1 Objectives, Terminology, and Overview of Pathogen Status
Scientific Objectives
Animal experiments are essential to progress in the biomedical sciences (NRC, 1985; Gay, undated). Like investigations in any field of science, the merit of animal experiments ultimately depends on rigid adherence to principles of the scientific method. Proper practice of these principles yields data that have both reliability and reproducibility, key objectives of all good experiments (Bernard, 1865).
Living systems are remarkably complex and often subject to large deviations in biologic response due to seemingly trivial influences, either endogenous or exogenous. Thus, the biological responsiveness of mice and rats, comprising 90% of the experimental animals used in the United States (ILAR, 1980), can be altered by genetic factors (Kahan et al., 1982; Hedrich, 1983) and numerous environmental influences, including physical, chemical, and microbial factors (Lindsey et al., 1978; Baker et al., 1979; Pakes et al., 1984).
As will be discussed in considerable detail later in this volume, the prevention of many natural infections (microbial factors) in mice and rats is of crucial importance for accomplishing a wide range of scientific objectives for which these rodent species are used.
Infection Versus Disease
A common misconception, even among senior scientists and laboratory animal specialists, is that infection is synonymous with disease. Bacterial
infections of rodents include pathogens, opportunists, and commensals, of which the last two are by far the most numerous as constituents of the normal flora on mucosal and body surfaces (Dubos et al., 1965; Savage, 1971). Similarly, the viral and parasite pathogens of rodents vary considerably in pathogenicity. Also, it is important to distinguish between subclinical (inapparent, covert, or silent) and clinically apparent infections. Two types of subclinical infections are recognized: dormant (the agent can be recovered) and latent (the agent cannot be recovered by direct methods and its presence must be inferred by indirect methods).
Most natural infections due to pathogens in mice and rats are subclinical. Thus, clinical manifestations of pathogen infections in these species have only limited diagnostic value. Also, it cannot be overemphasized that aberrations in research results due to natural infections often occur in the absence of clinical disease. Thus, prevention of infection, not merely prevention of clinical disease, is essential (Lindsey et al., 1986a).
Terminology of Microbial and Pathogen Status
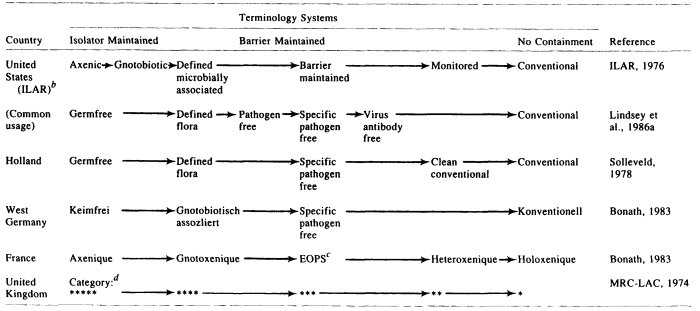
The development of gnotobiotic methodology in the 1940s and 1950s (Gustafsson, 1948; Reyniers, 1957a,b; Trexler and Reynolds, 1957; Luckey, 1963; Trexler, 1963, 1983; Newton, 1965; ILAR, 1970; Weisbroth, 1972; Myers, 1980) provided the basic technological armamentarium for controlling the microbial and pathogen status of mice and rats. The use of cesarean derivation and gnotobiotic isolator techniques was found to be highly practical in eliminating pathogens from rodent stocks for institutional (Trexler and Barry, 1958; Van Hoosier et al., 1966) and commercial (Cumming and Elias, 1957; Foster, 1958; Foster et al., 1963) purposes. The subsequent widespread application of these methods to the production of mice and rats for research resulted in a bewildering array of terms for the designation of microbial and pathogen status (Table 1). Some of the terms have etymological clarity and consistently convey clear meanings. However, many of the terms have proved unfortunate; they convey imprecise meanings that lead to confusion about animal quality. The resulting state of affairs has been detrimental to the continuing quest for improvement in the quality of rodents for research.
