3
Fuels and Chemicals from Biomass via Biological Routes
“We have to drive a substantial amount of cost out of both operating and capital costs in order to make this work.”
Chris Somerville
“It should be hard to displace a mature trillion dollar industry.”
Chris Somerville
Using biological systems to convert biomass into fuels and chemicals is already feasible, but the costs of doing so make the resulting products uncompetitive economically at present with those produced from petroleum. According to Chris Somerville, professor of alternative energy and director of the Energy Biosciences Institute at the University of California in Berkeley, currently, biological conversion processes are done in batch mode, which has a substantial impact on capital costs and throughput. Successfully developing continuous flow processes could have a marked positive effect on the economic competitiveness of biomass-derived fuels and chemicals. Creating such processes, however, will require significant advances in pretreatment and separations technologies, and realizing those advances requires recruiting chemists and chemical engineers to attack these problems.
BIOLOGICAL ROUTES TO FUELS AND CHEMICALS
The biological conversion of cellulosic biomass into fuels and chemicals should be straightforward, said Chris Somerville, but as companies have begun building commercial-scale bioconversion facilities it has become clear that putting theory into practice is more challenging than expected. One challenge is dealing with the complexity of the overall process of converting lignocellulosic biomass into ethanol. Using the National Renewable Energy Laboratory (NREL) Aspen model for bioconversion as an example, he noted that there are 21 unit processes that require engineering solutions and equipment. As a result, the capital recovery costs for a bioconversion plant are substantial, totaling about $1 per gallon of ethanol.
A closer look at these costs reveals some surprises, said Somerville. Lignin drying boilers and wastewater treatment account for 55 percent of the capital costs. A 50-million-gallon facility built using the NREL process could produce up to one billion gallons a year of wastewater that contains as much as 2 percent solids, necessitating the construction of a large wastewater treatment facility. Boiler costs are so high because it must handle solids, and constructing a solids boiler for a 50-million-gallon facility makes little sense economically. In fact, it might be more efficient to eliminate the boiler, pelletize the lignin and other solids, and sell them to a coal power plant. Figure 3-1 shows the contribution to the overall cost by process area and capital, operations, and fixed costs.
Turning to process costs, feedstock costs are reasonable, about $0.74 per gallon, but pretreatment, hydrolysis, and cellulase enzyme total about $0.83 per gallon, with total operating costs of about $2.15 per gallon of ethanol. Somerville said that given that ethanol has two-thirds the energy density of gasoline, this scheme does not work economically. A substantial amount of cost must be driven out of both operating and capital in order to make this work, and the first step that is needed to do that, he said, is to realize that all of the existing processes are fundamentally batch processes. Though he admitted that this is a radical view, he believes that ethanol production facilities need to follow in the footsteps of petrochemical plants and turn to continuous processes, which the chemical industry has shown is the only way to operate economically at commercial scale.
Turning Batch Processes into a Continuous Process
Batch processes, he explained, are inherently inefficient because they never operate at an optimal place in terms of sugar concentration, lignin inhibition, or microorganism
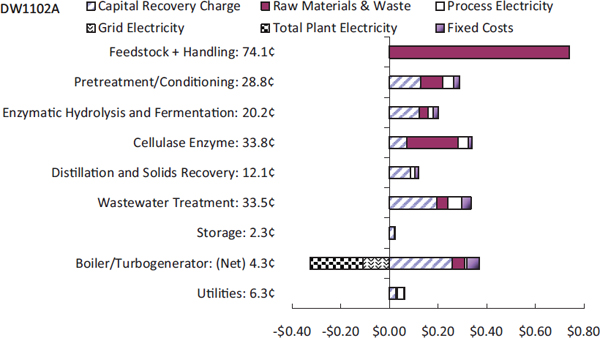
FIGURE 3-1 Contribution to minimum selling price of corn-derived ethanol from each process area.
SOURCE: Humbird et al. (2011). NREL/TP-5100-47764 (Somerville presentation).
performance. Batch processes produce dilute fuel that needs to be concentrated, catalysts and microorganisms are basically burned after each batch is processed, and wastewater generation is substantial. In addition, the entire process is shut down after each batch is completed, during which time capital equipment sits unused.
In the ideal process scheme, sugars are loaded into the fermentation tanks at an optimal rate and fuel is removed continuously. One scheme for reaching this optimum is to use a plugged reactor concept based on the continuous removal of fuel (see Figure 3-2). This concept relies on liquid/liquid extraction or an ethanol-selective membrane to achieve fuel separation under continuous operating conditions, and depending on the concentration of the sugar input, there will be little or no waste water to handle. Developing liquid/liquid extraction processes and new ethanol-selective membranes are areas in which the chemical sciences can make substantial contributions.
