Chapter 3
THE FOOD WE EAT
You are at the dinner table. On your plate are a roasted skinless chicken breast half topped with 1/2 cup spaghetti sauce, 1/2 cup brown rice, and a large pile (2 cups) of fresh, steamed string beans. At least that is what your eyes see and your nose smells—and your taste buds are about to experience. But just for a moment, let's look at this delicious dinner as your body sees it: a huge collection of matter to process, providing both energy and nutrients.
|
Food energy: 475 calories |
Calcium: 174 mg |
|
Protein: 36.4 g* |
Phosphorus: 413 mg |
|
Carbohydrate: 63 g |
Sodium: 690 mg |
|
Fat: 11 g |
Potassium: 1522 mg |
|
Saturated fat: 2.2 g |
Vitamin A: 1522 I.U.† |
|
Monounsaturated fat: 4.4 g |
Vitamin C: 38 mg |
|
Polyunsaturated fat: 3.0 g |
Thiamin: 0.42 mg |
|
Cholesterol: 73 mg |
Riboflavin: 0.42 mg |
|
Iron: 5.4 mg |
Niacin: 16.5 mg |
|
* There are approximately 28 grams (g) in 1 ounce, or approximately 0.035 ounce in 1 g; 1 mg equals one-thousandth of a gram. † I.U. stands for international unit, a standard measurement of vitamin A content. |
|
In addition, other vitamins and minerals are present in small amounts.
This meal is not only delicious but also nutritious, and if most of your meals were as healthful as this one you would be doing a great deal to help to reduce your risk for many chronic diseases. Fat in this meal accounts for only 21 percent of the calories, and saturated fat under 5 percent. Both of those figures are well below the guidelines in Chapter 2. The cholesterol content of the meal is low, too. In addition, there is relatively little salt in the meal, but it is high in potassium and vitamin A.
To choose healthful meals like this—and avoid the perils of a diet high in fat, cholesterol, sugar, and salt, and low in carbohydrates, potassium, and calcium—you must know something about the nutrients food contains.
WHAT'S IN FOOD?
As far as the body is concerned, food is made of various nutrients, and a large number of substances that have no nutrient value. Nutrients are the materials the body needs to build itself and stay in top working order. Some of these nutrients—primarily carbohydrates and fats, but also protein—provide energy. Others—protein and minerals—are building materials. Still others—vitamins and some trace elements and fatty acids—are necessary for the chemical reactions that produce energy to move muscles or carry out the regulation of body metabolism. To operate at its best, the body needs these nutrients in the right amounts. Too much or too little can—and often does—result in the body dysfunctioning. That's what we know as disease.
Understanding what food is made of is important if you are to provide your body with the right balance of nutrients. But you do not have to know every last detail of the nutritional composition of your food, or even the biochemical
processes your body uses to convert food nutrients into energy and muscle and bones. In a way, you can treat learning how to eat better as you would approach learning to drive a car. To drive, you need to know the traffic laws and you need to know how to operate the gas pedal, brake pedal, steering wheel, and turn signals. But you do not need to understand how gasoline burns in a car's engine or how the transmission works.
So here is a brief primer on nutrition that will help you follow the guidelines in Chapter 2.
Food Energy
Just as a car needs gasoline to run, your body needs fuel to do all the things it must do every day. That fuel, of course, comes from food. The usable energy in food is measured in units called kilocalories or, simply, calories. About two-thirds of the energy the body uses goes to keeping body temperature constant, repairing internal organs and skin, keeping the heart beating and lungs breathing, and ensuring the proper chemical balance inside and outside the body's cells. Most adults need between 1300 and 1800 calories a day just to stay alive without any physical activity at all. The other third of the energy is used for moving the body through its daily activities, dressing, walking, sitting, exercising, and all the other muscle-using activities that we do.
How many additional calories we need to eat depends on what we do during the day. The number of calories your body burns to carry out an activity depends on your weight, how long you do the activity, and how much work the activity takes.
For example, washing the dishes uses about one-half calorie an hour for every pound of body weight. So when a 150-pound man washes the dishes for 15 minutes, his body burns almost 19 calories. Reading aloud to his children for half an hour requires another 15 calories. He uses about the
same amount of energy every day just to eat, and the 15 minutes it takes him to get dressed in the morning and undressed at night takes an additional 11 or so calories. But riding his bicycle at a moderate pace for an hour burns about 165 calories. His wife, who weighs only 120 pounds, uses about 130 calories riding at his side.
