Current Capabilities to Remove or Contain Contamination
Part of the Committee’s statement of task was to discuss what is technically feasible in terms of removing a certain percentage of the total contaminant mass from the subsurface (and by association, reducing concentrations of target chemicals below drinking water standards). These questions were addressed comprehensively in the 2005 National Research Council (NRC) report that focused on source removal technologies, and previous NRC reports (NRC, 1994, 1997, 1999) provided professional judgment as to the potential effectiveness of various remedial technologies. This chapter reviews more recent data and reports on the ability of currently available remedial technologies to meet remedial action objectives for groundwater restoration. It is noted at the outset that poor design, poor application, and/or improper post-application monitoring at some sites makes evaluation of these technologies challenging, and reported performance results often appear in non-peer-reviewed documents.
Since the 2005 NRC report, technologies have evolved and more pilot-scale tests and full-scale remediation system performance data are available to help determine technology effectiveness (e.g., Johnson et al., 2009; Krembs et al., 2010; Stroo and Ward, 2010; Triplett Kingston et al., 2010a,b; Siegrist et al., 2011; Stroo et al., 2012). Technical information available for relevant case studies, however, is still often inadequate, particularly post-treatment monitoring, which severely constrains our ability to reach definitive statements regarding the effectiveness of a particular technology to meet remedial action objectives (RAOs). Critical evaluations of
remedial technologies have been performed in the last six years for thermal and in situ chemical oxidation (ISCO) applications (Triplett Kingston et al., 2009, 2010a,b; Siegrist et al., 2011). For dissolved chlorinated solvent plumes, information on remedial technologies may be found in Stroo and Ward (2010).
Based on what is known about the effectiveness of remediation technologies (as described in this chapter), the Committee concluded that regardless of the technology used, the complete removal of contaminant mass at complex sites is unlikely. Furthermore, the Committee discovered no transformational remedial technology or combination of technologies that can overcome the current challenges associated with restoring contaminated groundwater at complex sites. At these sites, some amount of residual contamination will remain in the subsurface after active remedial actions cease, requiring long-term management.
To evaluate the effectiveness of remediation, performance metrics need to be specified, along with monitoring to measure progress toward achieving the metrics. Performance metrics are discussed in several publications (e.g., see EPA, 2003; NRC, 2005; Kavanaugh and Deeb, 2011). They include metrics that are commonly used and can be reliably measured, such as (1) source mass removal and (2) change in dissolved concentrations, as well as metrics that can be measured but are not commonly used, such as (3) contaminant mass remaining, (4) change in dense nonaqueous phase liquid (DNAPL) distribution (residual versus pooled), (5) change in DNAPL composition and properties, and (6) physical, microbial, and geochemical changes. Metrics that are under development include (7) changes in contaminant mass flux distribution, (8) change in contaminant mass discharge rate downgradient from source areas, and (9) change in stable isotope ratios. Change in contaminant mass discharge in particular is receiving greater attention (see ITRC, 2010; CDM, 2009). The appropriate performance metrics for a given site are both technology and site specific.
Conceptual Model
In this report, groundwater remedial technologies are categorized based on their primary target: the contaminant source zone or the dissolved groundwater plume (see Figure 4-1). The source zone can include (1) residual DNAPL, (2) pooled DNAPL, (3) sorbed contaminants, and (4) dissolved contaminants that may have diffused into fine-grained media. All of these compartments represent long-term continuing sources of contaminants to the dissolved or aqueous plume. The dissolved plume is typically located downgradient from the source, and may be extensive (i.e., miles in length for recalcitrant chemicals). Chlorinated solvents—the primary recalcitrant organic contaminants at complex sites—can occur in four phases (organic
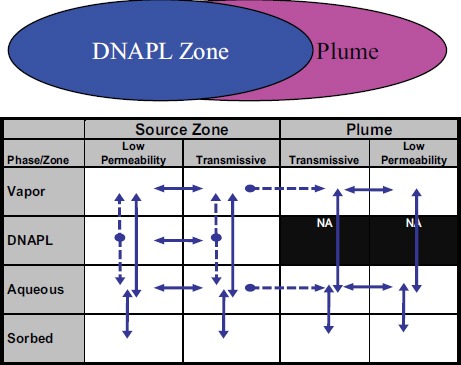
FIGURE 4-1 Conceptual model showing source zone and dissolved plume. The lower portion of the figure shows the 14-compartment model with common contaminant fluxes between compartments (solid arrows are reversible fluxes, dashed arrows are irreversible fluxes).
SOURCE: ESTCP (2011).
liquid, aqueous, solid-sorbed, and vapor) in the source zone and in three phases in the plume (there is no DNAPL phase in plumes). Each of these phases can occur in areas that can be classified as “transmissive” (mobile) or “low permeability” (immobile). This has led to a 14-compartment conceptual model depicting where contaminant mass could reside (Sale and Newell, 2011), which is discussed further in Chapter 6 of this report.
Because remedy selection and effectiveness depend, in part, on the contaminant mass distribution among phases and media (e.g., fine-grained media versus more permeable media, vadose zone versus saturated zone, DNAPL versus dissolved contaminants, etc.), a prerequisite for remediation is thorough site characterization, including the development of a conceptual site model that identifies, as much as possible, where DNAPL resides. As noted in Stroo et al. (2012), “source remediation is only as effective as the source delineation.” The technology reviews found in Triplett Kingston
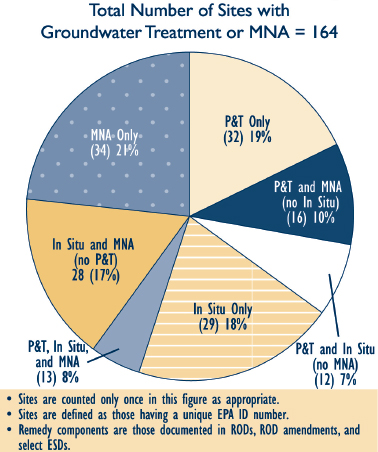
FIGURE 4-2 Sites with P&T, in situ treatment, or MNA as part of the groundwater remedy (FY 2005-2008).
SOURCE: EPA (2010a).
et al. (2009, 2010a,b) highlight the risks of inadequate site characterization: approximately two-thirds of the 14 thermal remediation case studies with sufficient data to evaluate technology performance ended up leaving mass in place because the treatment zone was smaller than the actual contaminant source zone. The reader is referred to Chapter 6 and particularly NRC (2005) for a more comprehensive discussion of site conceptual model development.
Dissolved plume remedies include pump and treat (P&T), bioremediation (including phytoremediation), permeable reactive barriers (PRBs), constructed wetlands (at the discharge point), monitored natural attenuation (MNA), and physical containment. As shown in Figure 4-2, MNA and P&T were used as groundwater remedies, either alone or in combination, at 82
TABLE 4-1 Generic Contaminant Removal or Containment Technologies and Common Applications
|
|
|
| Technology | Application |
|
|
|
| Thermal | Source Zone |
| Chemical Oxidation | Source Zone |
| Surfactant Flushing | Source Zone |
| Cosolvent Flushing | Source Zone |
| Pump & Treat | Source Zone/Plume |
| Physical Containment | Source Zone/Plume |
| Bioremediation | Source Zone/Plume |
| Permeable Reactive Barrier | Source Zone/Plume |
| Monitored Natural Attenuation | Source Zone/Plume |
|
|
|
percent of 164 Superfund facilities between 2005 and 2008. Several of the dissolved plume remedial technologies also can be applied to source zones (e.g., bioremediation, barriers, or hydraulic containment). A summary of the technologies discussed in this chapter and their most common application is provided in Table 4-1.
The goal of this chapter is to provide brief reviews of the major remedial technologies used in current remediation practice that can be applied to complex hazardous waste sites, particularly those with DNAPL source zones and/or large downgradient dissolved plumes. These reviews discuss our current knowledge regarding performance and limitations of the technologies, identify remaining gaps in knowledge, and provide case studies supporting these assessments. It is assumed that the reader is familiar with the material found in the NRC (2005) report, for which this chapter serves primarily as an update. The well-established technologies of excavation, soil vapor extraction/air sparging, and solidification/stabilization are not discussed because they have been presented in prior publications, and minimal advancements in these technologies have occurred during the past five to ten years. However, because of the potential importance of containment of source areas and plumes for long-term management, pump and treat for hydraulic containment is discussed.
In situ thermal treatment technologies, including electrical resistance heating (ERH), conductive heating, steam-based heating, radio frequency heating (RFH), and in situ soil mixing combined with steam and hot air injection, have continued to be developed and applied in the last five to ten years (see Table 4-2 and Baker and Bierschenk, 2001; Beyke and Fleming, 2005; Davis, 1998; de Percin, 1991; EPA, 1995a,b, 1999; Farouq Ali and
TABLE 4-2 Summary of Thermal Technology Applications by Technology Type (1988-2007)
| Technology | Number of Applications | Pilot-Scalea | Full-Scalea | Number Since Year 2000 |
| Steam-Based | 46 | 26 | 19 | 15 |
| Electrical Resistance Heating | 87 | 23 | 56 | 48 |
| Conduction | 26 | 12 | 14 | 17 |
| Other/Radio-Frequency | 23 | 14 | 9 | 4 |
| Total | 182 | 75 | 98 | 84 |
aSome sites have an unknown application size and thus are not included in the pilot- and full-scale count.
SOURCE: Reprinted, with permission, from Triplett Kingston (2008).
Meldau, 1979; Vinegar et al., 1999). All involve raising the temperature of the subsurface to enhance the removal of contaminants by separate-phase liquid extraction, mobilization, volatilization, and in situ destruction. Relative to other technologies, some in situ thermal treatment technologies (e.g., ERH) applications result in preferential heating and contaminant removal from lower permeability media.
A review of the application of these technologies was conducted by Triplett Kingston (2008) and Triplett Kingston et al. (2009, 2010a,b, 2012). Data and documents from 182 thermal treatment applications conducted between 1988 and 2007 were reviewed, including 87 ERH, 46 steam-based heating, and 26 conductive heating applications. The applications were categorized based on the hydrogeology of the site, using the five generalized hydrogeologic scenarios developed in NRC (2005). These include relatively homogeneous and permeable unconsolidated sediments (Scenario A), largely impermeable sediments with inter-bedded layers of higher permeability material (Scenario B), largely permeable sediments with inter-bedded lenses of low-permeability material (Scenario C), competent, but fractured bedrock (Scenario D), and weathered bedrock, limestone, sandstone (Scenario E). The majority (72 percent) of thermal remediation applications reviewed were conducted in settings containing layers of high- and low-permeability media (Scenarios B and C).
ERH applications accounted for about 50 percent of all thermal applications since 2000 and outnumbered each of the other technology applications by about a factor of 3; there also appeared to be increasing use of conductive heating and decreasing use of steam-based heating (Table 4-2). These trends are reflective of underlying technical factors controlling performance, as well as design and operating challenges and vendor avail-
ability. ERH is attractive for volatile and semi-volatile chemicals in heterogeneous settings because its ability to achieve targeted energy delivery is less sensitive to subsurface heterogeneities than steam injection, and the energy delivery and contaminant recovery systems are arguably less complex to design and operate. Conductive heating has likely increased in use because it is the only thermal technology that can achieve in situ temperatures significantly greater than the boiling point of water and that is sometimes a desired operating condition. The study did not provide remediation costs because the cost data reviewed varied greatly and were thought to be unreliable, especially given some of the suboptimal designs.
Most relevant to this report are the post-treatment performance data from in situ thermal treatment sites. Interestingly, post-treatment groundwater monitoring data that could be used to evaluate technology performance were available for only 14 of the 182 sites (8 percent) reviewed by Triplett Kingston et al. (2010a,b, 2012), reflecting the overall industry-wide lack of sufficient post-treatment monitoring at many remediation sites. Most of the sites for which adequate data were available correspond to hydrogeologic setting Scenario C, with little or no performance data available for the other settings. Table 4-3 presents the estimated order-of-magnitude reductions in concentration and mass discharge for the 14 sites that had sufficient data for the analysis. Note that mass reduction data are not provided in Table 4-3 because initial mass in place was rarely known with certainty. For six of the 14 sites (43 percent), at least a 100-fold reduction in mass discharge was observed. For five of the 14 sites, detailed analysis revealed that post-treatment groundwater concentrations ranged from about 10 to 10,000 μg/L and source zone mass discharges ranged from about 0.1 to 100 kg/y.
The following factors should be considered in interpreting the widely varying performance results shown in Table 4-3:
1. As noted by Johnson et al. (2009), the criteria or rationale used to set the duration of treatment operation was usually not documented, and “in most cases it appeared that the duration was determined prior to start-up or may have been linked to a time–temperature performance criterion (i.e., operate for two months once a target temperature is reached in situ). There was little indication that the duration of operation was selected based on mass removal-, groundwater quality-, or soil concentration-based criteria” or performance monitoring.