In a hypothetical continuous process that Somerville discussed, biomass would be ground and fed into a lignin removal process that feeds polysaccharides into a polysaccharide depolymerization process using enzymes or chemical catalysts that can be recycled. He made note of ongoing research that has produced heterogeneous platinum on carbon catalysts with extremely high activities. A concentrated sugar solution would then be fed in a fermentation reactor, with fuel separation and volume adjustment being performed continuously. And while there are challenges remaining to optimize this process, the only real stumbling block today is implementing lignin removal at the beginning of the process in a way that minimizes polysaccharide loss and recycles the lignin solved at very high efficiency.
In reviewing some of the approaches being explored today, Somerville said that each has limitations. Acid pretreatment is inexpensive, but it removes valuable hemicelluloses as well as lignin and it also produces some downstream inhibition. Various ionic liquids will dissolve cellulose and also separate polysaccharide-lignin mixtures, but ionic solvents are currently too expensive and would require recycling efficiencies of 99.999 percent to work economically. A more promising approach, one that needs the attention of the chemical sciences, is to develop catalysts that will partially depolymerize lignin. He noted that there have been some successes reported with model systems, but that this is still an area of research that is underexplored. Studies using supercritical water also look promising, though the engineering challenges of working at the required high pressures are substantial.
Studies on continuous pretreatment technologies have examined weak acid/high temperature and strong acid/low temperature combinations, as well as the use of aqueous bases, with or without ammonia and with or without added oxidants. So far, the strong acid/low temperature approach appears to be the most promising, and it produces a very pure sugar solution after a solid/liquid separation step. Both hydrochloric acid and the extraction solvent are recycled efficiently. Somerville said a major issue is building durable and safe process equipment that works with strong, concentrated acids such as 40 percent hydrochloric acid.
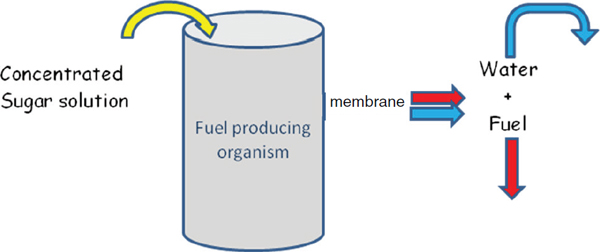
FIGURE 3-2 Plugged reactor concept for continuous fuel production.
SOURCE: Image taken from Somerville presentation.
In concluding his talk, Somerville noted two other challenges that require the attention of chemists and chemical engineers. The first is producing optimized depolymerization catalysts, whether they are enzymes or chemical catalysts, in very large quantities at an affordable price. The second is to develop large-scale anaerobic processes that produce gasoline and diesel fuel from sugars.
Discussion
In response to a question about how much progress was being made on developing a continuous fermentation process, Somerville said that Yong-Su Jin at the University of Illinois would be publishing the results of a study demonstrating efficient continuous fermentation of C12 sugars rather than C6 sugars. Tom Richard asked Somerville to discuss some of his work on developing new enzymes of pretreatment, and Somerville noted that his group has identified enzymes from cow rumen that operate efficiently at high temperature, but that the major limitation in developing those enzymes for industrial use today is the inability to engineer their favorite production hosts to produce them in the necessary quantities. He remarked that this is a fundamental piece of science that needs the attention of the research community.
Somerville then expanded on the need to develop ways of converting sugars into fuels. In his opinion, the most promising long-term strategy is to make short-chain alcohols and then use long-established chemistry to produce mixtures of the longer-chain isoalkanes that are used in jet fuel and diesel. He noted that chemists at his institute are exploring old chemistries that had largely been forgotten because they produce mixtures, but since fuels are mixtures of products, those chemistries may actually be useful today. Getting chemists to change their way of thinking to appreciate reactions that produce mixtures was a real challenge because that idea goes against the paradigm of modern chemical science.
This breakout session was led by Leonard Katz, associate professor at the University of California, Berkeley, and a member of the scientific advisory board of Lygos. The discussion about biological technologies for biomass conversion focused on whether the biological conversion of biomass into chemical and fuel should be conducted in an integrated biorefinery or whether it should be broken into components. The argument was made that preparing biomass for processing should be one component, that deconstruction to produce sugars would be a second component, and that conversion of sugar into fuel or chemicals would be a third process. Developing these three processes together into an integrated biorefinery was considered by many breakout group members to be too risky at this stage of technology and capacity development. However, the discussion also raised the possibility that it might be economically and technologically attractive to combine biomass preprocessing and deconstruction into an integrated process or to combine deconstruction and fermentation in an integrated process. Many breakout group members said that determining the optimal configuration is an area that needs further study and analysis.