In general, you can figure your approximate daily energy needs using one of the following equations:
|
For women: |
little physical activity: 960 + 3.8 times weight moderate activity: 1120 + 4.5 times weight regular exercise or manual labor: 1280 + 5.1 times weight |
|
For men: |
little physical activity: 1080 + 5.5 times weight moderate activity: 1260 + 6.4 times weight regular exercise or manual labor: 1440 + 7.3 times weight |
For example, if our 150-pound man rides his bicycle every day for an hour, his daily energy need is a little over 2500 calories a day (1440 + 7.3 times 150 = 2535). Similarly, his 120-pound wife needs almost 1900 calories a day if she exercises every day as well. If they were both couch potatoes who worked at desk jobs all day, their energy needs would drop significantly: he would need 1900 calories a day, and she would only need about 1400 calories.
Not all nutrients contain the same amount of calories (see Table 3.1). One gram of protein provides 4 calories of energy. So does 1 gram of carbohydrate. One gram of fat, however, provides 9 calories, and 1 gram of alcohol yields 7 calories.
Let's look at the practical consequences of the different calorie contents of carbohydrates, protein, and fat by comparing the calorie and nutrient content of a cup of ice cream, a cup of frozen yogurt, and a cup of ice milk (see Figure 3.1).
TABLE 3.1 Energy Yield of Fat, Protein, Carbohydrate, and Alcohol
|
|
Calories Provided Per Gram |
|
Fat |
9 |
|
Protein |
4 |
|
Carbohydrate |
4 |
|
Alcohol |
7 |
Ice cream, frozen yogurt, and ice milk are not much different in the amount of protein they contain, but the fat and carbohydrate content of the three desserts is quite different: 14 g of fat in ice cream compared with only 8 g of fat in frozen yogurt, and 7 g in ice milk. That is a difference of 6 or 7 g of fat, or about 54 to 63 calories (6 g of fat times 9 calories per gram equals 54 calories). Most foods high in calories are also high in fat.
It is not just desserts that contain a lot of fat. In the dinner at the beginning of this chapter, the food that contributed the majority of the fat was the chicken. Even so, a skinless chicken breast has only about 5.5 g of fat.
The human body uses fat as its primary means of storing energy. When the body needs to take energy out of its reserves, it preferentially uses its fat stores. It does not convert protein as a primary fuel source until most of the fat is gone. If on a given day your body needs to burn more energy than you eat, it converts some of the stored fat, at 9 calories per gram. That is why exercising regularly can help a person lose weight. On the other hand, if you eat more calories on a given day than your body uses, the body uses the surplus energy to make fat molecules and stores them in the various fatty tissues in your body. That is why you gain weight when you regularly eat more calories than your body uses.
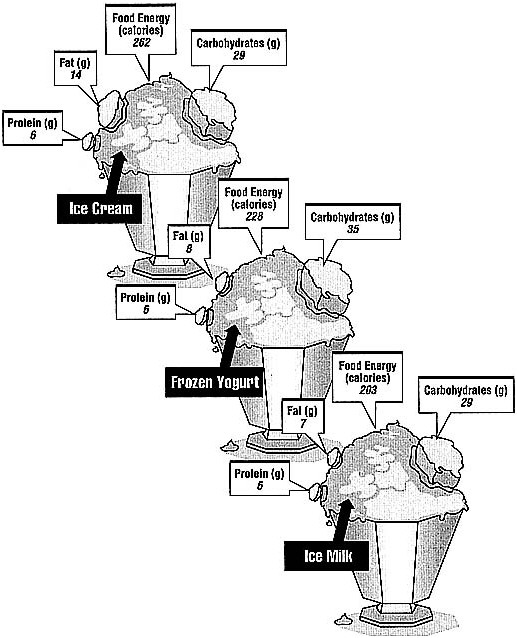
FIGURE 3.1 Comparison of calorie and nutrient content in 1 cup of ice cream, frozen yogurt, and ice milk.
SOURCE: U.S. Department of Agriculture, Human Nutrition Information Service.
Carbohydrates
In the Eat for Life eating pattern, the most important source of food energy is carbohydrates. They are also the least expensive source of calories, which is why the great majority of the world's population relies on carbohydrates to meet much of their daily energy needs.
Carbohydrates are among the most plentiful substances in the world, particularly in the plant kingdom. Plants are about 10 to 15 percent carbohydrate, whereas animals, including humans, contain a mere 1 percent carbohydrate.
