee). 2011. National Vaccine Advisory Committee draft white paper on the United States vaccine safety system. National Vaccine Advisory Committee, Washington, DC.
Omer, S.B., W.K. Pan, N.A. Halsey, S. Stokley, L.H. Moulton, A.M. Navar, M. Pierce, and D.A. Salmon. 2006. Nonmedical exemptions to school immunization requirements: Secular trends and association of state policies with pertussis incidence. Journal of the American Medical Association 296(14):1757-1763.
Opel, D.J., R. Mangione-Smith, J.A. Taylor, C. Korfiatis, C. Wiese, S. Catz, and D.P. Martin. 2011. Development of a survey to identify vaccine-hesitant parents: The parent attitudes about childhood vaccines survey. Human Vaccines 7(4):419-425.
Siegrist, C.A. 2008. Vaccine immunology. In Vaccines, vol. 5, edited by S.A. Plotkin, W.A. Orenstein, and P.A. Offit. Philadelphia, PA: Saunders Elsevier.
Smith, J.C. 2010. The structure, role, and procedures of the US Advisory Committee on Immunization Practices (ACIP). Vaccine 28:A68-A75.
Smith, J.C., D.E. Snider, L.K. Pickering, and Advisory Committee on Immunization Practices. 2009. Immunization policy development in the United States: The role of the Advisory Committee on Immunization Practices. Annals of Internal Medicine 150(1):45-49.