2. Triplett Kingston et al. (2010a,b, 2012) discovered that treatment system footprints (areas treated) were often smaller than the source zones that had been treated. The main reason for this was that the pre-treatment extent of the source zone was larger than what it was conceptualized to be at the time that the remediation system was
TABLE 4-3 Effect of Application of In Situ Thermal Technology on Dissolved Groundwater Concentrations and Mass Discharge (Flux) from the Treatment Zone to the Aquifer
| Site No. | Heating Technology | Generalized Scenario/Site | Dissolved Groundwater Concentration Reduction | Mass Discharge Reduction | ||||
| <10× | 10× | 100× | 1000× | >1000× | ||||
| 1 | ERH | Generalized Scenario A(SDC) | 10× | × | ||||
| 2 | ERH | Generalized Scenario Ba(SDC) | <10× | x | x | |||
| 3 | ERH | Generalized Scenario C | 10× | x | ||||
| 4 | ERH | Generalized Scenario Cb(SDC) | >10× to <100× | x | ||||
| 5 | ERH | Generalized Scenario Cc | <10× | x | ||||
| 6 | ERH | Generalized Scenario Cc | <10× | x | x | |||
| 7 | ERH | Generalized Scenario C | <10× | x | ||||
| 8 | ERH | Generalized Scenario C(SDC) | 10× | x | ||||
| 9 | ERH | Generalized Scenario C(SDC) | 100× | x | ||||
| 10 | ERH | Generalized Scenario C | 1000× | x | ||||
| 11 | SEE | Generalized Scenario C | 100× | x | ||||
| 12 | SEE | Generalized Scenario C | 10× | x | ||||
| 13 | SEE | Generalized Scenario Cc | 10000× | x | x | |||
| 14 | SEE | Generalized Scenario Db | <10× | x | ||||
NOTE: SDC, supplemental data collection site for this project. Site 1 = Hunter Army Airfield, Site 2 = Air Force Plant 4, Site 4 = Camp LeJeune Site 89, Site 8 = Fort Lewis EGDY Area 3, Site 9 = NAS Alameda Site 5-1.
aMass discharge assessment involved two calculations using first only the post-treatment field investigation data and then the post-treatment field investigation data supplemented with data from a set of monitoring wells that were directly in line with the field investigation transect.
bPilot application appeared to encompass the entire source zone based on documentation reviewed.
cSite used two different vertical intervals to calculate mass discharge: (1) only shallow geology and (2) shallow and deep geology.
SOURCE: Triplett Kingston et al. (2010b).
designed. This points to a need to consider uncertainty in, and verification of, source zone extent when designing thermal remediation systems. It also suggests that decision makers and designers should weigh the incremental costs of additional source zone characterization data versus the costs of a larger system footprint and costs of failure of achieving remedial goals. Triplett Kingston et al. (2010a,b) found that sampling dissolved groundwater concentration transects perpendicular to groundwater flow and immediately downgradient of a source zone was a valuable approach for verifying source zone width.
In summary, the data in Table 4-3 are indicative of state-of-the-practice performance, but are likely not indicative of the technologies’ true capabilities. Site No. 9 is probably most indicative of what thermal technologies can achieve in simple geologic settings because of the way it was designed and operated. At that site, dissolved chlorinated solvent concentrations were reduced from >10,000 µg/L to <100 µg/L levels, with final concentrations being <1 µg/L in many parts of the plume transect immediately downgradient of the source zone.
CHEMICAL TRANSFORMATION PROCESSES
Chemical transformation processes used for the treatment of both organic and inorganic contaminants have advanced significantly since 2005. There are three basic approaches to the use of abiotic chemical amendments to treating groundwater: (1) ISCO, (2) chemical reduction (discussed in the permeable reactive barriers section) using zero-valent iron (ZVI), bi-metallic reductants (BMRs), and other reductants (e.g., iron minerals such as magnetite), and (3) newer methods like the application of ISCO in permeable reactive barriers and the use of in situ generation of ozone using electrodes, which are discussed in Chapter 6. In most cases chemical transformation processes result in the formation of by-products that are either less toxic or amenable to subsequent degradation or natural attenuation. In a few cases, however, there is the potential to form undesirable and toxic by-products. Thus, multiple approaches may be required to ensure that complete detoxification can occur at the targeted site. In many cases, chemical transformation requires the injection and delivery of a reactant-containing fluid to the treatment zone, and is subject to the same limitations experienced by all flushing technologies—most notably the bypassing contaminants stored in low-permeability media.
In Situ Chemical Oxidation
ISCO relies upon the injection and activation of powerful chemical oxidants into subsurface sites that react with contaminants and oxidize them into carbon dioxide, carbon monoxide, or other substances less deleterious than the target contaminant. ISCO is relatively nonselective and capable of remediating a broad spectrum of contaminants. The technology has limitations including a finite amount of available oxidant, undesirable side reactions (e.g., oxidation of naturally occurring substances and the formation of precipitates), and the sometimes poor delivery of the oxidant into complex subsurface media. Thus, each site must be assessed for its biogeochemical complexity as well as hydrologic properties (e.g., fractured media, the presence of clay lenses, etc.) prior to implementing ISCO.
There are four oxidants routinely used in ISCO: catalyzed hydrogen peroxide (CHP or Fenton’s reagent), persulfate, permanganate, and ozone (see Table 4-4 for a summary of their application, advantages, and disadvantages). Two other oxidants have received limited usage (peroxone and percarbonate). The number of ISCO applications has steadily increased for all the major oxidants over the past decade (Krembs et al., 2010, 2011).
Siegrist et al. (2011) examined all aspects of ISCO remediation including field applications, performance, and challenges at complex sites. Highlights from that report include the fact that delivery of the oxidant can be problematic, especially if more than one ingredient is required. Additionally, there is a risk that ISCO treatment will mobilize other contaminants of concern (e.g., chromate, selenate). Other limitations depend on the specific oxidant used. For example, reduction of permanganate results in the formation of manganese oxides that can alter aquifer permeability (although paradoxically this can also benefit remediation if the manganese oxides’ high surface reactivity further attenuates contaminants through surface mediated oxidation—Loomer et al., 2010). Persulfate leads to generation of large amounts of sulfate, which can alter the biogeochemical environment of the aquifer through the generation of reduced sulfur (and even lead to an environment conducive to reductive dehalogenation). Lastly, the highly reactive nature and short half-life (∼20 minutes in water) of ozone render it difficult to deliver in a stable form.
ISCO can be an effective treatment strategy, but like most other remediation technologies its success is dependent upon the complexity of the site and the nature of the contaminant. A 1999 ESTCP report summarizing 42 pilot- and full-scale ISCO projects deemed only 19 to be “successful.” Another more recent but smaller evaluation of ISCO used at 29 chlorinated solvent sites found that mass was reduced (1) by 55 to 95 percent with a median reduction of 90 percent, and (2) by 75 to 90 percent with a median
TABLE 4-4 Chemicals Used in ISCO Applications, Including Advantages and Disadvantages
| Specific ISCO | Principal Oxidant | Pathway | Advantages | Disadvantages |
| Catalyzed Hydrogen Peroxide Propagation (Fenton’s Reaction) | Hydroxyl radical (OH•) | Fe+2 + H2O2 |
Highly non-selective oxidant, no fouling, temperature increase | Nonproductive side reactions, cost, rapidly consumed, potential for excessive heating, gas generation. |
| Permanganate | MnO4– [Mn(VII)] | MnO4– |
Somewhat non-selective, can be used in PRB (Ch. 6) | Aquifer fouling from MnO2 precipitation, density issues, toxic metals mobilization |
| Persulfate | Sulfate radical (SO4•), reactive oxygen species (ROS) | Heat activated S2O8–2 |
Somewhat non-selective, no fouling, fairly easy to deliver | Activation can be tricky (heat or add sodium hydroxide), quickly deactivates under acidic conditions, persistence in source dependent on delivery duration and groundwater flow |
| Ozone | O3 | From O2 using corona discharge ozone generator | Non-selective toward hydrocarbons, oxygenates | Decomposes to oxygen quickly, delivered as a gas, need for on-site generator, continuous inputs |
TABLE 4-5 ISCO Performance Metrics (1) for All Oxidants Organized by Contaminant Class (First Four Columns) and (2) for All Contaminants Organized by Oxidant (Last Five Columns)
| Goals | Chloroethenes | BTEX | TPH | MTBE |
| % of closed sites | 20 (n = 50) | 43 (n = 7) | 38 (n = 8) | 63 (n = 8) |
| % of sites achieving MCLs | 3 (n =105) | 0a (n = 12) | 25 (n =12) | 60 (n = 5) |
| % of sites with rebound | 72 (n = 54) | 38 (n = 8) | 43 (n = 7) | 29 (n = 7) |
| Median % total contaminant reduction in groundwater | nd | nd | nd | nd |
NOTE: Numbers in parentheses are total number of sites for each category. Not all sites had clearly stated goals for each category in Column 1. nd = not determined.
a The incidence of meeting MCLs was only entered in the affirmative after attempting to discuss the case study with the regulatory official. This percentage shown should not be interpreted to mean that ISCO has never reached MCLs at a BTEX site.
SOURCE: Adapted from Krembs et al. (2011).
reduction of 80 percent, for sites treated with Fenton’s reagent and permanganate, respectively (Stroo et al., 2012).
Krembs et al. (2010, 2011) surveyed 242 sites variously contaminated with petroleum hydrocarbons and chlorinated solvents that have used some form of ISCO (five different oxidants were evaluated). The performance results, organized by contaminant class and oxidant, are summarized in Table 4-5. Of the 242 ISCO projects, only 15 percent achieved maximum contaminant levels (MCLs), while about 39 percent were able to reach alternative concentration limits. Approximately 80 percent of the applications were successful in reducing contaminant mass; typically, fuel-related contaminants were more amenable to ISCO treatment relative to chlorinated solvents and less expensive to treat. The DNAPL sites were more likely to require treatment beyond ISCO, which increases costs. Indeed, none of the sites containing free-phase DNAPLs were able to attain MCLs and only three sites with very dilute chloroethene-contaminated plumes achieved MCLs. Ultimately Krembs et al. (2011) found that site closure was attained at 24 percent of the full-scale sites, mostly through meeting alternative concentration limits. Of these closures, 89 percent required a combination of ISCO and some other technology either post- or pre-ISCO treatment, such as SVE, excavation, etc. In examining Table 4-5, it should be remembered that peroxone was only tested at two sites, while permanganate and Fenton’s reagent were deployed at more and diverse types of sites,
| Permanganate | CHP or Fenton’s reagent | Ozone | Persulfate | Peroxone |
| 16 (n = 32) | 27 (n = 22) | 50 (n =12) | 0 (n = 4) | 50 (n = 2) |
| 0 (n = 55) | 2 (n = 45) | 31 (n =13) | 0 (n = 8) | 50 (n = 2) |
| 78 (n = 32) | 57 (n = 21) | 27 (n =11) | 50 (n = 2) | nd |
| 51 (n = 27) | 56 (n = 26) | 96 (n = 5) | 24 (n = 5) | nd |
which likely accounts for their lower success percentages. Furthermore, the hydrogeology of the sites also played an important role in determining success. For example, 33 percent of the relatively homogeneous sites (hydraulic conductivities of > 10–5 cm/s) attained MCL goals, while none of more heterogeneous sites (K < 10–5 cm/s) attained MCLs (Krembs et al., 2011).
Select ISCO field-scale applications that removed a significant amount of contaminant mass are summarized in Table 4-6. The Kings Bay Naval Base was a targeted application using Fenton’s reagent designed to achieve considerable source zone treatment so that natural attenuation could be utilized to address any remaining contaminants. Although mostly successful, the presence of iron shifted the redox conditions from sulfate- to iron-reducing, rendering natural attenuation less effective (Chapelle et al., 2005). The Nebraska Ordnance Plant is a good example of the use of permanganate to treat the chemical explosive RDX. A performance assessment demonstrated the difficulties in controlling the actual application of permanganate in the field compared to laboratory experiments. Scotch Cleaners, where a DNAPL plume was treated with permanganate, highlights the variable success rate of using permanganate, as well as the difficulties of oxidizing tetrachloroethene (PCE) that is strongly sorbed to the solid phase. LNAPL at Edwards AFB should have been amenable to ISCO, but delivery issues made remedy execution difficult. Air sparging was used to improve ISCO performance by enhancing the contact of persulfate with the target contaminants in the capillary fringe. Finally, the Pine Barrens case study
TABLE 4-6 Select Applications of ISCO Indicating Mass Reduced
| Field Site | ISCO Treatment | Contaminant | Mass Reduced | References |
| Naval Base, Kings Bay, GA | CHP (50% H2O2 + ferrous sulfate) | PCE, vinyl chloride | Variable with significant PCE and VC reductions (> 90%) | Chapelle et al., 2005 |
| Nebraska Ordnance Plant, Mead, NE | Permanganate (40% by weight) | RDX | Variable, sometimes > 70% reduction | Albano et al., 2010 |
| The Scotch Cleaners, Topeka, KS | Permanganate (3% by weight) | PCE | Variable with ~ 90% reduction, but significant rebound of PCE in places | Heseman and Hildebrandt, 2009 |
| Edwards Air Force Base, CA | Base activated persulfate (~8000 pounds) and air sparging | LNAPL | Decreases in total hydrocarbons, but not BTEX. BTEX dropped after air sparging | Siegal et al., 2009 |
| Gas Station, Pine Barrens, NJ | Ozone Sparge | BTEX and MTBE | Approximately 95% reduction in both, but re-release has contaminated the site again | Dey et al., 2002 |
is an example of using ozone to treat a site contaminated with BTEX and MTBE where, in addition to oxidation, enhanced aerobic biodegradation contributed to the success of the remedy.
