Technology Development to Support Long-Term Management of Complex Sites
Despite years of characterization and implementation of remedial technologies, many complex federal and private industrial facilities with contaminated groundwater will require long-term management actions that could extend for decades or longer. As discussed in Chapter 2, the Department of Defense (DoD) manages a substantial number of such sites. Chapter 4 concluded that the further application of existing remediation technologies is likely to provide only incremental progress in achieving restoration at the most complex sites. Thus, for these sites the management challenges include optimization of active remedies, reducing mass flux/mass discharge of contaminants from source areas such that natural attenuation may be effective, or ensuring that any active or passive engineered containment system will remain effective over the long term. This chapter discusses technological developments that can aid in addressing these management challenges—in particular, providing the scientific and technical bases for transitioning from active remediation to more passive strategies where applicable.
Optimization of remedial technologies, transitioning to active or passive containment, and improving long-term management can be achieved through (1) better understanding of the spatial distribution of contaminants, exposure pathways, and processes controlling contaminant mass flux and attenuation along exposure pathways; (2) improved spatio-temporal monitoring of groundwater contamination through better application of conventional monitoring techniques, the use of proxy measurements, and development of sensor-based monitoring technologies; and (3) application of emerging diagnostic and modeling tools. In addition to these topics, the
chapter explores emerging remediation technologies that have yet to receive extensive field testing and evaluation, and it reviews the state of federal funding for relevant research and development and provides recommendations on research topics relevant to the future management of complex sites where groundwater restoration is unlikely.
The decision to transition a site from active remediation to long-term management requires a thorough understanding of the geologic framework, history of contamination events, the current location and phase distribution of contaminants, temporal processes that affect groundwater flow and chemical migration, and interactions at hydrogeologic and compliance boundaries. The combined understanding of these factors, referred to here as site conceptualization, supports the development of specific management tools such as the conceptual site model (CSM, see Chapter 4) and mathematical models. Typically, the site conceptualization and associated tools are updated as the project progresses from discovery of contamination through closure or transition to long-term management, with the degree of detail dependent on the nature of the contamination and the physical dimensions of the site. The development and enhancement of an accurate and suitably detailed site conceptualization is an important component of addressing future management challenges at these sites including the transition to long-term management.
The current cleanup paradigm distinguishes the source zone from the downgradient plume, in terms of treating each region differently with respect to characterization and remediation, and it acknowledges the dominant role of geologic heterogeneity in controlling contaminant removal from both regions. In NRC (2005), hydrogeologic heterogeneity was conceptually captured by identifying five generic geologic environments ranging from nearly uniformly homogeneous, unconsolidated porous media (Type I) to fractured rock and carbonate aquifers (Types IV and V). More recently, a 14-compartment model has been proposed (Figure 4-1; Sale and Newell, 2011; ITRC, 2011), in which contaminants can reside in groundwater, sorbed, and vapor phases, either within the source zone or the plume, and which are further subdivided into high- and low-permeability regions. In the high-permeability regions, advective transport will control contaminant migration, while in the low-permeability regions, the dominant transport mechanism is molecular diffusion. The advantage of such multi-compartment conceptual models is the ability to focus on the exchange of contaminant mass between specific compartments that can limit the rate and extent of remediation, recognizing that the controlling processes can change over time.
The 14-compartment framework highlights characterization challenges that significantly influence optimization of remedial actions and the transition to long-term management, including the source/plume distinction, spatial heterogeneity in hydraulic conductivity, and the potential role of the vapor pathway when volatile organic compounds (VOCs) are present. A more comprehensive application of the framework that fully accounts for the relative magnitudes of contaminant mass in each of the compartments and the rates of mass transfer between compartments will require further development to better understand (1) the potential roles of desorption and of back-diffusion from low-permeability compartments to advective zones, (2) the variety of aquifer materials and conditions that comprise the “less transmissive” compartments, (3) the reactive characteristics of the aquifer that control the potential success of long-term strategies such as monitored natural attenuation, and (4) the complex factors that control the fate of volatile contaminants, which can exhibit markedly different behavior at seemingly similar sites because of variability in subsurface conditions, building characteristics at the soil interface, and climate conditions. Each of these issues is further explored below.
Back-Diffusion and Desorption
For many complex sites that have been subject to partial or complete source removal, the transition to long-term management is largely controlled by volatilization into the vapor phase (if applicable) and transport into the aqueous phase plume, as these two phases are the primary media for both off-site contaminant migration and the biotic and abiotic transformation processes associated with natural attenuation. Current conceptualizations of the plume have focused on three potential sources of contaminant mass influx in the groundwater: (1) discharge from undetected mass remaining in the upgradient source zone, (2) aqueous back-diffusion from aquifer materials to the pore water within low-permeability plume material and subsequent diffusive transport to advective zones, and (3) mass transfer (desorption) from aquifer sediments within both transmissive and low-permeability plume materials. For successful transition to long-term management, the contaminant influx from these three processes must be balanced by natural attenuation processes or controlled by physical/hydraulic containment.
The potential loading of dissolved mass from the source zone to the plume has received considerable attention and is straightforward to assess because the mass discharge occurs at the boundary of, rather than within, the plume compartment. However, back-diffusion and desorption of contaminants from materials within the plume are much more difficult to analyze because they are spatially nonuniform, dependent on the history
of the source and plume migration, and are not easily measurable. In particular, measured groundwater concentrations provide only limited insight into the processes responsible for the persistence of dissolved contaminant plumes because it is difficult to distinguish the relative influence of flow field heterogeneity, back-diffusion, and desorption.
The potential importance of back-diffusion is supported by conceptual and modeling analysis (e.g., MacKay and Cherry, 1989; Wilson, 1997; Parker et al., 2008) and a limited number of field investigations that have directly sampled aquitard material (Ball et al., 1997; Chapman and Parker, 2005). Sorption processes are typically included in contaminant transport models and estimates of time to remediate, although the common use of the retardation factor reflects the optimistic assumptions of a single sorbent and rapid linear partitioning. A considerable body of research over the past two decades has demonstrated that, for many aquifer materials, sorption processes are in fact spatially heterogeneous, nonlinear, and potentially limited by solute diffusion to sorbent material located within the interior of soil particles (e.g., as reviewed by Allen-King et al., 2002). As with back-diffusion, conceptual and modeling analyses have shown that nonlinear and/or rate-limited desorption can potentially contribute to plume persistence over decades (e.g., Ball and Roberts, 1991; Rabideau and Miller, 1994; Rivett et al., 2006). However, at the time of this writing, there is a lack of field data and characterization techniques to distinguish desorption processes from other nonideal effects. A modest step toward better understanding the potential role of sorption processes would be to routinely characterize the organic content of collected soil samples (Simpkin and Norris, 2010), a task that could be accomplished at relatively low cost.
Understanding whether back-diffusion and desorption are occurring at a site is challenging because the relative importance of each process is highly dependent on the site-specific contamination history and the presence and distribution of low-permeability and/or strongly sorbing materials. And yet, current site characterization techniques typically do not fully delineate the structure of these materials, particularly when they are distributed over small spatial scales within the plume interior. Furthermore, there are no proven remedial techniques to preferentially target and accelerate the removal of contaminants from localized sites that are desorption/diffusion limited. Finally, currently used mathematical models are difficult to configure to provide realistic predictions of time to remediation when desorption/diffusion processes are the limiting factor because of the need to assign initial conditions that properly represent the mass located in immobile compartments. Additional research is needed to develop strategies for long-term management that focus on plume zone processes that contribute to plume longevity rather than the processes that occur in the source zone.
Representing Complex Geologic Environments
The 14-compartment model of Sale and Newell (2011) assigns “low- permeability” compartments to both the source and plume domains, highlighting the potential role of back-diffusion in both domains. Such an approach is conceptually similar to the classification scheme proposed by NRC (2005), which included a hierarchy of five geologic environments ranging from nearly uniformly homogeneous, unconsolidated porous media (Type I) to fractured rock and carbonate aquifers (Types IV and V). While both schemes distinguish between contaminants in “mobile” and “immobile” groundwater, the five-region classification recognizes two subtle but potentially significant differences not captured by the 14-compartment model. First, the diffusion rate and storage capacity of contaminants in low-permeability geologic materials can differ substantially among clays, fractures, and/or intrinsic porosity of indurated rock. Second, in addition to providing potential sinks for diffusive exchange of contaminants, some complex domains (highly heterogeneous unconsolidated porous media, fractured rock, karst) are often characterized by large variations in the groundwater velocity. Hence efforts to characterize “complexity” understood in terms of spatial variability must consider both groundwater flow and contaminant transport within and between discrete compartments, regardless of how such compartments are delineated.
Differences in the diffusion process are relatively straightforward to account for, but require appropriate specification of the geometry and diffusion characteristics of the low-permeability material. In some cases, the necessary information is provided by field characterization, but for many problems of interest, such as diffusion out of thin clay lenses, the relevant diffusion path length is difficult to determine. Similarly, accounting for variation in advective transport pathways typically requires a very detailed conceptualization of the groundwater flow field, particularly the low-permeability features. For example, spatial variations in the hydraulic conductivity of unconsolidated media can lead to preferential pathways in aquifers over significant distances, similar to characteristics associated with fractured rock and karst formations. Such paths of preferential groundwater flow often control the distribution of contaminant mass in both source areas and downgradient plumes, and must be properly considered in the design and implementation of containment and remediation strategies. Chapman et al. (2010) present an example of how information from detailed site characterization can be incorporated into a remedial design that yields good performance despite the presence of preferential flow paths. However, while available modeling tools are increasingly capable of incorporating detailed descriptions of hydraulic conductivity heterogene-
ity (e.g., see Guilbeault et al., 2005), the requirements for additional site characterization can represent a considerable burden on site management.
Transformation Capacity
As discussed in Chapter 7, monitored natural attenuation (MNA) is the dominant process during long-term management at sites not relying on physical or hydraulic containment. Knowledge of the biogeochemical environment and the identification of potentially important reactive pathways for the target contaminants are necessary prerequisites for initiating MNA after the transition to long-term management has occurred. Relevant considerations include bulk aquifer properties such as mineral composition and pore water chemical constituents, as well as the presence of the necessary microbial consortia. Contaminant transformation during MNA can occur through microbial pathways, abiotic mechanisms, or in many cases a combination of both.
Of critical importance to the aquifer “transformation capacity” for MNA is the spatial pattern of redox zonation. Redox zonation occurs as a result of microbial metabolism where in a homogeneous system terminal electron acceptors with the most favorable free energies are preferably used before the next one can be utilized (termed the “redox ladder” by Borch et al., 2010). Complex sites, however, may have areas of overlapping or patchy redox zonation whereby microbial communities that utilize different terminal electron acceptors can co-exist. Determining whether the site is fully oxic, has extensive zones of anoxia, or is comprised of these patchy suboxic/anoxic regions in conjunction with the target contaminant composition is critical to determining the appropriateness of MNA (Rugge et al., 1998; Hofstetter et al., 1999).
Another important parameter in contaminant transformation is the presence of reactive minerals associated with aquifer solids, such that characterizing these chemical factors can yield clues about the potential effectiveness of MNA. A variety of naturally occurring iron and manganese oxides, iron sulfide minerals, and clays with iron moieties have been shown to be highly reactive and can act as respective reductants and oxidants in abiotic attenuation pathways (Kappler and Straub, 2005; Hofstetter et al., 2003; Neumann et al., 2009; He et al., 2009). Microorganisms play an important role in the controlling both the type and stability of these minerals since many organisms are capable of utilizing mineral oxides as terminal electron acceptors (Lovley, 1993; Tebo et al., 2004). Under some circumstances the microbial population can convert iron oxides to reactive media useful for MNA by producing Fe(II), which can either be chelated by natural ligands, be adsorbed to the remaining iron oxides to create highly potent reductants, or react with sulfides (if sulfate is in abundance as a ter-
minal electron acceptor) to produce potentially reactive iron sulfide minerals (Hakala et al., 2007; Hakala and Chin, 2010). In other cases, however, reduction of manganese oxides (which can mediate oxidation reactions) may result in a decrease in potential MNA. In aquifer pore waters, reactive species such as natural organic matter and reduced sulfur species (bisulfide, polysulfides, and organic thiols) play an important role in MNA by acting as reductants and electron mediators (Kappler and Haderlein, 2003; Hakala and Chin, 2010). Natural organic matter significantly increases the reactivity of reduced sulfur species by acting as an electron mediator, and is an important reductant in sulfur-rich aquifers (Dunnivant et al., 1992).
An example of a well-characterized site with high transformation capacity amenable to MNA is Altus Air Force Base, which has abundant levels of both sulfate and Fe(III) (Kennedy et al., 2006). Microbial metabolic activity at this site produced potent reactive reductants such as reduced sulfur compounds, Fe(II), and iron sulfide minerals, which were capable of abiotically transforming TCE and its derivatives. These investigators reported the absence of sulfate in the area of the TCE plume and the existence of abundant iron sulfide minerals. Further they found no TCE in the area where iron sulfides are abundant and only trace levels of by-products, suggesting that MNA was occurring.
While much is known about the biological/abiotic conditions necessary to effect contaminant transformation during MNA, there is not yet a complete protocol to determine the extent to which such conditions are present at a site and whether contaminants are being degraded. The tools discussed later in this chapter represent important initial steps toward the development of such a protocol.