Carbohydrates are a family of compounds made solely of the three elements carbon, hydrogen, and oxygen. These elements are arranged into rings, and the rings can be strung together into chains that are two rings to thousands of rings long (see Figure 3.2).
Scientists distinguish carbohydrates as being either simple carbohydrates (sugars)—containing one or two rings (monosaccharides or disaccharides )—or complex carbohydrates—with many rings (polysaccharides). In addition, complex carbohydrates can be digestible (starches) or indigestible (fiber or roughage), depending on how the rings are hooked together.
Glucose, sucrose, fructose, maltose, and lactose are common sugars. The body can covert all of these sugars directly into energy, or it can use them to make fats. Glucose and fructose are made of one ring and are called monosaccharides. They are found in honey and fruit.
Sucrose, maltose, and lactose have two rings and are called disaccharides. When the body digests these sugars, it splits them into monosaccharides. Sucrose, made of one molecule of glucose hooked to one molecule of fructose, is what we call table sugar. It is found in molasses, in maple syrup, and in fruits. Maltose, consisting of two glucose molecules
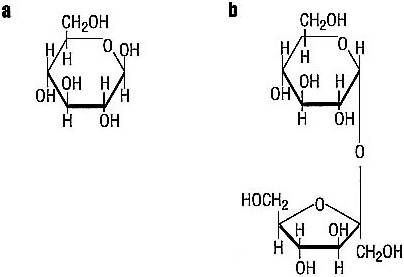
FIGURE 3.2 Structure for (a) monosaccharide glucose, and (b) disaccharide sucrose.
hooked together, is found in sprouting grains, malted milk, malted cereals, and some corn syrups. Lactose, or milk sugar, consists of one molecule of glucose and one molecule of another monosaccharide called galactose.
Sucrose and fructose are the sugars added during food processing. Corn syrups, used in many baked goods, get their sweetness from glucose and maltose. In recent years, food processors have learned how to make high-fructose corn syrups by rearranging the glucose ring to make fructose. High-fructose corn syrups have replaced much of the sucrose in most soda pops.
Starches, made of hundreds, even thousands, of glucose molecules, are the most common digestible polysaccharides in the diet. They are also the major source of energy in the human diet. Grains, beans, and some fruits and vegetables are rich sources of starch. For example, almost 90 percent of the calories in a potato and 73 percent of the calories in pinto beans come from starch.
In the digestive system, the large starch molecules are broken down, or digested, into individual glucose molecules. Glucose, not the original starch molecules, is absorbed from the digestive tract into the blood stream. When a person says he has ''high blood sugar," he is talking about the amount of glucose in the blood stream, that is the blood sugar level.
Fiber is a complex mixture of many indigestible substances—most of which are nonstarch polysaccharides—that make up the structural material of plants. Among these are cellulose, hemicellulose, pectin, lignin, gums (such as guar and locust bean, common food additives used to improve the texture of some foods), and mucilage. Some processed foods contain carrageenan and alginates, indigestible polysaccharides produced by algae. Lignin is an indigestible plant product, but it is not a carbohydrate.
Pectins, gums, mucilages, and some hemicelluloses dissolve in water and are sometimes called soluble fiber. At least part of the fiber in oat and rice bran is soluble fiber.
Cellulose, most hemicelluloses, and lignins do not dissolve in water and are known as insoluble fiber. Wheat bran, for example, is mostly insoluble fiber.
The main difference between indigestible polysaccharides and starch is that in both soluble and insoluble fiber the chemical links that hold the individual molecules together as a chain are resistant to the processes of the human digestive system. Thus they provide little food energy to the body. Humans cannot digest grass because it is mostly indigestible complex carbohydrate. Cows and sheep can use grass as food because their stomachs contain bacteria that digest these carbohydrates, releasing simple sugars that are absorbed into the animals' blood streams. Though fiber has little energy value for humans, it seems to be necessary for the large intestine to function at its peak.
In general, foods with a high fiber content include wholegrain breads and cereals, fruits, vegetables, beans, peas, and nuts. Fruit skins, seeds, berries, and the bran layers of cereal grains are richer sources of fiber than the rest of these foods.
Fats
In the Eat for Life eating pattern, fats take second place to carbohydrates as an energy source. Fats are a large family of compounds that are made mostly of the elements carbon and hydrogen, with a small amount of oxygen. The major fat in food is triglyceride, a molecule of glycerol with three fatty acids attached (see Figure 3.3).