The application of extraction technologies for subsurface remediation typically involves the injection of an ingredient that can mobilize the contaminant in the treatment zone, which is then extracted from the subsurface along with the delivered active ingredient. When applied appropriately, extraction technologies can be extremely effective for removing large amounts of contaminant mass from the subsurface in relatively short periods of time, but they are best suited for Scenario A sites where the injected fluid can be delivered and recovered efficiently. Bypassing of contaminants in low-permeability material will occur when using extraction technologies. Additionally, active ingredient costs and the need to manage large volumes of delivered and extracted fluids render these technologies most suitable for Scenario A source zones of limited size. Two in situ extraction technologies, surfactant flushing and cosolvent flushing, are discussed in the following sections, with emphasis on lessons learned from field-scale applications.
Surfactant Flushing
Based on the lessons learned from laboratory and pilot-scale tests conducted in the 1980s and 1990s (e.g., see summaries in AATDF, 1997; Roote, 1998), subsequent field trials of surfactant flushing showed substantial success. A number of well-controlled, field-scale tests of surfactant flushing indicate that DNAPL recoveries in the range of 60 to 70 percent can be expected, and that mass recoveries of greater than 90 percent are achievable (e.g., Hasegawa et al., 2000; Londergan et al., 2001; Ramsburg et al., 2005). For example, after flushing a DNAPL-contaminated source zone at Hill AFB OU2 with approximately 2.5 pore volumes of surfactant solution, it was estimated that 99 percent of the DNAPL mixture was recovered from the test cell at an average cost of $793/L DNAPL (Londergan et al., 2001). Representative examples of surfactant enhanced aquifer remediation (SEAR) field demonstrations, including the surfactant formulation and estimated mass recovery, are summarized in Table 4-7. Recent field applications by commercial vendors have focused on the mobilization (displacement) of light NAPLs (e.g., gasoline) using relatively low concentration surfactant formulations (< 2% wt) to minimize active ingredient costs.
Despite the mass reductions evident in Table 4-7, the use of surfactants to treat source zones has declined markedly in the past five to ten years and is linked to problems in delivering the surfactant solution to the in-
TABLE 4-7 Representative Examples of SEAR Field Demonstrations
| Field Site | Surfactant Formulation | NAPL | Amount Recovered (Estimated Mass Recovery)a | References |
| Dover Air Force Base | 3.3% Aerosol® MA + 3.3% isopropanol + 0.4% CaCl2 | PCE | 46 L (68%) | Childs et al., 2006 |
| Bachman Road Oscoda, MI | 6% Tween® 80 | PCE | 19 L (greater than 90%) | Abriola et al., 2005; Ramsburg et al., 2005 |
| Alameda Point, CA | 5% Dowfax® 8390 + 2% Aerosol® MA + 3% NaCl + 1% CaCl2 | TCA, TCE, DCA, DCE | 325 kg (97%) | Hasegawa et al., 2000 |
| Camp Lejeune Marine Corps Base | 4% Alfoterra® 145-4PO sulfate + 16% propanol + 0.2 % CaCl2 | PCE | 288 L (72%) | Delshad et al., 2000; Holzmer et al., 2000 |
| Hill Air Force Base OU1 | 3% Brij® 97 + 2.5% pentanol | Jet fuel, chlorinated solvents | 396 L (72%) | Jawitz et al., 1998, 2001 |
| Hill Air Force Base OU2 | 7.6% Aerosol® MA + 4.5% isopropanol + 0.7% NaCl | TCE, TCE, PCE, CT | 363 L (98.5%) | Londergan et al., 2001 |
| Hill Air Force Base OU1 (Cell 6) | 4.3% Dowfax® | Jet fuel, chlorinated solvents | 1.5 kg (58%, 68%)b | Knox et al., 1999 |
| Hill Air Force Base OU1 (Cell 5) | 2.2% Aerosol® OT + 2.1% Tween® 80 + 0.4% CaCl2 | Jet fuel, chlorinated solvents | 14.4 kg (42%, 97%)b | Knox et al., 1999 |
| Coast Guard Station, Traverse City, MI | 3.6% Dowfax® 8390 | PCE, jet fuel | 3.3 g PCE + 47 kg THc | Knox et al., 1997 |
| Thouin Sand Quarry, Quebec, Canada | 9.2% butanol + 9.2 % Hostapur® SAS 60 + 13.2% toluene + 13.2% d-limonene | TCE, PCE, waste oil | 532 kg (86%) | Martel et al., 1998 |
| Canadian Forces Base Borden | 2% 1:1 Rexophos® 25/97 + Alkasurf® NP10 | PCE | 67 L (69%) | Fountain et al., 1996 |
a Except when DNAPL was released intentionally, percent mass recovery values are based on estimates of the initial mass present at the site.
b The first value is based on pre- and post-treatment soil cores, while the second value is based on pre- and post-treatment partitioning interwell tracer tests (PITTs).
c Total hydrocarbons monitored but initial mass not reported.
tended target zone, and the need for subsequent extraction and treatment of the effluent waste stream (contaminant and surfactant). Injections may require Underground Injection Control permits, and extraction and treatment can be time consuming and expensive. Additionally, selection of a surfactant formulation that is both safe and effective requires laboratory and/or pilot-scale treatability tests. For example, due to their emulsifying properties, surfactants tend to disperse fine particles, which can lead to particle mobilization and pore clogging (Liu and Roy, 1995; Rao et al., 2006). Surfactants may be lost via adsorption on the solid phase and/or partitioning into NAPL, which can lead to the formation of viscous emulsions (Jain and Demond, 2002; Zimmerman et al., 1999). Low interfacial tension surfactant formations (< 1 dyne/cm) also can lead to uncontrolled mobilization of dense NAPLs (Pennell et al., 1996).
Another drawback accounting for SEAR’s decrease in popularity is that the up-front costs for active ingredients and effluent treatment systems can be substantial. To minimize surfactant costs, it is advisable to minimize the amount of active ingredient required and to consider surfactants that are used in other commercial applications, such as food products, detergents, and pharmaceuticals. For example, sorbitan ethoxylates (e.g., Tween® 80), which are used in whipped toppings and other food products, typically cost less than $2/lb (Ramsburg and Pennell, 2001). In contrast, specialty surfactants can cost $20 to $40/lb.
Cosolvent Flushing
Cosolvent (alcohol) flushing is similar to SEAR in objective, mode of action, and field application, except that cosolvents increase contaminant dissolved-phase concentrations by making the aqueous phase less polar. Several field-scale demonstrations of cosolvent flushing have been conducted, most notably at Hill Air Force Base, Dover Air Force Base, and the former Sages dry cleaner site in Jacksonville, FL (Table 4-8). Two field demonstrations of cosolvent flushing were conducted at Hill Air Force Base Operable Unit 1 (OU1), which recovered approximately 80 and 85 percent of the NAPL mass from separate 3 m × 5 m test cells, which were vertically confined by interlocking sheet pile walls (Falta et al., 1999; Rao et al., 1997). At the Sages dry cleaner site, injection of a 95 percent ethanol solution was able to achieve similar PCE mass recovery (62 to 65 percent) without test cell confinement, while downgradient aqueous PCE concentrations were reduced by up to 92 percent following cosolvent flushing (Jawitz et al., 2000). A subsequent field demonstration of cosolvent flushing was conducted at the Dover Air Force Base, where a known amount of PCE was released into a test cell that was subsequently flushed with a 70 percent
ethanol solution, recovered approximately 64 percent of initial PCE mass (Brooks et al., 2004).
Although cosolvent flushing showed initial promise as an effective strategy for NAPL source zone remediation, the technology faces a number challenges that have limited implementation beyond the cases shown in Table 4-8. First, the lower density of the concentrated cosolvent solution (70 to 90 percent active ingredient) relative to water requires careful design of injection and extraction systems. Gradient injection (increasing the concentration of cosolvent delivered over time) can minimize density over-ride effects (Rao et al., 1997), while careful placement of injection wells promotes upward migration of the miscible effluent in unconfined systems (Jawitz et al., 2000). Second, due to the relatively high concentration of active ingredient required, material costs can be substantial even though ethanol can be purchased without federal and state alcohol consumption taxes. For example, ethanol typically costs approximately $3/gallon, but can increase to $5/gallon due to market fluctuations and demands for ethanol as an alternative fuel. In addition, the use of alcohols other than ethanol (e.g., iso-propanol) to enhance performance, even at relatively low concentrations (e.g., 2 to 5 percent), can greatly increase material costs. Finally, cosolvents are highly flammable liquids and require special safety procedures, both during mixing and injection as well as during treatment or disposal of the effluent waste stream.
Some of the problems associated with cosolvent flushing are illustrated by additional work that was performed at the former Sages dry cleaner site. Following a second cosolvent flush, monitoring revealed that chlorinated ethenes and ethanol had migrated from shallow groundwater to surface water in a nearby drainage ditch. This triggered the need for additional remediation consisting of an air sparge/soil vapor extraction (AS/SVE) system. Consequently, it became necessary to remove and treat the ethanol and PCE breakdown products. The SVE system was subsequently expanded and a dewatering groundwater pump-and-treat system was initiated (Levine-Fricke-Recon, 2008). Although the AS/SVE system was eventually terminated, the dewatering system continued to operate to reduce discharge to the drainage ditch with plans to expand groundwater extraction to include intermediate groundwater (Levine-Fricke-Recon, 2008). This expansion to deeper groundwater was performed because PCE DNAPL was found at depths below the original shallow treatment zone discussed in Jawitz et al. (2000).
To the Committee’s knowledge there have been no new publications on surfactant or cosolvent flushing since about 2005. Almost all of the recent field-scale implementations that it is aware of have been performed by the company Surbec Environmental, LLC using low-IFT, low-concentration floods applied to LNAPLs (diesel/gasoline). While the low-IFT Surbec appli-
TABLE 4-8 Representative Field-Scale Demonstrations of Cosolvent Flushing
| Field Site | Co-Solvent | DNAPL | Amount Recovered (Estimated Mass Recovery) | References |
| Dover Air Force Base Test Cell | 70% Ethanol | PCE | 53 L (64%) | Brooks et al., 2004 |
| Sages Dry Cleaner, Jacksonville, FL | 95% Ethanol | PCE | 30 L (63%) | Jawitz et al., 2000 |
| Hill Air Force Base OU1 | 80% t-Butanol + 15% Hexanol | Jet fuel, chlorinated solvents | 99 mg/kga (80%) | Falta et al., 1999 |
| Hill Air Force Base OU1 | 70% Ethanol + 12% Pentanol | Jet fuel, chlorinated solvents | ~300 L (85%) | Rao et al., 1997 |
a Solid phase concentration reported, based on sum of targeted compounds.
cations are gaining traction, other surfactant/cosolvent flushing applications have received limited attention since the disappointing results obtained during full-scale implementation at the former Sages dry cleaners (cosolvent flushing) and Site 88, Marine Corps Base Camp Lejeune, NC (surfactant flushing) (Battelle and Duke Engineering, 2001), both of which necessitated additional source zone treatment.
Pump-and-treat (P&T) systems use extraction wells to remove groundwater containing dissolved contaminants. The extracted water is treated on-site and/or discharged to a publicly owned treatment works, then re-injected to the aquifer or reused for industrial or potable purposes. The design and operation of P&T systems are based on (1) hydraulic containment to prevent further plume migration, and/or (2) contaminant mass removal to restore the aquifer to drinking water conditions. These goals are not mutually exclusive, as many systems follow a hybrid approach that combines containment with limited mass removal.
As noted in the previous NRC study on P&T (NRC, 1994), this technology is effective at hydraulic containment, but less effective in removing mass. Of the 77 sites evaluated in that study, only six sites reported achieving MCLs, and more than half of the sites were candidates for very long term management. However, despite long-standing concerns regarding cost and performance with regards to contaminant mass removal, P&T remains one of the most widely applied groundwater remediation technologies, appearing in 20 to 30 percent of CERCLA groundwater decision documents, approximately the same proportion as in situ technologies (EPA, 2010b). The emphasis of this section is on containment because efficient and effective containment is a key consideration for long-term management of complex sites.
In 2000 EPA began implementing a systematic review and modification of P&T systems within Superfund, based on the Remediation System Evaluation (RSE) process developed by the U.S. Army Corps of Engineers (EPA, 2000). Over the ensuing decade, the application of this procedure to most of the approximately 75 EPA-led P&T sites has generated dozens of recommendations expected to significantly reduce O&M costs (EPA, 2008). Many of these savings are associated with more efficient operation of monitoring and above-ground treatment systems, but in some cases savings were partially offset by investments needed to increase confidence in the achievement of plume capture. In parallel with these evaluations, the EPA has produced an extensive set of guidance documents to support more cost-effective P&T design and operation (EPA, 2002, 2005a,b,c, 2007a,b,c)
and support greater confidence in the achievement of successful hydraulic containment (EPA, 2008).
P&T has several desirable attributes, including its straightforward application, a relatively long track record of operation, and the ability to achieve plume hydraulic containment with a high degree of confidence. While further improvement in the design and operation of P&T containment systems is likely to be incremental, additional work is needed to (1) refine and deploy more cost-effective above-ground treatment systems and monitoring networks, (2) improve the ability to accurately predict P&T duration, and (3) develop “greener” P&T systems that more effectively utilize renewable energy sources and perform better with respect to sustainability metrics such as energy consumption and carbon footprint (e.g., Environmental Management Support Inc., 2008).
As was discussed in NRC (1994), P&T is capable of cleaning up groundwater to health-based standards in a relatively short time for dissolved, weakly sorbing contaminants in simple geology, especially if aided by biodegradation. However, at complex sites like those considered in this report, the earlier NRC report concluded that P&T is not expected to be effective in attaining health-based standards for many decades due to the slow processes of dissolution from DNAPL, desorption, and back-diffusion from fine-grained media.