Vapor Intrusion Issues
As described in Chapter 5, the vapor intrusion pathway is increasingly considered at complex sites with DNAPL contamination. This pathway can be conceptualized as three distinct zones (Figure 6-1): (1) the source zone where contaminant is immobilized, (2) the subsurface migration pathway, and (3) the influence zone of the building. The management of vapor intrusion requires expanded site characterization, an interpretation of the several types of vapor concentration measurements in the context of site-specific conditions, and, if necessary, development of appropriate mitigation strategies if source removal measures are insufficient to reduce exposure to acceptable levels.
Characterization of the vapor pathway is challenged by the fact that each component is subject to considerable spatial and temporal variability. Fluctuating water table conditions controlled by recharge, pumping, and stream–aquifer interactions can result in transient vapor flux generation at
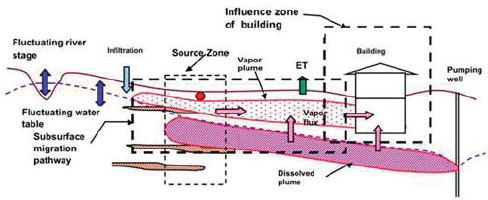
FIGURE 6-1 Vapor intrusion pathways.
the sources. The migration pathway from source to building is significantly affected by changes in soil moisture, temperature, wind, and ambient pressure, and in some cases, biogeochemical transformation processes. Vertical migration is also influenced by changes in building ventilation and heating, ventilation, and air conditioning systems operation. Finally, attempts to characterize the pathway via indoor air sampling can be confounded by indoor sources of contamination.
Among the available guidance for assessing vapor intrusion (e.g., Johnson et al., 1999; Hay-Wilson et al., 2005; McAlary et al., 2005; NYSDOH, 2006; ITRC, 2007), federal guidance is evolving toward an approach based on multiple lines of evidence that involves sampling of indoor air, subslab soil gas, deeper soil gas, groundwater, and soil—in combination with screening-level modeling and empirical assessment (e.g., EPA, 2002, 2011a,b, 2012a,b,c). This reflects experiences with conflicting lines of evidence at some sites, recognition that there will likely be spatial variability in pathway sampling results, low confidence in our ability to correctly interpret the data, and a limited peer-reviewed knowledge base to rely upon. This also suggests that assessment paradigms that rely on too few samples (in space and time) are limited.
Vapor intrusion from groundwater plumes with chlorinated solvents is especially challenging to characterize, partly because such plumes can vary widely in size. Where large plumes encompass an entire neighborhood, assessment of all potentially affected buildings may be impracticable. Furthermore, it is not always the case that the greatest indoor air impacts are found in buildings overlying the highest groundwater concentrations. Groundwater-related vapor intrusion has been documented in some buildings overlying dissolved chlorinated solvent groundwater concentrations as
low as 10 μg/L, and no impacts have been observed in other buildings overlying groundwater concentrations as great as 10–100 mg/L (EPA, 2012b).
A number of commercial products can serve as indoor sources of chlorinated solvent vapors, so that interpreting indoor air quality and sub-slab soil gas data is not always straightforward (Gorder and Dettemmaier, 2011). As a case in point, approximately 3,000 residences overlie chlorinated solvent groundwater plumes originating from Hill Air Force Base, although monitoring has indicated that a very small percentage of the residences have indoor air impacts attributable to groundwater contamination. Detailed study beyond typical pathway assessment monitoring identified numerous indoor air sources of contaminants, including household cleaning products, craft supplies, gun cleaners, and holiday ornaments—leading to a list of 72 household products known to contain TCE and almost another 2,000 products known or suspected of containing chlorinated solvents.
A solid technical basis is lacking for determining which scenarios require indoor sampling and what sampling frequency and duration are appropriate, both over the short term (i.e., daily) and long term (i.e., seasonal). Studies suggest that vapor intrusion emissions into buildings can fluctuate on time scales ranging from days to weeks (Luo, 2009; Luo et al., 2010; Johnson et al., 2012). Research by McHugh et al. (2010) suggests that changes in indoor air concentrations may be different for chemicals emanating from groundwater than those emanating from indoor chemical sources, such that temporal data might be used to distinguish between indoor air impacts from these two sources. However, even with detailed indoor air monitoring data, the issue of temporal variability is further complicated by the dynamics of volatilization from the groundwater plume, which is affected by groundwater table elevation, moisture infiltration rates, moisture profiles, and other climate factors (Sakaki et al., 2013). In general, the temporal changes in the vapor emission rates from groundwater have yet to be studied in great detail and further study is needed to more intelligently design sampling plans.
Because the costs and complexity of vapor intrusion assessment have been increasing without a commensurate increase in the mechanistic understanding of the exposure pathway, the resulting response actions reflect a conservative management approach.
Monitoring of groundwater is conducted over the entire life cycle of a complex site and can represent a significant percentage of life-cycle costs if residual contamination remains after active remediation has been completed, especially when monitoring extends over multiple decades. Traditionally, the monitoring of temporal changes in groundwater contamination
relied on conventional well sampling, which is labor intensive and requires costly laboratory analyses. Given that tens to hundreds of monitoring wells are present at most sites, and standard quarterly sampling is often required, estimates of monitoring can exceed $100 million per year at DoD facilities alone, which represents a significant percentage of the financial resources dedicated to remediation efforts. Furthermore, the traditional two-dimensional resolution of monitoring well networks (which produce vertically averaged concentration values) may be insufficient to support an accurate site conceptualization, particularly for highly heterogeneous formations.
Continued development of conventional monitoring techniques has led to more detailed characterization of the distribution of dissolved contaminants, particular in the vertical dimension. However, to support a cost-effective transition to long-term management, additional tools are needed. This section addresses ongoing developments in (1) optimization of conventional monitoring systems, (2) techniques for measuring contaminant flux, (3) sensor technology, and (4) new tools that can be applied to better understand whether MNA is working.
Improved Application of Conventional Monitoring Tools
The deployment of conventional site characterization tools has evolved in a manner that has emphasized greater spatial resolution in regions where contamination is significant. In particular, multi-level monitoring and nested well systems now enable the collection of hydraulic head data and groundwater samples over relatively short vertical intervals (ITRC, 2004; Einarson, 2006; Einarson et al., 2010; Kavanaugh and Deeb, 2011). Although more costly than conventional 2-D monitoring, multi-level monitoring systems can lead to more streamlined and accurate remedial investigations and long-term management.
Formal simulation/optimization techniques have been developed to improve the design of monitoring programs—a process sometimes termed long-term monitoring optimization (LTMO). These applications are in a relatively early stage of development and a variety of approaches are available to formulate and solve the optimization problem. For example, one approach might be to analyze the value of information provided by an existing monitoring network to identify monitoring wells that are spatially redundant and could be removed (e.g., Reed et al., 2000, 2001; Babbar-Sebens and Minsker, 2008). Most work to date has focused on monitoring frequency and spatial resolution of well networks, with less attention given to issues such as the number and selection of analytes, sampling analytical techniques, and data processing. In a pilot study comparing two software-driven LTMO systems, the U.S. Environmental Protection Agency (EPA)
suggested that annual savings of a few hundred to tens of thousands of dollars might be achievable, particular for sites where more than 50 samples are collected and analyzed annually (EPA, 2004). EPA subsequently issued a “road map” to assist managers with developing a site-specific LTMO program (EPA and USACE, 2005), including user-friendly software tools. Although the underlying concepts are fairly well established, additional documentation of successful case studies would clarify the range of potentially achievable cost savings.
Monitoring of Source Zone Contamination
The successful design of a source zone remediation program depends on sufficiently detailed knowledge of the spatial pattern of immobile source materials. A number of recent reviews have evaluated the variety of tools available to quantify the magnitude and spatial distribution of DNAPL (e.g., NRC, 2005; Mercer et al., 2010). These tools range from low-cost methods to infer the presence of DNAPL (as reviewed by Kram et al., 2001) to more extensive methods designed to delineate the spatial distribution of NAPL saturation to guide source zone remediation (e.g., Saenton and Illangasekare, 2004; Moreno-Barbero and Illangasekare, 2005, 2006). For the latter purpose, the partitioning interwell tracer test (PITT) has proven to be relatively effective (e.g., Annable et al., 1998; Brooks et al., 2002), although its deployment is hindered by high cost and need for relatively sophisticated interpretive tools.
As it is unlikely that complete removal of contaminant source material will be feasible for many complex sites, the transition to long-term management will depend not only on the amount of source mass removed, but on the rate at which mass is transferred between the source and plume compartments during the post-remediation period. One of the most promising recent developments in source zone management is the development of tools for measuring contaminant mass flux, either at localized monitoring points or as an integrated mass discharge across a control plane. Such knowledge of contaminant discharge is particularly useful in evaluating the potential for downgradient natural attenuation processes.
Conceptually, contaminant discharge is a calculated parameter that reflects both temporal and spatial averaging of the product of groundwater discharge (length per area per time) and contaminant concentration (mass per volume). Field methods include synoptic sampling (e.g., Einarson, 2006), passive flux meters (Annable et al., 2005; Basu et al., 2006), steady-state pumping (e.g., Buschek, 2002), recirculation flux measurements (Goltz et al., 2007), integral pumping tests (Bockelmann et al., 2001; Bauer et al., 2004), and modified integral pumping tests (Brooks et al., 2008). The use of flux measurements as an alternative to concentration-based metrics
offers several advantages relevant to long-term management, including less sensitivity to spatial/temporal variability and correspondence with screening models that attempt to correlate source zone mass removal with downgradient plume behavior.
Several recent reviews have explored the relative performance of various techniques for measuring mass flux, which appear to be highly site-specific (EPA, 2009; ITRC, 2010; Kavanaugh and Deeb, 2011). Additional field research is needed to support the more widespread adoption of flux-based performance metrics, including (1) further clarification of the range of uncertainty associated with mass flux and mass discharge measurements, (2) continued refinement of specific aspects of the various techniques, including a better definition of the necessary preliminary site characterization, and (3) new measurement techniques.
Sensor Technology
Because existing monitoring and performance assessment tools are expensive, slow, and consist of point measurements, real-time measurements could provide data for management decisions including optimization of active remedies and assurance that either active or passive containment is effective. Recent advances in microelectronics, wireless communication technologies, and information technologies have produced potentially low-cost techniques to gather and process large amounts of data at very high spatial and temporal resolution. Such wireless sensor networking could be applied to a variety of subsurface systems.
Advances in the development of wireless sensor networks have been successfully applied to problems in infrastructure monitoring, weather and storms, volcanoes, air quality, agriculture, forestry, and ecology (e.g., Culler et al., 2004; Haenggi, 2005; Werner-Allen et al., 2006). Much of this work has highlighted the advantages of deploying a large number of inexpensive sensors to replace of a few highly accurate, but expensive sensors. While environmental monitoring has been considered an ideal application since the field’s inception, only a few projects have combined wireless sensing with subsurface monitoring, largely because of the technical difficulty and cost associated with monitoring VOCs in groundwater environments. For example, a study by EPA (2003) concluded that although a sensor might cost as little as $100 to manufacture, a fully developed multiparameter sensor suitable for long-term management applications would cost around $7,500. More recently, an ESTCP-sponsored project (Lieberman, 2007) evaluated sensors for monitoring VOCs, including Halogen-Specific Detector/Membrane Interface Probe systems and laser-induced fluorescence, based on ROST (rapid optical screening tool). However, despite some advances in detection capabilities, the relatively large costs (thousands of dol-
lars per sensor) inhibit deployment in a wireless sensor network setting, and such developments have not advanced to the stage of commercialization. In addition to cost, other outstanding issues must be resolved if wireless sensor network technology is to be adopted for long-term monitoring of groundwater plumes, including the scope and accuracy of contaminant-specific sensors, signal transmission issues in subsurface environments, the mode and frequency of sensor failure, and the availability and efficiency of power sources.
New developments in sensor technology for vapor monitoring could contribute to more effective management of the vapor intrusion pathway. Point-in-time sample collection using Summa Canisters is the standard indoor-air sampling approach. These are limited to time-integration periods of a few days at best, which is likely inadequate for pathway assessment (Luo, 2009; Luo et al., 2010). The ideal vapor intrusion sensing system would be capable of assessing (1) whether conditions exist that can cause unacceptable vapor intrusion (i.e., periods of building under-pressurization, or contaminants present in soil gas at levels above concentrations of concern), (2) the actual impact of vapor intrusion on indoor air quality (i.e., indoor air monitoring), (3) the actual exposure of building occupants to vapor intrusion (i.e., simulated uptake monitoring), and, in the case of mitigation systems, (4) whether the mitigation system is meeting operational goals that eliminate the vapor intrusion pathway (i.e., maintaining a building over-pressurization condition). These sensing systems would need to measure pressure differentials of 0 to 5 Pa without drift for extended periods of time and reliably quantify vapor contaminant concentrations in the 0.1–100 ppbv range, under a range of humidity conditions and for sampling durations of a few minutes to a few days and over periods of many months. One vision for future sensing systems is something like a household CO monitor with real-time data communication to a home computer, tablet, or PDA to increase occupants’ awareness of their indoor air quality.