Six terminology systems for laboratory rodent microbiological status, representing the major systems in use in the Western world, are shown in Table 1. Based on the microbial exclusion method(s) required, the different types of animals in Table 1 are separable into three major groupings:
- Isolator maintained. An isolator is a sterilizable life-support chamber, usually constructed of stainless steel or plastic, in which gnotobiotes may be housed and maintained free from contamination (ILAR, 1970). "Isolator maintained" as used here means continuous, uninterrupted maintenance of animals in an isolator so as to prevent any microbial contamination.
TABLE 1 Terminology for Microbiological Status of Laboratory Rodentsa
- Barrier maintained. A barrier is a housing "system that combines construction features, equipment, and operating methods to stabilize the enclosed environment to minimize the probability that pathogens or other undesirable organisms will contact or infect the enclosed animal population" (ILAR, 1976a). Barriers may be provided at the facility, room, rack, and/or cage levels. "Barrier maintained" as used here means continuous, uninterrupted maintenance of animals in one or more barriers so as to prevent contamination by any pathogen.
- No containment. The term no containment means the housing of animals without measures to prevent pathogen contamination. Animals housed in this manner are generally designated conventional (see below).
The individual types of animals included in Table 1 are defined as follows:
- Germfree (or axenic, keimfrei, axenique) animal. Derived by hysterectomy, reared and maintained in an isolator by germfree techniques, and demonstrably free of associated forms of life including viruses, bacteria, fungi, protozoa, and other saprophytic or parasitic forms (ILAR, 1970,1976a; Bonath, 1983). Endogenous viruses, e.g., leukemia viruses that occur in all mice, are present (ILAR, 1970).
- Gnotobiotic animal. Derived by hysterectomy, reared and maintained in an isolator by germfree techniques, and has one or more associated nonpathogenic agent(s), all of which are known (ILAR, 1970, 1976).
- Defined flora (or defined microbially associated, gnotobiotisch assozliert, gnotoxenique) animal. A germfree animal that has been intentionally associated with one or more microorganisms (e.g., the "Schaedler cocktail," consisting of eight nonpathogenic bacteria) and maintained continuously in an isolator to prevent contamination by other agents (ILAR, 1976a; Bonath, 1983). This term may be used synonymously with gnotobiote.
- Pathogen-free (PF) animal. Free of all demonstrable pathogens (Sacquet, 1965). This term is frequently abused because there is no universal agreement on which agents are pathogens, which tests should be done for them, how the animal populations should be sampled, or how frequently testing should be done. A pathogen (in the context of laboratory animal quality) may be defined as an infectious agent that can cause overt disease and/or alter biologic response(s) during experimentation (Lindsey et al., 1986a). Proper usage of the term pathogen-free requires that the pathogen-free status of a given subpopulation of animals be supported by current test results from a battery of tests appropriate for all pathogens of the rodent species in question. In actual practice, a list of pathogens must be specified. Thus, this term differs very little from specific pathogen free (Lindsey et al., 1986a).
- Specific pathogen free (SPF) (or barrier maintained, EOPS) animal. Free of a specified list of pathogens. This term also is frequently abused.