Physical containment relies on a barrier that prevents transport by groundwater flow and/or contaminant diffusion. Physical containment is not a removal technique, but prevents contaminant migration from the contaminated area and passing groundwater from interacting with the source zone. Containment requires that contaminant migration is prevented both vertically and horizontally. To prevent horizontal migration, an impermeable vertical wall is installed that surrounds the contamination source. Vertical migration is prevented by ensuring that the vertical cutoff walls are embedded in a confining layer and, if desired, a cap at the ground surface also is installed. In many applications, physical containment draws on experience derived from the design of landfills, specifically the use of low-permeability clay (e.g., bentonite or soil-bentonite) and polymer geomembrane liners. Other materials are also used including sheet pile or cement/grout. Physical containment can be used for any contaminant as long as the material used to construct the wall is chemically compatible with the contaminants. Degradation of the containment barrier by either the chemical pollutants or via natural processes will lead to failure (Jefferis, 2008).
In the Treatment Technologies for Site Cleanup: Annual Status Report (Twelfth Edition) (EPA, 2007d), the number of cover systems and vertical
walls at Superfund facilities was quantified and evaluated. For the 71 covers designed to contain source or groundwater contamination, 52 were functioning as intended. At the remaining sites, the covers had been removed, had not yet been constructed, or were too new to have reliable data. From 1982 to 2005, there were 67 installations of vertical cutoff walls at 55 facilities (or 3.6 percent of Superfund facilities). Of 16 representative containment systems evaluated (Appendix H of EPA, 2007d), 13 were functioning as intended. Of the remaining three, one had been removed, one was under construction, and one had insufficient data to evaluate its performance.
A 1998 EPA report provided design guidance and performance evaluation for subsurface engineered barriers and evaluated 36 sites (EPA, 1998). At 25 of the 36 sites, effective containment was achieved, seven sites had insufficient data, and four sites had failures (leaks). Sites reporting leakage, however, were reparable. The EPA (1998) report concludes “subsurface engineered barriers are effective containment systems for the short- and middle-term, if properly designed” and “[t]he most likely pathway for leaking of continuous subsurface barriers is in the vicinity of their keys” (i.e., their connection to the aquitard). A recent NRC review, however, concluded that available field data are insufficient to provide a robust assessment of the potential for or actual occurrence of failure in vertical barriers, particularly over times scales of 100 years or longer (NRC, 2007).
Failure assessment of physical containment systems is made difficult for two reasons. First, the vertical barrier is often installed in a manner that allows some downgradient contamination to be outside the barrier, such that downgradient detections post-barrier may be residual contamination and not representative of a failure. Second, the vertical barrier eliminates or greatly reduces groundwater flow, making groundwater velocities outside the wall so low that it could take years for a failure to reach a downgradient monitoring well only tens of feet away.
When designed, installed, and monitored properly, physical containment can be an effective technique for preventing contaminant migration. Contaminant mass is not reduced, but containment can be combined with treatment technologies as long as the treatment applied does not lead to physical damage of the containment barrier or alter it in a chemically adverse way. Various in situ techniques (ISCO, bioventing, etc.) can be performed in a contained area. Vertical barriers are often combined with low-rate pumping from inside the contained area, and hence perfect installation may not be essential. A case study of this approach is provided in Box 4-1.
BOX 4-1
Physical Containment at Former Koppers Company Wood Treating Plant, Salisbury, MD
In 1936, wood-treating operations began in Salisbury, MD; the facility closed in 1984. The site, which contained a large amount of creosote, was located adjacent to the Wicomico River. The groundwater flow is in the direction of nearby Wicomico River. The site is underlain by sand and peat containing a large, shallow DNAPL source. The DNAPL sits ∼50 feet at depth on top of a confining layer of clay and slit (type III).
Sixteen years passed between the initial site environmental assessment and implementation of the remedy, the goal of which was to contain DNAPL. The remedy consisted of (1) a barrier wall encircling 41.3 acres, (2) Keens Creek reroute, (3) planting new trees (phytoremediation), (4) a shallow hydraulic gate (with an air sparging system just downgradient), (5) in situ biological groundwater treatment, (6) wetland mitigation, (7) product (creosote) recovery, and (8) soil cover. The main component of the remedy was the barrier; other components mainly supplemented containment. The capital cost of the remedy was about $10–$11 million with an annual operation and maintenance cost of approximately $200,000.
In 2008, the barrier was found to be effective based on evaluation of (a) existing water-level data from monitoring wells inside and outside the barrier wall, and (b) tidal influences observed in monitoring wells located inside and outside the barrier wall. The successful implementation of the remedy at this site demonstrates that containment for large sources may be the most appropriate approach and that it can be combined with other technologies. It also shows that regulators are willing to accept a remedy including containment as part of the remedial plan. Containment, however, is not inexpensive, and the monitoring to ensure it is working correctly leads to recurring costs.
In the context of remediation technologies, bioremediation refers to the transformation of contaminants by microorganisms, to benign by-products. Effective bioremediation requires the presence of appropriate organisms in sufficient densities for meaningful reaction rates, along with adequate supplies of electron donors or acceptors (depending on the type of reactions desired) and nutrients to support the desired biological reactions. In addition, the geochemical environment must be controlled to ensure appropriate conditions (e.g., pH, temperature, redox potential) for optimal bacterial growth. In contrast to natural attenuation, enhanced bioremediation typically involves biostimulation and/or bioaugmentation. Biostimulation can be achieved by the delivery of electron acceptors (e.g., oxygen for aerobic metabolism), electron donors (e.g., hydrogen for reductive dehalogena-
tion), or suitable precursors that are utilized by organisms to support the biochemical transformation of the target contaminant. For example, active and passive schemes have been used to introduce oxygen into the subsurface to support the aerobic transformation processes, while hydrogen delivery methods (primarily through the injection of soluble nontoxic organic materials such as emulsified oil or molasses that serve as the microbially produced hydrogen) have been developed to enhance biological reductive dehalogenation. Bioaugmentation, which refers to the amendment of the subsurface with certain microorganisms, is used when native microbial populations are insufficient or incapable of transforming the contaminant regardless of system conditions.
Enhanced In Situ Bioremediation
Enhanced in situ bioremediation (ISB) is one of the most widely used technologies for the treatment of contaminated source zones and groundwater plumes for a variety of organic contaminants. Initially, the treatment of source zones by ISB was not considered to be feasible due to the relatively high (e.g., close to solubility level) contaminant concentrations. Laboratory studies conducted in the early 1990s indicated that biodegradation could occur in source zones contaminated with chlorinated solvents, and this bioactivity was able to enhance the rate of PCE dissolution from highly concentrated NAPL pools (Seagren et al., 1993, 1994). Subsequent studies demonstrated that the bacteria responsible for reductive dechlorination could survive in close proximity to residual TCE- and PCE-DNAPL (Cope and Hughes, 2001; Yang and McCarty, 2000, 2002). These findings served as the basis for several well-characterized pilot-scale tests (e.g., Hood et al., 2008) and full-scale remediation efforts (e.g., Wymore et al., 2006) that further demonstrated the ability of ISB to effectively treat chlorinated solvent source zones.
Under anoxic conditions (less than 0.1 to 0.5 mg/L dissolved oxygen), several bacteria (e.g., Geobacter sp. strain SZ) are able to chloro-respire PCE and TCE to form DCE, while only bacteria in the genus Dehalococcoides have been shown to obtain energy from the dechlorination of DCE and VC to ethene (Bradley and Chapelle, 2010). Due to this specificity, bioaugmentation is widely used for remediation of chlorinated solvent source zones. Several commercially available bacterial consortia are available for this purpose, including KB-1® and BDI®. In addition, an adequate supply of electron donors is necessary to support chloro-respiration, and a number of soluble (e.g., lactate) and less soluble (e.g., emulsified vegetable oil, molasses, and HRC®) electron donors are delivered to the subsurface to support reductive dechlorination. Some of the complications that can result are (1) substantially lowering of the pH, which can inhibit Dehalococ-
coides, (2) incomplete dechlorination due to competition from alternative electron acceptors (e.g., nitrate), (3) interspecies competition for hydrogen (methanogens), and (4) accumulation or presence of inhibitory compounds (Bradley and Chapelle, 2010).
Recent surveys of source zone remediation technologies reveal that ISB has been used at approximately 25 percent of the sites considered and is one of the most commonly applied in situ treatment methods along with thermal treatment and in situ chemical oxidation (NAVFAC, 2004; ESTCP, 2011). The primary reasons for this widespread adoption of ISB compared to other in situ technologies include relatively low capital costs, minimal infrastructure requirements, ability to treat a wide range of contaminants, and absence of an effluent waste stream that requires above-ground treatment and/or disposal. ISB has proven to be particularly effective for low-strength source zones (Newell et al., 2006) and was shown to have the lowest median cost ($29/yd3) when compared to other in situ technologies (McDade et al., 2005; McGuire et al., 2006). Additionally, ISB has the potential to be coupled with more aggressive remediation technologies to achieve a “polishing” or “combined remedy” approach (e.g., Ramsburg et al., 2005; Sleep et al., 2006). Performance of ISB at selected sites is summarized in Table 4-9.
While much of the above discussion focused on chlorinated solvents, the range of contaminants to which the technology can be applied is quite broad, and very few common groundwater contaminants remain that cannot be treated biologically. For example, considerable success has been achieved for a range aerobic and anaerobic biological treatment methods applied to petroleum hydrocarbons (e.g., BTEX), polyaromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and pesticides (see Stroo et al., 2012, for comprehensive listing of references summarizing in situ biological treatment methods). Nevertheless, several notable contaminants, including chloroform, and 1,4-dioxane, remain relatively recalcitrant from a bioremediation perspective. The identification and evaluation of mixed consortia and pure cultures that are capable of functioning at high contaminant concentrations and in the presence of co-contaminants is needed to further extend the applicability of ISB to source zones.
During the past five to ten years ISB has remained a widely used technology for source zone and plume treatment as costs have remained competitive compared to other treatment technologies, effectiveness has improved through more targeted applications and refinement of electron donor delivery and pH control, and successes in source zone treatment have been reported. The data shown in Table 4-9 serve to illustrate the implementation of ISB for treatment of highly contaminated chlorinated solvent source zones. Another recent review of data from 32 sites using in situ bioremediation for chlorinated solvent sites found that mass was
TABLE 4-9 Representative In Situ Bioremediation Field-Scale Demonstrations
| Field Site | ISB Treatment | Contaminant(s) | Concentration Reduction | Reference(s) |
| Tarheel Army Missile Plan | Emulsified Oil Substrate | TCE, petroleum hydrocarbons | From 1 mg/L to < 5 µg/La | ITRC, 2007 |
| Dry Cleaner Site, Portland, OR | Hydrogen Release Compound | PCE, TCE, cis-DCE | From 7,000 µg/L to 50 µg/L (PCE) after 1 yrb | ITRC, 2007 |
| Cape Canaveral Launch Complex 34 (LC 34) | Ethanol, KB-1® | TCE | From 155 mg/L to 0.2 mg/Lc | Hood et al., 2008 |
| Kelly AFB, Texas | Methanol, Acetate, KB-1® | TCE | From 1 mg/L to < 5 µg/L | Major et al., 2002 |
| Dover National Test Site | Lactate, Ethanol, KB-1® | PCE (100 L release) | NAd | ESTCP, 2007 |
| INEEL Test Area North | Lactate, Whey | TCE | From <10 mg/L to 150-550 µg/Le | Wymore et al., 2006 |
aConcentrations of VC remained above the MCL (0.15 µg/L) after one year.
bConcentrations of TCE and cis-DCE also exhibited substantial decreases followed by rebound and cis-DCE stall was apparent, all of which indicates the need for an additional electron donor treatment.
cApproximately 98% reduction in TCE mass in the upper unit based on pre- and post-treatment soil samples, consistent with average ethene concentrations measured.
dConcentrations were not provided. However, 77 kg of PCE were removed during the test, of which 15 kg were estimated to be recovered as degradation byproducts (TCE, cis-DCE, VC, ethene).
eConcentrations at injection well before and after whey injection. Concentration 25 ft downgradient from the injection well decreased to 150-400 µg/L.
reduced at these sites by 60 to 90 percent, with a median reduction of 81 percent (Stroo et al., 2012). Concentration reduction ranged from 75 to 95 percent, with a median of 91 percent. Both Stroo et al. (2012) and Table 4-9 suggest that ISB is likely to achieve substantial reductions in mass and dissolved-phase concentrations, but will have difficulty reaching drinking water standards (MCLs) in highly contaminated source zones (e.g., Cape Canaveral Launch Complex 34). As newly developed gene-based monitoring tools become more commonplace, more sophisticated and successful implementation of ISB technologies is anticipated.
Permeable reactive barriers (PRBs) are highly permeable zones comprised of material that is either abiotically reactive or that encourages the development of biological reactivity. PRBs are placed so as to capture a plume of subsurface contamination (i.e., installed to be perpendicular to the hydraulic gradient). PRBs may be a trench completely filled with reactive material or consist of a “funnel and gate” system in which impermeable barriers are used to direct flow through the reactive material. As groundwater passes through the PRB, dissolved contaminants are chemically or biologically transformed, or removed by sorption or precipitation, so that concentrations exiting the PRB are below risk-based thresholds. Because a PRB affects contaminants only as they are transported through it, its usage reflects a strategy of long-term site management, implemented because removal of source zone contamination is infeasible and/or the lack of ongoing operation and maintenance costs leads to a favorable economic evaluation. PRBs are often compared against P&T for hydraulic containment, and, depending on the lifetime of the barrier, may offer cost advantages compared to P&T (see e.g., ITRC, 2011).