Evaluating Monitored Natural Attenuation
Chapter 7 discusses the possibility, at many complex sites, of a transition from active source zone remediation to more passive strategies such as MNA or natural attenuation without monitoring. Critical needs in implementing MNA are verification that contaminant transformation is occurring and that the required bacteria are present and active (if biodegradation is the principal attenuation mechanism).
Verification of contaminant transformation can be accomplished by direct groundwater monitoring for the contaminants of concern or alternatively via geophysical techniques, which provide a noninvasive means of identifying changes in biogeochemical conditions in groundwater. Several
geophysical parameters are sensitive to redox gradients, microbial activity, and changes in pore-fluid chemistry. For example, changes in electrical resistivity can reflect changes in contaminant concentrations, microbial abundances, and the distribution of amendments that promote contaminant degradation; electrodic potential is sensitive to the local redox chemistry; self-potential can measure natural electrical current sources arising from redox zonation due to contaminant degradation; and induced polarization provides evidence of processes near fluid-grain boundaries to infer microbial abundances. Although geophysical measurements can be conducted in existing monitoring wells using removable sensors, the underlying geologic variability in the aquifer may yield subtle variations that do not provide a distinct geophysical signal for particular biogeochemical conditions. Thus, it is usually necessary to monitor geophysical parameters over time and compare parameter values to background conditions.
The additional new technologies reviewed below could enable a more immediate and focused observation of transformation processes relevant to MNA. Not all of these techniques will be required at all sites to document the occurrence of MNA. Laboratories at research universities are able to perform such analyses, and commercial laboratories are beginning to offer such services as well.
Tools from Molecular Biology
New molecular biology tools have facilitated the direct observation of the relevant microbial processes and have enhanced the discovery of new enzymes and biochemical pathways that can be applied to MNA. Determining if site-specific bacteria are capable of degrading the target contaminants can be accomplished using genomic tools to detect and quantify gene copies, such as quantitative polymerase chain reaction (qPCR) and PCR-denaturing gradient gel electrophoresis (PCR-DGGE). Similarly, proteomics can be used to identify and quantify protein biomarkers that are produced as a stress response during the degradation of contaminants (Nesatyy and Suter, 2007). Box 6-1 describes recent field research using these methods.
Another method for measuring gene expression is transcriptomics, which creates complementary DNA from extracted mRNA. Transcriptomics is not as representative of microbial activity as proteomics (Belle et al., 2006), but it does not have the same bias due to database limitations. If new sequences are discovered via transcriptomics, it is then possible to detect them using proteomics.
Metabolomics is the study of the small molecules (e.g., metabolites) produced by cellular processes in response to the environment; monitoring their changes may be a means to verify groundwater contaminant biotransformation (Singh, 2006). For example, metabolic biomarkers for BTEX and
BOX 6-1
“Omics” in the Laboratory and Field
Genomics. Microbial communities at contaminated sites often contain the genes necessary for degradation of BTEX (Hendrickx et al., 2006; Beller et al., 2008; Kao et al., 2010) and chlorinated solvents (Hendrickson et al., 2002; Carreon-Diazconti et al., 2009), and resistance to metals (Waldron et al., 2009). The presence/absence of genes has also been correlated to the rates of reductive dehalogenation of chlorinated ethenes at sites where natural attenuation (Lu et al., 2006; Burgmann et al., 2008) and active bioremediation (Lee et al., 2008) were occurring. Changes in microbial communities due to abiotic treatment schemes (e.g., zero-valent iron) have also been observed (Da Silva et al., 2007), but it is unclear if these changes enhance remediation system performance. The techniques are not foolproof, however, for in studying the biodegradation of RDX, Fuller and Stefan (2008) were unable to find genes associated with RDX degradation in samples in which RDX loss was occurring.
Proteomics. Quantification of specific proteins known to be involved in compound degradation could be used to assess the potential for relevant microbial activity at a site and to verify that bacteria are actively degrading site contaminants. This is promising for cis-DCE, which has been shown to lead to the up-regulation in Polaromonas sp. Strain JS666 of specific proteins important in cis-DCE transformation (Jennings et al., 2009). The proteins associated with bacteria responsible for aerobic biodegradation of vinyl chloride have also been identified (Chuang et al., 2010). Proteomic studies have also focused on bacteria capable of facilitating reductive dehalogenation of groundwater pollutants, such Dehalococcoides species that reduce TCE (Werner et al., 2009). Differences in the proteomics of different strains of Dehalococcoides can allow evaluation of which dehalogenases are being expressed, which is linked to the capability of the bacteria to degrade specific contaminants (Morris et al., 2007). The proteins associated with the biodegradation of MTBE by a specific bacterial strain have been identified (Eixarch and Constanti, 2010). Similarly, the proteins involved in anaerobic benzene biodegradation have been characterized, and they are different if the bacteria are grown on benzene vs. benzoate (Benndorf et al., 2009).
Proteomics can also be used to evaluate the bioremediation of metal contaminated sites. Wilkins et al. (2009) evaluated the proteins produced by Geobacter strains during a biostimulation effort focused on uranium reduction. The proteins associated with metal reduction in Shewanella onidensis MR-1 have also been identified (Elias et al., 2007). Use of proteomics could be used to evaluate the success of biostimulation efforts at metal contaminated sites and to monitor changes over time in the microbial consortia responsible for the metal reduction.
PAH include benzylsuccinate for toluene and napthoic acid for naphthalene (Bombach et al, 2010). The use of metabolomics, however, requires knowledge of potential metabolites of the organism under different conditions. One tool to evaluate biodegradation potential and possible metabolites is a database of transformation pathways (e.g., Singh, 2006; Gao et al.,
2010), but substantial effort is required to build the appropriate databases of metabolic profiles for contaminant-degrading organisms.
Isotope Analysis
Another emerging tool for understanding the effectiveness of MNA is compound-specific isotope analysis (CSIA), which is used to monitor the changes in stable isotope ratios of elements within molecules (e.g., 13C/12C, 2H/1H) over time. The technique uses isotopes in compounds present at natural abundance—i.e., isotopically labeled compounds are not used. As a transformation reaction proceeds, a molecule of a contaminant containing the lighter isotope (e.g., 12C) will usually react more quickly than the molecule with the heavier one (e.g., 13C) if this atom is included in the bond being broken (i.e., it is at the reactive center). Thus, the remaining parent compound is depleted in the light isotope (and enriched in the heavy isotope) and the reverse is true for reaction products. The change is quantified via the isotope fractionation factor (α), the isotope enrichment factor (e), and/or the apparent kinetic isotope effect. Most studies on contaminant transformation report enrichment factors (in %o), which can be related to the extent of contaminant transformation. In contrast non-transformative processes such as sorption or dilution result in no fractionation (Pooley et al., 2009; Beller et al., 2008; McKelvie et al., 2007; Amaral et al., 2009).
A guide for the use of CSIA in the assessment of biodegradation of contaminants in groundwater is available from EPA (Hunkeler et al., 2008). Starting with the recommendations of Sherwood Lollar et al. (1999), the EPA report lays out six criteria that must be met to provide evidence for biodegradation of contaminants in groundwater, which would also presumably apply to abiotic reactions. Aelion et al. (2010) also provides detailed information about CSIA and its utility in evaluating biodegradation of contaminants. Several field studies have shown the potential utility of CSIA, particularly in the verification of contaminant degradation during MNA. A summary is given in Box 6-2.
While CSIA is a powerful tool, it has several limitations. As outlined by Blessing et al. (2008), care must be taken in choosing sampling locations and in preserving samples prior to analysis. CSIA is also currently limited to pollutants that have sufficient volatility to be analyzed using gas chromatography, and the isotope ratio mass spectrometer itself often has a limited linear response range and is not particularly sensitive, requiring either large sample sizes or sample preconcentration techniques (Amaral et al., 2009). Furthermore, the relative bioavailability of the contaminant may affect several “masking” processes that alter measured enrichment factors (Elsner et al., 2005; Thullner et al., 2008; Kampara et al., 2008; Aeppli et al. 2009). As outlined in the recommendations, additional research is
needed to advance the use of CSIA as a robust and routine measurement for groundwater sampling to support MNA.
MODELING FOR LONG-TERM MANAGEMENT
The implementation of mathematical models to simulate subsurface flow and transport has become an increasingly important component of long-term management. Models can provide insight into the relative importance of the processes that control remediation, although the prediction of the time to meet remediation goals remains an ongoing challenge. Recent advances in computing hardware and computational methods have significantly broadened the scope of available models, including models capable of simulating very complex biogeochemical processes at high resolution, as well as screening models that utilize a simplified representation of site geometry and/or secondary transport processes to provide an approximate representation of processes believed to control remediation and/or attenuation.
Predicting Source Zone Mass Removal during Remediation
Although active source removal typically occurs prior to the transition to long-term management, new developments in remedial technology and/or site characterization might result in scenarios where additional source zone activity is undertaken. Modeling of source zone processes remains challenged by a number of technical constraints, including the need to represent second-order processes such as pore clogging by biofilms and/or precipitated reaction products, reactions with natural organic matter and other non-target compounds, gas production, and other changes in aquifer properties (e.g., Heiderscheidt et al., 2008; Glover et al., 2007). Furthermore, practical applications are almost always constrained by incomplete knowledge of site-specific conditions needed to appropriately assign geologic heterogeneity, NAPL architecture, and/or other contaminant initial conditions (e.g., Illangasekare et al., 1995; Fagerland et al., 2007a,b). In some cases, advanced DNAPL characterization technologies such as PITT could provide the desired initial conditions for modeling (e.g., Basu et al., 2008) and better support the performance assessment of source remediation.
In light of the inherent challenges of modeling source zone processes at field sites, sophisticated process-oriented models have been more commonly applied in the interpretation of laboratory studies, where the relevant input parameters are more straightforward to obtain and assess (e.g., EPA, 2009), and not in the field. In reviewing the current state of remediation technology (Chapter 4), the Committee observed that for many complex sites, the engineering design for source zone remediation is frequently ac-
BOX 6-2
Field Implementation of CSIA Techniques
Biological reductive dechlorination of PCE and TCE was deduced in contaminated groundwater at Dover Air Force base by determining the isotopic enrichment of PCE and TCE in wells downgradient from the source zone and by identifying reaction products (Sherwood Lollar et al., 2001). Imfeld et al. (2008) showed that changes in isotope fractionation of DCEs in a treatment wetland could be related to changes in the hydrogeochemistry of a wetland. Under oxic conditions, the enrichment factor was –1.7%o but reached –32.6%o once the wetland became methanogenic. In an aerobic fractured bedrock aquifer contaminated with chlorinated ethenes, Pooley et al. (2009) used a combination of CSIA and reactive transport modeling to verify aerobic biodegradation of TCE and cis-DCE and demonstrated the recalcitrance of PCE.
Using two-dimensional CSIA (carbon and hydrogen), Fisher et al. (2007) demonstrated that the degradation process at a BTEX-contaminated site was anaerobic biodegradation. As shown in Figure 6-2, the enrichment factors clearly fall in the region of anaerobic biodegradation. In a controlled field study, Beller et al. (2008) used a combination of CSIA, genomic analysis (qPCR), and metabolite identification to study natural attenuation of BTEX, including the effects of ethanol. Amaral et al. (2010) used CSIA to demonstrate a lack of natural attenuation of TNT and DNT in an oxic contaminated aquifer.
CSIA is also applicable to sites undergoing active groundwater remediation. Song et al. (2002) used isotope effects to verify effectiveness of enhanced in situ bioremediation of chlorinated ethenes. After determining the carbon isotope patterns of the reaction of chlorinated ethenes with nano-zero valent iron (nZVI) in the laboratory (Elsner et al., 2008), it was possible to assess the effectiveness of nZVI in the field (Elsner et al., 2010). In the Elsner et al. (2010) study, natural attenuation via biodegradation of TCE and 1,1,1-TCA in the groundwater were confirmed by the extent of isotopic fractionation between the source zone and downgradient wells. After injection of nZVI, an increase of the magnitude of the enrichment factor (and the detection of dechlorination products) was used to confirm the nZVI was reacting with the target contaminants.
complished without the use of available process-oriented models, in part because of their mathematical and computational complexity, but in large measure because of the substantial amount of characterization and parameter information required to implement such models with confidence. Indeed, Siegrest et al. (2011), recognizing the potential cost and complexity associated with applying detailed models such as the in situ chemical oxidation (ISCO) simulator CORT3D (Heiderscheidt, 2005; Illangasekare
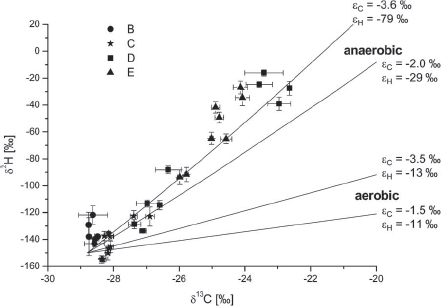
FIGURE 6-2 Concurrent carbon and hydrogen isotope ratios of benzene measure at various sampling depths (B through E) together with isotope patterns for aerobic and anaerobic benzene degradation calculated from published enrichment factors for carbon and hydrogen as well as the isotope signature of the contaminant source using the Rayleigh equation.
SOURCE: Reprinted, with permission, from Fisher et al. (2007). © 2007 by American Chemical Society.
et al., 2007), highlighted the need for “medium range” models that could be applied to address basic design questions applicable to most source zone technologies. While research targeting such “intermediate complexity” models could support more effective remedial designs, it is important that model developers work closely with practitioners to develop tools that balance theoretical rigor, mathematical complexity, data requirements, and user-friendliness.