One idea that was raised during the discussion was that there may be a market for lignin as a carbon-neutral source of energy and that there may be advantages to removing lignin from biomass at local facilities close to the biomass source. Such an approach might improve the economics of biomass
conversion by reducing the amount of material that would need to be transported to larger processing facilities for conversion to sugars while also providing an energy source to support local industry.
The major technological and commercial barriers to scaling up sustainable technologies involve moving from batch processing to continuous processing, at least up to the stage of sugar production. It was noted by several breakout group members that batch processing of biomass to produce sugar on a scale needed to produce 21 billion gallons of ethanol a year by 2022 was untenable economically. Producing that much ethanol in batch fermentation plants would require 525 40-million-gallon plants at $40 million apiece.
Most breakout group members believe that fuel production and chemical production should be considered separate pathways, much like they are in the petrochemical industry. While many breakout group members agreed that fuel production from sugars via fermentation should be done through a continuous process to be truly economical and scalable, production of most chemicals is best done in batch mode, at least based on the extensive experience of the chemical industry. It was noted during the discussion from a member of the group that 90 percent of organic chemicals used today are made in batch operations. This percentage reflects the production of the many hundreds of specialty chemicals which are done in relatively small batches, whereas the top 100 commodity chemicals, which represent by mass the bulk of synthesized organic chemicals, are produced in continuous-flow batches. The chemical industry is already exploring the production of chemicals from biomass independent of fuel production.
The breakout group discussed the need to solve technological issues involving economical production of enzymes to meet a variety of demands. Many members of the group concurred that technology development was needed to design enzymes with higher activities, that could pretreat biomass prior to sugar production, that would resist inhibition by lignin or organic acids, and that will function in alternative environments, such as in ionic solvents or under pressure. There was substantial discussion, with no consensus, about whether it was better to build better and less expensive enzymes or to engineer microorganisms to that can perform multiple steps in the conversion process. The suggestion was made that the biomass field could learn from the pharmaceutical industry, which makes extensive use of secondary metabolism by engineered microorganisms to produce high-value products. The breakout group also noted the need to develop methods for conducting large-scale anaerobic fermentation to achieve more efficient conversion of sugars to product.
Addressing the issue of needed skills, the breakout group concurred that fundamental process engineering is “a dead field” that attracts little interest among researchers and few funding opportunities, but that this field should be reinvigorated if technological barriers are to be addressed. What little process engineering research does occur is largely conducted overseas. The same appears to be true for separations technology and surface chemistry, and the breakout group agreed that chemists need to receive better training in these key technological fields. Some members of the group highlighted the need for universities to establish biofuels courses which has already been done at the University of California, Berkeley. Some group members noted the need for biochemists to receive more training in enzymology, a field that once flourished.
According to Katz, members of the group said chemical engineers also need a new set of skills to contribute to the development of a biomass-based industry. Chemical engineers today receive very little training in batch processing or in the design and operation of continuous enzymatic processes. Both of these deficits need to be addressed immediately, according to the breakout group members, Katz said.
Turning to issues of transportation infrastructure, the individual breakout group members concurred that the costs of biomass collection and transportation needed to be addressed if biomass is to make a significant contribution to the production of fuel and chemicals. The members of the group said that the biomass industry will have to depend on the existing transportation infrastructure given the huge expense of creating a new one. It was noted during the breakout group discussion that $4 billion was invested in an ethanol pipeline system that is only at 25 percent of capacity now.
One idea from the group was that it may be necessary to subsidize stover collection by secondary harvesting services to meet supply considerations if the market develops for the products of biomass conversion given that there is little economic incentive today for farmers to collect stover. The group also noted that storage of corn stover or corn cobs, which could also be a good source of biomass, is expensive and is actually a significant economic barrier that needs to be addressed. The breakout group raised the idea of developing a slurry-based pipeline system for biomass or a system for converting biomass into pellets for easier transport.
The breakout group concluded its discussions with a comment about the idea of converting biomass to so-called drop-in fuels versus expanding the amount of ethanol produced. The group said that it is hard to compete with ethanol in terms of net energy return from sugar because ethanol’s high oxygen, low carbon content closely matches that of sugars. For advanced biofuels based on hydrocarbons, fatty acids produced by algae or oil crops are likely to be the better feedstock.