PRBs used in the early 1990s relied on zero-valent iron (ZVI, i.e., iron metal or Fe0) to reduce chlorinated solvents. The application of ZVI has gone beyond the reduction of chlorinated solvents and is now being studied as a treatment technology for a variety of subsurface contaminants including chromium (Lo et al., 2006) to ordnance chemicals such as RDX (Wanaratna et al., 2006). ZVI can also be mixed into soils to achieve contaminant reduction. In addition to ZVI, various other materials (including activated carbon, zeolites, metal oxides and other minerals, and organoclays) have been used to achieve the abiotic transformation of organic compounds and transform/sequester inorganic contaminants (Thiruvenkatachari et al., 2008). A few sorbing barriers (e.g., use of granular activated carbon or ion exchange materials) have been installed that will require replacement when the capacity is exhausted. A recent DOE installation at West Valley (which consisted of 850 feet of zeolite) was designed
for a 20-year lifespan (Chamberlain et al., 2011), although future source removal activity could extend the life of the wall.
One advantage of PRBs is that the reactive material can be tailored to specific contaminants. Additionally, barriers comprised of organic material can be conducive to the development of biological consortia that are able to treat the groundwater contaminants (Davis and Patterson, 2003; ITRC, 2011). It is also possible to generate reactive zones in the subsurface by chemically altering the geologic materials themselves via in situ redox manipulation. For example, the injection of dithionite to reduce iron minerals present in the subsurface can be used to generate a reactive zone. Barriers of different chemistries may also be placed sequentially. Thus, treatment of a variety of organic and inorganic pollutants is possible with PRBs, as long as an appropriate reactive material is available or the necessary conditions for biological degradation can be generated in the PRB.
Critical needs in the design of PRBs (ITRC, 2005, 2011) are knowledge of the local hydrology, the nature and extent of the plume to be treated, the depth to a confining layer, and the concentration(s) of the contaminant(s) to be treated. Additionally, because groundwater chemistry (pH, alkalinity, hardness, other chemical species) may affect barrier performance and longevity, these parameters need to be known and the effects on the reactive media tested prior to installation. While PRBs remove contaminants from groundwater via various mechanisms, they are generally considered a containment technology because they prevent further migration of contaminants from the source zone.
Environmental Technologies, Inc. reports 156 PRBs have been installed to treat VOCs around the world (113 in the United States, nine in Canada, 19 in Europe, 14 in Japan, and two in Australia), but the company does not maintain a database on performance. The 2005 ITRC PRB guidance document complied data for 113 PRBs, and those data are summarized in Table 4-10.
For a properly designed barrier, it is likely that the ultimate cause of failure will be loss in media reactivity (unless it is regenerated or replaced) or failure to maintain the necessary conditions for biodegradation (ITRC, 2005). Thus, all barriers eventually will need to be excavated and replaced if the upstream contamination remains. While some ZVI barriers have been operating for over 15 years, currently no ZVI PRB has been taken out of operation, and their operational lifetime is unknown. For biowalls, performance will be dictated by the availability of substrates/nutrients, which will need to be replenished over time (ITRC, 2011).
TABLE 4-10 Summary of PRBs for Treatment of Contaminated Groundwater (1994–2004)
| Type of Site | |||||
| Barrier Media | Superfund | Industrial | U.S. Government | International | Total |
| Zero-valent iron | 5 | 36 | 26 | 16 | 83 |
| Non-iron reactive materials | 1 | 6 | 1 | 1 | 9 |
| Bio-barrier | 0 | 6 | 5 | 4 | 15 |
| Combination/sequenced a | 0 | 4 | 2 | 0 | 6 |
a Five of the six in this category include zero-valent iron.
SOURCE: Based on data from ITRC (2005).
Monitored natural attenuation (MNA) relies on natural processes to degrade or immobilize groundwater contaminants. For source zones, natural dissolution can result in the transfer of the contaminant from the nonaqueous liquid phase to the dissolved phase, where contaminants are amenable to additional attenuation reactions. The attenuation may be biological, abiotic, or biogenic (i.e., bacteria are required to create the necessary abiotic reagent(s)). Monitoring is required to verify that transformation or immobilization is occurring (as opposed to decreases in concentration resulting from dilution or dispersion), that the contaminants are not migrating, and that attenuation continues to occur over time. The success of remediating a contaminated site using MNA is dependent upon a number of biogeochemical variables and the types of contaminants present. These include the type, amount, and distribution of terminal electron acceptors and other important chemical species (e.g., nutrients); the composition of the contaminants; the nature of the hydrogeologic media (particle composition, organic content, hydraulic conductivity, etc.); and some knowledge of the in situ microbial fauna.
Natural attenuation is now well established for the degradation of many petroleum based compounds such as BTEX (e.g., Wiedenmeir et al., 1999) and PAHs (e.g., Neuhauser et al., 2009). Laboratory studies have demonstrated the potential for (abiotic or biological) reduction of halogenated ethenes, under conditions in which iron sulfide or green rusts are generated (Scherer et al., 2009; Butler et al., 2009; Wilson, 2010), but these studies also show the complexity of these processes. The degradation of chlorinated solvents has been shown at a site with reduced iron minerals present (see Box 4-2). With chlorinated solvents, it must be verified that reaction products that are of similar or greater toxicity than the parent compound are not produced. For other organic compounds (halogenated aromatics, oxygenated hydrocarbons) evidence for natural attenuation is still minimal. For selected inorganic pollutants, natural attenuation via biodegradation is established (Coates and Achenbach, 2004).
Because there is no hydraulic, physical, or chemical containment of the pollutants during MNA, site hydrology and the extent of contamination must be known in detail so that it can be verified that the contamination is not spreading. As described in NRC (2000), a conceptual site model must be built that characterizes the groundwater flow, accounts for temporal and spatial variability and uncertainties in the flow, delineates the contaminant source and plume, and includes terms for contaminant loss. Site characterization needs to be carefully done and can be expensive; monitoring typically occurs over longer time spans (Kennedy et al., 2006, Vaneglas et al., 2006). As discussed in Chapter 6, new chemical and microbiological
BOX 4-2
MNA at the Twin Cities Army Ammunition Plant (TCAAP) New Brighton/Arden Hills, Minnesota
The TCAAP site was built by the U.S. Army to manufacture ammunition for World War II. Starting in the late 1950s, Alliant Techsystems began manufacturing of ammunition at the site (MPCA, 2010). Degreasing operations are the cause of the major groundwater contamination. Up to 20 million pounds total of TCE and 1,1,1-TCA were disposed of on-site in a sandy glacial outwash (Mark Ferrey, personal communication, July 2, 2010).
The unsaturated zone under the disposal area has a depth of 150 feet to groundwater. The TCE and 1,1,1-TCA migrated rapidly to the groundwater, which is in the Prairie du Chien aquifer (a high-yielding fractured dolomite bedrock). The aquifer is highly permeable and manganese- or iron-reducing. The Jordan Sandstone, which lies below the Prairie du Chien, was also contaminated. While the solvent disposal area is known, no DNAPL sources have been identified in the overlying material or aquifer (Mark Ferrey, personal communication, July 2, 2010). The total length of the contaminated groundwater plume is approximately 5.5 miles. A pump-and-treat system is used to capture water, treat it with activated carbon to non-detect levels, and then use it in the municipal drinking water system. A soil-vapor extraction system was also installed in the suspected source zone (MPCA, 2010; EPA, 2010c; Mark Ferrey, personal communication, July 2, 2010).
Because the redox conditions in the plume were neither sulfate reducing or methanogenic, it was assumed that biodegradation would be minimal. After the installation of the pump-and-treat and soil vapor extraction systems, concentrations in the plumes were observed to fall, and concentrations at the containment
analyses (e.g., compound specific isotope analysis, genomics, proteomics) are being developed that can help verify that degradation of contaminants is actively occurring during MNA.
Ten years ago the consensus was that MNA was an option “for only a few types of contaminants” under certain circumstances. Over time, MNA has become a more prevalent component of remedial systems for contaminated groundwater. As reported in Superfund Remedy Report Thirteenth Edition (EPA, 2010c), the use of MNA at NPL sites steadily increased through the 1990s and is selected as a component in a remedy at approximately 30 to 40 percent of NPL facilities annually. From 2005 to 2008, 56 percent of NPL sites implementing groundwater treatment used MNA alone or as a component of the remedy. MNA is likely to be a component of the remediation plan when more aggressive treatments leave residual contamination in place.
An evaluation of 52 temporal records at 23 sites containing chlorinated
wells also decreased. Groundwater modeling predicted concentrations that were 20 to 30 times higher than those observed, suggesting that degradation of the chlorinated solvents was occurring. Investigations by scientists at the Minnesota Pollution Control Agency and EPA (Ferrey et al., 2004; Brown et al., 2007) demonstrated that abiotic reduction of chlorinated solvents (including dichloroethenes) occurred at this site due to the presence of reduced iron minerals. It was hypothesized that degradation was occurring in the groundwater, and once the plume was no longer fed by the source zones the plumes began to recede.
This was the first site to show that abiotic degradation could be an important component of monitored natural attenuation for chlorinated ethenes like TCE or DCE (which was unexpected at this site). The site altered the paradigm regarding whether and how abiotic reactions should be considered and under what prevailing redox conditions. When MNA became a component of the remediation plan (i.e., it was superimposed on the larger regulatory response), there was initial public concern from New Brighton that the Minnesota Pollution Control Agency was backing away from the goal of being protective of public health and that it would give the U.S. Army a means to argue that treatment via activated carbon at the drinking water treatment plant was no longer necessary. The agreement, however, still states that the activated carbon beds will be used until contaminants are non-detectable (at levels obviously below MCLs) in the source water. The site is currently scheduled for delisting from the NPL in 2040. Initial estimates were that cleanup would take 80 years. Models that include the observed abiotic degradation suggest that groundwater cleanup may be completed in 17 to 25 years (MPCA, 2010; EPA, 2010c; Mark Ferrey, personal communication, July 2nd, 2010).
Additional detail is available in Wilson (2010).
solvent contamination showed median decreases in contaminant concentration of 74 percent over five to 15 years (Newell et al., 2006). It should be noted, however, that these were sites where source treatment was not necessary, and it cannot be ruled out the reductions observed are due to dilution or plume migration. That said, the results are promising for MNA of chlorinated solvents at sites with low levels of contamination. A recent analysis of 35 sites with chlorinated aliphatic hydrocarbon contamination (Brauner et al., 2008) was used to develop a sustainability assessment framework for MNA, including (1) analysis of plume stability (using statistical analysis of measured concentration), and (2) estimation of remediation timeframes (mathematical modeling). The study was unable, however, to find a robust method to assess the longevity of specific degradation processes.
Given the right combination of contaminants present and site hydrology and biogeochemistry, MNA can be an effective remediation technique. However, specific chemical conditions and/or bacteria are required and the
necessary reactions must occur on time scales that are faster than contaminant transport and must be sustainable over long periods of time (NRC, 2000). Thus, MNA might ultimately achieve restoration at many sites but probably not within a timeframe of less than 100 years. Although implementation can be straightforward if an accurate conceptual site model and appropriate site conditions exist, verification of degradation with multiples lines of evidence is necessary for MNA to survive scientific, regulatory, and public scrutiny. Indeed, NRC (2000) documented that public opinion of MNA is decidedly wary, with many communities considering it a “do nothing approach.”
It is now widely recognized that even successful application of remedial technologies will not completely remove all of the contaminant mass from most DNAPL source zones. In fact, aggressive source zone treatments are likely to increase the mobility and distribution of the residual mass or stable pools, which may lead to increased aqueous phase concentrations in the short term. As a result, attention has shifted from developing the most effective stand-alone single technology to the development and testing of complementary in situ remediation technologies that can be combined, at the same time (in parallel) or sequentially (in series) to more efficiently treat contaminant source zones (Amos et al., 2007; Christ et al., 2005; Costanza et al., 2009; Friis et al., 2006; Ramsburg et al., 2004). In a sequential or “treatment train” approach, an aggressive in situ treatment technology, such as electrical resistive heating or surfactant flushing, is used to remove a large fraction of contaminant mass in a relatively short timeframe, while a second “polishing” technology such as microbial reductive dechlorination is then applied to remove or detoxify the remaining contaminant mass. Such sequential remediation strategies have the potential to take advantage of efficient mass removal achieved by aggressive treatment technologies, while addressing limitations associated with an individual technology (e.g., flow bypassing) that lead to incomplete mass removal. When designing a combined remedy, it must be kept in mind that physical-chemical treatments may alter geochemical conditions and microbial ecology, which could be either detrimental (e.g., aquifer clogging, reduced microbial diversity) or beneficial (e.g., enhanced electron donor availability) to the overall secondary remediation process (Christ et al., 2005; Stroo et al., 2003).
There are several examples of combined technologies relevant to complex sites contaminated with DNAPLs. The combination of thermal treatment and bioremediation has been recently demonstrated (Costanza et al., 2009; Friis et al., 2005). In this application, ERH was used both for source removal and to release electron donors to stimulate bioremediation. (It
should be noted that in regions where the temperature exceeded 50°C for prolonged periods of time, bioaugmentation may be required to achieve meaningful levels of microbial reductive dechlorination; Friis et al., 2006). Another example involves the use of surfactants and surfactant + cosolvent formulations to enhance ISCO. The primary advantages of this approach are increased contaminant availability and stabilization of the oxidant and catalyst during delivery. For example, a patent (USPTO 7,976,421 B2) was recently issued for surfactant enhanced in situ chemical oxidation (S-ISCO®); however, this technology has received limited testing.