Modeling Plume Processes
Except for the special case of vapor intrusion, modeling for long-term management is primarily concerned with the transport and reaction of dissolved contaminants in groundwater. The most commonly utilized tools for this purpose are numerical models that couple a solution to the groundwater flow equation in saturated media (e.g., MODFLOW, McDonald and Harbaugh, 1988) with a solution to the advective-dispersive-reactive equation (e.g., MT3DMS, Zheng and Wang, 1999). Modern groundwater flow models are capable of representing considerable detail in the flow field, utilizing millions of computational nodes to represent spatially variable aquifer properties, typically over horizontal scales of meters to kilometers (e.g., DOE, 2009) although finer resolution is increasingly feasible. In conjunction with the finer spatial resolution of flow models, reactive transport models are increasingly able to incorporate a wide variety of biogeochemical reactions. Of particular relevance to long-term management, a considerable body of work has addressed the simulation of natural attenuation processes for both petroleum and halogenated organic contaminants, including sequential parent/daughter reactions and multiple electron acceptor/donors. In particular, the modular code structure employed by MT3DMS has facilitated a number of extensions that address reaction scenarios typical of those found at complex sites, including SEAM3D (Waddill and Widdowson, 2003), BioRedox (Carey et al., 1999), and RT3D (Clement, 1997).
Despite the above advances, the prediction of time-to-complete remediation for dissolved plumes remains an elusive goal. The primary reason for this disconnect is that most commonly employed aqueous-phase simulation models lack the capability to represent the various nonideal processes that release contaminants from immobile phases, particularly nonideal desorption and back-diffusion (note: NAPL dissolution is typically not associated with the “plume” compartment). Furthermore, although it would be conceptually straightforward to incorporate such features in reactive transport models, full implementation would require detailed knowledge of the spatial distribution of localized sources, as well as the governing mass transfer processes (e.g., diffusion path lengths, desorption rates). One possible approach is the dual porosity or dual domain formulation of the advective–dispersive equation (e.g., as in MT3D, Zheng and Wang, 1999; AFCEE, 2007), which can be configured to represent back-diffusion, although this approach has not been widely utilized and would require further refinement to represent highly localized zones of low permeability.
In the short term, credible predictions of time-to-complete remediation based on current modeling tools would be expected only for sites where back-diffusion and desorption are not expected to be significant factors. Such sites, if they exist, would probably be associated with relatively recent
contaminant releases. For example, Rivett et al. (2006) generated reasonable modeling predictions of a pump-and-treat field experiment associated with a relatively short (less-than-two-year) plume history. In contrast, Parker et al. (2008) identified back-diffusion as the primary factor responsible for the inability of aqueous transport models to simulate a controlled pump-and-treat study of a more typical (decades-old) plume.
Modeling Hydraulic Containment
While the prediction of time-to-complete remediation remains an elusive goal for mass removal technologies, the common application of pump and treat for hydraulic containment has been supported by ongoing developments to apply groundwater flow/transport models in an optimization framework. A variety of software packages are now available to identify well configurations that provide hydraulic containment while minimizing the overall extraction rates and/or treatment costs, including tools based on the MODFLOW/MT3D simulators. Recent applications of simulation/optimization to P&T design at several DoD facilities were summarized in Chapter 4 (EPA, 1999a,b, 2005). In general, the optimized designs were expected to yield an approximate average life-cycle savings of 10 to 20 percent over trial-and-error designs. However, the models used in these studies were based on conventional advective-dispersive-sorptive transport and did not include the various nonideal processes described above. Thus, while providing design guidance for efficient hydraulic containment, predictions of the time to achieve remedial objectives were likely optimistic.
Although the use of simulation/optimization techniques for hydraulic containment design is relatively mature, continuing developments will provide more realistic cost functions for scenarios in which expected costs are not proportional to the volume of extracted water. Also, because one goal of optimization is to find the least-cost solution to achieve hydraulic containment, optimized designs typically reflect a reduced margin of safety for plume capture, particular if the underlying flow model treats the aquifer as relatively homogeneous. Thus, it is important to account for uncertainty and spatial variability in a robust manner, and there are a number of promising techniques under development that have not yet been widely embraced by practitioners (e.g., Aly and Peralta, 1999; Gopalakrishnan et al., 2003; Guan and Aral, 2004; Bau and Mayer, 2007; Peralta, 2011).
Modeling Natural Attenuation
The decision to switch from an aggressive remediation strategy to MNA or natural attenuation without monitoring requires an estimate of post-remediation plume development, including (1) the amount of plume expansion (if any) that would occur under MNA, (2) the “steady state”
plume dimensions, and (3) the rate at which the resulting plume would be depleted. Although complex groundwater flow fields can affect plume development under MNA, for many sites the dominant concerns are biogeochemical reaction processes, which depend on the aquifer transformation capacity and reaction rates for the contaminants of interest. Thus, a simplified one-dimensional steady flow field can often be assumed, which facilitates a closed-form analytical solution to the governing equations for an idealized plane source coupled with multi-solute transport and biodegradation (e.g., BIOCHLOR, Aziz et al., 2000). Although widely used, the success of such screening models is dependent on accurate information concerning reaction rates, appropriate handling of the scale-dependent dispersion process, and accurate assessment of the source term, which is usually represented in terms of known contaminant concentrations or fluxes distributed over a vertical plane source. Recently, the plane source concept has been extended to include the time-dependent mass flux from a DNAPL source zone based on a power function approach, as implemented in the REMCHLOR software.
Screening models such as REMCHLOR represents a significant step forward in the practical application of the 14-compartment model, but its implementation emphasizes mass transfer from the source area to the transmissive plume. The release of contaminants from other potentially problematic compartments, such as low-permeability zones and the sorbed phase, has not yet been studied sufficiently to support implementation of these processes in a screening mode. In particular, the process of back-diffusion from the downgradient plume region is more difficult to conceptualize and approximate mathematically relative to source-zone release, as the geometry and history of the matrix diffusion process is more complex. Furthermore, for both source and plume regions, the potential influence of nonlinear and/or rate-limited desorption has received less attention, although researchers have long recognized the potential influence of nonideal sorption processes (e.g., Brusseau and Rao, 1989; Ball and Roberts, 1991; Rabideau and Miller, 1994; Allen-King et al., 2002; Rivett et al., 2006).
***
Predicting the trajectory of any remediation activity at complex sites (not just MNA) will require further research to clarify the conditions for which back-diffusion and desorption are likely to be contributing factors in the plume zone, development of better characterization tools to establish the necessary initial conditions for modeling, efficient computational power to incorporate the limiting nonideal processes into contaminant transport models, and the careful design of field studies to evaluate the resulting predictive capability of the models.
EMERGING REMEDIATION TECHNOLOGIES
Although the pace of remediation technology development has slowed considerably since the most recent NRC evaluation of source zone strategies (NRC, 2005), a few emerging technologies are in various stages of testing and could eventually provide additional cost-effective tools for managing complex sites. This section provides a snapshot of several emerging technologies, none of which, with exception of nanotechnologies, have received extensive field testing.
The preponderance of research on nanoparticles used in groundwater remediation has focused on nanoscale zero-valent iron (nZVI) and zero-valent iron doped with a catalytic metal (such as palladium). Contaminants amenable to treatment with nZVI include chlorinated methanes, ethanes, and ethenes (Lien and Zhang, 1999, 2005; Liu et al., 2005; Song and Carraway, 2005, 2006, 2008; Liu and Lowry, 2006), chlorinated phenols (Cheng et al., 2007), PCBs (Wang and Zhang, 1997), hexachlorocyclohexanes (Elliott et al., 2008), TNT (Welch and Riefler, 2008), nitrate (Sohn et al., 2006), perchlorate (Cao et al., 2005), chromate (Xu and Zhao, 2007; Hoch et al., 2008), arsenic (Ramos et al., 2009), and heavy metals (ZnII, CdII, PbII, NiII, CuII, and AgI; Li and Zhang, 2007). While the high surface area leads to high reaction rates, the nZVI particles tend to aggregate (Phenrat et al., 2007) when injected into the subsurface, which limits their transport in porous media (Hong et al., 2009). For this reasons, much effort has focused on the development of surface coatings that allow the nZVI to be injected into the subsurface and reach the contaminated area.
An EPA compilation of pilot- and full-scale tests with nZVI1 shows concentration reductions in the target zone of 50 to 90 percent (but sometimes lower), and there was evidence of contaminant rebound once the nZVI is exhausted. The location and amount of nZVI, flow rate, and DNAPL dissolution rate are all critical design parameters, such that emplacement of nZVI downstream of the DNAPL zone provides the best performance (Taghavy et al., 2010; Fagerlund et al., 2012). This latter finding suggests that use of larger-sized iron particles would be more cost effective unless the selectivity/lifetime of nZVI can be improved (Fagerlund et al., 2012). Overall, it appears that nZVI is best applied in limited situations to treat zones where the most contaminant could be removed in a short period of time. It should be kept in mind that while nanoparticles show some promise for remediation of groundwater pollutants, nanoparticles may also present a future environmental risk (Wiesner et al., 2006) and require additional research regarding their fate, transport, and toxicity.
For source zones that contain contaminants amenable to treatment
__________________
1 See http://www.clu-in.org/download/remed/nano-site-list.pdf.
with zero-valent iron, the in situ mixing of contaminated soil with zero-valent iron and clay (ZVI-clay) could afford two advantages: (1) the iron may accelerate the destruction of source zone contaminants, and (2) the clay may reduce contaminant transport from the source zone and redirect upgradient groundwater away from the source zone. While conceptually straightforward, ZVI-clay has received limited field testing (Shackelford et al., 2005; Bozzini et al., 2006; Olson et al., 2012). However, if proven successful, the general approach could be tailored to other contaminants using different reactive media.
The self-sustaining treatment for active remediation (STAR) technique has been proposed for treatment of creosote, petroleum hydrocarbons, and other combustible NAPLs. STAR is a controlled burning reaction (self-sustaining smoldering) that can be performed ex situ or in situ, even under fully saturated conditions. A heating element is introduced to the NAPL and heated to the NAPL ignition temperature. Air is then injected to initiate ignition. The heat released then serves to heat NAPL farther away, and the combustion process continues as long as sufficient air is supplied. Although the approach is best suited to readily combustible contaminants, laboratory studies have shown up to 99.9 percent removal of coal tar or crude oil (Switzer et al., 2009; Pironi et al., 2011), with pilot-scale tests ongoing.2
An alternative to traditional in situ chemical oxidation (ISCO) is the encapsulation of reactive agents in a permeable reactive ISCO barrier. This allows the slow release of the oxidant over time, supporting nearly continuous treatment of the plume until the oxidant is exhausted (Ross et al., 2005; Luster-Teasley et al., 2010; Liang et al., 2011). The release rate is dependent upon whether the design is based upon oxidant diffusion or erosion of the encapsulating polymer. In some cases, both processes control the release rate. The amount of oxidant added to the polymer matrix coupled with the release rate will dictate both its life cycle and effectiveness as an oxidant. The major challenge in utilizing this technology is optimizing the dose of oxidant needed to degrade the target contaminant with a slow enough release rate to minimize frequent media replacement.
In situ electrodes can be deployed for the sequential reduction and oxidation of contaminants (Wani et al., 2005, 2006) or for the generation of ozone (Vera et al., 2009). The electrode approach allows one to change the potentials of a cathode and anode such that the process responsible for the degradation of the contaminant of concern can be set to either reduction (e.g., production of hydrogen) or oxidation (e.g., generation of ozone or other reactive species). The mode of operation is dependent upon the target contaminants; many halogenated substances are more amenable to reduction processes, while BTEX and PAHs are better suited for oxidation.
__________________
This approach was shown to be effective for the reduction of both RDX and TNT (Wani et al., 2006) and does not require the use of chemical additives. The use of electrodes to produce ozone in situ (Vera et al., 2009) has an advantage over on-site ex situ ozone generators in that it is passive in nature, which circumvents the logistical difficulties of pumping ozone- saturated water into the contaminated zone, and it can continuously generate ozone at the site, which maintains a constant level of oxidant. None of these in situ electrode methods, however, has been tested at field sites, and both cost and scaling issues may be important.
As the focus at complex sites shifts from active remediation to long-term management, the development and effective deployment of appropriate concepts and tools may require a redirection of research efforts. The majority of support for research applicable to groundwater remediation has been provided by federal agencies, particularly EPA, the DoD, the National Science Foundation (NSF), the Department of Energy (DOE), and the National Institute for Environmental Health Sciences (NIEHS). The funding estimates shown in Table 6-1 were obtained primarily by searching
TABLE 6-1 Federal Research Funding for Groundwater Remediation
| Federal Program | Estimated Cumulative Funding ($M) (1996–2011)a | Number of Projects (1996–2011)a |
| Department of Defense | 315 | 250–300 |
| National Science Foundation (HS) | 2–4 | < 10 |
| National Science Foundation (CBET) | 25 | 80–110 |
| Department of Energy (including actinide research) | 138 | 200 |
| Environmental Protection Agency (including ten Hazardous Substance Research Centers) | 14 | 85 projects plus centers |
| National Institute for Environmental Health Sciences | 500–800b | Unknown |
aEstimated by the Committee through searches of public databases.
bThe $500-$800 million in research funding for NIEHS was primarily for research on human health impacts of contaminant exposure. Although relevant to groundwater remediation, the funding was not specifically directed to groundwater-specific contamination issues, and is thus not directly comparable to the other federal programs.
public databases using appropriate keywords (e.g., groundwater, remediation, etc.) but, in some cases, considerable judgment was required to distinguish relevant remediation research from more general environmental programs. Furthermore, some relevant projects were primarily focused on non-groundwater issues such as sediment remediation. It should be noted that other federal agencies not shown in Table 6-1, such as the U.S. Geological Survey (USGS), occasionally fund projects that have relevance to groundwater remediation (e.g., the USGS Water Resources Institutes). Although the Committee found it difficult to quantify historic research funding specifically applicable to groundwater remediation, its opinion is that such funding has generally declined over the past decade, with the single exception of the DoD. Additional details related to the primary agencies are given below.