Foods that are almost pure fat include cooking oil, lard, butter, margarine, and shortening. Foods that contain significant amounts of fat include meat, dairy products, chocolate, cakes, pies, cookies, nuts, and a few fruits and vegetables—coconut and avocado, for example.
One characteristic of fats is that they do not mix with or dissolve in water. Instead, fat molecules tend to cluster
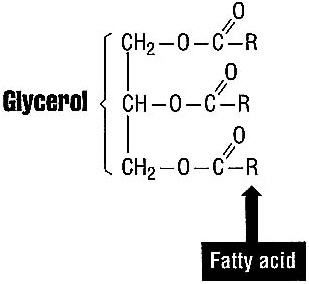
FIGURE 3.3 A triglyceride. The "R" represents fatty acids chemically bound to the glycerol backbone of the triglyceride.
together with other fat molecules. Fats are soluble in organic solvents like benzene or ether.
Fatty acids come in a variety of sizes, and all fat in foods contains a mixture of these various fatty acids. Some fatty acids contain as few as 4 carbon atoms, whereas others are made of as many as 20 or more strung together in a line. The other way in which fatty acids differ from one another is in the number of hydrogen atoms they contain per carbon atom. For example, the four fatty acids shown in Figure 3.4 all contain 16 or 18 carbon atoms and 2 oxygen atoms. Oleic, linoleic, and linolenic acids all have 18 carbons and 2 oxygens, but they have different numbers of hydrogen atoms.
Palmitic acid (Figure 3.4a) is found in meats, butter fat, shortening, and some vegetable oils. Palmitic acid is a saturated fatty acid, one that has the maximum number of hydrogen atoms—2—attached to every carbon atom, except for those on each end. In effect, the molecule is "saturated" with hydrogen atoms. Other saturated fatty acids found in food include stearic acid (18 carbon atoms), myristic acid (14 carbon atoms), and lauric acid (12 carbon atoms). Fats high in saturated fatty acids are usually solid at room temperature and certainly at refrigerator temperature.
In oleic acid (Figure 3.4b), there are 2 hydrogen atoms missing in the middle of the molecule, 1 from each of 2 adjoining carbon atoms. The place where this occurs is called an unsaturation. Since oleic acid has one unsaturation, it is called a monounsaturated fatty acid. Oleic acid is the predominant monounsaturated fatty acid in food. Olive oil, peanut oil, and canola oil are particularly good sources of oleic acid.
The last two fatty acids in Figure 3.4 are missing still more hydrogen atoms, again from adjacent pairs of carbon atoms. Since these fatty acids have more than one unsaturation, they are called polyunsaturated fatty acids. The polyunsaturated fatty acid with two unsaturations is called linoleic acid, and the polyunsaturated fatty acid with three unsaturations is called linolenic acid. Linoleic acid is called an essential fatty
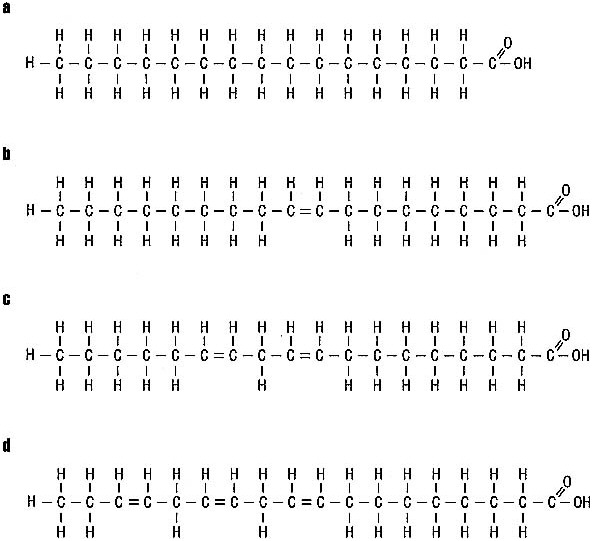
FIGURE 3.4 Comparison of the structure of four fatty acids: (a) palmitic, (b) oleic, (c) linoleic, and (d) linolenic acids.
acid because the body cannot make it and must meet its need for it from food sources. Scientists believe that linolenic acid may prove to be an essential nutrient as well.
Foods from the plant kingdom, with the notable exceptions of palm oil and coconut, are good sources of polyunsaturated fatty acids. Seafood is rich in monounsaturated fatty acids and polyunsaturated fatty acids. Fats with a large percentage of monounsaturated fatty acids and polyunsaturated fatty acids are usually liquid even when refrigerated.