Third, pilot- and field-scale trials conducted at former dry cleaning facilities indicate that surfactants and cosolvents can enhance biological reductive dechlorination to treat residual contaminants (Ramakrishnan et al., 2005; Ramsburg et al., 2004). Post-treatment monitoring of the Bachman Road site, which was flushed with Tween® 80, provided evidence of stimulated microbial reductive dechlorination (see Box 4-3). Within the treated PCE-DNAPL source zone, residual Tween 80 provided suitable electron donors that stimulated native microbial dechlorination activity (Ramsburg et al., 2004). These findings were supported by laboratory studies that further defined the effects of Tween 80 on reductive dechlorination (Amos et al., 2007). When combining surfactant flushing and in situ bioremediation, selection of compatible surfactants and appropriate concentrations is critical due to potential surfactant toxicity or inhibition toward microbial populations relevant to the desired degradation pathway (e.g., McGuire and Hughes, 2003; Yeh et al., 1999). Christ et al. (2005) performed a modeling analysis of source zone treatments, demonstrating that bioremediation alone provides minimal benefits when compared to natural gradient dissolution, while aggressive treatment of the source zone with surfactants followed by bioremediation dramatically reduced source longevity by several orders of magnitude (see Box 4-3).
In the Committee’s opinion, combined remedy approaches offer substantial opportunities to reduce the costs associated with the remediation of complex sites. For example, using a combined remedy can capitalize on the beneficial aspects of specific technologies, such as source zone flushing (e.g., steam, surfactant) coupled with ERH to address contamination existing in high- and low-permeability media, respectively. In some cases, it is appropriate to develop a combined remedy at the initiation of a remedial action (e.g., surfactant-enhanced in situ chemical oxidation or thermal treatment followed by bioremediation). When the combined remedy involves aggressive source removal activity followed by bioremediation or monitored natural attenuation for the residual/dilute contamination, it is critical to have prior knowledge of the biogeochemical environment and the potentially important reactive pathways for the target contaminants. More often, combining remedies is done sequentially at sites where the initial rem-
BOX 4-3
Combined Remedies at the Bachman Road Dry Cleaner Site
In surfactant flushing applications, some fraction of the initial contaminant mass and the introduced surfactant is likely to remain in the subsurface following source treatment. Long-term monitoring of the Bachman Road site, which was flushed with a biodegradable, food-grade, nonionic surfactant (Tween® 80), provided evidence of stimulated microbial reductive dechlorination (Figure 4-3a). Within the treated PCE-DNAPL source zone, fermentation of residual Tween 80, detected at concentrations of 50 to 2,750 mg/L 450 days after SEAR, provided suitable electron donors(s) that stimulated native microbial dechlorination activity in the oligotrophic aquifer (Ramsburg et al., 2004). These findings were complemented by laboratory-based studies that further demonstrated the ability of low levels (< 1,000 mg/L) of Tween® 80 to support reductive dechlorination of chlorinated ethenes (Amos et al., 2007).
To further evaluate the potential benefits of surfactant flushing coupled with subsequent bioremediation relative to bioremediation or natural gradient dissolution, Christ et al. (2005) performed a number of numerical simulations based on the conditions observed at the Bachman Road site. Results of the model predictions clearly demonstrate that bioremediation alone provides only minimal benefits when compared to natural gradient dissolution, while aggressive treatment of the source zone with SEAR followed by bioremediation dramatically increased the rate of mass removal and reduced source longevity by several orders of magnitude (Figure 4-3b). These findings clearly demonstrate the potential benefits of coupling aggressive source zone mass removal technologies with compatible bioremediation processes.
FIGURE 4-3 (A) Plan view diagram of the Bachman Road site source zone and downgradient PCE-contaminated groundwater plume. SOURCE: Reprinted, with permission, from Abriola et al. (2005) ©2005 by the American Chemical Society. (B) Percent DNAPL mass remaining as a function of time for three alternative remediation strategies: natural gradient dissolution alone; bioremediation alone; and SEAR (4% Tween® 80) followed by bioremediation. SOURCE: Reprinted, with permission, from Christ et al. (2005) ©2005 by Environmental Health Perspectives.
edy has reached an asymptote (in terms of performance) prior to reaching cleanup goals. In such cases, there can be substantial cost savings in transitioning to less aggressive and less expensive technologies (see Chapter 7 for further discussion of this transition). Whether planned from the beginning
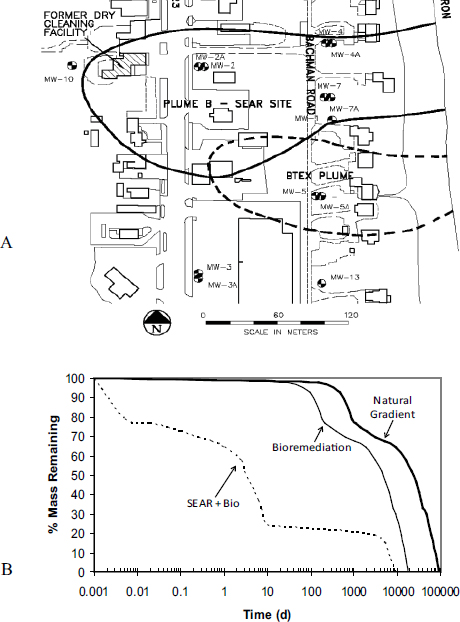
or turned to after initial remedy failure, the design and implementation of combined remedies should be undertaken on a case-by-case basis. In practice, selection of combined remedies is likely to require additional technical expertise, field sampling and analysis, and laboratory-scale treatability tests to ensure that the combination is feasible and yields added value.
CONCLUSIONS AND RECOMMENDATIONS
Over the last two decades, remedial technologies have matured and evolved, especially in the area of DNAPL source-zone remediation. Indeed, development of the technologies discussed in this chapter has advanced to the accepted-use stage (although many of the technology applications have been at simple sites or only a small portion of a complex site). Summaries of the effectiveness of source zone and plume technologies are provided in Tables 4-11 and 4-12, respectively, drawing on results discussed in the preceding sections.
Given Tables 4-11 and 4-12 and the best professional judgment of the Committee, the capabilities of the technologies described in this chapter are often not sufficient to meet the conventional objective of meeting MCLs at complex sites, such that contamination is likely to remain in place following treatment for a large number of complex sites. That is, significant technical limitations persist that make achievement of MCLs throughout the aquifer unlikely at most complex groundwater sites for many decades. Furthermore, future improvements in these technologies are likely to be incremental, such that long-term monitoring and stewardship at sites with groundwater contamination should be expected.
The Committee could identify only limited data upon which to base a scientifically supportable comparison of remedial technology performance for the technologies reviewed in this chapter. There have been a few well-studied demonstration projects and lab-scale research studies, but adequate performance documentation generated throughout the remedial history at sites either is not available or does not exist for the majority of completed remediation efforts. This has hindered attempts to perform empirical analyses of technology performance and how that relates to design parameters, operating conditions, monitoring and optimization plans, and site characteristics. Furthermore, poor design, poor application, and/or poor post-application monitoring at typical (i.e., non-research or demonstration) sites makes determination of the best practicably achievable performance difficult.
There is a clear need for publicly accessible databases that could be used to compare the performance of remedial technologies at complex sites (performance data could be concentration reduction, mass discharge reduction, cost, time to attain drinking water standards, etc.). The Committee envisions a database with much more comprehensive performance data than is found, for example, in the CERCLIS database of Superfund facilities. To ensure that data from different sites can be pooled to increase the statistical power of the database, a standardized technical protocol regarding data collection and analysis would be needed, although it goes beyond
Table 4-11 Source Zone Technology Summaries
| Technology | Performance | Comments |
| Thermal | <10X to 100,000X concentration and flux reductiona 95 to 99+ percent mass reductionb |
Can be effective in heterogeneous media; potentially high energy consumption; limited number of vendors to perform work |
| In Situ Chemical Oxidation | 55 to 90 percent mass reductionb | Most applications have been at small sites in permeable media; applications to DNAPL are very challenging (potential for DNAPL mobilization and contaminant bypassing); side reactions could render ISCO less effective |
| Surfactant Flushing | 65 to 90+ percent mass recoveryc | Can bypass contaminants in heterogeneous media; risk of uncontrolled DNAPL mobilization and migration; low IFT formulations suitable for LNAPL recovery |
| Cosolvent Flushing | 65 to 85 percent mass recoveryc | Can bypass contaminants in heterogeneous media; risk of uncontrolled DNAPL mobilization and migration; requires large volumes of cosolvents thereby driving up costs |
| In Situ Bioremediation | 60 to 90 percent mass reductionb | Problematic conditions include pooled DNAPL, the potential for high methane levels, and groundwater velocity <10 ft/y or >10 ft/d; potential for biofouling and metals solubilization |
Note: The summary table includes only those technologies for which significant new performance information has become available since NRC (2005). For complete descriptions of contaminant source remediation technologies, see NRC (2005).
bFrom Stroo et al. (2012).
cFrom Tables 4-7 and 4-8. Mass recovery percentages should be interpreted with caution because initial mass in place is uncertain.
the scope of this report to provide the details of such a protocol. Federal agencies with nationwide responsibility for complex sites (EPA, DoD, DOE) should take the lead on developing such databases.
Additional independent reviews of source zone technologies are needed to summarize their performance under a wide range of site characteristics. Since NRC (2005), only thermal and ISCO technologies have undergone a thorough, independent review. Other source zone technologies should also be reviewed by an independent scientific group (e.g., SERDP/ESTCP, ITRC,
Table 4-12 Plume Technology Summaries
| Technology | Performance | Comments |
| Pump & Treat | Containment reduces or eliminates downgradient mass flux; some mass removal achieved | Assessing capture can require extensive monitoring; long-term management required with associated operation-and-maintenance costs; extensive guidance available; technology is robust and flexible; treated water can be used as resource |
| Physical Containment | Can reduce or eliminate downgradient mass flux | Needs natural low-permeability bottom; long-term monitoring and maintenance required; water management (and possible treatment) inside the containment area likely required |
| Permeable Reactive Barrier | Containment reduces or eliminates downgradient mass flux; some mass removal achieved | Usually needs natural low-permeability bottom; treatment occurs in the subsurface; treatment is passive; monitoring can be focused; barrier replacement eventually required |
| Monitored Natural Attenuation | Significant mass reduction can be achieved, reducing mass flux downgradient | Often considered a polishing step in treatment train; can require extensive long-term monitoring to ensure requisite biogeochemical conditions persist |
or ASTM). Such reviews should include a description of the state of the practice, performance metrics, and sustainability information of each type of remedial technology so that there is a trusted source of information for use in the remedial investigation/feasibility study process and optimization evaluations.
Research is needed on how to better combine existing or new remediation technologies to address complex contaminated sites. There is the potential at most complex sites to combine multiple technologies in space and time to cost-effectively remove/treat contamination in both the source zone and the downgradient dissolved plume. However, additional research is needed to examine combinations of in situ remediation technologies to optimize removal and cost effectiveness.
AATDF (Advanced Applied Technology Demonstration Facility). 1997. Technology Practices Manual for Surfactants and Cosolvents. Houston, TX: AATDF.
Abriola, L. M., C. D. Drummond, E. J. Hahn, T. C. G. Kibbey, L. D. Lemke, K. D. Pennell, C. A. Ramsburg, and K. M. Rathfelder. 2005. A pilot-scale demonstration of surfactant-enhanced PCE solubilization at the Bachman Road site (1) Site characterization and test design. Environmental Science & Technology 39:1778-1790.
Albano, J., S. D. Comfort, V. Zlotnik, T. Halihan, M. Burbach, C. Chokejaroenrat, and W. Clayton. 2010. In situ chemical oxidation of rdx-contaminated groundwater with permanganate at the Nebraska Ordnance Plant. Ground Water Monitoring & Remediation 30(3):96-106.
Amos, B. K., R. C. Daprato, J. B. Hughes, K. D. Pennell, and F. E. Loffler. 2007. Effects of the nonionic surfactant Tween 80 on microbial reductive dechlorination of chlorinated ethenes. Environmental Science & Technology 41:1710-1716.
Baker, R. S., and J. M. Bierschenk. 2001. In-Situ Thermal Destruction Makes Stringent Soil and Sediment Cleanup Goals Attainable. Proceedings of the Fourth Tri-Service Environmental Technology Symposium, San Diego, CA. June 18-20, 2001.
Battelle and Duke Engineering & Services. 2001. Final Technical Report for Surfactant-Enhanced DNAPL Removal at Site 88 Marine Corps Base Camp Lejeune, North Carolina. Prepared for Naval Facilities Engineering Service Center, Post Hueneme, California.
Beyke, G., and D. Fleming. 2005. In situ thermal remediation of DNAPL and LNAPL using electrical resistance heating. Remediation Summer:5-22.
Bradley, P. M., and F. H. Chapelle. 2010. Biodegradation of chlorinated ethenes. Pp. 39-67 in In Situ Remediation of Chlorinated Solvent Plumes. H. F. Stroo and C. H. Ward, Eds. Springer. New York.