DoD’s primary research mechanism is its Strategic Environmental Research and Development Program (SERDP) and Environmental Security and Technology Certification Program (ESTCP). SERDP, in partnership with EPA, supports a wide range of projects related to DoD-generated environmental issues, including diverse topics such as ecosystem effects to innovative subsurface remediation strategies. ESTCP constitutes DoD’s environmental technology demonstration program and involves no other federal partners.
Relevant NSF programs include the Division of Geosciences program in Hydrologic Sciences (HS), and several programs within the Division of Chemical Bioengineering, Environmental, and Transport Systems (CBET). Much of the Hydrologic Sciences research is focused on nano- to global-scale hydrologic and/or chemical processes. A review of this database yielded only a handful of projects that deal directly or indirectly with groundwater remediation over the past decade. Significantly more projects related to subsurface remediation/characterization have been funded through the NSF Division of Chemical Bioengineering, Environmental, and Transport Systems to address the development of tools for understanding contaminant behavior in the subsurface and remediation strategies. While the cumulative funding is significantly more than for the Hydrologic Sciences, the annual average is on the order of $1.4 million/year for remediation-focused research, with some projects addressing contaminants that are not the primary drivers for complex hazardous sites.
DOE funding for research on subsurface contamination is currently administered through the Office of Biological and Environmental Research. While a large number of proposals have been funded through this office, many of the projects are focused on actinide and inorganic contaminants, with fewer projects focused on chlorinated solvents and hydrocarbons, although a large number of funded projects have addressed microbial activity in the subsurface. Most of the sponsored research is conducted at the
National Labs (e.g., Oak Ridge, Pacific Northwest), often in conjunction with academic partnerships.
EPA has funded research on groundwater remediation through a variety of mechanisms, including its Science to Achieve Results (STAR) basic research program, several agency research laboratories, and externally funded Hazardous Substance Research Centers (which have been discontinued). The available database records were insufficient to provide more than an approximate estimate of overall funding levels across the external programs and it was difficult to distinguish in-house research from those projects awarded to universities.
Since 1987, NIEHS has operated the Superfund (Basic) Research Program, which is similar to the EPA Hazardous Substance Research Centers program in that it supports the operation of stand-alone research centers. Remediation-oriented projects have received a very small fraction of the overall funding, which has been largely directed toward human health rather than technology research.
In addition to federal agencies, some research funding for subsurface science and technology has been provided by state agencies and the private sector. In general, individual corporations fund both internal and external programs to address specific challenges important to fulfilling their obligations to protect human health and the environment via remediation. Contracting strategies vary from site-specific feasibility experiments to university contracts of sufficient duration to support full Ph.D. programs, often targeting basic “first-principles” research. As U.S. government research funding has declined, corporate research programs have sought international partners to provide matching funds, such as the Source Area in situ BioREmediation (SABRE) program centered in the United Kingdom.
It is important to note that research investments by government agencies and the private sector have yielded several innovative approaches to site remediation such as ZVI, ISCO, and thermal methods. Nonetheless, given budget constraints facing both government agencies and companies that fund remediation research, the development of more effective treatment technologies is likely to occur at a much reduced pace. Some private sector organizations are working with universities to pursue targeted and applied research on new solutions to legacy site issues. In addition, companies selling products and services in the remediation business continue to develop innovative technical strategies and products to improve all components of groundwater remediation. Whether this level of funding and other market-driven technical innovations will be sufficient to address the challenges of long-term management is uncertain. Other consequences of the lack of government funding are a reduction in support of graduate programs and the migration of students away from the remediation field. This may lead
to a shortage of qualified personnel over the next decade to respond to the long-term management issues of these complex sites.
CONCLUSIONS AND RECOMMENDATIONS
Many complex federal and private industrial facilities with contaminated groundwater will require long-term management actions that could extend for decades or longer. Technological developments can aid in the transition from active remediation to more passive strategies and provide more cost-effective and protective long-term management of complex sites. Further improvements in long-term site management are likely to emphasize more cost-effective containment, new diagnostic tools for performance and compliance monitoring, and modeling strategies that can be used for decision making. The following conclusions and recommendations are offered.
Long-term management of complex sites requires an appropriately detailed understanding of geologic complexity and the potential distribution of contaminants among the aqueous, vapor, sorbed, and NAPL phases, as well as the unique biogeochemical dynamics associated with both the source area and the downgradient plume. Recent improvements to the understanding of subsurface biogeochemical processes have not been accompanied by cost-effective site characterization methods capable of fully distinguishing the distribution of contaminants between different subsurface zones or compartments (as described by the recently proposed conceptual 14-compartment model). Management of residual contamination to reduce the exposure risks via the vapor intrusion pathway is challenged by the highly variable nature of exposure, as well as uncertain interactions between subsurface sources and indoor background contamination.
Existing protocols for assessing monitored natural attenuation and other remediation technologies should be expanded to integrate compound-specific isotope analysis and molecular biological methods with more conventional biogeochemical characterization and groundwater dating methods. The development of molecular and isotopic diagnostic tools has significantly enhanced the ability to evaluate the performance of degradation technologies and monitored natural attenuation at complex sites.
Mathematical models are increasingly important tools for key decision points in the management of complex sites, despite the inherent difficulty in predicting the time-to-complete remediation. In particular, the use of more realistic models that can account for processes that significantly decrease the rate of mass removal during remediation is critical for deciding whether to transition to active or passive long-term management. The implemen-
tation of site-specific models is often constrained by the lack of spatially detailed information about contaminant distributions and/or reaction processes. While modeling predictions will always be subject to uncertainty due to the inherent limitations in site characterization and the high degree of heterogeneity, accurate models can bound the likely timeframes for restoration and provide another line of evidence needed to make decisions on the ultimate disposition of complex sites.
Although the Committee did not attempt a comprehensive assessment of research needs, research in the following areas would help address technical challenges associated with long-term management at complex contaminated sites:
• Remediation Technology Development. Additional work is needed to advance the development of emerging and novel remediation technologies, improve their performance, and understand any potential broader environmental impacts. A few developing remediation techniques could provide more cost-effective remediation for particular combinations of contaminants and site conditions at complex sites, but they are in the early stages of development.
• Tools for Characterizing Complex Subsurface Conditions. More refined concepts and tools are needed to better delineate and manage localized contamination associated with the processes of back-diffusion and desorption, complex geologic environments, and aquifer transformation capacity. These include better characterization of subsurface media to assess the magnitude of back-diffusion and desorption processes and to identify the type and abundances of naturally occurring reactive chemical species and microorganisms that are involved in natural biodegradation processes.
• Tools to Assess Vapor Intrusion. Further research and development should identify, test, and demonstrate tools and paradigms that are practicable for assessing the significance of vapor intrusion, especially for multi-building sites and preferably through short-term diagnostic tests. Development of real-time unobtrusive and low-cost air quality sensors would allow verification of those short-term results over longer times at buildings not needing immediate mitigation.
• Molecular Biological Tools and Databases. Robust databases are necessary to conduct molecular biological analyses for various contaminant degradation pathways, in conjunction with further refinement of field sampling protocols. There is also a need for better methods of protein extraction from environmental matrices, as well as more cost effective methods to detect specific peptides that do
not require knowledge of the exact gene sequence. For CSIA, data analysis tools for enrichment factors should be expanded to address a wider range of groundwater pollutants at low concentrations and should consider the effects of bioavailability and mass transfer limitations.
• Modeling. Additional targeted modeling research and software development that will benefit the transition of sites from active remediation to long-term management should be initiated. Particular needs include concepts and algorithms for including the processes of back-diffusion and desorption in screening and plume models, and the development of a larger suite of intermediate-complexity modeling tools to support engineering design for source remediation.
Overall, research and development have been unable to keep pace with the needs of practitioners trying to conduct remediation on complex sites. Currently, a national strategy for technology development to support long-term management of complex sites is lacking. It is not clear that the pertinent federal agencies will be capable of providing the funding and other support for the fundamental research and development that is necessary to meet the challenges facing complex sites. A comprehensive assessment of future research needs, undertaken at the federal level and involving coordination between federal agencies, would allow research funding to be allocated in an efficient and targeted manner.
Aelion, C. M., R. Aravena, D. Hunkeler, and P. Hoehnener (Eds.). 2010. Environmental Isotopes in Biodegradation and Bioremediation. Boca Raton, FL: CRC Press.
Aeppli, C., M. Berg, O. A. Cirpka, C. Holliger, R. P. Schwarzenbach, and T. B. Hofstetter. 2009. Influence of mass-transfer limitations on carbon isotope fractionation during microbial dechlorination of trichloroethene. Environmental Science & Technology 43(23):8813-8820.
AFCEE (Air Force Center for Engineering and the Environmental). 2007. AFCEE Source Zone Initiative, Final Report, Colorado State University, Boulder CO, 234 pp.
Allen-King, R. M., P. Grathwohl, and W. P. Ball. 2002. New modeling paradigms for the sorption of hydrophobic organic chemicals to heterogeneous carbonaceous matter in soils, sediments, and rocks. Advances in Water Resources 25:985-1016.
Aly, A. H., and R. C. Peralta. 1999. Optimal design of aquifer cleanup systems under uncertainty using a neural network and genetic algorithm. Water Resources Research 35(8):2523-2532.
Amaral, H. I. F., J. Fernades, M. Berg, R. P. Schwarzenbach, and R. Kipfer. 2009. Assessing TNT and DNT groundwater contamination by compound-specific isotope analysis and 3H–3He groundwater dating: A case study in Portugal. Chemosphere 77:805-812.
Amaral, H. I. F., M. Berg, M. S. Brennwald, M. Hofer, and R. Kipfer. 2010. 13C/12C analysis of ultra-trace amounts of volatile organic contaminants in groundwater by vacuum extraction. Environmental Science & Technology 44(3):1023-1029.
Annable, M. D., P. S. C. Rao, K. Hatfield, W. D. Graham, A. L. Wood, and C. G. Enfield. 1998. Partitioning tracers for measuring residual NAPL: Field-scale test results. Journal of Environmental Engineering 124:498-503.
Annable, M. D., K. Hatfield, J. Cho, H. Klammler, B. L. Parker, J. A. Cherry, and P. S. C. Rao. 2005. Field-scale evaluation of the passive flux meter for simultaneous measurement of groundwater and contaminant fluxes. Environmental Science & Technology 39(18):7194-7201.
Aziz, C. E., C. J. Newell, J. R. Gonzales, P. Haas, T. P. Clement, and Y. Sun. 2000. BIOCHLOR: Natural Attenuation Decision Support System Version 1.0, User’s Manual. EPA/600/R-00/008. Ada, OK: EPA ORD. http://www.epa.gov/ada/csmos/models/biochlor.html.
Babbar-Sebens, M., and B. Minsker. 2008. Standard interactive genetic algorithm—comprehensive optimization framework for groundwater monitoring design. Journal of Water Resources Planning and Management 134(6):538-547.
Ball, W. P., and P. V. Roberts. 1991. Long-term sorption of halogenated organic-chemicals by aquifer material. 1. Equilibrium. Environmental Science & Technology 25(7):1223-1237.
Ball, W., C. Liu, G. Xia, and D. F. Young. 1997. A diffusion-based interpretation of tetrachloroethene and trichloroethene concentration profiles in a groundwater aquitard. Water Resources Research 33(12):2741-2757.
Basu, N. B., P. S. C. Rao, I. C. Poyer, M. D. Annable, and K. Hatfield. 2006. Flux-based assessment at a manufacturing site contaminated with trichloroethylene. Journal of Contaminant Hydrology 86(1-2):105-127.
Basu, N. B., A. D. Fure, and J. W. Jawitz. 2008. Predicting DNAPL dissolution using a simplified source depletion model parameterized with partitioning tracers. Water Resources Research 44(7):W07414.1-13, doi:10.1029/2007WR006008.
Bau, D. A., and A. S. Mayer. 2007. Data-worth analysis for multiobjective optimal design of pump-and-treat remediation systems. Advances in Water Resources 30(12):1815-1830.
Bauer, S., M. Bayer-Raich, T. Holder, C. Kolesar, D. Muller, and T. Ptak. 2004. Quantification of groundwater contamination in an urban area using integral pump tests. Journal of Contaminant Hydrology 75(3-4):183-213.
Belle, A., A. Tanay, L. Bitincka, R. Shamir, and E. K. O’Shea. 2006. Quantification of protein half-lives in the budding yeast proteome. Proceedings of the National Academy of Sciences 103(35):13004-13009.