TABLE 2 Prevalence of Murine Virus Infections in Mouse Coloniesa
|
Virus |
United States |
|
Canada |
United Kingdom |
West Germany and Others |
Japan |
|
|
|
|
Commercial Breedersb |
Commercial Breeders and Research Institutionsc |
Breeders and Research Institutionsd |
Breeders and Research Institutionse |
Breeders and Research Institutionsf |
Commercial Breeders and Research Institutionsg |
Breedersh |
Research Institutionsi |
|
PVM |
36 |
95 |
83 |
42 |
14 |
|
|
|
|
Sendai |
73 |
86 |
47 |
34 |
23 |
52 |
46 |
54 |
|
Mouse hepatitis |
83 |
81 |
73 |
40 |
60 |
26 |
19 |
36 |
|
Minute |
73 |
81 |
50 |
0 |
33 |
|
|
|
|
Theiler's |
5 |
62 |
40 |
0 |
33 |
|
|
|
|
Reovirus-3 |
14 |
52 |
33 |
4 |
13 |
8 |
|
|
|
Adenovirus |
19j |
0 |
0 |
0 |
|
0 |
|
|
|
Polyoma |
0 |
5 |
14 |
0 |
0 |
|
|
|
|
Mousepox |
0 |
0 |
14 |
0 |
0 |
|
|
|
|
LCMVk |
0 |
0 |
0 |
0 |
0 |
|
|
|
|
No. of colonies |
77 |
21 |
12-18/agent |
1-35/agent |
2-60/agent |
89 |
196 |
61 |
|
a Data are percentages of colonies found to be infected Serologic methods were used except where specified otherwise. b From Lindsey et al., 1986a. c From Parker, 1980. d From Lussier and Descoteaux, 1986. e From Gannon and Carthew, 1980. f From Kraft and Meyer, 1986. g From Nakagawa et al., 1984. h From Suzuki et al., 1982. i From Fujiwara, 1980. j Diagnosis was based on the finding of typical intranuclear inclusions in intestinal epithelium (Luethans and Wagner, 1983). All animals were serologically negative for adenovirus. k Lymphocytic choriomeningitis virus. |
||||||||
TABLE 3 Prevalence of Murine Virus Infections in Rat Coloniesª
|
Virus |
United States |
Canada |
United Kingdom |
West Germany and Others |
Japan |
|
|
|
|
|
Commercial Breedersb |
Commercial Breeders and Research Institutionsc |
Breeders and Research Institutionsd |
Breeders and Research Institutionse |
Breeders and Research Institutionsf |
Commercial Breeders and Research Institutionsg |
Breedersh |
Research Institutionsh |
|
PVM |
44 |
64 |
70 |
72 |
40 |
|
|
|
|
Sendai. |
61 |
52 |
55 |
30 |
30 |
28 |
3 |
6 |
|
SDAVi |
44 |
68 |
75 |
45 |
41 |
8 |
41 |
43 |
|
KRVi |
44 |
71 |
74 |
60 |
|
32 |
|
|
|
Toolan H-1 |
11 |
52 |
71 |
0 |
27 |
|
|
|
|
Reovirus-3 |
6 |
44 |
44 |
0 |
|
16 |
|
|
|
Adenovirus |
6j |
36 |
17 |
|
|
8 |
|
|
|
Theiler's |
|
44 |
58 |
|
|
|
|
|
|
LCMVi |
|
|
7 |
0 |
|
|
|
|
|
No. of colonies |
18 |
25 |
15-24/agent |
1-33/agent |
36-45/agent |
71 |
34 |
49 |
|
a Data are percentages of colonies found to be infected. Serologic methods were used except where specified otherwise. b From Lindsey et al., 1986a. c From Parker, 1980. d From Lussier and Descoteaux, 1986. e From Gannon and Carthew, 1980. f From Kraft and Meyer, 1986. g From Suzuki et al., 1982. h From Fujiwara, 1980. i SDAV = Sialodacryoadenitus virus KRV = Kilham rat virus LCMV = Lymphocytic choriomeningitus virus j Diagnosis was based on the finding of typical intranuclear inclusions in intestinal epithelium (Luethans and Wagner, 1983). All animals were serologically negative for adenovirus. |
||||||||
- Proper usage requires that the absence of certain specified pathogens from a given subpopulation of animals be supported by current test results from a battery of tests appropriate for those pathogens (Lindsey et al., 1986a).
- Virus antibody-free animal. Free of antibodies to viral pathogens. Proper usage requires that the absence of viral pathogens for a given subpopulation of animals be supported by current test results from a battery of appropriate serologic tests for a list of specified pathogens (Lindsey et al., 1986a).
- Monitored (or clean conventional, heteroxenique) animal. Housed in a low-security barrier and demonstrated by sequential monitoring to be free of major pathogens (ILAR, 1976a; Bonath, 1983). These terms are even less specific than pathogen-free and specific pathogen free. They imply a standard of quality without defining the standard, and their usage is to be discouraged.
- Conventional (or konventionell, holoxenique) animal. Microbial burden is unknown and uncontrolled, and housing is generally in open rooms that have unrestricted access (ILAR, 1976a; Bonath, 1983).