Brauner, J. S., D. C. Downey, and R. Miller. 2008. Using advanced analysis approaches to complete long-term evaluations of natural attenuation processes on the remediation of dissolved chlorinated solvent contamination. Final report for the Strategic Environmental Research and Development Program.
Brooks, M. C., M. D. Annable, P. S. C. Rao, K. Hatfield, J. W. Jawitz, W. R. Wise, A. L. Wood, and C. G. Enfield. 2004. Controlled release, blind test of DNAPL remediation by ethanol flushing. Journal of Contaminant Hydrology 69:281-297.
Brown, R. A., J. T. Wilson, and M. Ferrey. 2007. Monitored Natural Attenuation Forum: The case for abiotic MNA. Remediation Journal 17:127-137.
Butler, E. C., Y. Dong, X. Liang, T. Kuder, R. P. Philp, and L. R. Krumholz. 2009. Abiotic reductive dechlorination of tetrachloroethylene and trichloroethylene in anaerobic environments. Final report for the Strategic Environmental Research and Development Program.
CDM. 2009. Final Focused Feasibility Study, Well 12A Superfund Site, Tacoma, Washington, EPA Region X. http://www.epa.gov/region10/pdf/sites/cb-stc/well12a_final_ffs_apr2009.pdf.
Chamberlain, J., L. Michalczak, and S. Seneca. 2011. Design and installation of a permeable treatment wall at the West Valley Demonstration Project to mitigate expansion of Strontium-90 contaminated groundwater. Waste Management 2011 Conference, Feb. 27–March 3, 2011.
Chapelle, F. H., P. M. Bradley, and C. C. Casey. 2005. Behavior of a chlorinated ethene plume following source-area treatment with Fenton’s Reagent. Ground Water Monitoring & Remediation 25(2):131-141.
Childs, J., E. Acosta, M. D. Annable, M. C. Brooks, C. G. Enfield, J. H. Harwell, M. Hasegawa, R. C. Knox, P. S. C. Rao, D. A. Sabatini, B. Shiau, E. Szekeres, and A. L. Wood. 2006. Field demonstration of surfactant-enhanced solubilization of DNAPL at Dover Air Force Base, Delaware. Journal of Contaminant Hydrology 82:1-22.
Christ, J. A., C. A. Ramsburg, L. M. Abriola, K. D. Pennell, and F. E. Löffler. 2005. Coupling aggressive mass removal with microbial reductive dechlorination for remediation of DNAPL source zones: A review and assessment. Environmental Health Perspectives 113(4):465-477. DOI: 10.1289/ehp.6932.
Coates, J. D., and L. A. Achenbach. 2004. Microbial perchlorate reduction: Rocket-fuelled metabolism. Nature Reviews Microbiology 2:569-580.
Cope, N., and J. B. Hughes. 2001. Biologically-enhanced removal of PCE from NAPL source zones. Environmental Science & Technology 35(10):2014-2020.
Costanza, J., K. E. Fletcher, F. E. Loffler, and K. D. Pennell. 2009. Fate of TCE in heated Fort Lewis soil. Environmental Science & Technology 43:909-914.
Davis, E. L. 1998. Steam Injection for Soil and Aquifer Remediation. Ground Water Issue No. EPA 540-S-97-505. Washington, DC: EPA.
Davis, G. B., and B. M. Patterson. 2003. Developments in permeable reactive barrier technology. Pp. 205-226 in Bioremediation: A Critical Review. I. M. Head, L Singleton, and M. G. Milner, eds. Horizon Scientific Press.
De Percin, P. R. 1991. Demonstration of in situ steam and hot-air stripping technology. Journal of Air and Waste Management 41(6):873-877.
Delshad, M., G. A. Pope, L. Yeh, and F. J. Holzmer. 2000. Design of the surfactant flood at Camp Lejeune. Second International Conference on Remediation of Chlorinated and Recalcitrant Compounds. Monterey, CA, May 22-25, 2000. Pp. 203-210.
Dey, J. C., P. Rosenwinkel, and K. Wheeler. 2002. In situ remediation of MTBE utilizing ozone. Remediation Journal 13(1):77-85.
Environmental Management Support, Inc. 2008. Energy Consumption and Carbon Dioxide Emissions at Superfund Cleanups. Draft report prepared for EPA Office of Superfund Remediation and Technology Innovation, Washington, D.C., EPA Contract EP W-07-037.
EPA (U.S. Environmental Protection Agency). 1995a. KAI Radio Frequency Heating Technology. EPA 540-R-94-528a. Washington, DC: EPA Government Printing Office.
EPA. 1995b. IITRI Radio Frequency Heating Technology. EPA 540-R-94-527a. Washington, DC: EPA Government Printing Office.
EPA. 1998. Evaluation of Subsurface Engineered Barriers at Waste Sites. EPA 542-R-98-005. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 1999. Cost and Performance Report: Six Phase Heating (SPH) at a Former Manufacturing Facility, Skokie Illinois. Washington, DC: EPA Office of Solid Waste and Emergency Response, Technical Innovation Office.
EPA. 2000. Superfund Reform Strategy, Implementation Memorandum: Optimization of fund-lead ground water pump and treat (P&T) systems. OSWER 9283.1-13. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2002. Elements for Effective Management of Operating Pump and Treat Systems. EPA 542-R-02-009. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2003. The DNAPL Remediation Challenge: Is There a Case for Source Depletion? EPA/600/R-03/143. Ada, OK: EPA NRMRL.
EPA. 2005a. Cost-Effective Design of Pump and Treat Systems. EPA 542-R-05-008. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2005b. Effective Contracting Approaches for Operating Pump and Treat Systems. EPA 542-R-05-009. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2005c. O&M Report Template for Ground Water Remedies (with Emphasis on Pump and Treat Systems). EPA 542-R-05-010. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2007a. Options for Discharging Treated Water from Pump and Treat Systems. EPA 542-R-07-006. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2007b. Optimization Strategies for Long-Term Ground Water Remedies (with Particular Emphasis on Pump and Treat Systems). EPA 542-R-07-007. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2007c. A Cost Comparison Framework for Use in Optimizing Groundwater Pump and Treat Systems. EPA 542-R-07-005. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2007d. Treatment Technologies for Site Cleanup: Annual Status Report (Twelfth Edition). Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2008. Ground Water Remedy Optimization Progress Report: 2006–2007. EPA 540/R-08/004. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2010a. Superfund Remedy Report, Thirteenth Edition. EPA-542-R-10-004. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2010b. O&M Report Template for Ground Water Remedies (with Emphasis on Pump and Treat Systems). EPA 542-R-05-010. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2010c. Summary of New Brighton/Arden Hills/TCAAP. http://www.epa.gov/region5superfund/npl/minnesota/MN7213820908.htm.
ESTCP (Environmental Security Technology Certification Program). 1999. Technology Status Review In Situ Oxidation. Washington, DC: ESTCP.
ESTCP. 2007. Biodegradation of Dense Non-Aqueous Phase Liquids (DNAPLS) through Bioaugmentation of Source Areas—Dover National Test Site, Dover, Delaware. ESTCP Project ER-0008, 101 pp.
ESTCP. 2011. In Situ Chemical Oxidation for Groundwater Remediation—Technology Practices Manual ESTCP Project ER-200623. Washington, DC: ESTCP.
Falta, R. W., C. M. Lee, S. E. Brame, J. T. Coates, C. Wright, A. L. Wood, and C. G. Enfield. 1999. Field test of high molecular weight alcohol flushing for subsurface nonaqueous phase liquid remediation. Water Resources Research 35(7):2095-2108.
Farouq Ali, S. M., and R. F. Meldau. 1979. Current steamflood technology. Journal of Petroleum Technology 31(10):1332-1342.
Ferrey, M. L., R. T. Wilkin, R. G. Ford, and J. T. Wilson. 2004. Nonbiological removal of cis-dichloroethylene and 1,1-dichloroethylene in aquifer sediment containing magnetite. Environmental Science & Technology 38:1746-1752.
Fountain, J. C., R. C. Starr, T. Middleton, M. Beikirch, C. Taylor, and D. Hodge. 1996. A controlled field test of surfactant-enhanced aquifer remediation. Ground Water 34(5): 910-916.
Friis, A. K., G. Heron, H. J. Albrechtsen, K. S. Udell, and P. L. Bjerg. 2006. Anaerobic dechlorination and redox activities after full-scale electrical resistance heating (ERH) of a TCE-contaminated aquifer. Journal of Contaminant Hydrology 88:219-234.
Friis, A. K., H. J. Albrechtsen, G. Heron, and P. L. Bjerg. 2005. Redox processes and release of organic matter after thermal treatment of a TCE-contaminated aquifer. Environmental Science & Technology 39:5787-5795.
Hasegawa, M. H., B.-J. Shiau, D. A. Sabatini, R. C. Knox, J. H. Harwell, R. Lago, and L. Yeh. 2000. Surfactant enhanced subsurface remediation of DNAPLs at the Former Naval Air Station Alameda, California. Pp. 219-226 in Treating Dense Nonaqueous-Phase Liquids (DNAPLs): Remediation of Chlorinated and Recalcitrant Compounds. G. B. Wickramanayake et al. (eds.). Columbus, OH: Battelle Press.
Hesemann, J. R., and M. Hildebrandt. 2009. Successful unsaturated zone treatment of PCE with sodium permanganate. Remediation Journal 19(2):37-48.
Holzmer, F. J., G. A. Pope, and L. Yeh. 2000. Surfactant-enhanced aquifer remediation of PCE-DNAPL in low-permeability sand. Second International Conference on Remediation of Chlorinated and Recalcitrant Compounds; Monterey, CA. May, 22-25, 2000. Pp. 187-193.
Hood, E. D., D. W. Major, J. W. Quinn, W.-S. Yoon, A. Gavaskar, and E. A. Edwards. 2008. Demonstration of enhanced bioremediation in a TCE source area at Launch Complex 34, Cape Canaveral Air Force Station. Ground Water Monitoring & Remediation 28(2):98-107.
ITRC (Interstate Technology and Regulatory Council). 2005. Permeable Reactive Barriers: Lessons Learned/New Directions. PRB-4. Washington, DC: ITRC Permeable Reactive Barriers Team. http://www.itrcweb.org/documents/prb-4.pdf.
ITRC. 2007. The Vapor Intrusion Pathway: A Practical Guideline. Washington, DC: ITRC. http://www.itrcweb.org/documents/VI-1.pdf.
IRTC. 2010. Use and Measurement of Mass Flux and Mass Discharge. Washington, DC: ITRC. http://www.itrcweb.org/Documents/MASSFLUX1.pdf.
ITRC. 2011. Permeable Reactive Barrier: Technology Update. PRB-5. Washington, DC: ITRC PRB Technology Update Team.
Jain, V., and A. H. Demond. 2002. Conductivity reduction due to emulsification during surfactant enhanced-aquifer remediation. 1. Emulsion transport. Environmental Science & Technology 36(24):5426-5433. Jawitz, J. W., M. D. Annable, P. S. C. Rao, and R. D. Rhue. 1998. Field implementation of a Winsor type I surfactant/alcohol mixture for in situ solubilization of a complex LNAPL as a single phase microemulsion. Environmental Science & Technology 32(4):523-530.
Jawitz, J. W., R. K. Sillan, M. D. Annable, P. S. C. Rao, and K. Warner. 2000. In situ alcohol flushing of a DNAPL source zone at a dry cleaner site. Environmental Science & Technology 34:3722-3729.
Jawitz, J. W., M. D. Annable, P. S. C. Rao, R. D. Rhue. 2001. Evaluation of remediation performance and cost for field-scale single-phase microemulsion (SPME) flushing. Journal of Environmental Science Health A36:1437-1450.
Jefferis, S. A. 2008. Reactive transport in cut-off walls and implications for wall durability. Geotechnical Special Publication 177(GeoCongress 2008):652-659.
Johnson, P. J., P. Dahlen, J. Triplett Kingston, E. Foote, and S. Williams. 2009. State-of-the-Practice Overview: Critical Evaluation of State-of-the-Art In Situ Thermal Treatment Technologies for DNAPL Source Zone Treatment. EXTCP Project ER-0312. May 2009.
Kavanaugh, M., and R. Deeb. 2011. Diagnostic Tools For Performance Evaluation of Innovative In-Situ Remediation Technologies at Chlorinated Solvent-Contaminated Sites. ESTCP Project ER-200318. Washington, DC: ESTCP.
Kennedy, L. G., J. W. Everett, and J. Gonzales. 2006. Assessment of biogeochemical natural attenuation and treatment of chlorinated solvents, Altus Air Force Base, Altus, Oklahoma. Journal of Contaminant Hydrology 83(3-4):221-236.
Knox, R. C., D. A. Sabatini, J. H. Harwell, R. E. Brown, C. C. West, F. Blaha, and C. Griffin. 1997. Surfactant remediation field demonstration using a vertical circulation well. Ground Water 35(6):948-953.
Knox, R. C., B. J. Shau, D. A. Sabatini, and J. H. Harwell. 1999. Field demonstration studies of surfactant-enhanced solubilization and mobilization at Hill Air Force Base, Utah. In Innovative Subsurface Remediation. Brusseau M. L., D. A. Sabatini, J. S. Gierke, M. D. Annable, eds. ACS Symposium Series 725, American Chemical Society, Washington, DC.
Krembs, F. J., R. L. Siegrist, M. L. Crimi, R. F. Furrer, and B. G. Petri. 2010. ISCO for groundwater remediation: Analysis of field applications and performance. Ground Water Monitoring & Remediation 30(4):42-53.