Beller, H. R., S. R. Kane, T. C. Legler, J. R. McKelvie, B. Sherwood Lollar, F. Pearson, L. Balser, and D. M. MacKay. 2008. Comparative assessments of benzene, toluene, and xylene natural attenuation by quantitative polymerase chain reaction analysis of a catabolic gene, signature metabolites, and compound-specific isotope analysis. Environmental Science & Technology 42(16):6065-6072.
Benndorf, D., C. Vogt, N. Jehmlich, Y. Schmidt, H. Thomas, G. Woffendin, A. Shevchenko, H. H. Richnow, and M. von Bergen. 2009. Improving protein extraction and separation methods for investigating the metaproteome of anaerobic benzene communities within sediments. Biodegradation 20(6):737-750.
Blessing, M., M. A. Jochmann, and T. C. Schmidt. 2008. Pitfalls in compound-specific isotope analysis of environmental samples. Analytical and Bioanalytical Chemistry 390(2):591-603.
Bockelmann, A., T. Ptak, and G. Teutsch. 2001. An analytical quantification of mass fluxes and natural attenuation rate constants at a former gasworks site. Journal of Contaminant Hydrology 53(3-4):429-453.
Bombach, P., H. H. Richnow, M. Kastner, and A. Fischer. 2010. Current approaches for the assessment of in situ biodegradation. Applied Microbiology and Biotechnology 86:839-852.
Borch, T., R. Kretzschmar, A. Kappler, P. Van Cappellen, M. Ginder-Vogel, A. Voegelin, and K. Campbell. 2010. Biogeochemical redox processes and their impact on contaminant dynamics. Environmental Science & Technology 44 (1):15-23.
Bozzini, C., T. Simpkin, T. Sale, D. Hood, and B. Lowder. 2006. DNAPL remediation at Camp Lejeune using ZVI-clay soil mixing. Proceedings of the International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, May 22-25.
Brooks, M. C., M. D. Annable, P. S. C. Rao, K. Hatfield, J. W. Jawitz, W. R. Wise, A. L. Wood, and C. G. Enfield. 2002. Controlled release, blind tests of DNAPL characterization using partitioning tracers. Journal of Contaminant Hydrology 59:187–210.
Brooks, M. C., A. L. Wood, M. D. Annable, K. Hatfield, J. Cho, C. Holbert, P. S. C. Rao, C. G. Enfield, K. Lynch, and R. E. Smith. 2008. Changes in contaminant mass discharge from DNAPL source mass depletion: Evaluation at two field sites. Journal of Contaminant Hydrology 102(1):140-153.
Brusseau, M. L., and P. S. C. Rao. 1989. Sorption nonideality during organic contaminant transport in porous media. CRC Critical Reviews in Environmental Control 19(1):33-99.
Burgmann, H., J. Kleikemper, L. Duc, M. Bunge, M. H. Schroth, and J. Zeyer. 2008. Detection and quantification of dehalococcoides-related bacteria in a chlorinated ethene-contaminated aquifer undergoing natural attenuation. Bioremediation Journal 12(4):193-209.
Buscheck, T. 2002. Mass Flux Estimates to Assist Decision-Making: Technical Bulletin. Version 1.0. ChevronTexaco Energy Research and Technology Company, June 2002.
Cao, J., D. Elliott, and W.-X. Zhang. 2005. Perchlorate reduction by nanoscale iron particles. Journal of Nanoparticle Research 7(4-5):499-506.
Carey, G. R., P. J. Van Geel, and J. R. Murphy. 1999. BIOREDOX-MT3DMS V2.0: A Coupled Biodegradation-Redox Model for Simulating Natural and Enhanced Bioremediation of Organic Pollutants – User’s Guide. Waterloo, Ontario: Conestoga-Rovers & Associates.
Carreon-Diazconti, C., J. Santamaria, J. Berkompas, J. A. Field, and M. L. Brusseau. 2009. Assessment of in situ reductive dechlorination using compound-specific stable isotopes, functional gene PCR, and geochemical data. Environmental Science & Technology 43(12):4301-4307.
Chapman, S. W., and B. L. Parker. 2005. Plume persistence due to aquitard back diffusion following dense nonaqueous phase liquid source removal or isolation. Water Resources Research 41. doi:10.1029/2005WR004224.
Chapman, S. W., B. Parker, J. Cherry, J. Langenbach, and S. Thotapalli. 2010. Performance of an Innovative Hydraulic Capture System for DNAPL Source Cutoff. 2010 Battelle Conference, Remediation of Chlorinated and Recalcitrant Compounds. May 25, 2010.
Cheng, R., J. L. Wang, and W.-X. Zhang. 2007. Comparison of reductive dechlorination of p-chlorophenol using Fe0 and nanosized Fe0. Journal of Hazardous Materials 144(1-2):334-339.
Chuang, A. S., Y. O. Jin, L. S. Schmidt, Y. Li, S. Fogel, D. Smoler, and T. E. Mattes. 2010. Proteomic analysis of ethene-enriched groundwater microcosms from a vinyl chloride-contaminated site. Environmental Science & Technology 44(5):1594-1601.
Clement, T. P. 1997. RT3D - A modular computer code for simulating reactive multi-species transport in 3-dimensional groundwater aquifers. Battelle Pacific Northwest National Laboratory Research Report, PNNL-SA-28967. http://bioprocess.pnl.gov/rt3d.htm.
Culler, D., D. Estrin, and M. Sirivastava. 2004. Overview of sensor networks. IEEE Computer Society 37(8):41-49.
Da Silva, M. L. B., R. L. Johnson, and P. J. J. Alvarez. 2007. Microbial characterization of groundwater undergoing treatment with a permeable reactive iron barrier. Environmental Engineering Science 24(8):1122-1127.
Davey, N. G., E. T. Krogh and C. G. Gill. 2011. Membrane-introduction mass spectrometry (MIMS). TrAC Trends in Analytical Chemistry 30(9):1477-1485.
DOE (Department of Energy). 2009. Draft Tank Closure and Waste Management Environmental Impact Statement for the Hanford Site, Richland, Washington. DOE/EIS-0391. Richland, WA: Department of Energy.
Dunnivant, F. M., R. P. Schwarzenbach, and D. L. Macalady. 1992. Reduction of substituted nitrobenzenes in aqueous solutions containing natural organic matter. Environmental Science & Technology 26(11):2133-2141.
Einarson, M. 2006. Multilevel ground-water monitoring. Pp. 808-845 In D.M. Nielsen (Ed.), Practical Handbook of Environmental Site Characterization and Ground-Water Monitoring (2nd ed.). Boca Raton, FL: CRC Press.
Einarson, M. D., D. M. Mackay, and P. J. Bennett. 2010. Sampling transects for affordable, high-resolution plume characterization and monitoring. Ground Water 48(6):799-808.
Eixarch, H., and M. Constanti. 2010. Biodegradation of MTBE by Achromobacter xylosoxidans MCM1/1 induces synthesis of proteins that may be related to cell survival. Process Biochemistry 45(5):794-798.
Elias, D. A., F. Yang, H. M. Mottaz, A. S. Beliaev, and M. S. Lipton. 2007. Enrichment of functional redox reactive proteins and identification of mass spectrometry results in several terminal Fe(III)-reducing candidate proteins in Shewanella oneidensis MR-1. Journal of Microbiological Methods 68(2):367-375.
Elliott, D. W., H.-L. Lien, and W.-X. Zhang. 2008. Zerovalent iron nanoparticles for treatment of ground water contaminated by hexachlorocyclohexanes. Journal of Environmental Quality 37(6):2192-2201.
Elsner, M., L. Zwank, D. Hunkeler, and R. P. Schwarzenbach. 2005. A new concept linking observable stable isotope fractionation to transformation pathways of organic pollutants. Environmental Science & Technology 39:6896-6916.
Elsner, M., M. Chartrand, N. VanStone, G. Lacrampe Couloume, and B. Sherwood Lollar. 2008. Identifying abiotic chlorinated ethene degradation: Characteristic isotope patterns in reaction products with nanoscale zero-valent iron. Environmental Science & Technology 42(16):5963-5970.
Elsner, M., G. Lacrampe-Couloume, S. Mancini, L. Burns, and B. Sherwood Lollar. 2010. Carbon isotope analysis to evaluate nanonscale Fe(0) treatment at a chlorohydrocarbon contaminated site. Groundwater Monitoring & Remediation 30(3):79-95.
EPA (U.S. Environmental Protection Agency). 1999a. Hydraulic optimization demonstration for groundwater pump and treat systems, volume I: Pre-optimization screening (method and demonstration). EPA/542/R 99/011A. Washington, DC: Office of Research and Development and Office of Solid Waste and Emergency Response.
EPA. 1999b. Hydraulic optimization demonstration for groundwater pump and treat systems, volume II: Application of hydraulic optimization. EPA/542/R-99/011B. Washington, DC: Office of Research and Development and Office of Solid Waste and Emergency Response.
EPA. 2002. Draft Guidance for Evaluating the Vapor Intrusion to Indoor Air Pathway from Groundwater and Soils (Subsurface Vapor Intrusion Guidance). Washington, DC: EPA OSWER. http://www.epa.gov/correctiveaction/eis/vapor/guidance.pdf.
EPA. 2003. A review of emerging sensor technologies for facilitating long-term ground water monitoring of volatile organic compounds. EPA/542/R-03/007. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2004. Demonstration of two long-term groundwater monitoring approaches. EPA/542/R-04/001A. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2005. O&M Report Template for Ground Water Remedies (with Emphasis on Pump and Treat Systems). EPA 542-R-05-010. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2009. Impacts of DNAPL Source Treatment: Experimental and Modeling Assessment of the Benefits of Partial DNAPL Source Removal. 600-09-R-096. Ada, OK: EPA ORD.
EPA. 2011a. Background Indoor Air Concentrations of Volatile Organic Compounds in North American Residences (1990–2005): A Compilation of Statistics for Assessing Vapor Intrusion. EPA 530-R-10-001. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2011b. Petroleum Hydrocarbons and Chlorinated Hydrocarbons Differ in Their Potential for Vapor Intrusion. Washington, DC: EPA Office of Underground Storage Tanks.
EPA. 2012a. Conceptual Model Scenarios for the Vapor Intrusion Pathway. EPA 530-R-10-003. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2012b. EPA’s Vapor Intrusion Database: Evaluation and Characterization of Attenuation Factors for Chlorinated Volatile Organic Compounds and Residential Buildings. EPA 530-R-10-003. EPA 530-R-10-002. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA. 2012c. Superfund Vapor Intrusion FAQs. Washington, DC: EPA Office of Solid Waste and Emergency Response.
EPA and USACE (U.S. Army Corps of Engineers). 2005. Road map to long-term monitoring optimization. EPA/542/R-05/003. Washington, DC: EPA Office of Research and Development.
Fagerlund, F., T. H. Illangasekare, and A. Niemi. 2007a. Nonaqueous-Phase Liquid Infiltration and Immobilization in Heterogeneous Media: 1. Experimental Methods and Two-Layered Reference. Vadose Zone Journal 6:471-482.
Fagerlund, F, T. H. Illangasekare, and A. Niemi. 2007b. Nonaqueous-phase liquid infiltration and immobilization in heterogeneous media: 2. Application to stochastically heterogeneous formations. Vadose Zone Journal 6:483-495.
Fagerlund, F., T. H. Illangaserkare, T. Phenrat, H.-J. Kim, and G. V. Lowry. 2012. PCE dissolution and simultaneous dechlorination by nanoscale zero-valent iron particles in a DNAPL source zone. Journal of Contaminant Hydrology 131:9-28.
Fisher, A., K. Theuerkorn, N. Stelzer, M. Gehre, M. Thullner, and H. H. Richnow. 2007. Applicability of stable isotope fractionation analysis for the characterization of benzene biodegradation in a BTEX-contaminated aquifer. Environmental Science & Technology 41(10):3689-3969.
Fuller, M. E., and R. J. Stefan. 2008. ER-1378 groundwater chemistry and microbial ecology effects on explosives biodegradation. Final report for the Strategic Environmental Research and Development Program.
Gao, J., L. B. M. Ellis, and L. P. Wackett. 2010. The University of Minnesota biocatalysis/biodegradation database: Improving public access. Nucleic Acids Research 38:D488-D491.
Glover, K., J. Munakata-Marr, and T. H. Illangasekare. 2007. Biologically-enhanced mass transfer of tetrachloroethene from dnapl in source zones: Experimental evaluation and influence of pool morphology. Environmental Science & Technology 41(4):1384-1389.
Goltz, M. N., Kim, S., Yoon, H., and Park J. 2007. Review of groundwater contaminant mass flux measurement. Environmental Engineering Research 12(4):176-193.
Gopalakrishnan, G., B. S. Minsker, and D. E. Goldberg. 2003. Optimal sampling in a noisy genetic algorithm for risk-based remediation design. Journal of Hydroinformatics 5:11-25.
Gorder, K. A., and E. M. Dettemaier. 2011. Portable GC/MS methods to evaluate sources of cVOC contamination in indoor air. Ground Water Monitoring & Remediation 31(4):113-119.
Guan, J., and M. M. Aral. 2004. Optimal design of groundwater remediation systems using fuzzy set theory. Water Resources Research 40(1):W01518.