Polyunsaturated fatty acids are further classified by where the unsaturations exist in the fatty acid molecule. You may have heard of omega-3 fatty acids, the main polyunsaturated fatty acid in many fish oils. All this term means is that the first unsaturation occurs at the third carbon atom from the omega end of the fatty acid, the end with 3 hydrogen atoms. Eicosapentaenoic acid (shown in Figure 3.5) is one of the chief omega-3 fatty acids in fish oil. If you count the carbon atoms starting at the omega end, you can see why linoleic acid is called an omega-6 fatty acid.
If you read food labels, you have probably seen the term "partially hydrogenated vegetable oil." This means that hydrogen atoms have been added to unsaturated fatty acids by means of a chemical process known as hydrogenation. This reaction eliminates some of the unsaturations; monounsaturated fatty acids become saturated fatty acids, and polyunsaturated fatty acids become monounsaturated fatty acids and saturated fatty acids. Vegetable oils are partially hydrogenated to make them solid at room temperature. Vegetable shortening and margarine are two examples of partially hydrogenated vegetable oils.
Unsaturated fatty acids in foods exist chiefly in the cis configuration, in which the hydrogen atoms are on the same side of the double bond. When fats and oils are partially hydrogenated during commercial processing, varying amounts of the trans configuration form. In the trans configuration, the hydrogen atoms are on opposite sides of the double bond.
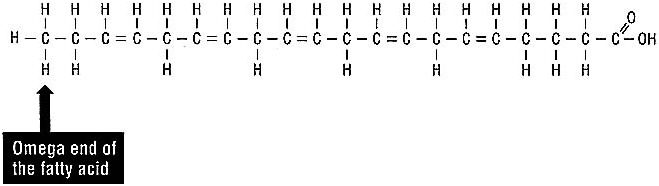
FIGURE 3.5 An omega-3 fatty acid: eicosapentaenoic acid.
Although small amounts of trans fatty acids occur naturally in milk and butter, the large increase in the use of partially hydrogenated vegetable oils has resulted in increases in trans fatty acids in foods. As yet, there are no reliable data on the trans fatty acid intake by the U.S. population, and estimates vary from 7 to 12 g per person per day.
Remember that all fats contain a mixture of fatty acids. Some have more of one kind of fatty acid than another kind. Beef fat, for example, contains more saturated fatty acids than monounsaturated or polyunsaturated fatty acids. Olive oil contains both saturated fatty acids and polyunsaturated fatty acids, but over half its fatty acids are monounsaturated, and most of that is oleic acid.
Cholesterol
There are a number of substances in food that are not exactly fats but, like fats, do not mix with water. One of these substances is cholesterol (see Figure 3.6).
Cholesterol exists in foods of animal origin and in the body, where it is an important component of many tissues, particularly the brain and nervous system. Cholesterol is found in all body cells as part of the structure of cell membranes. The body also uses cholesterol to make bile acids, various hormones, and vitamin D. Cholesterol is not an essential nutrient, however, because the body can manufacture all it needs.
FIGURE 3.6 Cholesterol.
Nevertheless, the U.S. diet has many sources of cholesterol, including egg yolks, liver, meat, certain shellfish, and whole-milk dairy products. Cholesterol is not found in plant foods.
Because cholesterol does not dissolve in water, it moves through the blood stream in clusters of molecules made of fat and protein called lipoproteins. Most of the cholesterol in the body is carried by three types of lipoproteins: high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very-low-density lipoprotein (VLDL). Cholesterol found in HDL is called HDL-cholesterol, and cholesterol found in LDL is called LDL-cholesterol. The term total serum cholesterol refers to the sum of HDL-, LDL-, and VLDL-cholesterol in the blood stream. Medical experts consider a total serum cholesterol level below 200 milligrams per deciliter (mg/dl) to be desirable.
Protein
If carbohydrates and fat are the body's energy sources, then proteins are the body's building blocks. If you do not count water, protein accounts for about three-quarters of the weight in most human tissues. Hair, skin, nails, and muscle are mostly protein, and bone contains a significant amount of protein as well. Certain proteins, called enzymes, perform the countless chemical reactions needed to produce energy to keep your body operating and to produce the thousands of different molecules that are present in muscle, bone, skin, hair, and organs.
Protein is composed of smaller chemical units called amino acids. Amino acids are made of the elements carbon, hydrogen, oxygen, nitrogen, and sometimes sulfur. There are 20 different amino acids that when hooked together in various numbers and combinations make up the thousands of different proteins in the human body. The body can make enough of all but 9 of these, the so-called essential amino acids.