Based on the foregoing information, it should be apparent that the terms used in defining rodent microbial status vary greatly in precision of meaning. Four terms (germfree, gnotobiotic, defined flora, and conventional), representing the extremes of microbial status, have clear definitions that are generally accepted and understood by scientists as well as technical personnel. However, major confusion is encountered in the definition and use of terms representing the middle ground of pathogen status. Herein lies a problem of major proportions. Pathogen-free, specific pathogen free, virus antibody-free, and clean conventional are relative terms that require explicit definition every time they are used. Their definitions must include the background of the rodent subpopulation in question (e.g., hysterectomy derived?, isolator maintained?, barrier maintained?); details of current housing (e.g., isolator, barrier); and data from laboratory tests, including the specific tests done for pathogens, the number of tests, the frequency of testing, and the results of all tests to date (Lindsey et al., 1986a).
Pathogen Status of Contemporary Rodents
The conceptual approaches and technological advances necessary for the prevention of pathogen infections in laboratory mice and rats have been available since the 1950s (Cumming and Elias, 1957; Reyniers, 1957a,b; Trexler and Reynolds, 1957; Foster, 1958; Trexler and Barry, 1958; Luckey, 1963). The prevalence of pathogens in contemporary rodent populations (colonies) provides an indication of the degree to which these concepts and methods have found practical application in the prevention of pathogen infections.
Tables 2 and 3 give the prevalence of virus infections in laboratory mice and rat populations in the United States, Canada, the United Kingdom, West
Germany, and Japan. [Additional prevalence data have been reported from the United States by Casebolt et al. (1988) and from Europe by Van Der Logt (1986)]. In mice, infections of pneumonia virus of mice (PVM), Sendai virus, mouse hepatitis virus, and minute virus of mice were commonly present in 50% or more of the colonies surveyed. Theiler's virus and reovirus-3 were not uncommon, and a few colonies had adenovirus, polyoma virus, or ectromelia virus. In rats, PVM, Sendai virus, SDA/RCV, Kilham rat virus (KRV), and Toolan H-1 virus were frequently found in more than 50% of colonies. Reovirus-3 and adenovirus were fairly common. LCMV was reported in 7% of rat colonies in Canada. Although antibodies to Theiler's virus were reported in 44% of rat colonies in one survey in the
TABLE 4 Prevalence of Bacterial Infections in Mouse Coloniesª
|
Bacterium |
United States |
United Kingdom |
West Germany and Others |
Japan |
|
|
|
|
Commercial Breedersb |
Accredited Institutionsc |
Breeders and Research Institutionsd |
SPF Breederse |
Conventional Breedersf |
Research Institutionsf |
|
Mycoplasma pulmonis |
19 |
17 |
|
0 |
24 |
20g |
|
Mycoplasma arthritidi |
3 |
|
|
|
|
|
|
Pseudomonas sp. |
57 |
|
|
33 |
32 |
|
|
Pasteurella pneumotropica |
18 |
66 |
|
0 |
74 |
|
|
Salmonella enteritidi |
6 |
|
|
0 |
5 |
|
|
Mycobacterium avium |
1 |
|
|
|
|
|
|
Citrobacter freundii (4280) |
1 |
|
|
|
|
|
|
Streptococcus moniliformis |
0 |
|
|
|
|
|
|
Corynebacterium kutscheri |
0 |
|
|
0 |
2 |
0g |
|
Bacillus piliformis |
0h |
|
0g |
0 |
6g |
5g |
|
No. of colonies |
77 |
96 |
8 |
33 |
89 |
61 |
|
a Data are percentages of colonies found to be infected. b From Lindsey et al., 1986a. c From Sparrow, 1976. d From Kraft and Meyer, 1986. e From Nakagawa et al., 1984. f From Fujiwara, 1980. g Results based on serologic methods. h Results based on absence of lesions, as determined by necropsy and histopathology. |