Guilbeault, M. A., B. L. Parker, and J. A. Cherry. 2005. Mass and flux distributions from DNAPL zones in sandy aquifers. Ground Water 43(1):70-86.
Haenggi, M. 2005. Opportunities and challenges in Wireless Sensor Networks. In: Handbook of Sensor Networks: Compact Wireless and Wired Sensing Systems. M. Ilyas and I. Mahgoub (eds.). Boca Raton, FL: CRC Press. Hakala, J. A., and Y.-P. Chin. 2010. Abiotic reduction of pendimethalin and trifluralin in controlled and natural systems containing Fe(ii) and dissolved organic matter. Journal of Agriculture and Food Chemistry 58(24):12840-12846.
Hakala J. A., Y.-P. Chin, and E. J. Weber. 2007. Influence of dissolved organic matter and Fe(ii) on the abiotic reduction of pentachloronitrobenzene. Environmental Science & Technology 41:7337-7342.
Hay-Wilson, L., P. C. Johnson, and J. Rocco. 2005. Collecting and interpreting soil gas samples from the vadose zone: A practical strategy for assessing the subsurface-vapor-to-indoor-air migration pathway at petroleum hydrocarbon sites. American Petroleum Institute. Publication Number 4741.
He, Y. T., C. Su, J. T. Wilson, R. T. Wilkin, C. Adair, T. Lee, P. Bradley, and M. Ferrey. 2009. Identification and characterization methods for reactive minerals responsible for natural attenuation of chlorinated organic compounds in ground water. EPA 600/R-09/115. Ada, OK: EPA Office of Research and Development.
Heiderscheidt, J. L. 2005. DNAPL Source Zone Depletion During In Situ Chemical Oxidation (ISCO): Experimental and Modeling Studies. PhD Dissertation, Environmental Science and Engineering Division, Colorado School of Mines, Golden, CO.
Heiderscheidt, J., R. L. Siegrist, and T. H. Illangasekare. 2008. Intermediate-scale 2D experimental investigation of in situ chemical oxidation using potassium permanganate for remediation of complex DNAPL source zones. Journal of Contaminant Hydrology 102(1-2):3-16.
Hendrickson, E. R., J. Payne, R. M. Young, M. G. Starr, M. P. Perry, S. Fahnestock, D. E. Ellis, R. C. Ebersole. 2002. Molecular analysis of dehalococcoides 16S ribosomal DNA from chloroethene-contaminated sites throughout North America and Europe. Applied and Environmental Microbiology 68(2):485-495.
Hendrickx, B., W. Dejonghe, F. Faber, W. Boenne, L. Bastiaens, W. Verstraete, E. M. Top, and D. Springael. 2006. PCR-DGGE method to assess the diversity of BTEX mono-oxygenase genes at contaminated sites. FEMS Microbiology Ecology 55(2):262-273.
Hoch, L. B., E. J. Mack, B. W. Hydutsky, J. M. Hershman, J. M. Skluzacek, and T. E. Mallouk. 2008. Carbothermal synthesis of carbon-supported nanoscale zero-valent iron particles for the remediation of hexavalent chromium. Environmental Science & Technology 42(7):2600-2605.
Hofstetter, T. B., C. G. Heijman, S. B. Haderlein, C. R. Holliger, and R. P. Schwarzenbach. 1999. Complete reduction of TNT and other (poly)nitroaromatic compounds under iron-reducing subsurface conditions. Environmental Science & Technology 33:1479-1487.
Hofstetter, T. B., R. P. Schwarzenbach, and S. B. Haderlein. 2003. Reactivity of Fe(II) species associated with clay minerals. Environmental Science & Technology 37:519-528.
Hong, Y., R. J. Honda, N. V. Myung, and S. L. Walker. 2009. Transport of iron-based nanoparticles: Role of magnetic properties. Environmental Science & Technology 43(23):8834-8839.
Hunkeler, D., R. U. Meckenstock, B. Sherwood Lollar, T. C. Schmidt, and J. T. Wilson. 2008. A Guide for Assessing Biodegradation and Source Identification of Organic Ground Water Contaminants using Compound Specific Isotope Analysis. EPA report 600/R-08/148.
Illangasekare, T. H., J. L. Ramsey, K. H. Jensen, and M. Butts. 1995. Experimental study of movement and distribution of dense organic contaminants in heterogeneous aquifers. Journal of Contaminant Hydrology 20:1-25.
Illangasekare, T. H., J. Munakata Marr, R. L. Siegrist, K. Soga, K. C. Glover, E. Moreno-Barbero, J. L. Heiderscheidt, S. Saenton, M. Matthew, A. R. Kaplan, Y. Kim, D. Dai, J. L. Gago, and J. W. E. Page. 2007. Mass Transfer from Entrapped DNAPL Sources Undergoing Remediation: Characterization Methods and Prediction Tools, SERDP Project CU-1294.
Imfeld, G., C. Estop Aragones, S. Zeiger, C. V. von Eckstadt, H. Paschke, R. Trabitzsch, H. Weiss, and H. H. Richnow. 2008. Tracking in situ biodegradation of 1,2-dichloroethenes in a model wetland. Environmental Science & Technology 42(21):7924-7930.
ITRC (Interstate Technology & Regulatory Council). 2004. Strategies for Monitoring the Performance of DNAPL Source Zone Remedies. http://www.itrcweb.org/Documents/DNAPLs-5.pdf.
ITRC. 2007. Vapor Intrusion Pathway: A Practical Guideline. Washington, DC: Interstate Technology Regulatory Council. http://www.itrcweb.org/gd_VI.asp.
ITRC. 2010. Use and Measurement of Mass Flux and Mass Discharge. MASSFLUX-1. Washington, DC: ITRC Integrated DNAPL Site Strategy Team. ITRC. 2011. Technical and Regulatory Guidance: Integrated Strategies for Chlorinated Solvent Sites; Interstate Technology & Regulatory Council: Washington, DC.
Jennings, L. K., M. M. G. Chartrand, G. Lacrampe-Couloume, B. Sherwood Lollar, J. C. Spain, and J. M. Gossett. 2009. Proteomic and transcriptomic analyses reveal genes upregulated by cis-dichloroethene in Polaromonas sp. strain JS666. Applied and Environmental Microbiology 75(11):3733-3744.
Johnson, P. C., M. W. Kemblowski, and R. L. Johnson. 1999. Assessing the significance of subsurface contaminant vapor migration to enclosed spaces: Site-specific alternatives to generic estimates. Journal of Soil Contamination 8(3):389-421.
Johnson, P. C., H. Luo, C. Holton, P. Dahlen, and Y. Guo. 2012. Vapor intrusion above a dilute CHC plume: Lessons-learned from two years of monitoring. EPA-AEHS Workshop, Recent Advances to VI Application & Implementation, 20 March 2012, San Diego. https://iavi.rti.org/WorkshopsAndConferences.cfm.
Kampara, M., M. Thullner, H. H. Richnow, H. Harms, and L. Y. Wick. 2008. Impact of bioavailability restrictions on microbially induced stable isotope fractionation. 2. Experimental evidence. Environmental Science & Technology 42(17):6552-6558.
Kao, C. M., H. Y. Chien, R. Y. Surampalli, C. C. Chien, and C. Y. Chen. 2010. Assessing of natural attenuation and intrinsic bioremediation rates at a petroleum-hydrocarbon spill site: Laboratory and field studies. Journal of Environmental Engineering 136(1):54-67.
Kappler, A., and S. B. Haderlein. 2003. Natural organic matter as reductant for chlorinated aliphatic pollutants. Environmental Science & Technology 37:2707-2713.
Kappler A., and K. L. Straub. 2005. Geomicrobiological cycling of iron. Reviews in Mineralogy and Geochemistry 59:85-108.
Kavanaugh, M., and R. Deeb. 2011. Diagnostic Tools for Performance Evaluation of Innovative In-Situ Remediation Technologies at Chlorinated Solvent-Contaminated Sites. ER-200318. Washington, DC: ESTCP.
Kennedy, L. G., J. W. Everett, and J. Gonzales. 2006. Assessment of biogeochemical natural attenuation and treatment of chlorinated solvents, Altus Air Force Base, Altus, Oklahoma. Journal of Contaminant Hydrology 83(3-4):221-236.
Kram, L. K., Keller, A. L, Rossabi, J., and Everett, L. G. 2001. DNAPL characterization methods and approaches, Part 1: Performance comparisons. Ground Water Monitoring and Remediation 21(4):109-123.
Lee, P. K. H., T. W. Macbeth, K. S. Sorenson Jr, R. A. Deeb, and L. Alvarez-Cohen. 2008. Quantifying genes and transcripts to assess the in situ physiology of Dehalococcoides spp. in a trichloroethene-contaminated groundwater site. Applied and Environmental Microbiology 74(9):2728-2739.
Li, X., and W.-X. Zhang. 2007. Sequestration of metal cations with zerovalent iron nanoparticles—A study with high resolution x-ray photoelectron spectroscopy (HR-XPS). Journal of Physical Chemistry C 111(19):6939-6946.
Liang, H., R. Falta, C. Newell, S. Farhat, S. Rao, and N. Basu. 2011. Decision and Management Tools for DNAPL Sites: Optimization of Chlorinated Solvent Source and Plume Remediation Considering Uncertainty. ESTCP Project ER-200704.
Lieberman, S. 2007. Direct Push Chemical Sensors for DNAPL. ER-0109. Washington, DC: ESTCP.
Lien, H. L., and W.-X. Zhang. 1999. Transformation of chlorinated methanes by nanoscale iron particles. Journal of Environmental Engineering 125(11):1042-1047.
Lien, H. L., and W.-X. Zhang. 2005. Hydrodechlorination of chlorinated ethanes by nanoscale Pd/Fe bimetallic particles. Journal of Environmental Engineering 131(1):4-10.
Liu, Y., and G. V. Lowry. 2006. Effect of particle age (Fe0 Content) and solution pH on NZVI reactivity: H2 evolution and TCE dechlorination. Environmental Science & Technology 40(19):6085-6090.
Liu, Y., S. A. Majetich, R. D. Tilton, D. S. Sholl, and G. V. Lowry. 2005. TCE dechlorination rates, pathways, and efficiency of nanoscale iron particles with different properties. Environmental Science & Technology 39(5):1338-1345.
Lovley, D. R., E. E. Roden, E. J. P. Phillips, and J. C. Woodward. 1993. Enzymatic iron and uranium reduction by sulfate-reducing bacteria. Marine Geology 113(1-2):41-53.
Lu, X., J. T. Wilson, and D. H. Kampbell. 2006. Relationship between dehalococcoides DNA in ground water and rates of reductive dechlorination at field scale. Water Research 40(16):3131-3140.
Luo, H., P. Dahlen, P. C. Johnson, T. Peargin, and T. Creamer. 2009. Spatial variability of soil-gas concentrations near and beneath a building overlying shallow petroleum hydrocarbon–impacted soils. Ground Water Monitoring & Remediation 29(1):81-91.
Luo, H., P. Dahlen, and P. C. Johnson. 2010. Hydrocarbon and oxygen transport in the vicinity of a building overlying a NAPL source zone. Air and Waste Management Association: Vapor Intrusion 2010:155-185.
Luster-Teasley, S., P. Onochie, and V. Shirley. 2010. Encapsulation of Potassium Permanganate Oxidant in Biodegradable Polymers to Develop a Novel Form of Controlled Release Remediation. In: Emerging Environmental Technologies, Volume 2. Vishal Shah (ed.) Springer.
MacKay, D. M., and J. A. Cherry. 1989. Groundwater contamination: Pump-and-treat remediation. Environmental Science & Technology 23(6):630-636.
MacMillan, D. K., and D. E. Splichal. 2005. A review of field technologies for long-term monitoring of ordnance-related compounds in groundwater. ERDC/EL TR-05-14. Vicksburg, MS: U.S. Army Engineer Research and Development Center. McAlary, T., R. A. Ettinger, and P. C. Johnson. 2005. Reference Handbook for Site-Specific Assessment of Subsurface Vapor Intrusion to Indoor Air. EPRI Technical Report 1008492. Palo Alto, CA.
McDonald, M. G., and A. W. Harbaugh. 1988. A modular three-dimensional finite-difference ground-water flow model. Techniques of Water-Resources Investigations. Book 6. U.S. Geological Survey.
McHugh, T., R. Davis, G. DeVaull, H. Hopkins, J. Menatti, and T. Peargin. 2010. Evaluation of vapor attenuation at petroleum hydrocarbon sites. Soil and Sediment Contamination: An International Journal 19(6):725-745.
McKelvie, J. R., S. K. Hirschorn, G. Lacrampe-Couloume, J. Lindstrom, J. Braddock, K. Finneran, D. Trego, and B. Sherwood Lollar. 2007. Evaluation of TCE and MTBE in situ biodegradation: Integrating stable isotope, metabolic intermediate, and microbial lines of evidence. Ground Water Monitoring & Remediation 27(4):63-73.
Mercer, J. W., R. M. Cohen, and M. R. Noel. 2010. DNAPL site characterization issues at chlorinated solvent sites. Pp. 217-280 in In Situ Remediation of Chlorinated Solvent Plumes. Stroo, H. F., Ward, C. H., Eds. New York: Springer.