In practice, protein is the only source of the essential amino acids in the diet. When you eat a piece of chicken, your digestive system breaks the protein molecules in that chunk of chicken muscle into individual amino acids. These are absorbed into the blood stream and transported to all the cells in the body. Enzymes and other biological molecules inside a cell reassemble the amino acids into those proteins that the cell needs.
The body does not store significant amounts of amino acids, and so we have to eat protein regularly. Meat is a rich source of protein, since muscle is protein with fat mixed in. So, too, are dairy products. Beans, nuts, and cereal grains are also good sources of protein. Worldwide, people get most of their protein from vegetable sources, rather than the animal sources that provide most of the protein in U.S. diets.
Vitamins
Vitamins are a group of diverse compounds that the body needs in small amounts to remain healthy. They are used in a variety of biochemical processes. Vitamin K, for example, plays an important role in blood clotting, and vitamin D is involved in absorbing calcium and thus maintaining the bones in good condition. Some of the functions of the vitamins are listed together with food sources in Table 3.2.
When they were first discovered around the turn of the century, vitamins were classified according to whether or not they dissolved in water. The fat-soluble vitamins are vitamins A, D, E, and K. The water-soluble vitamins are vitamin
TABLE 3.2 Vitamins
|
Vitamin |
Functions |
Food Sources |
|
Fat-Soluble Vitamins |
||
|
Vitamin A |
Maintains normal vision and healthy skin and mucous membranes; necessary for normal growth and for reproduction |
Liver, butter, whole milk, egg yolks; margarine, skim milk, and certain breakfast cereals are fortified with vitamin A; the body also makes vitamin A from carotenoids, compounds present in dark-green leafy vegetables, yellow and orange vegetables, and fruit |
|
Vitamin D |
Promotes calcium and phosphorus absorption from the intestines; influences bone growth |
Liver, butter, fatty fish, egg yolks; milk is fortified with vitamin D |
|
Vitamin E |
Antioxidant that prevents cells from being damaged by various biochemical reactions that occur naturally |
Best sources are vegetable oils; also found in nuts, seeds, whole grains, and wheat germ |
|
Vitamin K |
Aids in blood clotting |
Best source is dark-green leafy vegetables; also found in cereals, dairy products, meat, and fruits |
|
Water-Soluble Vitamins |
||
|
Vitamin C |
Promotes growth of connective tissues; antioxidant |
Citrus fruits, tomatoes, broccoli, green peppers, strawberries, melons, cabbage, and leafy green vegetables; vitamin C is destroyed when foods are overcooked or cooked in large amounts of water |
|
Thiamin (Vitamin B1) |
Aids in obtaining energy from carbo-hydrates |
Meat, eggs, beans, and whole grains; enriched breads and cereals |
|
Vitamin |
Functions |
Food Sources |
|
Riboflavin (Vitamin B2) |
Aids in energy production and in many other biochemical processes |
Liver, milk, and dark-green leafy vegetables; whole grains and enriched breads and cereals |
|
Niacin |
Aids in energy production from fats, proteins, and carbohydrates; aids in manufacture of fatty acids |
Grain products, meat, poultry, fish, nuts, and beans |
|
Pyridoxine (Vitamin B6) |
Aids in manufacture of amino acids; aids in energy production from protein |
Meat, poultry, fish, grain products, and fruits and vegetables |
|
Cobalamin (Vitamin B12) |
Aids in DNA synthesis, and in energy production from fatty acids and carbohydrates; aids in production of amino acids |
Meat, milk and milk products, and eggs |
|
Folacin |
Aids in manufacture of genetic material in cells; aids in production of amino acids |
Dark-green leafy vegetables, liver, and fruits |
|
Biotin |
Aids in energy production |
Egg yolks, liver, beans, and nuts |
|
Pantothenic acid |
Aids in energy production; aids in the manufacture of fatty acids; participates in a wide variety of other biochemical processes |
Most foods |
C and the eight B vitamins—thiamin, riboflavin, niacin, pyridoxine, pantothenic acid, biotin, folacin, and cobalamin.
Fat-soluble vitamins are found in association with fats in foods, and they are also absorbed out of the digestive
system along with fats. The body can store variable amounts of the fat-soluble vitamins. It is possible to consume too much of some fat-soluble vitamins so that they build up in the body to toxic levels. Vitamin A and vitamin D poisonings are not unknown and can cause serious illness, and even death.