||||||
TABLE 5 Prevalence of Bacterial Infections in Rat Coloniesª
|
Bacterium |
United States |
United Kingdom |
West Germany and Others |
Japan |
|
|
|
|
Commercial Breedersb |
Accredited Institutionsc |
Breeders and Research Institutionsd |
SPF Breederse |
Conventional Breedersf |
Research Institutionsf |
|
Mycoplasma pulmonis |
17 |
24 |
23g |
0 |
38 |
18g |
|
Mycoplasma arthritidi |
6 |
|
|
|
|
|
|
Pseudomonas sp. |
50 |
|
|
36 |
39 |
|
|
Pasteurella pneumotropica |
33 |
58 |
|
10 |
70 |
|
|
Salmonella enteritidis |
0 |
|
|
0 |
0 |
|
|
Streptococcus moniliformis |
0 |
|
|
|
|
|
|
Corynebacterium kutscheri |
0 |
|
|
0 |
8 |
6g |
|
Bacillus piliformi |
0h |
|
41g |
0 |
17g |
47g |
|
No. of colonies |
18 |
67 |
34-40/agent |
31 |
64 |
49 |
|
a Data are percentages of colonies found to be infected. b From Lindsey et al., 1986a. c From Sparrow, 1976. d From Kraft and Meyer, 1986. e From Nakagawa et al., 1984. f From Fujiwara, 1980. g Results based on serologic methods. h Results based on absence of lesions, as determined by necropsy and histopathology. |
||||||
United States (Parker, 1980) and 58% of rat colonies in Canada (Lussier and Descoteaux, 1986), the significance of these findings appears uncertain.
The reported prevalences of bacterial infections in laboratory mouse and rat populations are given in Tables 4 and 5. Infections of Pseudomonas sp. and Pasteurella pneumotropica are quite common. The data for Mycoplasma pulmonis and Mycoplasma arthritidis are very deceiving. Most of the reported data were obtained by culture methods that are insensitive. Thus, the true prevalence of M. pulmonis is probably at least 5 to 10% greater than the percentage reported for each survey that used culture methods. The situation for M. arthritidis is far worse in that subclinical infections due to this agent are extremely difficult to demonstrate through cultures and are presently thought to be extremely common in contemporary mouse and rat populations (Cassell et al., 1986; Lindsey et al., 1986b). Similarly, the known insensitivity of culture methods for Salmonella enteritidis and Corynebacterium kutscheri
TABLE 6 Prevalence of Parasitic Infections and Infestations in Mice and Ratsa
|
Parasite |
United States: Commercial Breedersb |
United Kingdom: Accredited Breedersc |
||
|
|
Mice |
Rats |
Mice |
Rats |
|
Entamoeba muris |
51 |
24 |
27 |
28 |
|
Spironucleus muris |
42 |
17 |
29 |
28 |
|
Pinworms |
38 |
17 |
35 |
39 |
|
Tritrichomonas muris |
26 |
17 |
24 |
27 |
|
Giardia muris |
12 |
11 |
8 |
10 |
|
Hymenolepis sp. |
4 |
0 |
2 |
|
|
Eimeria sp. |
1 |
0 |
3 |
|
|
Mites |
29 |
0 |
26 |
8 |
|
Polyplax sp. |
|
0 |
|
5 |
|
No. of colonies |
77 |
18 |
96 |
67 |
|
a Data are percentages of colonies found to be infected. bFrom Lindsey et al., 1986a. cFrom Sparrow, 1976. |
||||
suggests that the data given in Tables 4 and 5 for these agents are very conservative estimates of the true prevalence. The true prevalence of Bacillus piliformis is unknown, but recent surveys by serologic methods in Japan (Fujiwara, 1980), Scandinavia (Fries, 1980), and West Germany (Kraft and Meyer, 1986) suggest that infection by this agent may be common in mice and rats.
The data from two surveys of parasitic infections and infestations in mice and rats are given in Table 6. Entamoeba muris, Spironucleus muris, pinworms, Tritrichomonas muris, Giardia muris, and mites were all fairly common.
Based on the data in Tables 2 through 6, it must be concluded that only very modest success has been achieved in the prevention of microbial infections and parasitic infections and infestations of contemporary mice and rats.