Moreno-Barbero, E., and T. H. Illangasekare. 2005. Simulation and performance assessment of partitioning tracer tests in heterogeneous aquifers. Environmental & Engineering Geoscience XI(4):395-404.
Moreno-Barbero, E., and T. H. Illangasekare. 2006. Influence of pool morphology on the performance of partitioning tracer tests: evaluation of the equilibrium assumption. Water Resources Research 42:11. doi:10.1029/2005WR004074.
Morris, R. M., J. M. Fung, B. G. Rahm, S. Zhang, D. L. Freedman, S. H. Zinder, and R. E. Richardson. 2007. Comparative proteomics of Dehalococcoides spp. reveals strain-specific peptides associated with activity. Applied and Environmental Microbiology 73(1):320-326.
Nesatyy, V. J., and M. J. F. Suter. 2007. Proteomics for the analysis of environmental stress responses in organisms. Environmental Science & Technology 41(20):6891-6900.
Neumann, A., T. B. Hofstetter, M. Skarpeli-Liati, and R. R. Schwarzenbach. 2009. Reduction of polychlorinated ethanes and carbon tetrachloride by structural Fe(II) in smectites. Environmental Science & Technology 43(11):4082-4089.
NRC (National Research Council). 2005. Contaminants in the Subsurface. Washington, DC: National Academies Press.
NYSDOH (New York State Department of Health). 2006. Final NYSDOH CEH BEEI Soil Vapor Intrusion Guidance. http://www.health.state.ny.us/environmental/investigations/soil_gas/svi_guidance/.
Olson, M. R., T. C. Sale, C. D. Shackelford, C. Bozzini, and J. Skeean. 2012. Chlorinated solvent source-zone remediation via ZVI-clay soil mixing: 1-year results. Ground Water Monitoring & Remediation 32:63–74. doi:10.1111/j.1745-6592.2011.01391.x.
Ouyang, G., and J. Pawliszyn. 2006. Recent developments in SPME for on-site analysis and monitoring. Trends in Analytical Chemistry 25(7):692-703.
Parker, B. L., S. W. Chapman, and M. A. Guilbeault. 2008. Plume persistence caused by back diffusion from thin clay layers in a sand aquifer following TCE source-zone hydraulic isolation. Journal of Contaminant Hydrology 102:86-104.
Peralta, R. C. 2011. Simulation/Optimization Modeling for Groundwater and Conjunctive Management. Boca Raton, FL: CRC Press.
Phenrat, T., N. Saleh, K. Sirk, R. D. Tilton, and G. V. Lowry. 2007. Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environmental Science & Technology 41(1):284-290.
Pironi, P., C. Switzer, J. I. Gerhard, G. Rein, and J. L. Torero. 2011. Self-sustaining smoldering combustion for NAPL remediation: Laboratory evaluation of process sensitivity to key parameters. Environmental Science & Technology 45:2980-2986.
Pooley, K. E., M. Blessing, T. C. Schmidt, S. B. Haderlein, K. T. B. MacQuarrie, and H. Prommer. 2009. Aerobic biodegradation of chlorinated ethenes in a fractured bedrock aquifer: Quantitative assessment by compound-specific isotope analysis (CSIA) and reactive transport modeling. Environmental Science & Technology 43(19):7458-7464.
Rabideau, A. J., and C. T. Miller. 1994. 2-Dimensional modeling of aquifer remediation influenced by sorption nonequilibrium and hydraulic conductivity heterogeneity. Water Resources Research 30(5):1457-1470.
Ramos, M. A. V., W. Yan, X. Q. Li, B. E. Koel, and W.-X. Zhang. 2009. Simultaneous oxidation and reduction of arsenic by zero-valent iron nanoparticles: Understanding the significance of the core-shell structure. Journal of Physical Chemistry C 113(33):14591-14594.
Reed, P., B. S. Minsker, and D. Goldberg. 2001. A multiobjective approach to cost effective long-term groundwater monitoring using an elitist nondominated sorted genetic algorithm with historical data. Journal of Hydroinformatics 3(2):71-90.
Reed, P. M., B. S. Minsker, and A. J. Valocchi. 2000. Cost-effective long-term groundwater monitoring design using a genetic algorithm and global mass interpolation. Water Resources Research 36(12):3731-3741.
Rivett, M. O., S. W. Chapman, R. M. Allen-King, S. Feenstra, and J. A. Cherry. 2006. Pump-and treat remediation of chlorinated solvent contamination at a controlled field-experiment site. Environmental Science & Technology 40(21):6770-6781.
Ross, C., L. C. Murdoch, D. L. Freedman, and R. L. Siegrist. 2005. Characteristics of potassium permanganate encapsulated in polymer. Journal of Environmental Engineering 131:1203-1211.
Rügge, K., T. B. Hofstetter, S. B. Haderlein, P. L. Bjerg, S. Knudsen, C. Zraunig, H. Mosbæk, and T. H. Christensen. 1998. Characterization of predominant reductants in an anaerobic leachate-contaminated aquifer by nitroaromatic probe compounds. Environmental Science & Technology 32(1):23-31.
Saenton, S., and T. H. Illangasekare. 2004. Determination of DNAPL entrapment architecture using experimentally validated numerical codes and inverse modeling. Proceedings of XVth Computational Methods in Water Resources 2004 International Conference (ed. C. T. Miller, M. W. Farthing and W. G. Gray), Elsevier.
Sakaki, T., P. E. Schulte, A. Cihan, J. A. Christ, and T. H. Illangasekare. 2013. Airflow pathway development as affected by soil moisture variability in heterogeneous soils. Vadose Zone Journal. In Press.
Sale, T. C., and C. Newell. 2011. Guide for Selecting Remedies for Subsurface Releases of Chlorinated Solvents. ESTCP Project ER-200530. Washington, DC: ESTCP.
Shackelford, C. D., T. C. Sale, and M. R. Liberati. 2005. In-situ remediation of chlorinated solvents using zero valent iron and clay mixtures: A case history. Proceedings of the Sessions of the Geo-Frontiers 2005 Congress. Geotechnical Special Publication. 142:5996.
Sherwood Lollar, B., G. F. Slater, J. M. E. Ahad, B. Sleep, J. Spivak, M. Brennan, and P. MacKenzie. 1999. Contrasting carbon isotope fractionation during biodegradation of trichloroethylene and toluene: Implications for intrinsic biodegradation. Organic Geochemistry 30(8):813-820.
Sherwood Lollar, B., G. F. Slater, B. Sleep, M. Witt, G. M. Klecka, M. Harkness, and J. Spivack. 2001. Stable carbon isotope evidence for intrinsic bioremediation of tetrachloroethene and trichloroethene at Area 6, Dover Air Force Base. Environmental Science & Technology 35(2):261-269.
Siegrist, R. L., M. Crimi, and R. A. Brown. 2011. In situ chemical oxidation: technology description and status. In In Situ Chemical Oxidation for Groundwater Remediation. Siegrist, R. L., Crimi, M., Simpkin, T. J., Eds. New York: Springer.
Simpkin, T. J., and R. D. Norris. 2010. Engineering and implementation challenges for chlorinated solvent remediation. Pp. 109-144 In In Situ Remediation of Chlorinated Solvent Plumes. Stroo, H. F., Ward, C. H., Eds. New York: Springer.
Singh, O. V. 2006. Proteomic and metabolomics: The molecular make-up of toxic aromatic pollutant bioremediation. Proteomics 6:5481-5492.
Sohn, K., S. W. Kang, S. Ahn, M. Woo, and S. K. Yang. 2006. Fe(0) nanoparticles for nitrate reduction: Stability, reactivity, and transformation. Environmental Science & Technology 40(17):5514-5519.
Song, D. L., M. E. Conrad, K. S. Sorenson, and L. Alvarez-Cohen. 2002. Stable carbon isotope fractionation during enhanced in situ bioremediation of trichloroethene. Environmental Science & Technology 36(10):2262-2268.
Song, H., and E. R. Carraway. 2005. Reduction of chlorinated ethanes by nanosized zero-valent iron: Kinetics, pathways, and effects of reaction conditions. Environmental Science & Technology 39:6237-6245.
Song, H., and E. R. Carraway. 2006. Reduction of chlorinated methanes by nanosized zero-valent iron: Kinetics, pathways, and effect of reaction conditions. Environmental Engineering Science 23:272-284.
Song, H., and E. R. Carraway. 2008. Catalytic hydrodechlorination of chlorinated ethenes by nanoscale zero-valent iron. Applied Catalysis B: Environmental. 78:53-60.
Switzer, C., P. Pironi, J. I. Gerhard, G. Rein, and J. L. Torero. 2009. Self-sustaining smoldering combustion: A novel remediation process for non-aqueous phase liquids in porous media. Environmental Science & Technology 43:5871-5877.
Taghavy, A., J. Costanza, K. D. Pennell, and L. M. Abriola. 2010. Effectivness of nanoscale zero-valent iron for treatment of a PCE-DNAPL source zone. Journal of Contaminant Hydrology 118:128-142.
Tebo, B. M., J. R. Bargar, B. G. Clement, G. J. Dick, K. J. Murray, D. Parker, R. Verity, and S. M. Webb. 2004. Biogenic manganese oxides: Properties and mechanisms of formation. Annual Review of Earth and Planetary Sciences 32:287-328.
Thullner, M., M. Kampara, H. H, Richnow, H. Harms, and L. Y. Wick. 2008. Impact of bioavailability restrictions on microbially induced stable isotope fractionation. 1. Theoretical calculation. Environmental Science & Technology 42(17):6544-6551.
Vera, Y. M., R. J. de Carvalho, M. L. Torem, and B. A. Calfa. 2009. Atrazine degradation by in situ electrochemically generated ozone. Chemical Engineering Journal 155(3):691-697.
Waddill, D. W., and M. A. Widdowson. 2003. SEAM3D: A numerical model for three-dimensional solute transport and sequential electron acceptor-based biodegradation in groundwater. ERDC/EL TR-00-18. Vicksburg, MS: U.S. Army Research and Development Center.
Waldron, P. J., L. Wu, J. D. Van Nostrand, C. W. Schadt, Z. He, D. B. Watson, P. M. Jardine, A. V. Palumbo, T. C. Hazen, and J. Zhou. 2009. Functional gene array-based analysis of microbial community structure in groundwaters with a gradient of contaminant levels. Environmental Science & Technology 43(10):3529-3534.
Wang, C. B., and W.-X. Zhang. 1997. Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environmental Science & Technology 31(7):2154-2156.
Wani, A. H., B. R. O’Neal, D. M. Gilbert, D. B. Gent, and J. L. Davis. 2005. Electrolytic Transformation of Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) and 2,4,6-Trinitrotoluene (TNT) in Aqueous Solutions. U.S. Army Corps of Engineers. ERDC/EL TR-05-10. Washington, DC.
Wani, A. H., B. R. O’Neal, D. M. Gilbert, G. B. Gent, and J. L. Davis. 2006. Electrolytic transformation of ordnance related compounds in groundwater: Laboratory mass balance studies. Chemosphere 62:689-698.
Welch, R., and R. G. Riefler. 2008. Estimating treatment capacity of nanoscale zero-valent iron reducing 2,4,6-trinitrotoluene. Environmental Engineering Science 25(9):1255-1262.
Werner, J. J., A. C. Ptak, B. G. Rahm, S. Zhang, and R. E. Richardon. 2009. Absolute quantification of Dehalococcoides proteins: Enzyme bioindicators of chlorinated ethene dehalorespiration. Environmental Microbiology 11:2687-2697.
Werner-Allen, G., K. Lorincz, M. Welsh, O. Marcillo, J. Johnson, M. Ruiz, and J. Lees. 2006. Deploying a wireless sensor network on an active volcano. IEEE Internet Computing 10(2):18-25.
Wiesner, M. R., G. V. Lowry, P. Alvarez, D. Dionysiou, and P. Biswas. 2006. Assessing the risks of manufactured nanomaterials. Environmental Science & Technology 40(14):4336-4345.
Wilkins, M. J., N. C. VerBerkmoes, K. H. Williams, S. J. Callister, P. J. Mouser, H. Elifantz, A. L. N’Guessan, B. C. Thomas, C. D. Nicora, M. B. Shah, P. Abraham, M. S. Lipton, D. R. Lovley, R. L. Hettich, P. E. Long, and J. F. Banfield. 2009. Proteogenomic monitoring of Geobacter physiology during stimulated uranium bioremediation. Applied and Environmental Microbiology 75(20):6591-6599.
Wilson, J. L. 1997. Removal of aqueous phase dissolved contamination: Non-chemically-enhanced pump and treat. In Subsurface Remediation Handbook. Ward, C. H., Cherry, J., Scalf, M., eds. Chelsea, MI: Ann Arbor Press.
Xu, Y., and D. Zhao. 2007. Reductive immobilization of chromate in water and soil using stabilized iron nanoparticles. Water Research 41(10):2101-2108.
Zheng, C., and P. P. Wang. 1999. MT3DMS, A Modular Three-Dimensional Multi-Species Transport Model for Simulation of Advection, Dispersion and Chemical Reactions of Contaminants in Groundwater Systems: Documentation and User’s Guide. SERDP-99-1. Vicksburg, MS: U.S. Army Corps of Engineers Engineer Research and Development Center.
This page intentionally left blank.










