Some vitamins are manufactured in the body. Vitamin D is made in the skin when exposed to sunlight. Bacteria that live in the human intestine produce vitamin K, which is then absorbed into the blood stream.
With the notable exception of vitamin B12, the body stores little of the water-soluble vitamins, and so you must get these substances in your diet regularly. Taking large amounts of these vitamins is futile, however, because the excess is carried in the blood stream to the kidneys and excreted in the urine.
Minerals
Like vitamins, minerals play a variety of roles in the body. Some minerals, such as calcium and phosphorus, are present in the body in relatively large amounts and are sometimes called macrominerals. Other minerals, such as iron and copper, are needed in much lower amounts and are called trace elements. Three macrominerals—sodium, potassium, and chloride—are sometimes called electrolytes, because they help maintain the proper electrical balance in cells and body fluids. Some of the functions of minerals are listed together with food sources in Table 3.3
Whereas all the other nutrients discussed so far are relatively big molecules, minerals are simple chemicals made of single atoms or salts made up of a few atoms. Together, minerals account for only about 4 percent of body weight.
Salts are associations of positive and negative components called ions. Table salt, for example, is made from positively charged sodium ions and negatively charged chloride ions—the chemical name for table salt is sodium chloride. When
TABLE 3.3 Minerals
|
Mineral |
Functions |
Food Sources |
|
Calcium |
The most abundant mineral in the body, 99 percent is in bones; is also important in muscle function |
Dairy products, bones of sardines and canned salmon, dark-green leafy vegetables, and lime-processed tortillas |
|
Chloride |
Is a component of stomach acid; aids in maintaining fluid balance in cells |
Table salt, eggs, meat, and milk |
|
Chromium |
Works with insulin to promote carbohydrate and fat metabolism |
Liver and other organ meats, brewer's yeast, whole grains, and nuts |
|
Copper |
Aids in energy production; aids in absorption of iron from digestive tract; forms dark pigment in hair and skin |
Liver, meat, whole grains, and nuts |
|
Fluoride |
Strengthens teeth and bones |
Some natural waters, fluoridated water, and tea |
|
Iodine |
Is part of the thyroid hormones that regulate metabolism |
Iodized table salt, ocean seafood, dairy products, and commercially made bread |
|
Iron |
Aids in energy production; helps to carry oxygen in the blood stream and muscles |
Meat, poultry, fish, nuts, whole and enriched grain products, and green vegetables |
|
Magnesium |
Is necessary for nerve function, bone formation, and general metabolic processes |
Grain products, vegetables, dairy products, fish, meat, and poultry |
|
Manganese |
Aids in regulation of carbohydrate metabolism and in general metabolic processes |
Cereals and most other plant foods |
|
Mineral |
Functions |
Food Sources |
|
Molybdenum |
Aids in energy production |
Meat, beans, and cereals |
|
Phosphorus |
Aids in bone formation, general metabolic processes, and energy production and storage |
Dairy products, meat, poultry, fish, and grain products |
|
Potassium |
Is necessary to maintain fluid balance in cells, transmit nerve signals, and produce energy |
Fruits and vegetables, nuts, grains, and seeds |
|
Selenium |
Protects cells against harmful reactions involving oxygen; aids in detoxifying toxic substances |
Meat, ocean fish, and wheat |
|
Sodium |
Is necessary to maintain fluid balance in cells, transmit nerve signals, and relax muscles |
Table salt and salt added to food during processing |
|
Zinc |
Is necessary for cell reproduction and tissue repair and growth |
Oysters and other shellfish, meat, poultry, eggs, hard cheeses, milk, yogurt, beans, nuts, and whole-grain cereals |
table salt is dissolved in water, it separates into sodium and chloride ions again. Sodium bicarbonate is the salt we call baking soda. In this book the term ''salt" will refer only to the compound sodium chloride. All other salts will be referred to by their chemical names.
HOW DIET HAS CHANGED OVER TIME
For most of human history, the quest for sufficient food was the chief occupation of the earth's people. Our early ancestors were hunter-gatherers, searching for edible plants and killing the occasional animal. Archeologists have found evidence suggesting that the early human diet was about 35 percent meat and 65 percent plant food, though little of it was cereal grains. These people ate very little fat—they ate no dairy products and the meat they ate contained only about 4 percent fat—and only low levels of sodium, but their diets were rich in dietary fiber, calcium, and vitamin C.
Two things happened to change the human diet. The first took place around 10,000 B.C., when some people became farmers. For the first time, dairy products and cereal grains became a part of the diet, and the supply of meat became more predictable.