Krembs, F. J., W. S. Clayton, and M. C. Marley. 2011. Evaluation of ISCO field applications and performance. Pp. 319-353 in In Situ Chemical Oxidation for Groundwater Remediation. Siegrist, R. L., M. Crimi, and T. J. Simpkin, eds. New York: Springer Science + Business Media, LLC. DOI: 10.1007/978-1-4419-7826-4.
Levine-Fricke-Recon. 2008. Cosolvent Flushing Pilot Test Report, Former Sages Dry Cleaner December 4, 1998, LFR Project Number: 6006.10-08.
Liu, M. W., and D. Roy. 1995. Surfactant-induced interactions and hydraulic conductivity changes in soil. Waste Management 15(7):463-470.
Lo, I. M., C. S. Lam, and K. C. Lai. 2006. Hardness and carbonate effects on the reactivity of zero-valent iron for Cr(VI) removal. Water Research 40(3):595-605.
Londergan, J. T., H. W. Meinardus, P. E. Mariner, R. E. Jackson, C. L. Brown, V. Dwarakanath, G. A. Pope, J. S. Ginn, and S. Taffinder. 2001. DNAPL removal from a heterogeneous alluvial aquifer by surfactant-enhanced aquifer remediation. Ground Water Monitoring & Remediation 21:57-67.
Loomer, D. B., T. A. Al, V. J. Banks, B. L. Parker, and K. U. Mayer. 2010. Manganese valence in oxides formed from in situ chemical oxidation of TCE by KMnO4. Environmental Science & Technology 44(15):5934-5939.
Major, D. W., M. M. McMaster, E. E. Cox, E. A. Edwards, S. M. Dworatzek, E. R. Hendrickson, M. G. Starr, J. A. Payne, and L. W. Buonamici. 2002. Field demonstration of successful bioaugmentation to achieve dechlorination of tetrachloroethene to ethene. Environmental Science & Technology 36(23):5106-5116.
Martel R., P. J. Gelinas, and L. Saumure. 1998. Aquifer washing by micellar solutions: 3. Field test at the Thouin Sand Pit (L’ Assomption, Quebec, Canada). Journal of Contaminant Hydrology 30:33-48.
McDade, J. M., T. M. McGuire, and C. J. Newell. 2005. Analysis of DNAPL source-depletion costs at 36 field sites. Remediation Journal 15(2):9-18.
McGuire, T., and J. B. Hughes. 2003. Effects of surfactants on the dechlorination of chlorinated ethenes. Environmental Toxicology and Chemistry 22(11):2630-2638.
McGuire, T. M., J. M. McDade, and C. J. Newell. 2006. Performance of DNAPL source depletion technologies at 59 chlorinated solvent-impacted sites. Ground Water Monitoring & Remediation 26(1):73-84.
MPCA. 2010. Twin Cities Army Ammunition Plant (TCAAP) Superfund Site. Minnesota Pollution Control Agency. http://www.pca.state.mn.us/index.php/waste/waste-and-cleanup/cleanup-programs-and-topics/topics/remediation-sites/twin-cities-army-ammunition-plant-tcaap-superfund-site.html NAVFAC (Naval Facilities Engineering Command). 2004. Guidance for Optimizing Remedy Evaluation, Selection, and Design.
Neuhauser, E. F., J. A. Ripp, N. A. Azzolina, E. L. Madsen, D. M. Mauro, and T. Taylor. 2009. Monitored natural attenuation of manufactured gas plant tar mono- and polycyclic aromatic hydrocarbons in ground water: A 14-year field study. Ground Water Monitoring & Remediation 29(3):66-76.
Newell, C. J., I. Cowie, T. M. McGuire, and W. McNab. 2006. Multi-year temporal changes in chlorinated solvent concentrations at 23 MNA sites. Journal of Environmental Engineering 132(6):653-663.
NRC (National Research Council). 1994. Alternatives for Groundwater Cleanup. Washington, DC: National Academy Press.
NRC. 1997. Innovations in Soil and Ground Water Cleanup. Washington, DC: National Academy Press.
NRC. 1999. Groundwater and Soil Cleanup. Washington, DC: National Academy Press.
NRC. 2000. Natural Attenuation for Groundwater Remediation. Washington, DC: National Academy Press.
NRC. 2005. Contaminants in the Subsurface: Source Zone Assessment and Remediation. Washington, DC: The National Academies Press.
NRC. 2007. Assessment of the Performance of Engineered Waste Containment Barriers. Washington, DC: The National Academies Press.
Pennell, K. D., G. A. Pope, and L. M. Abriola. 1996. Influence of viscous and buoyancy forces on the mobilization of residual tetrachloroethylene during surfactant flushing. Environmental Science & Technology 30(4):1328-1335.
Ramakrishnan, V., A. V. Ogram, and A. S. Lindner. 2005. Impacts of co-solvent flushing on microbial populations capable of degrading trichloroethylene. Environmental Health Perspectives 113(1):55-61.
Ramsburg, C. A., and K. D. Pennell. 2001. Experimental and economic assessment of two surfactant formulations for source zone remediation at a former dry cleaning facility. Ground Water Monitoring & Remediation 21:68-82.
Ramsburg, C. A., L. M. Abriola, K. D. Pennell, F. E. Loffler, M. Gamache, B. K. Amos, and E. A. Petroviskis. 2004. Stimulated microbial reductive dechlorination following surfactant treatment at the Bachman road site. Environmental Science & Technology 38:5902-5914.
Ramsburg, C. A., K. D. Pennell, L. M. Abriola, G. Daniels, C. D. Drummond, M. Gamache, H. U. Hsu, E. A. Petrovskis, C. M. Rathfelder, J. L. Ryder, and T. P. Yavaraski. 2005. Pilot-scale demonstration of surfactant-enhanced PCE solubilization at the Bachman Road site. 2. System operation and evaluation. Environmental Science & Technology 39(6):1791-1801.
Rao, P. H., M. He, X. Yang, Y.-C. Zhang, S.-Q. Sun, and J.-S. Wang. 2006. Effect of an anionic surfactant on hydraulic conductivities of sodium- and calcium-saturated soils. Pedosphere 16(5):673-680.
Rao, P. S. C., M. D. Annable, R. K. Sillan, D. Dai, K. Hatfield, W. D. Graham, A. L. Wood, and C. G. Enfield. 1997. Field-scale evaluation of in situ cosolvent flushing for enhanced aquifer remediation. Water Resources Research 33:2673-2686.
Roote, D. S. 1998. Technology Status Report In Situ Flushing (TS-98-01). Pittsburgh, PA: Ground-Water Remediation Technologies Analysis Center.
Sale, T. C., and C. Newell. 2011. Guide for Selecting Remedies for Subsurface Releases of Chlorinated Solvents. ESTCP Project ER-200530. Scherer, M. M., E. O’Loughlin, G. F. Parkin, R. Valentine, H. Al-Hosney, R. Handler, C. Just, P. Larese-Casanova, T. Pasakarnis, and S. L. Smith. 2009. S.L. ER-1369 Sustainability of Long-Term Abiotic Attenuation of Chlorinated Ethenes. Final report for the Strategic Environmental Research and Development Program.
Seagren, E. A., B. E. Rittman, and A. J. Valocchi. 1993. Quantitative evaluation of flushing and biodegradation for enhancing in situ dissolution of non-aqueous phase liquids. Journal of Contaminant Hydrology 12(1-2):103-132.
Seagren, E. A., B. E. Rittmann, and A. J Valocchi. 1994. Quantitative evaluation of the enhancement of NAPL-pool dissolution by flushing and biodegradation. Environmental Science & Technology 28(5):833-839.
Siegal, J., A. A. Rees, K. W. Eggers, and R. L. Hobbs. 2009. In situ chemical oxidation of residual LNAPL and dissolved-phase fuel hydrocarbons and chlorinated alkenes in groundwater using activated persulfate. Remediation Journal 19(2):19-35.
Siegrist, R. L., M. Crimi, T. J. Simpkin (eds). 2011. In Situ Chemical Oxidation for Remediation of Contaminated Groundwater. New York: Springer Science+Business Media, LLC.
Sleep, B. E., D. J. Seepersad, K. Mo, C. M. Heidorn, L. Hrapovic, P. L. Morrill, M. L. McMaster, E. D. Hood, C. LeBron, B. Sherwood Lollar, D. W. Major, and E. A. Edwards. 2006. Biological enhancement of tetrachloroethene dissolution and associated microbial community changes. Environmental Science & Technology 40(11):3623-3633.
Stroo, H. F., M. Unger, C. H. Ward, M. C. Kavanaugh, C. Vogel, A. Leeson, J. A. Marquesee and B. P. Smith. 2003. Remediation of chlorinated solvent source zones. Environmental Science & Technology 37:224A-230A.
Stroo, H. F., and C. H. Ward. 2010. In situ remediation of chlorinated solvent plumes. New York: Springer.
Stroo, H. F., A. Leeson, J. A. Marquesee, P. C. Johnson, C. H. Ward, M. C. Kavanaugh, T. C. Sale, C. J. Newell, K. D. Pennell, C. A. Lebron, and M. Unger. 2012. Chlorinated ethene source remediation: Lessons learned. Environmental Science & Technology 46:6438-6447.
Thiruvenkatachari, R., S. Vigneswaran, and R. Naidu. 2008. Permeable reactive barrier for groundwater remediation. Journal of Industrial and Engineering Chemistry (Amsterdam, Netherlands) 14(2):145-156.
Triplett Kingston, J. L. 2008. A Critical Evaluation of In Situ Thermal Technologies. Ph.D. Dissertation, Department of Civil and Environmental Engineering, Arizona State University.
Triplett Kingston, J. L., P. R. Dahlen, P. C. Johnson, E. Foote, and S. Williams. 2009. State of the Practice Overview: Critical Evaluation of State-of-the-Art In Situ Thermal Treatment Technologies for DNAPL Source Zone Treatment. ESTCP Project ER-0314. May 2009. http://www.serdp.org/content/download/4577/67309/version/2/file/ER-0314-State-of-the-Practice+Overview.pdf.
Triplett Kingston, J. L., P. Dahlen, and P. C. Johnson. 2010a. State-of-the-practice review of in-situ thermal technologies. Ground Water Monitoring & Remediation 30(4):64-72.
Triplett Kingston, J. L., P. R. Dahlen, P. C. Johnson, E. Foote, and S. Williams. 2010b. Critical Evaluation of State-of-the-Art In Situ Thermal Treatment Technologies for DNAPL Source Zone Treatment. ESTCP Project ER-0314. January 2010. http://www.serdp.org/content/download/4576/67301/file/ER-0314-FR.pdf.
Triplett Kingston, J. L., P. R. Dahlen, and P. C. Johnson. 2012. Assessment of ground-water quality improvements and mass discharge reductions at five in situ electrical resistance heating remediation sites. Ground Water Monitoring & Remediation. DOI:10.1111/j.1745-6592.2011.01389.x Vangelas, K. M., B. B. Looney, T. O. Early, T. Gilmore, F. H Chapelle, K. M. Adams, and C. H. Sink. 2006. Monitored natural attenuation of chlorinated solvents—moving beyond reductive dechlorination. Remediation Journal 16(3):5-23.
Vinegar, H. J., G. L. Stegemeier, F. G. Carl, J. D. Stevenson, and R. J. Dudley. 1999. In Situ Thermal Desorption of Soils Impacted with Chlorinated Solvents. Proceedings of the Annual Meetings of the Air and Waste Management Association, Paper No. 99-450.
Wanaratna, P., C. Christodoulatos, and M. Sidhoum. 2006. Kinetics of RDX degradation by zero-valent iron (ZVI). Journal of Hazardous Materials 136(1):68-74.
Wiedenmeir, T. H., H. S. Rifai, C. J. Newell, and J. T. Wilson. 1999. Natural Attenuation of Fuel Hydrocarbons and Chlorinated Solvents in the Subsurface. New York: John Wiley and Sons.
Wilson, J. T. 2010. Monitored natural attenuation of chlorinated solvent plumes. Chapter 11 in In Situ Remediation of Chlorinated Solvent Plumes. H. Stroo and C. H. Ward (eds.). New York: Springer.
Wymore, R. A., T. W. Macbeth, J. S. Rothermel, L. N. Peterson, L. O. Nelson, K. S. Sorenson, N. Akladiss, and I. R. Tasker. 2006. Enhanced anaerobic bioremediation in a DNAPL residual source zone: Test Area North case study. Remediation 16(4):5-22.
Yang, Y., and P. L. McCarty. 2000. Biologically enhanced dissolution of tetrachloroethene DNAPL. Environmental Science & Technology 34(14):2979-2984.
Yang, Y., and P. L. McCarty. 2002. Comparison between donor substrates for biologically enhanced tetrachloroethene DNAPL dissolution. Environmental Science & Technology 36(15):3400-3404.
Yeh, D. H., K. D. Pennell, and S. G. Pavlostathis. 1999. Effect of Tween surfactants on methanogenesis and microbial reductive dechlorination of hexachlorobenzene. Environmental Toxicology and Chemistry 18:1408-1416.
Zimmerman, J. B., T. C. G. Kibbey, M. A. Cowell, and K. F. Hayes. 1999. Partitioning of ethoxylated nonionic surfactants into nonaqueous-phase organic liquids: Influence on solubilization behavior. Environmental Science & Technology 33(1):169-176.
















