The second important change accompanied the Industrial Revolution of the late eighteenth century. The rise of factories gave birth to a new middle class of merchants and managers who had the money to afford a variety of foods. This demand prompted farmers to improve their methods and grow a wider variety of crops. The result was that the amount of food available to all people, both middle class and poor, increased. At the same time, the cost of food dropped significantly.
In the two centuries that have passed since then, the U.S. diet has undergone remarkable changes. In 1800, 95 percent of all Americans ate food that was straight off the farm, or fresh from the sea, with little processing done to it. Today, 95 percent of all Americans depend on others to produce, process, and distribute food to supermarkets. What we can eat depends mostly on what we can afford to buy.
Today, refrigerated railcars, trucks, and cargo planes make seasonal foods available year-round. A cornucopia of canned, frozen, fermented, and dried foods appears on grocery store
shelves, as do the foods seen nowhere in nature that come out of our country's food laboratories, such as "fruit" drinks that have no fruit juice and "meats" made from soybeans or wheat gluten. Supermarkets now carry as many as 30,000 different items, making the U.S. diet the most varied in the world.
Given the changes that have occurred in the U.S. food supply during this century, it is surprising that Americans today have available about the same level of calories as our grandparents did at the turn of the century. At the same time, we weigh more than our grandparents, suggesting that we do not get as much exercise as was once common.
The source of the calories in the food supply has changed. Fats now provide about 40 percent of the calories in the U.S. diet, compared with only 32 percent in 1909 (see Figure 3.7). The amount of polyunsaturated fatty acids and monounsaturated
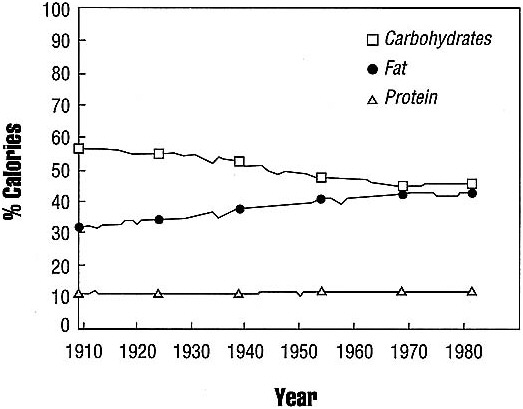
FIGURE 3.7 Percent of calories from carbohydrate, fat, and protein in the U.S. food supply since 1909. SOURCE: U.S. Department of Health and Human Services and U.S. Department of Agriculture. 1986. Nutrition Monitoring in the United States. U.S. Government Printing Office, Washington, D.C.
fatty acids in the U.S. diet increased more than the amount of saturated fatty acids during that period. Americans today eat about 385 mg of cholesterol a day, which is probably less than our grandparents ate because we consume fewer eggs and less butter than they did.
We also eat proportionately fewer carbohydrates than Americans did at the turn of the century; carbohydrates today account for 43 percent of the calories in the food supply, compared with 57 percent in 1909. In 1909, two-thirds of the carbohydrates in the food supply were complex polysaccharides, and one-third were simple sugars. Today, over half the carbohydrate calories are from sugars, with less than half coming from starches.
Calories from protein have remained fairly constant during this century, at about 17 percent of total calories.
What foods provide the calories in the U.S. diet? The largest share of calories comes from grain products and from meat, poultry, and fish. Fats, sweets, and beverages combined contribute about as many calories as fruit and vegetables or dairy products. Eggs, beans, nuts, and seeds combined account for the smallest proportion of calories in the U.S. diet.
Americans have also changed their eating patterns over the years. Whereas breakfast used to be the most important meal of the day, today only 53 percent of adults—and only 85 percent of children age five or younger—eat breakfast. In addition, eating out has become more common. One survey found that over half the women questioned ate out on a given day, and 88 percent ate out at least once over a 4-day period. Snacks now provide about 18 percent of the calories in the average U.S. diet.
It is commonly thought that the U.S. diet has become healthier since the last century. We live longer, grow taller, and enjoy better health than our ancestors. Diet has had some part in the health benefits we have realized over the years. Deficiency diseases are rarely seen in the United States
today. But we now live longer primarily because of improved sanitation, antibiotics, and vaccinations—infectious diseases are no longer the major killers they once were. Many diseases linked in some way to diet—heart disease, high blood pressure, cancer, diabetes, dental cavities, and others—still plague us.
























